Abstract
Design
There is a strong correlation between dietary intake and allergic diseases. Ultra-processed foods (UPFs) are gradually becoming dominant worldwide and causing health problems for children and adults. We hope to determine whether links exist between UPFs and allergic symptoms.
Methods
We investigated data from 2,736 children (16–19 years) and 4,256 adults (≥20 years) from the National Health and Nutritional Examination Survey (NHANES) 2005–2006. The associations between the mean UPFs contribution to total energy intake and all allergic symptoms (IgE, current asthma, allergy, rash, sneeze, wheeze, eczema, and hay fever) were estimated by weighted multivariate logistic regression.
Results
Logistic regression analysis showed UFPs were negatively associated with IgE levels in children. Those with higher quartiles had a reduced risk from 16% (OR, 0.84, 95%CI, 0.55 to 1.28) to 34% (OR, 0.66, 95%CI, 0.49 to 0.89), p for trend = 0.006. UPFs were also positively related to current asthma in children with an increased risk of 11% (OR, 1.11, 95%CI, 0.79 to 1.56) to 76% (OR, 1.76, 95%CI, 1.10 to 2.82), p for trend = 0.0393. UPFs were also associated with eczema in girls. But there was no association observed between UPFs and allergic symptoms in adults.
Conclusion
Our results suggested that UPFs assessed by the NOVA system were associated with IgE, current asthma in children, and eczema in girls. These results further support the need to test the association of modern dietary patterns with allergic symptoms.
Keywords: IgE, allergy, asthma, eczema, UPFs, NHANES
Introduction
Food allergies are a serious health problem (1), and they are commonly reported worldwide and continue to increase (2, 3). Data from the National Health and Nutrition Examination Survey (NHANES, 2007–2010) show the prevalence of food allergy was 6.5% in children and 10% in adults in the USA (4). While in the HealthNuts cohort, a study conducted in the Australian state of Victoria, there were unique insights into the accurate prevalence of IgE-mediated and oral food challenge-confirmed food allergies, which is 11.0% at age 1 year (5). A nonnegligible annual 5.7% increase in food allergy admissions was noticed in the UK (6). They can result in a wide range of combinations of signs and symptoms and can be involved in any organ system including the skin, the gastrointestinal tract, the respiratory system, and the cardiovascular (1). It is likely to be linked to an increasing preference for a more modern and western diet (7), which is characterized by a high intake of saturated fat, processed foods, pre-packaged foods, and added sugar in dairy products (8). Currently, ultra-processed foods (UPFs), as defined by the NOVA system, have already become a significant part of the western diet (9). They are industrial formulations typically composed of substances derived from additives and foods, including large amounts of added sugar, and usually require no cooking (9). The consumption of UPFs has increased rapidly among children and adults (10, 11). Epidemiological studies indicate that a high intake of UPFs is correlated with the development of several chronic diseases such as obesity (12, 13), insulin resistance (14), metabolic syndrome (15), dyslipidemia (16), hypertension (17), and cardiovascular disease (18). As one of the proxies for a low-quality diet, UPFs have gradually become an important concept in modern and western diets (19).
Substantial correlations have been established between unhealthy or pro-inflammatory diets and the pathogenesis of food allergy or asthma (20, 21). These include the association between a high intake of free fructose-containing beverages and allergy (20). Additionally, studies involving UPFs have suggested that biscuits, sweets or candies, processed meats, drinks, and packaged snacks enhance the risk of asthma and wheeze among Brazilian adolescents (22). However, there is also evidence to suggest the opposite. According to the data from the Pelotas birth cohort study, there were no significant associations found between UPFs consumption and asthma or wheeze during childhood or adolescence (23). To date, associations between UPFs and all sites of allergy-related symptoms have not been well demonstrated. In this article, we examine the relationship between UPFs and IgE and various allergic symptoms based on the data from the NHANES between 2005 and 2006.
Methods
Design
The national cohort study was conducted and utilized the data from 2005 to 2006 from the National Health and Nutrition Examination Survey (NHANES). It was designed as a large, stratified, multistage probability cluster sampling study by the National Center for Health Statistics (NCHS) and CDC to represent the US population.
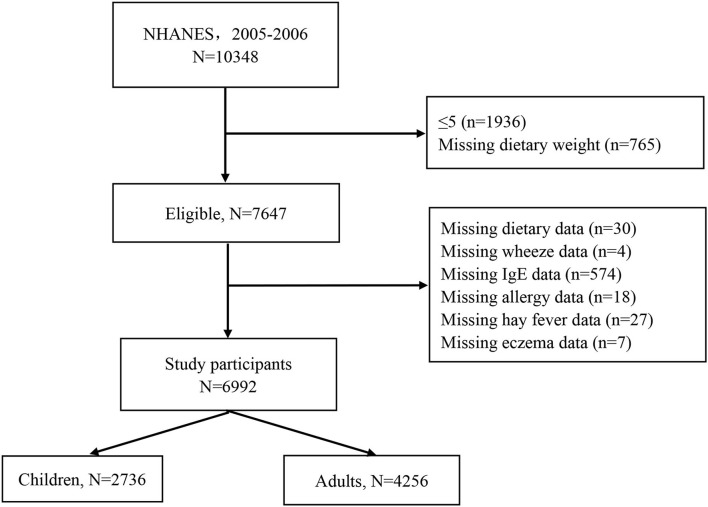
During the 2005–2006 cycle, a total of 10,348 participants were interviewed. We first sequentially excluded 1,936 participants ≤5 years old and with missing dietary weight data (n = 765). Then, children and adults missing dietary data (n = 30), wheeze (n = 4), serum specific IgE (n = 574), allergy (n = 18), hay fever (n = 27), and eczema (n = 7) were excluded. Finally, 2,736 children and 4,256 adults were left for analysis (Figure 1).
Figure 1.
Participants flowchart.
Assessment of UPFs
Trained interviews collected 24-h dietary recalls in-person following the U.S. Department of Agriculture (USDA) Automated Multiple-Pass Method, described in detail in the previous literature (24, 25). The 24-h dietary recall has been proven to be valid in previous studies (10, 11, 26). UPFs intake was assessed by administering two 24-h dietary recalls. All study samples (n = 9,169) had a first-day dietary recall by in-person household interview, and 90.1% (n = 8,264) had a second-day dietary recall by telephone interview. Nutrients were assigned to foods following the USDA Dietary Research Food and Nutrition Database (FNDDS) (27). The 8-digit food code was used to determine the food composition. Food and beverages consumed were recorded in grams, and this was then converted into calories by the FNDDS. UPFs intake was reported as the average intake to the total energy intake from two 24-h diet recalls according to the NOVA system.
NOVA has four food processing levels, namely, unprocessed foods, minimally processed foods, processed culinary in gradients' processed foods, and UPFs. All food items that matched the features of UPFs by the NOVA food classification system were listed and then applied to the FNDDS data (28). We pooled the energy of each individual's UPFs intake. A detailed description of UPFs and calorie estimation has been presented elsewhere (26, 29, 30). We referred to these studies to ensure the consistency and accuracy of food classification with previous studies. A more detailed description of the NOVA classification used in this analysis is shown in Supplementary Table S1.
Assessments of allergic symptoms and sensitization
Allergy sensitization is indicated by a total IgE level of ≥150 kU/L. Self-reported allergic diseases were assessed using allergy, medical conditions, and respiratory health questionnaires during in-personal household interviews. We selected seven primary outcomes as this study's allergic symptoms, including hay fever, allergy, itchy rash, sneeze, wheeze, eczema, and current asthma. In some cases, allergic symptoms could be identified when participants provided both positive responses to questions such as “has a doctor told that you have hay fever/allergy/itchy rash/sneeze/wheeze/ eczema?” and “during the past 12 months, have you had an episode of hay fever/allergy/ itchy rash/ sneeze/ wheeze/ eczema?”. Current asthma was identified by two questions, both with positive responses to questions such as “has a doctor told that you have asthma?” and “do you still have asthma?”.
Covariates
Potential confounding variables were chosen and adjusted in multivariate models. These included sociodemographic data, such as sex (male and female); age (categorized into 6–11 y, 12–19 y, 20–40 y, 40–59 y, and ≥60 y); race (non-Hispanic white, non-Hispanic black, Mexican, and others); family income ratio (<1 and ≥1); BMI [categorized into normal weight <25, 25 ≤ overweight<30, and 30 ≤ obesity by the World Health Organization (WHO) BMI classifications for adults and normal weight (<28.97 kg/m2), overweight (28.97 to <34.57 kg/m2), and obesity (≥34.57 kg/m2) for children according to the age- and gender-specific criteria by a previous study (31)]; and smoke status (categorized into the current smoker, former smoker, and never smoker by self-reported questionnaire for adults). Tobacco exposure among children was assessed and categorized by serum cotinine (32), active smoker (cotinine ≥ 10 ng/ml), second-hand smoker (0.015 ng/ml ≤ cotinine<10 ng/ml), and non-smoker (cotinine < 0.015 ng/ml). The housing characteristics were the presence of mildew smell (yes or no), furry animals (yes or no), and cockroaches (yes or no) in the home.
Statistical analysis
To reflect the characteristics of the NHANES multiple sampling survey (33), data analysis was performed by the R (version 4.2.0, “survey” package). We used a Taylor-series linearization approach to express mean ± standard errors (SEs) for all continuous variables and mean (95%CI) for all categorical variables following the official guidance of NHANES. Then, the data were compared by Student's t-test and Cochran–Mantel–Haenszel Chi-square test. We then used multiple logistic regression models to estimate the odds ratio (OR) for all sites of allergy sensitization and various allergic symptoms comparing quartiles 2, 3, and 4 (a higher intake of UPFs) with quartile 1 of UPFs (%Kcal) among children and adults, respectively. P for trend was calculated using the median value of each quartile as a continuous variable in each model. Stratified analysis was conducted to further explore the consistency of UPFs consumption's effects on allergies in the relevant confounding groups, such as age, gender, and race/ethnicity. Finally, interaction analysis was conducted between UPFs and covariates by including the interaction terms in the multiple logistic regression.
Results
Characteristics of the study participants
The participant characteristics by quartile of daily UPFs energy intake are shown in Table 1. The study population comprised 2,734 children (6–19 years) and 4,257 adults (≥ 20 years). The mean ± SE values of UPFs were 62.1 ± 0.9% for children and 53.3 ± 0.6% for adults. The participants with a higher intake of UPFs were likely to be non-Hispanic black in adults but were less likely to be Mexican American in both children and adults. They were also more likely to be the younger (<60 years) and less likely to be the elder (≥60 years) adults. The participants with a higher intake of UPFs consumption had a lower prevalence of IgE-sensitization in children, from 26.64 to 21.41%, p trend = 0.0162. However, they had a higher prevalence of allergy in children, from 18.22 to 28.46%, p trend = 0.0452, and a higher prevalence of current asthma in children, from 17.30 to 33.31%, p trend = 0.0194. They also had a higher prevalence of wheeze in adults, from 20.16 to 28.31%, p trend = 0.0326.
Table 1.
Characteristics of the study participants according to the quartiles of UPFs (%Kcal) among children and adults.
| Characteristics | by quartiles of UPFs(%Kcal) in children | by quartiles of UPFs(%Kcal) in adults | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | P trend | Q1 | Q2 | Q3 | Q4 | P trend | |
| Energy | 2016.54 (71.27) | 2196.47 (55.31) | 2337.41 (93.99) | 2241.69 (66.26) | 0.0044 | 2120.66 (40.28) | 2139.39 (42.36) | 2225.55 (38.56) | 2304.95 (56.16) | 0.0005 |
| Sex | 0.9444 | 0.743 | ||||||||
| Female | 23.45(2.09) | 26.91(1.73) | 24.30(1.61) | 25.34(1.98) | 23.00(1.06) | 25.60(1.51) | 25.54(1.19) | 25.86(1.31) | ||
| Male | 21.21(1.85) | 28.31(1.53) | 27.95(1.89) | 22.53(1.65) | 25.23(1.88) | 23.44(1.04) | 25.02(1.83) | 26.30(0.93) | ||
| Age | 0.3717 | <0.0001 | ||||||||
| 6–11 | 21.36(2.56) | 31.82(2.47) | 25.63(1.61) | 21.19(1.96) | NA | NA | NA | NA | ||
| 12–19 | 22.93(2.76) | 24.72(1.47) | 26.60(2.30) | 25.75(2.52) | NA | NA | NA | NA | ||
| 20–39 | NA | NA | NA | NA | 20.16(1.72) | 22.91(1.24) | 25.89(1.89) | 31.04(1.24) | ||
| 40–59 | NA | NA | NA | NA | 23.62(1.59) | 24.48(1.32) | 25.17(1.29) | 26.73(1.37) | ||
| ≥ 60 | NA | NA | NA | NA | 30.66(1.77) | 27.21(1.88) | 24.57(0.74) | 17.56(1.37) | ||
| Race/Ethnicity | 0.3877 | 0.0033 | ||||||||
| Non-Hispanic white | 20.06(2.37) | 26.72(1.76) | 28.77(1.76) | 24.44(2.12) | 22.89(1.39) | 24.72(0.86) | 26.59(1.49) | 25.80(0.72) | ||
| Non-Hispanic black | 23.77(2.04) | 27.44(2.13) | 22.18(2.12) | 26.61(2.47) | 16.35(1.35) | 22.05(1.28) | 25.17(1.51) | 36.44(1.90) | ||
| Mexican | 33.18(2.89) | 30.04(2.87) | 22.07(2.55) | 14.71(2.31) | 33.25(2.24) | 28.68(2.79) | 20.74(1.43) | 17.33(1.68) | ||
| Others | 19.40(3.12) | 30.36(5.28) | 21.94(3.73) | 28.30(4.76) | 37.44(2.80) | 22.46(2.98) | 17.52(1.93) | 22.57(3.05) | ||
| FIR | 0.2065 | 0.0414 | ||||||||
| <1 | 30.22(3.06) | 27.28(2.23) | 21.74(2.54) | 20.76(3.18) | 28.57(3.17) | 25.36(2.04) | 25.00(1.81) | 21.07(1.61) | ||
| ≥1 | 20.31(1.96) | 27.60(1.66) | 27.08(1.82) | 25.02(1.94) | 23.46(1.18) | 24.69(0.82) | 25.31(1.17) | 26.54(0.67) | ||
| BMI | 0.6317 | 0.1189 | ||||||||
| Normal | 22.39(1.72) | 28.59(1.42) | 25.64(1.50) | 23.37(1.55) | 24.00(2.04) | 25.79(1.18) | 26.04(2.03) | 24.17(1.26) | ||
| Obesity | 22.13(5.38) | 22.42(4.58) | 31.12(10.22) | 24.33(9.87) | 23.69(1.33) | 23.69(1.01) | 22.83(1.16) | 29.80(1.29) | ||
| Overweight | 21.28(3.66) | 20.08(4.26) | 30.47(5.21) | 28.16(5.52) | 24.80(2.06) | 24.52(1.53) | 26.25(1.73) | 24.43(1.57) | ||
| Tobacco exposure | 0.231 | 0.1421 | ||||||||
| No exposure | 17.01(2.23) | 29.46(2.76) | 27.91(4.13) | 25.62(3.57) | 26.07(2.39) | 26.41(2.49) | 23.32(2.37) | 24.20(2.88) | ||
| Secondhand smoke | 23.21(1.68) | 28.46(1.39) | 25.27(1.58) | 23.07(1.39) | 24.10(1.77) | 24.65(1.32) | 25.46(1.53) | 25.78(1.31) | ||
| Active smoker | 24.81(4.69) | 18.19(3.59) | 28.05(4.42) | 28.95(5.64) | 23.01(2.39) | 23.19(1.89) | 26.02(2.18) | 27.78(1.42) | ||
| Smoke Status | 0.5208 | |||||||||
| Never | NA | NA | NA | NA | 22.97(1.60) | 23.25(1.37) | 26.07(1.05) | 27.72(1.47) | ||
| Former | NA | NA | NA | NA | 26.77(1.55) | 27.88(1.89) | 23.58(1.73) | 21.77(1.87) | ||
| Active | NA | NA | NA | NA | 23.57(2.08) | 23.98(1.70) | 25.42(2.10) | 27.02(1.34) | ||
| Animals | 21.62(5.24) | 31.89(6.56) | 26.66(7.98) | 19.83(4.59) | 0.5238 | 17.14(5.22) | 26.15(5.20) | 25.13(5.24) | 31.59(4.18) | 0.1416 |
| Cockroaches | 28.24(3.39) | 27.65(2.41) | 23.59(4.66) | 20.52(2.41) | 0.0785 | 28.31(1.50) | 21.58(2.09) | 25.95(3.65) | 24.16(2.34) | 0.1849 |
| Mildew | 24.12(2.91) | 26.51(3.47) | 23.05(4.27) | 26.32(4.16) | 0.9465 | 20.69(2.19) | 24.95(2.57) | 26.87(2.43) | 27.49(1.67) | 0.1433 |
| IgE | 26.64(3.55) | 28.50(2.84) | 23.45(2.63) | 21.41(2.41) | 0.0162 | 25.60(2.35) | 25.35(2.30) | 22.35(2.12) | 26.70(1.95) | 0.5597 |
| Allergy | 18.22(2.07) | 25.54(3.50) | 27.78(3.35) | 28.46(3.35) | 0.0452 | 21.46(1.94) | 22.31(2.26) | 28.11(2.03) | 28.13(2.23) | 0.1211 |
| Current asthma | 17.30(2.40) | 24.59(3.93) | 24.80(4.82) | 33.31(3.73) | 0.0194 | 18.54(2.44) | 22.22(2.75) | 30.28(2.56) | 28.96(3.75) | 0.0497 |
| Wheeze | 21.40(2.73) | 27.73(3.46) | 24.49(4.20) | 26.38(3.53) | 0.6914 | 20.16(2.55) | 22.34(2.44) | 29.19(2.47) | 28.31(2.39) | 0.0326 |
| Rash | 19.97(4.30) | 34.07(5.87) | 27.53(5.16) | 18.44(4.02) | 0.3797 | 18.82(3.17) | 23.44(2.94) | 28.01(3.03) | 29.73(3.34) | 0.1291 |
| Sneeze | 21.60(3.41) | 27.37(3.01) | 21.55(2.15) | 29.48(2.76) | 0.2178 | 22.27(1.69) | 24.31(1.21) | 24.46(1.63) | 28.96(1.52) | 0.0957 |
| Hay fever | 15.81(4.56) | 36.13(6.27) | 20.37(6.72) | 27.69(7.22) | 0.522 | 29.45(3.50) | 22.39(3.09) | 23.70(2.54) | 24.46(2.53) | 0.2104 |
| Eczema | 16.74(3.51) | 26.51(3.91) | 29.92(3.54) | 26.82(3.75) | 0.1353 | 20.46(3.72) | 29.51(3.77) | 27.74(4.06) | 22.28(2.88) | 0.795 |
Weighted mean ± Se and Student's t-test for continuous variables.
Weighted %, mean (95% CI), and Cochran–Mantel–Haenszel Chi-square test for categorical variables. Trends across each quartile of UPFs consumption were assessed by t-test.
FIR, family income ratio.
Association of UPFs with allergic symptoms
After conducting the multiple logistic regression analysis, the associations of consumption of UPFs with all sites of allergy-related outcomes among children and adults are shown in Table 2. We found negative and significant associations between UPFs and IgE sensitization among children in all three models. In the fully adjusted models (Model 3), when compared with the lowest quartile, participants with higher quartiles (Q2–Q4) had a decrease in the risk of IgE-sensitization event from 16% (Q2, OR, 0.84, 95%CI, 0.55 to 1.28) to 34% (Q4, OR, 0.66, 95%CI, 0.49 to 0.89), p for trend = 0.006. Additionally, all three models found significant and positive associations with current asthma. The ORs (95% CIs) across the increasing quartiles in Model 3 were 1.11 (0.79, 1.56), 1.12 (0.70, 1.80), and 1.76 (1.10, 2.82) compared with Q1, p for trend = 0.0393. However, we did not find any significant association between UPFs and allergy-related outcomes among adults.
Table 2.
Odds ratios of the associations between UPFs and allergic symptoms in children and adults, NHANES (2005–2006).
| Allergic symptoms | Children | Adults | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model 1 | p trend | Model 2 | p trend | Model 3 | p trend | Model 1 | p trend | Model 2 | p trend | Model 3 | p trend | |
| IgE | 0.0147 | 0.0295 | 0.0062 | 0.5529 | 0.6329 | 0.7133 | ||||||
| Q1 | ref = 1.00 | ref = 1.00 | ||||||||||
| Q2 | 0.82(0.57,1.18) | 0.85(0.57,1.27) | 0.84(0.55,1.28) | 0.96(0.69,1.34) | 1.00(0.72,1.40) | 1.07(0.75,1.52) | ||||||
| Q3 | 0.68(0.43,1.08) | 0.72(0.45,1.17) | 0.68(0.42,1.12) | 0.80(0.55,1.16) | 0.83(0.57,1.22) | 0.89(0.59,1.35) | ||||||
| Q4 | 0.68(0.50,0.93) | 0.69(0.51,0.95) | 0.66(0.49,0.89) | 0.95(0.72,1.26) | 0.96(0.71,1.31) | 0.98(0.68,1.40) | ||||||
| Allergy | 0.0437 | 0.1019 | 0.0797 | 0.1344 | 0.2476 | 0.2477 | ||||||
| Q1 | ref = 1.00 | ref = 1.00 | ||||||||||
| Q2 | 1.14(0.77,1.69) | 1.13(0.75,1.69) | 1.09(0.71,1.69) | 1.02(0.74,1.41) | 0.98(0.71,1.37) | 0.99(0.70,1.39) | ||||||
| Q3 | 1.35(0.90,2.03) | 1.29(0.83,2.02) | 1.27(0.78,2.06) | 1.33(1.04,1.69) | 1.25(0.98,1.61) | 1.28(1.01,1.62) | ||||||
| Q4 | 1.56(1.03,2.35) | 1.49(0.95,2.32) | 1.46(0.96,2.23) | 1.26(0.87,1.82) | 1.19(0.82,1.72) | 1.17(0.80,1.72) | ||||||
| Current asthma | 0.0175 | 0.0317 | 0.0393 | 0.0556 | 0.1181 | 0.1665 | ||||||
| Q1 | ref = 1.00 | ref = 1.00 | ||||||||||
| Q2 | 1.16(0.81,1.67) | 1.16(0.82,1.63) | 1.11(0.79, 1.56) | 1.19(0.92,1.54) | 1.15(0.88,1.51) | 1.17(0.90,1.54) | ||||||
| Q3 | 1.25(0.74,2.10) | 1.21(0.73,2.00) | 1.12(0.70, 1.80) | 1.61(1.20,2.17) | 1.56(1.12,2.16) | 1.54(1.10,2.17) | ||||||
| Q4 | 1.96(1.24,3.08) | 1.87(1.19,2.95) | 1.76(1.10, 2.82) | 1.48(0.99,2.23) | 1.40(0.91,2.15) | 1.38(0.86,2.22) | ||||||
| Eczema | 0.1407 | 0.2003 | 0.2607 | 0.792 | 0.4103 | 0.8288 | ||||||
| Q1 | ref = 1.00 | ref = 1.00 | ||||||||||
| Q2 | 1.31(0.68,2.51) | 1.22(0.61,2.41) | 1.15(0.55,2.44) | 1.46(0.82,2.57) | 1.37(0.79,2.39) | 1.53(0.84,2.79) | ||||||
| Q3 | 1.60(0.94,2.71) | 1.52(0.87,2.65) | 1.54(0.88,2.69) | 1.32(0.83,2.09) | 1.19(0.73,1.96) | 1.30(0.80,2.13) | ||||||
| Q4 | 1.57(0.82,2.99) | 1.48(0.76,2.87) | 1.33(0.69,2.56) | 1.01(0.64,1.58) | 0.91(0.58,1.42) | 1.05(0.63,1.73) | ||||||
| Hay fever | 0.4822 | 0.614 | 0.8363 | 0.2198 | 0.1772 | 0.1653 | ||||||
| Q1 | ref = 1.00 | ref = 1.00 | ||||||||||
| Q2 | 1.87(0.77,4.57) | 1.90(0.78, 4.66) | 1.86(0.79, 4.34) | 0.73(0.48,1.09) | 0.69(0.46,1.03) | 0.70(0.45, 1.08) | ||||||
| Q3 | 1.10(0.44,2.73) | 1.09(0.43, 2.74) | 1.06(0.43, 2.62) | 0.75(0.48,1.17) | 0.68(0.43,1.09) | 0.62(0.39, 0.97) | ||||||
| Q4 | 1.65(0.66,4.15) | 1.55(0.62, 3.90) | 1.37(0.56, 3.35) | 0.75(0.52,1.08) | 0.70(0.46,1.05) | 0.72(0.47, 1.10) | ||||||
| Rash | 0.4231 | 0.5524 | 0.6024 | 0.1009 | 0.0872 | 0.1071 | ||||||
| Q1 | ref = 1.00 | ref = 1.00 | ||||||||||
| Q2 | 1.40(0.77,2.55) | 1.44(0.80,2.58) | 1.52(0.78, 2.96) | 1.24(0.76,2.02) | 1.28(0.77,2.13) | 1.26(0.71,2.22) | ||||||
| Q3 | 1.18(0.68,2.07) | 1.25(0.71,2.20) | 1.38(0.78, 2.44) | 1.46(0.92,2.30) | 1.51(0.96,2.37) | 1.52(0.94,2.44) | ||||||
| Q4 | 0.86(0.47,1.56) | 0.88(0.48,1.63) | 0.88(0.42, 1.85) | 1.50(0.91,2.47) | 1.58(0.95,2.63) | 1.47(0.88,2.46) | ||||||
| Sneeze | 0.208 | 0.3582 | 0.3872 | 0.0899 | 0.1342 | 0.0985 | ||||||
| Q1 | ref = 1.00 | |||||||||||
| Q2 | 1.03(0.62,1.70) | 1.02(0.62,1.68) | 1.04(0.61,1.79) | 1.11(0.87,1.41) | 1.08(0.85,1.37) | 1.09(0.85,1.40) | ||||||
| Q3 | 0.81(0.49,1.33) | 0.77(0.45,1.31) | 0.79(0.44,1.43) | 1.07(0.82,1.40) | 1.03(0.79,1.34) | 1.06(0.80,1.40) | ||||||
| Q4 | 1.41(0.96,2.06) | 1.33(0.90,1.95) | 1.31(0.86,2.01) | 1.33(0.99,1.79) | 1.30(0.97,1.74) | 1.30(0.98,1.74) | ||||||
| Wheeze | 0.6782 | 0.7729 | 0.9468 | 0.0312 | 0.0517 | 0.0658 | ||||||
| Q1 | ref = 1.00 | ref = 1.00 | ||||||||||
| Q2 | 1.05(0.72,1.53) | 1.04(0.71,1.52) | 1.10(0.76, 1.58) | 1.10(0.76,1.60) | 1.10(0.76,1.59) | 1.16(0.77,1.75) | ||||||
| Q3 | 0.97(0.61,1.54) | 0.95(0.58,1.55) | 0.87(0.53, 1.43) | 1.46(0.99,2.16) | 1.45(0.98,2.13) | 1.53(1.02,2.31) | ||||||
| Q4 | 1.17(0.62,2.21) | 1.13(0.59,2.17) | 1.09(0.58, 2.05) | 1.36(1.00,1.84) | 1.35(1.00,1.82) | 1.37(0.94,1.99) | ||||||
Model 1 adjusted for none.
Model 2 adjusted for gender, age, and race/ethnicity.
Model 3 adjusted for gender, age, race/ethnicity, family income ratio, BMI (categorical variables), smoke status (self-reported in adults), tobacco exposure (defined by cotinine), animals, cockroaches, and mildew.
P trend was calculated by using the median value of each quartile as a continuous variable in each model.
Ref, reference.
In addition, when using IgE as a continuous variable (Supplementary Table S2), the multiple linear regression also showed there is a significant association among children with β coefficient (95% CIs), −17.46 (−90.42, 55.50), −68.75 (−116.33, −21.16), and −56.12 (−115.09, 2.86), p for trend = 0.02.
Subgroup and interaction analyzes
Subgroup analyzes were performed by sex, age, and race/ethnicity (white, the others) among children and adults respectively (Table 3 and Supplementary Table S3). A significant interaction was observed between UPFs and the prevalence of eczema in children when stratified by sex (p = 0.02). We, thus, repeated the multiple logistic regression analysis in children after stratification by sex (Table 4). In this analysis, girls with higher UPFs consumption (Q2–Q4) had significantly increased odds of eczema (p for trend = 0.0114). In contrast, no significant association was found between UPFs and eczema among male children.
Table 3.
The association between UPFs and allergic symptoms, stratified by selected subgroups in children.
| IgE | Allergy | Current asthma | Eczema | Hay fever | Rash | Sneeze | Wheeze | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Age | 6–11 | Q1 | ref = 1.00 | ref = 1.00 | ref = 1.00 | ref = 1.00 | ref = 1.00 | ref = 1.00 | ref = 1.00 | ref = 1.00 |
| Q2 | 1.05(0.38, 2.86) | 1.33(0.49,3.62) | 2.02(0.94, 4.36) | 1.23(0.51, 2.98) | 3.91(0.82, 18.59) | 1.34(0.49, 3.66) | 1.18(0.50, 2.82) | 1.13(0.56, 2.30) | ||
| Q3 | 0.50(0.20, 1.26) | 0.95(0.31,2.88) | 1.59(0.49, 5.15) | 2.57(1.15, 5.75) | 1.61(0.15, 17.20) | 2.01(0.75, 5.39) | 0.79(0.28, 2.24) | 0.93(0.48, 1.81) | ||
| Q4 | 0.94(0.49, 1.79) | 2.69(1.28,5.66) | 2.12(0.78, 5.75) | 1.13(0.48, 2.70) | 2.50(0.39, 15.94) | 0.56(0.15, 2.14) | 1.46(0.69, 3.09) | 1.38(0.60, 3.17) | ||
| 12–19 | Q2 | 0.77(0.56,1.07) | 0.93(0.50,1.75) | 0.69(0.36, 1.33) | 1.08(0.48, 2.44) | 1.26(0.37, 4.25) | 1.67(0.76, 3.68) | 0.94(0.51,1.72) | 1.10(0.59, 2.05) | |
| Q3 | 0.80(0.50,1.28) | 1.46(0.74,2.90) | 0.93(0.51, 1.69) | 0.84(0.39, 1.80) | 0.73(0.25, 2.15) | 0.96(0.36, 2.59) | 0.78(0.44,1.38) | 0.85(0.34, 2.12) | ||
| Q4 | 0.59(0.45,0.78) | 1.00(0.50,2.00) | 1.64(0.89, 3.02) | 1.41(0.53, 3.76) | 0.93(0.29, 3.03) | 1.11(0.61, 2.04) | 1.24(0.80,1.92) | 0.99(0.37, 2.62) | ||
| p for interaction | 0.65 | 0.14 | 0.8 | 0.65 | 0.26 | 0.97 | 0.76 | 0.71 | ||
| Sex | female | Q1 | ref = 1.00 | ref = 1.00 | ref = 1.00 | ref = 1.00 | ref = 1.00 | ref = 1.00 | ref = 1.00 | ref = 1.00 |
| Q2 | 1.11(0.61, 2.01) | 1.44(1.11,1.87) | 1.20(0.68, 2.14) | 2.46(0.80, 7.58) | 4.64(2.02, 10.65) | 1.29(0.58, 2.89) | 1.06(0.57, 1.99) | 0.88(0.41, 1.89) | ||
| Q3 | 0.71(0.42, 1.19) | 1.85(1.10,3.14) | 2.01(0.85, 4.75) | 4.40(1.87,10.33) | 2.17(0.49, 9.53) | 1.60(0.83, 3.09) | 0.88(0.45, 1.73) | 1.54(0.63, 3.77) | ||
| Q4 | 0.65(0.36, 1.20) | 1.87(1.03,3.41) | 1.55(0.71, 3.41) | 2.88(1.05, 7.95) | 2.58(0.79, 8.39) | 0.66(0.28, 1.57) | 1.39(0.89, 2.15) | 1.31(0.58, 2.97) | ||
| male | Q2 | 0.66(0.35,1.26) | 0.77(0.36,1.64) | 1.03(0.59, 1.79) | 0.66(0.28, 1.55) | 0.47(0.11, 2.06) | 1.76(0.54, 5.69) | 0.98(0.49, 1.96) | 1.17(0.70, 1.95) | |
| Q3 | 0.68(0.36,1.27) | 0.81(0.40,1.64) | 0.60(0.29, 1.22) | 0.64(0.29, 1.40) | 0.45(0.14, 1.49) | 0.85(0.27, 2.66) | 0.72(0.33, 1.59) | 0.50(0.21, 1.19) | ||
| Q4 | 0.68(0.38,1.22) | 1.09(0.59,2.02) | 2.04(1.08, 3.83) | 0.72(0.29, 1.82) | 0.65(0.14, 2.96) | 1.04(0.35, 3.10) | 1.27(0.61, 2.63) | 0.91(0.44, 1.88) | ||
| p for interaction | 0.89 | 0.16 | 1.0 | 0.02 | 0.16 | 0.93 | 0.38 | 0.23 | ||
| Race/ | White | Q1 | ref = 1.00 | ref = 1.00 | ref = 1.00 | ref = 1.00 | ref = 1.00 | ref = 1.00 | ref = 1.00 | ref = 1.00 |
| Ethnicity | Q2 | 0.57(0.34,0.96) | 0.93(0.52, 1.66) | 1.21(0.64, 2.29) | 1.09(0.42, 2.85) | 2.79(1.05, 7.38) | 2.11(0.60, 7.49) | 1.04(0.51, 2.15) | 1.41(0.62, 3.18) | |
| Q3 | 0.65(0.28,1.49) | 1.15(0.54, 2.43) | 1.27(0.52, 3.08) | 1.24(0.64, 2.40) | 1.26(0.40, 3.98) | 1.67(0.58, 4.85) | 0.68(0.30, 1.55) | 1.09(0.40, 2.95) | ||
| Q4 | 0.68(0.44,1.05) | 1.28(0.75, 2.19) | 2.08(0.99, 4.37) | 1.08(0.50, 2.36) | 1.03(0.30, 3.49) | 0.89(0.25, 3.20) | 1.15(0.63, 2.09) | 1.17(0.36, 3.84) | ||
| the others | Q2 | 1.25(0.81, 1.92) | 1.44(0.73,2.81) | 0.94(0.58, 1.52) | 1.36(0.61,3.03) | 0.54(0.13, 2.31) | 1.06(0.47, 2.36) | 0.96(0.58,1.60) | 0.74(0.44, 1.26) | |
| Q3 | 0.78(0.50, 1.21) | 1.63(0.91,2.94) | 0.89(0.43, 1.86) | 2.35(1.05,5.29) | 0.75(0.17, 3.40) | 1.06(0.69, 1.64) | 1.11(0.67,1.83) | 0.60(0.36, 1.00) | ||
| Q4 | 0.70(0.45, 1.11) | 1.91(1.02,3.60) | 1.33(0.72, 2.46) | 2.32(0.98,5.47) | 2.27(0.50, 10.26) | 0.86(0.42, 1.79) | 1.70(1.26,2.28) | 1.01(0.66, 1.55) | ||
| p for interaction | 0.67 | 0.35 | 0.32 | 0.28 | 0.14 | 0.98 | 0.28 | 0.8 | ||
All models were adjusted for gender, age, race/ethnicity, family income ratio, BMI (categorical variables), smoke status (self-reported in adults), tobacco exposure (defined by cotinine), animals, cockroaches, and mildew except the subgroup variable.
Table 4.
The associations between UPFs and eczema by sex in children, NHANES 2005–2006.
| UPFs | Eczema in female | Eczema in male |
|---|---|---|
| Q1 | ref = 1.00 | ref = 1.00 |
| Q2 | 2.39 (0.70, 8.15) | 0.60 (0.24, 1.50) |
| Q3 | 4.81 (1.87, 12.38) | 0.55 (0.24, 1.27) |
| Q4 | 2.90 (0.97, 8.74) | 0.67 (0.26, 1.71) |
| p for trend = 0.0114 | p for trend =0.4107 |
All models were adjusted for age, race/ethnicity, family income ratio, BMI, tobacco exposure, animals, cockroaches, and mildew.
Discussion
In this nationwide population-based cross-sectional study, UPFs were significantly and negatively associated with IgE sensitization and were positively related to the prevalence of current asthma in children. Although no significant association was found for eczema, further stratified and interaction analyzes showed that the association was significant in girls.
Ultra-processed foods are not considered “real food” but affect human health by complex mechanisms involving the synergic effects of additives and nutrients lacking in them (34). However, information regarding their role in the allergic immune response is limited. IgE-mediated (allergic) food reaction is one of the major mechanisms of allergic reactions (35). The reaction is characterized by adverse immunological responses to specific food proteins. Considering the results from previous studies, higher levels of total IgE at baseline in participants were associated with increased intake of food proteins, such as egg, milk, peanut, and tree nuts (36). Because of the characteristics of UPFs (37), in patients with a higher intake of UPFs, it is difficult for the body to produce an allergic reaction (38, 39). On the other hand, it was reported that IgE sensitization and food hypersensitivity declined with increasing age (40). Our study found that IgE association was present in adults.
Allergic asthma is increasing, and diet changes represent a key factor in the increasing allergies (41). Fast food, processed food, and processed meat consumption in childhood have been proven to be associated with asthma development (42). Vitamin A, vitamin D, trace elements, and fiber could play a defensive role in airway immune reactions (43). A large retrospective study of 109,104 Brazilian adolescents [Brazilian study (22)] found positive associations between UPFs consumption and asthma. Another study of 971 adults reported that a high intake of processed meat causes worse asthma symptoms (44). Studies have provided positive associations between excessive fructose and the development of asthma among children (45, 46). These results expand on the knowledge about the relationship between UPFs and asthma. Our study also provided such a significant association. However, a crucial assumption underlying this analysis is that participants' exposure to UPFs is unlikely to vary heavily over the past year. Thus, we need to study more prospectively to reveal their association.
Eczema is a common chronic inflammatory skin disease in children (41, 47). Based on the data from the Isle of Wight Birth Cohort, the previous result suggested that men and women may experience various courses of eczema (48). The developmental trajectories indicated that the prevalence of eczema is equal to or slightly higher in men during adolescence but predominates in women during post-puberty (48). Our finding of a positive association between UPFs and eczema in girls suggests that girls with eczema are more susceptible to such low-quality diets. Besides, it is noticed that the dose–response trend declined in the highest quartile which suggests a potential threshold effect.
The possible and major mechanisms of food-induced hypersensitivity reactions include dual allergen exposure, the vitamin D hypothesis, and the hygiene hypothesis (35). A high intake of UPFs represents a high intake of processed foods, saturated fat, and sugar, but fewer proteins (allergens) (10, 11). It may contribute to decreased immediate-related hypersensitivity reactions (22). However, this does favor the formation of allergic immune defense in childhood (43). Previous studies have shown a reduction in allergic symptoms by repeated exposures to low doses of the allergen in children (49, 50). Recently, the Learning Early About Peanut Allergy (LEAP) study has validated that the onset of peanut allergy declines after low- and moderate-risk children (4–11 months of age) are exposed to peanuts at an early stage (51, 52). It has also shown that the effect is long-lasting after 1 year of continuing avoidance (53). In addition, available evidence has shown that the gut microbiome is always implicated in the development of food allergies (54, 55). Commensal microbiota can modulate immune development and the formation of a healthy immune response to food (56). On the other hand, various diets influence intestinal permeability and gut microbiome (57, 58). Some observational studies have provided insight into the association between vitamin D deficiency and food allergies in children (59). The insufficiency of vitamin D is identified frequently in multiple food allergies compared with children with single food allergies (60). At the same time, vitamins and trace elements, including vitamin D, are impossible to acquire from UPFs. Additionally, the development of food allergies depends on the combination of the dose, method of administration, and duration of exposure at an early time (36).
There are two main strengths of our study. The first is that all UPFs were identified from the individual's daily dietary intake rather than some specific components such as free fructose (20) and cookies (22). The second strength is that the data were based on an excellent, large, nationally representative sample to better present the associations between UPFs and allergic outcomes in this study. Due to the observational and cross-sectional nature of the NHANES database study, there are still several limitations. First, it was restricted to making causal inferences, as it is a cross-sectional study. Second, the reliability of such information based on self-reported questionnaire data is always an issue. Such questionnaires might be limited by recall bias, untruthful answers, etc. Third, IgE sensitization was simply defined as total IgE > 150 KU/L, as it was impossible to distinguish IgE levels caused by food allergy and/or other pathologies such as parasitic infection, auto-immune disorders, and neoplastic disease in samples of NHANES; it is needed to know that IgE sensitization has a certain expansion in this paper. Finally, UPFs were classified by NOVA, but the NHANES dietary survey was not specially designed to distinguish them according to NOVA. In the secondary classification, there is a certain misclassification bias that inevitably exists.
In conclusion, our study provided some evidence for the hypothesis that significant associations exist between UPFs and allergy symptoms in children and adolescents. As a considerable component of the modern and western diet, UPFs are new and important concepts based on the NOVA classification; awareness of its impact on allergy might help prompt the public about natural dietary patterns, which could improve the allergic immune defense in childhood.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found here: https://www.cdc.gov/nchs/nhanes/search/default.aspx.
Ethics statement
Detailed methods and protocols for the NHANES study were approved by the CDC/NCHS Research Ethics Review Board. They are publicly available through the CDC.gov website; this includes informed consent procedures for all participants. All methods in this study were performed according to the relevant guidelines and regulations. This study was exempt from human subject ethical review as the data are freely available in the public domain.
Author contributions
WK conceived and designed the study. WK and YX extracted the data and analyzed and interpreted the data. WK and JZ completed the statistical analyzes and analyzed the data. WK and CC contributed to drafting and editing the paper and full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. All authors have given the final approval of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The data from NHANES collection were sponsored by the CDC.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2022.1038141/full#supplementary-material
References
- 1.Renz H, Allen K, Sicherer S, Sampson H, Lack G, Beyer K, et al. Food allergy. Nat Rev Dis Primers. (2018) 4:17098. 10.1038/nrdp.2017.98 [DOI] [PubMed] [Google Scholar]
- 2.Clarke A, Elliott S, St Pierre Y, Soller L, La Vieille S, Ben-Shoshan M. Temporal trends in prevalence of food allergy in Canada. J Allergy Clin Immunol Pract. (2020) 8:1428–30.e5. 10.1016/j.jaip.2019.10.021 [DOI] [PubMed] [Google Scholar]
- 3.Keet C, Savage J, Seopaul S, Peng R, Wood R, Matsui E. Temporal trends and racial/ethnic disparity in self-reported pediatric food allergy in the United States. Ann Allergy Asthma Immunol. (2014) 112:222–9.e3. 10.1016/j.anai.2013.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McGowan E, Keet C. Prevalence of self-reported food allergy in the National Health and Nutrition Examination Survey (NHANES) 2007-2010. J Allergy Clin Immunol. (2013) 132:1216–9.e5. 10.1016/j.jaci.2013.07.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peters R, Koplin J, Gurrin L, Dharmage S, Wake M, Ponsonby A, et al. The prevalence of food allergy and other allergic diseases in early childhood in a population-based study: HealthNuts age 4-year follow-up. J Allergy Clin Immunol. (2017) 140:145–53.e8. 10.1016/j.jaci.2017.02.019 [DOI] [PubMed] [Google Scholar]
- 6.Baseggio Conrado A, Ierodiakonou D, Gowland M, Boyle R, Turner P. Food anaphylaxis in the United Kingdom: analysis of national data, 1998-2018. BMJ (Clinical research ed). (2021) 372:n251. 10.1136/bmj.n251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Trompette A, Gollwitzer E, Yadava K, Sichelstiel A, Sprenger N, Ngom-Bru C, et al. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat Med. (2014) 20:159–66. 10.1038/nm.3444 [DOI] [PubMed] [Google Scholar]
- 8.Cordain L, Eaton S, Sebastian A, Mann N, Lindeberg S, Watkins B, et al. Origins and evolution of the Western diet: health implications for the 21st century. Am J Clin Nutr. (2005) 81:341–54. 10.1093/ajcn.81.2.341 [DOI] [PubMed] [Google Scholar]
- 9.Monteiro C, Moubarac J, Cannon G, Ng S, Popkin B. Ultra-processed products are becoming dominant in the global food system. Obes Rev. (2013) 14(Suppl. 2):21–8. 10.1111/obr.12107 [DOI] [PubMed] [Google Scholar]
- 10.Juul F, Parekh N, Martinez-Steele E, Monteiro C, Chang V. Ultra-processed food consumption among US adults from 2001 to 2018. Am J Clin Nutr. (2022) 115:211–21. 10.1093/ajcn/nqab305 [DOI] [PubMed] [Google Scholar]
- 11.Wang L, Martínez Steele E, Du M, Pomeranz J, O'Connor L, Herrick K, et al. Trends in consumption of ultraprocessed foods among US youths aged 2-19 years, 1999-2018. JAMA. (2021) 326:519–30. 10.1001/jama.2021.10238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vedovato G, Vilela S, Severo M, Rodrigues S, Lopes C, Oliveira A. Ultra-processed food consumption, appetitive traits and BMI in children: a prospective study. Br J Nutr. (2021) 125:1427–36. 10.1017/S0007114520003712 [DOI] [PubMed] [Google Scholar]
- 13.Mendonça R, Pimenta A, Gea A, de la Fuente-Arrillaga C, Martinez-Gonzalez M, Lopes A, et al. Ultraprocessed food consumption and risk of overweight and obesity: the University of Navarra Follow-Up (SUN) cohort study. Am J Clin Nutr. (2016) 104:1433–40. 10.3945/ajcn.116.135004 [DOI] [PubMed] [Google Scholar]
- 14.Duan M, Vinke P, Navis G, Corpeleijn E, Dekker L. Ultra-processed food and incident type 2 diabetes: studying the underlying consumption patterns to unravel the health effects of this heterogeneous food category in the prospective Lifelines cohort. BMC Med. (2022) 20:7. 10.1186/s12916-021-02200-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Costa de Miranda R, Rauber F, Levy R. Impact of ultra-processed food consumption on metabolic health. Curr Opin Lipidol. (2021) 32:24–37. 10.1097/MOL.0000000000000728 [DOI] [PubMed] [Google Scholar]
- 16.Donat-Vargas C, Sandoval-Insausti H, Rey-García J, Moreno-Franco B, Åkesson A, Banegas J, et al. High consumption of ultra-processed food is associated with incident dyslipidemia: a prospective study of older adults. J Nutr. (2021) 151:2390–8. 10.1093/jn/nxab118 [DOI] [PubMed] [Google Scholar]
- 17.Mendonça R, Lopes A, Pimenta A, Gea A, Martinez-Gonzalez M, Bes-Rastrollo M. Ultra-Processed food consumption and the incidence of hypertension in a mediterranean cohort: the seguimiento universidad de navarra project. Am J Hypertens. (2017) 30:358–66. 10.1093/ajh/hpw137 [DOI] [PubMed] [Google Scholar]
- 18.Castro-Barquero S, Estruch R. Ultra-processed food consumption and disease: the jury is still out. Eur Heart J. (2022) 43:225–7. 10.1093/eurheartj/ehab795 [DOI] [PubMed] [Google Scholar]
- 19.Monteiro C. Nutrition and health. The issue is not food, nor nutrients, so much as processing. Public Health Nutr. (2009) 12:729–31. 10.1017/S1368980009005291 [DOI] [PubMed] [Google Scholar]
- 20.Yu R, Yang B, Cai L, Lu X, Wang X. Excess free fructose beverages and allergy in children and adolescents: results from NHANES 2005-2006. Ann Fam Med. (2018) 16:408–18. 10.1370/afm.2292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Han Y, Forno E, Shivappa N, Wirth M, Hébert J, Celedón J. The dietary inflammatory index and current wheeze among children and adults in the United States. J Allergy Clin Immunol Pract. (2018) 6:834–41.e2. 10.1016/j.jaip.2017.12.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Melo B, Rezende L, Machado P, Gouveia N, Levy R. Associations of ultra-processed food and drink products with asthma and wheezing among Brazilian adolescents. Pediatr Allergy Immunol. (2018) 29:504–11. 10.1111/pai.12911 [DOI] [PubMed] [Google Scholar]
- 23.Machado Azeredo C, Cortese M, Costa C, Bjornevik K, Barros A, Barros F, et al. Ultra-processed food consumption during childhood and asthma in adolescence: data from the 2004 Pelotas birth cohort study. Pediatr Allergy Immunol. (2020) 31:27–37. 10.1111/pai.13126 [DOI] [PubMed] [Google Scholar]
- 24.Moshfegh A, Rhodes D, Baer D, Murayi T, Clemens J, Rumpler W, et al. The US department of agriculture automated multiple-pass method reduces bias in the collection of energy intakes. Am J Clin Nutr. (2008) 88:324–32. 10.1093/ajcn/88.2.324 [DOI] [PubMed] [Google Scholar]
- 25.Blanton C, Moshfegh A, Baer D, Kretsch M. The USDA automated multiple-pass method accurately estimates group total energy and nutrient intake. J Nutr. (2006) 136:2594–9. 10.1093/jn/136.10.2594 [DOI] [PubMed] [Google Scholar]
- 26.Zhang Z, Jackson S, Martinez E, Gillespie C, Yang Q. Association between ultraprocessed food intake and cardiovascular health in US adults: a cross-sectional analysis of the NHANES 2011-2016. Am J Clin Nutr. (2021) 113:428–36. 10.1093/ajcn/nqaa276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Service . UAR. Food and Nutrient Database for Dietary Studies. [Internet]. Available online at: https://wwwarsusdagov/northeast-area/beltsville-md-bhnrc/beltsville-human-nutrition-research-center/food-surveys-research-group/docs/fndds/ (accessed July 31,2022).
- 28.Martínez Steele E, Baraldi L, Louzada M, Moubarac J, Mozaffarian D, Monteiro C. Ultra-processed foods and added sugars in the US diet: evidence from a nationally representative cross-sectional study. BMJ Open. (2016) 6:e009892. 10.1136/bmjopen-2015-009892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Neri D, Martinez-Steele E, Monteiro C, Levy R. Consumption of ultra-processed foods and its association with added sugar content in the diets of US children, NHANES 2009-2014. Pediatr Obes. (2019) 14:e12563. 10.1111/ijpo.12563 [DOI] [PubMed] [Google Scholar]
- 30.Zheng L, Sun J, Yu X, Zhang D. Ultra-Processed food is positively associated with depressive symptoms among United States adults. Front Nutr. (2020) 7:600449. 10.3389/fnut.2020.600449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Llorca-Colomer F, Murillo-Llorente MT, Legidos-García ME, Palau-Ferré A, Pérez-Bermejo M. Differences in classification standards for the prevalence of overweight and obesity in children. A Systematic Review and Meta-Analysis. Clin Epidemiol. (2022) 14:1031–52. 10.2147/CLEP.S375981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hukkanen J, Jacob P, 3rd, Benowitz NL. Metabolism and disposition kinetics of nicotine. Pharmacol Rev. (2005) 57:79–115. 10.1124/pr.57.1.3 [DOI] [PubMed] [Google Scholar]
- 33.CDC . NHANES Survey Methods and Analytic Guidelines. [Internet]. [cited July 31,2022] Available from: https://wwwn.cdc.gov/nchs/nhanes/analyticguidelines.aspx#analytic-guidelines. [Google Scholar]
- 34.Srour B, Chazelas E, Touvier M. [Ultra-processed food : from research to guidelines]. Rev Prat. (2021) 71:1107–12. Available online at: https://www.larevuedupraticien.fr/article/aliments-ultra-transformes-de-la-recherche-aux-recommandations [PubMed] [Google Scholar]
- 35.Sindher S, Long A, Chin A, Hy A, Sampath V, Nadeau K, et al. Food allergy, mechanisms, diagnosis and treatment: innovation through a multi-targeted approach. Allergy. (2022) 77:2937–48. 10.1111/all.15418 [DOI] [PubMed] [Google Scholar]
- 36.Yang H, Qu Y, Gao Y, Sun S, Ding R, Cang W, et al. Role of the dietary components in food allergy: a comprehensive review. Food Chem. (2022) 386:132762. 10.1016/j.foodchem.2022.132762 [DOI] [PubMed] [Google Scholar]
- 37.Gibney M. Ultra-processed foods: definitions and policy issues. Curr Dev Nutr. (2019) 3:nzy077. 10.1093/cdn/nzy077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sdona E, Ekström S, Andersson N, Håkansson N, Wolk A, Westman M, et al. Dietary fibre in relation to asthma, allergic rhinitis and sensitization from childhood up to adulthood. Clin Transl Allergy. (2022) 12:e12188. 10.1002/clt2.12188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alexiou A, Höfer V, Dölle-Bierke S, Grünhagen J, Zuberbier T, Worm M. Elicitors and phenotypes of adult patients with proven IgE-mediated food allergy and non-immune-mediated food hypersensitivity to food additives. Clin Exp Allergy. (2022) 10.1111/cea.14203 [DOI] [PubMed] [Google Scholar]
- 40.Patelis A, Gunnbjörnsdottir M, Borres M, Burney P, Gislason T, Torén K, et al. Natural history of perceived food hypersensitivity and IgE sensitisation to food allergens in a cohort of adults. PLoS ONE. (2014) 9:e85333. 10.1371/journal.pone.0085333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu W, Cai J, Sun C, Zou Z, Zhang J, Huang C. Time-trends for eczema prevalences among children and adults from 1985 to 2015 in China: a systematic review. BMC Public Health. (2022) 22:1294. 10.1186/s12889-022-13650-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hancu A, Mihaltan F, Radulian G. Asthma and ultra-processed food. Maedica. (2019) 14:402–7. 10.26574/maedica.2019.14.4.402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gozzi-Silva S, Teixeira F, Duarte A, Sato M, Oliveira L. Immunomodulatory role of nutrients: how can pulmonary dysfunctions improve? Front Nutr. (2021) 8:674258. 10.3389/fnut.2021.674258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li Z, Rava M, Bédard A, Dumas O, Garcia-Aymerich J, Leynaert B, et al. Cured meat intake is associated with worsening asthma symptoms. Thorax. (2017) 72:206–12. 10.1136/thoraxjnl-2016-208375 [DOI] [PubMed] [Google Scholar]
- 45.DeChristopher L, Tucker K. Excess free fructose, high-fructose corn syrup and adult asthma: the framingham offspring cohort. Br J Nutr. (2018) 119:1157–67. 10.1017/S0007114518000417 [DOI] [PubMed] [Google Scholar]
- 46.DeChristopher L, Tucker K. Excess free fructose, apple juice, high fructose corn syrup and childhood asthma risk-the National Children's Study. Nutr J. (2020) 19:60. 10.1186/s12937-020-00578-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Futamura M, Hiramitsu Y, Kamioka N, Yamaguchi C, Umemura H, Nakanishi R, et al. Prevalence of infantile wheezing and eczema in a metropolitan city in Japan: a complete census survey. PLoS ONE. (2022) 17:e0268092. 10.1371/journal.pone.0268092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ziyab A, Mukherjee N, Zhang H, Arshad S, Karmaus W. Sex-specific developmental trajectories of eczema from infancy to age 26 years: a birth cohort study. Clin Exp Allergy. (2022) 52:416–25. 10.1111/cea.14068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ulfman L, Tsuang A, Sprikkelman A, Goh A, van Neerven R. Relevance of early introduction of cow's milk proteins for prevention of cow's milk allergy. Nutrients. (2022) 14:2659. 10.3390/nu14132659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yakaboski E, Robinson L, Arroyo A, Espinola J, Geller R, Sullivan A, et al. Early introduction of food allergens and risk of developing food allergy. Nutrients. (2021) 13:2318. 10.3390/nu13072318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Du Toit G, Roberts G, Sayre P, Plaut M, Bahnson H, Mitchell H, et al. Identifying infants at high risk of peanut allergy: the learning early about peanut allergy (LEAP) screening study. J Allergy Clin Immunol. (2013) 131:135–43.e1-12. 10.1016/j.jaci.2012.09.015 [DOI] [PubMed] [Google Scholar]
- 52.Feeney M, Du Toit G, Roberts G, Sayre P, Lawson K, Bahnson H, et al. Impact of peanut consumption in the LEAP Study: feasibility, growth, and nutrition. J Allergy Clin Immunol. (2016) 138:1108–18. 10.1016/j.jaci.2016.04.0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.du Toit G, Sayre P, Roberts G, Lawson K, Sever M, Bahnson H, et al. Allergen specificity of early peanut consumption and effect on development of allergic disease in the learning early about peanut allergy study cohort. J Allergy Clin Immunol. (2018) 141:1343–53. 10.1016/j.jaci.2017.09.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee K, Guo J, Song Y, Ariff A, O'Sullivan M, Hales B, et al. Dysfunctional gut microbiome networks in childhood ige-mediated food allergy. Int J Mol Sci. (2021) 22:2079. 10.3390/ijms22042079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fazlollahi M, Chun Y, Grishin A, Wood R, Burks A, Dawson P, et al. Early-life gut microbiome and egg allergy. Allergy. (2018) 73:1515–24. 10.1111/all.13389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhao W, Ho H, Bunyavanich S. The gut microbiome in food allergy. Ann Allergy Asthma Immunol. (2019) 122:276–82. 10.1016/j.anai.2018.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wolter M, Grant E, Boudaud M, Steimle A, Pereira G, Martens E, et al. Leveraging diet to engineer the gut microbiome. Nat Rev Gastroenterol Hepatol. (2021) 18:885–902. 10.1038/s41575-021-00512-7 [DOI] [PubMed] [Google Scholar]
- 58.McKenzie C, Tan J, Macia L, Mackay C. The nutrition-gut microbiome-physiology axis and allergic diseases. Immunol Rev. (2017) 278:277–95. 10.1111/imr.12556 [DOI] [PubMed] [Google Scholar]
- 59.Nowak S, Wang H, Schmidt B, Jarvinen K. Vitamin D and iron status in children with food allergy. Ann Allergy Asthma Immunol. (2021) 127:57–63. 10.1016/j.anai.2021.02.027 [DOI] [PubMed] [Google Scholar]
- 60.Suaini N, Zhang Y, Vuillermin P, Allen K, Harrison L. Immune modulation by vitamin D and its relevance to food allergy. Nutrients. (2015) 7:6088–108. 10.3390/nu7085271 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Publicly available datasets were analyzed in this study. This data can be found here: https://www.cdc.gov/nchs/nhanes/search/default.aspx.