Abstract
Cell-mediated immune (CMI) responses to Bordetella pertussis antigens (pertussis toxin [PT], pertactin [PRN], and filamentous hemagglutinin [FHA]) were assessed in 48-month-old recipients of acellular pertussis [aP] vaccines (either from Chiron-Biocine [aP-CB] or from SmithKline Beecham [aP-SB]) and compared to CMI responses to the same antigens at 7 months of age, i.e., 1 month after completion of the primary immunization cycle. None of the children enrolled in this study received any booster of pertussis vaccines or was affected by pertussis during the whole follow-up period. Overall, around 75% of 4-year-old children showed a CMI-positive response to at least one B. pertussis antigen, independently of the type of aP vaccine received, and the proportion of CMI responders were at least equal at 48 and 7 months of age. However, longitudinal examination of individual responses showed that from 20 (against PT) to 37% (against FHA) of CMI responders after primary immunization became negative at 48 months of age. This loss was more than compensated for by conversion to positive CMI responses, ranging from 36% against FHA to 69% against PRN, in other children who were CMI negative at 7 months of age. In 60 to 80% of these CMI converters, a lack of decline or even marked elevation of antibody (Ab) titers against B. pertussis antigens also occurred between 20 and 48 months of age. In particular, the frequency of seropositivity to PRN and FHA (but not to PT) was roughly three times higher in CMI converters than in nonconverters. The acquisition of CMI response to B. pertussis antigens in 48-month-old children was not associated with a greater frequency of coughing episodes lasting ≥7 days and was characterized by a prevalent type 1 cytokine profile, with high gamma interferon and low or no production of interleukin-5, reminiscent of cytokine patterns following immunization with whole-cell pertussis vaccine or natural infection. Our data imply that vaccination-induced systemic CMI may wane by 4 years of age but may be acquired or naturally boosted by symptomless or minor clinical infection by B. pertussis. This might explain, at least in part, the persistence of protection against typical pertussis in aP vaccine recipients despite a substantial waning of both Ab and CMI responses induced by the primary immunization.
Despite recent experimental and clinical investigations of induction and expression of immune responses against Bordetella pertussis, the mechanisms underlying protection from pertussis are not completely understood (8, 15, 25). Although data from experimental infections suggest that both T and B memory cell compartments are involved (18, 20, 23, 26), it is not clear which component(s) of adaptive immune responses mediates protection induced by vaccination in infants and to what extent the mechanisms of protection induced by whole-cell pertussis (wP) or acellular pertussis (aP) vaccines differ from each other (4, 7, 13, 29, 36). Conflicting information has been generated about the existence of serologic correlates of protection (1, 9, 10, 21, 22, 32, 33). Early waning of humoral immunity in children who received highly efficacious aP vaccines with persistent protection against typical World Health Organization (WHO)-defined disease has consistently been reported (7, 11, 30), but recent serological investigations have suggested that high titers of antibodies against some B. pertussis antigens correlate with protection from disease (9, 33). We have recently addressed the study of cell-mediated immunity (CMI) against B. pertussis antigens in children participating in the Italian Efficacy Trial of wP and aP vaccines (12). We have shown that CMI persisted in these children up to 20 months of age (4, 7, 29, 36), and a correlation between clinical efficacy and percentage of vaccinees acquiring CMI to pertussis toxin (PT) was apparent (7). Coupled with evidence from experimental models of infection (18, 23, 26), the above-mentioned studies suggested that CMI induction could play a role in protection induced by vaccination and in its persistence despite the early fall of antibody (Ab) levels (4, 7, 11, 13, 29, 36).
To further substantiate this role, we have now extended CMI assessment to 4-year-old aP vaccine recipients who remained clinically protected from pertussis in the absence of booster vaccination (30). The data showed an apparently preserved, if not increased, level of CMI to the aP vaccine antigens. Unexpectedly, however, longitudinal examination of individual responses suggested a complex interplay between waning of vaccination-induced CMI and gain of CMI, probably due to asymptomatic infection by B. pertussis.
MATERIALS AND METHODS
Subjects under study.
Forty-one aP vaccine recipients were examined for CMI responses at 7 and 48 months of age. All of the children belonged to the CMI cohort within the double-blind, randomized controlled clinical trial of pertussis vaccine efficacy in Italy (Progetto Pertosse [7, 12]). Only aP recipients were included in this study because those receiving the wP vaccine (of low efficacy in the Italian trial) and those in the placebo group (receiving only diphtheria and tetanus toxoids) were vaccinated against pertussis at the unblinding of the trial (30), so they could not be further assessed for vaccine-induced CMI response. Nonetheless, it was possible to obtain blood specimens from three children of the placebo arm, whose parents declined vaccination with any pertussis vaccine. The general study design and details of the pertussis clinical trial have been reported elsewhere (7, 12). Two aP vaccines were used, one manufactured by Chiron Biocine, Siena, Italy (aP-CB), and one manufactured by SmithKline Beecham, Rixensart, Belgium (aP-SB). Each vaccine contained inactive PT, filamentous hemagglutinin (FHA), and pertactin (PRN) at 5, 2.5, and 2.5 μg in aP-CB vaccine and 25, 25, and 8 μg in aP-SB vaccine, respectively. Because of the limited amount of blood obtained, in several cases not all antigens could be tested for each response studied here.
Informed, written consent was obtained by parents or guardians of children enrolled in this study. The study was approved by the bioethical committee of the Italian Efficacy Trial of Pertussis Vaccine.
Antigens and mitogen.
PT, FHA, and PRN (all kindly donated by Chiron-Biocine) were used as antigens unless otherwise indicated. To avoid any potential mitogenicity, they were heat inactivated (96°C; 1 h). Mitogenic stimulation was induced by phytohemagglutinin (PHA) (HA16; Wellcome, Dartford, United Kingdom).
PBMC isolation and culture.
Venous heparinized blood samples were taken from each child for assessment of immune response. The blood samples from 7-month-old children were obtained and processed as previously described (4, 7). Blood specimens from 48-month-old vaccine recipients were collected at the Local Healthy Units of Piemonte, Friuli, and Puglia, all regions participating in the Italian Vaccine Efficacy Clinical Trial (12), and sent unseparated to the Laboratory of Bacteriology and Medical Mycology, Istituto Superiore di Sanità, Rome, Italy, where they were processed by 24 h after delivery. Each sample consisted of 6 ml of venous blood, of which 5 ml was used to assess CMI in the lymphoproliferation assay and 1 ml was used to measure Ab titers to the three antigen components of the aP vaccines (see below).
PBMC were isolated by centrifugation on density gradient (Lymphoprep; Nyegaard, Oslo, Norway), washed twice, and suspended in RPMI medium (Gibco, Grand Island, N.Y.) supplemented with 5% pooled AB serum and antibiotics (penicillin, 100 IU/ml; streptomycin, 0.1 mg/ml [Gibco]), hereafter referred to as complete medium (2). The recovery of PBMC from four children exceeded the amount needed for setting up the cultures; thus, it was possible to freeze an aliquot of them in dimethyl sulfoxide according to routine procedures (4). The frozen PBMC samples were stored in liquid nitrogen. For the CMI assays, PBMC were quickly thawed at 37°C, washed as described previously (4, 7), and then processed exactly as described above for the fresh PBMC. In addition, frozen PBMC from healthy adults responsive to B. pertussis antigens were tested, as CMI assay positive controls, in all experiments performed to assess the CMI in PBMC from the study children. To estimate the interassay reproducibility, frozen PBMC of the five donors were tested for lymphoproliferation 6, 7, 9, 13, and 14 times each. Standard error (SE) of the mean was in any case lower than 20%, considering all antigens used in the CMI assays.
Cell proliferation assay and definition of CMI positivity.
PBMC proliferation was measured by culturing 2 × 105 cells/well in 0.2 ml of complete medium, in triplicate, in flat-bottom 96-microwell trays (Falcon; Becton Dickinson, Lincoln Park, N.J.) in the presence of the predetermined optimal doses of stimulant: PT, 10 μg/ml; FHA, 20 μg/ml; and PRN, 20 μg/ml. PHA (1.5 μg/ml) was the positive mitogenic control for each PBMC sample tested. The cultures were harvested after 7 or 3 days for antigenic or mitogenic stimulation, respectively. DNA synthesis was evaluated by counting [3H]thymidine incorporation (7). The data are shown as the mean values (± SE) of the difference (in counts per minute) between the antigen-stimulated and unstimulated PBMC cultures. Mean values of unstimulated cultures of PBMC from the 41 aP vaccine recipients were (0.7 ± 0.1) × 103 cpm. A CMI-proliferative response was considered positive (CMI+) when the difference between the antigen-stimulated and unstimulated PBMC cultures was at least 3 × 103 cpm, corresponding to a mean stimulation index of ≥4. The evaluation criteria of CMI responses with frozen PBMC samples (at 7 months of age) were previously detailed (7). In particular, the assay was considered valid only when the cultures proliferated in the presence of the mitogen PHA (at >3.9 × 104 cpm) in order to rule out any influence of cell viability on CMI response to B. pertussis antigen. The mitogenicity control was particularly important in this study, which compares frozen (at 7 months of age) to fresh (at 48 months of age) PBMC samples. In a few cases, a direct comparison between fresh and frozen PBMC from 48-month-old children was also performed (see above).
Cytokine determination.
Production of cytokines was measured in 34 aP vaccine recipients. Cytokines were assayed in cultures of 2 × 106 cells/ml in 0.5 ml of complete medium, in the presence of B. pertussis antigen, at the concentration used for cell proliferation (7). Culture supernatants were collected at 48 h and used to measure gamma-interferon (IFN-γ), tumor necrosis factor alpha (TNF-α), and interleukin-5 (IL-5) by enzyme-linked immunosorbent assay (ELISA) (Quantikine; R&D systems, Inc., Minneapolis, Minn.) (3, 4). ELISA threshold detection levels were 3, 3, and 4.4 pg/ml for IL-5, IFN-γ, and TNF-α, respectively. The basal (unstimulated) secretion levels for IFN-γ, IL-5, and TNF-α obtained in PBMC cultures from all aP vaccine recipients studied were 4.5 ± 2, 1.9 ± 0.9, and 206 ± 59 pg/ml.
Ab determinations.
Ab (immunoglobulin G [IgG]) titers to B. pertussis antigens were assessed in 36 of 41 aP vaccine recipients by standardized ELISA, as previously described (7, 11). A serologic response to each pertussis antigen was defined as positive when the ELISA unit value was four times higher than the minimal level of detection (MLD), which was set at 2 U/ml for IgG to PT and FHA and 3 U/ml for IgG to PRN.
Pertussis surveillance.
Active surveillance of pertussis was carried out as previously described (7, 12, 30, 31). Briefly, an active monthly telephone surveillance of coughing of any type was implemented. This was done by purposely recruited and hired, full-time study nurses in charge of continuous contact with study families and trained to detect and immediately investigate any coughing episode lasting ≥7 days. All these episodes were investigated microbiologically and serologically by collection of nasopharyngeal mucus and acute- and convalescent-phase serum specimens. A confirmed B. pertussis infection was defined as an illness with coughing in which B. pertussis was isolated from the nasopharynx or when the convalescent-phase serum specimen demonstrated an increase of at least 100% in IgG or IgA titer against PT or FHA compared to that of the acute-phase serum, provided that the convalescent serum level was four times higher than the MLD. A case of pertussis (whooping cough) was defined, according to the WHO, as paroxysmal cough lasting at least 21 consecutive days associated with laboratory-confirmed B. pertussis infection.
Statistics.
Data from all proliferation, Ab titer, and cytokine determination experiments were recorded in a computerized database. Statistical descriptive analyses were carried out with the SPSS statistical package as previously reported (7). Differences in proportions were assessed by the chi-square test or Fisher’s exact test, as appropriate, while differences in mean values were assessed by Student’s t test.
RESULTS
Duration of CMI and Ab responses induced by acellular pertussis vaccines.
All children tested for CMI were under strict surveillance for pertussis, and none of them was found to be affected by the typical disease or confirmed B. pertussis infection (as defined in Materials and Methods) during the study period, although most of them suffered from one or more coughing episodes of ≥7 days duration per year (see also below).
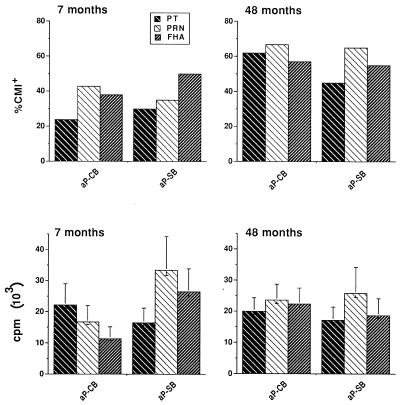
Figure 1 (top panels) cross-sectionally compares the overall CMI positivity to the three B. pertussis antigens at 7 and 48 months of age. The percentage of CMI+ children to each antigen tested (in particular against PT) was generally higher at the second time point, and the percentage of responders to at least one antigen was also higher at the second CMI assay (73.2 versus 58.5). However, none of the differences was statistically significant. The mean magnitude of the proliferative responses at 48 months of age for all antigens was comparable to that measured at the previous time point and was also comparable between aP-SB and aP-CB recipients (Fig. 1, bottom panels).
FIG. 1.
Cross-sectional analysis of percent positivity (CMI+) (top panels) and magnitude (mean counts per minute ± SE) of proliferative responses (bottom panels) to B. pertussis antigens in aP-CB and aP-SB vaccine recipients at the indicated ages. Proliferation assays were performed by using frozen and fresh PBMC in 7- and 48-month-old children, respectively. In both cases, PBMC (2 × 105/well in 0.2 ml) were cultured in the presence of PT (10 μg/ml), FHA (20 μg/ml), and PRN (20 μg/ml). DNA synthesis was measured after 7 days by counting incorporation. A CMI+ response was that occurring in the antigen-stimulated PBMC culture with a [3H]thymidine incorporation value of ≥3,000 cpm with respect to that of the antigen-unstimulated control culture. No statistically significant differences were noticed either between the responses to each antigen at the two ages or between the aP-CB and aP-SB recipients.
A longitudinal analysis of CMI responses of each subject at the two time points showed a number of interesting features. A loss of the CMI positivity detected at 7 months of age, and almost equally for all three B. pertussis antigens, was found in a number of 48-month-old children (Table 1). Conversely, CMI+ responses to B. pertussis antigens were found in a high proportion (from 36.4% to FHA to around 70% to PRN in aP-SB recipients) of 48-month-old children who were negative at the previous CMI measurement. Thus, the whole population of 48-month-old CMI+ subjects consisted of children who apparently had their CMI status preserved and children who acquired positivity (CMI converters) later on, as shown in Table 1.
TABLE 1.
Losses and gainsa of CMI responses to B. pertussis antigens in 48-month-old pertussis vaccine recipients
| Antigen | Loss of CMI+ (%)
|
Gain of CMI+ (%)
|
||
|---|---|---|---|---|
| aP-CB | aP-SB | aP-CB | aP-SB | |
| PT | 33 | 20 | 65.2 | 55.5 |
| PRN | 28 | 22 | 50 | 69.2 |
| FHA | 30 | 37.5 | 54.4 | 36.4 |
Calculated with respect to the proliferative PBMC responses of the same subjects at 7 months of age. CMI was defined as positive (CMI+) when the difference between the lymphoproliferative response (measured as [3H]thymidine incorporation and expressed in counts per minute) of the antigen-stimulated PBMC culture and that of the unstimulated control culture was ≥3,000 (corresponding to a stimulation index of ≥4).
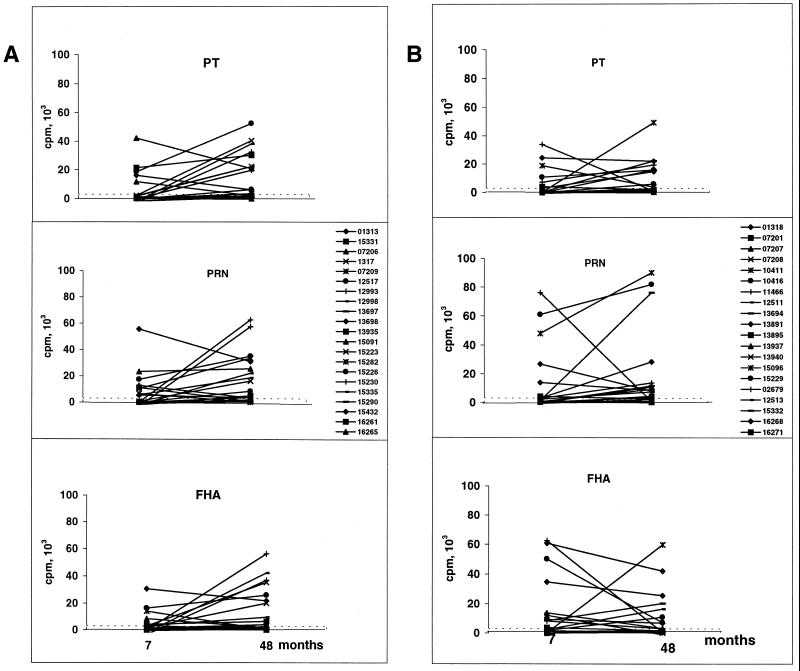
Figure 2 shows the individual magnitude of each response for each subject whose CMI was tested at both time points. Some of the highest cell proliferation values at 48 months of age were shown by the lymphocytes of children who did not respond at 7 months of age (see, in particular, the magnitude of the proliferative responses to PRN in aP-SB vaccine recipients).
FIG. 2.
Longitudinal CMI analysis. PBMC (2 × 105/well in 0.2 ml) from 21 aP-CB (A) and 20 aP-SB (B) recipients were assayed for proliferation at 7 and 48 months of age, as indicated. The magnitudes of cell proliferation induced by PT, PRN, and FHA antigens are shown. DNA synthesis was measured after 7 days by counting [3H]thymidine incorporation. Data are expressed as counts per minute of the differences between the antigen-stimulated PBMC culture and the unstimulated culture. On the right of the middle panels, the codes of the subjects tested are given.
Since the antigen-induced proliferative assays in children 48 months of age were performed with fresh PBMC whereas those of 7-month-old children were done with frozen cells (4, 7), which might have influenced our findings, a direct comparison between the proliferative responses to B. pertussis antigens of fresh and frozen PBMC from four children 48 months of age (two CMI+ and two CMI−) was made. As shown in Table 2, PBMC freezing and thawing not only did not affect the CMI-positive or -negative status, it did not even result in a lower magnitude of the proliferative response to any B. pertussis antigen, including the whole bacterial cells. In addition, the mitogenic response to PHA was definitely higher at 7 than at 48 months of age [(147.2 ± 8.3) and (145.2 ± 9.4) × 10−3 cpm in aP-CB and aP-SB recipients, respectively, compared to (88.3 ± 10.2) and (91.6 ± 10.4) × 10−3 cpm] (P < 0.001).
TABLE 2.
Comparison between fresh and frozena PBMC for their proliferative responses to B. pertussis antigens in 48-month-old children
| Antigen | Lymphoproliferative responseb
|
|||
|---|---|---|---|---|
| CMI+
|
CMI−
|
|||
| Fresh | Frozen | Fresh | Frozen | |
| None | 0.5 ± 0.1 | 0.35 ± 0.07 | 0.4 ± 0.0 | 0.2 ± 0.05 |
| PT | 21.4 ± 1.3 | 32.7 ± 5.7 | 2.8 ± 1.1 | 2.1 ± 1.5 |
| PRN | 16.9 ± 2.4 | 35.2 ± 9.9 | 1.1 ± 0.4 | 0.7 ± 0.1 |
| FHA | 12.1 ± 1.8 | 18.3 ± 0.8 | 0.8 ± 0.1 | 0.4 ± 0 |
| BPc | 10.0 ± 2.3 | 20.1 ± 6.0 | 1.3 ± 0.0 | 1.7 ± 0.3 |
Fresh and frozen PBMC from four children (two CMI+ and two CMI−) were assayed for proliferation as described in Materials and Methods and in the legend to Table 1.
Expressed as mean (triplicate samples from both children) ± SE of thymidine incorporation values (103 cpm).
B. pertussis cells (strain 18323; ATCC 9797) were heat inactivated (96°C; 1 h) and used at a concentration of 106 CFU/ml.
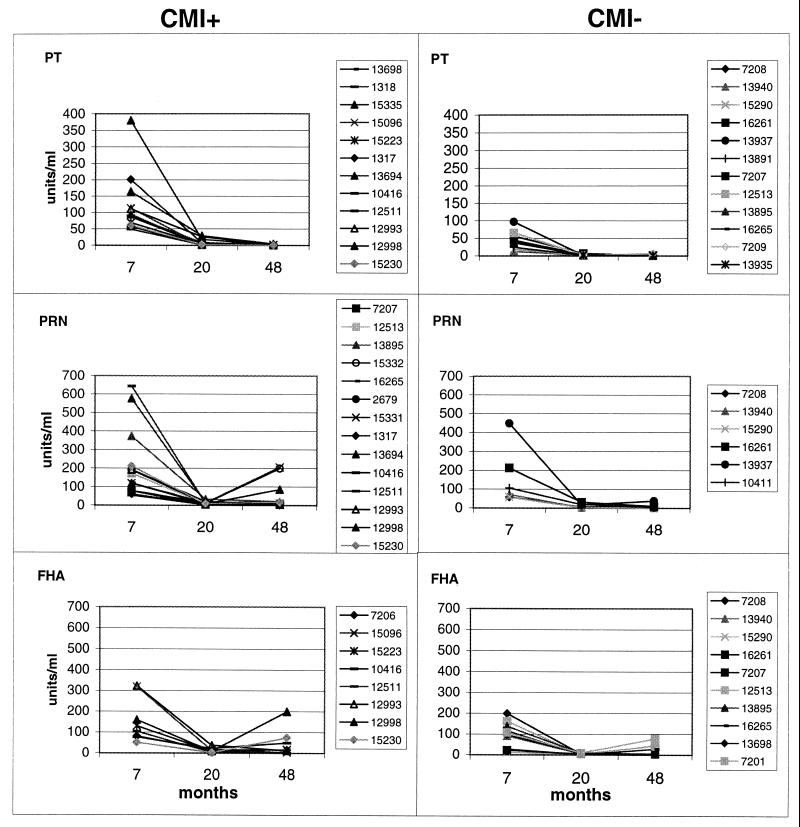
Finally, we measured Ab titers against B. pertussis antigens in the sera of 48-month-old aP vaccine recipients and compared these titers to those of the same children at 7 and 20 months of age. Table 3 shows that from 60 to 80% of the CMI converters also acquired or maintained anti-PRN and anti-FHA (but not anti-PT) Ab titers. The frequency of seropositivity to the two antigens mentioned above was roughly three times higher in CMI converters than in nonconverters (Table 3).
TABLE 3.
Number of aP vaccine recipients showing positive Ab responses according to CMI status
| CMI statusa | B. pertussis antigen | No. of subjects tested | Seropositivity (%)b |
|---|---|---|---|
| Converters | PT | 13 | 1 (7.6) |
| PRN | 15 | 9 (60) | |
| FHA | 9 | 7 (77.7) | |
| Nonconverters | PT | 15 | 1 (6.6) |
| PRN | 8 | 2 (25) | |
| FHA | 13 | 5 (38) |
At 48 months of age; all negative at 7 months of age.
To the B. pertussis antigen showing CMI conversion or nonconversion. Seropositivity was defined as a value of ELISA units four times higher than the MLD, set at 2 U/ml for IgG to PT and FHA and 3 U/ml for IgG to PRN.
Figure 3 shows individual results. As expected, Ab titers markedly declined between 7 and 20 months of age, reaching the lowest limit values in all children regardless of CMI acquisition, but rebounded to appreciable levels in a remarkable proportion of CMI+ subjects. Again, no differences in anti-PT serum titers were observed in those who acquired CMI positivity to PT compared to those remaining CMI− to this antigen.
FIG. 3.
Serum antibody titers at 7, 20, and 48 months of age against B. pertussis antigens indicated in each panel in children who were CMI− at 7 months of age and acquired (CMI+; left panels) or did not acquire (CMI−; right panels) CMI at 48 months of age. Seropositivity was defined as a value of ELISA units four times higher than the MLD, which was set at 2 U/ml for IgG to PT and FHA and 3 U/ml for IgG to PRN. For the determination of Ab titers and other technical details, see the text. The numeric codes individuate all children tested.
To evaluate whether the conversion to CMI+ response was consequent to or paralleled an increase in the number of coughing episodes, we surveyed all coughing episodes lasting ≥7 days in children who were CMI− at 7 months of age and converted to CMI positivity at 48 months of age compared to those in children who were CMI− at both ages. Table 4 shows that the two categories of children suffered from a comparable number of these coughing episodes, irrespective of the B. pertussis antigen against which the CMI response was assessed.
TABLE 4.
Number of coughing episodes (≥7 days) in relation to CMI status
| CMI statusa | B. pertussis antigen | No. of subjects tested | Mean no. (range) of coughing episodes/person/yrb |
|---|---|---|---|
| Converters | PT | 13 | 0.6 (0–4) |
| PRN | 15 | 0.7 (0–4) | |
| FHA | 9 | 0.6 (1–4) | |
| Nonconverters | |||
| PT | 15 | 0.5 (0–5) | |
| PRN | 8 | 0.6 (0–5) | |
| FHA | 13 | 0.7 (0–5) |
At 48 months of age; all negative at 7 months of age.
Lasting ≥7 days.
Cytokine profile induced by B. pertussis antigens in PBMC of 48-month-old vaccine recipients.
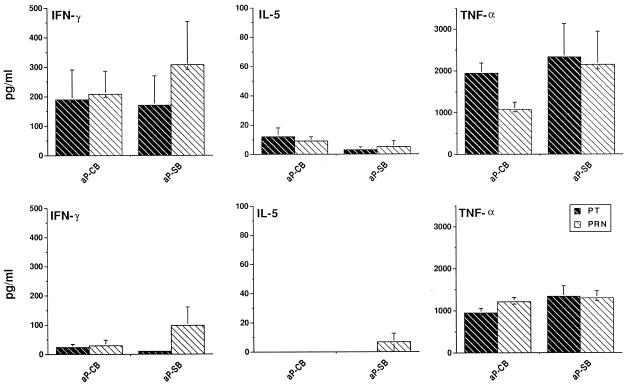
Since cytokine patterns are critical regulatory components of CMI responses, we also measured the production of typical type 1 (IFN-γ) or type 2 (IL-5) cytokines in supernatants of B. pertussis antigen-stimulated cultures of PBMC from 48-month-old vaccinees. TNF-α was also measured as a control cytokine. Cytokine production in mitogen (PHA)-stimulated PBMC served as an assay responsiveness control (see Materials and Methods). Figure 4 shows the amount of IFN-γ, IL-5, and TNF-α secreted in PT- and PRN-stimulated cultures of PBMC from CMI+ or CMI− children (top and bottom panels, respectively).
FIG. 4.
IFN-γ, IL-5, and TNF-α production in B. pertussis antigen-stimulated PBMC of 48-month-old vaccine recipients. There was an average of 21 (13 CMI+ [top panels] and 8 CMI− [bottom panels]) aP-CB and 13 (8 CMI+ and 5 CMI−) aP-SB recipients. PBMC from CMI+ and CMI− children were cultured in the presence of PT and PRN; after 48 h, the supernatants were collected and cytokine production was assayed by ELISA. The data are expressed as means ± SE. All differences in IFN-γ production between data in the top panels and bottom panels for both PRN and PT and both vaccines were highly significant (P < 0.01; Student’s t test). All comparisons of IL-5 and TNF-α production gave nonsignificant differences.
IFN-γ production, independently of the stimulatory antigen and the type of vaccine received, was consistently produced only by CMI+ subjects. Low levels of IL-5 (up to 20 pg/ml in aP-CB recipients) were produced by the minority of CMI+ children. TNF-α was consistently produced by PBMC of both categories of children, demonstrating that the absence of IFN-γ or IL-5 production by CMI− children was not due to a general hyporesponsiveness of the PBMC culture (TNF-α is essentially produced by nonproliferating cells in these cultures). It was possible to assay for mitogen (PHA)-induced IFN-γ, IL-5, and TNF-α secretion in 7 of 34 aP vaccine recipients. The values found were 1,042 ± 280, 59.7 ± 13, and 3,521 ± 568 pg/ml for IFN-γ, IL-5, and TNF-α, respectively. No significant differences in cytokine production were found between aP-CB and aP-SB recipients. Overall, the cytokine profile of CMI+ subjects showed a trend toward a preferential activation of type 1 response.
DISCUSSION
In this investigation, we initially attempted to validate our proposal that CMI rather than Ab levels could correlate with the apparent long-lasting protection induced by aP vaccines, as in our previous studies (4, 7). To this end, we further examined a number of children participating in the Italian Efficacy Trial of Pertussis Vaccines for their CMI responses to B. pertussis antigens at the fourth year of age in comparison with the same responses at 7 months of age. None of them was affected by pertussis or confirmed B. pertussis respiratory infection (as defined by WHO [see Materials and Methods]) during the study period. Several logistic problems connected with the nature of the trial, the lack of consent to donate blood, or even the small amount of PBMC obtained in most cases impeded an extensive investigation with a higher number of subjects, as for the primary CMI assessment (7), and with an extended panel of B. pertussis antigens, including some (e.g., fimbriae) which were not present in the vaccine formulation. Despite these limitations, 41 children who had been immunized with one of the two aP vaccines (aP-CB and aP-SB) could be studied for their CMI responses, and an almost-equal number (36) could be studied for their Ab responses to the three antigen constituents of the vaccine (PT, FHA, and PRN).
Two findings of this study provided some unexpected indications about the persistence of CMI induced by primary vaccination with aP vaccines. The lymphoproliferative responses to B. pertussis antigens indicated that several children acquired a CMI+ response in the time interval between the primary immunization and the fourth year of age. Conversely, a substantial loss of CMI responsiveness by the 48th month of age in a nonneglectable number of children who were CMI+ soon after immunization was also detected. CMI losses and gains were independent of the type of aP vaccine employed and occurred, though to different degrees, against all the antigens present in the vaccine formulation. Moreover, although the magnitudes of the CMI responses at 48 months of age, as a population average, were not different from that measured in 7-month-old children, for some vaccinees they were much higher than the postvaccination responses. Thus, a number of children developed a strong anti-B. pertussis CMI long after the primary immunization period, a fact that would hardly be explained in terms of age-dependent variations in the timing of CMI appearance following the initial priming (7).
We offer here the alternative explanation that at least some of the more pronounced late CMI responses are independent of vaccination itself and are likely acquired by asymptomatic infection by B. pertussis. If this interpretation is correct, it necessarily follows that systemic CMI induced by aP vaccines wanes in most children after primary immunization, although possibly later than serum antibody levels (7, 11), and the long-lasting protection against typical pertussis induced by the primary immunization with these vaccines (30) is not simply due to the persistence of vaccination-induced immunity but is also contributed to by the natural boosting or neoacquisition of that immunity.
The above interpretation must critically consider several possible alternative explanations. The first, and most naive, is technical, as for logistic reasons (7) frozen PBMC were used to assess CMI response in 7-month-old children whereas the present study was conducted with fresh PBMC, making it possible to underestimate CMI positivity at the earlier age. There are several observations, however, which appear to rule out this possibility as the sole, or even the main, explanation of our findings. First, it could not explain the loss of CMI response by fresh PBMC at 48 months of age in children whose frozen PBMC efficiently responded at 7 months of age. This loss cannot be neglected, as it involved around one-third of all CMI responders to FHA or PT (in aP-CB recipients) in a situation where not all immunized children had acquired CMI responsiveness after primary immunization (7). Second, the average magnitude of the proliferative response to the mitogenic stimulant PHA was greater at 7 than at 48 months of age, a finding that would not be expected if cell viability and proliferative potential were substantially affected by freezing. Third, and more important, a direct comparison of cell proliferation to B. pertussis antigens in frozen and fresh PBMC samples from four children did not reveal any difference either in the quality (positive or negative) or in the magnitude of CMI response to all B. pertussis antigens tested, including the highly stimulating B. pertussis whole bacterial cells. Equal efficiency in the proliferative cytokine responses to cytomegalovirus antigens by fresh and cryopreserved PBMC from human immunodeficiency virus-infected or noninfected subjects has very recently been reported by Weinberg et al. (35).
Two other findings are in favor of asymptomatic infection as the likely explanation for the acquisition of CMI responses in 48-month-old children. In most of these subjects, there were appreciable antibody titers higher than those detected in CMI− subjects against PRN and FHA at 48 months of age. In a number of subjects, there was marked elevation of these titers between 20 and 48 months, and some of them corresponded to high PBMC proliferative responses. In addition, an increased orientation toward a type 1 cytokine profile induced by pertussis antigen stimulation was observed in PBMC from 48-month-old aP vaccinees. In particular, IL-5 levels were low or not detectable in aP-SB recipients, who included the highest IL-5 producers at the earlier age (4). In previous observations of ours (4) and others (13, 28), a mixed type 1-type 2 profile in PBMC of aP vaccine recipients was found. It should be stressed here that the interpretation of these data is necessarily limited by the cross-sectional rather than longitudinal nature of the study, as all CMI+ children 48 months of age who were examined belonged to a subcohort of children who were not examined or were CMI− at 7 months of age and thus did not produce cytokines in antigen-stimulated PBMC (4).
Clearly, the best unequivocal proof that late CMI responses were due to exposure rather than vaccination would have been the detection of CMI or Ab response to B. pertussis in children who were not vaccinated with pertussis vaccines, or even responsiveness to B. pertussis antigens (e.g., fimbriae) not contained in the aP vaccines studied. Unfortunately, for the reasons given above, only the former possibility could be tested in three children of the same age who were known to have received only a diphtheria-tetanus vaccine and who had not been affected by pertussis. Remarkably, one of the three had excellent CMI responses against all three antigen constituents of aP vaccines (data not shown).
If disease-free infection is the major explanation for the apparent persistence of CMI response in aP vaccine recipients, other critical issues concern the nature and consequences of exposure. As shown by Isacson et al. (16), anti-PRN and anti-FHA IgGs are easily acquired in children with no symptoms of pertussis. Our children were carefully monitored for any coughing episode lasting ≥7 days over the investigation period, and none of them fulfilled the definition of pertussis or confirmed infection by B. pertussis. This does not completely rule out the possibility that milder coughing disease, potentially ascribable to infection by B. pertussis, might have occurred. In this context, the comparable frequency of coughing episodes in CMI converters and nonconverters and the recognized sensitivity of the surveillance system implemented in the Italian efficacy trial (30% observed rate of cough detection in the pertussis-unvaccinated control group and 96% of microbiology-serology investigations of the detected cough episodes; about 70% of pertussis cases confirmed by culture) are noteworthy (12, 31).
Another possibility is that the children were not really exposed to B. pertussis but rather to Bordetella parapertussis or nonencapsulated Haemophilus influenzae strains, which are known to possess FHA- and probably also PRN-cross-reacting antigens (6, 14, 16, 24). B. parapertussis infections have recently been found to be very common in young Finnish children immunized with wP vaccine (14). An initial survey (19) has shown that the circulation of this latter agent in Italy is not high enough to cause a significant rise in anti-FHA and -PRN Ab titers in most subjects. Moreover, PT is still regarded as a truly specific B. pertussis antigen, and the acquisition of CMI response to PRN and FHA in our children was generally paralleled by anti-PT CMI acquisition. Thus, exposure to the above-mentioned agents seems less likely than exposure to B. pertussis in explaining our CMI data, although it could still play a part. Interestingly, no child examined showed elevation of Ab titer against PT, as occurs during disease, suggesting that disease-free infection with B. pertussis (or even cross-reacting bacteria) may more easily recall CMI than Ab response to PT in vaccinated children. In this context, the recent observation by He et al. (13) that CMI and Ab responses to PRN and FHA in schoolchildren who were recipients of aP vaccines were significantly correlated whereas their PBMC-proliferative and IgG Ab responses to PT were not is interesting.
That asymptomatic or subclinical infection by B. pertussis may result in extensive priming of immunity to this agent is not a novel finding. Ryan et al. (28, 29) and Tran Minh et al. (34) reported that a proportion of unvaccinated, uninfected children displayed specific T-cell responses to B. pertussis antigens. Interestingly, this cellular response was typically Th1 (28, 29). In adults with no record of pertussis, we have recently found that 80 to 100% of individuals tested were CMI+ to B. pertussis antigens, including PT (5, 27), a percentage hardly attributable to the coverage level of pertussis vaccination in Italy (17, 27). The cytokine profile induced in purified T cells by B. pertussis antigens was essentially Th1 (5).
Overall, vaccine efficacy in 48-month-old aP recipients was still high in the Italian trial (reference 30 and data not shown). The waning of vaccination-acquired cellular immunity measured in the blood does not rule out the persistence of vaccination-induced memory in other body sites (e.g., lungs and lymph nodes), as was also suggested by a recent study with an experimental model of infection (20). This memory could be the source of at least partial protection during natural exposure to B. pertussis, which would then constitute a strong CMI booster. In conclusion, our findings suggest that waning of vaccination-dependent CMI responses in blood occurs in children receiving aP vaccines, although probably later than serum antibody waning. However, most vaccinees appear to maintain a sufficient memory of the primary immunization to be prone to reacquire a potent CMI upon natural exposure to B. pertussis or cross-reacting bacteria. We suggest that a significant portion of the long-lasting protection against pertussis noticed in the recipients of a primary immunization cycle with aP vaccines in Italy may be due to natural boosters. These findings should be considered for future antipertussis vaccination policies.
ACKNOWLEDGMENTS
This work was partially supported by grants from NIH-NIAID (Contract N01-A1-25138), Istituto Superiore di Sanità (Proper project; contract 97A/P), and CNR Target Project on Biotechnology.
We are grateful to the Progetto Pertosse study group and in particular to A. Barale, L. Oberto, F. Rosa, R. Manzino, P. Ferrero, G. De Ambrosi, L. Marotta, G. Pittolo, M. Cimatoribus, and A. Londero for very valuable cooperation in this study. We are also grateful to A. Giammanco (University of Palermo, Palermo, Italy) for her help in the serology work and to F. Girolamo and A. Botzios for help in the preparation of the manuscript.
REFERENCES
- 1.Ad Hoc Group for the Study of Pertussis Vaccines. Placebo controlled trial of two acellular pertussis vaccines in Sweden; protective efficacy and adverse events. Lancet. 1998;ii:955–960. [PubMed] [Google Scholar]
- 2.Ausiello C M, Urbani F, Gessani S, Spagnoli G C, Gomez M J, Cassone A. Cytokine gene expression in human peripheral blood mononuclear cells stimulated by mannoprotein constituents of Candida albicans. Infect Immun. 1993;61:4905–4911. doi: 10.1128/iai.61.10.4105-4111.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ausiello C M, la Sala A, Ramoni C, Urbani F, Funaro A, Malavasi F. Secretion of IFN-γ, IL-6, granulocyte-macrophage colony-stimulating factor and IL-10 cytokines after activation of human purified T lymphocytes upon CD38 ligation. Cell Immun. 1996;173:192–197. doi: 10.1006/cimm.1996.0267. [DOI] [PubMed] [Google Scholar]
- 4.Ausiello C M, Urbani F, la Sala A, Lande R, Cassone A. Vaccine and antigen-dependent type 1 and type 2 cytokine induction in children after primary vaccination with whole-cell or acellular pertussis vaccines. Infect Immun. 1997;65:2168–2174. doi: 10.1128/iai.65.6.2168-2174.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ausiello C M, Lande R, la Sala A, Urbani F, Cassone A. Cell-mediated immune response of healthy adults to B. pertussis vaccine antigens. J Infect Dis. 1998;178:466–470. doi: 10.1086/515628. [DOI] [PubMed] [Google Scholar]
- 6.Barenkamp S J, Leininger E. Cloning, expression, and DNA sequence analysis of genes encoding nontypeable Haemophilus influenzae high-molecular-weight surface-exposed proteins related to filamentous hemagglutinin of Bordetella pertussis. Infect Immun. 1992;60:1302–1313. doi: 10.1128/iai.60.4.1302-1313.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cassone A, Ausiello C M, Urbani F, laSala A, Lande R, Piscitelli A, Giuliano M, Salmaso S The Progetto Pertosse-CMI Working Group. Cell-mediated and antibody responses to B. pertussis antigens in children vaccinated with acellular or whole-cell pertussis vaccines. Arch Pediatr Adolesc Med. 1997;151:283–289. doi: 10.1001/archpedi.1997.02170400069013. [DOI] [PubMed] [Google Scholar]
- 8.Cherry J D. Pediatr. Infect. Dis. J. S90–S96. 1997. Comparative efficacy of acellular pertussis vaccines: an analysis of recent trials. [DOI] [PubMed] [Google Scholar]
- 9.Cherry J D, Gornbein J, Heininger U, Stehr K. A search for serologic correlates of immunity to Bordetella pertussis cough illnesses. Vaccine. 1998;16:1901–1906. doi: 10.1016/s0264-410x(98)00226-6. [DOI] [PubMed] [Google Scholar]
- 10.Edwards K M, Meade B D, Decker M D, Reed G F, Rennels M B, Steinhoff M C, Anderson E L, Enguland J A, Pichichero M E, Deloria M A. Comparison of thirteen acellular pertussis vaccines: overview and serological response. Pediatrics. 1995;96:548–557. [PubMed] [Google Scholar]
- 11.Giuliano M, Mastrantonio P, Giammanco A, Piscitelli A, Salmaso S, Wassilak S G F. Antibody responses and persistence in the 2 years following immunization with two acellular and one whole-cell vaccines against pertussis. J Pediatr. 1998;132:973–978. doi: 10.1016/s0022-3476(98)70395-6. [DOI] [PubMed] [Google Scholar]
- 12.Greco D, Salmaso S, Mastrantonio P, Giuliano M, Tozzi A G, Anemona A, Ciofi Degli Atti M L, Giammanco A, Panei P, Blackwelder W C, Klein D L, Wassilak S G F the Progetto Pertosse Working Group. A controlled trial of two acellular vaccines and one whole-cell vaccine against pertussis. N Engl J Med. 1996;334:341–348. doi: 10.1056/NEJM199602083340601. [DOI] [PubMed] [Google Scholar]
- 13.He Q, Minh N N T, Edelman K, Viljanen M K, Arvilommi H, Mertsola J. Cytokine mRNA expression and proliferative responses induced by pertussis toxin, filamentous hemagglutinin, and pertactin of Bordetella pertussis in the peripheral blood mononuclear cells of infected and immunized schoolchildren and adults. Infect Immun. 1998;66:3796–3801. doi: 10.1128/iai.66.8.3796-3801.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.He Q, Viljanen M K, Arvilommi H, Aittanen B, Mertsola J. Whooping cough caused by Bordetella pertussis and Bordetella parapertussis in an immunized population. JAMA. 1998;280:635–637. doi: 10.1001/jama.280.7.635. [DOI] [PubMed] [Google Scholar]
- 15.Hewlett E L. Pediatr. Infect. Dis. J. S78–S84. 1997. Pertussis: current concepts of pathogenesis and prevention. [DOI] [PubMed] [Google Scholar]
- 16.Isacson J, Trollfors B, Taranger J, Lagergard T. Acquisition of IgG serum antibodies against two Bordetella antigens (filamentous hemagglutinin and pertactin) in children with no symptoms of pertussis. Pediatr Infect Dis J. 1995;14:517–521. doi: 10.1097/00006454-199506000-00009. [DOI] [PubMed] [Google Scholar]
- 17.Levy-Bruhl D, Pebody R, Veldhuijzen I, Valenciano M, Osborne K. ESEN: a comparison of vaccination programmes—part 2: pertussis. Eurosurv Weekly. 1998;3:107–110. doi: 10.2807/esm.03.11.00086-en. [DOI] [PubMed] [Google Scholar]
- 18.Mahon B P, Sheanhan B J, Griffin F, Murphy G, Mills K H G. Atypical disease after B. pertussis respiratory infection of mice with targeted disruptions of interferon-γ receptor or immunoglobulin μ chain genes. J Exp Med. 1997;186:1843–1851. doi: 10.1084/jem.186.11.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mastrantonio P, Stefanelli P, Giuliano M, Herrera Rojas Y, Ciofi degli Atti M, Anemona A, Tozzi A E. Bordetella parapertussis infection in children: epidemiology, clinical symptoms, and molecular characteristics of isolates. J Clin Microbiol. 1998;36:999–1002. doi: 10.1128/jcm.36.4.999-1002.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mc Guirk P, Mahon B P, Griffin F, Mills K H G. Compartmentalization of T cell responses following respiratory infection with B. pertussis: hyporesponsiveness of lung T cells is associated with modulated expression of the co-stimulatory molecule CD28. Eur J Immunol. 1998;28:153–163. doi: 10.1002/(SICI)1521-4141(199801)28:01<153::AID-IMMU153>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 21.Medical Research Council. Vaccination against whooping cough: relation between protection in children and results of laboratory tests. Br Med J. 1956;2:454–462. [PMC free article] [PubMed] [Google Scholar]
- 22.Miller E, Ashworth L A E, Redhead R, Thornton C, Waight P A, Coleman T. Effect of schedule on reactogenicity and antibody persistence of acellular and whole-cell pertussis vaccines: value of laboratory tests as predictors of clinical performance. Vaccine. 1997;15:51–60. doi: 10.1016/s0264-410x(96)00112-0. [DOI] [PubMed] [Google Scholar]
- 23.Mills K H G, Ryan M, Ryan E, Mahon B P. A murine model in which protection correlates with pertussis vaccine efficacy in children reveals complementary roles for humoral and cell-mediated immunity in protection against B. pertussis. Infect Immun. 1998;66:594–602. doi: 10.1128/iai.66.2.594-602.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mooi F R, van der Heide H G J, TerAvest A R, Welinder K G, Livey I, van der Zeisj B M A, Gaastra V. Characterization of fimbrial subunits from Bordetella species. Microb Pathog. 1987;3:1–8. doi: 10.1016/0882-4010(87)90054-4. [DOI] [PubMed] [Google Scholar]
- 25.Pichichero M E. Pertussis and the pertussis vaccines. Curr Opin Infect Dis. 1993;6:558–564. [Google Scholar]
- 26.Redhead K, Watkins J, Barnard A, Mills K H G. Effective immunization against B. pertussis respiratory infection in mice is dependent on induction of cell-mediated immunity. Infect Immun. 1993;61:3190–3198. doi: 10.1128/iai.61.8.3190-3198.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rota M C, Ausiello C M, D’Amelio R, Cassone A, Giammanco A, Molica C, Lande R, Greco D, Salmaso S. Prevalence of markers of exposure to Bordetella pertussis among Italian young adults. Clin Infect Dis. 1998;26:297–302. doi: 10.1086/516293. [DOI] [PubMed] [Google Scholar]
- 28.Ryan M, Murphy G, Gothefors L, Nilsson L, Storsaeter J, Mills K H G. Bordetella pertussis respiratory infection in children is associated with preferential activation of type 1 T helper cells. J Infect Dis. 1997;175:1246–1250. doi: 10.1086/593682. [DOI] [PubMed] [Google Scholar]
- 29.Ryan M, Murphy G, Nilsson L, Shackley F, Gothefors L, Omar K, Miller E, Storsaeter J, Mills K H G. Distinct T-cell subtypes induced with whole cell and acellular pertussis vaccines in children. Immunology. 1998;93:1–10. doi: 10.1046/j.1365-2567.1998.00401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Salmaso S, Mastrantonio P, Wassilak S G F, Giuliano M, Anemona A, Giammanco A, Tozzi A E, Ciofi degli Atti M L, Greco D the Stage II Working Group. Persistence of protection through 33 months of age provided by immunization in infancy with two three-component acellular pertussis vaccines. Vaccine. 1998;16:1270–1275. doi: 10.1016/s0264-410x(98)00040-1. [DOI] [PubMed] [Google Scholar]
- 31.Salmaso, S., A. E. Tozzi, and M. L. Ciofi degli Atti. Observer bias in acellular pertussis vaccine trials. Pediatrics, in press. [DOI] [PubMed]
- 32.Storsaeter J, Blackwelder W C, Hallander H O. Pertussis antibodies, protection, and vaccine efficacy after household exposure. Am J Dis Child. 1992;146:167–172. doi: 10.1001/archpedi.1992.02160140033016. [DOI] [PubMed] [Google Scholar]
- 33.Storsaeter J, Hallander H O, Gustafsson L, Olin P. Levels of anti-pertussis antibodies related to protection after household exposure to Bordetella pertussis. Vaccine. 1998;16:1907–1916. doi: 10.1016/s0264-410x(98)00227-8. [DOI] [PubMed] [Google Scholar]
- 34.Tran Minh N N, Edelman K, He Q, Viljanen M K, Arvilommi H, Mertsola J. Antibody and cell-mediated immune responses to booster immunization with a new acellular pertussis vaccine in school children. Vaccine. 1998;16:1604–1610. doi: 10.1016/s0264-410x(98)00072-3. [DOI] [PubMed] [Google Scholar]
- 35.Weinberg A, Zhang L, Brown D. Abstracts of the 6th Conference on Retroviruses and Opportunistic Infections. 1999. Effect of cryopreservation on cell-mediated immunity assay, abstr. 450; p. 152. [Google Scholar]
- 36.Zepp F, Knuf M, Habermehl P, Schimitt H J, Rebsch C, Schmidtke P, Clements R, Slaoui M. Pertussis-specific cell-mediated immunity in infants after vaccination with a tricomponent acellular pertussis vaccine. Infect Immun. 1996;64:4078–4084. doi: 10.1128/iai.64.10.4078-4084.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]