Abstract
Per- and polyfluoroalkyl substances (PFAS) are a diverse class of fluorinated anthropogenic chemicals that include perfluoroalkyl acids (PFAA), which are widely used in modern commerce. Many products and environmental samples contain abundant precursors that can degrade into terminal PFAA associated with adverse health effects. Fish consumption is an important dietary exposure source for PFAS that bioaccumulate in food webs. However, little is known about bioaccumulation of PFAA precursors. Here, we identify and quantify PFAS in recreational fish species collected from surface waters across New Hampshire, US, using a toolbox of analytical methods. Targeted analysis of paired water and tissue samples suggests that many precursors below detection in water have a higher bioaccumulation potential than their terminal PFAA. Perfluorobutane sulfonamide (FBSA), a short-chain precursor produced by electrochemical fluorination, was detected in all fish samples analyzed for this compound. The total oxidizable precursor assay interpreted using Bayesian inference revealed fish muscle tissue contained additional, short-chain precursors in high concentration samples. Suspect screening analysis indicated these were perfluoroalkyl sulfonamide precursors with three and five perfluorinated carbons. Fish consumption advisories are primarily being developed for perfluorooctane sulfonate (PFOS), but this work reinforces the need for risk evaluations to consider additional bioaccumulative PFAS, including perfluoroalkyl sulfonamide precursors.
Keywords: PFAS precursors, targeted analysis, total oxidizable precursor (TOP) assay, Bayesian inference, suspect screening, bioaccumulation, consumption advisories, seafood
Short abstract
Perfluoroalkyl sulfonamide precursors with high bioaccumulation were detected in all fish samples but are not considered by present fish advisories.
Introduction
Per- and polyfluoroalkyl substances (PFAS) are a diverse class of anthropogenic chemicals with thousands of potential structures.1,2 Human exposure to PFAS has been associated with many adverse health effects,3 and seafood is known to be an important dietary PFAS source.4,5 Many regions are developing fish consumption guidelines to reduce exposure risks for some of the most bioaccumulative legacy PFAS, predominantly perfluorooctane sulfonate (PFOS).6,7 However, per- and polyfluoroalkyl precursors (hereon referred to as precursors) make up most of the PFAS mass in consumer products8,9 and many contaminated aquatic ecosystems.10 Prior work suggests some precursors have enhanced propensity for biological uptake relative to the terminal perfluoroalkyl acids (PFAA).11
PFAS precursors released to the environment may undergo abiotic or biotic transformation and eventually form PFAA as terminal products. Precursors that originate from the electrochemical fluorination (ECF) process have a fully fluorinated backbone in their chemical structure, while those manufactured by the fluorotelomerization (FT) process are not fully fluorinated.12 Targeted mass spectrometry methods (LC-MS/MS) only capture a small fraction of the PFAS used in commerce and released to the environment.13,14 It is challenging to detect most precursors using targeted methods because many analytical standards are not commercially available. High-resolution mass spectrometry (HRMS) can be used to confirm the presence of specific precursors and assign probable structures to unknown PFAS. However, these results are not quantitative and are difficult to interpret when diverse precursors are present at low concentrations, which is often the case with environmental samples. Semi-quantification of PFAS from HRMS measurements has been used to estimate the concentrations of analytes that lack matched analytical standards, but uncertainties are not quantifiable and could span an order of magnitude or more.15,16
To address some of the challenges associated with PFAS precursor detection, Ruyle et al.17 developed a statistical method for interpreting results from the total oxidizable precursor (TOP) assay that groups precursors by their perfluorinated carbon chain length and manufacturing origin (ECF or FT) using Bayesian inference (hereon referred to as TOP + BI). The TOP assay transforms oxidizable precursors to perfluoroalkyl carboxylates (PFCA) with known perfluorinated carbon chain lengths that are detectable at trace levels using targeted LC-MS/MS analysis. The TOP + BI method is preferred over analytically detected changes in PFCA concentrations (only TOP) because it explicitly accounts for analytical uncertainties, incomplete recoveries, and variability in product yields following precursor degradation.
Many sites across the United States (US) have been contaminated by ECF- and FT-based aqueous film-forming foams (AFFF) that contain large quantities of precursors.18,19 Some precursors, like per- and polyfluoroalkyl ether acids (PFEA), are known to be resistant to oxidation by the TOP assay.20 Nonetheless, the TOP + BI method performs well at sites affected by AFFF chemistries that have many precursors present at low abundance.10
The main objective of this work was to better understand the bioaccumulation potential of PFAS and precursors present in inland surface waters. To do this, we used a toolbox of analytical and statistical methods to measure PFAS in muscle tissues from eight species of freshwater fish commonly caught by recreational fishers in New Hampshire (NH), US. Targeted analysis (LC-MS/MS) was used to detect a suite of up to 37 PFAS in paired surface water and fish tissue samples. Concentrations of PFAS precursors in fish grouped by perfluorinated carbon chain length (Cn where n = number of perfluorinated carbons) and manufacturing origin were interpreted using the TOP + BI method. Suspect screening was used to confirm the presence of additional precursors in fish muscle tissue. The combined data set provides insights into the accumulation of precursors in freshwater food webs. We discuss implications for developing and enhancing fish consumption advisories.
Materials and Methods
Field Sample Collection
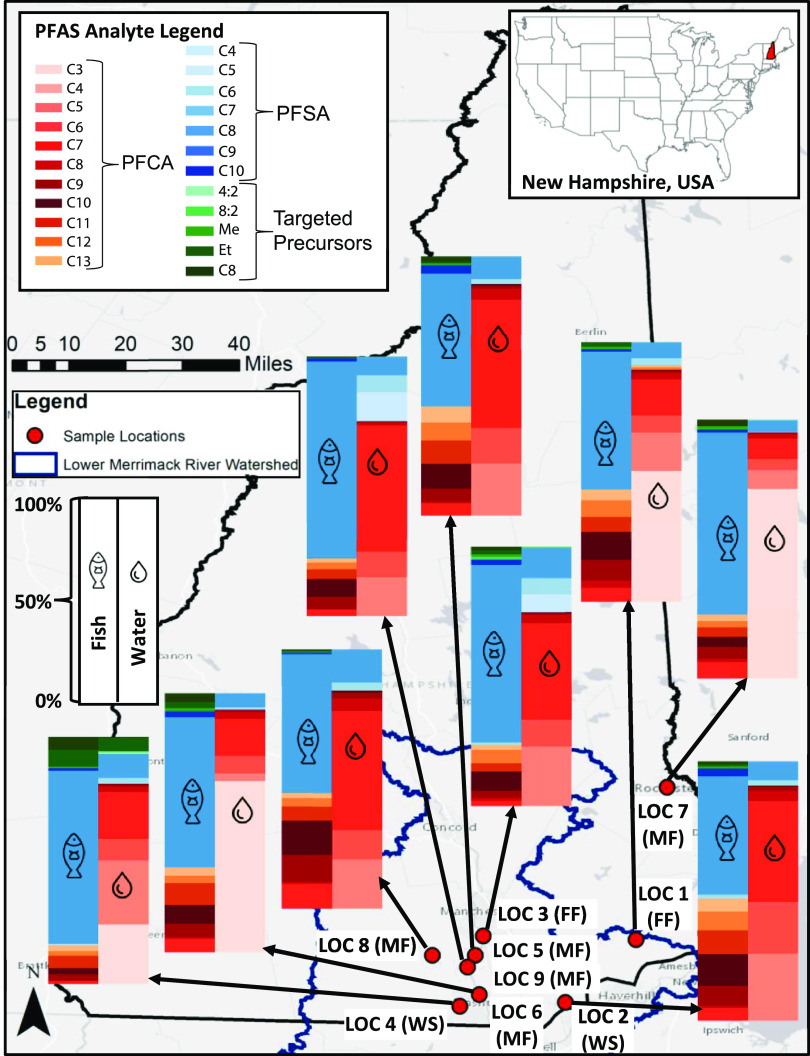
We collected paired water and fish samples from nine freshwater ecosystems in southern New Hampshire, US, in September–October 2017. Locations (denoted LOC) were selected based on proximity to suspected PFAS sources, including AFFF use (FF) (LOC 1, 3), waste disposal sites (WS) (LOC 2, 4), and plastics and textile manufacturing (MF) point sources (LOC 5–9, Figure S1). We were unable to differentiate PFAS profiles in fish based on potential sources due to limited sample sizes associated with each category.
A total of 23 surface water grab samples, 7 field duplicates, and 2 field blanks were collected in precleaned 1 L HDPE bottles and transported to Harvard University. Water samples were stored at 4 °C and analyzed within a month. Local recreational fishers and the NH Fish and Game Department assisted with fish harvesting. Fish (n = 62, 1–3 fish per species per location) were stored frozen at −20 °C and analyzed within a month, followed by reanalysis in 2021. Fish species included yellow perch: Perca flavescens; lake whitefish: Coregonus clupeaformis; bluegill: Lepomis macrochirus; pumpkinseed: Lepomis gibbosus; brown bullhead: Ameiurus nebulosus; chain pickerel: Esox niger; largemouth bass: Micropterus salmoides; and smallmouth bass: Micropterus dolomieui. The Supporting Information (SI) contains additional information on sampling (Sections 1.1–1.2, Table S1).
Chemicals and Reagents
Targeted analysis (LC-MS/MS) was used to detect up to 37 PFAS analytes, denoted by their perfluorinated carbon chain length (Cn where n = number of perfluorinated carbons). This list included eighteen PFAA [eleven PFCA (C3–C13) and seven perfluoroalkyl sulfonates (PFSA: C4–C10)] and up to nineteen targeted PFAS precursors [four fluorotelomer sulfonates (FTSA: 4:2, 6:2, 8:2, 10:2), six perfluoroalkyl sulfonamides (FASA: FBSA, FHxSA, FOSA, FDSA, N-MeFOSA, N-EtFOSA), two perfluoroalkyl sulfonamidoethanols (FASE: N-MeFOSE, N-EtFOSE), three perfluoroalkyl sulfonamidoacetic acids (FASAA: FOSAA, N-MeFOSAA, N-EtFOSAA), three fluorotelomer carboxylates (FTCA: 3:3, 5:3, 7:3), and one polyfluoroalkyl ether carboxylate (PFPE: ADONA)]. The SI Section 1.3 contains additional details on chemicals and materials used for analysis (Table S2).
Sample Extraction
Subsamples (500 mL) of 1 L water samples were obtained by sonicating and inverting the sample several times. Samples were extracted and analyzed for 25 targeted PFAS at Harvard University in 2017. Samples were spiked with 2 ng isotopically labeled internal standard followed by offline weak anion exchange (WAX) solid phase extraction (SPE), following established methods.21 Fish muscle tissues were also extracted and analyzed for 25 targeted PFAS in 2017. In 2021, some fish tissue samples were re-extracted and analyzed for a larger suite of 37 targeted PFAS to compare with TOP assay results. For both fish extractions, 0.5 g of homogenized wet-weight muscle tissue fortified with internal standards was subjected to ion-pairing extraction, following established methods.22 Bluegill muscle tissues were analyzed as composites (n = 3), and all other fish were analyzed individually. The SI Section 1.4 contains additional details on extraction methods.
Targeted Analysis
Water and fish muscle tissue extracts were analyzed for targeted PFAS using an Agilent (Santa Clara, CA) 6460 triple quadrupole liquid chromatograph-tandem mass spectrometer (LC-MS/MS) equipped with an Agilent 1290 Infinity Flex Cube online SPE, with slight modifications to previously published methods.21 Each 100–300 μL extract was loaded onto an Agilent Zorbax SB-Aq (4.6 mm × 12.5 mm; 5 μm) online SPE cartridge with 0.85 mL of 0.1% aqueous formic acid at a flow rate of 1 mL min–1. Analytes were eluted from the SPE cartridge and loaded onto an Agilent Poroshell 120 EC-C18 (3.0 mm × 50 mm; 2.7 μm) reversed-phase HPLC column using ammonium acetate (2 mM) in methanol and ammonium acetate (2 mM) in Milli-Q water at a flow rate of 0.5 mL min–1 and column temperature of 50 °C. Analytes were ionized with an electrospray ionization (ESI) source in negative ion mode and introduced to the tandem mass spectrometer at a temperature of 300 °C, gas flow rate of 13 L min–1, and nebulizer pressure of 45 psi. Additional details are provided in SI Section 1.5.
Targeted PFAS were quantified using both isotopic dilution and extracted internal standard quantification with 7- to 11-point calibration curves. For PFAS without matched isotopically labeled standards, the internal standard closest in retention time and/or within the same functional group was used for quantification (Table S3). Milli-Q water was used for procedural blanks, and two to three blanks were included with each water and fish tissue extraction (Table S4). Average (±standard deviation) spike recoveries using Milli-Q water as the spiking matrix were 105 ± 23% for the water extraction and 88 ± 10 and 104 ± 25% for the fish extractions. Average spike recoveries using fish muscle as the spiking matrix was 115 ± 33% for the fish extractions (Table S5). Sections 1.6–1.7 of the SI contain additional details on blanks, duplicates, spikes, and internal standard recoveries (Table S6).
Limits of detection (LODs) were calculated based on the average concentration at which the sample signal-to-noise ratio (S/N) was three. Method detection limits (MDLs) were determined based on sample dilution volumes or weight, and only values >MDL are reported here. MDLs for fish samples ranged between 0.001 and 1.27 ng g–1 (SI Section 1.8 and Table S7). Method trueness was assessed using NIST SRM 1947 (Lake Michigan fish tissue). Relative percent differences between NIST SRM 1947 analyzed in this study and the reference concentrations were within ±30% for all detectable PFAS, which compares favorably with other studies (SI Section 1.9 and Table S8).23,24
Total Oxidizable Precursor (TOP) Assay and Statistical Interpretation
The TOP assay uses hydroxyl radicals formed by heated persulfate under basic pH conditions to oxidize precursors into PFCA of the same or shorter perfluorinated carbon chain lengths that can be detected using targeted analysis.25 The TOP assay was applied to one sample of fish muscle tissue from each location. We chose the fish species at each location that had the highest targeted PFAS concentrations (Table S9). The extract oxidation procedure was adapted from an aqueous oxidation procedure,10 which itself is modified from the original method developed by Houtz and Sedlak.25 Following ion-pairing extraction with ENVI-carb cleanup, extracts were transferred to 50 mL polypropylene tubes and evaporated to dryness. The tubes were vigorously shaken following addition of Milli-Q water (20 mL) and 0.12 M potassium persulfate and 0.25 M sodium hydroxide solution (20 mL). Samples were heated in an 85 °C water bath for ≥12 h and then neutralized to pH 7, as needed. Samples were processed similarly to water samples using SPE and prepped for targeted analysis (SI Section 1.10).
Precursor oxidation efficiency in the presence of fish tissue was evaluated with each sample batch by spiking fish muscle tissue with targeted precursors prior to extraction and oxidation. Complete oxidation of targeted precursor concentrations (concentrations < MDL) was verified after every batch of samples. Internal standards were added after the TOP assay to avoid oxidation of the isotopically labeled precursors. Molar yields of several targeted precursors oxidized in the presence of fish tissue to the corresponding PFCA were compared to literature data for other matrices (Table S10).10,24−29 Targeted PFAS recovery spikes were included to assess the stability and recovery of PFAS after ion-pairing extraction and the TOP assay (Table S11). Low recoveries for the longer-chain PFCA (C > 8) indicated that PFAS loss occurred during the ion-pairing extraction and TOP assay, so they were omitted from further interpretation. The addition of internal standards after oxidation meant they could not be recovery-corrected. Instead, spike recoveries were used to correct oxidized C3–C8 PFCA concentrations with average recoveries ≥50%.30 The change in C3–C8 PFCA after the TOP assay was calculated based on the recovery-corrected difference. Uncertainty in recoveries was accounted for in the Bayesian inference. We did not include modifications to the TOP assay to detect C < 3 PFCA26 because the method used here is based on prior work17 that did not require it to complete the PFAS mass balance, but this could be explored in future work. Additional details on the TOP assay validation are provided in SI Section 1.10.
Precursor concentrations (grouped by perfluorinated carbon chain length) were based on the recovery-corrected measured increases in C3–C8 PFCA (Table S9) produced by the TOP + BI method previously developed for aqueous samples.10,17 Manufacturing origins [ECF vs FT] of precursors are identifiable based on their unique yields. FT precursors have n perfluorinated carbons followed by two or three aliphatic hydrocarbons (n:2, n = 4, 6, 8; n:3, n = 5, 7) and oxidize to form multiple PFCA analytes in the TOP assay (Table S10). ECF precursors include those with Cn (n = 4–8) perfluorinated carbons and generally oxidize to form one Cn–1 PFCA with ∼100% yield (Table S10). Ten ECF and FT precursor groups with perfluorinated carbon chain lengths ranging from 4–8 were included in the statistical interpretation. Longer-chain precursor groups (C > 8) were not included due to reduced recovery of the longer-chain PFCA in the TOP assay. Inferred precursor classes based on this method incorporate those with analytical standards (i.e., targeted precursors) and others without that require suspect screening and/or nontargeted analysis to be identified. Precursors were inferred using their oxidation yields (Table S10) and measurements of their oxidation products by Markov chain Monte Carlo (MCMC) analysis implemented in Python 3.7.4 using emcee 3.0.2.31 The likelihood of precursor concentrations, given the measurements, was determined by sampling the posterior distribution of precursor concentrations generated from the least-squares of the log difference between the model and measurements (yields of terminal PFCA generated by the TOP assay). We used a noninformative Jeffrey’s prior because little is known about the presence of precursors in fish tissue and other biotic tissues (SI Section 1.11). Probability density functions were based on the nonparametric kernel density of oxidizable precursor concentrations. Here, we report the expected value (hereon referred to as the expected mean) and 95% confidence intervals (CI) of inferred precursor concentrations.
Bioaccumulation Factors (BAF)
Field-measured BAF (μg PFAS kg–1 wet-weight fish tissue/μg PFAS L–1 water) were calculated for each sampling site. This calculation relies on detectable PFAS concentrations (>MDL) in both water and fish. Some longer-chain PFAS are frequently below detection in water but are known to be bioaccumulative and were detectable in fish muscle in this study. We therefore divided the measured tissue concentrations of PFAS detectable in fish by the MDL for each analyte in water to estimate the lower bound of their BAF (referred to as “potential BAF”).
Suspect Screening and Nontargeted Analysis
Suspect screening and nontargeted analysis were performed on a subset of fish muscle tissue extracts at the University of Rhode Island using a SCIEX ExionLC AC UHPLC system coupled to a SCIEX X500R quadrupole time-of-flight tandem mass spectrometer (QTOF MS/MS). Each 20 μL extract was loaded onto a Phenomenex Gemini C18 analytical column (3 μm, 110 Å, 50 mm × 2 mm) preceded by a Phenomenex SecurityGuard cartridge at a flow rate of 0.3 mL min–1 and column temperature of 45 °C using ammonium acetate (10 mM) in methanol and ammonium acetate (10 mM) in Milli-Q water. An additional Phenomenex Gemini C18 column (5 μm, 110 Å, 50 mm × 4.6 mm) was used as the delay column for PFAS instrumental contribution. MS data were collected using both IDA and SWATH acquisitions in negative ESI mode at a temperature of 450 °C, curtain gas pressure of 30 psi, ion source gas 1 at 40 psi, and ion source gas 2 at 60 psi. Raw data were screened using the SCIEX Fluorochemical HRMS/MS Spectral Library 2.0. For quantitative comparison between targeted LC-MS/MS and suspect screening QTOF MS/MS results, a targeted HRMS/MS method was used, with the IS operated under the same conditions as for suspect screening. The SI Section 1.12 contains additional details on analyte parameters (Table S12) and suspect screening identification (Table S13).
Statistical Analyses
Statistical analyses were performed in R version 4.0.2 using NADA32 and python version 3.7.4 using SciPy33 and statsmodels.34 We used hierarchical clustering (Figure S2) to group locations with similar PFAS profiles and then tested for statistically significant differences in fish PFAS concentrations among clusters using analysis of variance (ANOVA) and Tukey’s HSD post hoc test (Table S14). Bluegill was the only fish species measured at every location. Samples with targeted PFAS measurements with ≤70% detection frequency were excluded from statistical summaries. For samples above this detection frequency that contained compounds < MDL, nondetects were imputed using robust regression on order statistics.35 7:3 FTCA was detected by LC-MS/MS in >90% of fish samples measured but was excluded from further evaluation due to the presence of a biological interference identified by HRMS (SI Section 1.13). Some biological molecules can interfere with quantification of certain PFAS if they have the same nominal mass in unit resolution.36 Complementary measurements using HRMS are useful since interfering molecules in biological samples can be distinguished from PFAS using exact mass measurements.
Results and Discussion
Concentrations of Targeted PFAS in Fish
Based on the reference dose value (RfD) derived by the state of New Hampshire for PFOS in 2019,37 all but two fish samples analyzed in this study exceeded the daily consumption (8 oz meal) limit for adults (≤1.1 ng g–1), and 21% of samples exceeded the weekly consumption limit (≤7.4 ng g–1, Table S15). No samples exceeded the adult or child-based consumption limits for other PFAS (PFOA, PFNA, PFHxS) with available RfD values (Table S15). Although, New Hampshire already has a consumption limit of ≤4 meals/month of wild-caught fish based on mercury (SI Section 2.1). Linear PFOS was the predominant PFAS detected in all fish samples (0.21–52 ng g–1, mean 5.1 ng g–1, Table S16). Only 21% of samples had detectable branched PFOS isomers, and these were present at much lower concentrations (0.21–3.0 ng g–1) than the linear isomer. This likely reflects preferential accumulation and retention of the linear isomer and/or reduced uptake and faster elimination of the branched isomers.38,39
The sum of targeted PFAS (∑PFAS) across all fish samples analyzed ranged from 0.95–60 ng g–1 (species averages: 1.1–11 ng g–1). The C7–C13 PFCA (PFOA to PFTeDA), linear PFOS, PFDS, and three ECF precursors (FOSA, L-N-MeFOSAA, L-N-EtFOSAA) were detected in ≥80% of samples (Figure S3). After PFOS, the C10 PFCA (PFUnDA) was the PFAA with the highest average concentration (0.55 ± 0.43 ng g–1), followed by the other long-chain PFCA (C9, C11–C12, Table S16). Limited sample sizes meant we were not able to assess statistically significant differences in PFAS concentrations among all locations. Instead, we grouped waterbodies with similar PFAS profiles using hierarchical clustering and tested differences among fish species within each cluster. Only a few statistically significant (p < 0.05) differences were observed among these clusters for individual PFAS (Table S14). LOC 4 (potential waste disposal site source) had higher ∑PFOS in bluegill, the only fish species measured at every location, compared to other locations (Table S16), but the difference was not statistically significant.
A short-chain perfluorobutane sulfonamide ECF precursor (FBSA) was detected using targeted analysis in every fish sample analyzed for this analyte. The average measured concentration of FBSA was greater than any other targeted precursor (1.1 ± 1.8 ng g–1). FBSA is a degradation product and major metabolite of other precursors in some AFFF formulations and surface treatment products.40,41 Detection of FBSA in environmental samples has only recently been reported.42,43 Concentrations similar to those measured in this study were detected in freshwater fish from different waterbodies across Canada and the Great Lakes region,44 suggesting widespread presence of FBSA in the environment.
Differences between Surface Water and Fish PFAS Composition
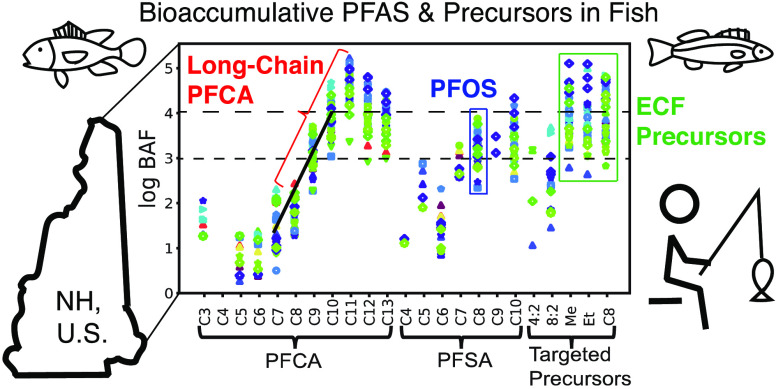
PFAS frequently detected in water (i.e., ∑PFOS, PFOA, and a few other short-chain PFAA) are the focus of current regulatory efforts across the US.45Figure 1 contrasts the PFAS composition between paired water and fish tissue samples. In New Hampshire surface waters, the shorter-chain PFCA (C3, C5–C7) are most abundant in water, whereas fish muscle tissue predominantly contains ∑PFOS (sum of linear and branched isomers) and longer-chain PFCA (C7, C9–C13) (Tables S16 and S17). The ECF precursors (FOSA, L-N-MeFOSAA, and L-N-EtFOSAA) were only above detection limits in one water sample but were detected in ≥84% of fish samples. This means relative PFAS abundance in water is not a good proxy for those detected in fish. Long-chain PFCA, in particular, are close to or below limits of detection in water but bioaccumulate in fish to levels that may be considered a human exposure risk.46,47 Presently, fish consumption advisories are focused mainly on PFOS as the predominant analyte detected in fish and overlook many of the other frequently detected compounds.6,7,48
Figure 1.
Composition of targeted PFAS measured in water and fish collected from the lower Merrimack River Watershed in New Hampshire, US, in 2017. PFAS are labeled by perfluorinated carbon (Cn) chain length: perfluoroalkyl carboxylates (PFCA) range from C3–C13, perfluoroalkyl sulfonates (PFSA) from C4–C10, and targeted precursors include 4:2 and 8:2 fluorotelomer sulfonates, perfluorooctane sulfonamide (C8), and N-methyl (Me) and N-ethyl (Et) perfluorooctane sulfonamidoacetic acids. Sample locations are denoted by LOC + site number with potential source types in brackets: FF = aqueous film-forming foam, WS = waste disposal site, and MF = plastics or textile manufacturing. The map was created using ArcGIS software by Esri.49 Sources: Esri, HERE, Garmin, OpenStreetMap contributors, and the GIS user community.
Field-Measured Bioaccumulation Factors (BAF) for Fish
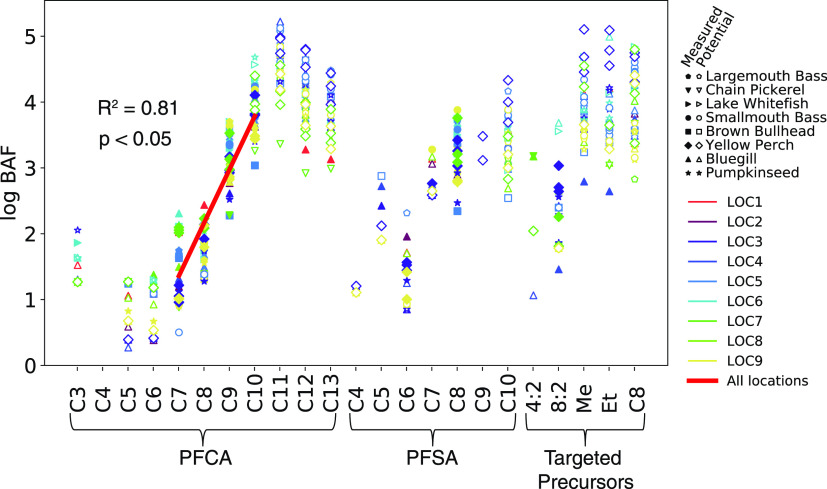
Many studies report liver tissue or whole-body fish PFAS concentrations to estimate risks to wildlife,50 but concentrations in fish muscle tissue are most relevant for human consumption. Figure 2 shows field-measured bioaccumulation factors (BAF) for muscle tissue for the eight freshwater fish species from this study (Table S18). BAF calculated for the C7–C10 PFCA and C8 PFSA (∑PFOS) were based on detectable concentrations in both water and muscle tissue for ≥70% of samples and are indicated by solid markers (Figure 2). A statistically significant (p < 0.05) linear relationship (R2 = 0.81) between measured BAF and PFCA chain length is evident for the C7–C10 PFCA, with an average increase in BAF by 0.78 ± 0.14 log units per perfluorinated carbon (Figures 2 and S4). A similar relationship could not be constructed for the PFSA because most were below detection other than ∑PFOS. The log BAF for the C7–C10 PFCA ranged from 0.9–4.3 and 2.5–3.9 (mean of 3.2) for the C8 PFSA (∑PFOS). Log BAF between 3 and 4 indicate substances with a tendency to bioaccumulate, while those with log BAF ≥ 4 are considered very bioaccumulative.51
Figure 2.
Empirically derived bioaccumulation factors (BAF, L kg–1) for different PFAS, fish species, and sampling locations in New Hampshire, US. Each marker indicates an individual measurement, and each marker type denotes the fish species. Solid markers show measured BAF based on detectable water and fish concentrations, while open markers show potential BAF calculated from method detection limits for water and measured fish concentrations. Abbreviations for precursors are: perfluorooctane sulfonamide (C8), N-methyl (Me), and N-ethyl (Et) perfluorooctane sulfonamidoacetic acids, and fluorotelomer sulfonates indicated by carbon number (n:2). The red line and R2 value are based on linear regression of the measured BAF data for the C7–C10 PFCA. Location-specific regressions are shown in Figure S4.
Potential log BAF represent the lower limit of bioaccumulation potential for analytes that were below detection in water (by substituting the MDL for the concentration in water, Figure 2). Potential log BAF ranged from 3.1–5.2 for C11–C13 PFCA, 2.7–4.3 for C10 PFSA (PFDS), and 2.6–5.1 for the ECF precursors (FOSA, L-N-MeFOSAA, and L-N-EtFOSAA), indicating they are all very bioaccumulative. High BAF have previously been reported for ECF and FT precursors, including FOSA, N-EtFOSA, 6:2 fluorotelomer phosphate diester (6:2 diPAP), and select PFECA.4,52,53We did not measure FBSA in water samples and therefore could not estimate a potential BAF in this study. Another study recently reported FBSA has a log BAF of 2.0–3.2,54 indicating a tendency to bioaccumulate.
A limitation of potential BAF calculations is that they reflect both the inherent properties of the chemicals to accumulate in fish and analytical detection limits. While a higher potential BAF for the C11 PFCA is consistent with the log linear increase observed for C7–C10 PFCA (Figure 2), it could also reflect the lower analytical MDL for water compared to the C12 and C13 PFCA (Table S7). Irrespective of detection limits, lower bioaccumulative potential for PFCA > C11 has been observed in other studies,53,55−57 supporting the trends indicated by potential BAF in this study (Figure 2). This may reflect reduced bioavailability of larger molecules due to a steric hindrance to uptake past a certain chain length.
Overall, these results suggest many precursors have enhanced propensity for bioaccumulation compared to their terminal degradation products. Potential BAF for precursors may be underestimated if any were biotransformed in vivo into intermediate and terminal PFAA. Conversely, BAF calculations may overestimate the accumulation potential of terminal PFAA if precursor biotransformation has contributed to observed tissue burdens.58
Oxidizable Precursors in Fish Muscle Tissue
Following the TOP assay, analytically detectable increases in concentrations of the C3–C8 PFCA of greater than 1 nmol L–1 (nM) were measured in 35% of tissue samples analyzed (Figure S5). TOP + BI results indicated ECF rather than FT precursors were the predominant class present in these samples (Figures 3 and S6). Similar distributions of inferred precursors were observed in multiple fish species from the same locations. This likely means that any cross-species differences in uptake were less important than aqueous exposures for observed tissue concentrations of precursors.
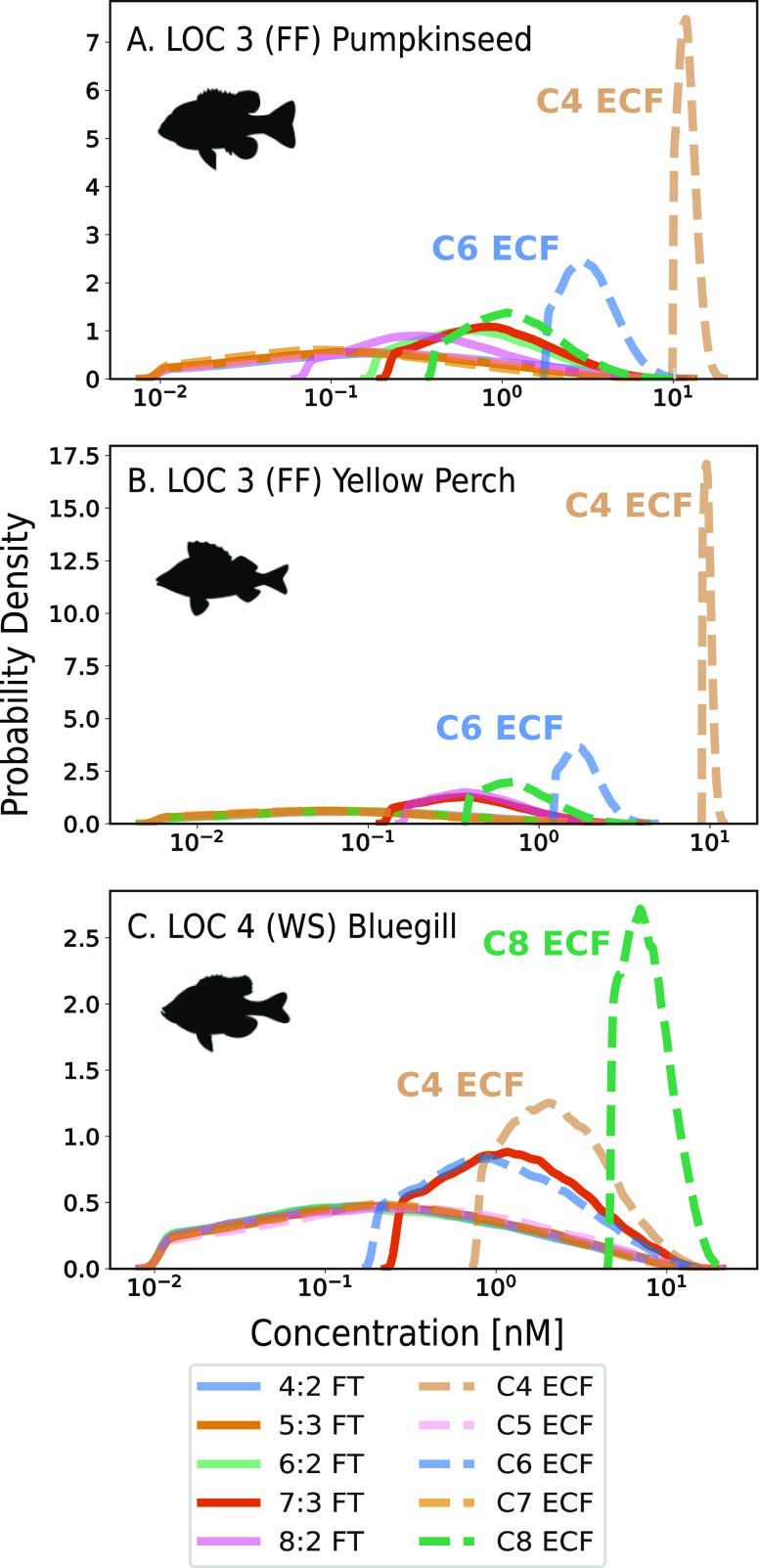
Figure 3.
Inferred concentrations (nM) of oxidizable precursors and their perfluorinated carbon chain length based on total oxidizable precursor (TOP) assay results interpreted using Bayesian inference (TOP + BI). Panels (A) and (B) show probability density functions for the concentrations of oxidizable precursors for two species (pumpkinseed (PS2) and yellow perch (YP1)) from location 3 (LOC 3) where AFFF (FF) is a potential source. Panel (C) shows bluegill (BGcomp) from location 4 (LOC 4) where waste disposal (WS) is a potential source. Higher peaks with narrower ranges indicate greater probability (less uncertainty) in inferred concentrations. Results for samples from LOC 5 and 9 are provided in the SI (Figure S6).
Results from the TOP + BI analysis for LOC 3 samples (Figure 3A,B) showed C4 ECF precursors had higher concentrations (x-axis) and probabilities of occurrence (indicated by higher, narrower peaks, y-axis) than the other C3–C8 precursors. The highest expected mean concentrations from the TOP + BI analysis for LOC 3 samples ranged from 10–13 nM for the C4 ECF precursors, followed by 1.9–3.7 nM for the C6 ECF precursors (Figure 3 and Table S19). Relatively small uncertainties (narrow probability distributions) in the concentration ranges of C4 ECF precursors were enabled by high measured concentrations of FBSA (a C4 ECF precursor) in the targeted analysis (9.5–11 nM, Table S20), which was used to constrain the inference. The expected mean concentrations of C4 ECF precursors from the TOP + BI analysis were within 18% of the targeted FBSA concentrations, suggesting FBSA was likely the only C4 ECF precursor present in fish muscle tissue from LOC 3. In the LOC 4 sample, the C8 ECF precursors had the highest probabilities of occurrence and were well-constrained by a narrow probability density function (Figure 3C).
Greater uncertainty in the TOP + BI results was apparent for other locations with lower concentrations of targeted and inferred (≤2 nM) precursors (e.g., LOC 5 and LOC 9) (Figure S6 and Tables S19 and S20). Fish tissue from these locations showed suggestive evidence of C4 ECF precursors (expected means of 2.2–4.0 nM, Table S19) but had lower targeted FBSA concentrations (≤1.7 nM, Table S20), which provides a measurement constraint for the inference. For these locations, the probability density functions for inferred C4 ECF precursors were shallow and broader (Figure S6), indicating greater uncertainty (Figure S7). Inferred C4 ECF precursor expected mean concentrations were 2–12 times higher than targeted concentrations of FBSA (Table S20). Given the uncertainty in the posteriors for the LOC 5 and LOC 9 samples, we do not consider this robust evidence for additional C4 ECF precursors. Detection of other C4 ECF precursors from HRMS would be needed to confirm such a finding.
Uncertainty in the TOP + BI results for the C4 ECF precursors highlights some of the limitations of standard analytical techniques. The TOP assay oxidizes FBSA to the C3 PFCA (PFBA), which can be challenging to measure at low concentrations in biological tissues. Short-chain PFAS coelute with many biological molecules in LC-MS/MS analysis due to their small size.36 This coelution with matrix interferences affects ionization efficiency and increases the background, leading to reduced and variable recovery and higher detection limits. Measurement uncertainties are considered in the TOP + BI method and propagate to uncertainty in the posterior probability distribution of inferred concentrations (Figures 3 and S6–S8). These results highlight some of the challenges associated with measuring low concentrations of PFAS and precursors in biological tissues.
In summary, we find the TOP + BI method is most informative when total precursor concentrations in samples exceed 9 nM (concentration ranges for LOC 3 and LOC 4 samples). For samples with lower total precursor concentrations (e.g., <2 nM for LOC 5 and LOC 9), large uncertainties in the inferred concentrations (broad posterior probability density functions) make results less informative (Table S20). In general, targeted precursor measurements are useful for constraining uncertainty in the statistical inference, emphasizing the need for additional commercially available standards.
Evaluation of Consistency in Precursor Detection across Analytical Methods
We compared the expected mean concentrations of all C4–C8 precursors from the TOP + BI analysis to the summed concentrations of targeted precursors of each chain length. Targeted analysis accounted for 75–92% of the expected mean concentration of precursors from the TOP + BI analysis in LOC 3 samples, 46% in LOC 4, and 8–22% in LOC 5 and LOC 9 (Table S20). The differences between targeted and mean inferred precursor concentrations were greatest for LOC 5 and LOC 9 samples that had relatively low concentrations of precursors compared to other sites but were in better agreement (3–69% difference) with the lower 95% CI of inferred concentrations (Table S20).
Results from suspect screening analysis for the same fish tissue samples subjected to the TOP + BI analysis confirmed the detection of a C3 ECF precursor, perfluoropropane sulfonamide (FPrSA), and a C5 ECF precursor, perfluoropentane sulfonamide (FPeSA) at a high confidence level (2a59) and high detection frequency (Table S13). High abundance of these precursors was determined based on peak area. In the LOC 3 samples, peak areas for FPeSA were almost double those of FBSA (Table S21). Concentrations of FPeSA were semi-quantitatively estimated to be 2× greater than FBSA in LOC 3 YP1 (20 nM FPeSA vs 8.7 nM FBSA) and 3× greater in LOC 3 PS2 (32 nM FPeSA vs 11 nM FBSA) (Figure 4 and Table S22). Standards for the even-chain PFSA were used for semi-quantification because no matched analytical standards are available for these compounds. Uncertainty in such measurements cannot be quantified but could be large. Concurrence or discrepancies with the TOP + BI results are therefore useful for establishing additional lines of evidence or uncertainty for the abundance of a particular nontargeted and semi-quantified compound.
Figure 4.
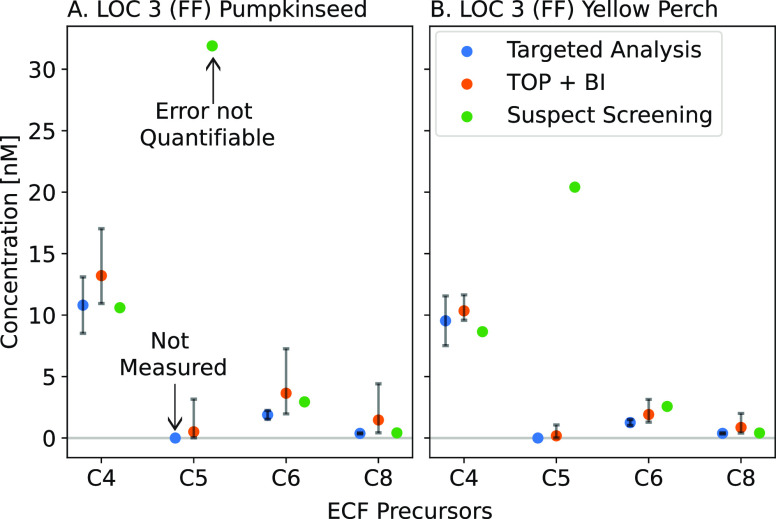
Comparison of measured, inferred, and semi-quantified concentrations of precursors. The C4, C5, C6, and C8 ECF precursors for LOC 3 samples are shown where the potential source is AFFF (FF). Panel (A) shows LOC 3 pumpkinseed (PS2), and panel (B) shows LOC 3 Yellow Perch (YP1). The blue circles show targeted analysis results for ECF precursors (FBSA (C4), FHxSA (C6), and FOSA/L-N-MeFOSAA (C8)) with concentrations quantified using analytical standards. C5 was not measured by targeted analysis since no C5 ECF precursor standards were available. Error bars for targeted analysis are based on the average relative percent difference between sample and spike duplicates (n = 2–6). The orange circles show expected mean molar concentrations of ECF precursor classes from the TOP+BI analysis and 95% confidence intervals of the inference. The green circles show results from suspect screening. The C5 ECF precursor (FPeSA) is semi-quantified (no C5 analytical standard). The suspect screening results do not have error bars as the error cannot be quantified for these measurements.
No additional PFAS analytes were identified using the nontargeted analysis. Fewer precursors in biological tissues were identified by suspect screening in this work compared to more contaminated locations such as those directly impacted by AFFF.60 This study is more representative of background levels of PFAS, likely from multiple environmental sources.
Results from targeted analysis, suspect screening analysis, and TOP + BI measurements showed reasonable agreement for the C4, C6, and C8 ECF precursors (Figure 4 and Table S20). In contrast, semi-quantified concentrations of FPeSA from the suspect screening analysis greatly exceeded even the upper 95% CI of inferred concentrations of C5 ECF precursors from the TOP + BI analysis (Figure 4 and Tables S19 and S21). For example, the semi-quantified concentrations of FPeSA (C5 ECF) in samples from LOC 3 exceeded 20 nM compared to the upper 95% CI TOP + BI concentration of 3.4 nM, which accounts for analytical uncertainty and variable recoveries (Figure 4). The maximum analytically measured increase in the C4 PFCA (PFPeA) following TOP (the oxidation product of FPeSA) was 3.1 nM. The TOP + BI comparison thus suggests that semi-quantified concentrations are overestimated.
A high bias in semi-quantified concentrations for some precursors in this study is consistent with past work that has shown semi-quantification using surrogate reference standards produces results that can be biased due to ionization or fragmentation differences.61 Past work suggests semi-quantified concentrations may be overestimated by up to four times the TOP assay results due to lower accuracy and limited analytical standards.15,62 Alternatively, the same studies have suggested TOP assay results can underestimate true concentrations due to incomplete oxidation and low recoveries.62 However, we have accounted for these factors in the Bayesian inference interpretation of the TOP assay (TOP + BI). HRMS/MS is not subject to the same interferences that can be problematic for LC-MS/MS due to exact mass measurements, so this is not expected to be a factor in the high bias in concentration. Instead, we attribute the variability to the lack of commercial analytical standards to quantify concentrations associated with instrumental results from the HRMS/MS analysis. These results emphasize the benefits of using a toolbox of methods to better understand the robustness of any given measurement, especially for compounds lacking commercially available standards.
In summary, both the TOP + BI results and suspect screening analysis indicate the presence of short-chain ECF precursors (perfluoroalkyl sulfonamides) in fish muscle samples that were not detected by targeted analysis. Analytical standards for additional short-chain sulfonamide compounds (e.g., C3: FPrSA and C5: FPeSA) are needed to quantify concentrations of these bioaccumulative precursors more accurately.
Implications
Results of this study reinforce the high bioaccumulation propensity of several long-chain PFAA63−65 that are frequently detected in human serum46 and breastmilk.47 Exposure to long-chain PFAA has been associated with adverse toxicological outcomes and is correlated with reported fish consumption, highlighting the importance of seafood as an exposure source.66,67 Results of this study also emphasize the bioaccumulative potential for ECF precursors, specifically short-chain perfluoroalkyl sulfonamides (C3–C5) such as FBSA (C4). The C1–C8 sulfonamide congeners have pKa values of 5.86–9.72 compared to <4 for PFAA,68 indicating the presence of more neutral species in solution that will have a greater propensity to partition into cells due to hydrophobic interactions.69 These precursors were detected in multiple species of recreational fish across New Hampshire, US. The widespread detection of perfluoroalkyl sulfonamide precursors in biota indicates that additional exposure and risk evaluations are needed for some understudied PFAS.
Federal and state regulatory efforts are presently focused on legacy PFAS predominantly detected in water and do not consider the full range of highly bioaccumulative terminal PFAA and precursors discussed in this work. Metabolism of precursors that exhibit a higher bioaccumulation potential than their terminal degradation products will enhance exposures to terminal PFAA of concern.11,70 Some studies have suggested sulfonamide precursors have greater bioactivity than PFAA of similar perfluorinated carbon chain length due to their higher pKa, greater fraction of neutral species at similar pH, and interactions with lipids and membranes facilitated by the sulfonamide head group.69,71−73 Additional physicochemical and toxicological data on diverse precursors, particularly the sulfonamides, are needed to better understand their bioaccumulation potential and toxicity. Our work suggests that more comprehensive fish advisories are needed to account for potential human exposures to the full suite of highly bioaccumulative longer-chain PFAA and ECF precursors.
We found reasonable agreement among analytical methods for measuring PFAS in biota (targeted analysis, semi-quantification, and TOP + BI) in samples that had relatively higher PFAS concentrations (total precursor concentrations > 9 nM). More uncertainty among analytical methods was apparent for samples with total precursor concentrations < 2 nM, in part reflecting challenges associated with detection and recoveries in a more complex tissue matrix at low PFAS concentrations. Matrix interferences that affect accurate quantification are a challenge for new measurement techniques that aim to characterize unknown PFAS present in environmental samples at low levels. The toolbox of analytical methods used in this study allowed us to identify additional precursors and quantitatively estimate the lower and upper bounds of their concentrations in these fish samples. However, without individual PFAS analytical standards, accurate quantification of the short-chain perfluoroalkyl sulfonamide precursors in biota will remain a challenge. Thus, additional commercially available standards for potentially bioaccumulative PFAS precursors are essential for more comprehensively characterizing PFAS exposures for all fish consumers.
Acknowledgments
This research was supported by the National Institute of Environmental Health Sciences Superfund Research Program (P42ES027706). H.M.P. was supported by a fellowship from the Natural Sciences and Engineering Research Council of Canada (NSERC). The authors thank NH Fish and Game guides (Hope Eagleson, Bill Horgan, Mike Ivone, Gardener Murphy, and Larry Murphy) for their assistance with field sampling, and staff at the NH DES for input on the data.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.est.2c03734.
Sampling information; supporting descriptions of extraction methods, analyses, and quality assurance/quality control; tables of concentration and BAF data; details of the Bayesian inference; and precursor evaluations across methods (PDF)
Fish concentrations (Table S16) (XLSX)
Water concentrations (Table S17) (XLSX)
The authors declare no competing financial interest.
Supplementary Material
References
- Organisation for Economic Co-operation and Development (OECD). Toward a New Comprehensive Global Database of Per- and Polyfluoroalkyl Substances (PFASs): Summary Report on Updating the OECD 2007 List of per- and Polyfluoroalkyl Substances (PFASs); ENV/ JM/MONO(2018)7; 2018.
- Wang Z.; DeWitt J. C.; Higgins C. P.; Cousins I. T. A Never-Ending Story of Per- and Polyfluoroalkyl Substances (PFASs)?. Environ. Sci. Technol. 2017, 51, 2508–2518. 10.1021/acs.est.6b04806. [DOI] [PubMed] [Google Scholar]
- Agency for Toxic Substances and Disease Registry. Toxicological Profile for Perfluoroalkyls; Atlanta, GA: U.S. Department of Health and Human Services, Public Health Service, 2021, 10.15620/cdc:59198. [DOI] [PubMed] [Google Scholar]
- Miranda D. A.; Benskin J. P.; Awad R.; Lepoint G.; Leonel J.; Hatje V. Bioaccumulation of Per- and Polyfluoroalkyl Substances (PFASs) in a Tropical Estuarine Food Web. Sci. Total Environ. 2021, 754, 142146 10.1016/j.scitotenv.2020.142146. [DOI] [PubMed] [Google Scholar]
- Augustsson A.; Lennqvist T.; Osbeck C. M. G.; Tibblin P.; Glynn A.; Nguyen M. A.; Westberg E.; Vestergren R. Consumption of Freshwater Fish: A Variable but Significant Risk Factor for PFOS Exposure. Environ. Res. 2021, 192, 110284 10.1016/j.envres.2020.110284. [DOI] [PubMed] [Google Scholar]
- Massachusetts Department of Public Health. Freshwater Fish Consumption Advisory List (2021). https://www.mass.gov/doc/public-health-freshwater-fish-consumption-advisories-2021/download (accessed August 8, 2022).
- Minnesota Department of Health. Great Lakes Consortium for Fish Consumption Advisories: Best Practice for Perfluorooctane Sulfonate (PFOS) Guidelines (2019). https://www.health.state.mn.us/communities/environment/fish/docs/consortium/bestpracticepfos.pdf (accessed September 13, 2022).
- Tokranov A. K.; Nishizawa N.; Amadei C. A.; Zenobio J. E.; Pickard H. M.; Allen J. G.; Vecitis C. D.; Sunderland E. M. How Do We Measure Poly- and Perfluoroalkyl Substances (PFASs) at the Surface of Consumer Products?. Environ. Sci. Technol. Lett. 2019, 6, 38–43. 10.1021/acs.estlett.8b00600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaider L. A.; Balan S. A.; Blum A.; Andrews D. Q.; Strynar M. J.; Dickinson M. E.; Lunderberg D. M.; Lang J. R.; Peaslee G. F. Fluorinated Compounds in U.S. Fast Food Packaging. Environ. Sci. Technol. Lett. 2017, 4, 105–111. 10.1021/acs.estlett.6b00435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruyle B. J.; Pickard H. M.; LeBlanc D. R.; Tokranov A. K.; Thackray C. P.; Hu X. C.; Vecitis C. D.; Sunderland E. M. Isolating the AFFF Signature in Coastal Watersheds Using Oxidizable PFAS Precursors and Unexplained Organofluorine. Environ. Sci. Technol. 2021, 55, 3686–3695. 10.1021/acs.est.0c07296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X.; Lohmann R.; Sunderland E. M. Poly- and Perfluoroalkyl Substances in Seawater and Plankton from the Northwestern Atlantic Margin. Environ. Sci. Technol. 2019, 53, 12348–12356. 10.1021/acs.est.9b03230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck R. C.; Franklin J.; Berger U.; Conder J. M.; Cousins I. T.; de Voogt P.; Jensen A. A.; Kannan K.; Mabury S. A.; van Leeuwen S. P. Perfluoroalkyl and Polyfluoroalkyl Substances in the Environment: Terminology, Classification, and Origins. Integr. Environ. Assess. Manage. 2011, 7, 513–541. 10.1002/ieam.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung L. W. Y.; Mabury S. A. Are Humans Exposed to Increasing Amounts of Unidentified Organofluorine?. Environ. Chem. 2016, 13, 102–110. 10.1071/EN15041. [DOI] [Google Scholar]
- Koch A.; Kärrman A.; Yeung L. W. Y.; Jonsson M.; Ahrens L.; Wang T. Point Source Characterization of Per- and Polyfluoroalkyl Substances (PFASs) and Extractable Organofluorine (EOF) in Freshwater and Aquatic Invertebrates. Environ. Sci.: Processes Impacts 2019, 21, 1887–1898. 10.1039/C9EM00281B. [DOI] [PubMed] [Google Scholar]
- Nickerson A.; Rodowa A. E.; Adamson D. T.; Field J. A.; Kulkarni P. R.; Kornuc J. J.; Higgins C. P. Spatial Trends of Anionic, Zwitterionic, and Cationic PFASs at an AFFF-Impacted Site. Environ. Sci. Technol. 2021, 55, 313–323. 10.1021/acs.est.0c04473. [DOI] [PubMed] [Google Scholar]
- Charbonnet J. A.; Rodowa A. E.; Joseph N. T.; Guelfo J. L.; Field J. A.; Jones G. D.; Higgins C. P.; Helbling D. E.; Houtz E. F. Environmental Source Tracking of Per- and Polyfluoroalkyl Substances within a Forensic Context: Current and Future Techniques. Environ. Sci. Technol. 2021, 55, 7237–7245. 10.1021/acs.est.0c08506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruyle B. J.; Thackray C. P.; McCord J. P.; Strynar M. J.; Mauge-Lewis K. A.; Fenton S. E.; Sunderland E. M. Reconstructing the Composition of Per- and Polyfluoroalkyl Substances in Contemporary Aqueous Film-Forming Foams. Environ. Sci. Technol. Lett. 2021, 8, 59–65. 10.1021/acs.estlett.0c00798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews D. Q.; Hayes J.; Stoiber T.; Brewer B.; Campbell C.; Naidenko O. V. Identification of Point Source Dischargers of Per- and Polyfluoroalkyl Substances in the United States. AWWA Water Sci. 2021, 3, e1252 10.1002/aws2.1252. [DOI] [Google Scholar]
- Leeson A.; Thompson T.; Stroo H. F.; Anderson R. H.; Speicher J.; Mills M. A.; Willey J.; Coyle C.; Ghosh R.; Lebrón C.; Patton C. Identifying and Managing Aqueous Film-Forming Foam-Derived Per- and Polyfluoroalkyl Substances in the Environment. Environ. Toxicol. Chem. 2021, 40, 24–36. 10.1002/etc.4894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C.; Hopkins Z. R.; McCord J.; Strynar M. J.; Knappe D. R. U. Fate of Per- and Polyfluoroalkyl Ether Acids in the Total Oxidizable Precursor Assay and Implications for the Analysis of Impacted Water. Environ. Sci. Technol. Lett. 2019, 6, 662–668. 10.1021/acs.estlett.9b00525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber A. K.; Barber L. B.; LeBlanc D. R.; Sunderland E. M.; Vecitis C. D. Geochemical and Hydrologic Factors Controlling Subsurface Transport of Poly- and Perfluoroalkyl Substances, Cape Cod, Massachusetts. Environ. Sci. Technol. 2017, 51, 4269–4279. 10.1021/acs.est.6b05573. [DOI] [PubMed] [Google Scholar]
- Dassuncao C.; Pickard H.; Pfohl M.; Tokranov A. K.; Li M.; Mikkelsen B.; Slitt A.; Sunderland E. M. Phospholipid Levels Predict the Tissue Distribution of Poly- and Perfluoroalkyl Substances in a Marine Mammal. Environ. Sci. Technol. Lett. 2019, 6, 119–125. 10.1021/acs.estlett.9b00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner J. L.; O’Connell S. G.; Butt C. M.; Mabury S. A.; Small J. M.; De Silva A. O.; Muir D. C. G.; Delinsky A. D.; Strynar M. J.; Lindstrom A. B.; Reagen W. K.; Malinsky M.; Schäfer S.; Kwadijk C. J. A. F.; Schantz M. M.; Keller J. M. Determination of Perfluorinated Alkyl Acid Concentrations in Biological Standard Reference Materials. Anal. Bioanal. Chem. 2012, 404, 2683–2692. 10.1007/s00216-012-5943-5. [DOI] [PubMed] [Google Scholar]
- Simonnet-Laprade C.; Budzinski H.; Maciejewski K.; Le Menach K.; Santos R.; Alliot F.; Goutte A.; Labadie P. Biomagnification of Perfluoroalkyl Acids (PFAAs) in the Food Web of an Urban River: Assessment of the Trophic Transfer of Targeted and Unknown Precursors and Implications. Environ. Sci.: Processes Impacts 2019, 21, 1864–1874. 10.1039/C9EM00322C. [DOI] [PubMed] [Google Scholar]
- Houtz E. F.; Sedlak D. L. Oxidative Conversion as a Means of Detecting Precursors to Perfluoroalkyl Acids in Urban Runoff. Environ. Sci. Technol. 2012, 46, 9342–9349. 10.1021/es302274g. [DOI] [PubMed] [Google Scholar]
- Janda J.; Nödler K.; Scheurer M.; Happel O.; Nürenberg G.; Zwiener C.; Lange F. T. Closing the Gap – Inclusion of Ultrashort-Chain Perfluoroalkyl Carboxylic Acids in the Total Oxidizable Precursor (TOP) Assay Protocol. Environ. Sci.: Processes Impacts 2019, 21, 1926–1935. 10.1039/C9EM00169G. [DOI] [PubMed] [Google Scholar]
- Göckener B.; Eichhorn M.; Lämmer R.; Kotthoff M.; Kowalczyk J.; Numata J.; Schafft H.; Lahrssen-Wiederholt M.; Bücking M. Transfer of Per- and Polyfluoroalkyl Substances (PFAS) from Feed into the Eggs of Laying Hens. Part 1: Analytical Results Including a Modified Total Oxidizable Precursor Assay. J. Agric. Food Chem. 2020, 68, 12527–12538. 10.1021/acs.jafc.0c04456. [DOI] [PubMed] [Google Scholar]
- Martin D.; Munoz G.; Mejia-Avendaño S.; Duy S. V.; Yao Y.; Volchek K.; Brown C. E.; Liu J.; Sauvé S. Zwitterionic, Cationic, and Anionic Perfluoroalkyl and Polyfluoroalkyl Substances Integrated into Total Oxidizable Precursor Assay of Contaminated Groundwater. Talanta 2019, 195, 533–542. 10.1016/j.talanta.2018.11.093. [DOI] [PubMed] [Google Scholar]
- Wang B.; Yao Y.; Wang Y.; Chen H.; Sun H. Per- and Polyfluoroalkyl Substances in Outdoor and Indoor Dust from Mainland China: Contributions of Unknown Precursors and Implications for Human Exposure. Environ. Sci. Technol. 2022, 56, 6036–6045. 10.1021/acs.est.0c08242. [DOI] [PubMed] [Google Scholar]
- Shoemaker J. A.; Tettenhorst D. R.. Method 537.1. Determination of Selected Per- and Polyflourinated Alkyl Substances in Drinking Water by Solid Phase Extraction and Liquid Chromatography/Tandem Mass Spectrometry (LC/MS/MS); EPA/600/R-18/352; Cincinnati, OH, 2018; Vol. 1. [Google Scholar]
- Foreman-Mackey D.; Hogg D. W.; Lang D.; Goodman J. Emcee: The MCMC Hammer. Publ. Astron. Soc. Pac. 2013, 125, 306–312. 10.1086/670067. [DOI] [Google Scholar]
- Lee L.NADA: Nondetects and DataAnalysis for Environmental Data. https://cran.r-project.org/web/packages/NADA/index.html (accessed June 03, 2020).
- Virtanen P.; Gommers R.; Oliphant T. E.; Haberland M.; Reddy T.; Cournapeau D.; Burovski E.; Peterson P.; Weckesser W.; Bright J.; van der Walt S. J.; Brett M.; Wilson J.; Millman K. J.; Mayorov N.; Nelson A. R. J.; Jones E.; Kern R.; Larson E.; Carey C. J.; Polat İ.; Feng Y.; Moore E. W.; VanderPlas J.; Laxalde D.; Perktold J.; Cimrman R.; Henriksen I.; Quintero E. A.; Harris C. R.; Archibald A. M.; Ribeiro A. H.; Pedregosa F.; van Mulbregt P.; et al. SciPy 1.0: Fundamental Algorithms for Scientific Computing in Python. Nat. Methods 2020, 17, 261–272. 10.1038/s41592-019-0686-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seabold S.; Perktold J.. Statsmodels: Econometric and Statistical Modeling with Python, American University, 2010; pp 92–96 10.25080/Majora-92bf1922-011. [DOI]
- Helsel D. R.Statistics for Censored Environmental Data Using Minitab and R; Proceedings of the Python in Science Conference, 2nd ed.; Wiley: Denver, Colorado, 2012. [Google Scholar]
- Bangma J. T.; Reiner J.; Fry R. C.; Manuck T.; McCord J.; Strynar M. J. Identification of an Analytical Method Interference for Perfluorobutanoic Acid in Biological Samples. Environ. Sci. Technol. Lett. 2021, 8, 1085–1090. 10.1021/acs.estlett.1c00901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- New Hampshire Department of Environmental Services (NHDES). Technical Background Report for Proposed Maximum Contaminant Levels (MCLs) and Ambient Groundwater Quality Standards (AGQSs) for Perfluorooctane Sulfonic Acid (PFOS), Perfluorooctanoic (PFOA), Perfluorononanoic Acid (PFNA), and Perfluorohexane Sulfonic Acid (PFHxS), 2019, https://www.des.nh.gov/sites/g/files/ehbemt341/files/documents/r-wd-19-29.pdf (accessed August 08, 2022).
- Houde M.; Czub G.; Small J. M.; Backus S.; Wang X.; Alaee M.; Muir D. C. G. Fractionation and Bioaccumulation of Perfluorooctane Sulfonate (PFOS) Isomers in a Lake Ontario Food Web. Environ. Sci. Technol. 2008, 42, 9397–9403. 10.1021/es800906r. [DOI] [PubMed] [Google Scholar]
- Zhong W.; Zhang L.; Cui Y.; Chen M.; Zhu L. Probing Mechanisms for Bioaccumulation of Perfluoroalkyl Acids in Carp (Cyprinus Carpio): Impacts of Protein Binding Affinities and Elimination Pathways. Sci. Total Environ. 2019, 647, 992–999. 10.1016/j.scitotenv.2018.08.099. [DOI] [PubMed] [Google Scholar]
- Barzen-Hanson K. A.; Roberts S. C.; Choyke S.; Oetjen K.; McAlees A.; Riddell N.; McCrindle R.; Ferguson P. L.; Higgins C. P.; Field J. A. Discovery of 40 Classes of Per- and Polyfluoroalkyl Substances in Historical Aqueous Film-Forming Foams (AFFFs) and AFFF-Impacted Groundwater. Environ. Sci. Technol. 2017, 51, 2047–2057. 10.1021/acs.est.6b05843. [DOI] [PubMed] [Google Scholar]
- Chu S.; Letcher R. J. In Vitro Metabolic Formation of Perfluoroalkyl Sulfonamides from Copolymer Surfactants of Pre- and Post-2002 Scotchgard Fabric Protector Products. Environ. Sci. Technol. 2014, 48, 6184–6191. 10.1021/es500169x. [DOI] [PubMed] [Google Scholar]
- Kaboré H. A.; Goeury K.; Desrosiers M.; Vo Duy S.; Liu J.; Cabana G.; Munoz G.; Sauvé S. Novel and Legacy Per- and Polyfluoroalkyl Substances (PFAS) in Freshwater Sporting Fish from Background and Firefighting Foam Impacted Ecosystems in Eastern Canada. Sci. Total Environ. 2022, 816, 151563 10.1016/j.scitotenv.2021.151563. [DOI] [PubMed] [Google Scholar]
- Kaboré H. A.; Goeury K.; Desrosiers M.; Vo Duy S.; Liu J.; Cabana G.; Munoz G.; Sauvé S. Fish Exhibit Distinct Fluorochemical and Δ15N Isotopic Signatures in the St. Lawrence River Impacted by Municipal Wastewater Effluents. Front. Environ. Sci. 2022, 10, 1–14. 10.3389/fenvs.2022.833164. [DOI] [Google Scholar]
- Chu S.; Letcher R. J.; McGoldrick D. J.; Backus S. M. A New Fluorinated Surfactant Contaminant in Biota: Perfluorobutane Sulfonamide in Several Fish Species. Environ. Sci. Technol. 2016, 50, 669–675. 10.1021/acs.est.5b05058. [DOI] [PubMed] [Google Scholar]
- Post G. B. Recent US State and Federal Drinking Water Guidelines for Per- and Polyfluoroalkyl Substances. Environ. Toxicol. Chem. 2021, 40, 550–563. 10.1002/etc.4863. [DOI] [PubMed] [Google Scholar]
- Hu X. C.; Dassuncao C.; Zhang X.; Grandjean P.; Weihe P.; Webster G. M.; Nielsen F.; Sunderland E. M. Can Profiles of Poly- and Perfluoroalkyl Substances (PFASs) in Human Serum Provide Information on Major Exposure Sources?. Environ. Health 2018, 17, 11 10.1186/s12940-018-0355-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng G.; Schreder E.; Dempsey J. C.; Uding N.; Chu V.; Andres G.; Sathyanarayana S.; Salamova A. Per- and Polyfluoroalkyl Substances (PFAS) in Breast Milk: Concerning Trends for Current-Use PFAS. Environ. Sci. Technol. 2021, 55, 7510–7520. 10.1021/acs.est.0c06978. [DOI] [PubMed] [Google Scholar]
- ECOS (Environmental Council of the States). State PFOS Fish Tissue Advisory Values, Compiled June 2020. https://www.ecos.org/wp-content/uploads/2020/09/PFOS-thresholds-in-state-fish-advisories-9_14_20B-ECOS.pdf.
- Esri. ″State Map″ [basemap]. Scale Not Given. “World Light Gray Base”. June, 2021, https://services.arcgisonline.com/ArcGIS/rest/services/Canvas/World_Light_Gray_Base/MapServer (accessed September 13, 2022).
- Burkhard L. P. Evaluation of Published Bioconcentration Factor (BCF) and Bioaccumulation Factor (BAF) Data for Per- and Polyfluoroalkyl Substances Across Aquatic Species. Environ. Toxicol. Chem. 2021, 40, 1530–1543. 10.1002/etc.5010. [DOI] [PubMed] [Google Scholar]
- United States Environmental Protection Agency (USEPA) EPA’s PBT Chemical Substances Initiative, 1998; Vol. 63.
- Chen M.; Zhu L.; Wang Q.; Shan G. Tissue Distribution and Bioaccumulation of Legacy and Emerging Per-and Polyfluoroalkyl Substances (PFASs) in Edible Fishes from Taihu Lake, China. Environ. Pollut. 2021, 268, 115887 10.1016/j.envpol.2020.115887. [DOI] [PubMed] [Google Scholar]
- Li Y.; Yao J.; Zhang J.; Pan Y.; Dai J.; Ji C.; Tang J. First Report on the Bioaccumulation and Trophic Transfer of Perfluoroalkyl Ether Carboxylic Acids in Estuarine Food Web. Environ. Sci. Technol. 2022, 56, 6046–6055. 10.1021/acs.est.1c00965. [DOI] [PubMed] [Google Scholar]
- Munoz G.; Mercier L.; Duy S. V.; Liu J.; Sauvé S.; Houde M. Bioaccumulation and Trophic Magnification of Emerging and Legacy Per- and Polyfluoroalkyl Substances (PFAS) in a St. Lawrence River Food Web. Environ. Pollut. 2022, 309, 119739 10.1016/j.envpol.2022.119739. [DOI] [PubMed] [Google Scholar]
- Ng C. A.; Hungerbühler K. Bioaccumulation of Perfluorinated Alkyl Acids: Observations and Models. Environ. Sci. Technol. 2014, 48, 4637–4648. 10.1021/es404008g. [DOI] [PubMed] [Google Scholar]
- Martin J. W.; Mabury S. A.; Solomon K. R.; Muir D. C. G. Bioconcentration and Tissue Distribution of Perfluorinated Acids in Rainbow Trout (Oncorhynchus Mykiss). Environ. Toxicol. Chem. 2003, 22, 196–204. 10.1002/etc.5620220126. [DOI] [PubMed] [Google Scholar]
- Inoue Y.; Hashizume N.; Yakata N.; Murakami H.; Suzuki Y.; Kikushima E.; Otsuka M. Unique Physicochemical Properties of Perfluorinated Compounds and Their Bioconcentration in Common Carp Cyprinus Carpio L. Arch. Environ. Contam. Toxicol. 2012, 62, 672–680. 10.1007/s00244-011-9730-7. [DOI] [PubMed] [Google Scholar]
- Lewis A. J.; Yun X.; Spooner D. E.; Kurz M. J.; McKenzie E. R.; Sales C. M. Exposure Pathways and Bioaccumulation of Per- and Polyfluoroalkyl Substances in Freshwater Aquatic Ecosystems: Key Considerations. Sci. Total Environ. 2022, 822, 153561 10.1016/j.scitotenv.2022.153561. [DOI] [PubMed] [Google Scholar]
- Charbonnet J. A.; McDonough C. A.; Xiao F.; Schwichtenberg T.; Cao D.; Kaserzon S.; Thomas K. V.; Dewapriya P.; Place B. J.; Schymanski E. L.; Field J. A.; Helbling D. E.; Higgins C. P. Communicating Confidence of Per- and Polyfluoroalkyl Substance Identification via High-Resolution Mass Spectrometry. Environ. Sci. Technol. Lett. 2022, 9, 473–481. 10.1021/acs.estlett.2c00206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch A.; Yukioka S.; Tanaka S.; Yeung L. W. Y.; Kärrman A.; Wang T. Characterization of an AFFF Impacted Freshwater Environment Using Total Fluorine, Extractable Organofluorine and Suspect per- and Polyfluoroalkyl Substance Screening Analysis. Chemosphere 2021, 276, 130179 10.1016/j.chemosphere.2021.130179. [DOI] [PubMed] [Google Scholar]
- Dubocq F.; Wang T.; Yeung L. W. Y.; Sjöberg V.; Kärrman A. Characterization of the Chemical Contents of Fluorinated and Fluorine-Free Firefighting Foams Using a Novel Workflow Combining Nontarget Screening and Total Fluorine Analysis. Environ. Sci. Technol. 2020, 54, 245–254. 10.1021/acs.est.9b05440. [DOI] [PubMed] [Google Scholar]
- Nickerson A.; Maizel A. C.; Kulkarni P. R.; Adamson D. T.; Kornuc J. J.; Higgins C. P. Enhanced Extraction of AFFF-Associated PFASs from Source Zone Soils. Environ. Sci. Technol. 2020, 54, 4952–4962. 10.1021/acs.est.0c00792. [DOI] [PubMed] [Google Scholar]
- Dassuncao C.; Hu X. C.; Nielsen F.; Weihe P.; Grandjean P.; Sunderland E. M. Shifting Global Exposures to Poly- and Perfluoroalkyl Substances (PFASs) Evident in Longitudinal Birth Cohorts from a Seafood-Consuming Population. Environ. Sci. Technol. 2018, 52, 3738–3747. 10.1021/acs.est.7b06044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conder J. M.; Hoke R. A.; Wolf W.; de Russell M. H.; Buck R. C. Are PFCAs Bioaccumulative? A Critical Review and Comparison with Regulatory Criteria and Persistent Lipophilic Compounds. Environ. Sci. Technol. 2008, 42, 995–1003. 10.1021/es070895g. [DOI] [PubMed] [Google Scholar]
- Muir D.; Bossi R.; Carlsson P.; Evans M.; De Silva A.; Halsall C.; Rauert C.; Herzke D.; Hung H.; Letcher R.; Rigét F.; Roos A. Levels and Trends of Poly- and Perfluoroalkyl Substances in the Arctic Environment – An Update. Emerging Contam. 2019, 5, 240–271. 10.1016/j.emcon.2019.06.002. [DOI] [Google Scholar]
- Skogheim T. S.; Villanger G. D.; Weyde K. V. F.; Engel S. M.; Surén P.; Øie M. G.; Skogan A. H.; Biele G.; Zeiner P.; Øvergaard K. R.; Haug L. S.; Sabaredzovic A.; Aase H. Prenatal Exposure to Perfluoroalkyl Substances and Associations with Symptoms of Attention-Deficit/Hyperactivity Disorder and Cognitive Functions in Preschool Children. Int. J. Hyg. Environ. Health 2020, 223, 80–92. 10.1016/j.ijheh.2019.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawabata K.; Matsuzaki H.; Nukui S.; Okazaki M.; Sakai A.; Kawashima Y.; Kudo N. Perfluorododecanoic Acid Induces Cognitive Deficit in Adult Rats. Toxicol. Sci. 2017, 157, 421–428. 10.1093/toxsci/kfx058. [DOI] [PubMed] [Google Scholar]
- Rayne S.; Forest K. A New Class of Perfluorinated Acid Contaminants: Primary and Secondary Substituted Perfluoroalkyl Sulfonamides Are Acidic at Environmentally and Toxicologically Relevant PH Values. J. Environ. Sci. Health, Part A 2009, 44, 1388–1399. 10.1080/10934520903217278. [DOI] [PubMed] [Google Scholar]
- Nouhi S.; Ahrens L.; Campos Pereira H.; Hughes A. V.; Campana M.; Gutfreund P.; Palsson G. K.; Vorobiev A.; Hellsing M. S. Interactions of Perfluoroalkyl Substances with a Phospholipid Bilayer Studied by Neutron Reflectometry. J. Colloid Interface Sci. 2018, 511, 474–481. 10.1016/j.jcis.2017.09.102. [DOI] [PubMed] [Google Scholar]
- Dassuncao C.; Hu X. C.; Zhang X.; Bossi R.; Dam M.; Mikkelsen B.; Sunderland E. M. Temporal Shifts in Poly- and Perfluoroalkyl Substances (PFASs) in North Atlantic Pilot Whales Indicate Large Contribution of Atmospheric Precursors. Environ. Sci. Technol. 2017, 51, 4512–4521. 10.1021/acs.est.7b00293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin T. A.; MacKillop E. A.; Meinick R. L.; Thayer K. A.; Seidler F. J. Developmental Neurotoxicity of Perfluorinated Chemicals Modeled in Vitro. Environ. Health Perspect. 2008, 116, 716–722. 10.1289/ehp.11253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J.; Gu W.; Barrett H.; Yang D.; Tang S.; Sun J.; Liu J.; Krause H. M.; Houck K. A.; Peng H. A Roadmap to the Structure-Related Metabolism Pathways of Per- and Polyfluoroalkyl Substances in the Early Life Stages of Zebrafish (Danio Rerio). Environ. Health Perspect. 2021, 129, 077004 10.1289/EHP7169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rericha Y.; Cao D.; Truong L.; Simonich M. T.; Field J. A.; Tanguay R. L. Sulfonamide Functional Head on Short-Chain Perfluorinated Substance Drives Developmental Toxicity. iScience 2022, 25, 103789 10.1016/j.isci.2022.103789. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.