Abstract
To investigate the association between time-of-day of stroke onset and functional outcome in patients with acute ischemic stroke(AIS) treated with endovascular thrombectomy(EVT). AIS patients treated with EVT between January 2013 and December 2018 were recruited and divided them into four 6-h interval groups according to the time-of-day of stroke onset. A total of 438 patients were enrolled, 3-month favorable outcome were achieved in 58.6%, 43.7%, 36.6%, and 30.5% of patients in the 00:00–06:00, 06:00–12:00, 12:00–18:00, and 18:00–24:00 groups, respectively (adjusted OR 0.61, 95% CI 0.40–0.93; p = 0.020). Compared with the 18:00–24:00 interval, patients in the 00:00–06:00 interval (adjusted OR 4.01, 95%CI 1.02–15.80, p = 0.047) and the 06:00–12:00 interval (adjusted OR 3.24, 95% CI 1.09–9.64, p = 0.034) were more likely to achieve favorable outcome. The time-of-day of stroke onset was not associated with 3-month mortality (adjusted p = 0.829), symptomatic intracerebral hemorrhage (sICH, adjusted p = 0.296), or early successful recanalization (adjusted p = 0.074). In conclusion, in AIS patients treated with EVT, those onsets either between 00:00 and 06:00 or between 06:00 and 12:00 appeared to be associated with a higher proportion of favorable outcomes at 3 months, but the time-of-day at stroke onset was not associated with the incidence of sICH, rate of early successful recanalization, or 3-month mortality.
Keywords: Ischemic stroke, endovascular therapy, circadian rhythm, functional outcome, time of day
Introduction
Circadian rhythms in humans are physiological, metabolic, and behavioral oscillations that are featured with an approximate 24-hour cycle. The intrinsic biological rhythms are orchestrated by circadian clock and has been demonstrated to be associated with risks of cerebrovascular disease(s). 1,2 Epidemiological studies have found that the incidence of ischemic stroke is higher in the early hours of the morning, which has been reported to be up to 49% higher than in the other 18 hours of the day. 3 Furthermore, clinical outcomes of patients with acute ischemic stroke (AIS) also follow a circadian curve, with nighttime stroke resulting in faster infarct progression, 4 higher presenting neurologic severity, more frequent early neurological deterioration and worse 3-month functional outcome. 5 These findings motivated studies that investigate the temporal effects on the reperfusion therapy. In patients with AIS who were treated with intravenous thrombolysis, diurnal administration of intravenous alteplase appeared to be associated with a higher recanalization rate and more favorable outcomes, while nocturnal fibrinolytic therapy was much safer. 6,7
Endovascular thrombectomy (EVT) has become the standard therapy for vessel recanalization after stroke, especially for those secondary to large vessel occlusion. Several recent studies have observed the time-of-day effects on functional outcomes in patients with AIS who underwent EVT. Hajdu et al. 8 founded that EVT procedures performed in the morning were associated with a better functional outcome, while performed at the end of work day resulted in poorer functional outcomes, and this phenomenon was attributed to fatigue among neurointerventionists and the stroke unit staff. Conversely, Benali et al. 9 found that AIS patients who were treated with EVT during nighttime experienced a better 3-month functional outcome, and the authors attributed this phenomenon to the age-variability between nighttime and daytime. Therefore, the temporal fluctuation of functional outcomes were generally attributed to external factors, such as efficiency of the workflow, performance of the operators, or staffing level. 10 In this study, however, we hypothesized that intrinsic circadian rhythm may also play a role in the prognosis of patients with AIS who undergo EVT. We aimed to investigate the association between the time of day at stroke onset and neurological outcomes in AIS patients who underwent EVT.
Material and methods
Data availability statement
All supporting data for this analysis are available from the corresponding author upon reasonable request with study protocol and statistical analysis methods provided.
Study design and data source
The present investigation was based on a prospective registry cohort study conducted at the Xuanwu Stroke Center. Data documenting characteristics of the registry study and the EVT protocol for AIS have been described elsewhere. 11,12 The timing of stroke onset was documented in the hospital charts by stroke neurologists who interviewed patients or their relatives that first observed neurological deficits. According to The International Meridian Conference in 1884, the midnight was set as 00:00. The 24 hours of a universal day was divided into four 6-h intervals as following: night (00:00–06:00), morning (06:00–12:00), afternoon (12:00–18:00), and evening (18:00–24:00). This study screened all AIS patients (≥18 years of age) who experienced proximal large vessel occlusion and underwent EVT between January 1, 2013, and December 31, 2018. Patients with a pre-stroke modified Rankin Scale (mRS) score ≥3, those without accurate data documenting the onset timing, and/or those whose stroke onset time could be allocated into ≥2 of the aforementioned 6-h intervals were excluded.
The study was conducted in accordance with the Helsinki declaration of 1975 (and as revised in 1983) and was approved by the local ethics committee (the Ethics Committee of Xuanwu Hospital, Capital Medical University). All patients or their legally authorized representatives provided written informed consent on admission to hospital.
Data collection
The following information was collected from the institutional database: demographic information (age, sex, body mass index); medical history, including diabetes mellitus, hypertension, hyperlipidemia, coronary heart disease, atrial fibrillation, valvular heart disease, transient ischemia attack (TIA), cerebral ischemic/hemorrhagic stroke, statins use, smoking and drinking history; laboratory investigations including blood platelet count, fibrinogen; radiological examinations like initial CT results evaluated using the Alberta Stroke Program Early CT Score (ASPECTS) or posterior circulation Alberta Stroke Program Early CT Score (pc-ASPECTS); clinical characteristics, including initial stroke severity assessed using the NIH Stroke Scale (NIHSS), location of occluded vessels, and etiology of stroke; work flow process time, including the time from stroke onset to groin puncture, and from onset to recanalization; and clinical outcomes, including 3-month functional outcomes, 3-month mortality, safety outcome, and early successful recanalization.
Outcome assessments
The primary outcome was favorable outcome at 3 months after EVT, which was defined as mRS score of 0–2. Secondary outcomes were as follows: initial severity of stroke, measured according to the NIHSS on admission; symptomatic intracerebral hemorrhage (sICH), as defined according to the European Cooperative Acute Stroke Study III criteria (any apparent extravascular blood in the brain or within the cranium associated with clinical deterioration [an increase ≥4 points in the NIHSS score, or that led to mortality]); 13 three-month mortality; and early successful recanalization, defined as Thrombolysis In Cerebral Infarction (TICI) scale scores of 2 b or 3.
Statistical analysis
Baseline data, demographic characteristics, and clinical outcomes were compared among the four 6-h time interval groups. The Kolmogorov-Smirnov test was used to test for normality. Continuous data are described as mean ± SD or median (interquartile range [IQR]), and one-way analysis of variance (ANOVA) or Kruskal-Wallis tests were used to assess between-group differences. Binary data are described as frequency and percentage, and the chi-squared test along with odds ratio (OR) and corresponding 95% confidence interval (CI) were used to evaluate between-group differences.
Multivariate analysis was performed to assess the association(s) between the time-of-day of stroke onset and clinical outcomes. The covariates included in this analysis model were selected from the univariate analysis. To examine the association between the time-of-day of stroke onset and 3-month functional outcome, linear and logistic regression analysis were performed by using the 3-month functional outcome as the dependent variable, and the following covariates were forced into the models: NIHSS on admission, intravenous rt-PA, TICI scores, onset to groin puncture, onset to recanalization, gender, age, hypertension, hyperlipidemia, diabetes mellitus, atrial fibrillation, previous stroke, and sICH. In addition, the NIHSS on admission, intravenous rt-PA, TICI scores, onset to groin puncture, onset to recanalization, gender, age, hypertension, hyperlipidemia, diabetes mellitus, atrial fibrillation, previous stroke, and sICH were forced into the model if 3-month mortality was the dependent variable. To assess the association between the time-of-day of stroke onset and sICH, logistic regression was performed using sICH as the dependent variable. NIHSS on admission, intravenous rt-PA, TICI scores, onset to groin puncture, onset to recanalization, gender, age, hypertension, hyperlipidemia, diabetes mellitus, atrial fibrillation, and previous stroke were forced into the model. To examine the association between the time-of-day of stroke onset and early recanalization, logistic regression was performed using TICI scores as the dependent variable. NIHSS on admission, intravenous rt-PA, onset to groin puncture, onset to recanalization, gender, age, hypertension, hyperlipidemia, diabetes mellitus, atrial fibrillation, previous stroke, and sICH were forced into the model as interaction factors.
Furthermore, to compare the 18:00–24:00 time interval with other three-time intervals, odds ratios (OR) with 95% confidence interval (CI) were calculated for the 00:00–06:00 vs. 18:00–24:00, 06:00–12:00 vs. 18:00–24:00, and 12:00–18:00 vs. 18:00–24:00, with 18:00–24:00 time interval served as reference. Logistic regression analysis was conducted to adjust for confounding factors.
To examine whether study results were changed by stroke etiology, we performed a sensitivity analysis for associations between the time-of-day of stroke onset and functional outcome in a subset of patients with large artery atherosclerosis (LAA), who accounted for 71% of all enrolled patients. In addition, to examine whether study results were confounded by circadian fluctuation of body temperature, which was lower between 00:00 and 12:00 and was higher between 12:00 and 24:00, 14 a sensitivity analysis was conducted by grouping all enrolled patients into two 12-hour time intervals (00:00–12:00 vs. 12:00–24:00), coinciding with the circadian variation of body temperature. Adjusted ORs and corresponding 95% CIs were calculated using logistic regression analysis.
All statistical analyses were performed using SPSS version 23.0 (IBM Corporation, Armonk, NY, USA); differences with a two-sided p < 0.05 were considered to be statistically significant.
Results
Between January 2013 and December 2018, a total of 438 patients with AIS who underwent EVT were enrolled in this study. Patients were divided to four-time interval groups as follows: 00:00–06:00 (n = 58 [13.2%]); 06:00–12:00 (n = 151 [34.5%]); 12:00–18:00 (n = 134 [30.6%]); and 18:00–24:00 (n = 95 [21.7%]). The circadian distribution of stroke onset showed that the peak of stroke onset occurred between 06:00 and 09:00 (Supplemental Figure S1).
Baseline characteristics
Demographic and clinical characteristics according to the time-of-day of stroke onset are summarized in Table 1. The mean (±SD) age at stroke onset was 62.1 ± 12.1 years, and 333 (76.0%) patients were male. Patients with hypertension were more likely to suffer from stroke between 12:00 and 18:00 (p = 0.003), whereas patients with hyperlipidemia were less likely to suffer from stroke between 12:00 and 18:00 (p = 0.003). The mean (±SD) NIHSS score on admission was 18.23 (±9.13), and the circadian dependence existed in the initial stroke severity. Compared with other time intervals, patients with stroke occurring between 12:00 and 18:00 were more likely to experience severe stroke (p = 0.004). Similar trend was observed in the ASPECTS or pc-ASPECTS scores (p = 0.042). The time from stroke onset to groin puncture for patients who had stroke onset between 18:00 and 24:00 was longer than other time intervals (p < 0.001). The time from onset to recanalization also displayed a circadian variation, rising from a nadir between 06:00 and 12:00 to a peak between 18:00 and 24:00 (p < 0.001). The other baseline variables, such as the location of occluded vessels, stroke etiology, and previous ischemic stroke, were not significantly associated with the time-of-day of stroke onset (Table 1).
Table 1.
Demographic and clinical characteristics.
| All patients (n = 438) | 00:00–06:00 (n = 58) | 06:00–12:00 (n = 151) | 12:00–18:00 (n = 134) | 18:00–24:00 (n = 95) | p | |
|---|---|---|---|---|---|---|
| Demographic characteristics | ||||||
| Age at onset, mean ± SD | 62.10 (±12.11) | 62.5 (±11.95) | 60.2 (±13.44) | 64.2 (±11.26) | 61.8 (±10.74) | 0.052 |
| Male, n (%) | 333 (76.0%) | 43 (74.1%) | 119 (78.8%) | 95 (70.9%) | 76 (80.0%) | 0.319 |
| BMI, kg/m2, mean ± SD | 25.50 (±3.35) | 26.12 (±3.17) | 25.64 (±3.09) | 25.71 (±3.62) | 24.75 (±3.32) | 0.100 |
| Medical History, n (%) | ||||||
| Diabetes mellitus | 104 (23.8%) | 11 (19%) | 35 (23.2%) | 35 (26.3%) | 23 (24.2%) | 0.740 |
| Hypertension | 291 (66.4%) | 40 (69%) | 84 (55.6%) | 102 (76.1%) | 65 (68.4%) | 0.003 |
| Hyperlipidemia | 96 (21.7%) | 17 (29.3%) | 28 (18.5%) | 19 (14.1%) | 32 (32.2%) | 0.003 |
| Coronary heart disease | 85 (19.2%) | 15 (25.9%) | 23 (15.2%) | 30 (22.6%) | 16 (16.9%) | 0.213 |
| TIA | 8 (1.8%) | 0 (0%) | 2 (1.3%) | 5 (3.8%) | 1 (1%) | 0.224 |
| Ischemic stroke | 105 (24%) | 11 (19%) | 31 (20.5%) | 37 (27.8%) | 26 (27.4%) | 0.327 |
| Cerebral hemorrhage | 2 (0.5%) | 1 (1.7%) | 1 (0.7%) | 0 | 0 | 0.359 |
| Atrial fibrillation | 116 (26.5%) | 16 (27.6%) | 37 (24.5%) | 39 (29.3%) | 24 (25.3%) | 0.811 |
| Valvular heart disease | 21 (4.8%) | 1 (1.7%) | 11 (7.3%) | 3 (2.2%) | 6 (6.1%) | 0.133 |
| Statins use | 108 (24.7%) | 20 (34.5%) | 48 (31.8%) | 28 (21.1%) | 12 (12.6%) | 0.001 |
| Smoking (recent or current) | 198 (44.6%) | 26 (44.8%) | 75 (49.7%) | 52 (39.4%) | 42 (44.7%) | 0.390 |
| Drinking (recent or current) | 163 (37.5%) | 19 (32.8%) | 63 (41.7%) | 47 (35.6%) | 34 (36.2%) | 0.577 |
| Laboratory investigations | ||||||
| Blood platelet count, median (IQR) | 199 (160; 234) | 198 (155; 233) | 193 (156; 234) | 192 (155; 234) | 207 (171; 241) | 0.389 |
| Fibrinogen, mean ± SD | 3.14 (±0.94) | 3.35 (±0.94) | 3.11 (±0.85) | 3.13 (±0.87) | 3.42 (±1.14) | <0.001 |
| Radiological examinations | ||||||
| ASPECTS/pc-ASPECTS, median (IQR) | 9 (8; 10) | 8 (8; 9) | 8 (7; 9) | 9 (8; 10) | 9 (7; 10) | 0.042 |
| Clinical characteristics | ||||||
| NIHSS on admission, mean ± SD | 18.23 (±9.13) | 15.47 (±5.62) | 16.46 (±6.75) | 20.02 (±9.86) | 18.98 (±10.98) | 0.004 |
| NIHSS ≥ 16 on admission, n(%) | 198 (52.8%) | 18 (47.4%) | 53 (47.3%) | 81 (62.3%) | 46 (48.4%) | 0.064 |
| Intravenous rt-PA, n(%) | 105 (24.2%) | 5 (8.8%) | 35 (23.3%) | 32 (24.1%) | 33 (34.7%) | 0.004 |
| Intra-arterial thrombolysis, n(%) | 80 (18.3%) | 27 (46.6%) | 36 (23.8%) | 17 (12.7%) | 0 | <0.001 |
| Location of occluded vessels, n (%) | 0.681 | |||||
| ICA | 114 (26%) | 11 (19%) | 41 (27.2%) | 39 (29.1%) | 23 (24.2%) | |
| MCA | 160 (36.5%) | 23 (39.7%) | 52 (34.4%) | 44 (32.8%) | 41 (43.2%) | |
| Basilar artery | 127 (29%) | 18 (31%) | 43 (28.5%) | 39 (29.1%) | 27 (28.4%) | |
| Other arteries | 37 (8.4%) | 6 (10.3%) | 15 (9.9%) | 12 (9%) | 4 (4.2%) | |
| Stroke subtype, n(%) | 0.061 | |||||
| Large–artery atherosclerosis | 313 (71.6%) | 44 (75.9%) | 101 (66.9%) | 100 (74.6%) | 68 (72.3%) | |
| Cardioembolism | 100 (22.9%) | 13 (22.4%) | 34 (22.5%) | 30 (22.4%) | 23 (24.5%) | |
| Other subtypes | 24 (5.5%) | 1 (1.7%) | 16 (10.6%) | 4 (3.0%) | 3 (3.2%) | |
| Treatment process time (min) | ||||||
| Onset to groin puncture, median (IQR) | 306 (227; 390) | 284 (231; 321) | 285 (217; 363) | 288 (224; 365) | 366 (255; 515) | <0.001 |
| Onset to recanalization, median (IQR) | 405 (330; 484) | 372 (330; 413) | 359 (294; 426) | 400 (333; 476) | 510 (410; 695) | <0.001 |
Data are displayed as n%, mean(±SD), or median(IQR). NIHSS: National Institutes of Health Stroke Scale; ASPECTS: Alberta Stroke Program Early CT score; pc-ASPECTS: posterior circulation Alberta Stroke Program Early CT Score; ICA: internal carotid artery; MCA: middle cerabral artery; TIA: transient ischemia attack; SD: standard deviation; IQR: interquartile range.
Functional outcomes
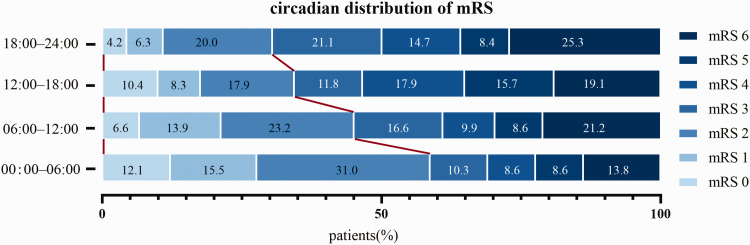
The functional outcomes of all enrolled patients at 3 months after EVT are summarized in Table 2. The mean 3-month mRS scores in the 00:00–06:00, 06:00–12:00, 12:00–18:00, and 18:00–24:00 time interval were 2.69 ± 1.91, 3.17 ± 1.94, 3.46 ± 1.93, and 3.67 ± 1.83, respectively (p = 0.012). After adjustment in a linear regression model, the time-of-day of stroke onset was independently associated with 3-month functional outcome (adjusted p = 0.007, Table 2 and Table S1). The distribution of mRS scores at 3 months after EVT based on the time-of-day of stroke onset was shown in Figure 1. The proportion of patients who achieved favorable outcomes at 3 months was 58.6%, 43.7%, 36.6%, and 30.5% in the 00:00–06:00, 06:00–12:00, 12:00–18:00, and 18:00–24:00 time interval, respectively (p = 0.004). After adjusting for confounding factors, the time-of-day of stroke onset was independently associated with functional outcomes at 3 months after EVT (adjusted OR 0.61 [95% CI 0.40–0.93]; adjusted p = 0.020, Table 2 and Table S2).
Table 2.
Clinical outcomes.
| All patients (n = 438) | 00:00–06:00 (n = 58) | 06:00–12:00 (n = 151) | 12:00–18:00 (n = 134) | 18:00–24:00 (n = 95) | p | 95% CI | Adjusted OR, (95% CI) | Adjusted p | |
|---|---|---|---|---|---|---|---|---|---|
| Efficacy outcomes | |||||||||
| 3-month mRS, Mean ± SD | 3.3 (±1.93) | 2.69 (±1.91) | 3.17 (±1.94) | 3.46 (±1.93) | 3.67 (±1.83) | 0.012 | NA | NA | 0.007a |
| 3-month mRS 0–2, n (%) | 178 (40.6%) | 34 (58.6%) | 66 (43.7%) | 49 (36.6%) | 29 (30.5%) | 0.004 | 2.63–2.86 | 0.611 (0.404–0.925)a | 0.020a |
| 3-month mortality, n (%) | 90 (20.8%) | 8 (13.8%) | 32 (21.2%) | 25 (19.1%) | 25 (27.2%) | 0.237 | 2.54–2.95 | 1.060 (0.627–1.791)a | 0.829a |
| Safety outcome | |||||||||
| Symptomatic ICH, n (%) | 40 (9.1%) | 1 (1.7%) | 18 (11.9%) | 14 (10.4%) | 8 (8.4%) | 0.142 | 2.45–2.96 | 0.684 (0.335–1.394)b | 0.296b |
| Early recanalization | |||||||||
| TICI ≥2b, n (%) | 376(86.2%) | 44 (75.9%) | 130 (86.1%) | 119 (90.2%) | 83 (87.4%) | 0.07 | 1.87–2.16 | 1.812 (0.944–3.479)c | 0.074c |
Data are displayed as n%, or mean(± SD); SD: standard deviation; OR: odds ratio; CI: confidence interval; TICI: thrombolysis in cerebral infarction; ICH: intracerebral hemorrhage; mRS: modified Rankin scale.
aAdjusted for NIHSS on admission, intravenous rt-PA, TICI scores, onset to groin puncture, onset to recanalization, gender, age, hypertention, hyperlipidemia, diabetes mellitus, atrial fibrillation, previous stroke, sICH.
bAdjusted for NIHSS on admission, intravenous rt-PA, TICI scores, onset to groin puncture, onset to recanalization, gender, age, hypertention, hyperlipidemia, diabetes mellitus, atrial fibrillation, previous stroke.
cAdjusted for NIHSS on admission, intravenous rt-PA, onset to groin puncture, onset to recanalization, gender, age, hypertention, hyperlipidemia, diabetes mellitus, atrial fibrillation, previous stroke, sICH.
Figure 1.
Distribution of modified Rankin Scale scores at 3-month after EVT in each 6-h interval group. The proportion of patients with favorable outcomes (mRS scores of 0–2) was 58.6% in the 00:00–06:00 time interval group, and was 43.7% in the 06:00–12:00 time interval group. mRS = modified Rankin Scale.
In addition, compared with the 18:00–24:00 time interval group, patients with stroke onset between 00:00 and 06:00 (adjusted OR, 4.01 [95% CI 1.02–15.80]; adjusted p = 0.047) and between 06:00 and 12:00 (adjusted OR, 3.24 [95% CI 1.09–9.64]; adjusted p = 0.034) were more likely to achieve favorable outcomes (Table 3).
Table 3.
Multivariable logistic regression for associations between clinical outcomes and the time of day at stroke onset.
| OR (95% CI) | p value | Adjusted OR (95% CI) | Adjusted p value | |
|---|---|---|---|---|
| 00:00–06:00 vs. 18:00–24:00 | ||||
| Favorable outcome (mRS 0–2)a | 3.224 (1.632–6.370) | 0.001 | 4.01 (1.021–15.801) | 0.047 |
| Mortalitya | 0.429 (0.179–1.030) | 0.058 | 0.536 (0.094–3.070) | 0.484 |
| sICHb | NA | 0.997 | NA | 0.998 |
| Early recanalization (TICI ≥2b)c | 0.454 (0.194–1.067) | 0.07 | 0.288 (0.038–2.187) | 0.229 |
| 06:00–12:00 vs. 18:00–24:00 | ||||
| Favorable outcome (mRS 0–2)a | 1.767 (1.027–3.039) | 0.04 | 3.241 (1.090–9.636) | 0.034 |
| Mortalitya | 0.721 (0.394–1.317) | 0.287 | 0.886 (0.244–3.214) | 0.854 |
| sICHb | 1.472 (0.613–3.533) | 0.387 | 5.605 (0.806–38.959) | 0.081 |
| Early recanalization (TICI ≥2b)c | 0.895 (0.418–1.915) | 0.775 | 0.329 (0.059–1.831) | 0.204 |
| 12:00–18:00 vs. 18:00–24:00 | ||||
| Favorable outcome (mRS 0–2)a | 1.312 (0.749–2.298) | 0.342 | 1.941 (0.645–5.838) | 0.238 |
| Mortalitya | 0.632 (0.336–1.191) | 0.156 | 0.327 (0.082–1.300) | 0.112 |
| sICHb | 1.269 (0.510–3.157) | 0.609 | 1.963 (0.252–15.291) | 0.52 |
| Early recanalization (TICI ≥2b)c | 1.323 (0.575–3.045) | 0.51 | 2.865 (0.252–32.554) | 0.396 |
Data are presented as odds ratios (OR) with 95% confidence interval (CI) . NIHSS: National Institutes of Health Stroke Scale; sICH: symptomatic intracerebral hemorrhage; mRS: modified Rankin scale; TICI: thrombolysis in cerebral infarction.
aAdjusted for NIHSS on admission, intravenous rt-PA, TICI scores, onset to groin puncture, onset to recanalization, gender, age, hypertention, hyperlipidemia, diabetes mellitus, atrial fibrillation, previous stroke, sICH.
bAdjusted for NIHSS on admission, intravenous rt-PA, TICI scores, onset to groin puncture, onset to recanalization, gender, age, hypertention, hyperlipidemia, diabetes mellitus, atrial fibrillation, previous stroke.
cAdjusted for NIHSS on admission, intravenous rt-PA, onset to groin puncture, onset to recanalization, gender, age, hypertention, hyperlipidemia, diabetes mellitus, atrial fibrillation, previous stroke, sICH.
Mortality
The 3-month mortality was 13.8%, 21.2%, 19.1%, and 27.2% in the 00:00–6:00, 06:00–12:00, 12:00–18:00, and 18:00–24:00 time interval, respectively (95% CI 2.54–2.95; p = 0.237, Table 2). No significant association between the time-of-day of stroke onset and the 3-month mortality was detected, and neither unadjusted nor adjusted p values showed significant difference if compared between the 18:00–24:00 time interval group and other three-time intervals (Table 3).
Symptomatic intracerebral hemorrhage and early recanalization
Symptomatic ICH and early successful recanalization results are summarized in Table 2. The rate of sICH was 9.1% for all enrolled patients, and incidences of sICH were 1.7%, 11.9%, 10.4%, and 8.4% in the 00:00–06:00, 06:00–12:00, 12:00–18:00, and 18:00–24:00 time interval, respectively (adjusted OR, 0.684 [95% CI 0.335–1.394]; adjusted p = 0.296, Table 2 and Table S3). In addition, the rate of early successful recanalization (TICI ≥ 2b) was 86.2% for all enrolled patients, and was 75.9%, 86.1%, 90.2%, and 87.4% in the 00:00–06:00, 06:00–12:00, 12:00–18:00, and 18:00–24:00 time interval, respectively (adjusted OR, 1.812 [95% CI 0.944–3.479]; adjusted p = 0.074, Table 2 and Table S3).
In addition, when compared with the 18:00–24:00 time interval group, neither unadjusted or adjusted p values showed significant difference between the time-of-day of stroke onset and the rate of sICH or the rate of early successful recanalization (Table 3).
Sensitivity analysis
Sensitivity analysis for enrolled patients with LAA (n = 313) showed that associations between the time-of-day of stroke onset and functional outcome were detected (Supplemental Table S4). The proportion of patients who achieved favorable outcome in the 00:00–06:00, 06:00–12:00, 12:00–18:00, and 18:00–24:00 time interval was 54.5%, 43.6%, 36%, and 29.4%, respectively (adjusted OR, 0.54 [95% CI 0.31–0.95]; adjusted p = 0.034). The rate of early successful recanalization was 72.7%, 85.1%, 91.8% and 88.2%, respectively (adjusted OR, 2.622 [95% CI 1.018–6.756]; adjusted p = 0.046). After adjusting for covariates, the time-of-day of stroke onset was also independently associated with functional outcome in AIS patients due to LAA. In addition, patients were also divided into the 00:00–12:00 and 12:00–24:00 time interval based on the physiological fluctuation of body temperature, the time-of-day of stroke onset was also independently associated with functional outcome at 3 months after EVT (Supplemental Table S5). The proportion of patients who achieved favorable outcomes was 47.8% and 34.1% in the 00:00–12:00, 12:00–24:00 time interval (adjusted OR, 0.424 [95% CI 0.189–0.952]; adjusted p = 0.037).
Discussion
In this study, we found that, in AIS patients who were treated with EVT, the time-of-day of stroke onset was associated with functional outcome at 3 months, and patients onset between 00:00 and 06:00 were more likely to achieve favorable outcomes. However, the time-of-day of stroke onset was not associated with the incidence of sICH, rate of early successful recanalization, or 3-month mortality.
Consistent with previous studies, this study found that circadian distribution existed in the incidence of stroke, with a much higher risk for stroke onset in the morning. This phenomenon may be attributable to a pro-thrombotic status in the morning, as previous studies have determined that the time to thrombotic vascular occlusion was shorter in the sleep-to-wake transition. 15 Moreover, hemodynamic stress (i.e., alterations in blood pressure) and increased sympathetic activity in the morning may also contribute to the higher risk for stroke by inducing plaque rupture. 2,16,17
Among this study population, we also found that stroke onset during 00:00 to 06:00 and 06:00 to 12:00 was associated with higher proportion of favorable outcomes at 3 months. One of the factors that may contribute to this result is the circadian variation in the penumbra area. In a rat model of cerebral ischemia/reperfusion (I/R) injury, the penumbra area was narrower in the active phase than in the resting phase, 18 indicating that timely reperfusion in the resting phase can salvage more cerebral infarction. Because the rate of successful revascularization was >80% for EVT in our study, the penumbra area is a critical factor for favorable outcomes of EVT at night. In addition, the circadian rhythm of body temperature may be another factor. Core body temperature falls to its lowest level during the night and rises to its highest in the evening. 19,20 A recent clinical study determined that body temperature during the intra-ischemia or post-ischemia phases was associated with clinical outcomes in patients undergoing EVT, and higher body temperature led to poorer functional outcomes. 21,22 Furthermore, although neither less robust collaterals nor the hypotension was ultimately translated into worse clinical outcome, the impact of the natural circadian rhythm of blood pressure should also be noticed, which reaches the highest level during the morning. It has been demonstrated that the intra-procedural hypotension had detrimental impact on collaterals, 23 and less robust collaterals predicted larger infarct size. 24 This might be a potential explanation for that clinical outcome of morning stroke was more favorable. The association between this phenomenon and blood-pressure should be evaluated in future trials. Therefore, we speculate that both circadian variation in the penumbra area and body temperature contribute to the association (s) between the time of day at stroke onset and functional outcome in AIS patients treated with EVT.
Consistent with previous studies (summarized in Tables 4 and 5), this study also found that patients onset between 12:00 and 18:00 experienced much severe stroke. This phenomenon may be attributed to the circadian secretion of melatonin. The pineal body mainly secretes melatonin at midnight (00:00) and peaks at 03:00. As a result, the serum level of melatonin after midnight is significantly higher than in the afternoon and evening. 33 Melatonin can protect the brain from I/R injury. Accumulating evidence has shown that both exogenous melatonin and melatonin agonists can reduce cerebral infarct size and promote the recovery of ischemic stroke. 34 –36 In addition, the circadian transcription and expression of clock genes may also contribute to this phenomenon. Experimental data have shown that I/R injury in mice induced at the middle of the resting phase resulted in smaller infarct volume, with increased expression of circadian proteins compared to other time periods. 25 Furthermore, circadian variation has also been reported in clinical studies of cardiovascular diseases. One study found that acute myocardial infarction size measured by cardiovascular magnetic resonance in a subset of ST segment elevated acute myocardial infarction patients treated with primary percutaneous coronary intervention, with the largest and smallest MI size occurring in patients with symptom onset between 00:00 and 01:00 and between 12:00 and 13:00 respectively. 37 Another study found that a circadian dependence of thrombus aspiration effectiveness with greatest myocardial salvage for patients with symptom onset between 06:00 and 17:59. 38
Table 4.
Effects of the time-of-day of stroke onset in rodent models.
| Reference | Year | Species, n for each group | Stroke model (I/R) | Time interval | Ischemic injury determination | Comments |
|---|---|---|---|---|---|---|
| Beker25 | 2017 | Balb/c mice(n = 7) | tMCAO, (1.5h/24 h) | ZT 0, ZT 6, ZT 12, ZT 18 | Cresyl violet | Less ischemic damage occurs in active phase (ZT18) |
| Esposito 18 | 2020 | C57BL/6 mice (n = 4) | tMCAO, (1h/24 h) | ZT 3–9ZT 15–21 | 2% TTC | Increased infarct size, but less infarct growth occurs in inactive phase (ZT 3–9) |
| Tischkau 26 | 2007 | SD rat (n = 6) | Global ischemia, (4 min/24 h) | ZT 6, ZT 14ZT 20 | Caspase activation | Increased ischemic damage occurs in active phase (ZT14) |
| Vinall 27 | 2000 | SD rat (n = 11–14) | tMCAO, (2 h/22 h) | ZT1, ZT4, ZT7, ZT10, ZT13, ZT16, ZT19, ZT22 | 2% TTC | Increased infarct size occurs in inactive phase (ZT4) |
tMCAO: transient middle cerebral artery occlusion; ZT: zeitgeber; TTC: 2,3,5–triphenyltetrazolium chloride; SD rat: Sprague Dawley rat; I/R: ischemia/reperfusion.
Table 5.
Effects of the time-of-day of stroke onset in AIS patients treated with reperfusion therapy.
| Study | Country | Year | Total patients | Age | Reperfusion therapy | Primary outcome | Secondary outcome | Findings |
|---|---|---|---|---|---|---|---|---|
| Curtze 28 | Finland | 2012 | 1427 | 69.0 | Thrombolysis | mRS at 3-months | DNTsICH | Negative |
| Cappellari 7 | Italy | 2014 | 476 | 71.2 | Thrombolysis | NIHSS at 24 h | HT | Administration of rt-PA during 12:00–24:00 was safer, while during 06:00–18:00 was less effective |
| Ding 29 | China | 2018 | 447 | 65.9 | Thrombolysis | NIHSS at 24 h | HT at 24 hmortality at 7-days | Negative |
| Lorenzano 30 | Italy | 2013 | 21513 | 69.0 | Thrombolysis | mRS at 3-months | sICH at 3-monthsmortality | Diurnal administration of rt-PA was more effective. |
| Rhoney 31 | USA | 2009 | 624 | NA | Thrombolysis | 3-months mortality | mRS at 3-monthssICH | 3-months mortality, mRS and lesion volume, asymptomatic hemorrhage was Negative, but less sICH between 4 am and 8am. |
| Vilas 6 | Spain | 2012 | 135 | 68.23 | Thrombolysis | Recanalization | mRS at 3-monthsNIHSS | Diurnal administration of rt-PA was beneficial for recanalization and favorable outcome |
| Hajdu 8 | Switzerland | 2021 | 1558 | 71.1 | EVT | mRS at 3-months | recanalization timemTICI scoresICH | Morning performance of EVT led to better favorable outcome |
| Korv 32 | Estonia | 2013 | 8878 | 64.5 | Rt-PA | mRS at 3-months; | sICH, NIHSS-24h | Negative |
| Benali 9 | France | 2020 | 169 | 75 | MT | mRS at 3-month | sICH, ENI, END, In-hospital mortality | Nighttime (6 pm–8 am) MT treatment was more effecitve. |
NIHSS: NIH Stroke Scale; mRS: modified Rankin scale; HT: hemorrhagic transformation; rt-PA: alteplase; sICH: symptomatic intracerebral hemorrhage; TICI: thrombolysis in cerebral infarction; DNT: door to needle time; EVT: endovascular therapy; MT: mechanical thrombectomy; ENI: early neurologic improvement; END: early neurologic deteration.
The present study has several limitations. First, this study was based on a prospectively registry cohort of patients with AIS who were treated with EVT, the inherent deficits of this study design may have introduced biases. Second, the onset timing of patients who experienced a wake-up stroke may be inaccurate. To minimize this deviation, all enrolled patients were assigned to the time frame of a 6-hour interval, and those without an accurate onset timing and whose stroke onset time could be allocated into ≥2 of the aforementioned 6-h intervals were excluded. Last, the early recanalization does not necessarily mean the affected brain circulation has been successfully reestablished, since numerous adverse events may compromise reperfusion following thrombectomy. 39 The no-reflow phenomenon may contribute to such futile recanalization, which is an infrequent phenomenon and could stand as an important therapeutic target. 40 Prospective studies are warranted to investigate that whether the stroke onset time is an important factor for tissue no-reflow.
Conclusion
In patients with AIS who underwent EVT, the time-of-day of stroke onset was associated with 3-month functional outcome, and those who experienced stroke onset either between 00:00 and 06:00 or between 06:00 and 12:00 were more likely to achieve favorable outcomes, but the time-of-day of stroke onset was not associated with the incidence of sICH, rate of early successful recanalization, or 3-month mortality. Further studies are needed to confirm these results and to investigate the underlying mechanisms.
Supplemental Material
Supplemental material, sj-pdf-1-jcb-10.1177_0271678X221111852 for Association between the time of day at stroke onset and functional outcome of acute ischemic stroke patients treated with endovascular therapy by Xian Wang, Xiaoyin Wang, Jin Ma, Milan Jia, Longfei Wu, Weili Li, Chuanhui Li, Chuanjie Wu, Changhong Ren, Xin Chen, Wenbo Zhao and Xunming Ji in Journal of Cerebral Blood Flow & Metabolism
Footnotes
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study has been supported by Beijing Nova Program (No. Z201100006820143), and National Natural Science Foundation of China (No. 82001257 and 82027802).
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions: Xian Wang, Wenbo Zhao, and Xunming Ji contributed to the study conception, Xiaoyin Wang, Jin Ma, Milan Jia contributed to the study design; Longfei Wu, Chuanhui Li, Weili Li, Chuanjie Wu, Changhong Ren, and Xin Chen collected the data; Xian Wang, Wenbo Zhao drafted the manuscript.
ORCID iDs: Xian Wang https://orcid.org/0000-0002-8263-8010
Longfei Wu https://orcid.org/0000-0002-0954-9126
Supplemental material: Supplemental material for this article is available online.
References
- 1.Scheer FA, Hu K, Evoniuk H, et al. Impact of the human circadian system, exercise, and their interaction on cardiovascular function. Proc Natl Acad Sci U S A 2010; 107: 20541–20546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lago A, Geffner D, Tembl J, et al. Circadian variation in acute ischemic stroke: a hospital-based study. Stroke 1998; 29: 1873–1875. [DOI] [PubMed] [Google Scholar]
- 3.Elliott WJ. Circadian variation in the timing of stroke onset: a meta-analysis. Stroke 1998; 29: 992–996. [DOI] [PubMed] [Google Scholar]
- 4.Reidler P, Brehm A, Sporns PB, et al. Circadian rhythm of ischaemic core progression in human stroke. J Neurol Neurosurg Psychiatry 2021; 1–4. [DOI] [PubMed] [Google Scholar]
- 5.Ryu WS, Hong KS, Jeong SW, et al. Association of ischemic stroke onset time with presenting severity, acute progression, and long-term outcome: a cohort study. PLoS Med 2022; 19: e1003910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vilas D, Gomis M, Blanco M, et al. Circadian rhythms in the efficacy of intravenous alteplase in patients with acute ischemic stroke and Middle cerebral artery occlusion. Chronobiol Int 2012; 29: 1383–1389. [DOI] [PubMed] [Google Scholar]
- 7.Cappellari M, Bovi P, Moretto G. Circadian variation in the effect of intravenous thrombolysis after non-lacunar stroke. J Thromb Thrombolysis 2014; 38: 253–259. [DOI] [PubMed] [Google Scholar]
- 8.Hajdu SD, Kaesmacher J, Michel PP, et al. Association of time of day when endovascular therapy for stroke starts and functional outcome. Neurology 2021; 96: e1124-1136–e1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Benali A, Moynier M, Dargazanli C, et al. Mechanical thrombectomy in nighttime hours: is there a difference in 90-Day clinical outcome for patients with ischemic stroke? Ajnr Am J Neuroradiol 2021; 42: 530–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ospel JM, Kashani N, Goyal M, et al. Time of day and endovascular treatment decision in acute stroke with relative endovascular treatment indication: insights from UNMASK EVT international survey. J Neurointerv Surg 2020; 12: 122–126. [DOI] [PubMed] [Google Scholar]
- 11.Zhao W, Shang S, Li C, et al. Long-term outcomes of acute ischemic stroke patients treated with endovascular thrombectomy: a real-world experience. J Neurol Sci 2018; 390: 77–83. [DOI] [PubMed] [Google Scholar]
- 12.Zhao W, Che R, Shang S, et al. Low-dose tirofiban improves functional outcome in acute ischemic stroke patients treated with endovascular thrombectomy. Stroke 2017; 48: 3289–3294. [DOI] [PubMed] [Google Scholar]
- 13.Hacke W, Kaste M, Bluhmki E, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med 2008; 359: 1317–1329. [DOI] [PubMed] [Google Scholar]
- 14.Joseph L, Anthony F, Dennis K, et al. Harrison's principles of internal medicine, https://accessmedicine.mhmedical.com/book.aspx?bookID=3095. 2022. (accessed 30 June 2022).
- 15.Westgate EJ, Cheng Y, Reilly DF, et al. Genetic components of the circadian clock regulate thrombogenesis in vivo. Circulation 2008; 117: 2087–2095. [DOI] [PubMed] [Google Scholar]
- 16.Sager HB, Husser O, Steffens S, et al. Time-of-day at symptom onset was not associated with infarct size and long-term prognosis in patients with ST-segment elevation myocardial infarction. J Transl Med 2019; 17: 180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao W, Wu C, Dornbos IID, et al. Multiphase adjuvant neuroprotection: a novel paradigm for improving acute ischemic stroke outcomes. Brain Circ 2020; 6: 11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Esposito E, Li W, T, Mandeville E, et al. Potential circadian effects on translational failure for neuroprotection. Nature 2020; 582: 395–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coiffard B, Diallo AB, Mezouar S, et al. A tangled threesome: circadian rhythm, body temperature variations, and the immune system. Biology (Basel) 2021; 10: 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tahsili-Fahadan P, Farrokh S, Geocadin R. Hypothermia and brain inflammation after cardiac arrest. Brain Circ 2018; 4: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Diprose WK, Liem B, Wang MTM, et al. Impact of body temperature before and after endovascular thrombectomy for large vessel occlusion stroke. Stroke 2020; 51: 1218–1225. [DOI] [PubMed] [Google Scholar]
- 22.Hoshide S, Kario K. Morning surge in blood pressure and stroke events in a large modern ambulatory blood pressure monitoring cohort: results of the JAMP study. Hypertension 2021; 78: 894–896. [DOI] [PubMed] [Google Scholar]
- 23.Raychev R, Liebeskind DS, Yoo AJ, et al. Physiologic predictors of collateral circulation and infarct growth during anesthesia – detailed analyses of the GOLIATH trial. J Cereb Blood Flow Metab 2020; 40: 1203–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rao VL, Mlynash M, Christensen S, et al. Collateral status contributes to differences between observed and predicted 24-h infarct volumes in DEFUSE 3. J Cereb Blood Flow Metab 2020; 40: 1966–1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beker MC, Caglayan B, Yalcin E, et al. Time-of-day dependent neuronal injury after ischemic stroke: implication of circadian clock transcriptional factor Bmal1 and survival kinase AKT. Mol Neurobiol 2018; 55: 2565–2576. [DOI] [PubMed] [Google Scholar]
- 26.Tischkau SA, Cohen JA, Stark JT, et al. Time-of-day affects expression of hippocampal markers for ischemic damage induced by global ischemia. Exp Neurol 2007; 208: 314–322. [DOI] [PubMed] [Google Scholar]
- 27.Vinall PE, Kramer MS, Heinel LA, et al. Temporal changes in sensitivity of rats to cerebral ischemic insult. J Neurosurg 2000; 93: 82–89. [DOI] [PubMed] [Google Scholar]
- 28.Sami C, Atte M, Satu M, et al. Does time of day or physician experience affect outcome of acute ischemic stroke patients treated with thrombolysis? A study from Finland. Int J Stroke 2012; 7: 511–516. [DOI] [PubMed]
- 29.Ding J, Bai Z, Zhou D, et al. Circadian rhythms may not influence the outcomes of thrombolysis in patients with ischemic stroke: a study from China. Chronobiol Int 2018; 35: 1533–1542. [DOI] [PubMed] [Google Scholar]
- 30.Lorenzano S, Ahmed N, Tatlisumak T, et al. Within-day and weekly variations of thrombolysis in acute ischemic stroke: results from safe implementation of treatments in stroke-international stroke thrombolysis register. Stroke 2014; 45: 176–184. [DOI] [PubMed] [Google Scholar]
- 31.Rhoney DH, Coplin WM, Lin Y, et al. Time of day, outcome, and response to thrombolytic therapy: the national institute of neurological disorders and stroke recombinant tissue plasminogen activator stroke trial experience. J Stroke Cerebrovasc Dis 2010; 19: 40–48. [DOI] [PubMed] [Google Scholar]
- 32.Kõrv J, Vibo R, Kadlecová P, et al. Benefit of thrombolysis for stroke is maintained around the clock: results from the SITS-EAST registry. Eur J Neurol 2014; 21: 112–117. [DOI] [PubMed] [Google Scholar]
- 33.Reiter RJ. Pineal melatonin: cell biology of its synthesis and of its physiological interactions. Endocr Rev 1991; 12: 151–180. [DOI] [PubMed] [Google Scholar]
- 34.Reiter RJ, Sainz RM, Lopez-Burillo S, et al. Melatonin ameliorates neurologic damage and neurophysiologic deficits in experimental models of stroke. Ann N Y Acad Sci 2003; 993: 35–47. discussion 48–53. [DOI] [PubMed] [Google Scholar]
- 35.Wu XL, Lu SS, Liu MR, et al. Melatonin receptor agonist ramelteon attenuates mouse acute and chronic ischemic brain injury. Acta Pharmacol Sin 2020; 41: 1016–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li W, Yang S. Targeting oxidative stress for the treatment of ischemic stroke: upstream and downstream therapeutic strategies. Brain Circ 2016; 2: 153–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bulluck H, Nicholas J, Crimi G, et al. Circadian variation in acute myocardial infarct size assessed by cardiovascular magnetic resonance in reperfused STEMI patients. Int J Cardiol 2017; 230: 149–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fournier S, Eeckhout E, Mangiacapra F, et al. Circadian variations of ischemic burden among patients with myocardial infarction undergoing primary percutaneous coronary intervention. Am Heart J 2012; 163: 208–213. [DOI] [PubMed] [Google Scholar]
- 39.Seners P, Turc G, Lion S, et al. Relationships between brain perfusion and early recanalization after intravenous thrombolysis for acute stroke with large vessel occlusion. J Cereb Blood Flow Metab 2020; 40: 667–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ter Schiphorst A, Charron S, Hassen WB, et al. Tissue no-reflow despite full recanalization following thrombectomy for anterior circulation stroke with proximal occlusion: a clinical study. J Cereb Blood Flow Metab 2021; 41: 253–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-jcb-10.1177_0271678X221111852 for Association between the time of day at stroke onset and functional outcome of acute ischemic stroke patients treated with endovascular therapy by Xian Wang, Xiaoyin Wang, Jin Ma, Milan Jia, Longfei Wu, Weili Li, Chuanhui Li, Chuanjie Wu, Changhong Ren, Xin Chen, Wenbo Zhao and Xunming Ji in Journal of Cerebral Blood Flow & Metabolism
Data Availability Statement
All supporting data for this analysis are available from the corresponding author upon reasonable request with study protocol and statistical analysis methods provided.