Abstract
Background
Circumferential resection margin (CRM) is very important in esophageal cancer, but its diagnostic criteria has not been unified. The College of American Pathologists (CAP) and the Royal College of Pathologists (RCP) provide two different criteria. The aim of this study is to evaluate the long-term prognostic significance of CRM status with different CRM criteria in esophageal squamous cell carcinoma (ESCC).
Methods
Influence of CRM status according to the CAP and RCP criteria on long-term survival of 838 patients with resected pT3 tumors and without neoadjuvant therapy was analyzed. Patients stratified into three groups on the basis of tumor distance from the CRM (CRM > 1 mm, 0-1 mm, and 0 mm) were also analysed.
Results
Positive CRM was found in 59 (7%) patients according to the CAP criteria and 317 (37.8%) patients according to the RCP criteria. Univariate and multivariate survival analysis showed that CRM status, according to three different criteria, was independent prognostic factor. However, subgroup analysis showed that the prognostic value of CRM status was limited to certain metastatic lymph node load. In pN0 subgroup, patients with CRM > 1 mm had better prognosis than patients with CRM 0-1 mm. Patients with CRM 0 mm had worse outcome than patients with CRM > 0 mm in pN1-2 subgroup. But CRM status had no prognosis value in pN3 subgroup.
Conclusions
The CRM status is an important prognostic factor in ESCC patients, but this effect was limited to patients without or with less lymph node metastasis (pN0-2). In clinical practice, we recommend the 1 mm-three-tier criteria as it provides more prognostic value than the traditional two-tier criteria.
Keywords: Esophagus, Squamous cell carcinoma, Circumferential resection margin, Prognosis
Background
Esophageal squamous cell carcinoma (ESCC) is one of the most common malignancies worldwide, especially in East Asia, such as China. In recent decades, the application of new technologies, devices, and neoadjuvant therapy has led to great progress in the diagnosis and treatment of esophageal cancer. Whether neoadjuvant therapy was undergone or not, surgery is still the cornerstone of the treatment of locally advanced esophageal cancer. As is well known, complete resection is the most important principle of surgical resection of the primary tumor. It has been reported that positive proximal and distal resection margins have significant association with worse prognosis in terms of recurrence and survival [1–3]. Even though the role of positive CRM in esophageal cancer has been investigated for decades, but it is still controversial. Many articles showed that there was significant relationship between positive CRM and local–regional recurrence, disease-free survival (DFS), and overall survival (OS) [4–9]. However, some studies were unable to show an effect of positive CRM on OS and tumor recurrence [10–12].
The College of American Pathologists (CAP) criteria and the Royal College of Pathologists (RCP) criteria are the two most commonly used criteria for the definition of positive or negative CRM. Tumor found at the resection margin is defined as positive CRM according to the CAP criteria [13], whereas tumor found at or within 1 mm of the resection margin is defined as positive CRM according to the RCP criteria [14]. The difference between the CAP and RCP criteria is whether CRM 0-1 mm should be interpreted as positive or not. If carcinoma is identified microscopically within 1 mm of the CRM after esophagectomy, physicians often face difficulties in selecting proper therapeutic options because little is known about the subspecialized cutoff points between 0 and 1 mm for positive CRM. To fulfill clinical needs and resolve disputes, some studies also proposed other CRM criteria. Yang et al. [4] suggested CRM 600 μm as the optimal cut-off point, and this modified CRM criteria had better prognostic power than the traditional criteria in ESCC patients. In addition to the above two-tier criteria, other studies also proposed the 1 mm-three-tier criteria (CRM > 1 mm, 0-1 mm, and 0 mm) [8] and the 500 μm-three-tier criteria (CRM > 500 μm, 0-500 μm, and 0 μm) [6]. However, most of the previous studies included patients with different pathologic T status and histologic subtypes, and various preoperative therapies (with or without neoadjuvant therapy), that could not represent the true significance of CRM status. As T1 or T2 tumor with CRM involvement is considered as surgical failure, and surgery is a rare treatment option in T4 tumor, so they should be separated from T3 tumor. Moreover, esophageal squamous cell carcinoma and adenocarcinoma are completely different disease entities [15–17], so they should be studied separately. In addition, neoadjuvant therapy can affect the prognosis [18, 19], which may affect the true value of a positive CRM on outcomes. The purpose of this study is to appraise the long-term prognostic significance of CRM status with different CRM criteria in ESCC and select the appropriate diagnostic criteria.
Methods
Patients
From March 1999 to July 2007, pT3 ESCC patients who underwent esophagectomy without prior neoadjuvant therapy at National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences, and Peking Union Medical College were consecutively analyzed. Exclusion criteria included incomplete resection (defined as cases with the presence of microscopic tumor within 1 mm of the proximal or distal resection margins), distant metastasis, postoperative mortality (within 30 days or in-hospital mortality), and patients with other malignancies that occurred before or after the diagnosis of primary esophageal cancer. In addition, specific sub-types of ESCC, including basaloid squamous cell carcinoma, spindle cell squamous cell carcinoma and verrucous squamous cell carcinoma were also excluded.
Surgical procedure
The most common procedures for tumors located in the middle-lower esophagus were modified Sweet through left thoracotomy or modified Ivor-Lewis through right thoracotomy approach with intrathoracic anastomosis. For tumors located in the upper esophagus, modified McKeown with cervical anastomosis were performed. All procedures involved two-field lymph node dissection.
Pathologic examination
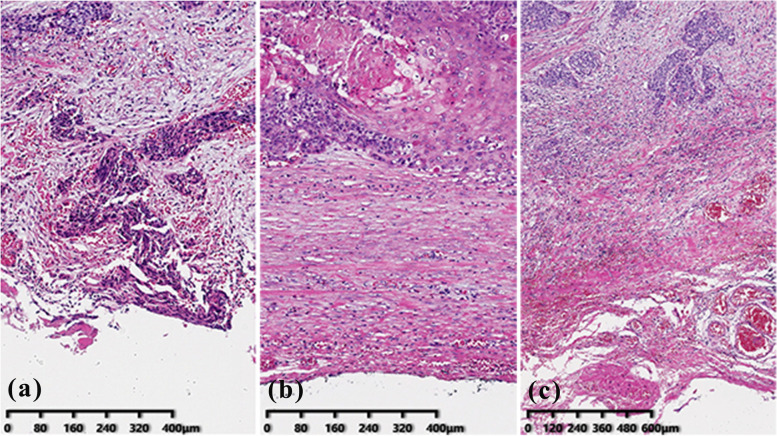
All original slides from the enrolled patients were reviewed by two certified pathologists. The CRM distance was measured from the deepest tumor cells to the vertical margin. The CRM status was identified by the CAP criteria (R0 and R1), the RCP criteria (R0 and R1), and the 1 mm-three-tier CRM criteria (CRM > 1 mm, 0-1 mm, and 0 mm), respectively (Fig. 1). In addition, all specimens were reviewed for lymph node status, extranodal invasion of lymph node metastasis, the degree of differentiation, lymphovascular invasion (LVI), and perineural invasion (PNI).
Fig. 1.
Photomicrographs of resected sections of esophageal cancer. a Tumor cells at the circumferential margin (CRM 0 mm), R1 according to CAP and RCP criteria. (Original magnification, × 100). b tumor cells within 1 mm of circumferential margin (CRM 0-1 mm), R0 according to CAP criteria, and R1 according to RCP criteria. (Original magnification, × 100). c tumor cells within more than 1 mm circumferential margin (CRM > 1 mm), R0 according to CAP and RCP criteria. (Original magnification, × 40)
Follow-up
Follow-up data were mainly gathered from clinical notes. Most patients were followed up every 3 months for the first two years after operation, every 6 months until the fifth year, and then annually for 10 years. For those patients who did not come for a follow-up visit, data were gathered by phone calls, and/or mail contact with patients or their next of kin. Survival was measured in months; cancer-related death was scored as an event; the death of any other causes was scored as the end of follow-up. OS time was recorded as the number of months from the date of surgery to the date when the death occurred or to the time of the last follow-up, at which point, the data were censored. DFS time was recorded as the number of months from the date of surgery to the date when the tumor recurred or death due to disease progression.
Statistical analysis
Patient’s age was analyzed after categorization. Pearson’s chi-square test was used for categorical variables. Kaplan–Meier method was used to plot the survival curves, and the log-rank analysis was used to evaluate differences in prognosis between groups. Prognostic factors for OS and DFS were calculated by using univariate and multivariate Cox regression analyses. Multivariate survival analysis was performed on factors that achieved statistical significance (P < 0.05) on univariate analysis. All statistical analyses were performed using the SPSS software package (version 25.0; IBM Corp, Armonk, NY, USA). A P value of less than 0.05 was considered statistically significant.
Results
A total of 939 patients with pT3 ESCC were included, but follow-up was completed in 838 patients (89.2%). Of these 838 patients, the median follow-up was 35 months (range 2–120 months). The male to female ratio was 4.1:1 (672:166) with the median age of 60 years at surgery (range 31–82 years). CRM positive (R1) was found in 59 (7%) patients according to the CAP criteria and 317 (37.8%) patients according to the RCP criteria. LVI, PNI, and lymph node metastasis were found in 446 (53.2%), 521 (62.2%), and 406 (48.4%) patients, respectively. Extranodal invasion occurred in 226 (52.2%) of the 406 patients with lymph node metastases. A total of 225 patients (26.8%) received post-operative adjuvant therapy, and 172 of these patients (76.4%) had lymph node metastasis. The clinicopathologic parameters of the entire cohort are summarized in Table 1.
Table 1.
Summary of clinical and histopathological characteristics of the 838 pT3N0-3M0 esophageal squamous cell carcinoma patients, the 5-year (5-yr), 10-year (10-yr) overall survival (OS) rate (%) and the 3-year (3-yr), 5-year (5-yr) disease-free survival (DFS) rate (%)
| Characteristic | N (%) | 5-yr OS (%) | 10-yr OS (%) | P value | 3-yr DFS (%) | 5-yr DFS (%) | P value | |
|---|---|---|---|---|---|---|---|---|
| Sex | Male | 672(80.2) | 45.7 | 39.9 | 0.881 | 46.2 | 40.9 | 0.704 |
| Female | 166(19.8) | 44.5 | 33.3 | 49.4 | 37.5 | |||
| Age (years) | ≤ 60 | 464(55.4) | 48.9 | 44.2 | 0.007 | 50.3 | 44.0 | 0.029 |
| > 60 | 374(44.6) | 41.2 | 31.7 | 42.5 | 35.3 | |||
| Tumor location | Upper thoracic | 119(14.2) | 47.6 | 39.6 | 0.694 | 49.6 | 41.9 | 0.893 |
| Middle thoracic | 486(58.0) | 45.9 | 41.7 | 46.8 | 40.2 | |||
| Lower thoracic | 233(27.8) | 43.7 | 33.3 | 45.6 | 39.2 | |||
| Degree of differentiation | Well | 376(44.9) | 49.1 | 41.8 | 0.001 | 53.1 | 44.9 | < 0.001 |
| Moderate | 392(46.8) | 44.8 | 37.6 | 43.5 | 37.9 | |||
| Poor | 70(8.4) | 29.9 | 27.8 | 32.6 | 27.9 | |||
| LVI | No | 392(46.8) | 55.9 | 48.5 | < 0.001 | 57.7 | 50.9 | < 0.001 |
| Yes | 446(53.2) | 36.4 | 29.7 | 37.5 | 30.9 | |||
| PNI | No | 317(37.8) | 54.3 | 46.2 | < 0.001 | 55.5 | 48.0 | 0.001 |
| Yes | 521(62.2) | 40.1 | 34.0 | 41.5 | 35.3 | |||
| pN | N0 | 405(48.3) | 61.6 | 52.6 | < 0.001 | 63.8 | 56.1 | < 0.001 |
| N1 | 238(28.4) | 40.3 | 34.2 | 41.1 | 34.6 | |||
| N2 | 140(16.7) | 21.1 | 16.9 | 22.6 | 16.9 | |||
| N3 | 55(6.6) | 13.7 | 13.7 | 9.0 | 6.8 | |||
| CRM CAP criteria | R0 | 779(93.0) | 47.2 | 39.8 | < 0.001 | 48.5 | 41.6 | < 0.001 |
| R1 | 59(7.0) | 22.9 | 22.9 | 26.0 | 20.8 | |||
| CRM RCP criteria | R0 | 521(62.2) | 50.6 | 44.2 | < 0.001 | 51.4 | 44.8 | 0.001 |
| R1 | 317(37.8) | 37.0 | 29.3 | 39.3 | 32.5 | |||
| CRM three-tier criteria | > 1 mm | 521 (62.2) | 50.6 | 44.2 | < 0.001 | 51.4 | 44.8 | < 0.001 |
| 0-1 mm | 258(30.8) | 40.3 | 30.6 | 42.4 | 35.2 | |||
| 0 mm | 59 (7.0) | 22.9 | 22.9 | 26.0 | 20.8 | |||
| Adjuvant therapy | No | 613(73.2) | 50.1 | 41.4 | < 0.001 | 44.9 | 38.4 | < 0.001 |
| Yes | 225(26.8) | 33.2 | 27.5 | 27.0 | 24.8 | |||
| Extranodal invasion | No | 207(47.8) | 37.8 | 30.8 | < 0.001 | 38.5 | 32.3 | < 0.001 |
| Yes | 226(52.2) | 24.3 | 21.7 | 24.6 | 19.2 |
CAP College of American Pathologists, CRM Circumferential resection margin, LVI Lymphovascular invasion, RCP Royal College of Pathologists, PNI Perineural invasion
Overall Survival and CRM status
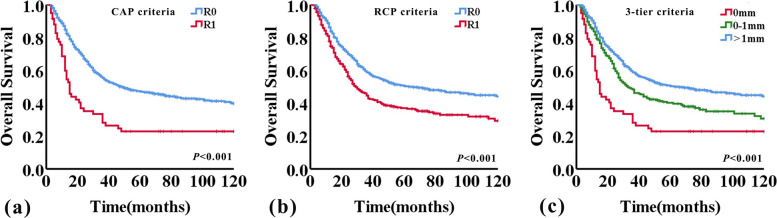
The median OS time for the entire study population was 44 months (95%CI 34.2–53.8 months). The 5- and 10-year OS rates were 45.5% and 38.6%, respectively. The median OS of patients who were diagnosed as R0 and R1 according to CAP criteria were 49 months (95%CI 35.7–62.3 months) and 15 months (95%CI 11.7–18.3 months), respectively (P < 0.001). Median OS of patients who were diagnosed as R0 and R1 according to the RCP criteria were 66 months (95%CI 39.3–92.7 months) and 29 months (95%CI 23.0–35.0 months), respectively (P < 0.001). The Kaplan–Meier survival curve is presented in Fig. 2. Patients with R1 had a significantly shorter OS than those with R0, according to either RCP or CAP criteria used (P < 0.001, both; log-rank test). When applying the 1 mm-three-tier stratification system for CRM status, the median OS of patients with CRM 0 mm, 0-1 mm, and > 1 mm were 15 months (95%CI 11.7–18.3 months), 33 months (95%CI 24.3–41.7 months), and 66 months (95%CI 39.3–92.7 months), respectively (P < 0.001). And there was significant difference between groups with CRM 0 mm versus CRM 0-1 mm, CRM 0 mm versus CRM > 1 mm, and CRM 0-1 mm versus CRM > 1 mm (P = 0.002, P < 0.001, and P = 0.001, respectively, log-rank test) (Fig. 2c).
Fig. 2.
Kaplan–Meier curves for overall survival according to CRM status
The OS of older patients was significantly worse compared with that of young patients. Similarly, cases with poor differentiation, LVI, PNI, higher number of lymph node metastasis, extranodal invasion, and received adjuvant therapy had worse outcome, and the relevant survival data are shown in Table 1.
Univariate Cox proportional hazards model indicated a significant relationship between OS and CRM status (according to either the CAP, or RCP, or 1 mm-three-tier criteria), the patient’s age, degree of tumor differentiation, LVI, PNI, pN, extranodal invasion, and adjuvant therapy (Table 2). Multivariate Cox regression analysis was performed with risk factors that were statistically significant on univariate analysis, except for extranodal invasion. Extranodal invasion is confined to the patients with lymph node metastasis, so it is not suitable for inclusion in multivariate survival analysis. The results of multivariate Cox proportional hazards analysis suggested that the patient’s age, tumor differentiation, pN and CRM status (according to the CAP and RCP criteria) were independent prognostic factors for OS (Table 2). But the difference between CRM 0-1 mm and CRM > 1 mm was not statistically significant (P = 0.100).
Table 2.
Univariate and Multivariate Cox proportional hazards analysis of various prognostic factors and their relationship to overall survival
| Univariate | Multivariate (CAP) | Multivariate (RCP) | Multivariate (three-tier criteria) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Parameter | HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | |
| Sex | Male/Female | 0.983(0.783–1.233) | 0.882 | ||||||
| Age (years) | ≤ 60/ > 60 | 1.285(1.069–1.545) | 0.007 | 1.017(1.006–1.028) | 0.002 | 1.018(1.007–1.029) | 0.001 | 1.018(1.007–1.029) | 0.001 |
| Tumor location | Upper thoracic | 1 | |||||||
| Middle thoracic | 0.995(0.756–1.312) | 0.974 | |||||||
| Lower thoracic | 1.086(0.806–1.464) | 0.586 | |||||||
| Degree of differentiation | Well and Moderate/Poor | 1.656(1.231–2.228) | 0.001 | 1.353(1.000–1.832) | 0.050 | 1.400(1.037–1.890) | 0.028 | 1.357(1.003–1.836) | 0.048 |
| LVI | No/Yes | 1.724(1.426–2.084) | < 0.001 | 1.200(0.977–1.473) | 0.082 | 1.206(0.983–1.479) | 0.073 | 1.189(0.969–1.460) | 0.098 |
| PNI | No/Yes | 1.500(1.233–1.825) | < 0.001 | 1.226(1.001–1.502) | 0.049 | 1.212(0.989–1.485) | 0.064 | 1.211(0.988–1.484) | 0.065 |
| pN | N0 | 1 | 1 | 1 | 1 | ||||
| N1 | 1.783(1.420–2.237) | < 0.001 | 1.697(1.338–2.151) | < 0.001 | 1.625(1.282–2.060) | < 0.001 | 1.673(1.319–2.123) | < 0.001 | |
| N2 | 3.219(2.519–4.114) | < 0.001 | 2.820(2.160–3.681) | < 0.001 | 2.721(2.084–3.553) | < 0.001 | 2.759(2.112–3.605) | < 0.001 | |
| N3 | 4.536(3.248–6.334) | < 0.001 | 3.727(2.615–5.311) | < 0.001 | 3.756(2.632–5.361) | < 0.001 | 3.694(2.590–5.268) | < 0.001 | |
| CRM CAP criteria | R0/R1 | 2.107(1.543–2.877) | < 0.001 | 1.814(1.321–2.492) | < 0.001 | ||||
| CRM RCP criteria | R0/R1 | 1.517(1.260–1.826) | < 0.001 | 1.295(1.072–1.564) | 0.007 | ||||
| CRM three-tier criteria | > 1 mm | 1 | 1 | ||||||
| 0-1 mm | 1.378(1.128–1.683) | 0.002 | 1.185(0.968–1.452) | 0.100 | |||||
| 0 mm | 2.350(1.706–3.238) | < 0.001 | 1.933(1.394–2.680) | < 0.001 | |||||
| Adjuvant therapy | No/Yes | 1.467(1.205–1.785) | < 0.001 | 1.072(0.869–1.323) | 0.516 | 1.079(0.875–1.331) | 0.478 | 1.073(0.870–1.324) | 0.511 |
| Extranodal invasiona | No/Yes | 1.518(1.206–1.912) | < 0.001 | ||||||
CAP College of American Pathologists, CRM Circumferential resection margin, LVI Lymphovascular invasion, RCP Royal College of Pathologists, PNI Perineural invasion
a This factor related to prognosis were not examined in the multivariate analysis
Disease-free survival
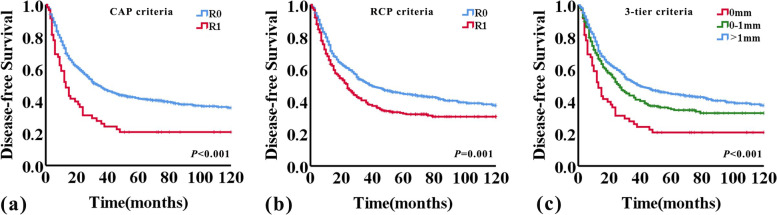
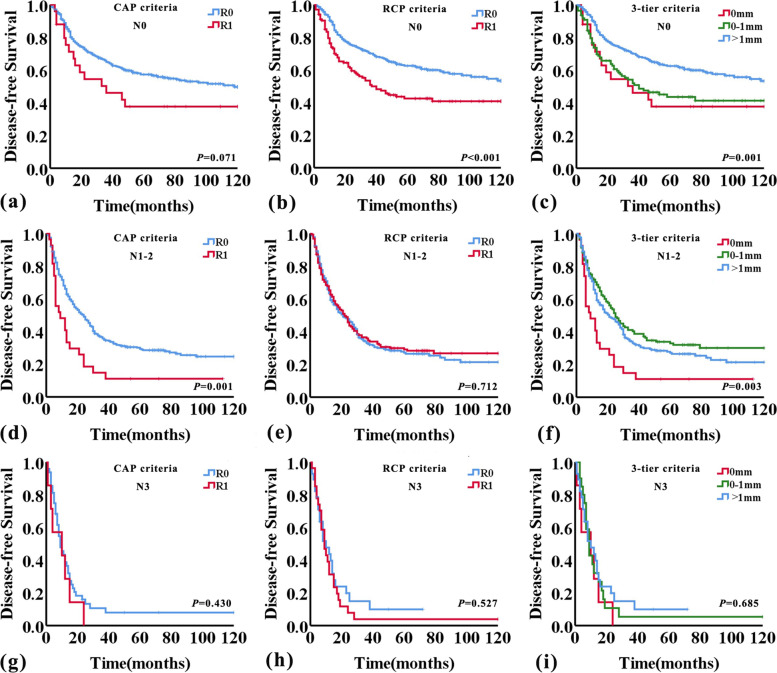
The median DFS time for the entire study population was 31 months (95%CI 26–36 months). The 3- and 5-year DFS rates were 46.9% and 40.2%, respectively. When using the CAP criteria, the median DFS was 34 months (95%CI 27.8–40.2 months) and 13 months (95%CI 8.9–17.1 months) for patients with R0 and R1 (P < 0.001), respectively, while using the RCP criteria, the median DFS was 39 months (95%CI 26.3–51.7 months) for R0 and 24 months (95%CI 19.2–28.8 months) for R1 (P = 0.001). The DFS of patients diagnosed as R1 was significantly shorter compared with that of patients diagnosed as R0, according to either CAP or RCP criteria used (P < 0.001 and P = 0.001, respectively; log-rank test) (Fig. 3a and b). When applying the 1 mm-three-tier criteria for CRM status, the median DFS for patients with CRM 0 mm, CRM 0-1 mm, and CRM > 1 mm was 13 months (95%CI 8.9–17.1 months), 27 months (95%CI 21.4–32.6 months), and 39 months (95%CI 26.3–51.7 months), respectively (P < 0.001). And there was significant difference between groups with CRM 0 mm versus CRM 0-1 mm, CRM 0 mm versus CRM > 1 mm, and CRM 0-1 mm versus CRM > 1 mm (P = 0.008, P < 0.001, and P = 0.018, respectively, log-rank test) (Fig. 3c).
Fig. 3.
Kaplan–Meier curves for disease-free survival according to CRM status
The DFS time of older patients was significantly shorter compared with that of young patients. Similarly, cases with poor differentiation, LVI, PNI, higher number of lymph node metastasis, extranodal invasion, and received adjuvant therapy had worse outcome, and the relevant survival data are shown in Table 1.
Univariate Cox proportional hazards model identified a significant relationship between DFS and CRM status (according to either the CAP, or RCP, or 1 mm-three-tier criteria), the patient’s age, degree of tumor differentiation, LVI, PNI, pN, extranodal invasion, and adjuvant therapy (Table 3). Multivariate analyses of the above-mentioned prognostic factors confirmed R1 using the CAP criteria as an independent predictor for DFS. Patient’s age, LVI, pN also remained an independent prognostic factor (Table 3). CRM status, according to the RCP criteria, failed to be an independent prognostic factor. Although, CRM status, according to the three-tier criteria, was an independent prognostic factor, but the difference between groups CRM 0-1 mm versus CRM > 1 mm was not statistically significant (P = 0.664).
Table 3.
Univariate and Multivariate Cox proportional hazards analysis of various prognostic factors and their relationship to disease-free survival
| Univariate | Multivariate (CAP) | Multivariate (RCP) | Multivariate (three-tier criteria) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Parameter | HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | |
| Sex | Male/Female | 0.960(0.775–1.188) | 0.707 | ||||||
| Age (years) | ≤ 60/ > 60 | 1.213(1.018–1.446) | 0.031 | 1.015(1.005–1.026) | 0.003 | 1.015(1.005–1.026) | 0.003 | 1.015(1.005–1.026) | 0.003 |
| Tumor location | Upper thoracic | 1 | |||||||
| Middle thoracic | 1.031(0.793–1.341) | 0.818 | |||||||
| Lower thoracic | 1.068(0.801–1.423) | 0.655 | |||||||
| Degree of differentiation | Well and Moderate/ Poor | 1.556(1.161–2.085) | 0.003 | 1.276(0.947–1.718) | 0.109 | 1.306(0.971–1.757) | 0.078 | 1.275(0.946–1.717) | 0.110 |
| LVI | No/Yes | 1.733(1.447–2.077) | < 0.001 | 1.248(1.025–1.518) | 0.027 | 1.261(1.037–1.534) | 0.020 | 1.245(1.022–1.515) | 0.029 |
| PNI | No/Yes | 1.363(1.132–1.640) | 0.001 | 1.079(0.890–1.308) | 0.438 | 1.072(0.884–1.299) | 0.482 | 1.076(0.887–1.305) | 0.458 |
| pN | N0 | 1 | 1 | 1 | 1 | ||||
| N1 | 1.813(1.462–2.247) | < 0.001 | 1.667(1.331–2.087) | < 0.001 | 1.615(1.290–2.023) | < 0.001 | 1.660(1.324–2.080) | < 0.001 | |
| N2 | 3.021(2.389–3.821) | < 0.001 | 2.581(1.998–3.335) | < 0.001 | 2.531(1.957–3.272) | < 0.001 | 2.565(1.983–3.319) | < 0.001 | |
| N3 | 4.882(3.529–6.753) | < 0.001 | 3.900(2.760–5.512) | < 0.001 | 3.852(2.721–5.452) | < 0.001 | 3.881(2.744–5.489) | < 0.001 | |
| CRM CAP criteria | R0/R1 | 1.803(1.331–2.443) | < 0.001 | 1.575(1.157–2.145) | 0.004 | ||||
| CRM RCP criteria | R0/R1 | 1.362(1.140–1.627) | 0.001 | 1.130(0.942–1.355) | 0.189 | ||||
| CRM three-tier criteria | > 1 mm | 1 | 1 | ||||||
| 0-1 mm | 1.255(1.036–1.521) | 0.020 | 1.044(0.859–1.270) | 0.664 | |||||
| 0 mm | 1.945(1.424–2.655) | < 0.001 | 1.601(1.166–2.199) | 0.004 | |||||
| Adjuvant therapy | No/Yes | 1.617(1.340–1.952) | < 0.001 | 1.216(0.996–1.485) | 0.055 | 1.231(1.009–1.503) | 0.041 | 1.216(0.996–1.485) | 0.055 |
| Extranodal invasiona | No/Yes | 1.556(1.247–1.943) | < 0.001 | ||||||
CAP College of American Pathologists, CRM Circumferential resection margin, LVI Lymphovascular invasion, RCP Royal College of Pathologists, PNI Perineural invasion
a This factor related to prognosis were not examined in the multivariate analysis
CRM status, lymph node status, OS, and DFS
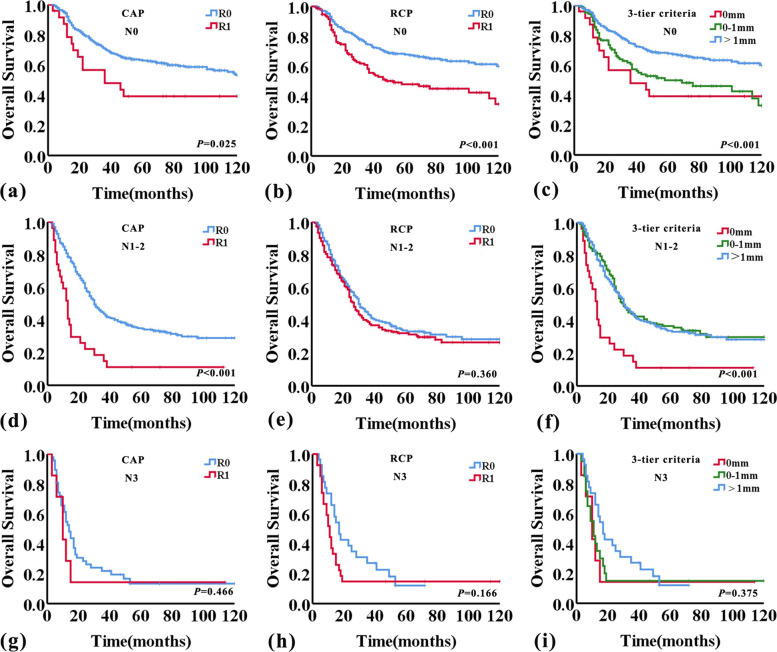
As previously described, pN and extranodal invasion were both important prognostic factors, and whether they would affect the predictive value of CRM status was uncertain. Then we analyzed the correlation of CRM status with pN and extranodal invasion of lymph nodes metastasis (Table 4). The result showed that CRM status had a significant correlation with pN (P = 0.005), but not extranodal invasion of lymph nodes metastasis (P = 0.056). Overall survival and disease-free survival curves were then analysed further for pN0, pN1-2, and pN3 subgroups using the CAP and RCP criteria, as well as the 1 mm-three-tier criteria. This analysis showed good separation of the OS and DFS curves within the pN0 and pN1-2 groups applying either the CAP or RCP, or the 1 mm-three-tier criteria, but not the pN3 group (Figs. 4 and 5). Within the pN0 group, patients with CRM > 1 mm had better survival than patients with CRM 0 mm and CRM 0-1 mm (OS, P = 0.005 and P < 0.001; DFS, P = 0.017 and P = 0.001; respectively, log-rank test) (Figs. 4 and 5). However, the difference in OS and DFS between CRM 0 mm and CRM 0-1 mm was not statistically significant within the pN0 group (P = 0.476 and P = 0.692, respectively, log-rank test). But in the pN1-2 group, patients with CRM 0 mm had worse survival than patients with CRM 0-1 mm and CRM > 1 mm (OS, P < 0.001 and P < 0.001; DFS, P = 0.001 and P = 0.005; respectively, log-rank test) (Figs. 4 and 5). And there was no significant difference between CRM 0-1 mm and CRM > 1 mm in OS and DFS within the pN1-2 group (P = 0.813 and P = 0.194, respectively, log-rank test). And the detailed univariate cox regression analyses data related to OS and DFS in pN0, pN1-2, and pN3 subgroups were shown in Table 5.
Table 4.
The correlation between CRM status and lymph nodes status and extranodal invasion of lymph nodes metastasis
| n | CRM status | |||||
|---|---|---|---|---|---|---|
| 0 mm | 0-1 mm | > 1 mm | P value | |||
| pN | N0 | 405 | 25(6.2%) | 109(26.9%) | 271(66.9%) | 0.005 |
| N1 | 238 | 11 (4.6%) | 77 (32.4%) | 150 (63.0%) | ||
| N2 | 140 | 16 (11.4%) | 52 (37.1%) | 72 (51.4%) | ||
| N3 | 55 | 7 (12.7%) | 20 (36.4%) | 28 (50.9%) | ||
| Extranodal invasion | No | 207 | 11(5.3%) | 66(31.9%) | 130(62.8%) | 0.056 |
| Yes | 226 | 23(10.2%) | 83(36.7%) | 120(53.1%) | ||
CRM Circumferential resection margin
Fig. 4.
Kaplan–Meier curves showing overall survival according to CRM status assessed by RCP, CAP, and 1 mm-three-tier criteria adjusted for lymph node status
Fig. 5.
Kaplan–Meier curves showing disease-free survival according to CRM status assessed by RCP, CAP, and 1 mm-three-tier criteria adjusted for lymph node status
Table 5.
CRM status and survival adjusted for lymph node status
| N0 | N1-2 | N3 | |||||
|---|---|---|---|---|---|---|---|
| HR (95%CI) | P value | HR (95%CI) | P value | HR (95%CI) | P value | ||
| OS | |||||||
| CAP criteria | R1 vs. R0 | 1.846(1.067–3.196) | 0.029 | 2.511(1.645–3.835) | < 0.001 | 1.367(0.576–3.246) | 0.478 |
| RCP criteria | R1 vs. R0 | 1.874(1.366–2.570) | < 0.001 | 1.122(0.874–1.441) | 0.366 | 1.499(0.830–2.705) | 0.179 |
| CRM three-tier criteria | 0 mm vs. 0-1 mm | 1.236(0.686–2.228) | 0.481 | 2.521(1.590–3.996) | < 0.001 | 1.112(0.437–2.833) | 0.824 |
| 0 mm vs. > 1 mm | 2.208(1.258–3.876) | 0.006 | 2.483(1.608–3.835) | < 0.001 | 1.596(0.642–3.967) | 0.314 | |
| 0-1 mm vs. > 1 mm | 1.801(1.283–2.529) | 0.001 | 0.970(0.741–1.270) | 0.825 | 1.468(0.776–2.777) | 0.238 | |
| DFS | |||||||
| CAP criteria | R1 vs. R0 | 1.615(0.952–2.740) | 0.076 | 1.936(1.271–2.949) | 0.002 | 1.369(0.611–3.071) | 0.446 |
| RCP criteria | R1 vs. R0 | 1.735(1.287–2.339) | < 0.001 | 0.956(0.751–1.218) | 0.716 | 1.196(0.676–2.115) | 0.538 |
| CRM three-tier criteria | 0 mm vs. 0-1 mm | 1.120(0.634–1.979) | 0.695 | 2.126(1.345–3.359) | 0.001 | 1.266(0.523–3.067) | 0.601 |
| 0 mm vs. > 1 mm | 1.884(1.097–3.232) | 0.022 | 1.820(1.184–2.800) | 0.006 | 1.440(0.613–3.380) | 0.403 | |
| 0-1 mm vs. > 1 mm | 1.700(1.233–2.345) | 0.001 | 0.845 (0.651–1.097) | 0.205 | 1.123(0.604–2.088) | 0.714 | |
CAP College of American Pathologists, CRM Circumferential resection margin, RCP Royal College of Pathologists
Discussion
The current study evaluated the long-term survival of 838 Chinese ESCC patients to clarify the prognostic value of CRM status. And, the results of our study demonstrated that CRM status was predictive of OS and DFS only in patients with a lower lymph node burden (pN0-2), and the 1 mm-three-tier criteria of CRM status was recommended in clinical practice.
The importance of CRM status in esophageal cancers has been discussed for decades, but remains controversial. Reviewing relevant articles, we found most of the previous reports analyzed different histologic types (mainly adenocarcinoma) as a whole of esophageal cancers [7, 10, 11, 20]. There were only five publications focused specifically on the relationship between CRM status and prognosis of ESCC patients [4–6, 21, 22]. The study by Okada et al. [5] showed that positive CRM was a significant prognostic factor for poor survival, judged by the CAP criteria or the RCP criteria in surgery alone subgroup. However, CRM status, according to the RCP criteria, had no association with survival in patients with neoadjuvant chemotherapy plus surgery [5]. Chao et al. [21] analyzed the relationship between CRM distance and survival in patients with neoadjuvant therapy, and confirmed the 1 mm-three-tier CRM criteria would provide more useful information for risk stratification in cancer recurrence and survival. Whereas, that study only included patients with small number of lymph node metastasis (pN0-1) [21]. Unlike Chao et al.’s results, Park et al. [22] found there were significant relationship between CRM status and loco-regional recurrence in patients with large number of lymph node metastasis (pN2-3), but not pN0-1 [22]. Although the study by Lee et al. [6] showed that patients with positive CRM had worse OS according to both the CAP criteria and the RCP criteria, the 500 μm-three-tier criteria of CRM status provided more detailed prognostic information. Notably, some patients in this study ever received neoadjuvant therapy [6]. Different from the above studies, Yang et al. [4] failed to demonstrate positive CRM, according to either the CAP criteria or the RCP criteria, had significant association with OS in pT3N0M0 ESCC patients, but they found patients with CRM more than 600 μm showed better OS than the ones with CRM less than 600 μm. Based on the previous findings, the impact of lymph node status as confounders of the predictive value of CRM status is uncertain.
Consistent with the previous studies [5, 6, 21], this current study showed CRM status, according to either the CAP criteria, the RCP criteria, or the 1 mm-three-tier criteria, was an independent risk factor affecting patient survival for the entire study. In addition, multivariate analysis showed lymph nodes metastasis was also related to the prognosis of ESCC patients. In order to investigate whether lymph node status would affect the prognostic significance of CRM status, we analyzed the relationship between CRM status and lymph node status. The result showed that there was a significant correlation between CRM status and pN. Subsequently, we performed additional subgroup analyses according to the pN status. Similar to the findings of Yang et al. [4], CRM status, according to either CAP or RCP criteria, was a risk factor affecting the prognosis of patients within the pN0 group, but the survival difference between CRM 0 mm and CRM 0-1 mm was not statistically significant. In the pN1-2 group, patients with CRM 0 mm had worse survival than ones with CRM 0-1 mm and CRM > 1 mm, and patients with CRM 0-1 mm had worse survival than ones with CRM > 1 mm, but the survival difference between patients with CRM 0-1 mm and CRM > 1 mm was not statistically significant. Notably, CRM status, according to either the CAP criteria or the RCP criteria, had no significant association with OS and DFS within the pN3 group. That may indicate that with the number of lymph node metastases increased, the impact of CRM status on prognosis decreased. Especially, the prognosis of patient's prognosis with pN3 is already very poor, the influence of other factors can be masked.
In spite of the present study is the largest number to evaluate the long-term prognostic value of CRM status in patients with pT3 ESCC without neoadjuvant therapy, there are several limitations in our study. First of all, this study failed to accurately collect the recurrence of the tumor and perform relevant statistical analysis. In addition, owing to the retrospective nature of this report, there was wide variation in adjuvant chemotherapy usage and radiation for which we could not account, and most patients received adjuvant therapy because of lymph node metastasis (76.4%). So, the efficacy of adjuvant therapy in positive CRM patients was not well established. Furthermore, given the retrospective nature of the study, and regional lymph nodes were dissected out and sent separately, the CRM status of lymph nodes metastasis could not be accurately assessed. Finally, we did not analyze whether neoadjuvant therapy would affect the prognostic significance of CRM status, because patients with neoadjuvant therapy were excluded in the study.
Conclusions
CRM status is associated with long-term outcome in ESCC, but only for patients with small number of lymph node metastasis (pN0-2). And we suggest adopting the 1 mm-three-tier criteria of CRM status in clinical practice.
Acknowledgements
Not applicable.
Abbreviations
- CRM
Circumferential resection margin
- CAP
The College of American Pathologists
- RCP
The Royal College of Pathologists
- ESCC
Esophageal squamous cell carcinoma
- DFS
Disease-free survival
- OS
Overall survival
- LVI
Lymphovascular invasion
- PNI
Perineural invasion
Authors’ contributions
Study conception and design: ZYY and LYX. Data acquisition and analysis: ZYY, HL, ZW, LLR, LW, JJQ, XMX, and XCZ. Data interpretation and manuscript writing: ZYY, ZW, and LYX. Revision of manuscript and contribution of intellectual content: ZYY, ZW, XCZ, YL, and LYX. All authors read and approved the final manuscript.
Funding
This work was supported by the CAMS Innovation Fund for Medical Sciences (CIFMS) (grant number 2016-I2M-3–005 and 2019-I2M-2–004). The funding source has no role in study design, data collection, analysis, interpretation, the writing of the manuscript, or the decision to submit the current study.
Availability of data and materials
All data generated or analysed during this study are included in this published article.
Declarations
Ethics approval and consent to participate
All included patients gave their written informed consent. The study was approved by the Ethics Committee (full name: Ethics Committee of National Cancer Center/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College) (reference number 20/184–2380).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Zhaoyang Yang, Hua Lin, Zhen Wang and Lulu Rong contributed equally to this work.
Contributor Information
Zhaoyang Yang, Email: 1060229646@qq.com.
Hua Lin, Email: linhuadoctor@126.com.
Zhen Wang, Email: wangzhen820306@163.com.
Lulu Rong, Email: 815519625@qq.com.
Xuchen Zhang, Email: xuchen.zhang@yale.edu.
Lin Wang, Email: wanglin16213@163.com.
Jianjun Qin, Email: 123114172@qq.com.
Xuemin Xue, Email: xuemin-xue@qq.com.
Yin Li, Email: liyin0825@aliyun.com.
Liyan Xue, Email: xuely@cicams.ac.cn, Email: xueliyan2003@126.com.
References
- 1.Wang YC, Deng HY, Wang WP, He D, Ni PZ, Hu WP, Wang ZQ, Chen LQ. Positive esophageal proximal resection margin: an important prognostic factor for esophageal cancer that warrants adjuvant therapy. J THORAC DIS. 2016;8(9):2512–2518. doi: 10.21037/jtd.2016.08.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mulligan ED, Dunne B, Griffin M, Keeling N, Reynolds JV. Margin involvement and outcome in oesophageal carcinoma: a 10-year experience in a specialist unit. Eur J Surg Oncol. 2004;30(3):313–317. doi: 10.1016/j.ejso.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 3.Tsutsui S, Kuwano H, Watanabe M, Kitamura M, Sugimachi K. Resection margin for squamous cell carcinoma of the esophagus. ANN SURG. 1995;222(2):193–202. doi: 10.1097/00000658-199508000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang YS, Wang YC, Deng HY, Yuan Y, Wang ZQ, He D, Chen LQ. Prognostic value of circumferential resection margin in T3N0M0 esophageal squamous cell carcinoma. Ann Transl Med. 2018;6(15):303. doi: 10.21037/atm.2018.06.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Okada N, Fujii S, Fujita T, Kanamori J, Kojima T, Hayashi R, Daiko H. The prognostic significance of the positive circumferential resection margin in pathologic T3 squamous cell carcinoma of the esophagus with or without neoadjuvant chemotherapy. SURGERY. 2016;159(2):441–450. doi: 10.1016/j.surg.2015.06.044. [DOI] [PubMed] [Google Scholar]
- 6.Lee GD, Lee SE, Kim KM, Kim YH, Ahn JH, Jung S, Choi YL, Kim HR, Park SI, Shim YM. New 3-tiered circumferential resection margin criteria in esophageal squamous cell carcinoma. ANN SURG. 2015;262(6):965–971. doi: 10.1097/SLA.0000000000001039. [DOI] [PubMed] [Google Scholar]
- 7.O’Neill JR, Stephens NA, Save V, Kamel HM, Phillips HA, Driscoll PJ, Paterson-Brown S. Defining a positive circumferential resection margin in oesophageal cancer and its implications for adjuvant treatment. Br J Surg. 2013;100(8):1055–63. [DOI] [PubMed]
- 8.Ahmad J, Loughrey MB, Donnelly D, Ranaghan L, Shah R, Napolitano G, Kennedy AJ. Prognostic value of added stratification of circumferential resection margin status in oesophageal carcinoma. Histopathology. 2013;62(5):752–763. doi: 10.1111/his.12078. [DOI] [PubMed] [Google Scholar]
- 9.Verhage RJ, Zandvoort HJ, Ten KF, van Hillegersberg R. How to define a positive circumferential resection margin in T3 adenocarcinoma of the esophagus. AM J SURG PATHOL. 2011;35(6):919–926. doi: 10.1097/PAS.0b013e31821a5692. [DOI] [PubMed] [Google Scholar]
- 10.Ghadban T, Reeh M, Koenig AM, Nentwich MF, Bellon E, Izbicki JR, Vashist YK, Kutup A. Prognostic Significant or Not? The Positive Circumferential Resection Margin in Esophageal Cancer: Impact on Local Recurrence and Overall Survival in Patients Without Neoadjuvant Treatment. ANN SURG. 2017;266(6):988–994. doi: 10.1097/SLA.0000000000001995. [DOI] [PubMed] [Google Scholar]
- 11.Quinn LM, Hollis AC, Hodson J, Elshafie MA, Hallissey MT, Whiting JL, Griffiths EA. Prognostic significance of circumferential resection margin involvement in patients receiving potentially curative treatment for oesophageal cancer. Eur J Surg Oncol. 2018;44(8):1268–1277. doi: 10.1016/j.ejso.2018.05.017. [DOI] [PubMed] [Google Scholar]
- 12.Theologou T, Diab M, Kyaw PA, Gosney JR, McShane J, Howes N, Page RD, Shackcloth M. The impact of positive circumferential margin on survival following oesophagectomy using the new 7th TNM classification. Eur J Cardiothorac Surg. 2013;44(5):855–859. doi: 10.1093/ejcts/ezt164. [DOI] [PubMed] [Google Scholar]
- 13.Chanjuan Shi, Jordan Berlin, Philip A. Branton, Patrick L. Fitzgibbons, Wendy L. Frankel, Wayne L. Hofstetter, Sanjay Kakar, David Kelsen, Veronica Klepeis, Jason Talmadge Lewis, Laura H. Tan, Mary K. Washington. Protocol for the Examination of Specimens From Patients With Carcinoma of the Esophagus. College of American Pathologists. 2020. https://documents.cap.org/protocols/cp-giupper-esophagus-20-4100.pdf. Accessed 15 Nov 2020.
- 14.Grabsch HI, Mapstone NP, Novelli M. Dataset for histopathological reporting of oesophageal and gastric carcinoma. The Royal College of Pathologists. 2019. https://www.rcpath.org/document-library-search.html. Accessed 15 Nov 2020.
- 15.Integrated genomic characterization of oesophageal carcinoma Nature. 2017;541(7636):169–175. doi: 10.1038/nature20805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gertler R, Stein HJ, Langer R, Nettelmann M, Schuster T, Hoefler H, Siewert JR, Feith M. Long-term outcome of 2920 patients with cancers of the esophagus and esophagogastric junction: evaluation of the New Union Internationale Contre le Cancer/American Joint Cancer Committee staging system. ANN SURG. 2011;253(4):689–698. doi: 10.1097/SLA.0b013e31821111b5. [DOI] [PubMed] [Google Scholar]
- 17.Siewert JR, Ott K. Are squamous and adenocarcinomas of the esophagus the same disease? SEMIN RADIAT ONCOL. 2007;17(1):38–44. doi: 10.1016/j.semradonc.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 18.Liu CY, Hsu PK, Hsu HS, Wu YC, Chuang CY, Lin CH, Hsu CP: Prognostic impact of circumferential resection margin in esophageal cancer with or without neoadjuvant chemoradiotherapy. DIS ESOPHAGUS 2020. [DOI] [PubMed]
- 19.Patrao AS, Papaxoinis G, Kordatou Z, Weaver JM, Owen-Holt V, Alkhaffaf B, Galloway S, Mansoor W. Prognostic significance of positive circumferential resection margin post neoadjuvant chemotherapy in patients with esophageal or gastro-esophageal junction adenocarcinoma. Eur J Surg Oncol. 2019;45(3):439–445. doi: 10.1016/j.ejso.2018.10.530. [DOI] [PubMed] [Google Scholar]
- 20.Dexter SP, Sue-Ling H, McMahon MJ, Quirke P, Mapstone N, Martin IG. Circumferential resection margin involvement: an independent predictor of survival following surgery for oesophageal cancer. Gut. 2001;48(5):667–670. doi: 10.1136/gut.48.5.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chao YK, Yeh CJ, Chang HK, Tseng CK, Chu YY, Hsieh MJ, Wu YC, Liu HP. Impact of circumferential resection margin distance on locoregional recurrence and survival after chemoradiotherapy in esophageal squamous cell carcinoma. ANN SURG ONCOL. 2011;18(2):529–534. doi: 10.1245/s10434-010-1244-4. [DOI] [PubMed] [Google Scholar]
- 22.Park HJ, Kim HJ, Chie EK, Kang CH, Kim YT. The influence of circumferential resection margin status on loco-regional recurrence in esophageal squamous cell carcinoma. J SURG ONCOL. 2013;107(7):762–766. doi: 10.1002/jso.23313. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analysed during this study are included in this published article.