Abstract
PfEMP1 is an antigenically variable molecule which mediates the adhesion of parasitized erythrocytes to a variety of cell types and which is believed to constitute an important target for naturally acquired protective immune responses in malaria. For 9 years we have monitored individuals living in an area of low-intensity, seasonal, and unstable malaria transmission in eastern Sudan, and we have used this database to study the acquisition, specificity, and duration of the antibody response to variant parasitized erythrocyte surface antigens. Both the levels and the spectrum of reactivity of these antibodies varied considerably among individuals, ranging from low levels of antibodies recognizing only few parasitized erythrocyte surface antigens to high levels of broad-specificity antibodies. In general, episodes of clinical malaria were associated with increases in the levels of parasitized erythrocyte surface-specific antibodies that subsided within months of the attack. This response was often, but not always, specific for the antigenic variants expressed by the parasite isolate causing disease. Our study provides evidence that Palciparum falciparum malaria is associated with a short-lived, variant-specific antibody response to PfEMP1-like antigens exposed on the surface of parasitized erythrocytes. Furthermore, our data suggest that the antigenic repertoires of variant antigens expressed by different parasite isolates show considerable overlapping, at least under Sahelian conditions of low-intensity, seasonal, and unstable malaria transmission. Finally, we demonstrate the existence of persistent differences among individuals in the capacity to mount antibody responses to variant surface antigens.
People living in areas of high Plasmodium falciparum malaria endemicity gradually develop substantial clinical protection against the disease over a period of several years; this is believed to reflect acquisition of protective immunity. However, although a variety of immune responses directed against numerous parasite antigens have been identified, it is still unclear which responses, and of what specificities, are essential for immunity. Recent evidence points to the importance of antibody responses specific for antigenically variable molecules expressed on the surface of P. falciparum-infected erythrocytes (4, 16). One such class of molecules, the PfEMP1 proteins, encoded by the var multigene family (2, 25, 28), are responsible of the sequestration of parasitized erythrocytes to the walls of postcapillary venules and certain other cytoadhesion phenotypes (14). The ability of infected cells to adhere to endothelial cells is thought to be central to the pathogenesis of P. falciparum malaria, and the acquisition of agglutinating antibodies, which mainly recognize PfEMP1-like molecules, has been linked to the development of protective immunity (4, 17). In addition to the ability of PfEMP1 to mediate endothelial sequestration, it is involved in the formation of rosettes, i.e., the binding of uninfected erythrocytes to a central parasitized cell (24, 32). Like sequestration, rosette formation has been implicated in malarial pathogenesis, and levels of antibodies capable of disrupting such rosettes have been reported to correlate with protective immunity (5, 6). The present study was undertaken to investigate the acquisition, specificity, and persistence of antibodies recognizing PfEMP1-like molecules under conditions of low-intensity, seasonal, and unstable malaria transmission; these conditions permit the analysis of human infections without the complicating effect of continuous superinfection often found in areas of high transmission intensity.
MATERIALS AND METHODS
Study area.
The study was carried out between 1988 and 1997 in the village of Daraweesh, Gedaref State, Sudan, located 430 km southeast of the capital Khartoum. The region is characterized by a short rainy season from July to October, whereas the remainder of the year is hot and dry. Essentially all malaria cases are seen during and shortly after the rainy season, from August to November. Malaria transmission in the region is unstable and is dependent on precipitation, which varies considerably between years. An epidemic of malaria followed unusually heavy rains in 1988, while very few cases were seen during the drought of 1990 and 1991. From 1992 to 1996 the annual incidence of malaria in Daraweesh has varied, with 24.7 to 35.2% of the population suffering at least one malaria attack (29). The predominant species of malaria parasite is P. falciparum (98% of cases), with P. vivax and P. malariae occasionally seen. The sole vector is Anopheles arabiensis.
Study cohort.
This study is part of a longitudinal study of infection and immunity to malaria in Daraweesh which has been under way since 1990. The study has received ethical approval from the ethical Committee of the University of Khartoum and national clearance from the Sudanese Ministry of Health. For the present study, we used data and samples from a cohort of 37 permanent residents (12 males, 25 females; age range in 1995, 7 to 51 years) monitored clinically and parasitologically by passive case detection for up to 9 years from 1988 to 1997. During the malaria season, all individuals feeling unwell reported to a health team that was present in the village on a daily basis. Blood smears were obtained from all individuals with a body temperature of >37.5°C and/or other symptoms suggestive of malaria. Individuals with clinical symptoms suggestive of malaria and patent parasitaemia in Giemsa-stained blood films were classified as having clinical episodes of malaria, and curative treatment was initiated.
Blood samples.
Venous blood samples were collected in heparinized Vacutainers (Becton Dickinson, Rutherford, N.J.). Following centrifugation, plasma was collected and stored at −20°C until use. From donors with malaria, the pellet of infected erythrocytes (IRBC) was collected, washed in RPMI 1640, resuspended in 28% glycerol, and snap-frozen in liquid nitrogen as described elsewhere (18). Informed consent was always obtained from the donors and/or their parents prior to the collection of blood samples.
Cultivation and characterization of parasite isolates.
Frozen IRBC were thawed, washed, diluted with fresh uninfected O Rh+ erythrocytes (RBC), and cultured by a modification (10) of the method originally described by Trager and Jensen (30). In brief, the isolates were grown in RPMI 1640 medium supplemented with 10% Albumax II (Life Technologies, Tåstrup, Denmark), 24 mM sodium bicarbonate, 2 mM HEPES buffer, and 50 mg of gentamicin (Gibco, Paisley, United Kingdom) per ml. Cultures were maintained at 37°C in an atmosphere of 94% nitrogen, 5% carbon dioxide, and 1% oxygen, and the culture medium was changed every other day. The growth rate was monitored by microscopic examination of Giemsa-stained smears. For the initial characterization of parasite isolates and levels of antibodies to PfEMP1-like antigens in plasma (see below), we used nine P. falciparum isolates obtained this way. Of these, six were primary isolates from Daraweesh (S9457, Z453, Z455, Z456, Y391, and Y395), one was a primary isolate from Ghana (L73), and the last two were long-term laboratory isolates (FCR3 and 3D7). For the detailed longitudinal analysis, we used four isolates from Daraweesh, collected during the malaria seasons of 1994 (S9457), 1995 (Z453 and Z455), and 1996 (Y372), respectively. All isolates were characterized genotypically by PCR analysis of polymorphic regions of three P. falciparum antigens, PfMSP1, PfMSP2, and GLURP as described elsewhere (22, 23).
Measurement of levels of PfEMP1-like antibodies in plasma by flow cytometry.
Levels in plasma of antibodies recognizing PfEMP1-like antigens expressed on the surface of RBC infected by late-stage P. falciparum parasites were measured by a flow cytometry assay developed by us and described in detail elsewhere (26). In brief, in vitro cultures with the majority of the parasites in the late trophozoite and schizont stages and a parasitemia of 2 to 3% were first labelled with ethidium bromide (40 μg/ml). Labelled cultures were subsequently enriched for late-stage parasites by exposure to a high-gradient magnetic field (MACS; Miltenyi Bio Tec, Bergisch Gladbach, Germany) (19, 26), and parasitemias of 40 to 60% late trophozoites and schizonts were found. Aliquots (100 μl) of late-stage-enriched IRBC were then sequentially incubated with test plasma (5 μl), goat anti-human immunoglobulin G (0.2 μl; DAKO, Glostrup, Denmark), and fluorescein isothiocyanate-conjugated rabbit anti-goat immunoglobulin G (DAKO). The duration of each incubation was 30 min, and the samples were extensively washed (in phosphate-buffered saline supplemented with 2% fetal calf serum) between incubations. All steps were done at room temperature. Finally, the samples were washed, resuspended in 300 μl of washing medium, and analyzed on an EPICS XL-MCL flow cytometer (Coulter, Miami, Fla.). IRBC were identified by their forward- and side-scatter properties and by their ethidium bromide fluorescence. Levels of PfEMP1-like antibodies in plasma were then quantified by determining the mean fluorescein isothiocyanate fluorescence of gated IRBC. All analysis of flow cytometric data was done with the PC Lysys software package (Becton Dickinson). For each parasite isolate tested, all test plasma samples were run in parallel, together with a pool of plasma samples obtained from Ghanaian adults living in an area of hyperendemic malaria transmission (positive control) and four individual plasma samples and a serum pool from nonexposed individuals (negative controls).
RESULTS
Characterization of parasite isolates.
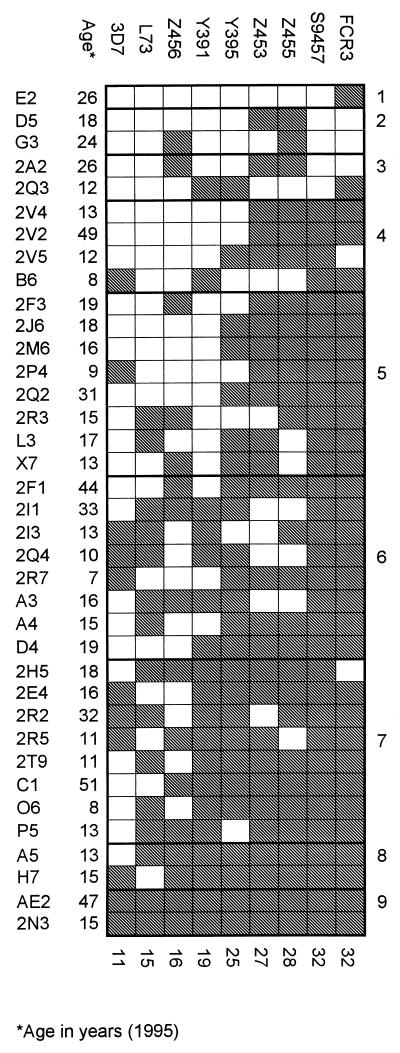
All the parasite isolates were genotypically distinct according to analysis of markers at polymorphic sites of PfMSP1, PfMSP2, and GLURP (Table 1). In accordance with this, serological typing with plasma samples obtained after the malaria season of 1995 to 1996 (January 1996) from the 37 members of the Daraweesh cohort (all healthy at that time point) showed discrete recognition patterns for each isolate (Fig. 1). However, among the field isolates the isolates Z453 and Z455 were genotypically identical at all markers except PfMSP2 (Table 1) and the recognition patterns obtained by serological typing of PfEMP1-like antigens on the surface of erythrocytes infected by these two isolates were very similar (Fig. 1). Isolates Z453 and Z455 were collected on the same day from siblings living in the same hut and developing malaria on the same day. It seems likely that Z453 and Z455 originated from the same mosquito that infected both children on the same occasion with an inoculation in which there was at least one common clone. A third isolate originating from the same hut and on the same day (Z456) was distinct from Z453 and Z455 by both genetic and serological typing (Table 1 and Fig. 1).
TABLE 1.
Characterization of parasite isolates
| Isolate | Donor | Origin, date obtained (day/mo/yr) | Genotype
|
Minimum no. of clones | |||
|---|---|---|---|---|---|---|---|
| PfMSP1 block 2 | PfMSP1 C terminus | PfMSP2 | GLURP | ||||
| 3D7 | Laboratory isolate | MAD20 | MAD20 | 3D7 | E | 1 | |
| FCR3 | Laboratory isolate | MAD20 | Wellcome, K1 | 3D7 | D | 2 | |
| L73 | Dodowa, Ghana, 30/11/94 | MAD20 | Wellcome, MAD20 | FC27, 3D7 | F | 2 | |
| Y391 | G12 | Daraweesh, 08/11/96 | K1 | Wellcome, K1 | FC27 | F | 2 |
| Y395 | 2D3 | Daraweesh, 11/11/96 | K1 | Wellcome, K1 | FC27 | C | 2 |
| S9457 | 2Q4 | Daraweesh, 12/10/94 | RO33, MAD20, K1 | MAD20, FC27 | 3D7 | F | 3 |
| Y372 | X5 | Daraweesh, 26/10/96 | MAD20 | MAD20, FC27 | 3D7 | E | 2 |
| Z453 | P11 | Daraweesh, 30/10/95 | RO33 | MAD20, FC27 | FC27 | B | 2 |
| Z455 | P8 | Daraweesh, 30/10/95 | RO33 | MAD20, FC27 | 3D7 | B | 2 |
| Z456 | P5 | Daraweesh, 30/10/95 | RO33 | Wellcome, K1 | 3D7 | B | 2 |
FIG. 1.
Levels of plasma antibodies recognizing PfEMP1-like molecules on the surface of intact RBC infected with late developmental stages of P. falciparum as detected by flow cytometry. Data from nine parasite isolates (3D7 to FCR3 [columns]) tested versus plasma samples obtained in January 1996 from 37 individuals (E2 to 2N3 [rows]) from Daraweesh village, Sudan, are shown. Shading indicates antibody levels above background (mean + 2 standard deviations of samples from unexposed individuals). Numbers along the right and bottom edges of the figure indicate the number of positive data points per plasma sample and parasite isolate, respectively.
Four of the Daraweesh isolates collected in 1994 (S9457), 1995 (Z453 and Z455), and 1996 (Y372) were frequently recognized by plasma of individuals from Daraweesh (Fig. 1 and data not shown) and were selected for the detailed longitudinal analysis of antibodies to PfEMP1-like antigens described below.
Screening of plasma samples for levels of antibodies to PfEMP1-like antigens.
In addition to revealing antigenic differences between the parasite isolates used, the serological typing demonstrated marked variation in the spectrum of antibodies recognizing PfEMP1-like antigens between individual plasma samples (Fig. 1). At one end of the spectrum was the sample obtained from donor E2, which showed only borderline recognition of the two laboratory isolates and no recognition of any of the primary field isolates (Fig. 1 and data not shown). At the other end, plasma from donor 2N3 contained high levels of antibodies to PfEMP1-like antigens expressed by all nine isolates (Fig. 1 and data not shown). We examined the relationship between the number of parasite isolates recognized by individual plasma samples and several possible explanatory variables. However, neither donor age [P(r) = 0.87, Pearson product moment correlation], number of clinical malaria episodes during 1993 to 1995 [P(r) = 0.96] or 1995 only [P(r) = 0.10], nor number of asymptomatic episodes of parasitemia, whether detected by Giemsa-stained blood films [P(r) = 0.2) or by PCR [P(r) = 0.17] showed a significant relationship. Asymptomatic parasitemia was detected during cross-sectional surveys in January, April, and June of each year from 1993 to 1995 as described elsewhere (23).
Based on these results (Fig. 1), three individuals, E2, 2P4, and 2N3, whose plasma samples appeared to contain qualitatively and quantitatively limited, intermediate, and high antibody recognition of PfEMP1-like antigens, respectively, were selected for more detailed longitudinal analysis.
Malaria-induced changes in levels of antibodies to PfEMP1-like antigens.
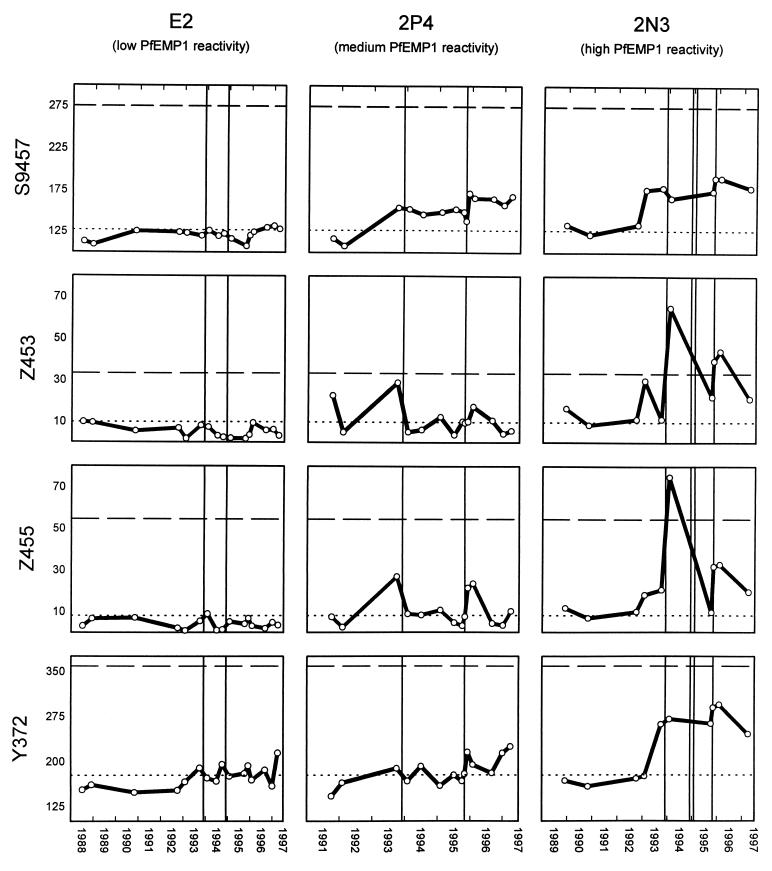
Individual E2 was a female aged 18 years at the time of the collection of the first blood sample (1988). A total of 16 plasma samples collected between June 1988 and March 1997 from this donor were analyzed for the presence of antibodies to parasitized RBC surface antigens expressed by four parasite isolates obtained at various time points from Daraweesh. In line with the data obtained with the nine isolates (Fig. 1), only very limited recognition of any of the isolates was seen at any time point with plasma samples from this donor, including plasma samples donated by this individual after each of two monitored malaria episodes (Fig. 2, left column).
FIG. 2.
Long-term changes in levels of plasma antibodies recognizing PfEMP1-like molecules on the surface of intact RBC infected with late developmental stages of P. falciparum as detected by flow cytometry. The reactivity of plasma from three individuals selected for low (E2, left column), medium (2P4, center column), and high (2N3, right column) antibody levels with four parasite isolates collected in 1994 (S9457, top row), 1995 (Z453, second row; Z455, third row), and 1996 (Y372, bottom row) are shown. The x axis shows the date. The y axis shows the anti-PfEMP1-like antibody reactivity (mean flow cytometer channel number). The negative cutoff (mean + 2 standard deviations for five plasma samples from unexposed individuals) is shown as a dotted horizontal line. The level of anti-PfEMP1-like antibodies in a pool of hyperimmune plasma (positive control) is shown as a dashed horizontal line. Solid vertical lines indicate clinical episodes of malaria.
A total of 10 plasma samples from individual 2P4, a male aged 8 years at the time of the collection of the first sample, were analyzed. During the period between collection of the first (September 1991) and last (March 1997) plasma samples, he experienced two clinical malaria episodes, both of which were associated with increases in titers of antibodies specific for PfEMP1-like antigens expressed on the surface of IRBC infected with any of the four isolates tested (Fig. 2, center column).
Individual 2N3 was a male aged 5 years at the time of collection of the first of 14 samples between November 1989 and March 1997. He suffered four malaria attacks between 1993 and 1995, but unfortunately no plasma samples were obtained at the times of the clinical attacks in November 1994 and January 1995. However, in the other two malaria episodes (November 1993 and October 1995), where plasma samples were available, these were both associated with marked increases in recognition of PfEMP1-like molecules expressed by each of the test isolates (Fig. 2, right column).
Isolate specificity of antibodies to PfEMP1-like antigens.
The availability of a series of plasma samples obtained at various time points before and after collection of a parasite isolate from the same individual offered a unique opportunity to investigate the specificity of antibodies recognizing variant IRBC antigens. Nine plasma samples were collected between June 1995 and March 1997 from individual P8, a female aged 8 years at the time of the collection of the first plasma sample. The two plasma samples obtained from this individual before a malaria episode in October 1995 (caused by isolate Z455) were unable to recognize any of the test isolates (including Z455) (Fig. 3, left column). While limited disease-induced increases in the responses to the heterologous isolates S9457 and Y372 were observed, the recognition of RBC infected by Z455 parasites increased considerably following the malaria episode caused by this isolate. Thus, the malaria attack known to have been caused by Z455 resulted in the development of antibodies specifically recognizing antigens on the surface of RBCs infected by this isolate but not by the unrelated isolates S9457 and Y372. In addition, the Z455-induced malaria episode caused an increase in the antibody reactivity to isolate Z453, slightly lower in magnitude but closely paralleling the response to Z455. As mentioned above, siblings P8 and P11 had malaria diagnosed on the same day, at which time Z455 was isolated from P8 and Z453 was isolated from P11.
FIG. 3.
Long-term changes in levels of plasma antibodies recognizing PfEMP1-like molecules on the surface of intact RBC infected with late developmental stages of homologous and heterologous P. falciparum isolates as detected by flow cytometry. The reactivity of plasma from two individuals (P8 [left column] and P11 [right column]) with four parasite isolates collected in 1994 (S9457, top row), 1995 (Z453, second row; Z455, third row), and 1996 (Y372, bottom row) is shown. Isolates Z455 and Z453 were collected during clinical malaria attacks in November 1995 from individuals P8 and P11, respectively. Symbols are as in Fig. 2.
In line with the above findings, the Z453-induced malaria episode in individual P11 (male, aged 4 years at the time of first sampling) was associated with markedly increased recognition of PfEMP1-like molecules expressed by this isolate, with little effect on the recognition of isolates S9457 and Y372 (Fig. 3, right panel). As with P8, recognition by antibodies in the plasma of P11 of the parasite isolate obtained from the sibling (Z455) was lower but otherwise strikingly similar to that of the autologous isolate, Z453 (Fig. 3, right panel).
Although these findings strongly suggest a close relationship between isolates Z453 and Z455, they were not identical, since recognition of the autologous isolate by antibodies in plasma of both P8 and P11 was stronger than that of the isolate obtained from the sibling (Fig. 3). The Z453 and Z455 isolates had different alleles of the PfMSP2 gene as well as showing different variants of the 3′ end of the PfMSP1 gene. The simplest explanation of the relationship between these isolates, each of which contains at least two clones, is that they contained a clone in common.
DISCUSSION
PfEMP1 is a variant molecule expressed on the surface of RBC infected with late developmental stages (mature trophozoites and schizonts) of P. falciparum parasites (31). PfEMP1 is encoded by the large var gene family (2, 25, 28), is the main molecule mediating the sequestration of IRBC to a variety of host receptors, and as such is believed to be a major factor in the pathogenesis of P. falciparum malaria (1, 20). Clonal variation in the expression of PfEMP1 can occur at around 2% per generation (21). The presence or absence of antibodies to particular PfEMP1 variants appears closely associated with susceptibility to infection with a particular P. falciparum isolate (4), and accumulation of a comprehensive repertoire of antibodies recognizing antigenically distinct PfEMP1 molecules may be the key to acquisition of clinical protection.
Studies of acquisition, decay, and specificity of anti-PfEMP1 antibodies in areas of intense parasite transmission are made difficult by the regular infectious challenge of individuals living in these areas and the consequent age-specific buildup of protective immunity. We have addressed these questions in a longitudinal study of individuals in a Sudanese village situated in an area of low-intensity, seasonal malaria transmission, where there are much less marked age-dependent differences in susceptibility to clinical disease and where individuals are exposed to only a few new infections per year. We have used plasma samples collected over periods of up to 9 years from permanent residents of this village to measure their capacity to recognize variant PfEMP1-like antigens expressed by RBC infected by a panel of different P. falciparum isolates. To permit such a study, which necessitates the analysis of a large array of plasma and parasite isolates, we have developed a flow cytometric assay that allows convenient and unbiased analysis of many samples (26). The assay detects antibodies on the surface of intact RBC infected with mature P. falciparum parasites, and the antigens recognized by these antibodies vary between isolates and have the molecular weight and properties expected of PfEMP1. However, we cannot formally rule out a contribution from antibodies recognizing other variant antigens, such as the rosettins and/or the STEVOR and RIF proteins (8, 13). Our flow cytometric assay correlates well with conventional assays for detection of agglutinating antibodies (12).
Our data show a marked interindividual variation in levels and specificities of antibodies recognizing PfEMP1-like antigens in this area of low-intensity malaria transmission. Neither the age of the plasma donors nor the number of previous episodes of clinical disease or asymptomatic parasitemia correlated significantly with the number of isolates recognized. Antibodies from plasma of some individuals, such as E2, showed only borderline recognition of few parasite isolates. Several mutually nonexclusive factors may contribute to this nonresponsiveness. Samples from E2 have generally shown minimal levels of antibody to other malaria antigen preparations, including PfMSP1 (7), RAP-1 (11), and crude schizont extract (12a), even in samples obtained shortly after documented clinical attacks. This result and the lack of recognition in E2 of PfEMP1-like antigens expressed by any of nine parasite isolates (Fig. 1) suggest that genetic factors are involved in determining host responsiveness. The limited antibody response in E2 to variant antigens expressed by any of four isolates at the time of the two malaria episodes in this individual supports this conclusion (Fig. 2, left column). Alternatively, it may reflect antigenic differences between the parasites causing these episodes and those present in the test isolates. However, both the present study (e.g., the broadly reactive antibody response following infection in 2N3 [Fig. 2, right column]) and our previous findings from the same village (12) suggest that the repertoires of variant antigens on the surface of RBC infected with parasite isolates present in our study area overlap considerably. This hypothesis is supported by the fact that although the four isolates used in the longitudinal study (Fig. 2 and 3) were obtained between 1994 and 1996 (Table 1), several plasma samples collected before the time of their isolation contained antibodies to variant antigens expressed by each of them (Fig. 2). Furthermore, the two malaria attacks occurring in individual 2P4 during the study period both produced an increase in levels of plasma antibodies recognizing all four parasite isolates tested (Fig. 2, center column). This finding clearly implies that the parasites causing these two disease episodes and those present in the test isolates were antigenically related. Similar data were obtained with plasma from individual 2N3 with respect to the two episodes, where plasma samples obtained within a few months of the attack were available (Fig. 2, right column). We found no evidence of disease-induced increases in antibodies to PfEMP1-like antigens in this donor in relation to two other, closely spaced malaria attacks by the turn of the year 1994. However, the next sample was obtained almost a year later, by which time the antibody levels may have decreased considerably. Several lines of evidence support the hypothesis of a relatively short half-life of such antibodies. First, none of the three individuals from whom samples obtained before 1992 were available showed any reactivity before this time. This probably indicates that antibodies recognizing PfEMP1-like antigens in these individuals were largely lost during the drought years of 1990 and 1991, when malaria was essentially absent from Daraweesh village. Second, the cases where several plasma samples were available within a limited period following malaria episodes all showed considerable reduction in antibody levels within a few months.
We have previously speculated that short-lived variant-specific antibodies may be involved in long-term maintenance of low-grade asymptomatic infections (27), whereas clinical disease may be the consequence of acquisition of new, antigenically different parasites displaying PfEMP1 variants to which insufficient specific antibodies are present (4, 9, 15). Indeed, our data on the antibody responses to homologous and heterologous parasites in the siblings P8 and P11 (Fig. 3) strongly support this hypothesis.
In conclusion, our study provides evidence that clinical disease episodes cause a marked and variant-specific antibody response, which usually subsides within months. The data also clearly suggest that the repertoires of variant antigens expressed by different isolates overlap to a considerable degree, in line with our own previous findings (12) as well as with data from a recent study in Kenya (3). Finally, the capacity to mount antibody responses to variant surface antigens varies considerably among individuals, possibly reflecting inherent, genetically restricted differences.
ACKNOWLEDGMENTS
The continuous support of the people of Daraweesh is gratefully acknowledged. Gitte Pedersen is thanked for excellent technical assistance.
The study received financial support from the ENRECA program of the Danish International Development Agency (Danida), the Danish Medical Research Council (SSVF), and the Danish Research Council for Development Research (RUF).
REFERENCES
- 1.Baruch D I, Ma X C, Singh H B, Bi X H, Pasloske B L, Howard R J. Identification of a region of PfEMP1 that mediates adherence of Plasmodium falciparum infected erythrocytes to CD36: conserved function with variant sequence. Blood. 1997;90:3766–3775. [PubMed] [Google Scholar]
- 2.Baruch D I, Pasloske B L, Singh H B, Bi X, Ma X C, Feldman M, Taraschi T F, Howard R J. Cloning the P. falciparum gene encoding PfEMP1, a malarial variant antigen and adherence receptor on the surface of parasitized human erythrocytes. Cell. 1995;82:77–87. doi: 10.1016/0092-8674(95)90054-3. [DOI] [PubMed] [Google Scholar]
- 3.Bull P C, Lowe B S, Kortok M, Marsh K. Antibody recognition of Plasmodium falciparum erythrocyte surface antigens in Kenya: evidence for rare and prevalent variants. Infect Immun. 1999;67:733–739. doi: 10.1128/iai.67.2.733-739.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bull P C, Lowe B S, Kortok M, Molyneux C S, Newbold C I, Marsh K. Parasite antigens on the infected red cell are targets for naturally acquired immunity to malaria. Nat Med. 1998;4:358–360. doi: 10.1038/nm0398-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carlson J, Helmby H, Hill A V, Brewster D, Greenwood B M, Wahlgren M. Human cerebral malaria: association with erythrocyte rosetting and lack of anti-rosetting antibodies. Lancet. 1990;336:1457–1460. doi: 10.1016/0140-6736(90)93174-n. [DOI] [PubMed] [Google Scholar]
- 6.Carlson J, Nash G B, Gabutti V, Al-Yaman F, Wahlgren M. Natural protection against severe Plasmodium falciparum malaria due to impaired rosette formation. Blood. 1994;84:3909–3914. [PubMed] [Google Scholar]
- 7.Cavanagh D, Elhassan I M, Roper C, Robinson V J, Giha H, Holder A A, Hviid L, Theander T G, Arnot D E, McBride J S. A longitudinal study of type-specific antibody responses to Plasmodium falciparum merozoite surface protein 1 in an area of unstable malaria in Sudan. J Immunol. 1998;161:347–359. [PubMed] [Google Scholar]
- 8.Cheng Q, Cloonan N, Fischer K, Thompson J, Waine G, Lanzer M, Saul A. stevor and rif are Plasmodium falciparum multicopy gene families which potentially encode variant antigens. Mol Biochem Parasitol. 1998;97:161–176. doi: 10.1016/s0166-6851(98)00144-3. [DOI] [PubMed] [Google Scholar]
- 9.Contamin H, Fandeur T, Rogier C, Bonnefoy S, Konate L, Trape J F, Mercereau-Puijalon O. Different genetic characteristics of Plasmodium falciparum isolates collected during successive clinical malaria episodes in Senegalese children. Am J Trop Med Hyg. 1996;54:632–643. doi: 10.4269/ajtmh.1996.54.632. [DOI] [PubMed] [Google Scholar]
- 10.Cranmer S L, Magowan C, Liang J, Coppel R L, Cooke B M. An alternative to serum for cultivation of Plasmodium falciparum in vitro. TransRSocTropMedHyg. 1997;91:363–365. doi: 10.1016/s0035-9203(97)90110-3. [DOI] [PubMed] [Google Scholar]
- 11.Fonjungo P N, Elhassan I M, Cavanagh D R, Theander T G, Hviid L, Roper C, Stüber D, Arnot D E, McBride J S. A longitudinal study of human antibody responses to Plasmodium falciparum rhoptry-associated protein 1 in a region of seasonal and unstable malaria transmission. Infect Immun. 1999;67:2975–2985. doi: 10.1128/iai.67.6.2975-2985.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giha, H. A., T. Staalsoe, D. Dodoo, I. M. Elhassan, C. Roper, G. M. H. Satti, D. E. Arnot, L. Hviid, and T. G. Theander. Overlapping antigenic repertoires of variant antigens expressed on the surface of erythrocytes infected by Plasmodium falciparum. Parasitology, in press. [DOI] [PubMed]
- 12a.Giha, H. A., et al. Unpublished data.
- 13.Helmby H, Cavelier L, Pettersson U, Wahlgren M. Rosetting Plasmodium falciparum-infected erythrocytes express unique strain-specific antigens on their surface. Infect Immun. 1993;61:284–288. doi: 10.1128/iai.61.1.284-288.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Howard R J. Malarial proteins at the membrane of Plasmodium falciparum-infected erythrocytes and their involvement in cytoadherence to endothelial cells. Prog Allergy. 1988;41:98–147. doi: 10.1159/000415221. [DOI] [PubMed] [Google Scholar]
- 15.Hviid, L. 24 June 1998, posting date. Clinical disease, immunity and protection against Plasmodium falciparum malaria in populations living in endemic areas. Exp. Rev. Mol. Med. [Online.] http://www-ermm.cbcu.cam.ac.uk. [24 June 1998, last date accessed.] [DOI] [PubMed]
- 16.Marsh K, Howard R J. Antigens induced on erythrocytes by P. falciparum: expression of diverse and conserved determinants. Science. 1986;231:150–153. doi: 10.1126/science.2417315. [DOI] [PubMed] [Google Scholar]
- 17.Marsh K, Otoo L, Hayes R J, Carson D C, Greenwood B M. Antibodies to blood stage antigens of Plasmodium falciparum in rural Gambians and their relation to protection against infection. TransRSocTropMedHyg. 1989;83:293–303. doi: 10.1016/0035-9203(89)90478-1. [DOI] [PubMed] [Google Scholar]
- 18.Merryman H T, Hornblower M. A method for freezing and washing red blood cells using a high glycerol concentration. Transfusion. 1972;12:145–156. doi: 10.1111/j.1537-2995.1972.tb00001.x. [DOI] [PubMed] [Google Scholar]
- 19.Paul F, Roath S, Melville D, Warhurst D C, Osisanya J O. Separation of malaria-infected erythrocytes from whole blood: use of a selective high-gradient magnetic separation technique. Lancet. 1981;ii:70–71. doi: 10.1016/s0140-6736(81)90414-1. [DOI] [PubMed] [Google Scholar]
- 20.Reeder J C, Cowman A F, Davern K M, Thompson J K, Thaus A L, Rogerson S J, Brown G V. BSP Malaria Meeting. 1997. Identification of a var gene associated with the adherence of Plasmodium falciparum to chondroitin sulfate A, abstr. 3.5. [Google Scholar]
- 21.Roberts D J, Craig A G, Berendt A R, Pinches R, Nash G, Marsh K, Newbold C I. Rapid switching to multiple antigenic and adhesive phenotypes in malaria. Nature. 1992;357:689–692. doi: 10.1038/357689a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roper C, Elhassan I M, Hviid L, Giha H, Richardson W, Babiker H, Satti G M H, Theander T G, Arnot D E. Detection of very low level Plasmodium falciparum infections using the nested polymerase chain reaction and a reassessment of the epidemiology of unstable malaria in Sudan. AmJTropMedHyg. 1996;54:325–331. doi: 10.4269/ajtmh.1996.54.325. [DOI] [PubMed] [Google Scholar]
- 23.Roper C, Richardson W, Elhassan I M, Giha H, Hviid L, Satti G M H, Theander T G, Arnot D E. Seasonal changes in the Plasmodium falciparum population in individuals and their relationship to clinical malaria: a longitudinal study in a Sudanese village. Parasitology. 1998;116:501–510. doi: 10.1017/s0031182098002650. [DOI] [PubMed] [Google Scholar]
- 24.Rowe J A, Moulds J M, Newbold C I, Miller L H. P. falciparum rosetting mediated by a parasite-variant erythrocyte membrane protein and complement-receptor 1. Nature. 1997;388:292–295. doi: 10.1038/40888. [DOI] [PubMed] [Google Scholar]
- 25.Smith J D, Chitnis C E, Craig A G, Roberts D J, Hudson-Taylor D E, Peterson D S, Pinches R, Newbold C I, Miller L H. Switches in expression of Plasmodium falciparum var genes correlate with changes in antigenic and cytoadherent phenotypes of infected erythrocytes. Cell. 1995;82:101–110. doi: 10.1016/0092-8674(95)90056-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Staalsoe T, Giha H A, Dodoo D, Theander T G, Hviid L. Detection of antibodies to variant antigens on Plasmodium falciparum infected erythrocytes by flow cytometry. Cytometry. 1999;35:329–336. doi: 10.1002/(sici)1097-0320(19990401)35:4<329::aid-cyto5>3.3.co;2-p. [DOI] [PubMed] [Google Scholar]
- 27.Staalsoe T, Hviid L. The role of variant-specific immunity in asymptomatic infections: maintaining a fine balance. Parasitol Today. 1998;14:177–178. doi: 10.1016/s0169-4758(98)01228-9. [DOI] [PubMed] [Google Scholar]
- 28.Su X, Heatwole V M, Wertheimer S P, Guinet F, Herrfeldt J A, Peterson D S, Ravetch J A, Wellems T E. The large diverse gene family var encodes proteins involved in cytoadherence and antigenic variation of Plasmodium falciparum-infected erythrocytes. Cell. 1995;82:89–100. doi: 10.1016/0092-8674(95)90055-1. [DOI] [PubMed] [Google Scholar]
- 29.Theander T G. Unstable malaria in Sudan: the influence of the dry season. Malaria in areas of unstable and seasonal transmission. Lessons from Daraweesh. TransRSocTropMedHyg. 1998;92:589–592. doi: 10.1016/s0035-9203(98)90775-1. [DOI] [PubMed] [Google Scholar]
- 30.Trager W, Jensen J B. Human malaria parasites in continuous culture. Science. 1976;193:673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- 31.Van Schravendijk M R, Pasloske B L, Baruch D I, Handunnetti S M, Howard R J. Immunochemical characterization and differentiation of two (approx.) 300-kD erythrocyte membrane-associated proteins of Plasmodium falciparum, PfEMP1 and PfEMP3. AmJTropMedHyg. 1993;49:552–565. doi: 10.4269/ajtmh.1993.49.552. [DOI] [PubMed] [Google Scholar]
- 32.Wahlgren M, Fernandez V, Scholander C, Carlson J. Rosetting. Parasitol Today. 1994;10:73–79. doi: 10.1016/0169-4758(94)90400-6. [DOI] [PubMed] [Google Scholar]