Abstract
Vaccination remains key to reducing the risk of COVID-19–related severe illness and death. Because of historic medical exclusion and barriers to access, Black communities have had lower rates of COVID-19 vaccination than White communities. We describe the efforts of an academic medical institution to implement community-based COVID-19 vaccine clinics in medically underserved neighborhoods in Philadelphia, Pennsylvania. Over a 13-month period (April 2021–April 2022), the initiative delivered 9038 vaccine doses to community members, a majority of whom (57%) identified as Black. (Am J Public Health. 2022;112(12):1721–1725. https://doi.org/10.2105/AJPH.2022.307030)
To improve COVID-19 vaccine access among medically underserved and vulnerable populations in Philadelphia, Pennsylvania, we implemented low-barrier vaccine clinics throughout Philadelphia, in collaboration with the Philadelphia Department of Public Health, the School District of Philadelphia, Philadelphia Parks and Recreation, faith-based institutions, community organizations, and professional sports organizations.
INTERVENTION AND IMPLEMENTATION
The University of Pennsylvania and the University of Pennsylvania Health Systems hosted large-scale COVID-19 vaccination clinics for Philadelphia residents in February 2021.1 In April 2021, when vaccine eligibility was expanded to include anyone aged 16 years or older, the Department of Family Medicine and Community Health began implementing community-based pop-up clinics in West and Southwest Philadelphia.
PLACE, TIME, AND PERSONS
The clinics targeted communities of color that faced financial and geographic barriers to vaccine access through health care centers and retail pharmacies and were primarily located in neighborhoods with low COVID-19 vaccination rates. From April 2021 to April 2022, we hosted 68 clinics in trusted neighborhood venues at the request of organizations with deep community ties. We typically aimed to host two clinics at three-week intervals to provide first and second doses. Our community-based hospital also maintained walk-in vaccine access five days a week.
PURPOSE
More than one third (33.7%) of the population of West Philadelphia is living in poverty, compared with 10.5% nationally.2 For many reasons (e.g., historic exclusion as a result of systemic racism, geographic barriers to access), Black adults and children have had lower COVID-19 vaccination rates than have those in White communities.3–5 The goal of this program was to implement frequent, low-barrier COVID-19 vaccine clinics in West and Southwest Philadelphia. We also aimed to promote patient choice by offering all available COVID-19 vaccinations (vs earlier mass vaccination efforts that typically offered single manufacturer vaccines).
Planning and Registration
We identified clinic locations through community partner requests and included K–12 schools, recreation centers, restaurants, religious institutions, and youth and athletic organizations. Requests exceeded our capacity to host clinics, so we prioritized locations that were accessible by public transit, were in low-vaccination neighborhoods, and had large indoor spaces to facilitate physical distancing. People could preregister via a text message–based system or walk in without appointments.1
Recruitment strategies included School District of Philadelphia–initiated robocalls and digital communications; SMS (short message service) campaigns in which all individuals who had previously registered for a vaccine clinic received a message in advance of our next clinic encouraging them to refer individuals for vaccination; physical and digital flyers shared with community partners; and virtual town halls with clinicians to answer questions.
Staffing
Clinics were staffed by volunteers who were recruited through listservs and personal outreach; volunteers signed up using a Web-based platform. Nonclinical volunteer roles included two operations leaders and five to 10 members of support staff (e.g., clinic navigation and registration). Clinical volunteers were Pennsylvania-licensed physicians, nurses, advanced practice providers, or pharmacists filling the following roles: one medical director, three to five vaccine preparation specialists, two to four postvaccine monitors, and five to 15 vaccinators. All vaccinators were required to complete a 10-minute Web-based training session before their first clinic. With clinical supervision, medical, dental, and pharmacy students also served as vaccinators. More than 500 staff members volunteered at 68 clinics, approximately 60% of whom were nonclinical and 40% clinicians.
Logistics
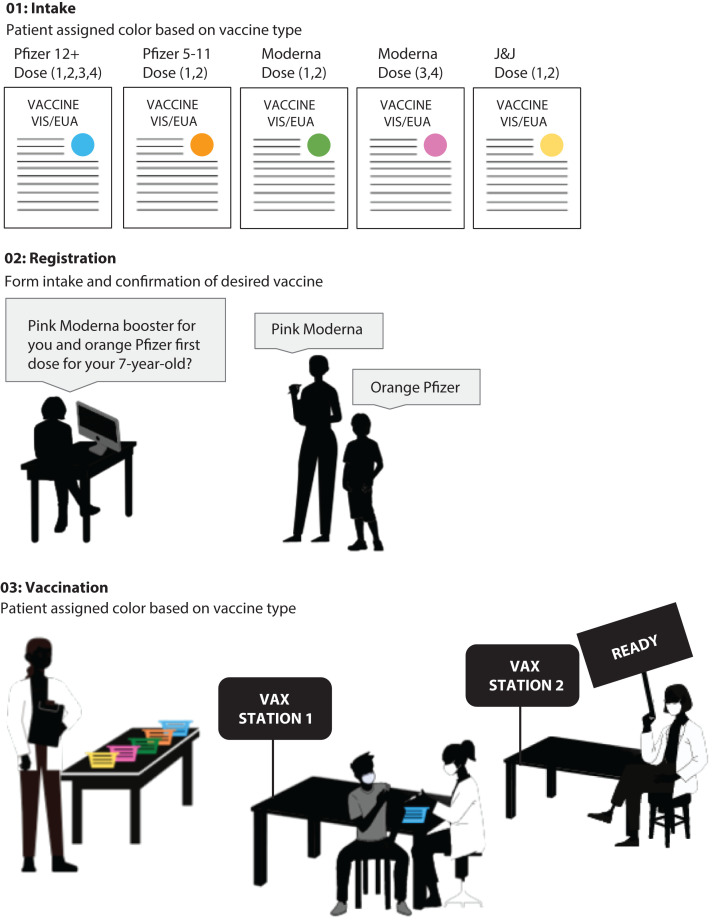
Clinics offered all COVID-19 vaccines approved formally or under US Food and Drug Administration emergency use authorization. Given the multiple manufacturers and doses available, we designed a color-coded system with safety protocols that included just-in-time training and multiple built-in color-coded checkpoints to ensure that patients received the correct vaccine (Figure 1). Upon entry to the vaccination clinic, patients were assigned color-coded paperwork indicating their designated vaccine:
FIGURE 1—
Clinic Color-Coded Safety System for Penn Medicine’s Pop-up COVID-19 Vaccine Clinics: Philadelphia, PA, April 2021–April 2022
Note. EUA = emergency use authorization; J&J = Johnson & Johnson; VAX = vaccination; VIS = vaccine information statement.
aColored stickers were placed on a clinic form for collecting basic demographic and health information (e.g., known allergies).
-
1.
Pfizer Blue—Pfizer-BioNTech 0.3-milliliter (mL) dose for those aged 12 years and older (primary series and booster doses);
-
2.
Pfizer Orange—Pfizer-BioNTech 0.2-mL dose for those aged five to 11 years;
-
3.
Moderna Green—Moderna 0.5-mL dose for primary series;
-
4.
Moderna Pink—Moderna 0.25-mL dose for booster (as of October 2021 approval); or
-
5.
JNJ Yellow—Johnson & Johnson/Janssen (primary series and booster doses).
Visual, written, and verbal communication all used the color-coded names. Vaccine storage, syringes, and labels were all similarly coded.
At check-in, patients received intake documents, including an emergency use authorization information packet, paper registration, and consent documents for patients younger than 18 years. Parents or guardians were required to accompany children aged five to 14 years receiving primary series and children aged 12 to 17 years receiving a booster. Parents or guardians of children aged 15 to 17 years receiving primary series were able to provide consent via telephone with an on-site physician.
Although the Philadelphia Department of Public Health allows minors aged 12 years and older to consent to their own COVID-19 immunization without the consent of a parent or guardian under an emergency use authorization, we included parent or guardian consent in our processes to prioritize community trust.6 After check-in, staff completed patient registration using a Web-based system, also serving as a second safety checkpoint to ensure that patients were assigned the correct vaccine. Because of the collection of protected health information, all registration and data input was completed on a secure, portable network connecting to a remote server.
Once check-in and registration were complete, patients were directed to a vaccine station. Clinicians displayed a “READY” sign to let volunteers know they were available to vaccinate the next patient. Vaccine vials were held in baskets of the assigned corresponding color. Before vaccination, clinicians completed a final confirmation of vaccine type with color-coded syringes. After vaccine administration, patients were directed to complete 15 to 30 minutes of clinical observation. Patients were not permitted to leave the clinic until the observer collected their registration paper and documented the time of observation completion.
EVALUATION AND ADVERSE EFFECTS
From April 2021 to April 2022, our team vaccinated 9038 patients across 68 clinics (Table 1). Most patients were Black/African American (57%), followed by White (23%), and Asian (7%); 59% of patients were aged 19 to 64 years, and nearly one quarter (24%) were aged five to 18 years. In the same period, the proportion of fully vaccinated residents in our eight target zip codes increased from 16% (50 627) to 62% (196 343) of the population—a 288% increase.7 We cannot attribute this total gain to our clinics because there were other vaccine providers in the area (e.g., select retail pharmacies). Nonetheless, the 9038 doses delivered—and unenumerated vaccine counseling—provided at our clinics for underserved populations contributed to the overall increase.
TABLE 1—
Self-Reported Characteristics of Penn Medicine Community COVID-19 Vaccine Clinic Participants: Philadelphia, PA, April 2021– April 2022
| Characteristic | No. (%) |
| Age, y | |
| 5–11 | 1329 (15) |
| 12–18 | 811 (9) |
| 19–64 | 5375 (59) |
| ≥ 65 | 1403 (16) |
| Not reported | 120 (1) |
| Race | |
| Black | 5124 (57) |
| White | 2054 (23) |
| Asian | 652 (7) |
| Other | 692 (8) |
| Not reported | 516 (6) |
| Gender | |
| Female | 4644 (51) |
| Male | 4243 (47) |
| Other | 63 (1) |
| Not reported | 88 (1) |
Note. The overall sample (n = 9038) includes data from the 68 community-based vaccine clinics (n = 6343) as well as walk-in clinics hosted at our community hospital (n = 2695).
A central challenge was ensuring that patients received the correct vaccine, which we addressed using a systems design model to develop the color-coded checkpoint system described previously. Logistical challenges included securing safe facility spaces that could accessibly accommodate high participant volumes with physical distancing. Other challenges included the physical setup and breakdown of a mobile clinic model that could scale to accommodate up to 500 vaccinations. Finally, the unknown sustainability of and ultimately end of funding from the federal government in March 2022 limited our reach.
SUSTAINABILITY
Our experience facilitating mobile, pop-up, community-based clinics could be adapted for other types of public health interventions, such as flu vaccination and school attendance–mandated immunizations. Color coding from registration limited administration errors and facilitated flow. These efforts, however, are only sustainable with appropriate funding, trustworthy community engagement, and institutional support.
PUBLIC HEALTH SIGNIFICANCE
Vaccination remains a key strategy to stem the tide of the COVID-19 pandemic. More than one year after emergency use authorization approval for vaccination among those aged 12 to 15 years and more than six months after emergency use authorization approval for those aged five to 11 years, vaccine uptake among children remains low. The implementation of centrally located community clinics at trusted venues such as public schools and recreation centers may reduce barriers to COVID-19 vaccination among medically underserved populations as well as children.
ACKNOWLEDGMENTS
This work was supported by the Philadelphia Department of Public Health and the Health Resources and Services Administration (HRSA) of the US Department of Health and Human Services (HHS; grant 1G32HS42684‐01‐00) as part of an award totaling $995 000.
The authors are grateful to all our partner organizations who helped facilitate vaccine clinics and who spent countless hours promoting the events in their networks. We would like to thank the Philadelphia Department of Public Health and the School District of Philadelphia for their tireless work to offer low-barrier vaccine clinics in Philadelphia’s underserved neighborhoods.
Note. The contents of this article are those of the authors and do not necessarily represent the official views of, nor an endorsement by, the HRSA, the HHS, or the US government.
CONFLICTS OF INTEREST
The authors have no potential or actual conflicts of interest to disclose.
HUMAN PARTICIPANT PROTECTION
This work was approved as quality improvement by the University of Pennsylvania’s institutional review board.
REFERENCES
- 1.Lee KC, Al-Ramahi N, Hahn L, et al. Operationalizing equity: a rapid-cycle innovation approach to COVID-19 vaccination in Black neighborhoods. NEJM Catalyst. 2021 https://catalyst.nejm.org/doi/pdf/10.1056/CAT.21.0094 [Google Scholar]
- 2.Semega J, Kollar M, Shrider EA, Creamer JF.2021. https://www.census.gov/content/dam/Census/library/publications/2020/demo/p60-270.pdf
- 3.Ndugga N, Hill L, Artiga S, Haldar S.2022. https://www.kff.org/coronavirus-covid-19/issue-brief/latest-data-on-covid-19-vaccinations-by-race-ethnicity
- 4.Johnson MS. Systemic racism is a cause of health disparities. J Fam Pract. 2021;70(4):162–164. doi: 10.12788/jfp.0189. [DOI] [PubMed] [Google Scholar]
- 5.City of Philadelphia. 2022. https://www.phila.gov/programs/coronavirus-disease-2019-covid-19/vaccines/vaccine-data
- 6.Lin A.2021. https://vax.phila.gov/index.php/notices/adolescents-12-may-consent-to-receive-pfizer-vaccine
- 7.Open Data Philly. 2022. https://www.opendataphilly.org/dataset/covid-vaccinations