Abstract
Background
In nature and in cultivated fields, plants encounter multiple stress factors. Nonetheless, our understanding of how plants actively respond to combinatorial stress remains limited. Among the least studied stress combination is that of flooding and herbivory, despite the growing importance of these stressors in the context of climate change. We investigated plant chemistry and gene expression changes in two heirloom tomato varieties: Cherokee Purple (CP) and Striped German (SG) in response to flooding, herbivory by Spodoptera exigua, and their combination.
Results
Volatile organic compounds (VOCs) identified in tomato plants subjected to flooding and/or herbivory included several mono- and sesquiterpenes. Flooding was the main factor altering VOCs emission rates, and impacting plant biomass accumulation, while different varieties had quantitative differences in their VOC emissions. At the gene expression levels, there were 335 differentially expressed genes between the two tomato plant varieties, these included genes encoding for phenylalanine ammonia-lyase (PAL), cinnamoyl-CoA-reductase-like, and phytoene synthase (Psy1). Flooding and variety effects together influenced abscisic acid (ABA) signaling genes with the SG variety showing higher levels of ABA production and ABA-dependent signaling upon flooding. Flooding downregulated genes associated with cytokinin catabolism and general defense response and upregulated genes associated with ethylene biosynthesis, anthocyanin biosynthesis, and gibberellin biosynthesis. Combining flooding and herbivory induced the upregulation of genes including chalcone synthase (CHS), PAL, and genes encoding BAHD acyltransferase and UDP-glucose iridoid glucosyltransferase-like genes in one of the tomato varieties (CP) and a disproportionate number of heat-shock proteins in SG. Only the SG variety had measurable changes in gene expression due to herbivory alone, upregulating zeatin, and O-glucosyltransferase and thioredoxin among others.
Conclusion
Our results suggest that both heirloom tomato plant varieties differ in their production of secondary metabolites including phenylpropanoids and terpenoids and their regulation and activation of ABA signaling upon stress associated with flooding. Herbivory and flooding together had interacting effects that were evident at the level of plant chemistry (VOCs production), gene expression and biomass markers. Results from our study highlight the complex nature of plant responses to combinatorial stresses and point at specific genes and pathways that are affected by flooding and herbivory combined.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12870-022-03911-3.
Keywords: Flooding stress, Herbivory, Gene expression, Heirloom tomatoes, Combined stresses, Phytochemistry, Beet Armyworm, Volatile Organic Compounds
Background
Within natural environments and in cultivated fields, plants often encounter diverse biotic and abiotic stressors such as herbivory, pathogen attack, drought, and flooding occurring individually, sequentially, or simultaneously [1–12]. To survive and reproduce, plants must appropriately respond to these stress factors [13]. Available evidence shows that the response of plants to stress combinations is unique and that it cannot be extrapolated from responses to individual stresses [4, 9, 11, 14–17]. When two or more stresses occur simultaneously, their effects can be additive, synergistic, or neutral. In some cases, the influence of one stress can become dominant [17]. The overall effect of stress combinations is further dependent on plant species, genotype, specific herbivore species, and severity of the co-occurring stresses [4, 11]. Numerous studies of combinatorial stresses have focused on interactions between abiotic and biotic stressors; for example: drought and herbivory [9, 18–20], drought and pathogen infection [10], salinity and ozone (O3) exposure [21], salinity and pathogen infection [21], ozone exposure and herbivory [22], elevated CO2 and herbivory [23], and high temperature and herbivory [9]. Despite the growing importance of flooding and herbivory in the context of climate change, the combined effects of these stressors are understudied.
Across North America, and in many regions of the world, flooding and waterlogging have become more frequent, severe, and economically damaging, with impacts on crops comparable in magnitude to drought and extreme temperatures [24, 25]. Waterlogging occurs when water is unable to drain away, leading to soil that is fully saturated. Flooding occurs when soil and roots are completely submerged under water. Both conditions challenge plants, due to oxygen deprivation in plant roots, which directly affects normal plant biochemical, molecular, and physiological processes [26–29]. Flooding may occur simultaneously or sequentially with biotic stressors such as insect herbivory [5, 21, 30, 31]. The simultaneous occurrence of flooding stress with herbivory may modify plant stress responses.
Genome-wide transcriptomic profiling studies on plants under combinations of biotic and abiotic stresses have been conducted in the past. For example, Rasmussen et al., [15] analyzed differences in gene expression patterns in ten Arabidopsis thaliana ecotypes challenged by single or dual (a)biotic stress combinations. Results from that study showed that changes in gene expression in response to combined stresses could not be predicted from single-stress treatments. Nguyen et al., [19] reported that flooding or drought pretreatment significantly modified the transcriptome signature of Solanum dulcamara plants that were already infested with S. exigua. Suzuki et al., [9] reviewed 33 different studies involving combinatorial stresses and found that simultaneous occurrence of different stresses generated unique responses, suggesting that plants dynamically respond to multiple co-occurring stress factors. Tamang et al., [32] reported that in soybeans, drought and flooding resulted in stress-specific and overlapping transcriptomic responses. Taken together, these studies suggest that transcriptomic responses/gene expression patterns of plants to combinatorial stresses cannot be predicted from responses to individual stresses.
Tomato (Solanum lycopersicum L.) is one of the most important vegetable crops in the world because of its high lycopene content, anti-oxidative properties, and as a source of micronutrients [33]. In 2018, globally, over 186 million tons of tomato were produced, worth over US $60 billion [34]. In addition, since the completion of its genome, tomato has been widely used as a model plant for studies investigating crop plant responses to biotic and abiotic stress [19, 33, 35–38] including flooding [29, 33, 39, 40]. Safavi-Rizi et al., [33], for example, investigated the effects of flooding stress associated hypoxia on the regulation of gene expression in tomato roots. They demonstrated transcriptome reprogramming in response to flooding stress and identified novel genes and pathways that potentially contribute to flooding stress tolerance. De Ollas et al., [29] investigated the role of abscisic acid (ABA) on the regulation of genetic and metabolic responses of tomato to soil flooding. They showed that ABA depletion in waterlogged tomato tissues acts as a positive signal, inducing several specific genetic and metabolic responses to flooding. In another study, Rodriguez-Saona et al., [41] investigated transcriptome changes in tomato in response to feeding by the potato aphid, (Macrosiphum euphorbiae), and the beet armyworm (Spodoptera exigua), individually or in combination. They demonstrated that herbivory resulted in the upregulation of several defense-related genes including genes encoding for threonine deaminase and numerous protease inhibitors. Considering the global diversity in the tomato germplasm and the contrasting and inherently complex and unpredictable combinations of stresses, there is an urgent need for studies aimed to understand how different tomato varieties diverge in their chemical and molecular response to stress combinations.
In this study, we investigated the effects of flooding, herbivory and their interaction on plant growth and plant chemistry and performed an unsupervised exploratory analysis of gene expression patterns. Our study focuses on two heirloom tomato varieties responding to waterlogging and/or herbivory by Spodotera exigua (the beet armyworm) larvae. We hypothesized that: 1) the combined stress of flooding and herbivory alters plant chemistry and negatively impacts plant growth, with a different effect than either of flooding or herbivory alone; and 2) the altered plant chemistry elicited during combinations of flooding and herbivory has a molecular underpinning detectable as differential gene expression.
Results
Volatile organic compounds
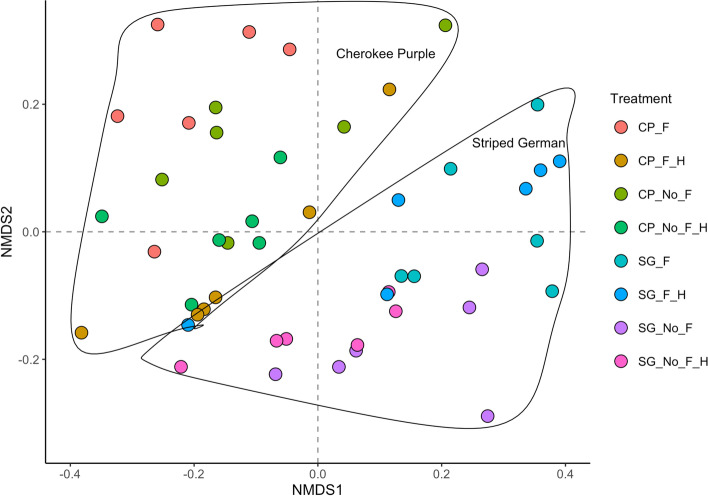
A total of 18 volatile organic compounds (VOCs) were detected and quantified from the headspace of the two heirloom tomato varieties subjected to four stress factors: (1) no flooding and no herbivory (control), (2) no flooding + herbivory, (3) flooding, and (4) flooding + herbivory. The detected VOCs, the majority of which were terpenoids, included the monoterpenes: α-pinene, o-cymene, σ-cymene, β-pinene, ( +)-4-carene, α-terpinene, β-phellandrene, trans-β-ocimene, p-cymene, β-ocimene, gamma-terpinene, and α-terpinolene; the sesquiterpenes: p-cresol (phenol), δ-elemene, β-elemene, caryophyllene, and humulene; and the alkane hydrocarbons: 1-pentadecane, and pentadecane. The non-metric dimensional scaling (NMDS) analysis of VOC emissions on Bray–Curtis dissimilarity matrix revealed strong clustering due to variety (Fig. 1).
Fig. 1.
Non-metric multidimensional scaling (NMDS) ordination constructed with a Bray–Curtis dissimilarity matrix of total volatile organic compounds emitted by two tomato varieties, Cherokee Purple (CP) and Striped German (SG), exposed to the following stress factors: (1) no flooding [No_F], (2) no flooding + herbivory [No_F_H], (3) flooding [F], and (4) flooding + herbivory [F_H]
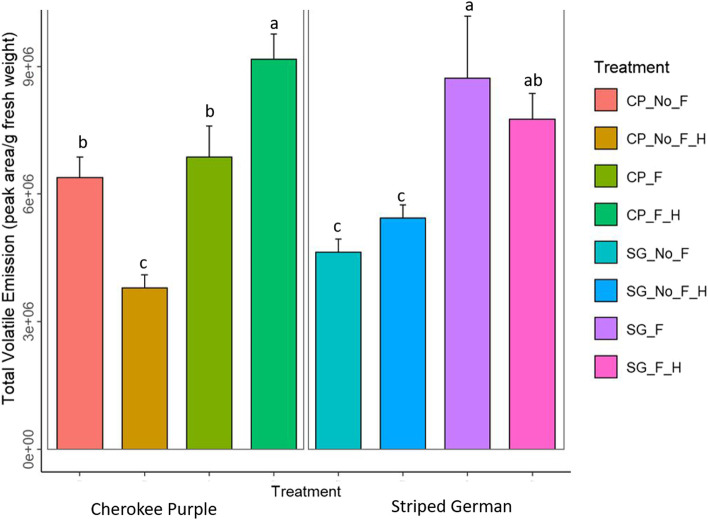
Three-way analysis of variance (ANOVA) revealed that total volatile organic compound emissions in the two heirloom varieties was influenced by flooding F[flooding] = 38.53, P[flooding] = < 0.0001 and the interaction of variety, flooding, and herbivory F[variety*flooding*herbivory] = 11.34, P[variety*flooding*herbivory] = 0.0017) (Table 1; Fig. 2). The highest VOCs emission rates, for both varieties, were recorded in the flooding treatments and the treatment involving the combinatorial stress of flooding and herbivory (Fig. 2). When considering the total volatile organic compounds emitted in plants exposed to flooding only, the Striped German (SG) variety emitted more volatiles than Cherokee Purple (CP) (Fig. 2).
Table 1.
Three-way analysis of variance table of treatment effect (variety, flooding, herbivory, and their interactions) on total volatile emissions
| Factors | DF | SS | MS | F- Value | P-Value |
|---|---|---|---|---|---|
| Variety | 1 | 8.329e + 10 | 8.329e + 10 | 0.028 | 0.8671 |
| Flooding | 1 | 1.133e + 14 | 1.133e + 14 | 38.536 | < .0001*** |
| Herbivory | 1 | 1.698e + 11 | 1.698e + 11 | 0.058 | 0.8112 |
| Variety x Flooding | 1 | 2.535e + 11 | 2.535e + 11 | 0.086 | 0.7705 |
| Variety x Herbivory | 1 | 1.696e + 10 | 1.696e + 10 | 0.006 | 0.9382 |
| Flooding x Herbivory | 1 | 7.350e + 12 | 7.350e + 12 | 2.501 | 0.1216 |
| Variety x Flooding x Herbivory | 1 | 3.332e + 13 | 3.332e + 13 | 11.337 | 0.0016** |
| Residuals | 40 | 1.176e + 14 | 2.939e + 12 |
Fig. 2.
Total volatile organic compounds emissions in two heirloom tomato varieties, Cherokee Purple (CP) and Striped German, (SG) exposed to the following stress factors: (1) no flooding [No_F], (2) no flooding + herbivory [No_F_H], (3) flooding [F], and (4) flooding + herbivory [F_H]. Tukey’s honest significance test was used to group means. Bars represent mean ± SE. Means with different letters are different (as determined by Tukey HSD, P < 0.05)
According to the random forest (RF) analysis, that ranked individual compounds by their importance in contributing to treatment separation and differences, the most important compounds were α-terpinolene, ( +)-4-carene, δ-elemene, α-pinene, humulene, β-phellandrene, caryophyllene, o-cymene, β-pinene and p-cymene (Table 2).
Table 2.
Ranking of volatile organic compounds (VOCs) that contribute to treatment differences. Random Forest was used. Compound rankings were determined using Mean Decrease Accuracy (MDA)
| Rank | Volatile Organic Compound | Mean Decrease Accuracy |
|---|---|---|
| 1 | p-cymene | 51.285543 |
| 2 | β-pinene | 48.739645 |
| 3 | o-cymene | 44.327346 |
| 4 | Caryophyllene | 43.056983 |
| 5 | β-phellandrene | 42.558952 |
| 6 | Humulene | 41.359839 |
| 7 | α-pinene | 38.821284 |
| 8 | δ-elemene | 37.039133 |
| 9 | ( +)- 4-carene | 35.596207 |
| 10 | α-terpinolene | 33.440834 |
| 11 | α-terpinene | 29.718382 |
| 12 | gamma-Terpinene | 22.976904 |
| 13 | β-elemene | 19.581877 |
| 14 | Pentadecane | 19.326385 |
| 15 | p-cresol | 13.409866 |
| 16 | 1-Pentadecane | 12.609018 |
| 17 | β-ocimene | 12.549250 |
| 18 | trans-β-ocimene | -0.951859 |
Results of three-way ANOVA of the effects of flooding, herbivory, variety, and their interactions on plant biomass characteristics (root length, root wet weight, root dry weight, shoot diameter, shoot length, shoot dry weight, and shoot dry weight) revealed significant differences for the main effects of variety and flooding on root length, root wet weight, root dry weight, shoot wet weight and shoot dry weight (Table 3). Flooding negatively affected plant growth. Wet root weight and dry root weight were significantly reduced by flooding in both heirloom varieties. Shoot dry weight was significantly reduced by flooding and by the interaction between flooding and herbivory in Striped German.
Table 3.
Three-way analysis of variance table of treatment effect (variety, flooding, herbivory, and their interactions) on plant growth characteristics
| Main Factor | Variety | Flooding | Herbivory | Var*F | Var*H | F*H | Var*F*H |
|---|---|---|---|---|---|---|---|
| Degrees of Freedom (Num, Den) | 1,40 | 1,40 | 1,40 | 1,40 | 1,40 | 1,40 | 1,40 |
| Parameter | F value and P value in parenthesis | ||||||
| Root length (cm) | 18.125 (< .0001) | 27.626 (< .0001) | 8.619 (0.0054) | 6.214 (0.0169) | 0.210 (0.6494) | 0.812 (0.3729) | 5.630 (0.0225) |
| Root wet weight (g) | 30.812 (< .0001) | 21.703 (< .0001) | 2.044 (0.1605) | 4.811 (0.0341) | 0.370 (0.5466) | 11.037 (0.0019) | 4.921 (0.0322) |
| Root dry weight (g) | 19.754 (< .0001) | 80.200 (< .0001) | 0.599 (0.4435) | 2.465 (0.1243) | 3.368 (0.0739) | 1.941 (0.1713) | 0.669 (0.4181) |
| Shoot diameter (cm) | 0.849 (0.362) | 45.660 (< .0001) | 0.377 (0.542) | 0.094 (0.760) | 0.849 (0.362) | 1.509 (0.226) | 2.358 (0.132) |
| Shoot length (cm) | 4.751 (0.0352) | 78.294 (< .0001) | 0.026 (0.8737) | 7.233 (0.0103) | 4.160 (0.0480) | 8.234 (0.0065) | 5.427 (0.0249) |
| Shoot wet weight (g) | 11.243 (0.0017) | 51.546 (< .0001) | 0.255 (0.6166) | 6.606 (0.0139) | 6.311 (0.0161) | 2.396 (0.1295) | 3.812 (0.0579) |
| Shoot dry weight (g) | 27.951 (< .0001) | 43.579 (< .0001) | 0.022 (0.8836) | 11.327 (0.0017) | 2.163 (0.1492) | 2.740 (0.1057) | 2.568 (0.1169) |
Gene expression analysis
Sequencing resulted in over 900 million high-quality reads (Phred score > 35 average along entire read), with an average of 37 million reads per library. There were 826 genes changing in expression between either of the factors analyzed and their interactions (fold change > 4, FDR-corrected p-value < 0.01).
Differential expression between varieties and its interaction with flooding
To investigate specific genes that are more likely to be differentially expressed between the two varieties, a more stringent cut off was used (fold-change > 8 p-val < 0.01) limiting the analysis to transcripts with very high differential expression and looking at the contrasts between varieties. This resulted in a set comprising 335 genes (Table S1) that were further used in a cluster analysis.
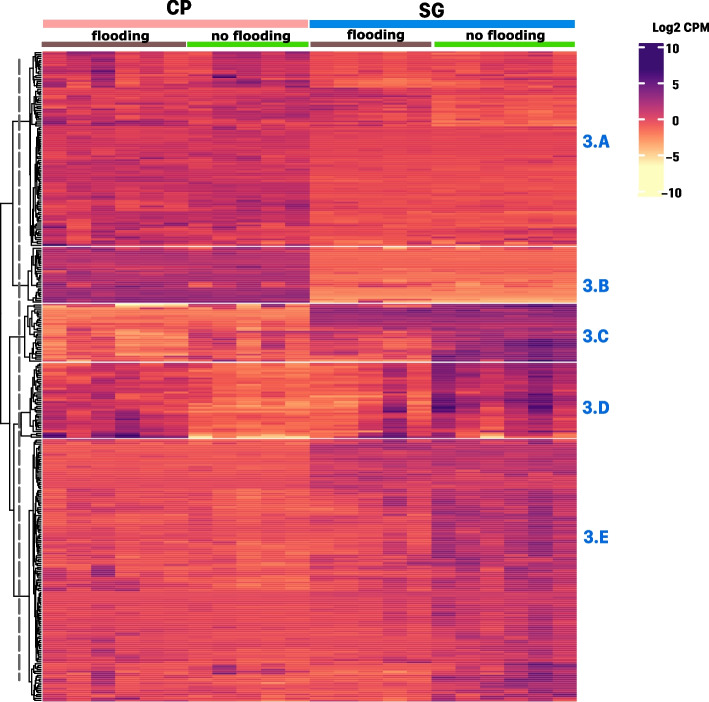
K-means clustering was applied to both: samples and genes. Clustering of samples confirmed two outlier samples that were further removed. The clustering separated five distinct groups of genes, designated 3.A, 3.B, 3.C, 3.D and 3.E (Fig. 3). Clusters 3.A and 3.B contained genes with higher expression in CP than in SG. There were 28 genes in cluster 3.B, a cluster showing marked and consistent high differences between CP and SG. Genes in this cluster included two phenylalanine ammonia-lyase (PAL), the enzyme in charge of the first committed step in the phenylpropanoid pathway. Also in the cluster were a gene encoding cinnamoyl-CoA reductase-like (LOC101262601), and one encoding phytoene synthase (Psy1) which catalyzes the first step in the carotenoid biosynthetic pathway. The top GO biological process terms in clusters A and B combined included: cinnamic acid biosynthetic process (GO:0009800), L-phenylalanine catabolic process (GO:0006559), trans-zeatin biosynthetic process (GO:0033466), glutamine family amino acid catabolic process (GO:0009065), and gibberellin biosynthetic process (GO:0009686) (Table S2). Taken together, the data suggests that both varieties might constitutively differ in the production of secondary metabolites including phenylpropanoids and terpenoids, where CP might have higher constitutive production of these compounds.
Fig. 3.
K-means clustering analysis of genes differentially expressed between two heirloom tomato varieties (fold-change > 8 and; FDR p-value < 0.01). CP: Cherokee Purple, SG: Striped German. The heatmap has treatments as columns and genes as rows. The top right inset shows the color scale for log2 counts per million (CPM) values with purple tones for up-regulated genes and yellow tones for down-regulated genes. The vertical striped line on dendrogram shows the level at which the tree was cut to separate groups based on k-means. Each separate cluster was named according to figure number followed by and alphabetic character. Each cluster gene content is described in detail in the text
Clusters 3.C and 3.E contained genes with lower expression in CP compared to SG (Fig. 3). Cluster 3.C had the most marked differences and comprised 29 genes. Genes in cluster 3.C with the most consistent and larger differences between both varieties (first half in top of the cluster) include several uncharacterized genes. GO biological processes terms in clusters 3.C and 3.E were enriched for disparate terms ranging from photosynthesis, secondary metabolism, and metabolism of high-molecular weight sugars (Table S1, Table S2).
Cluster 3.D contains transcripts that were affected by the interaction of flooding and variety where the flooding treatment most extremely affected varieties in opposite ways, with transcripts increasing in expression upon flooding in CP but decreasing in SG. Cluster 3.D contained 38 genes including 10 uncharacterized products and several transcription factors. The cluster is enriched for biological processed related to abscisic acid (ABA). Genes include several negative regulators of ABA signaling and genes known to respond to increased ABA in the cell environment. CP leaves appear to be responding to root flooding by repressing ABA production. SG, in turn, might have constitutively high ABA production or ABA-dependent signaling (Table S1, Table S2).
Changes in gene expression due to flooding
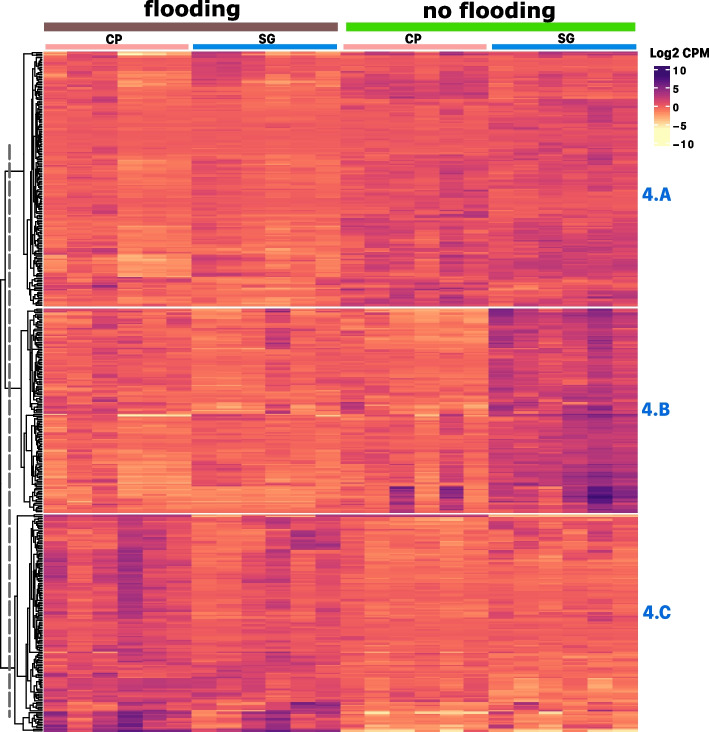
A subset of 404 genes were identified as highly differentially expressed at fold-change > 8, FDR p-value < 0.01 (Table S1) using contrasts between flooded and non-flooded samples. Clustering of samples successfully separated both treatments, and clustering of genes resulted on three distinct gene clusters (Fig. 4). Roughly, the subcluster (4.A) contains 152 genes that were generally downregulated with flooding, this cluster is significantly enriched for GO biological terms in cytokinin catabolism and general defense response (Table S2, Table S3). The differences in response to flooding between both varieties is evident in Cluster 4.B, with 122 genes, it includes genes that were downregulated in SG upon flooding. The top GO biological process term that was significantly enriched in 4.B was photosynthesis and the second was ABA signaling. Other GO biological processes that were enriched comprised response to water deprivation (GO:0009414), and wounding (GO:009611). There were two zeatin O-glucosyltransferase and one zeatin O-xylosyltransferase (Table S2). In general, this subcluster shows that varieties had different response to flooding stress.
Fig. 4.
K-means clustering analysis of genes differentially expressed between flooded tomato plants and non-flooded controls (fold-change > 8 and; FDR p-value < 0.01). CP: Cherokee Purple, SG: Striped German. The heatmap has treatments as columns and genes as rows. The top right inset shows the color scale for log2 counts per million (CPM) values with purple tones for up-regulated genes and yellow tones for down-regulated genes. The vertical striped line on dendrogram shows the level at which the tree was cut to separate groups based on k-means. Each separate cluster was named according to figure number followed by and alphabetic character. Each cluster gene content is described in detail in the text
Finally, cluster 4.C contained genes that were upregulated with flooding in both varieties and with no evident effect of herbivory (Fig. 4). Thus, this sub-cluster represents the general response to flooding. In this set of 130 genes, there were two genes encoding for enzymes in the first dedicated steps in the biosynthesis of ethylene: 1-aminocyclopropane-1-carboxylate synthase 3 (ACS3) and 1-aminocyclopropane-1-carboxylate oxidase homolog (ACO3), and. Increased ethylene biosynthesis is a well-documented process in waterlogged plants, including tomato [27, 39, 42]. We could also identify four genes in the flavonoid pathway that indicate increased anthocyanin production: a putative flavonoid 3′5' hydroxylase (LOC100736504), dihydroflavonol 4-reductase (LOC544150), a flavonoid 3',5'-methyltransferase (AnthOMT), and leucoanthocyanidin dioxygenase (LOC101251607). Notably, the set also comprised four genes encoding enzymes in the latest oxygenation steps of gibberellic acid (GA) that give rise to active gibberellin forms: gibberellin 2-beta-dioxygenase 1 (LOC101249786), gibberellin 2-oxidase 2 (GA2ox2), gibberellin 20-oxidase-3 (gene-20ox-3), and gibberellin 3-beta-dioxygenase 1-like (LOC101257892). The most significantly enriched GO biological process term was “protein folding” (GO:0006457), followed by “fruit ripening” (GO:0009835), and anthocyanin-containing compounds (GO:0009718) (Table S3). Protein folding enrichment is the result of the presence of eight (8) heat-shock proteins in the set. Heat-shock proteins are also markers of defense and response to various stresses.
Changes in gene expression due to herbivory
The response to herbivory was the weakest. Using the stringer criteria as in previous sections, only 36 genes were differentially expressed between plants responding to S. exigua feeding and no herbivory controls. Clustering analysis of this set did not show clear partitioning between treated and control samples. To further characterize the effects of herbivory, the analysis was repeated with the less stringent cut-off (fold-change > 4 FDR-corrected p-value < 0.01) and separately within each variety.
Gene expression due to herbivory in the Cherokee Purple variety
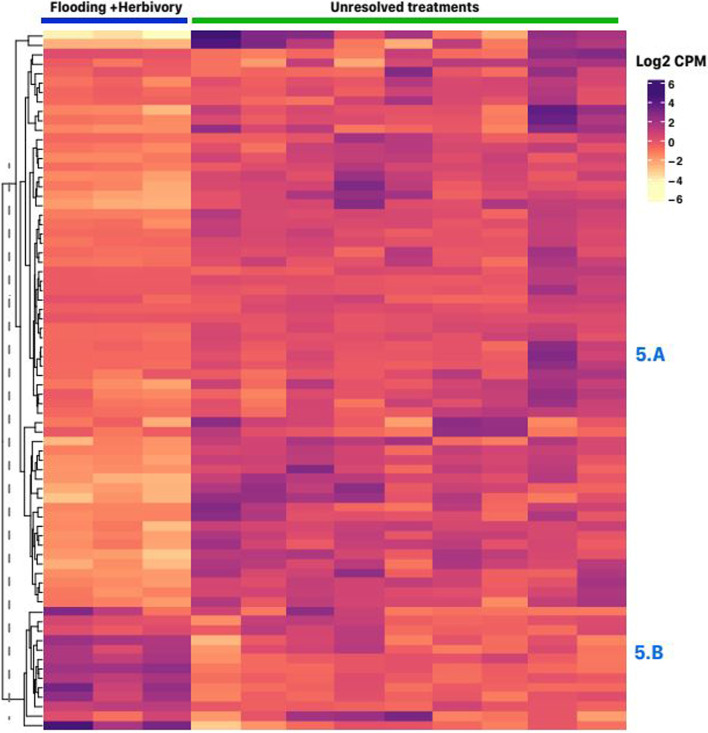
Seventy-four genes were differentially expressed between samples subjected to herbivory and controls in CP. The effect of herbivory was evident in flooded samples only (Fig. 5), and only two gene clusters could be distinguished in this set: Cluster 5.A, with 61 genes consistently downregulated upon herbivory, and Cluster 5.B with 13 genes that were upregulated with herbivory (different only in flooded samples). The top significantly enriched GO biological process in cluster 5.A was lignin biosynthesis (GO:0009809) owing the presence of transcripts encoding caffeoylshikimate esterase (LOC101259602), cinnamyl alcohol dehydrogenase (LOC101245999), caffeoyl-CoA O-methyltransferase (LOC101265977), among others. Cluster 5.A also include five transcripts encoding proteins from the plant GDSL esterase/lipase superfamily (CD1, LOC101262291, LOC101251962, LOC101266652, and LOC101267033) (Tables S1, S2 and S3); these hydrolytic enzymes have been implicated in the response to several biotic and abiotic stress response in plants [43].
Fig. 5.
K-means clustering analysis of genes differentially expressed in leaf tissue of tomato plants challenged with herbivory and controls in the tomato variety “Cherokee Purple” (fold-change > 4 and; FDR p-value < 0.01). The heatmap has treatments as columns and genes as rows. The top right inset shows the color scale for log2 counts per million (CPM) values with purple tones for up-regulated genes and yellow tones for down-regulated genes. The vertical striped line on dendrogram shows the level at which the tree was cut to separate groups based on k-means. Each separate cluster was named according to figure number followed by and alphabetic character. Each cluster gene content is described in detail in the text
Cluster 5.B includes chalcone synthase (CHS1) and PAL (LOC112941051) indicating up-regulation of the phenylpropanoid pathway. A gene encoding for a BAHD acyltransferase (gene-LOC101256185) was also present in the set. BAHD acyltransferase acylates phenolics compounds to change their transporting and reactivity properties. Also in the set was a UDP-glucose iridoid glucosyltransferase-like, suggesting the involvement of iridoid terpenes in the response to herbivore in flooded samples. In addition, there were two heat-shock proteins in cluster 5.B.
Gene expression due to herbivory in the Striped German variety
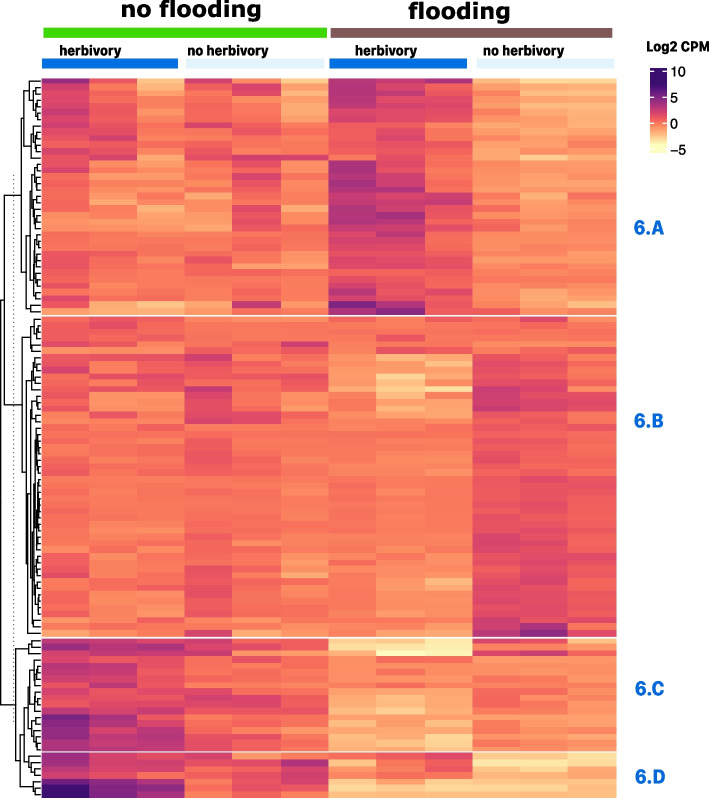
Within SG, there were 112 genes differentially expressed due to herbivory (fold-change > 4 and FDR corrected p-value 0.01). Clustering effectively separated herbivory and no herbivory samples but only within the flooding or no flooding groups (Fig. 6). Cluster 6.A shows genes that were upregulated with flooding and herbivory together but not with either stress alone. Seventeen out of 37 of the genes in cluster 6.A code for heat shock proteins (46%).
Fig. 6.
K-means clustering analysis of genes differentially expressed in leaf tissue of tomato plants challenged with herbivory and controls in the tomato variety “Cherokee Purple” (fold-change > 4 and; FDR p-value < 0.01). The heatmap has treatments as columns and genes as rows. The top right inset shows the color scale for log2 counts per million (CPM) values with purple tones for up-regulated genes and yellow tones for down-regulated genes. The vertical striped line on dendrogram shows the level at which the tree was cut to separate groups based on k-means. Each separate cluster was named according to figure number followed by and alphabetic character. Each cluster gene content is described in detail in the text
Cluster 6.B shows genes that were mildly downregulated by herbivory in flooded samples, including genes in primary metabolism, development, and photosynthesis (Table S3). Herbivory did not have apparent effects in non-flooded plants. Cluster 6.C contained 18 genes that were downregulated with herbivory in flooded samples but upregulated by herbivory alone. Among them were 2 genes encoding zeatin, O-glucosyltransferases, a GDSL esterase/lipase (see previous section), and several proteinases and proteinase inhibitors. Cluster 6.D, with 7 members, contains genes that were upregulated with herbivory, at a higher extent in non-flooded plants compared to flooded plants (Tables S1, S2, S3) Cluster 6.D included on gene encoding for a heat shock protein (hsc70), a thioredoxin (LOC101244843), and two peptidase/proteinase inhibitors (mcpi and LOC101246961).
Discussion
Identifying plant varieties and cultivars that can tolerate co-occurring biotic and abiotic plant stressors is critical to maintaining crop productivity in the context of a changing climate. Understanding how plants prioritize and respond to combinatorial stresses at the physiological and molecular levels and identifying the key genes and traits that underly adaptive responses remains a fundamental task for crop improvement and breeding of climate-resilient crops. To date, our knowledge about how crop plants like tomato respond to the combinatorial stress of flooding and herbivory remains limited. Available studies show that plant responses to combinatorial stresses are complex and hard to predict. In this study, we investigated volatile production levels and gene expression in two varieties of tomato plants following exposure to flooding, herbivory, and their combination.
Volatiles produced by plants undergoing biotic and abiotic stressors have been widely documented. Individually, biotic or abiotic stress factors, such as herbivory [20, 44–46], elevated O3 [21], drought [20, 45, 47], and salinity [47, 48], are known to alter volatile emissions. Much less is known about how volatiles that mediate ecological interactions among plants, insects and their natural enemies are influenced by concurrent stress factors. Furthermore, results from the few studies that investigated the influence of combinatorial stresses on plant volatile emissions are variable. For instance, the combination of drought and feeding by the potato aphid increased volatile emissions in tomato [20]. Ngumbi and Ugarte [31] reported an increase in VOC emission in maize exposed to flooding and herbivory by the fall armyworm, Spodoptera frugiperda. By contrast, Tariq et al., [18] reported a decrease in the emission of volatiles when Brassica oleracea plants were subjected drought and root herbivory. In our study, we showed that individually, flooding induced greater total volatile emissions in one of the tomato varieties (SG), with no effect in CP. Herbivory alone significantly decreased VOC emissions in CP, with no significant effect in SG. Combined flooding and herbivory significantly increased VOC emissions in both varieties compared to the controls. These results are in agreement with studies that have reported increased emissions of volatiles in response to interacting biotic and abiotic stresses in plants of agricultural importance including tomato and maize [20, 30, 31]. There is evidence that the induction of volatile emissions in plants undergoing stress is part of a programmed plant response to alleviate and mitigate the negative consequences of stress [4, 49, 50]. In our study, we identified 18 volatiles, mainly terpenoids, including α-pinene, o-cymene, β-pinene, ( +)-4-carene, β-phellandrene, caryophyllene and humulene. Increased amounts of these compounds were detected in plants exposed to the combined stress of flooding and herbivory. These VOCs have been reported to play important ecological and physiological functions that ultimately increase plant fitness by attracting natural enemies of insect herbivores and relieving plants of ongoing oxidative stresses [51]. Plants that are exposed to heat, high temperatures, or drought, for example, have been documented to increase volatile emissions; released volatiles act as thermoprotectants and help in stabilizing chloroplast membranes [52, 53]. Alternatively, these induced volatiles serve as signal molecules that activate regulatory genes involved in stress tolerance and plant defense [54]. The ecological roles of the volatiles we identified to be increased in the combinatorial stress treatments remains to be determined.
In our study, most of the released volatiles, were mono- and sesquiterpenes. We did not collect green leaf volatiles. There are several possible reasons that could have contributed to the lack of detection of green leaf volatiles across the treatments. The quality and quality of the blend of volatiles that is released by stressed plants can be influenced by many factors including the method used to collect headspace volatiles, the time of sampling, the herbivore used to elicit volatile organic compound production, the genotype or variety of plant used, and the microbiome of the plant [44, 55–57]. It is possible that the tomato varieties we used in this study, Cherokee Purple and Striped German, may not be prolific emitters of green leaf volatiles. It is also possible that because we sampled volatiles 48 h after the herbivore was introduced, we missed the short window in which green leaf volatiles are emitted. Future studies will investigate time-dependent volatile emissions in tomato plants experiencing flooding, herbivory and the combinatorial stress of flooding and herbivory. Moreover, in our study, in the treatments that involved herbivory by S. exigua, we did not detect a strong response in volatile emissions and gene expression. The varieties used in this study may have high levels of constitutive defenses. In this study, we did not starve the insects before allowing them to feed on experimental tomato plants to induce herbivore-induced volatiles and gene expression. In future, we will starve insects for 6 h before placing them on plants.
Biomass measurements showed that flooding detrimentally impacted plant growth, in agreement with previous studies that have investigated the impact of flooding on plant growth characteristics [35, 58–60]. In general, below-ground tissues were affected by flooding but not by herbivory, whereas above-ground tissues were affected by flooding alone and flooding combined with herbivory. Reduced plant growth is a direct result of shutting down of primary plant processes such as photosynthesis, and alterations in soil nutrients availability [61, 62]. Ultimately, reduced plant growth leads to poor quality crops and a reduction of crop yields. Our results showing that flooding negatively impacts plant growth add to the growing body of evidence documenting the detrimental impacts of flooding and further emphasize on the need for more research to understand the negative impacts of flooding on agricultural crops including tomato.
Gene expression analyses indicate that some key differences between the two heirloom tomato varieties reside in secondary metabolism pathways. CP had higher constitutive levels of genes encoding Phenylalanine ammonia-lyase (PAL). PAL converts L-phenylalanine to trans-cinnamate and ammonia is the first committed step in the phenylpropanoid pathway, redirecting large amounts of fixed carbon from primary to secondary metabolism [63, 64]. Phenylpropanoid derived metabolites including anthocyanins, flavonoids, isoflavonoids, phytoalexins, lignans and terpenoids have important functions in plant resistance mechanisms against biotic and abiotic stress, in signal transduction and communication between plants and other organisms, and as regulators of primary and secondary metabolism [63, 65–70]. Differential PAL gene expression patterns in response to biotic and abiotic stressors can result in plant phenotypes that differ in their stress adaptive responses and stress tolerance levels [68, 71, 72]. Psy1, also showing higher expression in CP, catalyzes the first step in the carotenoid biosynthetic pathway. Our results showed that CP may have increased constitutive production of phenylpropanoid compounds and terpenoids. Huang et al., [68] demonstrated that wild type Arabidopsis plants and pal mutants that differed in their pal gene expression patterns exhibited remarkable differences in their adaptive responses and sensitivity to biotic and abiotic stressors.
Our goal was to characterize the interactions between different stressors. We identified a group of genes that responded to flooding in different ways in the two varieties. In general CP plants subjected to flooding showed a down-regulation of ABA production, SG, in turn appears to have constitutively higher expression of genes in either ABA biosynthesis or in downstream ABA signaling. Abscisic acid (ABA) is an important phytohormone that regulates plants growth and development [73] and has been reported to be a key regulator of plant responses to abiotic stresses [74] because its accumulation controls multiple gene response networks, ultimately leading to tolerance and adaptation to abiotic stress [28, 29, 73, 75–77]. Genes involved in ABA biosynthesis and catabolism could be activated or decreased by abiotic stress [78, 79]. Studies on the role of ABA in modulating plant responses to flooding report variable results. For instance, using wild type and ABA-deficient tomato plants, [29] demonstrated that flooding reduced ABA content in wild type tomatoes and suggested that depletion of ABA serves as a positive signal that leads to the induction of several specific genetic and metabolic responses to flooding. Similar results have been reported in other plants including in Arabidopsis, soybeans, and tomato [42, 80–84]. In contrast, an increase in ABA content and levels in response to flooding has been reported in leaves of alfalfa and pea [85, 86]. In our study, consistent with [29], we documented different expression patterns of genes associated with ABA signaling in the two tomato varieties. Results from our study further confirm the involvement of ABA in plant responses to flooding stress.
Flooding alone affected several hormonal pathways and photosynthesis. Consistent with our biomass data, the downregulation of photosynthesis genes in plants undergoing flooding stress has been documented, with a subsequent impact on plant growth [19, 29, 87]. Reduced photosynthesis in plants actively going through flooding stress can be correlated with the production of reactive oxygen species (ROS) and cell damage [88–90]. High concentrations of ABA and increased xylem pH have been correlated with reduced stomatal conductance [91]. High foliar concentrations of auxins [92] and ABA [93] may build up and create an accumulation message in leaf tissues of flooded plants due to stomatal closure, resulting from root oxygen deficiency initiating loss of root hydraulic conductance, or from an increase in pH [94]. Crosstalk between plant stress-response pathways likely reduces fitness costs, by reducing production of redundant hormones. Indeed, studies have shown gibberellic acid (GA) mediates gene expression of jasmonic acid (JA) [95], while JA and salicylic acid (SA) mediate immune response [96].
In our study, flooding initiated down-regulation of cytokinin-related genes, in addition to up-regulation of ethylene biosynthesis and multiple gene encoding enzymes used in the latest oxygenation steps of GA, which give rise to active gibberellins. Consistent with our results, flooding has been shown to depress cytokinin activity [97, 98], and lead to accumulation of ethylene [99, 100]. Ethylene is likely critical for plants to initiate signals that prolong their survival under abiotic stress [101]. For example, epinasty (downward leaf growth), a response to flooding in tomato, arises in response to signals from oxygen‐deficient roots and upregulation of 1‐aminocyclopropane‐1‐carboxylic acid (ACC), the immediate precursor of ethylene [102]. Ethylene-mediated ROS signaling is critical in regulating hormonal pathways that lead to adaptive formation of lysigenous aerenchyma, used for gas diffusion in roots during flooding [103]. Submergence-induced accumulation of ethylene has also been implicated in upregulation of internode elongation [100]. GA regulates plant growth and abiotic stress tolerance through mediating growth and stress responses to abiotic stress [104]. One mechanism, demonstrated in Arabidopsis under salinity stress by [105] showed growth-repressing DELLA proteins promoted survival, through downregulation of root-hair growth and reduction of reactive oxygen species (ROS) levels. In deep-water rice, Kuroha et al., [106] reported increased GA production upon plant waterlogging, underlying the adaptation of rice to periodic flooding. Plausibly, a similar signaling mechanism is utilized by heirloom tomato plants. Finally, flooding upregulated eight genes encoding heat-shock proteins Broadly, heat shock proteins are regarded as dynamic biomolecules that help plants to counter biotic and abiotic stresses [107] via several mechanisms including enhancing membrane stability and detoxifying the ROS by positively regulating the antioxidant enzymes system.
We studied herbivory by looking at the effects upon feeding of leaf tissue by Spodoptera exigua, a leaf chewing caterpillar. The effects of herbivory were mild, and only flooded plants had measurable response to herbivory in both varieties, with a very small set of genes upregulated by herbivory alone in only one of the varieties (SG). This indicates that flooding might weaken some aspects of the plant stress response while priming others. In CP, several stress response proteins were downregulated by herbivory (in flooded plants) but key genes in the phenylpropanoid pathway were upregulated. Among the few genes that were upregulated in CP flooded plants was also an UDP-glucose iridoid glucosyltransferase-like, suggesting the involvement of iridoid or iridoid-like terpenes in the response to herbivore in flooded CP plants. In SG, a disproportionately large number of heat-shock proteins were upregulated by flooding and herbivory together. Heat shock proteins are one of the important and significant molecular chaperones that play key roles in abiotic and biotic stress tolerance by actively participating in protein quality control, enhancing membrane stability, regulating diverse signaling pathways, and detoxifying the ROS [53, 107–114]. In agreement with previous studies, including studies done on maize, soybeans, and tomato, that have revealed the upregulation of various heat shock proteins by flooding, our results revealing an upregulation of heat shock protein genes, suggest that these proteins likely play a significant role in protecting proteins from denaturation and degradation and detoxifying ROS during flooding stress [115]. Studies on flooding in plants including maize, soybean and tomato have revealed that pathways involving various heat shock proteins are triggered and upregulated by flooding [90, 115]. Heat shock protein responses to biotic and abiotic stress and their combinations are shaped by the type of the insect herbivore, plant type and variety, and plants development stage when stress factors are applied [107].
Finally, as we were able to identify some specific VOCs and gene pathways that seem to be important in the plant response to flooding and the combination of flooding and herbivory, we should consider some of the factors and limitations that could have affected the results and prevent generalizations. For example, in our study the plants were first exposed to flooding before being exposed to herbivory, this order is not trivial since the plants can prime a defense response based on the first stress they encounter. In addition, is likely that the responses of the plants to a chewing insect are different from those to insects with different feeding strategies. Finally, the chemical profiling method for this study focused on volatile compounds; many, if not most, phenylpropanoid compounds that are part not only of the response to the stresses but as part of the constitutive differences between the two tomato varieties are not volatiles. With those considerations, this study sheds light into combinatorial stress responses and addresses our initial hypotheses; that the combined effects of flooding and herbivory differ from either stress alone, and that different tomato varieties can have better tolerance to those stressors.
Conclusions
We demonstrated that flooding and the combination of flooding and herbivory elevated VOCs production, the majority of which were terpenes. Gene expression data demonstrated an upregulation of genes involved in the phenylpropanoid and terpenoid production pathways in at least one of the tomato heirloom varieties (CP) in response to these stresses. Plant volatiles mediate numerous plant and insect interactions [116]. How stress combinations alter plant VOCs can be better explained by looking at the genetic underpinning of the response to those stressors. Our study highlights the importance of studying combinatorial stresses and sheds light on key pathways that might be mediating this response as well as the complex dynamics that might at play during combined stresses. How these documented changes in gene expression patterns and plant chemistry affect plant and insect interactions deserves further investigation.
Methods
Tomato plant varieties
Two organic heirloom tomato (Solanum lycopersicum L.) varieties were used in this study; Cherokee Purple (CP) and Striped German (SG). We selected the heirloom varieties based on results from an unpublished survey by our lab of most popular tomato varieties grown organically by farmers in Central Illinois. Moreover, heirloom crops are prized by farmers, chefs and consumers for their diverse coloration, distinct flavor, and culinary qualities [117–120]. After performing numerous exploratory flooding experiments on four different tomato varieties commonly grown by Central Illinois farmers, we selected two heirlooms, Cherokee Purple (CP) and Striped German (SG) as most suitable to greenhouse experiments. CP grows relatively short vines, and produces a flattened, medium-large fruit, that is dusty pink with dark shoulders at approximately 72 days; the fruit has a rich, sweet flavor. SG grows medium-tall vines, and produces a flat, medium-large fruit, with variable shoulder ribbing in yellow and red at approximately 78 days; the fruit has a complex fruity flavor and smooth texture. Our selection was also informed by previous studies which indicate the broad genetic diversity found in heirloom tomato cultivars are better able to resist fungal pathogens [121], bacterial diseases [120, 122], and can maintain nutritional quality and yields under abiotic stressors such as drought [123] and salinity [124]. Seeds were obtained from Johnny’s seeds (Johnny’s Seeds, Winslow, Maine, USA). Seeds were germinated in seed trays (planting trays (model 1020), in 72-cell trays; 25 × 51 cm) containing potting soil (Berger BM2 Seed Germination & Propagation Mix; Berger, Saint-Modeste, Quebec, CA). Seedlings were grown at the greenhouse facilities of the University of Illinois at Urbana-Champaign plant care facility (PCF) at 25 °C ± 5 °C, 50 ± 5% relative humidity and 14L:10D photoperiod for two weeks.
Insect herbivore
The beet armyworm, Spodoptera exigua (Lepidoptera: Noctuidae), a generalist herbivore caterpillar, was used as the herbivore species in this study. S. exigua caterpillars were purchased from Benzon Research (Carlisle, Pennsylvania, USA) and maintained in a constant temperature incubator at 26 °C and exposed to a 16L:8D photoperiod until the start of the experiments.
Experimental design
Two weeks after germination, seedlings were transplanted into individual plastic pots (12.5 cm high, 16.5 cm diam) (Hummert International, Earth City, Missouri, USA) containing field-collected soil (Drummer silty clay loam) from Champaign County, Illinois, USA. One tomato seedling of each variety was transplanted into an individual pot. Plants were grown for three weeks at 25 ± 5 °C, 50 ± 5% relative humidity and 14:10-h (L/D). Three weeks after transplanting, plants were randomly assigned into four treatment groups: 1) no flooding + no herbivory, 2) flooding + no herbivory, 3) no flooding + herbivory, and 4) flooding + herbivory. Flooding was imposed by placing the pots containing tomato plants within a secondary larger white plastic bucket (16.5 cm high, 21.5 cm diam) (Consolidated Plastics, Stow, Ohio, USA) and filling it with water up to 5 cm above the soil surface. Plants in the no flooding treatments were watered regularly to maintain field capacity.
For the treatment factors involving insect herbivory, preliminary experiments showed that third instar S. exigua larvae acclimate and start feeding within 12 to 24 h of placement on a plant. Two third instar caterpillar larvae per plant were introduced three days post-flooding and allowed to feed for 48 h prior to volatile collection and collection of samples for gene expression analysis.
RNA sequencing and analysis
Samples were taken from 40-day old tomato plants that were subjected to flooding for 5 days and/or herbivory for 2 days. Plants that were subjected to both treatments were exposed to herbivory on the last 2 days of the 5-day flooding period. Control samples consisted of 40-day old tomato plants with no flooding or herbivory. Samples were obtained by cutting the tip of the leaflet closest to the stem from the second true leaf, using sanitized micro-dissecting scissors (re-sanitized for each sampling). Samples were individually collected in sealable plastic sleeves, sleeves were heat sealed, and immediately flash-frozen by submerging in liquid nitrogen.
Total RNA from each sample was extracted using a Nucleospin® RNA kit (Macherey–Nagel, Düren, Germany) according to the manufacturer’s protocol. There were three biological replications per treatment (flooding or herbivory) across two tomato varieties. The RNA was sequenced as single reads on one S1 lane for 101 cycles on a NovaSeq 6000 (Illumina, San Diego, CA). After sequencing, reads were pre-processed, mapped to the tomato reference genome (SL3.0) using STAR aligner [125], and quantified for differential expression with the EdgeR Bioconductor package [126]. Clusters for all sets were generated with the K-means method in R (v. 4.1.1) using the Hartigan and Wong algorithm [127] with a maximum of 100 iterations and 10 initial random sets. The number of clusters (k) to partition the data was visually determined after generating heatmaps and dendrograms with the Complex Heatmap package from R Bioconductor [128]. Gene ontology analyses were performed using topGO with the ‘elim’ and ‘weight’ algorithms to prevent redundant GOs from inflating the significance [129].
Collection and analysis of headspace volatiles
Aboveground headspace volatiles were collected using the solid phase micro-extraction (SPME) technique. Exhaustive preliminary experiments showed that the SPME method gave reliable and consistent results, that were comparable to other dynamic headspace volatile collection techniques. For the treatments without herbivore damage, plants were wrapped with an odor-blocking oven bag (Arcadia INTL, El Monte, California, USA) and wrapped for one hour to allow for volatile concentration. After one hour, a SPME fiber (65 µm polydimethdimethylsiloxane-divinylbenzezne (PDMS/DVB) fused silica, and stainless-steel fiber (Millipore Sigma®, Milwaukee, Wisconsin, USA) was inserted into each bag for 40 min, withdrawn, and then run immediately through coupled gas chromatography-mass spectrometry (GC–MS) for volatile identification and analysis. For the treatments involving herbivore damage, two third instar S. exigua larvae were allowed to feed on each tomato plant for 48 h before volatile collection. After 48 h with larvae still feeding, plants were wrapped with an odor-blocking oven bag, and similar headspace volatile collection protocols as described in treatments without herbivore damage were used.
Volatiles were identified using GC–MS, Hewlett-Packard (HP) 6890 GC (Hewlett-Packard, Sunnyvale, California, USA) in splitless mode, interfaced to an HP 5973 mass selective detector (MSD) with helium carrier gas. The GC oven was programmed as follows: inject at 40 °C, hold at 40 °C for 2 min, and then increase by 5 °C/min to 200 °C, for a total of 40 min. Injector and transfer line temperatures were 200 °C. Peak identification was performed using the NIST 98 library and by comparing published GC profiles of tomato headspace volatiles [130]. The structures of identified compounds were further confirmed by using synthetic standards that are commercially available purchased from Millipore Sigma® (St. Louis, Missouri, USA).
Plant growth parameters
At the end of the experiment, shoots and roots were harvested. Fresh shoot and root weight was recorded, and the shoots and roots were placed in paper bags, oven dried at 70 °C for 3 days, and their dry weights recorded.
Statistical analyses
Statistical analyses were performed in R software version 4.1.2 (R Core Team, 2021). Figures were produced using ggplot2 v.3.3.5 [131]. To visualize differences in total volatile organic compound emissions among treatments, non-metric dimensional scaling (NMDS) was used on a Bray–Curtis dissimilarity matrix using the metaMDS function of the vegan package v.2.5–7. The influence of variety, flooding, herbivory and their interactions on gene expression patterns, plant volatile organic compound emissions and plant growth characteristics were analyzed and compared using a three-way analysis of variance (ANOVA) followed by Tukey–Kramer HSD at P < 0.05. To identify the set of important volatile compounds that distinguish among treatment combinations, Random Forest (RF) algorithm for classification as outlined in [132, 133] was used. The RF analysis was performed using the package “randomForest” within the R environment using the function randomForest [134]. The model ran for 1,000 iterations for variable selection. VOCs importance was ranked with mean decrease in accuracy (MDA), where a higher MDA indicates higher importance in classification. The RF analysis generates the average out-of-bag (OOB) error, with a low OOB error suggesting a greater ability of the variable to differentiate treatment factors.
Supplementary Information
Acknowledgements
We thank Rosalie Metallo, Benjamin Harbaugh, and Monte Flack, UIUC Plant Care Facility for maintaining experimental plants. We thank Aaron Mleziva for helping with statistical analysis.
Experimental research on plants
Experimental research was carried out with relevant institutional, national, and international guidelines and legislation.
Authors’ contributions
EN conceived the experiment and design, EN and ED designed and carried out the greenhouse experiments and applied the treatments; EN and ED carried out sampling for VOC emission tests; EN analyzed and wrote results for VOC emission; BC sampled leaf tissue; BC extracted RNA; BC analyzed RNA-seq data and wrote RNA-seq results; EN, ED, and BC wrote the manuscript. The author(s) read and approved the final manuscript.
Funding
This research was funded by the University of Illinois at Urbana-Champaign.
Availability of data and materials
All raw RNA sequence data are available a NCBI under project accession: PRJNA850397.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors state no conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Rizhsky L, Liang H, Shuman J, Shulaev V, Davletova S, Mittler R. When defense pathways collide the response of arabidopsis to a combination of drought and heat stress. Plant Physiol. 2004;134(4):1683–96. doi: 10.1104/pp.103.033431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mittler R. Abiotic Stress, the Field Environment and Stress Combination. Trends Plant Sci. 2006;11(1):15–19. doi: 10.1016/j.tplants.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 3.Atkinson NJ, Urwin PE. The Interaction of Plant Biotic and Abiotic Stresses: From Genes to the Field. J Exp Bot. 2012;63(10):3523–3543. doi: 10.1093/jxb/ers100. [DOI] [PubMed] [Google Scholar]
- 4.Holopainen JK, Gershenzon J. Multiple Stress Factors and the Emission of Plant VOCs. Trends Plant Sci. 2010;15(3):176–184. doi: 10.1016/j.tplants.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 5.Prasch CM, Sonnewald U. Simultaneous application of heat, drought, and virus to Arabidopsis plants reveals significant shifts in signaling networks. Plant Physiol. 2013;162(4):1849–1866. doi: 10.1104/pp.113.221044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sewelam N, Oshima Y, Mitsuda N, Ohme-Takagi M. A Step towards understanding plant responses to multiple environmental Stresses: a genome-wide study. Plant, Cell Environ. 2014;37(9):2024–2035. doi: 10.1111/pce.12274. [DOI] [PubMed] [Google Scholar]
- 7.Osthoff A, dalle RoseBaldaufPiephoHochholdinger. PDJAHPF. Transcriptomic Reprogramming of Barley Seminal Roots by Combined Water Deficit and Salt Stress. BMC Genomics. 2019;20(1):325. doi: 10.1186/s12864-019-5634-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kissoudis C, van de Wiel C, Visser RGF, van der Linden G. Enhancing Crop Resilience to Combined Abiotic and Biotic Stress through the Dissection of Physiological and Molecular Crosstalk. Frontiers in Plant Science 5 (2014). https://www.frontiersin.org/article/10.3389/fpls.2014.00207. [DOI] [PMC free article] [PubMed]
- 9.Suzuki N, Rivero RM, Shulaev V, Blumwald E, Mittler R. Abiotic and Biotic Stress Combinations. New Phytol. 2014;203(1):32–43. doi: 10.1111/nph.12797. [DOI] [PubMed] [Google Scholar]
- 10.Ramegowda V, Senthil-Kumar M. The interactive effects of simultaneous biotic and abiotic stresses on plants: mechanistic understanding from drought and pathogen combination. J Plant Physiol. 2015;176:47–54. doi: 10.1016/j.jplph.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 11.Pandey P, Ramegowda V, Senthil-Kumar M. Shared and unique responses of plants to multiple individual stresses and stress combinations: physiological and molecular mechanisms. Front Plant Sci. 2015;6:723. doi: 10.3389/fpls.2015.00723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Desaint H, Aoun N, Deslandes L, Vailleau F, Roux F, Berthomé R. Fight Hard or Die Trying: When Plants Face Pathogens under Heat Stress. New Phytol. 2021;229(2):712–734. doi: 10.1111/nph.16965. [DOI] [PubMed] [Google Scholar]
- 13.Berens ML, Wolinska KW, Spaepen S, Ziegler J, Nobori T, Nair A, Krüler V, Winkelmüller TM, Wang Y, Mine A, Becker D, Garrido-Oter R, Schulze-Lefert P, Tsuda K. Balancing trade-offs between biotic and abiotic stress responses through leaf age-dependent variation in stress hormone cross-talk. Proc Natl Acad Sci USA. 2019;116(6):2364–73. doi: 10.1073/pnas.1817233116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Atkinson NJ, Lilley CJ, Urwin PE. Identification of genes involved in the response of arabidopsis to simultaneous biotic and abiotic stresses. Plant Physiol. 2013;162(4):2028–41. doi: 10.1104/pp.113.222372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rasmussen S, Barah P, Suarez-Rodriguez MC, Bressendorff S, Friis P, Costantino P, Bones AM, Nielsen HB, Mundy J. Transcriptome Responses to Combinations of Stresses in Arabidopsis. Plant Physiol. 2013;161(4):1783–1794. doi: 10.1104/pp.112.210773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Riley AC, Ashlock DA, Graether SP. Evolution of the Modular, Disordered Stress Proteins Known as Dehydrins. PLOS One. 2019;14(2):e0211813. doi: 10.1371/journal.pone.0211813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sewelam N, Brilhaus D, Bräutigam A, Alseekh S, Fernie AR, Maurino VG. Molecular Plant Responses to Combined Abiotic Stresses Put a Spotlight on Unknown and Abundant Genes. J Exp Bot. 2020;71(16):5098–5112. doi: 10.1093/jxb/eraa250. [DOI] [PubMed] [Google Scholar]
- 18.Tariq M, Wright DJ, Bruce TJA, Staley JT. Drought and Root Herbivory Interact to Alter the Response of Above-Ground Parasitoids to Aphid Infested Plants and Associated Plant Volatile Signals. PLOS One. 2013;8(7):e69013. doi: 10.1371/journal.pone.0069013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nguyen D, D’Agostino N, Tytgat TOG, Sun P, Lortzing T, Visser EJW, Cristescu SM, Steppuhn A, Mariani C, van Dam NM, Rieu I. Drought and flooding have distinct effects on herbivore-induced responses and resistance in Solanum dulcamara. Plant Cell Environ. 2016;39(7):1485–1499. doi: 10.1111/pce.12708. [DOI] [PubMed] [Google Scholar]
- 20.Catola S, Centritto M, Cascone P, Ranieri A, Loreto F, Calamai L, Balestrini R, Guerrieri E. Effects of Single or Combined Water Deficit and Aphid Attack on Tomato Volatile Organic Compound (VOC) Emission and Plant-Plant Communication. Environ Exp Bot. 2019;153:54–62. doi: 10.1016/j.envexpbot.2018.05.001. [DOI] [Google Scholar]
- 21.Mittler R, Blumwald E. Genetic Engineering for Modern Agriculture: Challenges and Perspectives. Annu Rev Plant Biol. 2010;61(1):443–62. doi: 10.1146/annurev-arplant-042809-112116. [DOI] [PubMed] [Google Scholar]
- 22.Khaling E, Agyei T, Jokinen S, Holopainen JK, Blande JD. The Phytotoxic Air-Pollutant O3 Enhances the Emission of Herbivore-Induced Volatile Organic Compounds (VOCs) and Affects the Susceptibility of Black Mustard Plants to Pest Attack. Environ Pollut. 2020;265:115030. doi: 10.1016/j.envpol.2020.115030. [DOI] [PubMed] [Google Scholar]
- 23.Guo H, Sun Y, Ren Q, Zhu-Salzman K, Kang L, Wang C, Li C, Ge F. Elevated CO2 Reduces the Resistance and Tolerance of Tomato Plants to Helicoverpa Armigera by Suppressing the JA Signaling Pathway. PLOS One. 2012;7(7):e41426. doi: 10.1371/journal.pone.0041426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Slater LJ, Villarini G. Recent Trends in U.S. Flood Risk. Geophysical Research Letters. 2016; 43(24:12,428–12,436. 10.1002/2016GL071199.
- 25.Li Y, Guan K, Schnitkey GD, DeLucia E, Peng B. Excessive Rainfall Leads to Maize Yield Loss of a Comparable Magnitude to Extreme Drought in the United States. Glob Change Biol. 2019;25(7):2325–2337. doi: 10.1111/gcb.14628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu P, Sun F, Gao R, Dong H. RAP2.6L Overexpression Delays Waterlogging Induced Premature Senescence by Increasing Stomatal Closure More than Antioxidant Enzyme Activity. Plant Mol Biol. 2012;79(6):609–22. 10.1007/s11103-012-9936-8. [DOI] [PubMed]
- 27.Voesenek LACJ, Bailey-Serres J. Flooding Tolerance: O2 Sensing and Survival Strategies. Curr Opin Plant Biol. 2013;16(5):647–653. doi: 10.1016/j.pbi.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 28.Voesenek LACJ, Bailey-Serres J. Flood Adaptive Traits and Processes: An Overview. New Phytol. 2015;206(1):57–73. doi: 10.1111/nph.13209. [DOI] [PubMed] [Google Scholar]
- 29.de Ollas C, González-Guzmán M, Pitarch M, Matus JT, Candela H, Rambla JL, Granell A, Gómez-Cadenas A, Arbona V. Identification of ABA-Mediated Genetic and Metabolic Responses to Soil Flooding in Tomato (Solanum Lycopersicum L. Mill). Front Plant Sci. 2021;12. https://www.frontiersin.org/article/10.3389/fpls.2021.613059. [DOI] [PMC free article] [PubMed]
- 30.Block AK, Hunter CT, Sattler SE, Rering C, McDonald S, Basset GJ, Christensen SA. Fighting on Two Fronts: Elevated Insect Resistance in Flooded Maize. Plant, Cell Environ. 2020;43(1):223–234. doi: 10.1111/pce.13642. [DOI] [PubMed] [Google Scholar]
- 31.Ngumbi EN, Ugarte CM. Flooding and herbivory interact to alter volatile organic compound emissions in two maize hybrids. J Chem Ecol. 2021;47(7):707–18. doi: 10.1007/s10886-021-01286-7. [DOI] [PubMed] [Google Scholar]
- 32.Tamang BG, Li S, Rajasundaram D, Lamichhane S, Fukao T. Overlapping and Stress‐specific Transcriptomic and Hormonal Responses to Flooding and Drought in Soybean. Plant J. 2021;107. 10.1111/tpj.15276. [DOI] [PubMed]
- 33.Safavi-Rizi V, Herde M, Stöhr C. RNA-Seq reveals novel genes and pathways associated with hypoxia duration and tolerance in tomato root. Sci Rep. 2020;10(1):1692. doi: 10.1038/s41598-020-57884-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.FAO. The State of Food and Agriculture 2019. Moving Forward on Food Loss and Waste Reduction. (2019). Rome. 10.4060/CA6030EN.
- 35.Vidoz ML, Loreti E, Mensuali A, Alpi A, Perata P. Hormonal Interplay during Adventitious Root Formation in Flooded Tomato Plants. Plant J. 2010;63(4):551–562. doi: 10.1111/j.1365-313X.2010.04262.x. [DOI] [PubMed] [Google Scholar]
- 36.The Tomato Genome Consortium. The Tomato Genome Sequence Provides Insights into Fleshy Fruit Evolution. Nature. 2012;485(7400):635–41. 10.1038/nature11119. [DOI] [PMC free article] [PubMed]
- 37.Gerszberg A, Hnatuszko-Konka K, Kowalczyk T, Kononowicz AK. Tomato (Solanum Lycopersicum L.) in the Service of Biotechnology. Plant Cell, Tissue and Organ Culture. 2015;120(3):881–902. doi: 10.1007/s11240-014-0664-4. [DOI] [Google Scholar]
- 38.de Pedro LF, Mignolli F, Scartazza A, Melana Colavita JP, Bouzo CA, Vidoz ML. Maintenance of Photosynthetic Capacity in Flooded Tomato Plants with Reduced Ethylene Sensitivity. Physiol Plant. 2020;170(2):202–217. doi: 10.1111/ppl.13141. [DOI] [PubMed] [Google Scholar]
- 39.Shiu OY, Oetiker JH, Yip WK, Yang SF. The Promoter of LE-ACS7, an Early Flooding-Induced 1-Aminocyclopropane-1-Carboxylate Synthase Gene of the Tomato, Is Tagged by a Sol3 Transposon. Proc Nat l Acad Sci. 1998;95(17):10334–39. doi: 10.1073/pnas.95.17.10334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ezin V, Yabi I, Ahanchede A. Impact of Salinity on the Production of Tomato along the Coastal Areas of Benin Republic. Afr J Environ Sci Technol. 2012;6(4):214–223. doi: 10.4314/ajest.v6i4. [DOI] [Google Scholar]
- 41.Rodriguez-Saona CR, Musser RO, Vogel H, Hum-Musser SM, Thaler JS. Molecular, Biochemical, and Organismal Analyses of Tomato Plants Simultaneously Attacked by Herbivores from Two Feeding Guilds. J Chem Ecol. 2010;36(10):1043–1057. doi: 10.1007/s10886-010-9854-7. [DOI] [PubMed] [Google Scholar]
- 42.Else MA, Hall KC, Arnold GM, Davies WJ, Jackson MB. Export of Abscisic Acid, 1-Aminocyclopropane-1-Carboxylic Acid, Phosphate, and Nitrate from Roots to Shoots of Flooded Tomato Plants (Accounting for Effects of Xylem Sap Flow Rate on Concentration and Delivery) Plant Physiol. 1995;107(2):377–84. doi: 10.1104/pp.107.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Volokita M, Rosilio-Brami T, Rivkin N, Zik M. Combining comparative sequence and genomic data to ascertain phylogenetic relationships and explore the evolution of the Large GDSL-Lipase Family in Land Plants. Mol Biol Evol. 2011;28(1):551–65. doi: 10.1093/molbev/msq226. [DOI] [PubMed] [Google Scholar]
- 44.Silva DB, Weldegergis BT, van Loon JJA, Bueno VHP. Qualitative and Quantitative Differences in Herbivore-Induced Plant Volatile Blends from Tomato Plants Infested by Either Tuta Absoluta or Bemisia Tabaci. J Chem Ecol. 2017;43(1):53–65. doi: 10.1007/s10886-016-0807-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pagadala Damodaram KJ, Gadad HS, Parepally SK, Vaddi S, Ramanna Hunashikatti L, Bhat RM. Low Moisture Stress Influences Plant Volatile Emissions Affecting Herbivore Interactions in Tomato Solanum Lycopersicum. Ecol Entomol. 2021;46(3):637–650. doi: 10.1111/een.13012. [DOI] [Google Scholar]
- 46.Paudel S, Lin PA, Foolad MR, Ali JG, Rajotte EG, Felton GW. Induced Plant Defenses Against Herbivory in Cultivated and Wild Tomato. J Chem Ecol. 2019;45(8):693–707. doi: 10.1007/s10886-019-01090-4. [DOI] [PubMed] [Google Scholar]
- 47.Cui LG, Shan JX, Shi M, Gao JP, Lin HX. The MiR156-SPL9-DFR Pathway Coordinates the Relationship between Development and Abiotic Stress Tolerance in Plants. Plant J. 2014;80(6):1108–1117. doi: 10.1111/tpj.12712. [DOI] [PubMed] [Google Scholar]
- 48.Tomescu D, Şumălan R, Copolovici L, Copolovici D. The Influence of Soil Salinity on Volatile Organic Compounds Emission and Photosynthetic Parameters of Solanum lycopersicum L Varieties. Open Life Sci. 2017;12(1):135–42. doi: 10.1515/biol-2017-0016. [DOI] [Google Scholar]
- 49.Vickers CE, Gershenzon J, Lerdau MT, Loreto F. A Unified Mechanism of Action for Volatile Isoprenoids in Plant Abiotic Stress. Nat Chem Biol. 2009;5(5):283–291. doi: 10.1038/nchembio.158. [DOI] [PubMed] [Google Scholar]
- 50.Loreto F, Schnitzler JP. Abiotic Stresses and Induced BVOCs. Trends Plant Sci. 2010;15(3):154–166. doi: 10.1016/j.tplants.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 51.Copolovici L, Niinemets Ü. Environmental Impacts on Plant Volatile Emission. In: Blande JD, Glinwood R (eds). Deciphering Chemical Language of Plant Communication. Part of Series: Signaling and Communication in Plants. Cham: Springer International Publishing. (2016):35–59. 10.1007/978-3-319-33498-1_2.
- 52.Sharkey T, Singsaas E. Why Plants Emit Isoprene. Nature. 1995;374:769–769. doi: 10.1038/374769a0. [DOI] [Google Scholar]
- 53.Loreto F, Barta C, Brilli F, Nogues I. On the induction of volatile organic compound emissions by plants as consequence of wounding or fluctuations of light and temperature. Plant, Cell Environ. 2006;29(9):1820–1828. doi: 10.1111/j.1365-3040.2006.01561.x. [DOI] [PubMed] [Google Scholar]
- 54.Scala A, Allmann S, Mirabella R, Haring MA, Schuurink RC. Green Leaf Volatiles: a Plant’s Multifunctional Weapon against Herbivores and Pathogens. Int J Mol Sci. 2013;14(9):17781–17811. doi: 10.3390/ijms140917781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Copolovici L, Kännaste A, Pazouki L, Niinemets Ü. Emissions of green leaf volatiles and terpenoids from Solanum lycopersicum are quantitatively related to the severity of cold and heat shock treatments. J Plant Physiol. 2012;169(7):664–672. doi: 10.1016/j.jplph.2011.12.019. [DOI] [PubMed] [Google Scholar]
- 56.Sharifi R, Lee SM, Ryu CM. Microbe-induced plant volatiles. New Phytol. 2018;220(3):684–691. doi: 10.1111/nph.14955. [DOI] [PubMed] [Google Scholar]
- 57.Sharifi R, Ryu CM. Social networking in crop plants: Wired and wireless cross-plant communications. Plant, Cell Environ. 2021;44(4):1095–1110. doi: 10.1111/pce.13966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kozlowski TT. Plant Responses to Flooding of Soil. BioSci. 1984;34(3):162–67. doi: 10.2307/1309751. [DOI] [Google Scholar]
- 59.Tariq MAUR, Farooq R, van de Giesen N. A Critical Review of Flood Risk Management and the Selection of Suitable Measures. Appl Sci. 2020;10(23):8752. doi: 10.3390/app10238752. [DOI] [Google Scholar]
- 60.Balke T, Webb EL, van den Elzen E, Galli D, Herman PMJ, Bouma TJ. Seedling Establishment in a Dynamic Sedimentary Environment: a Conceptual Framework Using Mangroves. J Appl Ecol. 2013;50(3):740–747. doi: 10.1111/1365-2664.12067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Irfan M, Hayat S, Hayat Q, Afroz S, Ahmad A. Physiological and Biochemical Changes in Plants under Waterlogging. Protoplasma. 2010;241(1–4):3–17. doi: 10.1007/s00709-009-0098-8. [DOI] [PubMed] [Google Scholar]
- 62.Jia W, Ma M, Chen J, Wu S. Plant Morphological, physiological and anatomical adaption to flooding stress and the underlying molecular mechanisms. Int J Mol Sci. 2021;22(3):1088. doi: 10.3390/ijms22031088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kim DS, Hwang BK. An Important Role of the Pepper Phenylalanine Ammonia-Lyase Gene (PAL1) in Salicylic Acid-Dependent Signalling of the Defence Response to Microbial Pathogens. J Exp Bot. 2014;65(9):2295–2306. doi: 10.1093/jxb/eru109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cass CL, Peraldi A, Dowd PW, Mottiar Y, Santoro N, Karlen SD, Bukhman YV, Foster CE, Thrower N, Bruno LC, Moskvin OV, Johnson ET, Willhoit ME, Phutane M, Ralph J, Mansfield SD, Nicholson P, Sedbrook JC. Effects of PHENYLALANINE AMMONIA LYASE (PAL) Knockdown on Cell Wall Composition, Biomass Digestibility, and Biotic and Abiotic Stress Responses in Brachypodium. J Exp Bot. 2015;66(14):4317–35. doi: 10.1093/jxb/erv269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dixon RA, Paiva NL. Stress-Induced Phenylpropanoid Metabolism. Plant Cell. 1995;7(7):1085–1097. doi: 10.1105/tpc.7.7.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ferrer JL, Austin MB, Stewart C, Noel JP. Structure and Function of Enzymes Involved in the Biosynthesis of Phenylpropanoids. Plant Physiology and Biochemistry: PPB. 2008;46(3):356–370. doi: 10.1016/j.plaphy.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vogt T. Phenylpropanoid Biosynthesis. Mol Plant. 2010;3(1):2–20. doi: 10.1093/mp/ssp106. [DOI] [PubMed] [Google Scholar]
- 68.Huang J, Gu M, Lai Z, Fan B, Shi K, Zhou YH, Yu JQ, Chen Z. Functional Analysis of the Arabidopsis PAL Gene Family in Plant Growth, Development, and Response to Environmental Stress. Plant Physiol. 2010;153(4):1526–38. doi: 10.1104/pp.110.157370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hou J, Lu D, Mason AS, Li B, Xiao M, An S, Fu D. Non-Coding RNAs and Transposable Elements in Plant Genomes: Emergence, Regulatory Mechanisms and Roles in Plant Development and Stress Responses. Planta. 2019;250(1):23–40. doi: 10.1007/s00425-019-03166-7. [DOI] [PubMed] [Google Scholar]
- 70.Anwar A, Wang K, Wang J, Shi L, Du L, Ye X. Expression of Arabidopsis Ornithine Aminotransferase (AtOAT) Encoded Gene Enhances Multiple Abiotic Stress Tolerances in Wheat. Plant Cell Reports. 2021;40(7):1155–70. doi: 10.1007/s00299-021-02699-0. [DOI] [PubMed] [Google Scholar]
- 71.Olsen KM, Lea US, Slimestad R, Verheul M, Lillo C. Differential Expression of Four Arabidopsis PAL Genes; PAL1 and PAL2 Have Functional Specialization in Abiotic Environmental-Triggered Flavonoid Synthesis. J Plant Physiol. 2008;165(14):1491–99. doi: 10.1016/j.jplph.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 72.Rasool F, Uzair M, Naeem MK, Rehman N, Afroz A, Shah H, Khan MR. Phenylalanine Ammonia-Lyase (PAL) Genes Family in Wheat (Triticum Aestivum L.): Genome-Wide Characterization and Expression Profiling. Agronomy. 2021;11(12):2511. doi: 10.3390/agronomy11122511. [DOI] [Google Scholar]
- 73.Zhu JK. Abiotic Stress Signaling and Responses in Plants. Cell. 2016;167(2):313–24. doi: 10.1016/j.cell.2016.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Khan N, Bano A, Ali S, Babar MA. Crosstalk amongst Phytohormones from Planta and PGPR under Biotic and Abiotic Stresses. Plant Growth Regulation. 2020;90(2):189–203. doi: 10.1007/s10725-020-00571-x. [DOI] [Google Scholar]
- 75.Shu K, Zhou W, Chen F, Luo X, Yang W. Abscisic Acid and Gibberellins Antagonistically Mediate Plant Development and Abiotic Stress Responses. Front Plant Sci. 2018;9:416 https://www.frontiersin.org/article/10.3389/fpls.2018.00416. [DOI] [PMC free article] [PubMed]
- 76.Gomez-Cadenas A, Vives V, Zandalinas SI, Manzi M, Sanchez-Perez AM, Perez-Clemente RM, Arbona V. Abscisic Acid: a Versatile Phytohormone in Plant Signaling and Beyond. Current Protein Pept Sci. 2015;16(5):413–34. doi: 10.2174/1389203716666150330130102. [DOI] [PubMed] [Google Scholar]
- 77.Zandalinas SI, Balfagón D, Arbona V, Gómez-Cadenas A, Inupakutika MA, Mittler R. ABA Is Required for the Accumulation of APX1 and MBF1c during a Combination of Water Deficit and Heat Stress. J Exp Bot. 2016;67(18):5381–90. doi: 10.1093/jxb/erw299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Song Y, Ci D, Tian M, Zhang D. Comparison of the Physiological Effects and Transcriptome Responses of Populus Simonii under Different Abiotic Stresses. Plant Mol Biol. 2014;86(1–2):139–156. doi: 10.1007/s11103-014-0218-5. [DOI] [PubMed] [Google Scholar]
- 79.Miao C, Xiao L, Hua K, Zou C, Zhao Y, Bressan RA, Zhu JK. Mutations in a Subfamily of Abscisic Acid Receptor Genes Promote Rice Growth and Productivity. Proc Natl Acad Sci. 2018;115(23):6058–63. doi: 10.1073/pnas.1804774115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sharp RE, LeNoble ME, Else MA, Thorne ET, Gherardi F. Endogenous ABA Maintains Shoot Growth in Tomato Independently of Effects on Plant Water Balance: Evidence for an Interaction with Ethylene. J Exp Bot. 2000;51(350):1575–84. doi: 10.1093/jexbot/51.350.1575. [DOI] [PubMed] [Google Scholar]
- 81.Hsu PK, Dubeaux G, Takahashi Y, Schroeder JI. Signaling Mechanisms in Abscisic Acid-Mediated Stomatal Closure. Plant J. 2021;105(2):307–321. doi: 10.1111/tpj.15067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shimamura S, Yoshioka T, Yamamoto R, Hiraga S, Nakamura T, Shimada S, Komatsu S. Role of Abscisic Acid in Flood-Induced Secondary Aerenchyma Formation in Soybean (Glycine Max) Hypocotyls. Plant Production Science. 2014;17(2):131–137. doi: 10.1626/pps.17.131. [DOI] [Google Scholar]
- 83.Dawood T, Yang X, Visser EJW, te Beek TAH, Kensche PR, Cristescu SM, Lee S, Floková K, Nguyen D, Mariani C, Rieu I. A Co-Opted Hormonal Cascade Activates Dormant Adventitious Root Primordia upon Flooding in Solanum Dulcamara. Plant Physiol. 2016;170(4):2351–64. doi: 10.1104/pp.15.00773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang Q, Wang L, Chandrasekaran U, Luo X, Zheng C, Shu K. ABA Biosynthesis and Signaling Cascades Under Hypoxia Stress. Front Plant Sci. 2021;12:661228. doi: 10.3389/fpls.2021.661228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Castonguay Y, Cloutier J, Bertrand A, Michaud R, Laberge S. SRAP Polymorphisms Associated with Superior Freezing Tolerance in Alfalfa (Medicago Sativa Spp. sativa) Theor Appl Genet. 2010;120(8):1611–19. doi: 10.1007/s00122-010-1280-2. [DOI] [PubMed] [Google Scholar]
- 86.Jackson MB, Young SF, Hall KC. Are Roots a Source of Abscisic Acid for the Shoots of Flooded Pea Plants? J Exp Bot. 1988;39(12):1631–37. doi: 10.1093/jxb/39.12.1631. [DOI] [Google Scholar]
- 87.Wang X, Liu T, Li C, Chen H. Effects of Soil Flooding on Photosynthesis and Growth of Zea Mays L. Seedlings under Different Light Intensities. Afr J Biotechnol. 2012;11(30):7676–85. doi: 10.4314/ajb.v11i30. [DOI] [Google Scholar]
- 88.Klok EJ, Wilson IW, Wilson D, Chapman SC, Rob M. Ewing RM, Somerville SC, Peacock WJ, Dolferus R, Dennis ES. Expression Profile Analysis of the Low-Oxygen Response in Arabidopsis Root Cultures. Plant Cell. 2002;14(10):2481–94. 10.1105/tpc.004747. [DOI] [PMC free article] [PubMed]
- 89.Bailey-Serres J, Juntawong P. Dynamic Light Regulation of Translation Status in Arabidopsis Thaliana. Frontiers in Plant Science. 2012;3:66. https://www.frontiersin.org/article/10.3389/fpls.2012.00066. [DOI] [PMC free article] [PubMed]
- 90.Chen W, Yao Q, Patil GB, Agarwal G, Deshmukh RK, Lin L, Wang B, Wang Y, Prince SJ, Song L, Xu D, An YC, Valliyodan B, Varshney RK, Nguyen HT. Identification and Comparative Analysis of Differential Gene Expression in Soybean Leaf Tissue under Drought and Flooding Stress Revealed by RNA-Seq. Front Plant Sci. 2016;7:1044. doi: 10.3389/fpls.2016.01044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Stoll M, Loveys B, Dry P. Hormonal changes induced by partial rootzone drying of irrigated grapevine. J Exp Bot. 2000;51(350):1627–1634. doi: 10.1093/jexbot/51.350.1627. [DOI] [PubMed] [Google Scholar]
- 92.Phillips IDJ. Root-shoot Hormone Relations II. Changes in Endogenous Auxin Concentration produced by Flooding of the Root System in Helianthus annuus. Ann Bot. 1964;28(1):37–45. doi: 10.1093/oxfordjournals.aob.a083893. [DOI] [Google Scholar]
- 93.Jackson MB, Hall KC. Early stomatal closure in waterlogged pea plants is mediated by abscisic acid in the absence of foliar water deficits. Plant, Cell Environ. 1987;10(2):121–130. doi: 10.1111/1365-3040.ep11602085. [DOI] [Google Scholar]
- 94.Jackson MB. Long-distance signalling from roots to shoots assessed: The flooding story. J Exp Bot. 2002;53(367):175–181. doi: 10.1093/jexbot/53.367.175. [DOI] [PubMed] [Google Scholar]
- 95.Cao D, Cheng H, Wu W, Soo HM, Peng J. Gibberellin Mobilizes Distinct DELLA-Dependent Transcriptomes to Regulate Seed Germination and Floral Development in Arabidopsis. Plant Physiol. 2006;142(2):509–525. doi: 10.1104/pp.106.082289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Navarro L, Bari R, Achard P, Lisón P, Nemri A, Harberd NP, Jones JDG. DELLAs Control Plant Immune Responses by Modulating the Balance of Jasmonic Acid and Salicylic Acid Signaling. Curr Biol. 2008;18(9):650–655. doi: 10.1016/j.cub.2008.03.060. [DOI] [PubMed] [Google Scholar]
- 97.Burrows WJ, Carr DJ. Effects of Flooding the Root System of Sunflower Plants on the Cytokin in Content in the Xylem Sap. Physiol Plant. 1969;22(6):1105–1112. doi: 10.1111/j.1399-3054.1969.tb09098.x. [DOI] [PubMed] [Google Scholar]
- 98.Neuman DS, Rood SB. Smit BA Does Cytokinin Transport from Root-To-Shoot in the Xylem Sap Regulate Leaf Responses to Root Hypoxia? J Exp Bot. 1990;41(10):1325–1333. doi: 10.1093/jxb/41.10.1325. [DOI] [Google Scholar]
- 99.Kende H, Knaap E, Cho HT. Deepwater Rice: A Model Plant to Study Stem Elongation. Plant Physiol. 1999;118:1105–1110. doi: 10.1104/pp.118.4.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bailey-Serres J, Voesenek LA. Life in the balance: A signaling network controlling survival of flooding. Curr Opin Plant Biol. 2010;13(5):489–494. doi: 10.1016/j.pbi.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 101.Sasidharan R, Hartman S, Liu Z, Martopawiro S, Sajeev N, van Veen H, Yeung E, Voesenek LACJ. Signal Dynamics and Interactions during Flooding Stress. Plant Physiol. 2018;176(2):1106–1117. doi: 10.1104/pp.17.01232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bradford KJ, Yang SF. Xylem Transport of 1-Aminocyclopropane-1-carboxylic Acid, an Ethylene Precursor Waterlogged Tomato Plants. Plant Physiol. 1980;65(2):322–326. doi: 10.1104/pp.65.2.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yamauchi T, Watanabe K, Fukazawa A, Mori H, Abe F, Kawaguchi K, Oyanagi A, Nakazono M. Ethylene and reactive oxygen species are involved in root aerenchyma formation and adaptation of wheat seedlings to oxygen-deficient conditions. J Exp Bot. 2014;65(1):261–273. doi: 10.1093/jxb/ert371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sponsel VM, Hedden P. Gibberellin Biosynthesis and Inactivation. In: Davies PJ (ed.). Plant Hormones: Biosynthesis, Signal Transduction, Action! Springer Netherlands. 2010:63–94). 10.1007/978-1-4020-2686-7_4.
- 105.Achard P, Cheng H, de Grauwe L, Decat J, Schoutteten H, Moritz T, van der Straeten D, Peng J, Harberd NP. Integration of Plant Responses to Environmentally Activated Phytohormonal Signals. Science. 2006;311(5757):91–94. doi: 10.1126/science.1118642. [DOI] [PubMed] [Google Scholar]
- 106.Kuroha T, Nagai K, Gamuyao R, Wang DR, Furuta T, Nakamori M, Kitaoka T, Adachi K, Minami A, Mori Y, Mashiguchi K, Seto Y, Yamaguchi S, Kojima M, Sakakibara H, Wu J, Ebana K, Mitsuda N, Ohme-Takagi M, Yanagisawa S, Yamasaki M, Yokoyama R, Nishitani K, Mochizuki Tamiya G, McCouch SR, Ashikari M. Ethylene-Gibberellin Signaling Underlies Adaptation of Rice to Periodic Flooding. Science. 2018;361(6398):181–86. doi: 10.1126/science.aat1577. [DOI] [PubMed] [Google Scholar]
- 107.ulHaq S, Khan A, Ali M, Khattak AM, Gai WX, Zhang HX, Wei AM, Gong ZH. Heat Shock Proteins: Dynamic Biomolecules to Counter Plant Biotic and Abiotic Stresses. Int J Mol Sci. 2019;20(21):5321. doi: 10.3390/ijms20215321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Swindell WR, Huebner M, Weber AP. Transcriptional Profiling of Arabidopsis Heat Shock Proteins and Transcription Factors Reveals Extensive Overlap between Heat and Non-Heat Stress Response Pathways. BMC Genomics. 2007;8:125. doi: 10.1186/1471-2164-8-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ahuja I, de Vos RCH, Bones AM, Hall RD. Plant molecular stress responses face climate change. Trends Plant Sci. 2010;15(12):664–674. doi: 10.1016/j.tplants.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 110.Xu ZS, Chen M, Li LC, Ma YZ. Functions and Application of the AP2/ERF Transcription Factor Family in Crop Improvement. J Integr Plant Biol. 2011;53(7):570–585. doi: 10.1111/j.1744-7909.2011.01062.x. [DOI] [PubMed] [Google Scholar]
- 111.Zhou J, Wang J, Yu JQ, Chen Z. Role and regulation of autophagy in heat stress responses of tomato plants. Front Plant Sci. 2014;5:174.https://www.frontiersin.org/article/10.3389/fpls.2014.00174. [DOI] [PMC free article] [PubMed]
- 112.Jacob P, Hirt H, Bendahmane A. The Heat-Shock Protein/Chaperone Network and Multiple Stress Resistance. Plant Biotechnol J. 2017;15(4):405–414. doi: 10.1111/pbi.12659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ahammed GJ, Li X, Zhou J, Zhou YH, Yu JQ. Role of Hormones in Plant Adaptation to Heat Stress. In: Ahammed GJ, Yu JQ (eds). Plant Hormones under Challenging Environmental Factors, Dordrecht: Springer Netherlands. 2016;1–21. 10.1007/978-94-017-7758-2_1.
- 114.Mishra D, Shekhar S, Singh D, Chakraborty S, Chakraborty N. Heat Shock Proteins and Abiotic Stress Tolerance in Plants. In: Asea AAA, Kaur P (eds). Regulation of Heat Shock Protein Responses. Heat Shock Proteins. Cham: Springer Int Publishing. 2018:41–69. 10.1007/978-3-319-74715-6_3.
- 115.Komatsu S, Makino T, Yasue H. Proteomic and Biochemical Analyses of the Cotyledon and Root of Flooding-Stressed Soybean Plants. PLOS One. 2013;8(6):e65301. doi: 10.1371/journal.pone.0065301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Jamieson MA, Burkle LA, Manson JS, Runyon JB, Trowbridge AM, Zientek J. Global Change Effects on Plant-Insect Interactions: The Role of Phytochemistry. Curr Opin Insect Sci. 2017;23:70–80. doi: 10.1016/j.cois.2017.07.009. [DOI] [PubMed] [Google Scholar]
- 117.Watson B. Taylor’s Guide to Heirloom Vegetables. Houghton Mifflin Harcourt Co. (1996).
- 118.Goldman A. The heirloom tomato: From garden to table: Recipes, portraits, and history of the world’s most beautiful fruit. New York: Bloomsbury; 2008. [Google Scholar]
- 119.Dwivedi S, Goldman I, Ortiz R. Pursuing the Potential of Heirloom Cultivars to Improve Adaptation, Nutritional, and Culinary Features of Food Crops. Agronomy. 2019;9(8):441. doi: 10.3390/agronomy9080441. [DOI] [Google Scholar]
- 120.Veluchamy S, Hind SR, Dunham DM, Martin GB, Panthee DR. Natural Variation for Responsiveness to flg22, flgII-28, and csp22 and Pseudomonas syringae pv. tomato in Heirloom Tomatoes. PLOS One. 2014;9(9):e106119. doi: 10.1371/journal.pone.0106119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Seidl Johnson AC, Jordan SA, Gevens AJ. Novel Resistance in Heirloom Tomatoes and Effectiveness of Resistance in Hybrids to Phytophthora infestans US-22, US-23, and US-24 Clonal Lineages. Plant Dis. 2014;98(6):761–765. doi: 10.1094/PDIS-06-13-0674-RE. [DOI] [PubMed] [Google Scholar]
- 122.Veluchamy S, Panthee DR. Differential expression analysis of a select list of genes in susceptible and resistant heirloom tomatoes with respect to Pseudomonas syringae pv. tomato European. J Plant Pathol. 2016;142(4):653–663. doi: 10.1007/s10658-015-0621-z. [DOI] [Google Scholar]
- 123.Tranchida-Lombardo V, Aiese Cigliano R, Anzar I, Landi S, Palombieri S, Colantuono C, Bostan H, Termolino P, Aversano R, Batelli G, Cammareri M, Carputo D, Chiusano ML, Conicella C, Consiglio F, D’Agostino N, de Palma M, di Matteo A, Grandillo S, Sanseverino W, Tucci M, Grillo S. Whole-genome re-sequencing of two Italian tomato landraces reveals sequence variations in genes associated with stress tolerance, fruit quality and long shelf-life traits. DNA Res. 2018;25(2):149–160. doi: 10.1093/dnares/dsx045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Massaretto IL, Albaladejo I, Purgatto E, Flores FB, Plasencia F, Egea-Fernández JM, Bolarin MC, Egea IRecovering Tomato Landraces to Simultaneously Improve Fruit Yield and Nutritional Quality Against Salt Stress. Frontiers in Plant Science. 2018;9:1778. https://www.frontiersin.org/articles/10.3389/fpls.2018.01778. [DOI] [PMC free article] [PubMed]
- 125.Dobin A, Davis CA, Schlesinger F, Drenkow F, Zaleski C, Jha S, Batut P, Chaisson M, Gingeras TR. STAR: Ultrafast Universal RNA-Seq Aligner. Bioinformatics. 2013;29(1):15–21. doi: 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Robinson MD, McCarthy DJ, Smyth GK. EdgeR. A Bioconductor Package for Differential Expression Analysis of Digital Gene Expression Data. Bioinformatics. 2010;26(1:139–40. 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed]
- 127.Hartigan JA, Wong MA. Algorithm AS 136: A K-Means Clustering Algorithm. Journal of the Royal Statistical Society. Series C (Applied Statistics). 1979;28(1):100–108. 10.2307/2346830.
- 128.Gu Z, Eils R, Schlesner M. Complex Heatmaps Reveal Patterns and Correlations in Multidimensional Genomic Data. Bioinformatics. 2016;32(18):2847–49. doi: 10.1093/bioinformatics/btw313. [DOI] [PubMed] [Google Scholar]
- 129.Alexa A, Rahnenfuhrer J. TopGO: Enrichment Analysis for Gene Ontology (version 2.46.0). Bioconductor version: Release. 2022;3(14):10.18129/B9.bioc.topGO.
- 130.Anastasaki E, Balayannis G, Papanikolaou NE, Michaelakis AN, Milonas PG. Oviposition Induced Volatiles in Tomato Plants. Phytochemistry Letters. 2015;13:262–66. doi: 10.1016/j.phytol.2015.07.007. [DOI] [Google Scholar]
- 131.Wickham H. Programming with Ggplot2. In: Wickham H (ed). Ggplot2: Elegant Graphics for Data AnalysisUse R! Cham: Springer International Publishing. 2016:241–53. 10.1007/978-3-319-24277-4_12.
- 132.Ranganathan Y, Borges RM. Reducing the Babel in Plant Volatile Communication: Using the Forest to See the Trees. Plant Biol. 2010;12(5):735–742. doi: 10.1111/j.1438-8677.2009.00278.x. [DOI] [PubMed] [Google Scholar]
- 133.Mccormick AC, Irmisch S, Reinecke A, Boeckler GA, Veit D, Reichelt M, Hansson BS, Gershenzon J, Köllner TG, Unsicker SB. Herbivore-Induced Volatile Emission in Black Poplar: Regulation and Role in Attracting Herbivore Enemies. Plant, Cell Environ. 2014;37(8):1909–1923. doi: 10.1111/pce.12287. [DOI] [PubMed] [Google Scholar]
- 134.Breiman L. Random Forests. Machine Learning. 2001;45(1):5–32. doi: 10.1023/A:1010933404324. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All raw RNA sequence data are available a NCBI under project accession: PRJNA850397.