Abstract
Objective
To assess changes in the prevalence of multidisciplinary cancer consultations (MDCc) over the last decade and examine patient, surgeon, hospital, and neighborhood factors associated with receipt of MDCc among individuals diagnosed with cancer.
Data Source
Surveillance, Epidemiology and End Results (SEER)–Medicare data from 2006 to 2016.
Study Design
We used time‐series analysis to assess change in MDCc prevalence from 2007 to 2015. We also conducted multilevel logistic regression with random surgeon‐ and hospital‐level effects to assess associations between patient, surgeon, neighborhood, and health care organization‐level factors and receipt of MDCc during the cancer treatment planning phase, defined as the 2 months following cancer diagnosis.
Data Collection/Extraction Methods
We identified Medicare beneficiaries >65 years of age with surgically resected breast, colorectal (CRC), or non‐small cell lung cancer (NSCLC) stages I–III (n = 103,250).
Principal Findings
From 2007 to 2015, the prevalence of MDCc increased from 35.0% to 61.2%. Overall, MDCc was most common among patients with breast cancer compared to CRC and NSCLC. Cancer patients who were Black, had comorbidities, had dual Medicare‐Medicaid coverage, were residing in rural areas or in areas with higher Black and Hispanic neighborhood composition were significantly less likely to have received MDCc. Patients receiving surgery at disproportionate payment‐sharing or rural‐designated hospitals had 2% (95% CI: −3.55, 0.58) and 17.6% (95% CI: −21.45, 13.70), respectively, less probability of receiving MDCc. Surgeon‐ and hospital‐level effects accounted for 15% of the variance in receipt of MDCc.
Conclusions
The practice of MDCc has increased over the last decade, but significant geographical and health care organizational barriers continue to impede equitable access to and delivery of quality care across cancer patient populations. Multilevel and multicomponent interventions that target care coordination, health system, and policy changes may enhance equitable access to and receipt of MDCc.
Keywords: health care delivery, inequities, multidisciplinary cancer care, multilevel
What is known on this topic
Multidisciplinary cancer consultations (MDCc) are associated with delivery of timely, guideline‐concordant cancer care.
There are persistent disparities in access to cancer care among minority and medically underserved populations.
Multilevel contextual measures of equitable access to MDCc have not been previously assessed.
What this study adds
The prevalence of MDCc increased from 35.0% to 61.2% from 2007 to 2015 across breast, colorectal, and non‐small‐cell lung cancer patients, albeit unequally for all.
Cancer patients who are Black, dual Medicare–Medicaid covered, residing in neighborhoods with high minority composition, and receiving surgery in rural‐designated and disproportionate sharing hospitals are significantly less likely to receive MDCc.
Using multilevel analyses, we found that 15% of the variation in receipt of MDCc was attributed to surgeons and hospitals.
1. INTRODUCTION
In 2021, many of the 1.8 million individuals diagnosed with cancer would have received multimodal treatments from various oncology specialists. Multidisciplinary care consultations (MDCc) refer to an array of care delivery approaches in which patients see or have their case evaluated by multiple health professionals, including medical oncologists, surgeons, and/or radiation oncologists, to identify treatment options, formulate optimized treatment plans, and initiate treatment. 1 Evidence from systematic reviews conducted over the last decade indicate that MDCc is associated with improvements in receipt of guideline‐concordant treatment, diagnostic accuracy, staging completeness, surgical techniques, and timeliness of care, with most studies focusing on lung/thoracic, head/neck, or gastrointestinal cancers. 2 , 3 , 4 , 5 , 6 , 7 , 8 MDCc has also been studied in the context of breast and prostate cancers given evolutions in multimodal management of these cancers. 8 , 9 Despite this research, the field lacks population‐based studies examining MDCc prevalence among some of the most commonly diagnosed cancers with the highest mortality rates such as breast, colorectal, and lung cancers.
Although clinical practice norms suggest MDCc is most common for patients with advanced cancers or complex cases, the use of models for delivering MDCc (e.g., multidisciplinary clinics) has grown across clinically diverse cancer populations as treatment options expand and patients seek consultations with multiple specialists to develop treatment plans. Even for patients with early stage cancers who may be recommended to receive surgery alone, engaging with a medical oncologist or radiation oncologist early in treatment planning may improve shared clinical decision making, care coordination, and receipt of timely, guideline‐concordant care. 3 , 9 , 10 , 11 , 12 , 13 , 14 , 15 However, although MDCc has been encouraged for nearly two decades, 16 it remains unclear whether MDCc is equitably delivered and/or received across diverse cancer patient populations.
Disparities in cancer outcomes experienced by racial and ethnic minorities and medically underserved populations (e.g., socioeconomically disadvantaged and rural populations) may arise from multilevel domains of influence including structural discrimination and system‐level barriers, such as limited availability of quality cancer care. 17 , 18 Limited prior research examining variation in receipt of MDCc suggests that patients who identify as Black, older, unmarried, and with comorbidities are less likely to receive MDCc. 9 , 10 , 13 , 14 Geographically, cancer patients residing in communities with large racial and ethnically minoritized populations, that are rural, and with higher poverty rates are more likely to experience limited access to cancer specialists and worse cancer outcomes. 13 , 19 Differences in the delivery of cancer care by physician specialty and health care settings has also been identified. Among Surveillance Epidemiology and Ends Results (SEER)‐Medicare beneficiaries with breast or colorectal cancer, 20% of cancer treatment variations were explained by factors beyond the patient level (i.e., between‐physician differences). 20 Additionally, among cancer patients receiving surgery, there is substantial between‐hospital variation in mortality and readmission rates with safety‐net hospitals, which often serve minority and medically underserved populations, having higher 90‐day readmission rates. 21 However, to our knowledge, no studies have concurrently examined patient, physician, hospital, and neighborhood factors associated with MDCc in a population‐based sample of patients with common high‐mortality cancers. 22 , 23 , 24 , 25
To address this gap, we used the SEER‐Medicare data to assess the prevalence of MDCc from 2007 to 2015, using a multilevel analysis to examine patient‐, neighborhood‐, surgeon‐, and hospital‐level factors associated with receipt of MDCc among Medicare beneficiaries with surgically resected (female) breast cancer, colorectal cancer (CRC), or non‐small‐cell lung cancer (NSCLC).
2. DATA AND METHODS
2.1. Data sources and study population
This retrospective population‐based cohort study used the SEER–Medicare linked data (2006–2016) to identify Medicare beneficiaries over 65 years of age with a pathologically staged (I, II, and III) single (female) breast cancer, CRC, or NSCLC as their first and only cancer. We identified patients who received surgical resection within 12 months after date of diagnosis, as confirmed by the first surgical claim for primary cancer (See Appendix S1). We excluded patients enrolled in a health maintenance organization or not enrolled in Medicare Parts A and B for at least 12 months before their cancer diagnosis and through study period, diagnosed at time of autopsy or death certificate, or died within 12 months from date of diagnosis, as we would not be able to fully capture MDCc claims. We also excluded patients missing month of diagnosis.
2.2. Measures
2.2.1. Outcome: Receipt of multidisciplinary cancer consultations (MDCc)
MDCc is operationalized as having encounters with two or more of the following oncology specialties within 2 months from diagnosis: surgery, medical oncology, and/or radiation oncology. Our approach aligns with Medicare claims guidelines and billing practices for physicians during the period of study. In the absence of specific guidelines for MDCc, the 2‐month time frame was chosen as it has been used as a quality indicator in other studies. 9 , 13 We also conducted sensitivity analyses using a 4‐month time frame. We secondarily assessed whether MDCc occurred on the same day.
We identified consultations with all oncology provider specialties using Carrier Claims with listed Current Procedural Terminology codes for patient encounters (See Appendix S1). We used National Provider Identifier information from these claims merged with American Medical Association (AMA) data to determine provider specialty. Provider specialty was ascertained using a hierarchical scheme using both primary and secondary specialty codes (See Appendix S1). 26 , 27
2.2.2. Multilevel predictors
Patient characteristics
Patient sociodemographic characteristics included age at diagnosis, race and ethnicity (non‐Hispanic [NH]‐Black, NH‐White, Hispanic, or Other), sex, marital status, and Medicare–Medicaid dual coverage status defined as being covered through a state buy‐in program at any point during study period (yes/no). Patient clinical characteristics included cancer type, staging, and number of comorbidities using the National Cancer Institute's (NCI's) comorbidity index. 28 Patient sociodemographic characteristics, including race and ethnicity, in the SEER‐Medicare database were obtained from the SEER cancer registries that collect data from various sources (e.g., administrative databases, patient intake forms, provider records, and imputation algorithms) and the Medicare database. 29 Per the SEER‐Medicare data use agreement, American Indian/Alaska Native, Asian, Pacific Islander, and unknown race or ethnicity were combined into a single “Other” category to protect confidentiality.
Neighborhood characteristics
Neighborhood characteristics included census‐tract‐level indices of racial and ethnic composition (e.g., percentage Black individuals, percentage Hispanic individuals), area deprivation, SEER registry geographic location, and rurality. Percent Black and Hispanic composition was categorized into quartiles based on the distribution of the study population within a census tract. Patient's zip code was linked to the Neighborhood Atlas's Area Deprivation Index (ADI) data to capture neighborhood socioeconomic disadvantage based on state‐level rankings. 30 SEER registry geographic location was categorized as Northeast, Midwest, Southwest, South, and West. Patient‐level zip codes were linked to the 2013 Rural–Urban Continuum Codes (RUCC) to obtain scores (1–3 classified as urban and 4–9 classified as rural). 31
Surgeon characteristics
For each surgeon, we identified surgical specialty (general, oncology, other), medical school graduation year (before 2000 or later), and sex (male, female). Individual provider information was obtained from the AMA data.
Hospital characteristics
Characteristics of the hospital where patients received their first surgery included NCI Cancer Center designation (yes/no), Commission on Cancer accreditation (yes/no), teaching status (yes/no), hospital type (non‐profit, private, government), rural hospital status (yes/no), and disproportionate share hospital (DSH) payment qualification (yes/no). Hospital characteristics were obtained from the NCI's SEER‐Medicare's data Hospital File. 32
2.2.3. Statistical analyses
To describe the study population, we reported frequencies and percentages of patients who received MDCc within 2 months from diagnosis over multiple days or on the same day of MDCc. Significant differences in the distribution of patients by MDCc (vs. no MDCc) and same‐day MDCc (vs. MDCc received over multiple days) were assessed using the Pearson Chi‐square test. Bivariate and multilevel mixed‐effects logistic regressions were performed to assess the associations between multilevel factors and MDCc with patients (Level 1) nested within surgeon (Level 2) and then clustered by the hospital where first surgery was performed (Level 3). Random intercepts were modeled for surgeons and hospitals to account for this clustering. We included the multilevel factors presented in Table 1 as fixed effects. These factors were identified a priori based on Andersen's Model of Healthcare Utilization 33 and prior research on MDCc. As measures of individual and neighborhood socioeconomic status, we assessed whether dual Medicare–Medicaid coverage and area deprivation, respectively, varied by race and ethnicity; these interaction terms were not significant on the basis of a Wald test and were excluded from the model. We conducted sensitivity analyses to assess the prevalence of MDCc by cancer type, stage, and receipt of MDCc within 4 months from diagnosis.
TABLE 1.
Characteristics of breast, colorectal, and non‐small‐cell lung cancer patients who received multidisciplinary cancer consultation (MDCc) and same‐day MDCc between 2007 and 2015
| Overall Cohort (N = 103,250) | MDCc (n = 58,017) | p‐Value (MDC vs. no MDC) | Same‐day MDCc (n = 6990) | p‐Value (same day vs. not same day) | ||||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |||
| Patient level | ||||||||
| Age (Mean ± SD) | 75.9 ± 4.0 | 74.8 ± 3.9 | <0.001 | 74.1 ± 3.9 | <0.001 | |||
| Race and ethnicity | <0.001 | 0.265 | ||||||
| Non‐Hispanic White | 86,394 | 83.7% | 48,830 | 56.5% | 5859 | 11.9% | ||
| Non‐Hispanic Black | 6619 | 6.4% | 3265 | 49.3% | 405 | 12.3% | ||
| Hispanic | 4832 | 4.7% | 2744 | 56.8% | 360 | 13.0% | ||
| Other | 5405 | 5.2% | 3036 | 56.2% | 379 | 12.4% | ||
| Sex | <0.001 | <0.001 | ||||||
| Male | 19,848 | 19.2% | 7593 | 38.3% | 757 | 9.5% | ||
| Female | 83,402 | 80.8% | 50,282 | 60.3% | 6246 | 12.4% | ||
| Marital status | <0.001 | 0.026 | ||||||
| Single | 8291 | 8.4% | 4310 | 52.0% | 511 | 11.8% | ||
| Married | 50,664 | 51.3% | 30,000 | 59.2% | 3735 | 12.3% | ||
| Other | 39,831 | 40.3% | 21,176 | 53.2% | 2453 | 11.6% | ||
| Medicaid coverage | <0.001 | 0.009 | ||||||
| No | 85,135 | 82.5% | 49,680 | 58.4% | 61,086 | 12.2% | ||
| Yes | 18,115 | 17.5% | 8195 | 45.2% | 917 | 11.2% | ||
| Cancer type | <0.001 | <0.001 | ||||||
| Breast | 58,184 | 56.4% | 41,643 | 71.6% | 5614 | 13.4% | ||
| Colorectal | 33,019 | 32.0% | 11,575 | 35.1% | 940 | 8.1% | ||
| Lung | 12,047 | 11.7% | 4657 | 38.7% | 449 | 9.6% | ||
| Stage | 0.292 | <0.001 | ||||||
| I | 52,281 | 50.6% | 29,407 | 56.3% | 3710 | 12.6% | ||
| II | 33,033 | 32.0% | 18,499 | 56.0% | 2187 | 11.7% | ||
| III | 17,936 | 17.4% | 9969 | 55.6% | 1106 | 11.0% | ||
| Comorbidities | <0.001 | <0.001 | ||||||
| 0 | 51,921 | 51.0% | 32,139 | 61.9% | 4.051 | 12.5% | ||
| 1 | 29,026 | 28.5% | 15,818 | 54.5% | 1855 | 11.7% | ||
| 2 | 7201 | 7.1% | 35,407 | 48.7% | 403 | 11.4% | ||
| 3+ | 13,650 | 13.4% | 5828 | 42.7% | 619 | 10.5% | ||
| Neighborhood level | ||||||||
| % Black composition | <0.001 | <0.001 | ||||||
| Lower quartile | 26,990 | 26.2% | 15,771 | 58.4% | 2061 | 13.0% | ||
| 2nd quartile | 26,444 | 25.6% | 15,133 | 57.2% | 1899 | 12.5% | ||
| 3rd quartile | 25,671 | 24.9% | 14,295 | 55.7% | 1704 | 11.9% | ||
| Highest quartile | 24,054 | 23.3% | 12,763 | 53.1% | 1332 | 10.4% | ||
| % Hispanic composition | <0.001 | 0.007 | ||||||
| Lower quartile | 25,159 | 24.4% | 14,039 | 55.8% | 1803 | 12.8% | ||
| Second quartile | 26,445 | 25.6% | 15,128 | 57.2% | 1783 | 11.7% | ||
| Third quartile | 26,340 | 25.5% | 14,942 | 56.7% | 1737 | 11.6% | ||
| Highest quartile | 25,212 | 24.4% | 13,709 | 54.4% | 1673 | 12.1% | ||
| Area deprivation index | <0.001 | <0.001 | ||||||
| Lowest quintile (lowest disadvantage) | 24,544 | 25.2% | 14,678 | 59.8% | 1945 | 13.2% | ||
| 2nd quintile | 21,353 | 21.9% | 12,264 | 57.4% | 1381 | 11.2% | ||
| 3rd quintile | 19,181 | 19.7% | 10,708 | 55.8% | 1226 | 11.4% | ||
| 4th quintile | 17,646 | 18.1% | 9575 | 54.3% | 1141 | 11.9% | ||
| Highest quintile (most disadvantage) | 14,747 | 15.1% | 7604 | 51.6% | 909 | 11.9% | ||
| Rurality (RUCC) | <0.001 | <0.001 | ||||||
| Urban | 86,377 | 83.7% | 48,628 | 56.3% | 5757 | 11.8% | ||
| Rural | 16,861 | 16.3% | 9239 | 54.8% | 1244 | 13.4% | ||
| SEER region | <0.001 | <0.001 | ||||||
| Northeast | 22,173 | 21.5% | 11,350 | 51.2% | 1043 | 9.1% | ||
| Midwest | 12,258 | 11.9% | 6928 | 56.5% | 1092 | 15.7% | ||
| Southwest | 4256 | 4.1% | 2656 | 62.4% | 404 | 15.1% | ||
| South | 26,178 | 25.4% | 14,585 | 55.7% | 1416 | 9.7% | ||
| West | 38,385 | 37.2% | 22,356 | 58.2% | 3048 | 13.5% | ||
| Surgeon level | ||||||||
| Surgeon specialty | <0.001 | <0.001 | ||||||
| General | 62,129 | 68.1% | 39,129 | 63.0% | 4649 | 11.8% | ||
| Oncology | 9877 | 10.8% | 6945 | 70.3% | 986 | 14.1% | ||
| Other surgeon specialty | 19,289 | 21.1% | 7943 | 41.2% | 699 | 8.8% | ||
| Surgeon graduation year | 0.028 | <0.001 | ||||||
| Before 2000 | 82,386 | 90.2% | 48,649 | 59.1% | 5549 | 11.3% | ||
| 2000 or later | 8909 | 9.8% | 5368 | 60.3% | 785 | 14.5% | ||
| Surgeon sex | <0.001 | <0.001 | ||||||
| Male | 68,174 | 74.7% | 37,398 | 54.9% | 3799 | 10.1% | ||
| Female | 23,121 | 25.3% | 16,619 | 71.9% | 2535 | 15.2% | ||
| Hospital level | ||||||||
| Hospital type | 0.019 | <0.001 | ||||||
| Non‐profit | 69,539 | 75.8% | 38,153 | 54.9% | 4676 | 12.2% | ||
| Private | 9532 | 10.4% | 5340 | 56.0% | 470 | 8.8% | ||
| Government | 12,669 | 13.8% | 6857 | 54.1% | 773 | 11.2% | ||
| NCI designation | 0.011 | <0.001 | ||||||
| None | 89,095 | 93.6% | 49,092 | 55.3% | 5580 | 11.3% | ||
| Yes (clinical and/or comprehensive) | 6047 | 6.4% | 3230 | 53.4% | 586 | 18.0% | ||
| CoC accreditation | <0.001 | 0.004 | ||||||
| No | 41,061 | 43.2% | 22,093 | 53.8% | 2.709 | 12.2% | ||
| Yes | 54,081 | 56.8% | 30,229 | 55.9% | 3457 | 11.4% | ||
| Teaching status | <0.001 | <0.001 | ||||||
| No | 42,810 | 46.7% | 23,870 | 55.8% | 2432 | 10.1% | ||
| Yes | 48,925 | 53.3% | 26,478 | 54.1% | 3487 | 13.1% | ||
| Disproportionate share payment qualification | 0.569 | <0.001 | ||||||
| No | 18,050 | 19.7% | 9876 | 54.7% | 1332 | 13.4% | ||
| Yes | 73,552 | 80.3% | 40,417 | 55.0% | 4583 | 11.3% | ||
| Rural primary status | <0.001 | <0.001 | ||||||
| No | 90,052 | 98.2% | 49,728 | 55.2% | 5883 | 11.8% | ||
| Yes | 1683 | 1.8% | 620 | 36.8% | 36 | 5.8% | ||
Abbreviations: CoC, Commission on Cancer; NCI, National Cancer Institute; RUCC, Rural–Urban Continuum Codes (2013); SD, standard deviation; SEER, Surveillance, Epidemiology, and End Results Program.
We calculated the variance inflation factor (mean VIF = 2.45) and could identify no significant evidence of multicollinearity between the multilevel factors in Table 1. Year of diagnosis was also included as a discrete covariate to account for the effect of calendar time on MDCc. We computed average marginal effects and report the (marginal) probability of receiving MDCc compared to the reference group, measured as the average changes in percentage (%) points, and 95% confidence intervals (CIs). To determine the variation in receipt of MDCc across surgeons and hospitals, we calculated the intraclass correlation (ICC) in a null (unadjusted) multilevel model without any patient, neighborhood, surgeon, and hospital factors and in the full model, which adjusted for all multilevel factors in Table 1. Statistical significance was assessed at the 0.05 level. All analyses were performed in STATA version 16.0 (STATA Corporation).
3. RESULTS
3.1. Patient and neighborhood characteristics
The study population included 103,250 patients with surgically resected breast, CRC, or NSCLC cancer from 2007 to 2015. Table 1 describes the characteristics of patients who received MDCc within 2 months from diagnosis. The mean patient age was 75 years. Significantly more Hispanic (56.8%), female (60.3%), and married (59.2%) patients, and patients without dual Medicare–Medicaid coverage (58.4%) received MDCc. Additionally, more patients without comorbidities (61.9%) received MDCc. Most patients residing in neighborhoods with a lower proportion of Black residents, lower area deprivation, urban areas, and in the Western part of the United States received MDCc. By cancer site, more breast cancer patients (71.6%) received MDCc than CRC (35.1%) or NSCLC patients (38.7%). Similar patient sociodemographic and clinical characteristics were observed for MDCc within 4 months from diagnosis and same‐day MDCc at both time periods.
3.2. Surgeon and hospital characteristics
There were 6456 unique surgeons and 1389 unique hospitals in this study. The number of patients per surgeon varied from 1 to 151 (median = 7.9), with 77.0% of the surgeons having at least two patients. The number of patients per hospital varied from 1 to 902 (median = 53.2), with only 13.7% of hospitals having only one patient. Most hospitals (78.8%) had at least two surgeons, and the number of surgeons per hospital varied from 1 to 73 (median = 7.2 surgeons per hospital). A higher proportion of patients who had their surgery performed by a surgeon specializing in oncology (70.3%), graduated from medical school after 2000 (60.3%), and by a female surgeon (71.9%) received MDCc. More patients who received surgery at a hospital that was private (56.0%), not NCI‐designated (55.3%), Commission on Cancer accredited (55.9%), and not designated as a rural hospital (55.2%) received MDCc.
3.3. Prevalence of MDCc
From 2007 to 2015, the prevalence of MDCc increased from 35.0% to 61.2%, while same‐day MDCc increased from 9.4% to 13.9% (p‐value <0.001) (Figure 1A). A total of 57,875 patients (56.1%) received MDCc, and of these, 12.0% received MDCc on the same day. Similar trends in prevalence of MDCc within 4 months from diagnosis were observed. Among patients who received MDCc, 91.8% had an encounter with a medical oncologist, 94.4% with a surgeon, and 49.1% with a radiation oncologist within 2 months of diagnosis. Consultation with both a medical oncologist and a surgeon was observed for 86.2% of patients with MDCc, while 35.7% of patients with MDCc had consultation with all three provider types. From 2007 to 2015, MDCc was highest among breast cancer patients overall and among stage II and III CRC or NSCLC patients compared to stage I (Figure 1B).
FIGURE 1.
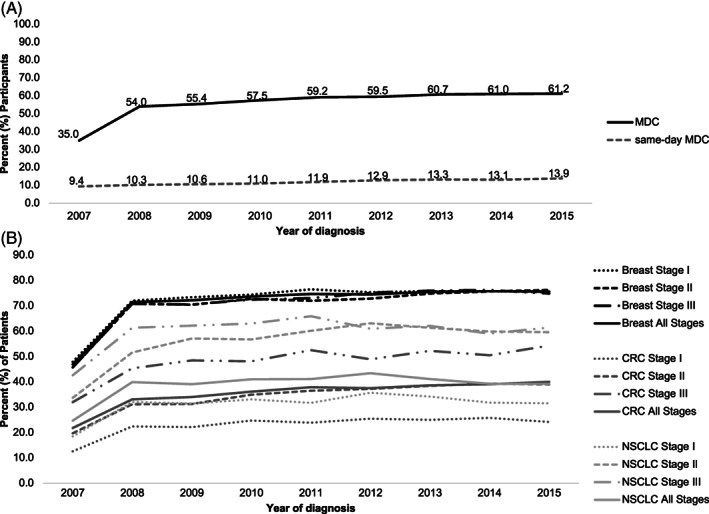
Prevalence of multidisciplinary cancer consultation (MDCc) from 2007 to 2015. (A) Prevalence of MDCc and same‐day MDCc by year of cancer diagnosis. (B) Prevalence of MDCc by year of cancer diagnosis stratified by cancer type and stage. CRC, colorectal cancer; MDCc, multidisciplinary cancer consultation; NSCLC, non‐small‐cell lung cancer.
3.4. Factors associated with receipt of MDCc
Results from the adjusted multilevel analysis indicated that patients who were older, single, diagnosed with CRC or NSCLC, and with at least one comorbidity had a lower probability of receiving MDCc (Table 2). Compared to NH‐White patients, NH‐Black patients were 3.3% less likely to have received MDCc (95% CI: −4.78, −1.83), while Hispanic patients were 1.8% more likely to have received MDCc (95% CI: 0.18, 3.40). Late‐stage cancer was also significantly associated with a higher likelihood of receiving MDCc compared to patients diagnosed with stage I (stage II: 6.6%, 95% CI: 5.85, 7.31; stage III: 16.3%, 95% CI: 15.47, 17.15). Patients with dual Medicare–Medicaid coverage were 7.2% less likely to have received MDCc compared to patients without dual coverage (95% CI: −8.09, −6.22).
TABLE 2.
Average marginal effects of receiving multidisciplinary cancer consultation (MDCc) among Medicare beneficiaries with surgically resected cancer from 2007 to 2015
| Unadjusted marginal effects | Adjusted marginal effects a | |||
|---|---|---|---|---|
| Percentage (%) points | (95% CI) | Percentage (%) points | (95% CI) | |
| Patient level | ||||
| Age | −1.24 | (−1.28, −1.20) | −0.96 | (−0.96, −0.86) |
| Race and ethnicity | ||||
| Non‐Hispanic White | Reference | Reference | ||
| Non‐Hispanic Black | −7.19 | (−8.44, −5.94) | −3.30 | (−4.78, −1.83) |
| Hispanics | 0.27 | (−1.17, 1.70) | 1.79 | (0.18, 3.40) |
| Other | −0.35 | (−1.71, 1.01) | 1.93 | (0.34, 3.52) |
| Sex | ||||
| Male | Reference | Reference | ||
| Female | 22.03 | (21.28, 22.79) | 0.13 | (−0.80, 1.07) |
| Marital status | ||||
| Single | Reference | Reference | ||
| Married | 7.23 | (6.07, 8.39) | 4.19 | (2.96, 5.42) |
| Other | 1.18 | (0.00, 2.36) | 1.13 | (−0.11, 2.37) |
| Medicaid coverage | ||||
| No | Reference | Reference | ||
| Yes | −13.12 | (−13.91, −12.32) | −7.16 | (−8.09, −6.22) |
| Cancer type | ||||
| Breast | Reference | Reference | ||
| Colorectal | −36.52 | (−37.15, −35.88) | −39.4 | (−40.37, −38.36) |
| Lung | −32.91 | (−33.86, −31.97) | −32.4 | (−34.25, −30.59) |
| Stage | ||||
| I | Reference | Reference | ||
| II | −0.25 | (−0.93, 0.44) | 6.58 | (5.85, 7.31) |
| III | −0.67 | (−1.51, 0.18) | 16.31 | (15.47, 17.15) |
| Comorbidities | ||||
| 0 | Reference | Reference | ||
| 1 | −7.40 | (−8.11, −6.69) | −2.05 | (−2.80, −1.31) |
| 2 | −1.32 | (−14.43, −11.97) | −4.11 | (−5.38, −2.83) |
| 3+ | −1.92 | (−20.13, −18.27) | −6.69 | (−7.71, −5.67) |
| Neighborhood level | ||||
| % Black composition | ||||
| Lower quartile | Reference | Reference | ||
| Second quartile | −1.20 | (−2.04, −0.36) | −0.19 | (−1.11, 0.73) |
| Third quartile | −2.77 | (−3.62, −1.93) | −0.19 | (−1.18, 0.81) |
| Highest quartile | −5.34 | (−6.20, −4.47) | −1.50 | (−2.73, −0.28) |
| % Hispanic composition | ||||
| Lower quartile | Reference | Reference | ||
| Second quartile | 1.40 | (0.55, 2.26) | 0.24 | (−0.72, 1.22) |
| Third quartile | 0.93 | (0.07, 1.78) | 0.35 | (−0.75, 1.44) |
| Highest quartile | −1.43 | (−2.29, −0.56) | −1.38 | (−2.71, −0.06) |
| Area deprivation index | ||||
| Lowest quintile (lowest disadvantage) | Reference | Reference | ||
| Second quintile | −2.37 | (−3.27, −1.46) | 0.65 | (−0.324, 1.62) |
| Third quintile | −3.98 | (−4.91, −3.04) | 0.41 | (−0.6, 1.46) |
| Fourth quintile | −5.54 | (−6.50, −4.58) | −0.05 | (−1.18, 1.08) |
| Highest quintile (most disadvantage) | −8.24 | (−9.25, −7.23) | −0.62 | (−1.86, 0.63) |
| Rurality (RUCC) | ||||
| Urban | Reference | Reference | ||
| Rural | −1.50 | (−2.32, −0.68) | −1.75 | (−3.00, −0.52) |
| SEER region | ||||
| Northeast | Reference | Reference | ||
| Midwest | 5.33 | (4.23, 6.43) | 8.13 | (5.41, 10.85) |
| Southwest | 11.22 | (9.62, 12.81) | 7.82 | (4.32, 11.32) |
| South | 4.53 | (3.63, 5.42) | 5.45 | (3.08, 7.83) |
| West | 7.05 | (6.23, 7.88) | 4.91 | (2.70, 7.12) |
| Surgeon level | ||||
| Surgeon specialty | ||||
| General | Reference | Reference | ||
| Oncology | 7.33 | (6.36, 8.31) | −0.55 | (−2.32, 1.23) |
| Other surgeon specialty | −21.80 | (−22.59, −21.01) | 0.71 | (−0.58, 1.99) |
| Surgeon graduation year | ||||
| Before 2000 | Reference | Reference | ||
| 2000 or later | 1.20 | (0.13, 2.27) | 0.79 | (−0.62, 2.21) |
| Surgeon sex | ||||
| Male | Reference | Reference | ||
| Female | 17.02 | (16.33, 17.71) | 0.49 | (−0.72, 1.70) |
| Hospital level | ||||
| Hospital type | ||||
| Non‐profit | Reference | Reference | ||
| Private | 1.16 | (0.09, 2.22) | −1.29 | (−3.17, 0.59) |
| Government | −0.74 | (−1.68, 0.20) | −1.66 | (−3.59, 0.26) |
| NCI designation | ||||
| No | Reference | Reference | ||
| Yes | −1.69 | (−2.98, −0.39) | −2.80 | (−6.20, 0.60) |
| CoC accreditation | ||||
| No | Reference | Reference | ||
| Yes | 2.09 | (1.45, 2.73) | 2.90 | (1.41, 4.40) |
| Teaching status | ||||
| No | Reference | Reference | ||
| Yes | −1.64 | (2.28, −0.99) | −0.44 | (−1.84, 0.95) |
| Disproportionate share payment qualification | ||||
| No | Reference | Reference | ||
| Yes | 0.24 | (−0.57, 1.05) | −2.06 | (−3.55, −0.58) |
| Rural primary status | ||||
| No | Reference | Reference | ||
| Yes | −18.38 | (−20.71, −16.06) | −17.57 | (−21.45, −13.70) |
Abbreviations: CI, Confidence Interval; CoC, Commission on Cancer; NCI, National Cancer Institute; RUCC, Rural–Urban Continuum Codes (2013); SEER, Surveillance, Epidemiology, and End Results Program.
Adjusted for age, race and ethnicity, sex, marital status, cancer type, cancer stage, comorbidities, percent Black and Hispanic neighborhood composition, area deprivation index, rurality, SEER region surgeon specialty, graduation year, sex, hospital type, NCI designation, Commission on Cancer accreditation, teaching status, disproportionate share payment qualification, rural primary status designation, year of diagnosis, and surgeon and hospital random effects.
Patients residing in neighborhoods with the highest Black (95% CI: −2.73, −0.28) or Hispanic (95% CI: −2.71, −0.06) composition had a 1.5% and 1.4% lower probability of receiving MDCc compared to patients residing in neighborhoods with lower (0%–25%) minority composition. Area deprivation was not significantly associated with receiving MDCc. Patients residing in rural areas had a 1.8% lower probability of receiving MDCc compared to patients residing in urban areas (95% CI: −3.00, −0.52).
Surgeon characteristics (i.e., specialty, sex, graduation year) were not significantly associated with MDC. However, patients receiving surgery at a Commission on Cancer accredited hospital had a significantly higher probability of receiving MDCc compared to patients receiving surgery at hospitals not accredited (2.9%, 95% CI: 1.41, 4.40). Receiving surgery at a hospital qualifying for DSH payment was associated with a 2.1% lower probability of receiving MDCc (95% CI: −3.55, −0.58), while patients receiving surgery at a rural primary status hospital had a 17.6% lower probability of receiving MDCc compared to patients receiving surgery at a non‐rural hospital (95% CI: −21.45, −13.70).
3.5. Variation in MDCc
In the null (unadjusted) multilevel model, the ICC for variation in receipt of MDCc attributed to the surgeon and hospital levels was 3.9% and 21.0%, respectively (Table 3). After accounting for patient, neighborhood, surgeon, and hospital characteristics, the variance in receipt of MDCc attributed to the hospital level reduced to 11.3% (95% CI: 10.3%, 12.4%).
TABLE 3.
Variance in receipt of multidisciplinary cancer consultations (MDCc) attributed to surgeon and hospital levels
| Null (unadjusted) model | Full (adjusted) model a | |||
|---|---|---|---|---|
| ICC | 95%CI | ICC | 95%CI | |
| Surgeon | 3.9% | (3.1%, 5.0%) | 4.1% | (3.3%, 5.1%) |
| Hospital | 21.0% | (19.9%, 22.2%) | 11.3% | (10.3%, 12.4%) |
Adjusted for age, race and ethnicity, sex, marital status, cancer type, cancer stage, comorbidities, percent Black and Hispanic neighborhood composition, area deprivation index, rurality, SEER region surgeon specialty, graduation year, sex, hospital type, NCI‐designation, Commission on Cancer accreditation, teaching status, disproportionate share payment qualification, rural primary status designation, year of diagnosis.
4. DISCUSSION
In this study, MDCc after diagnosis almost doubled from 2007 to 2015, representing an uptake in an important cancer care quality metric. During this period, most of the 57,875 patients with MDCc (86.2%) saw both a surgeon and medical oncologist, while 35.7% had encounters with surgical, medical oncology, and radiation oncology providers. As expected, MDCc varied by cancer type and stage, with MDCc being most prevalent among breast cancer patients and adjusted analyses finding higher probability of MDCc among patients diagnosed with late‐stage cancers.
Consistent with prior studies, we found that patients who are older, single, and Black were less likely to receive MDCc even after accounting for neighborhood‐, surgeon‐, hospital‐, and other patient‐level factors. 10 , 13 , 14 , 34 The disparity between Black patients and NH‐White patients may be partly explained by differences in system‐level factors such as limited access to quality cancer care, mistrust of providers and health care systems, and/or lack of patient–provider communication. 35 , 36 Conversely, and similar to Simpson et al., 13 Hispanic patients in our study had a 1.8% higher probability of receiving MDCc compared to NH‐White patients, which may be associated with stronger cultural‐related advantages of residing in Hispanic enclaves (e.g., access to social networks, resilience, and the presence of patient navigators or promatoras to enhance care delivery). 37 , 38 However, we found that residing in neighborhoods with the highest Black or Hispanic composition was associated with a 1.4% and 1.5%, respectively, lower probability of receiving MDCc. 39 As a measure of racial composition, the percentage of residents who identify as Black or Hispanic within a specific geographical area is a potential reflection of neighborhood racial context where neighborhoods with higher proportions of racial and ethnic minoritized groups may experience structural and systemic barriers such as inadequate transportation, limited availability of quality cancer care resources, and increased exposures to stress, bias, and trauma that may impede utilization of cancer‐related health services. 39 Neighborhood characteristics not assessed in this study, such as social cohesion, housing‐pattern‐based segregation, neighborhood mobility and isolation, and other social risks, are also likely to play a role in access to and receipt of MDCc and should be assessed in future research. 40 , 41 , 42 , 43
We also found that the probability of receiving MDCc was 7.2% lower among patients with dual Medicare–Medicaid coverage. Patients with Medicaid coverage may experience access barriers to specialty care due to inadequate provider reimbursement and high administrative burden (e.g., preauthorization, payment delays, claim rejection) that may lead some specialists to limit their Medicaid patient panels. 44 These findings suggest opportunities for clinic‐, health‐system‐, and policy‐level interventions that facilitate access to high‐quality cancer care for patients with Medicaid, particularly for patients from racially and ethnically minoritized and medically underserved groups who are more likely to experience greater financial burden after a cancer diagnosis. 45 Such approaches may include value‐based care delivery models and aligning reimbursement with equity‐driven quality measures.
Additionally, we found a lower probability of receiving MDCc with increasing comorbidity. Newly diagnosed older adult cancer patients are likely to have concurrent conditions, such as heart disease and diabetes, and compounded disease severity that may adversely affect cancer treatment options and contribute to poorer survival. 46 Populations that experience health disparities, including racial and ethnic minorities, are more likely to have comorbid medical and mental health conditions compared to NH‐Whites. 47 While there is an important, growing body of research examining integration of geriatric assessment, multimorbidity, and multidisciplinary approaches for comprehensive care for older adults with cancer, 48 , 49 relatively limited evidence to date examines MDCc approaches among populations facing cancer and other comorbid conditions. 50 More research is needed to better understand the impact of MDCc on care management, coordination, and outcomes for patients with concurrent and competing health care needs throughout their cancer journey. Future research should evaluate effects of MDCc on important outcomes such as treatment‐related toxicities, recurrence, and progression‐free survival in these populations.
Geographically, rural patients had 1.8% lower probability of receiving MDCc compared to urban patients. Less than 15% of oncologists practice in rural settings, and rural cancer patients experience limited access to local clinical settings that provide cancer treatment and longer travel distances to oncology care. 51 , 52 , 53 Although only 12% of patients with MDCc had same‐day MDCc, a post hoc analysis (data not shown) indicated that rural patients with MDCc had a higher probability of receiving MDCc on the same day compared to urban patients. Same‐day multidisciplinary clinics may enhance timely cancer care delivery and increase adherence of provider recommendations, especially for underserved populations. 54 , 55 Given the travel and financial barriers associated with residing in rural areas, 53 our findings underscore the need for provider‐ and system‐focused interventions that leverage telehealth resources and promote same‐day MDCc in populations or areas with limited cancer resources.
We found that—after adjusting for patient, neighborhood, surgeon, and hospital characteristics—approximately 15% of the variation in receipt of MDCc was attributable to surgeons and hospitals. This highlights the important roles providers and health care organizations play in care delivery, which may be modifiable points for interventions to improve equitable delivery of quality cancer care and outcomes. For example, interprofessional skills trainings and policies that promote teamwork competencies, collaboration, and building referral networks across care delivery organizations may be avenues to increase MDCc. 56 Accreditation by the Commission on Cancer may also play another important role at both the surgeon and hospital level as it was associated with receipt of MDCc. Commission on Cancer accredits hospitals based on the standards that patients will receive cancer care using a multidisciplinary team approach. 57 Understanding how best to implement MDCc across diverse cancer care delivery settings with limited availability of specialty providers, variable knowledge about cancer treatments, and experiencing financial pressures (e.g., rural or DSH‐designated hospitals) are important next steps. 58 For example, although many rural hospitals and oncologists may not be able to provide onsite multidisciplinary services, there may be opportunities to draw on telehealth, virtual consultation, and visiting specialist models to facilitate guideline‐recommended cancer care. 53
Several limitations must be considered when interpreting these findings. First, surgery is standard of care for all three cancer types, and our analyses were limited to patients with surgical resection. However, we recognize that surgery is only one treatment modality, and future analyses should examine other health care organizational factors associated with receipt of guideline‐recommended radiation and systemic therapies. Next, we used a claims‐based algorithm to capture MDCc but acknowledge that claims‐based encounters with multiple providers do not necessarily equate to optimal care coordination. Nevertheless, prior reviews demonstrate that MDCc are associated with receipt of guideline‐concordant treatment, diagnostic accuracy, staging completeness, and timeliness. 3 Additionally, our 2‐month post‐diagnosis timeframe for capturing MDCc may underestimate specialist encounters after diagnosis and before surgical resection, 9 but we found no significant differences in receipt of MDCc within 2 months compared to 4 months or in the factors associated with MDCc at both time periods. We also were unable to capture discussion of clinical cases at multidisciplinary tumor boards or similar non‐billable conferences, which have been considered standard clinical practice in certain cancer care delivery settings (e.g., NCI‐designated Cancer Centers). Presentation at tumor boards, however, often remains reserved for particularly complex, unusual, or novel cases because of time and resource constraints.
Our neighborhood race and ethnicity measures were also limited to compositional measures (e.g., percentage of Black individuals). Future research should examine effects of comprehensive measures of systemic and structural inequities on receipt of MDCc and related outcomes, such as segregation based on housing patterns. 59 We also did not conduct geospatial analyses of factors, such as distance to tertiary care or specialty availability in a given geographical area, which may partially mediate disparities in the receipt of MDCc and contribute to disparities in access, receipt, and outcomes of cancer care among rural populations. 60 , 61 , 62 Finally, other factors not assessed in this study may influence receipt of MDCc, including patient and provider preferences, hospital organizational structures and processes, financial incentives, and referral policies. Despite these limitations, our findings can inform future research and interventions aiming to improve access to and receipt of MDCc among populations that experience health disparities.
5. CONCLUSION
The prevalence of MDCc is growing, albeit unequally for all cancer patients, with persistent racial, ethnic, and geographical disparities. Given that the surgeon‐ and hospital‐level factors explained 15% of the variation in MDCc, our findings can be used to inform future strategies that aim to synergistically address barriers at the patient, provider, health system, and neighborhood level to enhance equitable access to and receipt of multidisciplinary cancer care.
AUTHOR CONTRIBUTIONS
All authors (Janeth I. Sanchez, Michelle Doose, Chris Zeruto, Veronica Chollette, Natalie Gasca, Dana Verhoeven, and Sallie J. Weaver) contributed to the acquisition, analysis, and interpretation of the data, contributed to the critical revision for important intellectual content, and provided final approval of the version to be published. Janeth I. Sanchez, Michelle Doose, Veronica Chollette, and Sallie J. Weaver. contributed to the study concept and design, drafting the article, and statistical analyses. Chris Zeruto and Natalie Gasca contributed to the statistical analyses.
FUNDING INFORMATION
This article was prepared as part of the authors' official duties as employees of the US Federal Government. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the National Cancer Institute, National Institute on Minority Health and Health Disparities, or other federal agencies.
Supporting information
Appendix S1. Supplemental table.
ACKNOWLEDGMENTS
The authors would like to thank Drs. Shakuntala Malik, Anand Singla, Edward Sauter, Lindsey Enewold, and Michael Halpern for providing clinical and research expertise to this study.
Sanchez JI, Doose M, Zeruto C, et al. Multilevel factors associated with inequities in multidisciplinary cancer consultation. Health Serv Res. 2022;57(Suppl. 2):222‐234. doi: 10.1111/1475-6773.13996
REFERENCES
- 1. American Society of Clinical Oncology, European Society for Medical Oncology . ASCO‐ESMO consensus statement on quality cancer care. Ann Oncol. 2006;17(7):1063‐1064. doi: 10.1093/annonc/mdl152 [DOI] [PubMed] [Google Scholar]
- 2. Pillay B, Wootten AC, Crowe H, et al. The impact of multidisciplinary team meetings on patient assessment, management and outcomes in oncology settings: a systematic review of the literature. Cancer Treat Rev. 2016;42:56‐72. doi: 10.1016/j.ctrv.2015.11.007 [DOI] [PubMed] [Google Scholar]
- 3. Prabhu Das I, Baker M, Altice C, Castro KM, Brandys B, Mitchell SA. Outcomes of multidisciplinary treatment planning in US cancer care settings. Cancer. 2018;124(18):3656‐3667. [DOI] [PubMed] [Google Scholar]
- 4. Kočo L, Weekenstroo HHA, Lambregts DMJ, et al. The effects of multidisciplinary team meetings on clinical practice for colorectal, lung, prostate and breast cancer: a systematic review. Cancers. 2021;13(16):4159. doi: 10.3390/cancers13164159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Basta YL, Bolle S, Fockens P, Tytgat K. The value of multidisciplinary team meetings for patients with gastrointestinal malignancies: a systematic review. Ann Surg Oncol. 2017;24(9):2669‐2678. doi: 10.1245/s10434-017-5833-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Taplin SH, Weaver S, Salas E, et al. Reviewing cancer care team effectiveness. J Oncol Pract. 2015;11(3):239‐246. doi: 10.1200/JOP.2014.003350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shao J, Rodrigues M, Corter AL, Baxter NN. Multidisciplinary care of breast cancer patients: a scoping review of multidisciplinary styles, processes, and outcomes. Curr Oncol. 2019;26(3):e385‐e397. doi: 10.3747/co.26.4713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Holmes A, Kelly BD, Perera M, Eapen RS, Bolton DM, Lawrentschuk N. A systematic scoping review of multidisciplinary cancer team and decision‐making in the management of men with advanced prostate cancer. World J Urol. 2021;39(2):297‐306. doi: 10.1007/s00345-020-03265-1 [DOI] [PubMed] [Google Scholar]
- 9. Churilla TM, Egleston BL, Murphy CT, et al. Patterns of multidisciplinary care in the management of non‐metastatic invasive breast cancer in the United States Medicare patient. Breast Cancer Res Treat. 2016;160(1):153‐162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Quyyumi FF, Wright JD, Accordino MK, et al. Factors associated with multidisciplinary consultations in patients with early stage breast cancer. Cancer Investig. 2019;37(6):233‐241. [DOI] [PubMed] [Google Scholar]
- 11. Brar SS, Hong NL, Wright FC. Multidisciplinary cancer care: does it improve outcomes? J Surg Oncol. 2014;110(5):494‐499. [DOI] [PubMed] [Google Scholar]
- 12. Epstein NE. Multidisciplinary in‐hospital teams improve patient outcomes: a review. Surg Neurol Int. 2014;5(Suppl 7):S295‐S303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Simpson DR, Martínez ME, Gupta S, et al. Racial disparity in consultation, treatment, and the impact on survival in metastatic colorectal cancer. J Natl Cancer Inst. 2013;105(23):1814‐1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Goulart BH, Reyes CM, Fedorenko CR, et al. Referral and treatment patterns among patients with stages III and IV non–small‐cell lung cancer. J Oncol Pract. 2013;9(1):42‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kesson EM, Allardice GM, George WD, Burns HJ, Morrison DS. Effects of multidisciplinary team working on breast cancer survival: retrospective, comparative, interventional cohort study of 13 722 women. BMJ. 2012;344:e2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Institute of Medicine (US) , National Research Council . Ensuring Quality Cancer Care. National Academies Press (US); 1999. [PubMed] [Google Scholar]
- 17. Alcaraz KI, Wiedt TL, Daniels EC, Yabroff KR, Guerra CE, Wender RC. Understanding and addressing social determinants to advance cancer health equity in the United States: a blueprint for practice, research, and policy. CA Cancer J Clin. 2020;70(1):31‐46. [DOI] [PubMed] [Google Scholar]
- 18. National Institute on Minority Health and Health Disparities . NIMHD Research Framework 2017.
- 19. Hung P, Deng S, Zahnd WE, et al. Geographic disparities in residential proximity to colorectal and cervical cancer care providers. Cancer. 2020;126(5):1068‐1076. doi: 10.1002/cncr.32594 [DOI] [PubMed] [Google Scholar]
- 20. Popescu I, Schrag D, Ang A, Wong M. Racial/ethnic and socioeconomic differences in colorectal and breast cancer treatment quality: the role of physician‐level variations in care. Med Care. 2016;54(8):780‐788. doi: 10.1097/MLR.0000000000000561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Haneuse S, Dominici F, Normand S‐L, Schrag D. Assessment of between‐hospital variation in readmission and mortality after cancer surgical procedures. JAMA Netw Open. 2018;1(6):e183038. doi: 10.1001/jamanetworkopen.2018.3038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Warnecke RB, Campbell RT, Vijayasiri G, Barrett RE, Rauscher GH. Multilevel examination of health disparity: the role of policy implementation in neighborhood context, in patient resources, and in healthcare facilities on later stage of breast cancer diagnosis. Cancer Epidemiol Biomark Prev. 2019;28(1):59‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zahnd WE, McLafferty SL. Contextual effects and cancer outcomes in the United States: a systematic review of characteristics in multilevel analyses. Ann Epidemiol. 2017;27(11):739‐748.e3. [DOI] [PubMed] [Google Scholar]
- 24. Gomez SL, Shariff‐Marco S, DeRouen M, et al. The impact of neighborhood social and built environment factors across the cancer continuum: current research, methodological considerations, and future directions. Cancer. 2015;121(14):2314‐2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Taplin SH, Anhang Price R, Edwards HM, et al. Introduction: understanding and influencing multilevel factors across the cancer care continuum. J Natl Cancer Inst Monogr. 2012;2012(44):2‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. White DP, Enewold L, Geiger AM, Banks R, Warren JL. Comparison of physician data in two data files available for cancer health services research. J Natl Cancer Inst Monogr. 2020;2020(55):66‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Warren JL, Barrett MJ, White DP, Banks R, Cafardi S, Enewold L. Sensitivity of Medicare data to identify oncologists. J Natl Cancer Inst Monogr. 2020;2020(55):60‐65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. National Cancer Institute . NCI comorbidity index overview. https://healthcaredelivery.cancer.gov/seermedicare/considerations/comorbidity.html
- 29. National Cancer Institute . SEER‐Medicare: Brief description of the SEER‐Medicare database. Updated May 16, 2022. https://healthcaredelivery.cancer.gov/seermedicare/overview/. Accessed March 21, 2022.
- 30. University of Wisconsin School of Medicine and Public Health . Area Deprivation Index v2.0. November 6, 2020. https://www.neighborhoodatlas.medicine.wisc.edu/
- 31. US Department of Agriculture Economic Research Service . Rural‐Urban Continuum Codes. https://www.ers.usda.gov/data-products/rural-urban-continuum-codes.aspx
- 32. National Cancer Institute . SEER‐Medicare: Provider files. https://healthcaredelivery.cancer.gov/seermedicare/aboutdata/provider.html. Accessed February 15, 2021.
- 33. Andersen R, Newman JF. Societal and individual determinants of medical care utilization in the United States. Milbank Q. 2005;83(4):95‐124. [PubMed] [Google Scholar]
- 34. Morris AM, Billingsley KG, Hayanga AJ, Matthews B, Baldwin L‐M, Birkmeyer JD. Residual treatment disparities after oncology referral for rectal cancer. J Natl Cancer Inst. 2008;100(10):738‐744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sanchez JI, Shankaran V, Unger JM, Madeleine MM, Selukar SR, Thompson B. Inequitable access to surveillance colonoscopy among Medicare beneficiaries with surgically resected colorectal cancer. Cancer. 2021;127(3):412‐421. [DOI] [PubMed] [Google Scholar]
- 36. Jolles MP, Richmond J, Thomas KC. Minority patient preferences, barriers, and facilitators for shared decision‐making with health care providers in the USA: a systematic review. Patient Educ Couns. 2019;102(7):1251‐1262. [DOI] [PubMed] [Google Scholar]
- 37. Aranda MP, Ray LA, Snih SA, Ottenbacher KJ, Markides KS. The protective effect of neighborhood composition on increasing frailty among older Mexican Americans: a barrio advantage? J Aging Health. 2011;23(7):1189‐1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. WestRasmus EK, Pineda‐Reyes F, Tamez M, Westfall JM. Promotores de salud and community health workers: an annotated bibliography. Fam Community Health. 2012;35(2):172‐182. [DOI] [PubMed] [Google Scholar]
- 39. White K, Borrell LN. Racial/ethnic residential segregation: framing the context of health risk and health disparities. Health Place. 2011;17(2):438‐448. doi: 10.1016/j.healthplace.2010.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Warner ET, Gomez SL. Impact of neighborhood racial composition and metropolitan residential segregation on disparities in breast cancer stage at diagnosis and survival between black and white women in California. J Community Health. 2010;35(4):398‐408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Peng TR, Navaie‐Waliser M, Feldman PH. Social support, home health service use, and outcomes among four racial–ethnic groups. Gerontologist. 2003;43(4):503‐513. [DOI] [PubMed] [Google Scholar]
- 42. Kolak M, Bhatt J, Park YH, Padrón NA, Molefe A. Quantification of neighborhood‐level social determinants of health in the continental United States. JAMA Netw Open. 2020;3(1):e1919928. doi: 10.1001/jamanetworkopen.2019.19928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Prestby T, App J, Kang Y, Gao S. Understanding neighborhood isolation through spatial interaction network analysis using location big data. Environ Plan A. 2020;52(6):1027‐1031. doi: 10.1177/0308518X19891911 [DOI] [Google Scholar]
- 44. Cunningham PJ, O'Malley AS. Do reimbursement delays discourage Medicaid participation by physicians? Simply raising fees might not be enough to entice physicians to take Medicaid patients, if they have to wait too long to receive payment for services rendered. Health Aff. 2008;27(Suppl1):w17‐w28. [DOI] [PubMed] [Google Scholar]
- 45. Shankaran V, Jolly S, Blough D, Ramsey SD. Risk factors for financial hardship in patients receiving adjuvant chemotherapy for colon cancer: a population‐based exploratory analysis. J Clin Oncol. 2012;30(14):1608‐1614. doi: 10.1200/jco.2011.37.9511 [DOI] [PubMed] [Google Scholar]
- 46. Stairmand J, Signal L, Sarfati D, et al. Consideration of comorbidity in treatment decision making in multidisciplinary cancer team meetings: a systematic review. Ann Oncol. 2015;26(7):1325‐1332. [DOI] [PubMed] [Google Scholar]
- 47. Ahmed N, Conway CA. Medical and mental health comorbidities among minority racial/ethnic groups in the United States. J Health Soc Behav. 2020;14(1):11. [Google Scholar]
- 48. Soo W‐K, King M, Pope A, Parente P, Darzins P, Davis ID. Integrated geriatric assessment and treatment (INTEGERATE) in older people with cancer planned for systemic anticancer therapy. J Clin Oncol. 2020;38(15_suppl):12011. doi: 10.1200/JCO.2020.38.15_suppl.12011 [DOI] [PubMed] [Google Scholar]
- 49. Presley CJ, Krok‐Schoen JL, Wall SA, et al. Implementing a multidisciplinary approach for older adults with cancer: geriatric oncology in practice. BMC Geriatr. 2020;20(1):231. doi: 10.1186/s12877-020-01625-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Weaver SJ, Jacobsen PB. Cancer care coordination: opportunities for healthcare delivery research. Transl Behav Med. 2018;8(3):503‐508. doi: 10.1093/tbm/ibx079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ballas LK, Elkin EB, Schrag D, Minsky BD, Bach PB. Radiation therapy facilities in the United States. Int J Radiat Oncol Biol Phys. 2006;66(4):1204‐1211. [DOI] [PubMed] [Google Scholar]
- 52. Payne S, Jarrett N, Jeffs D. The impact of travel on cancer patients' experiences of treatment: a literature review. Eur J Cancer Care. 2000;9(4):197‐203. [DOI] [PubMed] [Google Scholar]
- 53. Levit LA, Byatt L, Lyss AP, et al. Closing the rural cancer care gap: three institutional approaches. J Oncol Pract. 2020;16(7):422‐430. [DOI] [PubMed] [Google Scholar]
- 54. Akhtar Z, Stearns V, Cartwright P, et al. The effect of 1‐day multidisciplinary clinic on breast cancer treatment. Breast Cancer Res Treat. 2020;1‐7:623‐629. [DOI] [PubMed] [Google Scholar]
- 55. Zhang J, Mavros M, Cosgrove D, et al. Impact of a single‐day multidisciplinary clinic on the management of patients with liver tumours. Curr Oncol. 2013;20(2):123‐131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Chollette V, Doose M, Sanchez J, Weaver SJ. Teamwork competencies for interprofessional cancer care in multiteam systems: a narrative synthesis. J Interprof Care. 2021;26:1‐9. doi: 10.1080/13561820.2021.1932775 [DOI] [PubMed] [Google Scholar]
- 57. American College of Surgeons . CoC Standards and Resources. https://www.facs.org/quality-programs/cancer/coc/standards. Accessed March 21, 2022.
- 58. Neuhausen K, Davis AC, Needleman J, Brook RH, Zingmond D, Roby DH. Disproportionate‐share hospital payment reductions may threaten the financial stability of safety‐net hospitals. Health Aff. 2014;33(6):988‐996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. United States Census Bureau . Housing patterns: Appendix B: Measures of residential segregation. Updated November 21, 2021. https://www.census.gov/topics/housing/housing-patterns/guidance/appendix-b.html. Accessed March 21, 2022.
- 60. Lin CC, Bruinooge SS, Kirkwood MK, et al. Association between geographic access to cancer care, insurance, and receipt of chemotherapy: geographic distribution of oncologists and travel distance. J Clin Oncol. 2015;33(28):3177‐3185. doi: 10.1200/jco.2015.61.1558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Onega T, Duell EJ, Shi X, Wang D, Demidenko E, Goodman D. Geographic access to cancer care in the U.S. Cancer. 2008;112(4):909‐918. doi: 10.1002/cncr.23229 [DOI] [PubMed] [Google Scholar]
- 62. Johnson KJ, Wang X, Barnes JM, Delavar A. Associations between geographic residence and US adolescent and young adult cancer stage and survival. Cancer. 2021;127(19):3640‐3650. doi: 10.1002/cncr.33667 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supplemental table.


