Abstract

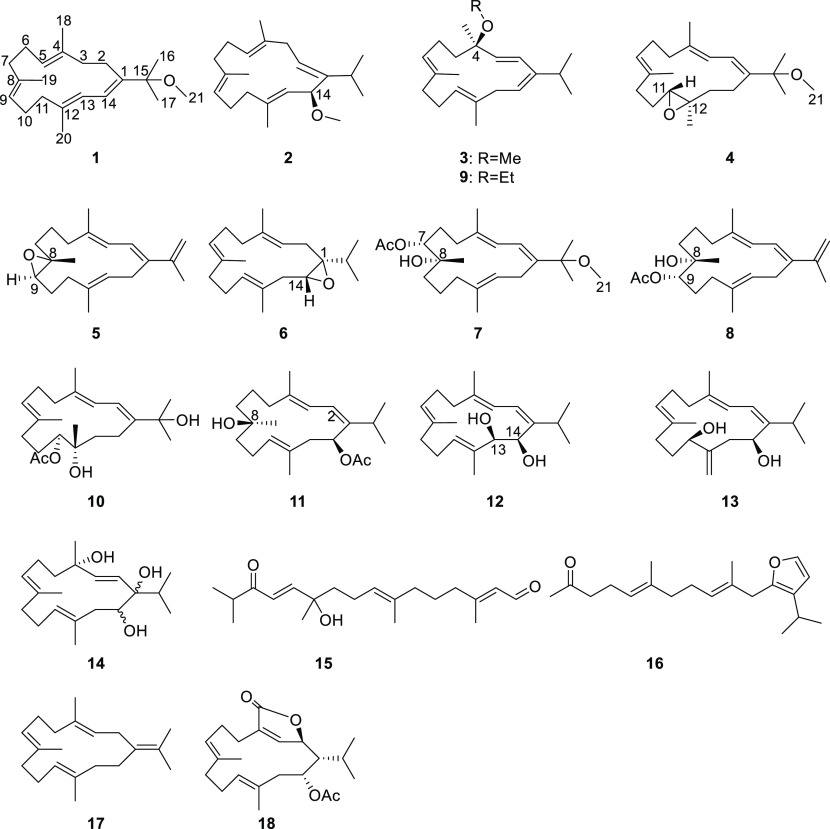
Eight new cembranoids (sarcophytembranoids A–H, 1–8) and 10 known terpenoids (9–18) were obtained from the soft coral Sarcophyton trocheliophorum of Ximao Island. Notably, 11, 15, and 16 were obtained from a natural source for the first time. The structures of the new isolates were elucidated by extensive spectroscopic analysis, optical rotatory dispersion, and X-ray diffraction experiments. Although the isolated compounds did not show significant activity against the tested tumor cell lines, compounds 3, 7, 8, and 10–15 exhibited anti-inflammatory activities at 10 μM, and compounds 17 and 18 showed moderate protein tyrosine phosphatase 1B inhibition activities with the minimum inhibitory concentrations of 22.19 and 11.26 μM, respectively.
Terpenoids are the most structurally diverse1 compounds to be isolated from all kingdoms of life. To date, over 80,000 terpenoids have been isolated2 with a variety of biological activities,3 for instance, antitumor, antibacterial, anti-inflammation, anti-dementia, antimalaria, and so forth. The 14-membered carbocyclic cembranoids are diterpenoids linking one isopropyl and three methyl groups, and the isopropyl group is often oxidized to carboxylic acids or hydroxymethyls. Various functions, including epoxide, ester, furan, lactone, and so forth, were frequently found among these compounds.4−6 Cembranoids are biologically synthesized from the C20 precursor geranylgeranyl diphosphate (GGPP).1,7 The biological activities of cembranoids have made them fascinating candidates for drug development and chemical synthesis.8 Over the past decades, nearly 1500 cembranoids have been discovered2 from various organisms, especially soft corals.9,10 Corals of the genus Sarcophyton are renowned for the production of a wide variety of bioactive terpenoids, especially cembranoids.11−20 More than 90 cembranoids have been isolated from Sarcophyton trocheliophorum in the past decades.13,14,16 In the course of our ongoing program toward the isolation of biologically active second metabolites from Chinese marine invertebrates, several geographically distinct soft coral S. trocheliophorum were chemically investigated, affording 33 new cembranoids from S. trocheliophorum collected off the Yalong Bay18,21−25 and Ximao Island.26 Among the isolates, sarcophytonolide N, sarcrassin E, 4Z,12Z,14E-sarcophytolide, sartrolide H, cembrene-C, and ketoemblide showed potential to moderate inhibitory activities against the human protein tyrosine phosphatase 1B (PTP1B) enzyme,18,22 which plays a major role in the dephosphorylation of the insulin receptor and the insulin receptor substrate (IRS-1) protein, representing a promising drug target for the treatment of type-II diabetes and obesity.27
Inspired by our and other research groups’ continuous isolation of bioactive and structurally diverse cembranoids from S. trocheliophorum, we chemically examined the soft coral S. trocheliophorum from Ximao Island. The current efforts let us identify 16 macrocyclic/linearized terpenoids, including 8 new cembranoids, sarcophytembranoids A–H (1–8), 8 known cembranoids 9–14 and 17–18, and 2 known linearized terpenoids 15 and 16 as shown in Chart 1. It is worth mentioning that compounds 11, 15, and 16 were isolated from a natural source for the first time. In addition, several isolates exhibited anti-inflammation or PTP1B inhibition activities. In this paper, the isolation, structure determination, and biological evaluation of the isolates are reported.
Chart 1. Chemical Structures of the Isolated Compounds.
Results and Discussion
The specimen of S. trocheliophorum was extracted using Me2CO in an ultrasonic bath. The ether-soluble portion of the Me2CO extract was subjected to silica, Sephadex LH-20, and reversed-phase high-performance liquid chromatography (RP-HPLC), affording 16 compounds 1–16, including 8 new cembranoids 1–8, 3 first-time isolated known compounds 11, 15, and 16, and 5 other compounds 9, 10, and 12–14. The 8 known compounds 9–16 were readily identified as (4R,1E,3Z,6E,10E)-14-ethoxy-3-isopropyl-6,10,14-trimethylcyclotetradeca-1,3,6,10-tetraene (9),28 sarcomililatol A (10),29 (1R,2Z,4E,7Z,9R,12E)-9-hydroxy-2-isopropyl-5,9,13-trimethylcyclotetradeca-2,4,7,12-tetraen-1-yl acetate (11),30 sarcophytol B (12),31 sarcophytol I (13),32 sarcophytol Q (14),28 (2E,7E,12E)-11-hydroxy-2,7,11,15-tetramethyl-14-oxohexadeca-2,7,12-trienal (15),30 (5E,9E)-11-(3-isopropylfuran-2-yl)-6,10-dimethylundeca-5,9-dien-2-one (16),30 (E,E,E)-1-isopropenyl-4,8,12-trimethylcyclotetradeca-3,7,11-triene (17),22 and sacrophytonolide I (18).33 We included the extensive structure elucidations of the new compounds below.
Sarcophytembranoid A (1) was isolated as a colorless crystal with a chemical formula of C21H34O as assigned by high-resolution electrospray ionization mass spectroscopy (HRESIMS) m/z: 325.2492 ([M + Na]+ calcd for C21H34ONa+, 325.2502). Its ultraviolet (UV) (λmax 286 and 204 nm) and infrared (IR) (νmax 1710 cm–1) spectra showed typical absorptions for a conjugated polyene pattern. The NMR spectra (Figures S2 and S3) of 1 revealed 21 carbon signals, including 5 methyls, 1 methoxy group, 6 sp3 methylenes, 4 sp2 methines, and 5 quaternary carbons. As only four trisubstituted double bonds could be found, as assigned by the following signals: δC 135.4 (qC), δH 4.99 (1H, t, J = 6.1 Hz)/δC 125.3 (CH); δC 134.2 (qC), δH 5.00 (1H, t, J = 5.3 Hz)/δC 126.1 (CH); δC 138.5 (qC), δH 5.92 (1H, d, J = 11.2 Hz)/δC 120.6 (CH); δC 144.0 (qC), δH 6.25 (1H, d, J = 11.2 Hz)/122.1 (CH), one monocyclic ring could be deduced, fitting the limit of five degrees of unsaturation. Further 2D NMR experiments helped establish the planar structure of 1 as a 14-membered cembrane. Briefly, the correlated spectroscopy (COSY) spectrum of 1 implicated four fragments: (i), H2-2 (δH 2.15, 2.22)/H2-3 (δH 2.10, 2.16); (ii), H-5 (δH 4.99)/H2-6 (δH 2.12, 2.25)/H2-7 (δH 2.09, 2.17); H-5 (δH 4.97)/H2-6 (δH 2.12, 2.25)/H2-7 (δH 2.09, 2.17); (iii), H-9 (δH 5.00)/H2-10 (δH 2.15, 2.22)/H2-11 (δH 2.09, 2.17); and (iv), H-13 (δH 5.92)/H-14 (δH 6.25). Further heteronuclear multiple bond correlation (HMBC) analysis established the connectivity of the four fragments, as evidenced by the following HMBC cross-peaks: from H3-18 to C-3/C-4/C-5, from H3-19 to C-7/C-8/C-9, from H3-20 to C-11/C-12/C-13, from H3-16 to C-1/C-15/C-17, from H3-17 to C-1/C-15/C-16, and from H-14 to C-2/C-15. The known compound sarcophytol V is a close analogue to sarcophytembranoid A, only different at the C-15 and C-16 positions. At C-15, our compound has a methoxy group (δC 50.4; δH 3.03, s) (C-15: δC 78.4, s); sarcophytol V has a hydroxy group (C-15: δC 76.4, s). At C-16, our compound has a methyl group (δC 26.3; δH 1.32, s); sarcophytol V has a −CH2OH group (δC 69.3; δH 3.43, d, 11.0 Hz/δH 3.63, d, 11.0 Hz).34
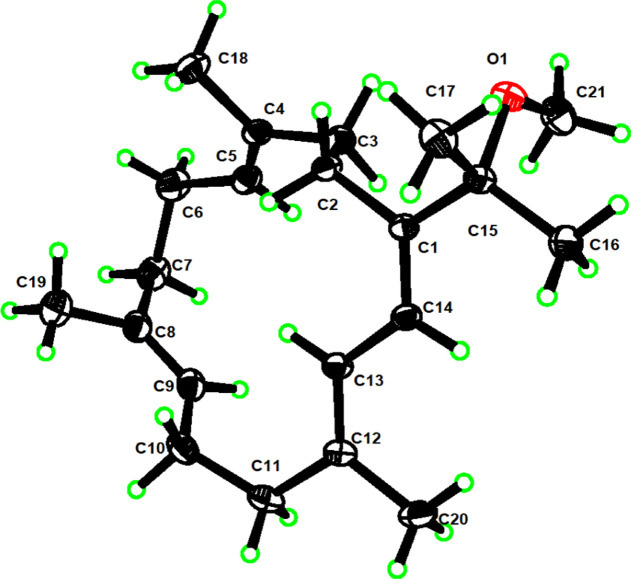
To determine the stereochemistry of the four double bonds, namely, Δ4, Δ8, Δ12, and Δ1(14), in 1, nuclear overhauser effect (NOE) analysis was implemented. The NOE correlations of H-6a/CH3-18, H-10a/CH3-19, H-11a/H-13, and H-14/H3-16 indicated that the four double bonds in 1 are all E-configured. The structure of 1 was also unambiguously confirmed by X-ray crystallography, as observed by Cu Kα radiation (Figure 1).
Figure 1.
Perspective ORTEP drawing of the X-ray structure of 1.
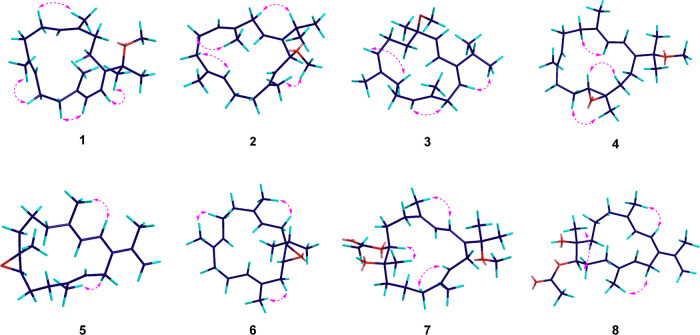
Sarcophytembranoid B (2) has a molecular formula of C21H34O based on HRESIMS (m/z: 325.2499 [M + Na]+, calcd for 325.2502). The signals of proton, carbon, and heteronuclear single quantum coherence spectra (Table 1) indicated the presence of four trisubstituted olefinic bonds: (i) [δH 5.52 (1H, dd, J = 9.1, 6.5 Hz)/δC 124.2 (CH), 146.6 (qC); (ii) δH 5.01 (1H, t, J = 5.8 Hz)/δC 122.8 (CH), δC 133.3 (qC); (iii) δH 4.94 (1H, d, J = 5.5 Hz)/δC 126.3 (CH), δC 134.1 (qC); and (iv) δH 5.26 (1H, d, J = 9.2 Hz)/δC 128.0(CH), δC 135.5 (qC)]. Extensive analysis of the 1H–1H COSY spectrum of compound 2 revealed four fragments: a–d (Figure 2) based on the evident correlations of H-2(δH 5.52)/H2-3(δH 2.56, 2.79) (a); H-5 (δH 5.01, t, J = 5.8 Hz)/H2-6 (δH 2.09, 2.09)/H-7 (δH 2.00, 2.19) (b); H-9 (δH 4.94, d, J = 5.5 Hz)/H2-10 (δH 2.18, 2.26)/H-11 (δH 2.00, 2.19) (c); and H-13 (δH 5.26, d, J = 9.2 Hz)/H-14 (δH 4.34, d, J = 9.2 Hz) (d). We then determined the planar macrocyclic structure by HMBC from H-2 to C-14/C-15, from H3-16 to C-1/C-15/C-17, from H3-17 to C-1/C-15/C-16, H3-18 to C-3/C-4/C-5, from H3-19 to C-7/C-8/C-9, from H3-20 to C-11/C-12/C-13, and from H3-21 to C-14. NOE data (Figure 4) revealed the geometries of four double bonds in 2, namely, Δ1, Δ4, Δ8, and Δ12, as E-configured, which is supported by the smaller chemical shifts of three methyl groups, CH3-18/19/20 (Table 2).
Table 1. 1H NMR Data (δH in ppm, J in Hz) for Compounds 1–8 in CDCl3.
| no. | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|---|
| 2a | 2.22, m | 5.52, dd (9.1, 6.5) | 5.94, d (16.6) | 6.15, d (10.1) | 6.28, d (11.0) | 2.78, m | 6.30, d (11.0) | 6.42, d (11.0) |
| 2b | 2.15, m | 1.83, m | ||||||
| 3a | 2.16, m | 2.79, dd (16.7, 9.1) | 5.72, d (16.6) | 5.83, d (10.1) | 5.98, d (11.0) | 5.16, t (7.8) | 6.16, d (11.0) | 6.43, d (11.0) |
| 3b | 2.10, m | 2.56, dd (16.7, 6.5) | ||||||
| 5a | 4.99, t (5.3) | 5.01, t (5.8) | 1.85, ddd (12.9, 9.7, 3.0) | 2.18, m | 2.23, m | 2.12, m | 2.20, m | 2.27, m |
| 5b | 1.66, ddd (12.9, 9.7, 3.0) | 2.11, m | 2.18, m | 2.07, m | 1.67, m | 2.27, m | ||
| 6a | 2.25, m | 2.09, m | 2.03, m | 2.27, m | 2.25, m | 2.20, m | 1.93, m | 2.95, m |
| 6b | 2.12, m | 2.09, m | 2.22, m | 2.17, m | 2.18, m | 2.20, m | 1.67, m | 2.25, m |
| 7a | 2.17, m | 2.19, m | 4.91, t (6.2) | 5.30, m | 2.06, m | 4.97, t (6.3) | 5.05, d (9.0) | 1.81, m |
| 7b | 2.09, m | 2.00, m | 1.51, m | 1.66, m | ||||
| 9a | 5.00, t (5.3) | 4.94, t (5.5) | 2.03, m | 2.28, m | 2.86, t (6.1) | 2.12, m | 2.20, m | 5.12, m |
| 9b | 2.03, m | 2.12, m | 2.07, m | 1.67, m | ||||
| 10a | 2.22, m | 2.26, m | 2.14, m | 2.00, m | 1.69, m | 2.20, m | 2.56, m | 1.97, m |
| 10b | 2.15, m | 2.18, m | 2.14, m | 1.46, m | 1.63, m | 2.15, m | 2.10, m | 1.53, m |
| 11a | 2.17, m | 2.19, m | 4.80, t (6.6) | 2.91, dd (9.2, 3.5) | 2.28, m | 5.22, t (6.9) | 2.19, m | 2.09, m |
| 11b | 2.09, m | 2.00, m | 2.15, m | 1.81, m | 1.81, m | |||
| 13a | 5.92, d (11.2) | 5.26, d (9.2) | 2.67, m | 2.10, m | 5.24, m | 2.15, m | 5.18, m | 5.17, m |
| 13b | 2.67, m | 1.35, m | 2.05, m | |||||
| 14a | 6.25, d (11.2) | 4.34, d (9.2) | 5.47, t (8.0) | 2.12, m | 2.39, m | 3.05, dd (9.3, 3.4) | 2.20, m | 2.27, m |
| 14b | 2.05, m | 2.22, m | 2.12, m | |||||
| 15 | 2.70, m | 2.56, m | 1.62, m | |||||
| 16a | 1.32, s | 0.94, d (7.0) | 1.08, d (6.8) | 1.31, s | 5.02, s | 1.09, d (6.9) | 1.35, s | 5.07, s |
| 16b | 4.96, s | 5.04, s | ||||||
| 17 | 1.32, s | 1.08, d (7.0) | 1.08, d (6.8) | 1.31, s | 1.92, s | 0.96, d (6.9) | 1.35, s | 1.94, s |
| 18 | 1.62, s | 1.63, s | 1.23, s | 1.75, s | 1.79, s | 1.58, s | 1.78, s | 1.81, s |
| 19 | 1.55, s | 1.55, s | 1.48, s | 1.67, s | 1.25, s | 1.59, s | 1.13, s | 1.06, s |
| 20 | 1.78, s | 1.66, s | 1.61, s | 1.27, s | 1.60, s | 1.66, s | 1.54, s | 1.48, s |
| 21 | 3.03, s | 3.24, s | 3.16, s | 3.04, s | 3.07, s | |||
| 22 | 2.11, s | |||||||
| 23 | 2.12, s |
Figure 2.
Key COSY (red) and HMBC (blue) correlations of 1–8.
Figure 4.
Key NOESY correlations of 1–8.
Table 2. 13C NMR Data (δC in ppm) for Compounds 1–8 in CDCl3.
| no. | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|---|
| 1 | 144.0, C | 146.6, C | 145.6, C | 143.0, C | 139.7, C | 67.2, C | 142.1, C | 142.6, C |
| 2 | 24.8, CH2 | 124.2, CH | 125.9, CH | 122.4, CH | 122.6, CH | 31.0, CH2 | 123.2, CH | 124.2, CH |
| 3 | 42.0, CH2 | 35.4, CH2 | 134.6, CH | 120.3, CH | 121.8, CH | 120.8, CH | 121.9, CH | 122.9, CH |
| 4 | 135.4, C | 133.3, C | 78.1, C | 138.9, C | 139.6, C | 135.7, C | 139.0, C | 141.3, C |
| 5 | 125.3, CH | 122.8, CH | 40.9, CH2 | 38.3, CH2 | 39.1, CH2 | 39.3, CH2 | 39.7, CH2 | 40.4, CH2 |
| 6 | 25.9, CH2 | 23.7, CH2 | 23.6, CH2 | 25.1, CH2 | 25.5, CH2 | 24.9, CH2 | 27.1, CH2 | 22.1, CH2 |
| 7 | 39.2, CH2 | 39.5, CH2 | 127.0, CH | 127.3, CH | 36.8, CH2 | 126.3, CH | 75.7, CH | 36.9, CH2 |
| 8 | 134.2, C | 134.1, C | 133.0, C | 133.6, C | 61.1, C | 133.6, C | 75.0, C | 75.2, C |
| 9 | 126.1, CH | 126.3, CH | 39.7, CH2 | 37.0, CH2 | 60.1, CH | 38.7, CH2 | 38.6, CH2 | 74.0, CH |
| 10 | 24.4, CH2 | 25.0, CH2 | 24.9, CH2 | 24.5, CH2 | 24.7, CH2 | 24.4, CH2 | 22.4, CH2 | 26.2, CH2 |
| 11 | 38.7, CH2 | 39.2, CH2 | 124.8, CH | 61.4, CH | 36.9, CH2 | 124.2, CH | 34.2, CH2 | 34.9, CH2 |
| 12 | 138.5, C | 135.5, C | 133.7, C | 61.4, CH | 133.8, C | 131.7, C | 134.6, C | 133.8, C |
| 13 | 120.6, CH | 128.0, CH | 37.1, CH | 38.9, CH2 | 126.9, CH | 36.3, CH2 | 126.0, CH | 126.4, CH |
| 14 | 122.1, CH | 77.6, CH | 121.3, CH | 23.1, CH2 | 22.1, CH2 | 62.5, CH | 25.7, CH2 | 26.3, CH2 |
| 15 | 78.4, C | 27.8, CH | 32.3, CH | 78.1, C | 143.5, C | 30.9, CH | 78.5, C | 139.4, C |
| 16 | 26.3, CH3 | 21.8, CH3 | 22.6, CH3 | 25.9, CH3 | 112.3, CH2 | 18.3, CH3 | 26.3, CH3 | 113.3, CH2 |
| 17 | 26.3, CH3 | 21.8, CH3 | 22.7, CH3 | 26.4, CH3 | 21.4, CH3 | 19.2, CH3 | 26.6, CH3 | 21.6, CH3 |
| 18 | 15.7, CH3 | 18.5, CH3 | 21.9, CH3 | 18.3, CH3 | 17.7, CH3 | 15.6, CH3 | 17.2, CH3 | 16.8, CH3 |
| 19 | 15.7, CH3 | 15.1, CH3 | 14.9, CH3 | 15.1, CH3 | 18.5, CH3 | 16.2, CH3 | 24.0, CH3 | 23.4, CH3 |
| 20 | 18.2, CH3 | 16.4, CH3 | 17.5, CH3 | 17.4, CH3 | 15.1, CH3 | 18.2, CH3 | 16.8, CH3 | 15.8, CH3 |
| 21 | 50.4, CH3 | 55.7, CH3 | 50.0, CH3 | 50.5, CH3 | 50.1, CH3 | 170.7, C | ||
| 22 | 170.9, C | 21.4, CH3 | ||||||
| 23 | 21.4, CH3 |
Optical rotatory dispersion (ORD) is a powerful method for determining absolute configuration by comparison of the experimental and calculated spectra.35,36 The specific rotation of 2 at the Na D-line (589 nm) is moderately large, [α]D20 +60.0 (c 0.20, MeOH), making the ORD comparison and the speculation of the absolute configuration more definitive. We calculated the specific rotations of 26 conformers of 2 (Figure S64) and averaged them by their relative abundance in the gas phase at 4 wavelengths (589, 546, 436, and 405 nm) at the 6-311+G(d) level. There is a satisfactory agreement of the overall patterns between the experimental and calculated data for 14S-2 (Figure 3), suggesting that the structure of compound 2 is as shown (Chart 1).
Figure 3.
Experimental ORD spectrum of 2 (red) and calculated ORD spectrum of 4S-2 (black) at different wavelengths.
Sarcophytembranoid C (3) possessed a molecular formula of C21H34O inferred from high-resolution electron ionization mass spectrometry (HREIMS) data m/z: 303.2620 ([M]+, calcd for 302.2604). 9 is an analogue of 3, with the only difference at the C-4 position. Through detailed comparison, we found that 9 contains an ethoxy group (OCH2–Me: δH 3.36, 2H, q, J = 7.0 Hz) in place of the methoxy group (OMe: δH 3.16, 3H, s) in 3. We further performed the electron capture dissociation (ECD) calculation to determine the stereochemistry of C-4 in 3 but did not achieve a satisfying result. As the NMR spectra of 9 and 3 are highly similar and considering that they should have the same biosynthetic precursors, the absolute configuration of 3 was thus tentatively assigned as the same as that of 9 (4R).
The HRESIMS ion peak at m/z: 341.2453 ([M + Na]+, calcd 341.2451) of sarcophytembranoid D (4) displayed a protonated molecule consistent with a molecular formula of C21H34O2. The NMR data of compound 4 highly matched those of 1 except for C-11 and C-12. Further comparison analysis revealed that the double bond Δ11,12 of 1 was oxidized to an epoxide to become 4. Then, the NOE experiment was conducted to elucidate the relative configuration of C-11 and C-12. The NOE correlations between H3-20/H-10 and H-11/H-13 and no prominent NOE cross-peaks between H3-20 (δH 1.27) and H-11(δH 2.91) indicated the trans-configuration between H3-20 and H-11. The relative configuration was then presumably identified as 11S*, 12R*.
The molecular formula of sarcophytembranoid E (5) was established as C20H30O by HRESIMS at m/z: 287.2365 ([M + H]+, calcd 287.2369). Detailed analysis of the 1H–1H COSY spectrum of compound 5 revealed the connectivity of four fragments: H-1 (δH 6.28)/H-2 (δH 5.97) (i); H2-5 (δH 2.18, 2.23)/H2-6 (δH 2.18, 2.25)/H2-7 (δH 1.51, 2.06) (ii); H-9 (δH 2.85)/H2-10 (δH 1.63, 1.69)/H2-11 (δH 2.15, 2.28) (iii); and H-13 (δH 5.24)/H2-14 (δH 2.22, 2.39) (iv). Then, the HMBC correlations from H3-18 to C-3/C-4/C-5, from H3-19 to C-7/C-8/C-9, from H3-20 to C-11/C-12/C-13, from H3-16 to C-1/C-15/C-17, from H3-17 to C-1/C-15/C-16, and from H-2 to C-14/C-15 built the planar structure. Furthermore, the clear NOE correlations of H-2 (δH 6.28)/H3-18 (δH 1.79), H3-20 (δH 1.60)/H-14b (δH 2.22), and H-3 (δH 5.98)/H2-14a (δH 2.39) suggested E-geometry for Δ1, Δ3, and Δ12. Considering that H3-19 (δH 1.25) and H-9 (δH 2.86) have no NOE correlation, the relative configuration of 5 was assigned as 8S*, 9R*. The absolute configuration of the epoxide was not determined due to the low yield of this compound.
Sarcophytembranoid F (6) was obtained as a colorless oil. Its molecular formula was determined according to the HRESIMS ion peak at m/z: 289.2527 ([M + H]+, calcd 289.2526). The NMR data of compound 6 was quite similar to that of 1 but with three differences: compound 6 contained an epoxide between C-1 and C-14, but 1 lacked an epoxide (i); 1 had a double bond between C-1 and C-2, but 6 did not (ii); there was a methoxy group at the C-15 position of 1, but not 6 (iii). The NOE interactions of H3-18 (δH 1.58)/H-2a (δH 2.78), H3-19 (δH 1.59)/H-6a (δH 2.20), and H3-20 (δH 1.66)/H-10b (δH 2.15) indicated that the double bonds Δ3, Δ7, and Δ11 are all E-configured. No NOE correlations were found between H-15 (δH 1.62) and H-14 (δH 3.05). Thus, the relative configuration was roughly determined as 1S*, 14R*.
Sarcophytembranoid G (7) was isolated as a colorless oil with a molecular formula of C23H38O4 deduced by the HRESIMS ion peak at m/z: 401.2660 ([M + Na]+, calcd 401.2662). The NMR data of 7 were similar to those of 4, except for C-7, C-8, and C-11 to C-13. Further analysis revealed that the original double bond Δ7 in the cembrane core of 4 is replaced in 7 with an acetoxy group at C-7 and a hydroxyl group at C-8. Additionally, the epoxide between C-11 and C-12 in compound 4 is replaced in 7 with a Δ12,13 double bond and full saturation at C-11. NOE correlations of H-2 (δH 6.30)/H3-16 (δH 1.35), H-2 (δH 6.30)/H3-18 (δH1.78), and H-11a (δH 2.19)/H-13 (δH 5.18) support E-geometry of Δ1, Δ3, and Δ12. Furthermore, C-11/12 in compound 10 shares the same chemical environment as C-7/8 in compound 7, which bears vicinal acetoxy and hydroxyl groups. By analyzing the chemical shifts, coupling constants, and peak patterns between two compounds, we reasoned that the relative configurations of C-7 and C-8 in 7 are probably all R*, as are C-11 and C-12 in 10.
Sarcophytembranoid H (8) has a molecular formula of C22H34O3 based on HREIMS data m/z: 346.2490 ([M]+, calcd for 346.2502). The NMR data of 8 partially matched those of 5, except for an additional acetyl signal in 8. Further analysis indicated that the chemical environment of C-8 and C-9 is the same as that of the hydroxy- and acetoxy-bearing carbons in compounds 7 and 10. Therefore, we did a similar chemical analysis for compound 8 as for compound 7 to determine the stereochemistry and concluded that the absolute configuration of 8 was most likely 1S, 2S. Furthermore, the NOE interactions of H-2 (δH 6.42)/H2-16 (δH 5.05), H-2 (δH 6.42)/H3-17 (δH 1.94), H-2 (δH 6.42)/H3-18(δH 1.81), and H-14a (δH 2.27)/H3-20 (δH 1.48) indicated the E-geometry of the double bonds Δ1, Δ3, and Δ12 in compound 8.
It also should be noted that although Masaru Kobayashi et al.30 had chemically synthesized compounds 11, 15, and 16, we isolated them from a natural source for the first time, highlighting the unlimited synthetic abilities among living organisms.
As the isolated compounds share a typical cembranoid skeleton, a plausible biosynthetic pathway was proposed (Figure S67). In brief, the cyclization of GGPP formed three different cembranoid scaffolds resulting from three deprotonations, forming double bonds: Δ1, Δ1(14), and Δ1(15). The isolated natural products would then be readily generated following a series of simple enzymatic reactions, for example, oxidation, acetylation, and methylation.
All the isolated compounds were screened for biological activities, including cytotoxicity, anti-inflammation, and PTP1B inhibition. Although no evident cytotoxic activity was found, compounds 3, 7, 8, and 10–15 displayed anti-inflammatory activities in 10 μM tests. Notably, compounds 7, 8, and 10 all exhibit anti-inflammatory activities and contain adjacent acetoxy and hydroxyl groups, suggesting that an epoxide ring-opening step could be essential to anti-inflammatory activity. In addition, it is worth mentioning that these compounds exhibited no evident cytotoxicity against BV-2 cells, suggesting that they are potential human-friendly lead compounds for anti-inflammation. Furthermore, compounds 17 and 18 showed moderate PTP1B inhibition activities with minimum inhibitory concentrations of 22.19 and 11.26 μM, respectively. However, cembranoids barely exhibit PTP1B inhibition activities. Despite rare exampled cembranoids being PTP1B inhibitors, the finding would provide new types of candidate leads in drug development for treating type II diabetes and obesity.
Previously reported cembranoids displaying PTP1B inhibition activities,22,37 for instance, sarcophytonolide N,22 sarcrassin E,22 ketoemblide,22 and jatrophainolides A–C,37 all have the methyl ester group or formyl group at C-18 (Figure S67), indicating that the oxidation of the methyl group at C-4 would probably significantly increase the PTP1B inhibitory activity. In addition, compounds 17 and cembrene-C22 showed moderate PTP1B inhibition activities, while their oxidized derivatives 1–14 did not, suggesting that oxidation at other positions may decrease the inhibitory activity.
Conclusions
In conclusion, 8 new cembranoids, sarcophytembranoid A–H (1–8), and 10 known compounds 9–18, including 3 previously chemically synthesized but not isolated from a natural source 11, 15, and 16, were isolated from the soft coral S. trocheliophorum collected from Ximao Island, China. In bioassays, compounds 3, 7, 8, and 10–15 displayed significant anti-inflammatory activities, and compounds 17 and 18 exhibited moderate PTP1B inhibition activities.
However, the biosynthesis of the diverse and bioactive cembranoids remains elusive. Recent reports on the identifications of terpene synthases, including cembrane synthase, from marine invertebrates,38−41 have proved that marine animals contain functional genes responsible for the biosynthesis of bioactive terpenoids, which are likely used as a “chemical weapon” against predators.5,42 These previous reports have opened the door to elucidate the biosynthesis of bioactive, soft coral-derived cembranoids. Because these compounds are often found in extremely low yield, understanding their biosynthetic pathways could allow for their overproduction in genetically engineered systems to assist downstream drug development of cembranoids and to assist efforts in chemical ecology to protect coral ecosystems.
Experimental Section
General Experimental Procedures
1D and 2D NMR spectra were acquired in CDCl3 with a Bruker AVANCE III 400, Bruker AVANCE III 500, or Bruker AVANCE III 600 spectrometer (Bruker Biospin AG, Fällanden, Germany) with residual CDCl3 (δH 7.26 ppm, δC 77.16 ppm) as the internal standard. HRESIMS spectra were measured using an Agilent G6520 Q-TOF mass spectrometer. A Nicolet 6700 spectrometer (Thermo Scientific, Waltham, MA, USA) was used to obtain the IR spectra. Circular dichroism spectra were measured using a JASCO 810 spectrometer. UV spectra were acquired using a Varian Cary 50 Bio spectrophotometer. Optical rotations were obtained on a PerkinElmer 241MC polarimeter. Column chromatography (CC) was performed using commercial silica gel (Sinopharm Chemical Reagent Co., Ltd., 200–300 and 300–400 mesh) and Sephadex LH-20 gel (GE Healthcare). Thin-layer chromatography was performed on precoated silica gel plates [Merck Chemicals (Shanghai) Co., Ltd., G60 F-254]. RP-HPLC was performed on an Agilent 1260 series liquid chromatography system (Agilent, Santa Clara, CA, USA) equipped with a DAD G1315D detector at 210 nm and an Agilent semi-preparative XDB-C18 column (5 μm, 250 × 9.4 mm). All solvents used for CC were of analytical grade (Shanghai Chemical Reagent Co., Ltd.) and for HPLC were of chromatographic grade (Dikma Technologies Inc).
Biological Material
The title animal, S. trocheliophorum, was collected from the coast of Ximao Island (stored in a −20 °C freezer until extraction), Hainan Province, China, and identified by Prof. Xiu-Bao Li (Hainan University). A voucher specimen (no. 18XD-19) is available for inspection at the Shanghai Institute of Materia Medica, CAS.
Extraction and Isolation
The soft coral (800 g, dry weight) of S. trocheliophorum was cut into pieces and extracted exhaustively with Me2CO at room temperature in an ultrasonic bath (4 × 5.0 L). The organic extract was evaporated to give a brown residue and then partitioned between Et2O and H2O. The upper layer was concentrated under reduced pressure to give a Et2O portion (21.0 g). The resulting residue was separated into five fractions (A–E) by gradient silica-gel CC (200–300 mesh, 0 → 50% Et2O) in petroleum ether (PE). Fraction B was then fractionated into subfractions (B1–B3) using a Sephadex LH-20 column (PE/CH2Cl2/MeOH = 2:1:1). Subfraction B1 was further purified by HPLC (100% CH3CN, 2.5 mL/min), yielding compounds 5 (3.0 mg), 6 (5.0 mg), 7 (2.0 mg), and 17 (3.0 mg). Fraction C was further isolated as compound 1 (2.0 mg), 2 (1.5 mg), 3 (3.0 mg), 8 (2.0 mg), 9 (1.0 mg), and 16 (0.7 mg) by Sephadex LH-20 CC (PE/CH2Cl2/MeOH, 2:1:1) and RP-HPLC (100% CH3CN, 2.5 mL/min). Fraction E was fractionated into subfractions E1–E3 using a Sephadex LH-20 column eluted with PE/CH2Cl2/MeOH (2:1:1). Subfraction E1 was further purified by HPLC (80% CH3CN, 2.5 mL/min) to give compound 4 (2.0 mg) and 11 (2.2 mg). Subfraction E2 was further purified by HPLC (80% CH3CN, 2.5 mL/min) to give compound 15 (1.5 mg). Subfraction E3 was further purified by HPLC (75% CH3CN, 2.5 mL/min) to give compound 12 (5.1 mg), 13 (2.1 mg), 14 (2.0 mg), and 18 (5.4 mg).
Sarcophytembranoid A (1): colorless crystals, mp 120.2–120.5 °C; IR (KBr) νmax: 3426, 2934, 1710, 1453, 1376, 1072 cm–1; UV (MeOH): λmax (log ε) 248.5 (4.35) nm; ECD (CH3CN): λmax (Δε) 395.0 (+0.01), 246.5 (−0.33) nm; 1H and 13C NMR data see Tables 1 and 2; HRESIMS m/z: 325.2492 [M + Na]+ (calcd for C21H34NaO, 325.2502).
Sarcophytembranoid B (2): colorless solid, [α]D20 +50.0 (c 0.15, MeOH); IR (KBr) νmax: 2959, 2923, 1443, 1383, 1260, 1087, 1031, 865, 803 cm–1; UV (MeOH): λmax (log ε) 217.0 (4.65) nm;1H and 13C NMR data see Tables 1 and 2; HRESIMS m/z: 325.2499 [M + H]+ (calcd for C21H34NaO, 325.2502).
Sarcophytembranoid C (3): colorless solid; [α]D20 +6.1 (c 0.30, MeOH); IR (KBr) νmax: 2959, 2928, 1434, 1381, 1155, 1080, 971, 860 cm–1; UV (MeOH): λmax (log ε) 232.0 (4.24) nm; ECD (CH3CN): λmax (Δε) 238 (+0.02), 200(−0.20) nm; 1H and 13C NMR data, see Tables 1 and 2; HREIMS m/z: 302.2620 [M]+ (calcd for C21H34O, 302.2604).
Sarcophytembranoid D (4): colorless solid; [α]D20 −12.4 (c 0.20, MeOH); IR (KBr) νmax: 2958, 2926, 1448, 1379, 1247, 1156, 1073, 826 cm–1; UV (MeOH): λmax (log ε) 248.5 (4.13) nm; ECD (CH3CN): λmax (Δε) 275.5 (−0.01), 246 (−0.20) nm; 1H and 13C NMR data, see Tables 1 and 2; HRESIMS m/z: 341.2453 [M + Na]+ (calcd for C21H34NaO2, 341.2451).
Sarcophytembranoid E (5): colorless solid; [α]D20 +3.3 (c 0.30, MeOH); IR (KBr) νmax: 2960, 2930, 2871, 1711, 1454, 1383, 1250, 1119, 980 cm–1; UV (MeOH): λmax (log ε) 216.0 (4.60) nm; ECD (CH3CN): λmax (Δε) 222.5 (−0.05), 210.5 (−2.79) nm; 1H and 13C NMR data, see Tables 1 and 2; HRESIMS m/z: 287.2365 [M + H]+ (calcd for C20H31O, 287.2369).
Sarcophytembranoid F (6): colorless solid; [α]D20 +6.0 (c 0.50, MeOH); IR (KBr) νmax: 2963, 2919, 1435, 1384, 1091, 1033 cm–1; UV (MeOH): λmax (log ε) 214.0 (4.83) nm; 1H and 13C NMR data, see Tables 1 and 2; HRESIMS m/z: 289.2527 [M + H]+ (calcd for C20H33O, 289.2526).
Sarcophytembranoid G (7): colorless solid; [α]D20 +7.5 (c 0.20, MeOH); IR (KBr) νmax: 3451, 2976, 2930, 2856, 1732, 1448, 1373, 1242, 1143, 1072, 1023 cm–1; UV (MeOH): λmax (log ε) 249.0 (4.37) nm; ECD (CH3CN): λmax (Δε) 224.5 (−0.09), 215 (+3.50) nm; 1H and 13C NMR data, see Tables 1 and 2; HRESIMS m/z: 401.2660 [M + Na]+ (calcd for C23H38NaO4, 401.2662).
Sarcophytembranoid H (8): colorless solid; [α]D20 −5.0 (c 0.20, MeOH); IR (KBr) νmax: 3440, 2973, 2932, 2870, 1732, 1668, 1448, 1374, 1241, 1029, 802 cm–1; UV (MeOH): λmax (log ε) 249.0 (4.35) nm; ECD (CH3CN): λmax (Δε) 233.0 (+0.02), 210 (−0.91) nm; 1H and 13C NMR data, see Tables 1 and 2; HR-EIMS m/z: 346.2490 [M]+ (calcd for C22H34O3, 346.2502).
Acknowledgments
This work was supported by the National Natural Science Foundation of China (NSFC) (no. 81991521), the SKLDR/SIMM Project (no. SIMM1903ZZ-06), and Zhejiang Province “Ten Thousand Talents Program” Science and Technology Innovation Leading Talent Project (2019R52009). B.X. is supported by the start-up fund from the Shanghai Institute of Materia Medica of the Chinese Academy of Sciences.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.2c05687.
Author Contributions
The manuscript was written with the contributions of all authors. All authors have given approval for the final version of the manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- Christianson D. W. Structural and Chemical Biology of Terpenoid Cyclases. Chem. Rev. 2017, 117, 11570–11648. 10.1021/acs.chemrev.7b00287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dictionary of Natural Products. http://dnp.chemnetbase.com, accessed July–September 2022.
- Jaeger R.; Cuny E. Terpenoids with Special Pharmacological Significance: A Review. Nat. Prod. Commun. 2016, 11, 1373–1390. 10.1177/1934578x1601100946. [DOI] [PubMed] [Google Scholar]
- Liang L. F.; Guo Y. W. Terpenes from the Soft Corals of the Genus Sarcophyton: Chemistry and Biological Activities. Chem. Biodiversity 2013, 10, 2161–2196. 10.1002/cbdv.201200122. [DOI] [PubMed] [Google Scholar]
- Li G.; Dickschat J. S.; Guo Y. W. Diving into the world of marine 2,11-cyclized cembranoids: a summary of new compounds and their biological activities. Nat. Prod. Rep. 2020, 37, 1367–1383. 10.1039/d0np00016g. [DOI] [PubMed] [Google Scholar]
- Liang L. F.; Lan L. F.; Taglialatela-Scafati O.; Guo Y. W. Sartrolides A–G and bissartrolide, new cembranolides from the South China Sea soft coral Sarcophyton trocheliophorum Marenzeller. Tetrahedron 2013, 69, 7381–7386. 10.1016/j.tet.2013.06.068. [DOI] [Google Scholar]
- Rudolf J. D.; Alsup T. A.; Xu B. F.; Li Z. N. Bacterial terpenome. Nat. Prod. Rep. 2021, 38, 905–980. 10.1039/d0np00066c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tius M. A. Synthesis of cembranes and cembranolides. Chem. Rev. 1988, 88, 719–732. 10.1021/cr00087a001. [DOI] [Google Scholar]
- Yang B.; Zhou X. F.; Lin X. P.; Liu J.; Peng Y.; Yang X. W.; Liu Y. H. Cembrane Diterpenes Chemistry and Biological Properties. Curr. Org. Chem. 2012, 16, 1512–1539. 10.2174/138527212800672583. [DOI] [Google Scholar]
- Nurrachma M. Y.; Sakaraga D.; Nugraha A. Y.; Rahmawati S. I.; Bayu A.; Sukmarini L.; Atikana A.; Prasetyoputri A.; Izzati F.; Warsito M. F.; Putra M. Y. Cembranoids of Soft Corals: Recent Updates and Their Biological Activities. Nat. Prod. Bioprospect. 2021, 11, 243–306. 10.1007/s13659-021-00303-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues I. G.; Miguel M. G.; Mnif W. A Brief Review on New Naturally Occurring Cembranoid Diterpene Derivatives from the Soft Corals of the Genera Sarcophyton, Sinularia, and Lobophytum Since 2016. Molecules 2019, 24, 781. 10.3390/molecules24040781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duh C. Y.; Hou R. S. Cytotoxic Cembranoids from the Soft Corals Sinularia gibberosa and Sarcophyton trocheliophorum. J. Nat. Prod. 1996, 59, 595–598. 10.1021/np960174n. [DOI] [Google Scholar]
- Shaaban M.; Ghani M. A.; Shaaban K. A. Unusual pyranosyl cembranoid diterpene from Sarcophyton trocheliophorum. Z. Naturforsch. B. 2016, 71, 1211–1217. 10.1515/znb-2016-0144. [DOI] [Google Scholar]
- Shaaban K. A.; Ghani M. A.; Shaaban M. New Cembranoid Diterpenes from Sarcophyton trocheliophorum. Br. J. Pharm. Res. 2014, 5, 192–201. 10.9734/bjpr/2015/14757. [DOI] [Google Scholar]
- Al-Footy K. O.; Alarif W. M.; Asiri F.; Aly M. M.; Ayyad S.-E. N. Rare pyrane-based cembranoids from the Red Sea soft coral Sarcophyton trocheliophorum as potential antimicrobial–antitumor agents. Med. Chem. Res. 2015, 24, 505–512. 10.1007/s00044-014-1147-1. [DOI] [Google Scholar]
- Hegazy M.-E. F.; Mohamed T. A.; Abdel-Latif F. F.; Alsaid M. S.; Shahat A. A.; Paré P. W. Trochelioid, A and B new cembranoid diterpenes from the Red Sea soft coral Sarcophyton trocheliophorum. Phytochem. Lett. 2013, 6, 383–386. 10.1016/j.phytol.2013.05.005. [DOI] [Google Scholar]
- Liu Z.; Cheng W.; Liu D.; van Ofwegen L.; Proksch P.; Lin W. H. Capnosane-type cembranoids from the soft coral Sarcophyton trocheliophorum with antibacterial effects. Tetrahedron 2014, 70, 8703–8713. 10.1016/j.tet.2014.09.034. [DOI] [Google Scholar]
- Liang L. F.; Kurtán T.; Mándi A.; Yao L. G.; Li J.; Lan L. F.; Guo Y. W. Structural, stereochemical, and bioactive studies of cembranoids from Chinese soft coral Sarcophyton trocheliophorum. Tetrahedron 2018, 74, 1933–1941. 10.1016/j.tet.2018.02.059. [DOI] [Google Scholar]
- Liang L. F.; Kurtán T.; Mándi A.; Yao L. G.; Li J.; Zhang W.; Guo Y. W. Unprecedented Diterpenoids as a PTP1B Inhibitor from the Hainan Soft Coral Sarcophyton trocheliophorum Marenzeller. Org. Lett. 2013, 15, 274–277. 10.1021/ol303110d. [DOI] [PubMed] [Google Scholar]
- Liang L. F.; Kurtán T.; Mándi A.; Gao L. X.; Li J.; Zhang W.; Guo Y. W. Sarsolenane and Capnosane Diterpenes from the Hainan Soft Coral Sarcophyton trocheliophorum Marenzeller as PTP1B Inhibitors. Eur. J. Org. Chem. 2014, 2014, 1841–1847. 10.1002/ejoc.201301683. [DOI] [Google Scholar]
- Yao L. G.; Zhang H. Y.; Liang L. F.; Guo X. J.; Mao S. C.; Guo Y. W. Yalongenes, A and B Two New Cembranoids with Cytoprotective Effects from the Hainan Soft Coral Sarcophyton trocheliophorum Marenzeller. Helv. Chim. Acta 2012, 95, 235–239. 10.1002/hlca.201100278. [DOI] [Google Scholar]
- Liang L. F.; Gao L. X.; Li J.; Taglialatela-Scafati T. S.; Guo Y. W. Cembrane diterpenoids from the soft coral Sarcophyton trocheliophorum Marenzeller as a new class of PTP1B inhibitors. Bioorg. Med. Chem. 2013, 21, 5076–5080. 10.1016/j.bmc.2013.06.043. [DOI] [PubMed] [Google Scholar]
- Chen W. T.; Liang L. F.; Li X. W.; Xiao W.; Guo Y. W. Further New Highly Oxidative Cembranoids from the Hainan Soft Coral Sarcophyton trocheliophorum. Nat. Prod. Bioprospect. 2016, 6, 97–102. 10.1007/s13659-016-0088-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang L. F.; Chen W. T.; Li X. W.; Wang H. Y.; Guo Y. W. New Bicyclic Cembranoids from the South China Sea Soft Coral Sarcophyton trocheliophorum. Sci. Rep. 2017, 7, 46584. 10.1038/srep46584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang L. F.; Chen W. T.; Mollo E.; Yao L. G.; Wang H. Y.; Xiao W.; Guo Y. W. Sarcophytrols, G. −L. Novel Minor Metabolic Components from South China Sea Soft Coral Sarcophyton trocheliophorum Marenzeller. Chem. Biodiversity 2017, 14, e1700079 10.1002/cbdv.201700079. [DOI] [PubMed] [Google Scholar]
- Chen Z. H.; Gao T. R.; Yang M.; Yao L. G.; Guo Y. W. Further new cembranoids from the South China Sea soft coral Sarcophyton trocheliophorum. Fitoterapia 2021, 151, 104902. 10.1016/j.fitote.2021.104902. [DOI] [PubMed] [Google Scholar]
- Goldstein B. J. Protein-Tyrosine phosphatases and the regulation of insulin action. J. Cell. Biochem. 1992, 48, 33–42. 10.1002/jcb.240480107. [DOI] [PubMed] [Google Scholar]
- Kobayashi M.; Iesaka T.; Nakano E. Marine Terpenes and Terpeoids. IX. : Structures of Six New Cembranoids, Sarcophytols F, K, P, Q, R and S, from the Soft Coral Sarcophyton glaucum. Chem. Pharm. Bull. 1989, 37, 2053–2057. 10.1248/cpb.37.2053. [DOI] [Google Scholar]
- Phan C. S.; Ng S. Y.; Kamada T.; Vairappan C. S. Two New Lobane Diterpenes from a Bornean Soft Coral Sinularia sp. Nat. Prod. Commun. 2016, 11, 899–900. 10.1177/1934578x1601100708. [DOI] [PubMed] [Google Scholar]
- Kobayashi M.; Kondo K.; Osabe K.; Mitsuhashi H. Marine Terpenes and Terpenoids. V. : Oxidation of Sarcophytol A, a Potent Anti-tumor-Promoter from the Soft Coral Sarcophyton glaucum. Chem. Pharm. Bull. 1988, 36, 2331–2341. 10.1248/cpb.36.2331. [DOI] [PubMed] [Google Scholar]
- Kobayashi M.; Nakagawa T.; Mitsuhashi H. Marine Terpenes and Terpenoids. I. Structures of Four Cembrane-type Diterpenes : SarCophytol-A, Sarcophytol-A Acetate Sarcophytol-B, and Sarcophytonin-A, from the Soft Coral. Sarcophyton glaucum. Chem. Pharm. Bull 1979, 27, 2382–2387. 10.1248/cpb.27.2382. [DOI] [Google Scholar]
- Kobayashi M.; Osabe K. Marine Terpenes and Terpenoids.VII. : Minor Cembranoid Derivatives, Structurally Related to the Potent Anti-tumor-Promoter Sarcophytol A, from the Soft Coral Sarcophyton glaucum. Chem. Pharm. Bull. 1989, 37, 631–636. 10.1248/cpb.37.631. [DOI] [PubMed] [Google Scholar]
- Yan X. H.; Li Z. Y.; Guo Y. W. Further new cembranoid diterpenes from the Hainan soft coral Sarcophyton latum. Helv. Chim. Acta 2007, 90, 1574–1580. 10.1002/hlca.200790164. [DOI] [Google Scholar]
- Cuong N. X.; Tuan T. A.; Kiem P. V.; Minh C. V.; Choi E. M.; Kim Y. H. New Cembranoid Diterpenes from the Vietnamese Soft Coral Sarcophyton mililatensis Stimulate Osteoblastic Differentiation in MC3T3-E1 Cells. Chem. Pharm. Bull. 2008, 56, 988–992. 10.1248/cpb.56.988. [DOI] [PubMed] [Google Scholar]
- Saito F.; Gerbig D.; Becker J.; Schreiner P. R. Absolute Configuration of trans-Perhydroazulene. Org. Lett. 2020, 22, 3895–3899. 10.1021/acs.orglett.0c01184. [DOI] [PubMed] [Google Scholar]
- Giorgio E.; Viglione R. G.; Zanasi R.; Rosini C. Ab Initio Calculation of Optical Rotatory Dispersion (ORD) Curves: A Simple and Reliable Approach to the Assignment of the Molecular Absolute Configuration. J. Am. Chem. Soc. 2004, 126, 12968–12976. 10.1021/ja046875l. [DOI] [PubMed] [Google Scholar]
- Zhang D. B.; Wang Z.; Liang Y. N.; Yu J. G.; Zhang D. Z.; Liu A. −C.; Zhang Z.; Song Z.-X.; Tang Z.-S.; Duan D.-Z. Jatrophainolides A-C, new cembrane-type diterpenoids with PTP1B inhibitory activity from the root bark of Jatropha integerrima. Phytochem. Lett. 2020, 36, 166–170. 10.1016/j.phytol.2020.02.007. [DOI] [Google Scholar]
- Burkhardt I.; de Rond T. D.; Chen P. Y.-T.; Moore B. S. Ancient plant-like terpene biosynthesis in corals. Nat. Chem. Biol. 2022, 18, 664–669. 10.1038/s41589-022-01026-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scesa P. D.; Lin Z.; Schmidt E. W. Ancient defensive terpene biosynthetic gene clusters in the soft corals. Nat. Chem. Biol. 2022, 18, 659–663. 10.1038/s41589-022-01027-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thimmappa R.; Wang S.; Zheng M.; Misra R. C.; Huang A. C.; Saalbach G.; Chang Y.; Zhou Z.; Hinman V.; Bao Z.; Osbourn A. Biosynthesis of saponin defensive compounds in sea cucumbers. Nat. Chem. Biol. 2022, 18, 774–781. 10.1038/s41589-022-01054-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F.; Lin Z.; Torres J. P.; Hill E. A.; Li D.; Townsend C. A.; Schmidt E. W. Sea Urchin Polyketide Synthase SpPks1 Produces the Naphthalene Precursor to Echinoderm Pigments. J. Am. Chem. Soc. 2022, 144, 9363–9371. 10.1021/jacs.2c01416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z.-H.; Guo Y. W.; Li X. W. Recent advances on marine mollusk-derived natural products: chemistry, chemical ecology and therapeutical potential. Nat. Prod. Rep. 2022, 10.1039/d2np00021k. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.