Abstract
Background and Aims
Rhododendron is a species-rich and taxonomically challenging genus due to recent adaptive radiation and frequent hybridization. A well-resolved phylogenetic tree would help to understand the diverse history of Rhododendron in the Himalaya–Hengduan Mountains where the genus is most diverse.
Methods
We reconstructed the phylogeny based on plastid genomes with broad taxon sampling, covering 161 species representing all eight subgenera and all 12 sections, including ~45 % of the Rhododendron species native to the Himalaya–Hengduan Mountains. We compared this phylogeny with nuclear phylogenies to elucidate reticulate evolutionary events and clarify relationships at all levels within the genus. We also estimated the timing and diversification history of Rhododendron, especially the two species-rich subgenera Rhododendron and Hymenanthes that comprise >90 % of Rhododendron species in the Himalaya–Hengduan Mountains.
Key Results
The full plastid dataset produced a well-resolved and supported phylogeny of Rhododendron. We identified 13 clades that were almost always monophyletic across all published phylogenies. The conflicts between nuclear and plastid phylogenies suggested strongly that reticulation events may have occurred in the deep lineage history of the genus. Within Rhododendron, subgenus Therorhodion diverged first at 56 Mya, then a burst of diversification occurred from 23.8 to 17.6 Mya, generating ten lineages among the component 12 clades of core Rhododendron. Diversification in subgenus Rhododendron accelerated c. 16.6 Mya and then became fairly continuous. Conversely, Hymenanthes diversification was slow at first, then accelerated very rapidly around 5 Mya. In the Himalaya–Hengduan Mountains, subgenus Rhododendron contained one major clade adapted to high altitudes and another to low altitudes, whereas most clades in Hymenanthes contained both low- and high-altitude species, indicating greater ecological plasticity during its diversification.
Conclusions
The 13 clades proposed here may help to identify specific ancient hybridization events. This study will help to establish a stable and reliable taxonomic framework for Rhododendron, and provides insight into what drove its diversification and ecological adaption. Denser sampling of taxa, examining both organelle and nuclear genomes, is needed to better understand the divergence and diversification history of Rhododendron.
Keywords: Himalaya–Hengduan Mountains, Rhododendron, genome skimming, plastid genome, phylogenomics, diversification, recent radiation
INTRODUCTION
A robust phylogeny is essential to understand the process of spatiotemporal evolution of a plant group (Jansen et al., 2007; Olofsson et al., 2019). For a species-rich group, intensive sampling of both genomes and taxa is necessary to recover a robust phylogeny (Barrett et al., 2013; Li et al., 2019), especially for groups where hybridization is common and gene trees may not correspond to species trees (Kong et al., 2021; Li et al., 2022). Until recently, resource limitation and costs forced most studies to choose between heavy sampling of either genomes or taxa, but the increasing accessibility and affordability of next-generation sequencing (NGS) data now permits both (Barrett et al., 2013; H. T. Li et al., 2021). Hence the phylogenomic method may be employed, using a large amount of genetic data from chloroplast, mitochondrial and nuclear genomes (Steele and Pires, 2011; Yu et al., 2018), using approaches such as genome skimming, transcriptome and target enrichment sequencing (e.g. Zeng et al., 2017; Villaverde et al., 2018; H. T. Li et al., 2021). Among these methods, genome skimming is now commonly used to cost-effectively and efficiently obtain the plastid genome. The plastid genome has numerous advantages for phylogenetic reconstruction, including uniparental inheritance, minimal recombination, and conservation of structure and evolutionary rate, but with sufficient characters for phylogenetic inference (Petit and Vendramin, 2007). Therefore, the plastid genome has been successfully used for molecular systematics at various taxonomic levels among angiosperms (Straub et al., 2012; Gitzendanner et al., 2018; Zhang et al., 2020; H. T. Li et al., 2021), notably within exceptionally species-rich genera such as Acacia (Williams et al., 2016) and Begonia (Li et al., 2022).
Rhododendron, a species-rich and taxonomically challenging genus in Ericaceae, comprises more than 1000 species (Chamberlain et al., 1996), making it the largest genus of woody plants in the Northern Hemisphere (Wu et al., 2003). Rhododendron is among the world’s most horticulturally valuable genera (Craven, 2011), but is also a vital component of montane ecosystems (Gibbs et al., 2011; Kumar, 2012), containing many dominant or ecologically important species that contribute to the stability of alpine or subalpine plant communities (Wu et al., 2003). One section (Vireya = Schistanthe) has radiated explosively in Southeast Asia, mainly in the Malay Peninsula, New Guinea and the islands between (Brown et al., 2006; Goetsch et al., 2011), whereas two subgenera (Hymenanthes and Rhododendron) have both diversified greatly in the Himalaya–Hengduan Mountains, together generating >90 % of the region’s >320 Rhododendron species, among which about two-thirds are endemic (Chamberlain et al., 1996; Fang et al., 2005; Yan et al., 2015; Fu et al., 2022). Diversification of Rhododendron species in the Himalaya–Hengduan Mountains was associated with uplifts of the Tibetan plateau and climate change during the Neogene (Shrestha et al., 2018; Ding et al., 2020; Xia et al., 2022).
A well-resolved phylogenetic tree would be of help to understand the diverse history of Rhododendron in the Himalaya–Hengduan Mountains, and shed light on the geological history of this area, as well as to the classification, conservation and utilization of this genus. However, rapid radiation into large numbers of species tends to generate very short phylogeny branches, hampering accurate phylogenetic resolution. Furthermore, resolution in many previous phylogenetic studies of Rhododendron has been hampered by insufficient sampling and/or the use of only a few DNA loci (Kurashige et al., 1998, 2001; Gao et al., 2002; Goetsch et al., 2005; Milne et al., 2010; Berry et al., 2018; Shrestha et al., 2018). Moreover, conflict among these studies probably reflects reticulate evolution, which might in turn have contributed to poor resolution of some key nodes. Numerous Rhododendron species occur sympatrically, and considerable interspecific hybridization/introgression events occur among them (Milne et al., 1999, 2003; Zhang et al., 2007; Milne and Abbott, 2008; Ma et al., 2010; Yan et al., 2017; Zheng et al., 2021). There are hence two particular challenges to generating a well-resolved and accurate phylogeny for Rhododendron: recent and rapid speciation, and reticulate evolution, both of which raise a challenge for phylogenetic inference and species identification in Rhododendron, whether based on morphology or molecular data (e.g. Yan et al., 2015; Fu et al., 2022).
There is agreement that subgenus Therorhodion is the first diverging group within Rhododendron (Gao et al., 2002; Goetsch et al., 2005; Xia et al., 2022), but the major divergence events that followed have tended to be neither well resolved nor agreed between studies. Xia et al. (2022) resolved deep phylogenetic relationships with strong support using 3437 orthologous nuclear genes from transcriptome data, but some species relationships had weak support, and there was conflict with their own plastid data, which were derived from 38 plastid protein-coding genes via transcriptome data. This cytonuclear discordance included both deep clade relationships and species relationships (e.g. within Hymenanthes), and there was also considerable missing plastid data, especially for key species such as R. semibarbatum and R. canadense (Xia et al., 2022). Only by combining a highly resolved plastid phylogeny with a nuclear one can the evolution of this genus be properly understood, because neither alone is likely to represent the species tree. Hence in the current study, near-complete plastid genomes of 161 sampled species were recovered using genome skimming data to reconstruct the plastid phylogeny, representing all 12 sections of eight subgenera recognized in Rhododendron (Chamberlain et al., 1996). Correct species identification is crucial for phylogenetic inference, but challenging within Rhododendron, so almost all species examined here were confirmed based on previous DNA barcoding studies (Yan et al., 2015; Fu et al., 2022). We used this phylogeny to estimate the timing and history of diversification in Rhododendron, especially in the Himalaya–Hengduan Mountains. In addition, we compared our plastid phylogeny with published nuclear phylogenies (especially Xia et al., 2022), to elucidate reticulate evolution events and clarify relationships at all levels within the genus, as well as to provide a resource for further research.
MATERIALS AND METHODS
Taxon sampling
A total of 161 species representing all eight subgenera (Hymenanthes, Rhododendron, Tsutsusi, Pentanthera, Azaleastrum, Therorhodion, Mumeazalea and Candidastrum) and 12 sections (Ponticum, Pogonanthum, Rhododendron, Vireya, Brachycalyx, Tsutsusi, Pentanthera, Rhodora, Sciadorhodion, Viscidula, Azaleastrum and Choniastrum) of Rhododendron recognized by Chamberlain et al. (1996) as well as the main lineages in other studies (Goetsch et al., 2005; Xia et al., 2022) were included in this study. Plastomes of 138 species from four subgenera, Hymenanthes, Rhododendron, Tsutsusi and Azaleastrum, were obtained from our previous study (Fu et al., 2022), and to these were added newly generated plastomes of 23 Rhododendron species from the other four subgenera, from genome skimming data, making 161 in total. Of these, 142 species occur in the Himalaya–Hengduan Mountains. Three species, Erica glandulosa, Diplarche multiflora and Empetrum nigrum, were selected as outgroups. Healthy and fresh leaves were collected and dried immediately in silica gel. Most vouchers were deposited at the Herbarium of Kunming Institute of Botany (KUN), Chinese Academy of Sciences. Detailed information of sampling, classification, vouchers and sources of data is provided in Supplementary Data Table S1.
DNA extraction, sequencing, assembly and annotation
Total genomic DNA was extracted from silica-gel-dried leaves using a modified CTAB method (Doyle and Doyle, 1987). Total DNA was quantified and sheared to a mean insert size of 500 bp for Illumina library construction following standard protocols (NEBNext® Ultra IITMDNA Library Prep Kit for Illumina®). The libraries were sequenced to generate ~2 Gb data for each species on the Illumina HiSeq X Ten platform (Illumina, San Diego, CA, USA) with 150-bp paired-end reads at BGI Wuhan, China.
Plastomes of the newly sampled species were de novo assembled from genome skimming data using the GetOrganelle toolkit (Jin et al., 2020). In this toolkit, target-associated plastomic reads were recruited via Bowtie2 v2.3.4 (Langmead and Salzberg, 2012), extracted from total genomic reads, and subsequently de novo assembled by SPAdes v3.15 (Bankevich et al., 2012). As previously described (Fu et al., 2022), it is extremely difficult to obtain the complete plastid genome of Rhododendron and the outgroups in Ericaceae from genome skimming data. Therefore, the plastid genome scaffolds were annotated and checked as implemented in Geneious v9.0.2 (Kearse et al., 2012) using as a reference the published plastome of Rhododendron delavayi (GenBank accession: NC_047438), which was assembled using Illumina and PacBio sequencing data.
Sequence alignment, substitution saturation and selective pressure analyses
The protein-coding genes, rRNA genes and non-coding regions (the last referring to both introns and intergenic regions between protein-coding genes and/or rRNA genes, throughout this paper) were separately extracted from the annotated plastid genome scaffolds using the Python script get_annotated_regions_from_gb.py (available from https://github.com/Kinggerm/PersonalUtilities/). Multiple sequence alignment for each locus was performed using MAFFT v7.471 (Katoh and Standley, 2013), the alignments were manually modified in Geneious, and the protein-coding genes were aligned using the ‘translation align’ option.
Substitutional saturation was assessed for each protein-coding gene in DAMBE v7.0.68 (Xia, 2018) and measured using the substitution saturation index (Iss). From this no substitution saturation was detected, so all protein-coding genes obtained here were included for subsequent analyses. Furthermore, the CodeML program implemented in PAML v4.9h (Yang, 2007) was used to estimate the ratio Ka/Ks (i.e. ω) of the non-synonymous substitution rate (Ka) to synonymous substitution rate (Ks) for each protein-coding gene.
Phylogenetic dataset construction and analysis
We obtained 72 protein-coding genes, 63 non-coding regions and four rRNA genes in total. By concatenating sequences in different combinations, three supermatrices (datasets) were formed: WP contained all 139 loci, NCS comprised the 63 non-coding regions, and PCS comprised 72 protein-coding genes plus four rRNA genes. To investigate the phylogenetic effect of genes under positive selection, two additional datasets, WP-ω and PCS-ω, were formed, by removing those genes under positive selection from the WP and PCS datasets, respectively.
Maximum likelihood (ML) and Bayesian inference (BI) methods were performed based on the WP dataset. ML analysis was conducted with a GTR + Γ substitution model and 1000 rapid bootstrap replicates, using RAxML v8.2.12 (Stamatakis, 2006). In addition, an ML tree of the WP dataset was also constructed using IQ-TREE v1.6.10 (Nguyen et al., 2015) under the MFP option with 1000 ultrafast bootstrap (UFBS) replicates (Hoang et al., 2018). For the BI method, two independent tree searches of PhyloBayes MPI analysis starting from a random tree were run until the likelihood of the sampled trees had stabilized and converged (maxdiff < 0.3), with constant sites removed (-dc), and trees and associated model parameters sampled every cycle under the CAT + GTR + Γ (four discrete gamma rates) substitution model, using PhyloBayes MPI v1.8c (Lartillot et al., 2013). ML analyses were also performed based on the NCS, PCS, WP-ω and PCS-ω datasets using RAxML and IQ-TREE respectively under the same parameters as before. Trees were visualized in FigTree v1.4.3 (available from http://tree.bio.ed.ac.uk/software/figtree/).
Divergence time estimation
To compare the divergence time estimated by different approaches, three methods (Bayesian, RelTime and penalized likelihood) were used in divergence time estimates. Divergence time was estimated using the full plastid dataset (WP) with the Bayesian approach conducted in BEAST v1.8.4 (Drummond et al., 2012). BEAST analysis was run under a relaxed molecular clock with uncorrelated, lognormally distributed substitution rates for each branch in the phylogenetic tree, the GTR + Γ + I nucleotide substitution model and a birth–death incomplete sampling speciation process tree prior. The dated tree was calibrated with two fossils. The leaf fossil of Rhododendron protodilatatum (Tanai and Onoe, 1961; Ozaki, 1980) dated to the start of the Pliocene [c. 5.3 million years ago (Mya)] was set as the minimum age constraint of the crown of sect. Brachycalyx [priors for the time to the most recent common ancestor (tMRCA): lognormal distribution with mean = 6, lognormal SD = 1 and offset = 5.3]. The seed fossil of R. newburyanum (Collinson and Crane, 1978) dated to the late Paleocene (c. 56 Mya) was set as the minimum age constraint of the Rhododendron crown group (priors for tMRCA: mean = 61, lognormal SD = 4 and offset = 56). All other priors were set to their default values. Two independent Markov chain Monte Carlo (MCMC) runs that were started with a random starting tree and sampled every 50 000 generations were conducted with the same parameters for a total of 2 × 109 generations. Stationarity and convergence were assessed using Tracer v1.7.1 (Rambaut et al., 2018), and Effective Sample Sizes (ESS) of all parameters exceeding 200 were considered convergent. The initial 25 % of trees sampled in each run were discarded as burn-in, and the remaining trees were combined into a single file using LogCombiner v1.8.4, and TreeAnnotator v1.8.4 (Drummond et al., 2012) was used to find the maximum clade credibility (MCC) tree, which was finally visualized using FigTree.
Penalized likelihood and RelTime (Tamura et al., 2012) approaches were also used to estimate divergence times for WP in treePL v1.0 (Smith and O’Meara, 2012) and MEGA X (Kumar et al., 2018), respectively. The same two fossil calibration points as for BEAST were used in both cases. For treePL analysis, 1000 ML bootstrap trees with branch length generated by RAxML were used as the input trees. A priming analysis was first performed to determine the best optimization parameter values, followed by a cross-validation analysis to determine the optimal smoothing parameter value. The RelTime method was performed based on the ML tree from the WP dataset, which which was built by RAxML, with the parameters set following Xia et al. (2022).
Diversification analyses
To test whether the choice of method would influence the results, three approaches (BAMM, LTT and MEDUSA) were used to estimate the diversification dynamics within Rhododendron. The outgroup taxa were discarded and only the species of Rhododendron were retained from the MCC tree generated by BEAST analysis. We utilized BAMM v2.5.0 (Rabosky, 2014) to assess the historical diversification rate change over time of Rhododendron. First, the setBAMMpriors function in BAMMtools v2.1.7 (Rabosky, 2014) was used to generate prior parameters for the ultrametric phylogenetic tree. If the calibrated chronogram was not fully sampled and only contained part of the species diversity of the genus, it may lead to biased estimates of diversification rates on molecular phylogenies (FitzJohn et al., 2009; Rabosky, 2014). Therefore, we performed BAMM analyses with a sampling fraction file to correct non-random incomplete taxon sampling. Species of Menziesia were treated as members of sect. Sciadorhodion, as proposed by Craven (2011). In the fraction file, tips (i.e. sampled species) were assigned to groups following a thorough survey, and group assignment was conducted as follows. First, all taxonomically recognized subgroups (Chamberlain et al., 1996) that were resolved as monophyletic had the species number of that group assigned, at the lowest possible taxonomic level, i.e. subsection or section where possible. However, within the subg. Hymenanthes, not all subsections were monophyletic, so species numbers had to be applied at the subgenus level. The same was true in the two major clades of subg. Rhododendron that did not contain the three basal groups (subsections Micrantha, Ledum and sect. Vireya), so the species number was set according to the total species number of all the section(s)/subsection(s) contained in each clade. In cases where sections were not monophyletic (e.g. Sciadorhodion and Rhodora), constituent clades were identified, and the taxonomic literature was used to estimate species numbers for each clade. The MCMC chain was then run for 2 × 107 generations and sampled every 10 000 generations in BAMM. Finally, BAMMtools was used to summarize rates over each branch and plot diversification rates over time from the output data of BAMM. The convergence (ESS > 200) was assessed, with the first 15 % of samples discarded as burn-in using the R package coda v0.19 (Plummer et al., 2006). With the expected number of shifts set to a prior value of 1, the single best shift configuration with the maximum a posteriori (MAP) probability was found for generating the phylorate plot. In addition, a rate-through-time (net diversification, speciation and extinction rates) curve was plotted using the plotRateThroughTime function. The divergence age and species diversification rate of the two major subgenera (Rhododendron and Hymenanthes) that are diverse in the Himalaya–Hengduan Mountains were extracted from the results of BEAST and BAMM respectively, and averages were taken to compare their diversification rate and species age (i.e. the time when a species diverged from its nearest sampled relative). The analyses were repeated with groups that occur entirely outside the Himalaya–Hengduan Mountains excluded, i.e. sect. Vireya and subsect. Ledum from subg. Rhododendron, and subsect. Pontica from Hymenanthes, to allow direct comparisons of diversification rates and species ages within the region.
The semi-logarithmic lineage through time (LTT) plot was drawn by APE v5.5 (Paradis and Schliep, 2019) to estimate the overall diversification pattern. A total of 2000 trees were randomly selected from the BEAST analysis to calculate the confidence intervals.
The diversification rate across the phylogeny of Rhododendron was also inferred, once again based on the MCC tree, using the R package MEDUSA v0.955 (Alfaro et al., 2009) applying default settings [i.e. the corrected Akaike information criterion (AICc) and mixed mode]. The species richness of each monophyletic group was consistent with the assignments of BAMM, and the MCC tree was pruned to contain the assigned groups so that each terminal reflected a monophyletic group. The species richness was assigned to each terminal branch.
RESULTS
Characteristics of the datasets
All Rhododendron species sampled here failed to provide a complete circular structure, but sequencing data could be assembled into many long plastome scaffolds. From these, annotation and extraction was achieved for 72 of the 75–78 protein-coding genes present in the Rhododendron plastome, plus 63 non-coding regions and four rRNA genes, ensuring that missing data for each species was <25 % (Supplementary Data Table S2). Dataset WP, containing all of these loci, had an aligned length of 108 666 bp, among which 14 078 (12.96 %) sites were variable and 7155 (6.58 %) were parsimony-informative (PI). Dataset PCS comprised 72 protein-coding and four rRNA genes; this was 58 063 bp in length, with 6114 variable (10.53 %) and 3067 PI (5.28 %) sites. The proportion of variable and PI sites remained the same or increased slightly when positively selected genes were excluded (datasets WP-ω and PCS-ω). Dataset NCS comprised the 63 non-coding regions with a combined length of 50 603 bp, and the highest proportions of both variable (15.74 %; 7964 total) and PI (8.08 %, 4088 total) sites among datasets (Table 1). Selective pressure analyses showed that four loci (cemA, rpl14, rps14 and rps15) were estimated to have experienced positive selection. There were ten, 18, five and two positively selected sites (M1a vs. M2a; P < 0.05) detected in cemA, rpl14, rps14 and rps15 respectively using the Bayes empirical Bayes (BEB) test. The cemA gene has the function of mediating CO2 uptake (Wicke et al., 2011). The rpl14 gene encodes a protein for the small ribosomal subunits, and the rps14 and rps15 genes encode large ribosomal subunit proteins, having the function as translation and protein-modifying enzymes (Wicke et al., 2011).
Table 1.
Comparison of the characteristics in the alignments of different datasets.
| Dataset | Length (bp) | Parsimony-informative sites (%) | Variable sites (%) | Identical sites (%) |
|---|---|---|---|---|
| WP | 108 666 | 7155 (6.58 %) | 14 078 (12.96 %) | 94 588 (87.04 %) |
| NCS | 50 603 | 4088 (8.08 %) | 7964 (15.74 %) | 42 639 (84.26 %) |
| PCS | 58 063 | 3067 (5.28 %) | 6114 (10.53 %) | 51 949 (89.47 %) |
| WP-ω | 106 977 | 7064 (6.60 %) | 13 905 (13.00 %) | 93 072 (87.00 %) |
| PCS-ω | 56 374 | 2976 (5.28 %) | 5941 (10.54 %) | 50 433 (89.46 %) |
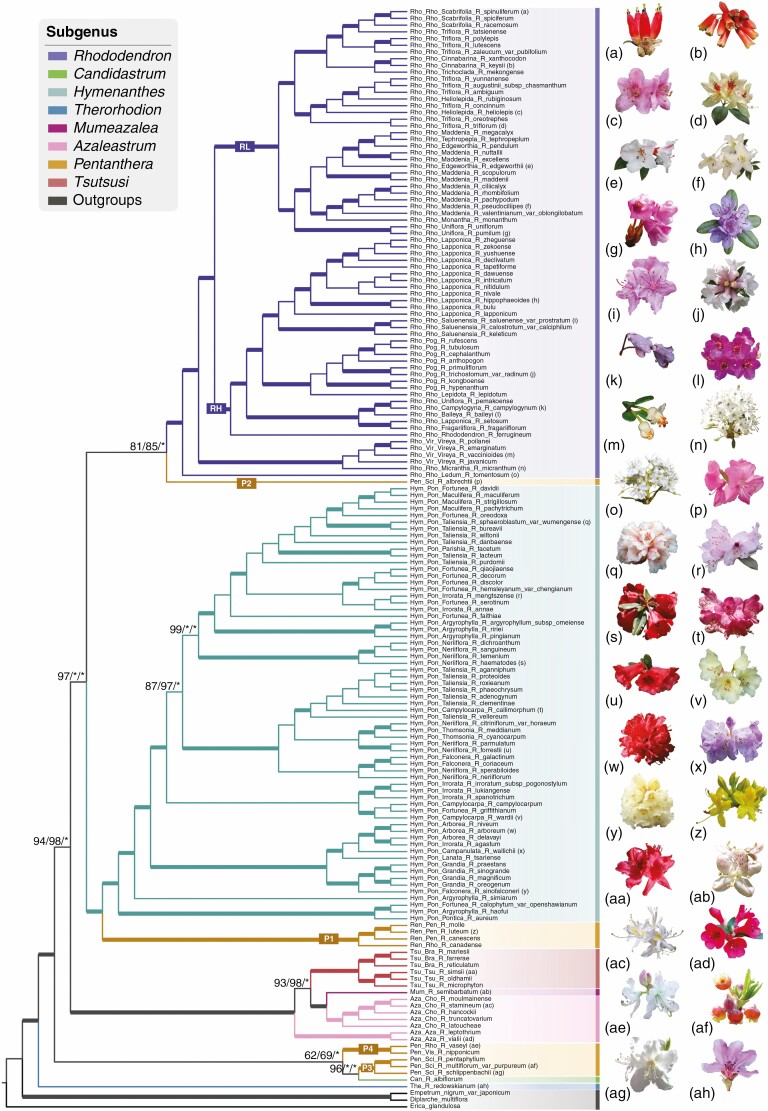
Inter- and intra-subgeneric relationships within Rhododendron
The phylogenetic relationships were highly consistent across all datasets (WP, NCS, PCS-ω and WP-ω) and all tree construction methods (ML and BI) except for PCS (Fig. 1; Supplementary Data Figs S1–11). The phylogenetic relationships based on dataset WP were unaffected by removing positively selected genes (Fig. 1; Figs S1–S3, S8 and S9), but the relationships resolved by the PCS dataset were slightly affected, mainly in the phylogenetic placement of subg. Candidastrum, which was sister to subg. Rhododendron + R. albrechtii based on the PCS dataset (Figs S6 and S7) but grouped with parts of subg. Pentanthera when positively selected genes were removed, and also in all other datasets (Figs S10 and -S11, and see below).
Fig. 1.
Phylogram of Rhododendron. Tree topology is the phylogenetic inference using RAxML for dataset WP. Branches of each subgenus are designated in different colours, and the corresponding subgeneric names are indicated in the key. Tip name contains abbreviations of the subgenus and section, full name of the subsection to which the species belongs, and species name. Support values shown on each branch indicate the phylogeny using RAxML, IQ-TREE and PhyloBayes respectively based on dataset WP. Branches with 100 % BS, 100 % UFBS and 1.0 Bayesian PP values are indicated by thick lines; otherwise, values are indicated along the deep branches (‘*’: 100 % or 1.0). Photographs of R. micranthum and R. tomentosum were taken by Mr Ze Wei, R. redowskianum by Dr Qinwen Lin, R. semibarbatum by Richard Milne, and the rest by Lianming Gao.
For the ML trees reconstructed by RAxML, both datasets WP and WP-ω had the highest phylogenetic resolution, with 80 % (128/161) of the internal nodes having bootstrap support (BS) ≥ 90 % (Supplementary Data Figs S1 and S8; Table 2). Dataset NCS had 75 % (121/161) of nodes with BS ≥ 90 % (Fig. S4; Table 2), but datasets PCS and PCS-ω only had 63 % and 62 % nodes with BS ≥ 90 %, respectively (Figs S6 and S10; Table 2). Here only the results from the WP dataset are reported, unless stated otherwise. Therorhodion was recovered as the basal group of Rhododendron in all analyses. The three largest subgenera – Rhododendron, Hymenanthes and Tsutsusi – were each resolved as monophyletic with strong support (Fig. 1; Figs S1–S9; Table S3), as were the two sections Tsutsusi and Brachycalyx of subg. Tsutsusi.
Table 2.
The frequency statistics of BS values in the ML tree based on different datasets using RAxML.
| Dataset | BS = 100 % | BS ≥ 90 % | BS ≥ 80 % | BS ≥ 75 % | BS ≥ 50 % | BS < 50 % |
|---|---|---|---|---|---|---|
| WP | 104 (65 %) | 128 (80 %) | 138 (86 %) | 143 (89 %) | 158 (98 %) | 3 (2 %) |
| NCS | 86 (53 %) | 121 (75 %) | 133 (83 %) | 139 (86 %) | 153 (95 %) | 8 (5 %) |
| PCS | 75 (47 %) | 101 (63 %) | 113 (70 %) | 116 (72 %) | 145 (90 %) | 16 (10 %) |
| WP-ω | 101 (63 %) | 128 (80 %) | 138 (86 %) | 140 (87 %) | 157 (98 %) | 4 (2 %) |
| PCS-ω | 70(43 %) | 100(62 %) | 111(69 %) | 114 (71 %) | 142 (88 %) | 19 (12 %) |
The values represent the frequency of the BS value falling within each interval.
Subgenera Azaleastrum and Pentanthera were recovered as polyphyletic, respectively comprising two (its sections Azaleastrum and Choniastrum) and four (clades P1, P2, P3 and P4, with P2 comprising only R. albrechtii, whereas the former genus Menziesia fell within clade P3) clades. Section Choniastrum was recovered as sister in turn to subg. Mumeazalea, subg. Tsutsusi and then sect. Azaleastrum. The positions and relationships of clades P2, P3 and P4 all varied slightly between certain datasets (Figs 1 and 4; Supplementary Data Figs S1–S11).
Fig. 4.
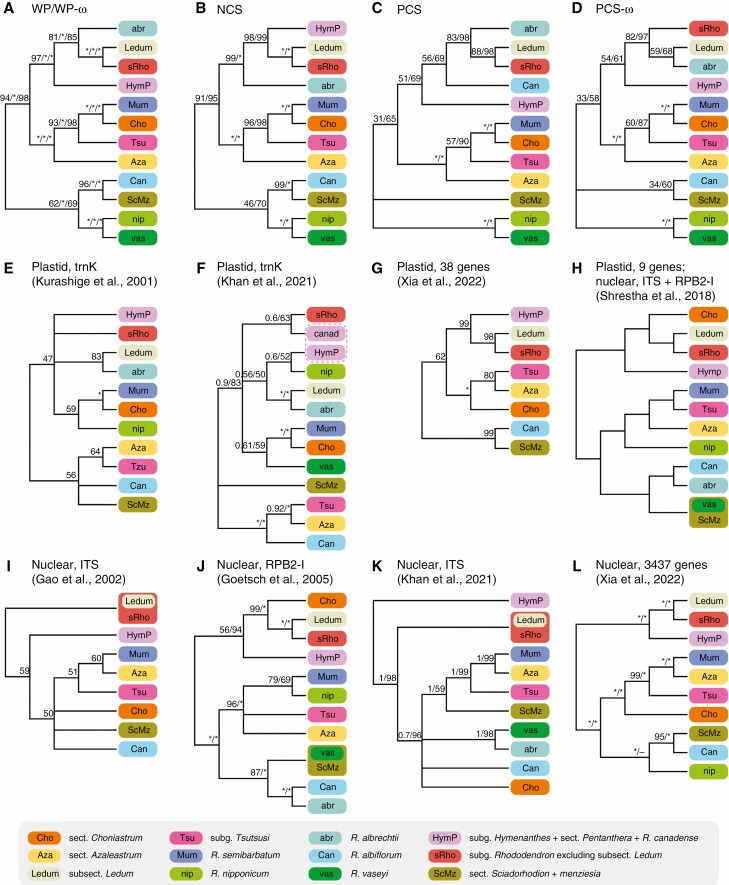
Comparisons of phylogenetic relationships of core Rhododendron between our analyses (A–D) and previous studies (E–L). In cases where multiple support values are shown, these are from different analysis methods and stated in the order they are mentioned for each tree, with values of 100 or 1 represented by an asterisk (*). (A) Phylogenetic relationships inferred from dataset WP using RAxML, PhyloBayes and IQ-TREE, which are also recovered from dataset WP-ω using RAxML and IQ-TREE; (B–D) phylogenetic relationships inferred from datasets NCS, PCS and PCS-ω respectively using RAxML and IQ-TREE; (E) phylogenetic relationships based on matK and trnK intron using PAUP (MP tree) summarized from figure 3 in Kurashige et al. (2001); (F) phylogenetic relationships based on trnK using MrBayes and IQ-TREE summarized from figure 1 in Khan et al. (2021); (G) phylogenetic relationships based on 38 plastid genes using IQ-TREE summarized from figure S3 in Xia et al. (2022); (H) phylogenetic relationships based on nine chloroplast genes plus ITS and RPB2-I regions using BEAST summarized from supporting information appendix S5 in Shrestha et al. (2018); (I) phylogenetic relationships based on ITS using PAUP (MP tree) summarized from figure 1 in Gao et al. (2002); (J) phylogenetic relationships based on RPB2-I using PAUP (MP tree) and MrBayes summarized from figure 2 in Goetsch et al. (2005); (K) phylogenetic relationships based on ITS using MrBayes and IQ-TREE summarized from figure 2 in Khan et al. (2021); (L) phylogenetic relationships based on 3437 nuclear orthologous genes using IQ-TREE and ASTRAL summarized from figures S1 and S2 in Xia et al. (2022).
Section Rhododendron was resolved as polyphyletic due to sections Pogonanthum and Vireya being embedded within it. Section Vireya itself was consistently monophyletic and sister to R. micranthum, a species of subsect. Micrantha in sect. Rhododendron. However, some analyses had a monophyletic sect. Pogonanthum as sister to R. lepidotum of sect. Rhododendron [i.e. ML trees of datasets WP, NCS and WP-ω (Fig. 1; Supplementary Data Figs S1, S3–S5, S8 and S9)], whereas in others R. lepidotum was nested within a paraphyletic Pogonanthum [BI tree of dataset WP and ML trees of datasets PCS and PCS-ω (Figs S2, S6, S7, S10 and S11)]. Other than these, and sections Rhodora and Sciadorhodion, all other sections from which more than one species sampled were strongly supported as monophyletic (Fig. 1; Figs S1–S11; Table S3). Notably subsect. Ledum, formerly treated as a distinct genus, was strongly supported as sister to the rest of subg. Rhododendron in all datasets except PCS-ω [which placed Ledum as sister to R. albrechtii (Clade P2) and then subg. Rhododendron].
Divergence time estimation
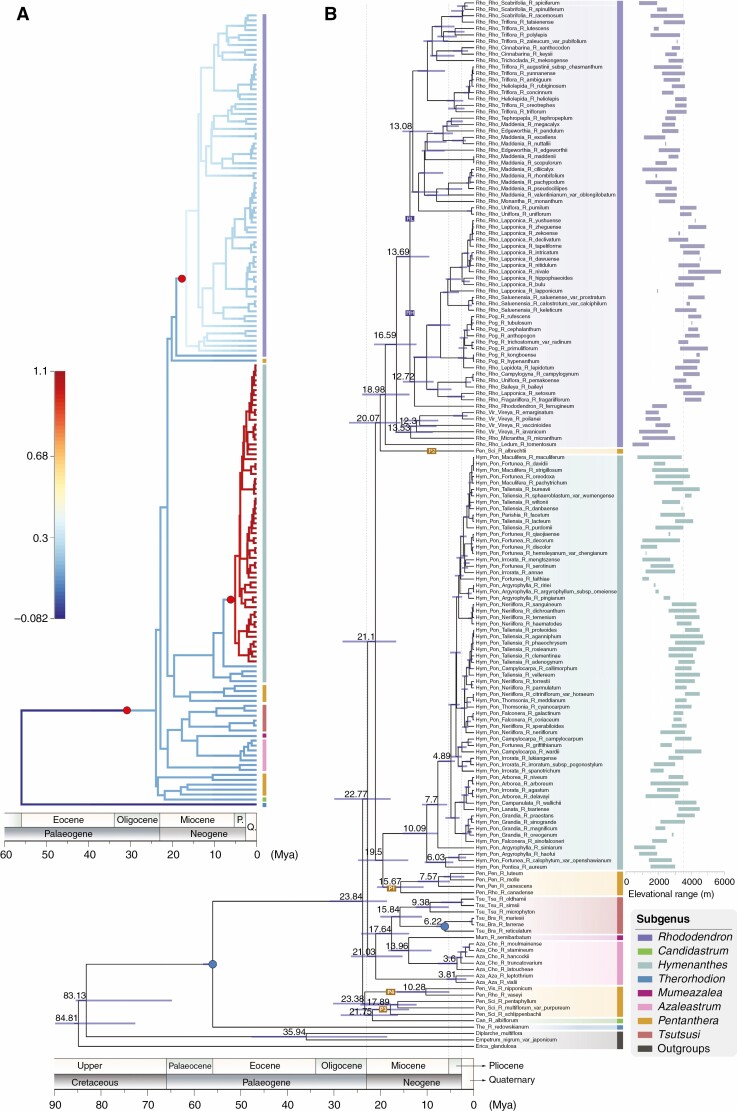
The divergence time estimates from all of BEAST, RelTime and TreePL analyses were very similar (Fig. 2B; Supplementary Data Figs S12–S14), so only the results from BEAST (Fig. 2B; Fig. S12) are described here. The first divergence, of subg. Therorhodion, from the MRCA of all other species occurred 56 Mya [95 % highest posterior density (HPD): 56–58.1 Mya]. After a period of >32 million years (Myr) with no divergence events, a clade comprising Candidastrum plus clades P3 and P4 then diverged in the late Oligocene at 23.8 Mya (95 % HPD: 18.6–30.9 Mya). This was the first of a sequence of ten divergence events during the 6.2 Myr between 23.8 and 17.6 Mya, across the Oligocene–Miocene boundary (Fig. 2). Among these, the first nine occurred during a period of 5 Myr, and hence by 18.98 Mya, the following groups had diverged: Candidastrum, Clade P3, Clade P4, Mumeazalea + subg. Tsutsusi + sect. Choniastrum, sect. Azaleastrum, Clade P1, Hymenanthes, Clade P2, subsect. Ledum, and all other subg. Rhododendron (Fig. 2). There followed a lag of around 10 Myr before crown divergence within Hymenanthes (10.1 Mya, 95 % HPD: 7.8–15.2 Mya), following which it diversified very rapidly. Conversely, diversification within subg. Rhododendron proceeded at a fairly continuous rate from its origin to the present, with two large but ecologically distinct clades RH (small shrubs occurring mostly >3500 m) and RL (shrubs to small trees occurring mostly < c. 3500 m) diverging 13.7 Mya (95 % HPD: 9.5–16.5 Mya) (Fig. 2B). Elsewhere in the tree, subg. Mumeazalea diverged from sect. Choniastrum at 14 Mya (95 % HPD: 9.1–18.4 Mya), after their MRCA diverged from subg. Tsutsusi 17.6 Mya (95 % HPD: 13.9–24.2 Mya). Species of sect. Choniastrum began to diversify in the Pliocene at 3.6 Mya (95 % HPD: 3.4–7.7 Mya). Within subg. Tsutsusi, the split between sections Brachycalyx and Tsutsusi was dated to 15.8 Mya (95 % HPD: 11.1–20.1 Mya) in the middle Miocene. Additionally, diversification within sections Pogonanthum, Vireya and Pentanthera initiated at 2.1 Mya (95 % HPD: 1.5–3.3 Mya), 12.3 Mya (95 % HPD: 8–15.7 Mya) and 7.6 Mya (95 % HPD: 5.1–12.1 Mya), respectively. The divergence between the two remaining lineages, R. nipponicum and R. vaseyi, occurred at 10.3 Mya (95 % HPD: 5.2–16.6 Mya).
Fig. 2.
Combined chronogram and phylorate plot of Rhododendron. (A) Phylorate plot with branches coloured according to the mean posterior density of net diversification rate (speciation rate minus extinction rate). Blue in the scale represents low rates and red represents high rates. Red circles mark the positions of rate shift in the MAP configuration. (B) Divergence time estimation based on BEAST analysis. The blue bars correspond to the 95 % HPD credibility intervals of age estimates. Nodes with solid blue circles are constrained with fossils.
Diversification analyses
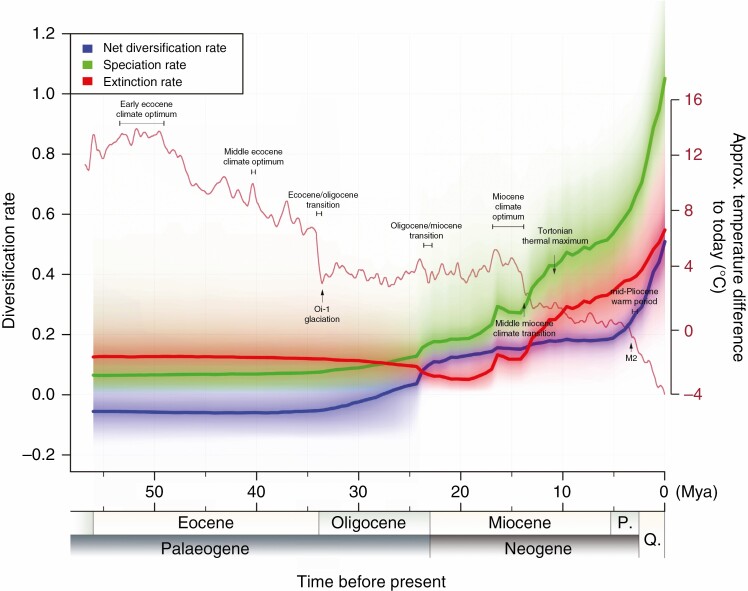
The phylorate plot from BAMM analysis indicated that the net diversification rate varied from low to high within Rhododendron (Fig. 2A). In total, three significant rate accelerations were detected (Fig. 2A). One was crown diversification of all Rhododendron except subg. Therorhodion, the second within subg. Rhododendron soon after its origin (c. 16.6 Mya) and the third within Hymenanthes but much later – c. 4.9 Mya, and hence around 14 Myr after its origin. The rate-through-time plot suggested that the net diversification, speciation and extinction rates were fairly constant up to 36 Mya, at which point the diversification and speciation rates began climbing slowly, then had a brief but substantial increase at ~24 Mya, after which the diversification rate climbed slowly until a remarkable acceleration from 5 Mya to the present. Meanwhile the speciation rate climbed slowly, followed by a significant increase and then a slight decline between 17 and 14 Mya, and then climbed rapidly until a remarkable acceleration from 5 Mya to the present. The extinction rate declined slowly from 36 to 20 Mya, and was indicated to be higher than the speciation rates until around 28 Mya, then from 20 to 5 Mya it tracked the speciation rate upwards, while always remaining >0.1 below it (Fig. 3). For the last 5 Myr it ceased to keep pace with speciation, leading to a steady increase in the net diversification rate from then to the present. The diversification rate shifts detected were concordant between the rate-through-time and the phylorate plot (Figs 2A and 3).
Fig. 3.
Rate-through-time plots for speciation, extinction and net diversification with 95 % confidence intervals indicated by shaded areas. Approximate annual air temperature differences from the present day are derived from Westerhold et al. (2020).
The LTT plot generated similar results as BAMM analysis and showed an accumulation of lineages since the late Oligocene, c. 24 Mya (Supplementary Data Fig. S15). In MEDUSA analysis, the background net diversification rate for Rhododendron was estimated as 0.0301 spp./Myr, and four significant changes of diversification rate were detected, comprising three increases and one decrease (Fig. S16). An increase from 0.0301 to 0.2343 spp./Myr occurred at crown divergence of the clade comprising all Rhododendron species except Therorhodion, Candidastrum, Clade P3 and Clade P4, then within this clade a further increase to 0.3558 spp./Myr was detected in the clade comprising subg. Rhododendron excluding subsect. Ledum. Elsewhere, an increase from 0.0301 to 0.1303 spp./Myr was detected within Clade P3. The detected decrease in the diversification rate, in Clade P2, involved drops in the rate from 0.2343 to 0 spp./Myr (Fig. S16).
For subg. Rhododendron, the mean net diversification rate was 0.1817 spp./Myr and was barely affected by the inclusion or exclusion of Vireya and/or Ledum (Supplementary Data Table S4); however, its mean species age of 2.81 drops to 2.29 Myr when both are excluded, with intermediate values when either one is excluded. Likewise, the mean net diversification rate and mean species age for Hymenanthes were 1.0156 spp./Myr and 0.98 Myr, whereas when the Tertiary relict species of subsect. Pontica were excluded the former increased marginally to 1.0292 spp./Myr whereas the latter dropped to 0.91 Myr. Hence when non Hengduan–Himalayan groups were excluded, then relative to Rhododendron the net diversification rate of Hymenanthes was more than five times faster, and its species age on average more than 60 % younger. The mean species age of clades within subg. Rhododendron showed that Clade RH (small shrubs, high elevation; 1.38 Myr) was younger than Clade RL (shrubs or small trees, relatively low elevation; 2.77 Myr), but the mean diversification rates of clades RH and RL were similar (0.1896 vs. 0.1780 spp./Myr).
DISCUSSION
Intensive sampling produces high resolution but reveals phylogenetic conflicts
Based on extensive sampling across taxa and the chloroplast DNA (cpDNA) genome, the full plastid dataset produced a well-resolved and well-supported phylogeny, yet several nodes were conflicted by partial datasets, and many conflicted with previous studies based on the matK region (Kurashige et al., 2001; Khan et al., 2021). However, very few topological conflicts existed between the different phylogenetic analysis methods used on our datasets, and most of the conflicting topologies were weakly supported.
Comparing the current analysis with all past analyses (Fig. 4), relatively few relationships are constant across all analyses, but subg. Therorhodion is always undisputedly sister to all other Rhododendron, and here the genus excluding Therorhodion is termed ‘core Rhododendron’ for ease of discussion. Subgenus Pentanthera (sensuChamberlain et al., 1996) was highly polyphyletic, whereas sect. Pentanthera plus R. canadense is always monophyletic and sister to subg. Hymenanthes. The two sections of subg. Azaleastrum (Azaleastrum and Choniastrum) are each always monophyletic but never sister to one another. Species from sect. Sciadorhodion of subg. Pentanthera (other than R. albrechtii) formed a clade here termed the ScMz clade, also including the former genus Menziesia (see also Goetsch et al., 2005; Craven, 2011; Xia et al., 2022). Subgenus Tsutsusi is always monophyletic as well as its two sections Tsutsusi and Brachycalyx. Subgenus Rhododendron is always monophyletic except that cpDNA sometimes places the former genus Ledum outside it (Supplementary Data Figs S10 and S11; Kurashige et al., 2001; Khan et al., 2021). The relationships of five individual species are inconsistent across all studies: these are R. vaseyi, R. nipponicum, R. albrechtii (all belonging to subg. Pentanthera sensuChamberlain et al., 1996), R. albiflorum (the monotypic subgenus Candidastrum) and R. semibarbatum (the monotypic subgenus Mumeazalea). Therefore, higher level relationships in core Rhododendron can be described across studies in terms of 12 clades of greatly varying sizes (Fig. 4): R. vaseyi, R. nipponicum, R. albrechtii, R. albiflorum, R. semibarbatum, the ScMz clade, Hymenanthes + sect. Pentanthera, subg. Rhododendron excluding Ledum, former genus Ledum (merged into Rhododendron by Kron and Judd, 1990), subg. Tsutsusi, sect. Azaleastrum and sect. Choniastrum. For ease of discussion, the last six are henceforth referred to as HymP, sRho, Ledum, Tsutsusi, Azaleastrum and Choniastrum. Many of these clades have already been recognized or suggested at the subgenus level (Chamberlain et al., 1996; Gao et al., 2002; Goetsch et al., 2005; Fu et al., 2022), but here we tentatively suggest that all 12 might ultimately merit recognition at this rank, once adequate data become available.
Relationships among these 12 core Rhododendron groups were fully resolved and generally well supported in our full (WP) plastid dataset. However, the position of R. albrechtii was altered relative to WP in the NCS and PCS-ω (but not PCS) datasets, and that of R. albiflorum shifted in the PCS (but not PCS-ω) dataset. Hence the positions of both species are sensitive to the inclusion or exclusion of genes under selection that might be subject to homoplasious adaptative changes (Fig. 4A–D; Supplementary Data Figs S6, S7, S10 and S11), and the differences involving the PCS and PCS-ω datasets are generally not strongly supported. However, regarding the conflict between NCS and WP, support for R. albrechtii branching before HymP is near maximum under NCS, but the reverse relationship has 81–85 % BS/UFBS support in the WP dataset, and slightly more with genes under selection removed (WP-ω; 82–87 % BS/UFBS). Therefore, coding genes not under detectable selection are responsible for the difference, and it is unclear which relationship better reflects the true plastid tree.
Our study strongly supported a clade of (Azaleastrum (Tsutsusi (Choniastrum + R. semibarbatum))), and generally this clade was consistent (Fu et al., 2022) or showed few conflicts (Xia et al., 2022) with recent phylogenies that sampled widely across the plastome and densely across taxa. Conversely, there were strong conflicts with previous phylogenies based on the plastid matK region (Kurashige et al., 2001; Khan et al., 2021), or on multiple cpDNA regions plus nuclear genes (Shrestha et al., 2018), mainly concerning the placement of Tsutsusi + Azaleastrum as sister to R. albiflorum, whereas Choniastrum + R. semibarbatum was sister to R. vaseyi or R. nipponicum but with weak support, hence breaking up groupings that are strongly supported in the current study. These matK-based analyses concurred with our PSC-ω dataset in placing R. albrechtii sister to Ledum (Fig. 4D).
These findings indicate strongly that a phylogeny based on a single plastid region, or even many, cannot be assumed to represent the true plastid tree, and even casts doubt on whether such a thing exists. The most well-supported discordance in our own datasets, concerning the position of R. albrechtii between our WP and NCS datasets, might result from plastid recombination, albeit probably involving more than one or two genes. An alternative hypothesis of incomplete lineage sorting cannot explain how this species appears in completely different clades in nuclear phylogenies, whereas both phylogenies are consistent with a past hybridization event.
Of nuclear phylogenetic studies of the whole genus, Xia et al. (2022) sampled by far the most of the genome, i.e. 3437 nuclear orthologous genes from transcriptome data, whereas others used single regions, i.e. RPB2 (Goetsch et al., 2005) or ITS (Gao et al., 2002; Khan et al., 2021). The positions of Choniastrum and (where included) each of R. albrechtii, R. vaseyi and R. nipponicum vary dramatically between these studies. If these four lineages are all removed, then our study (except dataset PCS), Xia et al.’s (2022) plastids and all these nuclear only analyses would resolve the same three clades: (HymP (sRho + Ledum)), (Azaleastrum + Mumeazalea + Tsutsusi) and (Candidastrum + ScMz). However, the former two are sister for all our plastome datasets, whereas the latter two are sister in all four nuclear studies, strongly indicating a reticulation event in the genus’ deep history. Together with all the other instances of discordance noted here, it seems very likely that numerous reticulate evolutionary events occurred during the history of this genus, and there can be no single correct species tree for it. Many of the five single species that have variable positions between phylogenies (R. albrechtii, R. albiflorum, R. semibarbatum, R. vaseyi and R. nipponicum) might have hybrid origins, and it is important that all of these are included in all future genus-level phylogenetic analyses if these issues are to be resolved.
The species barrier within Rhododendron is very fragile and numerous natural hybridization events have been detected (Milne et al., 1999, 2010; Zhang et al., 2007; Zha et al., 2008, 2010; Ma et al., 2010; Yan et al., 2017, 2019; Zheng et al., 2021). Hybridization/introgression will result in shared maternally inherited genotypes between closely related species (Du et al., 2009), which may lead to conflicts between nuclear and plastid phylogenies. Xia et al. (2022) obtained a well-resolved phylogeny based on 3437 orthologous nuclear genes, but some species relationships still conflicted with those inferred from plastid sequences in their study and the present study. However, they had issues with missing data in the 38 plastid protein-coding genes, and some key species were missing from their plastid analysis. Our phylogeny represented all subgenera and sections but only 35 of 59 Rhododendron subsections (c. 59 %), and ~45 % of Rhododendron species present in the Himalaya–Hengduan Mountains were sampled. Hence denser sampling of taxa, examining both organelle and nuclear genomes, is needed to better understand the divergence and diversification history of Rhododendron.
Divergence time and diversification history
We obtained a younger estimated age of diversification for most extant lineages as compared with Xia et al. (2022) and Shrestha et al. (2018). All three methods used (BEAST, Reltime and treePL) gave very similar results (Fig. 2B; Supplementary Data Figs S12–S14), indicating that sensitivity to the method used becomes small when enough taxa and genomes are sampled. Hence the results discussed here are from BEAST unless stated otherwise. However, compared to Xia et al. (2022), who also used Reltime with high taxon and genome coverage, we had fewer taxa but many more plastid protein-coding genes and in particular included non-coding regions.
We estimated the crown age of Rhododendron (i.e. divergence of Therorhodion) at 56 Mya, as inferred by Rose et al. (2018) and Xia et al. (2022). Fossil evidence indicates that early lineages of Rhododendron went extinct before this, during the Cretaceous–Palaeogene mass extinction event (Collinson and Crane, 1978), and the above date indicates that all extant taxa derive from a single surviving lineage. Crown divergence of core Rhododendron from our data was >30 Myr later, around the Oligocene–Miocene boundary at 23.8 Mya (Fig. 2B), a little older than Rose et al.’s (2018) 18.3 Mya estimation, but much younger than the 35.9 Mya estimation of Xia et al. (2022); the ~56 Mya estimation of Shrestha et al. (2018) appears to be an outlier.
Our data indicate that, during a brief 6.2-Myr period from 23.8 to 17.6 Mya (Fig. 2B), coinciding with climate cooling and intensification of the Asian summer monsoon around the Oligocene–Miocene transition (Deng et al., 2019; Su et al., 2019; S. F. Li et al., 2021), core Rhododendron diversified from one into ten lineages. Eight of the 12 component clades listed above had split, and HymP had itself split into deciduous and evergreen clades. Of the other four, R. semibarbatum diverged from Choniastrum at 13.96 Mya and R. nipponicum from R. vaseyi at 10.28 Mya. Of course, this is not the complete picture as hybridization events not detectable from this data were probably involved too. For example, here Mumeazalea diverged from Choniastrum 1.88 Myr after crown divergence in Tsutsusi, whereas Xia et al.’s (2022) nuclear data have it diverging from Azaleastrum earlier than crown divergence in Tsutsusi – hence a hypothesis to test is that it derived from a cross between sister lineages of Choniastrum and Azaleastrum.
Unsurprisingly given this rapid expansion of lineage numbers, crown divergence in core Rhododendron formed the first of three significant increased rate shifts in Rhododendron diversification detected by BAMM analysis (Fig. 2A), with the rate-through-time plot giving similar results (Fig. 3). The other two shifts were detected in the species-rich subgenera Hymenanthes and Rhododendron. The rate shift in subg. Rhododendron occurred c. 16.6 Mya when the species of the sRho clade began to diversify, after which Clade RH diverged from Clade RL at 13.7 Mya. This might have been an ecological speciation event, because Clade RH comprises small, narrow-leaved shrubs of thickets or open alpine habitats mostly above 3500 m, whereas Clade RL comprises larger leaved shrubs/small trees from in or around forests below 3500 m. This coincides with the Himalayas nearing present-day elevations at c. 17–14 Mya, driven by ongoing tectonic events (Wang et al., 2012; Su et al., 2019; Ding et al., 2020), generating complex terrain and heterogeneous habitats. Subsequent diversification in both clades might have been promoted by ongoing orogeny (Kapp and DeCelles, 2019), the intensification of the Asian summer monsoon in the Himalaya–Hengduan Mountains from ~14 Mya onwards (Farnsworth et al., 2019; S. F. Li et al., 2021; Spicer et al., 2021), and increasing moisture availability, leading to deeper valleys through river incision (Wang et al., 2012; Nie et al., 2018). All this would have promoted habitat diversity and barriers to dispersal, promoting parallel speciation in both clades.
Despite their similar mean net diversification rate (0.1896 vs. 0.1780 spp./Myr), the average species age in Clade RH is younger than in RL (1.38 vs. 2.77 Myr), indicating more recent radiation within Clade RH, which could be because their alpine habitats were only recently generated by mountain uplift and Quaternary global cooling (Ding et al., 2020). However, the mean divergence age across the whole of Hymenanthes was even younger (0.98 Myr), and it has a higher mean net diversification rate (1.0292 vs. 0.1827 spp./Mya) than subg. Rhododendron in the Himalaya–Hengduan Mountains. Hence despite both subgenera having a clear centre of diversity in this region, the timing and manner of diversification clearly differs between them. Both Hymenanthes and sRho diverged from their sister groups around 19.5 Mya, but while diversification in sRho was fairly continuous, crown divergence in Hymenanthes did not initiate until ~10 Mya (Fig. 2B; Milne, 2004). Furthermore, the first diverging clade of Hymenanthes comprises low-altitude Tertiary relict species (mostly not sampled here but see Milne, 2004; Milne et al., 2010) with a nested NE Himalayan subclade. Therefore, Hymenanthes may not have entered the Himalaya until after this clade diverged, hence much later than subg. Rhododendron. Furthermore, the next diverging species (R. simiarum at c. 7.7 Mya) is also a low-altitude species. The rate of diversification increased significantly c. 4.9 Mya according to BAMM analysis, with most species diverging after that (Fig. 2B; Milne, 2004). This sudden acceleration of diversification might have resulted from its invasion of the Himalaya region. Other possible contributors around that time include gradual global cooling (Milne, 2004; Milne and Abbott, 2002), and a period of high monsoon intensification (Ding et al., 2020; Xia et al., 2022), which together facilitated ecological and evolutionary opportunities for diversification in other groups (Luo et al., 2016; Ye et al., 2019). Hence, although a few clades in Hymenanthes are high-altitude only, overall altitudinal preference appears more plastic in Hymenanthes than in subg. Rhododendron despite the former having diversified over a shorter period.
Compared to our results, the best nuclear data available (Xia et al. 2022) indicate that crown diversification in core Rhododendron began considerably earlier, around 36 Mya, and diversification within the Tsutsusi–Azaleastrum–Choniastrum–Mumeazalea–ScMz–R. nipponicum–R. vaseyi–Candidastrum clade has proceeded at a steady rate since then. Early nodes involving subgenera Hymenanthes and Rhododendron are likewise around 8.8–10.3 Myr older than ours. Consequently, their analysis allows more time for diversification, and so rate shifts are much less apparent.
CONCLUSIONS AND FUTURE DIRECTIONS
Rhododendron is a large genus that is taxonomically difficult for two reasons. The first issue, recent rapid radiation, means that some clades may be supported by only a few apomorphic markers, and hence wide genomic coverage, as in this paper and Xia et al. (2022), will be necessary to resolve some clades, especially within Hymenanthes where much of the radiation has been very recent (Fig. 2B; Milne, 2004). Second, hybridization is rampant, and discordance between phylogenies based on different markers indicates that multiple reticulate evolution events may have occurred, and that no single marker can reconstruct the true species tree. Our phylogeny, sampling heavily across both taxa and the plastid genome, provides a major advance, yet also indicates that recombination might have occurred, due to hybridization/introgression, even within the plastid genome.
The identification of clades at both higher and lower levels that are consistently monophyletic across all markers and analyses is an important step towards unravelling Rhododendron evolution. The 12 clades of core Rhododendron identified here represent a step towards this, but even some of these are challenged by certain analyses, though this could occur due to undersampling of the genome (e.g. Ledum nests within sRho for ITS; Gao et al., 2002; Khan et al., 2021), or very uneven marker sampling across taxa (as in Shrestha et al., 2018). A study that samples all 12 clades with at least the nuclear genome coverage of Xia et al. (2022) is urgently needed, and from such data it would be possible to test which clades are retained when different portions of the nuclear genome are sampled. With clades demonstrated, or even tentatively assumed, to be monophyletic, then approaches such as integrated single copy gene (SCG) trees and phylonet-based network analysis (e.g. M. J. Li et al., 2021) can be used to begin to uncover patterns of reticulate evolution, and hence identify clades of hybrid origin.
Numerous natural hybridization events have been detected, and hence populations sampled for phylogenetic analysis (either directly or via material taken for cultivation) might have acquired cpDNA or nuclear material from other species. Therefore, sampling of multiple populations from different points in each species’ range is desirable where possible (Wang et al., 2022). While this will increase the resources required for sampling, species can be pruned to one individual for phylogenetic analysis if no introgression is detected.
Comparing the current study with Xia et al. (2022), clade ages throughout the genus seem to differ depending on which genome is examined, in spite of wide sampling of both taxa and genomes. More research is needed to determine why this difference exists, before truly reliable node age estimates can be obtained. Nonetheless, both studies found that Hymenanthes began to diversify 7–9 Myr after subg. Rhododendron, but diversified faster, so despite the two subgenera both having centres of diversity in and around the eastern Himalaya, it is clear that they did not diversify simultaneously. Our data indicate that highly heterogeneous habitats caused by active orogeny, plus climate cooling and the intensification of the Asian summer monsoon from the late Oligocene onwards were probably significant for diversification in subg. Rhododendron, whereas Hymenanthes might have invaded the mountains late in their history and radiated as a result. The two subgenera were also shown to differ in the ecological patterns of their divergence, with far more transitions between high and low altitudes in Hymenanthes than in Rhododendron. Studies like these will help with the development of a stable and reliable taxonomic framework for Rhododendron, as well as help us to understand what drove its diversification and ecological adaption, all of which will aid the conservation of Rhododendron.
SUPPLEMENTARY DATA
Supplementary data are available online at https://academic.oup.com/aob and consist of the following. Table S1. Taxa included in this study with classification, locality and voucher information. Table S2. Genes and intergenic regions recovered in the sampled taxa. Table S3. Summary of the monophyly and corresponding support values of subgenera, sections and subsections in Rhododendron with multiple sampled species by phylogenetic analyses. Table S4. Mean net diversification rate and species age of the clades in subgenera Rhododendron and Hymenanthes. Figure S1. ML tree inferred from dataset WP using RAxML. Figure S2. BI tree inferred from dataset WP using PhyloBayes. Figure S3. ML tree inferred from dataset WP using IQ-TREE. Figure S4. ML tree inferred from dataset NCS using RAxML. Figure S5. ML tree inferred from dataset NCS using IQ-TREE. Figure S6. ML tree inferred from dataset PCS using RAxML. Figure S7. ML tree inferred from dataset PCS using IQ-TREE. Figure S8. ML tree inferred from dataset WP-ω using RAxML. Figure S9. ML tree inferred from dataset WP-ω using IQ-TREE. Figure S10. ML tree inferred from dataset PCS-ω using RAxML Figure S11. ML tree inferred from dataset PCS-ω using IQ-TREE. For figures S1 to S11, support values are given on branches. Figure S12. Divergence times of Rhododendron estimated from dataset WP using BEAST. Figure S13. Divergence times of Rhododendron estimated from dataset WP using treePL. Figure S14. Divergence times of Rhododendron estimated from dataset WP using RelTime. Figure S15. LTT plots in Rhododendron. Figure S16. Diversification patterns of major lineages inferred from MEDUSA analyses based on the MCC tree from BEAST analysis.
ACKNOWLEDGMENTS
We thank Drs Lijun Yan, Jiayun Zou, Linjiang Ye, Jianjun Jin, Ting Zhang, Zhirong Zhang, Jing Yang and Chunxia Zeng for providing plant samples and/or lab assistance. The authors are also grateful to Dr Qinwen Lin and Mr Ze Wei for providing some photos of Rhododendron. Molecular experiments and data analysis were performed at the Laboratory of Molecular Biology and iFlora High Performance Computing Center of Germplasm Bank of Wild Species in Southwest China (GBOWS) respectively, Kunming Institute of Botany. P.M.H. acknowledges funding support from the Scottish Government’s Rural and Environment Science and Analytical Services division. The sequence alignments and all trees for this study are available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.8cz8w9grq..
Contributor Information
Zhi-Qiong Mo, CAS Key Laboratory for Plant Diversity and Biogeography of East Asia, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming 650201, Yunnan, China; Germplasm Bank of Wild Species, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming 650201, Yunnan, China; University of the Chinese Academy of Sciences, Beijing 100049, China.
Chao-Nan Fu, CAS Key Laboratory for Plant Diversity and Biogeography of East Asia, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming 650201, Yunnan, China; Germplasm Bank of Wild Species, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming 650201, Yunnan, China.
Ming-Shu Zhu, CAS Key Laboratory for Plant Diversity and Biogeography of East Asia, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming 650201, Yunnan, China; University of the Chinese Academy of Sciences, Beijing 100049, China.
Richard I Milne, Institute of Molecular Plant Sciences, School of Biological Sciences, University of Edinburgh, Edinburgh EH9 3JH, UK.
Jun-Bo Yang, Germplasm Bank of Wild Species, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming 650201, Yunnan, China.
Jie Cai, Germplasm Bank of Wild Species, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming 650201, Yunnan, China.
Han-Tao Qin, CAS Key Laboratory for Plant Diversity and Biogeography of East Asia, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming 650201, Yunnan, China; University of the Chinese Academy of Sciences, Beijing 100049, China.
Wei Zheng, CAS Key Laboratory for Plant Diversity and Biogeography of East Asia, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming 650201, Yunnan, China; University of the Chinese Academy of Sciences, Beijing 100049, China.
Peter M Hollingsworth, Royal Botanic Garden Edinburgh, Edinburgh EH3 5LR, UK.
De-Zhu Li, CAS Key Laboratory for Plant Diversity and Biogeography of East Asia, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming 650201, Yunnan, China; Germplasm Bank of Wild Species, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming 650201, Yunnan, China; University of the Chinese Academy of Sciences, Beijing 100049, China.
Lian-Ming Gao, CAS Key Laboratory for Plant Diversity and Biogeography of East Asia, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming 650201, Yunnan, China; Lijiang Forest Biodiversity National Observation and Research Station, Kunming Institute of Botany, Chinese Academy of Sciences, Lijiang 674100, Yunnan, China.
CONFLICT OF INTEREST
The authors declare on competing interests.
FUNDING
This study was supported by the Strategic Priority Research Program of Chinese Academy of Sciences (XDB31000000), the Large-scale Scientific Facilities of the Chinese Academy of Sciences (2017-LSFGBOWS-02), the National Natural Science Foundation of China (32000173, 91631101, 31670213), the Key Basic Research program of Yunnan Province, China (202101BC070003), and the Postdoctoral Directional Training Foundation of Yunnan Province (E132711261).
LITERATURE CITED
- Alfaro ME, Santini F, Brock C, et al. 2009. Nine exceptional radiations plus high turnover explain species diversity in jawed vertebrates. Proceedings of the National Academy of Sciences of the United States of America 106: 13410–13414. doi: 10.1073/pnas.0811087106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bankevich A, Nurk S, Antipov D, et al. 2012. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. Journal of Computational Biology 19: 455–477. doi: 10.1089/cmb.2012.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett CF, Davis JI, Leebens-Mack J, Conran JG, Stevenson DW. 2013. Plastid genomes and deep relationships among the commelinid monocot angiosperms. Cladistics 29: 65–87. doi: 10.1111/j.1096-0031.2012.00418.x. [DOI] [PubMed] [Google Scholar]
- Berry E, Sharma SK, Pandit MK, Geeta R. 2018. Evolutionary correlation between floral monosymmetry and corolla pigmentation patterns in Rhododendron. Plant Systematics and Evolution 304: 219–230. [Google Scholar]
- Brown GK, Craven LA, Udovicic F, Ladiges PY. 2006. Phylogeny of Rhododendron section Vireya (Ericaceae) based on two non-coding regions of cpDNA. Plant Systematics and Evolution 257: 57–93. [Google Scholar]
- Chamberlain D, Hyam R, Argent G, Fairweather G, Walter KS. 1996. The genus Rhododendron: its classification and synonymy. Oxford: Alden Press. [Google Scholar]
- Collinson ME, Crane PR. 1978. Rhododendron seeds from the Palaeocene of southern England. Botanical Journal of the Linnean Society 76: 195–205. doi: 10.1111/j.1095-8339.1978.tb02361.x. [DOI] [Google Scholar]
- Craven LA. 2011. Diplarche and Menziesia transferred to Rhododendron (Ericaceae). Blumea 56: 33–35. [Google Scholar]
- Deng T, Wu FX, Wang SQ, Su T, Zhou ZK. 2019. Significant shift in the terrestrial ecosystem at the Paleogene/Neogene boundary in the Tibetan Plateau. Chinese Science Bulletin 64: 2894–2906. [Google Scholar]
- Ding WN, Ree RH, Spicer RA, Xing YW. 2020. Ancient orogenic and monsoon-driven assembly of the world’s richest temperate alpine flora. Science 369: 578–581. doi: 10.1126/science.abb4484. [DOI] [PubMed] [Google Scholar]
- Doyle JJ, Doyle JL. 1987. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochemistry Bulletin 19: 11–15. [Google Scholar]
- Drummond AJ, Suchard MA, Xie D, Rambaut A. 2012. Bayesian phylogenetics with BEAUti and the BEAST 1.7. Molecular Biology and Evolution 29: 1969–1973. doi: 10.1093/molbev/mss075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du FK, Petit RJ, Liu JQ. 2009. More introgression with less gene flow: chloroplast vs. mitochondrial DNA in the Picea asperata complex in China, and comparison with other Conifers. Molecular Ecology 18: 1396–1407. doi: 10.1111/j.1365-294X.2009.04107.x. [DOI] [PubMed] [Google Scholar]
- Fang MY, Fang RZ, He MY, Hu LZ, Yang HB, Chamberlain DF. 2005. Ericaceae. In: Flora of China. Beijing and St. Louis, MO:Science Press, and Missouri Botanical Garden Press, 260–455. [Google Scholar]
- Farnsworth A, Lunt DJ, Robinson SA, et al. 2019. Past East Asian monsoon evolution controlled by paleogeography, not CO2. Science Advances 5: eaax1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FitzJohn RG, Maddison WP, Otto SP. 2009. Estimating trait-dependent speciation and extinction rates from incompletely resolved phylogenies. Systematic Biology 58: 595–611. [DOI] [PubMed] [Google Scholar]
- Fu CN, Mo ZQ, Yang JB, et al. 2022. Testing genome skimming for species discrimination in the large and taxonomically difficult genus Rhododendron. Molecular Ecology Resources 22: 404–414. [DOI] [PubMed] [Google Scholar]
- Gao LM, Li DZ, Zhang CQ, Yang JB. 2002. Infrageneric and sectional relationships in the genus Rhododendron (Ericaceae) inferred from ITS sequence data. Acta Botanica Sinica 44: 1351–1356. [Google Scholar]
- Gibbs D, Chamberlain D, Argent G. 2011. The Red List of Rhododendrons. Richmond: Botanic Gardens Conservation International. [Google Scholar]
- Gitzendanner MA, Soltis PS, Yi TS, Li DZ, Soltis DE. 2018. Plastome phylogenetics: 30 years of inferences into plant evolution. In: Chaw SM, Jansen RK, eds.: Plastid genome evolution. New York: Academic Press, 293–313. [Google Scholar]
- Goetsch LA, Craven LA, Hall BD. 2011. Major speciation accompanied the dispersal of Vireya Rhododendrons (Ericaceae, Rhododendron sect. Schistanthe) through the Malayan archipelago: evidence from nuclear gene sequences. Taxon 60: 1015–1028. [Google Scholar]
- Goetsch LA, Eckert AJ, Hall BD. 2005. The molecular systematics of Rhododendron (Ericaceae): a phylogeny based upon RPB2 gene sequences. Systematic Botany 30: 616–626. [Google Scholar]
- Hoang DT, Chernomor O, von Haeseler A, Minh BQ, Vinh LS. 2018. UFBoot2: improving the ultrafast bootstrap approximation. Molecular Biology and Evolution 35: 518–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen RK, Cai Z, Raubeson LA, et al. 2007. Analysis of 81 genes from 64 plastid genomes resolves relationships in angiosperms and identifies genome-scale evolutionary patterns. Proceedings of the National Academy of Sciences of the United States of America 104: 19369–19374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin JJ, Yu WB, Yang JB, et al. 2020. GetOrganelle: a fast and versatile toolkit for accurate de novo assembly of organelle genomes. Genome Biology 21: 241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapp P, DeCelles PG. 2019. Mesozoic–Cenozoic geological evolution of the Himalayan-Tibetan orogen and working tectonic hypotheses. American Journal of Science 319: 159–254. [Google Scholar]
- Katoh K, Standley DM. 2013. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Molecular Biology and Evolution 30: 772–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearse M, Moir R, Wilson A, et al. 2012. Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 28: 1647–1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan G, Nolzen J, Schepker H, Albach DC. 2021. Incongruent phylogenies and their implications for the study of diversification, taxonomy, and genome size evolution of Rhododendron. American Journal of Botany 108: 1957–1981. [DOI] [PubMed] [Google Scholar]
- Kong HH, Condamine FL, Yang LH, et al. 2021. Phylogenomic and macroevolutionary evidence for an explosive radiation of a plant genus in the Miocene. Systematic Biology 71: 589–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kron KA, Judd WS. 1990. Phylogenetic-relationships within the Rhodoreae (Ericaceae) with specific comments on the placement of Ledum. Systematic Botany 15: 57–68. [Google Scholar]
- Kumar P. 2012. Assessment of impact of climate change on Rhododendrons in Sikkim Himalayas using Maxent modelling: limitations and challenges. Biodiversity and Conservation 21: 1251–1266. [Google Scholar]
- Kumar S, Stecher G, Li M, Knyaz C, Tamura K. 2018. MEGA X: molecular evolutionary genetics analysis across computing platforms. Molecular Biology and Evolution 35: 1547–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurashige Y, Etoh JI, Handa T, Takayanagi K, Yukawa T. 2001. Sectional relationships in the genus Rhododendron (Ericaceae): evidence from matK and trnK intron sequences. Plant Systematics and Evolution 228: 1–14. [Google Scholar]
- Kurashige Y, Mine M, Kobayashi N, Handa T, Takayangi K, Yukawa T. 1998. Investigation of sectional relationships in the genus Rhododendron (Ericaceae) based on matK sequences. The Journal of Japanese Botany 73: 143–154. [Google Scholar]
- Langmead B, Salzberg SL. 2012. Fast gapped-read alignment with Bowtie 2. Nature Methods 9: 357–U354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lartillot N, Rodrigue N, Stubbs D, Richer J. 2013. PhyloBayes MPI: phylogenetic reconstruction with infinite mixtures of profiles in a parallel environment. Systematic Biology 62: 611–615. [DOI] [PubMed] [Google Scholar]
- Li LF, Chen XL, Fang DM, et al. 2022. Genomes shed light on the evolution of Begonia, a mega-diverse genus. New Phytologist 234: 295–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li HT, Luo Y, Gan L, et al. 2021. Plastid phylogenomic insights into relationships of all flowering plant families. BMC Biology 19: 232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li SF, Valdes PJ, Farnsworth A, et al. 2021. Orographic evolution of northern Tibet shaped vegetation and plant diversity in eastern Asia. Science Advances 7: eabc7741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li HT, Yi TS, Gao LM, et al. 2019. Origin of angiosperms and the puzzle of the Jurassic gap. Nature Plants 5: 461–470. [DOI] [PubMed] [Google Scholar]
- Li MJ, Zheng ZY, Liu JC, et al. 2021. Evolutionary origin of a tetraploid Allium species on the Qinghai-Tibet Plateau. Molecular Ecology 30: 5780–5795. [DOI] [PubMed] [Google Scholar]
- Luo D, Yue JP, Sun WG, et al. 2016. Evolutionary history of the subnival flora of the Himalaya-Hengduan Mountains: first insights from comparative phylogeography of four perennial herbs. Journal of Biogeography 43: 31–43. [Google Scholar]
- Ma YP, Milne RI, Zhang CQ, Yang JB. 2010. Unusual patterns of hybridization involving a narrow endemic Rhododendron species (Ericaceae) in Yunnan, China. American Journal of Botany 97: 1749–1757. [DOI] [PubMed] [Google Scholar]
- Milne RI. 2004. Phylogeny and biogeography of Rhododendron subsection Pontica, a group with a Tertiary relict distribution. Molecular Phylogenetics and Evolution 33: 389–401. [DOI] [PubMed] [Google Scholar]
- Milne RI, Abbott RJ. 2002. The origin and evolution of Tertiary relict floras. Advances in Botanical Research 38: 281–314. [Google Scholar]
- Milne RI, Abbott RJ. 2008. Reproductive isolation among two interfertile Rhododendron species: low frequency of post-F1 hybrid genotypes in alpine hybrid zones. Molecular Ecology 17: 1108–1121. [DOI] [PubMed] [Google Scholar]
- Milne RI, Abbott RJ, Wolff K, Charberlain DF. 1999. Hybridization among sympatric species of Rhododendron (Ericaceae) in Turkey: Morphological and molecular evidence. American Journal of Botany 86: 1776–1785. [PubMed] [Google Scholar]
- Milne RI, Davies C, Prickett R, Inns LH, Chamberlain DF. 2010. Phylogeny of Rhododendron subgenus Hymenanthes based on chloroplast DNA markers: between-lineage hybridisation during adaptive radiation? Plant Systematics and Evolution 285: 233–244. [Google Scholar]
- Milne, RI, Terzioglu S, Abbott RJ. 2003. A hybrid zone dominated by fertile F1s: maintenance of species barriers in Rhododendron. Molecular Ecology 12: 2719–2729. [DOI] [PubMed] [Google Scholar]
- Nguyen LT, Schmidt HA, von Haeseler A, Minh BQ. 2015. IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Molecular Biology and Evolution 32: 268–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie J, Ruetenik G, Gallagher K, et al. 2018. Rapid incision of the Mekong River in the middle Miocene linked to monsoonal precipitation. Nature Geoscience 11: 944–948. [Google Scholar]
- Olofsson JK, Cantera I, Van de Paer C, et al. 2019. Phylogenomics using low-depth whole genome sequencing: a case study with the olive tribe. Molecular Ecology Resources 19: 877–892. [DOI] [PubMed] [Google Scholar]
- Ozaki K. 1980. Late Miocene Tatsumitoge flora of Tottori Prefecture, southwest Honshu, Japan (III). Science Reports of the Yokohama National University II: Biological and Geological Sciences 27: 19–45. [Google Scholar]
- Paradis E, Schliep K. 2019. ape 5.0: an environment for modern phylogenetics and evolutionary analyses in R. Bioinformatics 35: 526–528. [DOI] [PubMed] [Google Scholar]
- Petit RJ, Vendramin GG. 2007. Plant phylogeography based on organelle genes: an introduction. In: Weiss S, Ferrand N, eds. Phylogeography of Southern European Refugia: Evolutionary perspectives on the origins and conservation of European biodiversity. Dordrecht: Springer Netherlands, 23–97. [Google Scholar]
- Plummer M, Best N, Cowles K, Vines K. 2006. CODA: convergence diagnosis and output analysis for MCMC. R News 6: 7–11. [Google Scholar]
- Rabosky DL. 2014. Automatic detection of key innovations, rate shifts, and diversity-dependence on phylogenetic trees. PLoS One 9: e89543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rambaut A, Drummond AJ, Xie D, Baele G, Suchard MA. 2018. Posterior summarization in Bayesian phylogenetics using Tracer 1.7. Systematic Biology 67: 901–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose JP, Kleist TJ, Lofstrand SD, Drew BT, Schoenenberger J, Sytsma KJ. 2018. Phylogeny, historical biogeography, and diversification of angiosperm order Ericales suggest ancient Neotropical and East Asian connections. Molecular Phylogenetics and Evolution 122: 59–79. [DOI] [PubMed] [Google Scholar]
- Shrestha N, Wang ZH, Su XY, et al. 2018. Global patterns of Rhododendron diversity: the role of evolutionary time and diversification rates. Global Ecology and Biogeography 27: 913–924. [Google Scholar]
- Smith SA, O’Meara BC. 2012. treePL: divergence time estimation using penalized likelihood for large phylogenies. Bioinformatics 28: 2689–2690. [DOI] [PubMed] [Google Scholar]
- Spicer RA, Su T, Valdes PJ, et al. 2021. Why ‘the uplift of the Tibetan Plateau’ is a myth. National Science Review 8: nwaa091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatakis A. 2006. RAxML-VI-HPC: Maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22: 2688–2690. [DOI] [PubMed] [Google Scholar]
- Steele PR, Pires JC. 2011. Biodiversity assessment: State-of-the-art techniques in phylogenomics and species identification. American Journal of Botany 98: 415–425. [DOI] [PubMed] [Google Scholar]
- Straub SCK, Parks M, Weitemier K, Fishbein M, Cronn RC, Liston A. 2012. Navigating the tip of the genomic iceberg: next-generation sequencing for plant systematics. American Journal of Botany 99: 349–364. [DOI] [PubMed] [Google Scholar]
- Su T, Farnsworth A, Spicer RA, et al. 2019. No high Tibetan Plateau until the Neogene. Science Advances 5: eaav2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K, Battistuzzi FU, Billing-Ross P, Murillo O, Filipski A, Kumar S. 2012. Estimating divergence times in large molecular phylogenies. Proceedings of the National Academy of Sciences of the United States of America 109: 19333–19338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanai T, Onoe T. 1961. A Mio-Pliocene flora from the Ningyo-toge area on the border between Tottori and Okayama prefectures, Japan. Geological Survey of Japan Report 187: 1–63. [Google Scholar]
- Villaverde T, Pokorny L, Olsson S, et al. 2018. Bridging the micro- and macroevolutionary levels in phylogenomics: Hyb-Seq solves relationships from populations to species and above. New Phytologist 220: 636–650. [DOI] [PubMed] [Google Scholar]
- Wang J, Fu CN, Mo ZQ, et al. 2022. Testing complete plastome for species discrimination, cryptic species discovery and phylogenetic resolution in Cephalotaxus (Cephalotaxaceae). Frontiers in Plant Science 13: 768810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang E, Kirby E, Furlong KP, et al. 2012. Two-phase growth of high topography in eastern Tibet during the Cenozoic. Nature Geoscience 5: 640–645. [Google Scholar]
- Westerhold T, Marwan N, Drury AJ, et al. 2020. An astronomically dated record of Earth’s climate and its predictability over the last 66 million years. Science 369: 1383–1387. [DOI] [PubMed] [Google Scholar]
- Wicke S, Schneeweiss GM, dePamphilis CW, Mueller KF, Quandt D. 2011. The evolution of the plastid chromosome in land plants: gene content, gene order, gene function. Plant Molecular Biology 76: 273–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams AV, Miller JT, Small I, Nevill PG, Boykin LM. 2016. Integration of complete chloroplast genome sequences with small amplicon datasets improves phylogenetic resolution in Acacia. Molecular Phylogenetics and Evolution 96: 1–8. [DOI] [PubMed] [Google Scholar]
- Wu ZY, Lu AM, Tang YC, Chen ZD, Li DZ. 2003. The families and genera of Angiosperms in China-a comprehensive analysis. Beijing: Science Press. [Google Scholar]
- Xia XH. 2018. DAMBE7: new and improved tools for data analysis in Molecular Biology and Evolution. Molecular Biology and Evolution 35: 1550–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia XM, Yang MQ, Li CL, et al. 2022. Spatiotemporal evolution of the global species diversity of Rhododendron. Molecular Biology and Evolution 39: msab314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan LJ, Burgess KS, Milne R, Fu CN, Li DZ, Gao LM. 2017. Asymmetrical natural hybridization varies among hybrid swarms between two diploid Rhododendron species. Annals of Botany 120: 51–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan LJ, Burgess KS, Zheng W, Tao ZB, Li DZ, Gao LM. 2019. Incomplete reproductive isolation between Rhododendron taxa enables hybrid formation and persistence. Journal of Integrative Plant Biology 61: 433–448. [DOI] [PubMed] [Google Scholar]
- Yan LJ, Liu J, Moeller M, et al. 2015. DNA barcoding of Rhododendron (Ericaceae), the largest Chinese plant genus in biodiversity hotspots of the Himalaya-Hengduan Mountains. Molecular Ecology Resources 15: 932–944. [DOI] [PubMed] [Google Scholar]
- Yang ZH. 2007. PAML 4: Phylogenetic analysis by maximum likelihood. Molecular Biology and Evolution 24: 1586–1591. [DOI] [PubMed] [Google Scholar]
- Ye XY, Ma PF, Yang GQ, et al. 2019. Rapid diversification of alpine bamboos associated with the uplift of the Hengduan Mountains. Journal of Biogeography 46: 2678–2689. [Google Scholar]
- Yu XQ, Yang D, Guo C, Gao LM. 2018. Plant phylogenomics based on genome-partitioning strategies: progress and prospects. Plant Diversity 40: 158–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng LP, Zhang N, Zhang QA, Endress PK, Huang J, Ma H. 2017. Resolution of deep eudicot phylogeny and their temporal diversification using nuclear genes from transcriptomic and genomic datasets. New Phytologist 214: 1338–1354. [DOI] [PubMed] [Google Scholar]
- Zha HG, Milne RI, Sun H. 2008. Morphological and molecular evidence of natural hybridization between two distantly related Rhododendron species from the Sino-Himalaya. Botanical Journal of the Linnean Society 156: 119–129. [Google Scholar]
- Zha HG, Milne RI, Sun H. 2010. Asymmetric hybridization in Rhododendron agastum: a hybrid taxon comprising mainly F1s in Yunnan, China. Annals of Botany 105: 89–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Wang YH, Jin JJ, et al. 2020. Exploration of plastid phylogenomic conflict yields new insights into the deep relationships of Leguminosae. Systematic Biology 69: 613–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JL, Zhang CQ, Gao LM, Yang JB, Li HT. 2007. Natural hybridization origin of Rhododendron agastum (Ericaceae) in Yunnan, China: inferred from morphological and molecular evidence. Journal of Plant Research 120: 457–463. [DOI] [PubMed] [Google Scholar]
- Zheng W, Yan LJ, Burgess KS, et al. 2021. Natural hybridization among three Rhododendron species (Ericaceae) revealed by morphological and genomic evidence. BMC Plant Biology 21: 529. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.