Abstract
Background and Aims
Seed cone traits are significant for understanding the evolutionary history of conifers. Podocarpaceae has fleshy cones with a distinct morphology compared with other conifers. However, we have a poor understanding of the seed cone morphology of the Prumnopityoid clade and within Podocarpaceae. This study presents detailed seed cone morpho-anatomy and the evolution of fleshy structures traits in the Prumnopityoid clade.
Methods
We investigated the detailed seed cone morpho-anatomy of selected species from the nine genera using the histological method. The evolution of morpho-anatomical traits was assessed using ancestral state reconstruction methods.
Key Results
The Prumnopityoid clade has evolved fleshy seed cones using different functional structures (e.g. aril, epimatium, bracts or receptaculum) and fleshiness is an ancestral trait in the clade. An epimatium is present in all genera except Phyllocladus, but with different structural morphologies (e.g. a fleshy asymmetrical cup-like epimatium or an epimatium that is fused with the integument, forming a fleshy sarcotesta-like seed coat). In all species with fleshy sarcotesta-like seed coats, the endotesta is hard and woody, forming a sclerotesta-like structure and the epimatium and exotesta are fused, forming a fleshy sarcotesta-like structure.
Conclusions
This study highlights that the Prumnopityoid clade has an amazing diversity of structures and complex evolutionary patterns. Fleshiness is an ancestral trait of the clade and has been achieved via diverse evolutionary pathways and structures. This clade has four distinct seed cone types, i.e. drupe-like, receptaculate, arilloid and dacrydioid cones, based on morpho-anatomical structures and traits. The macrofossil record also demonstrates the presence of several structures and traits.
Keywords: Australasia, conifers, fleshy structures, seed dispersal, palaeobotany, reproductive morphology, trait evolution
INTRODUCTION
Gymnosperm evolution has a key significance in understanding the origin and the evolution of seed plants. The evolution and mechanism of fleshy seed cones in lineages of surviving gymnosperms is an open topic of discussion (Contreras et al., 2017; Leslie et al., 2017; Khan and Hill, 2021; Nigris et al., 2021).
The Podocarpaceae have an amazing diversity of seed cone morphology and possess different morphotypes across all major clades. Several studies have investigated seed cone evolution in conifers (Tomlinson, 1992; Mundry, 2000; Restemeyer, 2002; Mill et al., 2004; Herting et al., 2020; Klaus and Matzke, 2020). Klaus and Matzke (2020) reported that the ancestral seed cones in Podocarpaceae were non-fleshy, and fleshy cone structures appeared seven times independently in podocarps. Herting et al. (2020) used a modern trait-evolution approach to investigate the ancestral conifer cone and suggested that the ancestral conifer seed cones were non-fleshy, but in podocarps fleshiness is an ancestral trait. Most of the recent studies are based on published data regarding species’ functional traits, but due to few detailed investigations on the morpho-anatomy and functional traits of the Prumnopityoid clade there has been misidentification of traits in several genera (e.g. seed cones of Manoao, Sundacarpus, Prumnopitys, Pectinopitys, Phyllocladus and Retrophyllum have been reported as non-fleshy, but they do in fact have fleshy structures) in these ancestral reconstruction studies (Klaus and Matzke, 2020; Herting et al., 2020). The living podocarps display diversity in functional structures (epimatium, bracts, aril and receptacle) and use these structures to achieve fleshiness (Khan and Hill, 2021). Some previous recent studies discussing the evolution of the coniferous seed scale in podocarps and conifers suggests that the epimatium is a homologous structure to the seed scale in other conifers (Herting and Stützel, 2020; Khan and Hill, 2021). Nigris et al. (2021) stated that these fleshy structures in gymnosperms are associated with ovule protection and seed dispersal.
The Podocarpaceae is a morphologically diverse family with 20 extant genera (several monotypic) and >200 species (Khan and Hill, 2021). Molecular studies divide the family into three major clades, i.e. Prumnopityoid, Podocarpoid, Dacrydioid, and one distinct grade (Biffin et al., 2011; Knopf et al., 2012). The Prumnopityoid clade contains the largest number of genera (nine). These genera can be placed into two broad subclades (Halocarpioid and Pectinopityoid) based on both morphological (the former typically scale-leaved and the latter broad-leaved) and molecular studies (although some genera are paraphyletic) (Leslie et al., 2017, 2018). The Halocarpioid subclade is also called the scale-leaved clade as most of the genera (Lepidothamnus, Manoao, Lagarostrobos and Parasitaxus) bear scale leaves (Andruchow-Colombo et al., 2019). However, Phyllocladus has developed specialized photosynthetic phylloclades and Halocarpus species show leaf dimorphism (scale leaves and flattened leaves). Phyllocladus species are morphologically unique among podocarps, having lost their true leaves (except in seedlings), and flattened the lateral short shoots into a two-dimensional flattened photosynthetic structure called a phylloclade (Keng, 1978; Dörken et al., 2021). Phylloclades are variable in shape and can be classified as simple (P. trichomanoides and P. aspleniifolius) and pinnately compound (P. trichomanoides, P. hypophyllus and P. toatoa). Some researchers have placed Phyllocladus in the separate family Phyllocladaceae (Keng, 1973; Tomlinson et al., 1997; Melikian and Bobrov, 2000), but molecular studies place them in the Halocarpioid subclade. The Halocarpioid subclade consists of six genera (12 species) predominantly in New Zealand, Australia and New Caledonia, but Lepidothamnus fonkii is found in Argentina and Chile and Phyllocladus hypophyllus is distributed in Brunei, Papua New Guinea, Malaysia, Indonesia and the Philippines.
The Pectinopityoid subclade was recently revised by Page (2019) and now includes three genera (Prumnopitys, Pectinopitys and Sundacarpus) and consists of ten species distributed in South America, Southeast Asia, New Caledonia, Papua New Guinea, New Zealand and Australia (Farjon, 2018). All species of the Pectinopityoid subclade have bifacially flattened leaves, which are hypostomatic, with a single resin canal. Both a hypodermis and accessory transfusion tissue are absent in Prumnopitys and Pectinopitys but are present in Sundacarpus (Salter, 2004; Knopf et al., 2012). Pectinopitys consists of six dioecious tree species, distributed in South America, New Zealand, New Caledonia and Australia (Page, 2019). Prumnopitys consist of three dioecious tree species distributed in South America and New Zealand. Sundacarpus is a monotypic genus (containing S. amarus) distributed in Australia, Papua New Guinea and into Southeast Asia. Pectinopitys and Sundacarpus are segregated from Prumnopitys based on both morphological and molecular data (Kelch, 2002; Page, 2019). Melikian and Bobrov (2000) proposed a new family, Prumnopityaceae, for this subclade, but this is not supported by molecular data.
The Prumnopityoid clade has diverse seed cone morphology and evolved fleshiness using several functional structures. However, comprehensive studies on the seed cone morpho-anatomy and functional traits of this clade are lacking. This resulted in miss-identifying both traits in several genera and ancestral reconstruction of the evolution of fleshy seed cones. . This study describes the detailed seed cone morpho-anatomy and combines it with available fossil seed cone records and evaluates the diversity and evolution of functional traits among the genera of the Prumnopityoid clade. This study will contribute to the complete understanding of the complicated evolutionary history of fleshiness and other functional traits of Podocarpaceae.
MATERIALS AND METHODS
Seed cone collections
Seed cones at different developmental stages were collected from the Royal Botanic Gardens Melbourne, Victoria; Mount Lofty Botanical Garden, South Australia; National Botanic Gardens, Canberra, ACT; The Tasmanian Arboretum, Devonport; and the Cairns Botanic Gardens, North Queensland, Australia (Table S1). For this study, we used living seed cones (ten seed cone replicates for each species) and we have stored four specimens of each species in the plant spirit collection with voucher numbers from UOA-61 to UOA-120 at the Department of Ecology and Evolution, University of Adelaide, Australia.
Morphology and distribution of the investigated taxa
Members of the Halocarpioid subclade are usually shrubs or sometimes trees with dimorphic leaves, while members of the Pectinopityoid subclade are usually large trees with flattened leaves and fleshy seed cones. In this study, we examined the following: Manoao colensoi (New Zealand); Lagarostrobos franklinii (Tasmania); Lepidothamnus fonkii, L. intermedius and L. laxifolius (Argentina, Chile and New Zealand); Parasitaxus usta (the only parasitic gymnosperm, New Caledonia); Falcatifolium taxoides (New Caledonia); Halocarpus bidwillii, H. biformis and H. kirkii (New Zealand); Phyllocladus aspleniifolius (Tasmania), P. toatoa and P. trichomanoides (both New Zealand); Phyllocladus hypophyllus (Brunei, Papua New Guinea, Malaysia, Indonesia and the Philippines); Prumnopitys andina (Chile and Argentina); Pectinopitys ferruginea (New Zealand); and Sundacarpus amarus (northeastern Australia through Papua New Guinea, the Philippines, to Brunei).
Taxon processing and sectioning
Specimens of 14 taxa (Table 1) were fixed in 200 mL of FAA (100 ml 95 % ethanol + 80 mL dH2O + 20 mL 37 % formaldehyde solution) immediately after collection. Whole seed cones, plus their longitudinal and cross sections were photographed with a Nikon-SMZ25 stereomicroscope. For histology, seed cones were fixed for 48–72 h and processed for a 48-h cycle using a Sakura Tissue-Tek VIP6 Vacuum Infiltration Tissue Processor. They were then embedded in paraffin wax (Sakura Tissue Tek Embedding Centre) and then longitudinal and cross sections of 8–10 µm thickness, were produced using a Leica RM 2235 Rotary Microtome and stained with H and E (Dako CoverStainer) and toluidine blue stains.
Table 1.
Seed cone morphological and anatomical qualitative and quantitative characters of the Prumnopityoid clade
| Characters | Halocarpus bidwillii | Halocarpus biformis | Phyllocladus trichomanoides | P. aspleniifolius | P. toatoa | P. hypophyllus | Lepidothamnus intermedius | L. laxifolius | Manoao colensoi | Lagarostrobos franklinii | Parasitaxus usta | Prumnopitys andina | Pectinopitys ferruginea | Sundacarpus amarus |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reproductive cycle | 2 years | 2 years | 1 year | 1 year | 1 year | 1 year | 2 years | 2 years | 2 years | 2 years | (1 year) | 2 years | 2 years | 2 years |
| Cone shape | Ellipsoid | Ellipsoid | Ellipsoid | Ellipsoid | Ellipsoid | Ellipsoid | Ellipsoid | Ellipsoid | Ovoid | Narrow cylindrical | Globose | Ellipsoid | Ellipsoid | Ellipsoid |
| Cone size (mm) | 3–5 × 2–2.5 | 2.5–4.5 × 1.5–2 | 5–8 × 1–2 | 5–8 × 2–2.5 | 6–8 × 1.2–2.6 | 7–10 × 1.6–2.5 | 5–8 × 2–3 | 6–7 × 2–3 | 4–6 × 1.5–2.5 | 4–5.5 × 2–3 | 3–5 × 3–4 | 18–25 × 16–20 | 14–20 × 10–16 | 25–35 × 22– 31 |
| Colour | White brownish | Yellow brownish | Reddish brown | Red | Reddish brown | Red purplish | Reddish brown | Reddish brown | Yellowish brown | Yellowish brown | Reddish brown | Yellowish | Reddish | Reddish |
| Number of seeds per cone | 1 or 2 | 1 or 2 | 2–4 | 2–5 | 2–8 | 1–3 | 1 or 2 | 1 or 2 | 1–3 | 5–8 | 1 | 4–12 | 1 or 2 | 1 or 2 |
| Seed size (mm) | 2.5–4.5 × 1.5–2 | 1–3 × 1.5–2 | 4–5 × 3 | 4–5 × 3.4 | 3.5–4.5 × 1.2–2.6 | 6–8 × 1.6–2.5 | 4–5 × 2–2.5 | 4–5 × 2.5 | 3–4 × 1.5–2.5 | 2.5–3 × 1.5–2 | 2.6–4.6 × 2.5–3.8 | 12–18 × 10–15 | 10–15 × 8–12 | 20–30 × 8–27 |
| Seed shape | Ellipsoid | Ellipsoid | Ellipsoid | Ellipsoid | Ellipsoid | Ellipsoid | Ellipsoid | Ellipsoid | Ovoid | Ovoid | Globose | Ellipsoid | Ellipsoid | Ellipsoid |
| Seed surface | Undulate | Undulate | Undulate | Undulate | Smooth | plicate | Undulate | Undulate | Undulate | Undulate | Rugose | Rugose | Rugose | Rugose |
| Seed colour | Greenish black | Greenish black | Greenish black | Greenish black | Dark brown | Brown | Purplish black | Purplish black | Black | Brown | Brown | Brownish | Brownish | Brownish |
| Ovule orientation | Inverted | Inverted | Erect | Erect | Erect | Erect | Erect | Erect | Inverted | Inverted | Inverted | Inverted | Inverted | Inverted |
| Aril | Present | Present | Present | Present | Present | Present | Absent | Absent | Absent | Absent | Absent | Absent | Absent | Absent |
| Aril shape | Cup-shaped | Cup-shaped | Cup-shaped | Cup-shaped | Cup-shaped | Cup-shaped | – | – | – | – | – | – | – | – |
| Aril colour | White | Orange | White | White | White | White | – | – | – | – | – | – | – | – |
| Aril structure | Fleshy | Fleshy | Fleshy | Fleshy | Fleshy | Fleshy | – | – | – | – | – | – | – | – |
| Aril cells | Isodiametric | Isodiametric | Rectangular | Isodiametric | Elongated oval | Elongated oval | – | – | – | – | – | – | – | – |
| Cuticle | thick | thick | Thin | Thin | Thin | Thin | Thin | Thin | Thin | Thin | Thin | Thin | Thin | Thin |
| Epidermal layers | 1 | 1 | 1 | 1–2 | 1 or 2 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Epidermal cell shape | Round | Round | Isodiametric | Isodiametric | Isodiametric | Isodiametric | Elongated triangular | Elongated triangular | Round | Elongated triangular | Isodiametric | Isodiametric | Isodiametric | Isodiametric |
| Exotesta | 4–6 | 3–5 | 2 | 1–2 | 3–4 | 3–4 | 3–5 | 2–5 | 4–6 | 2–4 | 10–16 | 16–22 | 12–16 | 14–26 |
| Mesotesta | – | – | 1–2 | 5–8 | 2–5 | 5–7 | – | – | – | – | – | 6–10 | 3–6 | 4–6 |
| Endotesta | 4–8 | 3–6 | 8–12 | 4–8 | 6–8 | 3–4 | 4–7 | 3–6 | 2–7 | 4–10 | 6–10 | 12–20 | 6–12 | 3–6 |
| Nucellus | 3–6 | 4–8 | 10–16 | 4–10 | 10–12 | 5–9 | 3–6 | 3–6 | 2–5 | 1–3 | 3–7 | 6–12 | 13–18 | 8–12 |
| Embryo shape | Straight | Straight | Straight | Straight | Straight | Straight | Straight | Straight | Straight | Straight | Straight | Straight | Straight | Straight |
| Embryo size (mm) | 0.36–0.8 × 0.25–0.45 | 0.52–0.95 × 0.29–0.55 | 0.6–0.7 × 0.2–0.3 | 0.35–0.62 × 0.15–0.25 | 0.3–0.7 × 0.2–0.25 | 0.5–0.6 × 0.2–0.3 | 0.42–0.85 × 0.38–0.62 | 0.4–0.82 × 0.33–0.58 | 0.4–0.6 × 0.2–0.3 | 0.3–0.5 × 0.15–0.2 | 0.2–0.5 × 0.1–0.2 | 0.6–0.9 × 0.2–0.4 | 0.6–1.0 × 0.3–0.5 | 0.5–1.1 × 0.2–0.4 |
| Bracts | 4–5 | 4–5 | 6–8 | 6–8 | 8–20 | 12–15 | 3–5 | 3–4 | 4–7 | 5–10 | 3–6 | 1 | 1–2 | 1–2 |
| Stomata on bracts | Present | Present | Present | Present | Present | Present | Present | Present | Present | Present | Present | Present | Present | Present |
| Epimatium | Present | Present | Absent | Absent | Absent | Absent | Present | Present | Present | Present | Present | Present | Present | Present |
| Epimatium colour | dark-Brown | dark-Brown | Absent | Absent | Absent | Absent | Red | Red | Yellowish | Brown | Brown | Yellowish | Red | Red |
| Epimatium morphology | coriaceous and not fused with testa | coriaceous and not fused with testa | Absent | Absent | Absent | Absent | Fleshy asymmetrical cup-like shape | Fleshy asymmetrical cup-like shape | Fleshy asymmetrical cup-like shape | Fleshy or dry, papery asymmetric | Fleshy and fused with testa | Fleshy and fused with testa | Fleshy and fused with testa | Fleshy and fused with testa |
| Receptaculum | Absent | Absent | Absent | Absent | Absent | Absent | Present | Present | Absent | Absent | Absent | Absent | Absent | Absent |
| Sclereids in testa | Present | Present | Present | Present | Present | Present | Present | Present | Present | Present | Present | Present | Present | Present |
| Resin canals | Present | Present | Present | Present | Present | Present | Present | Present | Present | Present | Present | Present | Present | Present |
| Resin ducts | Absent | |||||||||||||
| Dispersal | Zoochory (birds) | Zoochory (birds) | Zoochory (birds) | Zoochory (birds) | Zoochory (birds) | Zoochory (birds) | Zoochory (birds) | Zoochory (birds) | Zoochory (birds) | Hydrochory/zoochory | Zoochory | Zoochory (birds) | Zoochory (birds) | Zoochory (birds) |
Measurements and character mapping
Many morpho-anatomical and embryological characters were recorded (Table 1). The terminology used in this study is based on Khan and Hill (2021). The anatomical layers were measured from mature seed cones using ImageJ 1.8.0_112 software. Data on seed cone size and other traits were obtained from herbarium specimens and Eckenwalder (2009), Farjon (2010, 2017, 2018) and Farjon and Filer (2013). For ancestral reconstruction, we used the dated phylogeny (Table S2) of Khan and Hill (2021) based on three chloroplast genes (rbcL, matK and trnL-trnF) and three nuclear genes (NEEDLY, PHYP and ITS2). The characters were mapped for their evolution using RASP 4.2-BayesTraits (Yu et al., 2020) and Mesquite 3.6 (Maddison and Maddison, 2019) with maximum likelihood and the Markov chain Monte Carlo reconstruction method.
RESULTS
Lepidothamnus seed cone morpho-anatomy
The Lepidothamnus intermedius seed cone is oblong (Fig. 1A–C), 5–8 mm long and 2–3 mm wide, consisting of one or two ovules each surrounded by a fertile bract and three to five sterile bracts. The ovules are obliquely erect with a recurved micropyle. The seed cone longitudinal and cross sections show six anatomical regions (Fig. 2A–D).
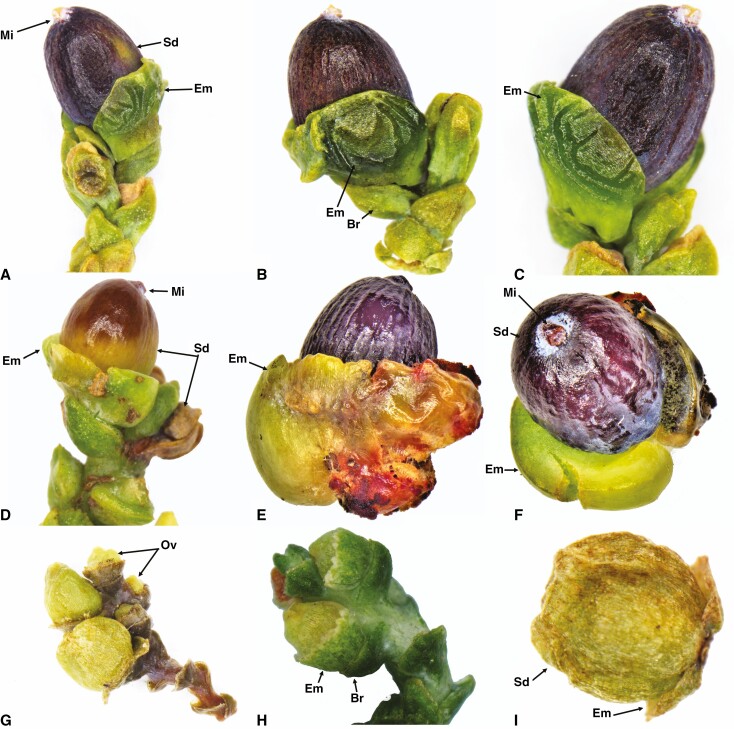
Fig. 1.
Seed cone showing morphological features of Lepidothamnus intermedius (A–C), Manoao colensoi (D–F) and Lagarostrobos franklinii (G–I). Sd, seed; Em, epimatium; Ov, ovule; Mi, micropyle; Br, bract.
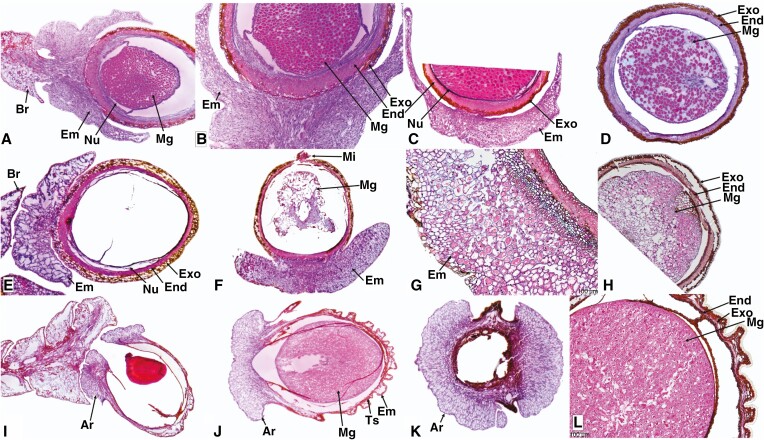
Fig. 2.
Seed cone longitudinal and cross sections showing morpho-anatomical features of Lepidothamnus intermedius (A–D), Manoao colensoi (E–H) and Halocarpus bidwillii (I–L). Br, bract; Ar, aril; Em, epimatium; Ts, testa; Exo, exotesta; End, endotesta; Nu, nucellus; Mg, megagametophyte; Mi, micropyle; Vb, vascular bundles. Scale bars (G, L) = 100 μm.
(1) Epimatium: 8–14 layers of elongate and rounded cells produce an epimatium that is free, fleshy, asymmetrical, and cup-shaped, covering one-third of the seed (Fig. 1A–C). ii. Exotesta: 3–5 layers of elongated and rounded cells. (3). Endotesta: 5–10 compact layers of sclerified cells. (4). Nucellus: 4–7 layers of cells arranged in rows above the megagametophyte. (5). Megagametophyte: straight; the embryo is 0.4–0.9 × 0.4–0.6 mm. vi. Receptacle: bright reddish and fleshy.
Manoao seed cone morpho-anatomy
Mature seed cones of Manoao colensoi are oblong, erect, glaucous purple-black and surrounded by five to seven spoon-shaped spirally arranged bracts (Fig. 1D–F). There are two to four fertile bracts per cone. Seed cones are oblong, about 4–6 mm long and 1.5–2.5 mm wide. The inverted ovules are surrounded by a fleshy, asymmetrical, cup-like, free epimatium and five to seven bracts. The seed cone longitudinal and cross sections show five major anatomical regions (Fig. 2E–H).
(1). Epimatium: 12–18 layers of large and small isodiametric cells produce a fleshy asymmetrical, cup-shaped epimatium that surrounds the seed (Fig. 1D–F). An enlarged vascular bundle and resin canal are present. (2). Exotesta: 4–6 layers of long and isodiametric cells. (3). Endotesta: 2–7 layers of compact, dense, sclerified cells. (4). Nucellus: 4–8 layers of cells arranged in rows around the megagametophyte. (5). Megagametophyte: about 10–16 layers of cells; the mature embryo is 0.4–0.6 × 0.2–0.3 mm.
Lagarostrobos seed cone morpho-anatomy
Lagarostrobos franklinii has a brown cylindrical (subglobose), strictly pendulous seed cone, about 4–5.5 mm long and 2–3 mm wide, containing five to eight seeds, each surrounded by a fertile bract. Each seed conelet contains a light brown, fleshy (initially) or dry, papery asymmetrical epimatium in the basal part (Fig. 1G–I). The ovule is inverted. The seed cone cross section shows five anatomical regions.
(1). Epimatium: 6–10 layers of large and small isodiametric cells produce an asymmetrical epimatium covering about half of one side of the seed. A vascular bundle and resin canal are present. (2). Exotesta: 2–4 layers of long, isodiametric cells. (3). Endotesta: 4–10 layers of compact, dense, sclerified cells. (4). Nucellus: 3–7 layers of cells arranged in rows above the megagametophyte. (5). Megagametophyte: straight; the embryo is 0.3–0.5 × 0.15–0.2 mm.
Parasitaxus seed cone morpho-anatomy
Parasitaxus usta has globose, red-purplish seed cones, 3–5 mm long and 3–4 mm wide. Each cone has a single seed surrounded by a fertile bract. The outer testa is fleshy. The ovule is inverted. The seed cone cross sections show five major zones.
(1). Epimatium: 3–6 layers of rectangular and rounded cells produce an epimatium that surrounds the testa and entirely fuses with it. (2). Exotesta: 10–16 layers of small and large round and elongated cells, with several vascular traces and resin canals, along with sclereids. (3). Endotesta: 6–10 compact layers of small dense cells. (4). Nucellus: 3–7 layers of dense and smaller cells. (5). Megagametophyte: straight; the embryo is 0.2–0.5 × 0.1–0.2 mm.
Halocarpus seed cone morpho-anatomy
The Halocarpus bidwillii seed cone is oblong, 3–5 mm long and 2–2.5 mm wide, with one or two inverted ovules surrounded by a fertile bract, four or five sterile bracts and one or two carpidia (a carpidium is a sub-terminal bract that is larger than the associated bract). A dark brown fleshy bract (also cited as the epimatium in the literature) is adnate to the carpidium. A white to yellowish v-shaped, fleshy free aril surrounds the seed at the proximal end. The seed cone cross section can be divided into six anatomical zones (Fig. 2I–L)
(1) Aril: free, 8–14 layers of small and elongated cells. (2) Epimatium: 2–4 layers of round-shaped cells. (3) Exotesta: 4–8 layers of small and long isodiametric cells with vascular bundles present. (4) Endotesta: 4–8 layers of compact dense cells. (5) Nucellus: 3–6 layers of cells around the megagametophyte. (6) Megagametophyte: straight; the mature embryo is 0.4–0.8 × 0.3–0.5 mm.
Phyllocladus seed cone morpho-anatomy
Phyllocladus trichomanoides seed cones occur on the phylloclades, either singly or in a pair. The seed cone is reduced and usually consists of one or two ovules in a cup-like elongated bract. The erect ovule is surrounded by a white, free arillus and two fused green fleshy bracts (one fertile and one sterile) (Fig. 3A–D). The seed cross section shows six anatomical regions (Fig. 4A–D).
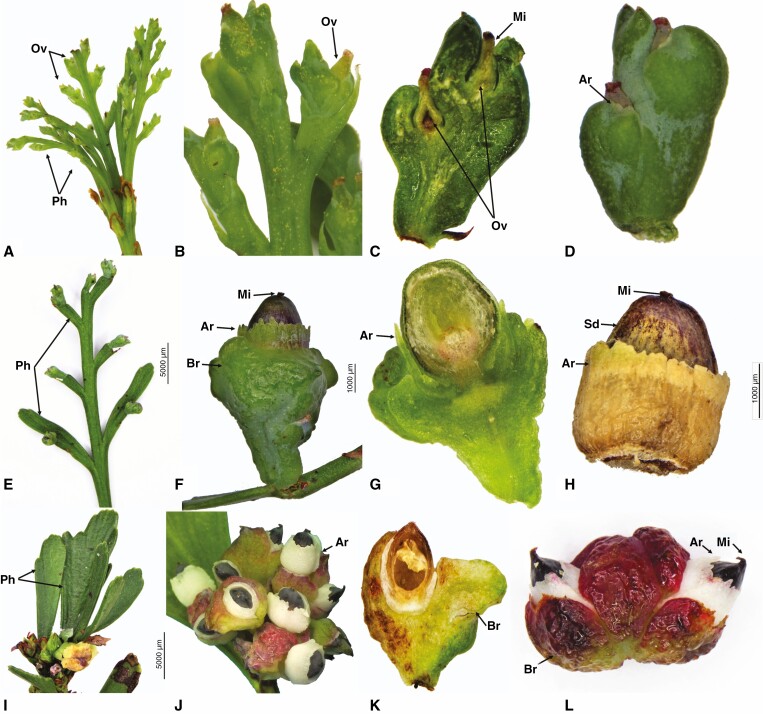
Fig. 3.
Seed cone morpho-anatomical features of Phyllocladus trichomanoides (A–D), P. hypophyllus (E–H) and P. aspleniifolius (I–L). Ph, phylloclades; Sc, seed cones; Ov, ovule; Ar, aril; Mi, micropyle; Br, bract. Scale bars (F, J) = 5000 μm; (G, H) = 1000 μm.
Fig. 4.
Seed cone longitudinal and cross-sections showing morpho-anatomical features of Phyllocladus trichomanoides longitudinal sections (A, B, D) and cross section (C), P. hypophyllus (E–H) and P. aspleniifolius (I–L). Br, bract; Sd= Seed ; Ov, ovule; Ar, aril; Mi, micropyle; Exo, exotesta; End, endotesta; Emb, embryo; Rd, resin duct; Vb, vascular bundles; Nu, nucellus (Nu); Mg, megagametophyte. Scale bars (H) = 200 μm; (L) = 100 μm.
(1) Aril: free, 3–8 layers of elongated-oval cells surround the ovule but do not fully enclose it. (2) Exotesta: 2 layers of irregular cells. (3) Mesotesta: 1 or 2 compact layers of sclerified cells. (4) Endotesta: 8–12 layers of isodiametric cells. (5) Nucellus: 10–16 layers of cells arranged in rows above the megagametophyte. (6) Megagametophyte: the mature embryo is about 0.6–0.7 × 0.2–0.3 mm.
Phyllocladus hypophyllus produces red-purple ovoid-oblong (7–10 × 1.6–2.5 mm) seed cones on the phylloclade. The ovule is erect, and the seeds are half covered by the aril (Fig. 3E–H). The seed cross section shows six anatomical regions (Fig. 4E–H).
(1) Aril: free, 10–16 layers of elongated-oval cells surround the ovule but do not fully enclose it. (2) Exotesta: 3 or 4 layers of irregular cells. (3) Mesotesta: 5–7 compact layers of sclerified cells. (4) Endotesta: 3 or 4 layers of isodiametric cells. (5) Nucellus: 6–12 layers of cells, arranged in rows above the megagametophyte. (6) Megagametophyte: the mature embryo is 0.5–0.6 × 0.2–0.3 mm.
Phyllocladus aspleniifolius produces pink-red fleshy multiovulate cones on the phylloclade. The cones are ovoid-oblong, 5–8 mm long and 2–2.5 mm wide with two to five greenish-black semi-ovoid seeds. Each seed is half enclosed by a white aril and fleshy bracts, which are intensely coloured (varying from pink to dark red) (Fig. 3I–L). The seed cross section shows six anatomical regions (Fig. 4I–L).
(1) Aril: free, 4–8 layers of rounded cells subtend the bracts but do not fully enclose the seed. (2) Exotesta: 1 or 2 layers of rounded cells. (3) Mesotesta: 5–8 compact layers of sclerified cells. (4) Endotesta: 3 or 4 layers of fibre cells. (5) Nucellus: 4–10 layers of cells surrounding the megagametophyte. (6) Megagametophyte: the mature embryo is 0.4–0.6 mm × 0.2–0.3 mm.
Prumnopitys andina seed cone morpho-anatomy
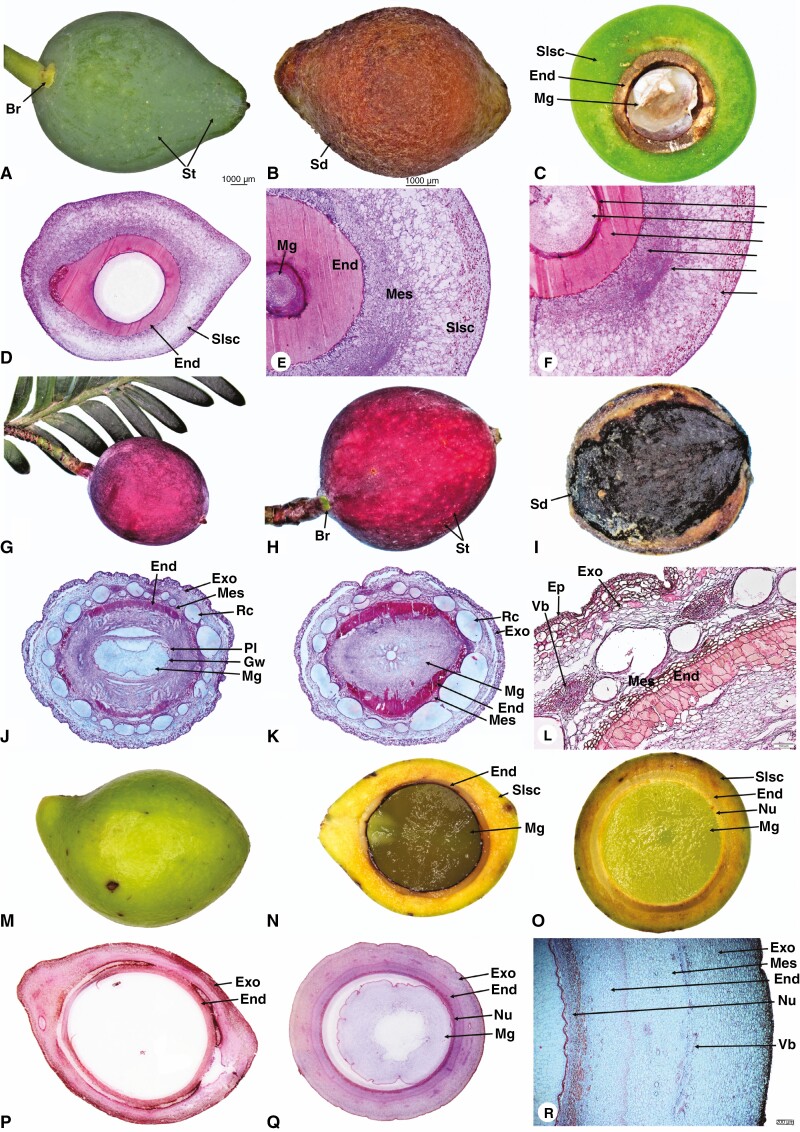
Prumnopitys andina produces yellow, oblong-subglobose seed cones with 4–12 inverted ovules. Each ovule is half surrounded by a fertile bract (Fig. 5A–C). The seed cone cross sections show six major zones (Fig. 5D–F).
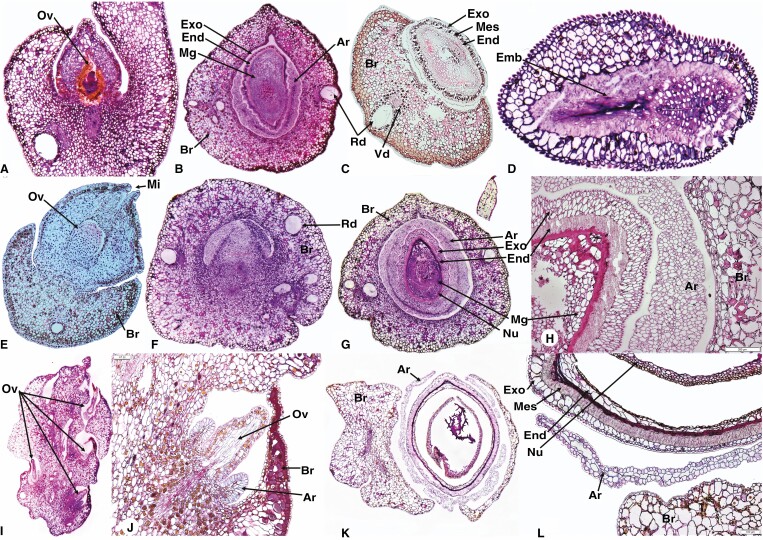
Fig. 5.
Seed cone photos (A, B, G, H, I and M), longitudinal and cross-sections showing morpho-anatomical features of Prumnopitys andina longitudinal (D) and cross sections (C, E, F); Pectinopitys ferruginea longitudinal (J) and cross sections (K, L); Sundacarpus amarus longitudinal (N, P) and cross sections (O, Q, R); Br, bract; St, stomata; Slsc, fleshy-sarcotesta-like seed coat; Sd, seed; Exo, exotesta; End, endotesta; Mg, megagametophyte; Nu, nucellus; Vb, vascular bundles; Mes, mesotesta; Rc, resin canal; Gw, gynospore wall; Pl, Parietal layers. Scale bars (A, B) = 1000 μm; (L) = 100 μm; (R) = 200 μm.
(1) Epimatium: fused, 4–12 layers of small isodiametric cells. (2) Exotesta: 16–22 layers of small and large round and elongated cells. The exotesta is fleshy and a connate part of the epimatium forms a fleshy sarcotesta-like seed coat. The outer layers have larger cells than the inner layer, which has smaller and dense cells. Vascular traces and resin canals are present in the inner layer. (3) Mesotesta: 6–10 layers of denser and compact isodiametric, parenchymatic cells. (4) Endotesta: 12–20 compact layers of small, dense, sclerified cells allow the woody part of the seed cone to form a sclerified sclerotesta-like structure. (5) Nucellus: 6–12 layers of dense and smaller cells constitute the protective cover of the embryo. (6) Gametophyte: 10–16 layers of cells; the mature embryo is 0.6–0.9 × 0.2–0.4 mm.
Pectinopitys ferruginea seed cone morpho-anatomy
Pectinopitys ferruginea produces red, ellipsoid seed cones. The seed cones have one or two inverted ovules each surrounded by a fertile bract (Fig. 5G–I). The seed cone cross sections show six major zones (Fig. 5J–L).
(1) Epimatium: fused, 4–6 layers of rectangular and rounded cells, including sclereids. (2) Exotesta: 12–16 layers of large and small elongated cells constitute the fleshy layers of the epimatium forming a fleshy sarcotesta-like seed coat, containing several vascular traces and resin canals, along with several enlarged resin ducts. (3) Mesotesta: 3–6 layers of dense and compact isodiametric, parenchymatic cells. (4) Endotesta: 6–12 compact layers of dense sclerified cells constitute the woody part of the seed cone, forming a sclerified sclerotesta-like structure. (5) Nucellus: 13–18 layers of smaller, dense cells constitute the protective cover of the embryo. (6) Gametophyte: straight; the mature embryo size is 0.6–1.0 × 0.3–0.5 mm.
Sundacarpus amarus seed cone morpho-anatomy
Sundacarpus amarus produces reddish, oblong-subglobose seed cones with an inverted ovule (Fig. 5M–O). The seed cone cross sections show six major zones (Fig. 5P–R).
(1) Epimatium: fused, 3–6 layers of rectangular and rounded cells. (2) Exotesta: 14–26 layers of small and large round and elongated cells. The exotesta is the fleshy part of the epimatium, forming a fleshy sarcotesta-like seed coat, with many vascular bundles and small and elongated resin canals. (3) Mesotesta: 4–6 layers of dense and compact round to elongated irregular, parenchymatic cells. Sclereids are not present. (4) Endotesta: 3–6 compact layers of small, dense, sclerified cells make the woody part of the seed cone, forming a sclerified sclerotesta-like structure. (5) Nucellus: 13–18 layers of dense and smaller cells constitute the protective cover of the embryo. (6) Gametophyte: straight; the mature embryo size is 0.7–1.3 × 0.3–0.5 mm.
Comparison of seed cone morpho-anatomical traits
Species of the Prumnopityoid clade show both morpho-anatomical affinities and variation of functional traits and structures within the clade and with other Podocarpaceae genera. The shape of the seed cone varies from ovoid (e.g. Manoao colensoi), narrow cylindrical (e.g. Lagarostrobos franklinii), globose (e.g. Parasitaxus usta) to ellipsoid. Seed cone sizes are the smallest in the scale-leaved Halocarpioid subclade species, while species of the broad-leaved Pectinopityoid subclade have the largest cone size (Supplementary Data Fig. 1). Manoao, Lagarostrobos and Lepidothamnus have a free, asymmetrical, cup-shaped epimatium, while in Halocarpus a coriaceous epimatium surrounds the ovule but is not fused with the testa (Figs 1 and 2). In Phyllocladus, the epimatium is absent but there is a well-developed free fleshy aril (Figs 3 and 4). Halocarpus has both an aril and an epimatium. The mesotesta is not evident in Lepidothamnus, Manoao or Lagarostrobos and the endotesta has sclerified cells but is not woody. The exotesta of mature seed cones of Pectinopitys is vascularized and has several enlarged resin ducts. Stomata are present on the seed cone surface of these genera. The seed cone arrangement varies from solitary (single-seeded) (e.g. Parasitaxus), both solitary and multiovulate (e.g. Manoao, Lepidothamnus, Halocarpus, Pectinopitys, Lagarostrobos and Phyllocladus) to multiovulate (e.g. Prumnopitys andina). Species of both subclades have differently coloured seed cones (Table 1). The morphology of the epimatium is thinly fleshy in Lepidothamnus, Manoao and Lagarostrobos, while in Parasitaxus, Prumnopitys, Pectinopitys and Sundacarpus the epimatium is fleshy and fused with the testa, forming a fleshy sarcotesta-like structure, with an endotesta that is hard and woody (sclerotesta) (Fig. 5B, I). Seed dispersal is mainly zoochorous except in Lagarostrobos, which also exhibits hydrochory.
DISCUSSION
Seed cone morpho-anatomical traits and structures
Both subclades of the Prumnopityoid clade have evolved diverse structures and traits in their seed cones.
Seed cone arrangement
In the Halocarpioid subclade, Parasitaxus has a single ovule per cone, Halocarpus and Lepidothamnus produce solitary cones (1 or 2 ovules per cone), and Phyllocladus, Manoao and Lagarostrobos have multiovulate cones. In the Pectinopityoid subclade, Prumnopitys subgenus Botryopitys (e.g. P. montana) and related genera (Pectinopitys and Sundacarpus) have simple multiovulate cones (2 seeds per cone, Page, 2019). Prumnopitys subgenus Prumnopitys has multiovulate cones (P. andina has about 4–12 seeds and P. taxifolia about 5–10 seeds; see also Mill et al., 2004).
Fleshy seed cones
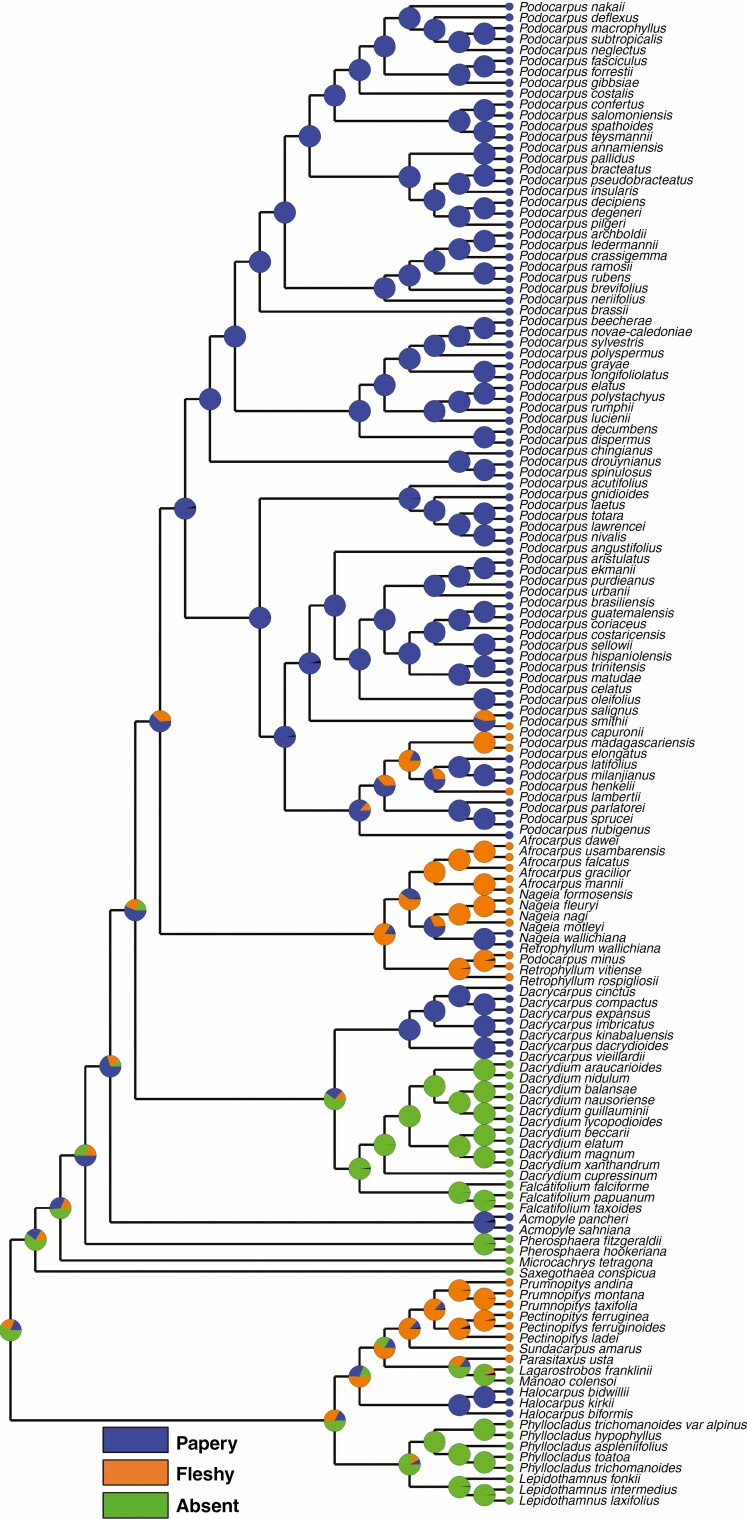
All genera of the Pectinopityoid and Halocarpioid subclades produce colourful fleshy seed cones (Figs 1, 3 and 5), although the mechanism and fleshy structures (e.g. epimatium, aril, receptaculum and bracts) differ between and within these subclades. Fleshiness is an ancestral trait in podocarps, and Pherosphaera and Saxegothaea are the only two extant genera with non-fleshy seed cones (Herting et al., 2020; Khan and Hill, 2021). Halocarpus produces fleshy cones using the aril, Phyllocladus uses the aril plus bracts, Lepidothamnus uses the receptaculum plus epimatium, and Manoao and Lagarostrobos use the epimatium, while in Prumnopitys, Pectinopitys, Sundacarpus and Parasitaxus fleshy seed cones are produced by the fusion of the epimatium and exotesta.
Prumnopitys, Pectinopitys, Sundacarpus and Parasitaxus produce drupe-like seed cones. The other two genera with similar drupe-like cones are Retrophyllum and Afrocarpus. Lepidothamnus produces Dacrydioid-type cones and has a fleshy receptaculum and asymmetrical epimatium. The other two genera with similar cone morphology are Falcatifolium and Dacrydium (Khan and Hill, 2021). Phyllocladus and Halocarpus have special arilloid-type cones with an aril and fleshy bracts. Manoao and Lagarostrobos have seed cones with fleshy, asymmetrical epimatium.
Epimatium morphology and occurrence
An epimatium is present in all genera except Phyllocladus and Pherosphaera (Tomlinson et al., 1991; Khan and Hill, 2021). The epimatium is either free or fused with the outer testa in this clade. In the taxa studied here, the epimatium is free and fleshy in Lepidothamnus, Manoao and Lagarostrobos. Dacrydium and Falcatifolium are the other two Podocarpaceae genera in the Dacrydioid clade with a similar free and fleshy epimatium (Khan and Hill, 2021). When the epimatium is fused with the testa it is either fleshy or papery, forming a sarcotesta-like seed coat (Fig. 6), such as in Parasitaxus, Pectinopitys, Prumnopitys and Sundacarpus, and this also occurs in Retrophyllum, Afrocarpus and some species of Podocarpus (i.e. P. smithii, P. henkelii, P. madagascariensis and P. capuronii) in the Podocarpoid clade (Mill et al., 2001, 2004; Khan and Hill, 2021).
Fig. 6.
Character mapping of the fused epimatium morphology in different genera of Podocarpaceae using maximum likelihood.
In Halocarpus, the epimatium is also fused with the outer testa but is papery, similar to Dacrycarpus, Acmopyle and most species of Nageia and Podocarpus (Khan and Hill, 2021). An epimatium is considered equivalent to an ovuliferous scale and is possibly eliminated in Phyllocladus by a reduction similar to that observed in Pherosphaera (Sinnott, 1913; Wilde, 1944; Tomlinson, 1992; Khan and Hill, 2021).
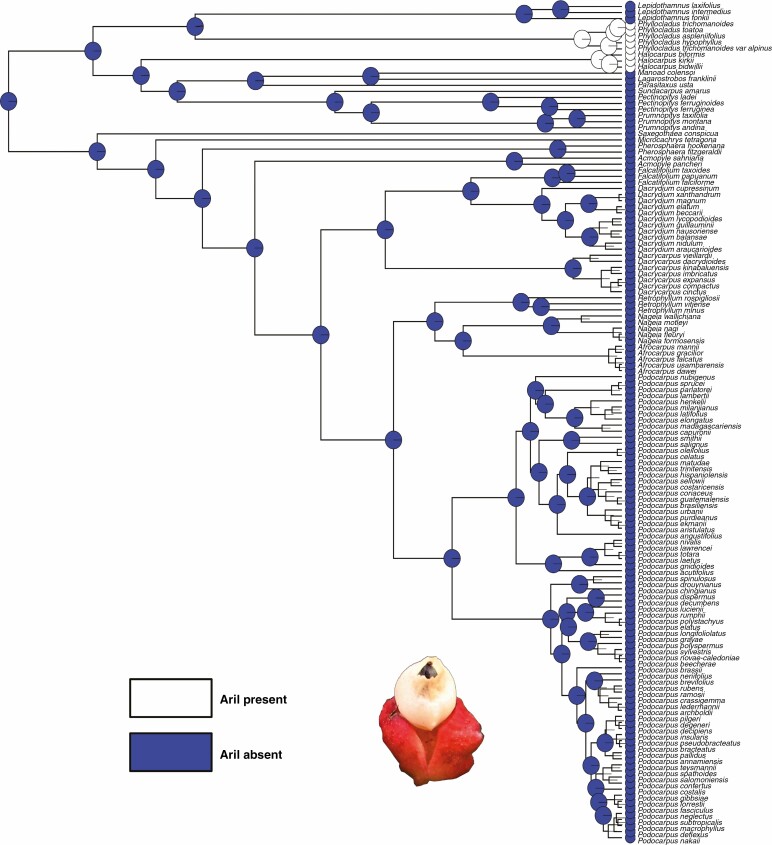
Aril morphology and occurrence
A fleshy, cup-shaped aril is only present in Phyllocladus (Fig. 3) and Halocarpus. The ancestral state reconstruction shows that an aril is a derived structure and evolved twice independently (Fig. 7). Halocarpus has an inverted ovule with a fused papery epimatium and a fleshy aril that develops at the proximal end of the bract (carpidium) and hence is the only genus with an aril, an epimatium and a distinct carpidium (Molloy, 1995). The only other conifer family with an aril is the Taxaceae and there the aril is interpreted as a fused pair of strongly swollen leaves (e.g. Pseudotaxus chienii) rather than a modified integument (Dörken et al., 2019). Hooker (1852) placed Phyllocladus in Taxaceae due to the similarity of their arils.
Fig. 7.
Character mapping of the presence of an aril in different genera of Podocarpaceae using maximum likelihood and BayesTraits.
Receptaculum morphology and occurrence
A fleshy receptaculum is present only in Lepidothamnus (Fig. 1A). The ancestral reconstruction shows that a fleshy receptaculum is a derived structure in the Prumnopityoid clade, where Lepidothamnus has evolved its fleshy receptaculum independently in the clade (Supplementary Data Fig. 2). In the initial developmental stages of the Lepidothamnus seed cone, the bracts are green and non-fleshy, but when the cone matures they develop into a red receptaculum, with a morphology similar to that reported for Falcatifolium and Dacrydium (Khan and Hill, 2021).
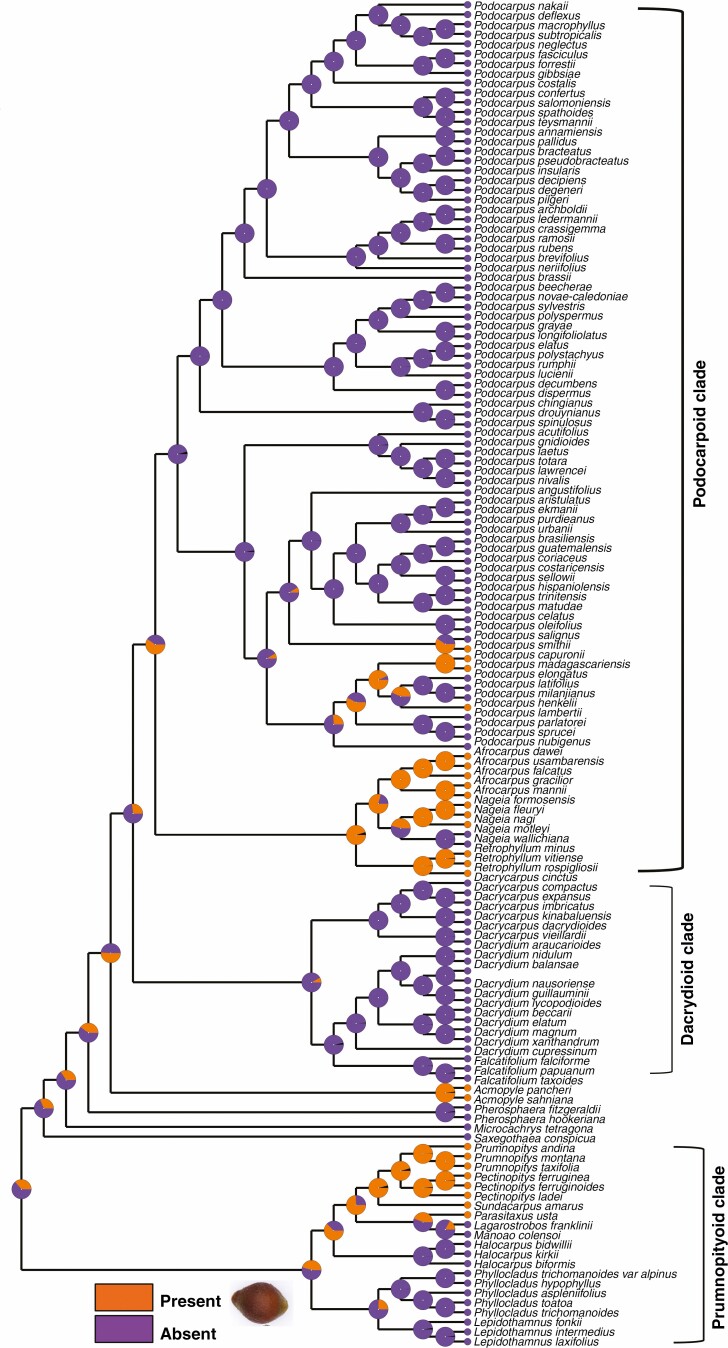
Testa morpho-anatomy
In Lepidothamnus, Halocarpus, Phyllocladus, Manoao and Lagarostrobos the testa is non-fleshy and has a papery structure (Supplementary Data Fig. 3). The endotesta in these genera is also non-woody. Other podocarp genera, e.g. Dacrydium, Dacrycarpus, Falcatifolium, Nageia (except N. wallichiana and N. motleyi) and Podocarpus (except P. smithii, P. henkelii, P. madagascariensis and P. capuronii), have a similar testa morphology (Khan and Hill, 2021), but in Acmopyle the epimatium and exotesta are papery and the endotesta is woody.
In Parasitaxus, Pectinopitys, Prumnopitys and Sundacarpus the outer testa is fused with the epimatium, forming a fleshy sarcotesta-like seed coat (Fig. 6). The endotesta is stony and woody and forms a sclerotesta-like structure. The ancestral state reconstruction shows that this sclerotesta-like structure has evolved multiple times in podocarps (Fig. 8) (e.g. Retrophyllum, Afrocarpus, Nageia and some species of Podocarpus) (Khan and Hill, 2021). The testa is resinous and vascularized in the Pectinopityoid subclade. Mill et al. (2004) also reported the presence of a vascularized testa (sarcotesta) in Pectinopitys, Prumnopitys and Afrocarpus. In all three genera, the endotesta is woody and stony (sclerotesta). Sclereids are present in the exotesta of Pectinopitys ferruginea. In both Prumnopitys andina and P. taxifolia the mesotesta is more or less sclerified and the seed cone anatomy shows the presence of simple vascular traces and few unbranched resin canals. In contrast, the seed cone of Pectinopitys ferruginea has an interconnected network of resin ducts (Mill et al., 2004).
Fig. 8.
Maximum likelihood character mapping of the presence of sclerotesta-like structures in Podocarpaceae.
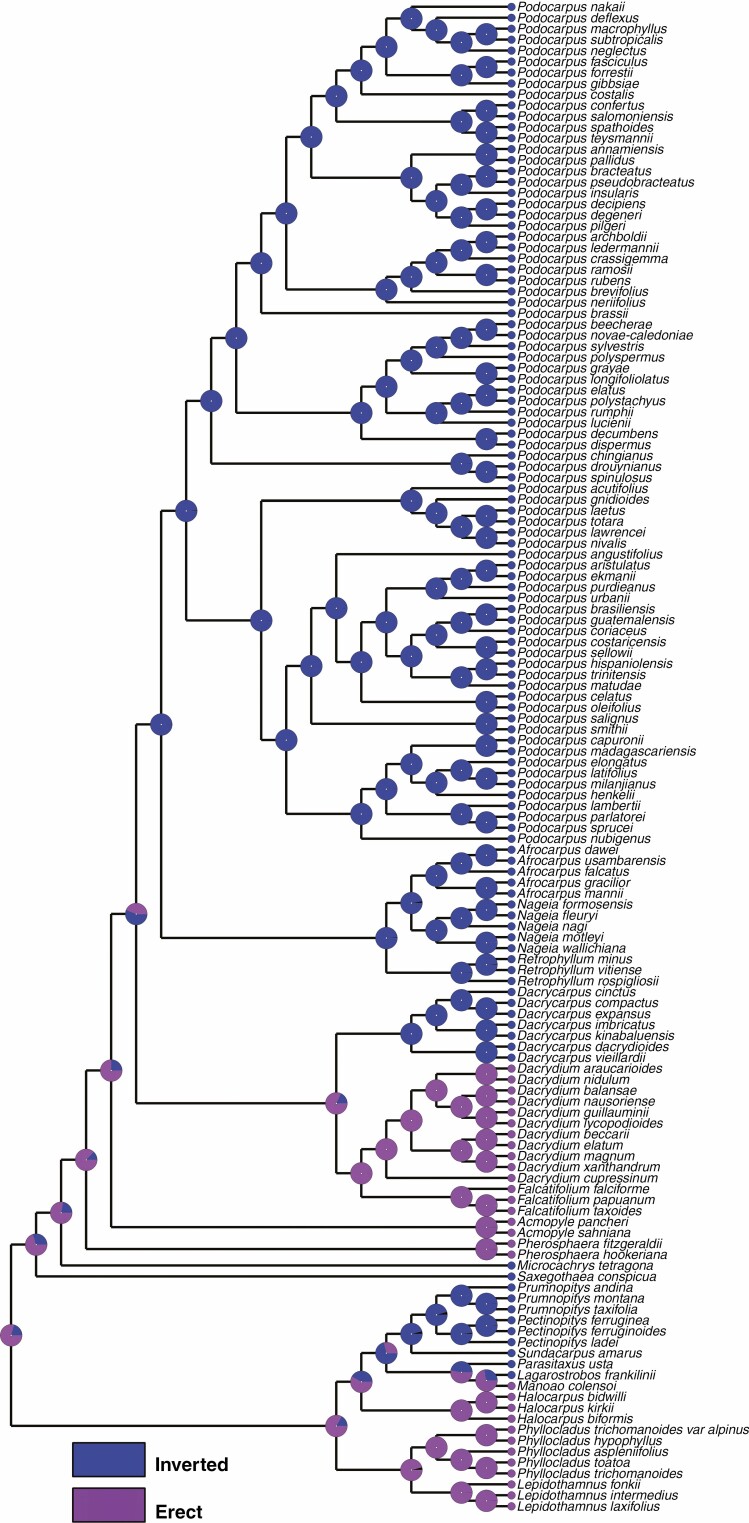
Ovule morphology and traits
All of the Prumnopityoid clade genera have inverted ovules except Lepidothamnus and Phyllocladus, where ovules are erect (i.e. ovules are vertical to the bract) (Tomlinson et al., 1991; Herting et al., 2020). Within the Podocarpaceae, Pherosphaera and Acmopyle are the only other genera with erect ovules. All species of Phyllocladus examined here have erect ovules (see also Möller et al., 2000, who reported erect ovules in P. hypophyllus). This pendulous position of the ovules in conifers is important for success in pollen capture and the pollination drop mechanism takes advantage of the saccate pollen grains in Podocarpaceae (Tomlinson et al., 1991; Owens et al., 1998; Dörken and Jagel, 2014). Ovule inversion occurs in the rest of the Prumnopityoid clade. Some early studies (Sahni, 1921; De Laubenfels, 1969) reported the presence of an inverted ovule in Acmopyle in the early developmental stages but Mill et al. (2001) reported that no developmental stage showed an inverted ovule in Acmopyle.
The micropyle orientation in the mature seed differs among the genera of the Prumnopityoid clade and both inverted and erect ovules are present in the early developmental stages (Fig. 9). The mature seed micropyle of Manoao, Lagarostrobos, Phyllocladus, Prumnopitys, Pectinopitys and Lepidothamnus is oriented towards the apex of the cone axis (Fig. 1). Acmopyle, Pherosphaera, Dacrydium and Falcatifolium share this orientation (Herting et al., 2020). Lepidothamnus and Manoao seeds have a hook-like micropyle, a character shared with Acmopyle (Tomlinson et al., 1997; Möller et al., 2000).
Fig. 9.
Character mapping of micropyle orientation in the mature seed in different genera of Podocarpaceae using RASP 4.2 and maximum likelihood.
Comparative reproductive biology
The reproductive cycle of the Halocarpioid subclade species is usually completed in 1–2 years, while in the Pectinopityoid subclade it is usually completed in ≥2 years (e.g. Tomlinson, 1992). The number of prosuspensor cells varies among the genera: Saxegothaea has 3 or 4, Phyllocladus has 4–6, Prumnopitys has 7–9, Dacrydium has 7–11 and Nageia has 18–23 (Doyle and Looby, 1939; Buchholz, 1941). The embryological development stages of Phyllocladus trichomanoides are similar to those of P. aspleniifolius (Kildahl, 1908; Quinn, 1986). The process of archegonia formation in P. trichomanoides is also similar to that in Prumnopitys andina (Looby and Doyle, 1944) and a megaspore (gynospore) membrane similar to that of Phyllocladus is also reported in Lepidothamnus laxifolius and Pectinopitys ferruginea (Quinn, 1965). The pattern of seed cone development of Manoao colensoi is closely related to that of Lepidothamnus laxifolius (Quinn, 1966), although the megaspore membrane of L. laxifolius is thinner than in M. colensoi (Quinn, 1965). In Sundacarpus, two embryo systems have been reported (Buchholz, 1941).
Diversity in functional traits of living podocarps and fossil evidence
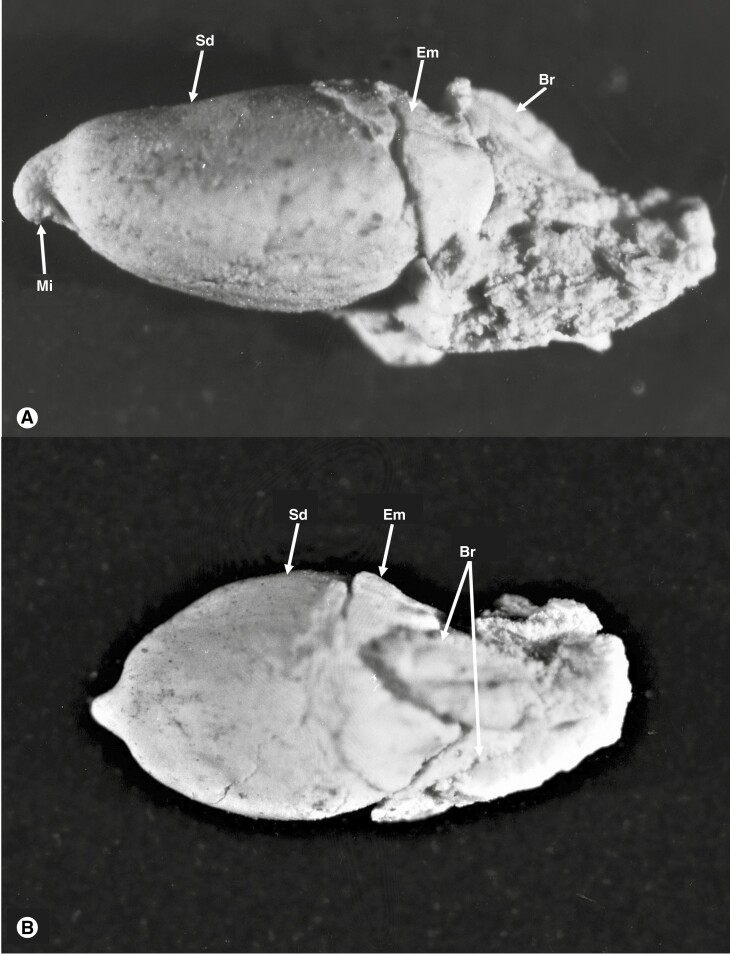
All species in the Prumnopityoid clade produce fleshy seed cones, although the fleshy structures differ among genera. Podocarps in general produce fleshy seed cones using different structures and they have evolved multiple times (Khan and Hill, 2021). Klaus and Matzke (2020) reported that the earliest transition from non-fleshy to fleshy seed cones in the Podocarpaceae occurred in the Cretaceous (∼82.4 Ma), but the fossil record contains fleshy seed cones much earlier than this. The fleshy structure in the fossilized seed cones of Lepidothamnus (Fig. 10) from the Middle Cretaceous of Winton, Queensland, Australia, also supports the presence of fleshy seed cones early in the fossil record (Peters, 1985). Similarly, a fleshy epimatium has been reported in the fossil seed cones of Harrisiocarpus gucikii and H. cracoviensis from the Middle Jurassic of Poland (Reymanówna, 1987). Similarly, fleshy seed cones of Podocarpospermum (Rajmahal Basin, India) are reported from the Lower Cretaceous (Banerji and Ghosh, 2006).
Fig. 10.
The seed cones (A, B) of an undescribed Lepidothamnus species from the mid-Cretaceous Winton, Queensland, shows morphological similarities, e.g. of the bract (Br), seed (Sd) and epimatium (Em), to those of living Lepidothamnus species. The photograph was taken by Dr M. D. Peters and is from the collection held in the David T. Blackburn Palaeobotany Collection at the University of Adelaide.
The presence of an epimatium is an ancestral trait in the Prumnopityoid clade and in Podocarpaceae and it is present in all genera of the clade except Phyllocladus. The ovuliferous scales of some extinct podocarps (e.g. Mataia and Nipaniostrobus) are folded over, covering half of the seed and are interpreted as an early evolutionary stage of epimatium evolution (Rao, 1943; Townrow, 1967; Miller, 1977; Stewart et al., 1993). Stalagma is another extinct genus from the Late Triassic in which a thin layer of tissue partially covers the seed and is considered the first evidence of an epimatium (Miller, 1977; Taylor et al., 2009; Contreras et al., 2017). The seed cones from the Middle Jurassic of Poland of two extinct fossil species (Harrisiocarpus gucikii and H. cracoviensis) resemble those of Microcachrys and show the presence of a fleshy epimatium (Reymanówna, 1987). Similarly, seed cones of an undescribed extinct species of Lepidothamnus from the middle Cretaceous of Winton, Queensland (Peters, 1985), show the presence of an asymmetrical cup-shaped epimatium (Fig. 10).
The Triassic and Jurassic fossil record shows the presence of seed cones with inverted ovules (e.g. Mataia, Harrisiocarpus and Nipaniostrobus) (Rao, 1943; Townrow, 1967; Reymanówna, 1987). In the undescribed species of Lepidothamnus from the mid-Cretaceous of Queensland, the micropyle of the seed is towards the seed cone apex (Peters, 1985).
Dispersal of seed cones
The gain or loss of traits within lineages influences their ecological dispersal through evolutionary time (Klaus and Matzke, 2020). Seed cones from the Prumnopityoid clade have predominantly zoochorous dispersal and the fleshy structures, e.g. aril, epimatium, receptaculum and sarcotesta-like seed coat, complement zoochory in extant species (Khan and Hill, 2021, 2022). Lagarostrobos is the only genus in the clade that shows both zoochory and hydrochory. Lagarostrobos franklinii is usually dispersed by water due to the flotation of seeds. However, green rosellas (Platycercus caledonicus) have also been observed to feed on Lagarostrobos and can potentially disperse the seeds (Shapcott, 1991). Lagarostrobos produces many seed cones in cycles of 5–6 years. Retrophyllum comptonii is another podocarp that shows both zoochory and hydrochory as a dispersal mechanism. Manoao has zoochorous seed dispersal and the Lepidothamnus seed cone morphology also favours zoochory (Molloy, 1995). Phyllocladus seeds are bird-dispersed and the birds digest the fleshy bracts and aril and pass the hard seeds in droppings (Tomlinson et al., 1989; Barker, 1995; Wagstaff, 2004; Jennings and Neyland, 2011).
The Pectinopityoid subclade also exhibits zoochory. Prumnopitys andina grows in mid-altitude forests in Argentina and Chile, where birds eat the seed cones, and the seeds are dispersed in their droppings (Beveridge, 1964). In the central North Island of New Zealand, Pectinopitys ferruginea exhibits frugivory (Beveridge, 1964). Prumnopitys taxifolia also exhibits zoochory in the Central North Island of New Zealand (Beveridge, 1964). The fleshy seed cones of the Prumnopityoid clade encourages zoochory, especially bird dispersal, and this is important for dispersal over long distances. Westcott et al. (2005) reported that Sundacarpus is probably dispersed by the flightless cassowary (Casuarius).
Evolutionary perspective
The Prumnopityoid clade has evolved considerable diversity in the fleshy and coloured seed cones. Four distinct types of fleshy seed cones (e.g. drupe-like, receptaculate, arilloid and dacrydioid-type cones) were recognized based on functional structures in the Prumnopityoid clade. Both free and fused epimatia have evolved in the clade (Fig. 6). An aril is a unique feature of this clade (only present in Halocarpus and Phyllocladus) in the Podocarpaceae. Similarly, both imbricate and flattened leaves are present in this clade and phylloclades are a unique trait present only in this clade. The presence of phylloclades could be a possible adaptation to cope with the competition and pressure (e.g. low light intensity) following the arrival of angiosperms, especially in the Palaeocene–Eocene (Dörken et al., 2021).
Fleshy and coloured seed cones seem likely to be an ancestral trait in this clade. The fact that the Prumnopityoid clade uses different pathways to produce fleshy seed cones demonstrates the ecological and evolutionary significance of this strategy. Klaus and Matzke (2020) stated that podocarps had probably evolved fleshy structures by the Late Cretaceous (∼82.4 Ma), correlating with the early diversification of birds (∼70–80 Ma) (Viseshakul et al., 2011; Klaus and Matzke, 2020). This evolutionary trait helps to attract birds and other animals for the dispersal of the seeds and could have played a role in long-distance dispersal in the Late Cretaceous (∼70–80 Ma) (Klaus and Matzke, 2020). Birds are considered to have evolved from theropod dinosaurs during the Jurassic (around 165–150 Ma) (Brusatte et al., 2015). The living genera of the clade are predominantly zoochorous and the production of fleshy seed cones is ancestral in the Prumnopityoid clade. However, most of these lineages demonstrate the presence of fleshy structures by the Triassic–Jurassic.
CONCLUSIONS
The reconstruction of the origin and drivers of the evolution of fleshy seed cones in podocarps is complex. The current study of the Prumnopityoid clade shows that fleshiness of seed cones is an ancestral trait that has been achieved using diverse mechanisms and functional structures. The Prumnopityoid clade now contains four main types of distinctive seed cones. The extant species of this clade are predominantly adapted to zoochorous dispersal. The evolution of different functional traits (e.g. fleshiness, aril, epimatium and receptaculum) shows multiple evolutions of fleshiness among these taxa. The seed cone morphology of the Pectinopityoid subclade is quite distinctive from that of the species of the Halocarpioid subclade.
SUPPLEMENTARY DATA
Supplementary data are available online at https://academic.oup.com/aob and consist of the following. Figure S1: seed cone size of both subclades shows that the Pectinopityoid subclade has larger cone size than the Halocarpioid subclade. Figure S2: character mapping of the presence of a receptaculum in different genera of Podocarpaceae using RASP 4.2 and maximum likelihood. Figure S3: character mapping of testa morphology in different genera of Podocarpaceae using maximum likelihood. Table S1: fossil taxa used for calibration of the phylogeny. Table S2: seed cone specimens collected from the Botanical Gardens.
ACKNOWLEDGEMENTS
We acknowledge Adelaide Microscopy, University of Adelaide, Australian National Botanic Gardens, Canberra, Mount Lofty Botanical Garden, The Tasmanian Arboretum Devonport, State Herbarium of South Australia and Australian National Herbarium, Canberra. We are also grateful to Dr David Tng, Centre for Rainforest Studies, Australia, for his help in providing plant material. R.K. did the laboratory work, analysis of data and writing of the first draft. V.M.D. contributed to first draft writing. E.B. helped in the phylogenetic inference and analysis of data. R.S.H. supervised the study and contributed to the designing, writing and revising of the final draft.
Contributor Information
Raees Khan, School of Biological Sciences, The University of Adelaide, SA, Australia.
Robert S Hill, School of Biological Sciences, The University of Adelaide, SA, Australia.
Veit M Dörken, Department of Biology, University of Konstanz, Konstanz, Germany.
Ed Biffin, School of Biological Sciences, The University of Adelaide, SA, Australia.
LITERATURE CITED
- Andruchow-Colombo A, Wilf P, Escapa IH.. 2019. A South American fossil relative of Phyllocladus: Huncocladus laubenfelsii gen. et sp. nov. (Podocarpaceae), from the early Eocene of Laguna del Hunco, Patagonia, Argentina. Australian Systematic Botany 32: 290–309. [Google Scholar]
- Banerji J, Ghosh AK.. 2006. Podospermum gen. et sp. nov., an Acmopyle-like dispersed silicified ovule/seed from Lower Cretaceous intertrappean beds of the Rajmahal Basin, India. Cretaceous Research 27: 707–711. doi: 10.1016/j.cretres.2006.03.003. [DOI] [Google Scholar]
- Barker P. 1995. Phyllocladus aspleniifolius: phenology, germination, and seedling survival. New Zealand Journal of Botany 33: 325–337. [Google Scholar]
- Biffin E, Conran JG, Lowe AJ.. 2011. Podocarp evolution: a molecular phylogenetic perspective. In: BL Turner, L Cernusak, eds. Ecology of the Podocarpaceae in tropical forests. Washington, DC: Smithsonian Institution Scholarly Press, 1–20. [Google Scholar]
- Beveridge A. 1964. Dispersal and destruction of seed in central North Island podocarp forests. Proceedings (New Zealand Ecological Society) 11: 48–55. [Google Scholar]
- Brusatte SL, O’Connor JK, Jarvis ED.. 2015. The origin and diversification of birds. Current Biology 25: R888–R898. [DOI] [PubMed] [Google Scholar]
- Buchholz JT. 1941. Embryogeny of the Podocarpaceae. Botanical Gazette 103: 1–37. doi: 10.1086/335023. [DOI] [Google Scholar]
- Contreras D, Duijnstee I, Ranks S, Marshall C, Looy C.. 2017. Evolution of dispersal strategies in conifers: functional divergence and convergence in the morphology of diaspores. Perspectives in Plant Ecology, Evolution and Systematics 24: 93–117. doi: 10.1016/j.ppees.2016.11.002. [DOI] [Google Scholar]
- Dörken VM, Jagel A.. 2014. Orientation and withdrawal of pollination drops in Cupressaceae s. l. (Coniferales). Flora – Morphology, Distribution, Functional Ecology of Plants 209: 34–44. [Google Scholar]
- Dörken VM, Nimsch H, Rudall PJ.. 2019. Origin of the Taxaceae aril: evolutionary implications of seed-cone teratologies in Pseudotaxus chienii. Annals of Botany 123: 133–143. doi: 10.1093/aob/mcy150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dörken VM, Hill RS, Jordan GJ, Parsons RF.. 2021. Evolutionary and ecological significance of photosynthetic organs in Phyllocladus (Podocarpaceae). Botanical Journal of the Linnean Society 196: 343–363. [Google Scholar]
- Doyle J, Looby W.. 1939. Embryogeny in Saxegothaea and its relation to other podocarps. Scientific Proceedings of the Royal Dublin Society 22: 127–147. [Google Scholar]
- Eckenwalder JE. 2009. Conifers of the world: the complete reference: Portland: Timber Press. [Google Scholar]
- Farjon A. 2010. A handbook of the world’s conifers, Vol. I and II. Leiden: Brill. [Google Scholar]
- Farjon A. 2018. The Kew review: conifers of the world. Kew Bulletin 73: 8. [Google Scholar]
- Farjon A. 2017. A Handbook of the world’s conifers: revised and updated edition. Leiden: Brill. [Google Scholar]
- Farjon A, Filer D.. 2013. An atlas of the world’s conifers: an analysis of their distribution, biogeography, diversity and conservation status. Leiden: Brill. [Google Scholar]
- Herting J, Stützel T.. 2020. Morphogenesis of the seed cone of Araucaria araucana (Molina) K. Koch and the evolution of the coniferous seed scale. Flora 273: 151719. doi: 10.1016/j.flora.2020.151719. [DOI] [Google Scholar]
- Herting J, Stützel T, Klaus KV.. 2020. The ancestral conifer cone: what did it look like? A modern trait-evolution approach. International Journal of Plant Sciences 181: 871–886. doi: 10.1086/710489. [DOI] [Google Scholar]
- Hooker DJ. 1852. Phyllocladus hypophyllus. In Icones Plantarum, II, 889. Cambridge University Press. https://www.jstor.org/stable/pdf/42907682.pdf?casa_token=p4_lxoRnqU0AAAAA:Kt_lI1kCDGNi0L9B9MT9UZwbt8LLAwJE3EJNDdy9BUevU1pIb-9vuPaXv-0f1-3G1ubTTudL2yVzmZXy65hquD6mGLyapAYvarvo0csdTguBz6Y9X-Gx [Google Scholar]
- Jennings S, Neyland M.. 2011. Seedling regeneration of celery-top pine (Phyllocladus aspleniifolius) after harvesting of rainforest in north-western Tasmania. Tasforests 19: 1–16. [Google Scholar]
- Kelch DG. 2002. Phylogenetic assessment of the monotypic genera Sundacarpus and Manoao (Coniferales: Podocarpaceae) utilising evidence from 18S rDNA sequences. Australian Systematic Botany 15: 29–35. [Google Scholar]
- Keng H. 1973. On the family Phyllocladaceae. Taiwania 18: 142–145. [Google Scholar]
- Keng H. 1978. The genus Phyllocladus (Phyllocladaceae). Journal of the Arnold Arboretum 59: 249–273. doi: 10.5962/bhl.part.22773. [DOI] [Google Scholar]
- Khan R, Hill R.. 2021. Morpho-anatomical affinities and evolutionary relationships of three paleoendemic podocarp genera based on seed cone traits. Annals of Botany 128: 887–902. doi: 10.1093/aob/mcab113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan R, Hill R.. 2022. Reproductive and leaf morpho-anatomy of the Australian alpine podocarp and comparison with the Australis subclade. Botany Letters 169: 237–249. doi: 10.1080/23818107.2022.2042381. [DOI] [Google Scholar]
- Kildahl NJ. 1908. The morphology of Phyllocladus alpinus. Botanical Gazette 46: 339–348. doi: 10.1086/329752. [DOI] [Google Scholar]
- Klaus KV, Matzke NJ.. 2020. Statistical comparison of trait-dependent biogeographical models indicates that Podocarpaceae dispersal is influenced by both seed cone traits and geographical distance. Systematic Biology 69: 61–75. doi: 10.1093/sysbio/syz034. [DOI] [PubMed] [Google Scholar]
- Knopf P, Schulz C, Little DP, Stützel T, Stevenson DW.. 2012. Relationships within Podocarpaceae based on DNA sequence, anatomical, morphological, and biogeographical data. Cladistics 28: 271–299. doi: 10.1111/j.1096-0031.2011.00381.x. [DOI] [PubMed] [Google Scholar]
- de Laubenfels D. 1969. A revision of the Malesian and Pacific rainforest conifers, I. Podocarpaceae, in part. Journal of the Arnold Arboretum 50: 315–369. [Google Scholar]
- Leslie AB, Beaulieu JM, Mathews S.. 2017. Variation in seed size is structured by dispersal syndrome and cone morphology in conifers and other nonflowering seed plants. New Phytologist 216: 429–437. doi: 10.1111/nph.14456. [DOI] [PubMed] [Google Scholar]
- Leslie AB, Beaulieu J, Holman G, et al. 2018. An overview of extant conifer evolution from the perspective of the fossil record. American Journal of Botany 105: 1531–1544. doi: 10.1002/ajb2.1143. [DOI] [PubMed] [Google Scholar]
- Looby W, Doyle J.. 1944. The gametophytes of Podocarpus andinus. Scientific Proceedings of the Royal Dublin Society 23: 222–237. [Google Scholar]
- Maddison W, Maddison D.. 2019. Mesquite: A modular system for evolutionary analysis. Version 3. 61. 2019. http://www.mesquiteproject.org/.
- Melikian A, Bobrov A.. 2000. Morphology of the female reproductive structures and an attempt to construct the phylogenetic system of the orders Podocarpales, Cephalotaxales and Taxales. Botanicheskiĭ Zhurnal 85: 50–68. [Google Scholar]
- Mill R, Möller M, Christie F, Glidewell S, Masson D, Williamson B.. 2001. Morphology, anatomy and ontogeny of female cones in Acmopyle pancheri (Brongn. & Gris) Pilg. (Podocarpaceae). Annals of Botany 88: 55–67. [Google Scholar]
- Mill RR, Möller M, Glidewell SM, Masson D, Williamson B.. 2004. Comparative anatomy and morphology of fertile complexes of Prumnopitys and Afrocarpus species (Podocarpaceae) as revealed by histology and NMR imaging, and their relevance to systematics. Botanical Journal of the Linnean Society 145: 295–316. doi: 10.1111/j.1095-8339.2004.00289.x. [DOI] [Google Scholar]
- Miller CN. 1977. Mesozoic conifers. Botanical Review 43: 217–280. doi: 10.1007/bf02860718. [DOI] [Google Scholar]
- Möller M, Mill R, Glidewell S, Masson D, Williamson B, Bateman R.. 2000. Comparative biology of the pollination mechanisms in Acmopyle pancheri and Phyllocladus hypophyllus (Podocarpaceae s. l.). Annals of Botany 86: 149–158. [Google Scholar]
- Molloy B. 1995. Manoao (Podocarpaceae), a new monotypic conifer genus endemic to New Zealand. New Zealand Journal of Botany 33: 183–201. [Google Scholar]
- Mundry I. 2000. Morphologische und morphogenetische Untersuchungen zur Evolution der Gymnospermen. Bibliotheca Botanica 152: 1–90. [Google Scholar]
- Nigris S, D’Apice G, Moschin S, Ciarle R, Baldan B.. 2021. Fleshy structures associated with ovule protection and seed dispersal in gymnosperms: a systematic and evolutionary overview. Critical Reviews in Plant Sciences 40: 285–302. doi: 10.1080/07352689.2021.1938397. [DOI] [Google Scholar]
- Owens JN, Takaso T, Runions CJ.. 1998. Pollination in conifers. Trends in Plant Science 3: 479–485. [Google Scholar]
- Page CN. 2019. New and maintained genera in the taxonomic alliance of Prumnopitys s. l. (Podocarpaceae), and circumscription of a new genus: Pectinopitys. New Zealand Journal of Botany 57: 137–153. doi: 10.1080/0028825x.2019.1625933. [DOI] [Google Scholar]
- Peters MD. 1985. A taxonomic analysis of a middle Cretaceous megafossil plant assemblage from Queensland, Australia. PhD Thesis, University of Adelaide, Australia. [Google Scholar]
- Quinn C. 1965. Gametophyte development and embryogeny in the Podocarpaceae. II. Dacrydium laxifolium. Phytomorphology 15: 37–45. [Google Scholar]
- Quinn C. 1966. Gametophyte development and embryogeny in the Podocarpaceae. III. Dacrydium bidwillii. Phytomorphology 16: 1–9. [Google Scholar]
- Quinn C. 1986. Embryogeny in Phyllocladus. New Zealand Journal of Botany 24: 575–579. [Google Scholar]
- Rao A. 1943. Nipaniostrobus, a new genus of Dacrydium-like seed-bearing cones, and other silicified plants from the Rajmahal series. Proceedings of the National Academy of Sciences of India 13: 113–133. [Google Scholar]
- Restemeyer J. 2002. Morphologische und morphogenetische Untersuchungen zur Phylogenie und Evolution der Podocarpaceae und Phyllocladaceae. PhD Thesis, Ruhr University Bochum,Germany. [Google Scholar]
- Reymanówna M. 1987. A Jurassic podocarp from Poland. Review of Palaeobotany and Palynology 51: 133–143. doi: 10.1016/0034-6667(87)90026-1. [DOI] [Google Scholar]
- Sahni B. 1921. VII.—On the structure and affinities of Acmopyle pancheri, Pilger. Philosophical Transactions of the Royal Society of London Series B 210: 253–310. [Google Scholar]
- Salter J. 2004. Comparative morphological, anatomical and embryological studies of Prumnopitys taxifolia and P. ferruginea (Podocarpaceae), and the hydrodynamics of their saccate pollen grains. PhD Thesis, University of Auckland, Australia. [Google Scholar]
- Shapcott A. 1991. Dispersal and establishment of Huon pine (Lagarostrobos franklinii). Papers and Proceedings of the Royal Society of Tasmania 125: 17–26. [Google Scholar]
- Sinnott EW. 1913. The morphology of the reproductive structures in the Podocarpineae. Annals of Botany 27: 39–82. [Google Scholar]
- Stewart WN, Stewart WN, Stewart WM, Rothwell GW.. 1993. Paleobotany and the evolution of plants. Cambridge, USA: Cambridge University Press. [Google Scholar]
- Taylor EL, Taylor TN, Krings M.. 2009. Paleobotany: the biology and evolution of fossil plants. Burlington, USA: Academic Press. [Google Scholar]
- Tomlinson P. 1992. Aspects of cone morphology and development in Podocarpaceae (Coniferales). International Journal of Plant Sciences 153: 572–588. [Google Scholar]
- Tomlinson P, Takaso T, Rattenbury J.. 1989. Cone and ovule ontogeny in Phyllocladus (Podocarpaceae). Botanical Journal of the Linnean Society 99: 209–221. [Google Scholar]
- Tomlinson P, Braggins J, Rattenbury J.. 1991. Pollination drop in relation to cone morphology in Podocarpaceae: a novel reproductive mechanism. American Journal of Botany 78: 1289–1303. [Google Scholar]
- Tomlinson PB, Braggins JE, Rattenbury JA.. 1997. Contrasted pollen capture mechanisms in Phyllocladaceae and certain Podocarpaceae (Coniferales). American Journal of Botany 84: 214–223. doi: 10.2307/2446083. [DOI] [PubMed] [Google Scholar]
- Townrow JA. 1967. Conifer from the Jurassic of east Antarctica. Papers and Proceedings of the Royal Society of Tasmania 101: 137–149. [Google Scholar]
- Viseshakul N, Charoennitikul W, Kitamura S, et al. 2011. A phylogeny of frugivorous hornbills linked to the evolution of Indian plants within Asian rainforests. Journal of Evolutionary Biology 24: 1533–1545. [DOI] [PubMed] [Google Scholar]
- Wagstaff SJ. 2004. Evolution and biogeography of the austral genus Phyllocladus (Podocarpaceae). Journal of Biogeography 31: 1569–1577. doi: 10.1111/j.1365-2699.2004.01066.x. [DOI] [Google Scholar]
- Westcott DA, Bentrupperbäumer J, Bradford MG, McKeown A.. 2005. Incorporating patterns of disperser behaviour into models of seed dispersal and its effects on estimated dispersal curves. Oecologia 146: 57–67. doi: 10.1007/s00442-005-0178-1. [DOI] [PubMed] [Google Scholar]
- Wilde MH. 1944. A new interpretation of coniferous cones: I. Podocarpaceae (Podocarpus). Annals of Botany 8: 1–41. doi: 10.1093/oxfordjournals.aob.a088549. [DOI] [Google Scholar]
- Yu Y, Blair C, He X.. 2020. RASP 4: ancestral state reconstruction tool for multiple genes and characters. Molecular Biology and Evolution 37: 604–606. doi: 10.1093/molbev/msz257. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.