Abstract
Microglia are critical for regulation of neuronal circuits that mature from adolescence to adulthood. The morphological complexity and process length of microglia can indicate different activation states. These states are sensitive to a variety of environmental and stress conditions. Microglia are sensitive to many factors that also regulate social behavior, and in turn, microglial manipulations can impact social function. Brief social isolation is one factor that can lead to robust social changes. Here, we explored the role of microglia in the effects of brief social isolation on social recognition memory. Using morphological measures of Iba1 to index microglial intensity, complexity, and process length, we identified different effects of brief isolation on microglial complexity in the basal region of the amygdala between adults and adolescents alongside overall increases in intensity of Iba1 in several cortical brain regions. Short-term social recognition memory is sensitive to the amount of social engagement, and provides an opportunity to test if social engagement produced by brief isolation enhances social learning in a manner that relies on microglia. We found that brief isolation facilitated social interaction across ages but had opposing effects on short-term social recognition. Isolation increased novel partner investigation in adolescents, which is consistent with better social recognition, but increased familiar partner investigation in adults. Depletion of microglia with PLX3397 prevented these effects of brief isolation in adolescents, and reduced them in adults. These results suggest that distinct changes in microglial function driven by the social environment may differentially contribute to subsequent social recognition memory during development.
Keywords: Development, Microglia, Isolation, Social memory
1. Introduction
Adolescence is a critical developmental period characterized by brain maturation, changes in social interaction, and sensitivity to the social environment. The social environment can drive neural changes that then shift social behavior across the lifespan, especially during a developmental period with heightened social sensitivity such as adolescence (Ferrara et al., 2021a). Social isolation has been used to understand how sensitivity to the social environment changes with age and how environment affects subsequent behavior and brain maturation. Long-term isolation (>3 weeks) during development results in social interaction deficits that can impair social learning and memory into adulthood (Kercmar, Büdefeld, Grgurevic, Tobet, & Majdic, 2011; Yusufishaq & Rosenkranz, 2013). However, shorter duration isolation has an opposing effect on social interaction. When limited to hours or days, isolation can facilitate age-specific social behaviors, like social play in adolescents and social investigation across ages (Ferrara, Trask, & Rosenkranz, 2021b). The degree of social interaction has been directly linked to better social fear memory retention, with higher interaction associated with better social fear learning (Twining, Vantrease, Love, Padival, & Rosenkranz, 2017). Therefore, shifts in the environment that promote social interaction may improve social memory across ages.
A network of brain regions can regulate and are sensitive to social behavior in both adolescents and adults (Matthews & Tye, 2019). The medial prefrontal cortex (mPFC), the prelimbic (PL) and anterior cingulate (ACC) regions in particular, and amygdala are critical for social engagement (Allsop et al., 2018; Ferrara, Mrackova, Loh, Padival, & Rosenkranz, 2020; Finlay et al., 2015; Huang, Zucca, Levy, & Page, 2020; Wang et al., 2011). These brain regions undergo pruning and increases in synaptic strength from adolescence to adulthood (Klune, Jin, & DeNardo, 2021; Koss, Belden, Hristov, & Juraska, 2014). Prolonged social isolation during this period causes lasting changes of neuronal morphology, excitatory and inhibitory neurotransmission, as well as several structural markers of plasticity in the mPFC and amygdala (Wang, Ho, Ko, Liao, & Lee, 2012) that may contribute to impairment of social processes. The neural substrates for effects of brief isolation are unclear, but may also include mPFC and amygdala (Ferrara et al., 2020; Matthews & Tye, 2019).
Microglia are critical regulators of synaptic transmission and they contribute to neuronal function across the lifespan. Signaling between microglia and neurons is essential for synaptic pruning and refinement as well as developmental changes in excitatory transmission, both of which are disrupted when microglia-neuron signaling is inhibited (Kim et al., 2017; Zhan et al., 2014). Neurodevelopmental disorders characterized by social dysfunction may include abnormalities in microglial activity (Boelen, Stassen, Steinbusch, Borchelt, & Streit, 2012; Kim et al., 2017; Zhan et al., 2014). Long-term disruption of microglia can impair social interaction in both young and adult rodents (Zhan et al., 2014). Further, social stress can impact microglial processes in adults and adolescents (Munshi et al., 2020; Zhang et al., 2019). Prior work has used microglial depletion approaches to understand the function of microglial activation during development on social behavior. When transiently depleted and allowed to repopulate in juveniles, microglia regulate aspects of social play in adolescents (post-natal day, PND, 28–35) and can increase social avoidance in adults (PND 60–120; Nelson & Lenz, 2017). Conversely, microglial activation as a result of stress or with pro-inflammatory endotoxin, lipopolysaccharide, reduces social interaction in adults (Munshi et al., 2020; Wohleb et al., 2012; Zhu, Zhang, Ding, Zhao, & Zheng, 2014). These findings suggest that microglial activation has a dynamic role in bidirectionally regulating social behavior and a sensitivity of microglia to the social environment. Additionally, microglia can respond rapidly to many stimuli, and therefore may be a mechanism through which brief social isolation impacts social behavior. However, the ways in which microglia are rapidly impacted by the social environment and contribute to subsequent socially-related memories during development is unclear.
Microglia shift through different profiles where they are engaged in different functions, including surveillance, pro-inflammatory and anti-inflammatory states. A shift between microglial states can be indexed using Iba1 (ionized calcium binding adaptor protein 1; found in microglia but not neurons or other brain glia) and quantified through dynamic morphological changes (Hinwood, Morandini, Day, & Walker, 2012; Rohan Walker, Nilsson, & Jones, 2013). This can be reflected with increases in Iba1 staining intensity, and notably, changes the degree of ramification (Ito et al., 1998; Sasaki et al., 2001). Alterations in Iba1 intensity can indicate an increase in microglial activity that governs alterations in morphological states through interactions with actin binding proteins to create membrane ruffling that contributes to cytoskeletal changes (Sasaki et al., 2001). During surveillance states, microglia processes via cytoskeletal changes are extended to interact with other cells in the brain and readily shift in response to alterations in the environment (Hanamsagar & Bilbo, 2017; Hanisch, 2002).
Here, we investigated morphological changes in microglia following brief isolation in maturing brain regions critical for the regulation of social behavior and memory. To understand the functional consequences of isolation-driven changes in microglia, we then depleted microglia during a discrete period of adolescence or adulthood, and tested the impact on social recognition training and testing (see Fig. 1 for experimental design).
Fig. 1.
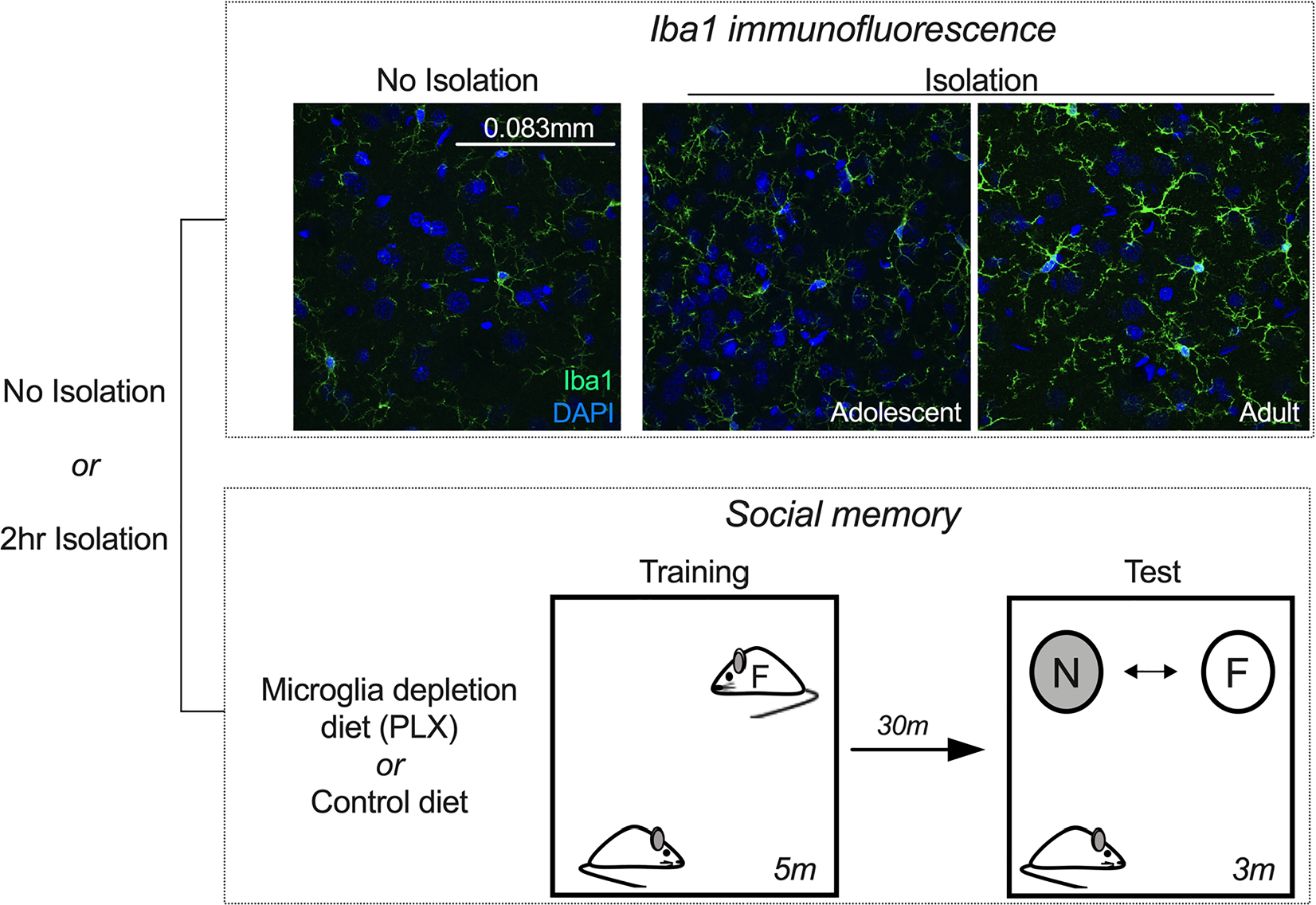
Experimental design for immunofluorescence and social recognition experiments. In one cohort, adult and adolescent rats were isolated or taken from their home cage (no isolation) and tissue was processed for Iba1 immunofluorescence. Images were captured in PL, ACC, and BLA regions. Imaged microglia (indexed with Iba1) were analyzed for changes in intensity, complexity, and process length. Representative 60x images displayed are from the basal amygdala. In another cohort, groups were fed a control or microglia depletion diet (PLX) for eight days. Groups were then isolated or taken from their home cage, and behavior was quantified during an open field test that was immediately followed by social interaction with an age-matched same-sex partner. Following the open field social interaction, groups were exposed to the same partner (familiar) or a novel partner under a wire cage. The time spent investigating novel and familiar partner at test was quantified.
2. Methods
Experiments were approved by the Institutional Animal Care and Use Committee at Rosalind Franklin University of Medicine and Science, and abided by the Guide for the Care and Use of Laboratory Animals (2011).
2.1. Subjects
Subjects were male Sprague Dawley rats purchased from Envigo (adolescent n = 53, adult n = 48; Indianapolis, IN) and housed 2–3 per cage in the Rosalind Franklin University animal facility. Rats had free access to food and water at all times and were maintained on a reverse light cycle (12 h light/dark). Adolescent rats arrived to the animal facility at postnatal day (PND) 20–28, and adults arrived at PND 64–69. At the time of brief isolation for behavior and immunofluorescence, adolescents were between the ages of PND 28–44, and adults were PND 71–133.
2.2. Microglia depletion
Groups habituated to the animal colony for one week prior to receiving Pexidartinib (PLX3397) or control diet mixed into rat chow by Research Diets Inc at a dose of 290 mg/kg of chow (as seen in Elmore et al., 2014; Torres et al., 2016). Rats were given altered diet for eight consecutive days prior to behavior. PLX3397 is a Colony-Stimulating Factor 1 Receptor (CSF1R) inhibitor, which substantially reduces the number of microglia (Oosterhof et al., 2018). This regimen of PLX3397 diet effectively reduces Iba1 expression within 7 days, but without impairment of social interaction or any observed health or gross locomotor consequences (Elmore et al., 2014; Torres et al., 2016).
2.3. Social recognition training and testing
Open field behavior and social interaction were measured in a dimly lit room (10–15 lx white light and dim red light). Rats were randomly assigned to brief isolation or control groups. Groups that were briefly isolated were placed individually into a transparent plexiglass transport cage (length: 28.58 cm, width: 17.78 cm, height: 20.32 cm) containing bedding and were at least 12 in. from all other cages in the animal colony during the dark cycle for 2 h prior to open field exposure (Ferrara et al., 2021). Control rats remained in their home cage during this period. All rats were individually placed into a black opaque plexiglass open field apparatus (100 cm × 100 cm) for acclimation immediately before social recognition training for 5 min. A novel age-matched same-sex conspecific partner was then placed into the apparatus and rats were allowed to freely interact for 5 min. This social interaction served as the familiarization phase for social recognition training. Following social interaction, rats were brought back to their home cages in the rat colony. After 30 min, these rats were returned to the open field and were allowed to investigate the previous stimulus rat (familiar) and a new age-matched same-sex rat (novel) under separate wire cages on the opposite sides of the open field apparatus.
All interactions were video recorded and captured with AnyMaze software (Stoelting, Wood Dale, IL). Videos were then uploaded into CowLog software (3.0.2, Helsinki, Finland; Hänninen & Pastell, 2009) and scored for social interaction consisting of nose-body contact, play, and chase behaviors during social recognition training. During the social recognition test, social investigation was scored as the nose touching the cage of novel or familiar partner. All scoring was by a rater blind to condition (as in Ferrara et al., 2021b). Data were exported in a CSV file and then transferred to an Excel file (Microsoft, Redmond, WA) where the sum and average duration of each interaction were calculated.
2.4. Immunofluorescence
Groups were deeply anesthetized following the brief isolation manipulation or control home cage and perfused with 0.1 M phosphate buffered saline (PBS) followed by 4% paraformaldehyde. Brains were sliced on a vibratome in 40 μm sections and mounted onto gelatinized slides. Slides were rehydrated in wash buffer (PBS + 0.05% Tween-20), endogenous peroxidase activity was blocked (PBS + 0.3% H2O2), and slices were permeabilized (PBS + 0.03% Triton X). Slices were then incubated in blocking solution for 1 h (PBS + 0.7% normal goat serum), and then Iba1 antibody (Wako Chemicals, Richmond, VA; 1:1000, #019-19741) overnight at 4 ◦C. Slices were then incubated in a secondary solution (1:500, Alexa Fluor 488, Invitrogen, Waltham, MA, catalog #: A32731) for 2 h, rinsed with wash buffer, and cover slipped with a DAPI counterstain.
2.5. Microscopy and image analysis
Cortical and amygdala regions were identified based on a rat brain atlas (Paxinos and Watson, 2007). Microglia in the prelimbic region of the medial prefrontal cortex were sampled from +3.7 to +2.5 anterior, in the anterior cingulate cortex from +1.9 mm to 0.2 mm anterior, and the basolateral amygdala from −2.0 to −3.3 posterior from bregma. All image capture and analyses were performed blind to condition, and all acquisition parameters, including illumination and exposure time, were held constant across brain sections and groups. Brain regions were captured on an Olympus Fluoview FV1200 confocal microscope (Shinjuku City, Tokyo, Japan) using a 20x or 60x objective lens. Serial z-stack images covered a depth of 4.55 μm through five consecutive sections (0.91 μm per section) and were acquired using Fluoview software (Olympus). Square sections in each region were captured and analyzed bilaterally by an individual blind to condition. Images were then exported as TIFF files, and intensity and skeletonization measures were completed using ImageJ software (NIH, Bethesda, MD, USA).
Mean intensity was measured for images captured with a 20x objective lens to capture the entire field of view for brain regions of interest using the “Measure” plugin in ImageJ. The mean intensity from individual sections was averaged bilaterally for each brain region. The same number of sections were analyzed from each animal for group analysis, and sampling was from matched rostral-caudal sections from each animal. Microglia were skeletonized with images captured from a 60x objective lens after Gaussian filtering (sigma = 0.75), using the “Gray Morphology” function, followed by image processing using native ImageJ functions in this order: despeckling, converting to binary, closing loops, and sharpening the image (similar to Young & Morrison, 2018). This resulted in a skeletonized Iba1-positive cell that best represented fluorescently labeled Iba1 expressing cells. When needed, Iba1 skeletonized images were manually edited to connect processes to best represent fluorescent images. To analyze process length of skeletonized Iba1 images, the “Analyze Skeleton” function was used in ImageJ (Morrison, Young, Qureshi, Rowe, & Lifshitz, 2017). Skeleton complexity was measured with box counting fractal analysis provided through the “FracLac” ImageJ plugin (Karperien, Ahammer, & Jelinek, 2013; Morrison et al., 2017). A minimum of six microglia for each rat were skeletonized for analysis, and analysis from skeletonized Iba1 cells were then averaged in each hemisphere. Images of single microglia found in Figs. 2–4 were enlarged to equal size to illustrate changes in intensity and complexity between ages and conditions.
Fig. 2.
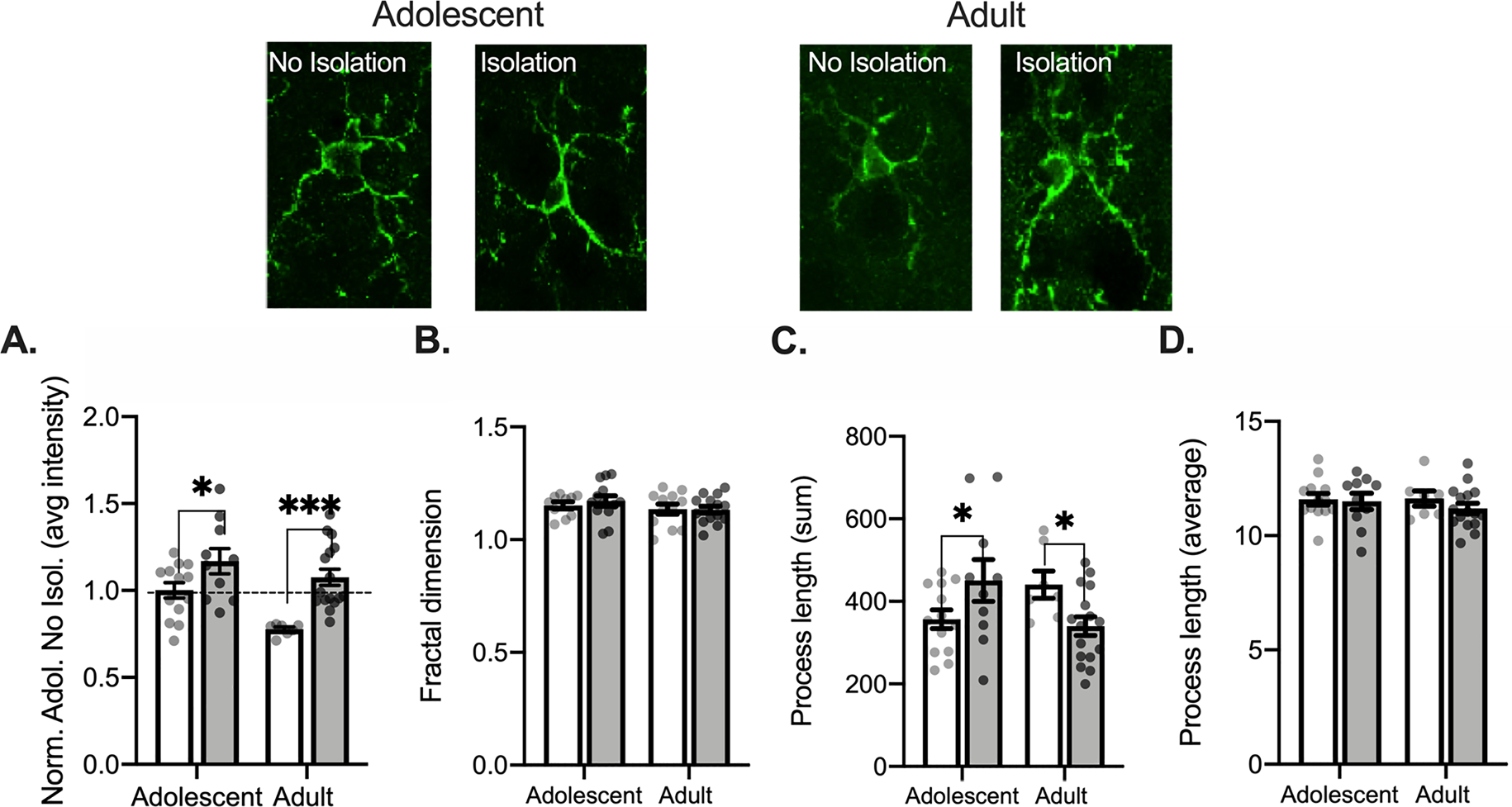
Isolation uniformly increases Iba1 intensity and bidirectionally changes process length in the anterior cingulate cortex of adult and adolescent rats. Representative Iba1 images for each age and condition are displayed above graphs. Adult and adolescents that were isolated for 2 h showed increases in Iba1 intensity (A). Skeletonized microglia show no change in complexity (B), total process length (C), or average process length (D). Non-Isolated adolescent n = 4, 13 bilateral slices averaged; Isolated adolescent n = 3, 10 bilateral slices averaged; Non-Isolated adult n = 3, 7 bilateral slices averaged for branch characteristic; Isolated adult n = 4, 16 bilateral slices averaged for branch characteristic. *p < 0.05.
Fig. 4.
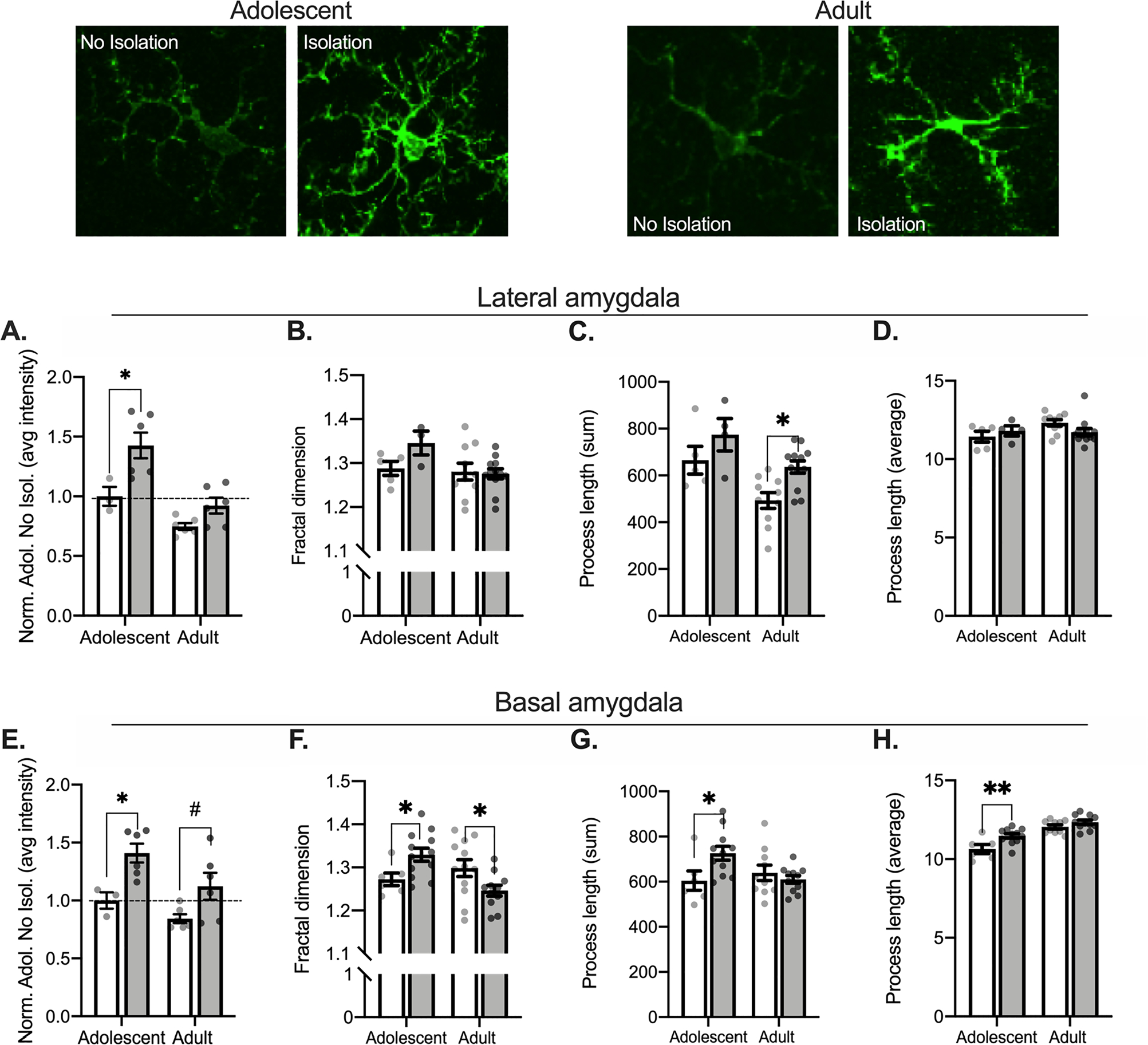
Isolation differentially alters Iba1 complexity in amygdala subregions between adults and adolescents. Representative Iba1 images from the basal amygdala are displayed above graphs. Isolation increases adolescent LA Iba1 intensity (A) but has no significant impact on Iba1 complexity in the LA (B). Isolated adults show an increase in the total process length (C) but no differences between groups for the average process length (D). In the BA, both adults and adolescents show an increase in Iba1 intensity (E). Isolation increases adolescent Iba1 complexity but reduces adult Iba1 complexity (F). Isolation also increases adolescent total process length (G) and the average process length (H). In the LA: Non-Isolated adolescent n = 1, 3 bilateral slices averaged for intensity, 5 bilateral slices averaged for branch characteristics; Isolated adolescent n = 2, 6 bilateral slices averaged for intensity, 4 bilateral slices averaged for branch characteristics; Non-Isolated adult n = 2, 6 bilateral slices averaged for intensity, 10 bilateral slices averaged for branch characteristics; Isolated adult n = 2, 6 bilateral slices averaged for intensity, 12 bilateral slices averaged for branch characteristics. In the BA: Non-Isolated adolescent n = 1, 3 bilateral slices averaged for intensity, 6 bilateral slices averaged for branch characteristics; Isolated adolescent n = 2, 6 bilateral slices averaged for intensity, 11 bilateral slices averaged for branch characteristics; Non-Isolated adult n = 2, 6 bilateral slices averaged for intensity, 10 bilateral slices averaged for branch characteristics; Isolated adult n = 2, 6 bilateral slices averaged for intensity, 11 bilateral slices averaged for branch characteristics. # = 0.053, *p < 0.05.
2.6. Statistical analyses
Graphpad Prism (San Diego, CA) was used for statistical analyses and to create figures. Behavioral statistical outliers two standard deviations above the mean during the social interaction training session were excluded from all subsequent analyses, these were one adult control diet non-isolated rat (Z = 2.54), one adult PLX non-isolated rat (Z = 2.37), and one adolescent PLX diet non-isolated rat (Z = −2.02). One control diet isolated adult and one control diet isolated adolescent were excluded for not investigating during the social recognition test. Data are presented as group averages with standard error of the mean (SEM). Immunofluorescence experiments were analyzed using Analysis of Variance (ANOVA) using age (adolescent, adult) and condition (no isolation, isolation) as factors. Behavioral experiments were analyzed using ANOVA with age (adolescent, adult) and condition (no isolation, isolation) as factors or a Repeated Measures (RM) ANOVA with condition (no isolation, isolation) and social target (familiar, novel) as factors. A one-sample t-test was used to compare discrimination index values to 0, a number that indicates equivalent time spent investigating a familiar and novel partner. Fisher’s LSD post hoc comparisons were used between groups as appropriate.
3. Results
3.1. Isolation uniformly increases Iba1 intensity and bidirectionally changes process length in the anterior cingulate cortex of adult and adolescent rats.
Microglia exist in a range of functional states and can be recruited to act in different states, even without entering a pro-inflammatory state. Sensitivity of microglia to the environment and involvement of microglia in ongoing behavior can increase microglial metabolic activity, which is often accompanied by rapidly increased Iba1 synthesis. A shift between different functional states is often accompanied by a change in the complexity of microglial processes and overall process length. Reduction in process length and complexity typically correspond to transition toward a pro-inflammatory state, as often seen with inflammation and stress exposure (Karperien et al., 2013; Munshi et al., 2018). To understand microglial-related changes in activity following brief isolation, we measured Iba1 intensity, microglial process length and process fractal dimensions as a proxy for process complexity (Fontainhas et al., 2011). A 2 (Age: Adolescent, Adult) × 2 (Condition: No Isolation, Isolation) ANOVA conducted to assess intensity of Iba1 staining in the ACC found a main effect of age, F(1, 42) = 18.26, p = 0.001, condition, F(1, 42) = 8.335, p = 0.006, but no interaction between the two, F(1, 42) = 1.434, p = 0.238 (Fig. 2A). Post hoc comparisons found that both adolescents (p = 0.029, adolescent control compared to isolation) and adults (p = 0.0006, adult control compared to isolation) showed increased Iba1 intensity following isolation, in addition to differences between naïve, non-isolated controls (p = 0.001, adult control compared to adolescent control). Isolation did not alter microglial skeleton complexity (age × condition two-way ANOVA, largest F: main effect of age F(1,46) = 1.943, p = 0.170; Fig. 2B). There were differences in the summed process length following isolation (age × condition two-way ANOVA, interaction F(1, 42) = 8.665, p = 0.005; but no effect of condition F(1,42) = 0.010, p = 0.921, or age F(1,42) = 0.167, p = 0.685; Fig. 2C). Post hoc comparisons found a significant increase in adolescent (p = 0.043) and a decrease in adult (p = 0.044) summed process length. However, there were no differences in average process length following isolation (largest F: main effect of condition F(1,42) = 0.790, p = 0.379; Fig. 2D). These results suggest that 2-hour isolation engages ACC microglia, indicated by increased Iba1 intensity, but in a manner that does not consistently produce morphological changes at this time point. Because Iba1 is a calcium-binding protein involved in many microglial functions, Iba1 intensity can be an indication of a functional change, even if not associated with a broad change in state, or indication of microglial activation that precedes morphological measures (Komorowska-Müller, Rana, Olabiyi, Zimmer, & Schmöle, 2021; Yang et al., 2013). Together, these results suggest that there are changes in microglia Iba1 proteins with isolation but not necessarily global morphological changes, indicative of increased microglial activity that do not lead to a shift in functional state at this time point.
3.2. Isolation increases Iba1 intensity in the adult but not adolescent prelimbic cortex
We next assessed Iba1 intensity in the PL. A 2 (Age: Adolescent, Adult) × 2 (Condition: No Isolation, Isolation) ANOVA found a main effect of age, F(1, 29) = 9.727, p = 0.004, but no interaction, F(1, 29) = 1.549, p = 0.223, and no effect of condition, F(1, 29) = 1.309, p = 0.262, (Fig. 3A). Isolation did not impact Iba1 intensity in adolescence (p = 0.908, adolescent control compared to isolation) but increased Iba1 intensity in adults (p = 0.0001, adult control compared to isolation). Isolation did not significantly alter skeleton complexity (largest F: main effect of age F(1,29) = 2.742, p = 0.109; Fig. 3B), summed process length (largest F: interaction F(1,29) = 3.945, p = 0.057; Fig. 3C), or average process length (largest F: main effect of age F(1,29) = 3.160, p = 0.086; Fig. 3D). Unlike the ACC, isolation does not substantially influence Iba1 intensity or morphology in PL. These results suggest that microglia in cortical brain regions critical for social behavior are not necessarily uniformly impacted, where microglia in the PL may not be as sensitive at a 2-hr time point as seen in the ACC.
Fig. 3.
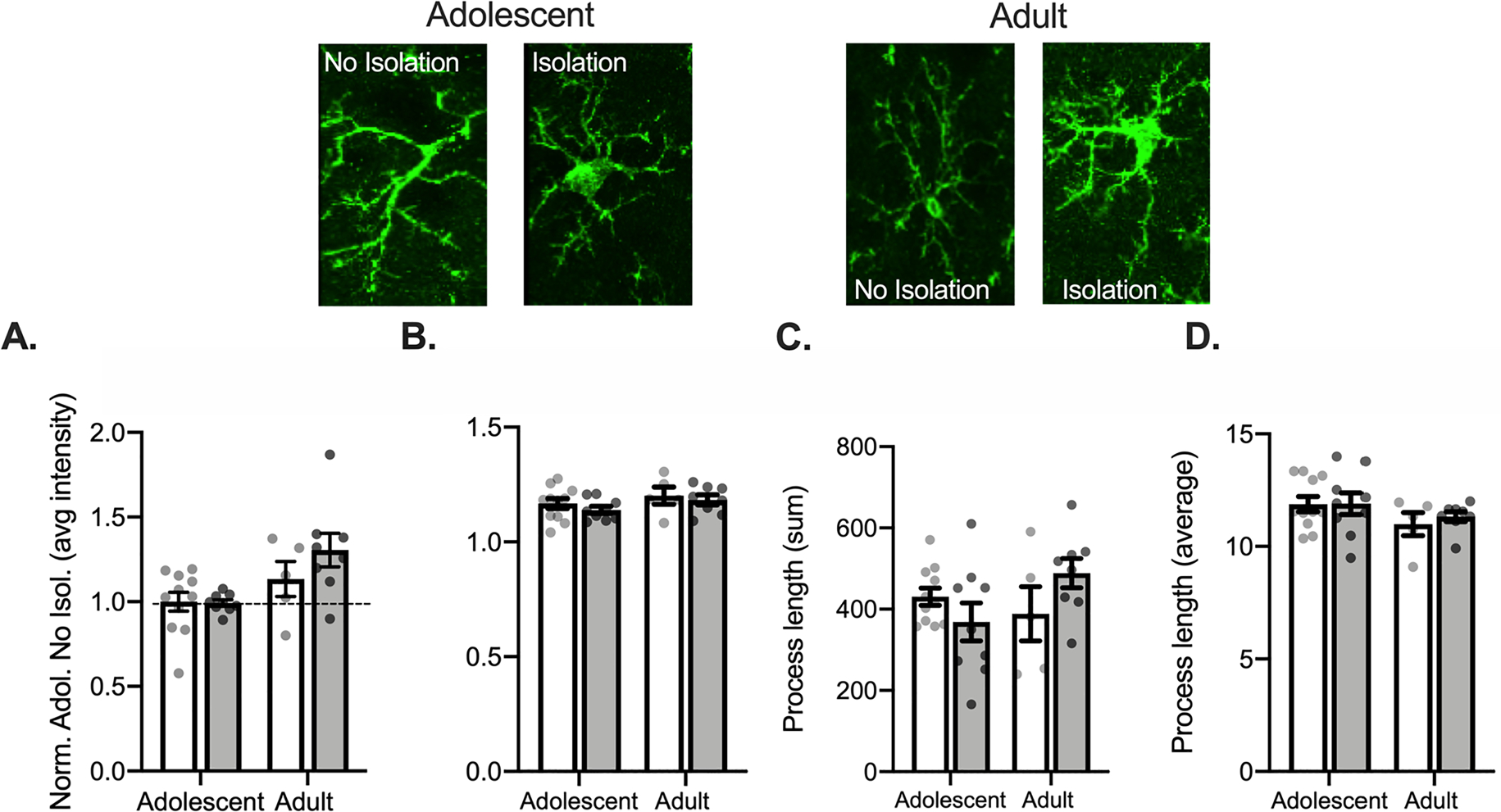
Isolation increases Iba1 intensity in the adult but not adolescent PL. Representative Iba1 images are displayed above graphs. There were no significant differences in Iba1 intensity (A), Iba1 complexity (B), total process length (C), or in the average process length (D) between isolated and non-isolated groups. Non-Isolated adolescent n = 4, 11 bilateral slices averaged; Isolated adolescent n = 3, 9 bilateral slices averaged for intensity; Non-Isolated adult n = 3, 5 bilateral slices averaged; Isolated adult n = 3, 11 bilateral slices averaged. *p < 0.05.
3.3. Isolation differentially alters Iba1 intensity and complexity in amygdala subregions between adults and adolescents.
In the lateral division of the BLA, a 2 (Age: Adolescent, Adult) × 2 (Condition: No Isolation, Isolation) ANOVA conducted to assess Iba1 intensity (Fig. 4A) found an effect of condition (F(1,17) = 13.62, p = 0.002) and a main effect of age (F(1,17) = 21.53, p = 0.0002), but no interaction (F(1,17) = 2.385, p = 0.1409). Post hoc comparisons show that isolation increased adolescent Iba1 intensity (p = 0.007, adolescent control compared to isolation) but not adult Iba1 intensity (p = 0.204, adult control compared to isolation). While there were differences in Iba1 intensity, there were not changes in skeleton complexity with isolation (age × condition two-way ANOVA; largest F: main effect of age F(1,26) = 3.618, p = 0.068; Fig. 4B). We next quantified process length. Isolation altered the total process length (age × condition ANOVA, main effects of age, F(1,27) = 12.83, p = 0.001, main effect of condition F(1,27) = 8.429, p = 0.007, no interaction F(1,27) = 0.153, p = 0.699; Fig. 4C). Post hoc comparisons show that total process length increased following isolation in adults (p = 0.005, adult control compared to isolation), but not adolescents (p = 0.148, adolescent control compared to isolation). There were no changes with the average process length in the lateral amygdala (age by condition ANOVA; largest F: interaction F(1,27) = 2.722, p = 0.111; Fig. 4D). Increased Iba1 in adolescents is consistent with engagement of microglia in adolescents after isolation. In contrast, the changes in adults are consistent with little change or a small shift away from a pro-inflammatory state towards a state of surveillance, indicated by increased process length in adults.
In the basal division of the BLA, isolation significantly impacted Iba1 intensity (age × condition two-way ANOVA, main effect of age, F(1,17) = 5.898, p = 0.027; main effect of condition, F(1,17) = 14.21, p = 0.002; no interaction, F(1,17) = 0.496, p = 0.491; Fig. 4E). Post hoc comparisons showed that isolation increased Iba1 intensity in both the adolescent (p = 0.020, adolescent control compared to isolation) and modestly in adults (p = 0.053, control compared to isolation) basal amygdala. When measuring skeleton complexity, a 2 (Age: Adolescent, Adult) × 2 (Condition: No Isolation, Isolation) ANOVA showed a significant interaction following isolation F(1,37) = 10.04, p = 0.003, with no main effects (largest F: age F(1,37) = 2.728, p = 0.107; Fig. 4F). Post hoc comparisons found that isolation increased skeleton complexity in adolescents (p = 0.038, adolescent control compared to isolation), but decreased skeleton complexity in adults (p = 0.024, adult control compared to isolation). Isolation also impacted total process length in the basal amygdala (age × condition two-way ANOVA, interaction F(1,34) = 5.861, p = 0.021; no significant main effects, largest F: main effect of condition F(1,34) = 2.189, p = 0.148; Fig. 4G) and the average process length (no interaction, F(1, 34) = 2.697, p = 0.110, but main effects of age F(1, 34) = 47.05, p < 0.0001, and condition F(1, 34) = 11.48, p = 0.002; Fig. 4H). Post hoc comparisons showed that isolation increased both adolescent total process length (p = 0.0146, control compared to isolation) and average process length (p = 0.002, control compared to isolation), but not adult total process length (p = 0.476, control compared to isolation) or average process length (p = 0.190, control compared to isolation). Together, these results indicate sensitivity of microglia within the basal region of the BLA to brief changes to the social environment. Increases in Iba1 intensity indicate that microglial activation is increased across age groups, but opposing changes in complexity indicate different activation states toward in which microglia may be shifted. We found increases in complexity and process length measures in adolescents, while reductions in complexity may indicate microglia shift toward a pro-inflammatory state in adults.
3.4. Microglial depletion interferes with effects of social isolation in adolescents
Brief isolation can transiently increase social engagement, and this brief isolation may also impact learning processes that require social engagement. Social recognition memory formation is one such process that requires social engagement. This provides an opportunity to test if the effects of social isolation on microglia are involved in behavioral effects of social isolation. Therefore, we next tested the role of isolation-related changes in microglia on social interaction and subsequent recognition. To test this, adults and adolescents were fed PLX3397 (PLX), to deplete microglia, or a control diet for eight days before social interaction measures. To verify prior reports of minimal effects of PLX diet on locomotion and exploration, behavior in an open field was measured. A 2 (Diet: Control, PLX) × 2 (Condition: No Isolation, Isolation) ANOVA was used to assess exploration in an open field apparatus, measured as time in center of the field. In adolescents, there were no differences between groups in center exploration during open field testing (largest F: interaction, F(1,40) = 1.474, p = 0.232; Fig. 5A).
Fig. 5.
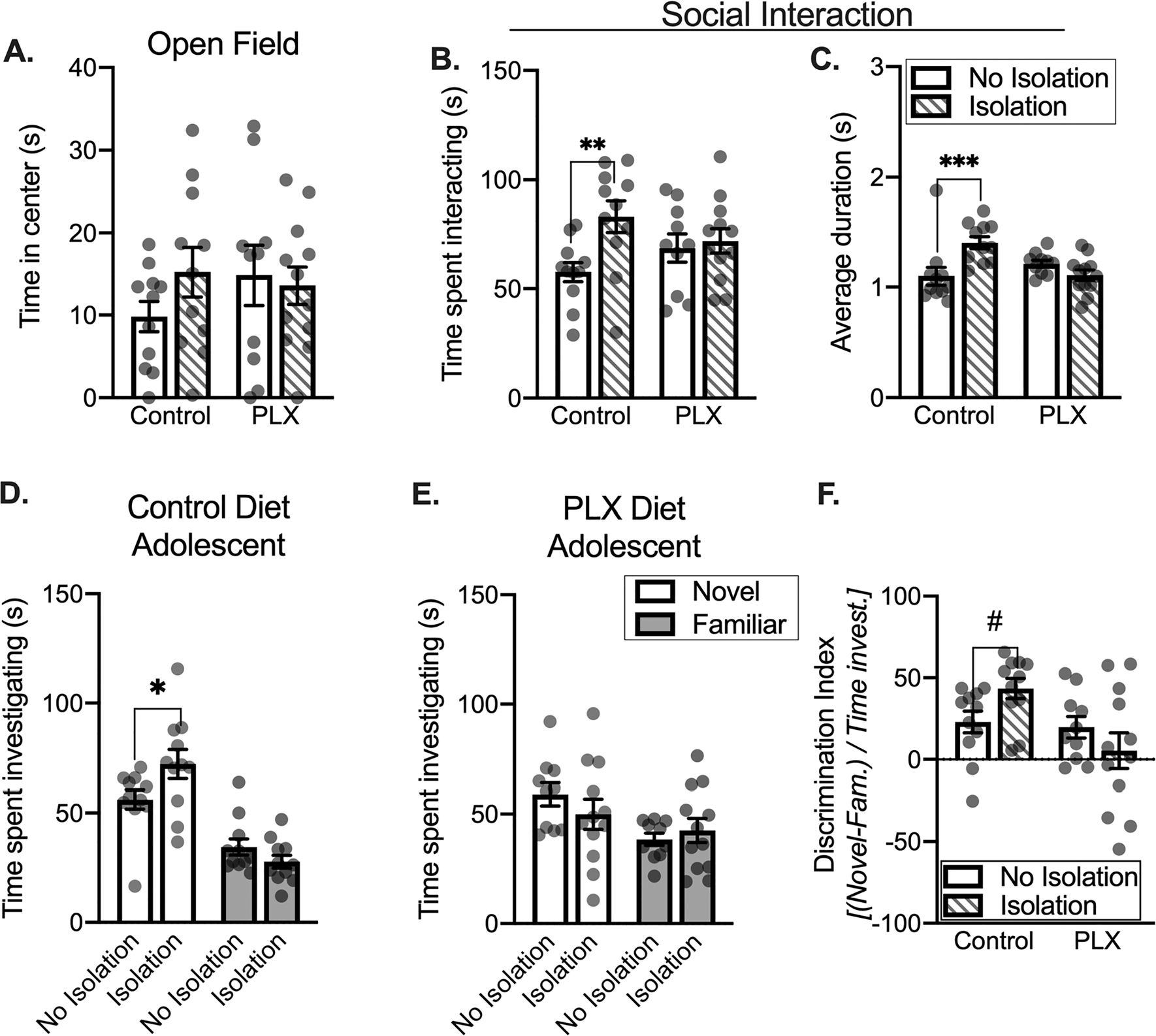
Microglial depletion interferes with effects of social isolation in adolescents. Isolation did not impact time in the center of the open field apparatus (A). Isolation increases time spent interacting with an age-matched partner in the control group (B). Isolated controls show a significant increase in the average duration of each social interaction relative to non-isolated controls (C). During a social recognition test, isolated control adolescents spend a greater amount of time investigating a novel partner (D). PLX groups do not show isolation-dependent changes in social investigation (E). When comparing each group to one another, isolated controls show a greater preference for novel partner relative to PLX groups (F). Control diet: No isolation n = 11, Isolation n = 11; PLX diet: No isolation n = 10, Isolation n = 12. # = 0.08, *p < 0.05, **p < 0.01.
Using a two-way ANOVA, we investigated the impact of isolation on social interaction in control and PLX groups. We found main effect of condition (F(1,40) = 5.742, p = 0.0213), but no main effect of diet (F < 1, p = 0.9993) and no interaction (F(1,40) = 3.437, p = 0.071). Similar to our prior work, brief isolation increased social interaction in adolescents (p = 0.0045 post hoc comparison between control diet isolated and non-isolated; Fig. 5B). We also quantified average duration of social interaction as a secondary measure (Fig. 5C). Isolation impacted the average duration of adolescent social interaction bouts (two-way ANOVA interaction F(1,40) = 12.50, p = 0.0010; no main effect of diet F(1,40) = 2.415, p = 0.128; no main effect of condition F(1,40) = 2.947, p = 0.0938). Isolation increased the average duration of each interaction in control diet animals (p = 0.0006, control compared to isolation) but not PLX diet animals (p = 0.207). Thus, in adolescents, microglial depletion does not impair baseline social interaction, but microglia are needed for the effects of brief isolation on social interaction.
Adolescent social recognition was measured as preference for a novel relative to a familiar conspecific using a 2 (target: Novel, Familiar) × 2 (Condition: No Isolation, Isolation) ANOVA. In control diet groups we found a significant interaction (F(1,20) = 5.783, p = 0.026) and a main effect of target F(1,20) = 53.20, p < 0.0001; Fig. 5D), but no main effect of condition (F(1,20) = 1.077, p = 0.312). Isolation further increased the time spent investigating a novel partner relative to non-isolated controls (p = 0.018, non-isolation control compared to isolation). While in PLX adolescent groups, we found a main effect target (F(1,20) = 4.539, p = 0.0457), we did not find a main effect of condition (F(1,20) = 0.328, p = 0.573) or an interaction (F(1,20) = 1.002, p = 0.329). There were no differences between isolated an non-isolated PLX groups for novel (p = 0.257) or familiar (p = 0.604) partner investigation (Fig. 5E). To more finely assess the impact of isolation and PLX, a discrimination index was used for a within-subjects metric of novel or familiar partner preference, with positive numbers reflecting greater novel partner preference and negative numbers indicating greater familiar partner preference (Fig. 5F). A 2 (diet: Control, PLX) × 2 (Condition: No Isolation, Isolation) ANOVA found a significant interaction (F1,40) = 4.603, p = 0.038) and a significant main effect of diet (F(1,40) = 6.434, p = 0.015), but no main effect of condition (F(1,40) = 0.146, p = 0.704). Isolation modestly increased novel partner preference in control diet groups (p = 0.081, control diet isolation group compared to control diet no isolation group). We also compared discrimination index values using a one sample t-test to assess changes in recognition of each group. Adolescent non-isolated control, t(10) = 3.419, p = 0.0066, adolescent isolated control, t(10) = 7.036, p < 0.0001, and non-isolated PLX, t(9) = 2.976, p = 0.0155, rats significantly differed from 0 (indicating equivalent familiar and novel partner investigation time). However, the adolescent isolated PLX, t(11) = 0.4927, p = 0.632, group did not differ from 0. These data suggest that microglial depletion in adolescents reduces isolation-driven changes in novel partner investigation, and subsequent novelty preference during social recognition.
3.5. Microglial depletion reduces the effects of social isolation in adults
Isolation had different effects on microglia in adults and adolescents (above). We next investigated if there were different effects of isolation on social interaction and social recognition, and the role of microglia, in adults. Adult cohorts were compared using a 2 (Diet: Control, PLX) × 2 (Condition: No Isolation, Isolation) ANOVA. There were no differences in center time exploration in the open field (no interaction: F(1,34) = 0.0036, p = 0.953; no main effect of diet: F(1,34) = 0.166, p = 0.686; no main effect of condition: F(1,34) = 0.363, p = 0.551; Fig. 6A). Therefore, similar to adolescents, microglial depletion had no gross effects on exploration.
Fig. 6.
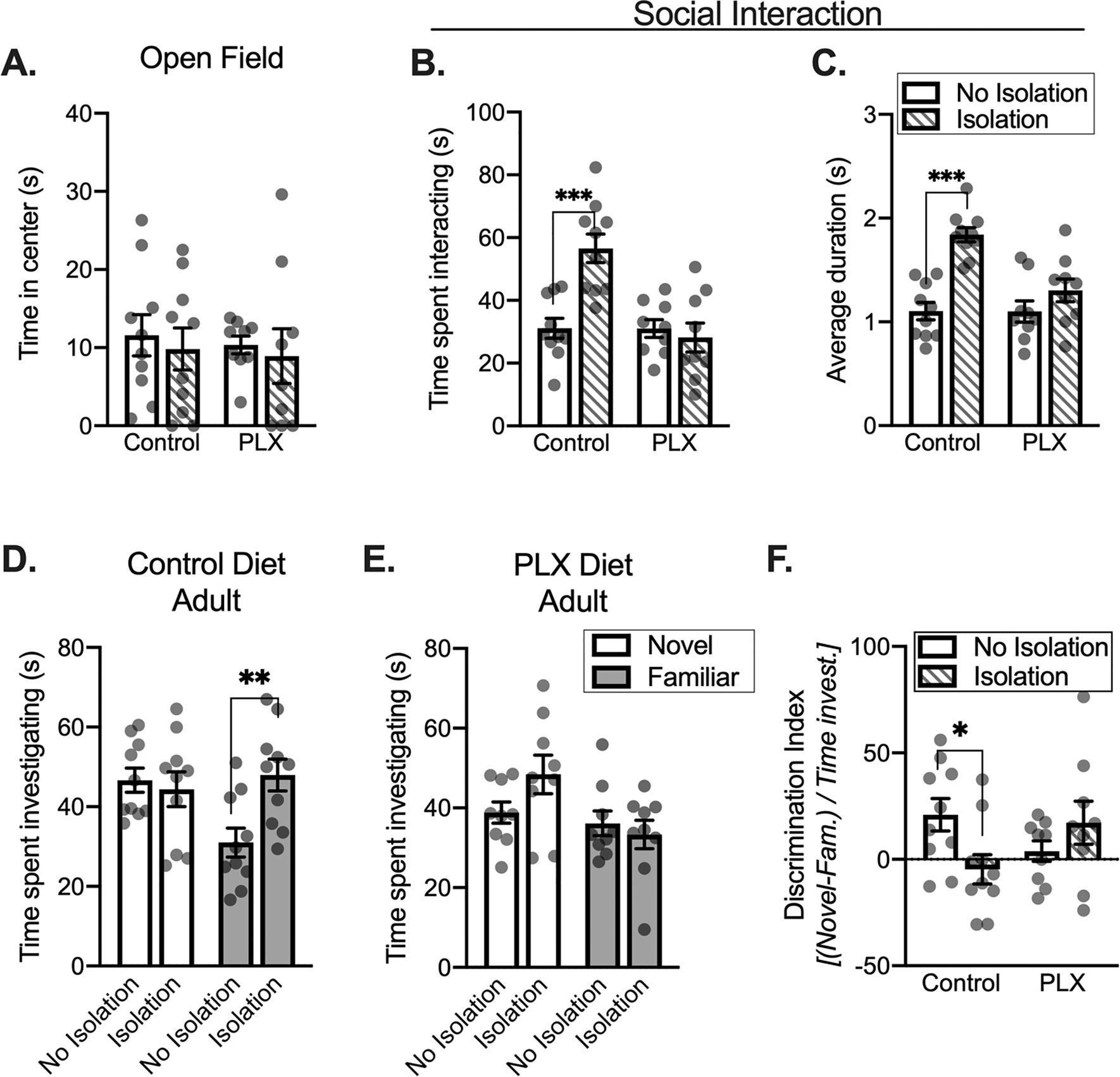
Microglial depletion reduces the effects of social isolation in adults. There were no differences between groups for the time in the center of the open field apparatus (A). Isolation increases time spent interacting between control diet conditions (B). Isolated controls have a greater average duration of each social interaction relative to non-isolated controls (C). During a social recognition test, isolated control adults spend a more time investigating a familiar partner (D). PLX groups do not show isolation-dependent changes in novel or familiar partner investigation during social recognition testing (E). Using a discrimination index, isolated controls show a relatively equivalent preference for novel and familiar partners relative to non-isolated controls groups (F). Control diet: No isolation n = 10, Isolation n = 10; PLX diet: No isolation n = 9, Isolation n = 9. *p < 0.05, **p < 0.01, ***p < 0.001.
When measuring the impact of isolation and microglial depletion on social interaction, a condition × diet two-way ANOVA revealed an interaction (F(1,34) = 13.19, p = 0.0009), a main effect of condition (F(1,34) = 8.43, p = 0.006), and a main effect of diet (F(1,34) = 13.34, p = 0.0009, Fig. 6B). Consistent with our previous work, brief isolation significantly increased total time spent interacting in control rats (p < 0.0001 post hoc control diet non-isolated compared to control diet isolated). However, PLX diet disrupted this effect of isolation, where PLX groups did not show isolation-driven increases the time spent interacting (p = 0.619, PLX non-isolated rats compared to PLX isolated rats). Similar to total time spent interacting, we also found significant main effects of diet (F(1,34) = 8.734, p = 0.0056) and condition (F(1,34) = 26.6, p < 0.0001) as well as an interaction (F(1,34) = 8.458, p = 0.0064) for the average duration of each interaction (Fig. 6C). While brief isolation significantly increased the average duration of each interaction bout in control diet conditions (p < 0.0001, control diet non-isolation compared to isolation rats), this was disrupted by PLX such that there was no longer an effect of isolation on average bout duration (p = 0.1303, PLX diet non-isolated compared to PLX diet isolated rats).
Subsequent social recognition was assessed with a 2 (target: Novel, Familiar) × 2 (Condition: No Isolation, Isolation) ANOVA of the time spent investigating a novel or familiar partner 30-minutes after the social interaction. When measuring the impact of isolation on novel and familiar partner investigation in control diet groups, we found a main effect of condition (F(1,18) = 5.329, p = 0.033) and an interaction (F(1,18) = 4.971, p = 0.039), but no main effect of target (F(1,18) = 1.971, p = 0.177, Fig. 6D). In control diet groups, there was more time investigating a novel partner than a familiar partner, indicating social recognition. However, unlike adolescents, isolation increased the time spent investigating a familiar partner in adults (p = 0.003, non-isolated compared to isolated rats), but did not change the time spent investigating a novel partner (p = 0.674). Microglial depletion disrupted the effects of isolation on investigation during this test (PLX diet groups, largest F: main effect of target F(1,16) = 3.954, p = 0.064; Fig. 6E). Preference index was compared between adult groups using a 2 (diet: Control, PLX) × 2 (Condition: No Isolation, Isolation) ANOVA. With this ANOVA, we found a significant interaction (F(1,34) = 6.554, p = 0.015), but no main effect of diet (F(1,34) = 0.098, p = 0.756) or condition (F(1,34) = 0.655, p = 0.424, Fig. 6F). Preference for a novel partner was disrupted by isolation (p = 0.020, post hoc comparison between preference in control rats compared to preference in isolation rats) but there were no differences between PLX treated groups (p = 0.236). We next used a one sample t-test to quantify changes in social recognition memory of each groups, where adult non-isolated control, t(9) = 2.747, p = 0.023, and isolated PLX, t(8) = 2.442, p = 0.041, rats significantly differed from 0 (equivalent familiar and novel partner investigation time). This is in contrast to adult isolated control, t(9) = 0.837, p = 0.424, and non-isolated PLX, t(8) = 0.796, p = 0.449, groups, which did not differ from 0.
4. Discussion
The present results demonstrate age- and region-specific effects of social isolation on microglial activity and morphology. While isolation increases Iba1 intensity in cortical regions as well as in the amygdala, changes in complexity and branching are only evident within the BLA immediately following brief isolation. Further, we found that microglial depletion prevents isolation-driven increases in social interaction in both age groups. While traditionally, rodents spend a greater proportion of time investigating novel partner after socially interacting, isolation has opposing effects on social recognition in adults and adolescent. Isolation reduced novelty preference in adults (demonstrated via increases in time spent investigating a familiar partner) but facilitated this in adolescents (demonstrated via increases in time spent investigating a novel partner). Microglial depletion reduced effects of isolation on recognition in both age groups. Suggesting that microglial-related changes during isolation influence social interaction as well as recognition in adults and adolescents.
Microglial morphology indexed with Iba1 change with a variety of environmental conditions that impact social behavior, like stress exposure. Here, we found that brief changes to the social environment increased Iba1 intensity in a number of different brain regions. Iba1 function and expression is activity-dependent, often due to increased intracellular calcium when microglia respond to activating stimuli (Ohsawa et al., 2000). While the current results do not test the exact function of isolation-driven alterations in Iba1 intensity, prior work has demonstrated that Iba1 interacts with cytoskeletal protein, actin (Sasaki et al., 2001). It is possible that increases in Iba1 intensity as a result of brief isolation are due to increase microglial calcium influx, leading to rapidly increased expression that subsequently drives morphological changes through interactions with actin. Here, increased Iba1 expression is a useful marker that microglia are responding, and possibly contributing to changes in the function of specific brain regions. In cortical brain regions, morphological changes may occur at later time points while basal amygdala microglia may be more sensitive to brief changes in the social environment. In line with this, we found alterations in microglial complexity and process length in adolescent basal amygdala. These alterations in microglia correspond with increases in microglial surveillance of the neural environment to maintain homeostasis (Carvalho-Paulo & Neto, 2021; Orr et al., 2009). Increases in microglial extension are regulated by AMPA and NMDA receptors, where glutamatergic neurotransmission can increase microglial process length and receptor antagonism can decrease process length (Fontainhas et al., 2011). Prior work has shown that this same brief isolation increases BLA neuronal activity (Ferrara et al., 2021). It is possible that isolation-driven increases in adolescent microglial complexity and process length are a result of interaction with active neurons. While adolescent microglia increase in complexity within the basal amygdala, microglial complexity is reduced in isolated adults. This reduction in complexity without change in process length suggest that more subtle changes in microglia occur during isolation in adults. Future work should investigate how brief isolation alters microglial interactions with neurons, particularly those activated by isolation.
Previous work has demonstrated that changes in activity within the ACC and BLA can regulate social behavior, and that BLA neuronal activity can increase following brief isolation to facilitate social interaction (Ferrara et al., 2021b; Jeon et al., 2010; Varlinskaya, Vogt, & Spear, 2013). Because we saw microglial changes following isolation in brain regions sensitive to changes in social circumstances, we next depleted microglia in adolescents and adults to understand the influence on social interaction and recognition. In control groups, brief isolation increased social interaction across ages; however, this was blocked with microglial depletion. Recent work has demonstrated that isolation over the course of days can impair social recognition memory in adults (Leser & Wagner, 2015; Shahar-Gold, Gur, & Wagner, 2013). We found that 2-hour isolation also impaired recognition in adults fed a control diet. Although microglial depletion had weaker effects in adults, and tended towards impairing social recognition, it partially rescued the impairing effects of social isolation on social recognition. One interpretation is that microglial activity in adults contributes to increased novelty preference during a social recognition test in control adults, but a change in social state driven by isolation can produce a different microglia-dependent effect that acts in opposition to novelty preference during a social recognition test.
In adolescents, we found an opposite effect of isolation on social investigation during recognition testing. Here, brief isolation facilitated time spent investigating a novel partner during social recognition testing and microglial depletion reduced this enhancing effect of isolation. This suggests that microglia in adolescents contribute to initial increased time spent in social investigation during a heightened social drive state that leads to subsequent increased preference for a non-familiar partner during social recognition testing. This interpretation links increases in a social drive state after isolation that then leads to greater social interaction during a familiarization phase. This would be expected to produce better encoding and subsequent recall of the familiarity of this partner to promote novel partner preference during testing, consistent with other forms of social learning that are directly correlated with social interaction (Twining et al., 2017; Yusufishaq & Rosenkranz, 2013). We favor this interpretation for two reasons. First, the social drive state produced by brief social isolation is transient and can be sated with as little as 15 min of interaction (Latane, Nesbitt, Eckman, & Rodin, 1972). In the current set of experiments, rats have the opportunity to socially engage both during the familiarization (training) phase and when returned to the home cage environment between training and testing prior to testing. Second, the average duration spent socially interacting mirrored the effects of brief isolation on novel partner preference during recognition testing in adolescents, with both average duration and novel partner preference increased in isolated groups. This suggests that isolation increased the adolescent social drive state, leading to improved social recognition at testing. This is in contrast to adults, where a high social drive state after isolation also increased the average duration of social interaction bouts, but this was associated with weaker preference for a novel partner during social recognition testing. It is unclear if this might be due to effects of social isolation that might influence social encoding that counter the formation of social recognition memory. While the behavioral mechanisms underlying opposing changes in social recognition memory between ages may be unclear, the corresponding isolation-related changes in microglial morphology within the BA implies a role for microglia in adult and adolescent high social drive states, where opposite changes in complexity may be reflect different microglial contributions that underlie opposing effects on social recognition.
The effects of social isolation on social recognition memory are opposite in adults and adolescents. In parallel, there are opposite effects of brief isolation on microglia in the basal subregion of the BLA with an increase in complexity in adolescents but a significant reduction in adults. Isolation similarly increases Iba1 intensity in the ACC region of mPFC in both adults and adolescents and may impact complexity at different time points following isolation. Synaptic inputs from the mPFC to the BLA increase in strength across development, are pruned, and increase their ability to recruit inhibitory control over the BLA between adolescence and adulthood (Arruda-Carvalho, Wu, Cummings, & Clem, 2017; Cressman et al., 2010; Selleck, Zhang, Samberg, Padival, & Rosenkranz, 2018). It is possible that as the cortical-amygdala circuits mature, the impact on BLA microglia diverges, and social behavior shifts, suggesting that microglial regulation of social behavior changes alongside brain maturation to differentially influence social memory.
Together, we provide evidence that social drive states induced by brief social isolation facilitates social interaction and has bidirectional age-dependent effects on social recognition. Isolation-dependent changes in microglia demonstrate their sensitivity to the social environment across ages, but may differentially contribute to social memory (Fig. 7). In adolescents, microglia contribute to process that increase social recognition but in adults the role of microglia depends on social state, where high social drive states can lead to a microglia state that opposes processes that contribute to social recognition. Overall, these results demonstrate distinct functional roles of microglia in social memory between adults and adolescents, and suggest that states of microglial activation, such as inflammation, may produce different effects on social behavior and memory across ages. Future work should investigate the impact of puberty as well as sex differences in microglial-mediated social recognition memory. The current work spans a postnatal window that encompasses early stages of puberty in male rats (Schneider, 2013). Puberty is partly defined by the beginning of hormonal changes that influence a number of different behaviors, although peak male testosterone levels are not achieved until near PND 70, not within the peripubertal time window examined here (Folib et al., 2011; Pignatelli, Xiao, Gouveia, Ferreira, & Vinson, 2006). Puberty also influences physiological responses to stress, and may therefore impact physiological responses to brief changes in the social environment (Folib et al., 2011). Additionally, the adolescent time period is characterized by sex differences in the impact of isolation on memory. For instance, isolation promotes social conditioned place preference in adolescent males but not females, and isolation has differential age-dependent effects on novel object induced conditioned place preference (Douglas, Varlinskaya, & Spear, 2003; Douglas, Varlinskaya, & Spear, 2004). We have previously demonstrated that social play increases with brief isolation in male adolescents, but other work has demonstrated that the nature of social play varies between males and females, with males showing increased instances of play-fighting behavior (Ferrara et al., 2021a; Pellis and Pellis, 1990). Based on this, isolation may serve to improve social recognition memory through facilitation of social play-fighting behavior, as this is more commonly seen in adolescent males and is increased with brief 2-hour isolation. It is critical for future work to link alterations in microglial-mediated changes in social recognition over the course of development with puberty in males and females, as this may shed light on specific social behaviors and maturational changes that influence opposing effects of social recognition memory seen in the present study.
Fig. 7.
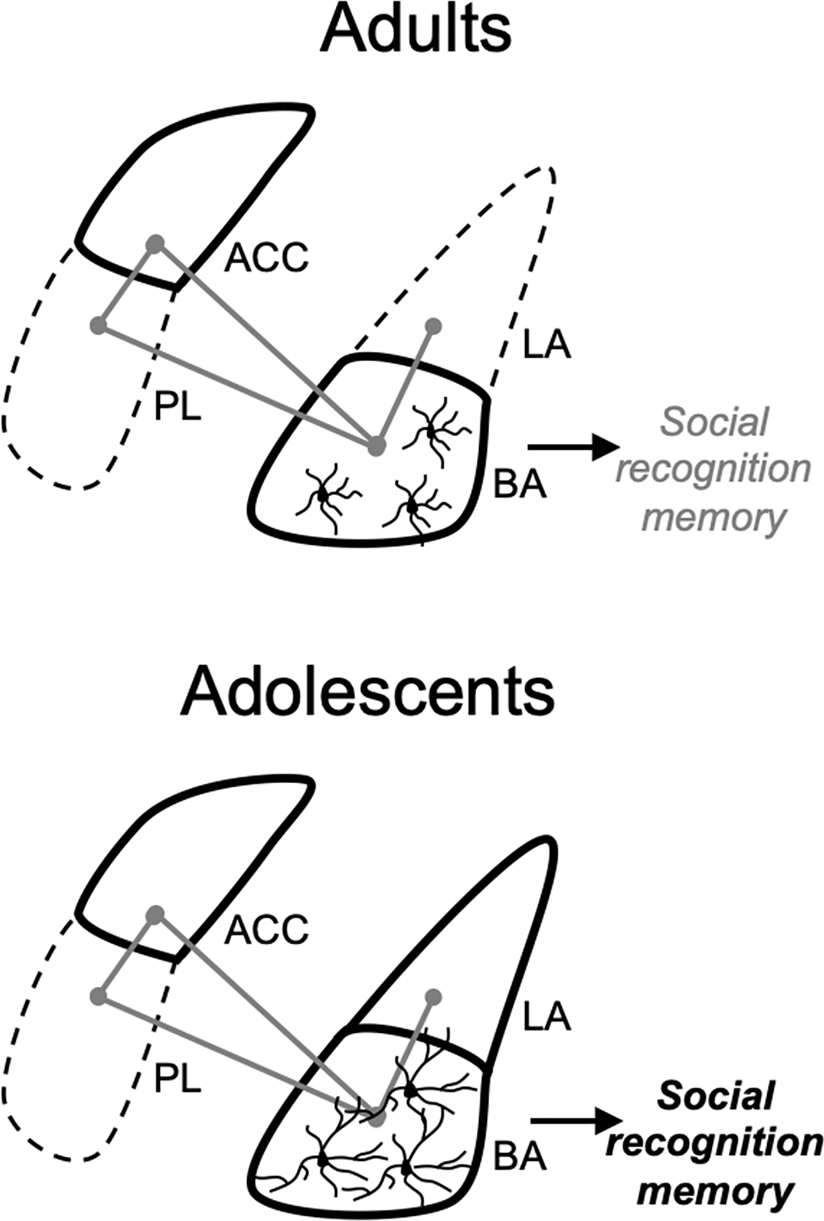
Proposed model. Brief social isolation increases social drive in both of adults and adolescents. Social isolation increases Iba1 intensity in cortical and amygdala subregions known to have a role in social behaviors. It produces a uniform increase in the ACC and BA in adults and adolescents, but adult-only increases in the PL and adolescent-only increase in LA (solid outline for isolation driven increases in Iba1 intensity). Increases in Iba1 intensity may correspond to recruitment of microglia that promote engagement of a social neural network to facilitate social interaction (projections and connectivity indicated with gray lines between brain regions). While there are similar increases in Iba1 intensity, there are distinct changes in microglia complexity between ages in the BA with, increases in complexity in adolescents and decreases in complexity in adults. These opposing changes in microglia complexity within the BA also correspond to opposing changes in social recognition memory between age groups. Therefore, recruitment of microglia across brain regions sensitive to social experiences may be critical for facilitation of social behavior (promoting a social drive state), while distinct changes in complexity within the BA may guide subsequent recognition memory.
Acknowledgements
This work was supported by National Institute of Health (NIH) grants: MH118237, MH109484 (JAR), MH069558 (FJH), and F32MH122092 (NCF).
Footnotes
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
CRediT authorship contribution statement
Nicole C. Ferrara: Conceptualization, Methodology, Data curation, Writing – original draft, Writing – review & editing. Sydney Trask: Data curation, Writing – review & editing. Lily Yan: Data curation. Mallika Padival: Data curation. Fred J. Helmstetter: Writing – review & editing. J. Amiel Rosenkranz: Conceptualization, Methodology, Writing – review & editing.
References
- Allsop SA, Wichmann R, Mills F, Burgos-Robles A, Chang CJ, Felix-Ortiz AC, … Tye KM (2018). Corticoamygdala transfer of socially derived information gates observational learning. Cell, 173(6), 1329–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arruda-Carvalho M, Wu WC, Cummings KA, & Clem RL (2017). Optogenetic examination of prefrontal-amygdala synaptic development. Journal of Neuroscience, 37(11), 2976–2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boelen E, Stassen FR, Steinbusch HW, Borchelt DR, & Streit WJ (2012). Ex vivo cultures of microglia from young and aged rodent brain reveal age-related changes in microglial function. Neurobiology of Aging, 33(1), 195–e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho-Paulo D, Bento Torres Neto J, de Oliveira TCG, de Sousa AA, dos Reis RR, dos Santos ZA, …, Picanço Diniz CW (2021). Microglial morphology across distantly related species: phylogenetic, environmental and age influences on microglia reactivity and surveillance states. Frontiers in immunology, 12, 2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cressman VL, Balaban J, Steinfeld S, Shemyakin A, Graham P, Parisot N, & Moore H (2010). Prefrontal cortical inputs to the basal amygdala undergo pruning during late adolescence in the rat. Journal of Comparative Neurology, 518(14), 2693–2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas LA, Varlinskaya EI, & Spear LP (2003). Novel-object place conditioning in adolescent and adult male and female rats: Effects of social isolation. Physiology & Behavior, 80(2–3), 317–325. [DOI] [PubMed] [Google Scholar]
- Douglas LA, Varlinskaya EI, & Spear LP (2004). Rewarding properties of social interactions in adolescent and adult male and female rats: Impact of social versus isolate housing of subjects and partners. Developmental Psychobiology: The Journal of the International Society for Developmental Psychobiology, 45(3), 153–162. [DOI] [PubMed] [Google Scholar]
- Elmore MRP, Najafi AR, Koike MA, Dagher NN, Spangenberg EE, Rice RA, … Green KN (2014). CSF1 receptor signaling is necessary for microglia viability, which unmasks a cell that rapidly repopulates the microglia-depleted adult brain. Neuron, 82(2), 380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrara NC, Mrackova E, Loh MK, Padival M, & Rosenkranz JA (2020). Fear learning enhances prefrontal cortical suppression of auditory thalamic inputs to the amygdala in adults, but not adolescents. International Journal of Molecular Sciences, 21(8), 3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrara NC, Trask S, Avonts B, Loh MK, Padival M, & Rosenkranz JA (2021a). Developmental shifts in amygdala activity during a high social drive state. Journal of Neuroscience, 41(45), 9308–9325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrara NC, Trask S, & Rosenkranz JA (2021b). Maturation of amygdala inputs regulate shifts in social and fear behaviors: A substrate for developmental effects of stress. Neuroscience & Biobehavioral Reviews. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay JM, Dunham GA, Isherwood AM, Newton CJ, Nguyen TV, Reppar PC, … Greene RW (2015). Effects of prefrontal cortex and hippocampal NMDA NR1-subunit deletion on complex cognitive and social behaviors. Brain Research, 1600, 70–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folib Allison, Lui Patina, & Romeo, Russell D (2011). The transformation of hormonal stress responses throughout puberty and adolescence. Journal of Endocrinology, 210 (3), 391–398. 10.1530/JOE-11-0206 [DOI] [PubMed] [Google Scholar]
- Fontainhas AM, Wang M, Liang KJ, Chen S, Mettu P, Damani M, … Wong WT (2011). Microglial morphology and dynamic behavior is regulated by ionotropic glutamatergic and GABAergic neurotransmission. PloS One, 6(1), e15973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanamsagar R, & Bilbo SD (2017). Environment matters: Microglia function and dysfunction in a changing world. Current Opinion in Neurobiology, 47, 146–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanisch UK (2002). Microglia as a source and target of cytokines. Glia, 40(2), 140–155. [DOI] [PubMed] [Google Scholar]
- Hänninen Laura, & Pastell Matti (2009). CowLog: Open-source software for coding behaviors from digital video. Behavior research methods, 41(2), 472–476. [DOI] [PubMed] [Google Scholar]
- Hinwood M, Morandini J, Day TA, & Walker FR (2012). Evidence that microglia mediate the neurobiological effects of chronic psychological stress on the medial prefrontal cortex. Cerebral Cortex, 22(6), 1442–1454. [DOI] [PubMed] [Google Scholar]
- Huang WC, Zucca A, Levy J, & Page DT (2020). Social behavior is modulated by valence-encoding mPFC-amygdala sub-circuitry. Cell Reports, 32(2), 107899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito D, Imai Y, Ohsawa K, Nakajima K, Fukuuchi Y, & Kohsaka S (1998). Microglia-specific localisation of a novel calcium binding protein, Iba1. Molecular Brain Research, 57(1), 1–9. [DOI] [PubMed] [Google Scholar]
- Jeon D, Kim S, Chetana M, Jo D, Ruley HE, Lin SY, … Shin HS (2010). Observational fear learning involves affective pain system and Ca v 1.2 Ca 2+ channels in ACC. Nature Neuroscience, 13(4), 482–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karperien A, Ahammer H, & Jelinek H (2013). Quantitating the subtleties of microglial morphology with fractal analysis. Frontiers in Cellular Neuroscience, 7, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kercmar J, Büdefeld T, Grgurevic N, Tobet SA, & Majdic G (2011). Adolescent social isolation changes social recognition in adult mice. Behavioural Brain Research, 216(2), 647–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, Cho MH, Shim WH, Kim JK, Jeon EY, Kim DH, & Yoon SY (2017). Deficient autophagy in microglia impairs synaptic pruning and causes social behavioral defects. Molecular Psychiatry, 22(11), 1576–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klune CB, Jin B, & DeNardo LA (2021). Linking mPFC circuit maturation to the developmental regulation of emotional memory and cognitive flexibility. Elife, 10, e64567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komorowska-Müller JA, Rana T, Olabiyi BF, Zimmer A, & Schmöle AC (2021). Cannabinoid receptor 2 alters social memory and microglial activity in an age-dependent manner. Molecules, 26(19), 5984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koss WA, Belden CE, Hristov AD, & Juraska JM (2014). Dendritic remodeling in the adolescent medial prefrontal cortex and the basolateral amygdala of male and female rats. Synapse, 68(2), 61–72. [DOI] [PubMed] [Google Scholar]
- Latane B, Nesbitt P, Eckman J, & Rodin J (1972). Long- and short-term social deprivation and sociability in rats. Journal of Comparative and Physiological Psychology, 81(1), 69. [Google Scholar]
- Leser N, & Wagner S (2015). The effects of acute social isolation on long-term social recognition memory. Neurobiology of Learning and Memory, 124, 97–103. [DOI] [PubMed] [Google Scholar]
- Matthews GA, & Tye KM (2019). Neural mechanisms of social homeostasis. Annals of the New York Academy of Sciences, 1457(1), 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison H, Young K, Qureshi M, Rowe RK, & Lifshitz J (2017). Quantitative microglia analyses reveal diverse morphologic responses in the rat cortex after diffuse brain injury. Scientific Reports, 7(1), 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munshi S, Loh MK, Ferrara N, DeJoseph MR, Ritger A, Padival M, … Rosenkranz JA (2020). Repeated stress induces a pro-inflammatory state, increases amygdala neuronal and microglial activation, and causes anxiety in adult male rats. Brain, Behavior, and Immunity, 84, 180–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munshi Soumyabrata, & Rosenkranz J Amiel (2018). Effects of peripheral immune challenge on in vivo firing of basolateral amygdala neurons in adult male rats. Neuroscience, 390, 174–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson LH, & Lenz KM (2017). Microglia depletion in early life programs persistent changes in social, mood-related, and locomotor behavior in male and female rats. Behavioural Brain Research, 316, 279–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oosterhof N, Kuil LE, van der Linde HC, Burm SM, Berdowski W, van Ijcken WF, … van Ham TJ (2018). Colony-stimulating factor 1 receptor (CSF1R) regulates microglia density and distribution, but not microglia differentiation in vivo. Cell Reports, 24(5), 1203–1217. [DOI] [PubMed] [Google Scholar]
- Orr AG, Orr AL, Li XJ, Gross RE, & Traynelis SF (2009). Adenosine A 2A receptor mediates microglial process retraction. Nature Neuroscience, 12(7), 872–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohsawa K, Imai Y, Kanazawa H, Sasaki Y, & Kohsaka S (2000). Involvement of Iba1 in membrane ruffling and phagocytosis of macrophages/microglia. Journal of Cell Science, 113(17), 3073–3084. [DOI] [PubMed] [Google Scholar]
- Paxinos George, & Watson Charles (2007). The rat brain in stereotaxic coordinates: hard cover edition. Elsevier. [DOI] [PubMed] [Google Scholar]
- Pellis Sergio, & Pellis Vivien (1990). Differential rates of attack, defense, and counterattack during the developmental decrease in play fighting by male and female rats. The Journal of the International Society for Developmental Psychobiology, 23 (3), 215–231. [DOI] [PubMed] [Google Scholar]
- Pignatelli D, Xiao F, Gouveia AM, Ferreira JG, & Vinson GP (2006). Adrenarche in the rat. Journal of Endocrinology, 191(1), 301–308. [DOI] [PubMed] [Google Scholar]
- Rohan Walker F, Nilsson M, & Jones K (2013). Acute and chronic stress-induced disturbances of microglial plasticity, phenotype and function. Current Drug Targets, 14(11), 1262–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki Y, Ohsawa K, Kanazawa H, Kohsaka S, & Imai Y (2001). Iba1 is an actin-cross-linking protein in macrophages/microglia. Biochemical and Biophysical Research Communications, 286(2), 292–297. [DOI] [PubMed] [Google Scholar]
- Schneider Miriam (2013). Adolescence as a vulnerable period to alter rodent behavior. Cell and tissue research, 354(1), 99–106. [DOI] [PubMed] [Google Scholar]
- Selleck RA, Zhang W, Samberg HD, Padival M, & Rosenkranz JA (2018). Limited prefrontal cortical regulation over the basolateral amygdala in adolescent rats. Scientific Reports, 8(1), 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahar-Gold H, Gur R, & Wagner S (2013). Rapid and reversible impairments of short-and long-term social recognition memory are caused by acute isolation of adult rats via distinct mechanisms. PloS One, 8(5), e65085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres L, Danver J, Ji K, Miyauchi JT, Chen D, Anderson ME, … Tsirka SE (2016). Dynamic microglial modulation of spatial learning and social behavior. Brain, Behavior, and Immunity, 55, 6–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twining RC, Vantrease JE, Love S, Padival M, & Rosenkranz JA (2017). An intra-amygdala circuit specifically regulates social fear learning. Nature Neuroscience, 20(3), 459–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varlinskaya EI, Vogt BA, & Spear LP (2013). Social context induces two unique patterns of c-Fos expression in adolescent and adult rats. Developmental Psychobiology, 55(7), 684–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Zhu J, Zhu H, Zhang Q, Lin Z, & Hu H (2011). Bidirectional control of social hierarchy by synaptic efficacy in medial prefrontal cortex. Science, 334(6056), 693–697. [DOI] [PubMed] [Google Scholar]
- Wang YC, Ho UC, Ko MC, Liao CC, & Lee LJ (2012). Differential neuronal changes in medial prefrontal cortex, basolateral amygdala and nucleus accumbens after postweaning social isolation. Brain Structure and Function, 217(2), 337–351. [DOI] [PubMed] [Google Scholar]
- Wohleb ES, Fenn AM, Pacenta AM, Powell ND, Sheridan JF, & Godbout JP (2012). Peripheral innate immune challenge exaggerated microglia activation, increased the number of inflammatory CNS macrophages, and prolonged social withdrawal in socially defeated mice. Psychoneuroendocrinology, 37(9), 1491–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang TT, Lin C, Hsu CT, Wang TF, Ke FY, & Kuo YM (2013). Differential distribution and activation of microglia in the brain of male C57BL/6J mice. Brain Structure and Function, 218(4), 1051–1060. [DOI] [PubMed] [Google Scholar]
- Young K, & Morrison H (2018). Quantifying microglia morphology from photomicrographs of immunohistochemistry prepared tissue using ImageJ. Journal of Visualized Experiments: JoVE, (136). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yusufishaq S, & Rosenkranz JA (2013). Post-weaning social isolation impairs observational fear conditioning. Behavioural Brain Research, 242, 142–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan Y, Paolicelli RC, Sforazzini F, Weinhard L, Bolasco G, Pagani F, … Gross CT (2014). Deficient neuron-microglia signaling results in impaired functional brain connectivity and social behavior. Nature Neuroscience, 17(3), 400–406. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Xu H, Zhang F, Shao F, Ellenbroek B, Wang J, & Wang W (2019). Deficiencies of microglia and TNFα in the mPFC-mediated cognitive inflexibility induced by social stress during adolescence. Brain, Behavior, and Immunity, 79, 256–266. [DOI] [PubMed] [Google Scholar]
- Zhu F, Zhang L, Ding YQ, Zhao J, & Zheng Y (2014). Neonatal intrahippocampal injection of lipopolysaccharide induces deficits in social behavior and prepulse inhibition and microglial activation in rats: Implication for a new schizophrenia animal model. Brain, Behavior, and Immunity, 38, 166–174. [DOI] [PubMed] [Google Scholar]


