Abstract
Arcuate nucleus (ARH) dopaminergic neurons regulate several biological functions, including prolactin secretion and metabolism. These cells are responsive to growth hormone (GH), but it is still unknown whether GH action on ARH dopaminergic neurons is required to regulate different physiological aspects. Mice carrying specific deletion of GH receptor (GHR) in tyrosine hydroxylase (TH)- or dopamine transporter (DAT)-expressing cells were produced. We investigated possible changes in energy balance, glucose homeostasis, fertility, pup survival and restraint stress-induced prolactin release. GHR deletion in DAT- or TH-expressing cells did not cause changes in food intake, energy expenditure, ambulatory activity, nutrient oxidation, glucose tolerance, insulin sensitivity and counter regulatory response to hypoglycemia in male and female mice. In addition, GHR deletion in dopaminergic cells caused no gross effects on reproduction and pup survival. However, restraint stress-induced prolactin release was significantly impaired in DAT- and TH-specific GHR knockout male mice, as well as in pegvisomant-treated wild-type males, whereas an intact response was observed in females. Patch-clamp recordings were performed in ARH DAT neurons, and differently than prolactin GH did not cause acute changes in the electrical activity of DAT neurons. Furthermore, TH phosphorylation at Ser40 in ARH neurons and median eminence axonal terminals was not altered in DAT-specific GHR knockout male mice during restraint stress. In conclusion, GH action in dopaminergic neurons is required for stress-induced prolactin release in male mice, suggesting the existence of sex differences in the capacity of GHR signaling to affect prolactin secretion. The mechanism behind this regulation still needs to be identified.
Keywords: DAT, hypothalamus, pituitary, TIDA, tyrosine hydroxylase
Graphical Abstract
1. Introduction
Catecholaminergic neurons are found in numerous brain areas and play important roles regulating different physiological aspects, including arousal, sympathetic nervous system, motivation/reward, locomotion, metabolism and neuroendocrine axes (1). Dopamine, noradrenaline and adrenaline are the final products of the catecholamine biosynthesis, depending on the expression of specific enzymes that allow the synthesis of each of these substances (1). Tyrosine hydroxylase (TH) is the rate limiting enzyme in the catecholamine biosynthesis and TH expression is a common marker of all catecholamine neurons (1). Among the different types of catecholaminergic neurons, there is a population of cells located in the arcuate nucleus of hypothalamus (ARH) known as tuberoinfundibular dopaminergic (TIDA) neurons. TIDA neurons are predominantly found in the dorsal part of the ARH and project to the external layer of the median eminence (ME) to regulate pituitary prolactin secretion (2, 3). TIDA neurons inhibit prolactin synthesis and release via activation of dopamine D2 receptor in lactotropic cells (4). Additionally, TIDA neurons are highly responsive to prolactin representing the major negative-feedback loop to control the hypothalamic-prolactin axis (5–9). ARH dopaminergic neurons are also implicated in other physiological functions. Of note, recent studies indicate that ARH TH-expressing cells regulate metabolism (10, 11).
Growth hormone (GH) receptor (GHR) expression is found in ARH neurons (12). Moreover, an acute systemic injection of GH produces a robust activation of the signal transducer and activator of transcription 5 (STAT5) signaling pathway in the ARH (13). Interestingly, STAT5 transcription factors represent the major intracellular pathway recruited by both prolactin and GH (14). Recent studies have investigated possible physiological roles played by GH in specific ARH neurons. GH action on ARH agouti-related peptide (AgRP)-producing neurons regulates metabolic and neuroendocrine adaptations trigged during food restriction (15), partially via STAT5-dependent mechanisms (16). GHR expression in proopiomelanocortin-expressing cells is required for the hyperphagia induced during glucoprivic situations (17). In contrast, ARH kisspeptin-producing neurons do not exhibit GH-induced STAT5 phosphorylation (pSTAT5), suggesting that these cells do not express GHR (18).
A significant percentage of ARH TH-expressing neurons exhibit GH-induced pSTAT5 and the importance of GH signaling in TH cells was recently investigated (19). Noteworthy, GHR deletion from TH-expressing cells leads to increased GH secretion and body growth in mice (19). Thus, TH neurons are implicated in the control of GH secretion via a negative-feedback loop (19). However, it is still unknown whether GH action on catecholaminergic neurons regulates other physiological functions, particularly metabolism and prolactin secretion. Thus, the objective of the present study was to investigate the importance of GH action on physiological functions regulated by ARH dopaminergic neurons. For this purpose, we produced two cell-specific knockout mice by ablating GHR either in TH- or dopamine transporter (DAT)-expressing cells. Of note, DAT is not expressed in noradrenergic or adrenergic neurons (20), which ensures that the response to GH in DAT-specific GHR knockout mice mainly affects subpopulations of dopaminergic neurons (19, 21). Thus, the combined results from DAT- and TH-specific GHR knockout mice can provide a most reliable idea of the importance of GHR signaling to the biological functions controlled by ARH dopaminergic neurons.
2. Materials and Methods
2.1. Mice
Cell-specific ablation of GHR was achieved by breeding mice carrying loxP-flanked Ghr alleles (22) with DAT-Cre mice (The Jackson Laboratory, Bar Harbor, ME; RRID: IMSR_JAX:006660) or TH-Cre mice (The Jackson Laboratory; RRID: IMSR_JAX:008601). Control (CON) and conditional knockout mice (Cre-expressing animals) were homozygous for the loxP-flanked Ghr alleles. To visualize DAT-expressing cells, a LoxP-STOP-LoxP-GAG-tdTomato reporter mouse (The Jackson Laboratory; RRID: IMSR_JAX:007909) was bred until producing DAT-Cre and DAT-Cre/GHRflox/flox mice carrying the reporter allele. Mice were in the C57BL/6 background, weaned at 3–4 weeks of age and their mutations were confirmed by genotyping the DNA that had been previously extracted from the tail tip using a commercially available kit (REDExtract-N-Amp™ Tissue PCR Kit, Sigma-Aldrich, St. Louis, MO). Mice received a regular rodent chow diet and filtered water ad libitum. The experiments performed were previously approved by the Ethics Committee on the Use of Animals of the Institute of Biomedical Sciences, University of São Paulo (protocol number: 73/2017).
2.2. Detection of GH-responsive dopaminergic neurons
Adult CON (2 males and 1 female), DAT- (3 males) and TH-specific (4 males and 2 females) GHR knockout mice received an acute intraperitoneal (i.p.) injection of 20 μg/g b.w. of porcine pituitary GH [from Dr. A.F. Parlow, National Hormone and Pituitary Program (NHPP), Torrance, CA]. Another group of CON mice (3 males) received i.p. injection of saline. After 90 minutes, mice were anesthetized with isoflurane and perfused with saline followed by 10% buffered formalin. Brains were cryoprotected overnight in 20% sucrose and cut in 30-μm thick sections using a freezing microtome. Brain slices were rinsed in 0.02 M potassium PBS, pH 7.4 (KPBS), followed by pretreatment in water solution containing 1% hydrogen peroxide and 1% sodium hydroxide for 20 min. After rinsing in KPBS, sections were incubated in 0.3% glycine and 0.03% lauryl sulfate for 10 min each. Next, slices were blocked in 3% normal donkey serum for 1 h, followed by incubation in a primary antibody cocktail containing anti-pSTAT5Tyr694 made in rabbit (Cell Signaling, Beverly, MA, Cat# 9351; RRID: AB_2315225; 1:1,000) and anti-TH made in mouse (Immunostar, Hudson, WI; #22941; RRID: AB_572268; 1:2,500) for 40 h. The anti-TH primary antibody was not used in brain sections of DAT-specific GHR knockout mice because these animals carried the tdTomato reporter protein, so no reaction was necessary to visualize DAT-expressing cells. Then, sections were rinsed in KPBS and incubated for 90 min in AlexaFluor488- and AlexaFluor594-conjugated secondary antibodies (1:500, Jackson ImmunoResearch Laboratories, Cambridge, MA). After rinses in KPBS, sections were mounted onto gelatin-coated slides that were covered with Fluoromount G mounting medium (Electron Microscopic Sciences, Hatfield, PA).
2.3. Evaluation of energy balance
Possible changes in energy balance were evaluated in 5-month-old male and female mice. Mice were housed in individual cages for one week for acclimation. Then, body weight and food intake were measured for approximately 5 consecutive days. O2 consumption, CO2 production, ambulatory activity (by infrared sensors) and respiratory exchange ratio (RER; calculated by CO2 production/ O2 consumption) were determined using the Oxymax/Comprehensive Lab Animal Monitoring System (Columbus Instruments, Columbus, OH) for approximately 7 days. The data from the first 2–3 days of analysis were discarded because we considered acclimation period. The results used for each animal were the average of the analyzed days.
2.4. Evaluation of glucose homeostasis and counter regulatory response to hypoglycemia
Glycemia was measured using a glucose meter (One Touch Ultra; Johnson & Johnson, Milpitas, CA) via blood samples collected from the tail tip. Food was removed from cage for 4 hours before each test. Five-month-old mice were subjected to a glucose tolerance test (GTT; 2 g glucose/kg b.w.; i.p.), insulin tolerance test (ITT; 1 IU insulin/kg b.w.; i.p.) and counter regulatory response induced by an i.p. injection of 0.5 g/kg 2-deoxy-D-glucose (2DG; Sigma-Aldrich). To avoid stress, there was a 5-day interval between the tests. Blood glucose levels were determined at 0, 20, 40, 60, 90 120 and 180 min after the injections.
2.5. Evaluation of fertility and pup survival
CON, DAT- and TH-specific GHR knockout female mice were bred with GHRflox/flox male mice and the number of days to give birth was measured. To determine pup survival, we counted the number of viable pups in the offspring at weaning time.
2.6. Restraint stress and blood collections
Five-month-old CON, DAT- and TH-specific GHR knockout mice were physically restrained for 120 min in 50-ml polypropylene centrifuge tubes, containing holes of 0.4 cm diameter to breathe. This immobilization allows limb movements, but the animal was unable to turn 180°. Additionally, a group of C57BL/6 wild-type mice that received an i.p. injection of the GHR antagonist pegvisomant (20 μg/g b.w.; Pfizer, Puurs, Belgium) just before the beginning of restraint stress was also evaluated. Blood samples (30 μL each) were obtained through a small cut in the tail tip, immediately following the onset of stress (0) and at intervals of 15, 30, 45, 60, 90 and 120 minutes, using heparinized capillaries (75 mm length and 1.5 mm diameter). After each collection, blood was centrifuged and plasma was collected and stored at −80°C.
2.7. Measurement of plasma prolactin levels
Plasma prolactin levels were determined using ultrasensitive prolactin enzyme-linked immunosorbent assay. The assay was performed as previously described (23), with the exception that mouse prolactin (AFP-6476C, NHPP) was used as reference preparation. Briefly, a 96-well high-binding plate (9018, Corning, Kennebunk, ME) was coated with 50 μL of guinea pig anti-rat prolactin antibody (AFP65191, NIDDK-NHPP; RRID: AB_2756841; 1:1,500) overnight at 4 ºC. The antibody was decanted and wells were incubated with 200 μL of 5% skim milk powder in 0.05% tween-20 phosphate-buffered saline for 2 h at room temperature (RT). A standard curve was generated using a 2-fold serial dilution of the mouse prolactin reference preparation (AFP-6476C). The wells were incubated with 50 μL of samples for 24 hours at RT. The plate was washed, and the wells were incubated with 50 μL of the rabbit anti-rat prolactin antibody (AFP131581570, NHPP; RRID: AB_2722653; 1:70,000) for 24 h at 4 ºC. After washing, wells were incubated with 50 μL of horseradish peroxidase-conjugated goat anti-rabbit IgG (P044801–2, Dako Pathology Solutions, Santa Clara, CA; RRID: AB_2617138; 1:2,000) for 90 min at RT. Following a final wash, wells were incubated with 100 μL of 2 mg/mL o-phenylenediamine dihydrochloride (P1526, Sigma-Aldrich) diluted in citrate-phosphate buffer (pH 5.0) containing 0.02% hydrogen peroxide for 45 min at RT. The absorbance was determined at 490 nm using a microplate reader, and the wavelength of 650 nm was used for background correction. The concentrations of prolactin were obtained by interpolating the optical density of samples against a nonlinear regression of the standard curve. The intra-assay and inter-assay coefficients of variation were 7.9% and 10.6%, respectively.
2.8. Electrophysiology
To analyze the potential effects of GH or prolactin on the membrane excitability of dopaminergic neurons in the dorsal ARH, whole-cell patch-clamp recordings were performed in hypothalamic slices of adult (8 to 12-week-old) DAT-Cre/tdTomato-reporter mice. Mice were decapitated, their brains collected and immediately submerged in ice-cold, carbogen-saturated (95% O2 and 5% CO2) artificial cerebrospinal fluid (aCSF; 124 mM NaCl, 2.8 mM KCl, 26 mM NaHCO3, 1.25 mM NaH2PO4, 1.2 mM MgSO4, 5 mM glucose and 2.5 mM CaCl2). Coronal sections (250 μM thick) from a hypothalamic block were cut with a vibratome (Leica Biosystems, model: VT1000S, Buffalo Grove, IL) and then incubated in oxygenated aCSF at RT for at least 1h before the recording. Slices were transferred to the recording chamber and allowed to equilibrate for 10–20 min before the recording. The slices were bathed in oxygenated aCSF (30°C) at a flow rate of 2 mL/min. In current-clamp mode, neurons were recorded under zero current injection (I = 0) in whole-cell patch-clamp configuration. The pipette solution was composed of: 120 mM K-gluconate, 1 mM NaCl, 10 mM KCl, 10 mM HEPES, 5 mM EGTA, 1 mM CaCl2, 1 mM MgCl2, 3 mM KOH and 4 mM (Mg)- ATP, pH 7.3. The pipettes had a resistance of 5–7 MΩ. The membrane potential was monitored for at least 5 min (Basal), followed by the addition of porcine GH (5 μg/mL) or ovine prolactin (6.25 μg/mL; Sigma-Aldrich) to the bath for approximately 5 min. The effects of GH and prolactin were monitored for up to 10 minutes. Only one cell was recorded in each brain slice. The membrane potential values were compensated to account for the junction potential (−8 mV). Average frequency of action potentials was determined offline using the Mini Analysis Program (Synaptosoft, Decatur, GA; RRID: SCR_002184), by analyzing 2 minutes before, during and after the administration of the hormone to the bath. Series resistance was < 20 MΩ. Data were discarded if a change of 20% or more occurred during the recording.
2.9. Immunofluorescence reaction to stain total TH and TH phosphorylation
CON and DAT-specific GHR knockout mice were perfused in basal conditions (non-stressed animals; n = 6/group) or after 45 min of restraint stress (n = 4–5/group). Two consecutive brain series were subjected to immunofluorescence reactions to stain total TH and TH phosphorylation at the Ser40 residue (pTH), which is known to increase TH activity (7, 24–26). For this purpose, brain slices were rinsed in KPBS, blocked in 3% normal donkey serum for 1 h, followed by incubation overnight in anti-TH antibody (Abcam, Cambridge, UK; #ab112; RRID: AB_297840; 1:1,000) or anti-pTHSer40 antibody (Thermo Fisher Scientific, Waltham, MA; #36–8600; RRID: AB_138590; 1:10,000). Subsequently, brain sections were rinsed in KPBS, incubated for 90 min in AlexaFluor488-conjugated secondary antibody (1:500, Jackson ImmunoResearch Laboratories) and mounted onto gelatin-coated slides. The slides were covered with Fluoromount G mounting medium (Electron Microscopic Sciences).
2.10. Image analysis
Photomicrographs were acquired with a Zeiss Axiocam HRc camera coupled to a Zeiss Axioimager A1 microscope (Zeiss, Munich, Germany). Images were digitized using Axiovision software (Zeiss). The ImageJ Cell Counter software (http://rsb.info.nih.gov/ij/) was used to manually count the number of single (TH or pTH)- or doubled-labeled cells (pSTAT5/TH) in three rostrocaudal levels of the ARH [Bregma: −1.25 to −1.55 mm, according to the Allen Brain Atlas (http://mouse.brain-map.org/)]. To determine the density of TH and pTH axonal terminals in the external layer of the ME, epifluorescence photomicrographs were acquired in three rostrocaudal levels (Bregma: −1.25 to −1.55 mm). Using ImageJ software, we drew a rectangle that comprised approximately one third of the external layer of the ME. Then, we used the Measure tool to obtain the integrated optical density (IOD), which represents the average staining intensity in the selected area. The IOD obtained in the external layer of the ME was subtracted from the background IOD. The values obtained at different rostrocaudal levels were averaged, representing the data for each animal.
2.11. Statistical analysis
Data were expressed as mean ± s.e.m. and analyzed with GraphPad Prism software (San Diego, CA). Comparisons between two groups were performed using unpaired two-tailed Student’s t-test. When three groups were compared simultaneously, the analysis was performed by one-way ANOVA with Tukey’s test for multiple comparisons. Changes along time in the GTT, ITT, 2DG test and stress-induced prolactin release were determined by repeated measures two-way ANOVA. The electrophysiological data between basal, treatment and washout periods were compared by repeated measures one-way ANOVA. Differences were considered significant if P ≤ 0.05.
3. Results
3.1. TIDA neurons are responsive to GH
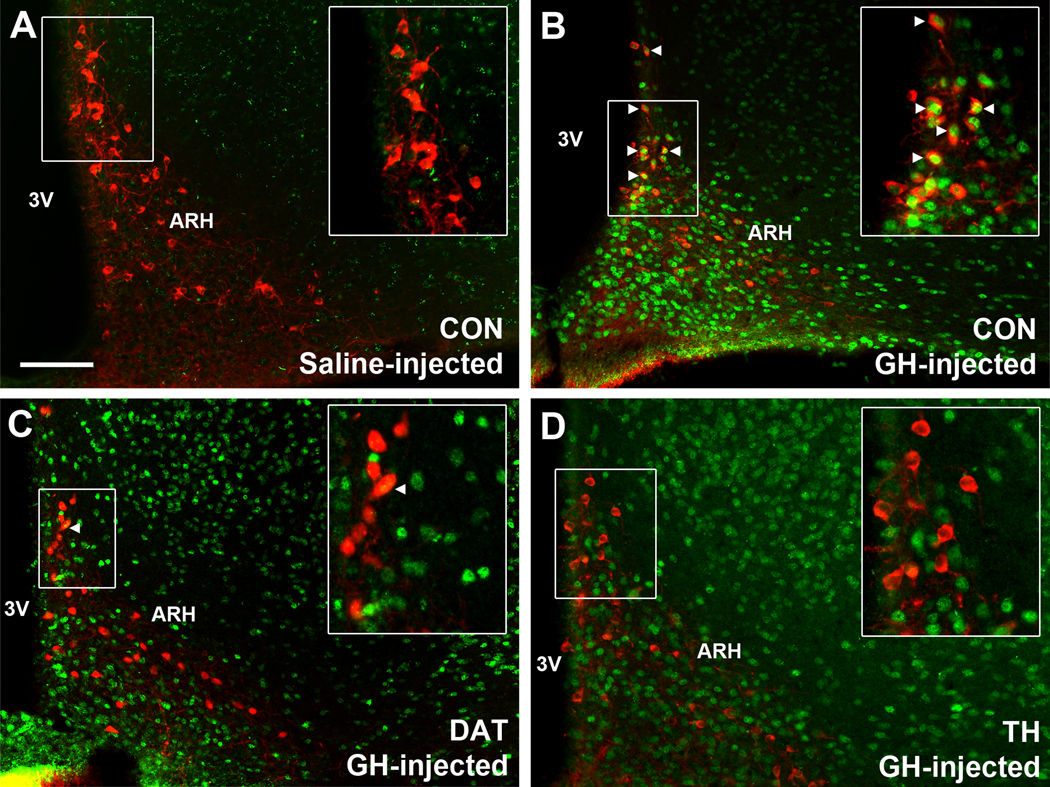
To demonstrate that TH-expressing neurons in the ARH are directly responsive to GH, CON mice received an acute i.p. injection of saline or GH and their brains were processed to detect pSTAT5 immunoreactivity (Fig. 1A–B). Whereas saline-treated mice (3 males) exhibited virtually no pSTAT5 (Fig. 1A), GH-injected CON mice (2 males and 1 female) showed a large amount of pSTAT5 immunoreactive cells in the ARH (Fig. 1B), ventromedial nucleus of the hypothalamus (VMH; Fig. 1B) and other brain areas (data not shown). Importantly, 52.6 ± 4.7% of ARH TH-expressing neurons co-expressed GH-induced pSTAT5, especially in its dorsal aspects (Fig. 1B), indicating that TIDA neurons are directly responsive to GH. The percentage of pSTAT5/TH co-localization in the ARH was similar between males (55 ± 6%) and the female (47%). To induce deletion of GHR in TIDA neurons, DAT-Cre and TH-Cre mice were crossed with GHR-flox animals. To demonstrate the specificity of the cell-specific GHR deletions, these knockouts received GH injections to induce pSTAT5 in the brain (Fig. 1C–D). In mice carrying ablation of GHR in DAT-expressing cells (3 males), only 7.8 ± 2.6% of ARH DAT neurons exhibited pSTAT5, whereas a normal distribution of GH-induced pSTAT5 was observed in ARH non-DAT cells and other brain nuclei (Fig. 1C). It is worth mentioning that only part of ARH TH neurons express DAT (21), so a group of TH-positive, but DAT-negative cells remain responsive to GH in the ARH of DAT-specific GHR knockout mice, as demonstrated earlier (19). In mice carrying ablation of GHR in TH-expressing cells (4 males and 2 females), we observed 2.4 ± 0.6% of ARH TH cells expressing pSTAT5 after an acute GH stimulus, whereas GH-responsive cells were normally observed in non-TH positive neurons (Fig. 1D). These results confirm the efficacy of the cell-specific GHR deletions.
Figure 1. TIDA neurons are responsive to GH.
A-D Epifluorescence photomicrographs showing the distribution of dopaminergic neurons (red) and pSTAT5 (green) in saline-injected CON mice (A), GH-injected CON mice (B), GH-injected DAT-specific GHR knockout mice (C) and GH-injected TH-specific GHR knockout mice (D). Examples of dopaminergic neurons that are responsive to GH are indicated by arrowheads. Abbreviations: 3V, third ventricle; ARH, arcuate nucleus; VMH, ventromedial nucleus of the hypothalamus. Scale bar = 100 μm.
3.2. GHR expression in dopaminergic neurons is not necessary for the maintenance of energy homeostasis
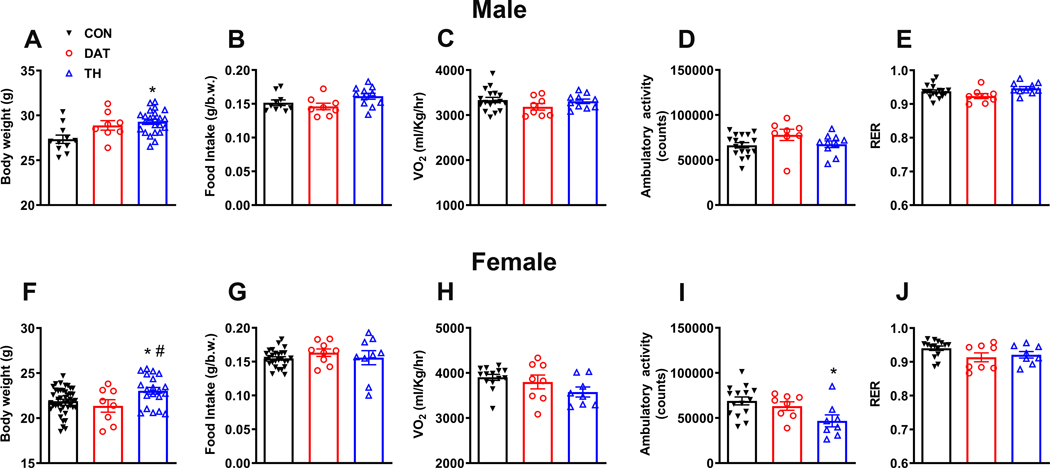
Previous studies indicate that hypothalamic dopaminergic neurons regulate metabolism (10, 11, 27, 28). GHR signaling in specific hypothalamic neurons also produces changes in food intake, body weight and energy expenditure (15, 17, 29–31). Thus, it is possible that GH action on hypothalamic dopaminergic neurons regulates different metabolic aspects. To investigate this possibility, we determined whether mice carrying ablation of GHR in DAT- or TH-expressing cells exhibit alterations in energy homeostasis. We observed in both males (F(2, 39) = 7.154, P = 0.0023; Fig. 2A) and females (F(2, 69) = 4.865, P = 0.0106; Fig. 2A) that TH-specific GHR ablation led to increased body weight. This phenotype was detailed characterized in a recent publication from our group showing that these animals exhibit increased GH secretion due to defects in hypothalamic negative feedback, without exhibiting alterations in body adiposity (19). In contrast, DAT-specific GHR deletion did not affect body weight (Fig. 2A, F). Inactivation of GHR in TH- or DAT-expressing cells did not cause changes in food intake (Fig. 2B, G). Additionally, GHR deletion in TH- or DAT-expressing neurons caused no significant changes in energy expenditure (Fig. 2C, H) and RER (Fig. 2E, J). However, ambulatory activity was reduced in female mice carrying ablation of GHR in TH-expressing cells (F(2, 28) = 4.759, P = 0.0166; Fig. 2I), whereas no alterations were observed in males (Fig. 2D). Thus, GHR expression in dopaminergic cells is not necessary for the maintenance of energy homeostasis.
Figure 2. GHR expression in dopaminergic neurons is not necessary for the maintenance of energy homeostasis.
A Body weight in CON (n = 11), DAT (n = 8) and TH (n = 23) male mice. B Daily food intake in CON (n = 10), DAT (n = 8) and TH (n = 11) male mice. C Oxygen consumption in CON (n = 17), DAT (n = 8) and TH (n = 10) male mice. D Ambulatory activity in CON (n = 17), DAT (n = 8) and TH (n = 10) male mice. E Respiratory exchange ratio (RER) in CON (n = 17), DAT (n = 8) and TH (n = 10) male mice. F Body weight in CON (n = 44), DAT (n = 8) and TH (n = 20) female mice. G Daily food intake in CON (n = 27), DAT (n = 9) and TH (n = 9) female mice. H Oxygen consumption in CON (n = 14), DAT (n = 8) and TH (n = 8) female mice. I Ambulatory activity in CON (n = 15), DAT (n = 8) and TH (n = 8) female mice. J RER in CON (n = 15), DAT (n = 8) and TH (n = 8) female mice. Differences between the groups were determined by one-way ANOVA and Tukey’s test for multiple comparisons (* P < 0.05, CON versus TH; # P < 0.05, DAT versus TH).
3.3. Glucose homeostasis is not altered by GHR deletion in dopaminergic cells
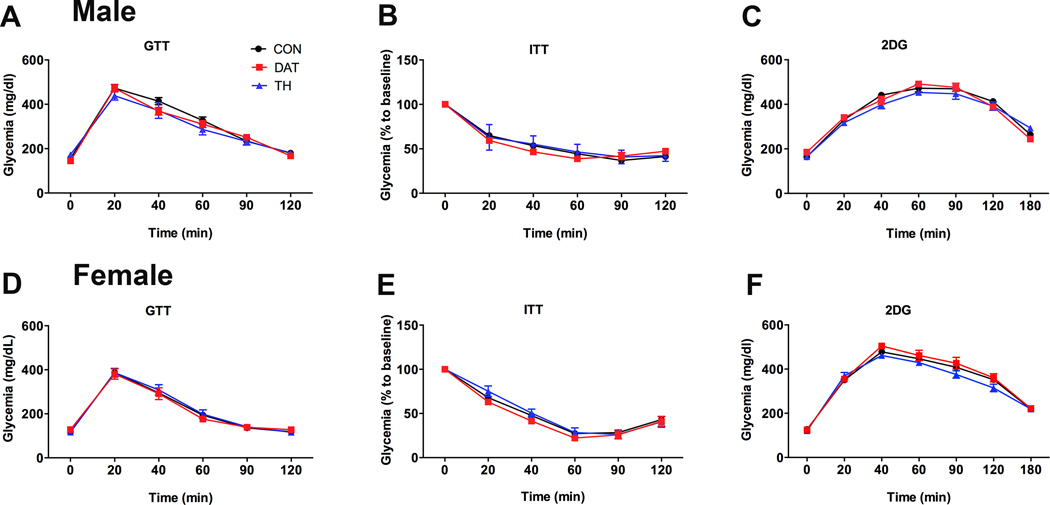
Genetic manipulations in dopaminergic neurons alter glucose homeostasis (28). Furthermore, dopaminergic drugs are known to affect glucose tolerance and insulin sensitivity, possibly via central mechanisms (27, 32–34). Thus, glucose homeostasis was also evaluated in mice carrying GHR deletion in dopaminergic cells. In male mice, no statistically significant effects of GHR deletion in DAT- or TH-expressing cells were observed in the glucose tolerance test (main effect of time [F(3, 135) = 298.0, P < 0.0001], main effect of GHR ablation [F(2, 46) = 0.694, P = 0.5047] and interaction [F(10, 230) = 2.01, P = 0.0333]; Fig. 3A) or insulin tolerance test (main effect of time [F(3, 115) = 446.1, P < 0.0001], main effect of GHR ablation [F(2, 46) = 0.3506, P = 0.7061] and interaction [F(10, 230) = 2.818, P = 0.0026]; Fig. 3B), although a significant interaction was found. When the area under the curve was calculated, no significant changes between the groups of males were observed in the glucose tolerance test (F(2, 46) = 0.7373, P = 0.484; data not shown) and insulin tolerance test (F(2, 46) = 0.4189, P = 0.6602; data not shown). Similar to the results found in males, GHR ablation in DAT or TH cells of female mice caused no significant effects on glucose tolerance (main effect of time [F(3, 115) = 215.2, P < 0.0001], main effect of GHR ablation [F(2, 40) = 0.063, P = 0.9387] and interaction [F(10, 200) = 0.3111, P = 0.9777]; Fig. 3D) or insulin sensitivity (main effect of time [F(3, 117) = 529.8, P < 0.0001], main effect of GHR ablation [F(2, 36) = 2.215, P = 0.1238] and interaction [F(10, 180) = 1.498, P = 0.1432]; Fig. 3E). Central GH signaling regulates the counter regulatory response to 2DG infusion (30). Thus, the glucoprivic effects induced by 2DG were also evaluated in the present study. In accordance with the absence of changes in glucose homeostasis, male (main effect of time [F(4, 173) = 261.1, P < 0.0001], main effect of GHR ablation [F(2, 41) = 0.3856, P = 0.6825] and interaction [F(12, 246) = 2.023, P = 0.0229]; Fig. 3C) and female (main effect of time [F(3, 117) = 529.8, P < 0.0001], main effect of GHR ablation [F(2, 36) = 2.215, P = 0.1238] and interaction [F(10, 180) = 1.498, P = 0.1432]; Fig. 3F) mice carrying GHR ablation in DAT- or TH-expressing cells exhibited no changes in the counter regulatory response caused by 2DG administration.
Figure 3. Glucose homeostasis is not altered by GH action on dopaminergic cells.
A Glucose tolerance test (GTT) in CON (n = 27), DAT (n = 10) and TH (n = 12) male mice. B Insulin tolerance test (ITT) in CON (n = 27), DAT (n = 10) and TH (n = 13) male mice. C Blood glucose levels during a counter-regulatory response induced by 2-deoxy-D-glucose (2DG) infusion in CON (n = 24), DAT (n = 9) and TH (n = 10) male mice. D GTT in CON (n = 25), DAT (n = 9) and TH (n = 9) female mice. E ITT in CON (n = 23), DAT (n = 7) and TH (n = 9) female mice. F Counter-regulatory response induced by 2DG infusion in CON (n = 25), DAT (n = 9) and TH (n = 9) female mice. Differences between the groups were determined by repeated measures two-way ANOVA.
3.4. GHR deletion in dopaminergic cells causes no gross effects on reproduction and pup survival
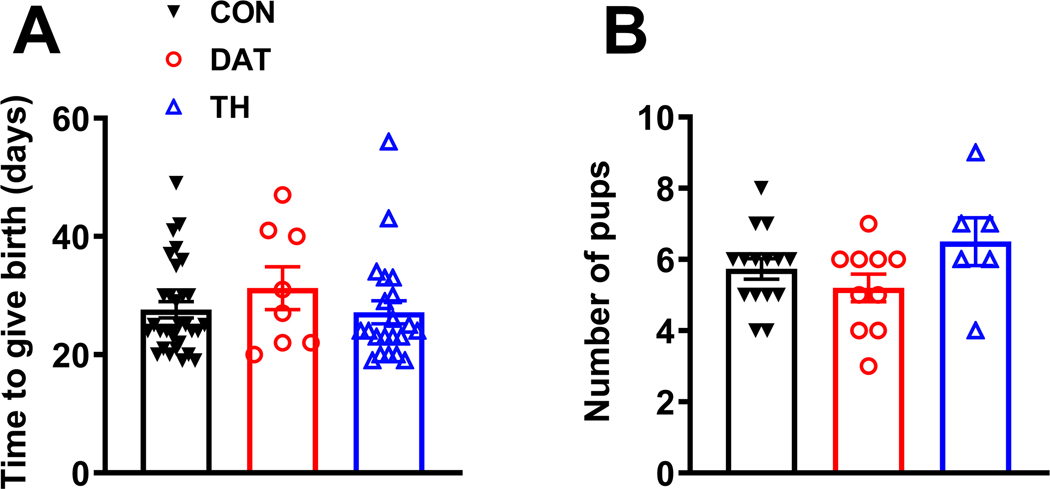
Prolactin secretion exerts an important function regulating reproduction and lactation (4, 35). Thus, we determined whether GHR deletion in TH- or DAT-expressing cells affects fertility and number of viable pups in the offspring. We found that the average time to give birth after the initiation of mating was similar among CON, DAT and TH female mice (Fig. 4A). Moreover, the average number of pups at weaning was not significantly affected by GHR deletion (Fig. 4B).
Figure 4. GHR deletion in dopaminergic cells causes no gross effects on reproduction and pup survival in female mice.
A Average time to give birth after the initiation of mating in CON (n = 31), DAT (n = 8) and TH (n = 21) female mice. B Average number of pups at weaning in CON (n = 15), DAT (n = 10) and TH (n = 6) female mice. Differences between the groups were determined by one-way ANOVA and Tukey’s test for multiple comparisons.
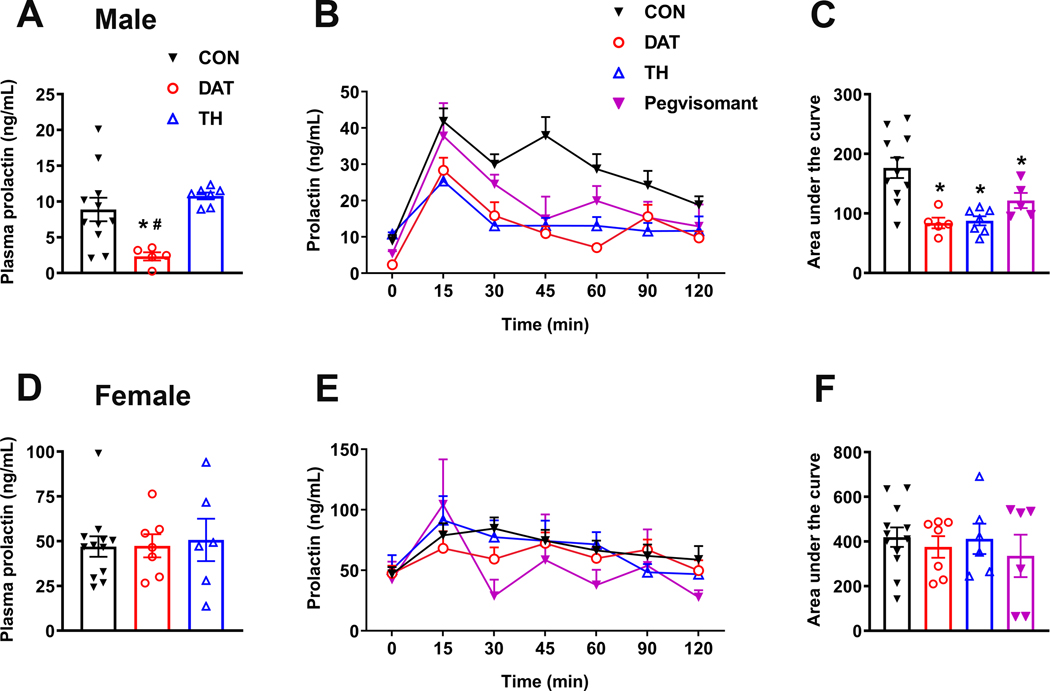
3.5. GH action on dopaminergic neurons regulates stress-induced prolactin release only in male mice
Since TIDA neurons are responsive to GH and they regulate pituitary prolactin secretion (2, 3), we determined whether GHR ablation in TH- or DAT-expressing cells affects basal prolactin levels. Basal prolactin levels, corresponding to time zero in the serial blood bleeding, were higher in females than in males (Fig. 5A, D), as expected. Interestingly, inactivation of GHR in DAT-, but not in TH-expressing cells, reduced basal prolactin levels in male mice (F(2, 20) = 7.13, P = 0.0046; Fig. 5A). On the other hand, plasma prolactin was not affected by GHR deletion in female mice (Fig. 5D). Previous studies have shown that GH action in specific hypothalamic neurons is necessary for triggering neuroendocrine responses during situations of metabolic stress (15, 17, 30). Restraint stress leads to a rapid increase in prolactin secretion, whereas GH release is inhibited (36–39). Given the fact that GHR signaling in TIDA neurons may modulate their function and consequently prolactin secretion, we investigated whether GH action on TH- or DAT-expressing cells is necessary for restraint stress-induced prolactin secretion. In CON mice, restraint stress induced a significant increase in plasma prolactin levels in males (main effect of stress [F(6, 144) = 24.41, P < 0.0001], main effect of GHR ablation [F(3, 24) = 9.2, P = 0.0003] and interaction [F(18, 144) = 2.405, P = 0.0022]; Fig. 5B) and females (main effect of stress [F(4, 96) = 6.8, P = 0.0001], main effect of GHR ablation [F(3, 27) = 0.4919, P = 0.6909] and interaction [F(18, 159) = 1.36, P = 0.1589]; Fig. 5E). Remarkably, male mice carrying deletion of GHR in DAT or TH cells exhibited an attenuated response of prolactin to restraint stress, leading to an area under the curve of plasma prolactin levels significantly lower than CON animals (F(3, 24) = 9.496, P = 0.0003; Fig. 5C). In contrast, GHR inactivation in female mice did not affect stress-induced prolactin secretion (Fig. 5F). To further evaluate the role of GH signaling in regulating stress-induced prolactin secretion, we tested a group of wild-type animals that were treated with pegvisomant, a GHR antagonist, before restraint stress. Similarly to the results found in mice carrying GHR ablation in dopaminergic neurons, pegvisomant significantly attenuated the restraint stress-induced prolactin secretion in male mice (Fig. 5B–C), whereas no effects were observed in females (Fig. 5E–F).
Figure 5. GH action on dopaminergic neurons regulates stress-induced prolactin release in male mice.
A Basal prolactin levels, corresponding to time zero in serial blood collection, in CON (n = 11), DAT (n = 5) and TH (n = 7) male mice. B Changes in plasma prolactin levels in male mice subjected to restraint stress for 120 minutes. C Area under the curve of prolactin levels in CON (n = 11), DAT (n = 5), TH (n = 7) and pegvisomant-treated wild-type (n = 5) male mice. D Basal prolactin levels, corresponding to time zero in serial blood collection, in CON (n = 12), DAT (n = 7) and TH (n = 6) female mice. E Changes in plasma prolactin levels in female mice subjected to restraint stress for 120 minutes. F Area under the curve of prolactin levels in CON (n = 12), DAT (n = 7), TH (n = 6) and pegvisomant-treated wild-type (n = 6) female mice. Differences between the groups in basal prolactin levels and area under the curve were determined by one-way ANOVA and Tukey’s test for multiple comparisons (* P < 0.05 versus CON; # P < 0.05, DAT versus TH). Differences in stress-induced prolactin secretion were determined by repeated measures two-way ANOVA.
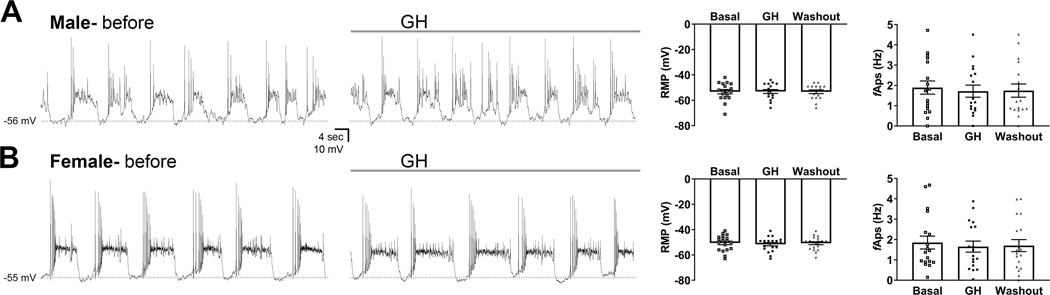
3.6. In contrast to prolactin, GH does not cause acute changes in the electrical activity of TIDA neurons
Prolactin administration leads to a robust depolarization in TIDA neurons (5–7), which is in accordance with the negative-feedback loop that controls prolactin secretion. To determine whether GH also affects the membrane potential oscillation nadir of TIDA neurons, whole-cell patch-clamp recordings were performed in DAT-expressing neurons in the dorsal ARH. We observed that GH application to the bath did not cause significant changes in the membrane potential or frequency of action potentials of ARH DAT-expressing neurons either in male mice (17 cells from 9 animals; Fig. 6A) or in females (19 cells from 9 animals; Fig. 6B). In addition, no significant changes were observed in the membrane/input resistance (data not shown). As previously demonstrated (5–7), prolactin induced a significant depolarization (+13.3 ± 2.3 mV) in all cells recorded (n = 3 cells from 2 mice) and this effect was not reversible during the washout period analyzed (data not shown).
Figure 6. GH does not induce acute changes in the membrane potential oscillation nadir or the frequency of action potentials of TIDA neurons.
A-B Representative current-clamp recordings of DAT-expressing neurons in the arcuate nucleus of male (A; 17 cells from 9 animals) and female mice (B; 19 cells from 9 animals) before and during GH administration. Dashed lines indicate the baseline membrane potential oscillation. * P < 0.05, significantly different from basal period. Differences between the groups were determined by repeated measures one-way ANOVA.
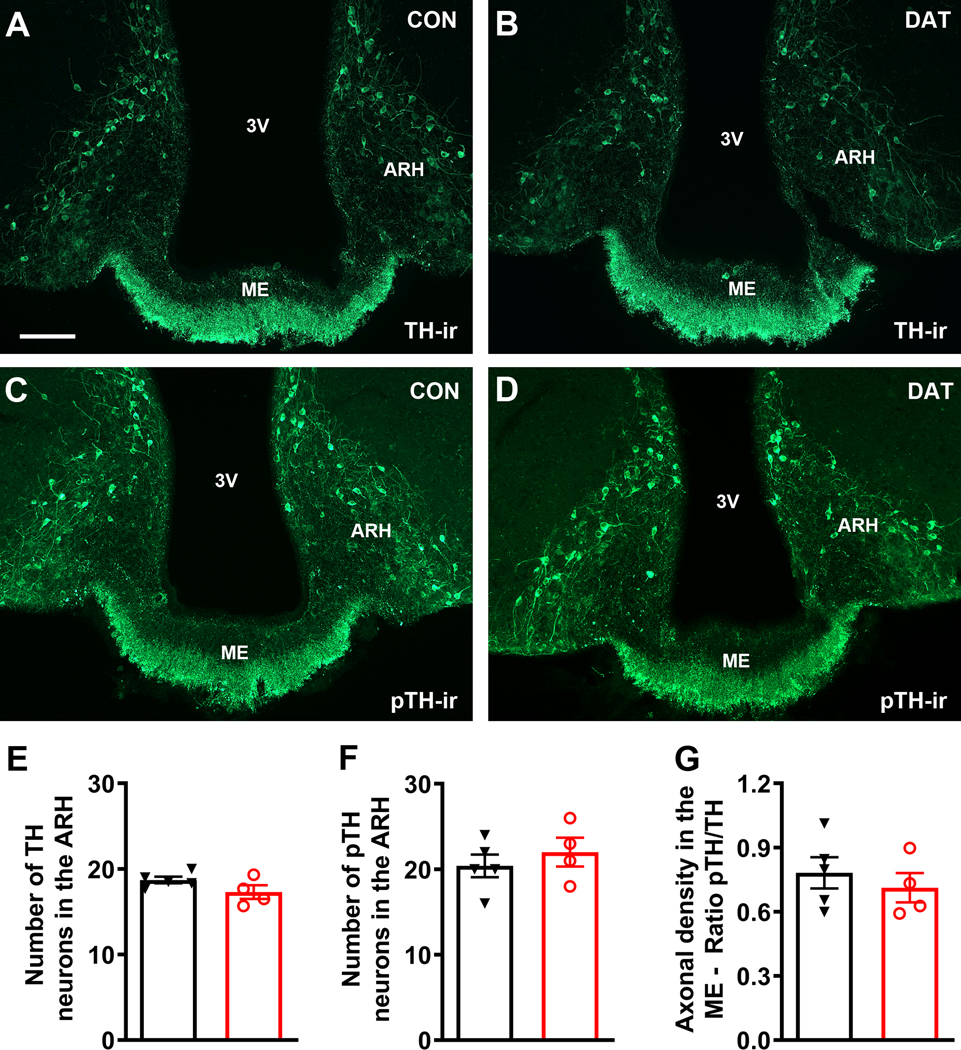
3.7. DAT-specific GHR ablation does not affect TH phosphorylation during restraint stress
Dopamine neurotransmission is not only controlled by the frequency of action potentials of dopaminergic neurons, but also by posttranslational modifications in the TH enzyme. In this regard, previous studies have shown that phosphorylation in specific TH residues can enhance its activity, increasing dopamine synthesis and release (7, 24–26). Before determining whether the blunted stress-induced prolactin release observed in DAT-specific GHR knockout male mice could be explained by changes in pTH, we analyzed the number of pTH immunoreactive neurons in the ARH and the density of pTH immunoreactive terminals in the ME of mice perfused in basal conditions (non-stressed animals). DAT-specific GHR knockout mice showed no changes in the density of pTH axonal terminals in the ME (CON: 9.1 ± 0.9; DAT: 10.3 ± 1.5; P = 0.4985; n = 6/group) or the number of pTH positive neurons in the ARH (CON: 32.3 ± 1.1; DAT: 32.0 ± 1.1; P = 0.8332), as compared to CON mice. Subsequently, male mice were evaluated after 45 minutes of restraint stress (Fig. 7). We observed that control and DAT-specific GHR knockout mice exhibited similar numbers of TH and pTH immunoreactive neurons in the ARH during restraint stress (Fig. 7A–F). Furthermore, the ratio in the density of pTH/TH axonal terminals in the ME was similar between the experimental groups (Fig. 7A–D, G).
Figure 7. DAT-specific GHR ablation does not affect TH phosphorylation during restraint stress in male mice.
A-B Epifluorescence photomicrographs showing tyrosine hydroxylase (TH)-immunoreactivity (TH-ir) in the arcuate nucleus (ARH) and median eminence (ME) of control (CON) and DAT-specific GHR knockout (DAT) male mice. C-D Epifluorescence photomicrographs showing the immunoreactivity against the phosphorylated form of TH (pTH-ir) in the ARH and ME of CON and DAT male mice. E Number of TH immunoreactive neurons in the ARH of CON (n = 5) and DAT (n = 4) male mice. The values obtained at different rostrocaudal levels were averaged. F Number of pTH immunoreactive neurons in the ARH of CON (n = 5) and DAT (n = 4) male mice. G Ratio in the density of pTH/TH axonal terminals in the ME of CON (n = 5) and DAT (n = 4) male mice. Abbreviation: 3V, third ventricle. Scale bar = 100 μm. Comparisons between the groups were performed using unpaired two-tailed Student’s t-test.
4. Discussion
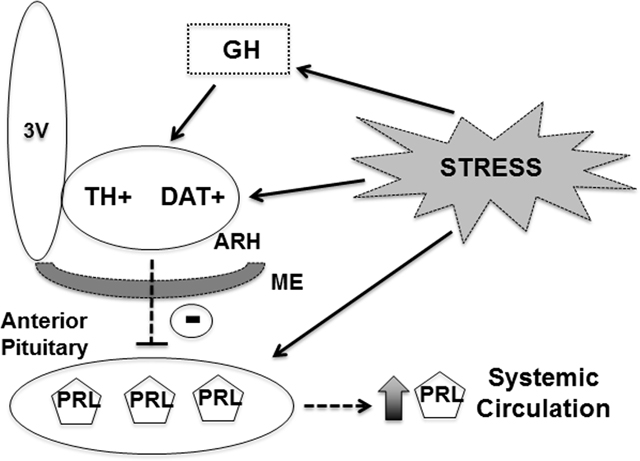
In the last few years, several studies started to uncover the physiological importance of GH action on specific neuronal populations (15, 17–19, 29–31, 40). These studies revealed that GH has a particularly important action during situations of stress or when homeostasis is challenged (41). For example, during food deprivation GHR signaling in AgRP-expressing neurons triggers energy-saving adaptations to decrease weight loss and increase the chances of survival (15, 16), whereas during hypoglycemia GH action on VMH neurons enhances the counter regulatory response to recover blood glucose levels (30). Interestingly, in most of the cases, GHR ablation in specific neuronal populations caused no or just minor physiological changes during basal conditions (15, 17, 29, 30), highlighting the importance of central GHR signaling as a cue during situations of stress to coordinate physiological adjustments to restore homeostasis. In the present study, we investigated the importance of GH action in functions regulated by ARH dopaminergic neurons. Similar to the findings of earlier studies, GHR deletion in DAT- or TH-expressing cells produced minor alterations in situations in which animals were not challenged, except for the evident increase in body weight of TH-specific GHR knockout mice that is secondary to increased GH secretion, as previously demonstrated (19). However, when DAT- and TH-specific knockout mice were subjected to restraint stress we observed a blunted stress-induced prolactin secretion.
GH-responsive neurons were identified in the present study via the expression of pSTAT5 in mice previously injected with GH. Although this method has been extensively used by our research group (13, 15–19, 29–31, 40–43), we cannot guarantee that all pSTAT5 positive cells express GHR. In fact, no study so far demonstrated that TIDA neurons express Ghr mRNA. However, GH-induced pSTAT5 positive cells in the dorsal ARH co-localize with Ghr mRNA, although no further co-localization with TH or DAT was performed (13). Additionally, using a novel GHR-Cre mouse model, a preprint study showed some co-localization between GHR and TH in the ARH, although in a lower extent compared to our study and with double-labeled cells located in the ventral ARH (44). Thus, it is desirable that future studies determine the percentage of DAT or TH neurons that express GHR.
ARH TH cells recently emerged as a novel neuronal population that controls metabolism (10, 11). Hypothalamic dopamine signaling regulates energy expenditure and brown fat thermogenesis (27). Optogenetic activation of ARH TH neurons increases food intake in mice, whereas their inhibition leads to body weight reduction (10). ARH TH neurons do not co-localize with AgRP/neuropeptide Y-expressing cells, but likewise they are activated by the appetite-stimulating hormone ghrelin (10, 11). Central GH injection also has orexigenic effects (15) and our results indicate that approximately half of ARH TH neurons are directly responsive to GH in both male and female mice. Nevertheless, GHR ablation in DAT- or TH-expressing cells did not cause significant changes in energy and glucose homeostasis. Thus, GH’s effects on metabolism likely rely on other neuronal populations. However, we did not evaluate our experimental animals under metabolic challenges, such as chronic high-fat diet intake. Thus, we cannot rule out the possibility that the absence of GHR signaling in dopaminergic cells may affect metabolism in situations of metabolic imbalance.
ARH dopaminergic neurons are classically involved in the control of prolactin secretion (4–9). Besides prolactin’s effect stimulating milk production, prolactin is necessary for postpartum maternal nursing behavior (45–47). Additionally, prolactin regulates reproduction and fertility (23, 48, 49). Our findings suggest that GHR deletion in DAT- or TH-expressing cells did not affect fertility and pup survival. However, since we did not perform a detailed evaluation of reproduction and pup growth, our results should be considered preliminary. Pup survival depends on lactation performance and maternal care, so the physiological patterns of prolactin secretion during lactation were probably not affected by the genetic ablations. However, DAT-specific, but not TH-specific GHR knockout male mice exhibited lower basal prolactin levels, whereas normal basal prolactin levels were observed in mutant females. Although the vast majority of DAT neurons express TH, there are some exceptions including neurons of the suprachiasmatic (SCH) and ventral premammillary (PMv) nuclei (21). SCH regulates circadian rhythms, and PMv neurons are known to mediate the effects of leptin on reproduction (50–52) and regulate inter-male aggression (53–55). Whether GH action on these DAT-positive, TH-negative neurons may regulate basal prolactin secretion in male mice remains to be determined.
Restraint stress-induced prolactin secretion was impaired in male DAT- and TH-specific GHR knockout mice, as well as in pegvisomant-treated wild-type mice. Several central mechanisms have been proposed to explain the activation of stress-induced prolactin secretion, including changes in the release of noradrenaline (36, 56), bombesin (38), dopamine (37, 57), histamine (58) and prolactin-release peptide (59). Another study showed that prolactin signaling is reduced in TIDA neurons during restraint stress, suggesting that a temporary loss in prolactin negative-feedback contributes with stress-induced prolactin release (60). Of note, circulating GH levels quickly falls during restraint stress (36–39). Thus, acute changes in GHR signaling in dopaminergic neurons may be necessary to fully induce prolactin secretion during restraint stress. Of note, although our manuscript focused on studying ARH dopaminergic neurons as putative mediators of GH action to regulate prolactin secretion, different hypothalamic and brainstem areas contain TH-expressing neurons that are responsive to GH, including the periventricular nucleus, paraventricular nucleus of the hypothalamus and locus coeruleus (19, 42). Additionally, we cannot rule out the participation of catecholaminergic cells of the periphery. Thus, it must be taken into account that the actions of GH to control stress-induced prolactin secretion may involve ARH TIDA neurons as well as other cell populations or indirect mechanisms that were not investigated in the present study.
Plasma prolactin levels exhibit a strong sexual dimorphism, in which females display higher values compared to males. Likewise, our findings indicate the existence of sex differences in the capacity of GHR to affect prolactin secretion. Interestingly, previous studies have also shown a differential response between male and female rats in the effects caused by acute restraint stress on TIDA neuronal activity (61, 62). In this sense, restraint stress induces a greater decrease in the activity of TIDA neurons in females compared to males (61). This difference is mediated by testosterone action (61). To determine how GHR signaling affects stress-induced prolactin release, we first investigated whether GH induces changes in ARH DAT neuronal activity. Differently than prolactin that induces a robust depolarization in TIDA neurons (5–7), GH did not cause significant alterations in the membrane potential, input resistance or frequency of action potentials of ARH TIDA neurons either in males or females. The absence of GH effect is interesting because prolactin and GH have a lot of similarities in the intracellular signaling pathways recruited by their receptors (14, 63). In pre-pubertal male rats, prolactin increases the activity of TIDA neurons via phosphoinositide 3-kinase (PI3K)-dependent and -independent mechanisms (5). It is unknown if GHR signaling is able to recruit PI3K signaling pathway in TIDA neurons of adult mice. Alternatively, we determined whether GH regulates pTH to affect stress-induced prolactin release since TH phosphorylation at Ser40 increases its enzymatic activity and several physiological conditions affect prolactin secretion via this posttranslational modification (7, 24–26). Nevertheless, our findings indicate that GHR signaling in TIDA neurons does not affect stress-induced prolactin release via modifications in TH phosphorylation. Therefore, the mechanism used by GHR to affect stress-induced prolactin secretion in male mice still needs to be identified.
What is the physiological importance of prolactin secretion during situations of stress? Previous studies have shown that prolactin has anxiolytic and anti-stress effects, reducing the acute activation of restraint stress-responsive neurons and the rise in plasma adrenocorticotropic hormone, corticosterone and noradrenaline (64–67). Prolactin possibly modulates stress response via its action in the adrenal cortex (60) or through the central nervous system since prolactin receptor is found in brain nuclei that control behavioral and neuroendocrine responses to stress (13, 45, 67, 68). Thus, more than just a milk-stimulating hormone, prolactin is a pleiotropic factor that coordinates multiple neurobiological adaptations, frequently related to pregnancy and lactation, but not necessarily restricted to those situations (4, 35, 69). Thus, the cross-talk between GH and prolactin is possibly related to fine adjustments in the perception and response to stressful stimuli. Accordingly, alterations in brain GH action can cause maladaptive changes to stress, impairing memory and stress resilience, predisposing individuals to excessive fear memory formation and ultimately leading to posttraumatic stress disorder (70–72). Therefore, our findings contribute to the identification of novel functions mediated by central GH action, demonstrating that GHR signaling, possibly via dopaminergic neurons, modulates the response to stress through the regulation of prolactin secretion, at least in male mice.
Acknowledgements
We thank Ana M.P. Campos for the technical assistance. This work was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP-Brazil; 16/20897-3 to F.W., 17/21840-8 to R.F., 17/21854-9 to F.M.C.,17/02983-2 to J.D., 17/22189-9 to N.S.M., 18/04956-5 to J.P.C. and 20/01318-8 to J.D.), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq/Brazil grant number: 409323/2018-7 to RES) and National Institutes of Health (NIA; grant number: R01AG059779 to E.O.L. and J.J.K.). J.D. is investigator of the CNPq.
Footnotes
Conflict of interest
The authors declare no conflicts of interest.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1.Goridis C, Rohrer H. Specification of catecholaminergic and serotonergic neurons. Nat Rev Neurosci 2002; 3: 531–41. [DOI] [PubMed] [Google Scholar]
- 2.Leong DA, Frawley LS, Neill JD. Neuroendocrine control of prolactin secretion. Annu Rev Physiol 1983; 45: 109–27. [DOI] [PubMed] [Google Scholar]
- 3.Kawano H, Daikoku S. Functional topography of the rat hypothalamic dopamine neuron systems: retrograde tracing and immunohistochemical study. J Comp Neurol 1987; 265: 242–53. [DOI] [PubMed] [Google Scholar]
- 4.Grattan DR. 60 YEARS OF NEUROENDOCRINOLOGY: The hypothalamo-prolactin axis. J Endocrinol 2015; 226: T101–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lyons DJ, Hellysaz A, Broberger C. Prolactin regulates tuberoinfundibular dopamine neuron discharge pattern: novel feedback control mechanisms in the lactotrophic axis. J Neurosci 2012; 32: 8074–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown RS, Piet R, Herbison AE, Grattan DR. Differential actions of prolactin on electrical activity and intracellular signal transduction in hypothalamic neurons. Endocrinology 2012; 153: 2375–84. [DOI] [PubMed] [Google Scholar]
- 7.Romano N, Yip SH, Hodson DJ, Guillou A, Parnaudeau S, Kirk S, Tronche F, Bonnefont X, Le Tissier P, Bunn SJ, Grattan DR, Mollard P, Martin AO. Plasticity of hypothalamic dopamine neurons during lactation results in dissociation of electrical activity and release. J Neurosci 2013; 33: 4424–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kokay IC, Grattan DR. Expression of mRNA for prolactin receptor (long form) in dopamine and pro-opiomelanocortin neurones in the arcuate nucleus of non-pregnant and lactating rats. J Neuroendocrinol 2005; 17: 827–35. [DOI] [PubMed] [Google Scholar]
- 9.Anderson GM, Beijer P, Bang AS, Fenwick MA, Bunn SJ, Grattan DR. Suppression of prolactin-induced signal transducer and activator of transcription 5b signaling and induction of suppressors of cytokine signaling messenger ribonucleic acid in the hypothalamic arcuate nucleus of the rat during late pregnancy and lactation. Endocrinology 2006; 147: 4996–5005. [DOI] [PubMed] [Google Scholar]
- 10.Zhang X, van den Pol AN. Hypothalamic arcuate nucleus tyrosine hydroxylase neurons play orexigenic role in energy homeostasis. Nat Neurosci 2016; 19: 1341–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Skov LJ, Ratner C, Hansen NW, Thompson JJ, Egerod KL, Burm H, Dalboge LS, Hedegaard MA, Brakebusch C, Pers TH, Perrier JF, Holst B. RhoA in tyrosine hydroxylase neurones regulates food intake and body weight via altered sensitivity to peripheral hormones. J Neuroendocrinol 2019; 31: e12761. [DOI] [PubMed] [Google Scholar]
- 12.Burton KA, Kabigting EB, Clifton DK, Steiner RA. Growth hormone receptor messenger ribonucleic acid distribution in the adult male rat brain and its colocalization in hypothalamic somatostatin neurons. Endocrinology 1992; 131: 958–63. [DOI] [PubMed] [Google Scholar]
- 13.Furigo IC, Metzger M, Teixeira PD, Soares CR, Donato J Jr. Distribution of growth hormone-responsive cells in the mouse brain. Brain Struct Funct 2017; 222: 341–63. [DOI] [PubMed] [Google Scholar]
- 14.Teglund S, McKay C, Schuetz E, van Deursen JM, Stravopodis D, Wang DM, Brown M, Bodner S, Grosveld G, Ihle JN. Stat5a and Stat5b proteins have essential and nonessential, or redundant, roles in cytokine responses. Cell 1998; 93: 841–50. [DOI] [PubMed] [Google Scholar]
- 15.Furigo IC, Teixeira PDS, de Souza GO, Couto GCL, Romero GG, Perello M, Frazao R, Elias LL, Metzger M, List EO, Kopchick JJ, Donato J Jr. Growth hormone regulates neuroendocrine responses to weight loss via AgRP neurons. Nat Commun 2019; 10: 662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Furigo IC, Teixeira PD, Quaresma PGF, Mansano NS, Frazao R, Donato J. STAT5 ablation in AgRP neurons increases female adiposity and blunts food restriction adaptations. J Mol Endocrinol 2020; 64: 13–27. [DOI] [PubMed] [Google Scholar]
- 17.Quaresma PGF, Teixeira PDS, Furigo IC, Wasinski F, Couto GC, Frazao R, List EO, Kopchick JJ, Donato J Jr. Growth hormone/STAT5 signaling in proopiomelanocortin neurons regulates glucoprivic hyperphagia. Mol Cell Endocrinol 2019; 498: 110574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Silveira MA, Zampieri TT, Furigo IC, Abdulkader F, Donato J Jr., Frazao R. Acute effects of somatomammotropin hormones on neuronal components of the hypothalamic-pituitary-gonadal axis. Brain Res 2019; 1714: 210–7. [DOI] [PubMed] [Google Scholar]
- 19.Wasinski F, Pedroso JAB, Dos Santos WO, Furigo IC, Garcia-Galiano D, Elias CF, List EO, Kopchick JJ, Szawka RE, Donato J Jr. Tyrosine Hydroxylase Neurons Regulate Growth Hormone Secretion via Short-Loop Negative Feedback. J Neurosci 2020; 40: 4309–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Augood SJ, Westmore K, McKenna PJ, Emson PC. Co-expression of dopamine transporter mRNA and tyrosine hydroxylase mRNA in ventral mesencephalic neurones. Brain Res Mol Brain Res 1993; 20: 328–34. [DOI] [PubMed] [Google Scholar]
- 21.Yip SH, York J, Hyland B, Bunn SJ, Grattan DR. Incomplete concordance of dopamine transporter Cre (DAT(IREScre))-mediated recombination and tyrosine hydroxylase immunoreactivity in the mouse forebrain. J Chem Neuroanat 2018; 90: 40–8. [DOI] [PubMed] [Google Scholar]
- 22.List EO, Berryman DE, Funk K, Gosney ES, Jara A, Kelder B, Wang X, Kutz L, Troike K, Lozier N, Mikula V, Lubbers ER, Zhang H, Vesel C, Junnila RK, Frank SJ, Masternak MM, Bartke A, Kopchick JJ. The role of GH in adipose tissue: lessons from adipose-specific GH receptor gene-disrupted mice. Mol Endocrinol 2013; 27: 524–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Silva JF, Henriques PC, Campideli-Santana AC, Araujo-Lopes R, Aquino NSS, Hipolito LTM, Lopes-Aguiar C, Reis AM, Grattan DR, Szawka RE. Estradiol Potentiates But Is Not Essential for Prolactin-Induced Suppression of Luteinizing Hormone Pulses in Female Rats. Endocrinology 2020; 161: 1–14. [DOI] [PubMed] [Google Scholar]
- 24.Dunkley PR, Bobrovskaya L, Graham ME, von Nagy-Felsobuki EI, Dickson PW. Tyrosine hydroxylase phosphorylation: regulation and consequences. J Neurochem 2004; 91: 1025–43. [DOI] [PubMed] [Google Scholar]
- 25.Feher P, Olah M, Bodnar I, Hechtl D, Bacskay I, Juhasz B, Nagy GM, Vecsernyes M. Dephosphorylation/inactivation of tyrosine hydroxylase at the median eminence of the hypothalamus is required for suckling-induced prolactin and adrenocorticotrop hormone responses. Brain Res Bull 2010; 82: 141–5. [DOI] [PubMed] [Google Scholar]
- 26.Aquino NS, Araujo-Lopes R, Batista IA, Henriques PC, Poletini MO, Franci CR, Reis AM, Szawka RE. Hypothalamic Effects of Tamoxifen on Oestrogen Regulation of Luteinising Hormone and Prolactin Secretion in Female Rats. J Neuroendocrinol 2016; 28. [DOI] [PubMed] [Google Scholar]
- 27.Folgueira C, Beiroa D, Porteiro B, Duquenne M, Puighermanal E, Fondevila MF, Barja-Fernandez S, Gallego R, Hernandez-Bautista R, Castelao C, Senra A, Seoane P, Gomez N, Aguiar P, Guallar D, Fidalgo M, Romero-Pico A, Adan R, Blouet C, Labandeira-Garcia JL, Jeanrenaud F, Kallo I, Liposits Z, Salvador J, Prevot V, Dieguez C, Lopez M, Valjent E, Fruhbeck G, Seoane LM, Nogueiras R. Hypothalamic dopamine signaling regulates brown fat thermogenesis. Nat Metab 2019; 1: 811–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Doan KV, Kinyua AW, Yang DJ, Ko CM, Moh SH, Shong KE, Kim H, Park SK, Kim DH, Kim I, Paik JH, DePinho RA, Yoon SG, Kim IY, Seong JK, Choi YH, Kim KW. FoxO1 in dopaminergic neurons regulates energy homeostasis and targets tyrosine hydroxylase. Nat Commun 2016; 7: 12733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Teixeira PDS, Couto GC, Furigo IC, List EO, Kopchick JJ, Donato J Jr. Central growth hormone action regulates metabolism during pregnancy. Am J Physiol Endocrinol Metab 2019; 317: E925–E40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Furigo IC, de Souza GO, Teixeira PDS, Guadagnini D, Frazao R, List EO, Kopchick JJ, Prada PO, Donato J Jr. Growth hormone enhances the recovery of hypoglycemia via ventromedial hypothalamic neurons. FASEB J 2019; 33: 11909–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bohlen TM, Zampieri TT, Furigo IC, Teixeira PD, List EO, Kopchick J, Donato J Jr., Frazao R. Central growth hormone signaling is not required for the timing of puberty. J Endocrinol 2019; 243: 161–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Furigo IC, Suzuki MF, Oliveira JE, Ramos-Lobo AM, Teixeira PDS, Pedroso JA, de Alencar A, Zampieri TT, Buonfiglio DC, Quaresma PGF, Prada PO, Bartolini P, Soares CRJ, Donato J Jr. Suppression of Prolactin Secretion Partially Explains the Antidiabetic Effect of Bromocriptine in ob/ob Mice. Endocrinology 2019; 160: 193–204. [DOI] [PubMed] [Google Scholar]
- 33.Gibson CD, Karmally W, McMahon DJ, Wardlaw SL, Korner J. Randomized pilot study of cabergoline, a dopamine receptor agonist: effects on body weight and glucose tolerance in obese adults. Diabetes Obes Metab 2012; 14: 335–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Luo S, Liang Y, Cincotta AH. Intracerebroventricular administration of bromocriptine ameliorates the insulin-resistant/glucose-intolerant state in hamsters. Neuroendocrinology 1999; 69: 160–6. [DOI] [PubMed] [Google Scholar]
- 35.Grattan DR, Kokay IC. Prolactin: a pleiotropic neuroendocrine hormone. J Neuroendocrinol 2008; 20: 752–63. [DOI] [PubMed] [Google Scholar]
- 36.Collu R, Du Rusisseau P, Tache Y. Role of putative neurotransmitters in prolactin, GH and LH response to acute immobilization stress in male rats. Neuroendocrinology 1979; 28: 178–86. [DOI] [PubMed] [Google Scholar]
- 37.Kawakami M, Higuchi T, Matsuura M. Immobilization stress and prolactin secretion in male rats. Possible roles of dopamine and TRH. Neuroendocrinology 1979; 29: 262–9. [DOI] [PubMed] [Google Scholar]
- 38.Tache Y, Brown M, Collu R. Effects of neuropeptides on adenohypophyseal hormone response to acute stress in male rats. Endocrinology 1979; 105: 220–4. [DOI] [PubMed] [Google Scholar]
- 39.Kawakami M, Higuchi T. Effect of partial deafferentation of the hypothalamus on stress-induced LH suppression and prolactin release. Neuroendocrinology 1981; 32: 278–84. [DOI] [PubMed] [Google Scholar]
- 40.Wasinski F, Furigo IC, Teixeira PDS, Ramos-Lobo AM, Peroni CN, Bartolini P, List EO, Kopchick JJ, Donato J Jr. Growth Hormone Receptor Deletion Reduces the Density of Axonal Projections from Hypothalamic Arcuate Nucleus Neurons. Neuroscience 2020; 434: 136–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wasinski F, Frazão R, Donato JJ. Effects of growth hormone in the central nervous system. Arch Endocrinol Metab 2019; 63: 549–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Quaresma PGF, Dos Santos WO, Wasinski F, Metzger M, Donato J Jr. Neurochemical phenotype of growth hormone-responsive cells in the mouse paraventricular nucleus of the hypothalamus. J Comp Neurol 2021. doi: 10.1002/cne.25017. [DOI] [PubMed] [Google Scholar]
- 43.Wasinski F, Klein MO, Bittencourt JC, Metzger M, Donato J Jr. Distribution of growth hormone-responsive cells in the brain of rats and mice. Brain Res 2021; 1751: 147189. [DOI] [PubMed] [Google Scholar]
- 44.de Lima JBM, Debarba LK, Khan M, Ubah C, Didyuk O, Ayyar I, Koch M, Sadagurski M. Growth hormone receptor (GHR)-expressing neurons in the hypothalamic arcuate nucleus regulate glucose metabolism and energy homeostasis. bioRxiv 20202020.08.17.254862. [Google Scholar]
- 45.Brown RSE, Aoki M, Ladyman SR, Phillipps HR, Wyatt A, Boehm U, Grattan DR. Prolactin action in the medial preoptic area is necessary for postpartum maternal nursing behavior. Proc Natl Acad Sci U S A 2017; 114: 10779–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bridges RS, DiBiase R, Loundes DD, Doherty PC. Prolactin stimulation of maternal behavior in female rats. Science 1985; 227: 782–4. [DOI] [PubMed] [Google Scholar]
- 47.Lucas BK, Ormandy CJ, Binart N, Bridges RS, Kelly PA. Null mutation of the prolactin receptor gene produces a defect in maternal behavior. Endocrinology 1998; 139: 4102–7. [DOI] [PubMed] [Google Scholar]
- 48.Donato J Jr., Frazao R. Interactions between prolactin and kisspeptin to control reproduction. Arch Endocrinol Metab 2016; 60: 587–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sonigo C, Bouilly J, Carre N, Tolle V, Caraty A, Tello J, Simony-Conesa FJ, Millar R, Young J, Binart N. Hyperprolactinemia-induced ovarian acyclicity is reversed by kisspeptin administration. J Clin Invest 2012; 122: 3791–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jr Donato J, Cravo RM, Frazão R, Gautron L, Scott MM, Lachey J, Castro IA, Margatho LO, Lee S, Lee C, Richardson JA, Friedman J, Chua S, Coppari R, Zigman JM, Elmquist JK, Elias CF. Leptin’s effect on puberty in mice is relayed by the ventral premammillary nucleus and does not require signaling in Kiss1 neurons. J Clin Invest 2011; 121: 355–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jr Donato J, Silva RJ, Sita LV, Lee S, Lee C, Lacchini S, Bittencourt JC, Franci CR, Canteras NS, Elias CF. The ventral premammillary nucleus links fasting-induced changes in leptin levels and coordinated luteinizing hormone secretion. J Neurosci 2009; 29: 5240–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ross RA, Leon S, Madara JC, Schafer D, Fergani C, Maguire CA, Verstegen AM, Brengle E, Kong D, Herbison AE, Kaiser UB, Lowell BB, Navarro VM. PACAP neurons in the ventral premammillary nucleus regulate reproductive function in the female mouse. eLife 2018; 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stagkourakis S, Spigolon G, Williams P, Protzmann J, Fisone G, Broberger C. A neural network for intermale aggression to establish social hierarchy. Nat Neurosci 2018; 21: 834–42. [DOI] [PubMed] [Google Scholar]
- 54.Soden ME, Miller SM, Burgeno LM, Phillips PEM, Hnasko TS, Zweifel LS. Genetic Isolation of Hypothalamic Neurons that Regulate Context-Specific Male Social Behavior. Cell reports 2016; 16: 304–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen AX, Yan JJ, Zhang W, Wang L, Yu ZX, Ding XJ, Wang DY, Zhang M, Zhang YL, Song N, Jiao ZL, Xu C, Zhu SJ, Xu XH. Specific Hypothalamic Neurons Required for Sensing Conspecific Male Cues Relevant to Inter-male Aggression. Neuron 2020; 108: 763–74 e6. [DOI] [PubMed] [Google Scholar]
- 56.Haanwinckel MA, Antunes-Rodrigues J, De Castro e Silva E. Role of central beta-adrenoceptors on stress-induced prolactin release in rats. Horm Metab Res 1991; 23: 318–20. [DOI] [PubMed] [Google Scholar]
- 57.Demarest KT, Moore KE, Riegle GD. Acute restraint stress decreases dopamine synthesis and turnover in the median eminence: a model for the study of the inhibitory neuronal influences on tuberoinfundibular dopaminergic neurons. Neuroendocrinology 1985; 41: 437–44. [DOI] [PubMed] [Google Scholar]
- 58.Knigge U, Matzen S, Warberg J. Histaminergic mediation of the stress-induced release of prolactin in male rats. Neuroendocrinology 1988; 47: 68–74. [DOI] [PubMed] [Google Scholar]
- 59.Maruyama M, Matsumoto H, Fujiwara K, Noguchi J, Kitada C, Fujino M, Inoue K. Prolactin-releasing peptide as a novel stress mediator in the central nervous system. Endocrinology 2001; 142: 2032–8. [DOI] [PubMed] [Google Scholar]
- 60.Kirk SE, Xie TY, Steyn FJ, Grattan DR, Bunn SJ. Restraint stress increases prolactin-mediated phosphorylation of signal transducer and activator of transcription 5 in the hypothalamus and adrenal cortex in the male mouse. J Neuroendocrinol 2017; 29. [DOI] [PubMed] [Google Scholar]
- 61.Demarest KT, Moore KE, Riegle GD. Acute restraint stress decreases tuberoinfundibular dopaminergic neuronal activity: evidence for a differential response in male versus female rats. Neuroendocrinology 1985; 41: 504–10. [DOI] [PubMed] [Google Scholar]
- 62.Lookingland KJ, Gunnet JW, Toney TW, Moore KE. Comparison of the effects of ether and restraint stress on the activity of tuberoinfundibular dopaminergic neurons in female and male rats. Neuroendocrinology 1990; 52: 99–105. [DOI] [PubMed] [Google Scholar]
- 63.Brooks CL. Molecular mechanisms of prolactin and its receptor. Endocr Rev 2012; 33: 504–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jaroenporn S, Nagaoka K, Kasahara C, Ohta R, Watanabe G, Taya K. Physiological roles of prolactin in the adrenocortical response to acute restraint stress. Endocr J 2007; 54: 703–11. [DOI] [PubMed] [Google Scholar]
- 65.Donner N, Bredewold R, Maloumby R, Neumann ID. Chronic intracerebral prolactin attenuates neuronal stress circuitries in virgin rats. Eur J Neurosci 2007; 25: 1804–14. [DOI] [PubMed] [Google Scholar]
- 66.Torner L, Toschi N, Pohlinger A, Landgraf R, Neumann ID. Anxiolytic and anti-stress effects of brain prolactin: improved efficacy of antisense targeting of the prolactin receptor by molecular modeling. J Neurosci 2001; 21: 3207–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gustafson P, Bunn SJ, Grattan DR. The role of prolactin in the suppression of Crh mRNA expression during pregnancy and lactation in the mouse. J Neuroendocrinol 2017; 29. [DOI] [PubMed] [Google Scholar]
- 68.Brown RS, Kokay IC, Herbison AE, Grattan DR. Distribution of prolactin-responsive neurons in the mouse forebrain. J Comp Neurol 2010; 518: 92–102. [DOI] [PubMed] [Google Scholar]
- 69.Bernard V, Young J, Binart N. Prolactin - a pleiotropic factor in health and disease. Nat Rev Endocrinol 2019; 15: 356–65. [DOI] [PubMed] [Google Scholar]
- 70.Vander Weele CM, Saenz C, Yao J, Correia SS, Goosens KA. Restoration of hippocampal growth hormone reverses stress-induced hippocampal impairment. Front Behav Neurosci 2013; 7: 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Meyer RM, Burgos-Robles A, Liu E, Correia SS, Goosens KA. A ghrelin-growth hormone axis drives stress-induced vulnerability to enhanced fear. Mol Psychiatry 2014; 19: 1284–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gisabella B, Farah S, Peng X, Burgos-Robles A, Lim SH, Goosens KA. Growth hormone biases amygdala network activation after fear learning. Transl Psychiatry 2016; 6: e960. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.