Abstract
Sleep and emotions are closely associated; however, the methodological challenges in the examination of sleep and the processes of emotion regulation in children and adolescents have not been investigated so far. Additionally, there is the demand to identify the levels of emotion regulating processes in which problematic or restricted sleep causes effect. Experimental sleep deprivation as well as prevalent sleep problems have been found to have negative influence on mental health and regulating functions. This review focuses first on the methodological protocols of the included studies. Subsequently, the results are summarized in the context of a multilevel model of emotion regulation. Thereafter, suggestions for future directions are given. Sleep problems and sleep deprivation are associated with a decrease of functional emotion regulating behavior and impaired emotion generation, and prolonged sleep enhances better mood and affect states, positive emotion expression, and faster sensory processing in response to emotional stimuli. This literature review highlights the limitations in current research, focusing on types of measurements, task characteristics, and data analysis. At the conclusion, suggestions are given for the future research direction in the field of sleep and emotion regulation in children and adolescents.
Keywords: Sleep, Sleep Deprivation, Emotions, Child, Emotional Regulation
INTRODUCTION
Problematic sleep and the risk for mental health
The relevance of sleep to emotional and affect regulating processes has been well established1,2. This becomes even more important, considering that about 15%-30% of children and adolescents experience difficulties with sleep, characterized by symptoms of insomnia such as sleep onset delay or prolonged nocturnal awakenings, and poor sleep quality3,4. This high prevalence is concerning, as the health risk associated with problematic sleep includes the development of affective and emotional problems1,5,6, and shortcomings in multiple domains of emotion regulation functioning7,8. According to cross-sectional data, inadequate sleep patterns seem to be associated with symptoms of childhood anxiety and impulsivity9, higher levels of hyperactivity and more conduct and peer problems10. It was also postulated that persistent sleep problems in childhood predicted adulthood anxiety disorders and affective dysregulation as long-term effects2,11. Furthermore, studies following an experimental design have brought evidence for a direct interconnectedness of sleep and emotion regulation processes; when sleep of healthy people has been manipulated with nights of sleep deprivation, evidence was found of more negative affect states12,13, less positive mood, with more feelings of tension and anxiety14, more symptoms of depression15, and less happiness16.
Whereas the enlightening of the associations between sleep and emotion regulation in children and adolescents is necessary to develop a better understanding about their impact on developmental processes, investigation of these associations is still challenging because emotion regulation is a broadly used construct, whose operationalization as well as outcomes are poorly defined17.
Emotion regulation - a multifaceted framework from developmental perspective
The process model of emotion regulation by Gross (1998)18 seems to be one of the most cited available theoretical frameworks to understand emotion regulation19. Whereas this well-established conceptualization of emotion regulation primarily focuses on intrinsic emotional response modifying processes to accomplish the individual goals, developmental aspects of emotion and affect regulation, as children’s social interaction with others are left out of consideration20. Especially developmental research favored a multilevel definition of emotion regulation, including different dimensions of regulation processes21, considering the intrapersonal, as well as social aspects of emotion regulation22. Within this conceptualization emotion regulation is considered as an adaptive system including physiological, attentional, emotional, behavioral, cognitive, and interpersonal levels22,23. However, the question of “what is regulated” per level remains open24. A multifaceted systemic scheme organizes the umbrella term of emotion regulation into a structured framework with an encompassing range of concrete objectives related to different levels of emotion regulation24 (see Table 1).
Table 1.
Summary of levels of emotion regulation (Calkins and Fox, 2002)22 and related process objectives (Thompson, 1994)24.
| Emotion regulation level | Emotion regulation processes/objectives |
|---|---|
| Physiological level | • Regulation of reactions of
the nervous system • Regulation of arousal through, e.g. response inhibition • Interpretation of biological cues related to emotional arousal |
| Attentional level | • Shifting/redirecting
attention • Behavioral distraction • Speed of processing |
| Emotional level | • Evaluation of positive or
negative affect • Regulation of upcoming tension |
| Behavioral level | • Controlling the intensity of
emotional reactions with consideration of environmental
demands • Estimation and implementation of appropriate behavioral reaction • Fight or flight decision |
| Cognitive level | • Cognitive
coping • Construal’s of emotionally arousing events • Reattribution of emotional content • Defense mechanisms |
| Interpersonal level | • Interpersonal
coping • Estimation of emotional requirements of familiar settings • Selecting settings with which being emotional comfortable |
Within neurophysiological processes, the activity of the nervous system to manage emotional arousal is central. Regions of the temporal cortex, particularly the amygdala is a key component of the cortical emotional processing25 known to show promptly responses of the nervous systems to a manifold of arousing stimuli26,27. The competence of e.g. inhibitory control over emotional arousal or executive cognitive functioning, growths proportional to the progress of cortical development28.
Governance of attentional processes is one of the first attempt of emotion regulation expected to appear during infancy and continues to be used in late adulthood29,30. Already young children are able to escape from emotionally arousing events through shifting their attention towards stimuli voluntarily to reduce their emotional reactivity30. Those regulation strategies of attention management become more complex with age and involve the internal redirection of attention, as thinking positive during distressing experience31 or behaviorally distraction32. Measurement of these attentional processes in response to emotional stimuli is somewhat difficult due to its internal character, but reaction times, as well as measuring accuracy to e.g. spatial cues with emotion eliciting content represent an opportunity to objectivate attentional processing33.
Whereas regulation of attention towards emotion eliciting events at young age can be governing extrinsic, e.g., through parental assistance, the intrinsic component of emotion regulation is represented by constructs of emotionally arousing events and emerge with age24. Such cognitive self-defense mechanisms include rationalization as well as reappraisal, which involves deliberately changing the way individual think about the meaning of an emotionally arousing stimulus or situation32,34. Therefore, these mechanisms are expected to result in modified personal causal attributions of affect and emotion arousing events24.
Next, the encoding of biological emotional cues is also an attempt to regulate emotional arousal. Biological indices for an advanced affective state are increased heart, and breathing rate or perspiration35,36. As an increased heart rate is the physiological response towards an external stimulus37 the emotional response of fear is the result of the perception process and an individual is willing to assume that fleeing will be the appropriate behavioral response38. Consequently, the interpretation of biological cues regulates the behavioral response.
Additionally, the access to coping strategies is an important facet of emotion regulation. When people believe they possess sufficient resources to cope with stressors, they experience a challenge response associated with positive outcomes such as mastering a challenging situation or feeling resilient39. In contrast, when situational demands are perceived as exceeding resources, individuals experience threat resulting in e.g. impairment of executive functioning and decision-making40. In early childhood material coping, such as playing with a favorite toy or listen to a radio play, as well as interpersonal coping mechanisms such as seeking (physical) proximity to caregivers under stressful situations, is common41. With increasing age, interpersonal coping becomes superior, e.g. peers are sought out for their expected emotional support42. Subsequently, individuals create their everyday life interactions, as well as their environmental life-style setting, as social relationships, work-place, family, memberships, etc., in accordance to their self-perceived needs, including emotional demands which is valued as comfortable and manageable43.
Finally, the process of choosing a functional expression of emotion means generating an appropriate behavioral reaction representing the individually perceived emotional arousal. For example, a careful analysis of the emotions of all parties involved in a peer conflict, combined with an insight into the negative interpersonal consequences of anger and aggression, may help a person to find a satisfactory way out of this challenging situation by trying to get others to support him or her and protest together against an unjust state of affairs instead of blindly lashing out in anger24.
The gap in methodological discussion
Recently, a variety of subjective and objective measurements was used in studies to assess the association of sleep and emotion regulation. In addition, different paradigms with stimuli that address affect and emotion, such as separation scenarios or puzzle tasks, emotional images or faces, have been implemented to assess how sleep influences children’s and adolescents’ responses to those stimuli in an objective manner. These reviews addressing sleep and emotion regulation processes in children have focused on sleep and psychopathological symptoms6, or on general consequences of sleep loss44. Of course, there are reviews investigating the association of sleep and emotion in adults1,2,45. These have concentrated on sleep effects on individual constructs of emotion, e.g. social emotion2. One integrative review concerned sleep effects on levels of emotion regulation in adults1, but a methodological discussion component was lacking. Especially this discussion is important, because differences in methods can cause different results.
In sum, the question remains open2 about the influences of sleep manipulation or sleep problems on different constructs of a multifaceted concept that represents emotion regulation processes for a young population, as well as the questions of methodological challenges to evaluate the association of sleep and emotion regulation. The present review has been initiated to fill this gap and, it is intended to be a practical, methodological assistance to researchers when planning and analyzing future research on the association of particular levels of emotion regulation processes and sleep in children and adolescents.
OBJECTIVES AND METHODS
The primary goal of this review was to initiate a systematic review focusing on the methodological protocols, thus involving different paradigms and designs of the current literature investigating the effect of sleep on levels of emotion regulation processes in children and adolescents. In detail, we began by systematically reviewing the sleep and emotion literature, including their subjective and objective outcome measures, as well as experimental tasks and their longitudinal and cross-sectional designs. Secondly, the results of the investigation are summarized and discussed in the context of the introduced processes of emotion regulation24. Additionally, key methodological limitations are discussed. This review concludes by suggesting some future directions for further research.
Criteria of study selection
Inclusion and exclusion criteria are based on the recommendation of the preferred reporting items for systematic reviews and meta-analysis (PRISMA) statement46 (see Table 2). It should be noted, that this systematic search was not restricted to a particular study design, due to our aim to figure out the complexity as well as the heterogeneity while investigating the association of sleep and emotion regulation.
Table 2.
Inclusion and exclusion criteria according to the PRISMA recommendation.
| Inclusion criteria | |
|---|---|
| Study characteristics | - Articles were available via the
chosen databases - Unpublished studies were included if they are available - Written in English - The title, abstract or keywords contained the listed search terms |
| Types of studies | - Experimental studies - Correlation studies - Data gathering based on parental, teacher or self-report - Objective and subjective measurement of sleep |
| Participants | - Families with healthy and normally
developed children - Adolescent persons - 0-18 years of age - Participants are in normal physical and psychological health and without medication - Participants suffer from problematic sleep |
| Types of intervention | - Trials investigating the effect of
experimentally induced sleep restriction or scheduled sleep
manipulation - Cross-sectional and longitudinal |
| Types of outcome measures | - Sleep duration, quality,
efficiency - Temperament, mood, emotional-responses, knowledge, problems, functioning, regulation |
| Exclusion criteria | |
| Types of studies | - Studies of sleep restriction
omitting the investigation of emotional components - Studies, that are no longer publicly available and there was no response from the corresponding authors |
| Types of publication | - Book chapters, commentaries and reviews |
| Participants | - Studies with samples of participants older than 18 years of age |
| - Samples with diagnosed psychological disorders, chronically diagnosed illness, or special medical circumstances |
Regarding the chosen outcome measure of emotion regulation processes, we attempt to outline differences in assessment and study results through the span from early childhood to adolescence. Because data assessment of infants or toddler’s emotion regulation includes usually subjective, parental report, objective measures are more common in older children and adolescents47. Therefore, we did not restrain our search to studies including either subjective or objective methods. Furthermore, different regulation processes are assessed differently, a constraint to certain methods, such as emotional tasks making use of emotional stimuli or stress inducing paradigms, would lead to fail the aim of the present review.
Additionally, to enlighten the influence of distinct assessment and parameters of sleep on the outcome measure of emotion regulation, the authors decided to include papers with subjective and objective assessment of sleep parameters as sleep quality, efficiency and duration, as well as experimental sleep manipulation as nap deprivation in toddlers and night sleep deprivation in older children and adolescents.
Strategy of search and sources of literature
A systematic literature search according to the recommendations of Moher et al. (2009)46 was conducted using the electronic databases considered appropriate for health and psychology. MEDLINE, PsychINFO, PsycARTICLES, PSYNDEX, and Google Scholar were chosen databases. Publications up to beginning of May 2021 were included. Keywords representing the part of sleep were “sleep”, “sleep deprivation”, “shortened sleep”, “sleep duration”, “sleep disturbances”, “sleep problems”, “sleep disorder”, and “insomnia”. Search terms for emotion regulation were “emotion”, “regulation”, and “affect”. To complete the search formula “toddler”, “children” and “adolescent” were added to represent the relevant age groups. Search terms were combined by the Boolean operators OR and AND. Titles, abstracts, and keywords were checked to ensure that only articles dealing with the specific terms were included. Additionally, we reviewed lists of suggestions from the search engines, and articles included in the references of the chosen documents were reviewed for their relevance.
Collection process and study information management
One author (FL) screened titles and abstracts of possible research papers. As a second stage, studies were evaluated for their eligibility according to the inclusion criteria by two authors (FL, MS, process was supervised by AS). The whole paper was read and information was collected in accordance with Bonvanie et al. (2017)48. This structure contains: 1) aim and study designs, 2) sample characteristics, 3) details of the study, 4) outcome measures, and 5) results. Figure 1 provides the flowchart of the literature search and selection process. The resulting 32 papers were organized to their measurement to assess emotion related processes in combination with and without experimental sleep manipulation, as well as to their longitudinal character. The papers are summarized in Table 3.
Figure 1.
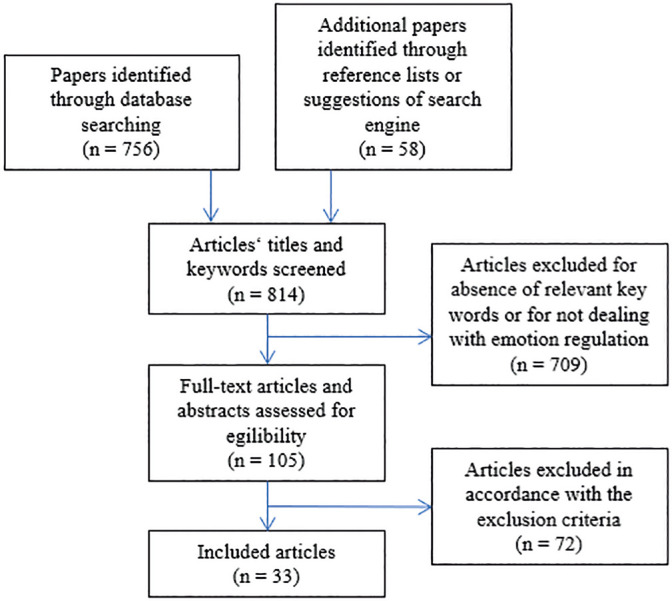
Summary of literature search and selection process.
Table 3.
Summary of studies included to the review.
| Author | Subjects N (Mage in years) |
Design | Task | Stimuli | Measures | Sleep Measures | Result | |
|---|---|---|---|---|---|---|---|---|
| 1 | Bastien et al. (2019)85 | 82 (2.1) | Longitudinal | - | - | Toddler behavior assessment questionnaire. | Actigraphy. | Shorter nighttime sleep duration and lower sleep efficiency at the age of 2 years predicted more anger at 3 years. Higher rates of social fear at 2 years predicted shorter day- and nighttime sleep duration at 3 years. |
| 2 | Baum et al. (2014)69 | 50 (15.5) | Between-subjects. SR: 6.5hrs of sleep SE: 10hrs of sleep for 4 nights. |
- | - | Vanderbilt assessment scale, Emotion control subscale of the behavior rating inventory of executive functioning, POMS. | Sleep diary, actigraphy. | SR predicted increased levels of
tension and anxiety, oppositionality, and less emotion
regulation. Mood dimensions deteriorate, except depressive mood. |
| 3 | Bayes and Bullock (2020)79 | 114 (8.4) | Cross-sectional | - | - | Conner’s behavior rating scale. | Sleep disorders inventory for students-children and adolescents. | Sleep problems seem to be moderately associated to emotional distress, aggressive behavior, and impulsivity/hyperactivity |
| 4 | Berger et al. (2012)62 | 10 (2.8) | Within-subjects. Afternoon nap deprivation. | Affective response
task. Unsolvable puzzle task. |
11 emotional images (5 positive, 3
neutral, 3 negative). Incorrect piece in the puzzle. |
Behavioral rating. | Sleep diary, CSHQ, actigraphy. | ND predicted significant more negative/less positive affect to emotional images, and duration of emotional responses during the puzzle is affected by ND. |
| 5 | Bolinger et al. (2018)49 | 16 (9,3) | Within-subjects. | Encoding and recognition task. |
444 emotional images of the IAPS. | PANAS, LPP, HRD. | Stanford sleepiness scale, PSG. | After nocturnal sleep, emotional responses that are automatic as HRD increase, and cognitive emotional responses as subjective behavioral ratings and neurological activity LPP decreased. |
| 6 | Cho et al. (2017)60 | 123 (2.0) | Longitudinal | A 5 min version of laboratory temperament assessment battery. Snack delay task. | 6 laboratory episodes Puppet show Clown interaction Stranger approach Stranger working Spider Robot |
Behavioral ratings, ITSEA, and ECG. | Sleep diary. | Longer sleep duration predicted fewer internalizing symptoms in children showing a higher RSA. |
| 7 | Cremone et al. (2017)52 | 43 (4.6) | Between-subjects | Dot-probe task. | 32 happy/neutral and angry/neutral
face pairs on a screen. Trial: fixation (500ms), stimuli presentation (1000ms), probe (1100ms). |
Accuracy and reaction times. | PSG measures. | No emotional attention bias
following N. ND exhibit bias to negative and positive stimuli. Greater SWA during N predicted faster responding to emotional stimuli. |
| Author |
Subjects
N (Mage in years) |
Design | Task | Stimuli | Measures | Measures-Sleep | Result | |
| 8 | Dagys et al. (2012)68 | 47 (13.1) | Within-subjects. SR: 2hrs of sleep SE: 8.5hrs of sleep for 2 nights. |
- | - | PANAS-C, children’s morningness-eveningness preferences scale. | Duke structured interview for sleep disorder, sleep diary, actigraphy. | SE predicted more positive affect,
positivity. No difference concerning negative affect between SE and SR. Evening as well as morning chronotypes displayed less positive affect after SR. |
| 9 | DeLeon and Karraker (2007)65 | 41 (0.7) | Cross-sectional | - | - | Revised infant temperament questionnaire, Infant/Toddler symptom checklist. | Infant care diary. | Rhythmic and adaptable infants took
longer naps and slept more at night. Distractible children took shorter and more frequent naps. |
| 11 | Foley and Weinraub (2017)82 | 1057 | Longitudinal. Assessment took place at the age of 54 months in grade 1, 3, and 5. | - | - | Generated questionnaire for
feelings, risky behavior and emotional regulation. Child behavior checklist. Children’s depression inventory. |
CBCL. | Early sleep problems predicted
anxious-depressed symptoms in the middle childhood, a higher
rate of emotional reactivity in the
preadolescence. Gender differences in temporal development of sleep and emotion problems exist. |
| 12 | Gregory and O’Connor (2002)81 | 490 Assessments from 3 to 15 years of age. |
Longitudinal | - | - | CBCL. | CBCL. | Early sleep problems at 4y predicted
depression/anxiety, attention problems, and aggression in
adolescent age No evidence of early depression/anxiety symptoms predicting later sleep problems. |
| 13 | Gruber et al. (2012)77 | 33 (8.6) | Within-subjects. SR/SE: 1hr later/earlier to bed for 5 nights. |
- | - | Connors’ global index-teacher. | SE predicted significant lower emotional lability and restless-impulsivity. | |
| 14 | Gruber et al. (2020)80 | 122 (8.6) | Cross-sectional | - | - | CBCL. | CSHQ, actigraphy. | Children scored above the cut-off of
the CSHQ had more emotional problems. Data is in consent with the subjective sleep data. |
| Author |
Subjects
N (Mage in years) |
Design | Task | Stimuli | Measures | Measures-Sleep | Result | |
| 15 | Han (2014)66 | 14 (4.8) | Within-subjects. Afternoon nap deprivation. | Affective response task. | 34 emotional images with appropriate
auditory stimuli (8 strong negative and positive, 8 weak
positive and negative). Trial: 11s, fixation (2s), cue to attention (2s), stimuli presentation (7s). |
fEMG. | Sleep diary, actigraphy. | ND predicted greater emotional responses to strong negative and positive stimuli. No change in affective responses to weak stimuli. Emotional responses to emotional pictures were lower after the N. |
| 16 | Kouros and El-Sheik (2015)72 | 142 (10.7) | Cross-sectional | - | - | Daily mood report, Personality inventory for children. | Actigraphy. | Sleep latency, efficiency, mood and behavioral problems were found to be interconnected significantly. |
| 17 | Lo et al. (2016)55 | 56 (16.6) | Between-subjects. SR: 5hrs of sleep SE: 9hrs of sleep for 7 nights. |
- | - | PANAS. | Karolinska sleepiness scale, Pittsburgh sleep quality index, actigraphy, PSG. | SR predicted a decrease of positive
affect with a lowest point at the last day of sleep restriction.
No significant change of negative mood through sleep restriction. |
| 18 | McMakin et al. (2016)67 | 48 (13.3) 16 (14.5) |
Within subjects. SR to 4hrs of sleep for 2 nights SR to 6hrs of sleep on 1 night, and 2hrs of sleep on the second night. SE: 10hrs of nocturnal sleep. |
Peer conflict task. Auditory valence identification task. Affective response task. |
Individual real-life disagreements.
42 emotion eliciting sound clips (14 positives, negative,
neutral). Trial: 15s, orientation (1s), stimuli presentation (6s), rating interval (8s). |
Behavioral rating, accuracy, reaction times, pupillography and by subjective self-report. | PSG | SR predicted more self-reported and
objective measured negative affect. SR predicted less positive affect in study 1, not in study 2. Negative affective behavior was significant higher after sleep restriction. |
| 19 | Miller et al. (2015)61 | 12 (2.8) | Within-subjects. Afternoon nap deprivation. |
Unsolvable puzzle. | Incorrect piece in the puzzle. | Rating observation. | Sleep diary, CSHQ, actigraphy. | ND predicted less skepticism, and
negative self-appraisal. ND predicted more physical self-soothing, perseveration, and tenancy. |
| 20 | Raynolds (2017)73 | 20 (15.7) | Within-subjects. SE: 1hr earlier to bed for 5 nights. |
Online social interaction task.
Paced auditory serial addition task. |
Getting to know an unknown
person. Fast calculating. |
Daily mood questionnaire. The self-assessment Manikin. Computer based linguistic inquiry and word count, facial expressions valence. |
Sleep diary, actigraphy. | SE predicted more negative facial
expression and higher levels of facial expression
variability. No change in emotional language, subjective report of emotion regulation, persistence or task performance. |
| Author |
Subjects
N (Mage in years) |
Design | Task | Stimuli | Measures | Measures-Sleep | Result | |
| 21 | Reddy et al. (2017)57 | 42 (14.8) | Between-subjects. SR: 2hrs later to bed. SE: 9.5hrs in bed. |
Emotion reactivity and regulation task. | 40 emotional images (8 positive and
neutral, 24 negative). Trial: 18s, 10s stimulus presentation, 8s rating interval. |
PANAS, State-trait anxiety inventory for children. Emotional reactivity and ER was assessed by subjective valence, intensity/arousal, and reappraisal ratings. | Epworth’s sleepiness scale, BEARS sleep screen, sleep diary, actigraphy. | SR predicts subjective decrease of
positive affect and increase of state and trait
anxiety. No change in emotional reactivity and regulation. |
| 22 | Ross and Karraker (1999)63 | 40 (1.3) | Between subjects. 20 subjects were assessed before The other 20 subjects were assessed after their regular nap. |
Rieser-Danner’s plexiglas barrier
task. Parts of the Laboratory temperament assessment battery. Ainsworth’s strange situation procedure. |
5 Stressing episodes, Toys in jar, Remote-controlled toy approach, Maternal separation, Attractive toy, Mother busy. |
Behavioral rating. Infant behavior questionnaire. |
- | Fatigue sensitizes infants to
certain stressors instead of simply increasing irritability and
interferes with infants’ coping responses. Exhausted children exhibited a higher degree of fatigue frustration. |
| 23 | Rubens et al. (2017)78 | 285 Assessments from 3rd to 5th grade | Longitudinal | - | - | Children’s emotion management
scales, Pediatric anxiety scale of the patient-reported outcomes measurement Information system, Short mood and feelings questionnaire, Affective reactivity index, Self-report scale for deviant behavior, Self-reported reactive/proactive social behavior. |
Sleep quality was assessed by subjective 4-item child self-report scale. | Better sleep quality predicted lower
self-reported emotional and behavioral
problems. Regarding gender effects girls scored higher on the anxiety scale and lower on irritability, delinquency engagement and reactive aggression. |
| 24 | Saenz et al. (2015)84 | 47 (1.6) | Longitudinal | - | - | BITSEA. | Sleep diary, actigraphy. | In girls, shorter sleep duration at the age of 3 months predicted significant more externalizing problems at the age of 18 months. |
| 25 | Schumacher et al. (2017)53 | 19 (3.8) | Between-subjects. SR: 3hrs later to bed for 1 night. |
A go/no-go task. Unsolvable puzzle. |
No-go trial (pig). Incorrect piece in the puzzle. |
Accuracy, rating observers. | Sleep diary, actigraphy. | No significant effects of sleep
restriction on response inhibition or
self-regulation. Interaction effect of response inhibition and sleep condition on adaptive self-regulation and maladaptive self-regulation. |
| Author |
Subjects
N (Mage in years) |
Design | Task | Stimuli | Measures | Measures-Sleep | Result | |
| 26 | Settineri et al. (2010)71 | 529 (17.1) | Cross-sectional | - | - | Mood was assessed by subjective measurement with an 8-item scale. | TST, napping and sleepiness was assessed by subjective measurement with a 4-item scale. | Well-being at awakening had a
negative correlation with sadness, apathy, anhedonia, and
pessimistic thoughts. Well-being at awakening was positively correlated with TST, negatively with afternoon naps and daytime drowsiness. |
| 27 | Short and Louca (2015)70 | 12 (16.2) | within-subjects. SR: 36hrs of wakefulness |
- | - | POMS - short form. | Sleep diary, Karolinska sleepiness scale, actigraphy, PSG. | Dimensions of mood significantly
deteriorate during a night of sleep
restriction. Increased anxiety in females but not in male participants after sleep restriction. Only girls reported an increase of depressive mood in response to SR. |
| 28 | Soffer-Dudek et al. (2011)56 | 94 (10.5 at the 1st assessment) | Longitudinal | Balloons task. | Faces on balloons showing different emotional expressions. | Accuracy on judgments. | Sleep diary, actigraphy. | More night awakenings predicted less task performance on the face-emotion processing task. |
| 29 | Troxel et al. (2013)76 | 776 Assessments at 1, 6, 24, and 36, and 54 months. |
Longitudinal | - | Neutral parent-child interaction at home was videotaped for 15 minutes during the visit. | Negative emotionality was behavioral rated by researcher. | CBCL-parent and teacher version. | Early sleep problems and negative emotionality predicted later internalizing behavior. |
| 30 | Vaughn et al. (2015)58 | 62 (4.1) | Cross-sectional | Denham’s emotion knowledge task. | Faces showing different emotional expressions. | Emotional knowledge was rated on the documented subjects’ ratings during the task. | Sleep diary, actigraphy. | Sleep duration had positive correlations with emotional knowledge. |
| 31 | Vriend et al. (2013)54 | 32 (9.8) | Within-subjects. 1hr SE/SR for 4 nights. |
Affective response task. | 33 emotional images | Subjective affect rating on visual analogue scales. | Child’s pictorial sleepiness scale,
CSHQ, Sleep evaluation questionnaire, Epworth sleepiness scale, actigraphy. |
SR predicted less positive affective response and poorer parental reported ER. No change in negative affect responses or ER. |
| 32 | Wang et al. (2019)83 | 1625 Assessments from 5 to 17 years of age | Longitudinal | - | - | Dysregulation profile of the CBCL. | CBCL | Persistent sleep problems, measured over a span from five to 17 years found to contribute to a ten-time increased risk for developing regulatory difficulties. |
| 33 | Weissbluth (1981)64 | 60 (0.6) | Cross-sectional | - | - | Carey infant temperament questionnaire. | Sleep interview. | Significant negative correlations
between TST and mood, adaptability, rhythmicity, withdrawal, and
persistence. Children described as “difficult” had shorter sleep duration than “easy” children. |
Abbreviations: Bedtime issues, Excessive daytime sleepiness, Night awakenings, Regularity and duration of sleep, Snoring (BEARS); (Brief) infant-toddler social emotional assessment (B)ITSEA); Child behavior checklist (CBCL); Children’s sleep habits questionnaire (CSHQ); Electrocardiography (ECG); Emotion regulation (ER); Facial electromyography (fEMG); Heart rate deceleration (HRD); International affective picture system (IAPS); Late positive potential (LPP); Napping (N); Nap deprivation (ND); Polysomnography (PSG); Positive and negative affect schedule (for children) (PANAS (-C); Profile of mood states (POMS); Respiratory sinus arrhythmia (RSA); Strengths and difficulties questionnaire (SDQ); Sleep extension (SE); Sleep restriction (SR); Slow wave activity (SWA); Total sleep time (TST).
It should be mentioned, that during the literature collection and selection process it was conspicuous, that almost 95% of the originally 814 studies were rejected because they were not eligible for the review. Reasons for that high rejection rate were, e.g., that despite defining a young population without appreciable health related limitations in the search formula, also a great number of studies with adults and participants suffering from chronical illness were found in the result pool of the literature search process.
Missing data
If full-texts were missing, correspondence authors were contacted once via mail and asked to provide the full text, if those papers were not provided, they have been excluded from further analysis in this review (n=2).
RESULTS
Effect of sleep on neurophysiological processes linked to emotion regulation
In order to assess the effect of sleep on emotion related neurophysiological activity researchers utilized EEG measurement.
Cortical activity. Bolinger et al. (2018)49 were interested in recordings of late positive potentials (LPP), which are recognized to be a neurophysiological marker for emotion regulation in children and is modulated by conscious cognitive processes50. They assessed the processing and recognition of neutral, as well as emotional visual stimuli. Two points of assessment were planned in a sample of children; first, an encoding phase included affective ratings of pictures with emotional content, second the recognition phase took place ten hours later, in the evening following encoding for the “no sleep” condition, and on the next morning for the “sleep” condition. The accuracy of recognition increased significantly after sleep. Thus, sleep seemed to enhance stimuli processing in a way that may preserve a person’s autonomic reactivity. It is striking, that while be tested in the evening (no-sleep condition), participants achieved better recognition accuracy to pictures with negative emotional content compared to be tested after sleep.
Researcher also implemented facial emotional stimuli to investigate the effect of napping on behavioural responses to emotion eliciting stimuli in pre-school children51. Cremone et al. (2017)52 utilized EEG recordings during a nap to assess the slow wave activity (SWA) reflecting neocortical oscillations, which contribute to emotional processing. During measurement of attention bias to emotional stimuli, the allocation of attention toward or away from emotional stimuli was assessed. Within the task, children had to click on the right or left button of the mouse to indicate the location of the stimulus as quickly and as accurately as possible. In contrast to the findings in school-aged children, accuracy and reaction times in pre-school children for negative and positive affective stimuli did not differ between the nap conditions. However, being tested before sleep was associated to greater attention bias. There was not a significantly difference for positive or negative trials. Furthermore, results indicated a greater slow wave activity (SWA) while napping was associated with a faster response to the stimuli.
Those results support the assumption that sleep seems to enhance cortical activity, and cognitive processes in school-aged children49, as well as the automatic direction of attention in response to presentation of emotional stimuli in pre-school children51. It would be very interesting to get more detailed insight into discriminating power of the emotional category of stimuli on neurophysiological reactions towards sleep deprivation in children.
Effect of sleep on attentional processing linked to emotion regulation
Whereas the effect of sleep on attentional regulation processes as defined by Thompson (1994)24 were not assessed in the included studies, measures as accuracy and speed of attentional processing are summed up.
Response inhibition. In order to investigate the effect of sleep on attentional processing, sleep deprived toddlers53, children54, and adolescents55 were investigated on their response inhibition. During a go/no-go task the subjects’ accuracy to inhibit the button response in the case of the presented no-go stimulus was assessed. No main effects of sleep deprivation on response inhibition were reported after one night going to bed three hours later than usual53 and after one hour of night sleep extension or restriction54. Contrary to the findings in children53,54, the reaction times and accuracy in discriminating behaviour in adolescents deteriorated significantly after night sleep deprivation and did not re-establish to the baseline performance after two nights of recovery55.
An additional study looked for indication of the effects of sleep quality on emotional information processing in early adolescents56. To meet this goal, subjects had to respond via mouse-click to gender or to particular positive and negative emotions represented by faces on balloons which arose from the bottom of a computer screen. More night awakenings and lower sleep efficiency were found to predict only lower success on the face-emotion task; accuracy in responses to gender trials seemed to be unaffected. These results demonstrated that emotion information processing change as a function of night awakenings and sleep efficiency.
Effect of sleep on reasoning processes linked to emotion regulation
Cognitive reappraisal and emotional reasoning. To assess the relation between sleep restriction and reasoning processes in response to emotion eliciting stimuli belonging to the IAPS, Reddy et al. (2017)57 assessed adolescent’s cognitive reappraisal techniques as distancing from the reality of the picture, thinking about improvement, or creation of a positive explanation. According to their results, one night of sleep deprivation had no main-effect on adolescent’s ability generating reappraisal statements as well as on their efficacy in regulating negative emotions. Contrasting results were presented by another study investigated the association of sleep parameter and emotional reasoning58. During the Denham’s affect knowledge task, children had to name and reason about emotion expressions represented on images59. Whereas sleep latency and efficiency had no significant correlation with emotion cause score, the parameter of sleep duration had positive correlations with the ability to reason about emotions58.
Effect of sleep on biological cues linked to emotion regulation
Evidence for associations between sleep and affect was also found in physiological measures.
Heart rate. To assess the internal biological cues of emotion regulation, Bolinger et al. (2018)49 recorded the participants’ heart rate deceleration (HRD) in response to emotion eliciting stimuli. In contrast to the already described LPP, the HRD response to emotional stimuli decreased in the case of wakefulness. Testing after night sleep led to an increase in the HRD response to pictures with negative emotional content, whereas the HRD seem to be unaffected in case of neutral stimuli49.
Parasympathetic activity. Cho et al. (2017)60 investigated the association between sleep duration and emotion regulation problems in toddlers. To assess the regulation capacity, children participated in a series of short, mildly stressing, social interaction tasks. Parents reported their children’s regulatory behavior responses and, for parasympathetic reactivity, the respiratory sinus arrhythmia reactivity (RSA) was assessed. The authors report evidence of increased RSA reactivity to any interaction episodes, in combination with longer sleep duration and less internalizing symptoms. Longer sleep duration predicted fewer internalizing symptoms in children showing a higher RSA reactivity to the fear-eliciting stimuli. Subjects with shorter sleep durations showed decreased parasympathetic response, which is associated with less capacity for regulation60.
Effect of sleep on coping processes linked to emotion regulation
From research in nap deprived children, it was also reported that toddlers react differently depending upon whether they are sleepy or well-rested.
Self-regulation. To assess coping processes, children were confronted with an age-appropriate, but unsolvable puzzle either one hour after their habitual nap or one hour after the habitual nap would normally have occurred (nap vs. nap deprivation)61,62. One minute after all pieces, except the incorrect one, were successfully placed, children were encouraged to finish. After nap deprivation, perceived scepticism about the piece would match, decreased. Physical self-soothing, as repetitive bodily-directed behaviors, and focussing on the piece that would not fit, thus perseverance and tenancy to complete increased after nap deprivation. Additionally, negative self-appraisal, as discrediting the competence to solve the puzzle in the unsolvable puzzle task, and display of confusion to the challenging situation, decreased after nap-deprivation61,62.
Another study found sleep deprivation had no direct effects on self-regulation strategies in three years old children53. Though, a mediating effect of response inhibition was assumed. It was reported that children with better response inhibition before sleep restriction were more likely to use adaptive self-regulation strategies, while poor response inhibition predicted increased use of maladaptive self-regulation strategies in response to the unsolvable puzzle task after sleep restriction.
To examine the effect of fatigue on infants’ emotional coping, visiting and assessment of children took place 1 hour after the infant’s regular nap would have occurred (nap deprivation) or actually occurred (nap condition)62. Another study used the time point of mother’s expectation of her infant to be awake or the time when the infant’s habitual morning or afternoon nap started to assess differences between alert and fatigued children63. The emotional stimuli included five mild stress inducing episodes. To assess children’s frustration toleration, infants were encouraged to play with toys placed in an unopenable jar. Fatigued children looked more often to the experimenter and were less persistent in exploring the jar. While separated from their mothers’, fatigued children were more focused on proximity seeking, and fatigued children showed also more self-soothing behaviour during an episode of prohibiting to play with an attractive toy. This study supplied evidence that, in response to different stressors, fatigued infants are more emotionally reactive and less mature in their emotion regulation capacities than rested infants.
These findings are supported by cross-sectional research by Weissbluth (1981)64. He found significant negative correlations between total sleep duration and infants’ resilience. Furthermore, children described as “difficult” were negative in mood, less adaptable, approachable, and rhythmical and had shorter total sleep times per day, than “easy” children. “Difficult” children also had a higher level of activity and lower sensory thresholds than “easy” children. This is in line with another study65, with the additional finding that infants, described as rhythmic and adaptable, also took longer naps than distractible children did. Furthermore, infants described with persistent night awakenings were described as displaying more maladaptive stress-coping, as dysregulation behavior, e.g. higher levels of separation distress, than was so with continuous sleepers.
Effect of sleep on emotion expression
According to Thompson (1994)24, emotion regulation encompasses generation of appropriate emotional expressions. Therefore, upcoming emotions and affects have to be processed and appropriate behavior has to be generated. Within the following paragraph the effect of sleep on the emergence of these affective responses will be summed up.
Affective response ratings. Focus has been on the effects of experimental sleep deprivation on affective responses to emotion eliciting stimuli. Therefore, sleep deprived and control subjects were compared on their affective arousal after confrontation with images or sounds representing pleasant, unpleasant, or neutral stimuli. To evaluate emotional responses of infants after nap restriction, emotion assessment was implemented at home62. To observe and quantify the children’s emotion expressions towards pictures from the International Affective Picture System (IAPS) on a screen, researchers analyzed the videotaped task sessions. Nap restriction contributed to more negative affect in response to neutral pictures and conversely positive pictures induced fewer positive reactions62. A comparable task was implemented in pre-school children66. The assessment contained strong and weak emotional visual stimuli of the IAPS, which were paired with an appropriate auditory stimulus. Affective responses were assessed by facial electromyography (fEMG). After nap-deprivation, results also demonstrated greater affective responses to strong negative and positive pictures. No sensitivity was reported of affect states to weak stimuli after nap-deprivation. Affective responses of nocturnal sleep restricted school-aged children were rated via self- and parental report after presentation of the IAPS stimuli54. Short sleep condition predicted less positive affective responses and more problems in emotion regulation. The study by Bolinger et al. (2018)49 also made use of IAPS stimuli and they assessed the subjective emotional response by asking their participants directly for their valence ratings of the stimuli after first presentation and after a recognition session following the night sleep or in the evening (sleep vs. wake condition). Whereas the valence rating leaves unchanged in the wake condition, in the sleep condition the negative valence rating of negative stimuli decreased from encoding to recognition session and neutral stimuli were rated as more negative49. One study protocol included the affective response task with only auditory stimuli67. During the task, adolescents were confronted with short sound clips. Measurement was based on subjective reports, as well as objective pupillography assessment. As reported in the visual stimuli studies, negative affect rating was significant higher in the restriction group and positive affect rating was lower after sleep restriction. Additionally, the puzzle task was implemented to evaluate the outcome measure of affective responses62. Nap restricted children had shorter positive emotion responses; they expressed less joy and pride after solving the puzzle. Without their afternoon nap, children also showed longer negative emotions, in particular worry and anger, when faced with an unsolvable puzzle62.
Another study displayed significant differences in subjective reports of positive affect between sleep extended and restricted adolescents68. Adolescents scored higher for positive affect when they were in the rested condition. No difference concerning negative affect was found between sleep conditions. Regarding participants’ chronotype, evening as well as morning chronotypes displayed less positive affect if they were sleep deprived. This result is congruent to the finding of a steadily decrease of positive affect during the seven-day period of sleep deprivation55. Data for negative affect showed no significant change due to sleep restriction. This suggests that subjective positive affect is more sensitive to sleep deprivation than subjective negative affect. Overall, studies of the effects of sleep on the affective responses to emotional stimuli reveal a rather heterogenous picture, reflecting the complexity of physiological as well as cognitive and behavioral processes of emotion regulation.
Mood dimensions. To assess different mood dimension, the profile of mood states with mood describing adjectives, was completed after sleep restriction in adolescents69,70. Whereas Baum et al. (2014)69 used the POMS only twice, after a baseline and after sleep restriction week, in the study by Short and Louca (2015)70 the (POMS)-short form was completed every two hours during the night of strict sleep deprivation. Participants of Baum et al. (2014)69 reported increased levels of different mood dimensions, except depression. The contrary findings of Short and Louca (2015)70 revealed that all dimensions of mood, inclusive depressive mood significantly deteriorate after sleep deprivation, whereas anxiety was found only in females, and depressive mood only marginally in male participants.
A short telephone inquiry was given to adolescents, to investigate the relationship between their mood states in the morning and their night sleep71. A good sleep quality was positively correlated to a better mood in the morning and negatively correlated to daytime drowsiness. Results were comparable to those in young children72. Children with longer sleep latency and low sleep efficiency had a decline in positive mood for the next day, which predicted higher sleep activity during the following night and longer sleep latencies again. Subjects showing longer sleep latencies also had an averaged negative mood stretching across the seven days of assessment, which was associated with higher levels of internalizing and externalizing symptoms in general.
While previous studies have investigated the emergence and association of positive or negative emotional states and sleep manipulation or sleep parameter, other authors have been focused on how emotions are expressed during social interaction.
Social interaction. Responses to tasks of social interaction seemed also to be sensitive to sleep deprivation67. By that method, individual recent disagreements with friends were sorted by their relevance. During the visit at the experimental laboratory, participants’ real-life friends were invited and asked to discuss one of the two most highly rated conflicts with the participants. Behavior during the task was rated by a researcher on facial expressions and on verbal content, and these summary scores of negative and positive affect behaviors were included in the analysis67. Observed negative affective behavior, such as conflict withdrawal and dominance during the peer conflict task, was significantly higher after sleep restriction.
The implemented task adopted in the study of Raynolds (2017)73 distinctly differed to those already described67. Within this study, the association of typical or extended sleep and emotion regulation was investigated. From a non-manipulated session, the task protocol consisted of adolescents getting to know an unfamiliar person for five minutes via an iPad. Thereafter adolescents were briefed that the next unfamiliar person had lost the phone, thus the subjects’ waiting time could be used to complete the Paced auditory serial addition task (PASAT)74. This task required participants to sum numbers sequentially as they appeared on the computer screen, and was designed to increase frustration and negative mood. After the task, participants did not have time to recover from the frustrating task before beginning the manipulated social interaction task, with the instruction to make the other person, who had lost the phone, feel better. Sleep extended adolescents had more negative facial expression and higher levels of facial expression variability than the typical sleep group throughout the manipulated task. Emotional language regulation, persistence, and the PASAT score did not achieve statistically significant effects.
In order to evaluate children’s negative emotionality, representing a predictor for being less confident and more vulnerable while faced with either positive or negative circumstances75, the mother-infant dyad was observed at a home visit during normal interaction76. Children with high scores for negative emotionality at the age of 6 months had more internalizing problems at the age of 54 months, when having had more sleep problems at the age of 36 months. Additionally, teachers were asked to value emotion regulation behavior of school-aged children to assess the influence of sleep manipulation77. The emotional outcome scores of emotional lability and restless-impulsive behavior improved after sleep extension, whereas these measures deteriorated in children experiencing sleep restriction. The finding that sleep deprivation affects facets of emotion regulation and oppositionality in a social context is supported by parental and self-report in adolescents69.
Whereas parental reporting is necessary while assessing data of infants and toddlers, school-aged children can give ratings on subjective measures on their own78. Better sleep quality was associated with less emotional and behavior problems79. Regarding gender effects, results indicate that girls score higher on the anxiety scale and lower on reactive aggression. Furthermore, emotional dysregulation was rated as low when children rate their sleep quality as high and young children’s dysregulation was rated high due to continues night awakenings. Thus, dysregulation seems to be sensitive to sleep parameters in infants64 as well as in school-aged children78.
The positive correlation between good sleep and emotion regulation ratings reached significance in the correlational studies including subjective measurements of sleep and affect in children and adolescents64,65,78-80. Because it is often asked if results regarding emotional outcome measures differ in consequence of making use of whether subjective or objective sleep measurement, one study displayed that parental reported presence of children’s sleep disturbances is a reliable predictor of objectively assessed inappropriate sleep schedules80. Unfortunately, results of these studies cannot investigate the interconnectedness of temperament, emotion regulation, and sleep, because assessment of affective constructs was at a single point of measurement. To assess the diverse mutual developmental trajectories of sleep and emotion regulation longitudinal research is needed.
Interconnectedness of sleep and development of emotion regulation competence
Within their longitudinal study, Gregory and O’Connor (2002)81 were interested in changes of sleep and behavioral problems over the period of childhood. Results suggest that early sleep problems predict behavioral problems, in particular emotional problems, in later lifetime. However, no evidence was found for early depression, anxiety or aggression symptoms predicting more sleep problems in mid-adolescence. Further, no differences between the sexes were detectable. Foley and Weinraub (2017)82 researched the topic of sleep and emotional adjustment in children, and found more early sleep problems predicted more anxious-depressed symptoms in the middle childhood in both boys and girls, and this was found to be associated with higher rates of emotional reactivity in the preadolescence. In contrast to Gregory and O’Connor (2002)81, gender differences were found. For boys, earlier anxious-depressed symptoms predicted more problematic sleep in the preadolescence; and more negative affective temperament in early childhood was correlated with more sleep problems and anxious-depressed symptoms at all points of measurement. For girls, more early sleep problems predicted less social competence in school and this was associated with more anxious-depressed symptoms in preadolescence. Additive, higher levels of sleep problems in the middle childhood in girls predict higher levels of emotional reactivity in preadolescence. The interconnectedness of sleep and emotional problems is also displayed by a longitudinal research based on parental-report data83. According to the published results, it was concluded that sleep problems and problems in emotion regulation are strongly associated in their development over time, and those participants suffering from persistent sleep problems have a 10 times increased risk to develop problems with emotion regulation83.
To overcome the limitation of exclusive subjective sleep measurement, a study in young children implemented actigraphy for five nights when infants were three months of age84. Emotional problems were assessed by subjective parental reports when infants were 20 months. Regression analysis of externalizing-, internalizing-, and dysregulation problems, sleep efficiency and sleep duration led to no detectable main effects in boys. In girls, shorter sleep duration at the age of 3 months predicted significantly more externalizing problems at the age of 18 months. Whereas the finding of existing gender differences was incongruent to another longitudinal study81, longitudinal research of Foley and Weinraub (2017)82 supported an association of insufficient sleep in infancy and later affective problems in girls, and in contrast with Saenz (2015)84, this was also found in boys. Differences might be explained by the source of data; parental report of sleep81,82 may be not associated with actigraphy assessment of sleep84. A further longitudinal study includes objective sleep measures in toddlers to investigate the mutual dependence of sleep and emotional outcomes85. They display that short sleep duration and low sleep efficiency at age two are associated to more frustration and anger one year later. There were also indices for high rates of social fear at the age of 2 being associated to shorter day- and nighttime sleep duration at measurement one year later. Nevertheless, it may be informative to include actigraphy standardly in future longitudinal research on sleep and affect and emotion.
Manipulation of sleep
To give a short insight to the differences in schedules of sleep manipulation we added this section to our review. This could be understand as hint for upcoming research, because differences in scheduling sleep for experimental research of emotion regulation could lead to different outcomes. However, a full discussion of this relationship will go beyond the scope of the review.
In sum 14 studies implemented sleep manipulation in their studies (for a detailed overview about the different protocols of sleep manipulation, they are summed up for the age groups of infants and young children, school-aged children, and adolescents in the Appendix).
Five studies implemented sleep restriction protocols or nap restriction in young children. Except one51, all of them implemented a sleep stabilization period of five or seven nights before sleep manipulation. Four studies restricted the afternoon nap to assess the effect of sleep loss on emotional reactions51,61,62,66. One study implemented a form of night-sleep restriction in young children53. Regarding the points of measurement, testing took place after respectively the normal and the sleep or nap restriction condition. Whereas Cremone et al. (2017)52 implemented the dot probe task, three studies included the unsolvable puzzle task to assess emotional regulation in toddlers. The assessment battery of Berger et al. (2012)62 as well as Han (2014)66 included emotional stimuli of the IAPS and the IADS.
In school-aged children, two studies implemented experimental sleep restriction. They implemented a stabilization period over four or six nights80. Within this period, children went to bed as they normally do. After stabilization, children were randomly assigned to the sleep restricted or sleep extended condition, with bedtimes one hour later or earlier than usual. Blinded teacher rated children’s emotional lability and restless-impulsivity, and the Conner’s global index-teacher was completed on the day after sleep stabilization period (baseline) as well on the last night of sleep manipulation period. Vriend et al. (2013)54 mixed objective (tasks) and subjective (questionnaires) measurements to assess emotion.
With regard to adolescents, five studies applied a stabilization period, which ranged from three nights55 to one week70. Instructions in the stabilization period were individual or normal, self-selected bedtimes68,69; advised bedtimes70,55 or the order to stay in bed for a minimum of 7.5 hours per night57. One study directly started with sleep restriction or extension67.
SUMMARY AND DISCUSSION
Although different methodological tasks and measurements have been summarized above, the measurements and results were discussed in accordance to the introduced multifaceted model of emotion regulation24.
Regarding neurophysiological processes, research utilizing EEG measurements49,51 supported the finding that sleep has a positive influence on the perception of emotional stimuli, as well as on the processing of automatic responses, due to an increase of neurological activity49,51. The reported results regarding cortical activity are congruent to comparable studies in adults86.
In contrast to adolescent’s55, children’s53,54 generation of a behavioral response to attention attracting neutral stimuli seem not to be affected by sleep manipulation. One explanation of the missing effect of sleep manipulation on attentional processes in children may relate to the small amount of sleep deprivation in children. It would be interesting to repeat the task by implementing longer periods of wakefulness to assess their consequences on children’s attention regulation processes. Regarding the results from research utilizing emotion eliciting stimuli, it could be concluded, that low sleep quality reduces the accuracy in processing emotional stimuli in adolescents56. Whereas studies including sleep manipulation utilize neutral stimuli, one study, concerning the influence of sleep quality on emotional processing, discriminated between the effect of neutral versus emotional stimuli. Unfortunately, the effect of either positive or negative emotional content of the stimuli was disregarded. Whereas recent research assessed attentional processes in an objective manner, future research should assess the influence of sleep deprivation as well as sleep parameters on children’s and adolescents’ use of attention regulation processes. Those processes according to Thompson (1994)24 encompass, e.g. the use of concrete strategies of conscious avoidance of emotion eliciting stimuli.
The effect of sleep deprivation on emotional reasoning is inconsistent to the effect of low sleep quality on it. Reddy et al. (2017)57 suggested that one night of sleep deprivation has no significant effect on reappraisal tactics and reasoning in adolescents, whereas a short sleep duration seemed to be linked to a decrease of emotional reasoning58. According to the results emotional reasoning seems not to be sensitive to sleep deprivation, whereas shortcomings in sleep quality cause lower performance in emotional reasoning. Regarding the operationalization of emotional reasoning, both studies made use of static pictures, differing in representing either emotion eliciting context57 or emotional facial expressions58. Unfortunately, in both studies, the emotion specificity of the results regarding the emotional reasoning did not report the differences in positive or negative reasoning distinctively. Future research should shed light on influence of longer periods of sleep restriction on emotional reasoning; consider reporting effect of sleep parameters on positive and negative reasoning about emotional states, and utilization of less static sources of stimuli, as short sequences of video clips with emotional content.
Results of studies concerning the physiological background of emotion regulation49,60 indicate a mutual dependence of emotional stimuli, physiological responses, and parameters of sleep. Findings support the idea, that rather than acting as a unity entity, emotion regulation and emotional responses emerge from interaction between automatic generating responses and cognitive processes, while both systems are sensitive for sleep. Beyond, future studies might wish to consider the implementation of behavioral ratings, as well as physiological and neuroimaging measures, to provide support to the body of literature regarding influencing factor of sleep on the association between physiological and emotion regulating processes in children and adolescents.
While focussing on coping, results of studies are supporting the assumption, that day and night sleep deprivation affects young children’s emotional coping competencies, whereas afternoon nap deprivation preserves increase use of maladaptive strategies in response to puzzle tasks61,62, as well as to stressing situations63. Findings of these observational studies are supported by findings from cross-sectional studies examining a longer night64 and day65 sleep duration to be associated positively to functional use of coping strategies. Another study found indirect, instead of direct effects of mild night sleep restriction on self-regulation strategies, mediated by children’s performance regarding response inhibition before being sleep restricted53. Therefore, future studies might wish to consider children’s’ coping predisposing factors as e.g. response inhibitation53 in more depth. Additionally, the incongruent finding of direct61,63 versus indirect53 effects of sleep manipulation on coping strategies may contribute to the difference in restricting day61,63 or night sleep53 in young children. Additionally, Berger et al. (2012)62 introduced the puzzle challenge with a “solving segment”, thus children finished the puzzle and were praised for their performance. How a previous successful event could be linked to self-regulation strategies, while performing the unsolvable puzzle task after restriction of night sleep is therefore a subject of debate. Furthermore, children’s reactivity when they were exposed to the unsolvable puzzle was significantly decreased after nap deprivation61,62. This may be an indication of reduced cognitive engagement and lowered motivation to retrieve information from the environment87.
Studies of affective response ratings with young children are with each other comparable to the result of greater negative responses to negative visual stimuli after nap-deprivation62,66; this is congruent with the results after nocturnal sleep deprivation in adolescents67. Discrepant to the results of lower positive responses on positive stimuli in toddlers62 was the result of greater positive responses towards positive pictures after nap-deprivation in young children66. Furthermore, ratings of negative affect were not influenced after sleep deprivation in school-aged children54. One explanation may relate to the selected stimuli, and their ratings of valence and arousal. The precise identification number of stimuli and the total ratings were not specified54,62,66 and cannot be consulted for discussion. Furthermore, two studies consulted additional objective measurements to rate subjects’ responses66,67, whereas another study used ratings by a human rater66, thus discrepancies in ratings can contribute to differences in evaluation of emotional responses. Finally, there was inclusion of an auditory stimulus; thus, the additionally activation of the auditory sense can have an influencing effect on the emotional response66,67. Future studies should investigate the listed reasons for incongruent results in more depth.
A decline in responses of subjective positive affect ratings on the positive and negative affect schedule (PANAS)88 was observable after two68 and also seven55 nights of sleep deprivation. Thus, different periods of nocturnal sleep restriction had no effects on the PANAS results. However, unlike in behavioral studies implementing the PANAS57, the effect on negative affect seemed to remain unchanged after sleep deprivation. Keeping in mind the subjective character of the PANAS, subjects may express test items representing a negative affect state, e.g. “guilty”, “scared” or “afraid” as irrelevant, thus negative affect stays unaffected55. Behavioral studies with additional activation of visual or auditory senses57,67 found sleep deprivation to increase ratings of negative affect stimuli and therefore support the presented explanation. In the case of this difference, future research should choose the implementation of different measures and stimuli to investigate the effect of sleep deprivation on affect states.
Whereas Baum et al. (2014)69 found no increase of the dimension of depression after partial sleep deprivation for four nights, Short and Louca (2015)70 reported that girls significantly, and boys instead of males marginally, reported an increase of the feeling of being depressed after a night of strict sleep restriction. In contrast to the other mood states assessed in the POMS89, depressed mood seemed not that sensitive to moderate sleep deprivation69 than to strict sleep deprivation70. Secondly, the items of the POMS depressive subscale are comparable to those of the negative affect states in the PANAS, thus, they may be valued as irrelevant and consequently depressive mood seemed nearly unaffected. However, female’s sensitivity regarding depression and anxiety following experimental strict sleep deprivation was only reported by Short and Louca (2015)70. This is in line with the results of a longitudinal study; girls with early sleep problems displayed more anxiety-depressive symptoms in preadolescence82. These findings demonstrate interrelatedness between the female gender and the sensitivity for problematic or restricted sleep. Taken together, these results suggest significant effects of sleep loss on subjective affect and mood states, but some striking results regarding sleep loss and indices of depression. Furthermore, the influence of female gender needs to be clarified in future research.
Due to the results, a mutual association between mood dimensions and sleep quality can be assumed71,72. These results are also supported by observational and behavioral studies in young children62,63 and adolescents67. Regardless of their diligence in the selection of instruments and sampling procedures, these studies cannot meet the aim of giving insight into long-term development of affect and sleep, due to its cross-sectional character.
Differences in the effect of sleep manipulation on social emotion regulation behavior are also somewhat striking. These differences can be explained with reasons, e.g. the deviating moment of assessment of social emotion regulation behavior as well as by different schedules of sleep manipulation. Whereas participants in the study by McMakin et al. (2016)67 were tested after restricting their sleep to respectively four and two hours on two consecutive nights, participants in the study by Raynolds (2017)73 were investigated after extending their sleep to one additional hour for five consecutive nights. Another explanation might contribute to the analysis’s outcome measures. Methodologically, McMakin et al. (2016)67 also assessed facial expressions, as well as verbal content but, in contrast to Raynolds (2017)73, the two composite scores were calculated by averaging one summary score. The question about the effects of sleep restriction on respectively affective facial expressions and language remains open in the study by McMakin et al. (2016)67. Additionally, differences between human rating and computer results of these measurements in the context of sleep manipulating studies have not been conducted yet. Future studies should investigate the listed reasons for incongruent results in more depth.
The finding of moderating effect of sleep problems on the association of a child’s early negative emotionality and later internalizing behavior must be considered in a critical light of methodological limitations. Negative emotionality was assessed when children were 6 months of age on a 15 minutes’ interaction. Biasing factors such as mood, representing the mental state that temporarily predisposes a person to act to a variety of events90, were not controlled. Whereas the longitudinal association of negative emotionality, sleep problems and behavior must be given more attention in future research, longitudinal research should also implement assessment of the variables at more frequent points of measurement to detect their individual development as well as their mutual associations. Similar points of criticisms are given to Gruber et al. (2012)77. Even if the children’s and adolescents’ emotion regulation behavior is affected by sleep loss, according to subjective measurement, this result is one-dimensional and can be biased by un-controlled day-to-day influences in their environment. Thus, future research should be interested in the implementation of more objective measurements and task paradigms67,73, to assess the effect of sleep loss or sleep extension on regulatory behavior in the school or daily-life context.
Results from longitudinal research provide inconsistent results regarding the mutual developmental pathways of sleep and emotion regulation81,82. Reasons for these striking results do not contribute to the measurement of sleep problems, because the child behavior checklist (CBCL)91 was employed and the sources of information were the subjects’ mothers in both cases. However, statistical analyses differed; whereas Gregory and O’Connor (2002)81 made use of hierarchical regression analysis, Foley and Weinraub (2017)82 proceeded through seven stages for testing longitudinal cross-lagged panel models, which is a more sensitive analysis because of the control of autoregressive effects and covariation among variables91,92.
An association between development of emotional problems due to early sleep problems can be assumed81,82,85, whereas the reverse association is still to debate81,82. According to Wang et al. (2019)83 the chance to rehabilitate from emotional problems increased in consequence of improvement of sleep problems.
On the one hand experimental sleep manipulation studies have the power to demonstrate cause and effect relationships because of its strict sleep schedules55,68,69, a few studies reduced the explanatory power due to unrealistic and extreme70 or short-term67 sleep manipulations. On the other hand, correlational studies have focused on natural circumstances, but have not allowed for cause-effect conclusions64. While the present review sums up results as well as the methodological approach the findings from experimental nap and sleep manipulation converge with each other and it could be assumed that real-world associations between sleep and emotion regulation reflect a true cause and effect association.
In sum, the reliance on self-, parental-, and teacher reports rather than objective measures of sleep and emotional constructs represented a considerable limitation in these longitudinal studies. Furthermore, there is still the request of research to address the temporal development of problematic sleep and behavioral regulation problems, and the influencing conditions under which sleep and regulation may develop into a negative cyclical pattern. The clarification of the hen egg problem remains a challenge for future studies.
Strengths and limitations
As demanded2, this review is the first attempt to identify the relationship between the impact of sleep manipulation as well as sleep parameters on different emotion regulation processes across the youth upcoming from a developmental, multifaceted model of emotion regulation. Studies methods and instruments are aggregated and serve as a structure for future research that is interested in the assessment of sleep and affect regulation in a young population. According to our selection criteria, studies addressing children and adolescents suffering from diagnosed mental or sleep disorders, chronic illness, or in special medical circumstances were excluded. Therefore, it should be kept in mind that results are representative only for healthy and normally developed children and adolescents.
Studies in infants or toddlers rely on parental report54,64,65 and observational methods62,76 in large part, because very young children have considerable limitations reporting intrapersonal emotional experiences17. It is important to consider, that reliance on parental report might lead on to misinterpretation and overestimation of the results due disregarding influence of potential parental covariates on their statements17.
Furthermore, as research has shown, studies assessing emotion regulation processes under controlled conditions increases the probability of activating special emotions39. A distinction of naturalistic or laboratory settings is essential to be able to assume that upcoming emotional affect regulate behavioural expressions, as a successful event can enforce a children’s resilience through frustrating situations62 or that emotional affect can be regulated e.g. adolescent’s positive reappraisal reduced their negative affect states57. Thus, disregarding the interference between a constructed setting and emotional responses prevent an integral understanding of the context of emotion regulation.
With respect to the implementation of emotional images from the IAPS54,62,66 the precise identification of chosen images was not specified in all cases; studies that implement images with increasingly gradients may exaggerate the effects of sleep deprivation and while IAPS is the source of stimuli in all studies, affective reactivity may differ in accordance with specific images, so methodological diligence is demanded for the transparency and validity of the research.
It must be stated that studies differ in their techniques of analysis as well as in data generation62,73. When comparing the studies, a change in a sum score in the affect relating variables62 does not have that interpretability as its individual reactivity to manipulated sleep73. To avoid a generalizing conclusion of the associations between sleep and affect, these differences and analytical details must be considered during the interpretation of results. Additional studies which did not report the significance of group differences are also less representable77.
However, some measures of implemented subjective affect57 were not as sensitive to sleep deprivation as others77, because the test items may not be relevant to the sample. Furthermore, assessment an infant’s manageability by one item might be somewhat insufficient64,65. Thus, when preparing research, it is necessary to be careful when verifying the suitability of the instruments for measurement in the target group.
Key limitation
A key limitation of the studies including sleep manipulation was that there were only two sleep or nap conditions. The contrast between the experimental groups of sleep or nap restriction and unmanipulated condition offers evidence of causation, but does not considering the individual preferences and sleep habits of participants. This negotiation may lead to exaggeration or underestimation of the effects of sleep or nap deprivation on emotion regulation outcomes. While the relationships between sleep manipulation and outcome measures of emotion regulation are debated, it seems important to attempt to identify the threshold at which sleep or nap deprivation affects emotions in young children and adolescents. Therefore, future experimental research should vary in sleep duration, as well as in bed and raise times to assess potential differences in impact on emotional outcomes.
Future directions
According to the remarks of this systematic review, the implications for future research are related to: 1) diversity of measurements and sources, 2) variety of task characteristics and procedure, 3) requirements for longitudinal research, and 4) gender vulnerabilities of sleep and affect.
Diversity of measurements and sources
Affect processes. Affective processing has several elements affected by sleep, such as the generation of affect states57, the duration of emotional expressions62, and emotional response behavior63. Therefore, individual measures may provide an incomplete picture of the interconnection of sleep and affect. Emotion regulation is complex, and only two studies assessed cortical activity, which contributes towards emotion regulation behavior49,51. Physiological as well as neuroimaging measurements provide information for the neural and physical responses while recepting an affective stimulus. Parental or teacher reports in toddlers and children, or self-report can be implemented to assess subjective measurements of affective and emotion states. Keeping in mind, that implementation of a specific questionnaire itself can have influence on the result69,70. It is the same for objective ratings and observations by humans, as well as by computer, to provide information on the emotion regulation, and emotion responding behaviors. To investigate the influencing effect of sleep on the whole process of affect processes, behavioral, physiological, neuroimaging, and subjective measurements need to be implemented.
Sleep assessment. This review provides the impression that, especially, longitudinal studies evaluate subjective sleep data more frequently than objective sleep data. Therefore, objective data, such as actigraphy should additionally be implemented in the research standard, due to the higher quality of data93. However, the use of actigraphy in infants or toddlers faces problems of appropriate algorithms94 to detect real night awakenings and, therefore possibly making use of behavioral sleep observation.
Variety of task characteristics and procedure
Stimuli presentation and sense activation. Additionally, it was reported that there are differences in the presentation of the stimulus (number, duration, rating-interval, and static image)62,66,67. This alone also can have an impact on the emotional response. To investigate the effect of these differences, experimental tasks could vary in the time of stimuli presentation or in the duration of the interval between stimuli. Additionally, there was evidence that emotional response ratings are sensitive to the content category of stimuli (neutral, negative, and positive). For precise results, future studies should always report differences, as well as missing difference in their subject’s responses towards neutral, positive, or negative stimuli. Furthermore, the activation of an additional sense during affect processes can also have an effect on the responses to the stimuli66,67. Differences in affect responses after stimulating different senses, and their sensitivity to sleep loss or sleep parameters may be interesting to investigate in the future. The relationships between different types of stimuli and presentation appear important to take into account.
Individual condition before assessment. Neural activity in response to emotional stimuli was affected by different sleep conditions49, and there is evidence that speed of generation of behavioral responses increased through enhanced cortical activity51. As such, the individual condition of brain activity could exert an additional effect on responses towards emotional stimuli. The role of psychological constitution before assessment seems to have an influential effect on the affect responses62,90. According to the finding from observational studies, the idea arose that the implementation of positive, successful events can enhance emotion regulation capacity during frustrating daily-life situations after sleep loss has emerged62. As such, future research aimed at these “mood manipulations” could give insight into the biasing influence of temporal mood, as well as subject’s internal factors as e.g. temperament65 on sensitivity to actual sleep loss and affective responses and, additionally, help to identify factors of resilience and better emotion regulation.
Sleep manipulation. In accordance with the results, individuals vary in their reactivity to sleep or nap-deprivation, and in their responses to different types of emotional measures. It is indisputable that the schedules of sleep deprivation alter in accordance with the subject’s age, but there were also differences in scheduling of sleep deprivation and manipulation within the group of toddlers53,62, school-aged children59, and adolescents66,67. These factors suggest that the perceived reactivity of sleep restriction on constructs of emotion regulation cannot be explained by performance observation or ratings alone; the procedure of sleep manipulation and the amount of sleep deprivation could also affect the gradient of reactivity. It is important to take into account the different schedules and durations of sleep loss, as well as their dose response costs on emotional processes.
Requirements for longitudinal research
Methodological demands. Longitudinal studies that examine the role of sleep parameters in the context of affect processes often limit the lack of assessment of affective and sleep related constructions simultaneously76. Research concerning the temporal development of the inter-relationship of sleep and affect, as well as influencing conditions under which sleep and affect regulation may develop into a negative pattern is underrepresented. Additionally, changes in the development of physiological responses to sleep deprivation in the context of the processing of emotional stimuli in children and adolescents should be considered in the future. The methodological challenge when planning longitudinal research contributes to the limited set of appropriate experimental tasks, because such a task needs to develop with the participant’s age. Therefore, within the scope of its variability of age-appropriate images of the IAPS, it seems to be a good option in regards to the affect stages, but in the scope of its increasing task demands, the Balloons task95, especially, allows the implementation of the same measure across a wide range of ages. Conducting such demanding research may be important for the understanding of the development of serious sleep, as well as affective problems and their mutual association.
Longitudinal outcomes. To bring back Thompson’s assumption, arguing that individuals construct their life-settings whilst taking into account the expected emotional load24,43, it is remarkable that none of the included studies was focused on the long-term consequence of sleep problems in childhood or adolescence for the later emotional load of their life-time settings, burdens or responsibilities. As reported in many studies there might be an association between healthy sleep early in life on better later mental health, resilience or performance8,9,11, early sleep could be expected to have impact on creation of the life setting, but to what extent remains open for future research. Evidence of this association could emphasize the importance of detection and treatment of problematic sleep during childhood and adolescence.
Vulnerability of gender to sleep and affect
Few studies have investigated the role of gender in the subjective report of noticeable behavior problems and less social competence82,84. The female gender was found to react with more emotional reactivity to problematic sleep and loss of sleep70,84. Possible reasons are for example gender related differences in hormonal regulation96. Furthermore, adolescent males are less willing to report and confess emotions such as depressive mood in their self-report97. Explanations of these findings were not investigated in the context of sleep and affect studies. Therefore, care needs to be taken in the interpretation of outcomes of this study and seen as hints towards differences, which should be evaluated in upcoming research.
CONCLUSION
Sleep seems to be associated to each level of emotion regulation processes. Sleep plays a role in the ongoing regulation of emotional arousal from infancy to adolescence. Affect processes in childhood and adolescents seems to be negatively affected by sleep problems or sleep deprivation, whereas prolonged sleep is a predictor for fewer behavioral problems, less negative affect states and better affect regulation. Sleep promotes sensory processing of emotional stimuli, and is most obvious in neuroimaging and physiological measures. Sleep deprivation impairs affect processing, with apparent results in subjective and objective measurements of affective reactions towards visual emotion eliciting stimuli. Observational studies suggest that sleep enhances the duration and strength of positive emotions towards successful events, as well as the ability for emotional reasoning, behavioral response, and regulation.
In general, associations of sleep and levels of affect in this review are restricted to young subjects. Furthermore, the types of measurements, tasks and data analyses may also have some influence on the results. Future studies should be based on more diverse measurement of affect and sleep; they should vary in their stimuli presentation and sense activation. Further, they should implement mood manipulation to investigate the mutual associations in some detail. The consideration of gender differences in affect processes and sleep are also important. Additionally, the role of different amounts of sleep loss during sleep deprivation requires clarification. Detailed and well-planned longitudinal research concerning temporal development of sleep and affect is needed. Following from the results, less sleep can be assumed to have a negative impact on affect processes, and is associated with notable short- and long-term psychological problems; therefore, understanding the relationships between affect processes and sleep is a central assignment for future research.
Regarding the practical implications the results indicate a direct relationship between sleep and emotional functioning in a young population. Therefore, prevention and intervention programs should increase sensitivity for sleep problems and its recognition. Moreover, psychoeducation regarding sleep and its positive and negative consequences among parents, children, adolescents, and health care professionals might provide faster recognition and a better opportunity for successful treatment of sleep irregularities and therefore prevent chronification and potential psychopathological consequences.
| Practice points | |
|---|---|
| • Sleep supports processing of emotional stimuli, obvious in physiological and physiological measures. | |
| • Sleep deprivation impairs affect processing, measured by subjective and objective measurements of affective reactions towards visual and auditory emotion eliciting stimuli. | |
| • Longer sleep duration, as well as undisturbed instead of quite sleep enhances the experience of positive emotions, as well as the capacity of emotional reasoning, behavioral responding and regulation. |
| Key research agenda |
|---|
| • Include diverse measurements of affect and sleep, and examine the presentation of stimuli and the activation of the senses. |
| • Be highly sensitive while choosing the methods of assessment and manipulation of sleep and emotion regulation. |
| • Differences in results have to be interpreted with regard to the used statistical analysis. |
| • Explicitly discriminate between assessment of emotion activation and emotion regulation. |
| • Investigate the role of different amounts of sleep loss during sleep deprivation on task performance, and involve the possible interference between a constructed setting and emotional responses in the interpretation of results. |
| • Assess the temporal development of sleep and emotion to clarify their mutual association during childhood and adolescence. |
| • Investigate influencing conditions under which sleep and affect regulation develop into a negative pattern. |
| • Implement and develop objective tests with age-appropriate and variable task demands for longitudinal research. |
| • While utilizing report of others, pay attention to possible confounders. |
| • Identify confounding intra-individual factors of better emotion regulation. |
| • Acknowledge of gender as an influencing factor. |
APPENDIX.
Table 1.
Sleep manipulation protocols of studies concerning infants and toddlers (n=5).
| Study | Days of stabilization period | Sleep duration/ bedtime |
Day(s) of manipulation period | Sleep condition | Sleep duration/ bedtime | Point of measurement | Content of measurement | ||
|---|---|---|---|---|---|---|---|---|---|
| Night | Day | Night | Day | ||||||
| Berger (2012)62 | 1-5 | 20:00-07:00 | 12:30-14:00 | 6 | Nap restriction | 20:00-07:00 | - | 15:00 | Emotional assessment,
video recorded (30min): • 11 emotion eliciting pictures • Puzzle task |
| 6-11 | 20:00-07:00 | 12:30-14:00 | 11 | Normal sleep | 20:00-07:00 | 12:30-14:00 | 15:00 | ||
| Cremone et al. (2017)52 | 0 | ns | ns | 1 | Nap restriction | ns | - | ≈15:00 | Emotional attention
bias • Dot probe task |
| 0 | ns | ns | 1 | Normal nap | ns | 13:00-15:00 | ≈15:00 | ||
| Han (2014)66 | 1-7 | 11-12hrs/day | 8 | Nap restriction | ns | - | ns | Emotional assessment
(11min) • 32 visual and auditory stimuli pairings |
|
| 9-11 | 11-12hrs/day | 12 | Normal nap | ns | Nap option in lab | ns | |||
| Miller (2015)61 | 1-5 | 20:00-07:00 | 12:30-14:00 | 6 | Normal sleep | 20:00-07:00 | 12:30-14:00 | 15:00 | Challenge task, video
recorded: • Puzzle task |
| 6-11 | 20:00-07:00 | 12:30-14:00 | 11 | Nap restriction | 20:00-07:00 | - | 15:00 | ||
| Schumacher (2017)53 | 1-5 | 20:00-07:00 | 12:30-14:00 | 6 | Normal sleep | 20:00-07:00 | 12:30-14:00 | 10:00 | Challenge task, video
recorded: • Go/no go task • Puzzle task |
| 6-10 | 20:00-07:00 | 12:30-14:00 | 11 | Sleep restriction | 23:00-07:00 | 10:00 | |||
Abbreviations: ns = Not specified.
Table 2.
Sleep manipulation protocols of studies concerning children (n=2).
| Study | Days of stabilization period | Sleep duration | Day(s) of manipulation period | Sleep condition | Sleep duration/ bedtime | Point of measurement | Content of measurement |
|---|---|---|---|---|---|---|---|
| Gruber (2012)77 | 1-5 | Individual bed/
sleeptimes Randomization after stab. period |
6-10 | Sleep extension | 1hr earlier than usual | • Last day of baseline and manipulation period | • The Connors’ global index-teachers with subscale “emotional lability” and “restless-impulsive behavior” |
| 6-10 | Sleep restriction | 1hr later than usual | |||||
| Vriend (2013)54 | 1 week | Individual bed/
sleeptimes Randomization after stab. period |
4 nights | Sleep extension | 1hr earlier than usual | • Baseline in
lab • In the morning after last night of sleep restriction • In the morning after last night of sleep extension |
Assessment battery (90-120
min): Parents: • Parents questionnaires Conners’ parent rating scale-revised • Emotion questionnaire Children: • Child’s pictorial sleepiness scale • Digit-span task • Finger windows task • Attention network test-interaction • Children’s colour trial test • Math fluency task • Emotion questionnaire for children |
| 4 nights | Sleep restriction | 1hr later than usual |
Table 3.
Sleep manipulation protocols of studies concerning adolescents (n=7).
| Study | Days of stabilization period | Sleep duration | Day(s) of manipulation period | Sleep condition | Sleep duration/ bedtime | Point of measurement | Content of measurement |
|---|---|---|---|---|---|---|---|
| Baum (2014)69 | 5 nights | Individual bed/ sleeptimes | • Baseline
assessment • Assessment after last night of SR • Assessment after last night of SE |
Parents: • Vanderbilt assessment scale • Emotion control subscale of the BRIEF Adolescents: • Profile of mood states Emotion control subscale of the BRIEF |
|||
| 2 nights | Individual bed/ sleeptimes | 4 nights | Sleep restriction | 6.5hrs in bed | |||
| 2 nights | Individual bed/ sleeptimes | 4 nights | Sleep extension | 10hrs in bed | |||
| Dagys et al. (2012)68 | 5 nights | Individual bed/
sleeptimes Randomization after stab. period |
1 night at home | Sleep restriction | In bed for 6.5hrs | ||
| 2nd night in lab | 03:00-05:00 | 22:00; 00:00; 02:00; 03:00; 05:00; 08:00 | • Positive and negative affect schedule-children | ||||
| 2 nights at home | Sleep extension | 8.5hrs in bed | 08:00 or 10:00 in lab | ||||
| Lo et al. (2016)55 | 3 nights | 23:00-08:00 | 7 nights | Sleep restriction | 01:00-06:00 | • Assessment every day at 10:00; 15:00; 20:00 | Cognitive performance test
battery (25min): • Karolinska sleepiness scale • Sustained attention to response task • Symbol digit modalities test • Verbal 1- and 3 back tasks • Mental arithmetic test • Positive and negative affect schedule • PVT |
| 7 nights | Normal sleep | 23:00-08:00 | |||||
| McMakin (2016)67 Study protocol 1 |
- | - | 2 nights | Sleep restriction | 01:00-05:00 | Assessment of affect three times during day one and one time in the morning after the 2nd night | •
PSG • Visual analogue scales • Auditory valence identification task, pupillography |
| 2 nights | Sleep extension | 22:00-08:00 | |||||
| McMakin (2016)67 Study protocol 2 |
- | - | 2 nights | Sleep restriction | 1st night 02:00-08:00 2nd night 02:00-04:00 |
Assessment of affect three times during day one and one time in the morning after the 2nd night | •
PSG • Visual analogue scales • Auditory valence identification task, pupillography • Peer conflict task in the afternoon of day 2 |
| 2 nights | Sleep extension | 22:00-08:00 | |||||
| 4 nights | Sleep extension | Go to bed as usual and stay in bed for 9.5hrs | |||||
| Study | Days of stabilization period | Sleep duration | Day(s) of manipulation period | Sleep condition | Sleep duration/ bedtime | Point of measurement | Content of measurement |
| Raynolds (2017)73 | 1-7 | Individual bed/
sleeptimes Randomization after stab. period |
5 nights | Normal sleep instead of unmanipilated | Go to bed as usual | Assessment in lab between 16:00 and 18:00 after last night of sleep manipulation | Computerized task battery,
videotaped Naturalistic online social interaction task • Frustrating PC game Manipulated online social interaction task |
| 5 nights | Sleep extension | 1hr earlier than usual | |||||
| Reddy et al. (2017)57 | 1-6 | Minimum of 7.5hrs in bed | 4 nights | Sleep restriction | 2hrs later than usual and only 4hrs in bed | Assessment in lab at 12:00 after last night of sleep manipulation | • Kiddie schedule
for affective disorders and schizophrenia for school-age
children-present and lifetime version • BEARS sleep screen • Positive and negative affect schedule-children • State-trait anxiety inventory for children • Epworth sleepiness scale • Emotion reactivity and regulation task based on the international affective pictorial system (16min, 40 trials) |
| 4 nights | Sleep extension | Go to bed as usual and stay in bed for 9.5hrs | |||||
| Short and Louca (2015)70 | 1 week | 22:00-07:00 | 2 nights | Normal sleep | 22:00-08:00 | 09:00; 11:00; 13:00; 15:00; 17:00; 19:00 | Neurobehavioral test
battery: • Psychomotor vigilance task • Digit symbol substitution task • Profile of mood scale-short form • Karolinska sleepiness scale |
| 1 night | Sleep restriction | 08:00-20:00 (36hrs awake) | Every 2 hours testing |
REFERENCES
- 1.Palmer CA, Alfano CA. Sleep and emotion regulation: an organizing, integrative review. Sleep Med Rev. 2017 Feb;31:6–16. doi: 10.1016/j.smrv.2015.12.006. [DOI] [PubMed] [Google Scholar]
- 2.Beattie L, Kyle SD, Espie CA, Biello SM. Social interactions, emotion and sleep: a systematic review and research agenda. Sleep Med Rev. 2015 Dec;24:83–100. doi: 10.1016/j.smrv.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 3.Calhoun SL, Fernandez-Mendoza J, Vgontzas AN, Liao D, Bixler EO. Prevalence of insomnia symptoms in a general population sample of young children and preadolescents: gender effects. Sleep Med. 2014 Jan;15(1):91–95. doi: 10.1016/j.sleep.2013.08.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schlarb AA, Gulewitsch MD, Weltzer V, Ellert U, Enck P. Sleep duration and sleep problems in a representative sample of German children and adolescents. Health. 2015 Nov;7(11):1397–1408. [Google Scholar]
- 5.Gaylor EE, Burnham MM, Goodlin-Jones BL, Anders TF. A longitudinal follow-up study of young children’s sleep patterns using a developmental classification system. Behav Sleep Med. 2005 Jun;3(1):44–61. doi: 10.1207/s15402010bsm0301_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gregory AM, Sadeh A. Annual research review: sleep problems in childhood psychiatric disorders-a review of the latest science. J Child Psychol Psychiatry. 2016 Mar;57(3):296–317. doi: 10.1111/jcpp.12469. [DOI] [PubMed] [Google Scholar]
- 7.Astill RG, Van Der Heijden KB, Van Ijzendoorn MH, Van Someren EJW. Sleep, cognition, and behavioral problems in school-age children: a century of research meta-analyzed. Psychol Bull. 2012 Nov;138(6):1109–1138. doi: 10.1037/a0028204. [DOI] [PubMed] [Google Scholar]
- 8.Chaput JP, Gray CE, Poitras VJ, Carson V, Gruber R, Old T, et al. Systematic review of the relationships between sleep duration and health indicators in school-aged children and youth. Appl Physiol Nutr Med. 2016 Jun;41(6 Suppl 3):S266–S82. doi: 10.1139/apnm-2015-0627. [DOI] [PubMed] [Google Scholar]
- 9.Alfano CA, Gamble AL. The role of sleep in childhood psychiatric disorders. Child Youth Care Forum. 2009 Dec;38(6):327–340. doi: 10.1007/s10566-009-9081-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wiater AH, Mitschke AR, Widdern SV, Fricke L, Breuer U, Lehmkuhl G. Sleep disorders and behavioral problems among 8-to 11-year-old children. Somnologie. 2005 Nov;9(4):210–214. [Google Scholar]
- 11.Gregory AM, Caspi A, Eley TC, Moffitt TE, O’Connor TG, Poulton R. Prospective longitudinal associations between persistent sleep problems in childhood and anxiety and depression disorders in adulthood. J Abnorm Child Psychol. 2005 Apr;33(2):157–163. doi: 10.1007/s10802-005-1824-0. [DOI] [PubMed] [Google Scholar]
- 12.Wickens CD, Hutchins SD, Laux L, Sebok A. The impact of sleep disruption on complex cognitive tasks: a meta-analysis. Hum Factors. 2015 Sep;57(6):930–946. doi: 10.1177/0018720815571935. [DOI] [PubMed] [Google Scholar]
- 13.Pilcher JJ, Huffcutt AI. Effects of sleep deprivation on performance: a meta-analysis. Sleep. 1996 May;19(4):318–326. doi: 10.1093/sleep/19.4.318. [DOI] [PubMed] [Google Scholar]
- 14.Dinges DF, Pack F, Williams K, Gillen KA, Powell JW, Ott GE, et al. Cumulative sleepiness, mood disturbance, and psychomotor vigilance performance decrements during a week of sleep restricted to 4-5 hours per night. Sleep. 1997 Apr;20(4):267–277. [PubMed] [Google Scholar]
- 15.Caldwell Junior JA, Caldwell JL, Brown DL, Smith JK. The effects of 37 hours of continuous wakefulness on the physiological arousal, cognitive performance, self-reported mood, and simulator flight performance of F-117A pilots. Mil Psychol. 2004 Nov;16(3):163–181. [Google Scholar]
- 16.Paterson JL, Dorrian J, Ferguson SA, Jay SM, Lamond N, Murphy PJ, et al. Changes in structural aspects of mood during 39-66 h of sleep loss using matched controls. Appl Ergon. 2011 Jan;42(2):196–201. doi: 10.1016/j.apergo.2010.06.014. [DOI] [PubMed] [Google Scholar]
- 17.Cole PM, Martin SE, Dennis TA. Emotion regulation as a scientific construct: Methodological challenges and directions for child development research. Child Dev. 2004 Mar/Apr;75(2):317–333. doi: 10.1111/j.1467-8624.2004.00673.x. [DOI] [PubMed] [Google Scholar]
- 18.Gross JJ. The emerging field of emotion regulation: an integrative review. Rev Gen Psychol. 1998 Sep;2(3):271–299. [Google Scholar]
- 19.Webb TL, Miles E, Sheeran P. Dealing with feeling: a meta-analysis of the effectiveness of strategies derived from the process model of emotion regulation. Psychol Bull. 2012 Jul;138(4):775–805. doi: 10.1037/a0027600. [DOI] [PubMed] [Google Scholar]
- 20.Fonagy P, Gergely G, Jurist EL, Target M. Affect regulation, mentalization and the development of the self. Abingdon: Routledge; 2018. [Google Scholar]
- 21.Thompson RA, Goodvin R. In: Socioemotional development in the toddler years: transitions and transformations. Brownell CA, Kopp CB, editors. New York: Guilford Press; 2007. Taming the tempest in the teapot; pp. 320–341. [Google Scholar]
- 22.Calkins SD, Fox NA. Self-regulatory processes in early personality development: a multilevel approach to the study of childhood social withdrawal and aggression. Dev Psychopathol. 2002 Aug;14(3):477–498. doi: 10.1017/s095457940200305x. [DOI] [PubMed] [Google Scholar]
- 23.Calkins SD. Commentary: conceptual and methodological challenges to the study of emotion regulation and psychopathology. J Psychopathol Behav Assess. 2010 Nov;32:92–95. [Google Scholar]
- 24.Thompson RA. Emotion regulation: a theme in search of definition. Monogr Soc Res Child. 1994;59(2-3):25–52. [PubMed] [Google Scholar]
- 25.Fox AS, Oler JA, Tromp DP, Fudge JL, Kalin NH. Extending the amygdala in theories of threat processing. Trends Neurosci. 2015 May;38(5):319–329. doi: 10.1016/j.tins.2015.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guillory SA, Bujarski KA. Exploring emotions using invasive methods: review of 60 years of human intracranial electrophysiology. Soc Cogn Affect Neur. 2014 Dec;9(12):1880–1889. doi: 10.1093/scan/nsu002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murray RJ, Brosch T, Sander D. The functional profile of the human amygdala in affective processing: insights from intracranial recordings. Cortex. 2014 Nov;60:10–33. doi: 10.1016/j.cortex.2014.06.010. [DOI] [PubMed] [Google Scholar]
- 28.Lewis MD, Lamm C, Segalowitz SJ, Stieben J, Zelazo PD. Neurophysiological correlates of emotion regulation in children and adolescents. J Cogn Neurosci. 2006 Mar;18(3):430–443. doi: 10.1162/089892906775990633. [DOI] [PubMed] [Google Scholar]
- 29.Mather M, Carstensen LL. Aging and motivated cognition: the positivity effect in attention and memory. Trends Cogn Sci. 2005 Oct;9(10):496–502. doi: 10.1016/j.tics.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 30.Rothbart MK, Sheese BE, Posner MI. Executive attention and effortful control: linking temperament, brain networks, and genes. Child Dev Perspect. 2007 Sep;1(1):2–7. [Google Scholar]
- 31.Compton RJ, Banich MT, Mohanty A, Milham MP, Herrington J, Miller GA, et al. Paying attention to emotion: an fMRI investigation of cognitive and emotional Stroop tasks. Cogn Affect Behav Neurosci. 2003 Jun;3(2):81–96. doi: 10.3758/cabn.3.2.81. [DOI] [PubMed] [Google Scholar]
- 32.Shafir R, Schwartz N, Blechert J, Sheppes G. Emotional intensity influences pre-implementation and implementation of distraction and reappraisal. Soc Cogn Affect Neurosci. 2015 Oct;10(10):1329–1337. doi: 10.1093/scan/nsv022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Prinzmetal W, McCool C, Park S. Attention: reaction time and accuracy reveal different mechanisms. J Exp Psychol Gen. 2005 Feb;134(1):73–92. doi: 10.1037/0096-3445.134.1.73. [DOI] [PubMed] [Google Scholar]
- 34.Case R, Hayward S, Lewis M, Hurst P. Toward a neo-Piagetian theory of cognitive and emotional development. Dev Rev. 1988 Mar;8:1–51. [Google Scholar]
- 35.Nardelli M, Valenza G, Greco A, Lanata A, Scilingo EP. Recognizing emotions induced by affective sounds through heart rate variability. IEEE Trans Comput. 2015 Oct;6:385–394. [Google Scholar]
- 36.Homma I, Masaoka Y. Breathing rhythms and emotions. Exp Physiol. 2008 Sep;93(9):1011–1021. doi: 10.1113/expphysiol.2008.042424. [DOI] [PubMed] [Google Scholar]
- 37.Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception II: implications for major psychiatric disorders. Biol Psychiatry. 2003 Sep;54(5):515–528. doi: 10.1016/s0006-3223(03)00171-9. [DOI] [PubMed] [Google Scholar]
- 38.Baumeister RF, Vohs KD, DeWall CN, Zhang L. How emotion shapes behavior: Feedback, anticipation, and reflection, rather than direct causation. Pers Soc Psychol Rev. 2007 May;11(2):167–203. doi: 10.1177/1088868307301033. [DOI] [PubMed] [Google Scholar]
- 39.Blascovich J, Mendes WB, Hunter SB, Salomon K. Social” facilitation” as challenge and threat. J Pers Soc Psychol. 1999 Jul;77(1):68–77. doi: 10.1037//0022-3514.77.1.68. [DOI] [PubMed] [Google Scholar]
- 40.Jefferson AL, Himali JJ, Beiser AS, Au R, Massaro JM, Seshadri S, et al. Cardiac index is associated with brain aging: the Framingham Heart Study. Circulation. 2010 Aug;122(7):690–697. doi: 10.1161/CIRCULATIONAHA.109.905091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Harrison LJ, Murray E. Stress, coping and wellbeing in kindergarten: children’s perspectives on personal, interpersonal and institutional challenges of school. J Early Child. 2015 Apr;47(1):79–103. [Google Scholar]
- 42.Skinner EA, Zimmer-Gembeck MJ. The development of coping. Annu Rev Psychol. 2007 Jan;58:119–144. doi: 10.1146/annurev.psych.58.110405.085705. [DOI] [PubMed] [Google Scholar]
- 43.Lerner RM, Busch-Rossnagel N A. New. York: Academic Press;; 2013. Individuals as producers of their development: a life-span perspective. [Google Scholar]
- 44.Sadeh A. Consequences of sleep loss or sleep disruption in children. Sleep Med Clin. 2007 Sep;2:513–520. [Google Scholar]
- 45.Cerolini S, Ballesio A, Lombardo C. Insomnia and emotion regulation: recent findings and suggestions for treatment. J Sleep Disord Manag. 2015 Aug;1:1. [Google Scholar]
- 46.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement. PLoS Med. 2009 Jul;6(7):e264–269. [PMC free article] [PubMed] [Google Scholar]
- 47.Döring N, Bortz J. Forschungsmethoden und evaluation. Wiesbaden: Springerverlag;; 2016. [Google Scholar]
- 48.Bonvanie IJ, Kallesøe KH, Janssens KAM, Schröder A, Rosmalen JG, Rask CU. Psychological interventions for children with functional somatic symptoms: a systematic review and meta-analysis. J Pediatr. 2017 Aug;187:272–81.e17. doi: 10.1016/j.jpeds.2017.03.017. [DOI] [PubMed] [Google Scholar]
- 49.Bolinger E, Born J, Zinke K. Sleep divergently affects cognitive and automatic emotional response in children. Neuropsychol. 2018 Aug;117:84–91. doi: 10.1016/j.neuropsychologia.2018.05.015. [DOI] [PubMed] [Google Scholar]
- 50.Herbert BM, Pollatos O, Schandry R. Interoceptive sensitivity and emotion processing: an EEG study. Int J Psychophysiol. 2007 Sep;65(3):214–227. doi: 10.1016/j.ijpsycho.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 51.Dennis TA, Hajcak G. The late positive potential: a neurophysiological marker for emotion regulation in children. J Child Psychol Psychiatry. 2009 Nov;50(11):1373–1383. doi: 10.1111/j.1469-7610.2009.02168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cremone A, Kurzdiel LB, Fratielli-Torres A, McDermott JM, Spencer RMC. Napping reduces emotional attention bias during early childhood. Dev Sci. 2017 Jul;20(4):e12411. doi: 10.1111/desc.12411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schumacher AM, Miller AL, Watamura SE, Kurth S, Lassonde JM, LeBourgeois MK. Sleep moderates the association between response inhibition and self-regulation in early childhood. J Clin Child Adolesc Psychol. 2017 Mar/Apr;46(2):222–235. doi: 10.1080/15374416.2016.1204921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vriend J, Davidson FD, Corkum PV, Rusak B, Chambers CT, McLaughlin EN. Manipulating sleep duration alters emotional functioning and cognitive performance in children. J Pediatr Psychol. 2013 Nov;38(10):1058–1069. doi: 10.1093/jpepsy/jst033. [DOI] [PubMed] [Google Scholar]
- 55.Lo JC, Ong JL, Leong RL, Gooley JJ, Chee MW. Cognitive performance, sleepiness, and mood in partially sleep deprived adolescents: the need for sleep study. Sleep. 2016 Mar;39(3):687–698. doi: 10.5665/sleep.5552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Soffer-Dudek N, Sadeh A, Dahl RE, Rosenblat-Stein S. Poor sleep quality predicts deficient emotion information processing over time in early adolescence. Sleep. 2011 Nov;34(11):1499–1508. doi: 10.5665/sleep.1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Reddy R, Palmer CA, Jackson C, Farris SG, Alfano CA. Impact of sleep restriction versus idealized sleep on emotional experience, reactivity and regulation in healthy adolescents. J Sleep Res. 2017 Aug;26(4):516–525. doi: 10.1111/jsr.12484. [DOI] [PubMed] [Google Scholar]
- 58.Vaughn BE, Elmore-Staton L, Shin N, El-Sheik M. Sleep as a support for social competence, peer relations, and cognitive functioning in preschool children. Behav Sleep Med. 2015 Feb;13(2):92–106. doi: 10.1080/15402002.2013.845778. [DOI] [PubMed] [Google Scholar]
- 59.Denham SA, Bassett HH, Way E, Mincic M, Zinsser K, Graling K. Preschoolers’ emotion knowledge: self-regulatory foundations, and predictions of early school success. Cogn Emot. 2012 Aug;26(4):667–679. doi: 10.1080/02699931.2011.602049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cho S, Philbrook LE, Davis EL, Buss KA. Sleep duration and RSA suppression as predictors of internalizing and externalizing behaviors. Dev Psychobiol. 2017 Jan;59(1):60–69. doi: 10.1002/dev.21467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Miller AL, Seifer R, Crossin R, Lebourgeois MK. Toddler’s self-regulation strategies in a challenge context are nap-dependent. J Sleep Res. 2015 Jun;24(3):279–287. doi: 10.1111/jsr.12260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Berger RH, Miller AL, Seifer R, Cares SR, Lebourgeois MK. Acute sleep restriction effects on emotion responses in 30-to 36-month-old children. J Sleep Res. 2012 Jun;21(3):235–246. doi: 10.1111/j.1365-2869.2011.00962.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ross CN, Karraker KH. Effects of fatigue on infant emotional reactivity and regulation. Infant Ment Health J. 1999 Feb;20(4):410–428. [Google Scholar]
- 64.Weissbluth M. Sleep duration and infant temperament. J Pediatr. 1981 Nov;99(5):817–819. doi: 10.1016/s0022-3476(81)80422-2. [DOI] [PubMed] [Google Scholar]
- 65.DeLeon CW, Karraker KH. Intrinsic and extrinsic factors associated with night waking in 9-month-old infants. Infant Behav Dev. 2007 Dec;30(4):596–605. doi: 10.1016/j.infbeh.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 66.Han HY. The impact of sleep restriction (nap restriction) on preschool childrens’ emotional response [dissertation] Hattiesburg: University of Southern Mississippi; 2014. [Google Scholar]
- 67.McMakin DL, Dahl RE, Buysse DJ, Cousins JC, Forbes EE, Silk JS, et al. The impact of experimental sleep restriction on affective functioning in social and nonsocial contexts among adolescents. J Child Psychol Psychiatry. 2016 Sep;57(9):1027–1037. doi: 10.1111/jcpp.12568. [DOI] [PubMed] [Google Scholar]
- 68.Dagys N, McGlinchey E, Talbot LS, Kaplan KA, Dahl RE, Harvey AG. Double trouble? The effects of sleep deprivation and chronotype on adolescent affect. J Child Psychol Psychiatry. 2012 Jun;53(6):660–667. doi: 10.1111/j.1469-7610.2011.02502.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Baum KT, Desai A, Field J, Miller LE, Rausch J, Beebe DW. Sleep restriction worsens mood and emotion regulation in adolescents. J Child Psychol Psychiatry. 2014 Jul;55(2):180–190. doi: 10.1111/jcpp.12125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Short MA, Louca M. Sleep deprivation leads to mood deficits in healthy adolescents. Sleep Med. 2015 Aug;16(8):987–993. doi: 10.1016/j.sleep.2015.03.007. [DOI] [PubMed] [Google Scholar]
- 71.Settineri S, Vitetta A, Mento C, Fanara G, Silvestri R, Tatì F, et al. Construction of a telephone interview to assess the relationship between mood and sleep in adolescence. Neurol Sci. 2010 Aug;31(4):459–465. doi: 10.1007/s10072-010-0255-z. [DOI] [PubMed] [Google Scholar]
- 72.Kouros CD, El-Sheikh M. Daily mood and sleep: reciprocal relations and links with adjustment problems. J Sleep Res. 2015 Feb;24(1):24–31. doi: 10.1111/jsr.12226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Raynolds KC. Impact of experimental sleep extension on adolescent social emotion regulation [dissertation] Houston: University of Houston; 2017. [Google Scholar]
- 74.Lazeron RH, Rombouts SA, Sonneville L, Barkhof F, Scheltens P. A paced visual serial addition test for fMRI. J Neurol Sci. 2003 Sep;213(1-2):29–34. doi: 10.1016/s0022-510x(03)00144-8. [DOI] [PubMed] [Google Scholar]
- 75.Belsky J, Steinberg LD, Houts RM, Friedman SL, DeHart G, Cauffman E. Family rearing antecedents of pubertal timing. Child Dev. 2007 Jul/Aug;78(4):1302–1321. doi: 10.1111/j.1467-8624.2007.01067.x. [DOI] [PubMed] [Google Scholar]
- 76.Troxel WM, Trentacosta CJ, Forbes EE, Campbell SB. Negative emotionality moderates’ associations among attachment, toddler sleep, and later problem behaviors. J Fam Psychol. 2013 Feb;27(1):127–136. doi: 10.1037/a0031149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gruber R, Cassoff J, Frenett S, Wiebe S, Carrier J. Impact of sleep extension and restriction on children’s emotional lability and impulsivity. Pediatrics. 2012 Nov;130(5):e1155–e61. doi: 10.1542/peds.2012-0564. [DOI] [PubMed] [Google Scholar]
- 78.Rubens SL, Evans SC, Becker SP, Fite PJ, Tountas AM. Self-reported time in bed and sleep quality in association with internalizing and externalizing symptoms in school-age youth. Child Psychiat Hum Dev. 2017 Jun;48(3):455–467. doi: 10.1007/s10578-016-0672-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bayes DM, Bullock B. Sleep problems in school aged children: a common process across internalising and externalising behaviours? Clocks Sleep. 2020 Mar;2(1):7–18. doi: 10.3390/clockssleep2010002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gruber R, Somerville G, Santisteban JA. Using parental report to identify children at risk for poor sleep and daytime problems. Behav Sleep Med. 2020 Jul/Aug;18(4):400–476. doi: 10.1080/15402002.2019.1613236. [DOI] [PubMed] [Google Scholar]
- 81.Gregory AM, O’Connor TG. Sleep problems in childhood: a longitudinal study of developmental change and association with behavioral problems. J Am Acad Child Adolesc Psychiatry. 2002 Aug;41(8):964–971. doi: 10.1097/00004583-200208000-00015. [DOI] [PubMed] [Google Scholar]
- 82.Foley JE, Weinraub M. Sleep, affect, and social competence from preschool to preadolescence: distinct pathways to emotional and social adjustment for boys and for girls. Front Psychol. 2017 May;8:711. doi: 10.3389/fpsyg.2017.00711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang B, Eastwood PR, Becker A, Isensee C, Wong JW, Huang RC, et al. Concurrent developmental course of sleep problems and emotional/behavioral problems in childhood and adolescence as reflected by the dysregulation profile. Sleep. 2019 Mar;42(3):zsy243. doi: 10.1093/sleep/zsy243. [DOI] [PubMed] [Google Scholar]
- 84.Saenz J, Yaugher A, Alexander GM. Sleep in infancy predicts gender specific social-emotional problems in toddlers. Front Paediatr. 2015;3:42. doi: 10.3389/fped.2015.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bastien L, Tétreault É, Bernier A. Disentangling the direction of associations between sleep and temperament in toddlers. Behav Sleep Med. 2020 Jul/Aug;18(4):523–536. doi: 10.1080/15402002.2019.1629442. [DOI] [PubMed] [Google Scholar]
- 86.Van Der Helm E, Yao J, Dutt S, Rao V, Saletin JM, Walker MP. REM sleep depotentiates amygdala activity to previous emotional experiences. Curr Biol. 2011 Dec;21(23):2029–2032. doi: 10.1016/j.cub.2011.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Silvia PJ. Looking past pleasure: anger, confusion, disgust, pride, surprise, and other unusual aesthetic emotions. Psychol Aesth Creat Arts. 2009 Nov;3(1):48–51. [Google Scholar]
- 88.Laurent J, Catanzaro SJ, Joiner Junior TE, Rudolph KD, Potter KI, Lambert S, et al. A measure of positive and negative affect for children: scale development and preliminary validation. Psychol Assess. 1999 Sep;11:326–338. [Google Scholar]
- 89.McNair DM, Lorr M, Droppleman LF. Manual for the profile of mood states (POMS) San Diego: Educational and Industrial Testing Service; 1971. [Google Scholar]
- 90.Payne RL, Cooper CL. Theory, research and applications for management. Chichester: Wiley & Sons; 2001. Emotions at work. [Google Scholar]
- 91.Achenbach TM, Edelbrock CS. Manual fort the child behavior checklist: and revised child behavior profile. Burlington: University of Vermont - Department of Psychology; 1983. [Google Scholar]
- 92.Little TD. Longitudinal structural equation modeling. New York: Guilford Press; 2013. [Google Scholar]
- 93.Kahn M, Bauminger Y, Volkowich E, Meiri G, Sadeh A, Tikotzky L. Links between infant sleep and parental tolerance for infant crying: longitudinal assessment from pregnancy through six months postpartum. Sleep Med. 2018 Oct;50:72–78. doi: 10.1016/j.sleep.2018.05.014. [DOI] [PubMed] [Google Scholar]
- 94.Schoch SF, Jenni OG, Kohler M, Kurth S. Actimetry in infant sleep research: an approach to facilitate comparability. Sleep. 2019 Jul;42(7):zsz083. doi: 10.1093/sleep/zsz083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rosenberg-Kima RB, Sadeh A. Attention, response inhibition, and face-information processing in children: the role of task characteristics, age and gender. Child Neuropsychol. 2010 Jul;16(4):388–404. doi: 10.1080/09297041003671192. [DOI] [PubMed] [Google Scholar]
- 96.Armitage R, Hoffmann R, Emslie G, Rintelman J, Moore J, Lewis K. Rest-activity cycles in childhood and adolescent depression. J Am Acad Child Adolesc Psychiatry. 2004 Jun;43(6):761–769. doi: 10.1097/01.chi.0000122731.72597.4e. [DOI] [PubMed] [Google Scholar]
- 97.Garrison CZ, Addy CL, Jackson KL, McKeown RE, Waller JL. The CES-D as a screen for depression and other psychiatric disorders in adolescents. J Am Acad Child Adolesc Psychiatry. 1991 Jul;30(4):636–641. doi: 10.1097/00004583-199107000-00017. [DOI] [PubMed] [Google Scholar]


