Abstract
Background:
Daily use of chlorhexidine gluconate (CHG) has been shown to reduce risk of healthcare-associated infections. We aimed to assess moving CHG bathing into routine practice using a human factors approach. We evaluated implementation in non-intensive care unit (ICU) settings in the Veterans Health Administration.
Methods:
Our multiple case study approach included non-ICU units from 4 Veterans Health Administration settings. Guided by the Systems Engineering Initiative for Patient Safety, we conducted focus groups and interviews to capture barriers and facilitators to daily CHG bathing. We measured compliance using observations and skin CHG concentrations.
Results:
Barriers to daily CHG include time, concern of increasing antibiotic resistance, workflow and product concerns. Facilitators include engagement of champions and unit shared responsibility. We found shortfalls in patient education, hand hygiene and CHG use on tubes and drains. CHG skin concentration levels were highest among patients from spinal cord injury units. These units applied antiseptic using 2% CHG impregnated wipes vs 4% CHG solution/soap.
Discussion:
Non-ICUs implementing CHG bathing must consider human factors and work system barriers to ensure uptake and sustained practice change.
Conclusions:
Well-planned rollouts and a unit culture promoting shared responsibility are key to compliance with daily CHG bathing. Successful implementation requires attention to staff education and measurement of compliance.
Keywords: Quality improvement, Systems engineering, Organizational culture, Contextual factors, Champions, Case study
BACKGROUND
Recent data indicate a downward trend in prevalence of health-care-associated infections (HAIs) with rates lower in 2015 than in 2011.1 However, HAIs remain a threat to patient safety, and are the most frequent adverse event in healthcare worldwide.1–6 There is strong evidence that daily treatment of hospitalized patients with broad-spectrum antiseptic chlorhexidine gluconate (CHG) can reduce risk of healthcare-associated bloodstream infections7–14 and colonization by multidrug resistant organisms, particularly in intensive care units (ICU).8,9,13 However, implementation of this evidence-based practice, and understanding of contextual factors have not been explored in non-ICU settings.
Contextual factors play a role in success of best practice interventions.15,16 In the case of CHG treatment, these factors may include staff and patient education, perceptions of teamwork, staffing levels and product acceptability – in this case, CHG wipes or CHG solution. The aim of this project was to examine processes and mechanisms to move CHG treatment into routine practice using a human factors and systems engineering approach.
METHODS
We used a multiple case study design17 to examine impact of contextual factors on CHG treatment implementation in 4 non-ICUs at Veteran Health Administration (VHA) settings, reporting methods according to the Consolidated Criteria for Reporting Qualitative Research.18 Mixed methods were used for data collection and analysis.19 Two data collection methods were employed, (1) focus groups and interviews and (2) measurements of intervention compliance-direct observations of CHG bathing (without corrections by observers) and assessment of CHG skin concentrations levels. The original plan was to use a sequential roll-out design with the research team as coaches with a plan to transfer lessons learned from site to site. We also planned for group phone calls to bring site participants together monthly– a virtual learning community. Due to setbacks in timing with each site, an individualized approach was adopted along with a flexible timeline for implementation start-up.
A “case” is defined as a VHA hospital unit in which CHG treatment was implemented. A case study design was chosen to ensure implementation of CHG treatment was explored through multiple lenses – to understand mechanisms to implement a new evidence-based practice in various hospital units and patient populations and within different facility and unit-level cultures.
A human factors framework called the Systems Engineering Initiative for Patient Safety (SEIPS),20 guided our project and data analysis.20–25 SEIPS focuses on 5 interacting work system elements — person, tasks, tools and technologies, physical environment, and organizational conditions. This framework allows for understanding interactions between elements which can impact care processes (eg, CHG bathing treatment).26 We used a deductive approach to content analysis using the SEIPS framework to guide analysis. A mentored implementation approach was used in which we paired subject matter experts who are also experienced in implementation (mentors) with local site team leaders (mentees).27
Setting
Four non-ICUs at 4 distinct VHA facilities participated in our Human-factors Engineering to Prevent Resistant Organismproject. This project was funded by the VHA National Center for Patient Safety and included 2 spinal cord injury units and 2 medical and/or surgical units. Participating hospitals are in the Midwestern United States (1 urban, 1 non-urban), the Pacific Northwest and East Coast (both urban). Unit characteristics and project participants are described in Table 1.
Table 1.
Characteristics of participating units
| Medical/surgical unit (Case A) | Spinal cord injury unit (Case B) | Medical/ surgical unit (Case C) | Spinal cord injury unit (Case D) | |
|---|---|---|---|---|
|
| ||||
| Total number of beds at hospital | 129 | 187 | 177 | 331 |
| Number of beds on intervention unit | 21 | 38 | 29 | 53 |
| Average patient days/month | 550 | 656 | 531 | 360 |
| HCWs performing CHG-treatment | CNAs during day shift | CNAs during day shift | CNAs and RNs during both day and PM shifts | CNAs both during day and PM shifts |
| Description of CHG treatment training | Research team provided on-site introduction and training to nursing staff and nurse managers | Training and education provided by nurse educators, researchers and patient safety nurse fellow. | Research team provided on-site introduction, shared training materials; unit nurse educator instructed HCWs on procedure in unit huddle and email | Research team shared training materials prior to on-site visit; research team instructed HCWs on procedure through Power-Point training, email and conference calls |
| Focus group: • # of participants • length in minutes |
• Two staff focus groups with 6 participants • Duration of focus group – 40 minutes |
• Two staff focus groups with 15 participants • Duration of focus group – 1 hour |
• Four staff focus groups with 9 participants • Duration of focus group – 30 minutes |
• Four participants • Duration of focus group – 45 minutes |
| Interviews: • # conducted (people interviewed) • total length in minutes |
• No individual interviews | • Two participants in 1 combined interview (nurse educator and nurse program manager) • Duration of interview – 1 hour |
• No individual interviews | • SCI unit medical director, nurse manager and registered nurse (team champion) • Duration of interviews - 30 minutes |
| Number of observations | 15 | 23 | 10 | 20 |
| Number of patients swabbed | 9 | 20 | 7 | 20 |
CNA, certified nursing assistant; HCW, health care worker; RN, registered nurse; SCI, spinal cord injury.
Intervention
Participating sites chose 1 of 3 CHG treatment procedures: (1) use of 2% CHG impregnated wipes (Sage Product LLC, Cary, IL), (2) direct application of CHG soap via washcloth, or (3) a combination of both methods. Sites also had a choice in how they rolled-out the intervention, use of unit champions, kick-off events, timing of rollout, and source of training (infection preventionists, unit staff, manufacturer representative or combination). The research team adapted to each site’s roll-out plan and assisted in problem-solving and developing timelines in a share decision-making process.
DATA COLLECTION AND ANALYSIS
Focus groups
Two researchers (LM and MJK) conducted focus groups with healthcare workers (HCWs) who were responsible for performing CHG treatments (nurses and certified nursing assistants). These HCWs volunteered to participate in the focus group. Researchers provided a project description and covered topics related to work system elements of SEIPS20,22 and participants identified barriers and facilitators to CHG treatment process. Focus groups were audio recorded and transcribed. Transcripts were coded by 2 researchers (MJK and LM) for relevant excerpts using a coding scheme (Table 2). The coding scheme was developed and agreed upon in advance by the research team. The 2 researchers convened and compared coding, discussing discrepancies. Once coded, the data were downloaded to Excel to allow for further analysis.
Table 2.
Codes and definitions for focus group coding
| Step in CHG process | Definitions |
|---|---|
|
| |
| Gather supplies | The healthcare worker (RN or CNA) gathering/collecting supplies and setting up including filling the Ziploc bag with water and any consequence of using the supply that occurs prior to or during the treatment. The supplies include: I. The basin and/or Ziploc bag II. Washcloths, towels III. The CHG soap IV. The CHG compatible lotion (Aloe Vesta or Medline) |
| Patient/caregiver education | Information shared (written and verbal) and teaching by the RN or CNA to patient and the caregivers including an explanation of the rationale, benefits and overall process associated with the CHG treatment. |
| Hand hygiene | Tasks performed by the RN or CNA gathering, prior to initiating the CHG treatment. This includes: I. Washing hands II. Donning/doffing gloves III. Using hand sanitizer IV. Wearing personal protective equipment (when necessary) |
| Treatment | The process of performing the CHG treatment including: I. Application of the CHG treatment II. Rinsing off the CHG treatment after the required amount of time III. Concluding the treatment by applying the compatible lotion (Aloe Vesta® or Medline®) |
| Documentation | Recording the CHG treatment in the electronic health record (EHR). |
| Barrier/Facilitator | |
| Barrier | A characteristic of a work system element or elements that interferes with a person’s ability to efficiently, appropriately and accurately accomplish a goal or activity (Carayon, et al. 2005). |
| Facilitator | A characteristic of a work system element(s) that makes it easy or easier to efficiently, appropriately and accurately accomplish a goal or activity (Carayon, et al., 2005). |
| Work system elements | |
| Task | The individual activities performed during or are associated with the infection prevention practice (eg, educating the patients and/or caregivers about the infection prevention (IP) practice, gathering supplies, following through on the on, documenting the IP practice in the EHR the time to complete the IP practice and the workload related to the IP practice. |
| Tool/technology | Supplies the healthcare worker uses for the IP practice, the characteristics of tools and technologies, and relevant electronic or paper media (eg, EHR, paper notes, notecards, contents of admission pack and YouTube videos). |
| Physical environment | The physical environment in which the IP practice takes place. This includes the distance to supplies; the layout of the room; the location of supplies and facilities (shower, sink, toilet, patent bed, etc.) where the IP practice occurs; the temperature of the room; noise. |
| Organization | The larger context in which the infection prevention practice occurs, including the hospital and/or unit culture (including level of RN or CNA involvement), policies and procedures, work schedules (including rounds), stocking schedules, availability of supplies, the training that the RN and CNA receive (including awareness or lack thereof, and standardization of IP practice). |
| Person | Characteristics including the education, skills, motivation, preference, needs and knowledge/experience of the healthcare team, individual healthcare professional, patient and/or the caregiver that influences their performance. |
CHG, chlorhexidine gluconate; CNA, certified nursing assistant; RN, registered nurse.
Interviews
We conducted interviews with nurse managers and educators, as well as an infection preventionists. All interviews were audio recorded and transcribed. For analysis, a similar procedure was used as described for focus groups.
Observations
Using a checklist, trained observers conducted direct observations of HCWs giving CHG treatments to determine compliance levels with steps in the bathing process. Data were entered in REDCap 8.1.1 a real-time online data collection platform. Our team has used this method in previous studies.28,29 We analyzed direct observation data by conducting descriptive analyses to assess completion of CHG treatment checklist items and duration of CHG treatment process.
Skin swabs
To assess compliance, we measured concentrations of CHG on patients’ skin. Three anatomical sites were sampled (jaw line to clavicle, antecubital fossae, and axillary area) 1 hour prior to, 1 hour after, and 24 hours after CHG treatment. Samples were collected by holding a swab vertical to the skin surface and rubbing across a 25 cm2 surface area of intact skin. We measured CHG concentration using a semiquantitative colorimetric assay described previously.30,31 Descriptive statistics were computed to determine the proportion of patients with any detectable CHG on 3 sites at the designated time intervals and we calculated time spent performing CHG treatment. Stata version 14 (Stata Corp, College Station, TX) was used for statistical analyses.
RESULTS
Nine focus groups were conducted with frontline HCWs who performed CHG treatments at 4 sites (cases) and 4 interviews with representatives from unit leadership and infection prevention. Sixty-eight direct observations of the CHG treatment process were conducted and we collected skin swabs from 56 patients. Each case is described below, presenting findings from each data collection method for respective cases (A, B, C, and D). Results for direct observations are summarized in Tables 3 and 4, while all-site swabbing results are summarized in Figure 1. Illustrative quotations are included within each case description.
Table 3.
Chlorhexidine treatment process compliance for 3 of 4 cases (project sites) that used 2% Chlorhexidine-impregnated wipes
| Characteristic | Site B | Site C | Site D |
|---|---|---|---|
|
| |||
| Total number of Observations | 23 | 10 | 20 |
| Mean duration of bath in minutes | 12.7 (SD = 1.8) | 11 (SD = 1.5) | 8.7 (SD = 1.3) |
| A. Gather Supplies (% yes) | |||
| CHG prepacked wipes pack | 100% | 100% | 100% |
| Gloves (and gown if used) | 100% | 100% | 95% |
| B. Patient or family education about CHG | 100% | 100% | 70% |
| C. Hand hygiene (% yes) | |||
| Hand hygiene performed | 70% | 100% | 100% |
| Don clean gloves | 100% | 100% | 100% |
| Personal Protective Equipment | 100% | N/A | 100% |
| D. Perform CHG Treatment (% yes) | |||
| Clean entire neck area including skin folds | 48% | 90% | 65% |
| Clean around any lines | 100% | 100% | 67% |
| Massage skin firmly with CHG cloth | 100% | 100% | 95% |
| Clean armpit | 87% | 100% | 80% |
| Clean back of knee | 82% | 100% | 70% |
| Clean in between toes | 81% | 88% | 60% |
| Clean in between fingers | 83% | 90% | 80% |
| Clean between all folds in groin | 100% | 100% | 85% |
| Cleans between all folds in gluteal area | 94% | 100% | 80% |
| Clean tubing and drains closest to body | 63% | 100% | 38% |
| Use CHG on superficial wounds (non-excoriated skin only, no treatment areas) | 100% | 100% | 100% |
| Use CHG on superficial skin rashes | 100% | 100% | 100% |
| Use CHG on stage 1 pressure ulcers | N/A | 100% | 100% |
| Allow to air dry/do not wipe off CHG | 91% | 100% | 95% |
| Goes from clean to dirty area (If in dirty area before bath completed, changes gloves before returning to clean area) | 87% | 100% | 85% |
CHG, chlorhexidine gluconate.
Table 4.
Chlorhexidine treatment process compliance for 1 VA site that used 4% CHG solution instead of 2% wipes
| Site A | |
|---|---|
|
| |
| Number of observations | 15 |
| Mean duration of bath in minutes | 15.8 (SD = 1.5) |
| A. Gather Supplies (% yes) | |
| Basin or Ziploc Bag | 73% |
| Washcloths | 100% |
| CHG soap | 100% |
| CHG compatible lotion | 93% |
| Patient or family education about CHG | 50%a,b |
| B. Hand hygiene (% yes) | |
| Hand hygiene performed | 93.3% |
| Don clean gloves | 100% |
| Personal Protective Equipment | 100% |
| C. Perform CHG Treatment (% yes) | |
| Wet washcloths | 100% |
| Wash patient’s face with non-CHG soap and water | 100% |
| Use 1 washcloth to wash each body part | 9% |
| Apply 2 pumps of CHG to each washcloth | 73% |
| Use different clean wet washcloth to rinse CHG off body part | 38% |
| Use non-CHG soap and water on genital area/perineum | 93% |
| Rinse genital area/perineum with clean wet washcloths | 88% |
| Avoid CHG soap on drains, lines, and/or dressings | 100% |
| Towel dry skin | 100% |
| Apply Medline or Aloe Vesta lotion | 73% |
Note. Denominator in calculations excludes cases where the step was not applicable, for example, the denominator for “Avoid CHG soap on drains, lines, and/or dressings” excludes patients who did not have IV lines, drains or dressings.
CHG, chlorhexidine gluconate; VA, Veterans Administration.
Calculated only for baths that were not first baths.
None of the baths were first baths, hence patient or family education about CHG was not observed.
Fig 1.
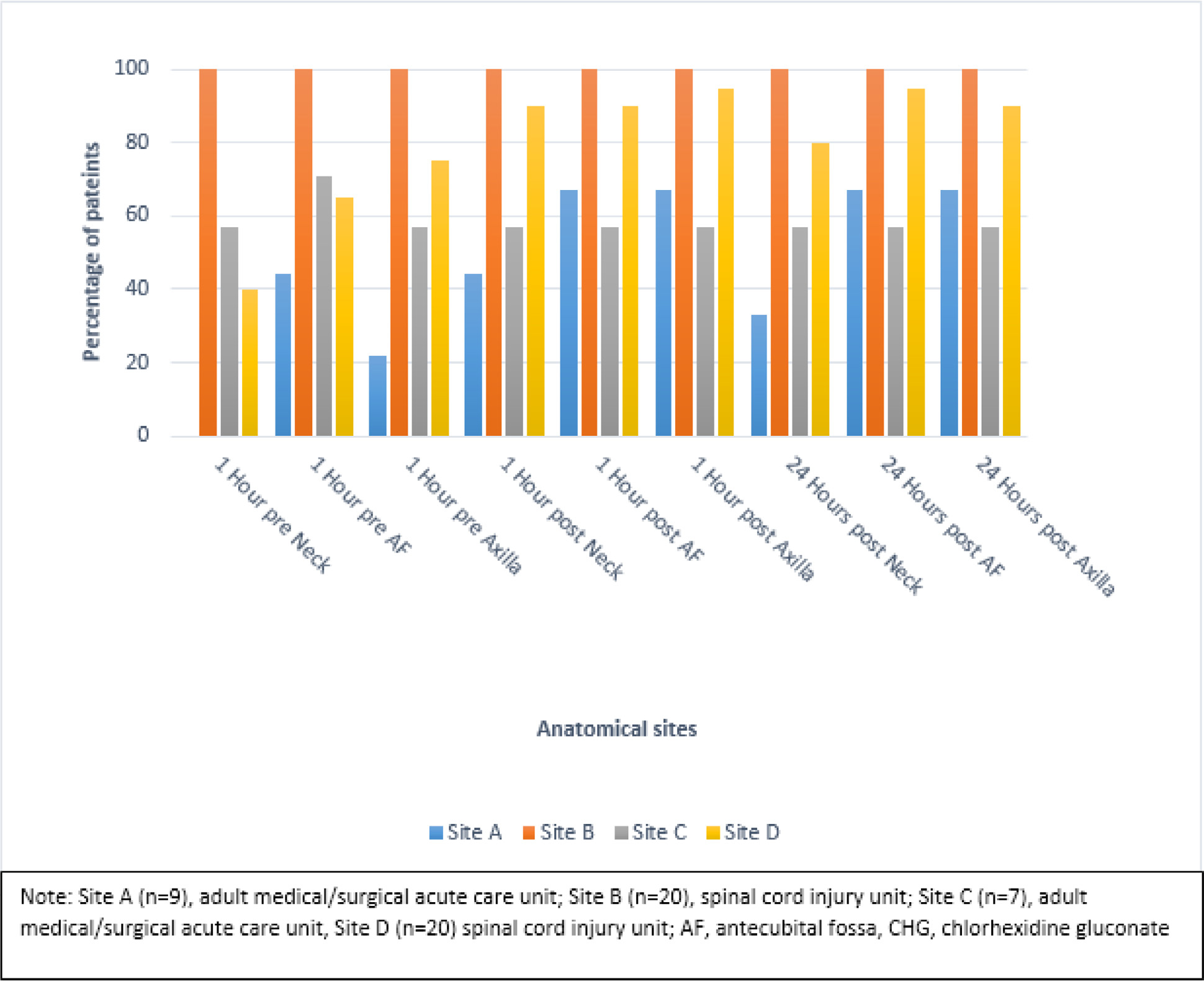
Proportion of patients with ANY detectable CHG concentration for 3 anatomic sites stratified by time of swab collection and project site.
Case A
Case A is a medical and/or surgical unit in a VHA hospital in the Midwest United States. The unit implemented direct application of CHG soap using pre-moistened reusable washcloths. The CHG rollout did not have a formal kick-off campaign and no champions were identified. Our project team provided an in-person training about CHG 1 month prior to the start date. A hospital epidemiologist and infectious disease physician attended this session. An infection preventionist provided staff education along with the CHG soap manufacturer representative during all shifts to nurses and nursing assistants. Due to a sluggish start-up, researchers conducted a focus group early in the process to explore staff perceptions about implementation of CHG bathing treatment. Lessons learned led to an implementation “restart” agreed upon by unit staff and researchers.
Interviews and focus groups
Staff training was a key issue for this site. Using the SEIPS work system framework,20,22 barriers (hindering the process) were identified 11 times during interviews and focus groups compared to facilitators (helping the process) identified 26 times. Those interviewed generally did not understand why they were being asked to participate in implementing CHG treatment; most staff assumed they were testing the CHG product. Once the actual purpose of implementation was discussed, 2 staff members previously most reluctant to using CHG volunteered to be unit champions. A staff member suggested conducting roundtable discussions about CHG product prior to implementation. The roundtable would need to provide enough information for staff to, in turn, adequately educate patients. Staff also indicated a need for a “big grand rollout” ushering in CHG bathing treatment as a new way to perform bathing. Lack of a formal and noticeable rollout appeared to lead to misinformation about the initiative. This perceived “haphazard” rollout not only led to questions and confusion but also minimal staff engagement in the implementation process. Product acceptability was another major barrier for staff. This group reported that CHG soap lacked a pleasant smell and the ability to produce “suds.” This was perceived, by staff, as a barrier for patient acceptance of the product. Staff raised concern about impact of CHG on patients’ skin such as drying and itching. Workflow did not appear to be a barrier for this unit.
“And whether it’s, you know, us educating the patients on the product itself, but obviously, we need to know the product ourselves in order to do that properly”
“You need a big grand rollout of what we were doing, but I think it kind of slipped by the wayside. And plus, you know, it depends, because we, not all of us work every single day during the weekday. And so sometimes, I think a couple of us might have missed out on maybe hearing about it . . .”
Observations and skin swabs for bathing compliance
We conducted 15 direct observations of the CHG treatment process at case A. The mean overall compliance was 82% (SD = 26%). Patient or family education about CHG treatment and using 1 washcloth for each body part were the most missed checklist items, occurring in only 50% and 9% of the CHG treatments, respectively. Mean duration of the CHG treatment was 15.8 (SD = 1.5) minutes (Table 4)
We collected skin swabs from 9 patients. The proportion of patients with detectable CHG 1-hour post and 24-hours post CHG treatment was greater than 60% for all anatomical sites except the neck (44% and 33%, respectively) (Fig 1).
Case B
Case B is a spinal-cord injury unit in a VHA hospital in the Midwest United States. Two percent CHG impregnated wipes were used on this unit. Rollout included a kick-off campaign with unit champions. Compared to other cases, this unit was different as implementation of CHG treatment was a sole responsibility of a new patient safety nurse fellow. In addition, this intervention had support from a facility-level nurse scientist who provided daily guidance on implementation aspects including data collection and analysis. This site also had the advantage of having 2 highly engaged champions (a registered nurse and a certified nursing assistant) who became leaders. These unit leaders, in conjunction with the nurse fellow, nurse scientist and nurse educators provided for extensive CHG treatment training and education of staff. Training did not appear to be a “one time” event; nurse leaders and unit champions were continually providing compliance updates to staff throughout the implementation period. Although this site was highly involved from the beginning, it took 1 year to become implementation-ready due to competing facility-level priorities. Nurse leaders managed all intervention planning, readiness, kick-off and orientation events. They also continuously developed tools to cope with barriers they encountered such as development of a list of common CHG-compatible lotions to use with Veterans who experience dry skin complications.
Interviews and focus groups
We conducted 2 focus groups of frontline staff (15 participants) involved in the actual CHG treatment process, including the unit champions. Overall, 107 barriers and 21 facilitators were identified during the interviews and focus groups. Most barriers were related to the CHG product. Time (n = 37) and workflow (n = 66) were also seen as barriers. Product barriers included temperature issues (did not retain warmth) and workflow barriers included staff being interrupted by physicians, other nurses or environmental services in the middle of a bathing treatment – leaving the CHG wipe and the patient to get cold. Another barrier perceived by staff was the “stickiness” of the product after application. Even with ongoing education reported by leaders, some staff were confused on the procedures and protocol related to use of CHG wipes for bathing.
“I thought that we were supposed to take the whole thing, like we couldn’t take one of the two-packs out. So, at first, I was just opening the package and the warmer and taking one two-pack out”
Many comments were made about CHG wipes packaging and warmers, product cost, expiration dates, waste, compatibility with lotions, when dressing could occur after a treatment and whether the CHG treatment was meant to replace a regular bath. Staff also emphasized the need for comprehensive staff education about the purpose of using CHG bathing treatment -as a means to better educate the patient. Staff in both focus groups appeared to be concerned about CHG bathing treatment creating “superbugs” for patients or disrupting normal skin microflora.
“I’m kind of worried, with the fact that with bathing, it’s kind of like something that’s been done for a long time. And I would think that you want to be creating like superbugs, but I always worry that maybe using the wipes, that might be causing problems down the line that I’m, that maybe we’re not aware of”
One combined interview was conducted with an inpatient program manager and a nurse educator. It was difficult to disentangle implementation responsibilities between the nurse manager and nurse educator due to the strong engagement of the nurse fellow, nurse scientist and unit champions. From the perspective of managers, it appeared staff education and engagement were the cornerstone of this unit’s implementation, and managers were quick to acknowledge the dedication of frontline staff and unit champions.
“I’ve seriously never worked with a group of people who are so committed to the patients they serve”.
Observations and skin swabs for bathing compliance
Twenty-three direct observations of CHG treatments were conducted at case B. The mean overall compliance was 89% (SD = 14%). Cleaning the tubing and drains closest to the body and cleaning the entire neck area including skin folds were the most missed checklist items, occurring in 63% and 48% of the CHG treatments, respectively. Nursing staff used these data to continue quality improvement efforts on bathing using audit and feedback. Mean duration of a treatment was 12.7 minutes (SD = 1.8) (Table 3).
We collected skin swabs from 20 patients. The proportion of patients with detectable CHG 1-hour post and 24-hours post CHG treatment was 100% for all anatomical sites (Fig 1).
Case C
Case C is a medical and/or surgical unit located in a large urban Pacific Northwest VHA hospital. The unit implemented a combination of direct application of CHG using reusable washcloths and CHG impregnated wipes. This unit was primarily a surgical unit and had already been familiar with use of CHG impregnated wipes for preoperative skin antisepsis. The CHG wipes were used for more dependent patients, while CHG soap and/or solution was used for patients who showered independently. The intervention was adopted as a unit-based quality improvement project. Researchers conducted an in-person visit 5 months prior to CHG treatment implementation with back-to-back sessions introducing the process and the SEIPS framework to nurses, nursing assistants, nurse managers, educators and infection control staff. We learned about a new initiative for the unit – a hygiene bundle – which coincided with CHG treatment implementation. The new hygiene bundle was initiated by the unit-level nursing council (part of a larger structure of shared governance) due to comments by patients on a patient satisfaction discharge survey which illustrated need for increased hygiene of patients on the unit. This new initiative allowed for a smooth integration of CHG treatment into routine care of patients because the CHG treatment was pulled into the hygiene bundle. Also, unique to this site was a heightened role of the infection preventionist during the start-up phase. Due to travel distance, all implementation training and mentoring from the research team was done virtually using phone calls, emails and videos on how to conduct observations and collect swabs. An on-site clinical nurse leader dedicated time to conduct all observations and swabbing. The research team provided protocols and checklists to staff, with phone call follow up.
Interviews and focus groups
Four focus groups (total of 9 participants) were conducted virtually using VHA Skype for Business. Two researchers (LM and MJK) led the focus groups. Overall, facilitators were identified 127 times and barriers identified 38 times with time being a barrier most often reported. Facilitators included staff education and training, staff engagement and staff support identified 26, 46 and 52 times respectively. Three themes appeared prominently and frequently – teamwork, pride in work, and staff incentives – all related to the organization element of the SEIPS model and reflecting unit culture. Both certified nursing assistants (CNAs) and registered nurses (RNs) assisted each other in completing CHG treatments. The unit held regular “potluck” meals in honor of nurses who were voted as examples of CHG treatment champions.
“It’s probably improved patient satisfaction as well, because we’re spending more time at the bedside talking to them, helping them change their gown. I think a lot of times they feel a little better after. I know like as a nurse, it helps me feel better about the work I do as well. I feel like I connect a little bit more with the patient when I’m able to help them with hygiene care”.
A unit culture of “equal responsibility” for CHG treatment appeared to influence uptake and sustainability of the CHG intervention. This unit also had a simple reward system in place that culminated in opportunities for staff to celebrate success and encourage teamwork.
“It’s only going to get done if the nurses and the CNAs are willing to buy in to its importance and be willing to put that on their list of things to do during the shift and help each other out. And so, you really need to have a good group effort”
Observations and skin swabs for bathing compliance
Staff from this facility conducted 10 direct observations. The mean overall compliance was 98% (SD=3 %). All the checklist items were completed with over 80% or greater compliance. Mean duration of a CHG treatment was 11 minutes (SD=1.5) (Table 3). We collected skin swabs from 7 patients. Four of 7 patients (57%) had detectable CHG 1-hour post and 24-hours post CHG treatment at all 3 anatomical sites (Fig 1).
Case D
Case D is a spinal-cord injury unit in a VHA hospital located in the East Coast United States. CHG (2%) impregnated wipes were used on this unit. CHG rollout included a kick-off campaign and identification of unit champions. Cases D and B were both spinal cord injury units. However, unlike case B where CHG implementation was spearheaded by nurses, implementation on this unit was led by a physician – the unit’s medical director. In addition, unlike the other 3 cases in which researchers initiated CHG bathing, this units’ medical director became interested in the project after learning about Human-factors Engineering to Prevent Resistant Organisms through a VHA webinar. Like other sites, time spent from initial contact to implementation start-up was approximately 1 year due to competing facility-level initiatives – including construction on the unit. CHG treatments began 2 months prior to an in-person visit by our research team to conduct observations, focus groups and collect swabs. Unit staff were familiar with using warmed bathing packages, so the addition of CHG warmed packages did not alter normal practice with the exception that CHG wipe solution was used as an after-bath treatment. Patients typically receive the CHG treatment following their shower, which occurs 3 times per week as part of their bowel program. On this unit, bowel programs occur on all 3 work shifts. CNA staff give showers and CHG application treatments.
Interviews and focus groups
Barriers to implementation were identified 61 times during interviews and focus groups compared to facilitators mentioned 30 times. Barriers related to the CHG treatment product (such as temperature of wipes, difficulty opening the packs, storage, causing dryness, causing itching, patients not feeling clean) and time (staffing issues, workload, patients wanting to be bathed first prior to CHG treatment) were key themes even though the process was familiar.
“Some patients are concerned that if I cannot put this product on my face, then why am I using this product if it’s a soap, like why can’t I use it? If I can’t use it on my face, then I shouldn’t be using it at all”
Organization themes identified as barriers were concern over cost-effectiveness, finding a consistent champion, confusion related to product preparation, staff communication, workflow, staff education and patient education. Concurrently, organization themes such as building staff awareness, ability to access educational materials and communication about MRSA transmission were key themes associated with facilitating the implementation process. Organization identified equally as a barrier and a facilitator (each 23 times).
“I think it’s going to be important to do observations, to continue doing observations every so often, and to have a champion, or somebody who really believes in it. . .”
The tools and technology element of the SEIPS model was identified as the number 1 barrier to implementation (primarily product barrier). Also, staff appeared to be well-aware that CHG treatment was high priority on the unit and indicated much communication about MRSA and felt that unit medical and nursing leadership was dedicated to the process. The overall perception was that CHG treatment improves patient care and outcomes, however there were some staff who felt that CHG treatment put patients at risk for developing resistance.
Interviews were conducted with the SCI unit medical director, the nursing manager, and a RN project team champion. These unit leaders reiterated many of the same frontline staff themes but pointed to 1 barrier – the lack of standardization and precision of the CHG bathing process- as a stumbling block to starting implementation. The medical director also discussed coming to the realization that the CHG process may never be precise but proceeding with implementation rollout needed to start sometime – no matter the circumstances on the unit at the time of implementation.
“Then we said, let’s start, because it’s never going to be perfect.
Observations and skin swabs for bathing compliance
Twenty direct observations of the CHG treatment process were conducted at case D. The mean overall compliance was 84% (SD = 17%). Cleaning tubing and drains closest to body and cleaning in between toes were the most missed checklist item, occurring in only 20% and 38% of the CHG treatments, respectively. Mean duration of a CHG treatment was 8.7 minutes (SD = 1.3) (Table 3)
We collected skin swabs from 20 patients. The proportion of patients with detectable CHG 1-hour post and 24-hours post CHG treatment was greater 80% or greater for all anatomical sites (Fig 1).
DISCUSSION
Examining contextual factors and identifying barriers and facilitators of the implementation process is a logical step in moving CHG bathing treatments into routine non-ICU care. We found frontline HCWs concerned about Veteran safety with use of CHG products which might increase antibiotic resistance or disrupt normal skin microflora. Staff indicated that if they are adequately educated as to why they are using CHG and if the training addresses safety concerns, it would be easier to educate and “sell” patients on the need for CHG bathing treatments. This supports a recent study indicating patient education and addressing patient self-efficacy as key to decreasing patient refusal of CHG bathing.32 Lack of coordinated and systematic training along with a haphazard approach to roll-out (some staff being trained and others not) appeared to negatively influence staff perception of intervention necessity and perceptions of their ability to provide education which has been associated with patient refusal of CHG bathing treatment.33 Work system facilitators across all sites were frequently related to unit culture and the perception that those performing CHG tasks had support from others on the unit for assistance with the process itself or educating staff and patients. These relate primarily to the organization element of the work system and have been shown in previous studies to improve compliance with CHG bathing treatment.34
Data from observations showed that the highest mean duration of a CHG treatment was 15.8 minutes (Case A – a medical and/or surgical unit using reusable wash cloths with washcloths moistened in a basin), while the lowest was 8.7 minutes (Case D – a spinal cord injury unit using CHG pre-packaged impregnated wipes). Highest proportions of patients with detectable CHG concentrations were observed in cases that used CHG impregnated wipes compared to cases that used CHG soap or solution (cases B and D). These results are consistent with recent studies that found improved CHG skin concentration with CHG impregnated wipes and less so with direct application of CHG soap using non-disposable washcloths.35,36
Implementation recommendations
Our staggered implementation timeline resulted in practical recommendations applicable to broad audiences. The following are key recommendations:
Facilitating implementation takes time
For 3 of the 4 sites, the time from initial contact with key staff to start-up of CHG bathing treatments was 1 year. One site proceeded quickly but had to restart due to confusion over intervention purpose. There were 2 sites (not included in this project) that devoted substantial time but ended up withdrawing. One site did not initially involve leadership and once “kick off” began, leadership questioned cost of CHG supplies and decided not to participate. The other site initially contacted our research team because, although they had been already implementing CHG bathing in non-ICU settings, they faced compliance issues. However, low infection prevention staffing at this site prevented participation.
To assist with start-up and follow-through issues, we recommend a mentored implementation approach. This strategy is appropriate for complex interventions requiring significant resources at the local level. Mentoring can enhance the chances of scalability by producing new experts (from local sites) to serve as mentors in future implementations.27,37–41
Consider organizational readiness
Unit and facility readiness should be assessed prior to implementing CHG bathing. We recommend addressing the following constructs of the Consolidated Framework of Implementation Research – a framework that provides a menu of constructs associated with effective implementation.42 In the case of CHG bathing treatment it is also important to weigh the different product considerations – and test for usability and acceptability by staff (and patients). Engaging staff in product choice and workflow considerations are important to an effective roll-out.
Champions at all levels
All stakeholders must be well-aware of the purpose for implementing CHG bathing treatment. Staff education cannot be a 1-time event. Participants revealed the need for ongoing staff education as a precursor to educating patients. To supplement staff and patient education efforts, we recommend formally appointing internal leaders or unit champions. These individuals may have influence on attitudes and beliefs of co-workers. Champions can be any individual who is a driving force in addressing barriers while boosting confidence of frontline staff in the implementation process.43,44
“That CNA, that champion took hold of it and was kind of a leader within the process and tried to champion it with her coworkers. You know, she really took it personally that, you know, she didn’t want people to get infections. . .
– Nurse Manager
It is important to acknowledge frontline staff questions and concerns about nursing time, product, and the risk/benefit ratio. Staff from 3 of 4 cases perceived this ratio to be narrow – asking questions about the benefit of HAI reductions vs the risk of resistance and disruption of normal flora for their Veteran patients. This concern should be addressed prior to implementation roll-out and continue to be discussed as staff members are hired.
Future study of implementation processes should illuminate how best to coordinate roll-out with other priorities at the facility and unit levels, best practices in educating staff from all shifts (for the sake of patient education), and how unit culture and leadership engagement impact sustained best practice.
A major strength of this project is our use of multiple data collection methods within a multiple case study design. This enabled us to explain factors influencing the implementation of CHG treatment in multiple sites.45 With this approach, we related qualitative data with compliance measures while exploring factors associated with implementation. This contributes to the body of evidence, since recent literature reports that CHG treatments are primarily performed in the ICU even though there is growing evidence for CHG benefit outside the ICU.46,47
Interviews and focus groups may have introduced bias due to convenience sampling. This is not a major limitation because focus groups involved HCWs from varying shifts and varying workforce levels (nurse managers, nurse educators, infection preventionists). We also recognize that bias may be introduced when RNs and CNAs were being observed during the bathing process. CHG skin swabs were limited at some sites due to distance issues. However, if travel distance prohibited site visits, we remotely trained hospital staff to conduct observations and obtain skin swabs. Even with the smaller number of samples, we demonstrated measurable compliance (observations) and CHG residual activity (skin swabs).
CONCLUSION
Continual and all-inclusive staff training in combination with leadership commitment and a sense of shared responsibility are key contextual factors impacting uptake and sustainability of CHG bathing treatment in non-ICUs. Organizations should ensure readiness and stakeholder buy-in from a work system and human factors perspective. It is important to assess compliance and provide feedback on a continuing basis. Audit and feedback of healthcare practices is an evidence-based practice found to improve healthcare behavior.48 Timing, education and buy-in from staff at every level may assist in overcoming barriers and perpetuating facilitators along the path from evidence to sustained practice.
Footnotes
Conflicts of interest: None to report.
References
- 1.Magill SS, O’Leary E, Janelle SJ, et al. Changes in prevalence of health care-associated infections in U.S. hospitals. N Engl J Med. 2018;379:1732–1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Magill SS, Edwards JR, Beldavs ZG, et al. Prevalence of antimicrobial use in US acute care hospitals, May-September 2011. Jama. 2014;312:1438–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roberts RR, Hota B, Ahmad I, et al. Hospital and societal costs of antimicrobial-resistant infections in a Chicago teaching hospital: implications for antibiotic stewardship. Clin Infect Dis. 2009;49:1175–1184. [DOI] [PubMed] [Google Scholar]
- 4.Roberts RR, Scott RD 2nd, Cordell R, et al. The use of economic modeling to determine the hospital costs associated with nosocomial infections. Clin Infect Dis. 2003;36:1424–1432. [DOI] [PubMed] [Google Scholar]
- 5.Roberts RR, Scott RD 2nd, Hota B, et al. Costs attributable to healthcare-acquired infection in hospitalized adults and a comparison of economic methods. Med Care. 2010;48:1026–1035. [DOI] [PubMed] [Google Scholar]
- 6.Zimlichman E, Henderson D, Tamir O, et al. Health care–associated infections: a meta-analysis of costs and financial impact on the us health care system. JAMA Intern Med. 2013;173:2039–2046. [DOI] [PubMed] [Google Scholar]
- 7.Climo MW, Sepkowitz KA, Zuccotti G, et al. The effect of daily bathing with chlorhexidine on the acquisition of methicillin-resistant Staphylococcus aureus, vancomycin-resistant Enterococcus, and healthcare-associated bloodstream infections: results of a quasi-experimental multicenter trial. Crit Care Med. 2009;37:1858–1865. [DOI] [PubMed] [Google Scholar]
- 8.Climo MW, Yokoe DS, Warren DK, et al. Effect of daily chlorhexidine bathing on hospital-acquired infection. N Engl J Med. 2013;368:533–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Evans HL, Dellit TH, Chan J, Nathens AB, Maier RV, Cuschieri J. Effect of chlorhexidine whole-body bathing on hospital-acquired infections among trauma patients. Arch Surg. 2010;145:240–246. [DOI] [PubMed] [Google Scholar]
- 10.Huang SS, Septimus E, Kleinman K, et al. Targeted versus universal decolonization to prevent ICU infection. N Engl J Med. 2013;368:2255–2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kassakian SZ, Mermel LA, Jefferson JA, Parenteau SL, Machan JT. Impact of chlorhexidine bathing on hospital-acquired infections among general medical patients. Infect Control Hosp Epidemiol. 2011;32:238–243. [DOI] [PubMed] [Google Scholar]
- 12.Milstone AM, Passaretti CL, Perl TM. Chlorhexidine: expanding the armamentarium for infection control and prevention. Clin Infect Dis. 2008;46:274–281. [DOI] [PubMed] [Google Scholar]
- 13.Vernon MO, Hayden MK, Trick WE, et al. Chlorhexidine gluconate to cleanse patients in a medical intensive care unit: the effectiveness of source control to reduce the bioburden of vancomycin-resistant enterococci. Arch Intern Med. 2006;166:306–312. [DOI] [PubMed] [Google Scholar]
- 14.O’Horo JC, Silva GL, Munoz-Price LS, Safdar N. The efficacy of daily bathing with chlorhexidine for reducing healthcare-associated bloodstream infections: a meta-analysis. Infect Control Hosp Epidemiol. 2012;33:257–267. [DOI] [PubMed] [Google Scholar]
- 15.Shekelle PG, Pronovost PJ, Wachter RM, et al. Advancing the science of patient safety. Ann Intern Med.154:693–696. [DOI] [PubMed] [Google Scholar]
- 16.Øvretveit J Understanding the conditions for improvement: research to discover which context influences affect improvement success. BMJ Qual Saf. 2011;20(Suppl 1):i18–i23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eisenhardt KM. Building theories from case study research. Acad Manag Rev. 1989;14:532–550. [Google Scholar]
- 18.Tong A, Sainsbury P, Craig J. Consolidated criteria for reporting qualitative research (COREQ): a 32-item checklist for interviews and focus groups. Int J Qual Health Care. 2007;19:349–357. [DOI] [PubMed] [Google Scholar]
- 19.Clack L, Zingg W, Saint S, et al. Implementing infection prevention practices across European hospitals: an in-depth qualitative assessment. BMJ Qual Saf. 2018;27:771–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carayon P, Schoofs Hundt A, Karsh BT, et al. Work system design for patient safety: the SEIPS model. Qual Saf Health Care. 2006;15(Suppl 1):i50–i58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carayon P Human factors in patient safety as an innovation. Appl Ergon. 2010;41:657–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carayon P, Wetterneck TB, Rivera-Rodriguez AJ, et al. Human factors systems approach to healthcare quality and patient safety. Appl Ergon. 2014;45:14–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shekelle PG, Wachter RM, Pronovost PJ, et al. Making health care safer II: an updated critical analysis of the evidence for patient safety practices. Evid Rep Technol Assess (Full Rep). 2013:1–945. [PMC free article] [PubMed] [Google Scholar]
- 24.Handbook of Human Factors in Health Care and Patient Safety. Mahwah, New Jersey: Lawrence Erlbaum Associates; 2011. [Google Scholar]
- 25.Carayon P, A X, S K. Human factors and ergonomics. Making Health Care Safer II: An Updated Critical Analysis of the Evidence for Patient Safety Practices. 211. Rockville, MD: Agency for Healthcare Research and Quality (US); 2013. [Google Scholar]
- 26.Rivera AJ, Karsh B-T. Human factors and systems engineering approach to patient safety for radiotherapy. Int J Radiat Oncol Biol Phys. 2008;71(1, Supplement):S174–S177. [DOI] [PubMed] [Google Scholar]
- 27.Li J, Hinami K, Hansen LO, Maynard G, Budnitz T, Williams MV. The physician mentored implementation model: a promising quality improvement framework for health care change. Acad Med. 2015;90:303–310. [DOI] [PubMed] [Google Scholar]
- 28.Caya T, Musuuza J, Yanke E, et al. Using a systems engineering initiative for patient safety to evaluate a hospital-wide daily chlorhexidine bathing intervention. J Nurs Care Qual. 2015;30:337–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Musuuza JS, Hundt AS, Zimbric M, Carayon P, Safdar N. Standardizing direct observation for assessing compliance to a daily chlorhexidine bathing protocol among hospitalized patients. Infect Control Hosp Epidemiol. 2016;37:1516–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Edmiston CE, Krepel CJ, Seabrook GR, Lewis BD, Brown KR, Towne JB. Preoperative shower revisited: can high topical antiseptic levels be achieved on the skin surface before surgical admission? J Am Coll Surg. 2008;207:233–239. [DOI] [PubMed] [Google Scholar]
- 31.Popovich KJ, Lyles R, Hayes R, et al. Relationship between chlorhexidine gluconate skin concentration and microbial density on the skin of critically Ill patients bathed daily with chlorhexidine gluconate. Infect Control Hosp Epidemiol. 2012;33:889–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Caya T, Knobloch MJ, Musuuza J, Wilhelmson E, Safdar N. Patient perceptions of chlorhexidine bathing: a pilot study using the health belief model. Am J Infect Control. 2019;47:18–22. [DOI] [PubMed] [Google Scholar]
- 33.Musuuza JS, Roberts TJ, Carayon P, Safdar N. Assessing the sustainability of daily chlorhexidine bathing in the intensive care unit of a veteran’s hospital by examining nurses’ perspectives and experiences. BMC Infect Dis. 2017;17:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hines AG, Nuss S, Rupp ME, Lyden E, Tyner K, Hewlett A. Chlorhexidine bathing of hospitalized patients: beliefs and practices of nurses and patient care technicians, and potential barriers to compliance. Infect Control Hosp Epidemiol. 2015;36:993–994. [DOI] [PubMed] [Google Scholar]
- 35.Rhee Y, Palmer LJ, Okamoto K, et al. Differential effects of chlorhexidine skin cleansing methods on residual chlorhexidine skin concentrations and bacterial recovery. Infect Control Hosp Epidemiol. 2018;39:405–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shah HN, Schwartz JL, Luna G, Cullen DL. Bathing with 2% chlorhexidine gluconate: evidence and costs associated with central line-associated bloodstream infections. Crit Care Nurs Q. 2016;39:42–50. [DOI] [PubMed] [Google Scholar]
- 37.Bunger AC, Doogan N, Hanson RF, Birken SA. Advice-seeking during implementation: a network study of clinicians participating in a learning collaborative. Implement Sci. 2018;13:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maynard GA, Budnitz TL, Nickel WK, et al. 2011 John M. Eisenberg patient safety and quality awards. Mentored implementation: building leaders and achieving results through a collaborative improvement model. Innovation in patient safety and quality at the national level. Jt Comm J Qual Patient Saf. 2012;38:301–310. [DOI] [PubMed] [Google Scholar]
- 39.Nickel W, Saint S, Olmsted RN, et al. The interdisciplinary academy for coaching and teamwork (I-ACT): a novel approach for training faculty experts in preventing healthcare-associated infection. Am J Infect Control. 2014;42(10 Suppl):S230–S235. [DOI] [PubMed] [Google Scholar]
- 40.Rogers KM, Childers DJ, Messler J, Nolan A, Nickel WK, Maynard GA. Glycemic control mentored implementation: creating a national network of shared information. Jt Comm J Qual Patient Saf. 2014;40:111–118. [DOI] [PubMed] [Google Scholar]
- 41.Varkey P, Antonio K. Change management for effective quality improvement: a primer. Am J Med Qual. 2010;25:268–273. [DOI] [PubMed] [Google Scholar]
- 42.Damschroder LJ, Aron DC, Keith RE, Kirsh SR, Alexander JA, Lowery JC. Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implement Sci. 2009;4:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Damschroder LJ, Banaszak-Holl J, Kowalski CP, Forman J, Saint S, Krein SL. The role of the champion in infection prevention: results from a multisite qualitative study. Qual Saf Health Care. 2009;18:434–440. [DOI] [PubMed] [Google Scholar]
- 44.Miech EJ, Rattray NA, Flanagan ME, Damschroder L, Schmid AA, Damush TM. Inside help: an integrative review of champions in healthcare-related implementation. SAGE Open Med. 2018;6: 2050312118773261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Szymczak JE. Beyond barriers and facilitators: the central role of practical knowledge and informal networks in implementing infection prevention interventions. BMJ Qual Saf. 2018;27:763–765. [DOI] [PubMed] [Google Scholar]
- 46.Frost SA, Alogso MC, Metcalfe L, et al. Chlorhexidine bathing and health care-associated infections among adult intensive care patients: a systematic review and meta-analysis. Crit Care. 2016;20:379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.O’Horo JC, Silva GLM, Munoz-Price S, Safdar N. The efficacy of daily bathing with chlorhexidine for reducing healthcare-associated bloodstream infections: a meta-analysis. Infect Control Hosp Epidemiol. 2012;33:257–267. [DOI] [PubMed] [Google Scholar]
- 48.Ivers N, Jamtvedt G, Flottorp S, et al. Audit and feedback: effects on professional practice and healthcare outcomes. Cochrane Database Syst Rev. 2012;13:Cd000259. [DOI] [PMC free article] [PubMed] [Google Scholar]


