Abstract
BACKGROUND
Clinical reports of multiple primary malignant tumors (MPMTs) in the digestive system are increasing. In China, although the survival rate of patients with MPMTs is increasing, the quality of life is very low. Many patients have reached the advanced stage when the second primary tumor is found, resulting in no early intervention and treatment. This is due to the misunderstanding of MPMTs by clinicians, who treat such tumors as metastases. Therefore, before a patient has a second primary tumor, doctors should understand some common combinations of digestive system MPMTs to provide clinical guidance to the patient.
AIM
To explore the high incidence combination of digestive system MPMTs under heterochronism and synchronization.
METHODS
A total of 1902 patients with MPMTs at Peking Union Medical College Hospital were analyzed retrospectively. They were divided into metachronous MPMT and synchronous MPMT groups, and then the high incidence combinations of the first primary cancer and the second primary cancer in metachronous cancer and synchronous cancer were sorted. Sex and age differences between metachronous and synchronous tumors were tested by the chi square test and t test, respectively. A P value < 0.05 was considered as statistically significant, and SPSS version 26.0 (SPSS Inc., Chicago, Illinois, United States) was used for statistical analysis.
RESULTS
Among the 1902 patients with MPMTs confirmed by pathology, 1811 (95.2%) cases were secondary primary cancers, 89 (4.7%) cases were tertiary primary cancers, and 2 (0.1%) cases were quaternary primary cancers. Most (88.2%) of the secondary primary cancers were identified as metachronous multiple primary cancers six months after diagnosis of the first primary cancer. The top ten most common MPMTs in the first primary cancer group ranged from high to low as follows: Breast cancer, thyroid cancer, nonuterine cancer, lung cancer, colon cancer, kidney cancer, uterine cancer, bladder cancer, rectal cancer, and gastric cancer. The highest incidence rate of the first primary cancer in male metachronous cancer was lung cancer (11.6%), the highest incidence rate of the second primary cancer was still lung cancer (24.9%), the highest incidence rate of the first primary cancer in female metachronous cancer was breast cancer (32.7%), and the highest incidence rate of the second primary cancer was lung cancer (20.8%). Among them, breast cancer, nonuterine cancer and uterine cancer were female-specific malignant tumor types, and thyroid cancer also accounted for 79.6% of female patients. The top five metachronous cancer combinations, independent of female-specific malignant tumor types and thyroid cancer, were colon cancer and lung cancer (26 cases), kidney cancer and lung cancer (25 cases), rectal cancer and lung cancer (20 cases), gastric cancer and lung cancer (17 cases), and bladder cancer and lung cancer (17 cases). The most common synchronous cancer combination was colon cancer and rectal cancer (15 cases).
CONCLUSION
Screening for lung cancer should be performed six months after the detection of colon cancer while rectal cancer screening should be performed within six months.
Keywords: Multiple primary malignant tumors, Colon cancer, Rectal cancer, Metachronous carcinoma, High incidence combinations, First primary carcinoma
Core Tip: This is a retrospective study to explore the high incidence combination of multiple primary malignant tumors (MPMTs). Among the 1902 patients with MPMTs confirmed by pathology, after excluding the effect of male-female specific malignancies, it was found that digestive system malignancies were very common as the first primary cancer. Therefore, the common combination of second primary cancers should be followed up at the limit of 6 mo after the detection of digestive system malignancies. Without excluding the influence of male-female specific malignancies, it was found that the combination of breast cancer and nonuterine cancer was the most common in metachronous multiple primary malignancies, and the combination of colon cancer and rectal cancer was the most common in synchronous multiple primary malignancies.
INTRODUCTION
Multiple primary malignant tumors (MPMTs) are defined as the simultaneous or successive occurrence of two or more primary malignant tumors in the same individual, which can be derived from the same organ, paired organs, different parts of the same system or different organs of different systems[1]. The diagnostic criteria were as follows: (1) Each tumor must have definite malignant histopathological changes; (2) Each tumor must have an independent pathological type; and (3) The possibility of invasion and metastasis of the second cancer as the first primary cancer must be excluded. MPMTs were divided into metachronous cancer and synchronous cancer. Synchronous MPMTs are defined as the second primary cancer that occurs within 6 mo of the first primary cancer, and metachronous MPMTs are the opposite of synchronous cancer.
In recent years, as many as two to five primary cancers have been reported in a single case[2-5]. However, double primary MPMTs are more common[6,7]. This study investigated the combined association between second primary cancer and first primary cancer. With the development of cancer nanotechnology[8], cancer patients can be clearly diagnosed and properly treated, and the survival rate of cancer patients has been greatly improved[9-11]. Due to the long-term side effects of chemotherapy and/or radiotherapy[12,13], the improvement of diagnostic sensitivity and the continuous influence of genetic and behavioral risk factors, cancer patients have an increased risk of developing a second cancer due to the improvement of survival rate[14], which can threaten their health. Due to the complex pathogenesis of cancer and the influence of the tumor microenvironment[15], it is difficult to find a perfect anticancer therapy that can resist the growth of malignant tumors without increasing toxicity and causing adverse pharmacological interactions. Predicting the occurrence of the second cancer among cancer survivors is conducive to the diagnosis and treatment of cancer patients, and some MPMTs seem to have a correlation with one another, as they appear in specific combinations. Understanding common cancer combinations is of significance for the clinical diagnosis and treatment of cancer patients.
MATERIALS AND METHODS
A total of 1902 patients were diagnosed with MPMTs at Peking Union Medical College Hospital from January 1, 2000, to June 1, 2021. According to the 3rd Edition description and definition[16], the primary cancer site is divided into 23 main types of solid cancer. The types are breast cancer; thyroid cancer; nonuterine cancer; lung cancer; colon cancer; rectal cancer; renal cancer; uterine cancer; bladder cancer; gastric cancer; head and neck cancer; prostate cancer; renal pelvis and ureter; oesophageal cancer; liver cancer; skin cancer; pancreatic cancer; thymus cancer; non-prostate cancer; small intestine cancer; adrenal cortex; sarcoma; bone and chondroma. Male genital cancer is divided into prostate cancer and non-prostate cancer. Nonuterine cancer includes cervical, vaginal, ovarian and fallopian tube cancers. Sex and age differences between metachronous and synchronous tumors were tested by the chi square test and t test, respectively. A P value < 0.05 was considered as statistically significant, and SPSS version 26.0 (SPSS Inc., Chicago, Illinois, United States) was used for statistical analysis.
RESULTS
The top ten, first-diagnosed cancers of metachronous and synchronous multiple primary cancers. Among 1902 patients with multiple primary cancers, including 681 males and 1221 females (1:1.79), 1678 cases (88.2%) were metachronous multiple primary cancers, and 224 cases (11.8%) were simultaneous multiple primary cancers. The majority of patients with multiple primary cancers were women (64.2%). There was a significant difference in the distribution of metachronous and simultaneous cancers between gender groups (tP < 0.001). The average age at diagnosis of the first metachronous and simultaneous cancers was 61 and 60 years, respectively (Table 1).
Table 1.
Clinical characteristics of multiple primary cancers patients. Clinical characteristics of patients with metachronous and synchronous multiple primary cancers
|
Clinical variable
|
Metachronous multiple primary cancers (n = 1687, 88.2%)
|
Synchronous multiple primary cancers (n = 224, 11.8%)
|
Total (n = 1902)
|
P
value
|
| Sex | < 0.0011 | |||
| Male | 562 (33.5%) | 119 (53.1%) | 681 (35.8%) | |
| Female | 1116 (66.5%) | 105 (46.9%) | 1221 (64.2%) | |
| Age | 0.3772 | |||
| Mean (SD) | 60.5 (11.7) | 59.8 (11.8) | 60.5 (11.7) | |
| Rang | 22.0-90.0 | 24.0-88.0 | 22.0-90.0 |
chi-squared test.
t-test.
The first and second primary cancers in the top ten MPMTs
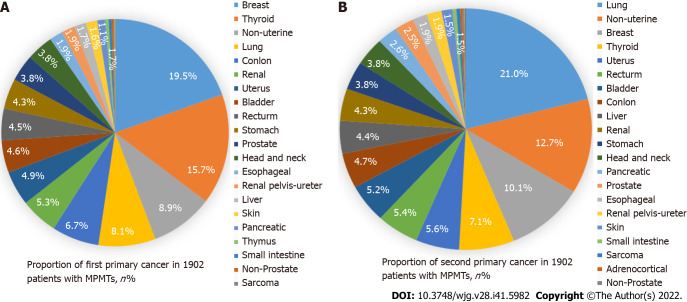
We found that the first primary cancer types of the top ten MPMTs were as follows (Figure 1A): Breast cancer (19.5%); thyroid cancer (15.7%); nonuterine cancer (8.9%); lung cancer (8.1%); colon cancer (6.7%); renal cell carcinoma (5.3%); uterine cancer (4.9%); bladder cancer (4.6%); rectal cancer (4.5%); gastric cancer (4.3%). The second primary cancer types of the top ten MPMTs were as follows (Figure 1B): Lung cancer (21.0%); nonuterine cancer (12.7%); breast cancer (10.1%); thyroid cancer (7.1%); uterine cancer (5.6%); rectal cancer (5.4%); bladder cancer (5.2%); colon cancer (4.7%); liver cancer (4.4%); and renal cell carcinoma (4.3%).
Figure 1.
Distribution of first and second primary cancers of multiple primary cancers. A: The first primary cancer; B: The second primary cancer.
The proportion of male to female first primary cancer and second primary cancer metachronous cases
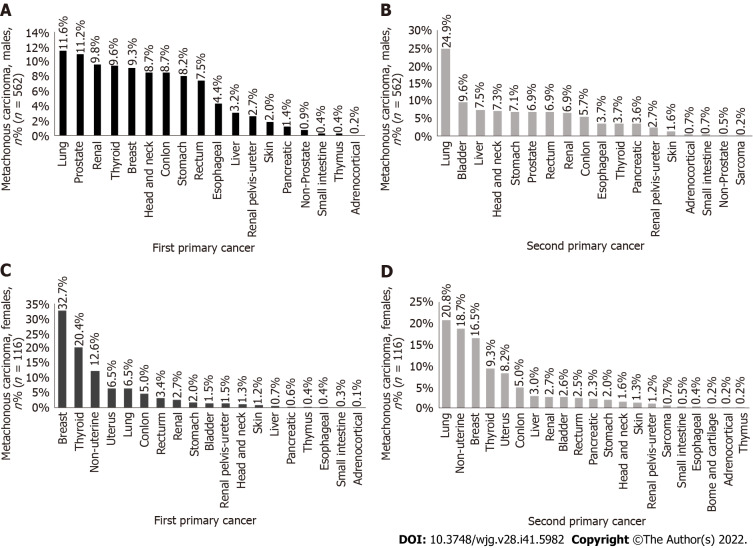
The incidence rate of lung cancer in metachronous MPMTs as the first primary cancer (Figure 2A) was the highest in men (11.6%), and the second primary cancer in male metachronous MPMTs was still the highest in lung cancer incidence rate (Figure 2B) (24.9%). The incidence rate of breast cancer as the first primary cancer in metachronous MPMTs (Figure 2C) was the highest in women (32.7%), and the incidence rate of lung cancer (Figure 2D) was the highest (20.8%) in female metachronous MPMTs. It should also be noted that most second primary cancers occur more than 6 mo after the diagnosis of the first primary cancer.
Figure 2.
The proportion of male to female first primary cancer and second primary cancer metachronous cases. A: The first primary cancer is distributed in men with metachronous cancer; B: The second primary cancer is distributed in men with metachronous cancer; C: The first primary cancer is distributed in women with metachronous cancer; D: The second primary cancer is distributed in women with metachronous cancer.
The proportion of male and female cancers of the first primary cancer and the second primary cancer in synchronous cancer
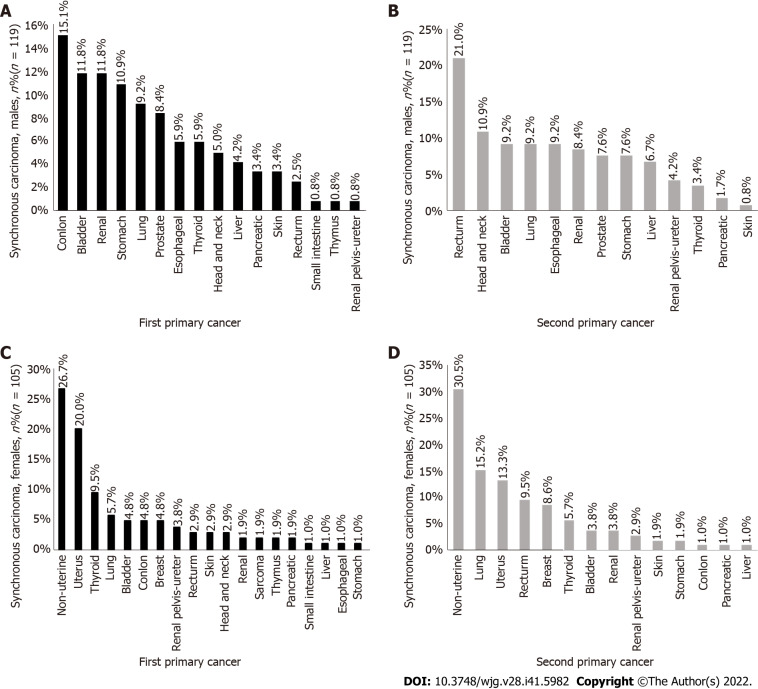
Among synchronous cancers, the incidence rate of colon cancer (Figure 3A) was the highest among the first primary cancers in men (15.1%), the incidence rate of rectal cancer (Figure 3B) was the highest among the second primary cancers (21.0%), the incidence rate of nonuterine cancer (Figure 3C) was the highest among the first primary cancers in women (26.7%), and the incidence rate of nonuterine cancer (Figure 3D) was the highest among the second primary cancers (30.5%).
Figure 3.
The proportion of male and female cancers of the first primary cancer and the second primary cancer in synchronous cancer. A: The distribution of the first primary cancer in men with synchronous cancer; B: The second primary cancer in men with synchronous cancer; C: The distribution of the first primary cancer in women with metachronous cancer; D: The second primary cancer in women with metachronous cancer.
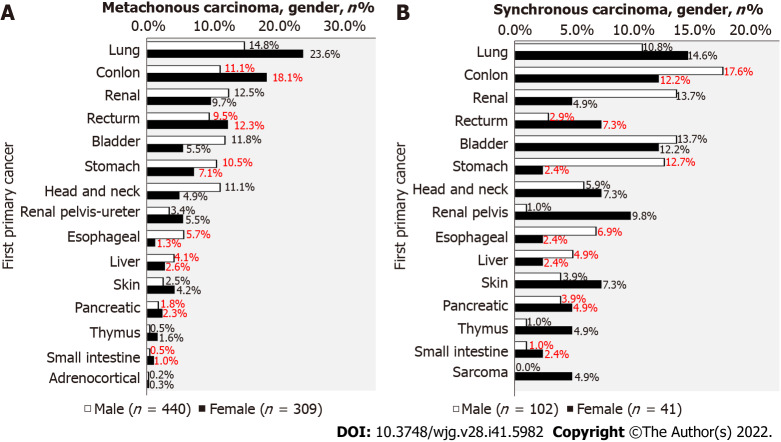
After excluding the influence of male-female specific malignant tumors, the proportion of male and female digestive system malignant tumors
After excluding male-female specific cancers, 749 patients (440 males and 309 females) were found to have metachronous cancers, of which 43.2% were digestive system malignancies in males and 44.7% were digestive system malignancies in females (Figure 4A). There were 143 patients (102 males and 41 females) with synchronous cancer. Among them, 49.9% of male and 34.0% of female digestive system malignancies were digestive system malignancies (Figure 4B).
Figure 4.
After excluding the influence of male-female specific malignant tumors, the proportion of male and female digestive system malignant tumors. A: The proportion of male and female primary malignant tumors after excluding the influence of male-female specific cancers, the proportion of male and female primary tumors of metachronous cancer; B: The proportion of male and female primary malignant tumors of synchronous cancer. The red numbers represent the percentage of digestive tumors.
The combination of the first primary cancer and the metachronous and synchronous second primary cancer in multiple primary cancers
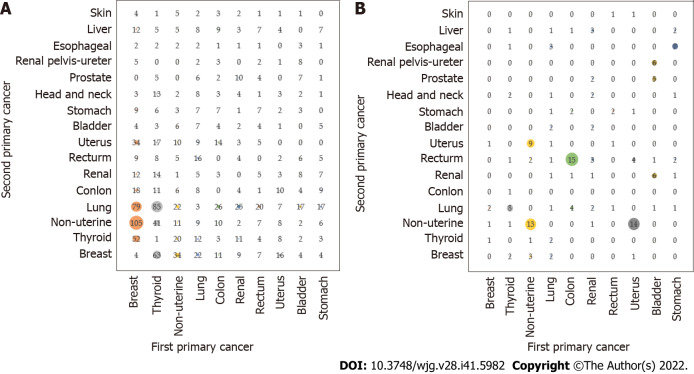
The combination of the first primary cancer and the second primary cancer in the top ten metachronous multiple primary cancers was as follows (Figure 5A): Breast cancer and nonuterine cancer (105 cases); thyroid cancer and lung cancer (85 cases); breast cancer and lung cancer (79 cases); thyroid cancer and breast cancer (63 cases); breast cancer and thyroid cancer (52 cases); thyroid cancer and nonuterine cancer (41 cases); nonuterine cancer and breast cancer (34 cases); breast cancer and uterine cancer (34 cases); colon cancer and lung cancer (26 cases); and renal cancer and lung cancer (25 cases). Breast or thyroid cancer may become the most prevalent second primary cancer among multiple primary cancers. The top five synchronous multiple primary cancers, the combination of the first primary cancer and the second primary cancer, were as follows (Figure 5B): Colon cancer and rectal cancer (15 cases); uterine cancer and nonuterine cancer (14 cases); nonuterine cancer and nonuterine cancer (13 cases); nonuterine cancer and uterine cancer (9 cases); and there were 8 cases of thyroid carcinoma.
Figure 5.
The top ten combined cases of first primary cancer and subsequent second primary cancer of metachronous and simultaneous multiple primary cancer. A: The combination of first primary cancer and second primary cancer of metachronous cancer; B: The combination of first primary cancer and second primary cancer of synchronous cancer.
Relationship between nonuterine cancer and nonuterine cancer in synchronous cancer
In the combination of nonuterine cancer and nonuterine cancer (Table 2), the results were as follows: Cervical cancer and ovarian cancer (5 cases); vaginal carcinoma and cervical carcinoma (3 cases); cervical vaginal cancer (2); fallopian tube carcinoma and cervical carcinoma (1 case); and ovarian cancer and cervical cancer (column 1). Nonuterine cancer and nonuterine cancer are not duplicates, as the term refers to any cancer occurring in the female reproductive system. Therefore, when a patient has one of the nonuterine cancers, doctors should check whether the other nonuterine organs have lesions at the time of diagnosis to avoid a missed diagnosis.
Table 2.
Combination of non-uterine cancer and non-uterine cancer
|
|
Cervix
|
Vagina
|
Ovary
|
| Cervix | 0 | 2 | 5 |
| Vagina | 3 | 0 | 0 |
| Fallopian tube | 1 | 0 | 1 |
| Ovary | 1 | 0 | 0 |
The row represents the first primary cancer and the column represents the second primary cancer.
DISCUSSION
In our study, female-specific malignant tumors accounted for the vast majority. Therefore, to balance the influence caused by the excess of certain malignant tumors between male and female malignant tumors, we tried to distinguish the malignant tumors specific to male and female patients from the malignant tumors likely to affect both sexes for discussion and found that the combination of colon cancer and lung cancer (26 cases) was the most common in metachronous cancer, followed by renal and lung cancer (25 cases), rectal and lung cancer (20 cases), gastric and lung cancer (17 cases), and bladder and lung cancer (17 cases). The combination of colon cancer and rectal cancer (15 cases) was the most common in synchronous cancer. There is evidence that breast-cancer susceptibility gene 1 (BRCA1)/BRCA2 and MMR genes are indeed closely related to the occurrence of first and second colon cancer[17]. For the combination of colon cancer and rectal cancer, it is hoped that future genetic testing will be performed. Colon cancer is more likely to appear as the first primary cancer in the combination of synchronous cancer and metachronous cancer. Since the majority of tumors occur in the digestive system, regular follow-up of the lungs should be performed 6 mo after the discovery of colon, rectal, or gastric cancer, and the rectum should be followed up and screened within 6 mo to prevent the occurrence of the second colorectal cancer[18-20].
We found that metachronous MPMTs are more common than synchronous MPMTs, which is consistent with a study conducted in Thailand last year[21]. However, Thailand has the highest incidence rate of liver cancer among metachronous cancer types, which is related to their eating habits. They like to eat sashimi, which leads to infection caused by parasitic liver flukes, resulting in a higher incidence of liver cancer than other cancer types[22]. On the other hand, we have less data on liver cancer because there are more cases of metastasis of other cancer species in the liver, so it was not included in the study. It is noteworthy that China has carried out systematic management of patients with hepatobiliary tumors in recent years, including clinical treatment and exploration methods (extended period), and proposed an ideal model (three-dimensional period) for future use[23]. Moreover, in recent years, China has made some progress in the treatment of hepatobiliary tumors, including the objective remission rate of lenvatinib and pembrolizumab in refractory biliary tract cancer reaching 25% and the disease control rate reaching 78.1%[24]. Stereotactic therapy is a feasible transformation therapy that can make patients with hepatocellular carcinoma with extrahepatic metastasis resectable[25]. In this new era for cancer treatment, targeted drugs, targeted immune checkpoint inhibitors or their combination bring new hope for the conversion of hepatocellular carcinoma to surgery and adjuvant therapy[26].
In this study, the number of single primary malignant tumors was not counted, so the incidence rate of MPMTs was not calculated, but this did not affect our study on the combination of the first primary cancer and the second primary cancer in MPMTs. Of all the statistics, we found that breast cancer is the most common primary cancer, which is also related to the high incidence of breast cancer in China[27]. The second most common primary malignant tumor is lung cancer, which has a high incidence in China[28]. Recent studies in the United States have shown that bladder cancer is the most common primary cancer, and lung cancer is the second most common primary cancer[29]. Compared with the first primary cancer, the cancer types in China and the United States are different. The reasons for this difference are as follows: (1) In China, there are more women than men with MPMTs, and the most common cancer among females is breast cancer. In contrast, in the United States, the incidence rate of bladder cancer is highest; (2) This difference is because the research subjects are different: We study Chinese people, mainly from some cities in northern China, while the main research subjects in Europe and America are Caucasian and Black people[29]; and (3) Different levels of development lead to different exposure factors, such as living habits, air pollution, occupational exposure, viruses, bacterial infection and other carcinogenic factors.
The incidence rate of breast cancer is the highest among the metachronous cancer types in China. Moreover, the male to female incidence rate of MPMTs is 1:1.79, which is different from the previously reported male to female incidence rate of cancer in China. The reason for this difference is that there are great differences in the natural ecological environment, lifestyle, and disease risk factors in the eastern, central and western regions of China, and there are regional differences in the incidence rate of malignant tumors between men and women[30]. This study is limited by the sample size, so it will have some impact on the study. It is worth noting that to facilitate the study, we regard each segment of the colon as a primary cancer because colon cancer seems to be segmented, but their pathogenesis is similar. Studies have shown that noncoding RNAs play a key role in the carcinogenesis and progression of the colon cancer[31].
In this study, for female patients, breast cancer and the second primary cancer nonuterine cancer had the most heterochronous combinations (105 cases). Gene analysis showed that the occurrence of breast cancer and ovarian cancer was related to the loss of BRCA1 or BRCA2 gene function leading to homologous recombination defects[32]. The common combinations of primary breast cancer and secondary breast cancer may be caused by chemotherapy and radiotherapy of primary tumors, genetic variation linking the two diseases, hormone signals from oestrogen, lifestyle and environmental factors[33], and the thyroid gland, whose cancer has a similar incidence pattern to breast cancer. The combination of the first or second primary cancer and thyroid cancer may be caused by thyroid hormone signals[33]. Therefore, when it is first discovered that a female patient has breast cancer, their vagina, cervix, fallopian tubes, ovaries and thyroid gland should be checked for lesions after 6 mo to achieve early detection and treatment[34-36]. Thyroid cancer was the second most common type of cancer in this study, and there were many combinations where the second primary cancer was lung cancer in metachronous MPMTs (85 cases). There is evidence that abnormalities in the oncogene rearrangement during transfection are the cause of lung cancer[37].
The metachronous combination of breast cancer and lung cancer (79 cases) should not be ignored. Lung cancer often appears in metachronous cancer in the form of a second primary cancer. There is evidence that the risk of primary lung cancer after treatment of breast cancer increases because smoking habits, age and the disease stage of breast cancer may affect the risk of secondary primary lung cancer in breast cancer patients[38]. Therefore, the regular follow-up of patients, including monitoring the lungs after 6 mo, should be carried out while breast cancer is treated. If lung cancer is found, it should be treated quickly, with both surgical treatment and adjuvant treatment[39,40].
We studied the high incidence combination of MPMTs, but there were some limitations. First, the population of this study is very limited. Most patients came from northern China, not all of China, and the basic data of the above foreign studies are from the Surveillance, Epidemiology and End Results program of the domestic population-based cancer registry or the data of many long-standing and highly reliable cancer centres on cancer diagnosis[41-42]. We recognize that this difference may also introduce bias to this study. Second, we have less synchronous cancer data, which is not enough to explain the strong association between synchronous cancer combinations. Third, we did not carry out genetic examination on patients, so the pathogenesis and aetiology of MPMTs were not discussed. Fourth, this study is a cross-sectional study involving 23 types of malignant tumors and describes the incidence of malignant tumors between men and women. It does not involve therapy, immune chemistry, next-generation sequencing, etc. Despite the limitations mentioned above, our results are informative. For patients treated with primary malignant tumors, attention should be given to the high incidence combination of the first and second primary cancers. In the absence of male-female specific cancer effects, attention should be given to the digestive system as a combination of primary malignancy and lung cancer. Without distinguishing between male-female specific malignancies, the possible metachronous cancer combination of breast cancer and nonuterine cancer and the synchronous cancer combination of colon cancer and rectal cancer should be given. After distinguishing synchronous cancer from metachronous cancer, the high incidence cancer combination should be followed up regularly for 6 mo. Through clinical experience and examination, some common cancers can be detected. If they can be removed surgically, they should be removed at the earliest stage possible. If the tumors cannot be removed, they should also be treated with the best adjuvant treatment, such as radiotherapy and chemotherapy, targeted therapy, and endocrine therapy, among others, to achieve the maximum therapeutic benefits[43,44].
CONCLUSION
In conclusion, after excluding the effect of cancers specific to men and women, screening for lung cancer should be performed 6 mo after detection of colon cancer and for rectal cancer within 6 mo.
ARTICLE HIGHLIGHTS
Research background
Multiple primary malignant tumors (MPMTs) of the digestive system are common clinically, usually presenting as a metachronous combination of colon cancer and lung cancer and a synchronous combination of colon cancer and rectal cancer.
Research motivation
Understanding some common combinations of multiple primary malignancies can help in providing clinical guidance to patients.
Research objectives
This study aimed to classify MPMTs of the digestive system and explore the combination of high incidence with the second primary malignant tumors.
Research methods
This retrospective study analyzed patients diagnosed with multiple primary malignancies in our centre over a 20-year period, classified the tumors, and further explored the high incidence of digestive system malignancies in the combination of multiple primary malignancies.
Research results
The most common metachronous combination pairs of multiple primary malignancies of the digestive system were colon cancer and lung cancer (26 cases), and synchronous combination pairs were colon cancer and rectal cancer (15 cases).
Research conclusions
Through our retrospective study, we found that when patients were diagnosed with colon cancer, they should be screened separately for lung cancer and rectal cancer at the limit of 6 mo.
Research perspectives
To provide clinical guidance to patients based on the combination of common multiple primary malignancies.
Footnotes
Institutional review board statement: This study was reviewed and approved by the Ethics Committee of Peking Union Medical College (Approval No. K22C0171).
Informed consent statement: Patients were not required to give informed consent to the study because the analysis used anonymous clinical data. The ethical Committee of Peking Union Medical College Hospital agreed to waive the informed consent.
Conflict-of-interest statement: All the authors report no relevant conflicts of interest for this article.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: August 24, 2022
First decision: September 26, 2022
Article in press: October 26, 2022
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Chen L, China; Dambrauskas Z, Lithuania S-Editor: Wang JJ L-Editor: A P-Editor: Wang JJ
Contributor Information
Xiao-Bo Yang, Department of Liver Surgery, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing 100006, China.
Long-Hao Zhang, Department of Liver Surgery, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing 100006, China; Digestive Disease Hospital Affiliated to Zunyi Medical University, Department of General Surgery, Affiliated Hospital of Zunyi Medical University, Zunyi 563099, Guizhou Province, China.
Jing-Nan Xue, Department of Liver Surgery, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing 100006, China; Digestive Disease Hospital Affiliated to Zunyi Medical University, Department of General Surgery, Affiliated Hospital of Zunyi Medical University, Zunyi 563099, Guizhou Province, China.
Yun-Chao Wang, Department of Liver Surgery, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing 100006, China.
Xu Yang, Department of Liver Surgery, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing 100006, China.
Nan Zhang, Department of Liver Surgery, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing 100006, China.
Dan Liu, Digestive Disease Hospital Affiliated to Zunyi Medical University, Department of General Surgery, Affiliated Hospital of Zunyi Medical University, Zunyi 563099, Guizhou Province, China.
Yan-Yu Wang, Department of Liver Surgery, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing 100006, China.
Zi-Yu Xun, Department of Liver Surgery, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing 100006, China.
Yi-Ran Li, Department of Liver Surgery, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing 100006, China.
Hui-Shan Sun, Department of Liver Surgery, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing 100006, China.
Li-Jin Zhao, Digestive Disease Hospital Affiliated to Zunyi Medical University, Department of General Surgery, Affiliated Hospital of Zunyi Medical University, Zunyi 563099, Guizhou Province, China.
Hai-Tao Zhao, Department of Liver Surgery, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing 100006, China. zhaoht@pumch.cn.
Data sharing statement
No additional data are available.
References
- 1.Warren S, Gates O. Multiple primary malignant tumors, a survey of the literature and statistical study. Am J Cancer. 1932;16:1358–1414. [Google Scholar]
- 2.Miao K, Yu S, Ni J, Zhang X, Zhang L. Second primary tumor after immune checkpoint inhibitor therapy: A case report. Thorac Cancer. 2022;13:1076–1078. doi: 10.1111/1759-7714.14327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhan X, He L, Song K, Cao S, Meng E, Wang Y. Case Report: Triple Primary Malignant Tumors of the Esophagus, Stomach, and Colon in a Patient With Genetic Analysis. Front Genet. 2021;12:676497. doi: 10.3389/fgene.2021.676497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abdeen Y, Al-Amer M, Taft E, Al-Halawani M. Four synchronous primary tumors in a male patient. J Cancer Res Ther. 2021;17:258–261. doi: 10.4103/jcrt.JCRT_187_18. [DOI] [PubMed] [Google Scholar]
- 5.Ying X, Zhang H, Chen B, Wu H, Bao L, Qian S, Ying X. Multiple metachronous rare primary malignant tumors: A case report. Thorac Cancer. 2019;10:2050–2053. doi: 10.1111/1759-7714.13182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feller A, Matthes KL, Bordoni A, Bouchardy C, Bulliard JL, Herrmann C, Konzelmann I, Maspoli M, Mousavi M, Rohrmann S, Staehelin K, Arndt V NICER Working Group. The relative risk of second primary cancers in Switzerland: a population-based retrospective cohort study. BMC Cancer. 2020;20:51. doi: 10.1186/s12885-019-6452-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang Z, Liu F, Qu Y, Qiu L, Zhang L, Yang Q. Second primary malignancy among malignant solid tumor survivors aged 85 years and older. Sci Rep. 2021;11:19748. doi: 10.1038/s41598-021-99260-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang Y, Li M, Gao X, Chen Y, Liu T. Nanotechnology in cancer diagnosis: progress, challenges and opportunities. J Hematol Oncol. 2019;12:137. doi: 10.1186/s13045-019-0833-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zou J, Wang E. Cancer Biomarker Discovery for Precision Medicine: New Progress. Curr Med Chem. 2019;26:7655–7671. doi: 10.2174/0929867325666180718164712. [DOI] [PubMed] [Google Scholar]
- 10.Santucci C, Carioli G, Bertuccio P, Malvezzi M, Pastorino U, Boffetta P, Negri E, Bosetti C, La Vecchia C. Progress in cancer mortality, incidence, and survival: a global overview. Eur J Cancer Prev. 2020;29:367–381. doi: 10.1097/CEJ.0000000000000594. [DOI] [PubMed] [Google Scholar]
- 11.Barazzuol L, Coppes RP, van Luijk P. Prevention and treatment of radiotherapy-induced side effects. Mol Oncol. 2020;14:1538–1554. doi: 10.1002/1878-0261.12750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schirrmacher V. From chemotherapy to biological therapy: A review of novel concepts to reduce the side effects of systemic cancer treatment (Review) Int J Oncol. 2019;54:407–419. doi: 10.3892/ijo.2018.4661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Copur MS, Manapuram S. Multiple Primary Tumors Over a Lifetime. Oncology (Williston Park) 2019;33 [PubMed] [Google Scholar]
- 14.Elia I, Haigis MC. Metabolites and the tumour microenvironment: from cellular mechanisms to systemic metabolism. Nat Metab. 2021;3:21–32. doi: 10.1038/s42255-020-00317-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arneth B. Tumor Microenvironment. Medicina (Kaunas) 2019;56 doi: 10.3390/medicina56010015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.World Health Organization. International classification of diseases for oncology, 3rd Edition (ICD-O-3). [cited 24 July 2022]. Available from: https://www.who.int/standards/classifications/other-classifications/international-classification-of-diseases-for-oncology .
- 17.Chan GHJ, Ong PY, Low JJH, Kong HL, Ow SGW, Tan DSP, Lim YW, Lim SE, Lee SC. Clinical genetic testing outcome with multi-gene panel in Asian patients with multiple primary cancers. Oncotarget. 2018;9:30649–30660. doi: 10.18632/oncotarget.25769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haria PD, Baheti AD, Palsetia D, Ankathi SK, Choudhari A, Guha A, Saklani A, Sinha R. Follow-up of colorectal cancer and patterns of recurrence. Clin Radiol. 2021;76:908–915. doi: 10.1016/j.crad.2021.07.016. [DOI] [PubMed] [Google Scholar]
- 19.Smith RA, Andrews KS, Brooks D, Fedewa SA, Manassaram-Baptiste D, Saslow D, Wender RC. Cancer screening in the United States, 2019: A review of current American Cancer Society guidelines and current issues in cancer screening. CA Cancer J Clin. 2019;69:184–210. doi: 10.3322/caac.21557. [DOI] [PubMed] [Google Scholar]
- 20.Kanth P, Inadomi JM. Screening and prevention of colorectal cancer. BMJ. 2021;374:n1855. doi: 10.1136/bmj.n1855. [DOI] [PubMed] [Google Scholar]
- 21.Tanjak P, Suktitipat B, Vorasan N, Juengwiwattanakitti P, Thiengtrong B, Songjang C, Therasakvichya S, Laiteerapong S, Chinswangwatanakul V. Risks and cancer associations of metachronous and synchronous multiple primary cancers: a 25-year retrospective study. BMC Cancer. 2021;21:1045. doi: 10.1186/s12885-021-08766-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sawanyawisuth K, Sashida G, Sheng G. Epithelial-Mesenchymal Transition in Liver Fluke-Induced Cholangiocarcinoma. Cancers (Basel) 2021;13 doi: 10.3390/cancers13040791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin J, Zhao H. Systemic management for patients with hepatobiliary tumors in a multi-dimensional view. Hepatobiliary Surg Nutr. 2019;8:626–628. doi: 10.21037/hbsn.2019.07.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin J, Yang X, Long J, Zhao S, Mao J, Wang D, Bai Y, Bian J, Zhang L, Wang A, Xie F, Shi W, Yang H, Pan J, Hu K, Guan M, Zhao L, Huo L, Mao Y, Sang X, Wang K, Zhao H. Pembrolizumab combined with lenvatinib as non-first-line therapy in patients with refractory biliary tract carcinoma. Hepatobiliary Surg Nutr. 2020;9:414–424. doi: 10.21037/hbsn-20-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang X, Xu H, Zuo B, Yang X, Bian J, Long J, Wang D, Zhang J, Ning C, Wang Y, Xun Z, Lu X, Mao Y, Sang X, Zhao H. Downstaging and resection of hepatocellular carcinoma in patients with extrahepatic metastases after stereotactic therapy. Hepatobiliary Surg Nutr. 2021;10:434–442. doi: 10.21037/hbsn-21-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao L, Zhao H. Conversion surgery for hepatocellular carcinoma in the new era of targeted and immune checkpoint inhibitor therapies. Hepatobiliary Surg Nutr. 2020;9:809–811. doi: 10.21037/hbsn-20-693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang X, Wang C, Guan J, Chen B, Xu L, Chen C. Progress of Breast Cancer basic research in China. Int J Biol Sci. 2021;17:2069–2079. doi: 10.7150/ijbs.60631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cao M, Chen W. Epidemiology of lung cancer in China. Thorac Cancer. 2019;10:3–7. doi: 10.1111/1759-7714.12916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Donin NM, Kwan L, Lenis AT, Drakaki A, Chamie K. Second primary lung cancer in United States Cancer Survivors, 1992-2008. Cancer Causes Control. 2019;30:465–475. doi: 10.1007/s10552-019-01161-7. [DOI] [PubMed] [Google Scholar]
- 30.Wu C, Li M, Meng H, Liu Y, Niu W, Zhou Y, Zhao R, Duan Y, Zeng Z, Li X, Li G, Xiong W, Zhou M. Analysis of status and countermeasures of cancer incidence and mortality in China. Sci China Life Sci. 2019;62:640–647. doi: 10.1007/s11427-018-9461-5. [DOI] [PubMed] [Google Scholar]
- 31.Chen S, Shen X. Long noncoding RNAs: functions and mechanisms in colon cancer. Mol Cancer. 2020;19:167. doi: 10.1186/s12943-020-01287-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sokol ES, Pavlick D, Khiabanian H, Frampton GM, Ross JS, Gregg JP, Lara PN, Oesterreich S, Agarwal N, Necchi A, Miller VA, Alexander B, Ali SM, Ganesan S, Chung JH. Pan-Cancer Analysis of BRCA1 and BRCA2 Genomic Alterations and Their Association With Genomic Instability as Measured by Genome-Wide Loss of Heterozygosity. JCO Precis Oncol. 2020;4:442–465. doi: 10.1200/PO.19.00345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bolf EL, Sprague BL, Carr FE. A Linkage Between Thyroid and Breast Cancer: A Common Etiology? Cancer Epidemiol Biomarkers Prev. 2019;28:643–649. doi: 10.1158/1055-9965.EPI-18-0877. [DOI] [PubMed] [Google Scholar]
- 34.Nebgen DR, Lu KH, Bast RC Jr. Novel Approaches to Ovarian Cancer Screening. Curr Oncol Rep. 2019;21:75. doi: 10.1007/s11912-019-0816-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bhatla N, Singhal S. Primary HPV screening for cervical cancer. Best Pract Res Clin Obstet Gynaecol. 2020;65:98–108. doi: 10.1016/j.bpobgyn.2020.02.008. [DOI] [PubMed] [Google Scholar]
- 36.Staples JN, Duska LR. Cancer Screening and Prevention Highlights in Gynecologic Cancer. Obstet Gynecol Clin North Am. 2019;46:19–36. doi: 10.1016/j.ogc.2018.09.002. [DOI] [PubMed] [Google Scholar]
- 37.Santoro M, Moccia M, Federico G, Carlomagno F. RET Gene Fusions in Malignancies of the Thyroid and Other Tissues. Genes (Basel) 2020;11 doi: 10.3390/genes11040424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Long Q, Wang Y, Che G. Primary Lung Cancer After Treatment for Breast Cancer. Int J Womens Health. 2021;13:1217–1225. doi: 10.2147/IJWH.S338910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Houston T. Screening for Lung Cancer. Med Clin North Am. 2020;104:1037–1050. doi: 10.1016/j.mcna.2020.08.005. [DOI] [PubMed] [Google Scholar]
- 40.Passiglia F, Bertaglia V, Reale ML, Delcuratolo MD, Tabbò F, Olmetto E, Capelletto E, Bironzo P, Novello S. Major breakthroughs in lung cancer adjuvant treatment: Looking beyond the horizon. Cancer Treat Rev. 2021;101:102308. doi: 10.1016/j.ctrv.2021.102308. [DOI] [PubMed] [Google Scholar]
- 41.Spada C, Hassan C, Bellini D, Burling D, Cappello G, Carretero C, Dekker E, Eliakim R, de Haan M, Kaminski MF, Koulaouzidis A, Laghi A, Lefere P, Mang T, Milluzzo SM, Morrin M, McNamara D, Neri E, Pecere S, Pioche M, Plumb A, Rondonotti E, Spaander MC, Taylor S, Fernandez-Urien I, van Hooft JE, Stoker J, Regge D. Imaging alternatives to colonoscopy: CT colonography and colon capsule. European Society of Gastrointestinal Endoscopy (ESGE) and European Society of Gastrointestinal and Abdominal Radiology (ESGAR) Guideline - Update 2020. Eur Radiol. 2021;31:2967–2982. doi: 10.1007/s00330-020-07413-4. [DOI] [PubMed] [Google Scholar]
- 42.Xiong J, Su Y, Bing Z, Zhao B. Survival between synchronous and non-synchronous multiple primary cutaneous melanomas-a SEER database analysis. PeerJ. 2020;8:e8316. doi: 10.7717/peerj.8316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chargari C, Levy A, Paoletti X, Soria JC, Massard C, Weichselbaum RR, Deutsch E. Methodological Development of Combination Drug and Radiotherapy in Basic and Clinical Research. Clin Cancer Res. 2020;26:4723–4736. doi: 10.1158/1078-0432.CCR-19-4155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tsimberidou AM, Fountzilas E, Nikanjam M, Kurzrock R. Review of precision cancer medicine: Evolution of the treatment paradigm. Cancer Treat Rev. 2020;86:102019. doi: 10.1016/j.ctrv.2020.102019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No additional data are available.