Abstract
This article comments on:
Raees Khan, Robert S. Hill, Veit M. Dörken and Ed Biffin, Detailed seed cone morpho-anatomy of the Prumnopityoid clade: an insight into the origin and evolution of Podocarpaceae seed cones, Annals of Botany, Volume 130, Issue 5, 1 November 2022, Pages 637–655 https://doi.org/10.1093/aob/mcac097
Conifers, as the name suggests, produce cones. A stereotypical conifer cone, like those of spruces or pines, is a robust structure composed of woody scales that support and protect developing seeds. However, not all conifers have such a cone; around 40 % of living species produce something closer to what one might informally call a ‘berry’ or a ‘drupe’, with white, orange, red or purple fleshy tissues surrounding a few seeds. A new study from Khan et al. (2022) investigates the evolution of these cones through a detailed morphological and anatomical study, revealing the range of their structural diversity and their deep evolutionary history.
If the typical woody conifer cone is adapted to keep seed predators out, the ‘berry’ or ‘drupe’-like cones analysed in their study seem to do the opposite: they produce colourful, succulent and often sweet tissues that attract animals to eat them. The purpose of these tissues is to link seed dispersal to the mobility of animals; seeds contained within the ingested cones eventually pass through the animal’s gut or will be regurgitated after the digestion of soft cone tissues, hopefully at some distance from the parent plant. These kinds of cones can be found among some familiar Northern Hemisphere conifers such as junipers and yews, but they are most abundant in the Podocarpaceae, a primarily tropical and Gondwanan family that has arguably evolved the most diverse reproductive structures of all living conifer groups. Khan et al. focus their study on cone evolution in the prumnopityoids, a major clade within Podocarpaceae (Fig. 1).
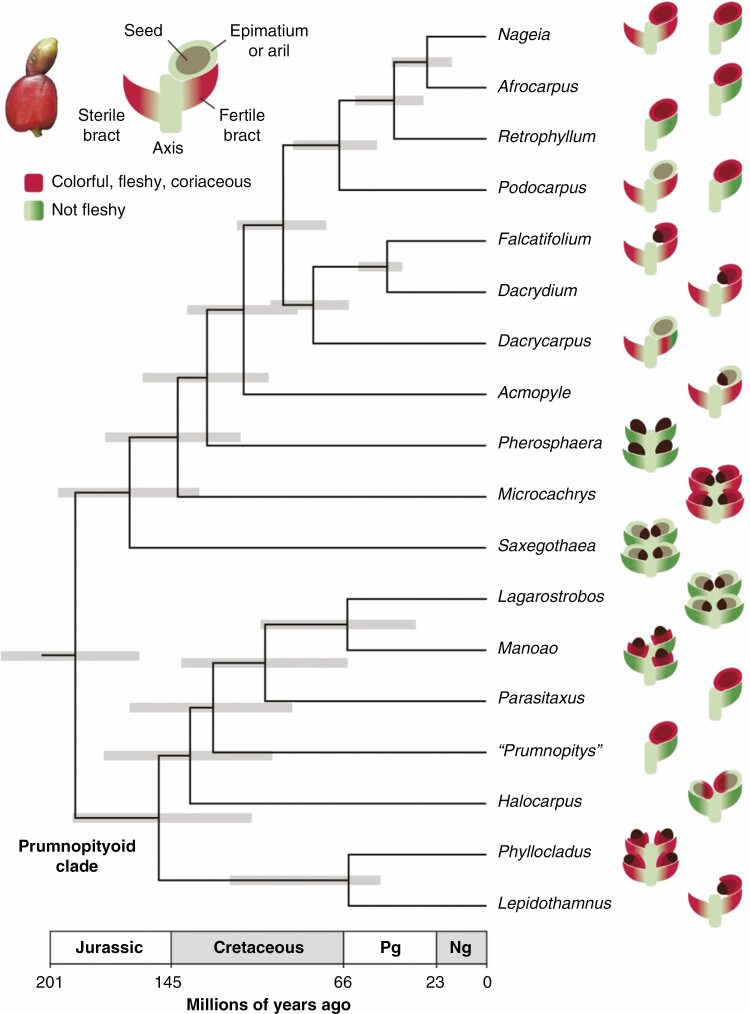
Fig. 1.
Time-calibrated molecular phylogeny of prumnopityoid and other Podocarpaceae genera with depictions of their cones. Depictions are based on an idealized schematic version of Podocarpaceae reproductive organs (labelled example of Podocarpus is shown in the upper left-hand key, with a photograph of a live specimen for comparison). The epimatium does not always surround the seed; here it is depicted as a cup around the seed base (e.g. Lepidothamnus). Red indicates the presence of colourful or fleshy tissues in an organ, although actual colours may vary. Seed orientation indicates whether they are erect (facing outwards) or inverted (facing the cone axis) in the mature cone. ‘Prumnopitys’ has been segregated into three genera, but these exhibit the same basic morphology. Phylogeny is taken from Leslie et al. (2018), with grey bars indicating uncertainty in estimated genus divergence ages (95% highest posterior density region). Pg = Palaeogene; Ng = Neogene.
Podocarpaceae are unusual conifers in general. They are one of the few living groups that are diverse in the tropics, with major centres of species richness in the montane forests of Borneo and New Guinea. Perhaps as a response to these environments, Podocarpaceae have evolved greater leaf diversity than most conifers, including long, flattened needles and large, multi-veined leaves that efficiently capture light in closed-canopy forests (Biffin et al., 2012). Their reproductive structures likewise show a high degree of variation; although most Podocarpaceae share a basic cone structure – their seeds are generally surrounded by an aril or an organ specific to the Podocarpaceae called an epimatium and are subtended by a fertile bract borne on a reproductive axis along with sterile bracts – different lineages have heavily modified these parts and their arrangement. Many have evolved a reduced number of fertile scales (often just one) and produce fleshy and/or colourful tissues across virtually every combination of reproductive organ (Fig. 1). What has driven the evolution of this diversity? One possibility is that these structures represent numerous independent adaptations for seed dispersal, where different lineages have utilized different combinations of available cone tissues to attract dispersal agents, which today are primarily birds. To the extent that molecular divergence dates are accurate (Fig. 1; see Leslie et al., 2018), the evolution of this disparity probably occurred over the Cretaceous as early birds were diversifying (Klaus and Matzke, 2020). The unusual, and unusually diverse, Podocarpaceae cone may therefore reflect a long history of avian interactions stretching back more than 140 million years.
Understanding the evolution of these cones, and their potential association with biotic and ecological changes over the Late Mesozoic, ultimately requires a phylogeny that samples deep Podocarpaceae lineages. Molecular studies (e.g. Knopf et al., 2012; Chen et al., 2022) have been critical in clarifying relationships among living taxa, but long morphological and molecular branch lengths (Fig. 1) make resolving the order and timing of reproductive trait evolution difficult. Fossil taxa would help to break up these long branches, but Podocarpaceae reproductive structures are not particularly common in the Mesozoic and the phylogenetic placements of those that exist are unclear. A fundamental step in resolving the position of these fossil taxa actually begins with living taxa: specifically, a more thorough understanding of their cone anatomy and morphology. Although there is a history of studies in this area (e.g. Florin, 1951; Tomlinson, 1992), Podocarpaceae cones are highly modified and most undergo substantial ontogenetic shape change as their tissues become fleshy. Even finding fresh samples for anatomical and developmental study is not easy; prumnopityoids, for example, include restricted endemics such as Tasmanian Lagarostrobus and New Caledonian Parasitaxus. A consistent understanding of what the basic parts of Podocarpaceae cones even are can therefore be difficult to achieve, but is essential to develop robust phylogenetic characters that can be applied to fossil taxa.
Khan et al. use staining and sectioning techniques to clarify the anatomical and morphological diversity of prumnopityoids, from the highly reduced drupe-like structures of Prumnopitys to the fleshy cones of Phyllocladus. Their results show that prumnopityoid reproductive diversity mirrors patterns found across the family Podocarpaceae (Fig. 1), and their analysis of trait evolution highlights the potential complexity of podocarp reproductive history. They use a likelihood-based character state reconstruction and a molecular phylogeny to infer that fleshiness is ancestral among crown Podocarpaceae, but their anatomical work clearly shows that fleshiness has been expressed in different organs and tissues across prumnopityoids. One interpretation of these results is that fleshiness and cone structural evolution are decoupled; there may have been a single origin of the capacity to form fleshy tissues in crown Podocarpaceae but this ability has been expressed across different parts of the cone in different lineages as their interactions with animal dispersal agents became stronger. Such an interpretation is not inconsistent with the fossil record; some of the earliest widely regarded Podocarpaceae from the Middle Jurassic are thought to have produced fleshy tissues (Reymanowna, 1987) but their cones are unlike those of any living lineage, with dozens of fertile scales arranged in a long spike. Smaller cones with a reduced number of seeds, which are more likely to function as single propagules dispersed by small animals, appear around the middle of the Early Cretaceous (~120 Ma; Vishnu-Mittre, 1959).
In addition to their work on extant taxa, Khan et al. (2022) also show one of these reduced cones, a previously undescribed fossil from the middle Cretaceous (~95 Ma) of Australia that may belong to the living genus Lepidothamnus. If correctly assigned, this material would represent the earliest known reproductive structures placed within an extant lineage. Fossils like these, when coupled with a detailed understanding of morphological and anatomical variation across living Podocarpaceae from studies such as that of Khan et al., provide exciting opportunities for exploring the evolutionary history of not only Podocarpaceae conifers, but also broader interactions between plants and animals in the Late Mesozoic.
LITERATURE CITED
- Biffin E, Brodribb TJ, Hill RS, Thomas P, Lowe AJ.. 2012. Leaf evolution in Southern Hemisphere conifers tracks the angiosperm ecological radiation. Proceedings of the Royal Society B 279: 341–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Jin WT, Liu XQ, Wang XQ.. 2022. New insights into the phylogeny and evolution of Podocarpaceae inferred from transcriptomic data. Molecular Phylogenetics and Evolution 166: 107341. doi: 10.1016/j.ympev.2021.107341. [DOI] [PubMed] [Google Scholar]
- Florin R. 1951. Evolution of cordaites and conifers. Acta Horti Berginal 17: 259–388. [Google Scholar]
- Khan R, Hill RS, Dörken VM, Biffin E.. 2022. Detailed seed cone morpho-anatomy of the Prumnopityoid clade; an insight into the origin and evolution of Podocarpaceae seed cones. Annals of Botany 130: 637–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaus KV, Matzke NJ.. 2020. Statistical comparison of trait-dependent biogeographical models indicates that Podocarpaceae dispersal is influenced by both seed cone traits and geographical distance. Systematic Biology 69: 61–75. doi: 10.1093/sysbio/syz034. [DOI] [PubMed] [Google Scholar]
- Knopf P, Schulz C, Little DP, Stützel T, Stevenson DW.. 2012. Relationships within Podocarpaceae based on DNA sequence, anatomical, morphological, and biogeographical data. Cladistics 28: 271–299. doi: 10.1111/j.1096-0031.2011.00381.x. [DOI] [PubMed] [Google Scholar]
- Leslie AB, Beaulieu J, Holman G, et al. 2018. An overview of extant conifer evolution from the perspective of the fossil record. American Journal of Botany 105: 1531–1544. doi: 10.1002/ajb2.1143. [DOI] [PubMed] [Google Scholar]
- Tomlinson PB. 1992. Aspects of cone morphology and development in Podocarpaceae (Coniferales). International Journal of Plant Sciences 153: 572–588. [Google Scholar]
- Reymanówna M. 1987. A Jurassic podocarp from Poland. Review of Palaeobotany and Palynology 51: 133–143. [Google Scholar]
- Vishnu-Mittre. 1959. Studies on the fossil flora of Nipania (Rajmahal Series), Bihar-Coniferales. Palaeobotanist 6: 82–112. [Google Scholar]