Abstract
Background
Land plants commonly produce red pigmentation as a response to environmental stressors, both abiotic and biotic. The type of pigment produced varies among different land plant lineages. In the majority of species they are flavonoids, a large branch of the phenylpropanoid pathway. Flavonoids that can confer red colours include 3-hydroxyanthocyanins, 3-deoxyanthocyanins, sphagnorubins and auronidins, which are the predominant red pigments in flowering plants, ferns, mosses and liverworts, respectively. However, some flowering plants have lost the capacity for anthocyanin biosynthesis and produce nitrogen-containing betalain pigments instead. Some terrestrial algal species also produce red pigmentation as an abiotic stress response, and these include both carotenoid and phenolic pigments.
Scope
In this review, we examine: which environmental triggers induce red pigmentation in non-reproductive tissues; theories on the functions of stress-induced pigmentation; the evolution of the biosynthetic pathways; and structure–function aspects of different pigment types. We also compare data on stress-induced pigmentation in land plants with those for terrestrial algae, and discuss possible explanations for the lack of red pigmentation in the hornwort lineage of land plants.
Conclusions
The evidence suggests that pigment biosynthetic pathways have evolved numerous times in land plants to provide compounds that have red colour to screen damaging photosynthetically active radiation but that also have secondary functions that provide specific benefits to the particular land plant lineage.
Keywords: Anthocyanin, antioxidant, auronidin, betalain, biosynthesis, evolution, flavonoid, photoprotection, photomodulation, stress
INTRODUCTION
When and how different specialized metabolite pathways may have evolved has been a focus of much research over recent years, especially with the availability of many more non-angiosperm whole-genome sequences. A specialized metabolite stress response observed across almost all land plant taxa is the production of red pigmentation. Red pigmentation can be induced by a variety of stresses and in most tissues. In some cases, the process has been extensively characterized, such as for the reddening of leaves on deciduous trees in autumn, but there are also many open questions on red pigment biosynthesis and function. In this review, we summarize current knowledge on the evolution and function of stress-induced red pigmentation in algae and land plants, including comparing the alternative theories for cellular functions. In particular, we examine the proposal that pigment biosynthetic pathways have evolved numerous times in land plants, to provide compounds that have red colour to screen excess photosynthetically active radiation (PAR) but that also have secondary functions providing specific benefits to the particular land plant lineage.
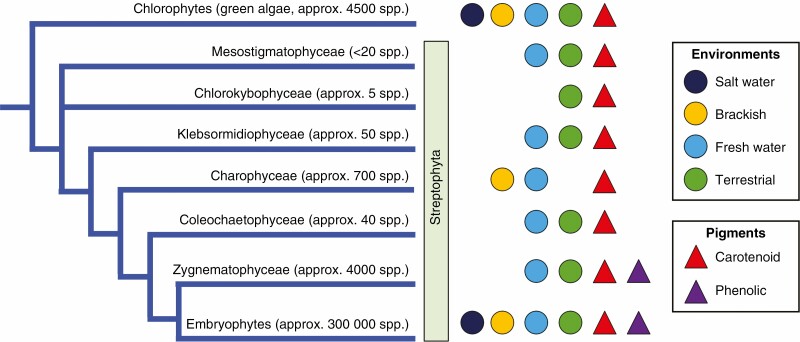
The Archaeplastida group of photosynthetic eukaryotes are thought to have originated about 1500 million years ago (MYA). This gave rise to the green algae (which included the ancestors of land plants), rhodophytes (red algae) and the less species-diverse glaucophytes and Prasinodermophyta (L. Li et al., 2020; Delaux and Schornack, 2021; Donoghue et al., 2021; Benton et al., 2022). Around 1000 MYA it is thought that the ancestors of chlorophyte algae and streptophytes arose from within the green algae lineage. The extant streptophytes are generally taken to include land plants (Embryophyta) and streptophyte algae, a group of freshwater and terrestrial algae (Fig. 1). The first land plants are thought to have arisen from a freshwater (or terrestrial) charophyte ancestor 550–470 MYA (Donoghue et al., 2021).
Fig. 1.
Species adapted to life on land are found in most lineages of the Viridiplantae. The currently proposed classes and relationships of the green plant lineages are shown, with estimates of the number of species within each clade. The Mesostigmatophyceae is taken as a clade containing Mesostigma and Spirotaenia. Whether species occur in the different environments is indicated. The majority of embryophyte species occur on the land, while generally low numbers of terrestrial species occur in the other clades, and often in ‘wet’ terrestrial habitats. The pigment occurrence refers to whether red- to purple-coloured pigments are produced. Many colourless simple phenolics and brownish phenolic polymers have been reported across both the Viridiplantae and more distant relatives such as the brown and red algae (e.g. phlorotannins, the oligomers of phloroglucinol common in brown algae).
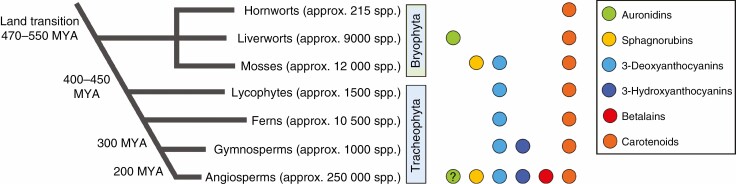
Early land plant evolution led to specializations for the terrestrial environment that resulted in embryophytes becoming the dominant terrestrial lineage. The extant land plants are classified into major groupings, notably the non-tracheophyte hornworts, liverworts and mosses (Bryophyta); lycophytes, ferns and fern allies (Monilophyta); gymnosperms; and flowering plants (Angiospermae) (Fig. 2). Species numbers within each of these groups vary greatly, with hornworts having the smallest estimated species diversity, at 220 (Frangedakis et al., 2021), and the largest groups being mosses and flowering plants, with species estimates of >12 000 and >300 000, respectively (Benton et al., 2022).
Fig. 2.
Occurrence of different red pigment types within land plants (Embryophyta). The currently proposed major classes and relationships of land plant lineages are shown, with estimates of the number of species within each clade. The occurrence of biosynthetic pathways to the betalain, carotenoid or various red phenolic pigments is indicated. The biosynthetic pathways may be of taxonomically restricted occurrence within the different groups (e.g. the restriction of betalains to the Caryophyllales), or may be reported as active only in reproductive tissues (e.g. the red carotenoid lycopene).
The early land plants adapted to take advantage of access to full sunlight and atmospheric CO2, which is available at concentrations higher than that dissolved in water and when plants first colonized land was at higher concentrations than today. However, both increased photosynthetic activity and the variable terrestrial conditions presented cellular and abiotic environment stresses different from those of the water environment, and required appropriate stress tolerance adaptations. All extant land plants (i.e. embryophytes) are thought to have evolved from a single event, resulting in the monophyletic land plant lineage. However, terrestrial colonization events have occurred independently within different algal lineages, and extant species belonging to the chlorophytes and the charophytes occupy terrestrial or semi-terrestrial habits (Fig. 1) (Holzinger and Pichrtová, 2016; Karsten et al., 2016; de Vries and Archibald, 2018; Fürst-Jansen et al., 2020). Some terrestrial algae are tolerant of relatively high amounts of light energy. Chlorophyte and charophyte species, plant lineages dominated by species that grow in ocean and freshwater habitats in which light penetration is reduced by the water, can tolerate >1400 μmol photons m−2 s−1 without any indication of photoinhibition (Karsten et al., 2016; Hoham and Remias, 2020), and algal species have been found living in snow and ice environments where they experience much higher amounts of light energy. Based on this, it has been argued that the early algae land plant ancestors were already well adapted to a terrestrial lifestyle, and the evolution of alternative life cycles was the more influential development leading to the dominance of land plants (see Harholt et al., 2016, and references therein). Another driver of the success of the land plant lineage is thought to be the development of symbiotic interactions with beneficial fungi coincident with, or soon after, the transition to the land, and it is estimated that 85 % of extant land plants form symbiotic interactions with fungi (Field and Pressel, 2018; Grosche et al., 2018). Such associations are widespread in the thalloid liverworts, hornworts and vascular plants, but are generally absent in the mosses and leafy liverworts (Field and Pressel, 2018; Grosche et al., 2018; Delaux and Schornack, 2021). Although algal ancestors may contain the genes that facilitate symbiosis (Delaux and Schornack, 2021), there is no evidence of fungal symbioses in charophyte green algae.
Among the abiotic stresses that are increased on land are desiccation, higher exposure to UVB radiation, and greater fluctuations in temperature, salinity and PAR. There have been recent reviews of the evolution of land plant traits as a response to these stresses (de Vries and Archibald, 2018; Fürst-Jansen et al., 2020; Markham and Greenham, 2021). In this review we focus on the contributions of red pigments to tolerance of the stresses faced in a terrestrial lifestyle, including the biosynthetic diversity of red pigments and the implications of this for pigment functions and the evolutionary origin of the biosynthetic pathways.
BIOSYNTHETIC AND STRUCTURAL DIVERSITY OF RED PIGMENTS IN PLANTS
Before discussing the diversity of red pigments in plants, it is useful to briefly cover some of the theories for the huge diversity of specialized metabolites in land plants in general, which includes >10 000 flavonoids, 12 000 alkaloids and 30 000 terpenoids (Desmet et al., 2021; Kulshrestha et al., 2022). A high frequency of gene duplication, with the associated opportunity for enzyme neofunctionalization to either accept new substrate combinations or generate novel products from existing substrates, is thought to have driven diversification of biosynthetic pathways (Rieseberg et al., 2022). This gave both biosynthetic potential for an abundance of different possible structures within an individual plant and many lineage-specific types of specialized metabolite. However, whether each structure has evolved to be the most effective for a specific function is uncertain. It is argued that the diversity of structures is so extensive that it is untenable to suggest each has evolved a specific structure–function activity (Desmet et al., 2021), and alternative theories have been proposed. The ‘Screening Hypothesis’ proposes that plants generate a diversity of structures of which only a few have biological activity. The remainder are ‘failed attempts’ at generating bioactives but are retained because the associated complexity of biosynthetic pathways increases the probability of generating new bioactives (Jones et al., 1991; Firn and Jones, 2003). An addition to this, which examines how specialized metabolite biosynthetic pathways may have arisen and subsequently diversified, proposes that specialized metabolites are principally produced as components of redox chemistry, particularly for scavenging of reactive oxygen species (ROS). In this case, the presence of specific functional groups (such as accessible unsubstituted hydroxyls, particularly ortho-hydroxyls) is more important than the overall structure, which is ‘free’ to diversify (Hadacek et al., 2010). These proposals could encompass some aspects of red pigment structural diversity. However, there is evidence for evolution favouring the production of specific complex structures for the flavonoid anthocyanin pigments, such as for generating blue flower colours (Yoshida et al., 2009; Houghton et al., 2021). Additionally, for Arabidopsis thaliana (hereafter ‘arabidopsis’), which lacks coloured flowers, >20 different anthocyanin structures have been reported (Kovinich et al., 2014) as well as at least 28 different flavonoids produced in response to UVB exposure (Tohge et al., 2016). The patterns of production of these are different depending on the plant growth conditions, and it has been suggested that the varied secondary modifications may promote functions suited to particular stress conditions (Kovinich et al., 2014). The different challenges individual stresses create are discussed in more detail later, when we examine the relative importance of flavonoids as antioxidants that remove ROS species vs. screening out PAR or UVB radiation that contribute to generating ROS in the first place. However, there is no doubt that ROS are a central component of the damage that results from many different plant stressors.
Reactive oxygen species can be generated by various biotic and abiotic stressors, the latter of which include UVB radiation, drought, salinity, cold and excess PAR (Devireddy et al., 2021; Sachdev et al., 2021). Light capture can exceed CO2 fixation rates, and this is commonly experienced by plants in the terrestrial environment, especially when plants are simultaneously undergoing other stresses such as cold or drought that reduce the photosynthetic efficiency. The excess photon energy can generate triplet excited chlorophyll states that react with O2 to yield singlet oxygen (1O2·), a highly reactive radical molecule, that can cause photosystem II (PSII) inactivation and photoinhibition. In addition, under stress. ROS are mainly produced by PSI (Pospíšil, 2009), i.e. via the reduction of molecular oxygen in the Mehler reaction, a coupled process leading to the formation of ATP without NADPH (Foyer, 2018).
There are various mechanisms to cope with excess PAR, many of which are conserved across land plants (Takahashi and Badger, 2011; Pinnola, 2019; Sandmann, 2019; Bassi and Dall’Osto, 2021). These include (1) mechanisms that reduce light capture (e.g. chloroplast movement, synthesis of screening compounds and light-harvesting complex state 1 to state 2 transition) and (2) mechanisms integrated into the photosynthetic machinery aimed at mitigating the excess energy due to supernumerary photons, such as alternative electron sinks [e.g. photorespiration, cyclic electron flow around PSI and non-photochemical quenching (NPQ)], the antioxidant apparatus (mainly consisting of enzymatic and non-enzymatic antioxidants belonging to the Foyer–Halliwell–Asada cycle in chloroplasts) and the production of other antioxidant compounds (e.g. hydroxyl-rich polyphenols) to quench ROS (Agati et al., 2020).
A conserved mechanism within the photosynthetic machinery for coping with excess PAR is NPQ, a multicomponent system that returns chlorophyll from the excited states back to the ground state, dissipating the excess energy as heat and thereby reducing the probability of ROS formation (for a review, see Demmig-Adams et al., 2014). Carotenoids, lipophilic yellow to red coloured pigments, integrated into the photosynthetic complex are important contributors to NPQ (Pinnola, 2019; Sandmann, 2019). NPQ mechanisms and the core carotenoid biosynthetic pathway are generally conserved across plants. However, some variations among the photosynthetic proteins involved have been identified among algae, bryophytes and vascular plants, in particular the presence of light-harvesting complex stress-related proteins in algae and mosses but not vascular plants (Pinnola, 2019). In addition to quenching excited chlorophyll, carotenoids can act as antioxidants that scavenge different forms of ROS after their release from the photosystem (D’Alessandro and Havaux, 2019; Sandmann, 2019). They are one of several non-flavonoid small metabolite antioxidants, such as tocopherols, ascorbate and glutathione. In addition to non-enzymatic antioxidant compounds, the antioxidant apparatus includes a range of antioxidant enzymes, including superoxide dismutase, ascorbate peroxidase and glutathione peroxidase, forming a well-orchestrated network able to balance ROS in plant tissues (Bassi and Dall’Osto, 2021).
The functions of some of the stress tolerance and antioxidant systems are disrupted by abiotic stresses such as UVB radiation that, as a downstream consequence of initial damage, alter the physiological ROS balance and promote oxidative stress (Choudhury et al., 2017). This may be one driver for the evolution during land colonization of flavonoids (and the accompanying initial phenylpropanoid pathway steps) as inducible stress tolerance compounds (Yonekura-Sakakibara et al., 2019; Davies et al., 2020; Fürst-Jansen et al., 2020; Desmet et al., 2021). UVB radiation is potentially a stronger stress factor in terrestrial than aquatic environments, as UVB is absorbed by water. The Charophyceae, which are algae of high morphological organization with a totally submerged lifestyle, seem to lack UVB tolerance compounds, indicating a reliance on absorbance of UVB by the surrounding water (Holzinger and Pichrtová, 2016). UVB radiation that penetrates into cells not only generates ROS but can directly damage membranes and DNA. Without protective mechanisms, the UVB radiation amounts commonly found in direct sunlight can cause significant cellular stress, and may lead to cell or plant death. Yet, as UVB is an integral part of the sunlight that contains the PAR required for photosynthesis, avoiding exposure through shading brings other disadvantages. The majority of land plants produce phenylpropanoids such as flavonoids (Fig. 3) as a defence against UVB radiation. Flavonoids have been shown to be key for UVB tolerance in liverworts and angiosperms (Clayton et al., 2018; Ferreyra et al., 2021). However, because the compounds induced by UVB radiation exposure are colourless in the great majority of cases (typically flavones and flavonols), UVB tolerance mechanisms are not discussed in detail in this review. In the remainder of the article, we examine further why non-photosynthetic red pigments may be important for stress tolerance despite the presence of a range of other stress tolerance systems. We summarize the variety of red non-photosynthetic pigment structures found in plants and whether these structural variations may reflect functional variations. We conclude by discussing the different proposals for how red pigments may improve stress tolerance, and whether there may be a common mechanism across all red pigments.
Fig. 3.
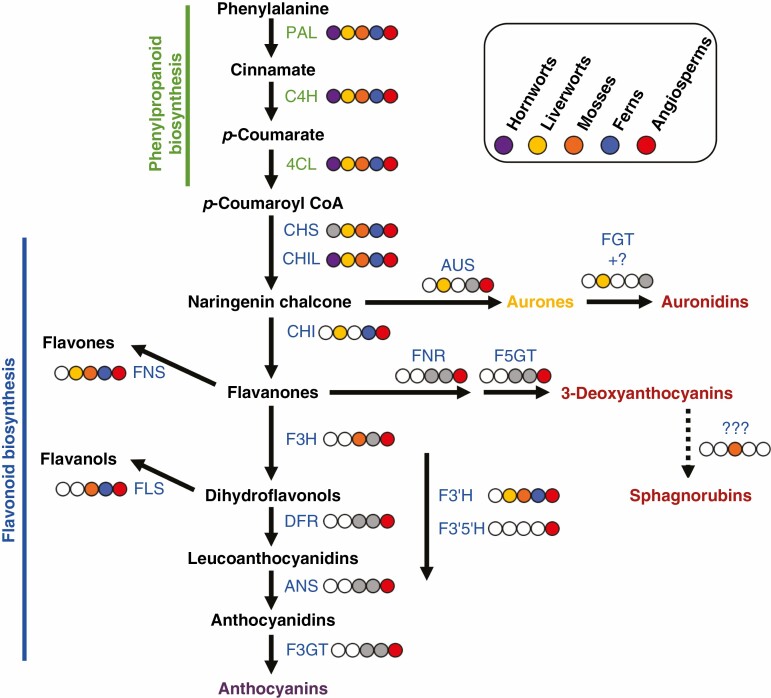
Schematic of the phenylpropanoid pathway leading to red flavonoid pigments and the occurrence of biosynthetic enzymes in different land plant lineages. A coloured circle next to an enzyme step indicates that that activity is present in the corresponding plant lineage (indicated by the colour key). A white circle with a grey outline indicates that it is not present in that lineage. A grey circle with a grey outline indicates that there is some support for the presence of the enzyme, from either sequence analysis or presence of the biosynthetic product, but that definitive evidence is lacking. With regard to proposed biosynthetic branches: the biosynthetic route to sphagnorubins is unknown but may be via 3-deoxyanthocyanidins; the biosynthetic route to auronidins is only partly characterized and, although auronidin 4-O-neohesperidoside can be present at relatively low amounts, auronidins principally accumulate as cell wall-bound aglycones. Enzyme abbreviations are: PAL, phenylalanine ammonia-lyase; C4H, cinnamate 4-hydroxylase; 4CL, 4-coumarate:CoA ligase; CHS, chalcone synthase; CHI, chalcone isomerase; CHIL, chalcone isomerase-like; F3H, flavanone 3-hydroxylase; DFR, dihydroflavonol 4-reductase; FNR, flavanone reductase; ANS, anthocyanidin synthase; FGTs, flavonoid O-glycosyltransferases; F3GT, flavonoid 3-O-glucosyltransferase; F5GT, flavonoid 5-O-glucosyltransferase; AUS, aureusidin synthase; FNS, flavone synthase; FLS, flavonol synthase; F3ʹH, flavonoid 3ʹ-hydroxylase; F3ʹ5 ʹH, flavonoid 3ʹ,5ʹ-hydroxylase.
Red pigments in algae
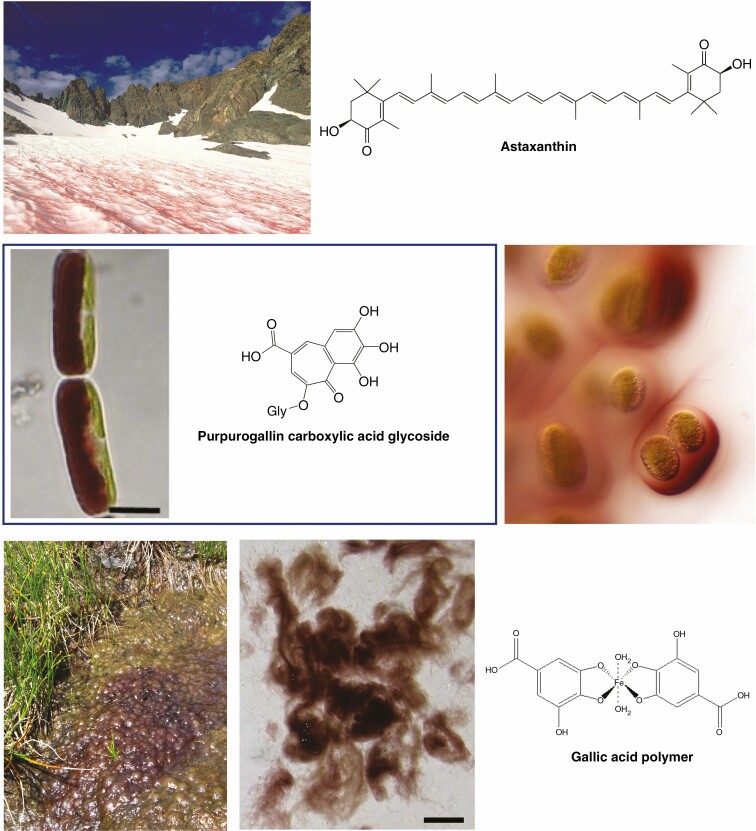
There are diverse red pigments in algae, many of which are yet to be structurally characterized. A common algal response to abiotic stress is the production of red non-photosynthetic carotenoids such as astaxanthin and astaxanthin esters (Fig. 4) (Holzinger et al., 2016; Hoham and Remias, 2020; Nakashima et al., 2021). A range of algae contribute to the phenomena known as ‘blood snows’ or ‘red tides’, when the snow surface turns red from algal carotenoid production (Fig. 4), with the Chlorophyceae genera Sanguina and Chlamydomonas commonly being among the causal species (Hoham and Remias, 2020; Nakashima et al., 2021). Some snow algae species produce phenolic compounds for UVB tolerance, but some are also known to produce astaxanthin in response to PAR stress, notably at the mature spore stage (Holzinger et al., 2016). PAR is thought to be the key environmental trigger for astaxanthin production, usually when combined with a secondary stress such as salinity, nitrogen deficiency or fluctuations in temperature (Hoham and Remias, 2020). The excess radiant energy absorbed by the carotenoids is partly dissipated as heat. Besides cellular benefits, this may assist the algae by melting snow and releasing nutrients (Hoham and Remias, 2020). However, it also accelerates glacier wastage and contributes to climate change-associated glacial loss (Williamson et al., 2020).
Fig. 4.
Examples of vegetative red pigmentation in terrestrial algae. The top image shows algae producing ‘blood snow’ in California, USA (USDA photograph by Paul Wade, https://commons.wikimedia.org/wiki/File:170828-FS-Inyo-PRW-001-MountRitter_(36217539154).jpg, Creative Commons Attribution 2.0 Generic licence). The middle row shows Ancylonema nordenskioeldii on the left and pigmented mucilage of a Serritaenia spp. on the right (both Zygnematophyceae). A derivative of purpurogallin has been identified as the phenolic pigment of Mesotaenium berggrenii, a relative of A. nordenskioeldii, with both these Zygnematophyceae species commonly found on alpine and Arctic glaciers and having purple-brown vacuoles containing phenolic pigments. The extracellular pigment of Serritaenia mucilage is also thought to be phenolic, but the structure has not been elucidated. The bottom row shows Zygogonium erictorum (also Zygnematophyceae), and its associated pigment thought to be a gallic acid-based polymer. Image of A. nordenskioeldii reprinted from Remias et al. (2012a). Image of Serritaenia courtesy of Dr Sebastian Hess (University of Cologne, Germany). Images of Z. erictorum reprinted from Aigner et al. (2013). Scale bars represent 10 µm (A. nordenskioeldii) and 1 cm (Z. erictorum).
Zygnematophyceae species, which are one of the most recently diverged algal lineages relative to land plants, also contribute to red pigmentation of snow and ice. However, stress-induced red carotenoid pigmentation has not been recorded for the Zygnematophyceae, neither has production of mycosporine-like amino acids (MAAs) been reported for this algal group. MAAs are colourless compounds with multiple stress tolerance functions, including UVB tolerance (Carreto and Carignan, 2011). They have been extensively characterized for red algae and reported for some groups of green algae. Rather than MAAs, in response to varied abiotic stresses, water-soluble reddish pigments have been observed in the vacuoles of varied Zygnematophyceae species from alpine and glacier environments (Remias et al., 2012a, b; Aigner et al., 2013; Herburger et al., 2016; Holzinger and Pichrtová, 2016; Williamson et al., 2020). Indeed, the red pigments of terrestrial algae have been a subject of discussion in this journal since the start of the 20th century (Fritsch, 1916). The red pigments observed are thought to represent a variety of compounds, although only a few individual structures have been defined, notably a purpurogallin-derived phenolic pigment (Remias et al., 2012a, b; Fig. 4). There are reports of a range of flavonoids in algal extracts (Yonekura-Sakakibara et al., 2019; Jiao et al., 2020). However, these have not included red flavonoid pigments, and it should be further noted that compound identification has usually been based on mass spectrometry (MS) data comparisons with databases, rather than characterization of pure compounds by structural identification methodologies such as nuclear magnetic resonance (NMR) spectroscopy. The chemistry data are supported by the proposed presence in genomes of extant algae of gene homologues for early steps of phenylpropanoid biosynthesis, although no complete gene set has been found (de Vries et al., 2017; Fürst-Jansen et al., 2020; Piatkowski et al., 2020). Thus, some of the phenylpropanoid biosynthetic genes may have been present in the common lineage prior to the origin of land plants, while flavonoid-specific genes may have arisen only within the land plant lineage. Alternatively, these gene sequences may have arisen by convergent evolution, which also probably explains the origin of a biosynthetic pathway for a range of flavonoids in the grape endophytic fungal genus Alternaria (Bilska et al., 2018; Lu et al., 2020, 2021), as the separation of the fungi and the algae/plant ancestors is thought to have been a relatively ancient event, prior to the divergence of fungi and animals (Burki et al., 2020). Convergent evolution has also given rise to the production of the red betacyanin pigments in both fungi and land plants, discussed in more detail later.
The stress-induced compound mixtures in Zygnematophyceae species may absorb in both UVB and PAR wavelengths, and pigment production can be induced by UVB radiation exposure and/or increased PAR, depending on the species (Aigner et al., 2013; Holzinger and Pichrtová, 2016). Thus, assigning cellular function is difficult, and physiological data are limited. Moreover, there are no direct comparisons of red vs. non-red near-isogenic lines. However, morphs of the high-alpine species Zygogonium ericetorum producing purple phenolic pigments had better photosynthetic performance than green morphs (Aigner et al., 2013). The red/purple morphs were dominant in the top layers of algal ‘mats’ and may help shade the green morphs underneath, which are better adapted to resist desiccation (Aigner et al., 2013). Overall, the data suggest that different Zygnematophycean lineages have evolved different inducible biosynthetic pathways for red vacuolar-located pigments, comparable in many respects with the evolutionary pattern for red pigments in land plants.
Recently, the production of extracellular photoprotective pigments ranging from blackish-violet to red-brown in colour, of undefined structure, has been reported for terrestrial Zygnematophyceae species in natural environments (Busch and Hess, 2022). In a laboratory experiment, there was directional secretion of red pigment-containing mucilage towards the area of the cell exposed to UVB radiation. The pigmented mucilage was able to absorb in the UVB spectrum, although it could not be determined whether this was specifically from the red pigment or from accompanying unidentified compounds. Pigmentation was not induced in response to additional PAR generated from light-emitting diodes (LEDs); therefore, it is uncertain as to whether the ability to absorb in the visible/PAR spectrum has physiological relevance. Some cyanobacteria also produce yellowish-brown pigments in response to abiotic stresses such as UVB radiation, excess PAR, desiccation and salinity, most notably the indole-alkaloid scytonemin that accumulates in extracellular polysaccharides (Storme et al., 2015; Ferreira and Garcia-Pichel, 2016). Scytonemin is able to absorb both PAR and UVB radiation, and has been widely studied in relation to UVB tolerance. As with the extracellular Zygnematophyceae pigment, these can also be produced in a directional manner towards the light stress, presenting a further example of convergent evolution for photoprotective pigmentation (Storme et al., 2015; Busch and Hess, 2022).
Flavonoid red pigments
The main red pigments of most land plants are anthocyanins (Figs 5–8). Anthocyanins are formed in a branch of the flavonoid pathway (Fig. 3), which has been reported from all land plant groups except hornworts. Flavonoids have a C6–C3–C6 core with two aromatic rings (named A- and B-rings) linked by a heterocyclic ring (C). The flavonoids are sub-divided into various classes based on the pattern of oxygenation of the core structure, with additional hydroxylation and/or methylation contributing to different core structures within each class. Classes commonly associated with tolerance to abiotic stress include the typically colourless flavones and flavonols, and the pigmented anthocyanins, 3-deoxyanthocyanins, auronidins and sphagnorubins. When considering land plants as a whole, anthocyanins are produced in a plethora of structures, in almost every type of plant organ, and in response to many different developmental and environmental signals. The variety of anthocyanin production in plants has given rise to a corresponding variety of theories on function, which include photomodulation, thermoregulation, anti-pathogen activity, defence against herbivores, sinks for excess carbon, osmoregulation, metal chelation and communication to pollen and seed dispersal agents.
Fig. 5.
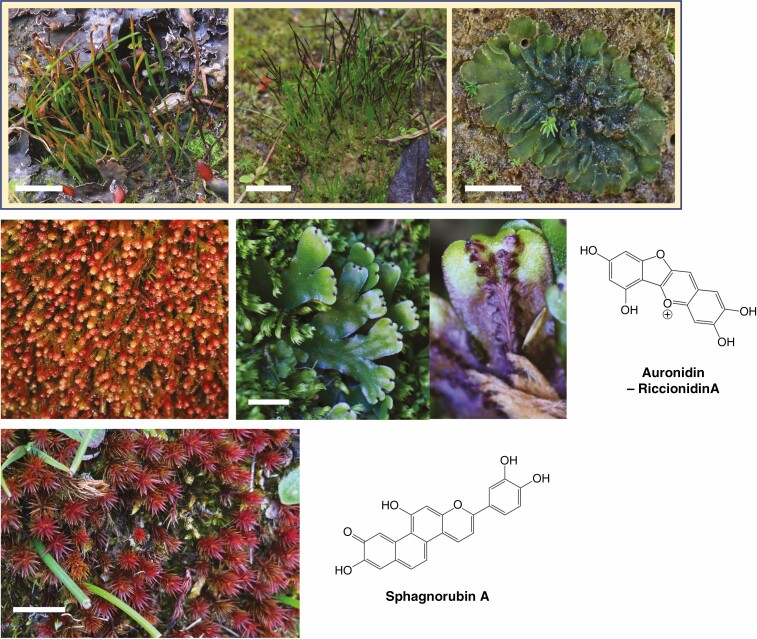
Examples of vegetative red pigmentation in non-tracheophyte plants. Example species are shown for hornworts (top row: from left to right; Phaeoceros carolinianus, Anthoceros laminifer and Phaeomegaceros hirticalyx), liverworts (middle row: Isotachis spp. on left and Marchantia foliacea on right) and mosses (bottom row: Polytrichum juniperinum/Juniper haircap moss) alongside structures of red pigments that are produced. The specific plant species shown may produce variations of the example core structure. No red pigment has been reported for hornworts. Scale bars represent 1 cm. All photographs by the authors.
Fig. 8.
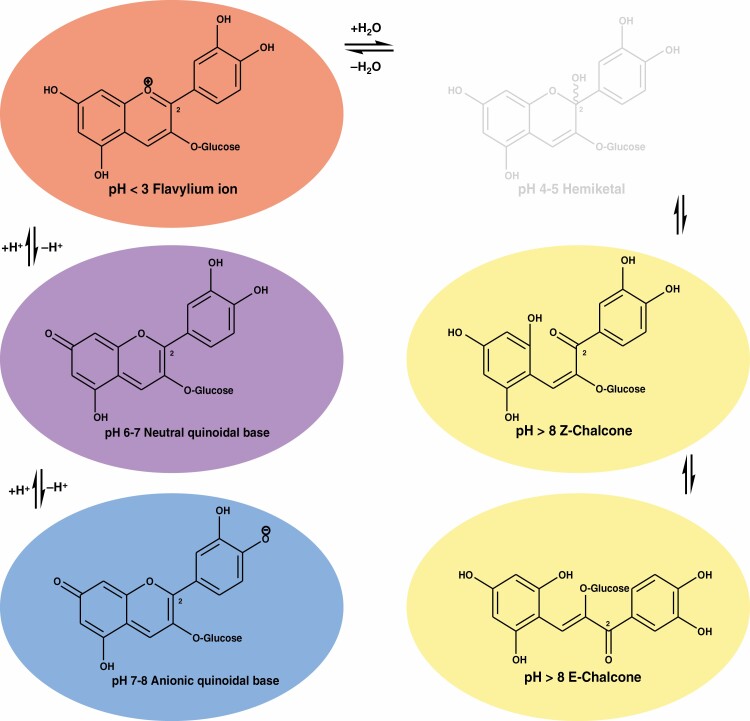
Structural modification and associated colours of the anthocyanin cyanidin-3-O-glucoside as a function of pH and hydration. Colour change from strong acid to base is shown, together with the causal shifts in chemical form.
The vast majority of anthocyanins are based around six core structures – anthocyanidin aglycones – which differ in the number and position of hydroxyl groups and whether those groups are methylated. The two major sub-groups are the ‘typical’ anthocyanins (3-hydroxyanthocyanins) and the 3-deoxyanthocyanins (Fig. 6). In this review, and commonly in the literature, unless otherwise specified, the term ‘anthocyanin’ refers to 3-hydroxyanthocyanins. A key feature of these is the number of hydroxyl groups on the B-ring, with 3ʹ-, 3ʹ,4ʹ- or 3ʹ,4ʹ,5ʹ-hydroxylation distinguishing pelargonidin-, cyanidin- and delphinidin-based anthocyanins, respectively. Extensive secondary modifications of the core anthocyanidin structure are known, principally glycosylation with a range of hexose moieties and secondary aromatic and aliphatic acylation of the carbohydrates. To date, around 800 different anthocyanin structures have been reported (Andersen and Jordheim, 2006; Berland and Andersen, 2021). Both the anthocyanidin aglycone structure and the patterns of secondary modifications influence the resultant colour of the plant tissue. Some of the structural modifications provide anthocyanins that can generate the colours preferred by specific pollinators, such as the highly modified delphinidin anthocyanins that are common to most blue flowers (Andersen and Jordheim, 2006; Yoshida et al., 2009). Whether the structural variations are important for stress tolerance properties is uncertain. The great majority of anthocyanins reported for vegetative tissues of vascular plants are cyanidin based, and confer red colour to the tissue. Similarly, in ferns, the 3-deoxyanthocyanins characterized in vegetative tissues are principally the 3ʹ,4ʹ-hydroxylated luteolinidin (Cohen et al., 2002b; Andersen and Jordheim, 2006).
Fig. 6.
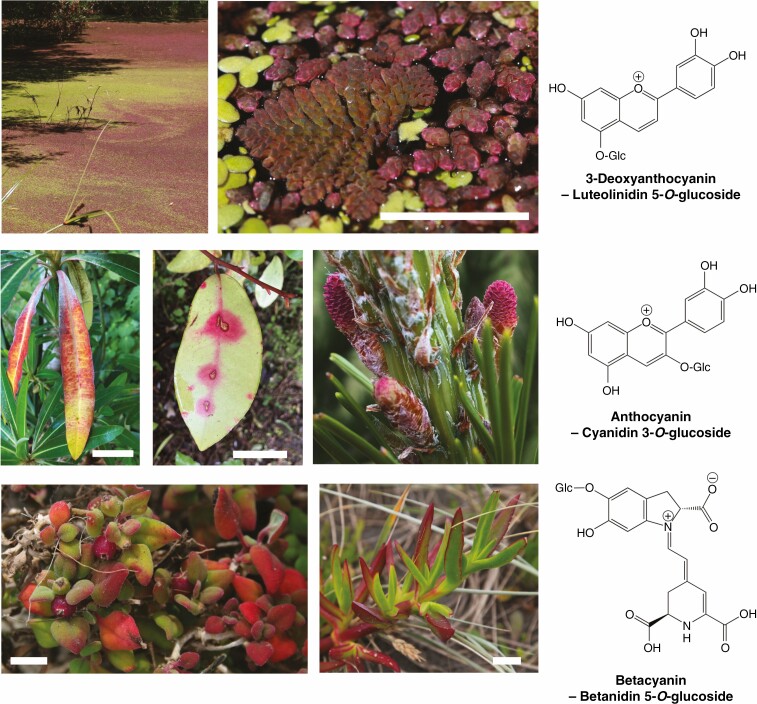
Examples of vegetative red pigmentation in vascular plants. Top row: the fern Azolla covering a pond in full sunlight and showing 3-deoxyanthocyanin production. The close-up image shows Azolla pinnata – triangular, regularly branched plant to the left of the image –and Azolla rubra, mixed with the green angiosperm Lemna minor (duckweed). Middle row: the angiosperms Euphorbia mellifera (Canary spurge/honey, left) and Pseudowintera colorata (centre), with pathogen/pest-induced anthocyanin production. Also shown are the red reproductive cones of the gymnosperm Pinus radiata (right). Bottom row: Tetragonia trigyna (left) and Carpobrotus spp. (right, ice plant, probably C. edulis, although hybrids can form with C. chilensis, which was co-occurring). The specific plant species shown may produce variations of the example core structure (such as glycosylation or other modifications). Scale bars represent 2.5 cm except for Azolla, where it is 1 cm. All photographs by the authors except P. radiata (Lorelle Phillips, Scion, New Zealand).
The 3-deoxyanthocyanins occur in only some angiosperm taxa, where they can provide bright orange flower colours and/or plant defence compounds (Winefield et al., 2005; Liu et al., 2010; Kariyat et al., 2019; Xiong et al., 2019). Reports of 3-deoxyanthocyanins from ferns or angiosperms are nearly all of simple glycosides, although there are a few reports of acylated compounds (Andersen and Jordheim, 2006). Bright red pigmentation from 3-deoxyanthocyanins is characteristic of the water fern Azolla (Cohen et al., 2002a, b). In addition to having a physiological in planta function, the pigments may also be part of the signalling pathway to the symbiotic cyanobacterium Nostoc (Cohen et al., 2002b). The 3-deoxyanthocyanins have also been reported to be present in some mosses (Andersen and Jordheim, 2006). The importance of these types of 3-deoxyanthocyanins to moss pigmentation is unclear, as mosses also produce another group of red pigments – the sphagnorubins, which are flavonoid based. Sphagnorubins have two additional aromatic rings connected to the A-ring (Fig. 5). As sphagnorubins contain a 3-deoxyanthocyanin component to their structure, it is possible that they are derived from 3-deoxyanthocyanins through the action of additional biosynthetic steps absent in other land plants. However, this is an assumption until the genetics of the sphagnorubin biosynthetic pathway are revealed. Phylogenetic analysis has suggested that mosses lack some of the biosynthetic genes required in angiosperms for 3-deoxyanthocyanin production (Piatkowski et al., 2020). Until recently, it was assumed the red flavonoid pigments of liverworts, as typified by riccionidin A (Fig. 5) were anthocyanins. However, Berland et al. (2019) demonstrated that the red pigments of liverworts are biosynthesized from a previously unreported branch of the flavonoid pathway. As this pathway proceeds via aurones, they named the pigments auronidins.
Sphagnorubins have been reported only as cell wall-bound compounds in planta. They are assumed to accumulate as aglycones, without secondary modifications. In contrast to the common anthocyanins, sphagnorubins are not soluble in water. Similarly the great majority of reports of auronidins also describe them as cell wall-bound compounds, presumed to be aglycones. A neohesperidose of auronidin (a glucose attached to a rhamnose that is attached to the auronidin) has been identified, although this was from a transgenic line with greatly increased auronidin biosynthesis (Berland et al., 2019).
Anthocyanins are by far the most common red pigments produced in the majority of gymnosperms and angiosperms. Common anthocyanins have been extensively characterized for the shifts in chemical form that occur, both in vitro and in planta, in response to the local hydration environment of the molecule and the effect of pH (Andersen and Jordheim, 2006; Alejo-Armijo et al., 2020). They convert between flavylium cation, quinoidal base, hemiketal, and cis- and trans-chalcone forms that differ in the perceived colour and pigment stability. Under the common in vitro conditions studied, the flavylium cation is red, the quinoidal base is purple and the mono-cationic quinoidal base is blue (Fig. 8). More recently, the in vitro chemistry of anthocyanins, 3-deoxyanthocyanins and auronidins (riccionidin A aglycone, classified as a furanoflavylium cation) have been compared for insights on what advantages each pigment could offer in planta (Alejo-Armijo et al., 2020; Pina et al., 2021). All three pigment types can undergo a similar sequence of chemical transformations, involving proton transfer, hydration, tautomerization and cis–trans isomerization. However, there are significant differences in kinetics and thermodynamics, as well as the resultant colours. Notably, the rates for interconversion between the auronidin species are dramatically slower. Sphagnorubin C has recently been found to behave in vitro as a two-component colour system between the reddish flavylium cationic and the yellow trans-chalcone forms, with the hemiketal and cis-chalcone forms appearing only transiently under equilibrium conditions (Berland and Andersen, 2021). The lack of the hydroxyl, and associated glycosyl, groups at the C-3 position in 3-deoxyanthocyanidins and sphagnorubins provides significantly increased stability against hydrophilic attack compared with 3-hydroxyanthocyanins (Berland and Andersen, 2021; Pina et al., 2021).
There are few studies on the vegetative production of anthocyanins in response to stress in gymnosperms, which could reflect its being a less common phenomenon. Anthocyanins are common in gymnosperm reproductive tissues, including the fleshy arils of species such as yew (Taxus baccata), fleshy false arils in species such as several of the Podocarpaceae of New Zealand, and seed cones of many conifer species (Rudall, 2020). Anthocyanins are generally produced during the earlier stages of cone development (Fig. 6). Amounts have been found to increase under environmental conditions that can generate more PAR-related stress, such as increasing altitude or periods of bright days and cold nights (Rudall, 2020). Theories for the anthocyanin function in cones are similar to those for anthocyanins in vegetative tissues – photomodulation, thermoregulation or defence against herbivores.
Evolution of the biosynthetic pathway for red flavonoid pigments
The sequencing of genomes from bryophytes and lycophytes, and the identification of the auronidin branch, has contributed to clearer proposals for how the flavonoid pathway evolved in land plants. The core phenylpropanoid pathway of PHENYLALANINE AMMONIA-LYASE (PAL), CINNAMATE 4-HYDROXYLASE (C4H) and 4-COUMAROYL-CoA LIGASE (4CL) that converts phenylalanine to the flavonoid precursor p-coumaroyl-CoA is conserved across land plants. The first committed flavonoid biosynthetic step is carried out by CHALCONE SYNTHASE (CHS), a POLYKETIDE SYNTHASE (PKS) Type III enzyme (Kim et al., 2013; Morita et al., 2019; Yonekura-Sakakibara et al., 2019; Naake et al., 2021). PKS genes with high sequence similarity to CHS are present in all land plant genomes examined to date (Yonekura-Sakakibara et al., 2019; Piatkowski et al., 2020; Davies et al., 2021; Naake et al., 2021). It is known for all major land plant groups, with the exception of hornworts, that at least some of these PKS genes encode CHS. The Anthoceros hornwort genomes (F.W. Li et al., 2020; Zhang et al., 2020) do contain PKS sequences (Davies et al., 2021), but they have yet to be examined by either assay of the encoded enzymatic activities or loss-of-function mutations. Analyses of PKS sequences in liverworts (Yu et al., 2018) and mosses (Kim et al., 2013) have identified authentic CHS genes but also other genes encoding different PKS activities. CHS activity cannot be assigned with certainty by sequence similarity alone, as minor alterations to the active site can switch the catalytic function. There are >20 different PKS enzymes identified to date in plants, and a few amino acid substitutions have been demonstrated to change activities from CHS to activities such as ACRIDONE SYNTHASE or STILBENE SYNTHASE (Morita et al., 2019; Naake et al., 2021). Thus, PKS sequences frequently form phylogenetic clades on a species, rather than a functional, basis (Kim et al., 2013; Davies et al., 2021).
Auronidins are red flavonoid pigments synthesized in a pathway branch that proceeds from chalcones via the yellow aurone intermediates (Fig. 2). It is thought that all other flavonoid pigments proceed via the conversion of chalcones to flavanones. This can occur spontaneously in vitro, but in planta the enzyme CHALCONE ISOMERASE (CHI) is generally required for significant flavanone formation. This is evidenced by the marked reduction or loss of flavanone-derived flavonoids in chi mutants in bryophyte (Clayton et al., 2018) or flowering plant species (Jiang et al., 2015). However, there are important open questions regarding CHI and the presence/absence of CHI genes in the bryophytes. As mentioned, liverworts have CHI genes and the loss-of-function mutants in Marchantia polymorpha lose production of flavanone-derived flavonoids. The published hornwort genome sequences (Anthoceros spp.) lack CHI genes (Davies et al. 2020, and the authors’ unpublished analyses). Thus, this is a possible basis for the lack of flavonoids in hornworts. However, moss genome sequences also lack CHI genes (Cheng et al., 2018), but mosses produce abundant flavonoids. A proposal we consider worthy of investigation is that mosses have evolved an alternative mechanism for conversion of chalcones to flavanones but hornworts have not.
Why hornworts and mosses lack CHI genes is not clear. Both contain the related CHALCONE ISOMERASE-LIKE (CHIL) genes, which share with CHI a common evolutionary origin from genes encoding fatty acid-binding proteins. Additionally, the intron/exon structure of CHIL, including the intron that interrupts the starting ATG, is conserved between all the bryophytes and arabidopsis (the authors’ unpublished analyses), as is the intron/exon structure of CHI between M. polymorpha and arabidopsis (Bowman et al., 2017). If the proposed monophyletic grouping of bryophytes is accepted, this suggests that both CHI and CHIL genes were present in the last common ancestor of extant land plants but CHI was lost during the evolution of mosses and hornworts. CHIL is a non-catalytic protein that can promote the production of various groups of phenylpropanoids, including flavonoids. In species examined to date, it can bind to CHS to direct the potentially promiscuous CHS activity towards formation of chalcones (Waki et al., 2020; Dastmalchi, 2021). There are loss-of-function chil mutants in both angiosperms (Morita et al., 2014) and liverworts (Clayton et al., 2018; Berland et al., 2019) that have greatly reduced flavonoid production. CHIL may also interact with other enzymes. In hops, CHIL promotes activity of both CHS and an aromatic prenyltransferase (Ban et al., 2018). In arabidopsis, it is proposed to promote flavonoid biosynthesis through interaction with CHS and CHI (Jiang et al., 2015). CHIL is unlikely to be the basis of flavonoid formation in the absence of CHI, as moss CHIL genes assayed in vitro do not have CHI activity (Cheng et al., 2018).
Flavanones are the starting point for pathway branches to 3-deoxyanthocyanins, 3-hydroxyanthocyanins, flavones and flavonols. Flavones are produced directly from flavanones by a range of enzymes (Yonekura-Sakakibara et al., 2019; Davies et al., 2020; D.D. Li et al., 2020). In angiosperms, 3-deoxyanthocyanidins are formed by FLAVANONE 4-REDUCTASE (FNR) converting flavanones to flavan-4-ols, which are substrates for ANTHOCYANIDIN SYNTHASE (ANS) and then UDP-GLYCOSIDE:FLAVONOID 5-O-GLYCOSYLTRANSFERASE (UF5GT) (Winefield et al., 2005; Shih et al., 2006; Ibraheem et al., 2015). Compared with 3-deoxyanthocyanins and flavones, the biosynthetic sequence to 3-hydroxyanthocyanins and flavonols includes an additional enzyme step following CHI: FLAVANONE 3-HYDROXYLASE (F3H), which makes dihydroflavonols (Fig. 3). The subsequent steps for 3-hydroxyanthocyanins are analogous to those for 3-deoxyanthocyanins: DIHYDROFLAVONOL 4-REDUCTASE (DFR), ANS and UDP-GLYCOSIDE:FLAVONOID 3-O-GLYCOSYLTRANSFERASE (UF3GT). Liverworts lack F3H genes, and do not produce either 3-hydroxyanthocyanins or flavonols (D.D. Li et al., 2020). F3H genes are present in moss genomes, and they produce flavonols. Despite having F3H, mosses do not produce 3-hydroxyanthocyanins. The reasons for this are unknown. Nor is the biosynthetic pathway to the red sphagnorubin pigments of mosses known.
Mosses, ferns and lycophytes produce 3-deoxyanthocyanins, but liverworts do not. However, neither the basis for their absence in liverworts nor the genes involved in their production in mosses, lycophytes or ferns has been established. The lack of an FNR gene has been suggested to be the basis for the absence of 3-deoxyanthocyanins in some plant groups (Piatkowski et al., 2020). Yet in the angiosperm species examined for 3-deoxyanthocyanin biosynthetic pathways to date, FNR and DFR are a single dual-functional enzyme. The FNR/DFR of Sinningia cardinalis, which has 3-deoxyanthocyanin-pigmented flowers, has similar activity to flavanones or dihydroflavonols (Winefield et al., 2005), and loss of function of the A1 FNR/DFR gene in maize causes loss of production of both types of anthocyanin (Halbwirth et al., 2003; Morohashi et al., 2012). Dual-function FNR/DFR genes have also been reported for Sorghum (Liu et al., 2010), although a gene encoding a protein with only FNR activity has been found, and mutations in that gene caused loss of 3-deoxyanthocyanin production (Kawahigashi et al., 2016).
All land plant groups (except hornworts) produce some flavonoids with ortho-hydroxylation of the B-ring (3ʹ4ʹ-hydroxylation), and those flavonoids associated with UVB tolerance or vegetative red pigmentation commonly have this hydroxylation pattern. In all characterized cases, this is catalysed by FLAVONOID 3ʹ-HYDROXYLASE (F3ʹH, the cytochrome P450 clade CYP75B), which accepts varied 3ʹ-hydroylated substrates. Some angiosperms also have the related enzyme FLAVONOID 3ʹ,5ʹ-HYDROXYLASE (F3ʹ5ʹH, CYP75A), and so produce flavonoids with 3ʹ4ʹ5ʹ-hydroxylation of the B-ring, most notably delphinidin-derived anthocyanins. Piatkowski et al. (2020) identified a F3ʹH/F3ʹ5ʹH orthogroup basal to land plants and charophyte green algae but absent from seedless plants. They also discuss the challenges of using sequence-based approaches for this gene clade. Indeed, despite several phylogenetics-based reports of F3ʹ5ʹH-like sequences in seedless plants, the only F3ʹ5ʹH genes verified by recombinant protein assays to date are from angiosperms. Zhang et al. (2020) found that the CYP71 subfamily was highly expanded in Anthoceros angustus, consisting of 56 gene models, including 32 genes encoding F3ʹH or F3ʹ5ʹH. However, the accompanying phylogenetic analysis did not place any sequences in the CYP75 clade, and our analysis also indicates the absence of strong F3ʹH or F3ʹ5ʹH candidates in hornworts. The analysis of de Vries et al. (2021) placed 72 Anthoceros CYP sequences within a larger clade containing, for example, arabidopsis sequences of CYP71, CYP75 and CYP76 (and a sub-clade of characterized bryophyte CYP98A CINNAMATE 3-HYDROXYLASE sequences). If bryophytes do lack F3ʹH genes, they may have evolved alternative hydroxylation activities, perhaps acting on the phenylpropanoid precursors before they become substrates for CHS. The majority of flavonoids are modified by the addition of one or more hexose groups (glycosylation), and may also have other secondary modifications through acylation, methylation, prenylation and sulfation. Patterns of flavonoid secondary modification vary among and within the major plant groups (Alseekh et al., 2020), but the evolutionary basis for this variation is generally unresolved.
The diversity of flavonoids across land plants makes it difficult to suggest what the flavonoid biosynthetic gene set may have been in the last common ancestor of extant land plants. Phylogenetics-based approaches to identify orthologue groups can help predict the shared genes among land plants that may represent the minimal set present in the last common ancestor, as comprehensively presented recently for flavonoid biosynthesis by Piatkowski et al. (2020). However, given the presence of genes within the flavonoid pathway with similar sequences but differing functions, functional characterization of the candidates revealed by phylogenetics is then often necessary. For example, phylogenetics identified F3H in liverworts but not mosses (Piatkowski et al., 2020), but assays of recombinant proteins suggest the reciprocal situation (D.D. Li et al., 2020). With regard to biosynthetic gene occurrence for red flavonoid pigments: hornworts possibly lack any flavonoids; non-tracheophyte plants lack 3-hydroxyanthocyanins; 3-deoxyanthocyanins have a sporadic distribution; and auronidins and sphagnorubins may be limited to liverworts and mosses, respectively. This pattern probably represents a mix of gains and losses of pathway branches since the last common ancestor. There are relatively few flavonoid biosynthesis components confirmed as common across all land plants, these being CHIL and PKS and the core phenylpropanoid pathway of PAL, C4H and 4CL. CHS and UDP-glycosyltransferase activities have been confirmed for all groups except hornworts to date. If it is assumed (based on the monophyly of bryophytes) that hornworts have lost the ability to make flavonoids, then it is probable that the last common ancestor had the genes required for formation of flavones, which could have been part of UVB tolerance. It is more difficult to draw conclusions for red pigmentation, except that the production of 3-hydroxyanthocyanins is a later evolutionary innovation (as is betalain biosynthesis). The evolutionary driver to have stress-related red pigmentation seems very strong, given its occurrence across many algae and nearly all land plants. As biosynthetic routes to red are present in almost all land plants, the last common ancestor of land plants may have had red pigmentation. However, there is insufficient information to propose which red pigment biosynthetic pathway may have been present. If the 3-deoxyanthocyanin biosynthetic genes of mosses and lycophytes are found to have a common genetic origin with those of angiosperms, this would support an early evolutionary origin of that pigment group. However, recombinant assays using the F3H genes of Psychomitrella patens (a moss) and Selaginella moellendorffii (a lycophyte) showed that they encode enzymes with dual FNSI/F3H activities (D.D. Li et al., 2020), suggesting that the seed plant F3H and non-seed plant FNSI/F3H genes are the result of independent evolutionary events from a common ancestral 2-oxoglutarate-dependent dioxygenase gene with a different function.
Blue, yellow and orange flavonoid pigments in relation to stress responses
Anthocyanins can confer blue pigmentation, commonly based on delphinidin production (Yoshida et al., 2009). There are also bright yellow flavonoid pigments – most notably aurones. However, neither delphinidin nor aurone production is a commonly characterized response to stress. The anthocyanins produced in response to stress are usually cyanidin based, rather than pelargonidin-, peonidin- (methylated cyanidin) or delphinidin-derived anthocyanins. The evidence suggests that biosynthesis of 3-hydroxyanthocyanin alternatives to cyanidin may have arisen only for plant communication with the beneficial biotic environment, specifically for pollination and seed dispersal. The simple delphinidin-based anthocyanins do not generate blue colours at physiological pH, and a great many adaptations that generate blue anthocyanin-based pigmentation have been characterized. These include anthocyanin secondary modifications and co-pigmentation changes that alter the local environment of the pigment, and genes that directly increase the vacuolar pH (Yoshida et al., 2009; Houghton et al., 2021). However, these are all associated with formation of blue flower or fruit colours rather than stress responses.
The auronidin aglycone can form colours from yellow through to purple in vitro, but only at about pH 1.0 and pH 12.0, respectively (Alejo-Armijo et al., 2020), and have only been reported as red compounds in planta. No blue flower colours from 3-deoxyanthocyanins have been reported from nature and, even in vitro at more basic pH values, luteolinidin does not generate blue colours (Alejo-Armijo et al., 2020). The retention of 3-deoxyanthocyanin biosynthesis in some angiosperms despite the evolution of the 3-hydroxyanthocyanin branch suggests some distinct functions. In some species, they confer bright orange/scarlet colours that favour bird-mediated pollination (Winefield et al., 2005; Xiong et al., 2019). Whether they are also the anthocyanins produced in response to abiotic stress in these species has not been examined in detail (Xiong et al., 2019). However, various 3-deoxyanthocyanin compounds are produced in vegetative tissues as phytoalexins, notably in the Andropogoneae grasses such as sorghum and maize (Liu et al., 2010; Kariyat et al., 2019; Xiong et al., 2019). They also contribute to feeding deterrence against snails and tadpoles in the fern Azolla, as well as promoting symbiosis with the nitrogen-fixing cyanobacterium Nostoc (Cohen et al., 2002a, b).
The yellow flavonoids – the chalcones, aurones and some substituted flavonols of infrequent occurrence (Boucherle et al., 2017) – have not been reported as stress-related pigments. Indeed, they are generally not reported as accumulating in significant amounts in non-reproductive tissues. The aurones are the most taxonomically widespread and brightest yellow of the flavonoid pigments. They can provide nectar guides to pollinators, but the reasons for production in non-floral structures such as liverwort sporophytes (Markham and Porter, 1978) are unknown. There is a similar knowledge gap for the function of yellow pigments in the next group of compounds we discuss – the betalains.
Betalains
Betalain pigments have limited distribution, with convergent evolution giving rise to their presence in only a few fungal and plant lineages (G. Li et al., 2019; Timoneda et al., 2019; Tossi et al., 2021). They are water-soluble nitrogen-containing compounds and, like anthocyanins, are stored in the vacuole. In plants, they have evolved to replace anthocyanins in several families within the core Caryophyllales (Brockington et al., 2011; Timoneda et al., 2019). The Caryophyllales taxa contain proportionally more species adapted to living in extreme (e.g. arid or saline) environments than reported for angiosperms overall, and these include many betalainic species (G. Li et al., 2019). The Caryophyllales are also known for the high frequency of occurrence of Crassulacean acid metabolism (CAM), an adaptation to periodic water supply, and C4 photosynthesis (Christin et al., 2015), as well as halophytism/Na+ hyperaccumulation (Flowers and Colmer, 2008; Moray et al., 2015; White et al., 2017). Indeed, salt tolerance may have evolved early in some betalain lineages and then been retained during species radiation (Moray et al., 2015). It is possible that betalain biosynthesis also evolved in response to the challenging environments that these Caryophyllales species occupy. There are several betalain-producing species that are generally familiar as food or ornamental crops, including beet/chard (Beta vulgaris), amaranth (Amaranthus caudatus) and quinoa (Chenopodium quinoa) of the Amaranthaceae; prickly pear (Opuntia spp) and dragon fruit (Hylocereus/Selenicereus spp.) of the Cactaceae; moss rose (Portulaca grandiflora, Portulacaceae); iceplants (various Aizoaceae spp.); and bougainvillea (Bougainvillea glabra) and four o’clock flower (Mirabilis jalapa) of the Nyctaginaceae.
The central chromophore of betalains is the pale yellow compound betalamic acid (BA), which is derived from tyrosine (Fig. 9). Although BA accumulates in some betalainic plants, it commonly is converted to red-violet betacyanin pigments through its condensation with cyclo-dihydroxyphenylalanine (cyclo-DOPA) (this may be BA aglycone or glucoside, or less commonly a decarboxylated form), and to yellow betaxanthin pigments when condensed with amino acids or amines. Betaxanthins and betacyanins have absorption maxima in the ranges 532–550 nm and 457–485 nm, respectively. The condensation reactions appear to be spontaneous rather than enzyme catalysed (Schliemann et al., 1999). Like anthocyanins, the core betacyanin structure is commonly modified by glycosylation and acylation. While the function of some secondary modifications is understood for anthocyanins, data for betacyanins are lacking. Unlike anthocyanins, glycosylation is not required for stable cellular accumulation of betalains, as in some Caryophyllales species and in some transgenics of anthocyanic species engineered to produce betalains the non-acylated aglycone betanidin and/or related isomers accumulate (Strack et al., 2003; Chung et al., 2015; Polturak et al., 2016, 2017; Grützner et al., 2021; Zhou et al., 2021). However, secondary modifications can alter the colour properties of betacyanin and may increase stability at varied pH.
Fig. 9.
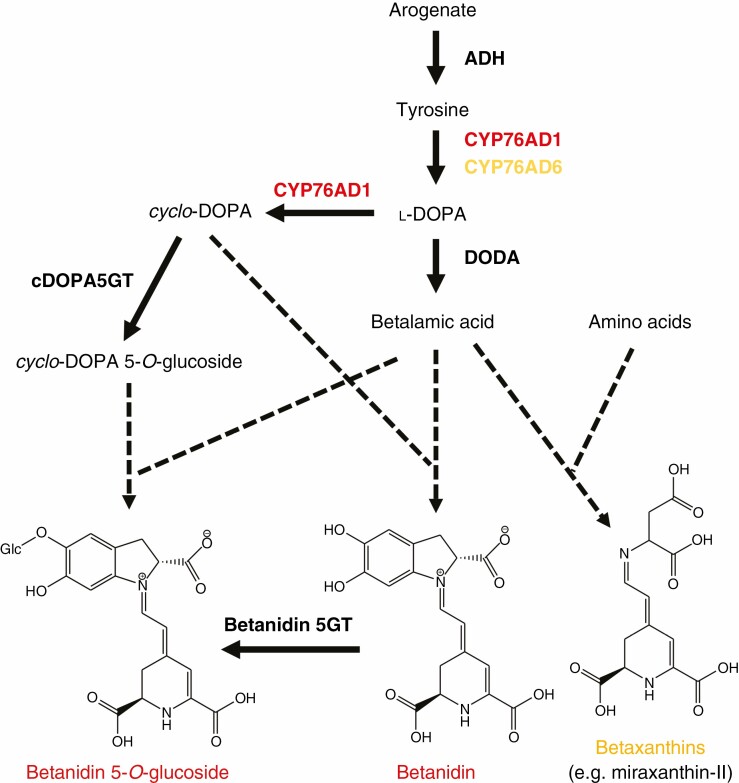
Schematic of the biosynthetic pathway to betacyanins and betaxanthins. Enzymes shown are the cytochrome P450 enzymes CYP76AD1 and CYP76AD6, DOPA 4,5-dioxygenase (DODA), cyclo-DOPA 5-O-glucosyltransferase (cDOPA5GT) and betanidin 5-O-glucosyltransferase (Betanidin 5GT). The condensation reactions to form betacyanin and betaxanthin pigments occur spontaneously (shown as dashed lines).
The core biosynthetic pathway is relatively simple (Fig. 9), involving only a few enzymatic steps (Vogt, 2002; Christinet et al., 2004; Sasaki et al., 2005; Hatlestad et al., 2012; Polturak et al., 2016; Sunnadeniya et al., 2016; Timoneda et al., 2019). l-DOPA is a key compound in the pathway, needed for the formation of both BA and cyclo-DOPA. In some plants, l-DOPA also condenses with BA to form the betaxanthin dopaxanthin. l-DOPA is derived from tyrosine through hydroxylation by CYP450 l-DOPA OXIDASE enzymes belonging to the CYP76AD clade (CYP76AD1/5/6/15). l-DOPA is converted to BA through an extradiol cleavage performed by l-DOPA 4,5-DIOXYGENASE (DODA). For betacyanin production, a CYP76AD enzyme (CYP76AD1/3) closely related to the l-DOPA OXIDASE is involved. It performs both the l-DOPA-forming reaction and the oxidation that converts l-DOPA to cyclo-DOPA. This dual functionality allows betacyanin and betaxanthin biosynthesis to be uncoupled so that either or both types of betalain can be produced in the same cell.
The biosynthetic enzymes are thought to have arisen from duplication of genes involved in other pathways and subsequently neofunctionalized for betalain biosynthesis. DODA is part of the LigB gene family, which is present in all land plant lineages. Phylogenetic, sequence and functional analyses suggest that an additional clade of LigB genes arose early in the evolution of the Caryophyllales order, and DODA evolved from within this additional clade (Christinet et al., 2004; Brockington et al., 2015; Chung et al., 2015; Bean et al., 2018). Some species are thought to have subsequently lost betalain biosynthesis and regained anthocyanin production; these have lost DODA genes (Brockington et al., 2015; Timoneda et al., 2019). Phylogenetic analysis indicates that the CYP76AD lineage underwent two gene duplication events within Caryophyllales, giving rise to three paralogous lineages that include the genes required for betalain biosynthesis (Brockington et al., 2015; Timoneda et al., 2019). Functional and phylogenetic data combined suggest that tyrosine hydroxylation alone was the ancestral function of the CYP76AD in the Caryophyllales lineage, and the ability to convert l-DOPA to cyclo-DOPA evolved later (Sunnadeniya et al., 2016). An additional deregulated version of AROGENATE DEHYDROGENASE that facilitates increased accumulation of the tyrosine precursor is thought to have arisen prior to the evolution of specific betalain biosynthetic enzymes (Lopez-Nieves et al., 2018; Timoneda et al., 2019). Some glycosylation enzymes are able to work on both flavonoid and betalain substrates, so the betalain enzymes were probably ‘co-opted’ from the flavonoid pathway (Vogt, 2002; Sasaki et al., 2005; Polturak et al., 2016; Timoneda et al., 2019).
Betalain production evolved in plant taxa that originally had anthocyanin production, and it is thought that betalain biosynthesis may have arisen multiple times (Brockington et al., 2011; Timoneda et al., 2019). The manner in which betalains arose to replace anthocyanins and the evolutionary drivers for it are unresolved, although a potential evolutionary path has been suggested recently (Timoneda et al., 2019). Betalain and anthocyanin production are mutually exclusive in extant taxa, although transgenics that can produce both types of pigment demonstrate that production of both together is not damaging to the cell (Polturak et al., 2017). Whether anthocyanin biosynthesis was lost before or after betalain biosynthesis arose, or whether there was a switch from one pathway to the other, is not known. As the R2R3MYB transcription factor genes that activate betalain biosynthesis are similar to those that regulate anthocyanin biosynthesis (Hatlestad et al., 2015), a mechanism is available for switching off one pathway and simultaneously activating the other. Betalainic species do retain some parts of the flavonoid pathway – such as for flavonol and proanthocyanidin biosynthesis. Thus, the genetic blocks preventing anthocyanin production are loss of function of a specific anthocyanin biosynthetic step and/or lack of transcriptional activation of the required genes.
ENVIRONMENTAL TRIGGERS AND PATTERNS OF RED PIGMENTATION
When considered from a general perspective, the environmental triggers for induction of red vegetative pigmentation are remarkably consistent among terrestrial algae and the many different groups of land plants. If species show stress-induced red pigmentation, then strong PAR in conjunction with a secondary stressor, such as cold, drought or nutrient imbalance, is commonly one of the triggering factors. In angiosperms, excess PAR, cold, drought, nutrient imbalance and pathogen or herbivore attack are all known inducing factors for both anthocyanin and betacyanin production. Indeed, the general patterns of production of anthocyanins and betacyanin are very similar with regard to both tissue localization and triggers for induction (Davies, 2015). This may, in part, reflect the co-option of the R2R3MYB transcription factor that originally regulated anthocyanin production to the regulation of betalain biosynthesis (Hatlestad et al., 2015). Fronds of the fern Azolla turn from green to red because of the production of large amounts of 3-deoxyanthocyanin in response to strong sunlight and temperature fluctuation (Fig. 6), as well as during interaction with microbial species (Cohen et al., 2002b). Similarly, many mosses, including Ceratodon purpureus and varied Sphagnum species, become intensely red pigmented by sphagnorubin when experiencing abiotic stress from excess PAR, dehydration, nutrient imbalance, temperature below 5 °C or pathogen attack (Gerdol et al., 1998; Bonnett et al., 2010; Manninen et al., 2011). Liverwort species may produce red pigmentation (presumably auronidin) in response to excess PAR, nutrient imbalance or during interaction with symbiotic or pathogenic microbes (Hooijmaijers and Gould, 2007; Humphreys et al., 2010; Albert et al., 2018; Kubo et al., 2018; Carella et al., 2019; Davies et al., 2020). Moreover, within terrestrial algae, excess PAR can induce the production of varied phenolic red pigments in a range of Zygnematophyceae species, and red carotenoids in some Chlorophyceae species. Under saturating PAR conditions, phenolic pigments of glacier-living algae are produced as the predominant light-absorbing compounds, at >10-fold the concentrations of chlorophyll a (Williamson et al., 2020). Thus, despite the type of red pigmentation differing greatly in both structure (e.g. anthocyanins, auronidins, sphagnorubins and carotenoids) and cellular localization (vacuole, cell wall, lipid bodies or external mucilage), the biosynthetic pathways are responding to the same general stressors.
It is much more common for flavones or flavonols rather than anthocyanins to be induced by UVB (Ferreyra et al., 2021). Simple anthocyanin-glycosides do absorb in the UVB region of the spectrum (Fig. 7), but with lower molar absorption coefficients than flavone- or flavonol-glycosides. Aromatic acylation of anthocyanins significantly adds to the ability to absorb UVB radiation (Giusti et al., 1999). However, most of the >300 different acylated anthocyanins reported are from floral tissues, and they have not been reported as UVB tolerance compounds in leaves or vegetative tissue (Andersen and Jordheim, 2006). The liverwort Cephaloziella varians becomes intensely red pigmented, presumably from auronidin, in response to UVB radiation in Antarctic conditions (Newsham et al., 2005). This is in contrast to the UVB induction of flavones in M. polymorpha (Clayton et al., 2018) or the common presence of cell wall-located colourless UVB-absorbing compounds in bryophytes (Clarke and Robinson, 2008; Soriano et al., 2018a, b). Overall, induction of colourless UVB-absorbing flavonoids or other compounds rather than red pigments seems to predominate as a response to UVB exposure.
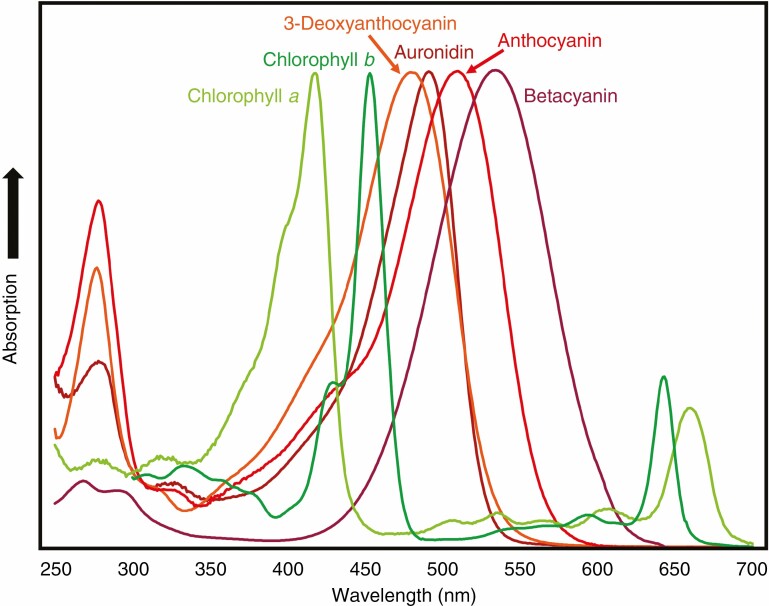
Fig. 7.
Comparative absorption spectra of chlorophyll a, chlorophyll b and different red pigments of land plants. Example spectra are shown for an auronidin (riccionidin A), a 3-deoxyanthocyanin (luteolinidin), a 3-hydroxyanthocyanin (kuromanin; cyanidin-O-glycoside) and a betalain (betanin; betanidin-5-O-glucoside).
As with many other aspects of pigmentation, data on environmental triggers for production, in planta localization and the specific pigment structures produced are abundant for angiosperms but lacking for other land plant lineages. For example, mosses are known to be able to produce both 3-deoxyanthocyanins and sphagnorubins, yet there is little knowledge about the signals activating the different biosynthetic pathways, or indeed if both, one or neither pathway is present in different branches of the moss phylogenetic tree.
Although general patterns and triggers of induction may be shared, when pigmentation is compared at a species level many differences emerge. Depending on the angiosperm species examined, anthocyanins may be prevalent in emerging foliage, senescing foliage, in areas of pest damage, following pathogen attack, in stems, in trichomes or in roots, tubers or other below-ground organs. Even when produced in the same tissues at similar times, the localization within tissues can vary, such as being only in the adaxial epidermis, being limited to sub-epidermal mesophyll cells or being only in the abaxial epidermis. Anthocyanins are also abundantly produced in trichomes (Jordheim et al., 2016) and stems (Cooney et al., 2015) of some species. Studies on environmental triggers for, and tissue localization of, betacyanin production are fewer than for anthocyanins. However, as mentioned earlier, the studies suggest that betacyanins have similar tissue distributions and inductive signals to those reported for anthocyanins (Lee and Collins, 2001; Mosco, 2012; Chung et al., 2015). This includes production in subterranean organs of some species, such as the tubers of the food crops beet (Beta vulgaris) and ulluco (Ullucus tuberosus), and in trichomes (including pigmented bladder cells that accumulate sodium and chloride ions in high concentrations; Dassanayake and Larkin, 2017). However, as with anthocyanins, varied triggers are reported depending on the species, including light quantity and quality, UVB radiation, elevated salinity (200 mmol L− 1), ROS and low temperature (below 10 °C), nitrogen or phosphorus deficiency, metal ion stress and plant senescence (Ibdah et al., 2002; Wang and Liu, 2007; Wang et al., 2007; Hayakawa and Agarie, 2010; Jain and Gould, 2015a, b; Jain et al., 2015; Klein et al., 2018; Parida et al., 2018; Henry et al., 2019; Izzo et al., 2019; reviewed in Davies et al., 2018; G. Li et al., 2019; Tossi et al., 2021). Although there are no published studies for stress functions in relation to autumnal senescence, abundant red pigmentation from betacyanins is a widely reported autumnal phenomenon of Salicornia species in saline environments (Cárdenas-Pérez et al., 2021). Betaxanthins often have different accumulation patterns from those of betacyanins. However, they may be produced in the same cells and can alter the observed colour (Chung et al., 2015). When comparing the pigments of vascular and non-tracheophyte plants, there is a marked difference in sub-cellular localization: anthocyanins and betacyanins are almost always located in the vacuoles while auronidins and sphagnorubins are cell wall bound.
With such varied patterns of production, it can be difficult to draw general conclusions that relate pigment localization to function. For anthocyanins, this has contributed to many alternative proposals about function that have made it almost impossible to find a unified explanation for their evolution. Nevertheless, whether located in the mesophyll (Hughes and Smith, 2007; Hughes et al., 2014) or adaxial epidermis (Hughes et al., 2007; Landi et al., 2014), or present in the abaxial epidermis of understorey plants in shade conditions (Hughes et al., 2014), trichomes (Jordheim et al., 2016) or stems (Cooney et al., 2015), anthocyanins can provide effective photoprotective screens. This supports a photomodulation function. However, some localizations suggest that certain pigments have other primary functions, which can be illustrated by two examples. In Marchantia species, auronidins are commonly produced in the ventral scales on the underside of the plant that provide a barrier between the meristem and soil (Fig. 5; Davies et al., 2020). This is not suited to protecting against damaging PAR, but does fit an anti-pathogen function. In some halophytes, betalains are induced in the bladder cells, which facilitate salt secretion (Dassanayake and Larkin, 2017). Notably, red carotenoids are not reported as stress-induced pigments in land plants, despite the presence of the biosynthetic pathways and their demonstrated benefits as PAR stress-related compounds in algae. This indicates some advantage offered by production of red phenolic pigments beyond direct PAR screening, even if they vary in structural aspects. The varied production and sub-cellular localizations of the different phenolic pigments may reflect specific functions delivered by the individual pigment structures in addition to screening PAR, and we examine this in the final section of this review.
HOW DOES PIGMENT STRUCTURE RELATE TO FUNCTION?
Anthocyanin pigments are based on the flavylium cation that allows more flexibility in structure than other non-charged chemical species and, since structure relates directly to function, there are numerous benefits in having a system that can adapt to changing environmental stimuli. Nature uses the versatility of the positively charged anthocyanin core structure to give rise to an abundance of colours through various multi-state chemical species (Fig. 8). The thermodynamic and kinetic properties of the flavylium multi-states are dependent on the localized pH as well as substituent effects of functional groups that decorate the core structure (Pina et al., 2021). In addition to pH, light and temperature can modulate a range of reactions mediated by acid–base equilibria, hydration (particularly at the C2 position), tautomerism and isomerization (cis–trans) reactions. As such, these compounds can vary from colourless to yellows, reds, purples and blues. A similar effect has been observed in the positively charged auronidins that give rise to colours ranging from yellow to red to purple (Alejo-Armijo et al., 2020). However, at physiological pHs, the colour range is likely to be more restricted. Unlike the anthocyanins, the auronidins have no equivalent C2 position available for hydration, so their structures are less flexible. Furthermore, the auronidins are stabilized in vivo through a close association with the cell wall, the mechanics of which are yet to be fully resolved.
Since flavylium ions are susceptible to degradation, there are a range of reported mechanisms that plants use to stabilize or change pigment colour, including acylation (particularly with aromatic precursors) and hydrogen bonding (enhanced through glycosylation); ionic interactions (e.g. metal ions); and hydrophobic (e.g. in molecular stacking) effects. These changes in both electron density (due to substituent effects) and electrostatic interactions also affect the hue of colours observed. Furthermore, through co-pigmentation, the colour of anthocyanins can be further intensified and their structures stabilized through interactions with other molecules (Pina et al., 2021). Anthocyanins are relatively planar in shape and can form intramolecular stacks with similarly shaped molecules, including simple phenolics when co-localized. Stacking and other physical measures can lead to hyperchromic (increase in absorbance) and/or bathochromic (increase in wavelength) shifts in absorbance. As mentioned earlier, metal ion addition generally leads to bluer hues while complex interactions involving co-pigmentation lead to the supramolecular structures found in some blue flowers, e.g. cornflowers (Houghton et al., 2021). Undoubtedly some of these intense colours have developed to attract pollinators as part of an ever-evolving chemical ecology, but whether there are further roles beyond photoprotection has yet to be determined.
Information is comparatively sparse for other red pigments. Betalains maintain more consistent spectral absorbance over a greater pH range than anthocyanins (Stintzing and Carle, 2004), and, as mentioned earlier, 3-deoxyanthocyanidins are more stable against hydrophilic attack. Whether the higher stability of these pigments is important for their functions in planta has not been explored. However, it is interesting that CAM photosynthesis, which is prevalent in betalainic species, can involve marked changes in cellular pH, with the vacuolar sap of these assimilatory cells in CAM reaching pH 4 or lower (Winter and Smith, 2022). Betalains could have an advantage over anthocyanins for pigmentation at these acidic pHs. Anthocyanins would require extensive co-pigmentation or other cellular interactions for stability at these pHs, but that would not be required for betalains (for which co-pigmentation has not been reported). Betalains also have higher extinction coefficients than anthocyanins, meaning less pigment is required to generate the same depth of colour, and betaxanthins autofluoresce (Gandía-Herrero et al., 2005). As with many other aspects of non-anthocyanin pigments, the significance of these pigment characteristics has not been explored, although it is suggested that fluorescence is unlikely to be a signal in biocommunication (Iriel and Lagoria, 2010). Similarly, there are no data to confirm whether the structures of auronidins and sphagnorubins offer advantages for binding in the cell wall or forming phenolic polymers. Auronidin–riccionidin dimers have been isolated (termed riccionidin B) (Kunz et al., 1993), providing a basis for formation of a polymer that could provide a physical barrier against pathogens, replicating one of the functions of lignin in vascular plants. Auronidin in the cell walls of M. polymorpha does indeed reduce penetration by hyphae of Phytophthora palmivora (Carella et al., 2019). Antioxidant functions are often proposed as central to anthocyanin stress tolerance properties. However, the anthocyanins considered in these studies are water-soluble, vacuolar-located glycosides, while auronidins and sphagnorubins are generally cell wall-bound aglycones, again suggesting differing cellular functions.
IS THERE A COMMON CORE FUNCTION OF RED PIGMENTS?
The hypothesis that anthocyanins protect leaves of plants facing biotic or abiotic stressors dates back to Pringsheim (1879), and is now a widely accepted function for foliar anthocyanins (Landi et al., 2015, 2021; Gould et al., 2018; Renner and Zohner, 2019, 2020). A direct PAR screening function of red pigments may seem self-evident – after all, colourless compounds are common to the UVB stress response while red pigments that can absorb PAR wavelengths are induced by excessive visible light exposure. However, whether this is the only cellular mechanism(s) underlying the mitigating effects of red pigments, or indeed their most important, is yet to be elucidated. Most debate arises from two properties of anthocyanins (and most red pigments) that contribute to their ability to reduce both the incidence and the severity of photo-oxidative damage and may account for their capacity to assist with a plethora of environmental stress: (1) intercepting a portion of supernumerary photons that would otherwise strike the chloroplasts (Feild et al., 2001), the so-called sunscreen prerogative; and (2) quenching stress-triggered ROS, thereby acting as powerful antioxidants. There is an analogous debate on how phenolics provide UVB tolerance. Flavones, flavonols and hydroxycinnamic acids all absorb in the UVB section of the light spectrum, allowing for a direct screening function to protect systems such as PSII (Booij-James et al., 2000; Takahashi and Badger, 2011). However, it has been argued that the antioxidant function of flavonoids is of more importance in reduction of UVB-related damage (Agati et al., 2020; Landi et al., 2015; Ferreyra et al., 2021). Measuring screening or ROS-scavenging functions separately in planta is challenging, as reduction of PAR or UVB radiation by direct screening may reduce production of ROS. Moreover, various additional functions not relating to these two cellular properties are also proposed for anthocyanins and add to the debate – including leaf warming, microbial defence, anti-feedants and, notably for current functional theories, communication with the biotic environment.
A heated debate has occurred in the last few decades concerning the functions of anthocyanins in senescing autumn leaves, in which the ‘co-evolution’ hypothesis has been proposed as an alternative to the photoprotection hypothesis, and the arguments put forward in the debate are useful for examining general functional theories for red pigments. Briefly, while the photoprotection hypothesis considers anthocyanins as capable of screening excessive light and/or preventing formation of and scavenging stress-triggered ROS (Landi et al., 2015; Gould et al., 2018), the co-evolution hypothesis suggests that anthocyanins in senescing leaves provide an honest warning signal of defence against insects that migrate to trees, generating preference for green leaves with lower contents of defensive chemicals (Archetti et al., 2009). Lately, new data have renewed the debate (Renner and Zohner, 2019; Pena-Novas and Archetti, 2020,2021a; Agati et al., 2021; Hughes et al., 2022; Joly, 2022). A comprehensive analysis by Renner and Zohner (2019) of 711 deciduous woody species confirmed a primary photoprotective function for anthocyanins and reported that cyanic leaves occur more frequently in species inhabiting areas characterized by lower temperatures and higher solar irradiance during autumn/winter, and hence suffering more severe ‘light stress’ during leaf senescence. In response, Pena-Novas and Archetti (2020) argued against Renner and Zohner’s results and in favour of the co-evolution hypothesis. However, we consider that the arguments made against photoprotective functions raise outdated misconceptions of anthocyanins that are worth addressing point by point. We examine the specific arguments in the context of both the co-evolution hypothesis and the relative importance of sunscreening or antioxidant action in contributing to photoprotection.
Anthocyanins are not optimally located for photoprotection in leaf tissues
The core argument here is that the vacuolar localization of anthocyanins reduces their usefulness for acting on chloroplast-generated ROS (Karageorgou and Manetas, 2006). However, the short-lived ROS generated by chloroplasts are rapidly converted into H2O2. H2O2 can quickly pass lipid bilayers such as the tonoplast, and the tonoplast is generally adjacent to chloroplasts as the vacuole accounts for >90 % of photosynthetic cells’ total volume (Yamasaki et al., 1997). Indeed, anthocyanin-rich cells accumulate less H2O2 than their acyanic counterparts (Gould et al., 2002; Zhang et al., 2012). In addition, Zipor and Oren-Shamir (2013) demonstrated that vacuolar anthocyanins are effective electron donors for vacuolar peroxidases, thereby suggesting a further mechanism for reducing ROS. Anthocyanins are frequently sub-epidermal in foliar tissues, while the concentration in epidermal cells might be suggested as optimal for light screening. However, even if sub-epidermal anthocyanins are principally antioxidants, they still screen some potentially damaging light.
Anthocyanins mainly absorb green light that is relatively harmless to chloroplasts
This is an old misconception (Manetas, 2006) that needs to be overcome. It is true that anthocyanins mainly absorb in the yellow–green portion of the PAR spectrum that is relatively poorly absorbed by chlorophylls (Fig. 7), although the carotenoids that assist chlorophylls in the light-harvesting process do extend the absorbance of PAR wavebands somewhat into the green region. However, green light is essential for plants under shade (Smith et al., 2017), as occurs for most anthocyanin-rich understorey species. Green light also interacts with photoreceptors (Landi et al., 2020) and is essential for photosynthesis of the deeper mesophyll layers (Liu et al., 2021), which are reached by red/blue-depleted light because of the strong absorbance of upper layers of the mesophyll. It has also been recently demonstrated that absorbance of blue light by anthocyanins has to be seriously considered (Landi et al., 2021), especially when anthocyanins are synthesized in their acylated forms under stress conditions (Jordheim et al., 2016). Finally, green light is used more efficiently than red or blue light at high photosynthetic photon flux densities (Landi et al., 2020), and supplementation with green light does cause higher photoinhibition (Hughes et al., 2005).
Photoprotection should translate to higher nutrient resorption in senescing leaves, and this does not consistently occur in red-leafed tree species
Observations that anthocyanins facilitate nutrient recovery during autumn (Hoch et al., 2003) led to a general assumption that, if the photoprotection hypothesis is correct, higher nutrient resorption in reddening compared with yellowing senescing leaves should be consistently observed (Pena-Novas and Archetti, 2021b), thereby conferring an ecological advantage from having anthocyanin-rich leaves. While it is true that resorbed nutrients are directly available for plant growth but nutrients recycled through litterfall may not become available for plant uptake for a long time (Aerts, 1996), nutrient resorption consequences of photoprotection need to be considered in the context of the specific ecosystem. Indeed, it is an open question whether nutrient-deficient species that evolved in low-nutrient availability environments should still exhibit greater resorption efficiency when not growing in their natural, low-fertile habitat (Hughes et al., 2022). Therefore, nitrogen resorption studies conducted with individuals out of their natural habitat (Pena-Novas and Archetti, 2021b) could generate misleading data. Additionally, nitrogen resorption analyses are usually conducted at ‘leaf level’, but a more robust approach would include an integrated overview at ‘whole-canopy level’, given that the progressive loss of foliage by plants could lead to changes in nitrogen recycling and a re-organization of morpho-anatomical and leaf photosynthetic traits (Escudero and Mediavilla, 2003). For example, the efficiency of nitrogen recovery before leaf fall in different woody species can be dependent on: duration of the abscission period (Del Arco et al., 1991); leaf life span and related different nitrogen recycling abilities (Pornon and Lamaze, 2007), especially when including evergreen species in the comparison (Pena-Novas and Archetti 2021b; Hughes et al., 2022); and leaf mass changes upon senescence (L. Li et al., 2019). Additionally, alternative analyses of the same current datasets have produced contrasting findings against (Pena-Novas and Archetti, 2021b) or in favour (Joly, 2022) of the photoprotection hypothesis. Future studies on nitrogen resorption capacity in red vs. yellow senescing leaves should therefore take a more comprehensive approach that includes consideration of the many influencing factors. It is also notable that autumn climatic conditions can generate red pigmentation of photosynthetic tissues that are not shed, such as in moss and fern species, arguing against nutrient reallocation – and co-evolution – hypotheses in such cases.
Several reports have failed to demonstrate photoprotection by anthocyanins
One of the initially more challenging arguments against the photoprotection hypothesis is the failure in some studies to find any advantage of red over green/yellow leaves for photoinhibition amelioration in plants under stress. However, the definition of the term ‘photoinhibition’ is not universally accepted. For example, in sensu lato it is described by ecophysiologists as the light-induced depression in the CO2 assimilation rate with a consequent reduction in the maximum quantum yields for CO2 uptake, a decrease in the convexity of the photosynthetic light response curve and, with prolonged exposure to excessive light, a decreased rate of light-saturated photosynthesis (Long et al., 1994). Nevertheless, in most studies on the photoprotective role of anthocyanins, the possible reduction/prevention of photoinhibition was simply estimated as the preservation of the PSII photochemical efficiency, either maximal (Fv/Fm) or operational (ΦPSII). This is too simplistic an approach, given that: PSII is only part of the photosynthetic apparatus and effects of other components such as PSI and/or Calvin–Benson cycle are not measured; photochemistry of PSII is only part of the dissipation mechanisms used for energy quenching; and chlorophyll fluorescence analyses provide an estimation of the leaf photosynthetic potential based on the analysis of just a shallow layer of chloroplasts over the leaf lamina. Therefore, it is not surprising that although many studies find that anthocyanins correlate with reduction of photoinhibition (i.e. higher Fv/Fm), others fail to uncover any photoprotective benefits (for a critical review, see Gould et al., 2018). It is conceivable that contemporary re-examination of older contradictory cases could find opposite results (some examples are reported in Hughes et al., 2022). Most reported studies were conducted in controlled conditions with low photon flux density (Gould et al., 2018). However, the term ‘photoprotection’ only has a real meaning in saturating light conditions, whether this be relatively high PAR or lower PAR amounts in conditions of compromised photosynthesis (such as extreme temperatures). In addition, the presence of anthocyanins implicates a suite of morpho-anatomical and biochemical changes which strongly affect the interception, entry and management of light by the leaf (Hughes, 2011). Finally, red vs. green leaf performance may differ during the day (Tattini et al., 2017).
In summary, only broader analyses of the photosynthetic process at the whole-leaf level (e.g. through gas exchange parameters and the real CO2 conductance to mesophyll in green compared with red individuals), as well as measurements of oxidative stress markers, can unequivocally provide information on the contribution to photoprotection of light screening by anthocyanins relative to antioxidant roles. This latter point has been controversial for decades (Neill and Gould, 2003) and, as mentioned earlier, is also debated for colourless flavonoids in terms of UVB radiation screening vs. antioxidant function. However, nowadays both the anthocyanin light screening and the antioxidant prerogative have started to be considered as two faces of the same coin (Landi et al., 2015). To further complicate the picture, anthocyanins are usually produced in young or senescent leaves, which are more vulnerable to environmental stress as they have reduced photosynthetic capacity, yet in some ecological studies these leaves are compared with mature green leaves regardless of the strong differences in photosynthetic apparatus (Manetas et al., 2002). Many of these considerations for examining the benefits of anthocyanins for photoprotection in the context of the alternative co-evolution hypothesis also apply to the question of why plants produce biosynthetically costly pigments when there is already an effective NPQ antioxidant system: it is necessary to accurately replicate the stress conditions experienced by anthocyanic tissues in nature and make appropriate experimental measurements. The use of genetic lines with constitutive red or green phenotypes and/or alterations to alternative stress tolerance mechanisms, preferably near-isogenic mutants or transgenics, would be a great advantage for such comparisons.
FUNCTIONS BEYOND PHOTOPROTECTION
The question we address in the final part of this review regards what additional functions red pigments may have beyond photoprotection (i.e. considered as light sunscreen and/or antioxidant function), and what this may indicate about evolution of the biosynthetic pathways. In relation to photosynthetic function, one possibility is that red pigments provide benefit as ‘carbon sinks’ to help avoid transitory feedback regulation of photosynthesis (Adams et al., 2013; Lo Piccolo et al., 2020). Indeed, there is an indissoluble link between sugar signalling and anthocyanin induction (Solfanelli et al., 2006), and anthocyanin biosynthesis can represent a valid carbon sink able to maintain the activity of the Calvin–Benson cycle, thereby avoiding energy (ATP) and reducing power (NADPH) accumulation in photosystems that would otherwise generate ROS. Indeed, the fact that photoinhibited leaves typically contain an overabundance of non-structural carbohydrates strongly suggests that photoinhibition of photosynthesis in those leaves might be reduced by the biosynthesis of carbon-based secondary metabolites, thereby possibly reducing the sugar accumulation and the feedback regulation of photosynthesis (Lo Piccolo et al., 2020). The carbon-buffering role exerted by anthocyanins can also represent a mechanism to balance the C/N ratio in senescing leaves, thereby extending the leaf life span of red- vs. green-leafed tree species (Lo Piccolo et al., 2018; Vangelisti et al., 2020). Therefore, it is conceivable that the carbon-buffering role should be included as another mechanism through which anthocyanins confer photoprotection, besides their antioxidant and screening capabilities (Gould et al., 2018; Lo Piccolo et al., 2018, 2020). This mechanism could also explain the accumulation of anthocyanins in abaxial leaf epidermises (which are not an optimal location for screening PAR) in species living in the understorey; for these shade-adapted species, transitory sunflecks can exert a severe photoinhibitory effect (Hughes et al., 2014). The carbon-buffering hypothesis also matches the recent nutrient-centred theory of anthocyanin function proposed by Hughes et al. (2022). The authors hypothesized that the evolution of species with red leaves in autumn was driven by soil fertility, with leaf reddening a response to low quantities of foliar nutrients.
For all the aforementioned reasons, the term ‘photomodulation’ rather than the classical ‘photoprotection’ seems to be more apt for future use, and better describes the role of anthocyanins as mediators in light-driven reactions. Whether this is the case for other red pigments has yet to be determined, since there have been few studies of this nature. For many of the pigments, an initial examination supports a core photoprotective function centred on direct screening, rather than antioxidant or secondary photomodulation functions. The cell wall-bound, probably insoluble, accumulation of auronidins and sphagnorubins and external accumulation in mucilage of some algal pigments argues against an antioxidant function. While betalain production has many similar stress triggers to those of anthocyanin production, since betalains contain nitrogen they are not suited to modulating the C/N balance and would not be expected to show the same responses to sugar status.
Understanding the physiological roles of the non-anthocyanin red pigments is important for proposing the possible evolutionary drivers of pigmentation in general, and research on non-anthocyanin model species is needed. The evidence reviewed here indicates that a core photoprotection function by direct screening is a major driver that has given rise to auronidin, sphagnorubin, 3-deoxyanthocyanin, anthocyanin, betalain and various algal red pigments that can all absorb in the visible wavelengths, i.e. generating a red pigment is the key evolutionary driver. That different lineages have evolved to produce different pigments could reflect the chances of mutation. However, as betalains are thought to have replaced anthocyanins on more than one occasion (Brockington et al., 2011), that at least suggests a functional evolutionary driver. We propose that each of the red pigment structures shares the core screening function, but they have additional functions for interactions with the abiotic and biotic environment. While it is possible these differing functionalities were factors influencing the initial evolution of the biosynthetic pathways, including co-evolution with other organisms (Rausher, 2001), it is perhaps more probable that once red pigment production had evolved for photoprotection, the pathway was adapted for secondary functions that conferred an evolutionary advantage. For anthocyanins, the diversification of angiosperms and anthocyanin secondary modifications, and the versatility of the core anthocyanin structure, have generated a myriad of proposed additional functionalities that have contributed to the challenge of finding a unified explanation for anthocyanin production. Some of these are well supported as replacing the photoprotection role of anthocyanins, such as blue flower colouration for pollination. For others, notably the co-evolution hypothesis for autumn leaf colour, it is necessary to find solid supporting evidence before they can replace the photoprotection hypothesis. For auronidins and 3-deoxyanthocyanidins, there is strong evidence of anti-pathogen or anti-herbivore functions. For betalains, evidence is growing of specific activities in salinity tolerance.
If red pigmentation has arisen many times, then what might the pigment status of the last common ancestor of land plants have been? The lack of anthocyanins in hornworts and liverworts, in the context of the proposed monophyly of bryophytes, makes it difficult to reach a conclusion. It is possible that the 3-deoxyanthocyanin pathway was present in the last common ancestor but lost in hornworts, liverworts and some mosses, with ‘replacement’ by auronidins or sphagnorubins. More whole-genome sequences from non-angiosperm species would greatly help in addressing this question.
ACKNOWLEDGEMENTS
Our thanks to Dr David Glenny (Manaaki Whenua–Landcare Research) and Dr Chris Cargill (Centre for Australian National Biodiversity Research) for helpful discussions and their ongoing assistance in identifying bryophytes of Aotearoa-New Zealand and Australia, Dr Lorelle Phillips (Scion, New Zealand) for the image of a pine bud, Dr Sebastian Hess (University of Cologne) and Dr Daniel Remias (University of Applied Sciences Upper Austria) for images of stress pigmentation in algae and comments on the manuscript, and Professor Fernando Pina (University of Lisbon) and Dr David Lewis (Plant & Food Research) for pigment spectral data.
Contributor Information
Kevin M Davies, The New Zealand Institute for Plant and Food Research Limited, Private Bag 11600, Palmerston North 4442, New Zealand.
Marco Landi, Department of Agriculture, Food and Environment, University of Pisa, Italy.
John W van Klink, The New Zealand Institute for Plant and Food Research Limited, Department of Chemistry, Otago University, Dunedin, New Zealand.
Kathy E Schwinn, The New Zealand Institute for Plant and Food Research Limited, Private Bag 11600, Palmerston North 4442, New Zealand.
David A Brummell, The New Zealand Institute for Plant and Food Research Limited, Private Bag 11600, Palmerston North 4442, New Zealand.
Nick W Albert, The New Zealand Institute for Plant and Food Research Limited, Private Bag 11600, Palmerston North 4442, New Zealand.
David Chagné, The New Zealand Institute for Plant and Food Research Limited, Private Bag 11600, Palmerston North 4442, New Zealand.
Rubina Jibran, The New Zealand Institute for Plant and Food Research Limited, Private Bag 92169, Auckland Mail Centre, Auckland 1142, New Zealand.
Samarth Kulshrestha, The New Zealand Institute for Plant and Food Research Limited, Private Bag 11600, Palmerston North 4442, New Zealand.
Yanfei Zhou, The New Zealand Institute for Plant and Food Research Limited, Private Bag 11600, Palmerston North 4442, New Zealand.
John L Bowman, School of Biological Sciences, Monash University, Melbourne, VIC, Australia.
FUNDING
Financial support was provided by the Marsden Fund of New Zealand/Te Pūtea Rangahau a Marsden (contracts PAF1701 and PAF2002) and a James Cook Research Fellowship (K.M.D., contract JCF-PAF2001).
LITERATURE CITED
- Adams WW, Muller O, Cohu CM, et al. 2013. May photoinhibition be a consequence, rather than a cause, of limited plant productivity? Photosynthesis Research 117: 31–44. doi: 10.1007/s11120-013-9849-7. [DOI] [PubMed] [Google Scholar]
- Aerts R. 1996. Nutrient resorption from senescing leaves of perennials: are there general patterns? Journal of Ecology 84: 597–608. [Google Scholar]
- Agati G, Brunetti C, Fini A, et al. 2020. Are flavonoids effective antioxidants in plants? Twenty years of our investigation. Antioxidants 9: 1098. doi: 10.3390/antiox9111098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agati G, Guidi L, Landi M, Tattini M.. 2021. Anthocyanins in photoprotection: knowing the actors in play to solve this complex ecophysiological issue. New Phytologist 232: 2228–2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aigner S, Remias D, Karsten U, Holzinger A.. 2013. Unusual phenolic compounds contribute to ecophysiological performance in the purple-colored green alga Zygogonium ericetorum (Zygnematophyceae, Streptophyta) from a high-alpine habitat. Journal of Phycology 49: 648–660. doi: 10.1111/jpy.12075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert NW, Thrimawithana AH, McGhie TK, et al. 2018. Genetic analysis of the liverwort Marchantia polymorpha reveals that R2R3MYB activation of flavonoid production in response to abiotic stress is an ancient character in land plants. New Phytologist 218: 554–566. [DOI] [PubMed] [Google Scholar]
- Alejo-Armijo A, Mendoza J, Parola AJ, Pina F.. 2020. Chemical evolution of the colour systems generated by riccionidin A, 3-deoxyanthocyanidins and anthocyanins. Phytochemistry 174: 112339. doi: 10.1016/j.phytochem.2020.112339. [DOI] [PubMed] [Google Scholar]
- Alseekh S, Perez de Souza L, Benina M, Fernie AR.. 2020. The style and substance of plant flavonoid decoration; towards defining both structure and function. Phytochemistry 174: 112347. doi: 10.1016/j.phytochem.2020.112347. [DOI] [PubMed] [Google Scholar]
- Andersen ØM, Jordheim M.. 2006. The anthocyanins. In: Andersen ØM, Markham KR, eds., Flavonoids chemistry, biochemistry and applications. Boca Raton, FL: CRC Press, 471–551. [Google Scholar]
- Archetti M, Döring TF, Hagen SB, et al. 2009. Unravelling the evolution of autumn colours: an interdisciplinary approach. Trends in Ecology and Evolution 24: 166–173. doi: 10.1016/j.tree.2008.10.006. [DOI] [PubMed] [Google Scholar]
- Ban Z, Qin H, Mitchell AJ, et al. 2018. Noncatalytic chalcone isomerase-fold proteins in Humulus lupulus are auxiliary components in prenylated flavonoid biosynthesis. Proceedings of the National Academy of Sciences, USA 115: E5223–E5232. doi: 10.1073/pnas.1802223115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassi R, Dall’Osto L.. 2021. Dissipation of light energy absorbed in excess: the molecular mechanisms. Annual Review of Plant Biology 72: 47–76. doi: 10.1146/annurev-arplant-071720-015522. [DOI] [PubMed] [Google Scholar]
- Bean A, Sunnadeniya R, Akhavan N, Campbell A, Brown M, Lloyd A.. 2018. Gain-of-function mutations in beet DODA2 identify key residues for betalain pigment evolution. New Phytologist 219: 287–296. [DOI] [PubMed] [Google Scholar]
- Benton MJ, Wilf P, Sauquet H.. 2022. The Angiosperm Terrestrial Revolution and the origins of modern biodiversity. New Phytologist 233: 2017–2035. [DOI] [PubMed] [Google Scholar]
- Berland H, Albert NW, Stavland A, et al. 2019. Auronidins are a previously unreported class of flavonoid pigments that challenges when anthocyanin biosynthesis evolved in plants. Proceedings of the National Academy of Sciences, USA 116: 20232–20239. doi: 10.1073/pnas.1912741116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berland H, Andersen OM.. 2021. Characterization of a natural, stable, reversible and colourful anthocyanidin network from Sphagnum moss based mainly on the yellow trans-chalcone and red flavylium cation forms. Molecules 26: 709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilska K, Stuper-Szablewska K, Kulik T, et al. 2018. Changes in phenylpropanoid and trichothecene production by Fusarium culmorum and F. graminearum sensu stricto via exposure to flavonoids. Toxins 10: 110. doi: 10.3390/toxins10030110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnett SAF, Ostle N, Freeman C.. 2010. Short-term effect of deep shade and enhanced nitrogen supply on Sphagnum capillifolium morphophysiology. Plant Ecology 207: 347–358. [Google Scholar]
- Booij-James IS, Dube SK, Jansen MAK, Edelman M, Mattoo AK.. 2000. Ultraviolet-B radiation impacts light-mediated turnover of the photosystem II reaction center heterodimer in Arabidopsis mutants altered in phenolic metabolism. Plant Physiology 124: 1275–1284. doi: 10.1104/pp.124.3.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucherle B, Peuchmaur M, Boumendjel A, Haudecoeur R.. 2017. Occurrences, biosynthesis and properties of aurones as high-end evolutionary products. Phytochemistry 142: 92–111. doi: 10.1016/j.phytochem.2017.06.017. [DOI] [PubMed] [Google Scholar]
- Bowman JL, Kohchi T, Yamato KT, et al. 2017. Insights into land plant evolution garnered from the Marchantia polymorpha genome. Cell 171: 287–304.e15. doi: 10.1016/j.cell.2017.09.030. [DOI] [PubMed] [Google Scholar]
- Brockington SF, Walker RH, Glover BJ, Soltis PS, Soltis DE.. 2011. Complex pigment evolution in the Caryophyllales. New Phytologist 190: 854–864. [DOI] [PubMed] [Google Scholar]
- Brockington SF, Yang Y, Gandia-Herrero F, et al. 2015. Lineage-specific gene radiations underlie the evolution of novel betalain pigmentation in Caryophyllales. New Phytologist 207: 1170–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burki F, Roger AJ, Brown MW, Simpson AGB.. 2020. The new tree of eukaryotes. Trends in Ecology & Evolution 35: 43–55. doi: 10.1016/j.tree.2019.08.008. [DOI] [PubMed] [Google Scholar]
- Busch A, Hess S.. 2022. Sunscreen mucilage: a photoprotective adaptation found in terrestrial green algae (Zygnematophyceae). European Journal of Phycology 57: 107–124. [Google Scholar]
- Cárdenas-Pérez S, Piernik A, Chanona-Pérez JJ, Grigore MN, Perea-Flores MJ.. 2021. An overview of the emerging trends of the Salicornia L. genus as a sustainable crop. Environmental and Experimental Botany 191: 104606. doi: 10.1016/j.envexpbot.2021.104606. [DOI] [Google Scholar]
- Carella P, Gogleva A, Hoey DJ, et al. 2019. Conserved biochemical defenses underpin host responses to oomycete infection in an early divergent land plant lineage. Current Biology 29: 1–13. [DOI] [PubMed] [Google Scholar]
- Carreto JI, Carignan MO.. 2011. Mycosporine-like amino acids: relevant secondary metabolites. Chemical and ecological aspects. Marine Drugs 9: 387–446. doi: 10.3390/md9030387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng A-X, Zhang X, Han X-J, et al. 2018. Identification of chalcone isomerase in the basal land plants reveals an ancient evolution of enzymatic cyclization activity for synthesis of flavonoids. New Phytologist 217: 909–924. [DOI] [PubMed] [Google Scholar]
- Choudhury FK, Rivero RM, Blumwald E, Mittler R.. 2017. Reactive oxygen species, abiotic stress and stress combination. The Plant Journal 90: 856–867. [DOI] [PubMed] [Google Scholar]
- Christin P-A, Arakaki M, Osborne CP, Edwards EE.. 2015. Genetic enablers underlying the clustered evolutionary origins of C4 photosynthesis in Angiosperms. Molecular Biology and Evolution 32: 846–858. [DOI] [PubMed] [Google Scholar]
- Christinet L, Burdet FX, Zaiko M, Hinz U, Zrÿd J-P.. 2004. Characterization and functional identification of a novel plant 4,5-extradiol dioxygenase involved in betalain pigment biosynthesis in Portulaca grandiflora. Plant Physiology 134: 265–274. doi: 10.1104/pp.103.031914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung H-H, Schwinn KE, Lewis DH, et al. 2015. Characterisation of betalain biosynthesis in Parakeelya flowers identifies the key biosynthetic gene DOD as belonging to an expanded LigB gene family that is conserved in betalain-producing species. Frontiers in Plant Science 6: 499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke LJ, Robinson SA.. 2008. Cell wall-bound ultraviolet-screening compounds explain the high ultraviolet tolerance of the Antarctic moss, Ceratodon purpureus. New Phytologist 179: 776–783. [DOI] [PubMed] [Google Scholar]
- Clayton WA, Albert NW, Thrimawithana AH, et al. 2018. UVR8-mediated induction of flavonoid biosynthesis for UVB tolerance is conserved between the liverwort Marchantia polymorpha and flowering plants. The Plant Journal 96: 503–517. [DOI] [PubMed] [Google Scholar]
- Cohen MF, Meziane T, Tsuchiya M, Yamasaki H.. 2002a. Feeding deterrence of Azolla in relation to deoxyanthocyanin and fatty acid composition. Aquatic Botany 74: 181–187. [Google Scholar]
- Cohen MF, Sakihama Y, Takagi YC, Ichiba T, Yamasaki H.. 2002b. Synergistic effect of deoxyanthocyanins from symbiotic fern Azolla spp. on hrmA gene induction in the cyanobacterium Nostoc punctiforme. Molecular Plant-Microbe Interactions 15: 875–882. [DOI] [PubMed] [Google Scholar]
- Cooney LJ, Schaefer HM, Logan BA, Cox B, Gould KS.. 2015. Functional significance of anthocyanins in peduncles of Sambucus nigra. Environmental and Experimental Botany 119: 18–26. [Google Scholar]
- D’Alessandro S, Havaux M.. 2019. Sensing β-carotene oxidation in photosystem II to master plant stress tolerance. New Phytologist 223: 1776–1783. [DOI] [PubMed] [Google Scholar]
- Dassanayake M, Larkin JC.. 2017. Making plants break a sweat: the structure, function, and evolution of plant salt glands. Frontiers in Plant Science 8: 406. doi: 10.3389/fpls.2017.00406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dastmalchi M. 2021. Elusive partners: a review of the auxiliary proteins guiding metabolic flux in flavonoid biosynthesis. The Plant Journal 108: 314–329. [DOI] [PubMed] [Google Scholar]
- Davies KM. 2015. Swapping one red pigment for another. Nature Genetics 47: 5–6. doi: 10.1038/ng.3174. [DOI] [PubMed] [Google Scholar]
- Davies KM, Albert NW, Zhou Y, Schwinn KE.. 2018. Functions of flavonoid and betalain pigments in abiotic stress tolerance in plants. Annual Plant Reviews Online 1: 21–62. doi: 10.1002/9781119312994.apr0604. [DOI] [Google Scholar]
- Davies KM, Jibran R, Zhou Y, et al. 2020. The evolution of flavonoid biosynthesis: a bryophyte perspective. Frontiers in Plant Science 11: 7. doi: 10.3389/fpls.2020.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies KM, Jibran R, Albert NW, Zhou Y, Schwinn KE.. 2021. Conservation and divergence between bryophytes and angiosperms in the biosynthesis and regulation of flavonoid production. In: Reed JD, Pereira de Freitas VA, Quideau S, eds. Recent advances in polyphenol research, Vol. 7. Chichester, UK: John Wiley & Sons, 227–263. [Google Scholar]
- Del Arco JM, Escudero A, Garrido MV.. 1991. Effects of site characteristics on nitrogen retranslocation from senescing leaves. Ecology 72: 701–708. [Google Scholar]
- Delaux PM, Schornack S.. 2021. Plant evolution driven by interactions with symbiotic and pathogenic microbes. Science 371: eaba6605. [DOI] [PubMed] [Google Scholar]
- Demmig-Adams B, Garab G, Adams W, Govindjee. 2014. Non-photochemical quenching and energy dissipation in plants, algae and cyanobacteria. Dordrecht: Springer. doi: 10.1007/978-94-017-9032-1 [DOI] [Google Scholar]
- Desmet S, Morreel K, Dauwe R.. 2021. Origin and function of structural diversity in the plant specialized metabolome. Plants 10: 2393. doi: 10.3390/plants10112393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devireddy AR, Zandalinas SI, Fichman Y, Mittler R.. 2021. Integration of reactive oxygen species and hormone signaling during abiotic stress. The Plant Journal 105: 459–476. [DOI] [PubMed] [Google Scholar]
- Donoghue PCJ, Harrison CJ, Paps Montserrat J, Schneider H.. 2021. The evolutionary emergence of land plants. Current Biology 31: R1281–R1298. [DOI] [PubMed] [Google Scholar]
- Escudero A, Mediavilla S.. 2003. Decline in photosynthetic nitrogen use efficiency with leaf age and nitrogen resorption as determinants of leaf life span. Journal of Ecology 91: 880–889. [Google Scholar]
- Feild TS, Lee DW, Holbrook NM.. 2001. Why leaves turn red in autumn. The role of anthocyanins in senescing leaves of red-osier dogwood. Plant Physiology 127: 566–574. [PMC free article] [PubMed] [Google Scholar]
- Ferreira D, Garcia-Pichel F.. 2016. Mutational studies of putative biosynthetic genes for the cyanobacterial sunscreen scytonemin in Nostoc punctiforme ATCC 29133. Frontiers in Microbiology 7: 735. doi: 10.3389/fmicb.2016.00735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreyra MLF, Serra P, Casati P.. 2021. Recent advances on the roles of flavonoids as plant protective molecules after UV and high light exposure. Physiologia Plantarum 173: 736–749. doi: 10.1111/ppl.13543. [DOI] [PubMed] [Google Scholar]
- Field KJ, Pressel S.. 2018. Unity in diversity: structural and functional insights into the ancient partnerships between plants and fungi. New Phytologist 220: 996–1011. [DOI] [PubMed] [Google Scholar]
- Firn RD, Jones CG.. 2003. Natural products – a simple model to explain chemical diversity. Natural Products Reports 20: 382–391. [DOI] [PubMed] [Google Scholar]
- Flowers TJ, Colmer TD.. 2008. Salinity tolerance in halophytes. New Phytologist 179: 945–963. [DOI] [PubMed] [Google Scholar]
- Foyer CH. 2018. Reactive oxygen species, oxidative signaling and the regulation of photosynthesis. Environmental and Experimental Botany 154: 134–142. doi: 10.1016/j.envexpbot.2018.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frangedakis E, Shimamura M, Villarreal JC, et al. 2021. The hornworts: morphology, evolution and development. New Phytologist 229: 735–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritsch FE. 1916. The morphology and ecology of an extreme terrestrial form of Zygnema (Zygogonium) ericetorum (Kuetz.). Hansg. Annals of Botany 30: 135–149. [Google Scholar]
- Fürst-Jansen JMR, de Vries S, de Vries J.. 2020. Evo-physio: on stress responses and the earliest land plants. Journal of Experimental Botany 71: 3254–3269. doi: 10.1093/jxb/eraa007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandía-Herrero F, Garcia-Carmona F, Escribano J.. 2005. Floral fluorescence effect. Nature 437: 334. [DOI] [PubMed] [Google Scholar]
- Gerdol R, Bonora A, Marchesini R, Gualandri R, Pancaldi S.. 1998. Growth response of Sphagnum capillifolium to nighttime temperature and nutrient level: mechanisms and implications for global change. Arctic and Alpine Research 30: 388–395. [Google Scholar]
- Giusti MM, Rodríguez-Saona LE, Wrolstad RE.. 1999. Molar absorptivity and color characteristics of acylated and non-acylated pelargonidin-based anthocyanins. Journal of Agriculture and Food Chemistry 47: 46311–44637. [DOI] [PubMed] [Google Scholar]
- Gould KS, Jay-Allemand C, Logan BA, Baissac Y, Bidel LPR.. 2018. When are foliar anthocyanins useful to plants? Re-evaluation of the photoprotection hypothesis using Arabidopsis thaliana mutants that differ in anthocyanin accumulation. Environmental and Experimental Botany 154: 11–22. [Google Scholar]
- Gould KS, McKelvie J, Markham KR.. 2002. Do anthocyanins function as antioxidants in leaves? Imaging of H2O2 in red and green leaves after mechanical injury. Plant, Cell & Environment 25: 1261–1269. [Google Scholar]
- Grosche C, Genau AC, Rensing SA.. 2018. Evolution of the symbiosis-specific GRAS regulatory network in bryophytes. Frontiers in Plant Science 9: 1621. doi: 10.3389/fpls.2018.01621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grützner R, Schubert R, Horn C, Yang C, Vogt T, Marillonnet S.. 2021. Engineering betalain biosynthesis in tomato for high level betanin production in fruits. Frontiers in Plant Science 12: 682443. doi: 10.3389/fpls.2021.682443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadacek F, Bachmann G, Engelmeier D, Chobot V.. 2010. Hormesis and a chemical raison d’être for secondary plant metabolites. Dose-Response 9: 79–116. doi: 10.2203/dose-response.09-028.Hadacek. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halbwirth H, Martens S, Wienand U, Forkmann G, Stich K.. 2003. Biochemical formation of anthocyanins in silk tissue of Zea mays. Plant Science 164: 489–495. [Google Scholar]
- Harholt J, Moestrup O, Ulvskov P.. 2016. Why plants were terrestrial from the beginning. Trends in Plant Science 21: 96–101. doi: 10.1016/j.tplants.2015.11.010. [DOI] [PubMed] [Google Scholar]
- Hatlestad GJ, Sunnadeniya RM, Akhavan NA, et al. 2012. The beet R locus encodes a new cytochrome P450 required for red betalain production. Nature Genetics 44: 816–820. doi: 10.1038/ng.2297. [DOI] [PubMed] [Google Scholar]
- Hatlestad GJ, Akhavan NA, Sunnadeniya RM, et al. 2015. The beet Y locus encodes an anthocyanin MYB-like protein that activates the betalain red pigment pathway. Nature Genetics 47: 92–96. doi: 10.1038/ng.3163. [DOI] [PubMed] [Google Scholar]
- Hayakawa K, Agarie S.. 2010. Physiological roles of betacyanin in a halophyte, Suaeda japonica Makino. Plant Production Science 13: 351–359. [Google Scholar]
- Henry JB, Perkins-Veazie P, McCall I, Whipker BE.. 2019. Restricted phosphorus fertilization increases the betacyanin concentration and red foliage coloration of Alternanthera. Journal of the American Society for Horticultural Science 144: 264–273. [Google Scholar]
- Herburger K, Remias D, Holzinger A.. 2016. The green alga Zygogonium ericetorum (Zygnematophyceae, Charophyta) shows high iron and aluminium tolerance: protection mechanisms and photosynthetic performance. FEMS Microbiology Ecology 92: fiw103. doi: 10.1093/femsec/fiw103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoch WA, Singsaas EL, McCown BH.. 2003. Resorption protection. Anthocyanins facilitate nutrient recovery in autumn by shielding leaves from potentially damaging light levels. Plant Physiology 133: 1296–1305. doi: 10.1104/pp.103.027631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoham RW, Remias D.. 2020. Snow and glacial algae: a review. Journal of Phycology 56: 264–282. doi: 10.1111/jpy.12952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzinger A, Allen MC, Deheyn DD.. 2016. Hyperspectral imaging of snow algae and green algae from aeroterrestrial habitats. Journal of Photochemistry and Photobiology B: Biology 162: 412–420. doi: 10.1016/j.jphotobiol.2016.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzinger A, Pichrtová M.. 2016. Abiotic stress tolerance of Charophyte green algae: new challenges for Omics techniques. Frontiers in Plant Science 7: 678. doi: 10.3389/fpls.2016.00678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooijmaijers CAM, Gould KS.. 2007. Photoprotective pigments in red and green gametophytes of two New Zealand liverworts. New Zealand Journal of Botany 45: 451–461. [Google Scholar]
- Houghton A, Appelhagen I, Martin C.. 2021. Natural blues: structure meets function in anthocyanins. Plants 10: 726. doi: 10.3390/plants10040726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes NM. 2011. Winter leaf reddening in ‘evergreen’ species. New Phytologist 190: 573–581. [DOI] [PubMed] [Google Scholar]
- Hughes NM, Smith WK.. 2007. Attenuation of incident light in Galax urceolata (Diapensiaceae): concerted influence of adaxial and abaxial anthocyanin layers on photoprotection. American Journal of Botany 94: 784–790. [DOI] [PubMed] [Google Scholar]
- Hughes NM, Neufeld HS, Burkey KO.. 2005. Functional role of anthocyanins in high-light winter leaves of the evergreen herb Galax urceolata. New Phytologist 168: 575–587. [DOI] [PubMed] [Google Scholar]
- Hughes NM, Morley CB, Smith WK.. 2007. Coordination of anthocyanin decline and photosynthetic maturation in juvenile leaves of three deciduous tree species. New Phytologist 175: 675–685. [DOI] [PubMed] [Google Scholar]
- Hughes NM, Carpenter KL, Keidel TS, Miller CN, Waters MN, Smith WK.. 2014. Photosynthetic costs and benefits of abaxial versus adaxial anthocyanins in Colocasia esculenta ‘Mojito’. Planta 240: 971–981. [DOI] [PubMed] [Google Scholar]
- Hughes NM, George CO, Gumpman CB, Neufeld HS.. 2022. Coevolution and photoprotection as complementary hypotheses for autumn leaf reddening: a nutrient-centered perspective. New Phytologist 233: 22–29. [DOI] [PubMed] [Google Scholar]
- Humphreys C, Franks P, Rees M, Bidartondo MI, Leake JR, Beerling DJ.. 2010. Mutualistic mycorrhiza-like symbiosis in the most ancient group of land plants. Nature Communications 1: 103. doi: 10.1038/ncomms1105. [DOI] [PubMed] [Google Scholar]
- Ibdah M, Krins A, Seidlitz HK, et al. 2002. Spectral dependence of flavonol and betacyanin accumulation in Mesembryanthemum crystallinum under enhanced ultra-violet radiation. Plant, Cell & Environment 25: 1145–1154. [Google Scholar]
- Ibraheem F, Gaffoor I, Qixian T, Chi-Ren S, Chopra S.. 2015. A Sorghum MYB transcription factor induces 3-deoxyanthocyanidins and enhances resistance against leaf blights in maize. Molecules 20: 2388–2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iriel A, Lagorio MG.. 2010. Is the flower fluorescence relevant in biocommunication? Naturwissenschaften 97: 915–924. [DOI] [PubMed] [Google Scholar]
- Izzo LG, Arena C, De Micco V, Capozzi F, Aronne G.. 2019. Light quality shapes morpho-functional traits and pigment content of green and red leaf cultivars of Atriplex hortensis. Scientia Horticulturae 246: 942–950. [Google Scholar]
- Jain G, Gould KS.. 2015a. Functional significance of betalain biosynthesis in leaves of Disphyma australe under salinity stress. Environmental and Experimental Botany 109: 131–140. [Google Scholar]
- Jain G, Gould KS.. 2015b. Are betalain pigments the functional homologues of anthocyanins in plants? Environmental and Experimental Botany 119: 48–53. [Google Scholar]
- Jain G, Schwinn KE, Gould KS.. 2015. Betalain induction by l-DOPA application confers photoprotection to saline-exposed leaves of Disphyma australe. New Phytologist 207: 1075–1083. [DOI] [PubMed] [Google Scholar]
- Jiang W, Yin Q, Wu R, et al. 2015. Role of a chalcone isomerase-like protein in flavonoid biosynthesis in Arabidopsis thaliana. Journal of Experimental Botany 66: 7165–7179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao C, Sørensen I, Sun X, et al. 2020. The genome of the charophyte alga Penium margaritaceum bears footprints of the evolutionary origins of land plants. Cell 181: 1097–1111. [DOI] [PubMed] [Google Scholar]
- Joly S. 2022. Red and yellow pigments in autumn leaves are associated with higher nitrogen resorption. Journal of Evolutionary Biology 35: 180–182. [DOI] [PubMed] [Google Scholar]
- Jones CG, Firn RD, Malcolm SB.. 1991. On the evolution of plant secondary chemical diversity. Philosophical Transactions of the Royal Society B: Biological Sciences 333: 273–280. [Google Scholar]
- Jordheim M, Calcott K, Gould KS, Davies KM, Schwinn KE, Andersen OM.. 2016. High concentrations of aromatic acylated anthocyanins found in cauline hairs in Plectranthus ciliatus. Phytochemistry 128: 27–34. [DOI] [PubMed] [Google Scholar]
- Karageorgou P, Manetas Y.. 2006. The importance of being red when young, anthocyanins and the protection of young leaves of Quercus coccifera from insect herbivory and excess light. Tree Physiology 26: 613–621. [DOI] [PubMed] [Google Scholar]
- Kariyat RR, Gaffoor I, Sattar S, et al. 2019. Sorghum 3-deoxyanthocyanidin flavonoids confer resistance against corn leaf aphid. Journal of Chemical Ecology 45: 502–514. [DOI] [PubMed] [Google Scholar]
- Karsten U, Herburger K, Holzinger A.. 2016. Living in biological soil crust communities of African deserts—physiological traits of green algal Klebsormidium species (Streptophyta) to cope with desiccation, light and temperature gradients. Journal of Plant Physiology 194: 2–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawahigashi H, Kasuga S, Sawada Y, et al. 2016. The sorghum gene for leaf color changes upon wounding (P) encodes a flavanone 4-reductase in the 3-deoxyanthocyanidin biosynthesis pathway. G3: Genes, Genomes, Genetics 6: 1439–1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SY, Colpitts CC, Wiedemann G, et al. 2013. Physcomitrella PpORS, basal to plant type III polyketide synthases in phylogenetic trees, is a very long chain 2ʹ-oxoalkylresorcinol synthase. Journal of Biological Chemistry 288: 2767–2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein FRS, Reis A, Kleinowski AM, et al. 2018. UV-B radiation as an elicitor of secondary metabolite production in plants of the genus Alternanthera. Acta Botanica Brasilica 32: 615–623. [Google Scholar]
- Kovinich N, Kayanja G, Chanoca A, Riedl K, Otegui MS, Grotewold E.. 2014. Not all anthocyanins are born equal: distinct patterns induced by stress in Arabidopsis. Planta 240: 931–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo H, Nozawa S, Hiwatashi T, et al. 2018. Biosynthesis of riccionidins and marchantins is regulated by R2R3-MYB transcription factors in Marchantia polymorpha. Journal of Plant Research 131: 849–864. [DOI] [PubMed] [Google Scholar]
- Kulshrestha S, Jibran R, van Klink JW, et al. 2022. Stress, senescence, and specialized metabolites in bryophytes. Journal of Experimental Botany 73: 4396–4411. doi: 10.1093/jxb/erac085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunz S, Burkhardt G, Becker H.. 1993. Riccionidins A and B, anthocyanidins from the cell walls of the liverwort Ricciocarpos natans. Phytochemistry 35: 233–235. [Google Scholar]
- Landi M, Guidi L, Pardossi A, Tattini M, Gould KS.. 2014. Photoprotection by foliar anthocyanins mitigates effects of boron toxicity in sweet basil (Ocimum basilicum). Planta 240: 941–953. [DOI] [PubMed] [Google Scholar]
- Landi M, Tattini M, Gould KS.. 2015. Multiple functional roles of anthocyanins in plant–environment interactions. Environmental and Experimental Botany 119: 4–17. [Google Scholar]
- Landi M, Zivcak M, Sytar O, Brestic M, Allakhverdiev SI.. 2020. Plasticity of photosynthetic processes and the accumulation of secondary metabolites in plants in response to monochromatic light environments: a review. Biochimica et Biophysica Acta 1861: 148131. [DOI] [PubMed] [Google Scholar]
- Landi M, Agati G, Fini A, Guidi L, Sebastiani F, Tattini M.. 2021. Unveiling the shade nature of cyanic leaves: a view from the ‘blue absorbing side’ of anthocyanins. Plant, Cell & Environment 44: 1119–1129. [DOI] [PubMed] [Google Scholar]
- Lee DW, Collins TM.. 2001. Phylogenetic and ontogenetic influences on the distribution of anthocyanins and betacyanins in leaves of tropical plants. International Journal of Plant Sciences 162: 1141–1153. [Google Scholar]
- Li DD, Ni R, Wang PP, et al. 2020. Molecular basis for chemical evolution of flavones to flavonols and anthocyanins in land plants. Plant Physiology 184: 1731–1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li FW, Nishiyama T, Waller M, et al. 2020. Anthoceros genomes illuminate the origin of land plants and the unique biology of hornworts. Nature Plants 6: 259–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Meng X, Zhu M, Li Z.. 2019. Research progress of betalain in response to adverse stresses and evolutionary relationship compared with anthocyanin. Molecules 24: 3078. doi: 10.3390/molecules24173078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Wang X, Manning WJ.. 2019. Effects of elevated CO2 on leaf senescence, leaf nitrogen resorption, and late-season photosynthesis in Tilia americana L. Frontiers in Plant Science 10: 1217. doi: 10.3389/fpls.2019.01217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Wang S, Wang H, et al. 2020. The genome of Prasinoderma coloniale unveils the existence of a third phylum within green plants. Nature Ecology & Evolution 4: 1220–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu HJ, Du YG, Chu H, et al. 2010. Molecular dissection of the pathogen-inducible 3-deoxyanthocyanidin biosynthesis pathway in Sorghum. Plant & Cell Physiology 51: 1173–1185. [DOI] [PubMed] [Google Scholar]
- Liu J, van Iersel MW.. 2021. Photosynthetic physiology of blue, green, and red light: light intensity effects and underlying mechanisms. Frontiers in Plant Science 12: 328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long SP, Humphries S, Falkowski PG.. 1994. Photoinhibition of photosynthesis in nature. Annual Review of Plant Physiology and Plant Molecular Biology 45: 633–662. [Google Scholar]
- Lopez-Nieves S, Yang Y, Timoneda A, et al. 2018. Relaxation of tyrosine pathway regulation underlies the evolution of betalain pigmentation in Caryophyllales. New Phytologist 217: 896–908. [DOI] [PubMed] [Google Scholar]
- Lo Piccolo E, Landi M, Pellegrini E, et al. 2018. Multiple consequences induced by epidermally-located anthocyanins in young, mature and senescent leaves of Prunus. Frontiers in Plant Science 9: 917. doi: 10.3389/fpls.2018.00917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo Piccolo E, Landi M, Massai R, Remorini D, Guidi L.. 2020. Girled-induced anthocyanin accumulation in red-leafed Prunus cerasifera: effect on photosynthesis, photoprotection and sugar metabolism. Plant Science 294: 110456. doi: 10.1016/j.plantsci.2020.110456. [DOI] [PubMed] [Google Scholar]
- Lu Y, Che J, Xu X, et al. 2020. Metabolomics reveals the response of the phenylpropanoid biosynthesis pathway to starvation treatment in the grape endophyte Alternaria sp. MG1. Journal of Agricultural and Food Chemistry 68: 1126–1135. [DOI] [PubMed] [Google Scholar]
- Lu Y, Song Y, Zhu J, et al. 2021. Potential application of CHS and 4CL genes from grape endophytic fungus in production of naringenin and resveratrol and the improvement of polyphenol profiles and flavour of wine. Food Chemistry 347: 128972. doi: 10.1016/j.foodchem.2020.128972. [DOI] [PubMed] [Google Scholar]
- Manetas Y. 2006. Why some leaves are anthocyanic and why most anthocyanic leaves are red? Flora 201: 163–177. [Google Scholar]
- Manetas Y, Drinia A, Petropoulou Y.. 2002. High contents of anthocyanins in young leaves are correlated with low pools of xanthophyll cycle components and low risk of photoinhibition. Photosynthetica 40: 349–354. [Google Scholar]
- Manninen S, Wood C, Leithc ID, Sheppard LJ.. 2011. Physiological and morphological effects of long-term ammonium or nitrate deposition on the green and red (shade and open grown) Sphagnum capillifolium. Environmental and Experimental Botany 72: 140–148. [Google Scholar]
- Markham KK, Greenham K.. 2021. Abiotic stress through time. New Phytologist 231: 40–46. [DOI] [PubMed] [Google Scholar]
- Markham KR, Porter LJ.. 1978. Production of an aurone by bryophytes in the reproductive phase. Phytochemistry 17: 159–160. [Google Scholar]
- Moray C, Hua X, Bromham L.. 2015. Salt tolerance is evolutionarily labile in a diverse set of angiosperm families. BMC Evolutionary Biology 15: 90. doi: 10.1186/s12862-015-0379-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita Y, Takagi K, Fukuchi-Mizutani M, et al. 2014. A chalcone isomerase-like protein enhances flavonoid production and flower pigmentation. The Plant Journal 78: 294–304. [DOI] [PubMed] [Google Scholar]
- Morita H, Wong CP, Abe I.. 2019. How structural subtleties lead to molecular diversity for the type III polyketide synthases. Journal of Biological Chemistry 294: 15121–15136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morohashi K, Casas MI, Ferreyra ML, et al. 2012. A genome-wide regulatory framework identifies maize Pericarp Color1 controlled genes. The Plant Cell 24: 2745–2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosco A. 2012. Tissue localization of betacyanins in cactus stems. Revista Mexicana de Biodiversidad 83: 413–420. [Google Scholar]
- Naake T, Maeda HA, Proost S, Tohge T, Fernie AR.. 2021. Kingdom-wide analysis of the evolution of the plant type III polyketide synthase superfamily. Plant Physiology 185: 857–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima T, Uetake J, Segawa T, Procházková L, Tsushima A, Takeuchi N.. 2021. Spatial and temporal variations in pigment and species compositions of snow algae on Mt. Tateyama in Toyama Prefecture, Japan. Frontiers in Plant Science 12: 689119. doi: 10.3389/fpls.2021.689119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neill SO, Gould KS.. 2003. Anthocyanins in leaves: light attenuators or antioxidants? Functional Plant Biology 30: 865–873. [DOI] [PubMed] [Google Scholar]
- Newsham KK, Geissler PA, Nicolson MJ, Peat HJ, Lewis-Smith RI.. 2005. Sequential reduction of UV-B radiation in the field alters the pigmentation of an Antarctic leafy liverwort. Environmental and Experimental Botany 54: 22–32. [Google Scholar]
- Parida AK, Kumari A, Panda A, Rangani J, Agarwal PK.. 2018. Photosynthetic pigments, betalains, proteins, sugars, and minerals during Salicornia brachiata senescence. Biologia Plantarum 62: 343–352. [Google Scholar]
- Pena-Novas I, Archetti M.. 2020. Biogeography and evidence for adaptive explanations of autumn colors. New Phytologist 228: 209–213. [DOI] [PubMed] [Google Scholar]
- Pena-Novas I, Archetti M.. 2021a. A comparative analysis of the photoprotection hypothesis for the evolution of autumn colours. Journal of Evolutionary Biology 33: 1669–1676. [DOI] [PubMed] [Google Scholar]
- Pena-Novas I, Archetti M.. 2021b. A test of the photoprotection hypothesis for the evolution of autumn colours: chlorophyll resorption, not anthocyanin production, is correlated with nitrogen translocation. Journal of Evolutionary Biology 34: 1423–1431. [DOI] [PubMed] [Google Scholar]
- Piatkowski BT, Imwattana K, Tripp EA, et al. 2020. Phylogenomics reveals convergent evolution of red-violet coloration in land plants and the origins of the anthocyanin biosynthetic pathway. Molecular Phylogenetics and Evolution 151: 106904. doi: 10.1016/j.ympev.2020.106904. [DOI] [PubMed] [Google Scholar]
- Pina F, Alejo-Armijo A, Clemente A, et al. 2021. Evolution of flavylium-based color systems in plants: what physical chemistry can tell us. International Journal of Molecular Sciences 22: 3833. doi: 10.3390/ijms22083833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinnola A. 2019. The rise and fall of light-harvesting complex stress-related proteins as photoprotection agents during evolution. Journal of Experimental Botany 70: 5527–5535. [DOI] [PubMed] [Google Scholar]
- Polturak G, Breitel D, Grossman N, et al. 2016. Elucidation of the first committed step in betalain biosynthesis enables the heterologous engineering of betalain pigments in plants. New Phytologist 210: 269–283. [DOI] [PubMed] [Google Scholar]
- Polturak G, Grossman N, Vela-Coria D, Aharoni A.. 2017. Engineered gray mold resistance, antioxidant capacity, and pigmentation in betalain-producing crops and ornamentals. Proceedings of the National Academy of Sciences, USA 114: 9062–9067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pornon A, Lamaze T.. 2007. Nitrogen resorption and photosynthetic activity over leaf life span in an evergreen shrub, Rhododendron ferrugineum, in a subalpine environment. New Phytologist 175: 301–310. [DOI] [PubMed] [Google Scholar]
- Pospíšil P. 2009. Production of reactive oxygen species by photosystem II. Biochimica et Biophysica Acta 10: 1151–1160. [DOI] [PubMed] [Google Scholar]
- Pringsheim N. 1879. Ueber lichtwirkung und chlorophyll funktion in der pflanze. Leipzig: Engelmann. [Google Scholar]
- Rausher MD. 2001. Co-evolution and plant resistance to natural enemies. Nature 411: 857–864. [DOI] [PubMed] [Google Scholar]
- Rieseberg TP, Dadras A, Fürst-Jansen JMR, et al. 2022. Crossroads in the evolution of plant specialized metabolism. Seminars in Cell & Developmental Biology S1084952122000738. doi: 10.1016/j.semcdb.2022.03.004. [DOI] [PubMed] [Google Scholar]
- Remias D, Holzinger A, Aigner S, Lütz C.. 2012a. Ecophysiology and ultrastructure of Ancylonema nordenskiöldii (Zygnematales, Streptophyta), causing brown ice on glaciers in Svalbard (high arctic). Polar Biology 35: 899–908. [Google Scholar]
- Remias D, Schwaiger S, Aigner S, Leya T, Stuppner H, Lütz C.. 2012b. Characterization of an UV- and VIS-absorbing, purpurogallin-derived secondary pigment new to algae and highly abundant in Mesotaenium berggrenii (Zygnematophyceae, Chlorophyta), an extremophyte living on glaciers. FEMS Microbiology Ecology 79: 638–648. [DOI] [PubMed] [Google Scholar]
- Renner SS, Zohner CM.. 2019. The occurrence of red and yellow autumn leaves explained by regional differences in insolation and temperature. New Phytologist 224: 1464–1471. [DOI] [PubMed] [Google Scholar]
- Renner SS, Zohner CM.. 2020. Further analysis of 1532 deciduous woody species from North America, Europe, and Asia supports continental-scale differences in red autumn colouration. New Phytologist 228: 814–815. [DOI] [PubMed] [Google Scholar]
- Rudall PJ. 2020. Colourful cones: how did flower colour first evolve? Journal of Experimental Botany 71: 759–767. [DOI] [PubMed] [Google Scholar]
- Sachdev S, Ansari SA, Ansari MI, Fujita M, Hasanuzzaman M.. 2021. Abiotic stress and reactive oxygen species: generation, signaling, and defense mechanisms. Antioxidants (Basel) 10: 277. doi: 10.3390/antiox10020277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandmann G. 2019. Antioxidant protection from UV- and light-stress related to carotenoid structures. Antioxidants 8: 219. doi: 10.3390/antiox8070219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki N, Wada K, Koda T, Kasahara K, Adachi T, Ozeki Y.. 2005. Isolation and characterization of cDNAs encoding an enzyme with glucosyltransferase activity for cyclo-DOPA from four o’clocks and feather cockscombs. Plant & Cell Physiology 46: 666–670. [DOI] [PubMed] [Google Scholar]
- Schliemann W, Kobayashi N, Strack D.. 1999. The decisive step in betaxanthin biosynthesis is a spontaneous reaction. Plant Physiology 119: 1217–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih CH, Chu IK, Yip WK, Lo C.. 2006. Differential expression of two flavonoid 3ʹ-hydroxylase cDNAs involved in biosynthesis of anthocyanin pigments and 3-deoxyanthocyanidin phytoalexins in Sorghum. Plant & Cell Physiology 47: 1412–1419. [DOI] [PubMed] [Google Scholar]
- Smith HL, McAusland L, Murchie EH.. 2017. Don’t ignore the green light: exploring diverse roles in plant processes. Journal of Experimental Botany 68: 2099–2110. [DOI] [PubMed] [Google Scholar]
- Solfanelli C, Poggi A, Loreti E, Alpi A, Perata P.. 2006. Sucrose-specific induction of the anthocyanin biosynthetic pathway in Arabidopsis. Plant Physiology 140: 637–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano G, Del-Castillo-Alonso MA, Monforte L, Nunez-Olivera E, Martinez-Abaigar J.. 2018a. First data on the effects of ultraviolet radiation on phenolic compounds in the model hornwort Anthoceros agrestis. Cryptogamie Bryologie 39: 201–211. [Google Scholar]
- Soriano G, Del-Castillo-Alonso MA, Monforte L, Nunez-Olivera E, Martinez-Abaigar J.. 2018b. Phenolic compounds from different bryophyte species and cell compartments respond specifically to ultraviolet radiation, but not particularly quickly. Plant Physiology and Biochemistry 134: 137–144. [DOI] [PubMed] [Google Scholar]
- Stintzing FC, Carle R.. 2004. Functional properties of anthocyanins and betalains in plants, food, and in human nutrition. Trends in Food Science & Technology 15: 19–38. [Google Scholar]
- Storme JY, Golubic S, Wilmotte A, Kleinteich J, Velázquez D, Javaux EJ.. 2015. Raman characterization of the UV-protective pigment gloeocapsin and its role in the survival of cyanobacteria. Astrobiology 15: 843–857. [DOI] [PubMed] [Google Scholar]
- Strack D, Vogt T, Schliemann W.. 2003. Recent advances in betalain research. Phytochemistry 62: 247–269. [DOI] [PubMed] [Google Scholar]
- Sunnadeniya R, Bean A, Brown M, et al. 2016. Tyrosine hydroxylation in betalain pigment biosynthesis is performed by cytochrome P450 enzymes in beets (Beta vulgaris). PLoS One 11: e0149417. doi: 10.1371/journal.pone.0149417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi S, Badger MR.. 2011. Photoprotection in plants: a new light on photosystem II damage. Trends in Plant Science 16: 53–60. [DOI] [PubMed] [Google Scholar]
- Tattini M, Sebastiani F, Brunetti C, et al. 2017. Dissecting molecular and physiological response mechanisms to high solar radiation in cyanic and acyanic leaves: a case study on red and green basil. Journal of Experimental Botany 68: 2425–2437. [DOI] [PubMed] [Google Scholar]
- Timoneda A, Feng T, Sheehan H, et al. 2019. The evolution of betalain biosynthesis in Caryophyllales. New Phytologist 224: 71–85. [DOI] [PubMed] [Google Scholar]
- Tohge T, Wendenburg R, Ishihara H, et al. 2016. Characterization of a recently evolved flavonol-phenylacyltransferase gene provides signatures of natural light selection in Brassicaceae. Nature Communications 7: 12399. doi: 10.1038/ncomms12399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tossi VE, Tosar LM, Pitta-Álvarez SI, Causin HF.. 2021. Casting light on the pathway to betalain biosynthesis: a review. Environmental and Experimental Botany 186: 104464. doi: 10.1016/j.envexpbot.2021.104464. [DOI] [Google Scholar]
- Vangelisti A, Guidi L, Cavallini A, et al. 2020. Red versus green leaves: transcriptomic comparison of foliar senescence between two Prunus cerasifera genotypes. Scientific Reports 10: 1959. doi: 10.1038/s41598-020-58878-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt T. 2002. Substrate specificity and sequence analysis define a polyphyletic origin of betanidin 5- and 6-O-glucosyltransferase from Dorotheanthus bellidiformis. Planta 214: 492–495. [DOI] [PubMed] [Google Scholar]
- de Vries J, Archibald JM.. 2018. Plant evolution: landmarks on the path to terrestrial life. New Phytologist 217: 1428–1434. [DOI] [PubMed] [Google Scholar]
- de Vries J, de Vries S, Slamovits CH, Rose LE, Archibald JM.. 2017. How embryophytic is the biosynthesis of phenylpropanoids and their derivatives in streptophyte algae? Plant & Cell Physiology 58: 934–945. doi: 10.1093/pcp/pcx037. [DOI] [PubMed] [Google Scholar]
- de Vries S, Fürst-Jansen JMR, Irisarri I, et al. 2021. The evolution of the phenylpropanoid pathway entailed pronounced radiations and divergences of enzyme families. The Plant Journal 107: 975–1002. [DOI] [PubMed] [Google Scholar]
- Waki T, Mameda R, Nakano T, et al. 2020. A conserved strategy of chalcone isomerase-like protein to rectify promiscuous chalcone synthase specificity. Nature Communications 11: 870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Liu T.. 2007. Involvement of betacyanin in chilling-induced photoinhibition in leaves of Suaeda salsa. Photosynthetica 45: 182–188. [Google Scholar]
- Wang C, Zhao J-Q, Chen M, Wang B-S.. 2007. Involvement of hydrogen peroxide in betacyanin accumulation induced by dark in leaves of Suaeda salsa. Journal of Plant Ecology 31: 748–752. [Google Scholar]
- White PJ, Bowen HC, Broadley MR, El-Serehy HA, et al. 2017. Evolutionary origins of abnormally large shoot sodium accumulation in nonsaline environments within the Caryophyllales. New Phytologist 214: 284–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson CJ, Cook JM, Tedstone A, et al. 2020. Algal photophysiology drives darkening and melt of the Greenland Ice Sheet. Proceedings of the National Academy of Sciences, USA 117: 5694–5705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winefield CS, Lewis DH, Swinny EE, et al. 2005. Investigation of the biosynthesis of 3-deoxyanthocyanins in Sinningia cardinalis. Physiologia Plantarum 124: 419–430. [Google Scholar]
- Winter K, Smith JAC.. 2022. CAM photosynthesis: the acid test. New Phytologist 233: 599–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Y, Zhang P, Warner RD, Fang Z.. 2019. 3-Deoxyanthocyanidin colorant: nature, health, synthesis, and food applications. Comprehensive Reviews in Food Science and Food Safety 18: 1533–1549. [DOI] [PubMed] [Google Scholar]
- Yamasaki H, Sakihama Y, Ikehara N.. 1997. Flavonoid-peroxidase reaction as a detoxification mechanism of plant cells against hydrogen peroxide. Journal of Plant Physiology 115: 1405–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonekura-Sakakibara K, Higashi Y, Nakabayashi R.. 2019. The origin and evolution of plant flavonoid metabolism. Frontiers in Plant Science 10: 943. doi: 10.3389/fpls.2019.00943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida K, Mori M, Kondo T.. 2009. Blue flower color development by anthocyanins: from chemical structure to cell physiology. Natural Products Reports 26: 884–915. [DOI] [PubMed] [Google Scholar]
- Yu H-N, Liu X-Y, Gao S, et al. 2018. Structural and biochemical characterization of the plant type III polyketide synthases of the liverwort Marchantia paleacea. Plant Physiology and Biochemistry 125: 95–105. [DOI] [PubMed] [Google Scholar]
- Zhang J, Fu XX, Li RQ, et al. 2020. The hornwort genome and early land plant evolution. Nature Plants 6: 107–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Su LJ, Chen JW, Zeng XQ, Sun BY, Peng CL.. 2012. The antioxidative role of anthocyanins in Arabidopsis under high-irradiance. Biologia Plantarum 56: 97–104. [Google Scholar]
- Zhou Y, Karl T, Lewis DH, et al. 2021. Production of betacyanins in transgenic Nicotiana tabacum increases tolerance to salinity. Frontiers in Plant Science 12: 653147. doi: 10.3389/fpls.2021.653147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipor G, Oren-Shamir M.. 2013. Do vacuolar peroxidases act as plant caretakers? Plant Science 199: 41–47. [DOI] [PubMed] [Google Scholar]