Learning objectives.
By reading this article, you should be able to:
-
•
Describe the mechanisms of radiofrequency (RF) lesioning for treating chronic pain.
-
•
Discuss the aspects of RF procedures that may affect outcome.
-
•
List the indications for RF procedures and potential risks.
-
•
Outline the key steps in RF lumbar medial branch denervation.
Key points.
-
•
Continuous radiofrequency (RF) uses RF alternating current to lesion target nerves by causing thermal lesions.
-
•
Radiofrequency denervation should be considered after unsuccessful conservative management.
-
•
Guidelines mandate careful selection of patients and use of diagnostic blocks before denervation.
-
•
Meticulous lesioning techniques are also key to success.
Radiofrequency (RF) techniques in chronic pain management are used to modulate sensory nerve transmission usually by producing thermal lesions. This article describes the common types of RF in clinical use for chronic pain management, the physics and technical aspects of the procedure and specific patient considerations and risks. Its role and evidence in the management of facetogenic lower back pain are discussed in further detail, as this is arguably the most studied RF technique.
In medical applications, RF refers to the use of medium or higher alternating electrical current frequencies, usually 500 kHz.1 Radiofrequency is a term used to describe the rate of oscillation of an alternating electrical current. The frequency range is the same as that in AM radio waves, part of the electromagnetic spectrum but is otherwise unrelated.
A circuit is formed from an RF generator to the patient, through monopolar or bipolar electrodes, with current returning to the RF generator. Alternating current within the RF spectrum passes between electrodes. For comparison, surgical diathermy also uses electrical current but with short-wave (0.5–1 MHz) current frequencies that generate heat via induction of electromagnetic fields.
In the 1960s, RF for pain management was originally developed for cervical cordotomy techniques. The 1970s saw its development for the treatment of trigeminal neuralgia, by lesioning the gasserian ganglion, and for axial spine pain targeting a variety of sites.2
Modern use of RF in chronic pain conditions is generally agreed to be suitable for consideration only after conservative approaches have failed. Consensus guidelines regarding lumbar facetogenic pain recommend starting with conservative treatments as a core principle of good medical practice, even if minimally invasive RF techniques may be more effective.3
Principles of RF denervation
Targeted application of RF energy to biological tissues results in heating and ultimately damage through protein denaturation, depending on the temperature reached and the duration the energy is applied for.2 The aim is to lesion a targeted nerve that innervates a structure believed to be contributing to pain. However, the lesion is not selective to specific tissue types, so precision is needed with RF needle placement. The mechanism uses continuous application of RF between electrodes via the patient's tissues. It is used therapeutically in interventional pain techniques to interrupt or modulate pain transmission by forming lesions on target nerves. This procedure is termed RF denervation or neurotomy, and it is also alternatively known as conventional RF.
Pulsed RF treatment
Pulsed RF (PRF) treatment was introduced in 1996 for treatment of radicular pain by application of current to lumbar dorsal root ganglia (DRG).4 Radiofrequency current is applied to tissues in short cycles, allowing any heat generated to dissipate and no nerve damage to occur, potentially giving a better risk profile than conventional denervation.5
Physics and equipment
Monopolar circuits comprise the active or lesioning electrode (also known as the ablation electrode), inserted within a cannula (Fig. 1) once positioned at the target. Radiofrequency energy is passed between this lesioning electrode and a dispersive electrode on the skin, also known as the grounding plate, through the patient's tissues. These electrodes, therefore, alternate as cathode and anode.
Fig 1.
Radiofrequency equipment. (a) Cannulae, straight and curved tips. (b) Electrode. (c) Simplicity bipolar cannula. (d) Trident multitined cannula. (a–c) Reproduced with permission from Abbott. (d) Reproduced with permission from Diros Technology.
The dispersive electrode is sited far enough away from the ablation electrode that peak current density across it will not cause significant heating. Practically, the common positioning sites for the ground electrode in interventional pain procedures are sufficient that thermal burns risk is negligible and no significant influence on the ablation electrode heating pattern is seen.6
Lesioning
Monopolar denervation
Radiofrequency current is applied continuously at 500 kHz to reach a target temperature of 80–90°C for 60–90 s. The ablation electrode has an integrated thermocouple (Fig. 2). This standard programme is usually repeated after small adjustments of the cannula position (e.g. multiple parallel lesions and 180° rotation of the curved needle tip) to maximise the chances of lesioning the nerve and to increase lesion volume.
Fig 2.
RF electrodes. Section through uninsulated tip. Inner RF probe with integrated thermocouple (black segment). Outer metal shell of the electrode. (a) Conventional and (b) cooled. Arrows demonstrating movement of cooled water around RF probe. Configuration otherwise the same as for conventional probes.
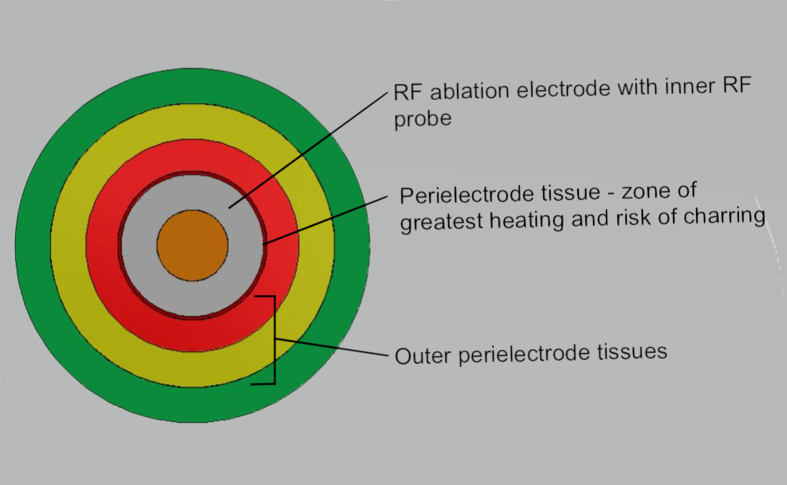
Current is carried via electrons in the RF circuit, generating an electric field around the electrode tip. Current flow through the patient's tissues is by ionic flow. The alternating RF current produces ion oscillation in the perielectrode tissues, with the resulting friction generating heat.
Impedance is defined as the resistance of a circuit to alternating or direct current. When tissues have sufficient impedance and the current density is adequate, thermal heating occurs. The resulting thermal damage is thought the primary mechanism for the therapeutic effect of destructive RF interventions.6
Critical lesioning temperature (CLT) is that necessarily to induce nerve damage to see clinical effect, usually permanent cessation of biological function. For an in-depth explanation, see Ball.6 The target 80°C for standard RF programmes was informed mostly from in vitro studies.7
Nerve damage occurs at 45°C. However, the time a specific temperature is maintained to create a lesion is also crucial and is displayed as a time temperature curve. So, a prolonged time at a lower temperature will achieve the same lesion as that performed for a few seconds at higher temperatures.6
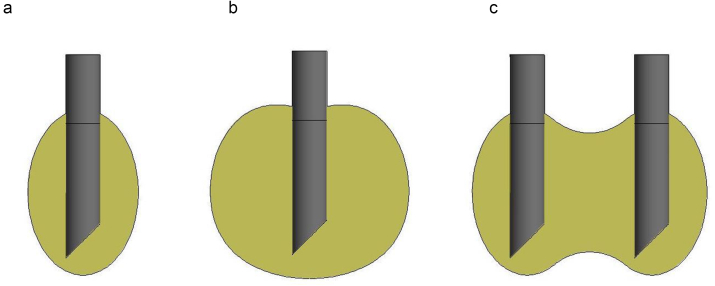
Tissue heating is greatest in the zone immediately adjacent to the electrode (Fig. S1). Intense heating causes desiccation and subsequent charring. Charred tissues have higher impedance, and thus limit heat spread, lesion size and shape producing non-uniform lesions. Typical RF programmes, therefore, attenuate this risk by slowing the rate of temperature rise to the target. Heating occurs around the electrode more proximally, producing a more ovoid lesion after 60–90 s (Fig. 3). Classically ovoid-shaped lesions are produced in homogeneous tissue, so a parallel approach, very close to the nerve, is warranted to maximise likelihood of capturing the target nerve.
Fig 3.
Lesion shapes. Diagrammatic representation of characteristic shapes with RF types. Lesion volume and morphology dependent on multiple variables. Not to scale. (a) Monopolar, (b) cooled and (c) bipolar. Bipolar lesions will vary with distance between electrodes and will result in two separate lesions if too far.
Impedance in biological tissue is generally low in fluids, blood and CSF, and higher in denser tissues such as spinal cord and bone. Impedance has an inverse relationship with lesion volume, so non-uniform density tissues, such as scar tissue, can result in irregular ablation patterns.6 Nearby large blood vessels may affect lesion volume and shape. Current flows preferentially down the path of least resistance so will be shunted away from areas of higher impedance. However, low impedance is not necessarily better. If the electrode is within a large volume of low-impedance tissue or fluid, current density will be lower as energy dissipates rapidly, and thus, a smaller area may only reach the CLT. It is important to note that the metal of the ablation electrode has low intrinsic impedance so will not heat directly, but it is warmed through conduction from perielectrode tissue heating.5,6 Heating of perielectrode fluid results in convection currents. Injection of a fluid before lesioning, usually local anaesthetic for the additional purpose of pre-emptive analgesia, is known to increase lesion volume via convection.6 Convection currents can shunt heat generated against gravity, so cannula angulation may also influence lesion shape and volume.
Current density is proportional to the surface area of the electrode. Given the cylindrical shape of electrodes, those with twice the diameter result in a doubling of the surface area so heating exponentially increases. Other explanations for a larger lesion volume achieved with large-gauge cannulae include tissue compression and displacement.6 Studies in animal models have demonstrated that continuous RF to DRG at 80°C results in Wallerian degeneration.8 Other explanations for its effect include damage to the vasa nervosum, Schwann cells or causing dysfunctional remyelination.6 Cell bodies are not targeted, so although denervation occurs, all nerves will regenerate with time. The factors influencing lesioning are summarised in Table 1.
Table 1.
Factors influencing lesioning. LA, local anaesthetic; RF, radiofrequency; US, ultrasound.
| Implications | ||
|---|---|---|
| Patient | ||
| Nerve course variations | Nerves opaque on fluoroscopy; US may be possible to aid identification at some sites; consider the following, as these may improve likelihood of capturing nerve target, further RF cycles after needle position adjustment (e.g. rotation of curved tip, approach target with tip parallel and cooled RF probe to increase lesion size) | |
| Perielectrode tissue | Irregularity, scar tissue and vasculature | Inverse relationship between impedance and lesion volume; irregular tissue; current will flow preferentially down the path of least resistance, shunting current and thus heat away from higher-impedance tissue |
| Equipment | ||
| Cannula gauge | Doubling of cannula gauge=surface area doubles and exponential increase in heating; current density is proportional to electrode surface area; larger gauge cannula may also help compress tissues and increase lesion volume | |
| Tip shape | Curved vs straight | Theoretically may mirror the path of the nerve and increase the likelihood of capturing the target |
| Circuit configuration | Monopolar | Ovoid-shaped lesions |
| Bipolar | Current over smaller area, possibly greater precision and less risk to adjacent structures | |
| Cooled | Reduces charring of peri-electrode tissue forming more spherical lesions in vitro | |
| Procedure | ||
| Target temperature | Critical lesioning temperature that is needed to induce nerve damage; 80°C standard target to cause Wallerian degeneration | |
| Rate of heat rise | Slower rate of increase to target temperature to minimise charring, which limits lesion size | |
| Cannula angle to target | As more ovoid shapes are produced with monopolar circuits, cannula tips should be aligned parallel to the course of the nerve | |
| Gravity | Convection currents generated with heating perielectrode fluid shunt heat against gravity; consider cannula angle to target | |
| Fluid injection before lesioning (e.g. LA and saline) | Convection currents in fluid can shunt heat; evidence supports increased lesion volume |
Bipolar denervation
Bipolar RF uses individual cathode and anode electrodes spatially placed close together with continuous RF current. The configuration of electrodes can vary depending on the therapeutic site, for example, they can be in series along the length of the same cannula, such as the Simplicity cannulae (Abbott Technologies, Chicago, IL, USA), developed for denervation of the lateral branches in management of sacroiliac joint (SIJ) dysfunction Click or tap here to enter text. (Fig. 1).9 The Simplicity probe has three electrodes so can produce two bipolar and three monopolar lesions.10 Bipolar RF can also be performed using palisade techniques, where the two electrodes can be moved between multiple parallel cannulae at different levels, again for lateral branch denervation to manage SIJ pain.
Radiofrequency current is therefore passed over a smaller area than with classical monopolar RF circuits using ablation and dispersive electrodes. Bipolar potentially allows for greater precision applying RF to specific targets, which may reduce risk of damage to adjacent structures because of the shorter current path through tissues. It can also be used to extend volume of lesioning producing strip lesions (Fig. 3).
Cooled RF denervation
Conventional RF techniques produce lesions that classically extend along the length of the uninsulated cannula tip, with little extending distally because of the charring of perielectrode tissue. Cooling the probe reduces the temperature at the tissue-tip interface to prevent charring and allows energy to spread more distally from the uninsulated tip in a controlled manner. This results in more uniform spherical and larger-volume lesions even in non-homogeneous target tissues (Fig. 3). This implies nerve targets can be approached in a more conventional perpendicular direction with cooled RF potentially simplifying access to deeper nerve targets or those with variable courses, such as the articular branches to the hip and knee.7
Cooled RF probes have a water current within the tip to cool the electrode (Fig. 2). These can be used via a mono- or bipolar circuit. The integrated thermistor serves the purpose to check that the cooling system is operational. Thus, the temperature measured via this thermistor should not rise significantly during lesioning. Radiofrequency programmes generally set the temperature to 60°C with time settings similar to standard conventional denervation RF programmes.7,11
Pulsed RF
Standard PRF generator programmes are usually 90 s duration, applying 500 kHz current for 20 ms with 480 ms pauses.12 Click or tap here to enter text. The equipment and cannulae are the same as for monopolar RF. The active electrode temperature is limited to 42°C, monitored via the integrated thermocouple. The RF generator automatically adjusts the voltage output and current frequency to ensure this temperature target is not breached. Target sites are treated with one to three cycles of this programme. Because of the short duration of current application, a relatively large voltage (45 V) can be applied without tissue damage. Electrodes are positioned perpendicular to the target nerve, as the electric field density is highest at the RF probe tip.5
The possible therapeutic effect of PRF is thought to be stimulated by the intermittent electric field with postulated effects on enhanced inhibition pathways, such as the opioid and descending noradrenaline (norepinephrine) and serotonin systems within the spinal cord.12 Lower electrical field strengths are thought to stimulate conditioning in target nerves with long-term depression of synaptic transmission in C-fibres.4 There is evidence that c-fos, a marker of neuronal activation in cell cultures exposed to electrical fields, is upregulated, postulated to be a sign of activation of some pain-inhibition mechanism.13 However, others have argued that this is not specific to either excitatory or inhibitory pathways.5 Electric fields induce transmembrane potentials. Transmembrane potentials can cause tissue disruption through electroporation (pore formation), and thus potentially trigger cell rupture.4 A further possible mechanism is through potential thermal damage. Although PRF programmes limit probe temperature to 42°C, in vitro studies have shown spikes above the 45°C threshold at which nerve damage can occur.4
Procedures
The following generic guide for RF procedures applies regardless of RF type, bipolar, cooled or pulsed. The Spine Intervention Society (SIS) suggests that i.v. access is established for procedures near the epidural space where there is a risk drugs may reach the cerebrospinal fluid.2 Consensus guidelines for lumbar medial branch denervation procedures mandate i.v. access.14 This has not gained unanimous agreement because of the variety of RF procedure sites, differences in drugs given with comparatively small doses of local anaesthetic agents and the use of contrast that minimises the risk of unrecognised dural puncture.
Routine sedation is not recommended by the SIS on the basis that patients are always sufficiently alert to enable monitoring, and thus guard against adverse events.2 Minimum monitoring standards for local and regional anaesthesia by an anaesthetist for operative procedures are stipulated by the Association of Anaesthetists as the same as that for procedures under sedation (to include HR, BP, ECG and peripheral oxygen saturations, with end-tidal CO2 monitoring if sedation is used).15 What constitutes an operative procedure is not defined, and thus, more peripheral sites of RF interventions representing lower risks of adverse events may arguably not warrant this level of monitoring. In the SIS guidelines, monitoring is only recommended when sedation is used.2
After usual attention to asepsis, a 16- to 22-gauge cannula is navigated to the target under fluoroscopic control, to align the tip parallel to the nerve, in the case of conventional RF for denervation procedures. The ‘gold standard’ technique is to use fluoroscopy to position the cannula, identify bony landmarks, position the cannula parallel to target nerves and enable the use of contrast. However, individual anatomy varies (e.g. variations in medial branch nerve length and distance from bony landmarks in the lumbar region) and ultrasound may be an effective alternative or adjunct to help avoid vascular structures and reduce radiation exposure.11,16,17
Cannulae are commonly Teflon coated, with an uninsulated curved or straight 2.5–10 mm Quincke tip (Fig. 1). The stylet is removed from the cannula once in the approximate target position and the RF ablation electrode inserted. A curved cannula tip theoretically helps maximise electrode proximity when aligning the electrode parallel to the nerve path. However, multiple lesions have not translated into improved outcomes.17 Multi-tined needles, such as the Trident cannula (Diros Technology, Markham, ON, Canada), have also been developed (Fig. 1) with the aim of maximising success in capturing the sensory nerve target and performing a complete lesion.
Radiofrequency generators include a nerve stimulation function. Sensory stimulation theoretically helps adjust electrode position to align with the target nerve as close as practically possible. Motor stimulation is performed at around 1–2 V at 2 Hz once the cannula is at its target, to ensure any motor roots are sufficiently far away to reduce risk of loss of motor function. Sensory stimulation is performed around 0.4–0.6 V at 50 Hz looking for paraesthesia, pain or vibration in the target nerve distribution. The interpretation of these effects can be ambiguous and maybe a result of direct muscle stimulation. The influence of stimulation on outcomes is not validated.6 Adequate sensory stimulation may be experienced regardless of whether the needle tip is parallel to the target or perpendicular, implying suboptimal positioning for denervation techniques.
Some authors recommend the routine use of stimulation for medial branch denervation, whereas others recommend it for a single-level denervation but not for multiple levels.3,14 There is consensus that motor stimulation should always be performed during RF denervation of the posterior rami medial branches for facetogenic pain, to avoid lesioning the anterior motor root.14
Local anaesthetic is used for infiltration of the skin and targeted area just before lesioning.14 This is to cover pain from the increase in temperature with denervation techniques and also used to increase lesion volume via convection spreading the zone of heating. Multiple RF lesions are performed by some practitioners to minimise the risk of missing the targeted nerve as a result of variable nerve paths.5 This is done by repeated cycles of RF programmes with cannula position adjustments, including rotation of curved cannulae if used. Further detail, such as RF generator programming, depends on the target nerve and type of RF used, so denervation of the L5 posterior rami is given as a specific example.
Denervation of L5 posterior rami
This technique was agreed unanimously when forming a consensus on current best-practice technique for RF denervation treating lumbar facet joint pain amongst a mostly UK faculty.3 The following 10-step technique is taken from that consensus document with illustrative images available (Supplementary Fig. S2).
-
(i)
Identify the L5–S1 disc interspace.
-
(ii)
Identify the L5 vertebral body.
-
(iii)
Rotate the image intensifier laterally to visualise the bony curvature between the transverse and articular processes (Supplementary Fig. S2A). Occasionally, the curvature is visible in the anteroposterior view, and no lateral rotation is necessary.
-
(iv)
Once the curvature is identified, tilt the image intensifier inferiorly, keeping the curvature clearly in view.
-
(v)
Tilt the image intensifier so as to view curvature as medially and inferiorly as possible.
-
(vi)
Once the aforementioned view is achieved, local anaesthetic is infiltrated at the skin entry point. This is in a line directly below curvature at lower border of transverse process of the same level.
-
(vii)
Aim to contact bone below curvature.
-
(viii)
Advance your RF cannula in a posterolateral view needle at 4 o'clock or 8 o'clock depending on right or left side. The target is the middle two-fourths of the lateral aspect of the articular process, aiming to contact the medial branch before it courses under the mamillo-accessory ligament in a trajectory parallel to the nerve (Supplementary Fig. S2B).
-
(ix)
Once the RF cannula is in position, use the tunnel view to confirm needle in curvature on bone.
-
(x)
Use the superior (cranial tilt) view with needle at 6 o'clock to assess depth of the cannula.
Fluoroscopy needle position should be confirmed in a minimum of two planes (e.g. anteroposterior [Supplementary Fig. S2C] and lateral [Supplementary Fig. S2D]). All procedure images in different image intensifier planes should be saved to the patient's record to ensure and document that needle tips are in contact with bone and not protruding into soft tissue to risk lesioning an unintended target. Lateral views are preferred by the authors to check depth of insertion, ensuring needle tips are clear of the foramina and thus DRG.18
Considerations in specific patients
Implanted devices
As for electrosurgery, pacemakers should be converted to a fixed rate for the duration of procedure, with interrogation checks and resumption of their usual programme after the procedure. Pacemakers present a particular issue, and guidance recommends consultation with the cardiology or pacing team; sometimes, the device is converted to a fixed rate for the duration of the procedure.17 Spinal cord stimulators should be switched off in close communication with the neuromodulation service. The ground plate must be placed away from the implanted pulse generator/pacemaker site.
Anticoagulation
Patients should be considered with similar precautions as for other needling techniques for pain, depending on the procedure site planned and an individual risk assessment. The SIS guidelines quote evidence for interventional spine procedures demonstrating no additional risk of haemorrhagic complications in patients continuing or stopping anticoagulants.2 They recommend that anticoagulation can be continued for extraspinal procedures, such as lumbar medial branch RF denervation. They cite key differences to anaesthesia spinal techniques, such as use of contrast with fluoroscopy to identify vascular puncture, smaller gauge needles and the rare use of catheters and prolonged infusions to justify this stance.
Anatomical variations and previous surgery
For patients with significant anatomical variations, such as scoliosis, and previous spinal surgery, including instrumented spinal fixation, technical difficulty and failure rates are increased.17 As for all RF procedures, meticulous attention should be given to the recommended technique and fluoroscopy imaging safety views, saving these images to the patient's record for future reference.2.
Risks
These are in line with other needling techniques and include bleeding, infection, intravascular injection, vasovagal syncope and allergic reactions to local anaesthetic agents. Those specific to RF include:
-
(i)
Failure: this can be minimised with meticulous needling techniques and the use of diagnostic blocks before selecting appropriate patients. For medial branch denervation, single or ideally two diagnostic blocks at different times, with careful assessment for positive response of 70–100% reduction in pain for at least the duration of action of the local anaesthetic is recommended.2 The evidence for this is discussed later.
-
(ii)
Lesion size: larger RF lesions are desirable to reduce risk of missing the target nerve and increase the number and extent of nerves captured by each lesion.19 However, this accompanies an increased risk of damage to non-target structures, including nerve roots, skin burns in thinner patients and post-procedural dysaesthesias.17
-
(iii)
Mixed nerve targets are to be avoided because of wholly undesirable motor fibre damage. The longer-term sequelae of medial branch RF denervation to the multifidus muscle (supplied by the medial branch) in terms of function are unclear. There is some evidence that no discernible segmental atrophy is seen on MRI at a mean 21 months after a successful RF denervation.20
-
(iv)
Pain during and after the procedure with RF techniques can be significant because of needle positioning and a higher level of technical difficulty for some procedures; however, it is usually transient.21 This is also related to cannula size when larger gauges are used to generate larger-volume lesions, resulting in more pain from the needle tract. Neuritis may be prevented by an injection of steroid after lesioning, but there is considerable variation in the use of steroid amongst practitioners, and the benefit is questionable.17
-
(v)
Burns: Assuming that skin contact is optimal, the risk of burns is negligible when using conventional sites for the dispersive electrode in monopolar RF. Radiofrequency generator programmes are designed to fault if conditions are detected that may imply a problem.6 Implanted spinal metalwork should be avoided, as contact with the RF probe does risk burns.17
Indications and evidence
Contemporary chronic pain management practice recognises the importance of biopsychosocial factors particularly with lower back pain. An isolated interventional approach without biopsychosocial assessment and consideration of its aetiology is therefore unlikely to be effective.17,22 Available guidelines agree that the decision to try an intervention is usually made after conservative approaches have failed. The main chronic pain conditions treated with RF are summarised in Table 2. Evidence supporting RF interventional pain procedures has been historically marred by non-standardised procedures and inherent difficulties in selecting trial participants. People with chronic pain comprise a heterogeneous group often with collections of symptoms that do not neatly fit strict diagnostic criteria. Research is difficult for these reasons and affects the level of evidence and certainty and limits meta-analysis.
Table 2.
Interventional pain procedures using RF techniques.
| Target | Examples of indications |
|---|---|
| Head and neck pain | |
| Gasserian ganglion | Trigeminal neuralgia |
| Sphenopalatine ganglion | Headache disorders; post-herpetic neuralgia |
| Greater occipital nerve | Occipital neuralgia |
| Spinal pain | |
| Dorsal root ganglia: cervical and lumbar | Radiculopathy |
| Medial branches of the posterior rami of spinal nerves: cervical and lumbar | Facetogenic pain |
| Lateral branches to posterior sacroiliac joint | Sacroiliac joint dysfunction |
| Others | |
| Lateral spinothalamic tract: unilateral cervical cordotomy | Intractable unilateral cancer pain below the shoulder |
| Articular supply (e.g. suprascapular and genicular nerves) | Rotator cuff injury; osteoarthritis of the knee |
| Splanchnic nerves | Pancreatic cancer pain |
| Lumbar sympathetic chain | Peripheral vascular disease; chronic regional pain syndrome |
| Spinal cord dorsal root entry zone | Brachial plexus ablation deafferentation pain |
| Thalamotomy/cingulotomy | Cancer-related pain |
| Ganglion impar | Pelvic pain |
Radiofrequency denervation of the posterior rami medial branches is arguably the most studied so is discussed in more depth as follows.
Posterior rami medial branch denervation
Spinal pain is one of the most common pain conditions, with facetogenic back pain accounting for 31–55%.23 Innervation of the facet joints is via the medial branches, which arise from the posterior ramus.24 Denervation of the posterior rami medial branches is an established procedure in the treatment of facetogenic pain. Key evidence of effectiveness from controlled trials was published originally in 1996 for the cervical region in the treatment of whiplash injury and in 2008 for lumbar back pain.18,21
Lord and colleagues studied 24 patients after road traffic collisions with pain in one or more cervical regions, using placebo-controlled local anaesthesia to select and then randomised participants to percutaneous RF denervation or a sham procedure, where the only difference was RF current application or not. The median time to return of pain was 263 days compared with 8 days in the sham group.18 Nath and colleagues also selected patients using repeated diagnostic blocks before randomisation to either sham or RF denervation, again only differing in whether RF current was applied.21 This study of 40 patients found statistically significant improvements in lumbar back pain at 6 months by a reduction of 2.1 (on an 11-point visual analogue score) compared with 0.4 in the sham group. There were also improvements in lumbar range of movement and reductions in sensory deficits and normalisation of weak or absent ankle reflexes. A multicentre study from the Netherlands reported on RF denervation interventions for lower back pain compared with a standardised exercise programme.25 The intervention groups underwent diagnostic blocks depending on examination findings to diagnose facetogenic, SIJ, intervertebral disc pain or a combination of these and then progressed to RF denervation. Results showed no significant difference in pain intensity, defined as minimum 2-point difference on numerical rating scale, at 3 months.25 However, the trial has been criticised for its methodology, including the technical aspects of the RF interventions (small-gauge cannulae and lack of published fluoroscopy images).26
Intra-articular (IA) facet joint injections of steroid have a contentious role in lower back pain management. The Facet Treatment Study was a multicentre RCT designed to test this, the diagnostic role of medial branch blocks and RF denervation outcomes.27 Two hundred and twenty-nine participants were randomised to three groups: intra-articular (IA) injection of steroid with bupivacaine into the facet joint; medial branch block with steroid and bupivacaine; with the final group undergoing the needling procedure for medial branch blocks but with a placebo injection of saline. Positive responses were ascertained by a 2-point decrease in average back pain scores with a patient's satisfaction score of 3 or more out of 5. Participants with a positive outcome at 1 month after IA injection of steroid and bupivacaine, medial branch block with steroid and those with a negative outcome after placebo progressed to RF ablation of the medial branches. The IA steroid and medial branch groups also progressed to RF ablation if the response became negative between 1 and 6 months follow-up. Results demonstrated no significant difference in benefit between the two intervention groups. Those with a positive facet joint IA injection had a 6.87 times increased odds of a positive outcome from RF ablation than participants with a negative response, suggesting these blocks may be an effective method to identify those likely to benefit from RF denervation.
A recent meta-analysis of 19 RCTs of RF treatment for facetogenic pain, SIJ dysfunction and intervertebral discs concluded size effects are generally small (<1 on an 11-point numerical rating scale) with both treated and controls, placebo or sham improving at 3 months.28 Of the six trials for facetogenic pain, the evidence was graded as low or very low with pain scores at 1–3 months showing similar statistically insignificant effect size. To address the ongoing debate, the National Institute for Health and Care Research has recently funded a multicentre double-blind RCT in NHS patients evaluating RF denervation for lower back pain against sham RF, due to complete in 2024.29
Bipolar and cooled RF
Bipolar RF in pain management has emerging therapeutic applications, such as IA application for knee osteoarthritis (OA), SIJ denervation and potentially may be a safer alternative to monopolar techniques in patients with implanted devices.7,28,30 An unblinded RCT of IA bipolar RF or monopolar for OA-related knee pain found, at 3 months, 84% compared with 50%, respectively, achieved at least 50% pain reduction from baseline measures.31 Evidence for SIJ denervation from an observation study in 43 patients refractory to conservative management supports quicker procedure times by 2.5 times with strip lesioning techniques than conventional separate cannula approach using cooled RF for each lateral branch. Both significantly improved pain scores at 1–3 months. However, there was less improvement in pain scores and disability at 6–12 months in the strip lesion group in comparison.32 Shorter procedure times may enable less radiation exposure and improve acceptability of the technique, given positioning required for longer times may be poorly tolerated for some patients.
Pulsed RF
Pulsed RF was first performed in 1996 on the DRG in the lumbar spine, and the current literature is limited.4 Pulsed RF has the potential for fewer adverse effects and risks than conventional monopolar RF, with evidence of a lack of tissue damage. However, available report that the duration of pain relief is shorter, up to 6 months compared with RF denervation techniques for the same nerve target.33 In a review of more than 200 articles in 2017, there were no published reports of nerve damage.33 There is some evidence supporting treatment to cervical and lumbar DRG for radiculopathy, demonstrating greater effectiveness at 3 months than sham RF and transforaminal epidural steroids.8
For chronic knee pain, PRF results are mixed in part because of variable joint innervation and no consensus on which nerves to target. Intra-articular PRF has shown some pain improvement albeit with less magnitude of effect than IA erythropoietin.33 The mechanism of IA PRF action is unclear. There is mixed evidence for PRF to the suprascapular nerve for shoulder pain. A recent meta-analysis of seven RCTs of PRF for chronic shoulder pain found no significant pain or functional improvement over conventional management with a low certainty of evidence.34 The nerves targeted were specified as the suprascapular in five of the RCTs. Pulsed RF to the greater and lesser occipital nerves for treatment of occipital nerve tenderness was compared with steroid in a double-blinded RCT with 81 participants selected via positive diagnostic blocks. Results demonstrated significant reduction in average occipital pain at 6 weeks (mean [range] visual analogue scale change from baseline –2.743 [2.487] compared with –1.377 [1.970]) than the steroid group, persisting at 6 months and regardless of the aetiology of migraine or occipital neuralgia.12
There is currently no consensus in over the role of PRF in clinical practice. Practitioners point at some benefit with no potential for tissue damage, whereas critics discuss the lack of robust evidence for benefit and trials regarding medial branch neurotomy showing inferiority compared with conventional RF.5
Guidelines
There are published guidelines for use of interventional techniques, including RF in the managment of axial spine pain, principally denervation of the posterior rami medial branches in facetogenic back pain.2 The National Institute for Health and Care Excellence (NICE) guidelines for low back pain and sciatica in patients aged over 16 yrs recommend RF denervation is considered when non-surgical management has failed, and the pain is moderate or severe (numerical rating scale 5/10 or more) and thought to be originating from structures innervated by the medial branches.35 Diagnostic blocks before RF denervation are crucial for selecting appropriate patients to identify those with a significant facetogenic contribution to their pain. There is debate over the benefit in terms of both assuring an effective outcome and resource implications of stipulating that one or more diagnostic blocks are undertaken first. The NICE guidelines recommend a positive response to single diagnostic medial branch blocks before lumbar medial branch RF.35 The SIS go further, recommending two sequential diagnostic injections with a minimum 80% reduction or complete relief, quoting a poor diagnostic certainty with single blocks because of their high false-positive rate (up to 41%).2 The 2020 consensus guidelines from Cohen and colleagues recommend a pragmatic compromise of a single prior diagnostic block.3,17
Outcomes
Radiofrequency denervation should provide significant improvement in pain and quality of life and reduce the need for analgesics, but it is important to counsel patients that it is not a cure. For medial branch RF denervation, there is a clear relationship between positive response to diagnostic blocks and success of RF treatment. More stringent criteria, such as requisite 70% or more relief of pain after diagnostic blocks, have been shown to result in success rates of more than 50% to subsequent RF denervation.2 Procedures can be repeated, but local funding arrangements often do not support this. The SIS guidelines discuss evidence supports a consistent duration of relief and degree of pain relief after subsequent RF denervation.2
Conclusions
Radiofrequency offers a longer-term effect by targeting nerves innervating pain generators to improve some chronic pain conditions. Understanding the science behind factors affecting lesion size is useful to help maximise the chances of a successful procedure coupled with careful selection of patients, diagnostic blocks and meticulous positioning of the ablation electrode. Large-scale RCT evidence supporting these techniques is lacking because of differences in patients studied, heterogeneity amongst patients and variations in RF techniques.
Declaration of interests
The authors declare that they have no conflicts of interest.
Biographies
Therese Walsh BSc FRCA PGCert FFPMRCA is completing a fellowship at Starship Children's Hospital, Auckland, New Zealand. She completed advanced pain training in Liverpool and is a module editor for e-Learning Anaesthesia.
Rajiv Malhotra FRCA FFPMRCA is a consultant in anaesthesia and pain medicine at Liverpool University Hospitals. He is a college tutor for anaesthesia and a committee member of the Pain Education Special Interest Group of The British Pain Society.
Manohar Sharma FRCA MSc FFPMRCA is a consultant in pain medicine and neuromodulation at The Walton Centre where he leads the interventional cancer pain service. He is a board member of the Faculty of Pain Medicine, honorary senior clinical lecturer at the University of Liverpool and is a member of the Spine Intervention Society and the Interventional Pain Special Interest Group of The British Pain Society.
Matrix codes: 1A03, 2E03, 2G02
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bjae.2022.08.004.
MCQs
The associated MCQs (to support CME/CPD activity) will be accessible at www.bjaed.org/cme/home by subscribers to BJA Education.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
figs1.
figs2.
figs3.
figs4.
figs5.
References
- 1.van Zundert J., Hambraeus J. In: Pain 2018: refresher course, 17th world congress on pain. Gold M., Pogatzki-Zahn E., Wallace M., editors. IASP Press; Seattle, WA: 2018. Injections for joint pain and radiofrequency lesioning. [Google Scholar]
- 2.Bogduk N., editor. Practice guidelines for spinal diagnostic and treatment procedures. 2nd Edn. International Spine Intervention Society; San Francisco, CA: 2013. [Google Scholar]
- 3.Cohen S.P., Bhaskar A., Bhatia A., et al. Consensus practice guidelines on interventions for lumbar facet joint pain from a multispecialty, international working group. Reg Anesth Pain Med. 2020;45:424–467. doi: 10.1136/rapm-2019-101243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chua N.H.L., Vissers K.C., Sluijter M.E. Pulsed radiofrequency treatment in interventional pain management: mechanisms and potential indications—a review. Acta Neurochir (Wien) 2011;153:763–771. doi: 10.1007/s00701-010-0881-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bogduk N. Pulsed radiofrequency. Pain Med. 2006;7:396–407. doi: 10.1111/j.1526-4637.2006.00210.x. [DOI] [PubMed] [Google Scholar]
- 6.Ball R.D. The science of conventional and water-cooled monopolar lumbar radiofrequency rhizotomy: an electrical engineering point of view. Pain Physician. 2014;17:E175–E211. [PubMed] [Google Scholar]
- 7.Kapural L., Deering J.P. A technological overview of cooled radiofrequency ablation and its effectiveness in the management of chronic knee pain. Pain Manag. 2020;10:133–140. doi: 10.2217/pmt-2019-0066. [DOI] [PubMed] [Google Scholar]
- 8.Koh W., Choi S., Karm M., et al. Treatment of chronic lumbrosacral radicular pain using adjuvant pulsed radiofrequency: a randomized controlled study. Pain Med. 2015;16:432–441. doi: 10.1111/pme.12624. [DOI] [PubMed] [Google Scholar]
- 9.Cohen S.P., Chen Y., Neufeld N.J. Sacroiliac joint pain: a comprehensive review of epidemiology, diagnosis and treatment. Expert Rev Neurother. 2013;13:99–116. doi: 10.1586/ern.12.148. [DOI] [PubMed] [Google Scholar]
- 10.Brennick C., Bickelhaupt B., Boies B., Nagpal A. Simplicity radiofrequency ablation demonstrates greater functional improvement than analgesia: a prospective case series. Pain Physician. 2021;24:E185–E190. [PubMed] [Google Scholar]
- 11.Bhatia A., Hoydonckx Y., Peng P., Cohen S.P. Radiofrequency procedures to relieve chronic hip pain: an evidence-based narrative review. Reg Anesth Pain Med. 2018;43:72–83. doi: 10.1097/AAP.0000000000000694. [DOI] [PubMed] [Google Scholar]
- 12.Cohen S.P., Lee Peterlin B., Fulton L., et al. Randomized, double-blind, comparative-effectiveness study comparing pulsed radiofrequency to steroid injections for occipital neuralgia or migraine with occipital nerve tenderness. Pain. 2015;156:2585–2594. doi: 10.1097/j.pain.0000000000000373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Racz G.B., Ruiz-Lopez R. Radiofrequency procedures. Pain Pract. 2006;6:46–50. doi: 10.1111/j.1533-2500.2006.00058.x. [DOI] [PubMed] [Google Scholar]
- 14.Eldabe S., Tariq A., Nath S., et al. Best practice in radiofrequency denervation of the lumbar facet joints: a consensus technique. Br J Pain. 2020;14:47–56. doi: 10.1177/2049463719840053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Checketts M.R., Alladi R., Ferguson K., et al. Recommendations for standards of monitoring during anaesthesia and recovery 2015: association of anaesthetists of great britain and Ireland. Anaesthesia. 2016;71:85–93. doi: 10.1111/anae.13316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shuang F., Hou S.X., Zhu J.L., et al. Clinical anatomy and measurement of the medial branch of the spinal dorsal ramus. Medicine. 2015;94:e2367. doi: 10.1097/MD.0000000000002367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cohen S.P., Bhaskar A., Bhatia A., et al. Consensus practice guidelines on interventions for lumbar facet joint pain from a multispecialty, international working group. Reg Anesth Pain Med. 2020;45:424–467. doi: 10.1136/rapm-2019-101243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lord S., Barnsley L., Wallis B., McDonald G., Bogduk N. Percutaneous radio-frequency neurotomy for chronic cervical zygapophyseal-joint pain. N Engl J Med. 1996;335:1721–1726. doi: 10.1056/NEJM199612053352302. [DOI] [PubMed] [Google Scholar]
- 19.Bogduk N., Dreyfuss P., Govind J. A narrative review of lumbar medial branch neurotomy for the treatment of back pain. Pain Med. 2009;10:1035–1045. doi: 10.1111/j.1526-4637.2009.00692.x. [DOI] [PubMed] [Google Scholar]
- 20.Dreyfuss P., Stout A., Aprill C., Pollei S., Johnson B., Bogduk N. The significance of multifidus atrophy after successful radiofrequency neurotomy for low back pain. PM R. 2009;1:719–722. doi: 10.1016/j.pmrj.2009.05.014. [DOI] [PubMed] [Google Scholar]
- 21.Nath S., Nath C.A., Pettersson K. Percutaneous lumbar zygapophysial (facet) joint neurotomy using radiofrequency current, in the management of chronic low back pain a randomized double-blind trial. Spine. 2008;33:1291–1297. doi: 10.1097/BRS.0b013e31817329f0. [DOI] [PubMed] [Google Scholar]
- 22.Baber Z., Erdek M.A. Failed back surgery syndrome: current perspectives. J Pain Res. 2016;9:979–987. doi: 10.2147/JPR.S92776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Manchikanti L., Boswell M., Singh V., Pampati V., Damron K., Beyer C. Prevalence of facet joint pain in chronic spinal pain of cervical, thoracic, and lumbar regions. BMC Musculoskelet Disord. 2004;28:15. doi: 10.1186/1471-2474-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Masini M., Paiva W.S., Araújo A.S. Anatomical description of the facet joint innervation and its implication in the treatment of recurrent back pain. J Neurosurg Sci. 2005;49:143–146. discussion 146. [PubMed] [Google Scholar]
- 25.Juch J.N.S., Maas E.T., Ostelo R.W.J.G., et al. Effect of radiofrequency denervation on pain intensity among patients with chronic low back pain. JAMA. 2017;318:68. doi: 10.1001/jama.2017.7918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McCormick Z.L., Vorobeychik Y., Gill J.S., et al. Guidelines for composing and assessing a paper on the treatment of pain: a practical application of evidence-based medicine principles to the mint randomized clinical trials. Pain Med. 2018;19:2127–2137. doi: 10.1093/pm/pny046. [DOI] [PubMed] [Google Scholar]
- 27.Cohen S.P., Doshi T.L., Constantinescu O.C., et al. Effectiveness of lumbar facet joint blocks and predictive value before radiofrequency denervation the Facet Treatment Study (FACTS), a randomized, controlled clinical trial. Anesthesiology. 2018;129:517–535. doi: 10.1097/ALN.0000000000002274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chappell M.E., Lakshman R., Trotter P., Abrahams M., Lee M. Radiofrequency denervation for chronic back pain: a systematic review and meta-analysis. BMJ Open. 2020;10 doi: 10.1136/bmjopen-2019-035540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Price C., Reeves B., Ahmad A., et al. Radiofrequency denervation of the lumbar facet joints: guidelines for the RADICAL randomised controlled trial. Br J Pain. 2021;15:251–258. doi: 10.1177/2049463720941053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bautista A., Dadabayev A., Rosenquist E., Cheng J. Bipolar radiofrequency neurotomy to treat neck and back pain in patients with automatic implantable cardioverter defibrillator. Pain Physician. 2016;19:E505–E509. [PubMed] [Google Scholar]
- 31.Gulec E., Ozbek H., Pektas S., Isik G. Bipolar versus unipolar intraarticular pulsed radiofrequency thermocoagulation in chronic knee pain treatment: a prospective randomized trial. Pain Physician. 2017;20:197–206. [PubMed] [Google Scholar]
- 32.Tinnirello A., Barbieri S., Todeschini M., Marchesini M. Conventional (Simplicity III) and cooled (SInergy) radiofrequency for sacroiliac joint denervation: one-year retrospective study comparing two devices. Pain Med. 2017;18:1731–1744. doi: 10.1093/pm/pnw333. [DOI] [PubMed] [Google Scholar]
- 33.Vanneste T., van Lantschoot A., van Boxem K., van Zundert J. Pulsed radiofrequency in chronic pain. Curr Opin Anaesthesiol. 2017;30:577–582. doi: 10.1097/ACO.0000000000000502. [DOI] [PubMed] [Google Scholar]
- 34.Pushparaj H., Hoydonckx Y., Mittal N., et al. A systematic review and meta-analysis of radiofrequency procedures on innervation to the shoulder joint for relieving chronic pain. Eur J Pain. 2021;25:986–1011. doi: 10.1002/ejp.1735. [DOI] [PubMed] [Google Scholar]
- 35.National Institute for Health and Care Excellence. Low back pain and sciatica in over 16s: assessment and management NICE guideline 2016. Available from: www.nice.org.uk/guidance/ng59. Accessed date: 03/10/2022. [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.