Abstract
The present opinion deals with the re‐evaluation of neohesperidine dihydrochalcone (E 959) when used as a food additive. It is obtained by catalytic hydrogenation of a flavanone – neohesperidine – which is naturally occurring and thus isolated by alcohol extraction in bitter oranges (Citrus aurantium). Based on in vivo data in rat, neohesperidine dihydrochalcone is likely to be absorbed, also in humans, and to become systemically available. It does not raise a concern regarding genotoxicity. The toxicity data set consisted of studies on subchronic and prenatal developmental toxicity. No human studies were available. The data set was considered sufficient to derive a new acceptable daily intake (ADI). Based on the weight of evidence (WoE) analysis, the Panel considered unlikely that neohesperidine dihydrochalcone would lead to adverse effects on health in animals in the dose ranges tested. The Panel also considered that a carcinogenicity study was not warranted and that the lack of human data did not affect the overall confidence in the body of evidence. The Panel derived an ADI of 20 mg/kg bodyweight (bw) per day based on a no observed adverse effect level (NOAEL) of 4,000 mg/kg bw per day from a 13‐week study in rat, applying the standard default factors of 100 for inter‐ and intraspecies differences and of 2 for extrapolation from subchronic to chronic exposure. For the refined brand‐loyal exposure assessment scenario, considered to be the most appropriate for the risk assessment, the exposure estimates at the mean ranged from < 0.01 to 0.09 mg/kg bw per day and at the 95th percentile (P95) from 0.01 to 0.24 mg/kg bw per day. Considering the derived ADI of 20 mg/kg bw per day, the exposure estimates were below the reference value in all age groups. Therefore, the Panel concluded that dietary exposure to the food additive neohesperidine dihydrochalcone (E 959) at the reported uses and use levels would not raise a safety concern.
Keywords: neohesperidine dihydrochalcone, E 959, food additive, sweetener
Summary
The present opinion deals with the re‐evaluation of neohesperidine dihydrochalcone (E 959) when used as a food additive.
Neohesperidine dihydrochalcone (E 959) is authorised as a food additive in the European Union (EU) in accordance with Annex II to Regulation (EC) No 1333/2008 on food additives and its specifications are defined in the Commission Regulation (EU) No 231/2012.
Neohesperidine dihydrochalcone was previously assessed by Scientific Committee on Food (SCF) in 1985 and 1989. Following these evaluations, an acceptable daily intake (ADI) of 5 mg/kg body weight (bw) per day was established, considering the lowest no observed adverse effect level (NOAEL) obtained in all the studies which were carried out and evaluated. Neohesperidine dihydrochalcone is authorised in the EU also as food flavouring ([FL‐no: 16.061]), in accordance with Regulation (EC) No 1334/2008 and it was evaluated within Flavouring Group Evaluation 32 (FGE.32) (EFSA CEF Panel, 2010). In addition, the EFSA FEEDAP Panel evaluated the safety of neohesperidine dihydrochalcone as a sensory feed additive for use in several species (EFSA FEEDAP Panel, 2011, 2014). The Joint FAO/WHO Expert Committee on Food Additives (JECFA) did not evaluate neohesperidine dihydrochalcone as a food additive, thus no JECFA specifications are available for E 959.
The current risk assessment was carried out based on structured protocols on hazard identification and characterisation (EFSA, 2020a) and on exposure assessment (EFSA, 2020b). The protocols defined upfront the strategy to be applied for collecting and selecting data, appraising the relevant evidence, analysing and integrating the evidence.
According to Commission Regulation (EU) No 231/2012 definition, neohesperidine dihydrochalcone (E 959) is obtained by catalytic hydrogenation of neohesperidin. Based on the information provided by the interested business operators, the source material neohesperidin is a flavanone naturally occurring in bitter oranges (Citrus aurantium) that is isolated by alcohol extraction. As laid down in the EU specifications, the purity assay for E 959 requires not less than 96% chemical purity. Structurally related flavonoid impurities can be present in E 959 (e.g. degradation products, unreacted starting material, compounds co‐extracted with the starting material). These impurities are also described in the EU Pharmacopeia monograph (European Pharmacopoeia 9.0, 2017). Considering the nature, the levels and the origin of the impurities, along with the recommendation that the source material for the food additive (i.e. neohesperidin obtained by alcohol extraction from bitter oranges) should be included in the EU definition of E 959, the Panel did not consider necessary to recommend the inclusion of limit values for these impurities in the EU specifications of E 959.
Based on the analytical data provided by the interested business operators and the dietary exposure estimation to the food additive, the Panel calculated the potential exposure to the toxic elements from the use of E 959. The resulting figures showed that the potential exposure to lead (Pb), cadmium (Cd), palladium (Pd) and mercury (Hg) from the uses and use levels of E 959 would not be of concern. For Pd, Cd and Hg, the Panel saw no need to introduce limits for these elements in the EU specifications for E 959. As the occurrence levels for Pb reported by both interested business operators are substantially below the current EU specification limit (not more than 2 mg/kg), the Panel noted that a lower limit for Pb is technologically feasible. For As, the lower end of the calculated margin of exposure (MOE) values fall below the target of 1,000, indicating that a lowering of the existing EU specification limit value of 3 mg/kg is recommended and technologically feasible based on the analytical data provided.
Because of the botanical origin of the source material – neohesperidin – analytical data on environmental contaminants (polycyclic aromatic hydrocarbons (PAHs), pesticides residues and mycotoxins) were provided by both interested business operators, together with adequate information on analytical techniques and methods used. In all analysed batches the contaminants were not detected above their limit of quantification (LOQ). In addition, the interested business operator provided microbiological analyses supporting the microbiological quality of the food additive.
Regarding water solubility, the Panel concluded that neohesperidine dihydrochalcone (E 959) can be considered slightly soluble in water at 20°C (230 mg/L) according to JECFA criteria (JECFA, 2006).
Based on the data on particle size distribution submitted by the interested business operators and the criteria set in the EFSA Guidance‐TR, the Panel concluded that the presence of small particles, including nanoparticles, cannot be excluded in the pristine food additive.
Taking into account the reported uses and use levels and the MPLs, the reported solubility, the increase of solubility of neohesperidine dihydrochalcone in water with temperature (Benavente‐Garcia et al., 2001) and the volume of gastric secretion (ranging from 215 mL within a single meal to 2,000 mL daily; ICRP, 2002; Mudie et al., 2014), the Panel considered that full dissolution of neohesperidine dihydrochalcone (E 959) is to be expected in the gastrointestinal (GI) tract and that ingested particles (if any) would not persist. Therefore, the Panel concluded there is no concern with regard the potential presence of small particles, including nanoparticles, in neohesperidine dihydrochalcone (E 959) when used as a food additive and considered that the risk assessment of neohesperidine dihydrochalcone (E 959) can be performed following the EFSA Guidance for submission for food additive evaluations (EFSA ANS Panel, 2012).
Detailed information on the manufacturing process have been submitted by the interested business operators. The process involves two main steps (i) hydroalcoholic extraction of the flavanone neohesperidin from immature bitter oranges (C. aurantium) and purification (ii) catalytic reduction of the purified neohesperidin to neohesperidine dihydrochalcone, using a palladium‐on‐charcoal (Pd/C) as catalyst, under alkaline conditions. Further purification processes (e.g. crystallisation) are also performed. Other types of manufacturing processes were not considered in the present assessment.
The Panel noted that no new data on the stability of E 959 under its currently permitted conditions of use/processing were provided by the interested business operators.
Based on the available in vivo studies in rats, the Panel considered that, also in humans, neohesperidine dihydrochalcone is likely to be absorbed, to become systemically available both as parent compound and as metabolites, which are excreted mainly in urine.
The published bacterial reverse mutation assays along with an in vitro micronucleus test in human lymphocytes, submitted by one interested business operator, provided sufficient evidence of lack of mutagenicity of neohesperidine dihydrochalcone (E 959) and lack of micronucleus formation in mammalian cells. Therefore, the Panel concluded that neohesperidine dihydrochalcone (E 959) does not raise a concern regarding genotoxicity.
The toxicity data set consisted of studies, assessed as relevant and reliable based on the criteria established in the draft protocol on hazard identification and characterisation of sweeteners (EFSA, 2020a), on subchronic and prenatal developmental toxicity in rodents. No human studies were available, neither retrieved in the literature nor submitted by the interested business operators. The Panel considered the available data sufficient to establish a new ADI.
Overall, no adverse effects on health were identified for neohesperidine dihydrochalcone based on the three toxicological studies considered. Based on the weight of evidence analysis, the Panel considered unlikely that neohesperidine dihydrochalcone would lead to adverse effects on health in animals in the dose ranges tested. The Panel also considered that the lack of human data does not affect the overall confidence in the body of evidence and that a carcinogenicity study was considered not warranted.
Based on a rat 13‐week NOAEL of 4,000 mg/kg bw per day, the highest dose tested, applying the standard default factor of 100 for inter‐ and intraspecies differences and the standard default factor of 2 for extrapolation from subchronic to chronic exposure (EFSA Scientific Committee, 2012), an ADI of 20 mg/kg bw per day was derived.
Dietary exposure to the food additive was estimated according to different exposure scenarios based on consumers‐only. The Panel considered the refined brand‐loyal exposure assessment scenario (with facets) to be the most appropriate exposure scenario for the risk assessment. For this scenario, the Panel considered use levels available for neohesperidine dihydrochalcone (E 959) for three out of 38 authorised food categories. This limited use of neohesperidine dihydrochalcone (E 959) as a sweetener was confirmed by data from literature. Also label information on the use of this food additive in foods from the Mintel GNPD supported that, in general, it has limited use in Europe, but is used in a few regularly consumed carbonated soft drinks.
In the refined brand‐loyal exposure assessment scenario, mean dietary exposure to neohesperidine dihydrochalcone (E 959) ranged from < 0.01 mg/kg bw per day in adults and the elderly to 0.09 mg/kg bw per day in toddlers. The 95th percentile (P95) of dietary exposure ranged from 0.01 mg/kg bw per day in the adults and the elderly to 0.24 mg/kg bw per day in toddlers.
Considering the ADI of 20 mg/kg bw per day as a reference value for the risk assessment, mean and P95 levels of dietary exposure to neohesperidine dihydrochalcone (E 959) in all age groups in the refined brand‐loyal exposure assessment scenario were below this reference value. In addition, the Panel noted that the exposure estimates for the regulatory maximum and the refined regulatory maximum level exposure assessment scenarios were also below the ADI.
The Panel concluded that dietary exposure to the food additive neohesperidine dihydrochalcone (E 959) at the reported uses and use levels would not raise a safety concern.
The Panel recommended that the European Commission consider amending existing EU specifications for neohesperidine dihydrochalcone (E 959) through:
including in the current definition the source of the starting material – neohesperidin – and how the starting material is obtained;
introducing the CAS number 20702‐77‐6;
introducing information on specific rotation;
lowering the current limits for arsenic and lead, taking into account the analytical data submitted by the interested business operators.
1. Introduction
The present opinion deals with the re‐evaluation of neohesperidine dihydrochalcone (E 959) when used as a food additive.
1.1. Background and Terms of Reference as provided by the requestor
1.1.1. Background
Regulation (EC) No 1333/2008 1 of the European Parliament and of the Council on food additives requires that food additives are subject to a safety evaluation by the European Food Safety Authority (EFSA) before they are permitted for use in the European Union (EU). In addition, it is foreseen that food additives must be kept under continuous observation and must be re‐evaluated by EFSA.
For this purpose, a programme for the re‐evaluation of food additives that were already permitted in the European Union before 20 January 2009 has been set up under the Regulation (EU) No 257/2010. 2 This Regulation also foresees that food additives are re‐evaluated whenever necessary in the light of changing conditions of use and new scientific information. For efficiency and practical purposes, the re‐evaluation should, as far as possible, be conducted by group of food additives according to the main functional class to which they belong.
The order of priorities for the re‐evaluation of the currently approved food additives should be set on the basis of the following criteria: the time since the last evaluation of a food additive by the Scientific Committee on Food (SCF) or by EFSA, the availability of new scientific evidence, the extent of use of a food additive in food and the human exposure to the food additive taking also into account the outcome of the Report from the Commission on Dietary Food Additive Intake in the EU of 2001. The report “Food additives in Europe 2000” submitted by the Nordic Council of Ministers to the Commission, provides additional information for the prioritisation of additives for re‐evaluation. As colours were among the first additives to be evaluated, these food additives should be re‐evaluated with a highest priority.
In 2003, the Commission already requested EFSA to start a systematic re‐evaluation of authorised food additives. However, as a result of adoption of Regulation (EU) 257/2010, the 2003 Terms of References are replaced by those below.
1.1.2. Terms of Reference
The Commission asks the European Food Safety Authority to re‐evaluate the safety of food additives already permitted in the Union before 2009 and to issue scientific opinions on these additives, taking especially into account the priorities, procedures and deadlines that are enshrined in the Regulation (EU) No 257/2010 of 25 March 2010 setting up a programme for the re‐evaluation of approved food additives in accordance with the Regulation (EC) No 1333/2008 of the European Parliament and of the Council on food additives.
1.2. Information on existing authorisations and evaluations
Neohesperidine dihydrochalcone (E 959) is authorised as a food additive in the EU in accordance with Annex II to Regulation (EC) No 1333/2008 on food additives and its specifications are defined in the Commission Regulation (EU) No 231/2012. 3
The SCF assessed the safety of neohesperidine dihydrochalcone (E959) in its initial review of sweeteners in 1984 (SCF, 1985). The SCF reviewed studies on metabolism, acute toxicity, subchronic toxicity, multigeneration reproduction, teratogenicity and chronic toxicity as well as several in vitro and in vivo mutagenicity studies. In the opinion it was stated that ‘neither of the chronic toxicity studies in rats and dogs established a clear no adverse effect level. The Committee was therefore unable to assess the safety of this sweetener until a no adverse effect level was established in an adequately conducted 90‐day study in rat’.
The safety of neohesperidine dihydrochalcone was re‐addressed in 1989 (SCF, 1989). It was stated that the previously evaluated studies showed variable and contradictory results, and a no‐effect level (NOEL) could not be determined for any of those. It became apparent that the diet used in some of the reviewed studies was nutritionally unbalanced and that some of the effects observed during the administration of high doses of neohesperidine dihydrochalcone disappeared when the diet was supplemented with various nutrients, in particular iodine. The SCF was provided two new 90‐days studies in rats: these studies were performed using a better‐balanced diet and did not produce the same effects that were observed in the previously evaluated studies. In these two studies, a no observed adverse effect level (NOAEL) of 1,000 mg/kg body weight (bw) per day and of about 900 mg/kg bw per day (average among 547–1,214 mg/kg bw per day) were established, respectively. The Committee reported that in a previously evaluated 2‐year study in dog a NOAEL of 1,000 mg/kg bw per day could be estimated, although it was considered by the authors to be preliminary due to the reduced number of animals used. Considering this observation and to ensure maximum protection, the SCF decided to use the lowest NOAEL obtained in all the studies which were carried out and evaluated: 500 mg/kg per day in the rat. Consequently, the SCF established an ADI of 5 mg/kg bw per day for neohesperidine dihydrochalcone.
Neohesperidine dihydrochalcone is authorised in the EU also as food flavouring ([FL‐no: 16.061]), in accordance with Regulation (EC) No 1334/2008 4 , and it was evaluated within Flavouring Group Evaluation 32 (FGE.32) on flavonoids (flavanones and dyhydrochalcones) by the former EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids (CEF Panel) in 2010 (EFSA CEF Panel, 2010). In that opinion, in which seven flavonoids including neohesperidine dihydrochalcone were assessed, the CEF Panel concluded that ‘the genotoxicity data available [did] not prevent the evaluation through the Procedure, the seven flavouring substances [could] be predicted to be metabolised to innocuous products […], it is considered […], that the seven flavouring substances [would] not give rise to safety concerns at the estimated levels of intake arising from their use as flavouring substances’. A tabulated summary of all the available toxicity data on neohesperidine dihydrochalcone was reported in the CEF Panel opinion: this included those previously considered by the SCF and a new prenatal developmental toxicity study (Waalkens‐Berendsen et al., 2004).
The EFSA Panel on Additives and Products or Substances used in Animal Feed Panel (FEEDAP Panel) evaluated the safety of neohesperidine dihydrochalcone as a sensory additive for use in several species (EFSA FEEDAP Panel, 2011, 2014). The Panel considered the proposed use levels of up to 35 mg neohesperidine dihydrochalcone/kg feed and 5 mg neohesperidine dihydrochalcone/L water for drinking to be safe for piglets (sucking and weaned), pigs for fattening, calves for rearing, calves for fattening, lambs for rearing, lambs for fattening, dairy sheep, ewes for reproduction, salmon and trout, and dogs, with a margin of safety ranging from 3 to 8. The exposure of consumers to neohesperidine dihydrochalcone in food would not be significantly increased by its use as a feed additive for mammals and poultry. However, the lack of data on metabolism and residues in fish precluded an assessment of consumer exposure from this source (EFSA FEEDAP Panel, 2011). In 2014, the EFSA FEEDAP Panel issued a complementary opinion on the safety of neohesperidine dihydrochalcone as a sensory additive for fish. Based on the data provided on the metabolism of structurally related compounds in fish, the FEEDAP Panel concluded that the use of neohesperidine dihydrochalcone as a feed additive for fish was safe for the consumer (EFSA FEEDAP Panel, 2014).
The Joint FAO/WHO Expert Committee on Food Additives (JECFA) did not evaluate neohesperidine dihydrochalcone as a food additive or as a food flavouring. However, in 2012 at its 67th meeting, toxicological data on neohesperidine dihydrochalcone were reported in the context of the safety evaluation of groups of related flavouring agents, including phenol and phenol derivatives (JECFA, 2012).
In the context of the Regulation (EC) No 1907/2006 5 of the European Parliament and of the Council on the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH), a registration dossier 6 on neohesperidine dihydrochalcone (E 959) is available. The dossier reports studies on acute toxicity on aquatic invertebrates and algae, with a reported endpoint obtained from a ‘read‐across from supporting substance (structural analogue or surrogate)’ of ≥ 105.5 mg/L for both organisms. In addition, the registration dossier reports a study on biodegradability, according to OECD TG 301 F and GLP criteria, concluding that neohesperidine dihydrochalcone (E 959) is readily biodegradable.
2. Data and methodologies
The current risk assessment was carried out by the EFSA Panel on Food Additives and Flavourings (FAF Panel) in the context of Regulation (EC) No 257/2010. Structured protocols on hazard identification and characterisation (EFSA, 2020a) and on exposure assessment (EFSA, 2020b) were developed in line with the principles of the EFSA PROMETHEUS project (PROmoting METHods for Evidence Use in Scientific assessments) (EFSA, 2015a). The protocols define the strategy to be applied for collecting and selecting data, appraising the relevant evidence, and analysing and integrating the evidence in order to draw conclusions that will form the basis for the scientific opinions.
2.1. Data
The FAF Panel was not provided with a newly submitted dossier. EFSA launched public calls for data 7 , 8 , 9 , 10 and contacted interested parties to collect relevant information.
The Panel based its assessment on information submitted to EFSA following the public calls for data, information from previous evaluations and additional available literature up to July 2022.
The steps followed for the acquisition of data and their selection are documented in detail in Appendix A.
Food consumption data used to estimate the dietary exposure to neohesperidine dihydrochalcone (E 959) were derived from the EFSA Comprehensive European Food Consumption Database (Comprehensive Database 11 ).
Mintel's Global New Products Database (GNPD) was checked to identify the use of neohesperidine dihydrochalcone (E 959) in food and beverage products and food supplements. Mintel's GNPD is an online database that contains the compulsory ingredient information present on the label of numerous products.
2.2. Methodologies
This opinion was formulated following the principles described in the EFSA Guidance on transparency with regard to scientific aspects of risk assessment (EFSA Scientific Committee, 2009) and following the relevant existing guidance documents from the EFSA Scientific Committee.
The FAF Panel assessed the safety of neohesperidine dihydrochalcone (E 959) as a food additive in line with the principles laid down in Regulation (EU) 257/2010 and in the relevant guidance documents: Guidance on submission for food additive evaluations by the Scientific Committee on Food (SCF, 2001) and the Guidance for submission for food additive evaluations (EFSA ANS Panel, 2012).
In animal studies, when the test substance is administered in the feed or in the drinking water, but doses are not explicitly reported by the authors as mg/kg bw per day based on actual feed or water consumption, the daily intake is calculated by the Panel using the relevant default values. In case of rodents, the values as indicated in the EFSA Scientific Committee Guidance document (EFSA Scientific Committee, 2012) are applied. In the case of other animal species, the default values used by JECFA (2000) are used. In these cases, the dose was expressed as ‘equivalent to mg/kg bw per day’. If a concentration in feed or drinking water was reported and the dose in mg/kg bw per day was calculated (by the authors of the study report or the Panel) based on these reported concentrations and on reported consumption data for feed or drinking water, the dose was expressed as ‘equal to mg/kg bw per day’.
The current risk assessment was carried out based on structured protocols on hazard identification and characterisation (EFSA, 2020a) and on exposure assessment (EFSA, 2020b). The protocols defined upfront the strategy to be applied for collecting and selecting data, appraising the relevant evidence, analysing and integrating the evidence.
The draft protocol for the hazard identification and characterisation of sweeteners was published on EFSA's website for comments, and the online public consultation was made available until 19 September 2019. A technical report on the outcome of this public consultation with the overview of the comments received and the general responses from EFSA was published (EFSA, 2020a).
A systematic approach was used for assessing hazard. The methods for hazard identification, including the assessment of internal validity for individual studies (risk of bias (RoB)) and the assessment of the body of evidence across all health outcomes, are detailed in Appendix A. In brief, following data retrieval and screening for relevance, RoB was performed and studies were classified into tiers from 1 to 3, corresponding to decreasing levels of internal validity. 12 Only tier 1 and tier 2 studies were included in the weight of evidence evaluation in the current opinion.
The overall evidence from the human and animal studies were weighted separately before being integrated. During that process ratings of initial confidence (expressed as high, moderate, low or very low) were assigned to all studies based on study design for each relevant, reported outcome. For each outcome across studies, the initial confidence rating could be downgraded based on either a concern for bias across studies, unexplained inconsistency, relevance of studies and/or imprecision; similarly, it could be upgraded based on the magnitude of effect, dose–response, consideration of residual confounding (human studies only) and consistency across study designs and experimental model systems (NTP‐OHAT, 2019). The following terms were used to express the level of confidence in the body of evidence, irrespective of whether an association between exposure to the substance and adverse health outcome(s) were identified: ‘high’, ‘moderate’, ‘low’ and ‘very low/no evidence identified’. For each level of confidence in the body of evidence, corresponding expressions for levels of evidence for adverse effects on health were denoted as ‘high’, ‘moderate’, ‘low’ and ‘inadequate’, respectively. Whereas when no adverse effects on health were identified, expressions for levels of evidence were denoted as ‘high’, ‘moderate’, ‘inadequate’ and ‘inadequate’, respectively. More details on the weight of evidence procedure are outlined in step 1.14 of the draft protocol (EFSA, 2020a) and the US National Toxicology Program (NTP) Handbook for conducting a literature‐based health assessment (NTP‐OHAT, 2019), with some modifications (Figure A.2, Appendix A). Integration of human and animal data were based on the highest level of evidence rating for an adverse or no adverse effect on health. Hazard identification conclusions i.e., expressions of likelihood of an association between intake of neohesperidine dihydrochalcone and adverse effect on health, were reached on groups of toxicological outcomes following a guidance (Figure A.3, Appendix A) developed by the FAF Panel.
Figure A.2.
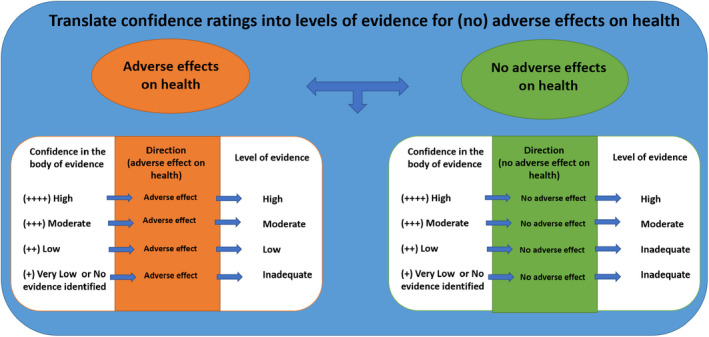
Translation of confidence ratings into level of evidence for conclusions of adverse effects on health or no adverse effects on health (adapted from NTP‐OHAT, 2019)
Figure A.3.
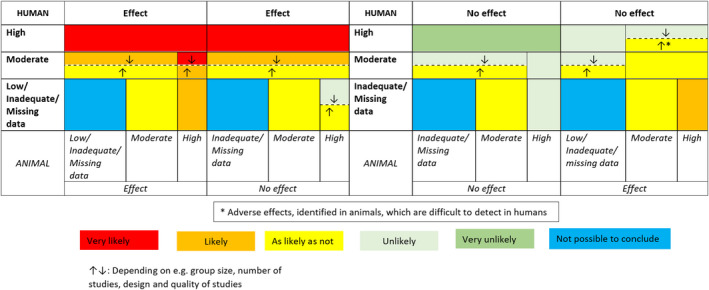
Scenarios for integration of animal and human evidence
Dietary exposure to neohesperidine dihydrochalcone (E 959) from its use as food additives was estimated combining food consumption data available within the Comprehensive Database with the maximum levels according to Annex II to Regulation (EC) No 1333/2008 13 and use levels submitted to EFSA following a call for data. Different scenarios were used to calculate the exposure (see Section 3.4). Uncertainties in the exposure assessment were identified and discussed.
The draft protocol for assessing dietary exposure to sweeteners was published on EFSA's website for comments, and the online public consultation was made available until 22 November 2019. A technical report on the outcome of this public consultation with the overview of the comments received and the general responses from EFSA was published (EFSA, 2020b).
3. Assessment
3.1. Technical data
3.1.1. Identity of the substance
According to the definition given in Commission Regulation (EU) No 231/2012, neohesperidine dihydrochalcone (E 959) is obtained by catalytic hydrogenation of neohesperidin. The food additive E 959 is identified as follows:
Chemical name: 2‐O‐α‐l‐rhamnopyranosyl‐4′‐β‐d‐glucopyranosyl hesperetin dihydrochalcone
Description: Off‐white, odourless, crystalline powder. Approximately between 1000 and 1800 times as sweet as sucrose
EINECS Number: 243‐978‐6
Chemical formula: C28H36O15
Molecular Weight: 612.6 g/mol
Synonyms: Neohesperidin dihydrochalcone; NHDC; Hesperetin dihydrochalcone‐4′‐β‐neohesperidoside; Neohesperidin DC
Solubility: Freely soluble in hot water, very slightly soluble in cold water, practically insoluble in ether and benzene
Following the EFSA calls for data, two interested business operators provided data and information to support the E 959 re‐evaluation. According to the interested business operators, the source material neohesperidin is a flavanone naturally occurring in bitter oranges (Citrus aurantium) and is isolated by alcohol extraction (Documentation provided to EFSA nr: 1 and 5). Additional identification numbers and names for neohesperidine dihydrochalcone (E 959), currently not reported in the EC Regulation No 231/2012, are the following:
CAS number: 20702‐77‐6
FEMA GRAS No: 3811
IUPAC name: 1‐[4‐[[2‐O‐(6‐deoxy‐α‐l‐mannopyranosyl)‐β‐d‐glucopyranosyl]oxy]‐2,6‐dihydroxyphenyl]‐3‐(3‐hydroxy‐4‐methoxyphenyl)propan‐1‐one
The chemical structure of neohesperidine dihydrochalcone (E 959) is given in Figure 1.
Figure 1.
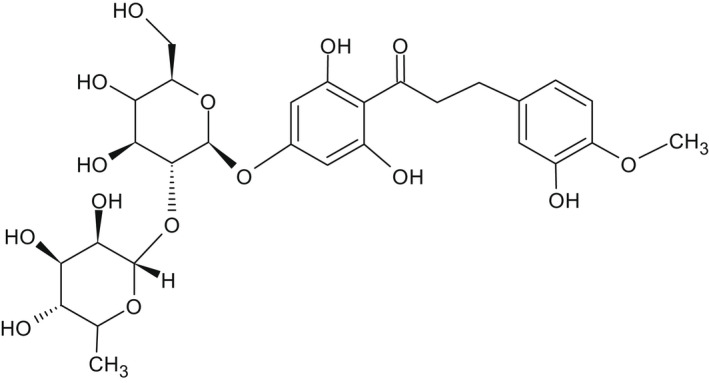
Chemical structure of neohesperidine dihydrochalcone (E 959)
The Panel noted that Fourier‐transform infrared (FT‐IR) and mass spectra were provided for a ‘commercial batch’ and a ‘working standard’ of E 959, consistent with the spectra of the authentic reference standard, i.e. ‘Ph. Eur. Neohesperidin Dihydrochalcone Chemical Reference Substance (CRS)’ (Documentation provided to EFSA nr: 3).
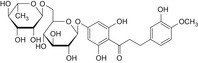
Structurally related flavonoid impurities can be present in E 959, they are: phloroacetophenone neohesperidoside (impurity A), neodiosmin (impurity B), neohesperidin (impurity C), naringin dihydrochalcone (impurity D), hesperidin dihydrochalcone (impurity E), hesperetin dihydrochalcone 7′glucoside (impurity F) and hesperetin dihydrochalcone (impurity G) (see Table 1 and Figure 2).
Impurities A, F and G are degradation products of neohesperidine dihydrochalcone; formed under the strong alkaline conditions or high temperatures of the manufacturing process (impurity A) or resulting from the hydrolytic cleavage of glycosidic bonds of neohesperidine dihydrochalcone (impurities F and G).
Impurity B is a flavone occurring in the source material bitter oranges and its structure is not altered during the hydrogenation step.
Impurity C is the unreacted starting material, substrate for the hydrogenation step.
Impurities D and E are produced by the side hydrogenation of flavanones naringin and hesperidin, respectively, which occur in bitter oranges and may be co‐extracted with the starting material neohesperidin.
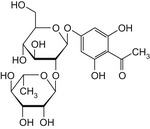
Table 1.
Chemical structures of E 959 impurities (Documentation provided to EFSA nr: 1, 3 and 5)
| Impurity | Chemical name | CAS No. | Structure |
|---|---|---|---|
| A | Phloroacetophenone neohesperidoside | – |
|
| B | Neodiosmin | 38665‐01‐9 |
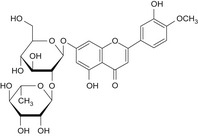
|
| C | Neohesperidin | 13241‐33‐3 |
|
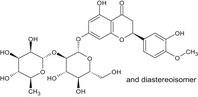
| D | Naringin dihydrochalcone | 18916‐17‐1 |
|
| E | Hesperidin dihydrochalcone | 35573‐79‐6 |
|
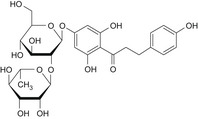
| F | Hesperetin dihydrochalcone 7′glucoside | – |
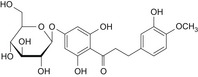
|
| G | Hesperetin dihydrochalcone | 35400‐60‐3 |
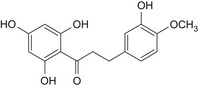
|
| H | Poncirin dihydrochalcone | – |
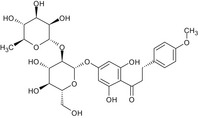
|
Figure 2.
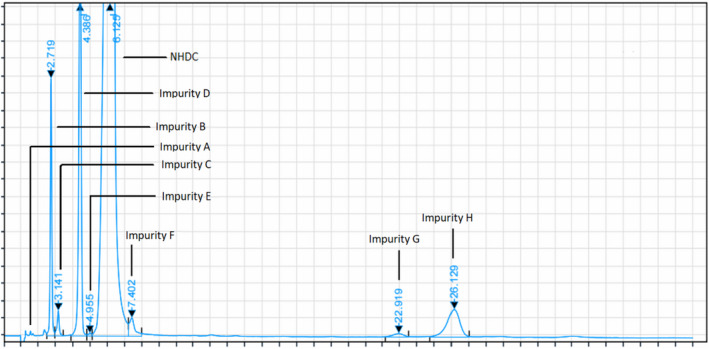
Representative chromatogram of E 959 with the individual impurities determined by HPLC analysis, as provided by one interested business operator (Documentation provided to EFSA nr: 3)
These impurities are described in the EU Pharmacopeia monograph for neohesperidine dihydrochalcone (European Pharmacopoeia 9.0, 2017).
The Panel noted that impurity D, naringin dihydrochalcone, is an authorised EU food flavouring ([FL‐no: 16.110]) and was evaluated within FGE.32 (EFSA CEF Panel, 2010).
In addition, one interested business operator tentatively identified another impurity based on its relative retention time (by high‐performance liquid chromatography (HPLC)) and ultraviolet (UV) absorption spectra, i.e. poncirin dihydrochalcone (impurity H). This impurity was consistently determined to be present in commercial batches of E 959. The chemical identity of this impurity was further investigated by ultrahigh‐performance liquid chromatography–electrospray ionisation multiple reaction monitoring tandem mass spectrometry (UHPLC‐ESI‐MS/MS) analysis (Documentation provided to EFSA nr: 3). According to the interested business operator, this impurity is the result of the hydrogenation of poncirin which is a flavonoid occurring in bitter oranges and thus possibly co‐extracted, and later hydrogenated, with the starting material neohesperidin. Horowitz and Gentili (1969), already described the synthesis of this compound during the development of neohesperidine dihydrochalcone.
Table 1 reports the chemical structures of the impurities discussed above and a representative chromatogram of E 959 with its individual impurities is presented in Figure 2.
3.1.2. Specifications
The EU specifications for neohesperidine dihydrochalcone (E 959), as laid down in the Commission Regulation (EU) No 231/2012, are listed in Table 2.
Table 2.
EU specifications for neohesperidine dihydrochalcone (E 959) according to Commission Regulation (EU) No 231/2012
| Commission Regulation (EU) No 231/2012 | |
|---|---|
| Synonyms | Neohesperidin dihydrochalcone; NHDC; Hesperetin dihydrochalcone‐4′‐β‐neohesperidoside; Neohesperidin DC |
| Definition | It is obtained by catalytic hydrogenation of neohesperidin. |
| Einecs | 243‐978‐6 |
| Chemical name | 2‐O‐α‐l‐rhamnopyranosyl‐4′‐β‐d‐glucopyranosyl hesperetin dihydrochalcone |
| Chemical formula | C28H36O15 |
| Molecular Weight | 612.6 |
| Assay | Content not less than 96% on the dried basis |
| Description | Off‐white, odourless, crystalline powder. Approximately between 1,000 and 1,800 times as sweet as sucrose |
| Identification | |
| Solubility | Freely soluble in hot water, very slightly soluble in cold water, practically insoluble in ether and benzene |
| Ultraviolet absorption maximum | 282–283 nm for a solution of 2 mg in 100 mL methanol |
| Neu's test | Dissolve about 10 mg of neohesperidine DC in 1 mL methanol, add 1 mL of a 1% 2‐aminoethyl diphenyl borate methanolic solution. A bright yellow colour is produced |
| Purity | |
| Loss on drying | Not more than 11% (105°C, 3 h) |
| Sulphated ash | Not more than 0.2% (expressed on dry weight basis) |
| Arsenic | Not more than 3 mg/kg (expressed on dry weight basis) |
| Lead | Not more than 2 mg/kg (expressed on dry weight basis) |
There are no JECFA specifications available for neohesperidine dihydrochalcone (E 959).
The Panel noted that the European Pharmacopeia contains monograph on neohesperidine dihydrochalcone (European Pharmacopoeia 9.0, 2017).
Based on the information provided by the interested business operators, the starting material neohesperidin is extracted in a hydroalcoholic solution from bitter oranges (C. aurantium) (Documentation provided to EFSA nr: 1 and 5). The Panel noted that the source of the starting material neohesperidin is not specified in the definition included in the current EU specifications of E 959. Therefore, the Panel is of the view that the current definition of E 959 should be revised to also include the source of the starting material neohesperidin and how the starting material is obtained.
The Panel also considered that the CAS number 20702‐77‐6 corresponding to neohesperidine dihydrochalcone should be included in the existing EU specifications for E 959.
Since neohesperidine dihydrochalcone (E 959) is optically active, the Panel considered that information on its specific rotation i.e., between −85 and −86 degrees (Documentation provided to EFSA nr: 1), should be included in the EU specifications. No information on the conditions at which the specific rotation was measured has been submitted.
The Panel noted that the Neu's test is not substance specific and thus not considered suitable to determine the identity of the neohesperidine dihydrochalcone (E 959). Therefore, the Panel is of the view that it could be removed from the EU specifications and could be replaced with an appropriate analytical method allowing the identification and quantification of neohesperidine dihydrochalcone (E 959).
The Panel noted that analytical data performed on at least five commercial batches of E 959, and supported by certificates of analysis, were provided by both interested business operators to demonstrate that E 959 is consistently produced within the established EU specifications as per Commission Regulation (EU) No 231/2012 (Documentation provided to EFSA nr: 1, 5 and 7).
Additionally, both interested business operators provided data on microbiological analyses performed in five commercial batches of E 959 supporting the microbiological quality of the food additive (total plate count not more than 1,000 CFU/g, yeast and mould not more than 100 CFU/g, absence of Escherichia coli in 1 g and absence of Salmonella in 25 g, Documentation provided to EFSA nr: 1 and 5).
One interested business operator provided analytical data on purity, impurities and water content for 67 batches of E 959 manufactured between January 2016 and March 2017 within the same manufacturing plant (Documentation provided to EFSA nr: 1). The purity of the 67 analysed batches, determined by HPLC‐UV, ranged from 97.0% to 98.8%. Regarding the impurities (see Table 1), the most abundant were naringin dihydrochalcone (impurity D), ranging from 1.11% up to 1.56%, neodiosmin (impurity B), ranging from 0.26% up to 0.55%, and poncirin dihydrochalcone (impurity H), ranging from 0.02% up to 0.4%. The Panel noted that the levels of impurities were estimated only based on peak areas (HPLC‐UV), relative to neohesperidine dihydrochalcone itself, except for impurity B (neodiosmin) for which an authentic standard was available as in accordance with the EU Pharmacopeia monograph for neohesperidin dihydrochalcone (Documentation provided to EFSA nr: 3). The interested business operator indicated that individual impurities did not exceed their maximum limits, as specified in the EU Pharmacopeia monograph for E 959 and presented in Table 3.
Table 3.
Maximum limits as specified in the EU Pharmacopeia monograph for neohesperidine dihydrochalcone (European Pharmacopoeia 9.0, 2017)
| Limit values based on the area of the principal peak in the chromatogram | |
|---|---|
| Phloroacetophenone neohesperidiside (Impurity A) | NMT 0.5%* |
| Neodiosmine (Impurity B) | NMT 2% |
| Neohesperidin (Impurity C) | NMT 0.5%* |
| Naringin dihydrochalcone (Impurity D) | NMT 2% |
| Hesperidin dihydrochalcone (Impurity E) | NMT 0.5%* |
| Hesperetin dihydrochalcone 7′glucoside (Impurity F) | NMT 0.5%* |
| Hesperetin dihydrochalcone (Impurity G) | NMT 0.5%* |
| Unknown (Impurity H)** | – |
| Total of all impurities apart from Impurity B | NMT 2.5% |
NMT: not more than.
These impurities are not explicitly quantified in the Ph. Eur. Rather, there is a general requirement that, other than impurities B and D which should each be not more than 2%, no other impurity (and therefore by inference, A, C, E, F and G in the Table) should individually be more than 0.5% using the HPLC‐UV method specified therein.
Included here for completeness since this impurity was reported by one interested business operator, although this impurity is not mentioned in the Ph. Eur.
The other interested business operator provided analytical data regarding purity, impurities and water content for ten batches of E 959 (Documentation provided to EFSA nr: 5 and 7). The purity of the ten batches was determined by HPLC with diode array detector (DAD) and ranged from 96.13% to 97.36% (w/w). Concerning impurities (see Table 1), the most abundant impurities detected were naringin dihydrochalcone (impurity D), ranging from 1.31% up to 2.32%, hesperetin dihydrochalcone (impurity G), ranging from 0.38% up to 0.61%, and neohesperidin (impurity C), ranging from 0.15% up to 0.32%. The Panel noted that the upper range of the most abundant impurities, i.e. impurity D and G, are slightly above the corresponding specification limits as set in the EU Pharmacopeia (see Table 3).
The Panel noted that impurity B, neodiosmin, which was the second most abundant impurity reported by the first interested business operator, was not detected in any of the 10 batches reported by this second interested business operator.
The Panel noted that the impurity profiles of commercial batches of E 959 differed within‐ and between the two interested business operators. The Panel considered that it cannot be assumed that these impurities were also present in the material(s) used in toxicity testing (see Appendices C and D) since they were reported only being compliant with the existing EU specifications for E 959 without any specific information on the impurities profile. On the other hand, the Panel noted that the impurities are all closely related to the chemical structure of neohesperidine dihydrochalcone (E 959) itself and that quantitative structure–activity relationship ((Q)SAR) analysis using the OECD QSAR Toolbox did not highlight any structural alerts in the impurities with regard to potential for genotoxicity (see Appendix E). The assay for E 959, as currently laid down in the EU specifications (EC Regulation 231/2012), requires not less than 96% chemical purity. Considering the nature, the levels and the origin of the impurities, along with the recommendation that the source material for the food additive (i.e. neohesperidin obtained from bitter oranges, C. aurantium) should be included in the EU definition of E 959, the Panel did not consider necessary to recommend inclusion of limit values for these impurities in the EU specifications of E 959.
Regarding toxic elements, the interested business operators provided analytical data on the levels of lead (Pb), arsenic (As), mercury (Hg) and cadmium (Cd) in commercial batches of E 959. Details of the analytical data provided are available in Appendix F (Documentation provided to EFSA nr: 1, 2, 3, 5 and 7). Given the differences in the quantification limits between the data sets, the results from the two interested business operators were considered to be consistent. The Panel calculated the potential exposure to the toxic elements from the use of E 959 assuming contamination of the food additive may be up to three times the highest reported level (or limit of quantification (LOQ) values) for the analysed batches (to account for representativeness, homogeneity and analytical measurement uncertainty) or up to the existing maximum limits for toxic elements, and then by calculation pro‐rata to the estimates of exposure to the food additive itself (regulatory maximum level exposure assessment scenario and refined brand‐loyal exposure assessment scenario; see Section 3.4.1). The exposure calculations to the toxic elements are presented and discussed in Appendix F.
The resulting figures showed that the potential exposure to Pb from the uses and use levels of E 959 would not be of concern using either the limit value calculated by the Panel as a possible EU specification limit (Table F.2, Appendix F) or the existing EU specification limit (Table F.3, Appendix F). As the occurrence levels for Pb reported by the interested business operators are substantially below the current EU specification limit (not more than 2 mg/kg), the Panel noted that a lower limit for Pb is technologically feasible.
Table F.2.
Risk assessment for toxic elements in E 959 based on the analytical data submitted by interested business operators and ‘modulated’ by the Panel
| Exposure to E 959 (mg/kg bw per day) (a) | MOE for As at 0.09 mg/kg | MOE for Pb at 0.12 mg/kg | % of the TWI for Cd at 0.03 mg/kg | % of the TWI for Hg at 0.03 mg/kg | % of the PDE for Pd at 2.5 mg/kg |
|---|---|---|---|---|---|
| 1.43 (b) | 2,331–62,160 | 2,914 | 0.012 | 0.01 | 0.18 |
| 0.24 (c) | 13,889–370,370 | 17,361 | < 0.01 | < 0.01 | 0.03 |
bw: body weight; MOE: margin of exposure; TWI: tolerable weekly intake; PDE: permitted daily exposure.
Refined regulatory maximum level exposure assessment scenario; Mean < 0.01–0.38; P95 0.02–1.43 mg/kg bw per day.
Refined brand‐loyal exposure assessment scenario; Mean < 0.01–0.09; P95 0.01–0.24 mg/kg bw per day.
Table F.3.
Risk assessment for toxic elements in E 959 based on the existing EU specifications
For As, the lower end of the margin of exposure (MOE) values falls below the target of 1,000 (Table F.3, Appendix F) at the current EU specifications limit, and this indicated that a lowering of the existing limit value of 3 mg/kg is recommended. A lowering seems technologically feasible, based on the analytical data provided.
For Cd and Hg, for which no maximum limits are set in the EU specifications, the resulting estimates of exposure are only a small fraction of their tolerable weekly intake (TWI) values (see Tables F.2 and F.3, Appendix F). Considering the occurrence levels reported by the interested business operators and the manufacturing process of E 959, the Panel sees no need to introduce specification limits for these two elements.
The Panel noted that the hydrogenation step in the manufacturing process for E 959, as described by the interested business operators (Documentation provided to EFSA nr: 1 and 5), is assisted by a heterogeneous palladium‐on‐charcoal (Pd/C) catalyst. In this respect, one interested business operator provided analytical data on the residual levels of palladium (Pd) in three batches of the food additive covering 3 production years. Pd was tested by inductively coupled plasma‐mass spectrometry (ICP‐MS) with an LOQ of 0.01 mg/kg. The levels of Pd reported were 0.42, 0.26 and 0.83 mg/kg (Documentation provided to EFSA nr: 3). The other interested business operator only declared that ‘the absence of palladium catalyst in the final product is ensured by the use of single‐use filters of a specific pore size, with which any remaining catalyst is removed’ (Documentation provided to EFSA nr: 7).
By using a modulation factor of 3 applied to the highest level reported (0.83 mg/kg), a value of 2.5 mg/kg of palladium in E 959 was used to perform the risk assessment for this element in the food additive (see Appendix F). The calculations provided in Appendix F indicated that, based on this concentration value, the estimated exposure to Pd coming from the uses and use levels of E 959 is only a small fraction of the permitted daily exposure (PDE) value (ICH, 2019). The Panel sees no need to introduce a limit for Pd in the EU specifications for E 959.
The Panel noted that the choice of maximum limits for toxic elements in the EU specifications is in the remit of risk management.
The interested business operators also provided data regarding contaminants, other than inorganic impurities, that might occur in the food additive given the botanical origin of the source material. One interested business operator provided analytical results for six batches of E 959 covering at least 5 years production, tested for polycyclic aromatic hydrocarbons (PAHs), pyrethrins, aflatoxins, melamine and a range of pesticides. In all analysed batches, the contaminants were not detected above the LOQ of the respective analytical methods applied (Documentation provided to EFSA nr: 1 and 3). The other interested business operator provided analytical data for five batches of E 959 tested for PAHs (benz(a)anthracene, benzo(a)pyrene, benzo(b)fluoranthene, chrysene), pesticide residues and mycotoxins. In all analysed batches, the contaminants were not detected above their LOQ (Documentation provided to EFSA nr: 5 and 7).
The Panel noted that both interested business operators provided adequate information on the analytical techniques and methods used for each potential contaminant determination along with the respective LOQ.
Solubility
Information on water solubility and particle size distribution of neohesperidine dihydrochalcone (E 959) was provided by the two interested business operators supporting E 959 re‐evaluation.
One interested business operator has provided information on the water solubility of neohesperidine dihydrochalcone based on the information reported by Benavente‐Garcia et al. (2001) (Documentation provided to EFSA nr: 4). Under the test conditions, the solubility of the test item at 20°C in water was determined to be 0.4 g/L and the solubility which was reached in 1 min after last addition. As reported by Benavente‐Garcia et al. (2001), the solubility of neohesperidine dihydrochalcone above 60°C rises sharply with the temperature and reaches 650 g/L at 80°C.
The same interested business operator has also provided information on the neohesperidine dihydrochalcone water solubility from a peer reviewed handbook data, the ‘Ullmann's Encyclopedia of Industrial Chemistry’ (von Rymon Lipinski, 2015) which reports the neohesperidine dihydrochalcone to have a solubility of ca. 0.5 g/L in water at room temperature (Documentation provided to EFSA nr: 4)
The Panel noted that the water solubility tests reported in the information submitted by this interested business operator has not been performed according to the requirements of the EFSA Guidance on technical requirements for regulated food and feed product applications to establish the presence of small particles including nanoparticles (EFSA Guidance on Particle‐TR) (EFSA Scientific Committee, 2021).
Following further to a request from EFSA for additional information, an interested business operator provided results of a water solubility test of neohesperidine dihydrochalcone performed using a shake flask method according to the method OECD TG 105 (Documentation provided to EFSA nr: 8). One batch of E 959 was analysed at 20°C and neohesperidine dihydrochalcone content was determined by HPLC technique with diode array detector (DAD) detector using external standard method. The validation of the method was conducted using the received sample of E 959. The LOQ and the limit of detection (LOD) were reported to be equal to 0.4015 and 0.0803 mg/L, respectively. Solubility of tested E 959 at 20 ± 0.5°C and at pH ranging 6.14–6.26 in water was determined to be 0.23 g/L. The Panel noted that the performed solubility test was not fully in line with the Guidance on Particle‐TR, as the recommended ultrafiltration step was not applied (EFSA Scientific Committee, 2021).
The Panel noted that, according to the data reported by the interested business operator, neohesperidine dihydrochalcone (E 959) can be considered very slightly soluble in water at 20°C (230 mg/L) according to the JECFA criterions (JECFA, 2006) and that the solubility increases with the temperature (Benavente‐Garcia et al., 2001).
Particle size distribution
One interested business operator provided information on particle size distribution (PSD) of 10 batches of E 959 determined by laser diffraction (LD) (Documentation provided to EFSA nr: 2). Based on the data measured, the interested business operator concluded that the particles with one dimension smaller than 100 nm are not present in E 959 batches. The calculated average median size of the analysed E959 particles is 12.5 ± 5.4 μm. The other interested business operator also provided data on particle size distribution generated by LD analysis of 6 batches of the food additive analysed in 2 different laboratories (3 batches each laboratory). The median mass aerodynamic diameter reported ranges from 9.38–12.51 μm (Documentation provided to EFSA nr: 6). The Panel noted that LD analysis is not considered a proper method to investigate the presence of nanosized particle as it does not provide information on the size of the constituent particles as required by the Guidance on Particle‐TR and is prone to errors for polydisperse materials (Rauscher et al., 2019; Mech et al., 2020a,b).
Following further to a request from EFSA for additional information, the two interested business operators also provided results from scanning electron microscopy (SEM) analysis on 5 and 10 batches of their E 959 products (Documentation provided to EFSA nr: 3, 7, 12 and 13). Both interested business operators performed the particle size analysis in the same accredited laboratory following the same method that was described. The 10 batches analysed by one interested business operator correspond to the ten batches analysed by means of LD (Documentation provided to EFSA nr: 2 and 13). Both interested business operators reported that the measured particles were of random shape (semi‐spherical, rod‐like, platelets and other shapes can be found on all the samples), and that the particle size was determined by measuring minimum Feret diameter of the particles (by using an image analysis software) as requested in the EFSA Guidance on Particle‐TR. For each batch of E 959, 200 representative particles were analysed, and number‐based size distributions and descriptive statistics were presented. The latter include the percentage of the particles smaller than 250 nm calculated based on the number of particles ≤ 500 nm. The results of analysis provided by one interested business operator shows that for 4 batches of E 959 analysed the percentage of particles with one dimension smaller than 250 nm was ranging from 12.50% to 22.62% and for the remining batch of 8.16%. The results provided by the other interested business operator show that for 3 batches the percentage of particles with one dimension smaller than 250 nm was ranging from 10.35% to 16.67% and for the remaining 7 batches from 4.05% to 9.58%.
The Panel noted that, based on the data provided and the criteria set in the EFSA Guidance on Particle‐TR (EFSA Scientific Committee, 2021), the presence of small particles including nanoparticles in the food additive cannot be excluded.
3.1.3. Manufacturing process
Two interested business operators provided detailed information on the manufacturing process of neohesperidine dihydrochalcone (E 959) (Documentation provided to EFSA nr: 1, 2 and 5).
In both manufacturing processes described by the interested business operators, the production of E 959 involves two main steps:
The first step involves the hydroalcoholic extraction of the flavanone neohesperidin from the source material (immature bitter oranges, C. aurantium) and purification. Information on the specifications of the starting material neohesperidin have been provided by both interested business operators.
The second step involves the catalytic reduction of the purified neohesperidin to neohesperidine dihydrochalcone, using palladium‐on‐charcoal (Pd/C) as catalyst under alkaline conditions. Further purification processes, such as crystallisation steps, are performed.
The Panel acknowledged that approaches other than extraction from bitter oranges (C. aurantium) might be used to obtain the precursor neohesperidin or neohesperidine dihydrochalcone itself. Grapefruit (Citrus paradisii) could be used to extract naringin which is then converted into phloroacetophenone‐4′‐β‐neohesperidoside and then condensed with isovanillin leading to the formation of neohesperidin that is hydrogenated to yield the dihydrochalcone (EFSA FEEDAP Panel, 2011). From the peer‐reviewed scientific literature, Frydman et al. (2005) proposed a three‐step process to convert hesperidin, a flavonoid extracted from orange peels, into neohesperidin (to be used as substrate for production of neohesperidine dihydrochalcone) using metabolic engineering and biotransformation: extraction of hesperidin from orange peels, hydrolysis of sugar moieties and biotransformation of hesperidin hydrolysis products into neohesperidin. Also, She et al. (2011) described a direct alcohol extraction of neohesperidine dihydrochalcone from Oxytropis myriophylla (genus of the family Leguminosae). In this respect, the Panel noted that according to the current existing EU specifications for E 959, neohesperidine dihydrochalcone (E 959) was manufactured by catalytic hydrogenation of neohesperidin. Thus, neohesperidine dihydrochalcone produced as described by She et al. (2011) does not comply with the current existing EU specifications for E 959.
The Panel noted that none of the interested business operators indicated using alternative production methods in the manufacture of neohesperidine dihydrochalcone (E 959), and therefore, these types of manufacturing process are not considered in the present assessment.
The interested business operator provided also information (Documentation provided to EFSA nr: 13) that, in the final step of the manufacturing process, neohesperidine dihydrochalcone (E 959) undergoes a milling process and a fine powder of E 959 of variable particle size distribution is obtained.
3.1.4. Methods of analysis in food
One interested business operator provided a compilation of references from the peer‐reviewed scientific literature describing a number of liquid chromatography (LC)‐based analytical methods, coupled with UV, mass spectrometry and/or (photo)diode array (DAD, PDA) as detection systems to determine neohesperidine dihydrochalcone (E 959) in a variety of food and beverages (Documentation provided to EFSA nr: 5). To mention some examples: LC‐based methods coupled with tandem mass spectrometry (MS/MS) described by Tsuruda et al. (2013) (LOQ 20 mg/kg, in solid foods such as biscuits, sausages, ice scream), by Ordoñez et al. (2015) (LOQ 0.17 mg/L, in soft drinks, nectars, mixed drinks) and by Lorenzo et al. (2015) using liquid chromatography electrospray ionisation tandem mass spectrometry (LC‐ESI‐MS/MS) and UPLC‐PDA in industrial beverages (LOQ 0.05 μg/L and LOQ 30 μg/L, respectively). Kubica et al. (2016) exploited hydrophilic interaction and reversed phase liquid chromatography (HILIC and RP‐LC, respectively) coupled with MS/MS for the determination of eight artificial sweeteners, including E 959, in alcoholic/non‐alcoholic beverages and instant drink powders. The LODs and LOQs were in the range 0.81–3.30 μg/L and 2.32–9.89 μg/L for HILIC and RP‐LC methods, respectively. Also, the use of capillary zone electrophoresis to quantify neohesperidine dihydrochalcone in low‐calorie soft drinks and foodstuffs was investigated by Pérez‐Ruiz et al. (2000), showing a LOD of 1.75 mg/L.
Similarly, the other interested business operator cited a number of relevant publications from the peer‐reviewed scientific literature, e.g. methods for the detection and quantification of neohesperidine dihydrochalcone in foods, using methods based on HPLC (Fisher, 1977; Castellar et al., 1997; Montijano et al., 1999; Zygler et al., 2011; Lim et al., 2013; Wang et al., 2015; Zhang et al., 2017). This business operator also referred to a European Committee for Standardization (CEN) standard for determination of neohesperidine dihydrochalcone (E 959) in foodstuffs (CEN/TS 14537:2003), which was validated in a collaborative test (BSI, 2003). Also, reference was made to a validated method for determination of neohesperidine dihydrochalcone in foods published by the European Commission (Wasik and Buchgraber, 2009) and to a method developed by the UK Food Standards Agency (FSA) for determination of sweeteners in food (FSA, 2014) reporting a LOD and LOQ as 1.8 and 6.1 mg/kg, respectively. According to the business operator, these analytical methods apply to all the food categories to which E 959 may be added (Documentation provided to EFSA nr: 1).
Other methods of analysis of E 959 in food described in the scientific literature are LC‐based methods using evaporative light‐scattering detection (ELSD) (Wasik et al., 2007; Buchgraber and Wasik, 2009) and (photo)‐diode array and charged aerosol detection systems (Ma et al., 2020; Sezgin et al., 2021). Also, the use of supercritical fluid chromatography coupled with ELSD was investigated for the determination of the sweetener in soft drinks (Lefler and Chen, 2008). Nambiar et al. (2018) used normal‐phase high performance thin‐layer chromatography for simultaneous densitometric determination of four sweeteners, including neohesperidine dihydrochalcone, in candies, jellies, beverages. Yang et al. (2014) applied cyclic voltammetry for quantitative determination of E 959 in beverages reaching a LOD of 2 × 10−8 mol/L (12 μg/L).
3.1.5. Stability of the substance, and reaction and fate in food
One interested business operator provided information on the stability of neohesperidine dihydrochalcone (E 959) to processing and storage based on the published literature (Documentation provided to EFSA nr: 5).
According to Borrego and Montijano (2001), the food additive is stable at room temperature for 3 years. Canales et al. (1993), studied the stability of neohesperidine dihydrochalcone (E 959) in aqueous buffer solutions at pH values from 1 to 7 and at temperatures ranging from 30 to 60°C for 140 days showing that, at room temperature conditions and pH > 2, the compound is resistant to hydrolysis. At higher temperature and lower pH, the glycosidic bond is hydrolysed, forming the aglycone hesperetin dihydrochalcone (impurity G, Table 1), glucose and rhamnose. The kinetics of degradation was first‐order. The half‐life values indicated that stability problems in the pH range (2–6) would not be expected and that the maximum stability of neohesperidine dihydrochalcone was at pH 4.
Other authors (Montijano and Borrego, 1999) tested the stability of neohesperidine dihydrochalcone in two water‐solvent mixture model systems (water‐ethanol and water‐glycerol) at a concentration of 300 mg/L under accelerated conditions (70, 80, 90°C) and buffered at pH 3. Samples were taken at different time intervals, until the concentration of neohesperidine dihydrochalcone was 75% or less. The results demonstrated that the stability of E 959 was improved in liquid media formulated with water and solvents with a dielectric constant lower than that of water, such as glycerol and ethanol, showing that the higher the ethanol concentration, the longer the half‐life is. Then, it would be expected that the shelf lives of alcoholic drinks and flavouring preparations would be higher than that of water‐based products.
The hydrolytic stability of neohesperidine dihydrochalcone has also been studied in solution (20 mg/L) during 65 days at various pHs at 20, 50, 70, and 90°C. The degradation follows a first‐order reaction with a half‐life at pH 4.5 of 195 days at 50°C, 62 days at 70°C and 23 days at 90°C. The time necessary to obtain a decrease of 10% of initial concentration of a nehoesperidin dihydrochalcone in aqueous diluted solution (3.26 × 10−5), at 20°C and pH 4.5 can be deduced and is of 164 days (Coiffard et al., 1998). Similarly, the stability of E 959 was tested in aqueous model solutions at different pHs and temperatures and the results showed a stability at room temperature and pH values over 2 (Inglett et al., 1969; Canales et al., 1993).
The stability of E 959 in different food and beverage matrices has been documented in several studies from the scientific literature. Tomás‐Barberán et al. (1995) tested E 959 in blackcurrant jam, boiled for 35–40 minutes at 102–106°C. The final pH of the product after addition of pectin was pH 3.08. The final product was packed in sterile jars and stored at ambient temperature for 18 months. Under these conditions, a degradation of 11% was observed at the end of storage. In yogurt fermented for 6 h at 43°C and 6 weeks under refrigerated storage (3°C), no significant decomposition was detected (Montijano et al., 1995). In non‐fermented milk products, sterilised at 120°C for 10 min, there was a 9–10% loss at pH 7 (Montijano et al., 1997a).
Montijano et al. (1996) studied the stability of neohesperidine dihydrochalcone in four fruit juice based soft drinks (orange, lemon, apple and pineapple), containing up to 25% fruit juice (15% for the lemon version) during pasteurisation at different times and temperatures (45 min, 100°C, 1 h at 90°C, 2 h at 80°C, 3 h at 70°C and 4 h at 70°C). The neohesperidine dihydrochalcone stability as a function of the pH was also studied in lemon drinks at different pHs (2.0, 2.5, 3.0, 3.5). All beverages contained 10 mg/L neohesperidine dihydrochalcone. The results showed that only at the lower pH tested (pH 2) a significant loss of neohesperidine dihydrochalcone (8%) was observed after 12 h at 90°C and hydrolysis products of neohesperidine dihydrochalcone were detected (hesperetin dihydrochalcone, hesperetin dihydrochalcone 4′‐B‐d‐glucoside and the sugars rhamnose and glucose). According to the authors, these extreme conditions are of no relevance to industrial pasteurisation of juice‐based beverages. In carbonated lemonade with a concentration of neohesperidine dihydrochalcone of 20 mg/L, at pH 3.3, a long‐term storage study is reported (1 year at room temperature either in the dark or light conditions) and two storage experiments under accelerated conditions (3 months at 40 and 90°C for 58 h in the dark) (Montijano et al., 1997b). The results showed no loss of neohesperidine dihydrochalcone after 1 year of storage. Similarly, the concentration of neohesperidine dihydrochalcone remained practically unchanged after 3 months at 40°C. A half‐life of 9.1 days was observed for the experiment at 90°C. However, this last drastic treatment is not representative of storage conditions for soft‐drinks.
The other interested business operator studied the storage stability of neohesperidine dihydrochalcone over 39 days at room temperature (20°C) (Documentation provided to EFSA nr: 1). Based on these results, the interested business operator declared that the dried food additive is stable over 3 years when stored in 5 kg metal drums with double polyethylene bag liners. The photostability was also tested using a xenon lamp, as light source, and quinine chemical actinometry was used to monitor exposure. The purity assay and loss on drying were measured before and after 12 h of irradiation in two different batches of E 959. The results showed that E 959 is photostable for the period measured (Documentation provided to EFSA nr: 1).
The Panel noted that no new data on the stability of E 959 under its currently permitted conditions of use/processing were provided by the interested business operators.
3.2. Authorised uses and use levels
Maximum levels of neohesperidine dihydrochalcone (E 959) have been defined in Annex II to Regulation (EC) No 1333/2008 on food additives, as amended. In this opinion, these levels are referred to as maximum permitted levels (MPLs).
Currently, neohesperidine dihydrochalcone (E 959) is an authorised food additive in the EU in 38 food categories (FCs) with MPLs ranging from 5 to 400 mg/kg and at quantum satis (QS) in three table‐top sweeteners food categories. Table 4 lists the food categories with their restrictions/exceptions that are permitted to contain added neohesperidine dihydrochalcone (E 959) and the corresponding MPLs as defined in Annex II to Regulation (EC) No 1333/2008.
Table 4.
MPLs of neohesperidine dihydrochalcone (E 959) in foods according to Annex II to Regulation (EC) No 1333/2008
| Food category number | Food categories | E‐number | Restrictions/exceptions | MPL (mg/L or mg/kg as appropriate) |
|---|---|---|---|---|
| 01.4 | Flavoured fermented milk products including heat‐treated products | E 959 | Only energy‐reduced products or with no added sugar | 50 |
| 02.2.2 | Other fat and oil emulsions including spreads as defined by Council Regulation (EC) No 1234/2007 and liquid emulsions | E 959 | Only as a flavour enhancer, only in the fat groups B & C in Annex XV to Regulation (EC) No 1234/2007 | 5 |
| 3 | Edible ices | E 959 | Only energy‐reduced or with no added sugar | 50 |
| 04.2.2 | Fruit and vegetables in vinegar, oil, or brine | E 959 | Only sweet‐sour preserves of fruit and vegetables | 100 |
| 04.2.3 | Canned or bottled fruit and vegetables | E 959 | Only fruit energy‐reduced or with no added sugar | 50 |
| 04.2.4.1 | Fruit and vegetable preparations excluding compote | E 959 | Only energy‐reduced | 50 |
| 04.2.5.1 | Extra jam and extra jelly as defined by Directive 2001/113/EC | E 959 | Only energy‐reduced jams jellies and marmalades | 50 |
| 04.2.5.2 | Jam, jellies and marmalades and sweetened chestnut puree as defined by Directive 2001/113/EC | E 959 | Only energy‐reduced jams, jellies and marmalades | 50 |
| E 959 | Only fruit jellies as flavour enhancer | 5 | ||
| 04.2.5.3 | Other similar fruit or vegetable spreads | E 959 | Only energy‐reduced fruit or vegetable spreads and dried‐fruit based sandwich spreads, energy‐reduced or with no added sugar | 50 |
| 5.1 | Cocoa and Chocolate products as covered by Directive 2000/36/EC | E 959 | Only energy‐reduced or with no added sugar | 100 |
| 5.2 | Other confectionery including breath freshening microsweets | E 959 | Only cocoa or dried fruit‐based, energy‐reduced or with no added sugar | 100 |
| E 959 | Only cocoa, milk, dried fruit or fat‐based sandwich spreads, energy‐reduced or with no added sugar | 50 | ||
| E 959 | Only starch‐based confectionery energy‐reduced or with no added sugar | 150 | ||
| E 959 | Only confectionery with no added sugar | 100 | ||
| E 959 | only breath‐freshening micro‐sweets, with no added sugar | 400 | ||
| 5.3 | Chewing gum | E 959 | Only with added sugar or polyols, as flavour enhancer (a) | 150 |
| E 959 | Only with no added sugars | 400 | ||
| 5.4 | Decorations, coatings and fillings, except fruit‐based fillings covered by category 4.2.4 | E 959 | Only starch‐based confectionery energy‐reduced or with no added sugar | 150 |
| Decorations, coatings and fillings, except fruit‐based fillings covered by category 4.2.4 | E 959 | Only confectionery with no added sugar | 100 | |
| Decorations, coatings and fillings, except fruit‐based fillings covered by category 4.2.4 | E 959 | Only cocoa or dried fruit‐based, energy‐reduced or with no added sugar | 100 | |
| Decorations, coatings and fillings, except fruit‐based fillings covered by category 4.2.4 | E 959 | Only sauces | 50 | |
| 6.3 | Breakfast cereals | E 959 | Only breakfast cereals with a fibre content of more than 15%, and containing at least 20% bran, energy‐reduced or with no added sugar | 50 |
| 7.2 | Fine bakery wares | E 959 | Only cornets and wafers, for ice‐cream, with no added sugar | 50 |
| 08.3.1 | Non‐heat‐treated meat products | E 959 | As a flavour enhancer only | 5 |
| 08.3.2 | Heat‐treated meat products | E 959 | As a flavour enhancer only, except for foie gras, foie gras entier, blocs de foie gras, Libamaj, libamaj ageszben, libamaj tombben | 5 |
| 9.2 | Processed fish and fishery products including mollusks and crustaceans | E 959 | Only sweet‐sour preserves and semi‐preserves of fish and marinades of fish, crustaceans and mollusks | 30 |
| 11.4.1 | Table‐top Sweeteners in liquid form | E 959 | Quantum satis | |
| 11.4.2 | Table‐top Sweeteners in powder form | E 959 | Quantum satis | |
| 11.4.3 | Table‐top Sweeteners in tablets | E 959 | Quantum satis | |
| 12.4 | Mustard | E 959 | 50 | |
| 12.5 | Soups and broths | E 959 | Only energy‐reduced soups | 50 |
| 12.6 | Sauces | E 959 | 50 | |
| 12.7 | Salads and savoury based sandwich spreads | E 959 | Only Feinkostsalat | 50 |
| 12.9 | Protein products, excluding products covered in category 1.8 | E 959 | Only vegetable protein products, only as flavour enhancer | 5 |
| 13.2 | Dietary foods for special medical purposes defined in Directive 1999/21/EC (excluding products from food category 13.1.5) | E 959 | 100 | |
| 13.3 | Dietary foods for weight control diets intended to replace total daily food intake or an individual meal (the whole or part of the total daily diet) | E 959 | 100 | |
| 14.1.3 | Fruit nectars as defined by Directive 2001/112/EC and vegetable nectars and similar products | E 959 | Only energy‐reduced or with no added sugar | 30 |
| 14.1.4 | Flavoured drinks | E 959 | Only energy reduced or with no added sugar, except milk and milk derivative based flavoured drinks | 30 |
| E 959 | Only milk and milk derivative based flavoured drinks, energy reduced or with no added sugar | 50 | ||
| 14.2.1 | Beer and malt beverages | E 959 | Only alcohol‐free beer or with an alcohol content not exceeding 1,2% vol; ‘Bière de table/Tafelbier/Table beer’ (original wort content less than 6%) except for ‘Obergäriges Einfachbier’; Beers with a minimum acidity of 30 milli‐equivalents expressed as NaOH; Brown beers of the ‘oud bruin’ type | 10 |
| E 959 | Only energy‐reduced beer | 10 | ||
| 14.2.3 | Cider and perry | E 959 | 20 | |
| 14.2.8 | Other alcoholic drinks including mixtures of alcoholic drinks with non‐alcoholic drinks and spirits with less than 15% of alcohol | E 959 | 30 | |
| 15.1 | Potato‐, cereal‐, flour‐ or starch‐based snacks | E 959 | 50 | |
| 15.2 | Processed nuts | E 959 | 50 | |
| 16 | Desserts excluding products covered in category 1, 3 and 4 | E 959 | Only energy‐reduced or with no added sugar | 50 |
| 17.1 | Food supplements supplied in a solid form, excluding food supplements for infants and young children | E 959 | 100 | |
| E 959 | Only food supplements in chewable form | 400 | ||
| 17.2 | Food supplements supplied in a liquid form, excluding food supplements for infants and young children | E 959 | 50 | |
| E 959 | Only food supplements in syrup form | 400 |
MPL: maximum permitted level.
If E 950, E 951, E 955, E 957, E 959 and E 961 are used in combination in chewing gum, the maximum level for each is reduced proportionally.
Use of neohesperidine dihydrochalcone (E 959) is not authorised according to Annex III to Regulation (EC) No 1333/2008 (Union list of food additives including carriers approved for use in food additives, food enzymes, food flavourings, nutrients and their conditions of use).
3.3. Exposure data
3.3.1. Concentration data
Most food additives in the EU are authorised at a specific MPL. However, a food additive may be used at a lower level than the MPL. Therefore, actual concentration data are required to perform a more realistic exposure assessment, especially for those food additives with an MPL at QS in at least one food category.
To obtain actual concentration data, EFSA issued a public call 14 for data (use levels and/or analytical data) on neohesperidine dihydrochalcone (E 959) in the framework of Regulation (EC) No 1333/2008 on food additives and of Commission Regulation (EU) No 257/2010 regarding the re‐evaluation of approved food additives.
In response to this public call, information on use levels of neohesperidine dihydrochalcone (E 959) in foods was made available to EFSA by 1 October 2018.
Analytical data of neohesperidine dihydrochalcone (E 959) in food and beverages submitted by 16 December 2020 were considered for the present assessment.
Reported use levels of neohesperidine dihydrochalcone (E 959) in foods
Industry provided EFSA with 59 use levels of neohesperidine dihydrochalcone (E 959) in foods for all the 38 food categories in which the use of this additive is authorised according to Annex II to Regulation (EC) No 1333/2008 (Table 4).
Information on these use levels was made available by Association of the European Self‐Medication Industry (AESGP), Food Drink Europe (FDE), Food Supplement Europe (FSE), Intertek Scientific & Regulatory Consultancy (Intertek) and Specialised Nutrition Europe (SNE).
The Panel noted that the majority of the use levels (n = 52) were provided by Intertek that did not report use levels from food industry but from food additive producers. Use levels reported by food additive producers are not considered in the refined exposure assessments as described in the protocol (EFSA, 2020b). These data are only used in the regulatory maximum level exposure assessment scenario in case of QS authorisation and when no data are provided for these uses by food industry. For neohesperidine dihydrochalcone (E 959), only the use levels provided by Intertek for the three food categories for table‐top sweeteners (FC 11.4.1, 11.4.2 and 11.4.3) were used in the regulatory maximum level exposure assessment scenario, because of QS authorisation (Table 4).
For the refined brand‐loyal scenario, only seven out of the 59 use levels were relevant. Among them, three belonged to FC 14.1.4 Flavoured drinks (non‐milk) which were reported as niche products. Since there were no other use levels from more widely consumed flavoured drinks, these three use levels were used in the refined scenario. The other four use levels referred to FC 17.1 Food Supplements in the solid form with the restriction of ‘only food supplements in chewable form’; of these four, three use levels referred to widely consumed food supplements and were therefore used in the exposure assessment for the refined brand‐loyal scenario.
Annex A, Table A.1 provides the use levels of neohesperidine dihydrochalcone (E 959) in foods as reported by industry.
Analytical results of neohesperidine dihydrochalcone (E 959) provided by Member States
In total 4,294 analytical results on neohesperidine dihydrochalcone (E 959) were reported to EFSA by national competent authorities of Austria (n = 1,346), Spain (n = 1,125), Slovakia (n = 905), Germany (n = 902) and Czechia (n = 16). The vast majority (99.9%) of these results were left‐censored (below LODs/LOQs).
In the remit of the re‐evaluation of sweeteners, the assessment of exposure to sweeteners is based on the assumptions that foods and beverages containing the sweetener are identified from the occurrence data set and the levels in these products are derived from the quantified analytical results only (EFSA, 2020b). Therefore, the left‐censored data were excluded from the exposure assessment (n = 4,289). Only five non left‐censored data remained. During data cleaning, one of these results was indicated by the data provider to be left‐censored and therefore it was also excluded. For three analytical results labelled as ‘Dietary supplements’ or ‘Combination of vitamins and minerals supplements’, the data provider could not confirm the analytical method that had been used to produce the data and therefore also these levels were excluded. One analytical result reported for chewing gum (FC 05.3) exceeded the MPL. As the data provider could not confirm the result, it was also excluded. Overall, no suitable analytical results for neohesperidine dihydrochalcone (E 959) in foods were available for the exposure assessment.
3.3.2. Summarised data extracted from the Mintel Global New Products Database
The Mintel Global New Products Database (GNPD) is an online database which monitors new introductions of packaged goods in the market worldwide. It contains label information of over 3.3 million food and beverage products of which more than 1,200,000 are or have been available on the European food market. Mintel started covering EU's food markets in 1996, currently having 24 out of its 27 member countries and Norway presented in the Mintel GNPD. 15
For the purpose of this opinion, the Mintel GNPD was used for checking the labelling of food and beverage products and food supplements for neohesperidine dihydrochalcone (E 959) 16 within the EU's food market as the database contains the required ingredient information on the label.
According to Mintel's GNPD, neohesperidine dihydrochalcone (E 959) was labelled on a few products (n = 122), mainly belonging to GNPD food subcategories Carbonated Soft Drinks (n = 36), Vitamins & Dietary Supplements (n = 32), and Nutritional & Meal Replacement Drinks (n = 15) between January 2016 and November 2021.
Annex A, Table A.2 lists the percentage of food products labelled to contain neohesperidine dihydrochalcone (E 959) out of the total number of food products per food subcategory according to Mintel's GNPD food classification. The percentages ranged from 0.01% in some food subcategories to 0.62% in Carbonated Soft Drinks. The average percentage of foods labelled to contain neohesperidine dihydrochalcone (E 959) was 0.13%. However, these percentages do not represent the market share of the products listed per food subcategory.
The Panel noted that among the beverages included in the subcategory Carbonated Soft Drinks labelled to contain neohesperidine dihydrochalcone (E 959), zero calorie beverages of three popular brands were listed. Within Vitamins and Dietary Supplements, products both in liquid (some in syrup form) and solid form (some as chewable) were present.
Annex A, Table A.2 also includes a match to the corresponding food categories in Annex II to Regulation (EC) No 1333/2008; however, this linkage is meant to be indicative since the food subcategories in the Mintel GNPD only partly correspond to the categories in the relevant legislation.
3.3.3. Food consumption data used for exposure assessment
EFSA Comprehensive European Food Consumption Database
Since 2010, the EFSA Comprehensive European Food Consumption Database (Comprehensive Database) has been populated with national data on food consumption at a detailed level. Competent authorities in European countries provide EFSA with data on the level of food consumption by the individual consumer from the most recent national dietary survey in their country (cf. Guidance of EFSA on the ‘Use of the EFSA Comprehensive European Food Consumption Database in Exposure Assessment’, EFSA, 2011). The version of the Comprehensive database taken into account in the exposure assessment of neohesperidine dihydrochalcone (E 959) was published in July 2021. 17 Data from EU Member States were considered for the estimations.
The food consumption data in the Comprehensive Database were collected by different methodologies and thus direct country‐to‐country comparisons of the exposure estimates may not be appropriate. Depending on the food category and the level of detail used for the exposure calculations, the exposure estimations may be influenced by subjects' underreporting and/or misreporting of the consumption amounts. Nevertheless, the EFSA Comprehensive Database includes the currently best available food consumption data across the EU.
Food consumption data from infants, toddlers, children, adolescents, adults and the elderly were used in the exposure assessment. For the present assessment, food consumption data were available from 41 different dietary surveys carried out in 22 EU Member States (Table 5). Not all Member States provided consumption information for all population groups, and in some cases the same country provided food consumption data from more than one consumption survey. In most cases, when for one country and age class different dietary surveys were available, only the most recent was used. However, when two national surveys from the same country gave a better coverage of the age range than using only the most recent one, both surveys were kept. For details on each survey, see Annex A, Table A.3.
Table 5.
Population groups considered for the exposure assessment of neohesperidine dihydrochalcone (E 959)
| Population | Age range | EU Member States with food consumption surveys covering more than 1 day |
|---|---|---|
| Infants | From more than 12 weeks up to and including 11 months of age | Bulgaria, Cyprus, Denmark, Estonia, Finland, France, Germany, Italy, Latvia, Portugal, Slovenia |
| Toddlers (a) | From 12 months up to and including 35 months of age | Belgium, Bulgaria, Cyprus, Denmark, Estonia, Finland, France, Germany, Hungary, Italy, Latvia, Netherlands, Portugal, Slovenia, Spain |
| Children (b) | From 36 months up to and including 9 years of age | Austria, Belgium, Bulgaria, Cyprus, Czechia, Denmark, Estonia, Finland, France, Germany, Greece, Hungary, Italy, Latvia, Netherlands, Portugal, Spain, Sweden |
| Adolescents | From 10 years up to and including 17 years of age | Austria, Belgium, Cyprus, Czechia, Denmark, Estonia, Finland, France, Germany, Greece, Hungary, Italy, Latvia, Netherlands, Portugal, Romania, Slovenia, Spain, Sweden |
| Adults | From 18 years up to and including 64 years of age | Austria, Belgium, Croatia, Cyprus, Czechia, Denmark, Estonia, Finland, France, Germany, Greece, Hungary, Ireland, Italy, Latvia, Netherlands, Portugal, Romania, Slovenia, Spain, Sweden |
| The elderly (b) | From 65 years of age and older | Austria, Belgium, Cyprus, Denmark, Estonia, Finland, France, Germany, Greece, Hungary, Ireland, Italy, Latvia, Netherlands, Portugal, Romania, Slovenia, Spain, Sweden |
The term ‘toddlers’ in the Comprehensive Database (EFSA, 2011) corresponds to ‘young children’ in Regulations (EC) No 1333/2008 and (EU) No 609/2013.
The terms ‘children’ and ‘the elderly’ correspond, respectively, to ‘other children’ and the merge of ‘elderly’ and ‘very elderly’ in the Comprehensive Database (EFSA, 2011).
Since 2018, all consumption records in the Comprehensive Database have been codified according to the FoodEx2 classification system (EFSA, 2015b) Nomenclature from the FoodEx2 classification system has been linked to the food categorisation system of Annex II to Regulation (EC) No 1333/2008, part D, to perform exposure assessments of food additives. In practice, the FoodEx2 food codes were matched to the food categories. For a detailed description of the methodology used to link FoodEx2 codes to the food categories, see Section 5.2.1 of EFSA (2020b). In FoodEx2, facets are used to provide further information about different properties and aspects of foods recorded in the Comprehensive Database. Facets were used in the exposure assessment of neohesperidine dihydrochalcone (E 959) to further identify foods to be included in the assessment (e.g. sweetener‐related facets for foods in relevant food categories, see details in Annex A, Table A.4).
Food categories considered for the exposure assessment of neohesperidine dihydrochalcone (E 959)
The food categories, for which MPLs are set and use levels of neohesperidine dihydrochalcone (E 959) were provided, were selected from the nomenclature of the Comprehensive Database (FoodEx2 classification system), at the most detailed level possible (up to FoodEx2 Level 7) (EFSA, 2015b).
Facets were used to identify eating events referring to foods assumed to contain sweeteners and to foods related to specific restrictions/exceptions defined in the legislation (see details in Table 4 and Annex A, Table A.4).
Facets were not used to identify relevant eating events for foods belonging to FC 11.4 Table‐top sweeteners and FC 05.3 Chewing gum; this is also true for ‘gum drops’ belonging to FC 05.2 Other confectionery including breath refreshening microsweets, ‘energy drinks’ belonging to FC 14.1.4 Flavoured drinks and ‘vitamin and mineral supplements’ belonging to FC 17 Food supplements as defined in Directive 2002/46/EC excluding food supplements for infants and young children. These foods and food categories are expected to be major contributors to the exposure to sweeteners according to the literature and present a relatively high percentage of products labelled to contain at least one sweetener. Thus, all eating events referring to these foods and food categories were included in the exposure assessment of neohesperidine dihydrochalcone (E 959) as described in the protocol (EFSA, 2020b).
As FC 17 does not consider food supplements for infants and toddlers as defined in the legislation, the exposure to neohesperidine dihydrochalcone (E 959) for these two population groups does not include the exposure via food supplements.
Eating occasions belonging to FC 13.2 (Dietary foods for special medical purposes), FC 13.3 (Dietary foods for weight control diets intended to replace total daily food intake or an individual meal) and FC 18 (Processed foods), were reclassified under food categories in accordance to their main component (e.g. gluten‐free pasta reclassified as pasta). Therefore, MPLs for FC 13.2 Dietary foods for special medical purposes and FC 13.3 Dietary foods for weight control diets categories were not considered in the exposure assessment.
For the following food categories, some restrictions/exceptions of the use of neohesperidine dihydrochalcone (E 959) in certain food categories (Table 4) are not referenced in the Comprehensive Database and therefore these restrictions were not taken into account. This may have resulted in an overestimation of the exposure in all scenarios.
For FC 2.2.2 Other fat and oil emulsions including spreads as defined by Council Regulation (EC) No 1234/2007 and liquid emulsions the restriction ‘only as flavour enhancer, only in the fat groups B & C in Annex XV to Regulation (EC) No 1234/2007’.
For FC 6.3 Breakfast cereals the restriction ‘only breakfast cereals with a fibre content of more than 15%, and containing at least 20% bran.’
For FC 8.3.1 Non‐heat‐treated meat products food category restriction ‘as flavour enhancer only’.
For FC 8.3.2 Heat‐treated meat product food categories, the restriction ‘except for foie gras, foie gras entier, blocs de foie gras, Libamaj, libamaj ageszben, libamaj tombben’, as well as the restriction ‘as flavour enhancer only’.
For FC 12.7 Salads and savoury based sandwich spreads, restriction ‘only Feinkostsalat’.
For FC 12.9 Protein products, excluding products covered in category 1.8, restriction ‘only as flavour enhancer’.
For FC 17.1 Food supplements supplied in a solid form, excluding food supplements for infants and young children', the restriction ‘only food supplements in chewable form’.
For FC 17.2 ‘Food supplements supplied in a liquid form, excluding food supplements for infants and young children’, the restriction ‘only food supplements in syrup form’.
Moreover, for FC 04.2.2 ‘Fruit and vegetables in vinegar, oil, or brine’ the restriction ‘only sweet‐sour preserves of fruit and vegetables’ was matched to ‘pickled’ to represent sweet and sour.
Furthermore, when specific restrictions/exceptions defined in the legislation within the same food category could not be identified in the Comprehensive Database, the highest MPL/use level among the authorised uses for that food category was extended to the whole food category, unless this MPL/use level referred to a niche food.
Full details are reported in Annex A, Table A.4 including a detailed description of the assumptions used for matching MPLs and use levels with the consumption events for all exposure scenarios.
For the refined scenarios (both the refined regulatory maximum level and the refined brand‐loyal exposure assessment scenarios), only three food categories were considered: FC 14.1.4 ‘Flavoured drinks’, FC 17.1 ‘Food supplements supplied in a solid form, excluding food supplements for infants and young children’ and FC 17.2 ‘Food supplements supplied in a liquid form, excluding food supplements for infants and young children’. In detail:
-
–
use levels reported for orange, lemon and mixed‐flavour soft drinks within FC 14.1.4 were matched to consumption of non‐milk‐based flavoured drinks only. No use levels were available for milk‐based flavoured drinks so they were not considered in the assessment. This may result in an underestimation of exposure.
-
–
use levels reported for FC 17.1 Food Supplements in the solid form with restriction to ‘only food supplements in chewable form’ were matched to the entire FC 17. Matching the use levels to the entire FC 17 may result in an overestimation of the exposure.
Overall, out of the 38 food categories in which neohesperidine dihydrochalcone (E 959) is authorised, 36 food categories were included in the regulatory maximum level exposure assessment scenario with their MPLs/highest use levels. For the refined scenarios (i.e. refined regulatory maximum level exposure assessment scenario and refined brand‐loyal exposure assessment scenario), three food categories were included based on the reported use levels and MPLs.
3.4. Exposure estimates
In the remit of the re‐evaluation of sweeteners, the Panel considered it appropriate to estimate chronic exposure (EFSA, 2020b). As suggested by the EFSA Working Group on Food Consumption and Exposure (EFSA, 2011), dietary surveys with only 1 day per subject were not considered as they are not adequate to assess repeated exposure. Similarly, subjects who participated only 1 day in the dietary studies, when the protocol prescribed more reporting days per individual, were also excluded from the chronic exposure assessment.
Exposure assessments of sweeteners under the re‐evaluation programme are carried out by the Panel based on two different sets of concentration data: (a) MPLs of use set down in the EU legislation (in the regulatory maximum level exposure assessment scenario) and (b) use levels and/or analytical data provided through the calls for data (in the refined brand‐loyal exposure assessment scenario).
To calculate the chronic dietary exposure to neohesperidine dihydrochalcone (E 959), food consumption and body weight data at the individual level were extracted from the Comprehensive Database and linked to the concentration data as described in Section 5.2.1 of the protocol (EFSA, 2020b).
Chronic dietary exposure was calculated by combining MPLs/use levels of neohesperidine dihydrochalcone (E 959) for each food with the average daily consumption of each food at individual level in each dietary survey and population group. Exposure estimates per food were summed and divided by the individual's body weight resulting in a distribution of daily individual average exposures per kilogram body weight. Based on these distributions, the mean and 95th percentile (P95) of exposure were calculated per survey and per population group. Means calculated over a number of less than six subjects and 95th percentiles calculated over a number of subjects of less than 60 subjects were removed from the final statistics as they may not be statistically robust (EFSA, 2011).
As stated in Section 5.2.3 of the protocol on dietary exposure assessment (EFSA, 2020b) supporting the present evaluation, the dietary exposure was assessed for consumers‐only of at least one food category that could contain neohesperidine dihydrochalcone (E 959) 18 for all scenarios. Exposure estimates for these population groups are assumed to be the best approximate reflecting the exposure levels in diabetics, which are considered to be the population with the highest exposure to sweeteners (EFSA, 2020b). Depending on the food categories considered in the exposure assessment, the exposure was estimated based on different numbers of consumers. Exposure estimates based on fewer food categories could be higher than those based on a larger number of food categories due to a higher number of non‐consumers within certain food categories.
Consumers‐only of a single food category may have a higher exposure than consumers‐only of at least one food category. To evaluate this, the exposure to neohesperidine dihydrochalcone (E 959) for consumers‐only of each single food category (but still considering the whole diet) was also calculated for the refined brand‐loyal exposure assessment scenario. These exposure estimates are discussed if they are higher than the exposure estimates for consumers‐only of at least one food category.
Regulatory maximum level exposure assessment scenario
The regulatory maximum level exposure assessment scenario is based on the MPLs as set in Annex II to Regulation (EC) No 1333/2008 and, in case of QS, on the maximum reported use level/the highest reliable percentile of the analytical levels when available. For neohesperidine dihydrochalcone (E 959), the MPLs used in the assessment are listed in Table A.4 of Annex A. For the three food categories of 11.4 Table‐top sweeteners in which neohesperidine dihydrochalcone (E 959) is authorised at QS, the maximum reported use level was used as no analytical data were available.
Refined regulatory maximum level exposure assessment scenario
Results of the regulatory maximum level exposure assessment scenario are not comparable to the exposure estimates of the refined scenarios since the food categories considered are different (n = 36 for the regulatory maximum level exposure assessment scenario and n = 3 for the refined scenarios) and therefore underlying populations of consumers‐only are not the same. For this reason, the Panel considered it appropriate to also perform a refined maximum level exposure assessment scenario based on the same population group as included in the refined brand‐loyal exposure assessment scenario.
The refined regulatory maximum level exposure assessment scenario considers only the three food categories for which use levels were provided to the Panel (Annex A, Table A.4). In this scenario, it is assumed that a consumer is exposed long‐term to neohesperidine dihydrochalcone (E 959) present at the MPL for these food categories, instead of at use level as in the refined brand‐loyal scenario.
Refined brand‐loyal exposure assessment scenario
The refined brand‐loyal exposure assessment scenario for neohesperidine dihydrochalcone (E 959) was based on use levels reported by food industry. This exposure scenario considers only those food categories for which these data were provided to the Panel. In this brand‐loyal consumers only scenario, it is assumed that a consumer is exposed long‐term to neohesperidine dihydrochalcone (E 959) present at the maximum reported use level for one food category and at the mean of typical use levels for the other authorised food categories as explained in the protocol (EFSA, 2020b).
Table A.4 of Annex A summarises the use levels of neohesperidine dihydrochalcone (E 959) used in the refined brand‐loyal exposure assessment scenario.
Additional exposure scenario for uncertainty analysis
In addition, to evaluate a possible uncertainty related to the quality of the facets, refined scenarios (refined regulatory maximum and brand‐loyal) without using the facets to select relevant foods were performed. Results for these scenarios are discussed in the uncertainty section.
3.4.1. Dietary exposure to neohesperidine dihydrochalcone (E 959)
Table 5 summarises the estimated dietary exposure to neohesperidine dihydrochalcone (E 959) from its use as a food additive in six population groups (Table 6) according to the different exposure scenarios among consumers only of at least one food category containing neohesperidine dihydrochalcone (E 959).
Table 6.
Summary of dietary exposure to neohesperidine dihydrochalcone (E 959) from its use as a food additive in the regulatory maximum level exposure assessment scenario and in the refined exposure scenarios, in six population groups among consumers only of at least one food category that could contain neohesperidine dihydrochalcone (E 959) (minimum–maximum across the dietary surveys in mg/kg bw per day and number of surveys in brackets) (a)
| Infants (12 weeks–11 months) | Toddlers (12–35 months) | Children (3–9 years) | Adolescents (10–17 years) | Adults (18–64 years) | The elderly (≥ 65 years) | |
|---|---|---|---|---|---|---|
| Regulatory maximum level exposure assessment scenario | ||||||
| Mean (b) |
0.01–0.22 (10) |
0.02–0.38 (15) |
0.02–0.32 (19) |
0.02–0.19 (21) |
0.02–0.15 (22) |
0.01–0.14 (22) |
| 95th percentile (c) |
0.02–0.47 (7) |
0.08–1.40 (13) |
0.10–1.01 (19) |
0.08–0.57 (20) |
0.10–0.50 (22) |
0.08–0.58 (21) |
| Refined regulatory maximum level exposure assessment scenario | ||||||
| Mean (b) |
0.06–0.48 (2) |
0.09–0.54 (7) |
0.03–0.26 (15) |
0.02–0.16 (19) |
0.01–0.15 (21) |
< 0.01–0.10 (19) |
| 95th percentile (c) | – |
0.25–1.43 (2) |
0.12–0.80 (12) |
0.18–0.53 (11) |
0.04–0.43 (17) |
0.02–0.43 (10) |
| Refined brand‐loyal exposure assessment scenario | ||||||
| Mean (b) |
0.01–0.08 (2) |
0.02–0.09 (7) |
0.01–0.05 (15) |
0.01–0.04 (19) |
< 0.01–0.02 (21) |
< 0.01–0.02 (19) |
| 95th percentile (c) | – |
0.04–0.24 (2) |
0.03–0.14 (12) |
0.03–0.15 (11) |
0.01–0.09 (17) |
0.01–0.07 (10) |
Results of the regulatory maximum level exposure assessment scenario and the refined exposure assessment scenarios are not comparable as the underlying populations of consumers are different.
Mean estimates based on dietary surveys/population classes with less than 6 consumers may not represent the population group and are thus not included in this table.
95th percentile estimates based on dietary surveys/population classes with less than 60 observations may not be statistically robust (EFSA, 2011), and are thus not included in this table.
For the regulatory maximum level exposure assessment scenario, the highest mean exposure to neohesperidine dihydrochalcone (E 959) was found in toddlers (0.38 mg/kg bw per day) as well as the highest P95 (1.40 mg/kg bw per day).
In the refined regulatory maximum exposure assessment scenario, mean exposure to neohesperidine dihydrochalcone (E 959) ranged from < 0.01 mg/kg bw per day in the elderly to 0.54 mg/kg bw per day in toddlers. The 95th percentile of exposure ranged from 0.02 mg/kg bw per day in the elderly up to 1.43 mg/kg bw per day in toddlers.
In the refined brand‐loyal exposure assessment scenario, mean exposure to neohesperidine dihydrochalcone (E 959) ranged from < 0.01 mg/kg bw per day in adults and the elderly to 0.09 mg/kg bw per day in toddlers. The 95th percentile of exposure ranged from 0.01 mg/kg bw per day in the adults and the elderly to 0.24 mg/kg bw per day in toddlers.
The Panel noted that the 95th percentile exposure levels for toddlers in the refined scenarios could be assessed for two dietary surveys only.
Detailed results per population group and survey for the refined regulatory maximum level exposure assessment scenario and the refined brand‐loyal scenario exposure assessment scenario are presented in Table A.5 of the Annex A.
Main food categories contributing to the exposure to neohesperidine dihydrochalcone (E 959)
The main contributor to the dietary exposure to neohesperidine dihydrochalcone (E 959) in the regulatory maximum level exposure assessment scenario was FC 14.1.4 Flavoured drinks in toddlers, children, adolescents and adults. For infants, the main food category contributing to the exposure was FC 12.6 Sauces and for the elderly, FC 11.4 Table‐top sweeteners.
In the refined regulatory maximum level exposure assessment scenario considering the facets, FC 14.1.4 Flavoured drinks was the main contributing food category in all population groups. For infants and toddlers, this food category was the only contributor. FC 17 Food supplements contributed more than 75% to the exposure in 4 surveys for the elderly. Similar results were noted for the refined brand‐loyal scenario.
For details on the contribution of each food category in the scenarios, see Tables A.6, A.7 and A.8 of Annex A, respectively.
Dietary exposure for consumers of a single food category containing neohesperidine dihydrochalcone (E 959)
The dietary exposure estimates for consumers‐only of a single food category were similar to the ones calculated for consumers‐only of at least one food category (refined brand‐loyal exposure assessment scenario) (Annex A, Table A.9).
3.4.2. Uncertainty analysis
In accordance with the guidance provided in the EFSA opinion related to uncertainties in dietary exposure assessment (EFSA, 2007), the following sources of uncertainties, that may result in an overestimation (+) or underestimation (−) of the exposure, have been considered and summarised in Table 7.
Table 7.
Qualitative evaluation of influence of uncertainties on the dietary exposure estimate
| Sources of uncertainties | Direction (a) |
|---|---|
| Consumption data | |
| Different methodologies/representativeness/underreporting/misreporting/no portion size standard/only a few days | +/– |
| Underreporting of food descriptors (facets) concerning the presence or potential presence of sweeteners | – (b) |
| Not considering some of the restrictions or all restrictions specified in the legislation (e.g. as flavour enhancer only) | + (b) |
| Food category(ies) not considered because the restriction was very specific | – |
| Concentration data | |
| Correspondence of reported use levels to the food items in the Comprehensive Database: uncertainties to which types of food the levels refer | +/– |
| Uncertainty in possible national differences in use levels of food categories | +/– |
| Refined regulatory maximum level exposure assessment and brand‐loyal scenario: three out of the 38 food categories authorised to contain neohesperidine dihydrochalcone (E 959) were considered in the exposure assessment | − (b) |
| Refined regulatory maximum level and brand‐loyal exposure assessment scenario: four out of 18 Mintel food subcategories in which neohesperidine dihydrochalcone (E 959) was labelled were included in the current exposure assessment. This represented 60% of the products labelled to contain neohesperidine dihydrochalcone (E 959) in the Mintel GNPD | − (b) |
| Regulatory maximum level exposure assessment scenario: exposure calculations are based on the MPLs | + |
| Methodology | |
| Use of data from food consumption surveys covering only a few days to estimate high percentile (95th) of long‐term (chronic) exposure | + |
+, uncertainty with potential to cause overestimation of exposure; –, uncertainty with potential to cause underestimation of exposure.
Uncertainty considerations on the direction (+/–) are made assuming the effect on the same underlying population of consumers.
The dietary exposure estimates of neohesperidine dihydrochalcone (E 959) in the refined scenarios were based on three food categories: FC 14.1.4 Flavoured drinks and two food categories of food supplements. Mintel GNPD confirmed the use of neohesperidine dihydrochalcone (E 959) in flavoured drinks (specifically in three widely known zero calorie/sugar free carbonated soft‐drinks) and in food supplements.
FC 14.1.4 Flavoured drinks also included the use of neohesperidine dihydrochalcone (E 959) in milk‐based flavoured drinks (see Table 4). Milk‐based flavoured drinks were not included in the exposure assessment because no use levels were provided for this subcategory. According to Mintel GNPD, only four products were labelled to contain neohesperidine dihydrochalcone (E 959) for ‘flavoured milk’, thus this limited use is not expected to have an impact on the overall dietary exposure.
For food supplements the reported use levels were assigned to the entire FC 17, without applying the restriction ‘only food supplements in chewable form’. This very likely resulted in an overestimation of the exposure.
Fifteen products belonging to Mintel subcategory Nutritional & Meal Replacement Drinks (FC 13.3), were labelled to contain neohesperidine dihydrochalcone (E 959). This use was not included in the exposure assessment, which may have resulted in an underestimation of the exposure to this food additive (Annex A, Table 5).
In the refined brand‐loyal exposure assessment scenario using facets, exposure was slightly lower at the upper end of the mean and P95 than when no facets were used to filter relevant foods (Annex A, Table A.9). This suggests that possible underreporting of facets did not significantly impact the exposure estimates.
Overall, the Panel considered the dietary exposure to neohesperidine dihydrochalcone (E 959) from its use as a food additive, to be overestimated in the refined regulatory maximum scenario, mainly because of the use of MPLs and not considering the restrictions for the food supplements.
Based on the data available, the Panel consider that the exposure estimates obtained with the refined brand‐loyal exposure assessment scenario (with facets) provides the most appropriate dietary estimates. This scenario is unlikely to result in a large underestimation of the real exposure to neohesperidine dihydrochalcone (E 959).
3.4.3. Concentrations of and dietary exposure to neohesperidine dihydrochalcone (E 959) in the literature
A literature search was carried out to collect data on levels of neohesperidine dihydrochalcone (E 959) in food, as well on dietary exposure to this sweetener. Bibliographic searches were conducted in bibliographic databases or scientific citation search terms (see Appendix A). In total, 20 papers were retrieved and reviewed. Of these papers, three reported on concentrations of neohesperidine dihydrochalcone (E 959) analysed in representative food samples available on the European market and two reported on dietary exposure to this sweetener in a European population.
Three studies that published data on concentrations of neohesperidine dihydrochalcone (E 959) in representative food samples were for the Spanish (Lorenzo et al., 2015), Italian (Janvier et al., 2015) and Polish (Zygler et al., 2012) markets. In all these studies, neohesperidine dihydrochalcone (E 959) was quantified in only a limited number of samples. In Spain, 66 food samples – energy drinks, soft drinks, juices, teas, soy beverages, dairy‐based drinks, beers, and spirit alcoholic drink – were analysed (Lorenzo et al., 2015). Neohesperidine dihydrochalcone (E 959) was quantified in only one sample belonging to dairy‐based drinks and one sample belonging to teas. In the Italian study, 290 foods – beverages, jams, ketchups, confectionery, dairy products, table‐top sweeteners and food supplements – were analysed for neohesperidine dihydrochalcone (E 959) and the sweetener was only quantified in one hard candy (Janvier et al., 2015). In 179 food samples– different brands of soft drinks, beverages, juices, yoghurts, jams, marmalades canned fruits, vegetable salads, preserved vegetables and fish products – from the Polish market, neohesperidine dihydrochalcone (E 959) was analysed and quantified in none of the samples (Zygler et al., 2012). Reported LOQs in these studies were 30, 10 and 40 μg/kg, respectively.
Two studies reported on the dietary exposure to neohesperidine dihydrochalcone (E 959). The first study estimated dietary exposure for children with type 1 diabetes mellitus aged 1–18 years (n = 242) from Belgium (Dewinter et al., 2016). Food consumption data of seven food categories – soft drinks; sweet bread spreads (e.g. jam or chocolate spread); dairy drinks; yoghurt and other dairy desserts; chocolate; sweets (e.g. candy and cake); table‐top sweeteners – were combined with MPLs of neohesperidine dihydrochalcone (E 959) as listed in Annex II of Regulation (EC) No 1333/2008. The mean exposure to neohesperidine dihydrochalcone (E 959) ranged from 0.24 mg/kg bw per day in children aged 13–18 years to 0.51 mg/kg bw per day in children aged 4–6 years. The corresponding P95 exposure estimates were 0.53 and 1.33 mg/kg bw per day, respectively. When including only consumers of foods that could contain neohesperidine dihydrochalcone (E 959), the P95 exposure estimate increased to 2.03 mg/kg bw per day in children aged 4–6 years (mean consumers only is not reported) based on MPLs.
The second study examined the dietary exposure to neohesperidine dihydrochalcone (E 959) in the general population aged 4 years and older in Italy (Le Donne et al., 2017). The assessment was based on the analysis of 326 foods including candies, chewing gum, table‐top sweeteners, beverages and food supplements. The study used a method with a LOQ of 10 μg/kg. Neohesperidine dihydrochalcone (E 959) was only quantified in three candies and one chewing gum. Dietary exposure was estimated by combining food consumption data from the Italian National Survey (INRAN‐SCAI 2005‐06) with MPLs, and with actual concentrations. The mean exposure to neohesperidine dihydrochalcone (E 959) was 0.06 mg/kg bw per day for the MPL scenario and much lower (7.4E‐7 mg/kg bw per day) for the scenario based on actual concentrations. The corresponding P95 exposure estimates were 0.21 and 0 mg/kg bw per day, respectively. In the MPL scenario, the assessment was also refined by including only foods labelled (confectionary) to contain neohesperidine dihydrochalcone (E 959). This resulted in a mean exposure of 0 mg/kg bw per day and a P95 exposure estimate of 0.01 mg/kg bw per day.
In summary, the analytical data reported for three national food markets in Europe are comparable to the analytical data reported to EFSA, confirming the limited use of E959. It is not possible to directly compare dietary exposure estimates from the literature to those reported in this opinion, because different food categories and concentration levels were included in the assessments.
3.5. Biological and toxicological data
The biological and toxicological studies that were assessed as relevant according to the inclusion criteria established in the draft protocol on hazard identification and characterisation of sweeteners (EFSA, 2020a), are listed in Appendix B. The identified studies were provided to EFSA following the public call for biological and toxicological data. 6 Additional relevant data were also identified from the literature. A list of studies that did not meet the inclusion criteria is provided in Annex B.
For studies on absorption, distribution, metabolism and excretion (ADME), acute toxicity and genotoxicity, the review approach was narrative. Information from mechanistic studies and studies in animal disease models available in the literature were also summarised as a narrative (3.5.5 Other studies).
For the other toxicological studies, an evaluation of the risk of bias (RoB) was performed (Appendix A, Table A.1) and a weight of evidence (WoE) approach for the reliable studies was applied for each health outcome (Appendix A, Annex C). The latter are also summarised in detail in Appendix C. A narrative synthesis of the WoE analysis is reported in Section 3.5.4.
Table A.1.
Results of the RoB evaluation
| RefID | Authors and year | Study type | Random sequence generation (selection bias) | Allocation concealment (selection bias) | Experimental conditions (Performance bias) | Blinding of research personnel (performance bias) | Incomplete outcome data (attrition bias) | Confidence in exposure characterisation (detection bias) | Confidence in outcome assessment (detection bias) | Selective reporting (reporting bias) | Appropriateness of statistical methods (other source of bias) | Tier (based on rules) | Tier (agreement with automatically generated Tier) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 339 | Western Regional Research Laboratory (1965) (a) (Documentation provided to EFSA nr: 10) | Sub‐chronic toxicity | − | − | − | − | + | −− | − | + | − | 3 | Yes |
| + | + | −− | |||||||||||
| 307 | T.N.O. (1986) (Documentation provided to EFSA nr: 9) (b) | Sub‐chronic toxicity | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | 1 | Yes |
| + | |||||||||||||
| 286 | Gumbmann et al. (1978) (Documentation provided to EFSA nr: 9) | Sub‐chronic toxicity | − | −− | − | − | + | − | + | + | − | 3 | Yes |
| − | + | − | |||||||||||
| 360 | Shi et al. (2021) | Sub‐chronic toxicity | + | + | ++ | + | − | + | − | ++ | ++ | 2 | Yes |
| − | + | ||||||||||||
| 286 | Gumbmann et al. (1978) (Documentation provided to EFSA nr: 9) | Chronic toxicity (1‐year rat) | − | − | − | + (c) | − | − | − | − | − | 3 | Yes |
| 286 | Gumbmann et al. (1978) (Documentation provided to EFSA nr: 9) | Chronic toxicity (2‐year rat) | − | + | − | + | + | − | − | − | − | 3 | Yes |
| − | + | + | |||||||||||
| 286 | Gumbmann et al. (1978) (Documentation provided to EFSA nr: 9) | Chronic toxicity (2‐year dog) | − | − | − | + | − | − | − | − | − | 3 | Yes |
| 230 | Waalkens‐Berendsen et al. (2004) | Prenatal developmental toxicity | + | + | + | + | + | ++ | + | + | ++ | 1 | Yes |
| ++ | ++ | ++ | ++ | ++ | |||||||||
| 286 | Gumbmann et al. (1978) (Documentation provided to EFSA nr: 9) | Reproductive and developmental toxicity | − | + | + | + | + | − | − | −− | − | 3 | Yes |
| − | − | + | − | −− | |||||||||
| 339 | Western Regional Research Laboratory (1965) (Documentation provided to EFSA nr: 10) | Reproductive toxicity | − | + | − | + | − | −− | − | − | −− | 3 | Yes |
| + | − | ++ | + |
Definitely low risk of bias (++), Probably low risk of bias (+), Probably high risk of bias (−), Definitely high risk of bias (−−).
Split cells reporting two different scorings for the same risk of bias question express the view of the two independent reviewers.
Study on which the derivation of the current acceptable daily intake (ADI) was based (SCF, 1989).
This unpublished study report was subsequently published as Lina BAR, Dreef‐van der Meulen HC, Leegwater DC, 1990. Subchronic (13‐week) oral toxicity of neohesperidin dihydrochalcone in rats. Food Chemistry and Toxicology, 26(7), 507–513. https://doi.org/10.1016/0278-6915(90)90121-3.
This question was cloned for a specific endpoint (preparation of organs for weighing) and scored, by the two reviewers, as a probably high risk of bias (−). The tier allocation for this study, related to this specific endpoint, was also tier 3.
3.5.1. Absorption, distribution, metabolism and excretion (ADME)
The following studies on ADME were considered relevant to be included in this opinion.
In vivo studies in rats
The plasma concentration–time profile of neohesperidine dihydrochalcone (purity > 98%) was analysed in six rats following oral administration of 20 mg/kg neohesperidine dihydrochalcone and in additional six rats following intravenous administration of 2 mg/kg neohesperidine dihydrochalcone, in both cases after withdrawal of diet 12 h prior to administration (Wang et al., 2014). The analytical methodology was validated following FDA guidelines with respect to specificity, recovery, within‐ and between‐day, precision, lower LOD, LLOQ and sample stability (US Food and Drug Administration, 2001). Only neohesperidine dihydrochalcone but not its metabolites were quantified in this study. Measurements were also performed in rat tissues (heart, liver, spleen, lung, kidney, brain, stomach) taken at 0.83, 0.5 and 1 h following intravenous dosing. After oral gavage, plasma neohesperidine dihydrochalcone concentrations increased very quickly within 0.2 h, followed by a decrease to lower (LLOQ) (10 ng/mL) by 24 h. The maximum plasma concentration (Cmax) of neohesperidine dihydrochalcone ranged from 801 to 1,100 ng/mL following oral administration; the half‐life (t1/2) was 1.0 ± 0.2 h and the volume of distribution 1.2 ± 0.49 L/kg. Based on a comparison of oral and intravenous administration, the authors calculated oral systemic bioavailability of intact neohesperidine dihydrochalcone to be 21.8%. There was no accumulation of neohesperidine dihydrochalcone in any of the organs examined.
Zhang et al. (2021) orally administered neohesperidine dihydrochalcone to male adult Sprague–Dawley rats in a dose of 5 mg/kg bw for 3 days. Blood was taken at time points 0, 0.5, 1 and 2 h post‐dose (at every time point n = 3). Urine was sampled for 48 h. At the end of the study, organs were removed and samples of heart, spleen, brain, lung and kidneys were used for analysis. After pretreatment, analysis and metabolite identification was performed partly in comparison with reference standards by ultrahigh‐performance liquid chromatography‐quadrupole time‐of‐flight mass spectrometry (UHPCL/QTOF‐MS). The fragmentation behaviour of neohesperidine dihydrochalcone under negative ion mode and positive ion mode was similar. A sum of 19 metabolites was identified or tentatively characterised in urine, 3 of them were found in plasma and 8 in faeces. Some of the metabolites identified in urine were also found in the investigated organs with the exception of spleen where none of the 19 urine metabolites was found. The metabolites were identified as being the result of six different reactions, namely hydrolysis, glucuronidation, sulfation, glutamination, N‐butyryl glycylation and lactylation. The reactions occurred at the hydroxyls of the C‐2’, C‐4’ and C‐6’ position (see Figure 1). Neohesperidine dihydrochalcone, neohesperidine dihydrochalcone glucuronide, neohesperidine dihydrochalcone sulfate, several hesperetin dihydrochalcone glucuronides, hesperetin dihydrochalcone di‐glucuronides, hesperetin dihydrochalcone sulfates, hesperetin dihydrochalcone glucuronides‐sulfates and hesperetin dihydrochalcone were reported by the authors as being present in urine.
In vitro studies
Braune et al. (2005) performed an in vitro study to investigate the degradation of neohesperidine dihydrochalcone by human intestinal microbiota. Neohesperidine dihydrochalcone (100 μL of a 50 mM DMSO‐solvated stock added to 10 mL of a solution inoculated with a faecal suspension or bacterial culture to give final concentration of 0.5 mM) was added separately to faecal slurries from 4 different human donors in a complex medium. In some of the experiments, a second dose of neohesperidine dihydrochalcone was added after 43–48 h of incubation. Scaled‐down fermentation experiments were performed with intermediates of neohesperidine dihydrochalcone degradation. Incubations were performed at 37°C in a tube rotator under anoxic conditions. Neohesperidine dihydrochalcone and aromatic metabolites were analysed by reversed‐phase HPLC. MS and nuclear magnetic resonance (NMR) spectroscopy were also selectively used to confirm structures. In basic buffer and glucose‐free medium, neohesperidine dihydrochalcone remained unchanged in all faecal slurries (up to 93 h). In contrast, addition of 20 mM glucose resulted in a disappearance of neohesperidine dihydrochalcone and the appearance of hesperetin dihydrochalcone, 3‐(3,4‐dihydroxyphenyl)propionic acid and 3‐(3‐hydroxy‐4‐methoxyphenyl)‐propionic acid in incubations within 22 h with all four faecal slurries. Using the complex medium, the time required for complete neohesperidine dihydrochalcone degradation ranged from 44 to more than 142 h. An additional transient metabolite – hesperetin dihydrochalcone 4′‐β‐d‐glucoside – was also identified. The demethylation of 3‐(3‐hydroxy‐ 4‐methoxyphenyl)propionic acid was observed in some of the fermentation experiments, giving rise to 3‐(3,4‐dihydroxyphenyl)propionic acid. Neohesperidine dihydrochalcone was also incubated at a concentration of 0.52 mM in complex medium with the gastrointestinal (GI) tract species Eubacterium ramulus or Clostridium orbiscindens. Neither of the strains converted neohesperidine dihydrochalcone within 43 h of incubation. However, E. ramulus completely converted hesperetin dihydrochalcone 4′‐β‐d‐glucoside within 18 h of incubation to an equimolar amount of 3‐(3‐hydroxy‐4‐methoxyphenyl)propionic acid. The aglycon hesperetin dihydrochalcone was transiently formed, reaching a maximum concentration after 4 h of fermentation. When hesperetin dihydrochalcone was used in fermentation experiments, E. ramulus cleaved the compound within 1 h to yield 3‐(3‐hydroxy‐4‐methoxyphenyl)‐propionic acid. C. orbiscindens was not able to convert hesperetin dihydrochalcone 4′‐β‐d‐glucoside within a period of 43 h, whereas the aglycon was cleaved.
The Panel considered that these limited data indicate that any neohesperidine dihydrochalcone not absorbed and remaining intact on its passage through the GI tract is relatively slowly catabolised by the GI microbiota in the colon in man.
In a Caco‐2 cell monolayer model, the bilateral permeation of flavonoids, among them neohesperidine dihydrochalcone (purity > 98%), was investigated (Fang et al., 2017). The tested concentration was 40 μM at which cell viability was 102.4 ± 6.35%. The concentration of neohesperidine dihydrochalcone was measured by HPLC; no quality assurance data were given. The cells were cultured in a medium to which enzymes present in the gut were not added. The apparent permeability coefficient Papp = DQ/Dt/AC0 was calculated where DQ/Dt is the rate of the flavonoid on the accepting chamber (μM/s), A is the surface area of the hanging insert (cm2) and C0 is the initial concentration μmol/mL. The authors stated that a low Papp‐value of < 6 × 10−6 cm/s suggested that the presence of a glycosidic group is unfavourable for the absorption of neohesperidine dihydrochalcone. Furthermore, the ratio of the bi‐directional Papp (ratio of Papp B to A to Papp A to B where A = apical and B = basolateral) of 1.2 indicated that neohesperidine dihydrochalcone is transported by passive diffusion. In this cell model, neohesperidine dihydrochalcone was relatively stable with a recovery of 80%. From this study a poor absorption of neohesperidine dihydrochalcone would be predicted.
In a later study (Fang et al., 2018), using the same experimental design as in Fang et al. (2017), the influence of the absence and presence of 100 μM verapamil, a P‐glycoprotein (P‐gp) inhibitor, was investigated. No influence of verapamil was found on the transport of neohesperidine dihydrochalcone indicating that neohesperidine dihydrochalcone is not a substrate of P‐gp and that P‐gp is not involved in the transport of neohesperidine dihydrochalcone.
Bozoğlan et al. (2014) examined the binding of neohesperidine dihydrochalcone to human serum albumin in vitro using UV, fluorescence, synchronous fluorescence and circular dichroism spectroscopic methods. Based on intrinsic fluorescence quenching of albumin at a physiologically‐relevant pH, the authors calculated a binding constant of 2.79 × 104 M−1 at 298.15 K.
Membrane vesicles from Sf9 cells expressing either human breast cancer resistance protein (BCRP), multidrug resistance associated protein 2 (MRP2) or P‐gp were used to examine the potential interaction of neohesperidine dihydrochalcone using the transporter substrates Lucifer yellow (LY), 5(6)‐carboxy‐2,7‐dichlorofluorescein and N‐methyl‐quinidine, substrates for BCRP, MRP2 and P‐gp respectively (Sjöstedt et al., 2017). Neohesperidine dihydrochalcone had an inhibitory effect on BCRP transport (IC50 = 0.86 μM), but almost no effect on P‐gp and MRP2.
Summary and conclusion on ADME
In vivo ADME data are only available from two studies in rats, with one assessing metabolites as well as the parent compound. Wang et al. (2014) estimated the systemic availability of neohesperidine dihydrochalcone in rats as being 21.8%. Zhang et al. (2021) identified 19 components in the urine, among them the parent compound besides several metabolites, mostly glucuronides, sulfates and other conjugates which were also formed after hydrolysis of neohesperidine dihydrochalcone to hesperetin dihydrochalcone. The putative metabolites were formed by six major reactions, namely hydrolysis, glucuronidation, sulfation, glutamination, N‐butyryl glycylation and lactylation. The reactions occurred at the hydroxyls of the C‐2’, C‐4’, and C‐6’ position. Wang et al. (2014) estimated a plasma half‐life of 1.0 ± 0.2 h and a volume of distribution of 1.2 ± 0.49 L/kg.
In vitro studies of Fang et al. (2017) support the findings of limited neohesperidine dihydrochalcone absorption. The Panel considered that neohesperidine dihydrochalcone interacts with human BCRP and may be either a substrate or an inhibitor but has no effect on P‐gp and MRP2, based on studies by Sjöstedt et al. (2017) and Fang et al. (2018).
Based on limited data in rats, the Panel considered that neohesperidine dihydrochalcone is likely to be absorbed in humans and to become systemically available intact and as metabolites. Further, based on studies in rats, neohesperidine dihydrochalcone and its metabolites are likely to be primarily excreted in urine.
3.5.2. Acute toxicity
The CEF Panel opinion reported an oral acute toxicity study on neohesperidine dihydrochalcone. The study was performed in rats (males and females) and resulted in an oral median lethal dose (LD50) of greater than 5,000 mg/kg bw (EFSA CEF Panel, 2010).
No new data on acute toxicity were received from the interested parties and no new data were identified in the literature.
3.5.3. Genotoxicity
Several published genotoxicity studies were previously considered by the SCF (1985, 1989) and by the CEF Panel in the context of the evaluation of FGE.32 dealing with flavonoids, including neohesperidine dihydrochalcone (EFSA CEF Panel, 2010). Concerning these studies, the CEF Panel noted that neohesperidine dihydrochalcone was negative in a valid bacterial reverse mutation assay (Ames test), with and without metabolic activation by rat and hamster liver S9 (Zeiger et al., 1987). Negative results in bacterial reverse mutation assays were also reported in several earlier studies (Batzinger et al., 1977; Bjeldanes and Chang, 1977; Brown et al., 1977; MacGregor and Jurd, 1978; Brown and Dietrich, 1979). In vivo positive results were reported in the micronucleus test in mouse bone marrow following intraperitoneal injection of neohesperidine dihydrochalcone in a study of insufficient reliability due to experimental shortcomings, in particular the use of only two mice per treatment (Sahu et al., 1981). In another in vivo micronucleus assay in mice, evaluated by the CEF Panel as ‘of limited validity but acceptable’, the oral administration of neohesperidine dihydrochalcone did not induce detectable increases in micronuclei (MacGregor et al., 1983). The CEF Panel concluded that the available data did not indicate a genotoxicity concern for neohesperidine dihydrochalcone which would prevent its evaluation through the procedure for the safety assessment of flavouring substances as defined in Commission Regulation (EC) 1565/2000 19 .
The FAF Panel agreed that the studies previously evaluated did not indicate a concern for genotoxicity but noted that exposure of the bone marrow could not be confirmed based on the determination of the polychromatic/normochromatic erythrocyte ratio in the study by MacGregor et al. (1983). In line with OECD TG 474 (2014) and the EFSA Scientific Committee opinion clarifying some aspects related to genotoxicity assessment (EFSA Scientific Committee, 2017), the result of this study is considered by the FAF Panel to be inconclusive rather than negative.
A literature search, covering the period subsequent to the last SCF evaluation (SCF, 1989), allowing a 1‐year overlap, did not identify any new data on genotoxicity of neohesperidine dihydrochalcone.
However, the original report of one unpublished genotoxicity study on neohesperidine dihydrochalcone was received through the call for data (Sequani Limited, 2018 in Documentation provided to EFSA nr: 9) by one interested business operator. This study is summarised in Table 8 and described in detail in Appendix D. The study consisted of an in vitro micronucleus test in human lymphoblastoid TK6 cells, with and without metabolic activation. Negative results were reported in the absence of metabolic activation while statistically significant increases in micronuclei, slightly above the historical negative control range, were observed in the presence of metabolic activation at the low and intermediate concentrations (200 and 650 μg/mL), but not at the highest concentration tested (2,000 μg/mL). According to OECD TG 487 (2014), based on such a data set, neohesperidine dihydrochalcone is evaluated as neither clearly negative nor clearly positive. Therefore, further investigation, such as a repeat experiment using modified experimental conditions, was requested to the interested parties, to allow a final evaluation of the activity of neohesperidine dihydrochalcone in this assay.
Table 8.
Summary table of newly available genotoxicity studies on neohesperidine dihydrochalcone (E 959)
| Study design (assay/cells/concentration) | Test material | Results | Reliability/relevance (a) | Reference |
|---|---|---|---|---|
|
In vitro micronucleus test in human lymphoblast TK6 cells 200, 650 and 2,000 μg/mL (3 h with and without metabolic activation); 65, 400, 650 and 750 μg/mL (27 h with and without metabolic activation) |
Neohesperidine dihydrochalcone, purity 97.8% | Equivocal | Reliable with restriction/limited | Sequani Limited, 2018 (Documentation provided to EFSA nr: 9) |
|
In vitro micronucleus test in human lymphocytes 0.5, 1.0 and 2.0 mg/mL (3–6 h with and without metabolic activation and 20–24 h without metabolic activation) |
Neohesperidine dihydrochalcone, purity 98.2% | Negative | Reliable without restriction/High | Bioneeds India Private Limited, 2022 (Documentation provided to EFSA nr: 11) |
A detailed approach for assessing relevance and reliability of genotoxicity studies has been developed and will be published as addendum to the protocol (EFSA, 2020a).
Consequently, one interested business operator submitted the original report of an unpublished genotoxicity study on neohesperidine dihydrochalcone (Bioneeds India Private Limited, 2022 in Documentation provided to EFSA nr: 11). This study is summarised in Table 8 and described in detail in Appendix D. The study is an in vitro micronucleus test in human lymphocytes in which no significant increase in the formation of micronuclei was reported. This study confirms that neohesperidine dihydrochalcone is negative in the in vitro micronucleus assay.
Summary and conclusion on genotoxicity
According to the EFSA strategy for genotoxicity testing, the bacterial reverse mutation assay and the in vitro micronucleus test are recommended as a basic test battery for hazard identification (EFSA Scientific Committee, 2011). The FAF Panel noted that the available data provide sufficient evidence of lack of mutagenicity of neohesperidine dihydrochalcone in the bacterial reverse mutation assays and a lack of micronucleus formation in mammalian cells. The Panel concluded that neohesperidine dihydrochalcone (E 959) does not raise a concern regarding genotoxicity.
As reported in Section 3.1.2, neohesperidine dihydrochalcone (E 959) contains a certain amount of impurities, which are present in the source material and/or generated during the hydrogenation step to obtain the food additive. These impurities are all closely structurally related to neohesperidine dihydrochalcone. For this purpose, a Q(SAR) analysis by the OECD Q(SAR) Toolbox on the impurities contained in E 959 was performed (Appendix E). Overall, the Q(SAR) analysis did not indicate relevant differences between the structural alert profile of E 959 and its impurities. This analysis supports the applicability of read across among E 959 and its impurities with regard to the genotoxicity evaluation.
3.5.4. Synthesis of systematically appraised evidence on biological and toxicological effects
3.5.4.1. Animal studies
Firstly, 746 references were screened based on title and abstract. These references included studies retrieved from the literature as well as biological and toxicological data received by the interested parties following the calls for data. 148 papers were included at the level of title and abstract and further screened based on full text, resulting in 5 animal studies included, whereas no human studies were identified (Appendix A, Figure A.1).
Figure A.1.
PRISMA flow chart (adapted from Moher et al., 2009)
Among five animal studies, two were retrieved from the literature (Waalkens‐Berendsen et al., 2004; Shi et al., 2021), while three were studies (Western Regional Research Laboratory, 1965 20 ; T.N.O., 1986 21 ; Gumbmann et al., 1978 in Documentation provided to EFSA nr: 9, 10) already considered in the previous evaluation by SCF (SCF, 1989). The latter were part of the submitted data by the interested parties and included the study on which the derivation of the current ADI was based (subchronic studies in Western Regional Research Laboratory, 1965 in Documentation provided to EFSA nr: 10).
As prescribed by the protocol (EFSA, 2020a), the previously considered pivotal study was evaluated for RoB together with any relevant literature published since the previous evaluation by the SCF, allowing 1 year of overlap (cut‐off date: 1988).
As a result, the previously considered pivotal study was allocated to tier 3, mainly because of low confidence in exposure characterisation. In light of this, the Panel considered appropriate to evaluate for the RoB the remaining toxicity studies received through the call for data. Among these, only one study was allocated to tier 1 (T.N.O., 1986 in Documentation provided to EFSA nr: 9), while the other studies were allocated to tier 3 (Gumbmann et al., 1978; reproductive toxicity study in Western Regional Research Laboratory, 1965 in Documentation provided to EFSA nr. 9, 10). The two studies retrieved through the literature were rated as tier 1 (Waalkens‐Berendsen et al., 2004) and tier 2 (Shi et al., 2021) (for RoB details see Table A.1, Appendix A).
The Panel agreed that studies with high risk of bias (Tier 3) could not be further considered for the derivation of a reference value for the safety assessment of neohesperidine dihydrochalcone (E 959).
Annex C reports the three evaluated animal studies, rated as tier 1 and tier 2 in the RoB evaluation, that were then gathered by endpoint within the different health outcome categories (HOC) for which a weight of evidence (WoE) analysis was performed. In this case, only animal studies were considered, as no human data on neohesperidine dihydrochalcone (E 959) were available (see Section 3.5.6).
The following animal oral toxicity studies have been considered (Table 9): a 13‐week toxicity study in rats (T.N.O., 1986 in Documentation provided to EFSA nr: 9) performed with 0%, 0.2%, 1%, 5% of neohesperidine dihydrochalcone in the diet (equal to 0, 150, 757, 4,011 mg/kg bw per day in males and 0, 166, 848, 4,334 mg/kg bw per day in females); an 11‐week study in mice (Shi et al., 2021) performed with a single dose of 0.1 mg/mL neohesperidine dihydrochalcone in water (equal to 10 mg/kg bw per day); a prenatal developmental toxicity study in rats (Waalkens‐Berendsen et al., 2004) performed with 0%, 1.25%, 2.5%, and 5% of neohesperidine dihydrochalcone in the diet (equal to 0, 800–900, 1,600–1,700, 3,100–3,400 mg/kg bw per day).
Table 9.
Summary table of the three animal oral toxicity studies that were rated as Tier 1 or 2
| RefID | Authors | Study type | Exposure duration | Species/strain | Dose level | Dose level in mg/kg bw per day |
|---|---|---|---|---|---|---|
| 307 | T.N.O., 1986 (a) (Documentation provided to EFSA nr: 9) | Subchronic toxicity | 13‐week | Rat/Wistar | 0%, 0.2%, 1.0%, 5.0% in the diet |
0, 150, 757, 4,011 (M) and 0, 166, 848, 4,334 (F) |
| 360 | Shi et al. (2021) | Subchronic | 11‐week | Mice/C57BL/6 | 0, 0.1 mg/mL in drinking water | 10 (F) (b) |
| 230 | Waalkens‐Berendsen et al. (2004) | Prenatal developmental toxicity | 21 days (gestation) | Rat/Wistar | 0%, 1.25%, 2.5%, and 5% in the diet | 0, 800–900, 1,600–1,700, 3,100–3,400 (F) |
M: males; F: females.
This unpublished study report was subsequently published as Lina BAR, Dreef‐van der Meulen HC, Leegwater DC, 1990. Subchronic (13‐week) oral toxicity of neohesperidin dihydrochalcone in rats. Food Chemistry and Toxicology, 26(7), 507–513. https://doi.org/10.1016/0278-6915(90)90121-3.
The nominal dose the authors aimed was 5 mg neohesperidine dihydrochalcone/kg bw per day. The test solution is reported to contain 0.1 mg neohesperidine dihydrochalcone/mL. Considering the reported drinking water intake of mice, the dose of neohesperidine dihydrochalcone is closer to 10 mg/kg bw per day.
The HOCs reported from the available animal studies, each encompassing of a number of endpoints, are described in Table 10.
Table 10.
Health outcome categories (HOCs) and related endpoints of the three systematically appraised animal studies subjected to WoE analysis
| Health outcome categories (HOCs) | Endpoints |
|---|---|
| General toxicity | Clinical signs; body weight; feed intake; feed efficiency (body weight gain/feed consumed); water intake; clinical chemistry; ophthalmoscopic examination |
| Haematotoxicity | Haematological parameters (haemoglobin, packed cell volume (PCV), red blood cells (RBCs) white blood cells (WBCs), differential WBCs count, thrombocytes, mean corpuscular volume (MCV), mean corpuscular haemoglobin (MCH), mean corpuscular haemoglobin concentration (MCHC)) |
| Liver toxicity | Liver weight; macro‐ and histopathology of liver; clinical chemistry |
| Nephrotoxicity | Kidney weight; macro‐ and histopathology of kidney; urinalysis; clinical chemistry |
| Other organ toxicity (a) | Weight and macro‐ and histopathology and of brain, caecum and testes and other organs (b) |
| Developmental toxicity | Uterus weight; ovaries weight; fertility index; gestation index; number of corpora lutea; number implantation sites; early and late resorptions, pre‐ implantation loss, post‐implantation loss, number of dead foetuses; number of live foetuses; sex ratio; placenta weight (foetuses); fetal weight, fetal external, skeletal and visceral abnormalities |
The organs included for assessment are those reported in the evaluated studies on neohesperidine dihydrochalcone, but will vary for other sweeteners depending on the included study types.
Relevant tissues and organs that are examined in standard subchronic toxicity studies, other than the ones already explicitly mentioned in the table (e.g. OECD TG 408, 1998, Section 35 for repeated dose 90‐day oral toxicity study in rodents).
The Panel drew conclusions for the level of evidence for adverse effects on health for each HOC based on the WoE analysis of the three considered studies. The level of evidence was based on the confidence ratings of each endpoint belonging to a specific HOC and corresponding rationales presented in the WoE tables (Annex C). A narrative synthesis is reported below.
Confidence in the body of evidence
Based on the available animal data, the Panel considered the confidence in the body of evidence to be high for the association between oral intake of neohesperidine dihydrochalcone and all health outcome categories listed in Table 11.
Table 11.
Summary table of the final ratings of confidence in the body of evidence (n = 3 studies) for each health outcome category based on the WoE analysis
| Health outcome categories (a) | Initial rating (no. of studies) (b) | Elements for downgrading | Elements for upgrading | Final rating of confidence | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Concern for risk of bias | Concern for unexplained inconsistency | Concern related to relevance of studies | Concern for imprecision | Downgrading | Magnitude of effect | Dose–response | Consistency across species/study design | Upgrading | |||
| General toxicity | High (3) | Not serious | Not serious | Not serious | Not serious | No | Not large | No | Yes | No | High |
| Haematotoxicity | High (1) | Not serious | N.A. (single study) | Not serious | Not serious | No | Not large | No | N.A. (single study) | No | |
| Liver toxicity | High (2) | Not serious | Not serious | Not serious | Not serious | No | Not large | No | Yes | No | |
| Nephrotoxicity | High (2) | Not serious | Not serious | Not serious | Not serious | No | Not large | No | Yes | No | |
| Other organs toxicity | High (2) | Not serious | Not serious (c) | Not serious | Not serious | No | Not large | No | Yes (c) | No | |
| Developmental toxicity | High (1) | Not serious | N.A. (single study) | Not serious | Not serious | No | Not large | No | N.A. (single study) | No | |
| Overall rating for all health outcome categories (a) | High (3) | Not serious | Not serious | Not serious | Not serious | No | Not large | No | Yes | No | High |
N.A.: not applicable.
As defined in Table 10 (on health outcome categories and related endpoints of the systematically appraised animal studies subjected to WoE analysis).
The total number of studies assessed was 3. The number in parentheses refers to studies reporting on the specific health outcome.
This is applicable for organs investigated in more than one studies (i.e. caecum); for organs investigated in only one study, this element was rated as ‘N.A. (single study)’ (i.e. for brain and testes, see Annex C).
General toxicity
No adverse effects related to general toxicity of neohesperidine dihydrochalcone in rats and mice were reported in three studies in doses up to 4,011 and 4,334 mg/kg bw per day in males and females, respectively (T.N.O., 1986 in Documentation provided to EFSA nr: 9; Waalkens‐Berendsen et al., 2004; Shi et al., 2021). Focal alopecia and transient diarrhoea were observed in both sexes in the high‐dose group (4,011 mg/kg bw per day in males and 4,334 mg/kg bw per day in females) of T.N.O. (1986) (Documentation provided to EFSA nr: 9). Body weight changes in rats and mice in two of the three evaluated studies were within 10% of control values and were considered to be non‐adverse (T.N.O., 1986 in Documentation provided to EFSA nr: 9; Shi et al., 2021). Likewise, a statistically significant 14% blood glucose increase observed at the lowest dose (150 mg/kg bw per day) in females only was considered incidental (T.N.O., 1986 in Documentation provided to EFSA nr: 9). The reported effects were transient, within the normal range of variation, and without a dose–response relationship.
Haematotoxicity
One study (T.N.O., 1986 in Documentation provided to EFSA nr: 9) reported a > 20% decrease in eosinophils in females and lymphocytes in males in the low‐dose group (equal to 150 mg/kg bw per day in males and 166 mg/kg bw per day in females), but these differences were not consistent between sexes or doses and were not accompanied by changes in the total number of white blood cells. No other effects were noted.
Liver toxicity
Two studies reported on endpoints relevant to assess hepatotoxicity in rats and mice (T.N.O., 1986 in Documentation provided to EFSA nr: 9; Shi et al., 2021). In the high‐dose group (4,011 mg/kg bw per day in males and 4,334 mg/kg bw per day in females) of T.N.O. (1986) (Documentation provided to EFSA nr: 9), plasma alkaline phosphatase (ALP) activity was slightly increased (20%, statistically significant) in females only, but the effect is considered not toxicologically relevant. Total bilirubin concentration was increased in both sexes with statistical significance only in females (225% or 3.2‐fold in females; 103% or 2‐fold in males), total plasma protein and cholesterol concentrations were decreased by 6% (statistically significant) and 22%, respectively, in males only. These changes were not accompanied by any biologically significant changes in liver weight (14% decrease), spleen weight, liver histopathology or ‐decrease in circulating erythrocytes. The increase in bilirubin was an isolated observation only seen at a very high dose. In the absence of any other indicator for liver injury, including histopathological changes, this effect in isolation was considered not adverse.
Nephrotoxicity
Clinical chemistry parameters were investigated in two studies (T.N.O., 1986 in Documentation provided to EFSA nr: 9; Shi et al., 2021). An increased glucose level (14%) in female rats at the lowest dose (166 mg/kg bw per day) in T.N.O. (1986) (Documentation provided to EFSA nr: 9) was considered incidental. This study also reported a moderate decrease (10%) in mean urinary pH (statistically significant) in the high‐dose group (4,011 mg/kg bw per day in males and 4,334 mg/kg bw per day in females) in both sexes, but the decrease is considered not biologically relevant and not adverse. Kidney absolute weight, but not relative weight, was reduced (15%) in males at the highest dose, probably related to the reduced growth of the animals. Furthermore, no kidney macro‐ or histopathological changes were observed. Thus, the reduction in kidney absolute weight was not considered adverse.
Other organ toxicity
Two studies reported on increased absolute and relative caecum weights (T.N.O., 1986 in Documentation provided to EFSA nr: 9; Waalkens‐Berendsen et al., 2004). The prenatal developmental toxicity study by Waalkens‐Berendsen et al. (2004) included data from dams only. In T.N.O. (1986) (Documentation provided to EFSA nr: 9), caecum weight was increased in all treatment groups up to 76%. However, weight increase in the two lower dose groups was not dose related and within the range of historical control values. Waalkens‐Berendsen et al. (2004) also reported on an increase in caecum weight (full) up to 42% in the dams in all treatment groups. Empty cecum weight was also significantly increased (25%) at the highest dose (3,100–3,400 mg/kg bw per day). An increase in caecum weight is a recognised physiological, adaptive response to the ingestion of high doses of low‐digestible substances and was considered as not toxicologically relevant. No macro‐ or histopathological changes in the caecum were reported. For other organs (see Table 10), no substance‐related changes, including in reproductive organs, were reported.
Developmental toxicity
Maternal and developmental endpoints were investigated by Waalkens‐Berendsen et al. (2004). No effects were observed at any dose tested (800–900, 1,600–1,700, 3,100–3,400 mg/kg bw per day).
Overall, no adverse effects on health were identified for neohesperidine dihydrochalcone based on the three toxicological studies that formed the basis of this assessment.
Conclusions on the level of evidence for no adverse effects on health
The level of evidence for no adverse effects on health across all health outcome categories was rated ‘high’. The Panel considered that there is a high confidence in the body of evidence that exposure to neohesperidine dihydrochalcone at the doses tested is not associated with adverse effects on health.
For translating the confidence rating into a level of evidence for no adverse effects on health, see Figure A.2 in Appendix A.
3.5.4.2. Human studies
No human studies on neohesperidine dihydrochalcone were received by the interested parties through the call for data and none were retrieved in the literature.
3.5.5. Other studies
3.5.5.1. Studies in animal disease models
The Panel considered that, in general, studies in animal disease models have only limited relevance for human health risk assessment because of their limitations in mimicking the situation in humans.
One study of this type was retrieved from the literature:
Choi et al. (2021) reported an in vivo study in high‐fat diet‐induced obese male C57BL/6J mice. The animals were divided into four groups (10/males per group) which were fed with a normal diet (ND) or a high‐fat diet (HFD). The latter were administered with 0%, 0.1% or 1% of neohesperidine dihydrochalcone in the diet (HFD + 0.1% neohesperidine dihydrochalcone and HFD + 1% neohesperidine dihydrochalcone). The treatment lasted 11 weeks. The body weight was weekly recorded. The HFD‐fed mice body weight gain was significantly increased (starting at week 2) compared to the ND‐fed mice. In contrast, in both HFD‐fed animals groups exposed to neohesperidine dihydrochalcone, the body weight gain was statistically significantly reduced in a dose‐dependent manner compared to the HFD‐fed group. In particular, in the HFD + 1% neohesperidine dihydrochalcone group, a similar body weight gain to that of the ND‐fed mice was reported. Instead, in the HFD + 0.1% neohesperidine dihydrochalcone group, the body weight gain was statistically significant increased (after week 7) compared to that of the ND‐fed mice. However, it was noted also that in the HFD + 0.1% neohesperidine dihydrochalcone group a statistically significant decrease of body weight gain was observed compared to the HFD‐fed group. In order to clarify that the body weight change was not affected by the dietary intake, the daily consumption of diet per cage was weighed and divided by the number of animals in the cage. It was observed that there was no difference among dietary intervention. According to the study authors, the obtained results showed that neohesperidine dihydrochalcone was able to suppress body weight gain.
3.5.5.2. Mechanistic studies
Mechanistic studies retrieved through the literature search were summarised below.
Han et al. (2018) reported inhibition of adipogenic differentiation in human adipose‐derived stem cells (hASCs) by a novel structural neohesperidine dihydrochalcone analogue, (E)‐3‐(4‐chlorophenyl)‐1‐(2,4,6‐trimethoxyphenyl)prop‐2‐en‐1‐one (CTP). CTP was cytotoxic (by MTS assay) to hASCs (1x1E04 cells/well) at ≤ 20 μM after incubation for 5 days, neohesperidine dihydrochalcone was not cytotoxic at up to 40 μM, the highest concentration tested. A concentration of 4 μM for both neohesperidine dihydrochalcone and CTP was therefore used for all subsequent experiments. hASC differentiation into adipocytes was induced by adding adipogenic differentiation medium and evaluated by intracellular incorporation of Oil Red O. Differentiation (Oil Red O staining) was reduced ‘slightly’ by subsequent addition of neohesperidine dihydrochalcone, and by 25% after addition of CTP. CTP significantly reduced the expression of peroxisome proliferator‐activated receptor‐γ (PPAR‐γ) and CCAAT‐enhancer binding protein‐α (C/EBP‐α) mRNA, consistent with down‐regulation of adipocyte differentiation. The expression of fatty acid synthase (FAS) and sterol regulatory element‐binding protein‐1 (SREBP‐1), intracellular lipogenic markers, was decreased in CTP‐treated cells (by 11.6% and 33.3%, respectively); no data were reported for neohesperidine dihydrochalcone. Intracellular reactive oxygen species (ROS) increased as adipogenic differentiation proceeds in hASCs; this was not affected by neohesperidine dihydrochalcone but was ‘strongly’ reduced by CTP. Nrf2 is a key transcriptional regulator of various antioxidant enzymes which regulates cellular ROS production; nuclear factor erythroid 2‐related factor‐2 (Nrf2) mRNA expression was slightly increased in both neohesperidine dihydrochalcone‐ and CTP‐treated cells. In contrast, heme oxygenase‐1 (HO‐1) and NAD(P)H: quinine oxidoreductase‐1 (NQO‐1) showed significant up‐regulation of mRNA expression (and protein gene products) only in CTP‐treated cells. It is unclear why CTP upregulates HO‐1 and NQO‐1 while neohesperidine dihydrochalcone does not. This study provides limited evidence on the effects of neohesperidine dihydrochalcone in adipocytes in vitro.
Johnston et al. (2005) reported that neohesperidine dihydrochalcone reduces glucose uptake by human intestinal Caco‐2 cells (TC7 subclone, passage numbers 30–40, 1 × 1E04 cells/well, grown for 19 days). D‐[6‐3H] glucose (125 kBq/mL in experimental solution) was used as the tracer; glucose transport was measured after 2 min incubation. Under sodium‐dependent conditions (favouring uptake via sodium/glucose cotransporter 1 (SGLT1)), 100 μM neohesperidine dihydrochalcone significantly reduced glucose transport. Under sodium‐free conditions (favouring uptake by glucose transporter (GLUT) proteins), 100 μM neohesperidine dihydrochalcone had no effect on glucose transport. The authors suggested that under sodium‐dependent conditions, hydrophobic interactions between neohesperidine dihydrochalcone and the surrounding bilayer contribute to the loss of active glucose transport. These limited data do not imply any adverse human health effects of neohesperidine dihydrochalcone.
Choi et al. (2021) examined also the effect of neohesperidine dihydrochalcone and dihydrocaffeic acid (DHCA), which is described as the metabolite of neohesperidine dihydrochalcone but without supporting references, on lipopolysaccharides (LPS)‐induced (500 ng/mL for 12 h) mouse macrophage (RAW 264.7) interleukin 6 (Il6) and tumour necrosis factor alpha (TNFα) secretion in vitro. Neohesperidine dihydrochalcone at 100 or 500 μM did not affect LPS‐induced Il6 secretion but it statistically significantly and dose‐dependently reduced secretion to 52% and 3% of LPS‐treated cells at 100 μM and 500 μM, respectively. Neohesperidine dihydrochalcone dose‐dependently reduced TNFα secretion to approximately 60% and 20% of LPS‐treated cells at 100 μM and 500 μM, respectively. The effects of LPS, neohesperidine dihydrochalcone and DHCA on RAW 264.7 oxygen consumption were also examined. The cells were pretreated with neohesperidine dihydrochalcone or DHCA for 24 h prior to exposure to LPS (500 ng/mL) for another 24 h. Basal respiration, ATP production and maximal respiration were significantly reduced by the addition of LPS to cells. These effects were marginally reduced by neohesperidine dihydrochalcone or DHCA; however, only a single undeclared concentration of each was examined. Lipid accumulation was also examined by Choi et al. (2021) using the 2T3‐L1 in vitro cell model. Pre‐adipocytes were differentiated into adipocytes over a 8 days period in the presence of either neohesperidine dihydrochalcone or DHCA for the last 6 days. Lipid accumulation was examined using a quantitative oil red staining protocol. Neohesperidine dihydrochalcone slightly but statistically significantly increased lipid accumulation in the cells by 14% whereas DHCA statistically significantly reduced lipid accumulation at day 8% to 75% when cells were exposed to 500 μM of either compound, the only concentrations tested. The authors concluded that these results indicate that neohesperidine dihydrochalcone and its claimed metabolite DHCA have the potential to suppress the inflammatory response and obese status, but that the molecular mechanisms involved in cellular signalling require additional investigation.
The Panel noted that no toxicologically relevant effects were observed in any of the available in vivo studies and that the concentrations in the in vitro studies are above the Cmax anticipated in rats when exposed at the level of the ADI. Therefore, the relevance for human health effects is probably very low.
Overall, because these mechanistic studies are considered not to contribute any substantive information relevant to the safety assessment of neohesperidine dihydrochalcone (E 959) as a food additive, they were not considered further by the Panel.
3.5.6. Integration of evidence and derivation of the ADI
The body of evidence of the considered animal studies presents a consistent pattern of findings indicating that oral intake of neohesperidine dihydrochalcone is not associated with adverse effects on health in the dose ranges tested. In the absence of human studies, the integration of evidence consisted of evaluation of toxicological studies in animals that formed the basis for derivation of an ADI. Based on the WoE analysis, it is unlikely that neohesperidine dihydrochalcone (E 959) would lead to adverse effects on health in animals in the dose ranges tested.
The Panel considered that the data from the 13‐week subchronic toxicity study in rat (T.N.O., 1986, in Documentation provided to EFSA nr: 9, GLP study, consistent with OECD TG 408), 11‐week study in mice (Shi et al., 2021) and prenatal developmental toxicity study (Waalkens‐Berendsen et al., 2004; GLP study, OECD TG 414) in rats were sufficient to establish a new ADI. Given that there was no concern for genotoxicity, a carcinogenicity study was considered not warranted. The Panel also considered that the lack of human data does not affect the overall confidence in the body of evidence. The Panel considered the highest dose tested of 4,000 mg/kg bw per day in rats as a NOAEL (T.N.O., 1986 in Documentation provided to EFSA nr: 9). Applying the standard default factor of 100 for inter‐ and intraspecies differences and the default factor of 2 for extrapolation from subchronic to chronic exposure (EFSA Scientific Committee, 2012; Guth et al., 2020), an ADI of 20 mg/kg bw per day was derived.
3.6. Environmental considerations
An extensive review collating published data on artificial sweeteners, including neohesperidine dihydrochalcone (E 959), to identify evidence of potential adverse effect on the environment was performed (Agriculture and Environment Research Unit, University of Hertfordshire, 2021). In this review, only a limited amount of relevant information relating to the environmental impact of neohesperidine dihydrochalcone (E 959) was identified. Some evidence was available suggesting that neohesperidine dihydrochalcone (E 959) is removed from wastewater by treatment facilities. In addition, neohesperidine dihydrochalcone (E 959) was reported as not routinely detected in several environmental compartments such as surface water, seawater and ground water (LODs ranging from 0.005 to 0.85 μg/L) (see Sections 10.3 and 13 of Agriculture and Environment Research Unit, University of Hertfordshire, 2021). This conclusion was confirmed in a follow‐up update of the literature search, where some additional papers dealing with the determination of neohesperidine dihydrochalcone (E 959) in surface water, seawater and waste‐water were retrieved (Ordonez et al., 2012; Tran et al., 2014; Arbelaez et al., 2015; Hernandez et al., 2015; Salas et al., 2015; Neale et al., 2017; Lakade et al., 2018; Xie et al., 2020; Gvozdic et al., 2020; Cardenas‐Soraca et al., 2020). The Panel also noted that in the context of the REACH Regulation (EC) 1907/2006, a registration dossier 6 on neohesperidine dihydrochalcone (E 959) is available, in which this substance is reported to be readily biodegradable based on experimental data. It is also noted that the FEEDAP Panel reported in its scientific opinions (EFSA FEEDAP Panel, 2011, 2014) that, although neohesperidine dihydrochalcone is not found as such in nature, the available experimental data demonstrate that its metabolism follows the same pathway as the metabolism of other flavonoids yielding metabolic products which are found in nature and that, in view of the fast degradation expected for neohesperidine dyhydriochalcone and its metabolites, no impact on the environment is foreseen from the use of neohesperidine dihydrochalcone in feed.
The available information was limited and no specific ecotoxicological and fate and behaviour studies on neohesperidine dihydrochalcone (E 959) were available. Overall, the Panel considered that it is not expected that neohesperidine dihydrochalcone (E 959) will reach the environment in measurable amounts and therefore a concern for the environment is not anticipated.
3.7. Discussion
Neohesperidine dihydrochalcone (E 959) is authorised as a food additive in the EU in accordance with Annex II to Regulation (EC) No 1333/2008 on food additives and its specifications are defined in the Commission Regulation (EU) No 231/2012.
Neohesperidine dihydrochalcone was previously assessed by SCF (1985; 1989). Following these evaluations, the Committee established an ADI of 5 mg/kg bw per day, considering the lowest NOAEL obtained in all the studies which were carried out and evaluated. Neohesperidine dihydrochalcone is authorised in the EU also as food flavouring ([FL‐no: 16.061]), in accordance with Regulation (EC) No 1334/2008 evaluated in FGE.32 (EFSA CEF Panel, 2010). In addition, the EFSA FEEDAP Panel evaluated the safety of neohesperidine dihydrochalcone as a sensory additive for use in animal feed for several species (EFSA FEEDAP Panel, 2011, 2014).
As specified in the Commission Regulation (EU) No 231/2012, neohesperidine dihydrochalcone (E 959) is obtained by catalytic hydrogenation of neohesperidin. According to the information provided by the interested business operators, the source material neohesperidine is a flavanone naturally occurring in bitter oranges (C. aurantium), isolated by alcohol extraction.
The Panel noted that the source of the starting material neohesperidin is not specified in the definition included in the current EU specifications of E 959. Therefore, the Panel is of the view that the current definition of E 959 should be revised to also include the source of the starting material neohesperidin and how the starting material is obtained.
Structurally related flavonoid impurities can be present in E 959 and their chemical names and structures are presented in Table 1. These impurities can be:
degradation products of neohesperidine dihydrochalcone (E 959) formed under (i) the strong alkaline conditions or high temperatures of the manufacturing process (impurity A) or (ii) resulting from the hydrolytic cleavage of glycosidic bonds of neohesperidine dihydrochalcone (impurity F and G);
side hydrogenation products of co‐extracted flavanones (naringin, hesperidin and poncirin) from the source material bitter oranges (impurity D, E and H);
the unreacted starting material neohesperidin (impurity C);
the unreacted (i.e. not hydrogenated) flavone neodiosmin occurring in the source material (bitter oranges) and co‐extracted with neohesperidin (impurity B).
These impurities are also described in the EU Pharmacopeia monograph for neohesperidine dihydrochalcone (European Pharmacopoeia 9.0, 2017).
Analytical data performed on at least five commercial batches of E 959, and supported by certificates of analysis, were provided by both interested business operators to demonstrate that E 959 is consistently produced within the established EU specifications as per Commission Regulation (EU) No 231/2012.
Both interested business operators provided analytical data on the impurity profiles of batches of E 959. In this respect, the Panel noted that the impurity profiles of the analysed batches of E 959 differed within‐ and between the two interested business operators. Also, it cannot be assumed that these impurities were also present in the material(s) used in the toxicity testing (Appendices C and D) since they were reported only being compliant with the existing EU specifications for E 959 without any specific information on the impurities profile. On the other hand, the Panel noted that the impurities are all closely related to the chemical structure of neohesperidine dihydrochalcone (E 959) itself and that Q(SAR) analysis using the OECD Q(SAR) Toolbox did not highlight any structural alerts in the impurities with regard to potential for genotoxicity (Appendix E). The purity assay for E 959, as currently laid down in the EU specifications, requires not less than 96% chemical purity. Considering the nature, the levels and the origin of the impurities, along with the recommendation that the source material for the food additive (i.e. neohesperidin obtained from bitter oranges, C. aurantium) should be included in the EU definition of E 959, the Panel did not consider necessary to recommend inclusion of limit values for these impurities in the EU specifications of E 959.
The Panel calculated the potential exposure to toxic elements from the uses of E 959 (Appendix F). The resulting figures showed that the exposure to Pb from the uses and use levels of E 959 would not be of concern using both the limit values calculated by the Panel (based on the analytical data provided by the interested business operators) as possible EU specification values (Table F.2, Appendix F) and the existing EU specifications (Table F.3, Appendix F). As the occurrence levels for Pb reported by both interested business operators are substantially below the current EU specification limit (not more than 2 mg/kg), the Panel noted that a lower limit for Pb is technologically feasible.
For As, the lower end of the calculated MOE values falls below the target of 1,000 (Table F.3, Appendix F), indicating that lowering the existing limit value of 3 mg/kg is recommended, which is technologically feasible based on the analytical data provided.
For Cd and Hg, for which no maximum limits are set in the EU specifications for E 959, the estimates of exposure are a very small fraction of their TWI values. Considering the occurrence levels reported by both interested business operators and the manufacturing process of E 959, the Panel sees no need to introduce limits for these two elements in the EU specifications for E 959.
The Panel noted that the hydrogenation step in the manufacturing process for E 959, as described by the interested business operators is assisted by a heterogeneous palladium‐on‐charcoal (Pd/C) catalyst. In this respect, one interested business operator provided analytical data on the residual levels of palladium in three batches of E 959. The Panel calculated the potential exposure to the palladium from the uses of E 959 (Appendix F) which resulted to be only a small fraction of the PDE value (ICH, 2019). The Panel sees no need to introduce a limit for Pd in the EU specifications for E 959.
The Panel noted that both interested business operator provided data on microbiological analyses supporting the microbiological quality of this food additive. In addition, analytical data on environmental contaminants (PAHs, pesticides residues and mycotoxins) were provided by both interested business operators. The Panel noted that both interested business operators provided adequate information on the analytical techniques and methods used in each contaminant determination along with the respective LOQ. In all analysed batches, the contaminants were not detected above their LOQ.
Regarding water solubility, the Panel concluded that neohesperidine dihydrochalcone (E 959) can be considered slightly soluble in water at 20°C (230 mg/L) according to JECFA criteria (JECFA, 2006). In addition, based on the data on particle size distribution submitted by the interested business operators and the criteria set in the EFSA Guidance‐TR, the Panel concluded that the presence of small particles, including nanoparticles, cannot be excluded in the pristine food additive.
The Panel noted that the maximum use levels of neohesperidine dihydrochalcone (E 959) reported by the food industry for various food categories (see Annex A) do not exceed 200 mg/L. Taking into account the water solubility of neohesperidine dihydrochalcone (E 959) (i.e. 230 mg/L at 20°C), the Panel considered that E 959 can be anticipated as fully dissolved when used as a food additive at the reported use levels in aqueous matrices (according to Section 2.3.4 of the EFSA Guidance on Particle‐TR). For matrices like sauces, chewing gum and chocolates, to which the food additive is allowed to be added, the Panel considered that neohesperidine dihydrochalcone (E 959) is expected to be fully dissolved in the GI tract. Table‐top sweeteners are not intended to be consumed as such and will be largely diluted in beverages and, accordingly, particles would be expected to dissolve.
Taking into account the reported uses and use levels and the MPLs, the reported solubility, the increase of solubility of neohesperidine dihydrochalcone in water with temperature (Benavente‐Garcia et al., 2001) and the volume of gastric secretion (ranging from 215 mL within a single meal to 2,000 mL daily; ICRP, 2002; Mudie et al., 2014), the Panel considered that full dissolution of neohesperidine dihydrochalcone (E 959) is to be expected in the GI tract and that ingested particles (if any) would not persist.
Therefore, the Panel concluded there is no concern with regard the potential presence of small particles, including nanoparticles, in neohesperidine dihydrochacone (E 959) when used as a food additive and considered that the risk assessment of neohesperidine dihydrochalcone (E959) can be performed following the EFSA Guidance for submission for food additive evaluations (EFSA ANS Panel, 2012).
Limited in vitro data indicated that unabsorbed neohesperidine dihydrochalcone is relatively slowly catabolised by the GI microbiota in the colon in humans. Based on the available in vivo studies in rats, the Panel considered that, also in humans, neohesperidine dihydrochalcone is likely to be absorbed, to become systemically available both as parent compound and as metabolites, which are excreted mainly in urine.
The Panel concluded that neohesperidine dihydrochalcone (E 959) does not raise a concern regarding genotoxicity.
Based on the weight of evidence analysis, it is unlikely that neohesperidine dihydrochalcone would lead to adverse effects on health in animals in the dose ranges tested. The Panel also considered that the lack of human data does not affect the overall confidence in the body of evidence. The Panel considered the highest dose tested of 4,000 mg/kg bw per day in rats as a NOAEL (T.N.O., 1986 in Documentation provided to EFSA nr: 9). Applying the standard default factor of 100 for inter‐ and intraspecies differences and the default factor of 2 for extrapolation from subchronic to chronic exposure (EFSA Scientific Committee, 2012; Guth et al., 2020), an ADI of 20 mg/kg bw per day was derived.
The Panel considered that the previous ADI of 5 mg/kg bw per day derived by the SCF (1989) was based on a study (subchronic studies in Western Regional Research Laboratory, 1965 in Documentation provided to EFSA nr: 10) that could not be considered as reliable based on current standards (low confidence in exposure characterisation). For other repeated dose toxicity studies considered by the SCF, including long‐term studies, high risk of biases was identified by the Panel. The only previously considered toxicity study appraised as of high reliability (Tier 1) was the 13‐week subchronic toxicity study in rat by T.N.O., 1986 (Documentation provided to EFSA nr: 9).
Dietary exposure to neohesperidine dihydrochalcone (E 959) was estimated according to different exposure scenarios based on consumers‐only as described in Section 3.4. The Panel considered the refined brand‐loyal exposure assessment scenario (with facets) to be the most appropriate exposure scenario for the risk assessment. For this scenario, the Panel considered use levels available for neohesperidine dihydrochalcone (E 959) for three out of 38 authorised food categories. This limited use of neohesperidine dihydrochalcone (E 959) as a sweetener was confirmed by data from literature. From literature, neohesperidine dihydrochalcone (E 959) was shown to be present in only one dairy‐based drink and one hard candy. Use levels used for the refined brand‐loyal exposure assessment scenario did not include these two food types. Also label information on the use of neohesperidine dihydrochalcone (E 959) in foods from the Mintel GNPD supported that, in general, it has limited use in Europe, but is used in a few regularly consumed carbonated soft drinks.
In the refined brand‐loyal exposure assessment scenario, mean dietary exposure to neohesperidine dihydrochalcone (E 959) ranged from < 0.01 mg/kg bw per day in adults and the elderly to 0.09 mg/kg bw per day in toddlers. The 95th percentile of dietary exposure ranged from 0.01 mg/kg bw per day in the adults and the elderly to 0.24 mg/kg bw per day in toddlers.
Considering the ADI of 20 mg/kg bw per day as a reference value for the risk assessment, mean and P95 levels of dietary exposure to neohesperidine dihydrochalcone (E 959) in all age groups in the refined brand‐loyal exposure assessment scenario were below this reference value. The Panel noted that the exposure estimates for the regulatory maximum and the refined regulatory maximum level exposure assessment scenarios were also below the ADI (see Table 6).
3.8. Uncertainty
The uncertainties related to the exposure assessment are summarised in Table 7 of Section 3.4.2.
Regarding the hazard identification and characterisation, the overall body of evidence assessed for this purpose consisted of only three studies, nevertheless it is concluded that the confidence in the body of evidence was sufficient to derive an ADI.
Despite the lack of replicative studies for some health outcome categories (such as haematotoxicity and developmental toxicity), based on consistent absence of adverse effects observed in all three studies, it is concluded that it is unlikely that further studies would have changed the outcome of this assessment. Therefore, the Panel considered that the ADI has been derived with high certainty. Furthermore, although a carcinogenicity study was not considered warranted, a lack of such studies may be considered a minor source of uncertainty. Overall, these uncertainties are considered minor and not influential for the conclusions on safety.
4. Conclusions
Based on the available toxicological data, the Panel derived an ADI of 20 mg/kg bw per day for neohesperidine dihydrochalcone (E 959). The Panel concluded that dietary exposure to the food additive neohesperidine dihydrochalcone (E 959) at the reported uses and use levels would not raise a safety concern.
5. Recommendation
The Panel recommended that the European Commission consider amending existing EU specifications for neohesperidine dihydrochalcone (E 959) through:
including in the current definition the source of the starting material – neohesperidin – and how the starting material is obtained;
introducing the CAS number 20702‐77‐6;
introducing information on specific rotation;
lowering the current limits for arsenic and lead, taking into account the analytical data submitted by the interested business operators.
6. Documentation as provided to EFSA
HealthTech BioActives (formely Interquim), June 2018. Reply to the EFSA call for technical and toxicological data on sweeteners authorised as food additive in the EU (EFSA‐Q‐2017‐00500). Technical data on neohesperidine dihydrochalcone (E 959): section 1.
HealthTech BioActives, May and July 2019. Reply to the EFSA call for technical data on sweeteners authorised as food additive in the EU (EFSA‐Q‐2019‐00318) and reply to EFSA clarifications request on technical data provided in response to EFSA call for technical and toxicological data on sweeteners authorised as food additive in the EU (EFSA‐Q‐2017‐00500).
HealthTech BioActives, January 2021. Reply to EFSA clarifications request on technical data provided in response to EFSA calls for data on sweeteners authorised as food additive in the EU (EFSA‐Q2017‐ 00500, EFSA‐Q‐2019‐00318).
HealthTech BioActives, January 2022. Reply to EFSA clarifications request on technical data provided in response to EFSA calls for data on sweeteners authorised as food additive in the EU (EFSA‐Q2017‐00500, EFSA‐Q‐2019‐00318).
Destillaciones Bordas, June 2018. Reply to the EFSA call for technical and toxicological data on sweeteners authorised as food additive in the EU (EFSA‐Q‐2017‐00500). Technical dossier on neohesperidine dihydrochalcone (E 959) and accompanying appendixes.
Destillaciones Bordas, September 2019. Reply to the EFSA call for technical data on sweeteners authorised as food additive in the EU (EFSA‐Q‐2019‐00318).
Destillaciones Bordas, March 2021. Reply to EFSA clarifications request on technical data provided in response to EFSA calls for data on sweeteners authorised as food additive in the EU (EFSA‐Q2017‐00500, EFSA‐Q‐2019‐00318).
Destillaciones Bordas, March 2022. Reply to EFSA clarifications request on technical data provided in response to EFSA calls for data on sweeteners authorised as food additive in the EU (EFSA‐Q2017‐00500, EFSA‐Q‐2019‐00318)
- HealthTech BioActives (formely Interquim), June 2018. Reply to the EFSA call for technical and toxicological data on sweeteners authorised as food additive in the EU (EFSA‐Q‐2017‐00500). Toxicological data on neohesperidine dihydrochalcone (E 959): section 2.
- Sequani Limited, 2018. Neohesperidin dihydrochalcone: In Vitro Mammalian Cell Micronucleus Test. Unpublished study report.
- T.N.O. – Division for Nutrition and Food Research, 1986. Sub‐chronic (90‐day) oral toxicity study with neohesperidine dihydrochalcone (NHDC) in rats. Unpublished study report. This unpublished study report was subsequently published in the literature and referred in EFSA CEF Panel, 2010 and in JECFA, 2012 as Lina BAR, Dreef-van der Meulen HC, Leegwater DC, 1990. Subchronic (13-week) oral toxicity of neohesperidin dihydrochalcone in rats. Food Chemistry and Toxicology, 26(7), 507–513. https://doi.org/10.1016/0278-6915(90)90121-3.
- Gumbmann MR, Gould DH, Robbins DJ and Booth AN, 1978. Toxicity studies of neohesperidin dihydrochalcone. In: Shaw, JH, Roussos, GG (eds.). Sweeteners and Dental Caries. Information Retrieval Inc., Washington D.C., pp. 301–310.
- HealthTech BioActives, September 2021. Reply to EFSA request for additional information in the context of the EFSA call for technical and toxicological data on sweeteners authorised as food additive in the EU (EFSA‐Q‐2017‐00500). The following unpublished study report was submitted:
- Western Regional Research Laboratory, 1965. Toxicity study of two flavanone dihydrochalcones (potential artificial sweetening agents). Unpublished report. This unpublished study is referred as Booth AN, Robbins DJ and Gagne WE, 1965 in EFSA CEF Panel, 2010 and in JECFA, 2012. The report includes several subchronic and reproductive toxicity studies.
HealthTech BioActives, April 2022. Reply to the EFSA call for data on genotoxicity data on sweeteners. Unpublished study report: Bioneeds India Private Limited, 2022. In vitro Mammalian Cell Micronucleus Test of NEOHESPERIDIN DC in Human Lymphocytes.
Destillaciones Bordas, July 2022, Reply to EFSA clarifications request on technical data provided in response to EFSA calls for data on sweeteners authorised as food additive in the EU (EFSA‐Q2017‐00500, EFSA‐Q‐2019‐00318).
HealthTech BioActives, July 2022, Reply to EFSA clarifications request on technical data provided in response to EFSA calls for data on sweeteners authorised as food additive in the EU (EFSA‐Q2017‐00500, EFSA‐Q‐2019‐00318).
Abbreviations
- ADI
acceptable daily intake
- ADME
absorption, distribution, metabolism, excretion
- AESGP
Association of the European Self‐Medication Industry
- As
arsenic
- BCRP
human breast cancer resistance protein
- BMDL
benchmark dose lower bound
- bw
bodyweight
- Cd
cadmium
- C/EBP‐α
CCAAT‐enhancer binding protein‐α
- CEF
EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids
- CEN
European Committee for Standardization
- Cfu
colony forming units
- CONTAM
EFSA Panel on Contaminants in Food Chain
- CTP
2,4,6‐trimethoxyphenyl)prop‐2‐en‐1‐one
- DAD
diode array detector
- DHCA
dihydrocaffeic acid
- ELSD
evaporative light‐scattering detection
- FAF
EFSA Panel on Food Additives and Flavourings
- FAS
fatty acid synthase
- FEEDAP
EFSA Panel on Additives and Products or Substances used in Animal Feed Panel
- FC
food category
- FDA
U.S. Food and Drug Administration
- FDE
Food Drink Europe
- FGE
Flavouring Group Evaluation
- FSA
Food Standards Agency
- FSE
Food Supplement Europe
- FT‐IR
Fourier‐transform infrared
- GD
gestational days
- GI
gastrointestinal
- GLUT
glucose transporter
- GNPD
Global New Products Database
- hASCs
human adipose‐derived stem cells
- HBGV
health‐based guidance value
- HFD
high‐fat diet
- Hg
mercury
- HILIC
hydrophilic interaction liquid chromatography
- HO‐1
heme oxygenase‐1
- HPLC
high‐performance liquid chromatography
- HPLC‐UV
high‐performance liquid chromatography with ultraviolet detection
- ICP‐MS
inductively coupled plasma‐mass spectrometry
- Il6
interleukin 6
- JECFA
Joint FAO/WHO Expert Committee on Food Additives
- LC
liquid chromatography
- LC‐ESI‐MS/MS
liquid chromatography electrospray ionisation tandem mass spectrometry
- LD
laser diffraction
- LD50
median lethal dose
- LOD
limit of detection
- LOQ
limit of quantification
- LPS
lipopolysaccharides
- LY
lucifer yellow
- MCHC
mean corpuscular haemoglobin concentration
- MCH
mean corpuscular haemoglobin
- MCV
mean corpuscular volume
- MOE
margin of exposure
- MPL(s)
maximum permitted level(s)
- MRP2
multidrug resistance associated protein 2
- MS
mass spectrometry
- ND
normal diet
- NHDC‐
Neohesperidine dihydrochalcone
- NMR
nuclear magnetic resonance
- NOAEL
no observed adverse effect level
- NQO‐1
NAD(P)H: quinine oxidoreductase‐1
- Nrf2
nuclear factor erythroid 2‐related factor‐2
- NTP
National Toxicology Program
- OECD TG
Organisation for Economic Co‐operation and Development Testing Guidelines
- (Q)SAR
quantitative structure–activity relationship
- PAHs
polycyclic aromatic hydrocarbons
- PCV
packed cell volume
- PDA
Photodiode Array
- PDE
permitted daily exposure
- Pb
lead
- Pd/C
palladium‐on‐charcoal
- P‐gp
P‐glycoprotein
- PPAR‐γ
peroxisome proliferator‐activated receptor‐γ
- P95
95th percentile
- QS
quantum satis
- RBC
red blood cells
- REACH
Registration, Evaluation, Authorisation and Restriction of Chemicals
- RP
reference point
- RoB
risk of bias
- ROS
reactive oxygen species
- RP‐LC
reversed phase liquid chromatography
- SCF
Scientific Committee on Food
- SEM
scanning electron microscopy
- SGLT1
sodium/glucose cotransporter 1
- SNE
Specialised Nutrition Europe
- SREBP‐1
sterol regulatory element‐binding protein‐1
- TNFα
tumour necrosis factor alpha
- TWI
tolerable weekly intake
- UHPLC‐ESI‐MS/MS
ultrahigh performance liquid chromatography–electrospray ionisation multiple reaction monitoring tandem mass spectrometry
- UHPCL/QTOF‐MS
ultra‐high performance liquid chromatography‐quadrupole time‐of‐flight mass spectrometry
- USDA
United States Department of Agriculture
- UV
ultraviolet
- WBC
white blood cells
- WoE
weight of evidence
Appendix A – Tailored protocol and its implementation for the assessment of hazard identification and characterisation of neohesperidine dihydrochalcone (E 959)
Please refer to steps 1.11 to 1.15 of the general protocol for hazard identification and characterisation of sweeteners (EFSA, 2020a).
Extensive literature search
Methodology
For step 1.11, open‐ended literature searches were conducted in the three selected databases with the search strings and criteria applied as follows:
Web of Science: TOPIC: ("Neohesperidine DC" OR "Neohesperidine dihydrochalcone" OR E959 OR "E 959" OR "20702‐77‐6") AND LANGUAGE: (English) Timespan: 1988–2022. 22 Indexes: SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, BKCI‐S, BKCI‐SSH, ESCI, CCR‐EXPANDED, IC.
Pubmed: (("Neohesperidine DC" OR "Neohesperidine dihydrochalcone" OR E959) AND (("1988"[Date ‐ Publication]: "2022 19 "[Date ‐ Publication]))) AND ("english"[Language]) Quoted phrases not found: "E 959", "20702‐77‐6"
SciFinder: Substance Identifier "20702‐77‐6">substances (1)>get references>refine "1988‐2022 19 ">refine "English">refine "Clinical Trial Journal Preprin…"
Additional data were submitted by the interested business operators through the call for biological and toxicological data on sweeteners.
Results
The final number of references that were screened, after removal of duplicates (based on title, year, author, journal, volume, issue and page numbers) was 746.
For step 1.11.1 (Screening of the studies for relevance), the general principles reported in the protocol applied. 148 papers were included at the level of title and abstract screening, whereas 598 papers were excluded. From the 148 papers included for the full text screening, 17 were considered as possibly relevant, whereas 131 were either excluded at the level of full text screening, or preliminarily categorised into technical data (49), exposure data (20), environmental data (20) or toxicological review (4) and further screened for confirmation of their relevance (see Figure A.1).
Evaluation of the risk of bias (RoB)
Methodology
For step 1.12, the criteria outlined in the protocol for the risk of bias evaluation of studies have been applied, including the rules for tier allocation. More detailed criteria (i.e. explanation on how to interpret response options for each question) were further developed and will be published as addendum to the protocol (EFSA, 2020a).
The studies evaluated for the RoB were allocated to a tier (from 1 to 3 corresponding to decreasing levels of internal validity 11 ) based on the rules as reported in step 1.12 of the protocol (EFSA, 2020a).
The evaluation of the RoB was conducted in parallel independently by two reviewers and, in case a conflict on tier allocation of a study was identified, ad hoc discussion between the reviewers took place prior to a final agreed tier allocation that was reached by consensus.
The tier was automatically generated by the DistillerSR tool, after input of the ratings for the individual elements of the study considered for the RoB. At the end of the evaluation, the reviewers were requested to express their agreement or disagreement on the tier generated by the tool based on their expert judgement. In case of disagreement, a clear justification should have been provided.
As prescribed by the protocol (EFSA, 2020a), the study on which the derivation of the ADI was based was included and evaluated according to the protocol together with any relevant literature published since the previous evaluation by the SCF, allowing 1 year of overlap.
Results
In the case of neohesperidine dihydrochalcone, the previous pivotal study (subchronic studies in Western Regional Research Laboratory, 1965 in Documentation provided to EFSA nr: 10) was allocated to tier 3, mainly because of low confidence in exposure characterisation. Therefore, the Panel considered appropriate to evaluate the RoB for all the existing toxicity studies available for this substance (Table A.1), that were received by interested parties through the call for data.
The results of the RoB evaluation of the studies performed by two reviewers are summarised in Table A.1. No disagreements with the tier generated by the tool were identified (see last two columns on the right of Table A.1).
Data extraction
Methodology
For step 1.13, information and data from the reliable animal studies as well as from the included genotoxicity studies were extracted and reported in tabular form. The data extraction forms outlined in the protocol (EFSA, 2020a) were considered as templates and, for this reason, were slightly revised and adapted during the data extraction.
Results
The data extraction forms of the studies are reported in Appendices C and D of the current opinion.
Weighing the body of evidence and synthesis of the evidence
Methodology
For step 1.14 (Weighing the body of evidence), a weight of evidence analysis for different health outcome categories, grouped by endpoint as appropriate, was performed on animal studies evaluated to have low risk of bias (Tiers 1 and 2 studies only) (see Section 2.2 Methodologies). The weight of evidence analysis was performed using Excel tables (see Annex C).
For a specific health outcome, animal studies were rated for initial confidence according to study design. The initial confidence in the body of evidence could be downgraded for the following properties: concern for bias across studies, unexplained inconsistency in effect estimates, relevance of the study design to address the topic of evaluation and imprecision (degree of certainty). Followed by possible downgrading, confidence could be upgraded for the following properties: large magnitude of effect, evidence for dose–response and consistency across study designs and experimental model systems. For a more detailed description of assessment criteria, see NTP Handbook for conducting a literature‐based health assessment using OHAT approach for systematic review and evidence integration OHAT (NTP‐OHAT, 2019).
In accordance with the draft protocol for hazard identification and characterisation of sweeteners (EFSA, 2020a), the confidence in the body of evidence was rated ‘high’, ‘moderate’, ‘low’ or ‘very low or no evidence identified’ corresponding to the symbols ‘++++’, ‘+++’, ‘++’, ‘+’, respectively (symbols used in the Excel tables, Annex C). Following the WoE analysis for each health outcome category, confidence ratings were translated into levels of evidence for adverse effects on health or no adverse effects on health. The following descriptors were used to rate the level of evidence for the presence of adverse effects on health: ‘high’, ‘moderate’, ‘low’ or ‘inadequate’ when the confidence in the body of evidence was ‘high’, ‘moderate’, ‘low’ or ‘very low or no evidence identified’, respectively. When no adverse effects on health could be identified, a modified version of the descriptors for level of evidence were used: ‘high’, ‘moderate’ and ‘inadequate’ corresponding to confidence in the body of evidence ratings of ‘high’, ‘moderate’ and ‘low/very low or no evidence identified’, respectively (Figure A.2, adapted from NTP‐OHAT, 2019).
For the final assessment of the likelihood of a substance to be a hazard, the level of evidence resulting from the human evidence and animal evidence streams are integrated as shown in Figure A.3. The following expressions were used: very likely, likely, as likely as not, unlikely, very unlikely and not possible to conclude. Identification or not of adverse effects on health in the human and animal data gives four scenarios to consider (Figure A.3).
The assessment takes into consideration that identification of human data and presence of adverse effects on health in contrast to animal data not identifying adverse effects increase the likelihood that the substance is a hazard to humans. On the other hand, in case of human data not identifying adverse effects on health in contrast to animal data outlining presence of adverse effects on health, the assessment takes into consideration that the unlikelihood of the substance to be a hazard to humans can decrease due to possible presence of adverse effects in animals that are difficult to detect in humans.
Results
The result of the weight of evidence analysis for neohesperidine dihydrochalcone is presented in Annex C and summarised in Section 3.5.4 on ‘Synthesis of systematically appraised evidence on biological and toxicological effect’ and Section 3.5.6 on ‘Integration of evidence and derivation of ADI’.
Appendix B – Biological and toxicological data
| RefID | Source | Author(s) | Title | Year |
|---|---|---|---|---|
| 24 | Literature | Bozoğlan BK, Tunç S and Duman O | Investigation of neohesperidin dihydrochalcone binding to human serum albumin by spectroscopic methods | 2014 |
| 25 | Literature | Braune A, Engst W and Blaut M | Degradation of Neohesperidin Dihydrochalcone by Human Intestinal Bacteria | 2005 |
| 57 | Literature | Fang Y, Cao W, Xia M, Pan S and Xu X | Study of structure and permeability relationship of flavonoids in Caco‐2 cells | 2017 |
| 58 | Literature | Fang Y, Liang F, Liu K, Qaiser S, Pan S and Xu X | Structure characteristics for intestinal uptake of flavonoids in Caco‐2 cells | 2018 |
| 75 | Literature | Han GE, Kang H‐T, Chung S, Lim C, Linton JA, Lee J‐H, Kim W, Kim S‐H and Lee JH | Novel neohesperidin dihydrochalcone analogue inhibits adipogenic differentiation of human adipose‐derived stem cells through the Nrf2 pathway | 2018 |
| 95 | Literature | Johnston K, Sharp P, Clifford M and Morgan L | Dietary polyphenols decrease glucose uptake by human intestinal Caco‐2 cells | 2005 |
| 209 | Literature | Sjöstedt N, Deng F, Rauvala O, Tepponen T and Kidron H | Interaction of Food Additives with Intestinal Efflux Transporters | 2017 |
| 230 | Literature | Waalkens‐Berendsen DH, Kuilman‐Wahls MEM and Bär A | Embryotoxicity and teratogenicity study with neohesperidin dihydrochalcone in rats | 2004 |
| 231 | Literature | Wang X, Pan Y, Ma J, Shi S, Zheng X and Xiang Z | Application of a liquid chromatography‐tandem mass spectrometry method to the pharmacokinetics, bioavailability and tissue distribution of neohesperidin dihydrochalcone in rats | 2014 |
| 286 | Call for data | Gumbmann MR, Gould DH, Robbins DJ and Booth AN (Documentation provided to EFSA nr: 9) | Toxicity studies of neohesperidin dihydrochalcone | 1978 |
| 306 | Call for data | Sequani Limited (Documentation provided to EFSA nr: 9) | Neohesperidin dihydrochalcone: In Vitro mammalian cell micronucleus test | 2018 |
| 307 | Call for data (a) | T.N.O. – Division for Nutrition and Food Research (Documentation provided to EFSA nr: 9) | Sub‐chronic (90‐day) oral toxicity study with neohesperidine dihydrochalcone (NHDC) in rats | 1986 |
| 339 | Call for data/Reply to EFSA clarifications request | Western Regional Research Laboratory (Documentation provided to EFSA nr: 10) | Toxicity study of two flavanone dihydrochalcones (potential artificial sweetening agents) | 1965 |
| 345 | Literature | Zhang FX, Yuan YL, Cui SS, Li M, Tan X, Qiu ZC and Li RM | Dissection of the potential pharmacological function of neohesperidin dihydrochalcone – a food additive – by in vivo substances profiling and network pharmacology | 2021 |
| 354 | Literature | Choi S, Yu S, Lee J and Kim W | Effects of Neohesperidin Dihydrochalcone (NHDC) on Oxidative Phosphorylation, Cytokine Production, and Lipid Deposition | 2021 |
| 360 | Literature | Shi ZJ, Lei HH, Chen G, Yuan PH, Cao Z, Ser HL, Zhu XH, Wu F, Liu CX, Dong MY, Song YC, Guo YY, Chen C, Hu KX, Zhu YF, Zeng XA, Zhou JL, Lu YJ, Patterson AD and Zhang LM | Impaired Intestinal Akkermansia muciniphila and Aryl Hydrocarbon Receptor Ligands Contribute to Nonalcoholic Fatty Liver Disease in Mice | 2021 |
| 789 | Call for data | Bioneeds India Private Limited (Documentation provided to EFSA nr: 11) | In vitro mammalian cell micronucleus test of neohesperidin DC in human lymphocytes | 2022 |
This unpublished study report was subsequently published as Lina BAR, Dreef‐van der Meulen HC, Leegwater DC, 1990. Subchronic (13‐week) oral toxicity of neohesperidin dihydrochalcone in rats. Food Chemistry and Toxicology, 26(7), 507–513. https://doi.org/10.1016/0278-6915(90)90121-3.
Appendix C – Data extraction forms for toxicological studies
Studies that are described in this Appendix are only reflecting the information provided in the study reports/papers.
Guideline studies : i.e. use of EPA, OECD, FDA or other guideline for study design.
Type of study : e.g. acute, short‐term (i.e. up to 28‐days, 4 weeks), subchronic (i.e. up to 90‐days, 13 weeks), chronic (i.e. up to 2‐years, 104 weeks), reproduction/developmental, carcinogenicity.
Dose regimen (dose level or concentration per group, and frequency) and achieved doses if available : based on analytical data and measured feed (water) intake (μg, mg or g/kg bw per day, mean for a whole study period, when the test compound is given in the diet (water)). In gavage studies ‘achieved doses’ are based on analytical data for a concentration of a test compound. When the doses were not explicitly reported by the authors as mg/kg bw per day and was not possible to be calculated from the analytical data and measured feed (water) intake, default factors were applied (see Section 2.2 Methodologies) and equivalence to mg/kg bw per day was reported.
Measured endpoints : in case of guideline studies, state only if there were any deviation from the guideline (e.g. missing and/or additional endpoints).
Time of measurement/observation period : for reproductive and developmental toxicity studies please indicate the life stage at which the measurement/observations were done (i.e. pre‐mating, mating, gestation, lactation, adult). For short‐term, subchronic, chronic and carcinogenicity study, it should be indicated in which week of the study or if at the beginning or the end of the treatment measurements/observations were done. In case of guideline studies, state only if there were any deviation from the guideline.
Methods to measure the endpoints : particularly relevant for new or non‐standard methods and/or for endpoints not covered by test guidelines. In case of guideline studies, state only if there were any deviation from the guideline.
No observed adverse effect level, lowest observed adverse effect level, benchmark dose/benchmark dose lower bound : if and as reported by the study author.
Subchronic toxicity studies
| Study ID | |
|---|---|
| RefID (DistillerSR) | 307 |
| Reference (authors, year, title, other info) | T.N.O. – Division for Nutrition and Food Research, 1986. Sub‐chronic (90‐day) oral toxicity study with neohesperidine dihydrochalcone (NHDC) in rats. |
| Source (published/unpublished) | Unpublished |
| Type of study and guideline | |
| Good laboratory practice (yes/no/No, but before establishment of GLP) | Yes |
| Guideline studies (if yes, specify) | No |
| Type of study | Subchronic toxicity |
| Animal model | |
| Species and strain | Rats, Wistar (breeding not reported) |
| Disease models (e.g. diabetes, allergy, obesity) | Not applicable |
| Housing conditions | |
| Housing condition | Housed under conventional conditions, 5 per sex per cage, in suspended stainless steel cages fitted with wire mesh floors and fronts. The cages were randomised over the cage‐racks. The temperature was kept at 22 ± 2°C, the relative humidity was at least 40%. Artificial light was provided for 12 h/day continuously, from 7.30 a.m. till 7.30 p.m. The number or air changes was about 10/h. Fed a powdered stock diet provided ad libitum from weighed feeders. Tap water was supplied ad libitum in glass bottles, cleaned weekly and filled with fresh water when necessary. |
| Diet name and source (if reported) | Powdered stock diet (van Eck, Cothen) |
| Treatment | |
| Test material | Neo‐DHC (Neohesperidine dihydrochalcone) |
| Provider | Sponsor |
| Compound purity | More than 99.5% |
| Vehicle used | None (direct admixture with powder rodent diet) |
| Dose regimen (dose level or concentration per group, and frequency) and achieved doses if available |
0%, 0.2%, 1.0%, 5.0% neohesperidine dihydrochalcone in the diet Equal to: 0, 150, 757, 4,011 mg/kg bw per day in males 0, 166, 848, 4,334 mg/kg bw per day in females |
| Route of administration (diet, drinking water, gavage) | Diet |
| Period of exposure (pre‐mating, mating, gestation, lactation, adult) | Adult |
| Duration of the exposure | 13 weeks (90 days) |
| Study design | |
| Sex and age at the start of the treatment | Males and females (3,5 weeks) |
| Number animals/sex per group |
20/males/group; 20/females/group For ophthalmoscopic examination: 20/males and 20/females of the control and highest dose group For haematology: 10/males/group and 10/females/group For clinical chemistry (aorta samples) and urinalysis: 10/males/group and 10/females/group. |
| Measured endpoints | Determination of neohesperidine dihydrochalcone in the diets, clinical signs, ophthalmoscopic examination, body weights, food consumption, water consumption, haematology, clinical chemistry, urinalysis, macroscopic and microscopic examination of organs and examination of intercurrent deaths. |
| Time of measurement/observation period |
The general condition and behaviour of all rats was checked daily. The eyes of all rats of the control and top‐dose group were examined prior to the start of the study and in week 13. Body weights were recorded initially then weekly. In addition, rats were weighed on the day of autopsy. Food intake was measured per cage weekly for 12 weeks. Water intake was measured per cage daily in week 7 and in week 12. For haematology, samples of blood were collected from males on day 84 and from females on day 85. For chemical chemistry, on day 87 blood was collected from the tail after deprivation of water for 24 h and of food during the last 16 h to determine glucose levels. Blood samples were collected from the aorta of males on day 91 and of females on day 92. For urinalysis, on day 87 rats were deprived of water 24 h and food 16 h and urine was collected during the last 16 h of deprivation period. On day 86, 3‐h urine samples were collected from unfasted rats and the pH was determined. Animals were killed on 4 successive days (91 and 93 males, 92 and 94 females) and examined macro/microscopically for pathological changes. |
| Methods to measure the endpoints | In line with standard sub‐chronic toxicity study. |
| Statistical analysis | |
| Statistical methods | Analysis of variance followed by Dunnett's test, analysis of variance followed by the LSD test, Mann–Whitney U‐test and Fisher's Test |
| Results | |
| Findings reported by the study author/s | The intake of neohesperidine dihydrochalcone in the various weeks calculated, showed the normal decrease with increasing study duration for each of the three dose groups. Focal alopecia and transient diarrhea were observed in the top‐dose group. No other treatment‐related changes in general condition or behaviour. One female rat of the mid‐dose group died in day 30, but not considered treatment related. Ophthalmoscopic examination did not reveal any treatment‐related changes. Body weights were decreased in the top‐dose group in males throughout the test period and in females in the first 2 weeks. Food intake was decreased in males of the top dose group in the first 2 weeks, whereas water intake was slightly increased in week 7 but not statistically significant. There were no treatment‐related changes in haematology. Alkaline phosphatase activity and bilirubin concentration were increased in females of the top‐dose group whereas in males of this group, total protein concentration was decreased. The changes in clinical chemistry variates observed in the top dose were not accompanied by other signs. It is not clear whether these changes are of toxicological significance. Urinary pH was statistically decreased in the top‐dose of both sexes. No treatment‐related changes in volume, density, composition or sediment of urine. The weights of the filled and empty caecum were increased in the top‐dose group in both sexes. This effect is not typical for NDHC and may be due to microbiological degradation of unabsorbed NDHC., but not considered a toxic effect. The tendency towards higher water intake, the occasional diarrhoea and the decrease in urinary pH may also be attributed to an increase amount of microbial degradation products. The relative weights of the brain and testicles were increased in males of the top‐dose group, but not considered a toxic effect. At autopsy, no treatment‐related gross abnormalities were observed. At microscopic examination, no histopathological changes were observed that could be considered to be treatment related. |
| No observed adverse effect level, lowest observed adverse effect level, benchmark dose/benchmark dose lower bound |
NOAEL: 1% neohesperidine dihydrochalcone in the diet. This level was equivalent to a nominal intake of approximately 750 and 850 mg/kg body weight per day for males and females respectively. |
| Further information | |
| Deviations from the protocol: analysis for the amount of test substance in the diets were not carried out by the sponsor, but at the TNO‐CIVO Institute. On day 15 and 86, the temperature and/or humidity in the animal room slightly exceeded the ranges mentioned, but this is not considered to have influenced the outcome of the study. Intake of the test substance and red blood cell indices were calculated, although this was not specifically mentioned in the protocol. Plasma cholesterol was determined in all groups. This determination was omitted from the protocol by mistake. Cholesterol was not determined in one female of the mid‐group because not enough sample could be obtained. PH was determined individually, in freshly voided, 3‐h urine samples, instead of in pooled, 16‐h, concentrated urine. This was done because in this way more representative pH values are obtained. A few organs of different rats were not weighed at autopsy for various reasons, viz: testicles – atrophy; kidneys – hydronephrosis; adrenal – enlargement; adrenal, ovary and thyroid – lost at autopsy. The organs of the rat that died during the study were not weighed. In addition to the protocol, the weight of the full and empty caecum was determined in all rats at study termination. A few organs or tissues of different rats could not be examined microscopically since, by oversight, they were not collected for fixation or they were lost during fixation and/or processing. The number of the various organs and tissues examined was given. The organs and tissues of one female rat of the mi‐dose group were examined microscopically, because this animal died during the study. To note that this unpublished study report was subsequently published as Lina BAR, Dreef‐van der Meulen HC, Leegwater DC, 1990. Subchronic (13‐week) oral toxicity of neohesperidin dihydrochalcone in rats. Food Chemistry and Toxicology, 26(7), 507–513. https://doi.org/10.1016/0278-6915(90)90121-3. | |
| Study ID | |
| RefID (DistillerSR) | 360 |
| Reference (authors, year, title, other info) | Shi ZJ, Lei HH, Chen G, Yuan PH, Cao Z, Ser HL, Zhu XH, Wu F, Liu CX, Dong MY, Song YC, Guo YY, Chen C, Hu KX, Zhu YF, Zeng XA, Zhou JL, Lu YJ, Patterson AD and Zhang LM, 2021 Impaired intestinal Akkermansia muciniphila and aryl hydrocarbon Receptor ligands contribute to nonalcoholic fatty liver disease in mice. mSystems 6(1): e00985‐20. https://doi.org/10.1128/mSystems.00985‐20 |
| Source (published/unpublished) | Published |
| Type of study and guideline | |
| Good laboratory practice (yes/no/no, but before establishment of GLP) | Yes |
| Guideline studies (if yes, specify) | Animal experimental procedures performed according to the Chinese National Guidelines |
| Type of study | Subchronic toxicity |
| Animal model | |
| Species and strain | Mice, C57BL/6 strain |
| Disease models (e.g. diabetes, allergy, obesity) | Not applicable |
| Housing conditions | |
| Housing condition | Housed in a specific pathogen‐free room with a 12 h light/dark cycle and a constant temperature (22°C ± 1°C) and humidity (40% to 60%). |
| Diet name and source (if reported) | Standard normal‐chow diet |
| Test material | Neohesperidine dihydrochalcone |
| Provider | Sigma‐Aldrich Chemical Co. |
| Compound purity | 96% |
| Vehicle used | Sterilised water or fresh sterilised solution |
| Dose regimen (dose level or concentration per group, and frequency) and achieved doses if available |
Solution of 0.1 mg/mL were used to meet the FDA‐approved acceptable daily intake (ADI) in humans (5 mg/kg per day) based on NAS exposure doses previously calculated. Equivalent to 10 mg/kg bw per day. |
| Route of administration (diet, drinking water, gavage) | Drinking water or solution |
| Period of exposure (pre‐mating, mating, gestation, lactation, adult) | Adult |
| Duration of the exposure | 11 weeks |
| Study design | |
| Sex and age at the start of the treatment | Females (6 weeks) |
| Number animals/sex/group | 10 females/group. 4 groups. |
| Measured endpoints | Food intake, water intake, body weight, liver histopathology and clinical biochemistry analyses: alanine aminotransferase (ALT), alkaline phosphatase (ALP), aspartate transaminase (AST), total bile acid (TBA), total bilirubin (TBIL), direct bilirubin (d‐BIL), glucose (GLC), triglycerides (TG), total cholesterol (TC), high‐density lipoprotein (HDL), low‐density lipoprotein (LDL), urea nitrogen (BUN), and creatinine (CREA) in serum. |
| Time of measurement/observation period | Food intake, water intake and the body weights of mice were monitored and recorded every week. Urine and faecal samples were collected every week over the experimental period. At the end of the experiments, the mice were sacrificed under isoflurane anaesthesia after 8 h of fasting. All the samples, including serum, liver, colon, ileum and caecal content samples, were immediately collected and stored at 280°C for the following experiments. |
| Methods to measure the endpoints | In line with standard sub‐chronic toxicity study |
| Statistical analysis | |
| Statistical methods | All experimental values are presented as means 6 standard deviations (SD). OriginLab software (Origin 2017) and GraphPad Prism software (Graph‐Pad 7.0) were used for data analysis and graphical illustrations. All data between different groups were statistically analysed by the Mann–Whitney U test or the Kruskal–Wallis analysis of variance (ANOVA) test. p Values of 0.05 were considered significant. |
| Results | |
| Findings reported by the study author/s |
No significant histopathological changes were observed in the livers of mice after NHDC consumption. Compared with controls, mice with NHDC consumption exhibited no marked changes in their volumes of drinking water, food intake, or body weights. It is noteworthy that NHDC consumption had minimal effects, including no change in LPS level, inflammatory cytokines, and lipid metabolism in the livers or sera of mice. NHDC had no significant impact on the bacterial community. NHDC consumption significantly increased levels of propionic acid and isobutyric acid (SCFAs). |
| No observed adverse effect level, lowest observed adverse effect level, benchmark dose/benchmark dose lower bound | Not reported |
| Further information | |
| Other measured endpoints: urine and faecal samples collection; quantification of tryptophan metabolites in faecal, serum, liver, and colon tissues; quantification of short‐chain fatty acids (SCFAs) in the caecal contents and of long‐chain fatty acids (LCFAs) in the livers and sera; gut microbiota analysis. | |
Prenatal developmental toxicity study
| Study ID | |
| RefID (DistillerSR) | 230 |
| Reference (authors, year, title, other info) | Waalkens‐Berendsen DH, Kuilman‐Wahls MEM & Bär A, 2004. Embryotoxicity and teratogenicity study with neohesperidin dihydrochalcone in rats. Regulatory Toxicology and Pharmacology, 40(1), 74–79. https://doi.org/10.1016/j.yrtph.2004.05.007 |
| Source (published/unpublished) | Published |
| Type of study and guideline | |
| Good laboratory practice (yes/no/no, but before establishment of GLP) | Yes |
| Guideline studies (if yes, specify) | OECD TG 414 (1981) |
| Type of study | Prenatal developmental toxicity |
| Animal model | |
| Species and strain | Rat, Wistar strain |
| Disease models (e.g. diabetes, allergy, obesity) | Not applicable |
| Housing conditions | |
| Housing condition | 21 days, during gestation, the dams were housed individually in suspended stainless steel cages fitted with wire‐mesh fronts and floors. Throughout the study, the temperature of the animal room ranged from 19 to 25°C and the relative humidity from 30% to 70%. A 12‐h light/dark cycle was maintained. |
| Diet name and source (if reported) | RM3 Diet (SDS Special Diets Services, Witham, UK) |
| Treatment | |
| Test material | Neohesperidine dihydrochalcone (Zoster S.A., Zeneta‐Murcia, Spain) |
| Provider | Sponsor |
| Compound purity | 96.9% |
| Vehicle used | None (direct admixture with powder rodent diet) |
| Dose regimen (dose level or concentration per group, and frequency) and achieved doses if available |
0%, 1.25%, 2.5%, and 5% neohesperidine dihydrochalcone in the diet. Equal to: 0, 800–900, 1,600–1,700, 3,100–3,400 mg/kg bw per day |
| Route of administration (diet, drinking water, gavage) | Diet |
| Period of exposure (pre‐mating, mating, gestation, lactation, adult) | Gestation |
| Duration of the exposure | 21 Gestation Days (GDs) |
| Study design | |
| Sex and age at the start of the treatment | Females (12–13 weeks) |
| Number animals/sex/group | 28 positively mated females/group. 4 groups. |
| Measured endpoints | Body weight, weight gain, food consumption, ovaries weight, gravid and empty uterus weight, cecum weight, number of corpora lutea, fetuses weight and sex, placentas weight, number of early and late resorptions, number of dead fetuses and number of implantations. |
| Time of measurement/observation period | The general condition and behaviour of the animals were observed twice daily. Body weight was determined on days 0, 7, 14, and 21 of gestation. Food consumption was determined during three consecutive periods (days 0–7, 7–14, and 14–21 of gestation). On day 21 of gestation the females were decapitated and examined for macroscopic abnormalities. |
| Methods to measure the endpoints | In line with OECD TG 414 |
| Statistical analysis | |
| Statistical methods | ANOVA followed by Dunnett's test, Kruskal–Wallis followed by the Mann–Whitney U test and Fisher's test. |
| Results | |
| Findings reported by the study author/s | The consumption of NHDC was well tolerated. No signs of ill health, abnormal behaviour or intolerance were noted in any treatment group. Mean maternal body weights and body weight changes during gestation were similar in all groups. Maternal food consumption, when expressed in g/kg bw per day, was slightly yet significantly increased in the mid and high‐dose group during the last week of pregnancy. Except for caecal enlargement, there were no changes observed at necropsy which could be related to the NHDC treatment. All dams had viable fetuses. There were no differences for the mean weight of the gravid and empty uterus, ovaries and placenta between the NHDC treatment groups and the controls. The fecundity and gestation index, the number of corpora lutea, implantation sites, live fetuses, early and late resorptions, pre‐ and post‐implantation losses, and sex‐ratio were not affected by the treatment. Mean fetal body weights of the viable fetuses were similar in all groups. Examination of the fetuses for external, visceral and skeletal changes did not reveal any fetotoxic, embryotoxic or teratogenic effects of NHDC. The observed caecal enlargement is a well‐known physiological, adaptive response to the ingestion of high doses of a low‐digestible substance and is generally accepted to lack toxicological relevance. |
| No observed adverse effect level, lowest observed adverse effect level, benchmark dose/benchmark dose lower bound |
NOAEL: 5% neohesperidine dihydrochalcone. This level corresponded to an intake of about 3,300 mg/kg bw per day. |
| Further information | |
| None | |
Appendix D – Data extraction forms for genotoxicity studies
| Study ID 306 | Sequani Limited, Neohesperidine dihydrochalcone: In Vitro mammalian cell micronucleus test, Unpublished report dated 08 February 2018 |
| Funding | Private |
| Good laboratory practice (GLP) compliance and guideline |
Good laboratory practice: yes Guideline studies: OECD TG 487 (2016) |
| Test system | In vitro micronucleus test in human lymphoblast TK6 cells. |
| Test material | Neohesperidine dihydrochalcone, batch number 017J017, purity 97.8% |
| Exposure/treatment conditions |
Short treatment: 3 h in the presence and absence of S9 mix and harvest after a recovery period of 41 h (3 cell cycles); extended treatment: 27 h (1.5–2 cell cycles). Doses: 200, 650 and 2,000 μg/mL (short treatment with and without S9 mix); 65, 400, 650 and 750 μg/mL (extended treatment). For each dose and vehicle (DMSO) and positive controls, approx. 20,000 nucleated events were scored by flow cytometry in duplicate cultures. |
| Results |
Equivocal with S9 mix, negative without S9 mix. Short treatments resulted in moderate cytotoxicity, with relative increases in cell counts (RICC) of 88% and 57% at the highest dose, with and without S9mix respectively. After treatment in the presence of S9mix, a statistically significant increase in micronucleus frequency, above the historical control range, was observed at the low and intermediate doses. No statistically significant increase in micronucleus frequency was observed without S9mix and there was no clear dose response. Extended treatment in the absence of S9 resulted in a reduction of RICC of approx. 55% (maximum recommended), and a slight increase in micronucleus frequency, within the historical control range, at one single dose (400 μg/mL). |
| Reliability/relevance | The study is considered reliable with restriction and its relevance limited. In the short‐term treatment the recovery time was longer (3 cell cycles) than recommended in OECD TG. In case of treatments inducing no cell cycle delay (as shown by RICC for treatment with S9mix) a delayed recovery of treated cells may result in the dilution of damage induced by treatment. |
| Study ID 789 | Bioneeds India Private Limited, In vitro Mammalian Cell Micronucleus Test of Neohesperidine DC in Human Lymphocytes, Unpublished report dated 8 April 2022 |
| Funding | Private |
| Good laboratory practice (GLP) compliance and guideline |
Good laboratory practice: yes Guideline studies: OECD TG 487 (2016) |
| Test system | In vitro micronucleus test in human lymphocytes. |
| Test material | Neohesperidine dihydrochalcone, batch number 021F043, purity 98.2%. Solvent DMSO. |
| Exposure/treatment conditions | Duplicate incubations. Concentrations tested were 0.5, 1.0 and 2.0 mg/mL (mild ppt seen at 2.0 mg/mL) 3–6 h exposures with and without metabolic activation. 20–24 h incubations without metabolic activation. Cytochalasin B used to block cytokinesis. |
| Results | Treatments induced dose‐related cytotoxicity, up to 40%–42% after short exposure (with and without S9, respectively), and 46% after extended treatment without S9. Under all exposure conditions there was no significant increase in micronuclei formation compared to negative controls. Vehicle (DMSO) and positive controls were within historical control ranges. |
| Reliability/relevance | Reliable without restriction and of high relevance. |
Appendix E – QSAR analysis of impurities of neohesperidine dihydrochalcone (E 959)
| Impurity | Chemical name | CAS No. | Structure | QSAR ToolBox |
|---|---|---|---|---|
| – | Neohesperidine dihydrochalcone (E 959) | 20702‐77‐6 |
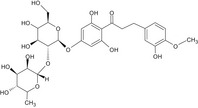
|
DNA binding OASIS: none DNA binding by OECD: Michael addition, P450‐mediated activation to quinone and quinone‐type chemicals (Hydroquinones) Carcinogenicity (gentox and nongentox): none DNA alerts for Ames, CA and MNT by OASIS: none In vitro mutagenicity (Ames) by ISS: none In vivo MN by ISS: Hacceptor‐path3‐Hacceptor (non‐covalent binding with DNA and/or proteins) Protein binding alerts for CA by OASIS: none |
| 1 | Phloroacetophenone neohesperidoside | – |
|
By read across with CAS 480‐66‐0; 1‐(2,4,6‐trihydroxyphenyl)ethenone: DNA binding OASIS: none DNA binding by OECD: none Carcinogenicity (gentox and nongentox): none DNA alerts for Ames, CA and MNT by OASIS: none In vitro mutagenicity (Ames) by ISS: none In vivo MN by ISS: Hacceptor‐path3‐Hacceptor (non‐covalent binding with DNA and/or proteins) Protein binding alerts for CA by OASIS: AN2 Michael addition to the quinoid‐type structures (Hydroxylated phenols) |
| 2 | Neodiosmin | 38665‐01‐9 |
|
DNA binding OASIS: AN2 Michael‐type addition, quinoid structure; radical mechanism via ROS formation (Flavonoids) DNA binding by OECD: Michael addition, P450‐mediated activation to quinone and quinone‐type chemicals (Hydroquinones) Carcinogenicity (gentox and nongentox): none DNA alerts for Ames, CA and MNT by OASIS: none In vitro mutagenicity (Ames) by ISS: none In vivo MN by ISS: Hacceptor‐path3‐Hacceptor (non‐covalent binding with DNA and/or proteins) Protein binding alerts for CA by OASIS: Michael addition to the quinoid type structures, Schiff base formation, ROS generation (Arenecarbonyl compounds) |
| 3 | Neohesperidin | 13241‐33‐3 |
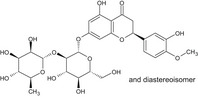
|
DNA binding OASIS: none DNA binding by OECD: Michael addition, P450‐mediated activation to quinone and quinone‐type chemicals (Hydroquinones) Carcinogenicity (gentox and nongentox): none DNA alerts for Ames, CA and MNT by OASIS: none In vitro mutagenicity (Ames) by ISS: none In vivo MN by ISS: Hacceptor‐path3‐Hacceptor (non‐covalent binding with DNA and/or proteins) (1,3‐dialkoxy‐benzene) Protein binding alerts for CA by OASIS: none |
| 4 | Naringin dihydrochalcone | 18916‐17‐1 |
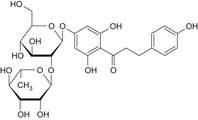
|
DNA binding OASIS: none DNA binding by OECD: Michael addition, P450‐mediated activation to quinone and quinone‐type chemicals (Alkyl phenols) Carcinogenicity (gentox and nongentox): none DNA alerts for Ames, CA and MNT by OASIS: none In vitro mutagenicity (Ames) by ISS: none In vivo MN by ISS: Hacceptor‐path3‐Hacceptor (non‐covalent binding with DNA and/or proteins) Protein binding alerts for CA by OASIS: none |
| 5 | Hesperidin dihydrochalcone | 35573‐79‐6 |
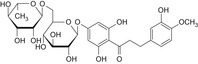
|
By read across from E959: DNA binding OASIS: none DNA binding by OECD: Michael addition, P450‐mediated activation to quinone and quinone‐type chemicals (Hydroquinones) Carcinogenicity (gentox and nongentox): none DNA alerts for Ames, CA and MNT by OASIS: none In vitro mutagenicity (Ames) by ISS: none In vivo MN by ISS: Hacceptor‐path3‐Hacceptor (non‐covalent binding with DNA and/or proteins) Protein binding alerts for CA by OASIS: none |
| 6 | Hesperetin dihydrochalcone 7′glucoside | – |
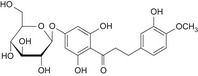
|
By read across from 35400‐60‐3: DNA binding OASIS: none DNA binding by OECD: Michael addition, P450‐mediated activation to quinone and quinone‐type chemicals (Hydroquinones) Carcinogenicity (gentox and nongentox): none DNA alerts for Ames, CA and MNT by OASIS: none In vitro mutagenicity (Ames) by ISS: none In vivo MN: Hacceptor‐path3‐Hacceptor (non‐covalent binding with DNA and/or proteins) Protein binding alerts for CA by OASIS: AN2, Michael addition to the quinoid‐type structure (hydroxylated phenols) |
| 7 | Hesperetin dihydrochalcone | 35400‐60‐3 |
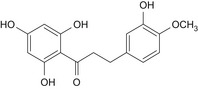
|
DNA binding OASIS: none DNA binding by OECD: Michael addition, P450‐mediated activation to quinone and quinone‐type chemicals (Hydroquinones) Carcinogenicity (gentox and nongentox): none DNA alerts for Ames, CA and MNT by OASIS: none In vitro mutagenicity (Ames) by ISS: none In vivo MN by ISS: Hacceptor‐path3‐Hacceptor (non‐covalent binding with DNA and/or proteins) Protein binding alerts for CA by OASIS: AN2, Michael addition to the quinoid‐type structure (hydroxylated phenols) |
| 8 | Poncirin dihydrochalcone | – |
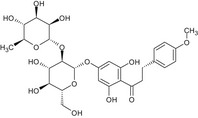
|
By read across from CAS 14941‐08‐3 (poncirin): DNA binding OASIS: none DNA binding by OECD: none Carcinogenicity (gentox and nongentox): none DNA alerts for Ames, CA and MNT by OASIS: none In vitro mutagenicity (Ames) by ISS: none In vivo MN by ISS: Hacceptor‐path3‐Hacceptor (non‐covalent binding with DNA and/or proteins) (1,3‐dialkoxy‐benzene) Protein binding alerts for CA by OASIS: none |
Summary and conclusions
Neohesperidine dihydrochalcone (E 959): the OECD QSAR ToolBox does not highlights E959 alerts for in vitro mutagenicity (Ames, chromosomal aberrations, micronucleus) or carcinogenicity (genotoxic and non‐genotoxic); one of the profilers for DNA binding (DNA binding by OECD) identifies an alert for DNA binding through nucleophilic (Michael) addition to quinone‐like structures formed by the oxidation of the hydroquinone part of E 959. This alert is generically associated to hydroquinone structures by the profiler. Another profiler (in vivo MN by ISS) identified a structural alert (Hacceptor‐path3‐Hacceptor) related to the capacity of forming non‐covalent binding with DNA and/or proteins. This alert was shown to lack the required specificity, as discussed in previous EFSA opinions, and it is not considered further.
Neohesperidine dihydrochalcone impurities: the alert for DNA binding through nucleophilic (Michael) addition was retained in the impurities 2, 3, 4, 5 (by read across), 6 and 7, which also bear hydroxyphenol or alkylphenol structures in their moieties. For the impurity 2 (neodiosmin) an alert for DNA binding was also highlighted by another profiler (DNA binding by OASIS), related to a possible mechanism of nucleophilic addition and ROS generation. For impurities 1, 2, 6, 7, an alert for covalent binding to proteins through Michael addition, and in case of 2 also Schiff base formation and ROS generation, was also identified. However, the relevance of such unspecific protein binding to genotoxicity is not elucidated. Finally, for the impurity 8 no structural alert was identified by read across with the structurally related poncirin.
Overall, the OECD QSAR grouping tool did not highlight relevant differences in the structural alert profile of E 959 and its impurities, which mainly consist in the possible binding to DNA and/or protein through Michael addition, with no alert for electrophilic genotoxicity, parametrised by the activity in genotoxicity tests in vitro (mainly in the Ames test). The analysis supports the applicability of read across with E 959 in the toxicological evaluation of E 959 impurities.
Appendix F – Exposure calculations to toxic elements from the use of neohesperidine dihydrochalcone (E 959) as a food additive
The interested business operators provided analytical data on the levels of lead (Pb), arsenic (As), mercury (Hg) and cadmium (Cd) in commercial batches of neohesperidine dihydrochalcone (E 959).
One interested business operator provided analytical data for five batches of neohesperidine dihydrochalcone (E 959) for As and Pb developed by ICP‐MS adapted from standard EN NF 15763. As and Pb were reported being below their LOQs, this being 0.01 mg/kg for both As and Pb in two batches and 0.03 and 0.04 mg/kg for As and Pb, respectively for the remaining three batches analysed. Upon clarifications request, the interested business operator indicated that the limit of detection was set as three times the standard deviation of the mean of the blank tests (n > 20) and the limit of quantification as the twice of the limit of detection (Documentation provided to EFSA nr: 1 and 3).
The other interested business operator provided analytical data for six batches of E 959 for As, Pb, Cd and Hg developed by ICP‐MS according to analytical method PNTe/LQM/FYQ/140. In all six batches the toxic elements were below the LOQ of 0.01 mg/kg except for one batch where Pb was present at the LOQ of 0.01 mg/kg (Documentation provided to EFSA nr: 5 and 7). Making an allowance to account for representativeness, homogeneity and analytical measurement uncertainty, and disregarding the two batches analysed with inferior LOQ for As and Pb (0.1 mg/kg), limit values for these four elements could be set at three times the highest LOQ values, giving As = 0.09, Pb = 0.12, Hg = 0.03 and Cd = 0.03 mg/kg.
One of the two interested business operators reported residual levels of the catalyst palladium (Pd) for three batches covering 3 production years, at 0.42, 0.26 and 0.83 mg/kg (Documentation provided to EFSA nr: 3). Setting a target value at approximately three times the highest level reported for the batches analysed gives Pd = 2.5 mg/kg.
The potential exposure to impurities from the use of neohesperidine dihydrochalcone (E 959) can be calculated by assuming that the impurity is present in the food additive up to a limit value, and then by calculation pro‐rata to the estimates of exposure to the food additive itself.
With regard to the dietary exposure to the food additive, the Panel considered values (Table 6, Section 3.4.1) of: (i) Refined regulatory maximum level exposure assessment scenario; Mean up to 0.54 and P95 up to 1.43 mg/kg bw per day; (ii) Refined brand‐loyal exposure assessment scenario; Mean up to 0.09 and P95 up to 0.24 mg/kg bw per day.
The levels of the impurities in E 959 combined with the estimated intakes of E959 could result in an exposure which can be compared with the following reference points (RPs) or health‐based guidance values (HBGVs) for the undesirable impurities potentially present in these food additives.
The risk assessment of the undesirable impurities helps inform whether there could be a possible health concern if these impurities would be present at the limit values in the food additive. The assessment is performed by calculating the MOE by dividing the reference point (e.g. BMDL Table F.1) by the exposure estimate, or by estimating the contribution of the use of the food additive to the HBGV (expressed as percentage of the HBGV).
Table F.1.
Reference points/health‐based guidance value for impurities potentially present in E 959
| Impurity/HBGV/RP (μg/kg bw) | Basis/reference |
|---|---|
| Arsenic (As)/0.3–8 (BMDL01) | The reference point is based on a range of benchmark dose lower confidence limit (BMDL01) values between 0.3 and 8 μg/kg bw per day identified for cancers of the lung, skin and bladder, as well as skin lesions. In general, the MOE should be at least 10,000 if the reference point is based on carcinogenicity in animal studies. However, as the BMDL for As is derived from human studies, an interspecies extrapolation factor (i.e. 10) is not needed (EFSA CONTAM Panel, 2009a; EFSA Scientific Committee, 2012) |
| Lead (Pb)/0.5 (BMDL01) | The reference point is based on a study demonstrating perturbation of intellectual development in children with the critical response size of 1 point reduction in IQ. The EFSA CONTAM Panel mentioned that a 1‐point reduction in IQ is related to a 4.5% increase in the risk of failure to graduate from high school and that a 1 point reduction in IQ in children can be associated with a decrease of later productivity of about 2%. A risk cannot be excluded if the exposure exceeds the BMDL01 (MOE lower than 1) (EFSA CONTAM Panel, 2010) |
| Cadmium (Cd)/2.5 (TWI) | The derivation of the reference point is based on a meta‐analysis to evaluate the dose–response relationship between selected urinary cadmium and urinary beta‐2‐microglobulin (B2M) as the biomarker of tubular damage recognised as the most useful biomarker in relation to tubular effects. A group‐based BMDL5 of 4 μg Cd/g creatinine for humans was derived. A chemical specific adjustment factor of 3.9 was applied to account for human variability in urinary cadmium within each dose subgroup in the analysis resulting in a reference point of 1.0 μg Cd per g creatinine. In order to remain below 1 μg Cd/g creatinine in urine in 95% of the population by age 50, the average daily dietary cadmium intake should not exceed 0.36 μg Cd/kg bw, corresponding to a weekly dietary intake of 2.5 μg Cd/kg bw (EFSA CONTAM Panel, 2009b) |
| Mercury (Hg)/4 (TWI) | The HBGV was set using kidney weight changes in male rats as the pivotal effect. Based on the BMDL10 of 0.06 mg/kg bw per day, expressed as mercury, and an uncertainty factor of 100 to account for inter and intra species differences, with conversion to a weekly basis and rounding to one significant figure, a TWI for inorganic mercury of 4 μg/kg bw, expressed as mercury was established (EFSA CONTAM Panel, 2012) |
| Palladium (Pd)/2 (PDE) | The PDE for oral exposure is based on a LOEL of 1.2 mg/kg bw per day in a lifetime study with mice taking into account 5 modifying factors and a human body weight of 50 kg (ICH, 2019) |
bw: body weight; RP: reference point; HBGV: health‐based guidance value; MOE: margin of exposure; TWI: tolerable weekly intake; PDE: permitted daily exposure.
The outcome of such an exercise (Tables F.2 and F.3) illustrates the health impact that could result if the maximum limits for toxic elements calculated by the Panel were to be used.
Since As and Pb already have limit values in the specifications of E 959, Table F.3 illustrates the health impact that could result if the existing EU specification limits for these 2 toxic elements were maintained.
For all five elements, the assessment of the uncertainty in the exposure showed a potential for overestimation of exposure for both scenarios used (see Section 3.4.2, Table 6). Also, the actual content of contaminants in E 959 would be lower on average than the limit values used for the calculations.
The Panel noted that when considering the current EU specifications limit value, the lower end of the calculated MOE values for As fall below 1,000 (Table F.3) and indicates that a lowering of the existing limit value for As from 3 mg/kg is recommended.
For Pb, the MOE values are well above 1 and for Cd, Hg and Pd the estimates of exposure are only a very small fraction of their respective TWI or PDE values, indication of no health concern using either of the two sets of concentration data (Tables F.2 and F.3).
Annex A – Exposure data and estimates
1.
Summary of reported use levels (mg/kg or mg/L as appropriate) of neohesperidine dihydrochalcone (E 959).
Number and percentage of food products labelled to contain neohesperidine dihydrochalcone (E 959)
Concentration data used in the exposure assessment scenarios of neohesperidine dihydrochalcone (E 959) (mg/kg or mL/kg as appropriate).
Summary of estimated exposure to neohesperidine dihydrochalcone (E 959) for the refined maximum level exposure assessment scenario and the refined brand‐loyal scenarios per population group and survey: mean and 95th percentile (mg/kg bw per day) among consumers of at least one food category that could contain the additive.
Main food categories contributing to the exposure of neohesperidine dihydrochalcone (E 959) (number of surveys by contribution class) in the MPL scenario.
Main food categories contributing to the exposure of neohesperidine dihydrochalcone (E 959) (number of surveys by contribution class) in the refined MPL scenario.
Main food categories contributing to the exposure of neohesperidine dihydrochalcone (E 959) (number of surveys by contribution class) in the brand‐loyal scenario
Annex A can be found in the online version of this output (‘Supporting information’ section): https://doi.org/10.2903/j.efsa.2022.7595.
Annex B – List of excluded studies
1.
Annex B can be found in the online version of this output (‘Supporting information’ section): https://doi.org/10.2903/j.efsa.2022.7595
Annex C – Weight of evidence (WoE) tables for animal studies
1.
Annex C can be found in the online version of this output (‘Supporting information’ section): https://doi.org/10.2903/j.efsa.2022.7595
Supporting information
Exposure data and estimates
List of excluded studies
Weight of evidence (WoE) tables for animal studies
Suggested citation: EFSA FAF Panel (EFSA Panel on Food Additives and Flavourings) , Younes M, Aquilina G, Castle L, Degen G, Engel K‐H, Fowler PJ, Frutos Fernandez MJ, Fürst P, Gundert‐Remy U, Gürtler R, Husøy T, Manco M, Mennes W, Moldeus P, Passamonti S, Shah R, Waalkens‐Berendsen I, Wright M, Batke M, Boon P, Bruzell E, Chipman J, Crebelli R, FitzGerald R, Fortes C, Halldorsson T, LeBlanc J‐C, Lindtner O, Mortensen A, Ntzani E, Wallace H, Cascio C, Civitella C, Horvath Z, Lodi F, Mech A, Tard A and Vianello G, 2022. Scientific Opinion on the re‐evaluation of neohesperidine dihydrochalcone (E 959) as a food additive. EFSA Journal 2022;20(11):7595, 81 pp. 10.2903/j.efsa.2022.7595
Requestor European Commission
Question number EFSA‐Q‐2011‐00726
Panel members Maged Younes, Gabriele Aquilina, Laurence Castle, Gisela Degen, Karl‐Heinz Engel, Paul J Fowler, Maria José Frutos Fernandez, Peter Fürst, Ursula Gundert‐Remy, Rainer Gürtler, Trine Husøy, Melania Manco, Wim Mennes, Peter Moldeus, Sabina Passamonti, Romina Shah, Ine Waalkens‐Berendsen and Matthew Wright.
Declarations of interest If you wish to access the declaration of interests of any expert contributing to an EFSA scientific assessment, please contact interestmanagement@efsa.europa.eu.
Acknowledgements The Panel wishes to thank the following for the support provided to this scientific output: Antonio Rivas Cornejo and the members of the FAF WG Specifications of Food Additives.
Annexes A, B and C are available under the Supporting Information section .
Adopted: 29 September 2022
Notes
Regulation (EC) No 1333/2008 of the European Parliament and of the Council of 16 December 2008 on food additives. OJ L 354, 31.12.2008, pp. 16–33.
Commission Regulation (EU) No 257/2010 of 25 March 2010 setting up a programme for the re‐evaluation of approved food additives in accordance with Regulation (EC) No 1333/2008 of the European Parliament and of the Council on food additives. OJ L 80, 26.3.2010, pp. 19–27.
Commission Regulation (EU) No 231/2012 of 9 March 2012 laying down specifications for food additives listed in Annexes II and III to Regulation (EC) No 1333/2008 of the European Parliament and of the Council. OJ L 83, 22.3.2012, pp. 1–295.
Regulation (EC) No 1334/2008 of the European Parliament and of the Council of 16 December 2008 on flavourings and certain food ingredients with flavouring properties for use in and on foods and amending Council Regulation (EEC) No 1601/91, Regulations (EC) No 2232/96 and (EC) No 110/2008 and Directive 2000/13/EC. OJ L 354, 31.12.2008, pp. 34–50.
Regulation (EC) No 1907/2006 of the European Parliament and of the Council of 18 December 2006 concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH), establishing a European Chemicals Agency, amending Directive 1999/45/EC and repealing Council Regulation (EEC) No 793/93 and Commission Regulation (EC) No 1488/94 as well as Council Directive 76/769/EEC and Commission Directives 91/155/EEC, 93/67/EEC, 93/105/EC and 2000/21/EC.
Call for technical and toxicological data on sweeteners authorised as food additives in the EU – Extended deadline for submitting data: 30/6/2018.
Call for technical data on sweeteners authorised as food additives in the EU – Updated deadline for submitting data:28/2/2020.
Call for food additives usage level and/or concentration data in food and beverages intended for human consumption (Batch 7).
Call for data on genotoxicity data on sweeteners – Updated deadline for submitting data: 31/3/2022.
Available online: http://www.efsa.europa.eu/en/food-consumption/comprehensive-database.
Risk of bias (or internal validity) is defined as ‘the extent to which the design and conduct of a study are likely to have prevented bias’. Risk of bias relates to the propensity of a study to be affected by systematic error. Biases can operate in either direction and can lead to underestimation or overestimation of the true intervention effect.
Commission Regulation (EC) No 1333/2008 of the European Parliament and of the Council of 16 December 2008 on food additives. OJ L 354, 31.12.2008, p. 16.
Missing Cyprus, Luxembourg and Malta.
Extraction date 30 November 2021 from https://www.gnpd.com/sinatra/gnpd/search/#
Available online: http://www.efsa.europa.eu/en/datexfoodcdb/datexfooddb.htm.
Part of the survey population may have no exposure to the additive because they did not report eating a food from one of the food categories that could contain the additive (these are ‘non‐consumers’). The sub‐group that reports eating these foods are called consumers.
Commission Regulation No 1565/2000 of 18 July 2000 laying down the measures necessary for the adoption of an evaluation programme in application of Regulation (EC) No 2232/96. OJ L 180, 19.7.2000, pp. 8–16.
This unpublished study is referred as Booth AN, Robbins DJ and Gagne WE, 1965 in EFSA CEF Panel, 2010 and in JECFA, 2012.
This unpublished study report was subsequently published in the literature and referred in EFSA CEF Panel, 2010 and in JECFA, 2012 as Lina BAR, Dreef‐van der Meulen HC, Leegwater DC, 1990. Subchronic (13‐week) oral toxicity of neohesperidin dihydrochalcone in rats. Food Chemistry and Toxicology, 26(7), 507–513. https://doi.org/10.1016/0278-6915(90)90121-3.
Until July 2022.
References
- Agriculture and Environment Research Unit, University of Hertfordshire , Lewis KA and Tzilivakis J, 2021. Review and synthesis of data on the potential environmental impact of artificial sweeteners. EFSA supporting publication 2021;EN‐6918, 127 pp. 10.2903/sp.efsa.2021.EN-6918 [DOI]
- Arbelaez P, Borrull F, Pocurull E and Marce RM, 2015. Determination of high‐intensity sweeteners in river water and wastewater by solid‐phase extraction and liquid chromatography‐tandem mass spectrometry. Journal of Chromatography A, 1393, 106–114. 10.1016/j.chroma.2015.03.035 [DOI] [PubMed] [Google Scholar]
- Batzinger RP, Ou S‐YL and Bueding E, 1977. Saccharin and other sweeteners: mutagenic properties. Science, 198, 944–946. 10.1126/science.337489 [DOI] [PubMed] [Google Scholar]
- Benavente‐Garcia O, Castillo J, Del Baño MJ and Lorente J, 2001. Improved water solubility of neohesperidin dihydrochalcone in sweetener blends. Journal of Agricultural and Food Chemistry, 49(1), 189–191. 10.1021/jf000186l [DOI] [PubMed] [Google Scholar]
- Bjeldanes LF and Chang GW, 1977. Mutagenic activity of quercetin and related compounds. Science, 197, 577–578. 10.1126/science.327550 [DOI] [PubMed] [Google Scholar]
- Borrego F and Montijano H, 2001. Neohesperidin Dihydrochalcone. In: Nabors LO (ed). Alternative Sweeteners. Marcel Dekker, Inc, New York, USA. pp. 85–99. [Google Scholar]
- Bozoğlan BK, Tunç S and Duman O, 2014. Investigation of neohesperidin dihydrochalcone binding to human serum albumin by spectroscopic methods. Journal of Luminescence, 155, 198–204. 10.1016/j.jlumin.2014.06.032 [DOI] [Google Scholar]
- Braune A, Engst W and Blaut M, 2005. Degradation of neohesperidin dihydrochalcone by human intestinal bacteria. Journal of Agriculture and Food Chemistry, 53, 1782–1790. 10.1021/jf0484982 [DOI] [PubMed] [Google Scholar]
- Brown JP and Dietrich PS, 1979. Mutagenicity of plant flavonols in the Salmonella/mammalian microsome test. Activation of flavonol glycosides by mixed glycosidases from rat cecal bacteria and other sources. Mutation Research, 66, 223–240. 10.1016/0165-1218(79)90083-1 [DOI] [PubMed] [Google Scholar]
- Brown JP, Dietrich PS and Brown RJ, 1977. Frameshift mutagenicity of certain naturally occuring phenolic compounds in the ‘salmonella/microsome’ test: activation of anthraquinone and flavonol glycosides by gut bacterial enzymes. Biochemical Society Transactions, 5, 1489–1452. 10.1042/bst0051489 [DOI] [PubMed] [Google Scholar]
- BSI (British Standards Institution) , 2003. Draft for development: foodstuffs – determination of neohesperidin‐dihydrochalcon. DD CEN/TS 14537:2003. Available online: https://shop.bsigroup.com/ProductDetail/?pid=000000000030084738
- Buchgraber M and Wasik A, 2009. Determination of 9 intense sweeteners in foodstuffs by high‐performance liquid chromatography and evaporative light‐scattering detection: interlaboratory study. Journal of AOAC International, 92, 208–222. 10.1093/jaoac/92.1.208 [DOI] [PubMed] [Google Scholar]
- Canales I, Borrego F and Lindley MG, 1993. Neohesperidin dihydrochalcone stability in aqueous buffer solutions. Journal of Food Science, 57, 589–591. 10.1111/j.1365-2621.1993.tb04330.x [DOI] [Google Scholar]
- Cardenas‐Soraca DM, Singh V, Nazdrajic E, Vasiljevic T, Grandy JJ and Pawliszyn J, 2020. Development of thin‐film solid‐phase microextraction coating and method for determination of artificial sweeteners in surface water. Talanta, 211, 120714. 10.1016/j.talanta.2020.120714 [DOI] [PubMed] [Google Scholar]
- Castellar MR, Iborra JL and Canales I, 1997. Analysis of commercial neohesperidin dihydrochalcone by high performance liquid chromatography. Journal of Liquid Chromatography and Related Technologies, 20, 2063–2073. 10.1080/10826079708005565 [DOI] [Google Scholar]
- Choi S, Yu S, Lee J and Kim W, 2021. Effects of neohesperidin dihydrochalcone (NHDC) on oxidative phosphorylation, cytokine production, and lipid deposition. Foods, 10, 1408–1418. 10.3390/foods10061408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coiffard C, Coiffard L, Peigne F and De Roeck‐Holtzhauer Y, 1998. Effect of pH on neohesperidin dihydrochalcone thermostability in aqueous solutions. Analusis, 26, 150–153. [Google Scholar]
- Dewinter L, Casteels K, Corthouts K, Van de Kerckhove K, Van der Vaerent K, Vanmeerbeeck K and Matthys C, 2016. Dietary intake of non‐nutritive sweeteners in type 1 diabetes mellitus children. Food Additives and Contaminants Part A – Chemistry Analysis Control Exposure & Risk Assessment, 33, 19–26. 10.1080/19440049.2015.1112039 [DOI] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) , 2007. Scientific opinion of the Scientific Committee related touncertainties in dietary exposure assessment. EFSA Journal 2007;5(1):438, 54 pp. 10.2903/j.efsa.2007.438 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) , 2011. Use of the EFSA Comprehensive European Food ConsumptionDatabase in Exposure Assessment. EFSA Journal 2011;9(3):2097, 34 pp. 10.2903/j.efsa.2011.2097 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) , 2015a. Scientific report on principles and process for dealing with data and evidence in scientific assessments. EFSA Journal 2015;13(5):4121, 35 pp. 10.2903/j.efsa.2015.4121 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) , 2015b. The food classification and description system FoodEx2 (revision 2). EFSA supporting publication 2015;EN‐804, 90 pp. 10.2903/sp.efsa.2015.EN-804 [DOI]
- EFSA (European Food Safety Authority) , 2020a. Outcome of the public consultation on a draft protocol for the assessment of hazard identification and characterisation of sweeteners. EFSA supporting publication 2020;17(2):EN‐1803, 25 pp. 10.2903/sp.efsa.2020.EN-1803 [DOI]
- EFSA (European Food Safety Authority) , 2020b. Outcome of the public consultation on a draft protocol for assessing exposure to sweeteners as part of their safety assessment under the food additives re‐evaluation programme. EFSA supporting publication 2020;EN‐1913, 52 pp. 10.2903/sp.efsa.2020.EN-1913 [DOI]
- EFSA ANS Panel (EFSA Panel on Food Additives and Nutrient Sources) , 2012. Guidance for submission for foodadditive evaluations. EFSA Journal 2012;10(7):2760, 60 pp. 10.2903/j.efsa.2012.2760 [DOI] [Google Scholar]
- EFSA CEF Panel (EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids) , 2010. Scientific opinion Flavouring Group Evaluation 32 (FGE.32): flavonoids (Flavanones and dihydrochalcones) from chemical groups 25 and 30. EFSA Journal 2010;8(9):1065, 61 pp. 10.2903/j.efsa.2010.1065 [DOI] [Google Scholar]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain) , 2009a. Scientific opinion on Arsenic in food. EFSA Journal 2009;7(3):980, 139 pp. 10.2903/j.efsa.2009.980 [DOI] [Google Scholar]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain) , 2009b. Scientific opinion on Cadmium in food. EFSA Journal 2009;7(10):1351, 199 pp. 10.2903/j.efsa.2009.1351 [DOI] [Google Scholar]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain) , 2010. Scientific opinion on Lead in food. EFSA Journal 2010;8(4):1570, 151 pp. 10.2903/j.efsa.2010.1570 [DOI] [Google Scholar]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain) , 2012. Scientific Opinion on the risk for public health related to the presence of mercury and methylmercury in food. EFSA Journal 2012;10(12):2985, 15 pp. 10.2903/j.efsa.2012.2985 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed) , 2011. Scientific Opinion on the on the safety and efficacy of neohesperidine dihydrochalcone when used as a sensory additive for piglets, pigs for fattening, calves for rearing and fattening, lambs for rearing and fattening, dairy sheep, ewes for reproduction, salmonids and dogs. EFSA Journal 2011;9(12):2444, 13 pp. 10.2903/j.efsa.2011.2444 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed) , 2014. Scientific Opinion on the on the safety of neohesperidine dihydrochalcone as a sensory additive for fish. EFSA Journal 2014;12(5):3669, 9 pp. 10.2903/j.efsa.2014.3669 [DOI] [Google Scholar]
- EFSA Scientific Committee , 2009. Guidance of the Scientific Committee on Transparency in the Scientific Aspects of Risk Assessments carried out by EFSA. Part 2: general principles. EFSA Journal 2009;7(7):1051, 22 pp. 10.2903/j.efsa.2009.1051 [DOI] [Google Scholar]
- EFSA Scientific Committee , 2011. Scientific Opinion on genotoxicity testing strategies applicable to food and feed safety assessment. EFSA Journal 2011;9(9):2379, 69 pp. 10.2903/j.efsa.2011.2379 [DOI] [Google Scholar]
- EFSA Scientific Committee , 2012. Guidance on selected default values to be used by the EFSA Scientific Committee, Scientific Panels and Units in the absence of actual measured data. EFSA Journal 2012;10(3):2579, 32 pp. 10.2903/j.efsa.2012.2579 [DOI] [Google Scholar]
- EFSA Scientific Committee , Hardy A, Benford D, Halldorsson T, Jeger M, Knutsen HK, More S, Naegeli H, Noteborn H, Ockleford C, Ricci A, Rychen G, Silano V, Solecki R, Turck D, Younes M, Aquilina G, Crebelli R, Gürtler R, Hirsch‐Ernst KI, Mosesso P, Nielsen E, van Benthem J, Carfì M, Georgiadis N, Maurici D, Parra Morte J and Schlatter J, 2017. Scientific Opinion on the clarification ofsome aspects related to genotoxicity assessment. EFSA Journal 2017;15(12):5113, 25 pp. 10.2903/j.efsa.2017.5113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA Scientific Committee , More S, Bampidis V, Benford D, Bragard C, Halldorsson T, Hernàndez‐Jerez A, Bennekou SH, Koutsoumanis K, Lambré C, Machera K, Naegeli H, Nielsen S, Schlatter J, Schrenk D, Silano V, Turck D, Younes M, Castenmiller J, Chaudhry Q, Cubadda F, Franz R, Gott D, Mast J, Mortensen A, Oomen AG, Weigel S, Barthelemy E, Rincon A, Tarazona J and Schoonjans R, 2021. Guidance on technical requirements for regulated food and feed product applications to establish the presence of small particles including nanoparticles. EFSA Journal 2021;19(8):6769, 48 pp. 10.2903/j.efsa.2021.6769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Pharmacopoeia , 2017. E. European Directorate for the Quality of Medicines and Health. 9th Edition, Volume 2.
- Fang Y, Cao W, Xia M, Pan S and Xu X, 2017. Study of structure and permeability relationship of flavonoids in Caco‐2 cells. Nutrients, 9, 1301–1315. 10.3390/nu9121301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang Y, Liang F, Liu K, Qaiser S, Pan S and Xu X, 2018. Structure characteristics for intestinal uptake of flavonoids in Caco‐2 cells. Food Research International, 105, 353–360. 10.1016/j.foodres.2017.11.045 [DOI] [PubMed] [Google Scholar]
- Fisher JF, 1977. High‐pressure liquid chromatographic method for the quantitation of neohesperidin dihydrochalcone. Journal of Agricultural and Food Chemistry, 25, 682–683. 10.1021/jf60211a029 [DOI] [Google Scholar]
- Frydman A, Weisshaus O, Huhman DV, Sumner Lloyd W, Bar‐Peled M, Lewinsohn E, Fluhr R, Gressel J and Eyal Y, 2005. Metabolic engineering of plant cells for biotransformation of hesperedin into neohesperidin, a substrate for production of the low‐calorie sweetener and flavor enhancer NHDC. Journal of Agricultural and Food Chemistry, 25, 9708–9712. 10.1021/jf051509m [DOI] [PubMed] [Google Scholar]
- FSA (Food Standards Agency) , 2014. Development of a robust and fully validated method for the simultaneous determination of sweeteners (including neotame and steviol glycosides) in food. Project Number FS241001; CPFC/2012/134/W202‐001. Available online: https://www.food.gov.uk/sites/default/files/WDixon%20Final%20report_sweeteners.pdf
- Gumbmann MR, Gould DH, Robbins DJ and Booth AN, 1978. Toxicity studies of neohesperidin dihydrochalcone. In: Shaw JH and Roussos GG (eds). Sweeteners and Dental Caries. Information Retrieval Inc., Washington D.C. pp. 301–310. [Google Scholar]
- Guth S, Roth A, Engeli B, Lachenmeier DW, Cartus AT, Hüser S, Baum M, Diel P, Eisenbrand G, Hengstler JG, Humpf H‐U, Joost H‐G, Lampen A, Leist M, Marko D, Steinberg P, Mally A and Zarn JA, 2020. Comparison of points of departure between subchronic and chronic toxicity studies on food additives, food contaminants and natural food constituents. Food and Chemical Toxicology, 146, 111784. 10.1016/j.fct.2020.111784 [DOI] [PubMed] [Google Scholar]
- Gvozdic E, Matic Bujagic I, Djurkic T and Grujic S, 2020. Trace analysis of artificial sweeteners in environmental waters, wastewater and river sediments by liquid chromatography‐tandem mass spectrometry. Microchemical Journal, 157, 105071. 10.1016/j.microc.2020.105071 [DOI] [Google Scholar]
- Han GE, Kang H‐T, Chung S, Lim C, Linton JA, Lee J‐H, Kim W, Kim S‐H and Lee JH, 2018. Novel neohesperidin dihydrochalcone analogue inhibits adipogenic differentiation of human adipose‐derived stem cells through the Nrf2 pathway. International Journal of Molecular Sciences, 19, 2215–2230. 10.3390/ijms19082215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez F, Ibanez M, Botero‐Coy A‐M, Bade R, Bustos‐Lopez MC, Rincon J, Moncayo A and Bijlsma L, 2015. LC‐QTOF MS screening of more than 1000 licit and illicit drugs and their metabolites in wastewater and surface waters from the area of Bogota, Colombia. Analytical and Bioanalytical Chemistry, 407, 6405–6416. 10.1007/s00216-015-8796-x [DOI] [PubMed] [Google Scholar]
- Horowitz RM and Gentili B, 1969. Taste and structure in phenolic glycosides. Journal of Agricultural and Food Chemistry, 17, 696–700. 10.1021/jf60164a049 [DOI] [Google Scholar]
- ICH (International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use) , 2019. Guideline for elemental impurities Q3D (R1). Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/international-conference-harmonisation-technical-requirements-registration-pharmaceuticals-human-use_en-32.pdf
- ICRP (International Commission on Radiological Protection) , 2002. Basic Anatomical and Physiological Data for Use in Radiological Protection Reference Values. ICRP Publication 89. Ann. ICRP, 32. Available online: https://journals.sagepub.com/doi/pdf/10.1177/ANIB_32_3-4 [PubMed]
- Inglett GE, Krbechek L, Dowling B and Wagner R, 1969. Dihydrochalcone sweeteners – sensory and stability evaluation. Journal of Food Science, 34, 101–103. 10.1111/j.1365-2621.1969.tb14371.x [DOI] [Google Scholar]
- Janvier S, Goscinny S, Le Donne C and Van Loco J, 2015. Low‐calorie sweeteners in food and food supplements on the Italian market. Food Additives & Contaminants: Part B, 8, 298–308. 10.1080/19393210.2015.1094829 [DOI] [PubMed] [Google Scholar]
- JECFA (Joint FAO/WHO Expert Committee on Food Additives) , 2000. Guidelines for the preparation of toxicologicalworking papers for the Joint FAO/WHO Expert Committee on Food Additives, Geneva, Switzerland.
- JECFA (Joint FAO/WHO Expert Committee on Food Additives) , 2006. Combined compendium of food additive specifications. Volume 4: analytical methods, test procedures and laboratory solutions used by and referenced in food additive specifications. Rome, Food and Agriculture Organization of the United Nations. ISBN 92‐5‐105569‐6.
- JECFA (Joint FAO/WHO Expert Committee on Food Additives) , 2012. Safety evaluation of certain food additives prepared by the seventy‐sixth meeting of the Joint FAO/WHO Expert Committee on Food Additives, WHO Food additives Series 67, Geneva. Available online: https://www.who.int/publications/i/item/9789241660679
- Johnston K, Sharp P, Clifford M and Morgan L, 2005. Dietary polyphenols decrease glucose uptake by human intestinal Caco‐2 cells. FEBS Letters, 579, 1653–1657. 10.1016/j.febslet.2004.12.099 [DOI] [PubMed] [Google Scholar]
- Kubica P, Namiesnik J and Wasik A, 2016. Comparison of hydrophilic interaction and reversed phase liquid chromatography coupled with tandem mass spectrometry for the determination of eight artificial sweeteners and common steviol glycosides in popular beverages. Journal of Pharmaceutical and Biomedical Analysis, 127, 184–192. 10.1016/j.jpba.2016.01.006 [DOI] [PubMed] [Google Scholar]
- Lakade SS, Zhou Q, Li A, Borrull F, Fontanals N and Marce RM, 2018. Hypercrosslinked particles for the extraction of sweeteners using dispersive solid‐phase extraction from environmental samples. Journal of Separation Science, 41, 1618–1624. 10.1002/jssc.201701113 [DOI] [PubMed] [Google Scholar]
- Le Donne C, Mistura L, Goscinny S, Janvier S, Cuypers K, D'Addezio L, Sette S, Catasta G, Ferrari M, Piccinelli R, Van Loco J and Turrini A, 2017. Assessment of dietary intake of 10 intense sweeteners by the Italian population. Food and Chemical Toxicology, 102, 186–197. 10.1016/j.fct.2017.02.014 [DOI] [PubMed] [Google Scholar]
- Lefler JL and Chen R, 2008. A feasibility study of using supercritical fluid chromatography (SFC)‐UV‐ELSD for food and beverage analyses. LCGC North America, 6, 42–47. [Google Scholar]
- Lim H‐S, Park S‐K, Kwak I‐S, Kim H‐I, Sung J‐H, Jang S‐J, Byun M‐Y and Kim S‐H, 2013. HPLC‐MS/MS Analysis of 9 Artificial Sweeteners in Imported Foods. Food Science and Biotechnology, 22, 233–240. 10.1007/s10068-013-0072-2 [DOI] [Google Scholar]
- Lina BAR, Dreef‐van der Meulen HC and Leegwater DC, 1990. Subchronic (13‐week) oral toxicity of neohesperidin dihydrochalcone in rats. Food Chemistry and Toxicology, 26, 507–513. 10.1016/0278-6915(90)90121-3 [DOI] [PubMed] [Google Scholar]
- Lorenzo RA, Pena MT and Fernández P, 2015. Artificial sweeteners in beverages by ultra‐performance liquid chromatography with photodiode array and liquid chromatography tandem mass spectrometry. Food Control, 47, 43–52. 10.1016/j.foodcont.2014.06.035 [DOI] [Google Scholar]
- Ma K, Li X, Zhang Y and Liu F, 2020. Determining high‐intensity sweeteners in white spirits using an ultrahigh performance liquid chromatograph with a photo‐diode array detector and charged aerosol detector. Molecules, 25, 40. 10.3390/molecules25010040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGregor JT and Jurd L, 1978. Mutagenicity of plant flavonoids: structural requirements for mutagenic activity in Salmonella typhimurium. Mutation Research, 54, 297–309. 10.1016/0165-1161(78)90020-1 [DOI] [PubMed] [Google Scholar]
- MacGregor JT, Wehr CM, Manners GD, Jurd L, Minkler JL and Carrano AV, 1983. In vivo exposure to plant flavonols. Influence on frequencies of micronuclei in mouse erythrocytes and sister‐chromatid exchange in rabbit lymphocytes. Mutation Research, 124, 255–270. 10.1016/0165-1218(83)90197-0 [DOI] [PubMed] [Google Scholar]
- Mech A, Rauscher H, Babick F, Hodoroaba VD, Ghanem A, Wohlleben W, Marvin H, Weigel S, Brüngel R and Friedrich CM, 2020a. The NanoDefine Methods Manual. Part 1: The NanoDefiner Framework and Tools, EUR29876 EN, Publications Office of the European Union, Luxembourg, 2020, ISBN 978‐92‐76‐11950‐0. 10.2760/55181 [DOI]
- Mech A, Rauscher H, Rasmussen K, Babick F, Hodoroaba VD, Ghanem A, Wohlleben W, Marvin H, Brüngel R and Friedrich CM, 2020b. The NanoDefine Methods Manual. Part 2: Evaluation of methods, EUR 29876 EN, Publications Office of the European Union, Luxembourg, 2020, ISBN 978‐92‐76‐11953‐1. 10.2760/071877 [DOI]
- Moher D, Liberati A, Tetzlaff J, Altman DG and the PRISMA Group , 2009. Preferred reporting items forsystematic reviews and meta‐analyses: the PRISMA statement. PLoS Medicine, 6, 6. 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montijano H and Borrego F, 1999. Hydrolysis of the intense sweetener neohesperidin dihydrochalcone in water‐organic solvent mixtures. International Journal of Food Science & Technology, 34, 291–294. 10.1046/j.1365-2621.1999.00259.x [DOI] [Google Scholar]
- Montijano H, Tomás‐Barberán FA and Borrego F, 1995. Stability of the intense sweetener neohesperidine DC during yogurt manufacture and storage. Zeitschrift für Lebensmittel‐Untersuchung und ‐Forschung, 201, 541–543. 10.1007/BF01201580 [DOI] [Google Scholar]
- Montijano H, Coll MD and Borrego F, 1996. Assessment of neohesperidine DC stability during pasteurization of juice‐based drinks. International Journal of Food Science & Technology, 31, 397–401. 10.1046/j.1365-2621.1996.00354.x [DOI] [Google Scholar]
- Montijano H, Tomás‐Barberán FA and Borrego F, 1997a. Accelerated kinetic study of neohesperidine DC hydrolysis under conditions relevant for high temperature processed dairy products. Zeitschrift für Lebensmittel‐Untersuchung und‐Forschung, 204, 180–182. 10.1007/s002170050058 [DOI] [Google Scholar]
- Montijano H, Borrego F, Tomás‐Barberán FA and Lindley MG, 1997b. Stability of neohesperidine dihydrochalcone in a lemonade system. Food Chemistry, 58, 13–15. [Google Scholar]
- Montijano H, Cano J, López‐Cremades FJ, Bañón J, Canales I and Borrego F, 1999. An estimation of the detection and quantitation limits of neohesperidin DC by high performance liquid chromatography. In: Low‐calories sweeteners: present and future. IUFoST world conference on low‐calorie sweeteners, April 1999, Barcelona, Spain. Ed Corti A. Karger AG Basel, Switz. World Review of Nutrition and Dietetics, 85, 125–128. 10.1159/000059690 [DOI] [PubMed] [Google Scholar]
- Mudie DM, Murray K, Hoad CL, Pritchard SE, Garnett MC, Gordon L, Amidon GL, Gowland PA, Spiller RC, Amidon GE and Marciani L, 2014. Quantification of Gastrointestinal Liquid Volumes and Distribution Following a 240 mL Dose of Water in the Fasted State. Molecular Pharmaceutics, 11, 3039–3047. 10.1021/mp500210c [DOI] [PubMed] [Google Scholar]
- Nambiar AP, Sanyal M and Shrivastav PS, 2018. Simultaneous densitometric determination of eight food colors and four sweeteners in candies, jellies, beverages and pharmaceuticals by normal‐phase high performance thin‐layer chromatography using a single elution protocol. Journal of Chromatography A, 1572, 152–161. 10.1016/j.chroma.2018.08.059 [DOI] [PubMed] [Google Scholar]
- Neale PA, Munz NA, Ait‐Aissa S, Altenburger R, Brion F, Busch W, Escher BI, Hilscherova K, Kienle C, Novak J, Seiler T‐B, Shao Y, Stamm C and Hollender J, 2017. Integrating chemical analysis and bioanalysis to evaluate the contribution of wastewater effluent on the micropollutant burden in small streams. Science of the Total Environment, 576, 785–795. 10.1016/j.scitotenv.2016.10.141 [DOI] [PubMed] [Google Scholar]
- NTP‐OHAT (Division of the National Toxicology Program, Office of Health Assessment and Translation) , 2019. Handbook for conducting a literature‐based health assessment using OHAT approach for systematic review and evidence integration. US Department of Health and Human Services, 101 pp. Available online: https://ntp.niehs.nih.gov/ntp/ohat/pubs/handbookmarch2019_508.pdf
- Ordonez EY, Quintana JB, Rodil R and Cela R, 2012. Determination of artificial sweeteners in water samples by solid‐phase extraction and liquid chromatography‐tandem mass spectrometry. Journal of Chromatography A, 1256, 197–205. 10.1016/j.chroma.2012.07.073 [DOI] [PubMed] [Google Scholar]
- Ordonez EY, Rodil R, Quintana JB and Cela R, 2015. Determination of artificial sweeteners in beverages with green mobile phases and high temperature liquid chromatography‐tandem mass spectrometry. Food Chemistry, 169, 162–168. 10.1016/j.foodchem.2014.07.132 [DOI] [PubMed] [Google Scholar]
- Pérez‐Ruiz T, Martínez‐Lozano C, Tomás V, Sanz A and Bravo E, 2000. Quantitative assay for neohesperidin dihydrochalcone in foodstuffs by capillary electrophoresis. Chromatographia, 51, 385–389. 10.1007/BF02490473 [DOI] [Google Scholar]
- Rauscher H, Mech A, Gibson N, Gilliland D, Held A, Kestens V, Koeber R, Linsinger T and Stefaniak E, 2019. Identification of nanomaterials through measurements, EUR 29942 EN, Publications Office of the European Union, Luxembourg, 2019, ISBN 978‐92‐76‐10371‐4, JRC118158. 10.2760/053982 [DOI]
- Sahu RK, Basu R and Sharma A, 1981. Genetic toxicological testing of some plant flavonoids by the micronucleus test. Mutation Research, 89, 69–74. 10.1016/0165-1218(81)90132-4 [DOI] [PubMed] [Google Scholar]
- Salas D, Borrull F, Fontanals N and Marce RM, 2015. Hydrophilic interaction liquid chromatography coupled to high‐resolution mass spectrometry to determine artificial sweeteners in environmental waters. Analytical and Bioanalytical Chemistry, 407, 4277–4285. 10.1007/s00216-014-8330-6 [DOI] [PubMed] [Google Scholar]
- SCF (Scientific Committee for Food) , 1985. Reports of the Scientific Committee for foods 16th series. EU Commission. Available online: https://ec.europa.eu/food/system/files/2020-12/sci-com_scf_reports_16.pdf
- SCF (Scientific Committee for Food) , 1989. Reports of the Scientific Committee for foods 21st series. EU Commission. Available online: https://ec.europa.eu/food/system/files/2020-12/sci-com_scf_reports_21.pdf
- SCF (Scientific Committee for Food) , 2001. Guidance on submissions for food additive evaluations by the Scientific Committee on Food. Opinion expressed on 11 July 2001. Available online: https://ec.europa.eu/food/fs/sc/scf/out98_en.pdf
- Sezgin B, Arli G and Can NO, 2021. Simultaneous HPLC‐DAD determination of seven intense sweeteners in foodstuffs and pharmaceuticals using a core‐shell particle column. Journal of Food Composition and Analysis, 97, 103768. 10.1016/j.jfca.2020.103768 [DOI] [Google Scholar]
- She G, Wang S and Liu B, 2011. Dihydrochalcone glycosides from Oxytropis myriophylla, 2011. Chemistry Central Journal, 5(1), 71. 10.1186/1752-153X-5-71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi ZJ, Lei HH, Chen G, Yuan PH, Cao Z, Ser HL, Zhu XH, Wu F, Liu CX, Dong MY, Song YC, Guo YY, Chen C, Hu KX, Zhu YF, Zeng XA, Zhou JL, Lu YJ, Patterson AD and Zhang LM, 2021. Impaired intestinal Akkermansia muciniphila and aryl hydrocarbon receptor ligands contribute to nonalcoholic fatty liver disease in mice. Msystems, 6, e00985‐20. 10.1128/mSystems.00985-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjöstedt N, Deng F, Rauvala O, Tepponen T and Kidron H, 2017. Interaction of food additives with intestinal efflux transporters. Molecular Pharmaceutics, 14, 3824–3833. 10.1021/acs.molpharmaceut.7b00563 [DOI] [PubMed] [Google Scholar]
- Tomás‐Barberán FA, Borrego F, Ferreres F and Lindley MG, 1995. Stability of the intense sweetener neohesperidine dihydrochalcone in blackcurrant jams. Food Chemistry, 53, 263–265. 10.1016/0308-8146(95)92821-Z [DOI] [Google Scholar]
- Tran NH, Nguyen VT, Urase T and Ngo HH, 2014. Role of nitrification in the biodegradation of selected artificial sweetening agents in biological wastewater treatment process. Bioresource Technology, 161, 40–46. 10.1016/j.biortech.2014.02.116 [DOI] [PubMed] [Google Scholar]
- Tsuruda S, Sakamoto T and Akaki K, 2013. Simultaneous determination of twelve sweeteners and nine preservatives in foods by solid‐phase extraction and LC‐MS/MS. Journal of the Food Hygienic Society of Japan (Shokuhin Eiseigaku Zasshi), 54, 204–212. 10.3358/shokueishi.54.204 [DOI] [PubMed] [Google Scholar]
- US Food and Drug Administration , 2001. Guidance for Industry – Bioanalytical Method Validation. US Department of Health and Human Services, CDER and CVM, Rockville, MD. [Google Scholar]
- von Rymon Lipinski GW, 2015. Sweeteners. Ullmann's Encyclopedia of Industrial Chemistry. [Google Scholar]
- Waalkens‐Berendsen DH, Kuilman‐Wahls MEM and Bär A, 2004. Embryotoxicity and teratogenicity study with neohesperidin dihydrochalcone in rats. Regulatory Toxicology and Pharmacology, 40, 74–79. 10.1016/j.yrtph.2004.05.007 [DOI] [PubMed] [Google Scholar]
- Wang X, Pan Y, Ma J, Shi S, Zheng X and Xiang Z, 2014. Application of a liquid chromatography‐tandem mass spectrometry method to the pharmacokinetics, bioavailability and tissue distribution of neohesperidin dihydrochalcone in rats. Xenobiotica, 44, 555–561. 10.3109/00498254.2013.861950 [DOI] [PubMed] [Google Scholar]
- Wang YH, Hu S, Avula B, Wang M, Sagi S and Khan IA, 2015. Simultaneous determination of ten high‐intensity sweeteners of regulatory interest using UHPLC‐UV‐ELSD/MS. Planta Medica, 81, 8. 10.1055/s-0035-1556239 [DOI] [Google Scholar]
- Wasik A and Buchgraber M, 2009. Analysis of sweeteners in foodstuff. Agro FOOD Industry Hi Tech, 20, 27–30. Available online: https://publications.jrc.ec.europa.eu/repository/handle/JRC50892 [Google Scholar]
- Wasik A, McCourt J and Buchgraber M, 2007. Simultaneous determination of nine intense sweeteners in foodstuffs by high performance liquid chromatography and evaporative light scattering detection – development and single‐laboratory validation. Journal of Chromatography A, 1157, 187–196. 10.1016/j.chroma.2007.04.068 [DOI] [PubMed] [Google Scholar]
- Xie H, Chen J, Huang Y, Zhang R, Chen C‐E, Li X and Kadokami K, 2020. Screening of 484 trace organic contaminants in coastal waters around the Liaodong Peninsula, China: occurrence, distribution, and ecological risk. Environmental Pollution, 267, 11546. 10.1016/j.envpol.2020.115436 [DOI] [PubMed] [Google Scholar]
- Yang L, Wang L, Li K and Ye B, 2014. Sensitive voltammetric determination of neohesperidin dihydrochalcone based on SWNTs modified glassy carbon electrode. Analitical Methods, 6, 9410–9418. 10.1039/C4AY02004A [DOI] [Google Scholar]
- Zeiger E, Anderson B, Haworth S, Lawlor T, Mortelmans K and Speck W, 1987. Salmonella mutagenicity tests: III. Results from the testing of 255 chemicals. Environmental and Molecular Mutagenesis, 9(S9), 1–110. 10.1002/em.2860090602 [DOI] [PubMed] [Google Scholar]
- Zhang J, Cai H‐Q, Yang J, Gai S‐Y, Zhao Y and Ran L‐J, 2017. Determination of neohesperidin dihydrochalcone in beverages by reversed phase high performance liquid chromatography. Science and Technology of Food Industry, 19, 256–260. [Google Scholar]
- Zhang FX, Yuan YL, Cui SS, Li M, Tan X, Qiu ZC and Li RM, 2021. Dissection of the potential pharmacological function of neohesperidin dihydrochalcone – a food additive – by in vivo substances profiling and network pharmacology. Food & Function journal, 12, 4325–4336. 10.1039/d1fo00104c [DOI] [PubMed] [Google Scholar]
- Zygler A, Wasik A, Kot‐Wasik A and Namieśnik J, 2011. Determination of nine high‐intensity sweeteners in various foods by high‐performance liquid chromatography with mass spectrometric detection. Analytical and Bioanalytical Chemistry, 400, 2159–2172. 10.1007/s00216-011-4937-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zygler A, Wasik A, Kot‐Wasik A and Namiesnik J, 2012. The content of high‐intensity sweeteners in different categories of foods available on the Polish market. Food Additives & Contaminants Part A, 29, 1391–1401. 10.1080/19440049.2012.699475 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Exposure data and estimates
List of excluded studies
Weight of evidence (WoE) tables for animal studies


