Abstract
Intracellular protozoan parasites of the genus Leishmania antagonize host defense mechanisms by interfering with cell signaling in macrophages. In this report, the impact of Leishmania donovani on mitogen-activated protein (MAP) kinases and nitric oxide synthase (NOS) expression in the macrophage cell line RAW 264 was investigated. Overnight infection of cells with leishmania led to a significant decrease in phorbol-12-myristate-13-acetate (PMA)-stimulated MAP kinase activity and inhibited PMA-induced phosphorylation of the MAP kinase substrate and transcription factor Elk-1. Simultaneously, leishmania infection markedly attenuated the induction of c-FOS and inducible nitric oxide synthase (iNOS) expression in response to PMA and gamma interferon (IFN-γ), respectively. These effects correlated with decreased phosphorylation of p44 and p42 MAP kinases on tyrosine residues. Consistent with the latter finding, lysates prepared from leishmania-infected cells contained an activity that dephosphorylated MAP kinase in vitro, suggesting the possibility of a phosphatase acting in vivo. Attenuation of both MAP kinase activity and c-FOS and iNOS expression was reversed by treatment of macrophages with sodium orthovanadate prior to infection. It was also found that the specific activity of the Src homology 2 domain containing tyrosine phosphatase (SHP-1) toward MAP kinase was markedly increased in leishmania-infected cells. These findings indicate that infection with L. donovani attenuates MAP kinase signaling and c-FOS and iNOS expression in macrophages by activating cellular phosphotyrosine phosphatases. This may represent a novel mechanism of macrophage deactivation during intracellular infection.
Protozoan organisms of the genus Leishmania are obligate intracellular parasites of macrophages (Mφ) that are responsible for severe morbidity and mortality in infected people in many parts of the world. A principal function of Mφ is to destroy intracellular pathogens. Hence, the manner in which Leishmania and other intracellular pathogens are able to survive and replicate within this ostensibly hostile intracellular milieu is an important question in cell biology and immunology. It is becoming increasingly evident that Leishmania evades host defense mechanisms by disrupting important target cell functions. For example, Leishmania and other intracellular pathogens have evolved mechanisms to modulate host signaling pathways in order to facilitate invasion and survival. Diverse lines of evidence indicate that Leishmania interferes with signal transduction in Mφ (23). It has been shown that Leishmania donovani infection of human monocytes selectively attenuates the gamma interferon (IFN-γ)-activated Jak-Stat1 signal pathway by inhibiting tyrosine phosphorylation of Jak1, Jak2, and Stat1 (15). It has also been shown that infection of macrophages with L. donovani significantly reduces protein phosphorylation in response to phorbol-12-myristate-13-acetate (PMA) which correlates with diminished protein kinase C (PKC) activity (19). Furthermore, it has been found that in murine macrophage infected with L. donovani, c-fos gene expression induced by PKC is impaired whereas protein kinase A (PKA)-mediated c-fos gene expression is unaffected (14).
Mitogen-activated protein (MAP) kinases are downstream targets of PKC (25), suggesting the possibility of impaired signaling through these enzymes in leishmania-infected cells. Two closely related MAP kinases, extracellular signal regulated-protein kinases 1 and 2, function as essential relays in many signal transduction processes. These kinases are in part responsible for regulating gene expression in response to diverse extracellular stimuli such as growth factors, cytokines, and hormones that influence cell proliferation, differentiation, and other functions (1, 21). MAP kinases have pleiotropic effects on processes in the cytoplasm, the nucleus, the cytoskeleton, and the plasma membrane (1, 21). Phosphorylation of MAP kinases on tyrosine and threonine is essential for their activation (20). Because of the broad range of effects of MAP kinases, their activities are tightly controlled by a family of dual-specificity enzymes known as MAP kinase phosphatases that dephosphorylate MAP kinases on both threonine and tyrosine, thereby rendering them inactive (7). It has also been reported that the Src homology 2 domain containing tyrosine phosphatase (SHP-1) is involved in deactivating MAP kinases via dephosphorylation of tyrosine residues (8).
In this study MAP kinase signaling and c-FOS and inducible nitric oxide synthase (iNOS) expression during infection with L. donovani were examined. The results show that infection with L. donovani attenuates both activation of and signaling through MAP kinases and the induction of c-FOS and iNOS. The data also show that leishmania infection brings about an increase in phosphotyrosine phosphatase activity, including the specific activity of SHP-1 toward MAP kinases. Inhibition of phosphotyrosine phosphatases reversed the effects of leishmania infection on signaling, c-FOS and iNOS expression. These findings are consistent with a model in which a phosphotyrosine phosphatase(s) becomes activated in response to leishmania infection, leading to impaired signaling through MAP kinases.
MATERIALS AND METHODS
Reagents.
The RAW 264.7 cell line was obtained from the American Type Culture Collection (Rockville, Md.). RPMI 1640 and Hanks balanced salt solution (HBSS) were from Stem Cell Technologies (Vancouver, British Columbia, Canada). Antiphosphotyrosine monoclonal antibody 4G10 was from Upstate Biotechnology Inc. (Lake Placid, N.Y.). Anti-MAP kinase-ct, MAP kinase glutathione S-transferase (GST), and MAP kinase kinase (MEK) were from KINETEK Pharmaceutical Inc. (Vancouver, British Columbia, Canada). Anti-SHP-1 and anti-iNOS antibodies were purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, Calif.). Horseradish peroxidase-conjugated goat anti-rabbit antibodies, protein A-agarose, and electrophoresis reagents and supplies were purchased from Bio-Rad (Hercules, Calif.). Enhanced chemiluminescence (ECL) reagents and ECL film were from Amersham International (Oakville, Ontario, Canada). PMA was from Sigma Chemical Co. (St. Louis, Mo.). Murine IFN-γ was a gift from Genentech Inc. (South San Francisco, Calif.). Lipopolysaccharide from Escherichia coli O127:B8 was from Difco (Detroit, Mich.).
Cell culture.
The murine Mφ cell line RAW 264.7 was cultured in RPMI 1640 medium supplemented with 10% heat-inactivated fetal calf serum, penicillin (100 U/ml), and streptomycin (100 μg/ml) at 37°C in a humidified atmosphere (5% CO2).
L. donovani.
Amastigotes of the Sudan strain 2S of L. donovani were maintained by serial intracardiac inoculation of amastigotes into female Syrian hamsters. Amastigotes were isolated from the spleens of hamsters infected 4 to 6 weeks earlier as previously described (18).
Infection of RAW 264.7 cells.
Exponentially growing RAW cells were infected with freshly isolated amastigotes of L. donovani at a parasite-to-Mφ ratio of approximately 15:1. After incubation at 37°C in a humidified atmosphere of 5% CO2 and 95% air, noningested amastigotes were removed by washing with HBSS. Rates of infection were determined by using cytospin preparations stained with Diff-Quik and were usually in the range of 90% with a mean of six organisms/cell for an overnight infection.
Cell incubation, immunoprecipitation, and immunoblotting.
After treatment, monolayers were washed twice with HBSS and immediately processed for immunoprecipitation. The cells were lysed in ice-cold modified RIPA buffer (50 mM Tris [pH 7.5], 1% Nonidet P-40, 0.25% sodium deoxycholate, 0.15 M NaCl, 1 mM EGTA, 1 mM NaF, 1 mM sodium orthovanadate, 1 mM phenylmethylsulfonylfluoride, 100 μM microcystine, 10 μg of aprotinin per ml, 10 μg of leupeptin per ml, 2 μg of pepstatin A per ml). The lysates were centrifuged in a microcentrifuge at maximum speed for 20 min at 4°C. The resulting supernatants were incubated with the desired antibodies, and 40 μl of protein A-Sepharose was added to recover immune complexes. Immune complexes were released by boiling agarose beads in sodium dodecyl sulfate (SDS) sample buffer. Samples were analyzed by SDS-polyacrylamide gel electrophoresis and electrophoretically transferred to nitrocellulose membranes by using a semidry electroblotting apparatus. The membranes were blocked with 3% bovine serum albumin in Tris-buffered saline–Tween 20 (TBS-T) (20 mM Tris [pH 7.6], 0.137 M NaCl, 0.1% Tween 20) for 12 to 16 h at 4°C. The blots were then incubated for 2 h with appropriate antibody at room temperature, washed in TBS-T, and incubated for 1 h with horseradish peroxidase-conjugated secondary antibody. After being washed again with TBS-T, the blots were developed with an ECL kit (Amersham) as recommended by the manufacturer. For reprobing, the membranes were stripped by incubation in 2% SDS–100 mM β-mercaptoethanol–62.5 mM Tris-HCl (pH 6.8) for 45 min at 50°C. After extensive washing and blocking, the membranes were probed with the appropriate antibody and developed.
Immune complex kinase assays.
Total-cell lysates prepared in modified RIPA buffer containing a cocktail of protease and phosphatase inhibitors (as described above) were incubated, while rotating, with 1 μg of anti-MAP kinase rabbit polyclonal antibody for 1 h at 4°C followed by addition of protein A-Sepharose and further incubation for 2 h at 4°C. Immunoprecipitated proteins were recovered by centrifugation in a microcentrifuge at 4°C. The immunoprecipitates were washed twice with 0.25 M Tris (pH 7.6) and once with 0.1 M NaCl containing 50 mM HEPES (pH 8.0). Samples were incubated at 30°C for 20 min in 100 μl of a mixture containing 1 μCi of [γ-32P]ATP, 50 μM ATP, 10 mM MgCl2, 1 mM dithiothreitol, 1 mM benzamidine, 0.3 mg of myelin basic protein (MBP), and 25 mM HEPES (pH 8.0). The reactions were stopped by adding 2× SDS sample buffer and boiling for 5 min. Samples were electrophoresed on 15% polyacrylamide gels and transferred to nitrocellulose membranes. After being stained with amido black, the blots were dried and exposed to X-ray film overnight. The same membranes were blocked and probed with anti-MAP kinase antibodies to assess the input of precipitated enzyme. Finally, the MBP bands were excised and subjected to liquid scintillation counting.
Dephosphorylation of MEK-phosphorylated MAP kinase-GST.
MAP kinase-1–GST coupled to glutathione-agarose was phosphorylated with MEK and [γ-32P]ATP for 30 min at 30°C in phosphorylation buffer (20 mM morpholinepropanesulfonic acid [MOPS; pH 7.2], 25 mM β-glycerophosphate, 10 mM MnCl2, 10 mM MgCl2, 2 mM NaF, 1 mM dithiothreitol, 10 μg of leupeptin per ml, 10 μg of aprotinin per ml, 200 μM ATP). MEK-phosphorylated MAP-kinase-1–GST agarose was washed twice with phosphatase assay buffer (25 mM imidazole [pH 7.0], 1 mM EDTA) and incubated for 45 min at 30°C with whole-cell lysates prepared in lysis buffer (20 mM Tris [pH 7.4], 1% Nonidet P-40, 50 mM NaCl, 10% glycerol, 1 mM phenylmethylsulfonyl fluoride 10 μg of leupeptin per ml, and 10 μg of aprotinin per ml without phosphatase inhibitors). Agarose beads were collected by centrifugation, washed, and boiled in SDS sample buffer for 5 min. Samples were electrophoresed on 10% polyacrylamide gels and then electrophoretically transferred to nitrocellulose membranes and probed for the phosphotyrosine content of MAP kinase-1–GST by using antiphosphotyrosine monoclonal antibody 4G10 (see above).
SHP-1 phosphatase assay.
The assay for protein tyrosine phosphatase activity was performed with phosphorylated MAP kinase-1–GST as the substrate. MAP kinase-1–GST coupled to glutathione-agarose was autophosphorylated in phosphorylation buffer with cold ATP for 30 min at 30°C. The agarose beads were washed twice in autophosphorylation buffer without ATP and twice in phosphatase assay buffer (25 mM imidazole [pH 7.0], 1 mM EDTA). Phosphorylated MAP kinase-1–GST was then resuspended in the desired volume of phosphatase assay buffer and used immediately to measure SHP-1 activity. SHP-1 was immunoprecipitated (see above) from cell lysates, washed three times in phosphatase buffer, and incubated with phosphorylated substrate for 1 h at 30°C. The reaction was stopped by the addition of an equal volume of 2× SDS sample buffer followed by immunoblotting with antiphosphotyrosine antibodies. The blots were then stripped and reprobed with anti-MAP kinase-1 and anti-SHP-1 to confirm equal input of substrate and phosphatase enzyme.
RESULTS
Leishmania infection attenuates PMA induced tyrosine phosphorylation in RAW 264.7 cells.
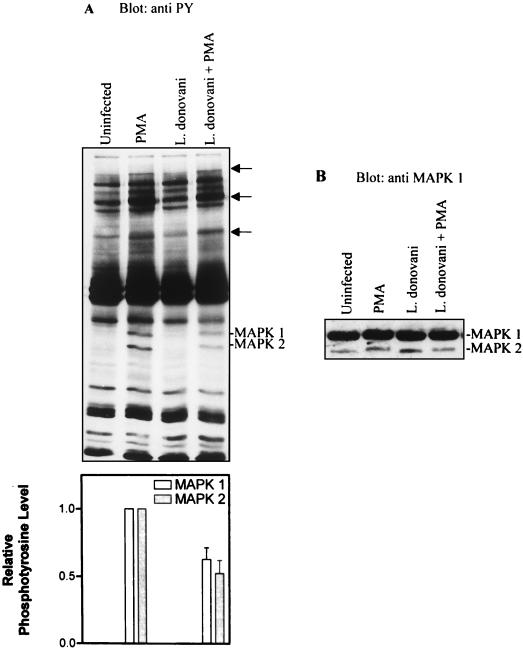
To examine whether leishmania infection modulates PMA-induced signaling, patterns of tyrosine-phosphorylated proteins were analyzed in cells infected with L. donovani. Control and infected cells were stimulated with 100 nM PMA for 15 min. PMA reproducibly induced tyrosine phosphorylation of multiple proteins including two with subunit Mr of 44,000 and 42,000 (Fig. 1A, lanes 1 and 2). Stripping and reprobing the membranes with anti-MAP kinase antibodies showed that the Mr 44,000 and 42,000 proteins were MAP kinase-1 and MAP kinase-2, respectively (Fig. 1B). Compared to control cells, in Mφ infected with leishmania the phosphorylation of MAP kinase-1 and MAP kinase-2 in response to PMA was significantly reduced (Fig. 1A, compare lanes 2 and 4). Based upon band shifting, the immunoblot shown in Fig. 1B discriminated between unphosphorylated and phosphorylated forms of both MAP kinase-1 and MAP kinase-2. Phosphorylated forms showed slower electrophoretic mobilities than did isoforms from non-PMA-treated cells (compare lanes 1 and 3 with lanes 2 and 4). Of particular interest was the finding that while infection with L. donovani significantly attenuated tyrosine phosphorylation of both MAP kinase isoforms, band shifting of the enzymes was still observed. These results suggested that although tyrosine phosphorylation of MAP kinases was markedly attenuated in infected cells, tyrosine phosphorylation per se does not appear to be essential for band shifting to occur. It is also of note that treatment of cells with PMA resulted in enhanced tyrosine phosphorylation of several other proteins (Fig. 1A [arrows]) in addition to MAP kinases-1 and -2. These proteins showed comparably enhanced tyrosine phosphorylation (in response to PMA) in both control and infected cells, indicating that the effects of leishmania on tyrosine phosphorylation of MAP kinases was selective.
FIG. 1.
L. donovani attenuates PMA-induced tyrosine phosphorylation of MAP kinases (MAPKs). Cells were either untreated or incubated with leishmania amastigotes at an approximate parasite-to-cell ratio of 15:1. After overnight incubation (17 h), control and infected cells were incubated in the absence or presence of 100 nM PMA for 15 min. (A) Cells were lysed in modified RIPA buffer as described in Materials and Methods. Whole-cell lysates were separated by SDS-polyacrylamide gel electrophoresis (10% polyacrylamide), transferred to nitrocellulose membranes, and probed with antiphosphotyrosine (anti PY) antibodies. Blots were developed by ECL, and an autoluminogram of a blot is shown. The tyrosine-phosphorylated bands with Mr of 44,000 and 42,000 corresponded to p44MAP kinase-1 and p42MAP kinase-2, respectively. In addition to MAP kinase-1 and MAP kinase-2, the positions of other PMA-induced phosphotyrosine-containing proteins are indicated by arrows. The autoluminogram was analyzed by densitometry in the region of p44 and p42 MAP kinases. (B) The same blot was stripped and reprobed with anti-MAP kinase antibodies. The data shown are from three independent experiments that yielded similar results. The values shown in the histogram represent mean and standard deviation.
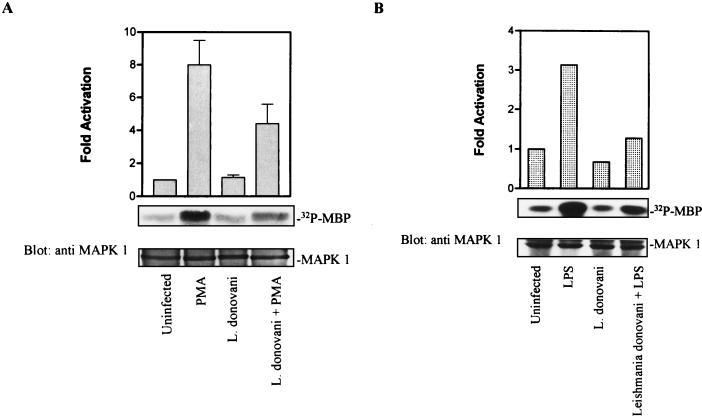
L. donovani infection attenuates MAP kinase activity.
To examine directly whether MAP kinase activity was affected by infection with leishmania, enzyme activity was assessed by an immune complex kinase assay with MBP as the substrate. Compared to basal activity in untreated control cells, incubation with PMA induced a significant increase in phosphotransferase activity toward MBP (Fig. 2A). In contrast, after infection with L. donovani, PMA-stimulated MAP kinase activity was markedly reduced. The effect of L. donovani on LPS-induced MAP kinase activity was also assessed. Activation of MAP kinase in response to LPS was also attenuated in infected cells (Fig. 2B). These effects were independent of any change in the abundance of MAP kinase proteins, since stripping and reprobing the membrane with anti-MAP kinase antibodies showed that equal amounts of enzyme had been immunoprecipitated from the various treatment groups (Fig. 2).
FIG. 2.
L. donovani infection attenuates phosphotransferase activity of MAP kinase. Cells were infected with L. donovani at an approximate parasite-to-cell ratio of 15:1. After overnight incubation (17 h), control and infected cells were stimulated with 100 nM PMA (A) or LPS (1 μg/ml) (B) for 15 min. The cells were lysed and immunoprecipitated with anti-MAP kinase (anti MAPK 1) antibody. MBP phosphorylating activity was measured in an immune complex kinase assay as described in Materials and Methods. Phosphorylated MBP was electrophoresed by SDS-polyacrylamide gel electrophoresis (15% polyacrylamide) and transferred to a nitrocellulose membrane. After being stained with amido black, the blot was dried and exposed to X-ray film. After autoradiography, the blot was blocked and probed with anti-MAP kinase antibody to assess the input of precipitated MAP kinase. After immunoblotting, the bands in the region of MBP were excised and counted by liquid scintillation counting. (A) The data shown are from one of three independent experiments that yielded similar results. Values in the histogram represent mean and standard deviation. (B) Values in the histogram represent the average of two determinations from independent experiments.
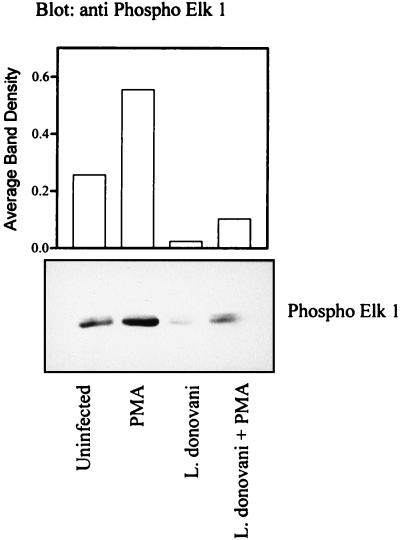
L. donovani infection inhibits the phosphorylation of Elk-1.
MAP kinases regulate gene expression in part through the phosphorylation and activation of transcription factors of the Ets family, including Elk-1 (10). To examine further the integrity of MAP kinase signaling in vivo, the phosphorylation status of Elk-1 was examined in intact cells. Phospho-Elk-1 was detected by immunoblotting with phospho-Ser383-specific Elk-1 antibody (New England BioLabs, Mississauga, Ontario, Canada). After overnight infection, the cells were stimulated with PMA and lysed for immunodetection of phosphorylated Elk-1. Treatment with PMA induced significantly enhanced phosphorylation of Elk-1 in control cells (Fig. 3). In contrast, PMA-induced phosphorylation of Elk-1 was markedly reduced in infected cells. Of note, phosphorylation of Elk-1 was also reduced in infected cells in the basal state. These findings suggest that leishmania infection may also modulate elements that regulate Elk-1 phosphorylation in resting cells.
FIG. 3.
L. donovani attenuates serine phosphorylation of Elk-1. Cells were incubated with or without amastigotes of L. donovani for 17 h and then stimulated with 100 nM PMA for 15 min. Detergent lysates were prepared with modified RIPA buffer as described in Materials and Methods, and soluble proteins were separated by SDS-polyacrylamide gel electrophoresis (7.5% polyacrylamide) followed by transfer to nitrocellulose membranes. The membranes were probed with phospho-Ser383-specific Elk-1 antibody. The blots were developed by ECL, and an autoluminogram is shown. The data shown are representative of two independent experiments that gave similar results; values in the histogram represent the average.
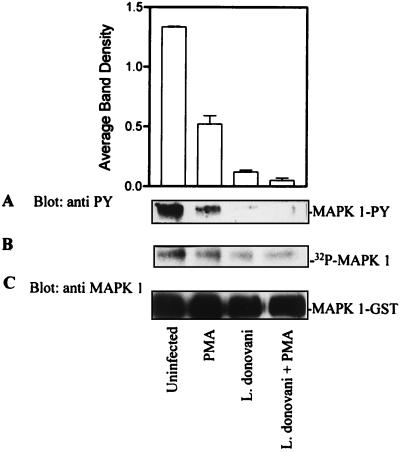
Leishmania infection stimulates an activity that dephosphorylates MEK-phosphorylated MAP kinase-1–GST.
Attenuation of MAP kinase activity in infected cells could have been due to direct inhibition of enzyme activity, activation of a phosphotyrosine phosphatase, or both. To investigate whether a phosphatase was involved, cells were infected with leishmania overnight and solubilized in lysis buffer without phosphatase inhibitors. Clarified total-cell lysates were incubated with MEK-phosphorylated MAP kinase-1–GST for 30 min at 30°C. MEK phosphorylation of MAP kinase-1–GST was performed in the presence of [γ-32P]ATP. Global dephosphorylation of MAP kinase-1–GST was examined by exposing the membrane to X-ray film (Fig. 4B). The extent of tyrosine dephosphorylation was also assessed by using phosphotyrosine antibodies in an immunoblot assay of the same membrane (Fig. 4A). As shown in Fig. 4A, infection with leishmania induced an activity that dephosphorylated MAP kinase-1–GST on tyrosine. Although lysates from infected cells also dephosphorylated 32P-MAP kinase-1–GST (Fig. 4B), this effect appeared to be less extensive than dephosphorylation of tyrosine residues per se (Fig. 4A). Stripping the membrane and reprobing with anti-MAP kinase antibodies showed comparable levels of input MAP kinase-1–GST (Fig. 4C). These results suggest that leishmania infection brings about an increase in phosphatase activity toward MAP kinase with a preference for dephosphorylating phosphotyrosine residues.
FIG. 4.
L. donovani infection stimulates an activity that dephosphorylates MEK-phosphorylated MAP kinase-1–GST. MAP kinase-1–GST (MAPK 1-GST) coupled to glutathione-agarose was phosphorylated with MEK and [γ-32P]ATP in phosphorylation buffer as described in Materials and Methods. Phosphorylated MAP kinase-1–GST agarose beads were incubated with whole-cell lysates prepared from control and leishmania-infected (17 h) cells treated with or without 100 nM PMA. The phosphatase assay was stopped by the addition of SDS sample buffer. (A) The phosphotyrosine content in MAP kinase-1 in PMA-treated control and infected cells was analyzed by immunoblotting with anti-phosphotyrosine (anti PY) antibodies. The autoluminogram shown was analyzed by densitometry. (B) The same membrane was exposed to X-ray film to determine the 32P content. (C) The membrane was stripped and probed with anti-MAP kinase antibody to assess the level of MAP kinase-1–GST input protein. The data shown are representative of three independent experiments that gave similar results; values in the histogram represent mean and standard deviation.
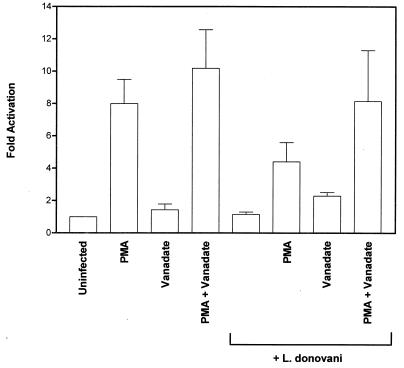
Attenuation of MAP kinase activity in leishmania-infected cells is abrogated by sodium orthovanadate.
The results presented above suggested that leishmania infection induced a protein tyrosine phosphatase (PTP) activity toward MAP kinase. To examine this further, cells were treated with the PTP inhibitor (6, 7) sodium orthovanadate for 3 h prior to infection. MAP kinase activity was then measured in an immune complex kinase assay with MBP as the substrate. As observed previously, PMA induced a significant increase in phosphotransferase activity toward MBP and treatment of cells with orthovanadate potentiated this response (Fig. 5). As expected, PMA-stimulated MAP kinase activity was significantly reduced in leishmania-infected cells. However, treatment of cells with sodium orthovanadate prior to incubation with leishmania prevented the infection-induced reduction in MAP kinase activity. These findings provide evidence to suggest that induction of PTP activity by leishmania leads to attenuation of MAP kinase activity in vivo.
FIG. 5.
Attenuation of MAP kinase activity in leishmania-infected cells is abrogated by sodium orthovanadate. Cells were preincubated with sodium orthovanadate (20 μM) for 3 h and then infected (17 h) with leishmania amastigotes at an approximate parasite-to-cell ratio of 15:1. Control and infected cells were then stimulated with PMA (100 nM) for 15 min. Cell lysates were immunoprecipitated with anti-MAP kinase antibody. MBP phosphorylation was assessed in an immune complex assay as described in Materials and Methods. Phosphorylated MBP was electrophoresed on SDS–15% polyacrylamide gels and electroblotted to nitrocellulose membranes. After being stained with amido black, the blots were dried and exposed to X-ray film. The bands in the region of MBP were excised and counted by liquid scintillation counting. The data shown are the mean and standard deviation of values obtained in three independent experiments.
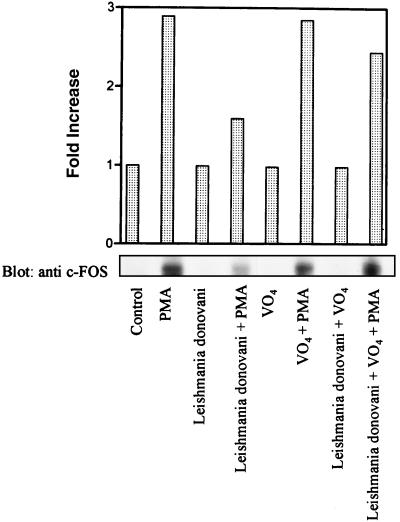
Leishmania infection inhibits the expression of c-FOS and iNOS: reversal by orthovanadate.
Phosphorylation of Elk-1 by MAP kinase is important for transcriptional activation of c-fos (5, 10). To assess if decreased phosphorylation of Elk-1 in leishmania-infected cells led to altered expression of c-fos, levels of c-FOS protein in control and infected cells were measured by immunoblotting. As expected, the level of c-FOS was significantly induced in PMA-treated cells compared to control cells (Fig. 6). In contrast, induction of c-FOS was markedly impaired in leishmania-infected cells. However, when cells were pretreated with sodium orthovanadate for 3 h prior to infection, the inducibility of c-FOS was restored (Fig. 6).
FIG. 6.
Pretreatment of cells with sodium orthovanadate reverses the effect of leishmania infection on c-FOS protein expression. Cells were either untreated or treated with orthovanadate (20 μM) for 3 h and then incubated with leishmania amastigotes at an approximate parasite-to-cell ratio of 15:1. After overnight incubation, control and infected cells were stimulated with PMA (100 nM) for 4 h. Cells were lysed in modified RIPA buffer as described in Materials and Methods. Whole-cell lysates were separated on SDS–7.5% polyacrylamide gels, transferred to nitrocellulose membranes, and probed with c-FOS antibodies. The blots were developed by ECL, and an autoluminogram of a blot is shown. The blot shown is from one of two independent experiments that yielded similar results, and the histogram shows the average values of the two experiments.
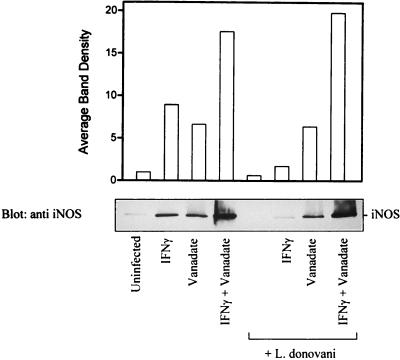
Additional experiments were done to investigate further the effects of infection and sodium orthovanadate on leishmania infection-related genes. Mononuclear phagocytes play a key role in controlling leishmania infection, and this is believed to be mediated in part by NO (29). In addition, IFN-γ increases the production of NO by upregulating the abundance of iNOS (16). As expected, the level of iNOS was significantly increased in IFN-γ treated cells compared to the basal level in control cells (Fig. 7). In contrast (Fig. 7), iNOS expression in IFN-γ-treated cells was markedly attenuated by leishmania infection. Notably, pretreatment of cells with sodium orthovanadate prior to infection reversed the inhibition of iNOS expression. These findings suggested that activation of a PTP activity by leishmania contributes to phagocyte deactivation.
FIG. 7.
Pretreatment of cells with sodium orthovanadate abrogates the effect of leishmania infection on iNOS protein expression. Cells were either untreated or treated with orthovanadate (20 μM) for 3 h and then incubated with leishmania amastigotes at an approximate parasite-to-cell ratio of 15:1. After overnight incubation (17 h), control and infected cells were stimulated with IFN-γ for 6 h. Cells were lysed in modified RIPA buffer as described in Materials and Methods. Whole-cell lysates were separated on SDS–7.5% polyacrylamide gels, transferred to nitrocellulose membranes, and probed with anti-iNOS antibodies. The blots were developed by ECL, and an autoluminogram of a blot is shown. The data shown are from two independent experiments that yielded similar results, and values in the histogram represent the average.
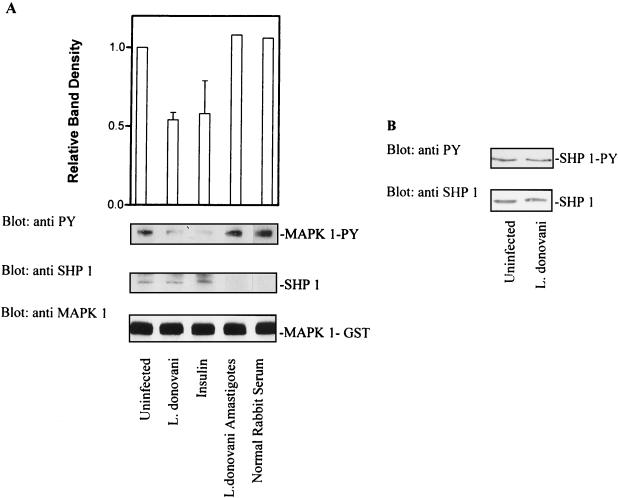
Leishmania infection activates SHP-1.
The results presented above indicated that infection with leishmania induced a phosphotyrosine phosphatase activity toward MAP kinase. SHP-1 is expressed primarily in hematopoietic cells and is known to be activated by phospholipids and mycobacterial lipoarabinomannan (8, 30). To examine whether leishmania infection induced enhanced expression of SHP-1, total cellular levels of SHP-1 were measured by immunoblotting. As shown in Fig. 8B, the levels of SHP-1 protein in control and infected cells were comparable. The activity of SHP-1 in cells infected overnight with leishmania was also examined. Lysates were prepared from control and infected cells, and SHP-1 was immunoprecipitated. PTP activity was measured by using autophosphorylated MAP kinase-1–GST as the substrate. As shown in Fig. 8A, incubation of phosphorylated MAP kinase-1–GST with SHP-1 immunoprecipitated from infected cells led to a significant decrease in signal intensity for tyrosine-phosphorylated MAP kinase. The magnitude of this effect was similar to that observed with insulin treatment alone, which is known to enhance the activity of SHP-1 in intact cells (28). Stripping of the membrane followed by immunoblotting for MAP kinase-1–GST (Fig. 8A) showed that the decrease in signal intensity for tyrosine-phosphorylated MAP kinase was not due to a decrease in the amount of enzyme protein. In addition, stripping and reprobing of the membrane for SHP-1 showed that equivalent amounts of the phosphatase had been immunoprecipitated from each treatment group (Fig. 8A). Of note, total-cell lysates prepared from freshly isolated leishmania amastigotes showed no dephosphorylating activity toward phosphorylated MAP kinase-1–GST. This result indicated that the presence of a leishmania-derived phosphatase with activity toward phosphorylated MAP kinase-1 was unlikely. Taken together, these results suggested that leishmania infection brought about increased specific activity of SHP-1 in infected cells. In some systems, SHP-1 activity is modulated by its state of tyrosine phosphorylation (2, 28). The tyrosine phosphorylation state of SHP-1 in leishmania-infected cells was also investigated. Lysates were prepared from control and infected cells, and SHP-1 was immunoprecipitated and then immunoblotted with antiphosphotyrosine antibodies. As shown in Fig. 8B, leishmania infection did not induce a change in the phosphotyrosine content of SHP-1. Thus, these results show that leishmania infection promotes increased specific activity of SHP-1 without altering either enzyme abundance or the tyrosine phosphorylation state of SHP-1.
FIG. 8.
L. donovani infection increases the specific activity of SHP-1 without affecting either tyrosine phosphorylation status or cellular levels of enzyme. (A) Cells were infected with leishmania for 17 h. Control and infected cells were lysed in buffer as described in Materials and Methods. Detergent-solubilized proteins were immunoprecipitated with antibody to SHP-1. Immunoprecipitated SHP-1 was incubated with autophosphorylated MAP kinase-1–GST (as described in Materials and Methods) for 1 h at 30°C. Reactions were stopped by adding SDS sample buffer. Phosphatase activity was assessed by immunoblotting with antiphosphotyrosine (anti PY) antibodies. The autoluminogram shown was analyzed by densitometry. The blots were then stripped and reprobed with anti-SHP-1 to determine the level of immunoprecipitated SHP-1. The same membranes were again stripped and reprobed with anti-MAP kinase (anti MAPK 1) to assess the input of MAP kinase-1–GST. (B) (Top) Cells infected with leishmania (17 h) were lysed in buffer, immunoprecipitated with anti-SHP-1, and immunoblotted with antiphosphotyrosine antibodies to assess the tyrosine phosphorylation status of SHP-1. (Bottom) Cells infected with leishmania (17 h) were lysed in modified RIPA buffer, and solubilized proteins were separated by SDS-polyacrylamide gel electrophoresis (10% polyacrylamide) followed by immunoblotting. Total cellular levels of SHP-1 were measured by immunoblotting with anti-SHP-1 antibody. The data shown in lanes with error bars are the mean and standard deviation of results obtained in three independent experiments. Other results are control results from one experiment.
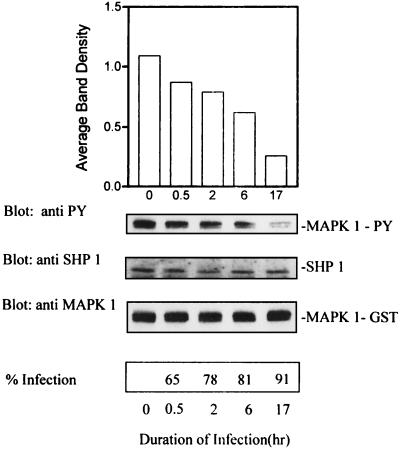
Time course of activation of SHP-1 by L. donovani.
To examine the kinetics of activation of SHP-1 by leishmania infection, cells were infected with leishmania for different times. The cells were then lysed in buffer without phosphatase inhibitors and processed for immunoprecipitation of SHP-1. Immunoprecipitated phosphatase activity was assayed against autophosphorylated MAP kinase-1–GST followed by immunoblot analysis with antiphosphotyrosine antibodies. As shown in Fig. 8, increased activity of SHP-1 was detectable as early as 30 min and this progressed significantly over a period of 17 h. The same membrane was stripped and reprobed to assess the relative inputs of immunoprecipitated SHP-1 and MAP kinase-1–GST for the various treatment groups. As shown in Fig. 9, essentially equivalent amounts of SHP-1 and MAP kinase-1–GST were present in each lane.
FIG. 9.
Time course of activation of SHP-1 by L. donovani. Cells were infected with leishmania for different time intervals as indicated, lysed in buffer without phosphatase inhibitors, and processed for immunoprecipitation of SHP-1. Immunoprecipitated SHP-1 was incubated with autophosphorylated MAP kinase-1–GST (MAPK 1-GST) for 1 h at 30°C as described in the legend to Fig. 5. Phosphatase activity was assessed by immunoblotting with antiphosphotyrosine (anti PY) antibody. The autoluminogram shown was analyzed by densitometry. The blot was then stripped and reprobed with anti-SHP-1 to assess the level of immunoprecipitated SHP-1. The same membrane was again stripped and probed with anti-MAP kinase to assess the input of MAP kinase-1–GST. The data shown are from one of two experiments that yielded similar results, and the values in the histogram represent the average.
DISCUSSION
L. donovani, like a number of other intracellular pathogens, promotes mononuclear phagocyte deactivation by impairing important functions of its target cells (11). Results previously reported have shown that L. donovani modulates several Mφ signaling pathways. For example, PKC-dependent stimulus-response coupling is defective in Mφs infected with amastigotes of L. donovani (19). Both the oxidative burst and protein phosphorylation in response to PMA are markedly reduced in leishmania-infected cells (19). The leishmania lipophosphoglycan (LPG), a major promastigote surface molecule, attenuates Mφ functions and modulates cell signaling through effects on PKC (27). It has also been reported that leishmania attenuates IFN-γ-induced activation of the Jak-Stat1 pathway in human monocytes (15). IFN-γ is one of the most important cytokines known to activate Mφ for enhanced microbicidal activity and for the expression of major histocompatibility complex class II genes (3, 4, 26), and these functions are impaired in leishmania-infected Mφs (9, 24).
In the present study, the impact of L. donovani on Mφ MAP kinase signaling and c-FOS and iNOS expression was investigated. MAP kinases are known to be involved in the regulation of a wide variety of fundamental cellular processes (1). Consistent with these important roles, MAP kinases are activated by a broad range of extracellular stimuli, including growth factors that bind to receptor tyrosine kinases, cytokines, whose receptors are coupled to cytoplasmic tyrosine kinases, and activators of PKC (21). MAP kinases become activated by rapid tyrosine and threonine phosphorylation brought about by MEK (25). PMA is a potent activator of many cell types and acts primarily via activation of multiple isoforms of PKC (17). PMA also activates MAP kinases as one of the principal events downstream of PKC, and this also involves phosphorylation of critical tyrosine and threonine residues in MAP kinases (25).
Given the evidence that leishmania leads to impaired signaling including effects on PKC, one of the objectives of the present study was to examine MAP kinase signaling in infected cells. Infection with L. donovani markedly reduced PMA-induced tyrosine phosphorylation of both p44 and p42 MAP kinases (Fig. 1A), and this was associated with reduced MAP kinase enzyme activity (Fig. 2). Similar effects of leishmania were observed when LPS was used as an agonist to activate MAP kinase. These findings were consistent with the requirement for tyrosine phosphorylation of MAP kinases for their activation (20). Notwithstanding this effect of diminished phosphotyrosine labeling, PMA-induced band shifting of MAP kinases in response to PMA was unaffected by leishmania infection (Fig. 1B). These results suggests that infection with L. donovani selectively affects tyrosine phosphorylation of MAP kinases and that band shifting is principally influenced by threonine phosphorylation, as has been observed in other systems (8). Of note, tyrosine phosphorylation of multiple other proteins in response to PMA was not diminished in leishmania-infected cells, suggesting that the effects of leishmania on tyrosine phosphorylation of MAP kinases were selective.
Elk-1, a member of the Ets family of transcription factors, is an important physiological substrate of MAP kinases in vivo and appears to be a direct target of activated MAP kinases (10). Elk-1 phosphorylation, therefore, is a useful measure of MAP kinase activity in vivo. Incubation of control cells with PMA induced increased phosphorylation of Elk-1 as determined by immunoblotting with an antibody specific for phospho-Elk-1 (Fig. 3). In contrast, induction of Elk1 phosphorylation in infected cells was significantly attenuated. Since phosphorylation of Elk-1, particularly at serine 383, is critical for Elk-1 to function as a transcriptional activator (5, 10), the finding of reduced phosphorylation of this regulatory protein in leishmania-infected cells provides direct evidence of functional impairment resulting from deactivation of MAP kinase. The findings that the expression of c-FOS in response to PMA and the expression of iNOS in response to IFN-γ were also markedly reduced by leishmania infection (Fig. 6 and 7) provided further evidence for the functional impairment of these cells.
Impaired tyrosine phosphorylation and activation of MAP kinase could potentially have been explained by the action of a tyrosine phosphatase. Indeed, incubation of cells with sodium orthovanadate prior to infection restored MAP kinase activation (Fig. 5) as well as the expression of both c-FOS and iNOS (Fig. 6 and 7). These findings are consistent with a model in which infection induces the activation of cellular phosphotyrosine phosphatases, leading to cell deactivation.
One potential candidate PTP for activation by leishmania is the hematopoietic cell phosphatase SHP-1, which is known to be involved in terminating activation signals (30). Moreover, it has been reported that MAP kinase is an in vitro substrate for SHP-1 (8). Indeed, the results of the present study indicate that leishmania increases SHP-1 activity toward MAP kinase. This conclusion is based upon two findings. First, in comparison to control cells, whole-cell lysates from leishmania-infected cells contained an enhanced activity that dephosphorylated MAP kinase on tyrosine in vitro (Fig. 4). Second, immunoprecipitates of SHP-1 from these samples showed that the specific activity of SHP-1 toward MAP kinase was increased as a result of infection (Fig. 8). The results of experiments to examine the kinetics of activation of SHP-1 showed that modulation of SHP-1 activity by leishmania infection was time dependent (Fig. 8). This progressive effect of leishmania on activation of SHP-1 and attenuation of MAP kinase may depend on the level of infection, a requirement for the synthesis of an activator, or both. To determine whether leishmania infection induced a phosphatase activity toward MAP kinase, other than SHP-1, efforts were made to immunodeplete SHP-1 from cell lysates. Attempts to quantitatively remove SHP-1 were not successful, however, and thus it was not possible to exclude the possibility of the presence of other phosphatases capable of dephosphorylating MAP kinase in these cells.
The exact mechanism of activation of SHP-1 and perhaps other PTP by leishmania remains to be identified. One potential mechanism to consider is that leishmania-derived molecules may either directly or indirectly modulate SHP-1 activity. SHP-1 is known to be directly activated by phospholipids (30) and mycobacterial lipoarabinomannan (LAM) (8). LAM purified from Mycobacterium tuberculosis inhibits MAP kinase signaling in vitro and in vivo, and this is associated with increased activity of SHP-1 (8). Distinct glycoinositol phospholipids (GIPLS) have been detected in leishmania amastigotes (13) and implicated as virulence factors (12). It has been proposed that GIPLS may function to promote immune system evasion during leishmania infection (22). Given that other lipid-like molecules activate SHP-1, GIPLS are attractive candidates for such a function during leishmania infection. The leishmania LPG inhibits PKC activity in Mφ (27) and could also be potentially involved in activation of SHP-1 or other cellular phosphotyrosine phosphatases. This seems somewhat unlikely, however, since significant amounts of promastigote-like LPG have not been detected in amastigotes of leishmania.
Although the results of this study provide strong evidence for deactivation of MAP kinase by a cellular phosphotyrosine phosphatase(s) in leishmania-infected cells, it was also possible that a leishmania-derived phosphatase with activity towards MAP kinase was responsible for some of the effects observed. This possibility seems highly unlikely, however, since total-cell lysates prepared from freshly isolated amastigotes showed no dephosphorylating activity toward phosphorylated MAP kinase-1–GST (Fig. 8A).
In summary, the results of this study show that L. donovani attenuates MAP kinase signaling and c-FOS and iNOS expression in macrophages. Attenuation of MAP kinase activity and inhibition of the expression of both iNOS and c-FOS appear to be accounted for by an increase in cellular PTP activity, since these effects are largely abrogated by orthovanadate. One candidate phosphatase is SHP-1. These findings suggest a possible mechanism for macrophage deactivation used by leishmania and possibly by other intracellular pathogens.
ACKNOWLEDGMENT
This work was supported by Medical Research Council of Canada grant MT-8633.
REFERENCES
- 1.Blenis J. Signal transduction via the MAP kinases: proceed at your own RSK. Proc Natl Acad Sci USA. 1993;90:5889–5892. doi: 10.1073/pnas.90.13.5889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bouchard P, Zhao Z, Banville D, Fischer E H, Shen S-H. Phosphorylation and identification of a major tyrosine phosphorylation site in protein tyrosine phosphatase 1C. J Biol Chem. 1994;269:19585–19589. [PubMed] [Google Scholar]
- 3.Flynn J L, Chan J, Triebold K J, Dalton D K, Stewart T A, Bloom B R. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J Exp Med. 1993;178:2249–2254. doi: 10.1084/jem.178.6.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gallin J I, Farber J M, Holland S M, Nutman T B. Interferon-gamma in the management of infectious diseases. Ann Intern Med. 1995;123:216–224. doi: 10.7326/0003-4819-123-3-199508010-00009. [DOI] [PubMed] [Google Scholar]
- 5.Gille H, Sharrocks A D, Shaw P E. Phosphorylation of transcription factor p62TCF by MAP kinase stimulates ternary complex formation at c-fos promoter. Nature. 1992;358:414–417. doi: 10.1038/358414a0. [DOI] [PubMed] [Google Scholar]
- 6.Gordon J A. Use of vanadate as protein-phosphotyrosine phosphatase inhibitor. Methods Enzymol. 1991;201:477–482. doi: 10.1016/0076-6879(91)01043-2. [DOI] [PubMed] [Google Scholar]
- 7.Hunter T. Protein kinases and phosphatases: the yin and yang of protein phosphorylation and signaling. Cell. 1995;80:225–236. doi: 10.1016/0092-8674(95)90405-0. [DOI] [PubMed] [Google Scholar]
- 8.Knutson K L, Hmama Z, Herrera-Velit P, Rochford R, Reiner N E. Lipoarabinomannan of Mycobacterium tuberculosis promotes protein tyrosine dephosphorylation and inhibition of mitogen-activated protein kinase in human mononuclear phagocytes. J Biol Chem. 1998;273:645–652. doi: 10.1074/jbc.273.1.645. [DOI] [PubMed] [Google Scholar]
- 9.Kwan W C, McMaster W R, Wong N, Reiner N E. Inhibition of expression of major histocompatibility complex class II molecules in macrophages infected with Leishmania donovani occurs at the level of gene transcription via a cyclic AMP-independent mechanism. Infect Immun. 1992;60:2115–2120. doi: 10.1128/iai.60.5.2115-2120.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marais R, Wynne J, Treisman R. The SRF accessory protein Elk-1 contains a growth factor-regulated transcriptional activation domain. Cell. 1993;73:381–393. doi: 10.1016/0092-8674(93)90237-k. [DOI] [PubMed] [Google Scholar]
- 11.Mauël J. Intracellular survival of protozoan parasites with special reference to Leishmania spp, Toxoplasma gondii and Trypanosoma cruzi. Adv Parasitol. 1996;38:1–51. doi: 10.1016/s0065-308x(08)60032-9. [DOI] [PubMed] [Google Scholar]
- 12.McConville M J. Glycosylated-phosphatidylinositols as virulence factors in Leishmania. Cell Biol Int Rep. 1991;15:779–798. doi: 10.1016/0309-1651(91)90033-f. [DOI] [PubMed] [Google Scholar]
- 13.McConville M J, Homans S W, Thomas-Oates J E, Dell A, Bacic A. Structures of the glycoinositolphospholipids from Leishmania major. A family of novel galactofuranose-containing glycolipids. J Biol Chem. 1990;265:7385–7394. [PubMed] [Google Scholar]
- 14.Moore K J, Labrecque S, Matlashewski G. Alteration of Leishmania donovani infection levels by selective impairment of macrophage signal transduction. J Immunol. 1993;150:4457–4465. [PubMed] [Google Scholar]
- 15.Nandan D, Reiner N E. Attenuation of gamma interferon-induced tyrosine phosphorylation in mononuclear phagocytes infected with Leishmania donovani: selective inhibition of signaling through Janus kinases and Stat1. Infect Immun. 1995;63:4495–4500. doi: 10.1128/iai.63.11.4495-4500.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nathan C F, Hibbs J B. Role of nitric oxide synthesis in macrophage antimicrobial activity. Curr Opin Immunol. 1991;3:65–70. doi: 10.1016/0952-7915(91)90079-g. [DOI] [PubMed] [Google Scholar]
- 17.Nishizuka Y. Studies and perspectives of protein kinase C. Science. 1989;233:305–312. doi: 10.1126/science.3014651. [DOI] [PubMed] [Google Scholar]
- 18.Olivier M, Baimbridge K G, Reiner N E. Stimulus-response coupling in monocytes infected with leishmania: attenuation of calcium transients is related to defective agonist-induced accumulation of inositol phosphates. J Immunol. 1992;148:1188–1196. [PubMed] [Google Scholar]
- 19.Olivier M, Brownsey R W, Reiner N E. Defective stimulus-response coupling in human monocytes infected with Leishmania donovani is associated with altered activation and translocation of protein kinase C. Proc Natl Acad Sci USA. 1992;89:7481–7485. doi: 10.1073/pnas.89.16.7481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Payne D M, Rossomando A J, Martino P, Erickson A K, Her J-H, Shabanowitz J, Hunt D F, Weber M J, Sturgill T W. Identification of the regulatory phosphorylation sites in pp42/mitogen-activated protein kinase (MAP kinase) EMBO J. 1991;10:885–892. doi: 10.1002/j.1460-2075.1991.tb08021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pelech S L, Sanghera J S. Mitogen-activated protein kinases: versatile transducers for cell signaling. Trends Biochem Sci. 1992;17:233–238. doi: 10.1016/s0968-0004(00)80005-5. [DOI] [PubMed] [Google Scholar]
- 22.Proudfoot L, O’Donnell C A, Liew F Y. Glycoinositolphospholipids of Leishmania major inhibit nitric oxide synthesis and reduce leishmanicidal activity in murine macrophages. Eur J Immunol. 1995;25:745–750. doi: 10.1002/eji.1830250318. [DOI] [PubMed] [Google Scholar]
- 23.Reiner N E. Altered cell signaling and mononuclear phagocyte deactivation during intracellular infection. Immunol Today. 1994;15:374–381. doi: 10.1016/0167-5699(94)90176-7. [DOI] [PubMed] [Google Scholar]
- 24.Reiner N E, Ng W, Ma T, McMaster W R. Kinetics of interferon-τ binding and MHC class II mRNA levels in leishmania-infected macrophages. Proc Natl Acad Sci USA. 1988;85:4330–4334. doi: 10.1073/pnas.85.12.4330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seger R, Ahn N G, Posada J, Munar E S, Jensen A M, Cooper J A, Cobb M H, Krebs E G. Purification and characterization of mitogen-activated protein kinase activator(s) from epidermal growth factor-stimulated A431 cells. J Biol Chem. 1992;267:14373–14381. [PubMed] [Google Scholar]
- 26.Ting J P Y, Baldwin A S. Regulation of MHC gene expression. Curr Opin Immunol. 1993;5:8–16. doi: 10.1016/0952-7915(93)90074-3. [DOI] [PubMed] [Google Scholar]
- 27.Turco S J, Descoteaux A. The lipophosphoglycan of Leishmania parasites. Annu Rev Microbiol. 1992;46:65–94. doi: 10.1146/annurev.mi.46.100192.000433. [DOI] [PubMed] [Google Scholar]
- 28.Uchida T, Matozaki T, Noguchi T, Yamao T, Horita K, Suzuki T, Fujioka Y, Sakamoto C, Kasuga M. Insulin stimulates the phosphorylation of Tyr538 and the catalytic activity of PTP1C, a protein tyrosine phosphatase with Src homology-2 domains. J Biol Chem. 1994;269:12220–12228. [PubMed] [Google Scholar]
- 29.Wei X Q, Charles I G, Smith A, Ure J, Feng G J, Huang F P, Xu D, Muller W, Moncada S, Liew F Y. Altered immune responses in mice lacking inducible nitric oxide synthase. Nature. 1995;375:408–411. doi: 10.1038/375408a0. [DOI] [PubMed] [Google Scholar]
- 30.Zhao Z, Shen S-H, Fischer E H. Stimulation by phospholipids of a protein-tyrosine-phosphatase containing two src homology domains. Proc Natl Acad Sci USA. 1993;90:4251–4255. doi: 10.1073/pnas.90.9.4251. [DOI] [PMC free article] [PubMed] [Google Scholar]