Abstract
Background & Aims
The burden of liver cancer varies across the world. Herein, we present updated estimates of the current global burden of liver cancer (incidence and mortality) and provide predictions of the number of cases/deaths to 2040.
Methods
We extracted data on primary liver cancer cases and deaths from the GLOBOCAN 2020 database, which includes 185 countries. Age-standardised incidence and mortality rates (ASRs) per 100,000 person-years were calculated. Cases and deaths up to the year 2040 were predicted based on incidence and mortality rates for 2020 and global demographic projections to 2040.
Results
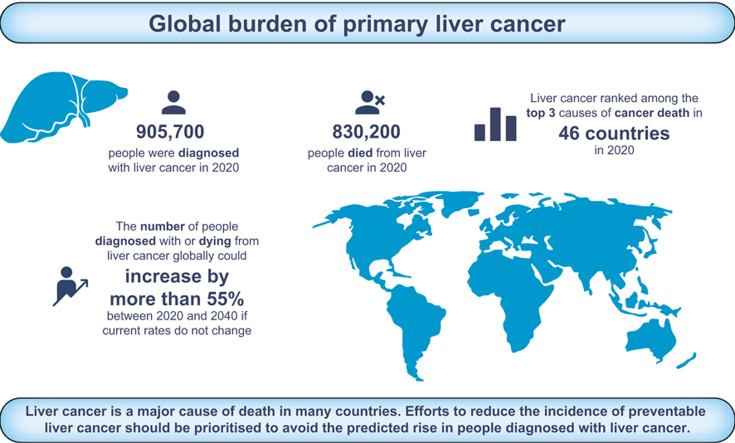
In 2020, an estimated 905,700 people were diagnosed with, and 830,200 people died from, liver cancer globally. Global ASRs for liver cancer were 9.5 and 8.7 for new cases and deaths, respectively, per 100,000 people and were highest in Eastern Asia (17.8 new cases, 16.1 deaths), Northern Africa (15.2 new cases, 14.5 deaths), and South-Eastern Asia (13.7 new cases, 13.2 deaths). Liver cancer was among the top three causes of cancer death in 46 countries and was among the top five causes of cancer death in 90 countries. ASRs of both incidence and mortality were higher among males than females in all world regions (male:female ASR ratio ranged between 1.2–3.6). The number of new cases of liver cancer per year is predicted to increase by 55.0% between 2020 and 2040, with a possible 1.4 million people diagnosed in 2040. A predicted 1.3 million people could die from liver cancer in 2040 (56.4% more than in 2020).
Conclusions
Liver cancer is a major cause of death in many countries, and the number of people diagnosed with liver cancer is predicted to rise. Efforts to reduce the incidence of preventable liver cancer should be prioritised.
Lay summary
The burden of liver cancer varies across the world. Liver cancer was among the top three causes of cancer death in 46 countries and was among the top five causes of cancer death in 90 countries worldwide. We predict the number of cases and deaths will rise over the next 20 years as the world population grows. Primary liver cancer due to some causes is preventable if control efforts are prioritised and the predicted rise in cases may increase the need for resources to manage care of patients with liver cancer.
Keywords: Hepatocellular carcinoma, intrahepatic cholangiocarcinoma, epidemiology, cancer registries
Graphical abstract
Highlights
-
•
905,700 people were diagnosed with and 830,200 people died from liver cancer globally in 2020.
-
•
Liver cancer was among the top three causes of cancer death in 46 countries.
-
•
The number of new cases and deaths from liver cancer could rise by >55% by 2040.
Introduction
The global burden of liver cancer is substantial. According to 2020 estimates, liver cancer is the sixth most commonly diagnosed cancer and the third most common cause of cancer death.1 Liver cancer also ranks as the second most common cause of premature death from cancer.2 Incidence and mortality rates of liver cancer have dropped in some Eastern Asian countries including Japan, China, and the Republic of Korea, but rates have increased in many previously low-incidence countries across the world, such as the US, Australia, and several European countries.3
Risk factors for liver cancer include older age and sex (higher risk among males than females), and there are some differences in risk by ethnicity.4 For example, in multi-ethnic populations such as the US, American Indians/Alaskan Natives, Hispanic persons, non-Hispanic Black persons and Asians/Pacific Islanders have higher rates than non-Hispanic White persons.4 Although HBV and HCV infections constitute the most important exogenous risk factors for primary liver cancer, excessive alcohol consumption and the related conditions of metabolic syndrome, type 2 diabetes, obesity, and non-alcoholic fatty liver disease have also become prominent causes of primary liver cancer.4,5 Further exogenous risk factors include cigarette smoking, ingestion of aflatoxin-contaminated food, and liver fluke infestation.5 Recent studies suggest that approximately 56% of liver cancer is related to HBV and 20% is related to HCV.6 A further 18% of liver cancer burden may be related to tobacco smoking,7 and an estimated 17% could be attributable to alcohol drinking globally,8 with the possibility of multiple risk factors being attributed to the same cases or deaths.
An updated evaluation of the global burden of liver cancer incidence and mortality is warranted due to the disparities in burden across populations and the availability of more recent estimates. In this analysis, we describe where liver cancer ranks amongst all cancer types for cancer diagnoses and deaths in nations across the world. We also present predictions of the future liver cancer burden to 2040.
Materials and methods
The number of new cases of, and deaths from, primary liver cancer (ICD-10 C22), were obtained from the GLOBOCAN 2020 database for 185 countries and territories, by sex and 18 age groups (0–4, 5–9, …, 80–84, 85 and over).1,2,9 Corresponding population data for 2020 were extracted from the United Nations (UN) website.10 The data sources and hierarchy of methods used in compiling the cancer estimates have been described in detail elsewhere.9 Briefly, the GLOBOCAN estimates are assembled at the national level using the best available sources of cancer incidence and mortality data within a given country.
We predicted the future number of primary liver cancer cases and deaths up to the year 2040 based on the medium-variant UN population projections and the current global-level incidence and mortality rates of primary liver cancer for 2020. The predicted number of new cancer cases or deaths was computed by multiplying the age-specific incidence or mortality rates for the world for 2020 by the corresponding projected world population estimate. These expected populations differ from that of 2020 in terms of age structure and size. The key assumption is that national rates, as estimated in 2020, will not change between 2020 and 2040 and thus changes in number of cases or deaths are solely due to the growth and aging of the population. To show the impact of changes in rates on the future primary liver cancer burden, we also predicted number of cases and deaths from seven scenarios of uniformly increasing or decreasing rates by 3%, 2%, and 1% annually from the baseline year of 2020 to 2040.
We present estimates of new cases and deaths and age-standardised incidence and mortality rates (ASRs) per 100,000 person-years based on the 1966 Segi-Doll World standard population.11,12 Male:female ratios (M:F) of incidence and mortality ASRs are presented. Cases, deaths, and ASRs of primary liver cancer are presented by country, by 19 world regions based on UN definitions,10 and by the UN’s four-tier Human Development Index (HDI) in 2020,13 the latter being a means to assess the burden, the strength of health systems, and the ability to report primary liver cancer cases and deaths at varying levels of development (low, medium, high and very high HDI). Rankings were based on number of new cancer cases and deaths by cancer type according to ICD-10 three-digit groupings and not including non-melanoma skin cancer (ICD-10 C44). For comparison of current liver cancer burden with the population prevalence of risk factors for liver cancer, the population attributable fractions of liver cancer due to HBV or HCV infection, alcohol consumption, and high body mass index were obtained from three global studies,[6], [7], [8] and are presented in Fig. S1.
Results
Global burden of liver cancer incidence and mortality
An estimated 905,700 people were diagnosed with, and 830,200 people died from, liver cancer globally in 2020 (Table 1). This equated to total ASRs for liver cancer of 9.5 and 8.7 new cases and deaths, respectively, per 100,000 people. More than half of the world’s estimated cases and deaths from liver cancer occurred in Eastern Asia (54.3% and 54.1%, respectively), which was home to 21.5% of the world’s population in 2020. China alone was home to 45.3% of the world’s liver cancer cases and 47.1% of liver cancer deaths.
Table 1.
Estimated number of primary liver cancer cases and deaths, and age-standardised incidence and mortality rates per 100,000 persons in 2020, by world region and HDI.
| Population |
Incidence |
Mortality |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total (thousands) | Percentage of world total (%) | Number of cases | Percentage of world total (%) | ASR | M:F | Number of deaths | Percentage of world total (%) | ASR | M:F | |
| Eastern Africa | 445,406 | 5.7 | 12,300 | 1.4 | 5.0 | 1.6 | 11,500 | 1.4 | 4.8 | 1.6 |
| Middle Africa | 179,595 | 2.3 | 6,100 | 0.7 | 6.1 | 2.3 | 5,700 | 0.7 | 5.9 | 2.3 |
| Northern Africa | 246,233 | 3.2 | 31,900 | 3.5 | 15.2 | 1.9 | 30,400 | 3.7 | 14.5 | 1.9 |
| Southern Africa | 67,504 | 0.9 | 2,600 | 0.3 | 4.6 | 2.2 | 2,400 | 0.3 | 4.3 | 2.3 |
| Western Africa | 401,861 | 5.2 | 17,600 | 1.9 | 8.4 | 2.0 | 16,900 | 2.0 | 8.2 | 2.0 |
| Caribbean | 43,532 | 0.6 | 3,400 | 0.4 | 5.5 | 1.6 | 3,200 | 0.4 | 5.0 | 1.6 |
| Central America | 179,670 | 2.3 | 11,800 | 1.3 | 6.3 | 1.2 | 11,200 | 1.4 | 5.9 | 1.2 |
| South America | 430,760 | 5.5 | 24,300 | 2.7 | 4.3 | 1.6 | 23,200 | 2.8 | 4.1 | 1.6 |
| Northern America | 368,870 | 4.7 | 46,600 | 5.1 | 6.8 | 2.7 | 34,800 | 4.2 | 4.7 | 2.4 |
| Eastern Asia | 1,678,090 | 21.5 | 491,700 | 54.3 | 17.8 | 3.0 | 449,500 | 54.1 | 16.1 | 3.1 |
| China | 1,447,470 | 18.6 | 410,000 | 45.3 | 18.2 | 3.1 | 391,200 | 47.1 | 17.2 | 3.0 |
| South-Eastern Asia | 668,620 | 8.6 | 99,300 | 11.0 | 13.7 | 3.0 | 95,700 | 11.5 | 13.2 | 3.0 |
| South-Central Asia | 2,014,709 | 25.8 | 54,700 | 6.0 | 3.0 | 2.0 | 52,800 | 6.4 | 2.8 | 2.0 |
| India | 1,380,004 | 17.7 | 34,700 | 3.8 | 2.6 | 2.3 | 33,800 | 4.1 | 2.5 | 2.3 |
| Western Asia | 278,429 | 3.6 | 11,300 | 1.3 | 4.7 | 1.9 | 10,900 | 1.3 | 4.5 | 1.9 |
| Central-Eastern Europe | 293,013 | 3.8 | 24,800 | 2.7 | 4.3 | 2.6 | 23,000 | 2.8 | 3.9 | 2.6 |
| Northern Europe | 106,261 | 1.4 | 11,900 | 1.3 | 5.0 | 2.1 | 10,500 | 1.3 | 3.9 | 2.1 |
| Southern Europe | 153,423 | 2.0 | 24,800 | 2.7 | 6.7 | 3.3 | 21,200 | 2.6 | 5.1 | 3.2 |
| Western Europe | 196,146 | 2.5 | 26,100 | 2.9 | 5.4 | 3.3 | 23,700 | 2.8 | 4.5 | 3.1 |
| Australia/New Zealand | 30,322 | 0.4 | 3,300 | 0.4 | 6.1 | 3.3 | 2,500 | 0.3 | 4.1 | 2.7 |
| Melanesia, Micronesia & Polynesia | 12,356 | 0.2 | 1,100 | 0.1 | 11.3 | 1.7 | 1,000 | 0.1 | 11.2 | 1.7 |
| Low HDI | 990,175 | 12.7 | 33,100 | 3.7 | 6.2 | 1.8 | 31,600 | 3.8 | 6.0 | 1.8 |
| Medium HDI | 2,327,556 | 29.9 | 100,000 | 11.0 | 4.7 | 2.3 | 95,900 | 11.5 | 4.5 | 2.3 |
| High HDI | 2,909,468 | 37.3 | 548,900 | 60.6 | 14.0 | 2.8 | 524,300 | 63.2 | 13.3 | 2.8 |
| Very high HDI | 1,564,286 | 20.1 | 223,300 | 24.7 | 7.0 | 2.8 | 178,100 | 21.5 | 5.1 | 2.8 |
| World | 7,794,799 | 100.0 | 905,700 | 100.0 | 9.5 | 2.7 | 830,200 | 100.0 | 8.7 | 2.7 |
ASR, age-standardised rate per 100,000; HDI, Human Development Index; M:F, male:female ASR ratio.
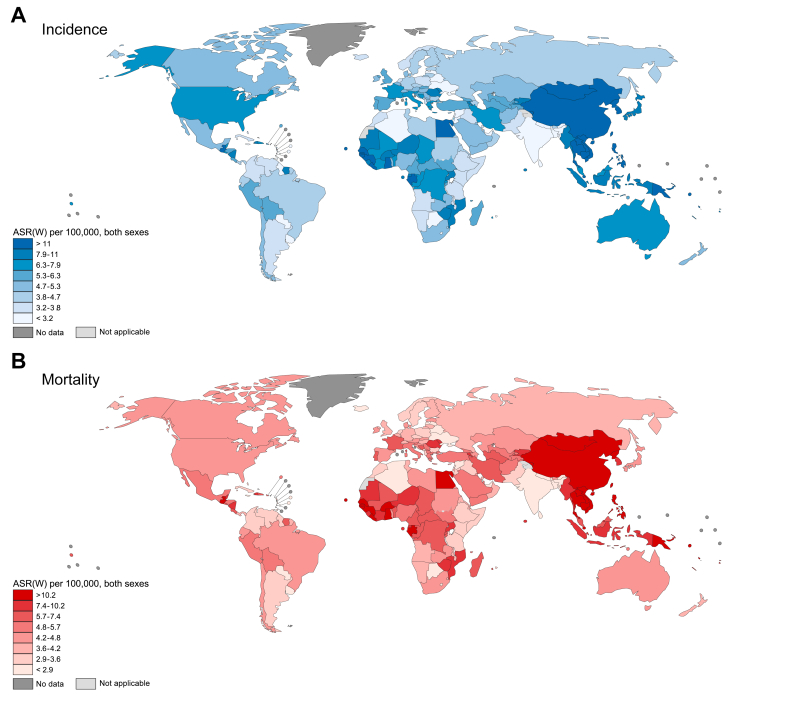
The ASRs of liver cancer incidence ranged 6-fold between world regions, from 3.0 new cases per 100,000 people in South-Central Asia to 17.8 in Eastern Asia. The pattern of mortality ASRs was similar. Eastern Asia had an ASR of 16.1 per 100,000 people compared with 2.8 in South-Central Asia, also resulting in a 6-fold difference. Elevated ASRs for incidence and mortality were also found in Northern Africa (15.2 new cases, 14.5 deaths) and South-Eastern Asia (13.7 new cases, 13.2 deaths). Disparities by sex were apparent, with liver cancer incidence and mortality ASRs higher among males than females in all regions. The incidence M:F ratio ranged from 1.2 in Central America to 3.3 in Southern and Western Europe, and Australia/New Zealand; the mortality M:F ratio was also lowest in Central America (1.2), and was highest in Southern Europe (3.2), Western Europe, and Eastern Asia (both 3.1).
At the national level, ASRs of liver cancer incidence were highest in Mongolia (85.6 new cases per 100,000 people), Egypt (34.1), Laos (24.4), and Cambodia (24.3), and lowest in Sri Lanka (1.2), Saint Lucia (1.3), Algeria (1.5), and Botswana (1.5) (Fig. 1). Mortality ASRs showed a similar pattern as incidence. The full results for number of cases and deaths, and ASRs of liver cancer by country are available in Table S1.
Fig. 1.
ASRs for primary liver cancer per 100,000 people in 2020, by country.
(A) Age-standardised incidence rate. (B) Age-standardised mortality rate. ASR(W), age-standardised rate. (This figure appears in color on the web.)
By HDI group, the largest burdens of liver cancer cases and deaths were in high HDI countries, representing 60.6% of new cases and 63.2% of deaths globally. The high HDI group also had the highest rates of incidence (14.0 new cases per 100,000 people) and mortality (13.3 deaths per 100,000 people). This large contribution to the world’s liver cancer burden was not unexpected as the high HDI group includes some of the countries with the highest rates of liver cancer incidence and mortality, such as Mongolia, Egypt and China. ASRs were similar across the remaining groups, ranging between 4.5 and 7.0. A correlation between a country’s HDI and ASRs for liver cancer incidence or mortality was not observed (Fig. S2).
Ranking of liver cancer diagnoses and deaths
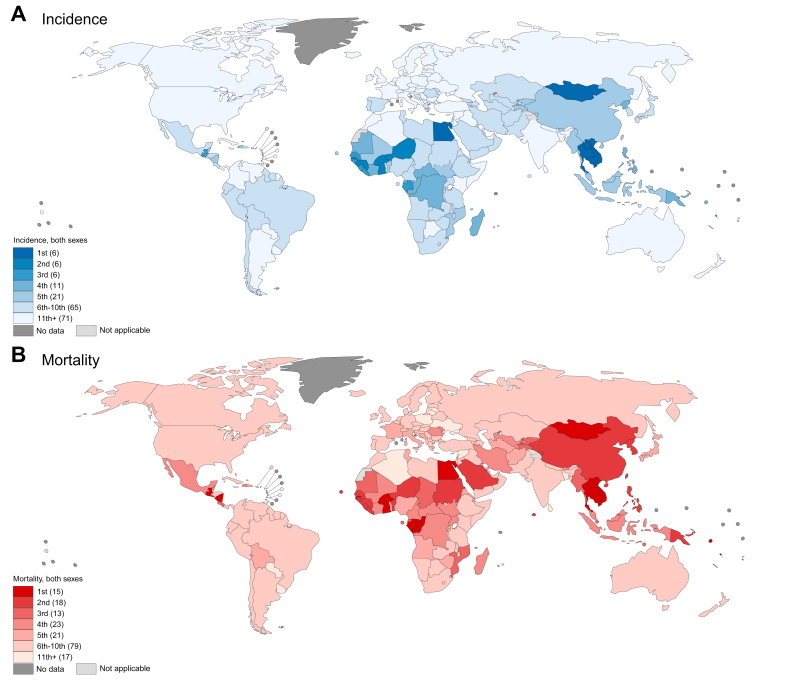
Globally, liver cancer ranked as the sixth most commonly diagnosed cancer and the third most common cause of cancer death in 2020. At the national level, liver cancer was the most commonly diagnosed cancer in six countries (Cambodia, Egypt, Laos, Mongolia, Thailand, and Vietnam) and was among the top three most commonly diagnosed cancers in a total of 18 countries (Fig. 2). In terms of mortality, liver cancer was the most common cause of cancer death in 15 countries (Burkina Faso, Cambodia, Egypt, Gabon, The Gambia, Ghana, Guatemala, Laos, Mongolia, Nicaragua, Republic of Congo, Solomon Islands, Thailand, Vanuatu, and Vietnam) and was among the top three causes of cancer death in a total of 46 countries worldwide. Liver cancer was among the top five causes of cancer death in 90 countries. Most of these countries were in Eastern and South-Eastern Asia, Northern and Western Africa, and Central America. However, liver cancer was also one of the top five causes of cancer mortality in some countries in Europe (Bosnia and Herzegovina, France, Italy, Republic of Moldova, and Romania) and Western Asia (Iran, Saudi Arabia, Turkmenistan, and Uzbekistan).
Fig. 2.
Ranking of primary liver cancer among other cancer types based on number of cases or deaths in 2020, by country.
(A) Number of cases. (B) Number of deaths. (This figure appears in color on the web.)
Predicted number and percentage increase of cases and deaths from liver cancer
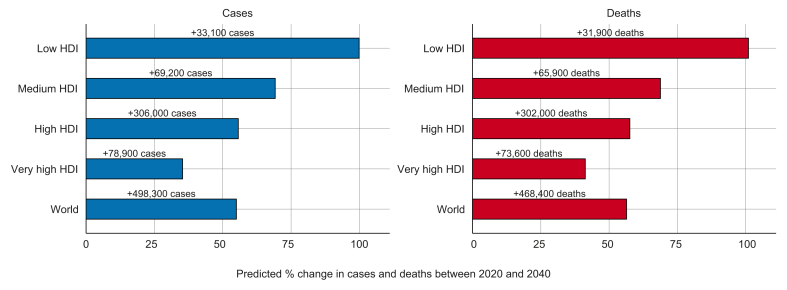
The number of new cases of liver cancer is predicted to increase by 55.0% between 2020 and 2040, with 1.4 million new diagnoses forecast for 2040 (Fig. 3). An estimated 1.3 million deaths are predicted to occur in 2040, an increase of 56.4%. By HDI group, the highest absolute increase in cases and deaths could occur in high HDI countries, with 55.7% more cases (306,000 additional cases) and 57.6% more deaths (302,000 additional deaths) per year by 2040, reflecting the already elevated rates in the high HDI group and its large population which is predicted to continue to grow. However, the largest relative increases in cases and deaths are predicted to occur in low HDI countries (99.9% and 101.0% increases, respectively) and medium HDI countries (69.2% and 68.8% increases, respectively), due to the predicted growth and aging of the population.
Fig. 3.
Predicted percentage change (absolute numbers are shown above bars) of new cases and deaths from primary liver cancer between 2020 and 2040, by HDI.
HDI, Human Development Index. (This figure appears in color on the web.)
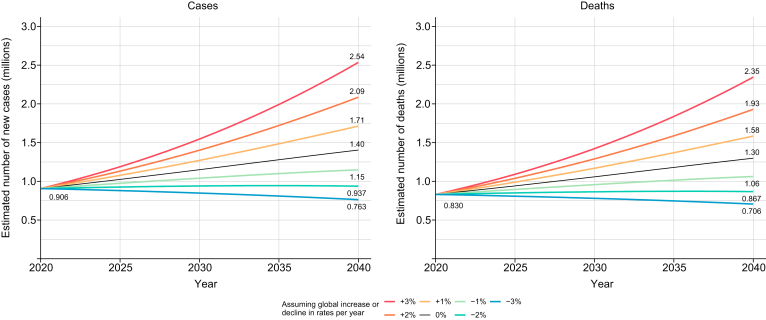
Predictions including annual changes in rates from seven scenarios (−3% to +3% annual change in ASRs) showed a potential increase in the annual number of liver cancer cases and deaths by 2040 in all scenarios except the scenario in which a 3% decrease in ASRs per year is achieved (Fig. 4).
Fig. 4.
Predicted number of new cases and deaths from primary liver cancer assuming seven scenarios of annual change in global rates between 2020 and 2040. (This figure appears in color on the web.)
Discussion
Globally, in 2020, an estimated 900,000 people were diagnosed with, and 830,000 people died from liver cancer. Liver cancer incidence and mortality rates were highest in Eastern Asia, Northern Africa, and South-Eastern Asia, and liver cancer was the most common cause of cancer death in 15 countries including several countries in South-Eastern Asia and sub-Saharan Africa. The number of new cases and deaths from liver cancer are predicted to rise by more than 50% over the next 20 years, assuming current rates do not change, with the burden set to increase unless a 3% or greater annual decrease in rates is achieved.
Liver cancer was among the top three causes of cancer death in 46 countries, and among the top five in 90 countries in 2020, despite not being the most commonly diagnosed cancer in the majority of countries across the world. Moreover, liver cancer was the second most common cause of premature death from cancer in 2020, after lung cancer, with more than 530,000 deaths among persons aged 30 to 69 years.2 Survival from liver cancer remains poor even in high-income countries. A recent study of seven high-income countries reported that the highest 3-year net survival from liver cancer occurred in Australia (28%) and the lowest occurred in Denmark (17%) in 2012–2014.14 The results of another study found that 5-year survival during 2010–2014 ranged from less than 10% in several European countries to 30% in Japan, and changed very little over a 20 year time-period.15 With few improvements in survival in recent decades, primary prevention of liver cancer is key in reducing its burden globally.
Liver cancer due to some major risk factors with large attributable fractions is potentially preventable. For example, chronic HBV infection, which is responsible for more than half of liver cancer cases globally,6 is most prevalent in sub-Saharan African countries, some South-East Asian countries, and Central Asia16 which is where the highest proportions of liver cancer attributable to HBV are found (Fig. S1A). HBV infection can be prevented by neonatal immunisation, which has now been introduced in 133 countries with global coverage of the full three vaccine doses estimated at 83% in 2020.17 A modelling study estimated that 1.5 million liver cancer deaths could be avoided between 2015 and 2030 by scaling up the coverage of neonatal HBV vaccination to 80% of newborns, as well as increasing coverage of infant HBV vaccination to 90% of infants, use of peripartum antivirals to 80% of HBV-positive mothers, and population-wide testing and treatment of 80% of eligible people.18 Many countries now have data on the first cohorts which received the HBV vaccine in infancy as they reach young adulthood; studies in Taiwan and Shanghai reported an 80% and 50% reduction in liver cancer incidence, respectively, among young adults vaccinated in infancy compared with previous or unvaccinated cohorts,19,20 and elimination of liver cancer has been achieved in Alaskan Native children since 1999 following universal neonatal immunisation coupled with a child catch-up programme.21
Another major risk factor for liver cancer is chronic HCV infection which causes approximately 20% of liver cancer cases globally, and more than 50% of liver cancer cases are attributable to HCV in the most affected countries including Egypt, the US, and Pakistan6 (Fig. S1B). There is no vaccine for HCV, but cure of chronic infection can be achieved with direct-acting antivirals (DAAs), and strategies to reduce HCV transmission can be applied worldwide.22 A prospective study of patients with HCV infection and cirrhosis in France observed a 70% reduction in risk of liver cancer incidence after a sustained virologic response, and suggested that DAA therapy will have a substantial effect on liver cancer rates in the future.23 This was further supported by a modelling study on patients with chronic HCV in England, which predicted an increase in liver cancer incidence unless there was a 115% increase in the number of eligible patients treated for HCV by 2018, which would have reduced the number of HCV-related liver cancer cases by 50% by 2020.24 In response to these trends, in 2016, the World Health Organization (WHO) set a goal of reducing HBV infections by 90% and reducing HBV- and HCV-related deaths by 65% by 2030; universal health coverage, with access to HBV immunisation and affordable DAAs, is essential to achieving this goal.25,26
Contamination of crops by the fungi Aspergillus flavus also poses a threat to public health in tropical and subtropical areas that lie in the global aflatoxin belt.27 Pre- and post-harvest strategies to decrease aflatoxin contamination including sorting crops and improving storage have been outlined,28 but many regions in the aflatoxin belt have limited resources to implement control measures. It has been estimated that populations in sub-Saharan Africa, South-East Asia, and China have the highest burdens of liver cancer attributable to aflatoxin exposure, particularly as there is a synergistic effect between aflatoxin and HBV infection.27 Additional causes of liver cancer must also be incorporated into planning for liver cancer control in various regions. For example, in Europe and North America excessive alcohol consumption was associated with an estimated 22% of liver cancer cases in 20208 (Fig. S1C), yet cost-effective policies exist to reduce consumption in the population.29
To explore the potential relationship between the development of a country and its rate of liver cancer incidence or mortality, we plotted HDI by liver cancer mortality rate and did not find a correlation. However, the current burden of liver cancer might be influenced by other demographic factors. For example, we found a strong male predominance for liver cancer across all world regions which has been reported previously and could be largely related to exposure to risk factors for liver cancer.4 Ethnic disparities in liver cancer incidence have also been observed in studies using cancer registry data in the US, finding the highest rates among American Indians/Alaskan Natives, Hispanics, and Asians/Pacific Islanders.4 Additional studies in three US states further disaggregated the ethnic groups and found the highest liver cancer incidence rates in California were among Vietnamese, Cambodian and Laotian groups,30 and the most elevated liver cancer mortality rates in California, Florida, and New York were among Vietnamese, Chinese and Korean groups.31 Furthermore, migration has likely influenced rates of liver cancer among ethnic minorities in Western countries, as observed in the US, Australia, Canada, and Western Europe, where the highest incidence rates were among migrants from high-risk countries.[31], [32], [33], [34] In addition, increasing age is directly correlated with liver cancer incidence in most populations,4 and population aging has already driven changes across the world, such as in Shanghai, China, where demographic changes, largely attributed to the aging population, accounted for 45% of the rise in liver cancer mortality between 1980 and 2019.35 Based on population projections, population aging will continue to drive the global burden of liver cancer.
As a baseline for control of liver cancer, we estimated the potential future number of cases and deaths resulting from several scenarios. If current rates remain the same, we predict the largest increases in liver cancer burden could occur in high HDI countries, including China, due to population growth and aging. The largest relative increases could occur in low HDI countries, where we predict that the number of liver cancer cases and deaths per year could double by 2040. Considering these changes, public health officials must prepare for the predicted increase in demand for resources to manage the care of patients with liver cancer throughout the cancer pathway, including improved access to palliative care. As our predictions are based on current rates and projected future populations, the impact of changes in risk factor exposure or national health programmes have not been taken into account, despite advances in HBV and HCV control. Recent successes include high immunisation coverage, testing, and treatment for HBV, and a reduction in new HCV infections in some regions, which has paralleled a rise in the number of people receiving curative treatment for HCV infections.36 While we would expect these promising achievements to result in a lower number of liver cancer cases in the future if current HBV and HCV control efforts are maintained, liver cancer incidence has increased over time in several areas with low HBV and HCV endemicity.3,37 This might be due to the growing obesity and diabetes epidemics37; thus, our baseline scenario of liver cancer predictions has possibly underestimated the future burden, if diabetes treatment and primary prevention of obesity are not addressed. Furthermore, focus on liver cancer prevention efforts must continue during and after the COVID-19 pandemic. Approximately 43% of countries that responded to the WHO Pulse survey reported disruption in HBV and HCV diagnosis and treatment during June 2020 to March 2021 due to the COVID-19 pandemic response.38 The impact of these disruptions could reverse some of the progress made in HBV and HCV control and might also be reflected in future liver cancer rates.
Our study provides a global snapshot of the estimated burden of liver cancer in 2020 and is an essential tool for planning of liver cancer control. The GLOBOCAN estimates presented here were compiled using national data from population-based cancer registries and vital registration systems wherever possible.9 While the estimation of rates is an extensive process using validated techniques, there are large gaps in data availability which could lead to a major underestimation of the burden of liver cancer in underrepresented populations. For example, only 15% of the world population and only 1% of the population in Africa were covered by the population-based cancer registries included in the latest volume of Cancer Incidence in Five Continents (vol. XI), a compilation of quality-assessed cancer registry data.39 The expansion of the African Cancer Registry Network has led to more accurate estimates of cancer burden in sub-Saharan Africa which were utilised in the GLOBOCAN methods, but data are still limited in many low- and middle-income countries.40 The Global Burden of Disease (GBD) Study has also produced estimates of liver cancer incidence and mortality up to 2019 using similar sources of cancer registry and vital registration data, but applying a different modelling method to obtain estimates in areas with less reliable or missing data.7 GBD estimated that, globally, 534,000 liver cancer cases and 485,000 liver cancer deaths occurred in 2019.7,41 These estimates were considerably lower than the 905,700 cases and 830,200 deaths in 2020 obtained from GLOBOCAN. At the national level, GBD estimates were much lower than GLOBOCAN for several of the countries which contributed the most cases and deaths to the global total; these included countries such as China which represented more than half of the difference between the GBD and GLOBOCAN estimates. For example, there were 187,700 liver cancer deaths in China according to GBD but 391,200 according to GLOBOCAN. Also, the crude rate of death from liver cancer in China according to GLOBOCAN was double that of GBD (27.0 vs.13.2 per 100,000). Two studies based on cancer registry data for China reported 422,1000 liver cancer deaths and a crude rate of 23.7 liver cancer deaths per 100,000 people in 2015.42,43 Large differences were also noted for Vietnam where GLOBOCAN estimated 25,300 liver cancer deaths in 2020 but GBD estimated 2,400 in 2019; the GLOBOCAN crude rate of death from liver cancer was also 10-times as high as the GBD estimate for Vietnam (26.0 vs. 2.5 per 100,000). Such discrepancies are the result of the differing modelling methods used by both studies to estimate cancer burden as well as potential differences in the data sources and the recency of the input data. As part of their modelling of all causes of death, the GBD also redistributed unspecified causes of death to produce additional deaths from cancer.7,41 Furthermore, the GBD methodology is based on global patterns of disease burden and uses covariates such as the prevalence of risk factors for liver cancer, e.g. HBsAg seroprevalence to impute missing cancer data, whereas the GLOBOCAN developers use a data-based approach and review available data for each country with respect to the local context and, if necessary, using information from neighbouring countries while ensuring that locally collected data form the basis of this process.9 We believe that producing cancer burden estimates based as closely as possible on the collected data is a priority, and that providing support and capacity building through such programs as the Global Initiative for Cancer Registry Development (https://gicr.iarc.fr/) is of utmost importance to ensure the sustainability and improved coverage of cancer registries, which will in turn produce more accurate measures of cancer burden.
The limitations of our liver cancer burden estimates include the reported change over time in methods of diagnosing liver cancer, with some areas of the world using imaging more commonly than biopsy, which might also be related to global variation in liver cancer diagnoses.14,44,45 In addition, the liver is a common site for metastasis so there is potential for some misclassification.46 Also, our 2040 predictions were not based on recent changes in liver cancer incidence and mortality rates or risk factor exposures and did not take into account heterogeneity in incidence and mortality trends between countries; thus, there is substantial uncertainty around our predictions. Finally, while our study estimated the total burden of liver cancer, distinct patterns are evident when examining liver cancer by histology.47 The major histologic types are hepatocellular carcinoma and intrahepatic cholangiocarcinoma and trends in the incidence of these histologic types differ: rates of hepatocellular carcinoma declined in high-risk countries, but increased in South-Central Asia, Europe, and North America between 1978 and 2012,37 with evidence of a decline in the US since 2015;48 rates of intrahepatic cholangiocarcinoma, however, increased in most countries between 1992 and 2012.49 It is estimated that hepatocellular carcinoma makes up 80% of liver cancer diagnoses globally; thus, addressing risk factors for hepatocellular carcinoma in regions with increasing rates would have the biggest impact on liver cancer burden.47
In summary, while the burden of liver cancer varies greatly, it is among the top three causes of cancer death in 46 countries, and among the top five causes of cancer death in 90 countries worldwide. Furthermore, the number of cases and deaths from liver cancer is predicted to increase by more than 50% over the next 20 years if global rates do not change, and will increase unless a 3% or greater annual decrease in rates is achieved. Liver cancer due to some major risk factors is preventable if control efforts are prioritised. While the impact of HBV and HCV elimination efforts is only beginning to be reflected in the burden of liver cancer today, increasing prevalence of other risk factors might drive future changes in liver cancer incidence. Considering these changes, public health officials must prepare for an increase in demand for resources to manage the care of patients with liver cancer throughout the cancer pathway.
Abbreviations
ASR, age-standardised rate; DAA, direct-acting antiviral; GBD, Global Burden of Disease; HDI, Human Development Index; M:F, male:female; UN, United Nations; WHO, World Health Organization.
Financial support
No direct funding was received.
Authors’ contributions
Study concept and design: HR, IS, JF. Analysis and interpretation of data: JV, ML, JF, HR. Drafting the manuscript: HR. Critical revision of the manuscript for important intellectual content: all authors.
Data availability statement
All cancer incidence, mortality, and population estimates are available to the public through the Global Cancer Observatory (www.gco.iarc.fr).
Conflict of interest
The authors declare no conflicts of interest.
Please refer to the accompanying ICMJE disclosure forms for further details.
Acknowledgements
We would like to thank all cancer registries and their staff who have contributed by sharing the data used to create global cancer estimates. The work reported by HR was undertaken during a PhD studentship at the International Agency for Research on Cancer.
Footnotes
Author names in bold designate shared co-first authorship
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhep.2022.08.021.
Disclaimer
Where authors are identified as personnel of the International Agency for Research on Cancer/World Health Organization, the authors alone are responsible for the views expressed in this article and they do not necessarily represent the decisions, policy or views of the International Agency for Research on Cancer/World Health Organization.
Supplementary data
The following are the supplementary data to this article:
References
- 1.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021 doi: 10.3322/caac.21660. [published Online First: 2021/02/05] [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J., Ervik M., Lam F., Colombet M., Mery L., Piñeros M., et al. International Agency for Research on Cancer; Lyon, France: 2021. Global cancer observatory: cancer today.https://gco.iarc.fr/today/home [Available from: ] [Google Scholar]
- 3.Arnold M., Abnet C.C., Neale R.E., Vignat J., Giovannucci E.L., McGlynn K.A., et al. Global burden of 5 major types of gastrointestinal cancer. Gastroenterology. 2020;159(1):335–349.e15. doi: 10.1053/j.gastro.2020.02.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McGlynn K.A., Petrick J.L., El-Serag H.B. Epidemiology of hepatocellular carcinoma. Hepatology. 2021;73:4–13. doi: 10.1002/hep.31288. [published Online First: 2020/11/24] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chuang S.-C., Vecchia C.L., Boffetta P. Liver cancer: descriptive epidemiology and risk factors other than HBV and HCV infection. Cancer Lett. 2009;286(1):9–14. doi: 10.1016/j.canlet.2008.10.040. [DOI] [PubMed] [Google Scholar]
- 6.Maucort-Boulch D., de Martel C., Franceschi S., Plummer M. Fraction and incidence of liver cancer attributable to hepatitis B and C viruses worldwide. Int J Cancer. 2018;142(12):2471–2477. doi: 10.1002/ijc.31280. [DOI] [PubMed] [Google Scholar]
- 7.Murray C.J.L., Aravkin A.Y., Zheng P., Abbafati C., Abbas K.M., Abbasi-Kangevari M., et al. Global burden of 87 risk factors in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396(10258):1223–1249. doi: 10.1016/S0140-6736(20)30752-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rumgay H., Shield K., Charvat H., Ferrari P., Sornpaisarn B., Obot I., et al. Global burden of cancer in 2020 attributable to alcohol consumption: a population-based study. Lancet Oncol. 2021;22(8):1071–1080. doi: 10.1016/S1470-2045(21)00279-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferlay J., Colombet M., Soerjomataram I., Parkin D.M., Piñeros M., Znaor A., et al. Cancer statistics for the year 2020: an overview. Int J Cancer. 2021 doi: 10.1002/ijc.33588. [published Online First: 2021/04/06] [DOI] [PubMed] [Google Scholar]
- 10.United Nations Statistics Division . 2021. Standard country or area codes for statistical use (M49) [Google Scholar]
- 11.Segi M., Fujisaku S. Department of Public Health, Tohoku University School of Medicine; 1960. Cancer mortality for selected sites in 24 countries (1950-1957) [Google Scholar]
- 12.Doll R., Payne P., Waterhouse J. Union for International Cancer Control; Berlin: 1966. Cancer incidence in five Continents: a technical report. [Google Scholar]
- 13.United Nations Development Programme . United Nations; New York: 2020. Human development report 2020. [Google Scholar]
- 14.Rutherford M.J., Arnold M., Bardot A., Ferlay J., De P., Tervonen H., et al. Comparison of liver cancer incidence and survival by subtypes across seven high-income countries. Int J Cancer. 2021;149(12):2020–2031. doi: 10.1002/ijc.33767. [DOI] [PubMed] [Google Scholar]
- 15.Allemani C., Matsuda T., Di Carlo V., Harewood R., Matz M., Niksic M., et al. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. 2018;391(10125):1023–1075. doi: 10.1016/S0140-6736(17)33326-3. [published Online First: 2018/02/06] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schweitzer A., Horn J., Mikolajczyk R.T., Krause G., Ott J.J. Estimations of worldwide prevalence of chronic hepatitis B virus infection: a systematic review of data published between 1965 and 2013. Lancet. 2015;386(10003):1546–1555. doi: 10.1016/S0140-6736(15)61412-X. [DOI] [PubMed] [Google Scholar]
- 17.World Health Organization (WHO) Global Health Observatory (GHO) 2021. Hepatitis B 3rd dose (HepB3) immunization coverage.https://www.who.int/data/gho/data/themes/immunization [Available from: [Google Scholar]
- 18.Nayagam S., Thursz M., Sicuri E., Conteh L., Wiktor S., Low-Beer D., et al. Requirements for global elimination of hepatitis B: a modelling study. Lancet Infect Dis. 2016;16(12):1399–1408. doi: 10.1016/S1473-3099(16)30204-3. [DOI] [PubMed] [Google Scholar]
- 19.Chiang C.-J., Yang Y.-W., You S.-L., Lai M.-S., Chen C.-J. Thirty-year outcomes of the national hepatitis B immunization program in Taiwan hepatitis B immunization program in Taiwan letters. JAMA. 2013;310(9):974–976. doi: 10.1001/jama.2013.276701. [DOI] [PubMed] [Google Scholar]
- 20.Yu S., Zi X., Zhu Q., Zheng Y., Wu C., Ren H., et al. Accelerating decreases in the incidences of hepatocellular carcinoma at a younger age in Shanghai are associated with hepatitis B virus vaccination. Front Oncol. 2022;12 doi: 10.3389/fonc.2022.855945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McMahon B.J., Bulkow L.R., Singleton R.J., Williams J., Snowball M., Homan C., et al. Elimination of hepatocellular carcinoma and acute hepatitis B in children 25 years after a hepatitis B newborn and catch-up immunization program. Hepatology. 2011;54(3):801–807. doi: 10.1002/hep.24442. [DOI] [PubMed] [Google Scholar]
- 22.Bailey J.R., Barnes E., Cox A.L. Approaches, progress, and challenges to hepatitis C vaccine development. Gastroenterology. 2019;156(2):418–430. doi: 10.1053/j.gastro.2018.08.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nahon P., Bourcier V., Layese R., Audureau E., Cagnot C., Marcellin P., et al. Eradication of hepatitis C virus infection in patients with cirrhosis reduces risk of liver and non-liver complications. Gastroenterology. 2017;152(1):142–156.e2. doi: 10.1053/j.gastro.2016.09.009. [DOI] [PubMed] [Google Scholar]
- 24.Cramp M.E., Rosenberg W.M., Ryder S.D., Blach S., Parkes J. Modelling the impact of improving screening and treatment of chronic hepatitis C virus infection on future hepatocellular carcinoma rates and liver-related mortality. BMC Gastroenterol. 2014;14(1):137. doi: 10.1186/1471-230X-14-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.World Health Organization (WHO) World Health Organization; 2016. Global health sector strategy on viral hepatitis 2016-2021. Towards ending viral hepatitis. [Google Scholar]
- 26.Tordrup D., Hutin Y., Stenberg K., Lauer J.A., Hutton D.W., Toy M., et al. Additional resource needs for viral hepatitis elimination through universal health coverage: projections in 67 low-income and middle-income countries, 2016–30. Lancet Glob Health. 2019;7(9):e1180–e1188. doi: 10.1016/S2214-109X(19)30272-4. [DOI] [PubMed] [Google Scholar]
- 27.Liu Y., Wu F. Global burden of aflatoxin-induced hepatocellular carcinoma: a risk assessment. Environ Health Perspect. 2010;118(6):818–824. doi: 10.1289/ehp.0901388. [published Online First: 2010/02/19] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wild C.P., Miller J.D., Groopman J.D. International Agency for Research on Cancer; 2015. Mycotoxin control in low-and middle-income countries. [PubMed] [Google Scholar]
- 29.World Health Organization . 2017. Tackling NCDs: best buys and other recommended interventions for the prevention and control of non-communicable diseases. Geneva, Switzerland. [Google Scholar]
- 30.Pham C., Fong T.-L., Zhang J., Liu L. Striking racial/ethnic disparities in liver cancer incidence rates and temporal trends in California, 1988–2012. JNCI: J Natl Cancer Inst. 2018;110(11):1259–1269. doi: 10.1093/jnci/djy051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pinheiro P.S., Callahan K.E., Jones P.D., Morris C., Ransdell J.M., Kwon D., et al. Liver cancer: A leading cause of cancer death in the United States and the role of the 1945–1965 birth cohort by ethnicity. JHEP Rep. 2019;1(3):162–169. doi: 10.1016/j.jhepr.2019.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yu X.Q., Feletto E., Smith M.A., Yuill S., Baade P.D. Cancer incidence in migrants in Australia: patterns of three infection-related cancers. Cancer Epidemiol Biomarkers Prev. 2022:OF1–OF8. doi: 10.1158/1055-9965.Epi-21-1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McDermott S., DesMeules M., Lewis R., Gold J., Payne J., Lafrance B., et al. Cancer incidence among Canadian immigrants, 1980–1998: results from a national cohort study. J Immigrant Minor Health. 2011;13(1):15–26. doi: 10.1007/s10903-010-9347-3. [DOI] [PubMed] [Google Scholar]
- 34.Arnold M., Razum O., Coebergh J.-W. Cancer risk diversity in non-western migrants to Europe: an overview of the literature. Eur J Cancer. 2010;46(14):2647–2659. doi: 10.1016/j.ejca.2010.07.050. [DOI] [PubMed] [Google Scholar]
- 35.Luo Z, Zou Y, Xie J, Cao H, Chen Y, Ding Y, et al. Influence of demographic factors on long-term trends of premature mortality and burden due to liver cancer: findings from a population-based study in Shanghai, China, 1973-2019. (2296-2565 (Electronic)). [DOI] [PMC free article] [PubMed]
- 36.World Health Organization (WHO) 2021. Global progress report on HIV, viral hepatitis and sexually transmitted infections. [Google Scholar]
- 37.Petrick J.L., Florio A.A., Znaor A., Ruggieri D., Laversanne M., Alvarez C.S., et al. International trends in hepatocellular carcinoma incidence, 1978–2012. Int J Cancer. 2020;147(2):317–330. doi: 10.1002/ijc.32723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.World Health Organization (WHO) 2021. Second round of the national pulse survey on continuity of essential health services during the COVID-19 pandemic. [Google Scholar]
- 39.Cancer incidence in five Continents, Vol. XI [Internet] International Agency for Research on Cancer; 2017. http://ci5.iarc.fr [cited 07/02/2022]. Available from: [Google Scholar]
- 40.The African Cancer Registry Network. http://www.afcrn.org/.
- 41.Global Burden of Disease 2019 Cancer Collaboration Cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life years for 29 cancer groups from 2010 to 2019: a systematic analysis for the global burden of disease study 2019. JAMA Oncol. 2022;8(3):420–444. doi: 10.1001/jamaoncol.2021.6987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen W., Zheng R., Baade P.D., Zhang S., Zeng H., Bray F., et al. Cancer statistics in China, 2015. CA: Cancer J Clin. 2016;66(2):115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 43.Wei W., Zeng H., Zheng R., Zhang S., An L., Chen R., et al. Cancer registration in China and its role in cancer prevention and control. Lancet Oncol. 2020;21(7):e342–e349. doi: 10.1016/S1470-2045(20)30073-5. [DOI] [PubMed] [Google Scholar]
- 44.European Association for the Study of the Liver EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69(1):182–236. doi: 10.1016/j.jhep.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 45.Marrero J.A., Kulik L.M., Sirlin C.B., Zhu A.X., Finn R.S., Abecassis M.M., et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68(2):723–750. doi: 10.1002/hep.29913. [DOI] [PubMed] [Google Scholar]
- 46.de Ridder J., de Wilt J.H.W., Simmer F., Overbeek L., Lemmens V., Nagtegaal I. Incidence and origin of histologically confirmed liver metastases: an explorative case-study of 23,154 patients. Oncotarget. 2016;7(34):55368–55376. doi: 10.18632/oncotarget.10552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rumgay H., Ferlay J., de Martel C., Georges D., Ibrahim A.S., Zheng R., et al. Global, regional and national burden of primary liver cancer by subtype. Eur J Cancer. 2022;161:108–118. doi: 10.1016/j.ejca.2021.11.023. [DOI] [PubMed] [Google Scholar]
- 48.Alvarez C.S., Petrick J.L., Parisi D., McMahon B.J., Graubard B.I., McGlynn K.A. Racial/ethnic disparities in hepatocellular carcinoma incidence and mortality rates in the United States, 1992-2018. Hepatology. 2022;76:589–598. doi: 10.1002/hep.32394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Florio A.A., Ferlay J., Znaor A., Ruggieri D., Alvarez C.S., Laversanne M., et al. Global trends in intrahepatic and extrahepatic cholangiocarcinoma incidence from 1993 to 2012. Cancer. 2020;126(11):2666–2678. doi: 10.1002/cncr.32803. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All cancer incidence, mortality, and population estimates are available to the public through the Global Cancer Observatory (www.gco.iarc.fr).