Abstract
Background
We investigated whether COVID-19 leads to persistent impaired pulmonary function, fibrotic-like abnormalities or psychological symptoms 12 months after discharge and whether severely ill patients (ICU admission) recover differently than moderately ill patients.
Methods
This single-centre cohort study followed adult COVID-19 survivors for a period of one year after discharge. Patients underwent pulmonary function tests 6 weeks, 3 months and 12 months after discharge and were psychologically evaluated at 6 weeks and 12 months. Computed tomography (CT) was performed after 3 months and 12 months.
Results
66 patients were analysed, their median age was 60.5 (IQR: 54–69) years, 46 (70%) patients were male. 38 (58%) patients had moderate disease and 28 (42%) patients had severe disease. Most patients had spirometric values within normal range after 12 months of follow-up. 12 (23%) patients still had an impaired lung diffusion after 12 months. Impaired pulmonary diffusion capacity was associated with residual CT abnormalities (OR 5.1,CI-95: 1.2–22.2), shortness of breath (OR 7.0, CI-95: 1.6–29.7) and with functional limitations (OR 5.8, CI-95: 1.4–23.8). Ground-glass opacities resolved in most patients during follow-up. Resorption of reticulation, bronchiectasis and curvilinear bands was rare and independent of disease severity. 81% of severely ill patients and 37% of moderately ill patients showed residual abnormalities after 12 months (OR 8.1, CI-95: 2.5–26.4). A minority of patients had symptoms of post-traumatic stress disorder, anxiety, depression and cognitive failure during follow-up.
Conclusion
Some patients still had impaired lung diffusion 12 months after discharge and fibrotic-like residual abnormalities were notably prevalent, especially in severely ill patients.
Keywords: Respiratory infection, Pulmonary fibrosis, Pulmonary function, Chest imaging, COVID-19
Take home message
Fibrotic-like abnormalities were highly prevalent (53%) one year after hospitalization with COVID-19 and were associated with impaired lung diffusion. Future studies are needed to clarify the temporal evolution of these fibrotic-like changes.
Introduction
The COVID-19 pandemic resulted in a very large number of hospitalizations with acute respiratory distress syndrome (ARDS) as major cause of morbidity and mortality [1, 2]. In the acute phase of infection, main imaging patterns concerned airspace opacification, but reticulation associated with bronchiolectasis and irregular interlobular or septal thickening have been reported, possibly indicative of early development of pulmonary fibrosis [3]. Radiological evidence of fibrotic-like abnormalities has been supported by histological evidence of fibrosis in multiple autopsy studies early on in the pandemic in 2020 [4]. Similarly, patients recovering from other coronaviruses also had radiographic evidence of pulmonary fibrosis during the acute phase of infection [5, 6]. However, long-term follow-up of severe acute respiratory syndrome (SARS) patients revealed that fibrotic abnormalities were only visible in 5% of patients after 15 years [7].
Both the alveolar inflammatory response to the viral infection and immune-mediated mechanisms are causes of lung injury in the acute phase of the disease, leading to activation of profibrotic pathways comparable with the wound healing process. In addition, SARS-CoV-2 is believed to have a direct role in promoting lung fibrosis, via its nucleocapsid protein and through the downregulation of Angiotensin-converting-enzyme-2 (ACE2), which promote transforming growth factor-beta (TGF-beta) signalling. In Intensive Care Unit (ICU) patients, mechanical ventilation induces mechanical stress that may play another important role in driving fibrotic pathways [8].
Previously reported follow-up studies of COVID-19 patients demonstrated residual abnormalities on computed tomography (CT) accompanied by both reduced lung volumes and impaired lung diffusion up to 12 months after discharge, especially in critically ill patients [9], [10], [11], [12]. Another study only found 5% of fibrotic changes in 5% of patients with moderate COVID-19 after 12 months, indicating the importance of disease severity as predictor of long-term consequences [13]. However, our current knowledge of long-term sequelae remains scarce, which makes it difficult to decide which patients are in need of standard follow-up to recognize fibrosis in time in case of new pandemic waves. Therefore, we aimed to evaluate to what extent COVID-19 leads to persistent impaired pulmonary function, fibrotic-like abnormalities or psychological symptoms one year after discharge and whether patients with severe disease recover differently than patients with moderate disease. Our secondary aim was to evaluate if radiologic abnormalities and functional outcomes were associated with persistent impaired lung diffusion.
Methods
Study design and population
This single-centre prospective cohort study followed 66 adult survivors of acute moderate or severe COVID-19 for a period of one year. Patients admitted to the Leiden University Medical Centre (LUMC) between March 23rd and June 23rd 2020 were included after confirmed diagnosis of COVID-19 based on a positive polymerase chain-reaction (PCR) test-result in combination with the presence of typical radiological findings according to the COVID-19 Reporting and Data System (CO-RADS). Patients who declined informed consent, patients who died, patients living outside of the Leiden area, patients who refused follow-up or who were lost to follow-up were excluded. Ethical approval was given by the local review board for COVID-19 related research prior to this study (protocolnumber 2020–059). All patients received a letter upon admission to state that clinical data could be used for research purposes and that they could opt out if they would not give informed consent. None of the admitted patients declined consent. Demographic and clinical characteristics were collected from electronic medical records.
Follow-up
Patients were invited for pulmonary evaluation 6 weeks, 3 months and 12 months after discharge. Forced vital capacity (FVC), forced expiratory volume in one second (FEV1) and diffusion capacity of the lungs for carbon monoxide adjusted for haemoglobin (DLCOc) were measured according to standard protocols using GLI (Global Lung Function Initiative) reference values. Radiological evaluation of COVID-19 pneumonia was performed at 3 and 12 months with non-enhanced chest CT, unless patients were previously diagnosed with pulmonary embolism as complication of COVID-19 infection, in which case pulmonary CT angiography with subtraction iodine maps was performed. HRCT scans were acquired at maximal inspiration in supine position on an Aquilion One or Aquilion One Genesis system (Canon Medical Systems, Ōtawara, Japan) using a standardized protocol at our radiology department, comprising a tube voltage = 120 kVp; tube current determined through automated exposure control; rotation time = 0.275–0.5 s; collimation = 80 × 0.5 mm; helical beam pitch = 0.8. In all patients, scans were obtained contiguously with a slice thickness of 1 mm and reconstructed with lung kernel (FC30). CT examinations were reviewed by one radiologist (L.S.) specialized in thoracic imaging and scored for the presence or absence of five distinctive findings: parenchymal consolidation, ground-glass opacities (GGO), reticulation, bronchiectasis and curvilinear bands. The percentage of affected parenchyma was estimated per individual lobe. Perceived breathlessness was assessed with the Medical Research Council (MRC) dyspnoea scale and dichotomized with a score of <2 or ≥2 . Functional limitations were assessed with the Post-COVID Functional Status (PCFS) scale and dichotomized with a score of <3 versus ≥3 [14]. Anxiety, depression, posttraumatic stress symptoms and cognitive functioning were measured 6 weeks and 12 months after discharge with respectively the GAD-7 (Generalized Anxiety Disorder Scale, cut-off score for moderate-severe anxiety of ≥10), PHQ-9 (Patient Health Questionnaire 9, cut-off score for moderate-severe depression ≥ 10), PCL_5 (PTSD Checklist for the DSM 5, cut-off score for PTSD ≥ 33) and the CFQ-25 (Cognitive Failure Questionnaire, cut-off score for cognitive impairments ≥ 44) [15], [16], [17], [18].
Statistical analysis
Demographic and clinical characteristics are presented with median and interquartile range (IQR) for continuous variables and with absolute values and percentages for categorical variables. Disease severity was categorized as severe in patients who received treatment on the ICU and as moderate in all other hospitalized patients. Baseline characteristics were compared based on disease severity with a Fischer's exact test for categorical variables and a Mann-Whitney U test for continuous variables.
A mixed model for repeated measurements was used for analyses of pulmonary function over time. Maximum likelihood was used for estimates with an unstructured covariance structure after selection based on the Akaike information criterion (AIC). Other covariance structures that were modelled were first-order autoregressive, diagonal and compound symmetry. The model included disease severity as fixed factor with time in months as a covariate to correct for the difference in time intervals between measurements. We evaluated whether time interacted with disease severity. Post-hoc pairwise comparisons were conducted for both groups with Bonferroni correction to analyse differences in FVC, FEV1 and DLCOc between 1.5 month and 3 months and between 6 and 12 months. The proportions of patients with abnormal spirometric values (<80% of predicted) and normalization of radiographic abnormalities were compared with a McNemar test in both groups. The percentage of affected parenchyma over time was analysed with a Wilcoxon Signed Rank test and differences in self-reported psychological symptoms during follow-up with an paired T-test. Variation in pulmonary function, chest CT abnormalities and psychological outcomes between patients with moderate and severe disease at 12 months were analysed with an unpaired T-test, Fischer's exact test or Mann-Whitney U test. Associations between impaired pulmonary function (DLCOc <80% of predicted), residual abnormalities, PCFS and MRC were analysed with a Chi-square test and presented as OR with 95-CI. The reported p-values are two-sided. Missing data were not imputed, complete-case analyses were performed. IBM SPSS Statistics, version 25 was used for al statistical analyses.
Role of the funding
This publication is part of the project COOP Study with project number 10430102110005 of the research program COVID-19 which is (partly) financed by the Netherlands Organisation for Health Research and Development (ZonMw).
Results
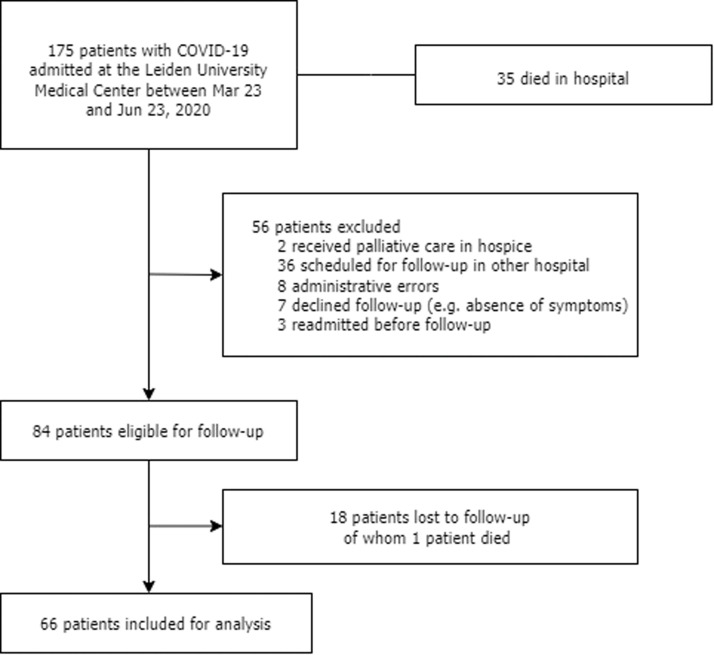
In total, 175 adult patients with PCR-confirmed COVID-19 were admitted to the LUMC between March 23rd and June 23rd 2020, of whom 109 (62%) patients were excluded (Fig. 1 ). Of these excluded patients, 35 (20%) patients died in hospital, two (1%) patients were discharged to a hospice for palliative care, 36 (21%) patients were scheduled for follow-up in another hospital (lived outside the Leiden area), eight (5%) patients were not invited for follow-up due to administrative errors, seven (4%) patients declined follow-up, and 3 (2%) patients were readmitted before follow-up visits. Of the 84 (48%) patients with planned follow-up visits, 18 (10%) were lost to follow-up. One of the patients lost to follow-up died within a year after discharge. The remaining 66 (38%) patients were included in this analysis.
Fig. 1.
Flowchart of this study.
The median age of patients was 60.5 (IQR: 54–69) years and 46 (70%) patients were male (Table 1 ). The median body mass index (BMI) was 27.3 kg/m2 (IQR: 24.2–30.6). 28 (44%) patients had severe disease and received treatment in the ICU with a median length of ICU stay of 15.5 days (IQR: 10–25). 27 (41%) patients required invasive mechanical ventilation. 38 (58%) patients had moderate disease and were treated with oxygen via nasal canula or masque on the general ward. Diabetes mellitus was more prevalent in severely ill patients (OR 4.7, CI-95: 1.3–17.2). Other comorbidities, as well as sex, age, BMI, smoking status and duration of symptoms before hospitalization were comparable between subgroups. There were no patients with a history of pulmonary fibrosis. Severe infection was reflected biochemically by higher levels of CRP, ferritin and d-dimer and radiographically by a higher percentage of affected parenchyma with corresponding higher CT-Severity Score. Severely ill patients had a longer median length of hospital stay compared to moderately ill patients (28.5 vs. 6.0 days; p<0.001) and were more frequently treated with (hydroxy‑)chloroquine (OR 14.7, CI-95: 4.1–52.4). Moderately ill patients were more often treated with remdesivir (OR 11.7, CI-95: 2.4–56.4).
Table 1.
Characteristics of COVID-19 patients according to disease severity.
| Total (N = 66) | Moderate (N = 38) | Severe (N = 28) | p-value | |
|---|---|---|---|---|
| Age, years | 60.5 (54.0–69.3) | 62.5 (54.0–70.3) | 60.0 (54.0–67.5) | 0.360 |
| Sex, male | 46 (70%) | 27 (71%) | 19 (68%) | 0.793 |
| Body mass index, kg/m2 | 27.3 (24.2–30.6) | 27.1 (23.4–28.7) | 27.7 (24.4–31.7) | 0.380 |
| Comorbidities | ||||
| Hypertension | 18 (27%) | 9 (24%) | 9 (32%) | 0.577 |
| Diabetes mellitus | 14 (21%) | 4 (11%) | 10 (36%) | 0.017 |
| Asthma | 9 (14%) | 3 (8%) | 6 (21%) | 0.153 |
| coronary artery disease | 7 (11%) | 3 (8%) | 4 (14%) | 0.446 |
| hypercholesterolaemia | 6 (9%) | 4 (11%) | 2 (7%) | 1.0 |
| Immunodeficiency | 4 (6%) | 3 (8%) | 1 (4%) | 0.631 |
| Heart valve anomaly | 4 (6%) | 2 (5%) | 2 (7%) | 1.0 |
| Chronic kidney disease | 4 (6%) | 2 (5%) | 2 (7%) | 1.0 |
| Atrial fibrillation or flutter | 3 (5%) | 2 (5%) | 1 (4%) | 1.0 |
| Cerebrovascular diseases | 2 (3%) | 0 | 2 (7%) | 0.176 |
| Chronic obstructive pulmonary disease | 2 (3%) | 2 (5%) | 0 | 0.504 |
| Pulmonary embolism (before COVID-19) | 1 (2%) | 0 | 1 (4%) | 0.424 |
| Smoking status | ||||
| Never | 32/57 (56%) | 18/33 (55%) | 14/24 (58%) | 0.794 |
| Former | 25/57 (44%) | 15/33 (45%) | 10/24 (42%) | .. |
| Active | 0 | 0 | 0 | .. |
| Clinical characteristics | ||||
| Duration of symptoms before admission, days | 10.0 (6.0–12.0) | 10.0 (6.5–14.0) | 9.0 (6.0–10.0) | 0.114 |
| C-reactive protein (max), mg/L | 212.1 (88.2–322.3) | 108.4 (66.0–188.7) | 331.4 (260.4–393.1) | <0.001 |
| D-dimer (max), ng/mL | 2490 (1081–5799) | 1135 (936–2214) | 4416 (2263–8314) | 0.001 |
| Ferritin (max), µg/L | 1245 (715–2088) | 948 (639–1370) | 1971 (817–2690) | <0.001 |
| CT Severity Score, total (0–25) | 10.5 (9.0–14.8) | 10.0 (8.0–11.3) | 15.0 (11.0–19.0) | <0.001 |
| Total affected parenchyma,% | 25.0 (19.5–44.3) | 20.0 (15.0–30.0) | 52.5 (28.8–63.8) | <0.001 |
| Pulmonary embolism (complication) | 17 (26%) | 6 (16%) | 11 (39%) | 0.046 |
| Treatment with corticosteroids | 2 (3%) | 0 | 2 (7%) | 0.176 |
| Treatment with remdesivir | 20 (30%) | 18 (47%) | 2 (7%) | <0.001 |
| Treatment with (hydroxy‑) chloroquine | 35 (53%) | 11 (29%) | 24 (86%) | <0.001 |
| Length of ICU stay, days | NA | NA | 15.5 (10.0–25.0) | NA |
| Invasive mechanical ventilation | NA | NA | 27 (96%) | NA |
| Duration of intubation, days | NA | NA | 13.0 (9.0–20.0) | NA |
| Length of hospital stay, days | 10.5 (5.0–24.3) | 6.0 (4.0–9.0) | 28.5 (18.5–37.3) | <0.001 |
| Discharge to rehabilitation clinic | 23 (35%) | 7 (18%) | 16 (57%) | 0.002 |
| Time from onset of symptoms to follow-up chest CT at 3 months, days | 122.0 (110.3–145.0) | 119.0 (108.5–126.5) | 144.0 (120.0–147.0) | 0.005 |
| Time from onset of symptoms to follow-up chest CT at 12 months, days | 413.0 (388.0–440.0) | 402.5 (386.0–418.0) | 422.0 (394.0–444.0) | 0.021 |
Data are median (IQR), n (%) or n/N (%) to indicate missing data. ICU=Intensive Care Unit. CT=Computed Tomography NA=Not applicable.
Dyspnoea and pulmonary function
One year after follow-up 8 (24%) patients with moderate disease and 10 (39%) patients with severe disease still perceived dyspnoea during normal activities (MRC≥2). Dyspnoea at 12 months was associated with persistent impaired pulmonary diffusion (OR 7.0, CI-95: 1.6–29.7) and not associated with residual chest CT abnormalities (OR 1.7, CI-95: 0.6–5.4). Of the 29 (45%) patients with moderate to severe limitations in everyday life (PCFS≥3) after 6 weeks, 20 (33%) patients still suffered from limitations after 12 months. Impaired pulmonary diffusion at 12 months was associated with these limitations (OR 5.8, CI-95: 1.4–23.8).
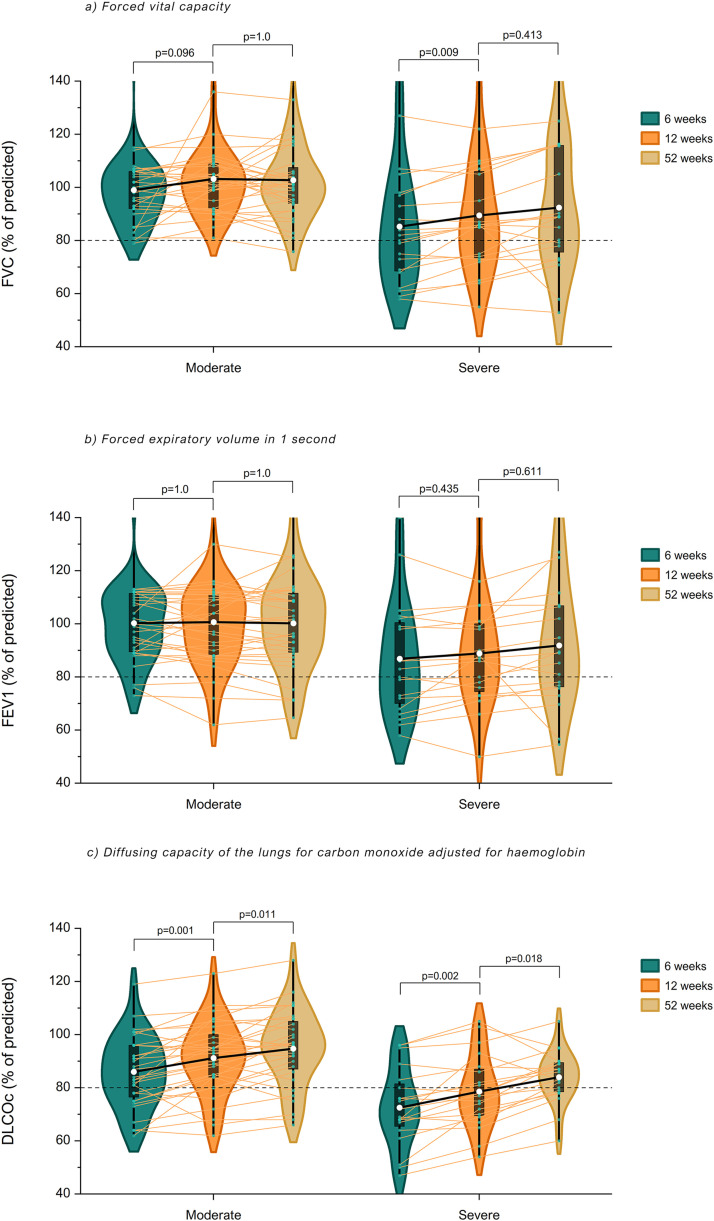
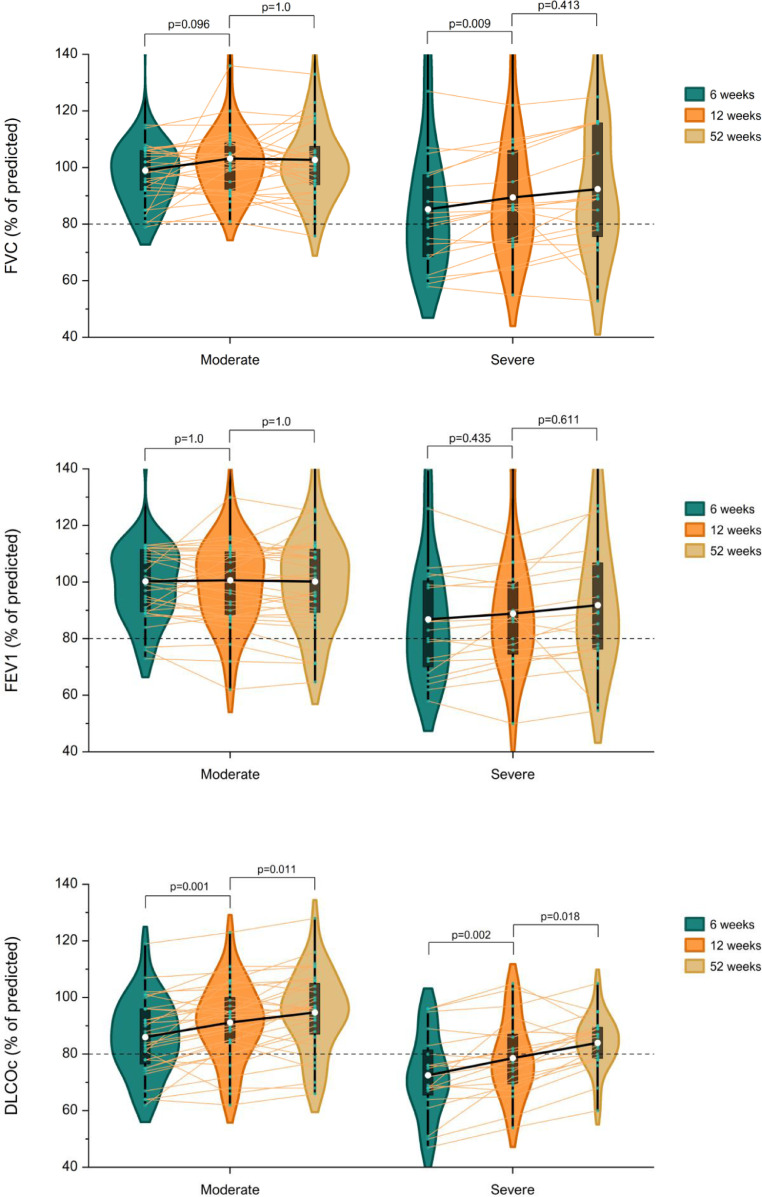
Spirometric values were within normal limits in most patients with moderate disease during follow-up (Table 2 ). The proportion of patients with reduced FVC, FEV1 or DLCOc did not change over time and 6 (19%) patients still had an impaired lung diffusion after 12 months. Severely ill patients had lower spirometric values during follow-up compared to patients with moderate disease (all p<0.001, Fig. 2 ). Mean DLCOc improved in severely ill patients with 6.1% (CI-95: 2.0–10.1) during the first 3 months of follow-up and continued to improve with 5.4% (CI-95: 0.8–10.0) afterwards. The proportion of severely ill patients with impaired lung diffusion decreased from 60% to 30% (p = 0.031) between 3 and 12 months of follow-up. Pulmonary function changed similarly in both groups over time as there was no interaction between time (in months) and disease severity.
Table 2.
Spirometry in COVID-19 patients with moderate or severe disease at 6 weeks, 12 weeks and 52 weeks follow-up.
| Moderate (N = 32/38) | Severe (N = 20/28) | Moderate vs. severea | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Spirometry | 6 weeks | 12 weeks | 52 weeks | p-valueb | p-valuec | 6 weeks | 12 weeks | 52 weeks | p-valueb | p-valuec | p-value |
| FVC, L | 4.26±0.96 | 4.41±0.90 | 4.39±1.04 | NA | NA | 2.91±0.91 | 2.97±0.84 | 3.05±0.94 | NA | NA | NA |
| FVC,% of predictedd | 99±14 | 103±15 | 103±15 | 0.096 | 1.0 | 85±22 | 89±22 | 92±24 | 0.009 | 0.413 | 0.100 |
| FVC, <80% of predicted | 1 (3%) | 0 | 1 (3%) | 1.0 | 1.0 | 9 (45%) | 7 (35%) | 7 (35%) | 0.500 | 1.0 | 0.003 |
| FEV1, L | 3.33±0.79 | 3.34±0.81 | 3.32±0.88 | NA | NA | 3.66±1.22 | 3.82±1.15 | 3.94±1.25 | NA | NA | NA |
| FEV1,% of predictedd | 100±15 | 100±18 | 100±17 | 1.0 | 1.0 | 87±21 | 89±20 | 92±23 | 0.435 | 0.611 | 0.146 |
| FEV1, <80% of predicted | 3 (9%) | 3 (9%) | 4 (13%) | 1.0 | 1.0 | 7 (35%) | 7 (35%) | 7 (35%) | 1.0 | 1.0 | 0.081 |
| Tiffeneau-indexd | 79±8 | 75±8 | 75±8 | NA | NA | 80±4 | 78±5 | 78±5 | NA | NA | NA |
| DLCOc,% of predictede | 86±13 | 91±14 | 95±14 | 0.001 | 0.011 | 73±15 | 79±14 | 84±10 | 0.002 | 0.018 | 0.005 |
| DLCOc, <80% of predicted | 10 (31%) | 5 (16%) | 6 (19%) | 0.125 | 1.0 | 14 (70%) | 12 (60%) | 6 (30%) | 0.625 | 0.031 | 0.5 |
Data are mean ± SD or n (%). FVC=Forced vital capacity. L=Liters. FEV1=Forced expiratory volume in one second. DLCOc=Diffusion capacity of the lungs for carbon monoxide adjusted for haemoglobin. NA= Not Applicable.
a. Comparison of moderate and severe patients at 52 weeks.
b. Comparison of 6 and 12 weeks.
c. Comparison of 12 and 52 weeks.
d. Quanjer GLI (2012) as reference values.
e. Stanojevic TLCO (2017) as reference values.
Fig. 2.
Change in pulmonary function during follow-up in COVID-19 patients with moderate or severe disease. Mean FVC, FEV1 and DLCOc (white dots) of COVID-19 patients with moderate and severe disease at 6 weeks, 12 weeks and 52 weeks are illustrated with violin plots and box plots. Individual values are shown with connected lines. A mixed model for repeated measures demonstrates that FVC only improved in severely ill patients between 6 and 12 weeks (p = 0.009). There was no change in FEV1 in both groups during follow-up. DLCOc improved between 6 and 12 weeks and continued to improve afterwards in both groups. Severely ill patients had lower spirometric values during follow-up compared to moderately ill patients (all p<0.001). There was no interaction between disease severity and time (FVC: pinteraction=0.160, FEV1: pinteraction =0.061, DLCOc: pinteraction =0.273).
Radiological evaluation
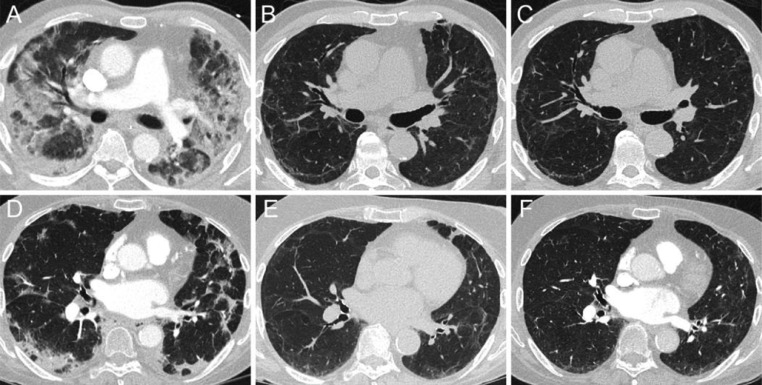
In total, 54 (84%) patients had residual abnormalities on chest CT 3 months after discharge and 34 (53%) patients still had residual abnormalities at 12 months (Table 3 ). Complete resorption of abnormalities between 3 and 12 months was seen in 16 (42%) moderately ill patients (p<0.001) and in 4 (15%) severely ill patients (p = 0.125). GGO resolved completely in 19 (50%) patients with moderate disease (p<0.001) and in 10 (38%) patients with severe disease (p = 0.006). Temporal changes in morphology of radiologic abnormalities are illustrated in Fig. 3 . The proportion of patients with reticulation, bronchiectasis or curvilinear bands was comparable between 3 and 12 months, independent of disease severity, highlighting persistence of these particular findings. At 12 months severely ill patients had a higher prevalence of residual abnormalities, reticulation and bronchiectasis compared to patients with moderate disease. The median percentage of affected parenchyma decreased in all patients between 3 and 12 months. Severely ill patients had a higher percentage of affected parenchyma at 12 months, ranging from 5 to 15%, compared to 0% in moderately ill patients. Enhanced CT was performed at least once in 15 (88%) patients with pulmonary embolism during admission and all these patients had complete resorption of pulmonary embolism. The presence of residual abnormalities at 12 months was associated with persistent impaired pulmonary diffusion capacity (OR 5.1,CI-95: 1.2–22.2) and with limitations in everyday life (OR 3.2, CI-95: 1.0–10.2).
Table 3.
Chest CT abnormalities in COVID-19 patients with moderate or severe disease at 12 weeks and 52 weeks follow-up.
| Moderate (N = 38) | Severe (N = 26/28) | Moderate vs. severea | |||||
|---|---|---|---|---|---|---|---|
| Chest CT | 12 weeks | 52 weeks | p-value | 12 weeks | 52 weeks | p-value | p-value |
| Enhanced CT | 3 (8%) | 3 (8%) | NA | 6 (23%) | 4 (15%) | NA | NA |
| Residual abnormalities | 29 (76%) | 13 (34%) | <0.001 | 25 (96%) | 21 (81%) | 0.125 | <0.001 |
| Parenchymal consolidation | 1 (3%) | 1 (3%) | 1.0 | 3 (12%) | 2 (8%) | 1.0 | 0.561 |
| Ground-glass opacities | 27 (71%) | 8 (21%) | <0.001 | 21 (81%) | 11 (42%) | 0.006 | 0.096 |
| Reticulation | 6 (16%) | 4 (11%) | 0.5 | 9 (35%) | 10 (39%) | 1.0 | 0.013 |
| Bronchiectasis | 8 (21%) | 7 (18%) | 1.0 | 17 (65%) | 16 (62%) | 1.0 | 0.001 |
| Curvilinear bands | 12 (32%) | 7 (18%) | 0.063 | 20 (77%) | 19 (73%) | 1.0 | <0.001 |
| Affected parenchyma RUL,% | 5 (0–15) | 0 (0–1) | <0.001 | 20 (5–40) | 7.5 (5–30) | <0.001 | <0.001 |
| Affected parenchyma ML,% | 5 (0–10) | 0 (0–5) | 0.016 | 15 (5–50) | 10 (0–20) | 0.001 | <0.001 |
| Affected parenchyma RLL,% | 10 (4–21) | 0 (0–5) | <0.001 | 20 (14–41) | 15 (5–32.5) | <0.001 | <0.001 |
| Affected parenchyma LUL,% | 5 (0–11) | 0 (0–1) | <0.001 | 15 (5–30) | 12.5 (4–21) | 0.001 | <0.001 |
| Affected parenchyma LLL,% | 5 (5–20) | 0 (0–5) | <0.001 | 20 (5–42.5) | 10 (4–25) | <0.001 | <0.001 |
Data are n (%) or mean (IQR). CT=Computed Tomography. RUL=Right upper lobe. ML=Middle lobe. RLL=Right lower lobe. LUL=Left upper lobe. LLL=Left lower lobe. NA=Not applicable.
a. Comparison of moderate and severe patients at 52 weeks.
Fig. 3.
Temporal evolution of typical radiological abnormalities in COVID-19 patients with moderate or severe disease. Axial CT-images illustrating the typical evolution of radiological abnormalities over time in a severely ill (A, B, C) and moderately ill (D, E, F) COVID-19 patient at baseline, 3 months and 12 months (left to right). During the acute phase of SARS-CoV-2-infection both patients predominantly demonstrate parenchymal consolidation in a pattern most resembling organizing pneumonia (pronounced peripheral involvement, bronchocentric distribution, perilobular pattern) (A, D). In time, parenchymal abnormalities also resolve in a manner similar to that of organizing pneumonia. At 3 months follow-up, resolution of prior consolidations leaves residual GGO, peripheral curvilinear bands and architectural distortion (B, E). At 12 months follow-up, these residual abnormalities have failed to fully resolve (C, F).
Psychological assessment
Most patients scored below cut-off values on the self-reported questionnaires for symptoms of anxiety, depression, post-traumatic stress and cognitive failures during follow-up (Table 4 ). Moderately ill patients had a mean reduction of −1.48 (CI-95: −0.33, −2.63) on the GAD-7 scale and a mean reduction of −2.17 (CI-95: −4.0, −0.36) on the PCL-5 questionnaire between 6 weeks and 12 months. Symptoms of depression or cognitive failure remained comparable in patients with moderate and severe disease. There were no differences in self-reported psychological symptoms based on disease severity at 12 months.
Table 4.
Psychological outcomes in COVID-19 patients with moderate or severe disease at 6 weeks and 52 weeks follow-up.
| Moderate (N = 38) | Severe (N = 28) | Moderate vs. severea | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Questionnaire | 6 weeks | 52 weeks | N | p-value | 6 weeks | 52 weeks | N | p-value | p-value |
| Anxiety, GAD-7 | 3.6 ± 3.7 | 2.1 ± 3.2 | 23 | 0.014 | 3.3 ± 4.0 | 1.7 ± 2.3 | 22 | 0.070 | 0.630 |
| Anxiety, GAD-7 ≥ 10 | 1 (4%) | 1 (4%) | 23 | NA | 2 (9%) | 0 | 22 | NA | NA |
| Depression, PHQ-9 | 5.2 ± 6.0 | 3.0 ± 3.1 | 23 | 0.021 | 5.1 ± 4.3 | 5.2 ± 4.6 | 21 | 0.966 | 0.075 |
| Depression, PHQ-9 ≥ 10 | 5 (22%) | 1 (4%) | 23 | NA | 4 (19%) | 5 (24%) | 21 | NA | NA |
| PTSS, PCL-5 | 13.6 ± 16.2 | 9.5 ± 12.9 | 16 | 0.088 | 12.1 ± 13.7 | 9.9 ± 10.3 | 17 | 0.316 | 0.926 |
| PTSS, PCL-5 ≥ 33 | 1 (6%) | 1 (6%) | 16 | NA | 5 (29%) | 1 (6%) | 17 | NA | NA |
| Cognitive failure, CFQ-25 | 18.2 ± 13.1 | 20.6 ± 13.2 | 16 | 0.214 | 21.6 ± 16.0 | 22.5 ± 13.3 | 14 | 0.747 | 0.701 |
| Cognitive failure, CFQ-25 ≥ 44 | 0 | 2 (13%) | 16 | NA | 1 (7%) | 1 (7%) | 14 | NA | NA |
Data are mean ± SD or n/N (%). GAD-7=Generalized Anxiety Disorder Scale. PHQ-9=Patient Health Questionnaire 9. PCL-5=Post-traumatic stress disorder checklist for the 5th Diagnostic and Statistical Manual of Mental Disorders. CFQ-25=Cognitive Failures Questionnaire. NA=Not applicable.
a. Comparison of moderate and severe patients at 52 weeks.
Discussion
In this observational study we evaluated pulmonary and psychological outcomes up to 12 months after hospitalization with COVID-19, wild-type (Wuhan-variant) exclusively, with the ultimate aim to evaluate whether infection with COVID-19 warrants standard long-term follow-up with spirometry and chest CT.
We found that most patients had clear signs of recovery with normalized pulmonary function and reduced radiographic involvement of affected parenchyma. Impaired lung diffusion and functional limitations, however, persisted in some patients and fibrotic-like changes remained highly prevalent.
The higher frequency of lung diffusion impairment shortly after the acute phase of infection in patients with severe disease compared to patients with moderate disease can be contributed to mechanisms like pulmonary oedema, alveolitis, pleural effusion, thrombotic events and endothelial inflammation which are often part of ARDS, the most common complication of COVID-19 that requires treatment on the ICU [19, 20]. Persistence of lung diffusion disorders was also reported in two other large cohort studies and can be explained by at least partially irreversible endothelial damage [11, 21]. COVID-19 induced acute lung injury has distinct histopathological features of this endothelial damage with diffuse microvascular involvement with intra- and extravascular fibrin deposition, (micro-)thromboemboli and formation of hyaline membranes even when other areas are already in a proliferative and healing phase. This heterogeneity of injury supports the theory of repeated viral injury and may explain why endothelial damage caused by COVID-19 is distinct from damage caused by other coronaviruses (SARS, MERS) or Influenza [22, 23]. However, persistent impairment of lung diffusion was similarly frequently reported, ranging from 11% to 34%, in outbreaks of previous coronaviruses [24, 25].
Psychological symptoms were reported in a minority of patients with lowest prevalence of symptoms of anxiety and cognitive symptoms and highest prevalence of depressive symptoms, especially in severely ill patients. Prevalence rates of these psychological symptoms were comparable to those previously reported in the general elderly Dutch population as well as with those reported by Heesakkers et al. in Dutch patients a year after ICU admission for COVID-19 [26], [27], [28]. It should be noted however that the response rate of psychological symptom assessment was relatively low and as such the findings may potentially be biased. Patients with more psychological symptoms were the most likely to not respond which could lead to an underestimation of symptoms.
Although fibrotic-like residual abnormalities on chest CT appeared to decrease in size independent of disease severity, the high prevalence one year after hospitalization is concerning. Other recently published post-COVID-19 cohorts support that patterns suggestive of fibrosis are frequent after one year follow-up with a prevalence ranging from 75% to 87% in the most severely ill patients [11, 21]. Subcutaneous emphysema, pneumomediastinum or pneumothorax which are associated with barotrauma, a frequently reported complication after invasive ventilation, were rare during hospitalization [29, 30]. However, ventilation induced injury (VILI) cannot be completely excluded as cause of a higher prevalence of fibrotic-like abnormalities in patients with severe disease.
Clinical relevance of these fibrotic-like changes is difficult to elucidate, because residual abnormalities were associated with impairment of lung diffusion and functional limitations but not with self-reported shortness of breath. Furthermore, a minimal clinical important difference for pulmonary function is not clearly defined and patients with severe disease, who had a higher prevalence of residual abnormalities and larger area of affected parenchyma, still seemed to have a lower FVC and DLCOc after one year compared to patients with moderate disease. That FVC only improved in the first 12 weeks could be attributed to recovery of muscle weakness and resorption of parenchymal consolidation, which occurs mostly in the acute phase of infection. In addition, FVC should be interpreted very cautiously in the presence of fibrotic-like patterns on chest CT because a normal FVC is seen in half of IPF patients at time of diagnosis and a decline in the acute phase could not be determined as a result of unknown pulmonary function pre-COVID [31]. The stabilization of FVC accompanied by fibrotic-like changes could mark that post-inflammatory fibrosis in COVID-19 is at least partially irreversible. Fortunately, a relative or absolute decline of ≥10% of FVC, which is associated with poor outcomes and mortality in idiopathic pulmonary fibrosis, was rare after 12 months of follow-up and we are yet to see any evidence of a progressive character of fibrosis after COVID-19 [32, 33]. Radiographic chest abnormalities were less prevalent in previous outbreaks of other coronaviruses, but the previously mentioned results of long-term follow-up of 80 SARS patients are reassuring in the fact that a progressive phenotype of fibrosis was very uncommon [7]. The high incidence of residual abnormalities in our cohort could partially by attributed by the fact that corticosteroids were infrequently administered, because it was believed to be harmful in ARDS caused by COVID-19 early on in the pandemic.
With a lack of therapies acting directly against SARS-CoV-2, patients in our cohort were treated with remdesivir and hydroxychloroquine according to Dutch guidelines at that time before evidence of a beneficial effect. Treatment with hydroxychloroquine got remarkable attention primarily due to in vitro data and immunomodulatory capacities, but a meta-analysis demonstrated an association with increased mortality and no clear benefit in hospitalized COVID-19 patients [34, 35]. In different clinical trials, treatment with remdesivir showed mixed results and seemed to shorten time to recovery [36].
The limited extent of affected parenchyma, lack of signs of progressive scarring and relatively normal pulmonary function in patients with moderate disease indicate that standard pulmonary follow-up with spirometry and chest CT is not always beneficial nor cost-effective, but future studies with larger patient cohorts are needed to support these findings. In our opinion, patients with persistent symptoms, severe disease (ICU admission) or a trigger for progression of fibrotic-like abnormalities (e.g. pulmonary infection, mechanical ventilation) would benefit from pulmonary evaluation. However, caution should be taken by the fact that infection with more recent SARS-CoV-2 variants (e.g. delta) can lead to a different prevalence of pulmonary sequelae as seen on baseline chest CT in hospitalized patients with the delta variant [37]. Currently, differences in long-term outcomes of COVID-19 caused by other SARS-CoV-2 variants opposed to the wild-type are unknown.
One of the strengths of this study was the evaluation of pulmonary function and radiologic chest abnormalities independent of pulmonary symptoms and previous measurements. Our study is limited by the relatively small number of patients, the use of self-reported questionnaires and its single-centre design which may hamper generalizability. In addition, the whole spectrum of post-COVID complaints were not clearly reported in our cohort due to lack of consensus on post-COVID syndrome at the time. Even so, we used the MRC and PCFS as surrogate marker for analysis of symptoms, the latter, being a validated instrument to monitor post-COVID-19 recovery and has been shown to closely correlate to intensity of a wide spectrum of symptoms as well as quality of life [38, 39].
In conclusion, fibrotic-like abnormalities were highly prevalent (53%) one year after hospitalization with COVID-19 and were associated with impaired lung diffusion. Future studies are needed to clarify the temporal evolution of these fibrotic-like changes.
Conflict of interest
None.
References
- 1.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shi H., Han X., Jiang N., Cao Y., Alwalid O., Gu J., et al. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis. 2020;20(4):425–434. doi: 10.1016/S1473-3099(20)30086-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Polak S.B., Van Gool I.C., Cohen D., von der Thusen J.H., van Paassen J. A systematic review of pathological findings in COVID-19: a pathophysiological timeline and possible mechanisms of disease progression. Mod Pathol. 2020;33(11):2128–2138. doi: 10.1038/s41379-020-0603-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Antonio G.E., Wong K.T., Chu W.C., Hui D.S., Cheng F.W., Yuen E.H., et al. Imaging in severe acute respiratory syndrome (SARS) Clin Radiol. 2003;58(11):825–832. doi: 10.1016/S0009-9260(03)00308-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Das K.M., Lee E.Y., Singh R., Enani M.A., Al Dossari K., Van Gorkom K., et al. Follow-up chest radiographic findings in patients with MERS-CoV after recovery. Indian J Radiol Imaging. 2017;27(3):342–349. doi: 10.4103/ijri.IJRI_469_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang P., Li J., Liu H., Han N., Ju J., Kou Y., et al. Long-term bone and lung consequences associated with hospital-acquired severe acute respiratory syndrome: a 15-year follow-up from a prospective cohort study. Bone Res. 2020;8:8. doi: 10.1038/s41413-020-0084-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cabrera-Benitez N.E., Parotto M., Post M., Han B., Spieth P.M., Cheng W.E., et al. Mechanical stress induces lung fibrosis by epithelial-mesenchymal transition. Crit Care Med. 2012;40(2):510–517. doi: 10.1097/CCM.0b013e31822f09d7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lerum T.V., Aalokken T.M., Bronstad E., Aarli B., Ikdahl E., Lund K.M.A., et al. Dyspnoea, lung function and CT findings 3 months after hospital admission for COVID-19. Eur Respir J. 2021;57(4) doi: 10.1183/13993003.03448-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang C., Huang L., Wang Y., Li X., Ren L., Gu X., et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397(10270):220–232. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang L., Yao Q., Gu X., Wang Q., Ren L., Wang Y., et al. 1-year outcomes in hospital survivors with COVID-19: a longitudinal cohort study. Lancet. 2021;398(10302):747–758. doi: 10.1016/S0140-6736(21)01755-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bellan M., Baricich A., Patrucco F., Zeppegno P., Gramaglia C., Balbo P.E., et al. Long-term sequelae are highly prevalent one year after hospitalization for severe COVID-19. Sci Rep. 2021;11(1):22666. doi: 10.1038/s41598-021-01215-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bocchino M., Lieto R., Romano F., Sica G., Bocchini G., Muto E., et al. Chest CT-based Assessment of 1-year Outcomes after Moderate COVID-19 Pneumonia. Radiology. 2022 doi: 10.1148/radiol.220019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klok F.A., Boon G., Barco S., Endres M., Geelhoed J.J.M., Knauss S., et al. The Post-COVID-19 Functional Status scale: a tool to measure functional status over time after COVID-19. Eur Respir J. 2020;56(1) doi: 10.1183/13993003.01494-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Donker T., van Straten A., Marks I., Cuijpers P. Quick and easy self-rating of Generalized Anxiety Disorder: validity of the Dutch web-based GAD-7, GAD-2 and GAD-SI. Psychiatry Res. 2011;188(1):58–64. doi: 10.1016/j.psychres.2011.01.016. [DOI] [PubMed] [Google Scholar]
- 16.Kroenke K., Spitzer R.L., Williams J.B. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blevins C.A., Weathers F.W., Davis M.T., Witte T.K., Domino J.L. The Posttraumatic Stress Disorder Checklist for DSM-5 (PCL-5): development and initial psychometric evaluation. J Trauma Stress. 2015;28(6):489–498. doi: 10.1002/jts.22059. [DOI] [PubMed] [Google Scholar]
- 18.Broadbent D.E., Cooper P.F., FitzGerald P., Parkes K.R. The Cognitive Failures Questionnaire (CFQ) and its correlates. Br J Clin Psychol. 1982;21(1):1–16. doi: 10.1111/j.2044-8260.1982.tb01421.x. [DOI] [PubMed] [Google Scholar]
- 19.Osuchowski M.F., Winkler M.S., Skirecki T., Cajander S., Shankar-Hari M., Lachmann G., et al. The COVID-19 puzzle: deciphering pathophysiology and phenotypes of a new disease entity. Lancet Respir Med. 2021;9(6):622–642. doi: 10.1016/S2213-2600(21)00218-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Batah S.S., Fabro A.T. Pulmonary pathology of ARDS in COVID-19: a pathological review for clinicians. Respir Med. 2021;176 doi: 10.1016/j.rmed.2020.106239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu X., Liu X., Zhou Y., Yu H., Li R., Zhan Q., et al. 3-month, 6-month, 9-month, and 12-month respiratory outcomes in patients following COVID-19-related hospitalisation: a prospective study. Lancet Respir Med. 2021;9(7):747–754. doi: 10.1016/S2213-2600(21)00174-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Calabrese F., Pezzuto F., Fortarezza F., Hofman P., Kern I., Panizo A., et al. Pulmonary pathology and COVID-19: lessons from autopsy. The experience of European Pulmonary Pathologists. Virchows Arch. 2020;477(3):359–372. doi: 10.1007/s00428-020-02886-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Borczuk A.C. Pulmonary pathology of COVID-19: a review of autopsy studies. Curr Opin Pulm Med. 2021;27(3):184–192. doi: 10.1097/MCP.0000000000000761. [DOI] [PubMed] [Google Scholar]
- 24.Hui D.S., Joynt G.M., Wong K.T., Gomersall C.D., Li T.S., Antonio G., et al. Impact of severe acute respiratory syndrome (SARS) on pulmonary function, functional capacity and quality of life in a cohort of survivors. Thorax. 2005;60(5):401–409. doi: 10.1136/thx.2004.030205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ahmed H., Patel K., Greenwood D.C., Halpin S., Lewthwaite P., Salawu A., et al. Long-term clinical outcomes in survivors of severe acute respiratory syndrome and Middle East respiratory syndrome coronavirus outbreaks after hospitalisation or ICU admission: a systematic review and meta-analysis. J Rehabil Med. 2020;52(5):jrm00063. doi: 10.2340/16501977-2694. [DOI] [PubMed] [Google Scholar]
- 26.Heesakkers H., van der Hoeven J.G., Corsten S., Janssen I., Ewalds E., Simons K.S., et al. Clinical outcomes among patients with 1-year survival following intensive care unit treatment for COVID-19. JAMA. 2022;327(6):559–565. doi: 10.1001/jama.2022.0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Zelst W.H., de Beurs E., Beekman A.T., Deeg D.J., van Dyck R. Prevalence and risk factors of posttraumatic stress disorder in older adults. Psychother Psychosom. 2003;72(6):333–342. doi: 10.1159/000073030. [DOI] [PubMed] [Google Scholar]
- 28.Blazer D.G. Depression in late life: review and commentary. J Gerontol A. 2003;58(3):249–265. doi: 10.1093/gerona/58.3.m249. [DOI] [PubMed] [Google Scholar]
- 29.McGuinness G., Zhan C., Rosenberg N., Azour L., Wickstrom M., Mason D.M., et al. Increased incidence of barotrauma in patients with COVID-19 on invasive mechanical ventilation. Radiology. 2020;297(2):E252–EE62. doi: 10.1148/radiol.2020202352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rajdev K., Spanel A.J., McMillan S., Lahan S., Boer B., Birge J., et al. Pulmonary barotrauma in COVID-19 patients with ARDS on invasive and non-invasive positive pressure ventilation. J Intensive Care Med. 2021;36(9):1013–1017. doi: 10.1177/08850666211019719. [DOI] [PubMed] [Google Scholar]
- 31.Cortes-Telles A., Forkert L., DE O'Donnell, Moran-Mendoza O. Idiopathic pulmonary fibrosis: new insights on functional characteristics at diagnosis. Can Respir J. 2014;21(3):e55–e60. doi: 10.1155/2014/825606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nathan S.D., Yang M., Morgenthien E.A., Stauffer J.L. FVC variability in patients with idiopathic pulmonary fibrosis and role of 6-min walk test to predict further change. Eur Respir J. 2020;55(5) doi: 10.1183/13993003.02151-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zappala C.J., Latsi P.I., Nicholson A.G., Colby T.V., Cramer D., Renzoni E.A., et al. Marginal decline in forced vital capacity is associated with a poor outcome in idiopathic pulmonary fibrosis. Eur Respir J. 2010;35(4):830–836. doi: 10.1183/09031936.00155108. [DOI] [PubMed] [Google Scholar]
- 34.Pecho-Silva S., Navarro-Solsol A.C., Panduro-Correa V., Rabaan A.A., Bonilla-Aldana D.K., Rodriguez-Morales A.J., et al. Non-recommended medical interventions and their possible harm in patients with COVID-19. Ther Adv Infect Dis. 2021;8 doi: 10.1177/20499361211034070. 20499361211034070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Axfors C., Schmitt A.M., Janiaud P., Van't Hooft J., Abd-Elsalam S., Abdo E.F., et al. Mortality outcomes with hydroxychloroquine and chloroquine in COVID-19 from an international collaborative meta-analysis of randomized trials. Nat Commun. 2021;12(1):2349. doi: 10.1038/s41467-021-22446-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu Z., Han Z., Liu B., Shen N. Remdesivir in treating hospitalized patients with COVID-19: a renewed review of clinical trials. Front Pharmacol. 2022;13 doi: 10.3389/fphar.2022.971890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yoon S.H., Lee J.H., Kim B.N. Chest CT findings in hospitalized patients with SARS-CoV-2: delta versus Omicron variants. Radiology. 2022 doi: 10.1148/radiol.220676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leite L.C., Carvalho L., Queiroz D.M., Farias M.S.Q., Cavalheri V., Edgar D.W., et al. Can the post-COVID-19 functional status scale discriminate between patients with different levels of fatigue, quality of life and functional performance? Pulmonology. 2022;28(3):220–223. doi: 10.1016/j.pulmoe.2022.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Machado F.V.C., Meys R., Delbressine J.M., Vaes A.W., Goertz Y.M.J., van Herck M., et al. Construct validity of the Post-COVID-19 functional status scale in adult subjects with COVID-19. Health Qual Life Outcomes. 2021;19(1):40. doi: 10.1186/s12955-021-01691-2. [DOI] [PMC free article] [PubMed] [Google Scholar]