Abstract
Introduction
Opioid use disorders (OUDs) constitute a major public health issue, and we urgently need alternative methods for characterizing risk for OUD. Electronic health records (EHRs) are useful tools for understanding complex medical phenotypes but have been underutilized for OUD because of challenges related to underdiagnosis, binary diagnostic frameworks, and minimally characterized reference groups. As a first step in addressing these challenges, a new paradigm is warranted that characterizes risk for opioid prescription misuse on a continuous scale of severity, i.e., as a continuum.
Methods
Across sites within the PsycheMERGE network, we extracted prescription opioid data and diagnoses that co-occur with OUD (including psychiatric and substance use disorders, pain-related diagnoses, HIV, and hepatitis C) for over 2.6 million patients across three health registries (Vanderbilt University Medical Center, Mass General Brigham, Geisinger) between 2005 and 2018. We defined three groups based on levels of opioid exposure: no prescriptions, minimal exposure, and chronic exposure and then compared the comorbidity profiles of these groups to the full registries and to those with OUD diagnostic codes.
Results
Our results confirm that EHR data reflects known higher prevalence of substance use disorders, psychiatric disorders, medical, and pain diagnoses in patients with OUD diagnoses and chronic opioid use. Comorbidity profiles that distinguish opioid exposure are strikingly consistent across large health systems, indicating the phenotypes described in this new quantitative framework are robust to health systems differences.
Conclusion
This work indicates that EHR prescription opioid data can serve as a platform to characterize complex risk markers for OUD using existing data.
Keywords: Opioids, Prescription data, Substance use disorders, Electronic health records, Opioid use disorder
Introduction
The opioid epidemic is a significant public health challenge in the USA, with continued high rates of hospitalizations and mortality as a result of misuse of prescription and illicit opioids [1, 2]. We urgently need studies that characterize risk for development of opioid use disorders (OUDs), but characterizing OUD is extremely challenging. Notably, OUD evolves in a chronological fashion, starting with exposure to opioids and continuing through intermittent and regular use, development of physical dependence, misuse, and relapse [3, 4]. Prevalence estimates for these phenotypes vary widely, in part due to variation in ascertaining and defining them [5]. One key challenge to defining opioid use and misuse is the need to differentiate individuals across this spectrum of overlapping features or stages. Another challenge is the need to acquire large enough sample sizes to study these phenotypes.
Electronic health records (EHRs) offer novel solutions for capturing opioid use behaviors in real-world healthcare settings as they contain medical data relevant to OUD from large cohorts of patients. While illicit opioid use is difficult to capture in a medical setting, EHR provides rich data related to prescription opioid use, as well as other comorbidities, that can help further dissect the risk for developing OUD. However, characterizing OUD in EHR settings is not trivial. First, most OUD case definitions used to date rely on diagnostic codes, but this approach is problematic because OUD tends to be underdiagnosed [6]. Second, by focusing on a binary case:control framework, we may be missing the spectrum of severity associated with various opioid use behaviors, which can have direct implications for treatment, diagnosis, and prevention mechanisms. Third, most reference/control groups fail to incorporate prior opioid exposure, which can result in biases [7].
To address these challenges, we shift our attention to a new paradigm that characterizes risk for opioid prescription misuse on a continuous scale of severity, i.e., as a continuum. Several opioid phenotype definitions have been developed to date that extend beyond diagnostic codes to include other sources of data, including prescription data available in the EHR (see online suppl. material at www.karger.com/doi/10.1159/000525313; [8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27]). However, little is known about patterns and correlates associated with different levels of prescription opioid exposure, including which factors distinguish patients across clinically distinct categories of exposure and whether these patterns are consistent across different health systems. In this project, we used millions of EHRs from the PsycheMERGE network to further our understanding of the progression from prescription to dependence and misuse. PsycheMERGE (www.psychemerge.com), an extension of the Electronic Medical Records and Genomics (eMERGE) network [28], leverages EHR and genomic data for mental health research, including substance use disorders (SUDs). Prior work from this network has demonstrated the value of using EHR to characterize the risk of developing various psychiatric disorders [29]. This study is one of the first to use large-scale EHR opioid prescription data across large health systems to characterize risk for opioid use and misuse.
In the present study, we defined three opioid risk groups based on patterns of prescription opioid use and a fourth group based on International Classification of Diseases (ICD) diagnostic codes for OUD. Using data from three large health systems, we sought to (1) evaluate and compare demographics and psychiatric and medical comorbidities across the four groups; (2) assess how the four groups differ in comparison to patients with no prescription data and the general population of patients from each system; and (3) compare consistencies and differences in results across the three healthcare systems. A better understanding of opioid use phenotypes and comorbidities across different levels of opioid exposure is beneficial in various data-driven studies, including clinical prediction, treatment outcomes, diagnosis, prevention, epidemiology, and genomics.
Materials and Methods
Data Sources
Our data sources included registries from three health systems: Vanderbilt University Medical Center (VUMC), Mass General Brigham (MGB), and Geisinger Health System. Details of each registry, including demographics and data sources, are listed in the Appendix in the online supplement. We acquired Institutional Review Board approval (VUMC: 201767, MGB: 2018P002642); consent was not required for review of deidentified medical records. The Geisinger Institutional Review Board deemed this research exempt because all variables were extracted and summarized using an approved data broker. This work was deemed nonhuman subjects research by the VUMC IRB (IRB# 160650).
Patients were included in the analyses if they had at least 3 years of medical history available between 2005 and 2018 and were 18 years of age or older on Decemeber 31, 2018. A minimum of 3 years of medical history was chosen to increase the likelihood that patients had enough prescription data to detect opioid prescription patterns. Patients younger than 18 years were excluded to reduce the likelihood of including individuals who had not yet developed OUD. We excluded patients with a cancer diagnosis (online suppl. Table 1) due to potential for long-term analgesia for cancer-related pain. There were 627,396 patients from VUMC, 1,272,880 patients from MGB, and 733,637 patients from Geisinger who met the inclusion and exclusion criteria. We then extracted relevant ordered (VUMC, MGB, Geisinger) or filled (Geisinger) opioid prescriptions using a list of commonly prescribed opioids (online suppl. Table 2). A preprint version of this article is available on medRxiv [30].
Prescription Opioid Phenotyping and Group Definitions
Five groups were included in the study (Table 1). First, we identified all patients from each health system who met inclusion/exclusion criteria (described above), which we refer to as the “Unscreened” group (aka the overall study sample). From this group, we next defined three subgroups using inpatient and outpatient medication records based on prescription opioid exposure levels, derived from previously published work [9, 10]. Patients in the “No Prescription” group had no documented opioid prescriptions during the period of observation. Patients in the “Minimal Exposure” group received two opioid prescriptions within 90 days at least once and no additional prescriptions within 9 months but did not have 3 or more prescriptions no more than 90 days apart at any point during the period of observation [31]. Patients in the “Chronic Exposure” group received 10 or more opioid prescriptions within a 12-month period [9, 10]. The final “OUD” group included patients with at least one ICD code for OUD (online suppl. Table 3). The definition of this group did not incorporate prescription data. Therefore, patients in this group could overlap with the three prescription-based groups (code used to determine group membership available here: https://github.com/sanchezroigelab/OUD_spectrum_PsycheMERGE). The No Prescription, Minimal Exposure, Chronic Exposure, and OUD groups cover only a subset of the patients in the Unscreened group.
Table 1.
Description of each group used in the analyses. Only patients ≥18 years of age, with no history of cancer, and over 3 years of medical record history were included in the analyses
| Group | Description |
|---|---|
| Unscreened | Every patient with available data in EHR who met inclusion/exclusion criteria |
|
| |
| No Prescription | No opioid prescription data |
|
| |
| Minimal Exposure | Two prescriptions no more than 90 days apart at least once and no third prescription within 9 months and no 3 prescriptions no more than 90 days apart |
|
| |
| Chronic Exposure | ≥10 prescriptions in a 12-month period |
|
| |
| OUD | At least 1 ICD code for OUD |
We avoid referring to the patients in the “No Prescription” group as having “no exposure” because exposure status is defined with prescription data and not verified by patient self-report.
Outcome Measures
Within each group, we characterized the length and density of EHR, patient demographics, opioid use patterns, and OUD diagnoses (online suppl. material). Density was defined as the total number of nonunique ICD codes a patient received between 2005 and 2018 divided by the number of years included in the analysis. We also identified diagnoses previously identified as comorbid with OUD, including other SUDs [32], psychiatric disorders [32], and other medical conditions, including human immunodeficiency virus, hepatitis C, and pain-related diagnoses [33] (relevant ICD codes in online suppl. Tables 4–16).
To further examine patterns of prescription opioid use, we defined periods of exposure or “bouts” as at least two opioid prescriptions that occurred no more than 90 days apart. A bout was considered to end when there were more than 90 days between opioid prescriptions for a particular patient. We calculated the average number and length (in days) of bouts.
Statistical Analyses
As this is the first application of this continuous framework across three health systems, we focused more on descriptive statistics in order to establish comorbidity profiles, which can be used in future experiments for hypothesis testing. Descriptive statistics (frequency, percent, mean [M], standard deviation) were used to describe and compare the different groups (online suppl. Table 17). Throughout the text, we present ranges (e.g., in percentages) across the three registries. Demographic characteristics and outcomes across the three prescription-based groups were compared using χ2 tests for categorical outcomes and independent t tests or Kruskal-Wallis tests for continuous outcomes. We regarded both a p < 0.05 and a 5% difference in prevalence between any of the three prescription opioid groups as clinically important. Given the large sample sizes, with only a few exceptions, the differences between outcomes were statistically significant across groups and therefore only qualitative descriptions are provided in the Results section. Full statistical results are described in online supplementary Table 18.
Results
Demographics
The demographic composition for age and sex was similar across the Unscreened groups from different health system registries (online suppl. Table 17). Average age at the time of the analysis was 50.8–53.3 years, with 23.1–27.0% of the patients under age 35, and 41.3–44.6% male patients. Race/ethnicity varied within the registries, reflecting the geographic regions from which each health system draws. Yet, the majority of the patients were identified in the EHR as White (74.1–95.4%), while only 3.2–12.6% were identified as Black or African American, 2.3–7.5% as Hispanic, 0.6–4.5% as Asian, and 0.4–9.0% as other race/ethnicity.
A higher proportion of patients in the OUD group were male (44.8–60.7%) compared to the prescription-based groups (30.9–46.2%; online suppl. Table 17). Patients in the OUD group were 5–8 years younger, on average, than the Unscreened group and 12–15 years younger than patients in the Chronic Exposure group. Only 5.7–7.6% of the patients in the Chronic Exposure group were under the age of 35, compared to 15.3–33.7% for the OUD, No Prescription, and Minimal Exposure groups. The Chronic Exposure and OUD groups were predominantly composed of patients identified as White (81.7–96.8%) and Black/African American (2.3–13.9%), with lower proportions of patients identified as Hispanic (1.0–5.0%) and Asian (0.1–0.9%) in comparison to the other groups (71.1–96.5% White, 2.7–16.0% Black/African American, 0.3–7.4% Hispanic, and 0.1–1.1% Asian).
Length of EHR was greater for the Minimal Exposure (11.0–13.9 years), Chronic Exposure (11.4–14.6 years), and OUD (11.7–13.0 years) groups compared to patients in the Unscreened (9.9–11.9 years) and No Prescription (9.3–11.0 years) groups. Density of EHR (visits/year) was highest for the Chronic Exposure group (24.9–59.3), followed by the OUD group (15.5–33.1).
Prevalence of OUD Diagnoses
The overall prevalence of an OUD diagnosis in the Unscreened group was 1.2–1.7% (Fig. 1); this number fluctuated during the study period (online suppl. Fig. 1), with a steeper increase around 2013 in VUMC and MGB and a later peak (2017) in Geisinger. OUD diagnoses were more common in patients identified as non-Hispanic White and increased with exposure to prescription opioids − from 0.3 to 0.6% for No Prescription, to 1.6–2.7% for Minimal Exposure, and 9.0–24.4% for Chronic Exposure. These patterns were consistent across health systems. In the OUD group, 82.1–96.8% of the patients were non-Hispanic White, which was higher than other racial/ethnic groups.
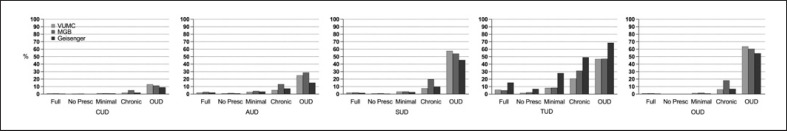
Fig. 1.
Rates of SUDs across the three registries (VUMC, MGB, Geisinger) by levels of opioid exposure (Unscreened, N (range across registries) = 627,396–1,272,880; No Prescription, N = 251,546–582,542; Minimal Exposure, N = 50,112–70,510; Chronic Exposure, N = 14,373–27,507; OUD, N = 8,673–21,489). Full, Unscreened group; No Presc, No Prescription group; CUD, cannabis use disorders; AUD, alcohol use disorders, SUD, substance use disorder; TUD, tobacco use disorders; OUD, opioid use disorder. Note that OUD as outcome pertains to having two or more OUD ICD codes on separate occasions.
Opioid Prescription Patterns
Average age at first opioid prescription ranged from 45.7 to 46.2 years in the Unscreened group across health systems, with the youngest average age of first prescription observed in the OUD group (34.8–39.0) and oldest among the Chronic Exposure group (46.8–52.5). The duration and number of periods of opioid exposure (“bouts”) increased as exposure to prescription opioids increased, with the Chronic Exposure group having the highest number (3.3–4.1) and length (224.1–566.5 days) of bouts. The OUD group had the second highest number (1.4–3.0) and length of bouts (135.8–185.0 days). In the Unscreened and Minimal Exposure groups, patterns of use diverged. For example, in the Unscreened group, the average number of bouts ranged from 0.4 to 1.8, and the length of bouts ranged from 12.6 to 97.0 days. In contrast, the Minimal Exposure group had a relatively higher number of bouts (1.5–1.8), but the length of use was shorter (10.0–15.9 days).
Substance Use Disorders
The prevalence of SUDs was highest in the OUD group (alcohol: 15.2–28.8%, tobacco: 47.0–68.6%, cannabis: 9.1–13.0%; Fig. 1). In contrast, the No Prescription group showed dramatically lower rates of SUDs (alcohol: 0.9–1.5%, tobacco: 2.3–6.8%, cannabis: 0.2–0.5%) than any other group, including the Minimal Exposure (alcohol: 3.1–4.1%, tobacco: 8.6–28.1%, cannabis: 0.8–1.2%) and the Chronic Exposure (alcohol: 6.1–13.2%, tobacco: 26.6–49.2%, cannabis: 1.9–5.1%) groups.
Psychiatric Disorders
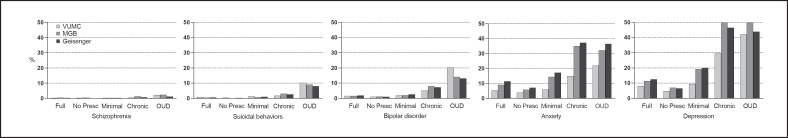
Prevalence of psychiatric disorders was highest in the Chronic Exposure and OUD groups (Fig. 2). For example, the prevalence of anxiety, one of the most common psychiatric disorders observed, was 19.3–37.2% and 25.7–36.6%, respectively, for the Chronic Exposure and OUD groups, compared to 8.0–17.3% for the Minimal Exposure group, 4.2–7.2% for the No Prescription group, and 6.5–11.5% for the Unscreened group. Depression prevalence was higher (36.4–50.4%) in the Chronic Exposure and OUD groups compared to the other groups (6.3–20.0%).
Fig. 2.
Rates of psychiatric disorders across the three registries (VUMC, MGB, Geisinger) and levels of opioid exposure (Unscreened, No Prescription, Minimal Exposure, Chronic Exposure, and OUD). Full, Unscreened group; No Presc, No Prescription group.
Bipolar disorder prevalence was highest in the OUD group (13.1–21.1%), higher than the Chronic Exposure group (5.2–8.0%), and dramatically higher than the Minimal Exposure (2.0–2.6%), No Prescription (1.0–1.3%), and Unscreened (1.6–1.9%) groups. Schizophrenia prevalence in the OUD group was slightly elevated (1.2–2.4%) compared to all other groups (0.23–1.29%). Suicidal behavior prevalence was higher in the OUD group (8.0–11.5%), compared to all other groups (0.2–3.1%).
Pain and Other Medical Conditions
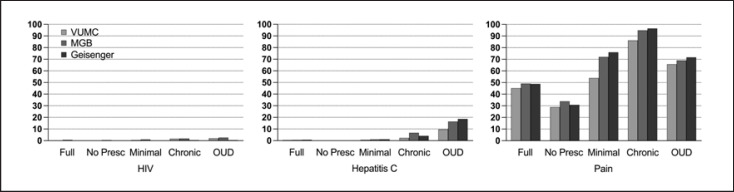
The prevalence of pain ICD codes was highest in the Chronic Exposure group (92.2–96.6%), higher than the Minimal Exposure (63.9–76.0%) and OUD groups (69.1–71.7%), and dramatically higher than the No Prescription or Unscreened groups (30.8–33.9% and 48.8–51.6%, respectively; Fig. 3). The prevalence of human immunodeficiency virus and hepatitis C was highest in the Chronic Exposure and OUD groups, particularly for hepatitis C (2.6–6.7% and 9.7–18.6%, respectively), compared to the other groups (0.1–1.2%).
Fig. 3.
Rates of medical conditions (HIV, hepatitis C, pain) known to be comorbid with OUDs across the three registries (VUMC, MGB, Geisinger) and levels of opioid exposure (Unscreened, No Prescription, Minimal Exposure, Chronic Exposure, and OUD diagnosis). Full, Unscreened group; No Presc, No Prescription group; HIV, human immunodeficiency virus.
Discussion
EHR represents a cost-effective strategy to study OUD using existing data from thousands to millions of patients. However, detecting OUD in EHR analyses is notoriously challenging. Diagnostic codes can be insufficient because OUD tends to be underdiagnosed [34, 35]. Furthermore, models often focus on extreme opioid use in service of predicting case:control classifications and thus miss the full spectrum of opioid exposures and use behaviors [36]. Our approach overcame these limitations by examining a continuum of opioid use behaviors based on levels of prescription opioid use. This approach is particularly important for defining opioid use phenotypes because behaviors range from appropriate use of prescribed opioids to nonmedical use of prescription (and illegal) opioids, and studies centered on examining each of the different transitions can have implications for prevention, diagnosis, and treatment.
This study systematically identified patterns and correlates of short- and long-term prescription opioid use and OUD across health systems, which facilitated important observations. First, we found that the OUD group had unique characteristics compared to the other groups, the most salient of which included comorbid psychiatric (anxiety, depression) and SUDs, particularly tobacco use disorders, in line with previous clinical studies [25, 27, 33, 37]. Prior studies estimated that 45–57% of individuals with OUD had at least one psychiatric disorder and reported that polysubstance abuse was exceedingly common [5], comparable to our findings. In addition, average age at first opioid prescription among the OUD group was approximately 10 years younger than that of other groups, highlighting the importance of age at first exposure to prescribed opioids and onset of OUD [38]. The demographic factor most noticeably associated with the OUD group was EHR identification as non-Hispanic White. This finding is consistent with the characterization of the opioid epidemic in the USA as primarily affecting rural and suburban individuals who identify as non-Hispanic White [39, 40].
Second, our findings emphasize the value of including opioid prescriptions in assessing risk for OUD [41, 42]. For example, with the exception of age at first opioid prescription, patients in the Chronic Exposure group most closely resembled the OUD group across most of the characteristics evaluated and may therefore represent the group with the highest risk of having or developing OUD [33, 43]. Consistent with this finding, the Chronic Exposure group also had a higher rate of OUD diagnosis than the No Prescription or Minimal Exposure groups. In addition, OUD diagnoses increased from the No Prescription to the Minimal Exposure group, providing further evidence for the relevance of assessing opioid prescriptions when determining risk for OUD [44].
Although findings were generally consistent across sites, we did observe some heterogeneity. This may have been due, in part, to differences in underlying patient populations, as evidenced by the differences in race/ethnicity proportions in the Unscreened groups; prior studies have documented varying correlates of prescription opioid misuse by race/ethnicity [45]. Differences across sites can also be due to a more rural population at Geisinger compared to MGB and VUMC [46] and the challenges of access to behavioral health services and treatment in rural populations. Nonetheless, the overall consistency in our findings suggests that correlates of opioid use phenotypes are shared among healthcare systems despite differences in data recording and patient populations and that opioid prescription EHR-based studies from different systems can be compared to one another, if using similar definitions.
Our findings have relevance for future EHR-based OUD research. First, our work re-emphasizes the importance of incorporating opioid prescriptions when defining the spectrum of opioid misuse and OUD. Prior algorithms have incorporated opioid prescriptions to identify opioid misuse (e.g., [10, 19, 47]), but additional efforts could place patients on a spectrum of opioid use behaviors. For example, to identify individuals at risk for developing OUD, phenotype risk scores [48, 49] could be constructed by agnostically training and testing a risk model using diagnosis codes (for OUD and other relevant predictors) and opioid prescriptions. Such a strategy would not only acknowledge OUD risk in the absence of an OUD diagnosis but could also allow for modeling of OUD risk trajectories over time [50]. Second, our descriptive data confirm the relevance of several predictors of opioid misuse (e.g., age, substance and psychiatric comorbidities) that have been previously incorporated into algorithms identifying opioid misuse but not necessarily validated [19, 51] into algorithms identifying opioid misuse. Third, the depth and breadth of data in EHR registries can be leveraged to clarify the phenotypic structure of OUD phenotypes. Future studies could use data-driven methods to integrate additional EHR components (e.g., prescriptions for other controlled substances, types of pain diagnoses, opioid dosages and types) and implement cluster-based methods such as latent profile analysis, k-means clustering, or principal component analysis to explore OUD sub-phenotypes [52].
Lastly, our work has implications for genetic studies, in particular informing selection of individuals for genetic analyses. A major roadblock in conducting genome-wide association studies of opioid use phenotypes is the lack of controls with characterized opioid use histories, resulting in the frequent use of unscreened individuals as controls. Consistent with previous work showing that using unscreened controls can introduce biases in genetic analyses [7], our work demonstrates that a Minimal Exposure group has a different set of clinical characteristics than an Unscreened or No Prescription group.
This study is subject to several limitations. We did not have complete information on opioid dosages [53] (and therefore morphine milligram equivalents) or information on the use of illicit opioids, and we were not able to differentiate between opioid types, which would have helped identify additional misuse phenotypes such as rapid dose escalation trajectories [41]. Further, we relied on opioid prescriptions to index opioid use, but this is likely an imperfect proxy for actual use. Reliance on OUD diagnosis for the OUD group could also have led to misclassification given the underdiagnosis of OUD [36]; this may have been particularly problematic in the data from the earlier years included in the study. Furthermore, the associations observed represent correlations and not causation; future studies may include sequences of events to disentangle potential trajectories of effect between the groups and the outcomes. Lastly, it is not known whether these results are generalizable to other populations, but the external validity of our findings is supported by consistencies observed across the three health systems, which serve diverse patient populations.
Conclusion
This work informs the selection of cases and controls for epidemiologic and genetic studies, demonstrates the utility of using levels of prescription opioid use in elucidating different aspects of OUD pathophysiology, and supports the appropriateness of combining EHR data across these health systems for future meta-analyses, despite known differences in geographical location, racial diversity, and potential differences in diagnostic practices across the systems.
Statement of Ethics
We acquired Institutional Review Board approval (VUMC: 201767, MGB: 2018P002642); consent was not required for review of deidentified medical records. The Geisinger Institutional Review Board deemed this research exempt because all variables were extracted and summarized using an approved data broker. This work was deemed nonhuman subjects research by the VUMC IRB (IRB# 160650).
Conflict of Interest Statement
Dr. Urman reports unrelated funding and/or fees from the NIH, AHRQ, NSF, Medtronic, Merck, AcelRx, Heron, and Pfizer. Dr. Smoller is a member of the Leon Levy Foundation Neuroscience Advisory Board and has received honoraria for an internal seminar at Biogen, Inc., and Tempus Labs. He is PI of a collaborative study of the genetics of depression and bipolar disorder sponsored by 23andMe for which 23andMe provides analysis time as in-kind support but no payments. He is also a member of the Scientific Advisory Board of Sensorium Therapeutics.
Funding Sources
Mariela V. Jennings, Sevim B. Bianchi, and Sandra Sanchez-Roige are supported by funds from the California Tobacco-Related Disease Research Program (TRDRP; Grant No. T29KT0526); Sevim B. Bianchi is also supported by P50DA037844; and Sandra Sanchez-Roige is also supported by NIDA DP1DA054394. Hyunjoon Lee is supported by funds from NIH 1R01MH118233-01. Brandon J. Coombes is supported by funds from NIMH R01 MH121924. Annika B. Faucon is supported by the Training Program On Genetic Variation And Human Phenotypes Training Grant (T32GM080178). Melissa N. Poulsen is supported by funds from NIDA K01DA04993. Rachel L. Kember is supported by funds from NIAAA (K01AA028292). Travis T. Mallard is supported by funds from NIH T32HG010464. Colin G. Walsh is supported by funds from NIMH R01 MH118233, R01 MH121455, R01 MH120122, R01 MH116269, DoD MSRC W81XWH-10-2-0181, and the FDA WO2006. Richard D. Urman is supported by NIH/NIDA − 1R34DA048268-01A1. Vanessa Troiani and Richard C. Crist are supported by funds from NIDA R01DA044015 and a grant from the Pennsylvania Department of Health. The PsycheMERGE consortium is supported by NIMH R01 MH118233 (J.W.S., L.K.D.). The project using VUMC data is supported by CTSA award No. UL1TR000445 from the National Center for Advancing Translational Sciences. The contents are solely the responsibility of the authors and do not necessarily represent official views of the National Center for Advancing Translational Sciences or the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author Contributions
Sandra Sanchez-Roige conceived the idea. Vanessa Troiani, Mariela V. Jennings, Hyunjoon Lee, Daniel B. Rocha, Brandon J. Coombes, Richard C. Crist, Yirui Hu, Maria Niarchou, Melissa N. Poulsen, and Richard D. Urman contributed to the design and implementation of the research. Mariela V. Jennings, Hyunjoon Lee, and Daniel B. Rocha led the main analyses. Lea K. Davis, Jordan W. Smoller, and Peter Straub contributed data. All authors contributed to the writing of the manuscript.
Data Availability Statement
Descriptive statistics are provided as online supplementary Tables. The code used to determine group membership is available here: https://github.com/sanchezroigelab/OUD_spectrum_PsycheMERGE.
Supplementary Material
Supplementary data
Supplementary data
Funding Statement
Mariela V. Jennings, Sevim B. Bianchi, and Sandra Sanchez-Roige are supported by funds from the California Tobacco-Related Disease Research Program (TRDRP; Grant No. T29KT0526); Sevim B. Bianchi is also supported by P50DA037844; and Sandra Sanchez-Roige is also supported by NIDA DP1DA054394. Hyunjoon Lee is supported by funds from NIH 1R01MH118233-01. Brandon J. Coombes is supported by funds from NIMH R01 MH121924. Annika B. Faucon is supported by the Training Program On Genetic Variation And Human Phenotypes Training Grant (T32GM080178). Melissa N. Poulsen is supported by funds from NIDA K01DA04993. Rachel L. Kember is supported by funds from NIAAA (K01AA028292). Travis T. Mallard is supported by funds from NIH T32HG010464. Colin G. Walsh is supported by funds from NIMH R01 MH118233, R01 MH121455, R01 MH120122, R01 MH116269, DoD MSRC W81XWH-10-2-0181, and the FDA WO2006. Richard D. Urman is supported by NIH/NIDA − 1R34DA048268-01A1. Vanessa Troiani and Richard C. Crist are supported by funds from NIDA R01DA044015 and a grant from the Pennsylvania Department of Health. The PsycheMERGE consortium is supported by NIMH R01 MH118233 (J.W.S., L.K.D.). The project using VUMC data is supported by CTSA award No. UL1TR000445 from the National Center for Advancing Translational Sciences. The contents are solely the responsibility of the authors and do not necessarily represent official views of the National Center for Advancing Translational Sciences or the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Gostin LO, Hodge JG, Noe SA. Reframing the opioid epidemic as a national emergency. JAMA. 2017 Oct;318((16)):1539. doi: 10.1001/jama.2017.13358. [DOI] [PubMed] [Google Scholar]
- 2.Shipton EA, Shipton EE, Shipton AJ. A review of the opioid epidemic: what do we do about it? Pain Ther. 2018 Jun;7((1)):23–36. doi: 10.1007/s40122-018-0096-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Strang J, Volkow ND, Degenhardt L, Hickman M, Johnson K, Koob GF, et al. Opioid use disorder. Nat Rev Dis Primer. 2020 Jan;6((1)):3. doi: 10.1038/s41572-019-0137-5. [DOI] [PubMed] [Google Scholar]
- 4.Kaye AD, Jones MR, Kaye AM, Ripoll JG, Galan V, Beakley BD, et al. Prescription opioid abuse in chronic pain: an updated review of opioid abuse predictors and strategies to curb opioid abuse: part 1. Pain Physician. 2017;20((2S)):18. [PubMed] [Google Scholar]
- 5.Freda PJ, Moore JH, Kranzler HR. The phenomics and genetics of addictive and affective comorbidity in opioid use disorder. Drug Alcohol Depend. 2021 Apr;221:108602. doi: 10.1016/j.drugalcdep.2021.108602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guy GP, Zhang K. Opioid prescribing by specialty and volume in the U.S. Am J Prev Med. 2018 Nov;55((5)):e153–5. doi: 10.1016/j.amepre.2018.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou H, Rentsch CT, Cheng Z, Kember RL, Nunez YZ, Sherva RM, et al. Association of OPRM1 functional coding variant with opioid use disorder: a genome-wide association study. JAMA Psychiatry. 2020 Oct;77((10)):1072. doi: 10.1001/jamapsychiatry.2020.1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brummett CM, Waljee JF, Goesling J, Moser S, Lin P, Englesbe MJ, et al. New persistent opioid use after minor and major surgical procedures in US adults. JAMA Surg. 2017 Jun;152((6)):e170504. doi: 10.1001/jamasurg.2017.0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun EC, Darnall BD, Baker LC, Mackey S. Incidence of and risk factors for chronic opioid use among opioid-naive patients in the postoperative period. JAMA Intern Med. 2016 Sep;176((9)):1286. doi: 10.1001/jamainternmed.2016.3298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Calcaterra SL, Scarbro S, Hull ML, Forber AD, Binswanger IA, Colborn KL. Prediction of future chronic opioid use among hospitalized patients. J Gen Intern Med. 2018 Jun;33((6)):898–905. doi: 10.1007/s11606-018-4335-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Polimanti R, Walters RK, Johnson EC, McClintick JN, Adkins AE, Adkins DE, et al. Leveraging genome-wide data to investigate differences between opioid use vs. opioid dependence in 41,176 individuals from the Psychiatric Genomics Consortium. Mol Psychiatry. 2020 Aug;25((8)):1673–1687. doi: 10.1038/s41380-020-0677-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Webster LR, Webster RM. Predicting aberrant behaviors in opioid-treated patients: preliminary validation of the opioid risk tool. Pain Med. 2005 Nov;6((6)):432–442. doi: 10.1111/j.1526-4637.2005.00072.x. [DOI] [PubMed] [Google Scholar]
- 13.Butler SF, Budman SH, Fernandez KC, Houle B, Benoit C, Katz N, et al. Development and validation of the current opioid misuse measure. Pain. 2007 Jul;130((1)):144–156. doi: 10.1016/j.pain.2007.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coyne KS, Barsdorf AI, Currie BM, Butler SF, Farrar JT, Mazière J-Y, et al. Construct validity and reproducibility of the prescription opioid misuse and abuse questionnaire (POMAQ) Curr Med Res Opin. 2021 Mar;37((3)):493–503. doi: 10.1080/03007995.2020.1865890. [DOI] [PubMed] [Google Scholar]
- 15.Knisely JS, Wunsch MJ, Cropsey KL, Campbell ED. Prescription opioid misuse index: a brief questionnaire to assess misuse. J Subst Abuse Treat. 2008 Dec;35((4)):380–386. doi: 10.1016/j.jsat.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 16.McLellan AT, Luborsky L, Woody GE, O'Brien CP. An improved diagnostic evaluation instrument for substance abuse patients. The addiction severity index. J Nerv Ment Dis. 1980 Jan;168((1)):26–33. doi: 10.1097/00005053-198001000-00006. [DOI] [PubMed] [Google Scholar]
- 17.Cochran BN, Flentje A, Heck NC, Van Den Bos J, Perlman D, Torres J, et al. Factors predicting development of opioid use disorders among individuals who receive an initial opioid prescription: mathematical modeling using a database of commercially-insured individuals. Drug Alcohol Depend. 2014 May;138:202–208. doi: 10.1016/j.drugalcdep.2014.02.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karhade AV, Ogink PT, Thio QCBS, Cha TD, Gormley WB, Hershman SH, et al. Development of machine learning algorithms for prediction of prolonged opioid prescription after surgery for lumbar disc herniation. Spine J. 2019 Nov;19((11)):1764–1771. doi: 10.1016/j.spinee.2019.06.002. [DOI] [PubMed] [Google Scholar]
- 19.Canan C, Polinski JM, Alexander GC, Kowal MK, Brennan TA, Shrank WH. Automatable algorithms to identify nonmedical opioid use using electronic data: a systematic review. J Am Med Inform Assoc. 2017 Nov;24((6)):1204–1210. doi: 10.1093/jamia/ocx066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hylan TR, Von Korff M, Saunders K, Masters E, Palmer RE, Carrell D, et al. Automated prediction of risk for problem opioid use in a primary care setting. J Pain. 2015 Apr;16((4)):380–387. doi: 10.1016/j.jpain.2015.01.011. [DOI] [PubMed] [Google Scholar]
- 21.Xu H, Stenner SP, Doan S, Johnson KB, Waitman LR, Denny JC. MedEx: a medication information extraction system for clinical narratives. J Am Med Inform Assoc. 2010 Jan;17((1)):19–24. doi: 10.1197/jamia.M3378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Webster LR. Risk factors for opioid-use disorder and overdose. Anesth Analg. 2017 Nov;125((5)):1741–1748. doi: 10.1213/ANE.0000000000002496. [DOI] [PubMed] [Google Scholar]
- 23.Brooner RK. Psychiatric and substance use comorbidity among treatment-seeking opioid abusers. Arch Gen Psychiatry. 1997 Jan;54((1)):71. doi: 10.1001/archpsyc.1997.01830130077015. [DOI] [PubMed] [Google Scholar]
- 24.Kidorf M, Disney ER, King VL, Neufeld K, Beilenson PL, Brooner RK. Prevalence of psychiatric and substance use disorders in opioid abusers in a community syringe exchange program. Drug Alcohol Depend. 2004 May;74((2)):115–122. doi: 10.1016/j.drugalcdep.2003.11.014. [DOI] [PubMed] [Google Scholar]
- 25.Barry DT, Cutter CJ, Beitel M, Kerns RD, Liong C, Schottenfeld RS. Psychiatric disorders among patients seeking treatment for co-occurring chronic pain and opioid use disorder. J Clin Psychiatry. 2016 Oct;77((10)):1413–1419. doi: 10.4088/JCP.15m09963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barth KS, Maria MM-S, Lawson K, Shaftman S, Brady KT, Back SE. Pain and motives for use among non-treatment seeking individuals with prescription opioid dependence: pain and prescription opioid dependence. Am J Addict. 2013 Sep;22((5)):486–491. doi: 10.1111/j.1521-0391.2013.12038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nazarian A, Negus SS, Martin TJ. Factors mediating pain-related risk for opioid use disorder. Neuropharmacology. 2021 Mar;186:108476. doi: 10.1016/j.neuropharm.2021.108476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McCarty CA, Chisholm RL, Chute CG, Kullo IJ, Jarvik GP, Larson EB, et al. The eMERGE network: a consortium of biorepositories linked to electronic medical records data for conducting genomic studies. BMC Med Genomics. 2011 Jan;4:13. doi: 10.1186/1755-8794-4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zheutlin AB, Dennis J, Karlsson Linnér R, Moscati A, Restrepo N, Straub P, et al. Penetrance and pleiotropy of polygenic risk scores for schizophrenia in 106,160 patients across four health care systems. Am J Psychiatry. 2019 Oct;176((10)):846–855. doi: 10.1176/appi.ajp.2019.18091085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jennings MV, Lee H, Rocha DB, Bianchi SB, Crist RC, Faucon A, et al. Identifying high-risk comorbidities of short and long-term opioid prescription use. medRxiv. 2021 Nov [Google Scholar]
- 31.Katzman C, Harker EC, Ahmed R, Keilin CA, Vu JV, Brummett CM. The association between preoperative opioid exposure and prolonged postoperative use. Ann Surg. 2020;7 doi: 10.1097/SLA.0000000000003723. [DOI] [PubMed] [Google Scholar]
- 32.Jones CM. Co-occurring substance use and mental disorders among adults with opioid use disorder. Drug Alcohol Depend. 2019;197:78–82. doi: 10.1016/j.drugalcdep.2018.12.030. [DOI] [PubMed] [Google Scholar]
- 33.Volkow ND, Jones EB, Einstein EB, Wargo EM. Prevention and treatment of opioid misuse and addiction: a review. JAMA Psychiatry. 2019 Feb;76((2)):208. doi: 10.1001/jamapsychiatry.2018.3126. [DOI] [PubMed] [Google Scholar]
- 34.Hallgren KA, Witwer E, West I, Baldwin L-M, Donovan D, Stuvek B, et al. Prevalence of documented alcohol and opioid use disorder diagnoses and treatments in a regional primary care practice-based research network. J Subst Abuse Treat. 2021;110:18–27. doi: 10.1016/j.jsat.2019.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kirson NY, Shei A, Rice JB, Enloe CJ, Bodnar K, Birnbaum HG, et al. The burden of undiagnosed opioid abuse among commercially insured individuals: the burden of undiagnosed opioid abuse. Pain Med. 2015 Jul;16((7)):1325–1332. doi: 10.1111/pme.12768. [DOI] [PubMed] [Google Scholar]
- 36.Palumbo SA, Adamson KM, Krishnamurthy S, Manoharan S, Beiler D, Seiwell A, et al. Assessment of probable opioid use disorder using electronic health record documentation. JAMA Netw Open. 2020;3((9)):e2015909. doi: 10.1001/jamanetworkopen.2020.15909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Edlund MJ, Martin BC, Devries A, Fan M-Y, Brennan Braden J, Sullivan MD. Trends in use of opioids for chronic noncancer pain among individuals with mental health and substance use disorders: the TROUP study. Clin J Pain. 2010 Jan;26((1)):1–8. doi: 10.1097/AJP.0b013e3181b99f35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.National Academies of Sciences, Engineering, and Medicine. Health and Medicine Division. Board on Health Sciences Policy. Committee on Pain Management and Regulatory Strategies to Address Prescription Opioid Abuse . Trends in prescription opioid use and misuse. In: Phillips JK, Ford MA, Bonnie RJ, editors. Pain management and the opioid epidemic: balancing societal and individual benefits and risks of prescription opioid use. Washington, DC: The National Academic Press; 2017. p. p. 188. [PubMed] [Google Scholar]
- 39.Keyes KM, Cerdá M, Brady JE, Havens JR, Galea S. Understanding the rural-urban differences in nonmedical prescription opioid use and abuse in the United States. Am J Public Health. 2014 Feb;104((2)):e52–9. doi: 10.2105/AJPH.2013.301709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Centers for Disease Control and Prevention . Annual surveillance report of drug-related risks and outcomes. 2019. [Google Scholar]
- 41.Rentsch CT, Edelman EJ, Justice AC, Marshall BDL, Xu K, Smith AH, et al. Patterns and correlates of prescription opioid receipt among US veterans: a national, 18-year observational cohort study. AIDS Behav. 2019 Dec;23((12)):3340–3349. doi: 10.1007/s10461-019-02608-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Naumann RB, Marshall SW, Gottfredson NC, Lund JL, Ringwalt CL, Skinner AC. Trajectories of dispensed prescription opioids among beneficiaries enrolled in a medicaid controlled substance “lock‐in” program. 2019;28((1)):16–24. doi: 10.1002/pds.4445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Klimas J, Gorfinkel L, Fairbairn N, Amato L, Ahamad K, Nolan S, et al. Strategies to identify patient risks of prescription opioid addiction when initiating opioids for pain: a systematic review. JAMA Netw Open. 2019 May;2((5)):e193365. doi: 10.1001/jamanetworkopen.2019.3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vowles KE, McEntee ML, Julnes PS, Frohe T, Ney JP, van der Goes DN. Rates of opioid misuse, abuse, and addiction in chronic pain: a systematic review and data synthesis. Pain. 2015 Apr;156((4)):569–576. doi: 10.1097/01.j.pain.0000460357.01998.f1. [DOI] [PubMed] [Google Scholar]
- 45.Nicholson HL. Correlates of prescription opioid misuse among Black adults: findings from the 2015 National Survey on Drug Use and Health. Drug Alcohol Depend. 2018;186:264–267. doi: 10.1016/j.drugalcdep.2017.12.006. [DOI] [PubMed] [Google Scholar]
- 46.Buettner-Schmidt K, Miller DR, Maack B. Disparities in rural tobacco use, smoke-free policies, and tobacco taxes. West J Nurs Res. 2019;41((8)):1184–1202. doi: 10.1177/0193945919828061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rough K, Huybrechts KF, Hernandez-Diaz S, Desai RJ, Patorno E, Bateman BT. Using prescription claims to detect aberrant behaviors with opioids: comparison and validation of 5 algorithms. Pharmacoepidemiol Drug Saf. 2019;28((1)):62–69. doi: 10.1002/pds.4443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bastarache L, Hughey JJ, Goldstein JA, Bastraache JA, Das S, Zaki NC, et al. Improving the phenotype risk score as a scalable approach to identifying patients with Mendelian disease. J Am Med Inform Assoc. 2019 Dec;26((12)):1437–1447. doi: 10.1093/jamia/ocz179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ruderfer DM, Walsh CG, Aguirre MW, Tanigawa Y, Ribeiro JD, Franklin JC, et al. Significant shared heritability underlies suicide attempt and clinically predicted probability of attempting suicide. Mol Psychiatry. 2020 Oct;25((10)):2422–2430. doi: 10.1038/s41380-018-0326-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Elmer J, Fogliato R, Setia N, Mui W, Lynch M, Hulsey E, et al. Trajectories of prescription opioids filled over time. PLoS One. 2019;14((10)):e0222677. doi: 10.1371/journal.pone.0222677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schirle L, Jeffery A, Yaqoob A, Sanchez-Roige S, Samuels DC. Two data-driven approaches to identifying the spectrum of problematic opioid use: a pilot study within a chronic pain cohort. Int J Med Inf. 2021 Dec;156:104621. doi: 10.1016/j.ijmedinf.2021.104621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nylund KL, Asparouhov T, Muthén BO. Deciding on the number of classes in latent class analysis and growth mixture modeling: a Monte Carlo simulation study. Struct Equ Model Multidiscip J. 2007 Oct;14((4)):535–569. [Google Scholar]
- 53.Morasco BJ, Duckart JP, Carr TP, Deyo RA, Dobscha SK. Clinical characteristics of veterans prescribed high doses of opioid medications for chronic non-cancer pain. Pain. 2010 Dec;151((3)):625–632. doi: 10.1016/j.pain.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data
Supplementary data
Data Availability Statement
Descriptive statistics are provided as online supplementary Tables. The code used to determine group membership is available here: https://github.com/sanchezroigelab/OUD_spectrum_PsycheMERGE.