Abstract
Pediatric autoimmune neuropsychiatric disorders associated with group A streptococcal infections (PANDAS) is a concept that is used to characterize a subset of children with neuropsychiatric symptoms, tic disorders, or obsessive-compulsive disorder (OCD), whose symptoms are exacerbated by group A streptococcal (GAS) infection. PANDAS has been known to cause a sudden onset of reward deficiency syndrome (RDS). RDS includes multiple disorders that are characterized by dopaminergic signaling dysfunction in the brain reward cascade (BRC), which may result in addiction, depression, avoidant behaviors, anxiety, tic disorders, and/or OCD. According to research by Blum et al., the dopamine receptor D2 (DRD2) gene polymorphisms are important prevalent genetic determinants of RDS. The literature demonstrates that infections like Borrelia and Lyme, as well as other infections like group A beta-hemolytic streptococcal (GABHS), can cause an autoimmune reaction and associated antibodies target dopaminergic loci in the mesolimbic region of the brain, which interferes with brain function and potentially causes RDS-like symptoms/behaviors. The treatment of PANDAS remains controversial, especially since there have been limited efficacy studies to date. We propose an innovative potential treatment for PANDAS based on previous clinical trials using a pro-dopamine regulator known as KB220 variants. Our ongoing research suggests that achieving “dopamine homeostasis” by precision-guided DNA testing and pro-dopamine modulation could result in improved therapeutic outcomes.
Keywords: PANDAS, CANS, Reward Deficiency Syndrome, Group A Beta-Hemolytic Streptococcal (GABHS), Pro-Dopamine Regulation, Dopamine Homeostasis, Molecular Mimicry, Lyme, Borrelia
1. Pediatric Autoimmune Neuropsychiatric Disorders Associated with Streptococcal Infections (PANDAS)
Pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections (PANDAS) are a group of emergent and problematic pediatric disorders. This group tends to involve a small subset of children, particularly in preadolescence, who develop an unknown rapid onset of reward deficiency syndrome (RDS), which includes addiction, depression, avoidant behaviors, anxiety, tic disorders, and/or obsessive-compulsive disorder (OCD) [1] [2] [3]. In addition, PANDAS in children can also manifest as emotional lability, separation anxiety, night-time fears, worsening handwriting, and learning regression. It has also been widely established that the dopamine receptor D2 (DRD2) dysfunction causes aberrant substance-seeking behaviors, including tobacco, alcohol, drugs, and food, as well as other related behaviors (i.e., pathological gambling, attention deficit hyperactivity disorder, and Tourette’s syndrome, including tics) [4]-[14]. The dopaminergic system, specifically DRD2, has been implicated in brain reward mechanisms in over 25,290 articles as of September 15, 2022. Additionally, research by Blum et al. has demonstrated that the DRD2 gene polymorphisms are important prevalent genetic determinants of RDS [15].
Group A beta-hemolytic streptococcal (GABHS) infections are thought to be the root cause of RDS and a variety of aggressive and depressive behaviors [3]. Cumulative data strongly suggests that infections such as Borrelia and Lyme, as well as other GABHS infections, cause an autoimmune reaction and associated antibodies target dopaminergic loci in the mesolimbic region of the brain, which disrupts brain function. Our group asserted in a previously published article that the principal vector of Lyme disease in the United States (US) is Ixodes scapularis (deer or black-legged ticks), and patients infected with this disease can present with anxiety and depression [16]. This study also discovered transcript coding for two putative cytosolic sulfotransferases, indicating that these ticks identified phenolic monoamines as their substrates. More specifically, dopamine and octopamine-targeting sulfotransferase activity were discovered in later recombinant proteins. Furthermore, it was demonstrated that, within the Ixodid tick’s salivary glands, the activation of Ixosc Sult 1 and Sult 2 may result in the deactivation of the salivation signal through the sulfonation of dopamine or octopamine. RDS behaviors can be caused by this phenomenon alone.
The original concept of PANDAS can be traced back to a clinical trial that was conducted at the National Institutes of Health (NIH) in the US [17]. The nosology has recently changed from PANDAS to Childhood Acute Neuropsychiatric Syndrome (CANS) [18]. At the moment, the diagnosis of PANDAS along with the hypothesis that infections can cause symptoms in a subgroup of children is disputed, particularly the proposed mechanism [19] [20] [21] [22]. Singer offered a more comprehensive concept to illustrate PANDAS by lowering the threshold assigned to PANDAS [22]. He claimed that CANS had a variety of unknown factors, which may or may not include GABHS and possibly hypodopaminergia [23].
2. Pandas Mechanisms
It is currently believed that PANDAS is an autoimmune reaction that, in part, mimics rheumatic fever and differs from the etiological links associated with spectrum disorders (e.g., attention deficit hyperactivity disorder, Tourette’s, autism, etc.) or RDS. As a result, rheumatic fever can be thought of as an autoimmune disorder caused by streptococcal infections in which antibodies affect the brain and induce neuropsychiatric conditions. In their article on Tourette’s Syndrome and OCD, Lombroso & Scahill introduced the idea of “molecular mimicry” as a potential explanation for PANDAS [24]. According to their theory, brain proteins and antigens on the cell wall of streptococcal bacteria are similar. As such, the produced antibodies cause an immunological reaction that targets and damages brain tissues, resulting in aberrant motor movements known as Sydenham chorea [25]. Some believe that, similar to Sydenham chorea, the antibodies cross-react with neuronal brain tissue, interrupting the brain reward circuit and causing tics and/or neuropsychiatric impairments that characterize PANDAS [26]. However, there is a lack of research supporting or disputing this hypothesis. The strongest supporting evidence comes from a controlled study that involved 144 children, but prospective longitudinal studies have not produced conclusive results [19].
In short, the following body of existing literature shows scientific support for the molecular basis of PANDAS-related mechanisms. The primary research credited with comprehending the molecular mechanisms implicated in PANDAS is from Madeleine Cunningham and colleagues at the University of Oklahoma Health Sciences Center. It is significant from a historical perspective that the original group, which consisted of the first 50 cases reported by Swedo et al. in 1998 [1], exhibited characteristics that were similar to Sydenham chorea, which provided evidence that assisted in distinguishing this original group from previously published OCD and tic cases [27]-[35].
As previously mentioned, GABHS infections are linked to a variety of neuropsychiatric disorders. The main theory regarding this association postulates that a GABHS infection generates auto-antibodies that, through molecular mimicry, cross-react with neuronal determinants (dopamine) in the brain. Cunningham’s group demonstrated in rats the link between GABHS-induced antibodies and the development of behavioral impairments [36]. To achieve this, over a period of 21 days, researchers administered immunoglobulin G (IgG) isolated from the sera of GABHS-exposed rats directly into the striatum of naïve rats. The most significant finding was that IgG from GABHS-exposed rats reacted with numerous receptors in vitro, including 5HT-2A, 5HT-2C, D1, and D2 receptors. However, it was discovered that, in vivo, specific brain proteins, such as serotonin transporters, dopamine receptors, and other neuronal proteins, colocalized with IgG deposits in the striatum of infused rats. Furthermore, Cox et al. conducted a study involving 311 subjects (aged 4 – 27 years, 66% male) who self-reported neuropsychiatric symptoms and also had concurrent group A streptococcal infection status [37]. When compared to healthy controls, the results revealed a significant increase in serum IgG antibodies against human dopamine receptor D1 (DRD1) and lysoganglioside. This seminal work, involving humans, supports the results from the rodent study [36]. The authors concluded that the results from this study showed a strong association between streptococcal-associated tics and OCD, elevated serum levels of anti-DRD1 and anti-lysoganglioside antineuronal antibodies, and higher CaMKII activation in human neuronal cells.
Further support has also been provided by other researchers and studies. For example, Quagliariello et al. demonstrated that streptococcal infections alter the bacterial populations in the gut and have an impact on the pro-inflammatory status by choosing particular bacterial strains linked to gut inflammation and immune response activation [38]. In terms of Sydenham chorea, Kirvan et al., in 2006, noted the pathogenesis of Sydenham chorea following group A streptococcal infection [39]. In this setting, Kirvan et al. believed Sydenham chorea was caused by antibodies that developed as a result of the infection and permeated into the brain, particularly the basal ganglia. Additionally, this group provided evidence that presumed antibodies present in acute chorea interact with the exterior of the neuronal cells, stimulate calcium calmodulin-dependent protein ki nase II, and increase tyrosine hydroxylase and the subsequent release of dopamine, which may result in a movement disorder.
In contrast to these results, Morris-Berry et al. assessed single-point-in-time optical densities for three putative antibodies identified in Sydenham chorea using enzyme-linked immunosorbent assays [40]. They found no differences between children with PANDAS (n = 44) or Tourette syndrome (n = 40) and controls (n = 24) for the streptococcal group A carbohydrate antigen, N-acetyl-beta-d-glucosamine, DRD2, or tubulin. However, a recent study by Chain et al. has successfully identified acute illness in both Sydenham chorea and PANDAS [41]. In particular, IgG autoantibodies against four neuronal autoantigens (lysoganglioside GM1, DRD1, DRD2, and tubulin) were observed, utilizing enzyme-linked immunosorbent assays, in Sydenham chorea sera (N = 8), sera and/or cerebrospinal fluid (CSF) from two groups of PANDAS cases (first group N = 25 and second group N = 35), sera from Tourette’s syndrome (N = 18), OCD (N = 25), attention deficit hyperactivity disorder (N = 18), and healthy controls (N = 28). While the reasons for this discrepancy in research findings are unclear, we recommend that interested readers scrutinize balanced reviews on related subjects [42]-[48].
3. Treatment of Pandas
With limited efficacy studies to date, the treatment of PANDAS remains controversial. However, standard treatment options for PANDAS include cognitive behavioral therapy, conventional tic therapy, and medications to treat OCD [49]. In the face of the arduous debate, there are off-label studies that demonstrate moderate to insufficient success when using antibacterial therapies [50] [51]. In addition, a single research study of individuals with PANDAS found, with limited to no supporting evidence, that immunomodulatory therapy, such as intravenous immunoglobulin (IVIG) or plasma exchange, is effective in treating symptoms [19] [24]. The NIH and American Academy of Neurology guidelines concluded in 2011 that there was “inadequate data to determine the efficacy of plasmapheresis in the treatment of acute OCD and tic symptoms in the setting of PANDAS”. Furthermore, Sigra et al. came to the conclusion in 2018 that rigorously conducted research regarding treatments for PANDAS is scarce, and published studies have a significant risk of bias [52].
Genomic testing, such as the Genetic Addiction Risk Score (GARS), could also help improve clinical outcomes and decision-making in individuals with PANDAS. GARS is a test that includes a panel of ten reward gene risk alleles and is able to accurately predict vulnerability to pain, addiction, and other compulsive behaviors that are defined as RDS and prevalent in PANDAS patients [53]. In addition, a unique therapeutic precision intervention utilizing the pro-dopamine regulator KB220 and associated variants, which includes a formulation of enkephalinase inhibitors, catabolic inhibitors, precursor amino-acids, and dopamine-releasing neuro-nutrients, can be used to induce dopamine homeostasis in individuals that have a genetic predisposition to developing RDS [54]. These genetically based formulations are based on the GARS test results. Therefore, coupling GARS testing with precision match KB220 pro-dopamine regulation could be used as a frontline modality to treat PANDAS/CANS in hypodopaminergic individuals with polymorphic risk alleles such as DRD1, DRD2, ANKK1 (Taq1A), etc.
4. Conclusion
We propose a novel putative treatment for PANDAS/CANS in the future, based on previous clinical trials utilizing KB220 variants as pro-dopamine regulators. Our hypothesis relates to the epigenetic repair of neuropsychiatric symptoms in a polymorphic dopamine D2 [-DRD2/ANKK1 (Taq1A)] and other anti-dopaminergic reward gene risk polymorphisms as measured by genetic risk assessment at the DNA level in compromised pre- and post-adolescence children with PANDAS/CANS. Our ongoing work posits a potential positive clinical outcome as a function of the induction of “dopamine homeostasis” with precision-guided DNA testing and pro-dopamine regulation (Figure 1).
Figure 1.
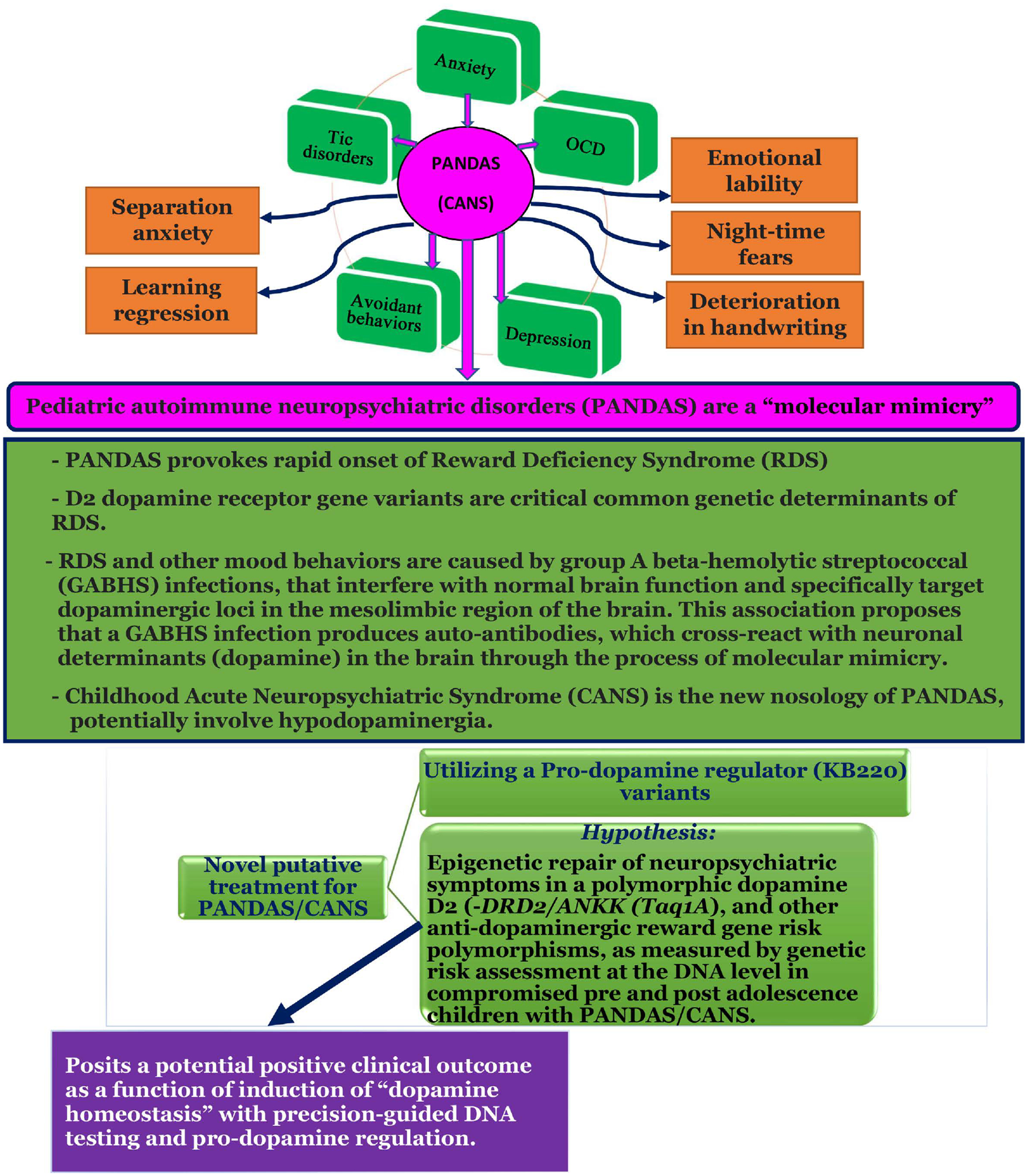
Hypothesizing that Pediatric Autoimmune Neuropsychiatric Associated Streptococcal (PANDAS) Causes Rapid Onset of (RDS) Behaviors and May Require Induction of “Dopamine Homeostasis”.
Acknowledgements
The authors express their gratitude to Margaret A Madigan for expert edits.
Funding
R41 MD012318/MD/NIMHD NIH HHS/United States and I01 CX000479/CX/CSRD VA/United States.
Footnotes
Conflicts of Interest
The authors declare no conflicts of interest regarding the publication of this paper.
References
- [1].Swedo SE, Leonard HL, Garvey M, Mittleman B, Allen AJ, Perlmutter S, Lougee L, Dow S, Zamkoff J and Dubbert BK (1998) Pediatric Autoimmune Neuropsychiatric Disorders Associated with Streptococcal Infections: Clinical Description of the First 50 Cases. American Journal of Psychiatry, 155, 264–271. 10.1176/ajp.155.2.264 [DOI] [PubMed] [Google Scholar]
- [2].Murphy TK, Storch EA, Lewin AB, Edge PJ and Goodman WK (2012) Clinical Factors Associated with Pediatric Autoimmune Neuropsychiatric Disorders Associated with Streptococcal Infections. The Journal of Pediatrics, 160, 314–319. 10.1016/j.jpeds.2011.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Moretti G, Pasquini M, Mandarelli G, Tarsitani L and Biondi M (2008) What Every Psychiatrist Should Know about PANDAS: A Review. Clinical Practice and Epidemiology in Mental Health, 4, Article No. 13. 10.1186/1745-0179-4-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Tsou CC, Chou HW, Ho PS, Kuo SC, Chen CY, Huang CC, Liang CS, Lu RB and Huang SY (2019) DRD2 and ANKK1 Genes Associate with Late-Onset Heroin Dependence in Men. The World Journal of Biological Psychiatry, 20, 605–615. 10.1080/15622975.2017.1372630 [DOI] [PubMed] [Google Scholar]
- [5].Zhang R, Manza P, Tomasi D, Kim SW, Shokri-Kojori E, Demiral SB, Kroll DS, Feldman DE, McPherson KL, Biesecker CL, Wang GJ and Volkow ND (2021) Dopamine D1 and D2 Receptors Are Distinctly Associated with Rest-Activity Rhythms and Drug Reward. Journal of Clinical Investigation, 131, e149722. 10.1172/JCI149722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Lachowicz M, Chmielowiec J, Chmielowiec K, Suchanecka A, Masiak J, Michałowska-Sawczyn M, Mroczek B, Mierzecki A, Ciechanowicz I and Grzywacz A (2020) Significant Association of DRD2 and ANKK1 Genes with Rural Heroin Dependence and Relapse in Men. Annals of Agricultural and Environmental Medicine, 27, 269–273. 10.26444/aaem/119940 [DOI] [PubMed] [Google Scholar]
- [7].Le Foll B, Gallo A, Le Strat Y, Lu L and Gorwood P (2009) Genetics of Dopamine Receptors and Drug Addiction: A Comprehensive Review. Behavioural Pharmacology, 20, 1–17. 10.1097/FBP.0b013e3283242f05 [DOI] [PubMed] [Google Scholar]
- [8].Deng XD, Jiang H, Ma Y, Gao Q, Zhang B, Mu B, Zhang LX, Zhang W, Er ZE, Xie Y and Liu Y (2015) Association Between DRD2/ANKK1 TaqIA Polymorphism and Common Illicit Drug Dependence: Evidence from a Meta-Analysis. Human Immunology, 76, 42–51. 10.1016/j.humimm.2014.12.005 [DOI] [PubMed] [Google Scholar]
- [9].Steiger H, Thaler L, Gauvin L, Joober R, Labbe A, Israel M and Kucer A (2016) Epistatic Interactions Involving DRD2, DRD4, and COMT Polymorphisms and Risk of Substance Abuse in Women with Binge-Purge Eating Disturbances. Journal of Psychiatric Research, 77, 8–14. 10.1016/j.jpsychires.2016.02.011 [DOI] [PubMed] [Google Scholar]
- [10].Stolf AR, Cupertino RB, Müller D, Sanvicente-Vieira B, Roman T, Vitola ES, Grevet EH, Von Diemen L, Kessler FHP, Grassi-Oliveira R, Bau CHD, Rovaris DL, Pechansky F and Schuch JB (2019) Effects of DRD2 Splicing-Regulatory Polymorphism and DRD4 48 bp VNTR on Crack Cocaine Addiction. Journal of Neural Transmission, 126, 193–199. 10.1007/s00702-018-1946-5 [DOI] [PubMed] [Google Scholar]
- [11].Boroń A, Śmiarowska M, Grzywacz A, Chmielowiec K, Chmielowiec J, Masiak J, Pawłowski T, Larysz D and Ciechanowicz A (2022) Association of Polymorphism within the Putative miRNA Target Site in the 3’UTR Region of the DRD2 Gene with Neuroticism in Patients with Substance Use Disorder. International Journal of Environmental Research and Public Health, 19, Article No. 9955. 10.3390/ijerph19169955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Blum K, Gold MS, Cadet JL, Baron D, Bowirrat A, Thanos PK, Brewer R, Badgaiyan RD and Gondré-Lewis MC (2022) Dopaminylation in Psychostimulant Use Disorder Protects Against Psychostimulant Seeking Behavior by Normalizing Nucleus Accumbens (NAc) Dopamine Expression. Current Psychopharmacology, 11, 11–17. 10.2174/2211556009666210108112737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Li Y, Xu H, Chitaman JM and Feng J (2022) Whole Genome DNA Methylation Profiling of D2 Medium Spiny Neurons in Mouse Nucleus Accumbens Using Two Independent Library Preparation Methods. Genes, 13, Article No. 306. 10.3390/genes13020306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Neuman J, Roeder N, Richardson B, Quattrin T, Hamilton J and Thanos PK (2022) High Fat Diet Increases [3H] Flunitrazepam Binding in the Mouse Brain That Is Dependent on the Expression of the Dopamine D2 Gene. Neurochemical Research, 47, 3003–3011. 10.1007/s11064-022-03644-7 [DOI] [PubMed] [Google Scholar]
- [15].Blum K, Sheridan PJ, Wood RC, Braverman ER, Chen TJ, Cull JG and Comings DE (1996) The D2 Dopamine Receptor Gene as a Determinant of Reward Deficiency Syndrome. Journal of the Royal Society of Medicine, 89, 396–400. 10.1177/014107689608900711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Blum K, Modestino EJ, Febo M, Steinberg B, McLaughlin T, Fried L, Baron D, Siwicki D and Badgaiyan RD (2017) Lyme and Dopaminergic Function: Hypothesizing Reduced Reward Deficiency Symptomatology by Regulating Dopamine Transmission. Journal of Systems and Integrative Neuroscience, 3, 1–4. 10.15761/JSIN.1000163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Pichichero ME (2009) The PANDAS Syndrome. In: Finn A, Curtis N and Pollard A, Eds., Hot Topics in Infection and Immunity in Children V, Vol. 634, Springer, New York, 205–216. 10.1007/978-0-387-79838-7_17 [DOI] [Google Scholar]
- [18].Marazziti D, Mucci F and Fontenelle LF (2018) Immune System and Obsessive-Compulsive Disorder. Psychoneuroendocrinology, 93, 39–44. 10.1016/j.psyneuen.2018.04.013 [DOI] [PubMed] [Google Scholar]
- [19].Leckman JF, Denys D, Simpson HB, Mataix-Cols D, Hollander E, Saxena S, Miguel EC, Rauch SL, Goodman WK, Phillips KA and Stein DJ (2010) Obsessive-Compulsive Disorder: A Review of the Diagnostic Criteria and Possible Subtypes and Dimensional Specifiers for DSM-V. Depression and Anxiety, 27, 507–527. 10.1002/da.20669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Kurlan R and Kaplan EL (2004) The Pediatric Autoimmune Neuropsychiatric Disorders Associated with Streptococcal Infection (PANDAS) Etiology for Tics and Obsessive-Compulsive Symptoms: Hypothesis or Entity? Practical Considerations for the Clinician. Pediatrics, 113, 883–886. 10.1542/peds.113.4.883 [DOI] [PubMed] [Google Scholar]
- [21].Shprecher D and Kurlan R (2009) The Management of Tics. Movement Disorders, 24, 15–24. 10.1002/mds.22378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Singer HS (2011) Tourette Syndrome and Other Tic Disorders. Handbook of Clinical Neurology, 100, 641–657. 10.1016/B978-0-444-52014-2.00046-X [DOI] [PubMed] [Google Scholar]
- [23].Singer HS, Gilbert DL, Wolf DS, Mink JW and Kurlan R (2012) Moving from PANDAS to CANS. The Journal of Pediatrics, 160, 725–731. 10.1016/j.jpeds.2011.11.040 [DOI] [PubMed] [Google Scholar]
- [24].Lombroso PJ and Scahill L (2008) Tourette Syndrome and Obsessive-Compulsive Disorder. Brain & Development, 30, 231–237. 10.1016/j.braindev.2007.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Bonthius DJ and Karacay B (2003) Sydenham’s Chorea: Not Gone and Not Forgotten. Seminars in Pediatric Neurology, 10, 11–19. 10.1016/S1071-9091(02)00004-9 [DOI] [PubMed] [Google Scholar]
- [26].Leckman JF, Bloch MH and King RA (2009) Symptom Dimensions and Subtypes of Obsessive-Compulsive Disorder: A Developmental Perspective. Dialogues in Clinical Neuroscience, 11, 21–33. 10.31887/DCNS.2009.11.1/jfleckman [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Giovannoni G (2006) PANDAS: Overview of the Hypothesis. Advanced Neurology, 99, 159–165. [PubMed] [Google Scholar]
- [28].Murphy TK, Kurlan R and Leckman J (2010) The Immunobiology of Tourette’s Disorder, Pediatric Autoimmune Neuropsychiatric Disorders Associated with Streptococcus, and Related Disorders: A Way Forward. Journal of Child and Adolescent Psychopharmacology, 20, 317–331. 10.1089/cap.2010.0043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].de Oliveira SK and Pelajo CF (2010) Pediatric Autoimmune Neuropsychiatric Disorders Associated with Streptococcal Infection (PANDAS): A Controversial Diagnosis. Current Infectious Disease Reports, 12, 103–109. 10.1007/s11908-010-0082-7 [DOI] [PubMed] [Google Scholar]
- [30].Shulman ST (2009) Pediatric Autoimmune Neuropsychiatric Disorders Associated with Streptococci (PANDAS): Update. Current Opinion in Pediatrics, 21, 127–130. 10.1097/MOP.0b013e32831db2c4 [DOI] [PubMed] [Google Scholar]
- [31].Toufexis MD, Hommer R, Gerardi DM, Grant P, Rothschild L, D’Souza P, Williams K, Leckman J, Swedo SE and Murphy TK (2015) Disordered Eating and Food Restrictions in Children with PANDAS/PANS. Journal of Child and Adolescent Psychopharmacology, 25, 48–56. 10.1089/cap.2014.0063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Murphy ML and Pichichero ME (2002) Prospective Identification and Treatment of Children with Pediatric Autoimmune Neuropsychiatric Disorder Associated with Group a Streptococcal Infection (PANDAS). Archives of Pediatrics and Adolescent Medicine, 156, 356–361. 10.1001/archpedi.156.4.356 [DOI] [PubMed] [Google Scholar]
- [33].Murphy TK, Parker-Athill EC, Lewin AB, Storch EA and Mutch PJ (2015) Cefdinir for Recent-Onset Pediatric Neuropsychiatric Disorders: A Pilot Randomized Trial. Journal of Child and Adolescent Psychopharmacology, 25, 57–64. 10.1089/cap.2014.0010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Swedo S, Leckman J and Rose N (2012) From Research Subgroup to Clinical Syndrome: Modifying the PANDAS Criteria to Describe PANS (pediatric Acute-Onset Neuropsychiatric Syndrome). Pediatrics & Therapeutics, 2, Article No. 113. 10.4172/2161-0665.1000113 [DOI] [Google Scholar]
- [35].Lepri G, Rigante D, Bellando Randone S, Meini A, Ferrari A, Tarantino G, Cunningham MW and Falcini F (2019) Clinical-Serological Characterization and Treatment Outcome of a Large Cohort of Italian Children with Pediatric Autoimmune Neuropsychiatric Disorder Associated with Streptococcal Infection and Pediatric Acute Neuropsychiatric Syndrome. Journal of Child and Adolescent Psychopharmacology, 29, 608–614. 10.1089/cap.2018.0151 [DOI] [PubMed] [Google Scholar]
- [36].Lotan D, Benhar I, Alvarez K, Mascaro-Blanco A, Brimberg L, Frenkel D, Cunningham MW and Joel D (2014) Behavioral and Neural Effects of Intra-Striatal Infusion of Anti-Streptococcal Antibodies in Rats. Brain, Behavior, and Immunity, 38, 249–262. 10.1016/j.bbi.2014.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Cox CJ, Zuccolo AJ, Edwards EV, Mascaro-Blanco A, Alvarez K, Stoner J, Chang K and Cunningham MW (2015) Antineuronal Antibodies in a Heterogeneous Group of Youth and Young Adults with Tics and Obsessive-Compulsive Disorder. Journal of Child and Adolescent Psychopharmacology, 25, 76–85. 10.1089/cap.2014.0048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Quagliariello A, Del Chierico F, Russo A, Reddel S, Conte G, Lopetuso LR, Ianiro G, Dallapiccola B, Cardona F, GABHSbarrini A and Putignani L (2018) Gut Microbiota Profiling and Gut-Brain Crosstalk in Children Affected by Pediatric Acute-Onset Neuropsychiatric Syndrome and Pediatric Autoimmune Neuropsychiatric Disorders Associated with Streptococcal Infections. Frontiers in Microbiology, 9, Article No. 675. 10.3389/fmicb.2018.00675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Kirvan CA, Swedo SE, Kurahara D and Cunningham MW (2006) Streptococcal Mimicry and Antibody-Mediated Cell Signaling in the Pathogenesis of Sydenham’s Chorea. Autoimmunity, 39, 21–29. 10.1080/08916930500484757 [DOI] [PubMed] [Google Scholar]
- [40].Morris-Berry CM, Pollard M, Gao S, Thompson C, Singer HS and Tourette Syndrome Study Group (2013) Anti-Streptococcal, Tubulin, and Dopamine Receptor 2 Antibodies in Children with PANDAS and Tourette Syndrome: Single-Point and Longitudinal Assessments. Journal of Neuroimmunology, 264, 106–113. 10.1016/j.jneuroim.2013.09.010 [DOI] [PubMed] [Google Scholar]
- [41].Chain JL, Alvarez K, Mascaro-Blanco A, Reim S, Bentley R, Hommer R, Grant P, Leckman JF, Kawikova I, Williams K, Stoner JA, Swedo SE and Cunningham MW (2020) Autoantibody Biomarkers for Basal Ganglia Encephalitis in Sydenham Chorea and Pediatric Autoimmune Neuropsychiatric Disorder Associated with Streptococcal Infections. Frontiers in Psychiatry, 11, Article No. 564. 10.3389/fpsyt.2020.00564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Cunningham MW and Cox CJ (2016) Autoimmunity against Dopamine Receptors in Neuropsychiatric and Movement Disorders: A Review of Sydenham Chorea and Beyond. Acta Physiologica, 216, 90–100. 10.1111/apha.12614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Pavăl D (2017) A Dopamine Hypothesis of Autism Spectrum Disorder. Developmental Neuroscience, 39, 355–360. 10.1159/000478725 [DOI] [PubMed] [Google Scholar]
- [44].Orefici G, Cardona F, Cox CJ and Cunningham MW (2016, February 10) Pediatric Autoimmune Neuropsychiatric Disorders Associated with Streptococcal Infections (PANDAS). In: Ferretti JJ, Stevens DL and Fischetti VA, Eds., Streptococcus Pyogenes: Basic Biology to Clinical Manifestations, University of Oklahoma Health Sciences Center, Oklahoma City. [PubMed] [Google Scholar]
- [45].Hayashi M (2013) Anti-Basal Ganglia Antibody. Brain Nerve, 65, 377–384. (In Japanese) [PubMed] [Google Scholar]
- [46].Macrì S, Onori MP, Roessner V and Laviola G (2013) Animal Models Recapitulating the Multifactorial Origin of Tourette Syndrome. International Review of Neurobiology, 112, 211–237. 10.1016/B978-0-12-411546-0.00008-1 [DOI] [PubMed] [Google Scholar]
- [47].Macerollo A and Martino D (2013) Pediatric Autoimmune Neuropsychiatric Disorders Associated with Streptococcal Infections (PANDAS): An Evolving Concept. Tremor and Other Hyperkinetic Movements, 3, Tre-03-167-4158-7. 10.5334/tohm.167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Singer HS, Mascaro-Blanco A, Alvarez K, Morris-Berry C, Kawikova I, Ben-Pazi H, Thompson CB, Ali SF, Kaplan EL and Cunningham MW (2015) Neuronal Antibody Biomarkers for Sydenham’s Chorea Identify a New Group of Children with Chronic Recurrent Episodic Acute Exacerbations of Tic and Obsessive Compulsive Symptoms Following a Streptococcal Infection. PLOS ONE, 10, e0120499. 10.1371/journal.pone.0120499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Kalra SK and Swedo SE (2009) Children with Obsessive-Compulsive Disorder: Are They Just “little Adults”? Journal of Clinical Investigation, 119, 737–746. 10.1172/JCI37563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Garvey MA, Perlmutter SJ, Allen AJ, Hamburger S, Lougee L, Leonard HL, Witowski ME, Dubbert B and Swedo SE (1999) A Pilot Study of Penicillin Prophylaxis for Neuropsychiatric Exacerbations Triggered by Streptococcal Infections. Biological Psychiatry, 45, 1564–1571. 10.1016/S0006-3223(99)00020-7 [DOI] [PubMed] [Google Scholar]
- [51].Snider LA, Lougee L, Slattery M, Grant P and Swedo SE (2005) Antibiotic Prophylaxis with Azithromycin or Penicillin for Childhood-Onset Neuropsychiatric Disorders. Biological Psychiatry, 57, 788–792. 10.1016/j.biopsych.2004.12.035 [DOI] [PubMed] [Google Scholar]
- [52].Sigra S, Hesselmark E and Bejeroti S (2018) Treatment of PANDAS and PANS: A Systematic Review. Neuroscience & Biobehavioral Reviews, 86, 51–65. 10.1016/j.neubiorev.2018.01.001 [DOI] [PubMed] [Google Scholar]
- [53].Blum K, Modestino EJ, Gondre-Lewis M, Chapman EJ, Neary J, Siwicki D, Baron D, Hauser M, Smith DE, Roy AK, Thanos PK, Steinberg B, McLaughlin T, Fried L, Barh D, Dunston GA and Badgaiyan RD (2018) The Benefits of Genetic Addiction Risk Score (GARS™) Testing in Substance Use Disorder (SUD). International Journal of Genomics and Data Mining, 1, Article No. 115. 10.29011/2577-0616.000115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Blum K, Baron D, McLaughlin, Thanos PK, Giordano J, Dennen, Ceccanti M and Badgaiyan RD (2022) Summary Document Research on RDS Anti-Addiction Modeling: Annotated Bibliography. Journal of Systems Integrative Neuroscience, 8, 2–35. [PMC free article] [PubMed] [Google Scholar]


