Abstract
Aromatic amines such as ortho-toluidine (o-Tol), 2-aminonaphthalene (2-AN), and 4-aminobiphenyl (4-ABP) are human bladder carcinogens and occur at various workplaces, in ambient air, in food products, as well as in tobacco smoke. In a clinical study comprising a period of 74 h under confinement, we investigated the exposure to these three aromatic amines as well as to 3-aminobiphenyl (3-ABP) by measuring them in urine of habitual users of combustible cigarettes (CCs), electronic cigarettes (ECs), heated tobacco products (HTPs), oral tobacco (OT), and nicotine replacement therapy products (NRTs). Non-users (NU) of any tobacco/nicotine products served as (negative) control group. Smokers (CC) exhibited the highest levels for all four aromatic amines measured, significantly elevated compared to NU and non-CC users. Urinary levels in users of EC, HTP, NRT (mostly nicotine gum), and OT (mostly snus) were not significantly different from those in NU. Users of HTP showed slightly elevated urinary excretion levels of o-Tol, 3-ABP, and 4-ABP compared to some other non-CC groups. Dose markers such as daily consumption, urinary nicotine equivalents (Nequ), and plasma cotinine (CotP) were found to be consistently and significantly correlated with the excretion of aromatic amines for smokers (CC) only. Excretion levels of 3- and 4-ABP in smokers were significantly lower in the urine collected overnight compared to that collected during the day, which is just the opposite of what we observed for other biomarkers in this study. The possible reason for this observation is discussed. In conclusion, in contrast to smoking of CCs, the use of ECs, HTPs, nicotine gum, and oral tobacco was not observed to be associated with significant exposure to the aromatic amines o-Tol, 2-AN, 3-ABP, and 4-ABP. The observed slight increase in o-Tol, 3-ABP, and 4-ABP excretions in HTP users has to be verified in larger studies.
Introduction
Tobacco use, particularly smoking of combustible cigarettes (CCs), is associated with an increased risk for diseases such as cancer, cardiovascular (CVD), and chronic obstructive pulmonary diseases (COPD).1,2 Cigarette smoking is an established risk factor for bladder cancer with a relative risk of about 5 compared to nonsmokers.3 Aromatic amines have been identified as causally related to the induction of bladder tumors in numerous human and animal studies.4,5 In this biomonitoring study, we focus on the four aromatic amine ortho-toluidine (o-Tol), 2-aminonaphthalene (2-AN), 4-aminobiphenyl (4-ABP), and 3-aminobiphenyl (3-ABP), the first three being classified as human carcinogen (Group 1) and 3-ABP not evaluated by the International Agency for Research on Cancer (IARC).4,5 The chemical structures of these four aromatic amines are shown in Figure 1.
Figure 1.
Chemical structures of the four aromatic amines investigated in this study.
Apart from tobacco smoke, exposure sources for the general population are emissions from cooking oil, hair dyes, and heated food.4,5 Exposure to o-Tol occurs ubiquitously, mostly of unknown origin. The local anesthetic prilocaine is immediately metabolized to o-Tol, leading to high exposure levels in the patients.6
Reported mainstream smoke yield ranges (derived with the ISO smoking regime) of market cigarettes were 8.6–144.3, 1.47–14.06, and 0.30–2.31 ng/cig for o-Tol, 2-AN, and 4-ABP, respectively.7 Yields of 3-ABP in the mainstream smoke of reference cigarettes were found to be in the range of 0.7–1.8 ng/cig.8 Releases per cigarette of these aromatic amines into sidestream smoke are usually 20–40 times higher than into mainstream smoke.9 Increases in indoor air concentrations of 4-ABP and 2-AN due to environmental tobacco smoke (ETS) were reported to be <1 ng/m3, whereas those for o-Tol can reach 10 ng/m3.10,11 In a recent review of the literature,12 the median daily intakes of o-Tol, 2-AN, and 4-ABP by smoking combustible cigarettes (CC) were estimated to amount to 750.8, 121.7 and 25.1 ng/day, respectively. From the concentrations in indoor air, an ETS-related intake in nonsmokers of <10 ng/day (for 4-ABP and 2-AN) and about 100 ng/day for o-Tol can be deduced. Only few data are available for the release of aromatic amines from the next-generation tobacco/nicotine products so that no reliable daily intakes can be estimated.12 Biomonitoring results of these aromatic amines in the various groups will be discussed below. More recently published biomarker data for users of various types of tobacco/nicotine products13−16 are also considered in the comparison with our data performed in the Discussion section.
With respect to the metabolism of the four aromatic amines dealt with in this study, primarily two competing pathways are of interest: (i) N-oxidation to the N-hydroxylamine by means of the inducible CYP 1A2 or peroxidases (e.g., the prostaglandin H synthase), in the case of o-Tol CYP 2A6 or 2E1 play the main role; (ii) N-acetylation by means of the N-acetyl transferase 2 (NAT2).4,17−19 Both the free and the N-hydroxylated aromatic amines can be N-glucuronidated and are transported together with the N-acetylated metabolite from the liver via the bloodstream into the bladder and finally excreted with the urine. O-Acetylation (via NAT1), O-sulfatation (via the sulfotransferase SULT1A1), or acidic pH can lead to the release of highly reactive nitrenium ions, which can undergo tautomerization to carbenium ions; both ions are capable of forming DNA adducts. Acidic pH in the bladder may release free and N-hydroxylated aromatic amines; the former can be again oxidized to the N–OH metabolite by an urothelial peroxidase.18,20 Formation of the N-acetylated amines can be viewed as a detoxification pathway. A minor part of aromatic amines absorbed may also reach the bladder lumen in unchanged (free) form.21
As suitable biomarkers of exposure to the four aromatic amines of interest, hemoglobin adducts as well as the free and N-acetylated amines excreted in urine have been established (for an overview, see Turesky and Lemarchand22). Hemoglobin adduct formation is preceded by the co-oxidation of oxy-hemoglobin and the N-hydroxy-metabolite to met-hemoglobin and the nitroso aryl compound, respectively, the latter of which covalently binds to cysteny-SH groups in hemoglobin.23−25 The adduct levels are, therefore, a direct dosimeter for the proximate carcinogen (the N–OH aryl amine) formed from the absorbed parent compounds in the liver. Biomonitoring the exposure to aryl amines in urine assesses the N-acetyl amines (after alkaline hydrolysis only19) as well as the N-glucuronidated and free aryl amines in urine, i.e., that part of the dose which is not activated to the proximate carcinogen, which represents the largest part. All of the metabolites mentioned above can be excreted in urine; the applied analytical method using acid hydrolysis assesses the N-glucuronidated and the free form of the aromatic amines.19,26
The objective of this study was to compare the exposure to o-Tol, 2-AN, 3-ABP, and 4-ABP by urinary biomarkers in a controlled clinical study over 74 h under confinement in habitual users of combustible cigarettes (CCs), electronic cigarettes (ECs), heated tobacco products (HTPs), oral tobacco (OT, primarily snus), and NRTs (nicotine replacement therapy products, primarily nicotine gum). As a (negative) control group, non-users (NU) of these products were included. In addition, the dose dependence of these biomarkers is studied in all user groups. Results were compared with corresponding published data.
Materials and Methods
Study Design and Biological Samples Collected for Analysis
A controlled, single-center, open-label trial was conducted. Five nicotine product user groups, including reportedly exclusive users of CC, EC, HTP, OT, and NRT with a control group of non-users (NU) recruited. The study design and the study population were described in a previous publication.27 The study protocol has been approved by the authorized ethics committee (Medical Association Hamburg, Germany). The purpose of the study was to identify specific biomarkers or biomarker profiles for the users of various tobacco/nicotine products by means of targeted and untargeted analytical methods. Briefly, 10 subjects per group, which were self-reported exclusive users of the respective products for at least 6 months, were confined for 74 h to a clinic. Product use (own brands, described in more detail elsewhere28,29) was allowed between 8 am and midnight during the subjects’ stay in the clinic.
On each study day, blood samples were collected at 7 am and 5 pm, starting in the evening of Day −1, when the subjects were admitted to the clinic. Throughout the course of the study, all urine voids were collected separately. For each void, the total volume and the time were recorded. For the analytical determinations, urine fractions were pooled, yielding six urine samples for each subject, three collected overnight (comprising 14 h) and three collected during the day (comprising 10 h). The “overnight” urine was collected from about 5 pm to 7 am (including the first morning urine) of the following day. The “during-the-day” urine was collected between 7 am (after the first morning urine) and 5 pm of the same day. A collection scheme for the biological samples has been published previously.28−30
Analytical Methods
The aromatic amines o-Tol, 2-NA, 3-ABP, and 4-ABP in urine were determined by means of gas chromatography/mass spectrometry (GC-MS) as described previously,31 with modifications. Briefly, to 5 mL of urine, the internal standards o-Tol-D9, 2-AN-13C6, and 4-ABP-D9 were added. The isotopic purity of labeled internal standards was >99%. The mixture was acidified with 1 mL of hydrochloric acid (37%) and hydrolyzed (1 h, 80 °C). After adjusting the pH to 6.0–6.4 by adding 1.25 mL of 10 N sodium hydroxide solution and 3 mL of 2-(N-morpholino)-ethanesulfonic acid buffer pH 6.0, the mixture was extracted twice with 5 mL of n-hexane. The combined extracts were dried over sodium sulfate and derivatized with 50 μL of pentafluoropropionic acid anhydride and 25 μL of pyridine (1 h, 80–85 °C). The derivatization mixture was washed with 3 mL of phosphate buffer (pH 8). After addition of 200 μL of toluene as keeper, the organic phase is reduced to 50 μL by means of a SpeedVac centrifuge. GC-MS analysis with negative-ion chemical ionization (NICI) was performed as described in the literature.31 The actual device was a DSQ GC-MS instrument (Thermo Scientific, Dreieich, Germany). An Optima 35 MS, 60 m, 0.25 mm ID, 0.25 μm df (Macherey-Nagel, Düren, Germany) was used as an analytical column. The quantifier ions used were m/z 233, 240, 269, 275, 295, 295, and 304 for o-Tol, 2-Tol-D9, 2-AN, 2-AN-13C6, 3-ABP, 4-ABP, and 4-ABP-D9, respectively. No qualifiers ions were available for the four analytes at the concentration ranges of interest. Limit of detection (LOD)/limit of quantification were: 0.8:10 (o-Tol), 0.6:1.7 (2-AN), 0.5:1.3 (3-ABP), and 0.5:1.5 (4-ABP) ng/L.
Urinary nicotine equivalents (Nequ), comprising the molar sum of nicotine and its 10 major metabolites, namely, cotinine, trans-3′-hydroxycotinine, nicotine-N-glucuronide, cotinine-N-glucuronide, trans-3′-hydroxycotinine-N,O-glucuronide, 4-OH-4-(3-pyridyl)-butanoic acid, nornicotine (NN), norcotinine, nicotine-N′-oxide, and cotinine-N-oxide, were determined by liquid chromatography with tandem mass spectrometry (LC-MS/MS) according to a published method,32 with modifications.28
Plasma cotinine was determined by an LC-MS/MS method as described earlier.33
All methods were fully validated according to FDA guidelines.34
Statistical Evaluation
Normal distribution tests of Shapiro–Wilk, D’Agostino & Pearson, and Kolmogorov–Smirnov were applied. Since the concentrations of the aromatic amines were mostly not normally distributed, the nonparametric Mann–Whitney U test (comparison of two groups) and Kruskal–Wallis–ANOVA (comparison of multiple groups) was used to determine statistical significances between the groups. The nonparametric Spearman rank test was utilized calculating correlations. p-Values of <0.05 were rated as significant. GraphPad Prism Software (San Diego, CA), Version 9.2.0 was utilized for statistical analysis and generation of graphs.
Results
Sixty (60) subjects completed the clinical study, 10 in each of the six groups (NU, CC, EC, HTP, NRT, OT). In all groups, the number of males and females were balanced, except for OT users, for which sex distribution was 9:1 (m/f). Group means for age ranged from 28.1 to 36.1 years and were not statistically different between groups. The reported average daily consumption prior to the study for users of CC, EC, HTP, NRT, and OT was 16.1 cigarettes, 9.75 mL of e-liquid, 15.5 sticks, 8.3 nicotine gums, and 6.9 g (mainly snus), respectively.
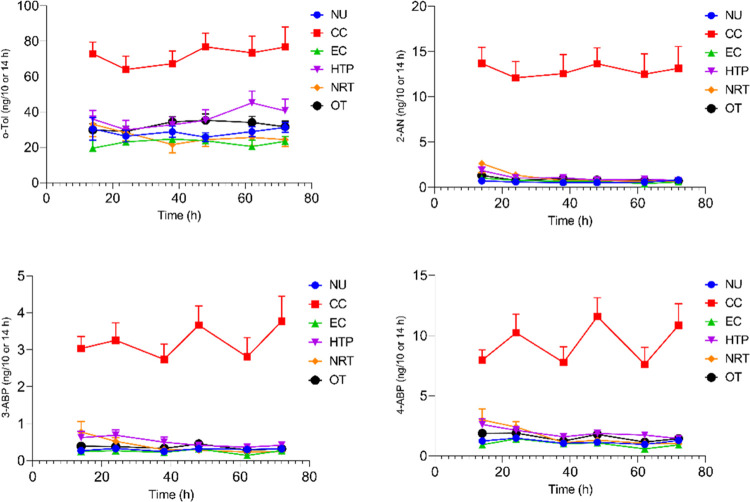
The time courses of the urinary excretion of o-Tol, 2-AN, 3-ABP, and 4-ABP for the six study groups during the confinement period of the study (74 h) are shown in Figure 2. Smokers (CC) exhibited the highest mean levels for all four aromatic amines at all time points. 3-ABP and 4-ABP excretions in the night and day urine fractions of smokers (CC) showed a characteristic pattern with lower levels in the urine fractions collected overnight compared to those collected during the day. This is notably different from all urinary biomarkers of exposure in smokers (CC), where a reverse pattern (if any) was observed.28,29,35 Reasons for this phenomenon are discussed below. Excretion levels for the five other groups (non-CC users including NU) were found to be similar with slight elevations in some urine fractions of all four aromatic amines in HTP users (Figure 2).
Figure 2.
Time courses of urinary excretion of o-toluidine (o-Tol), 2-aminonaphthalene (2-AN), 3-aminobiphenyl (3-ABP), and 4-aminobiphenyl (ABP) by groups over the confined study period of 31:2 days. Symbols and error bars in graphs represent means and standard errors of the means (SEMs), respectively. Time 0 h corresponds to about 5 pm or study Day −1 (admission to the clinic).
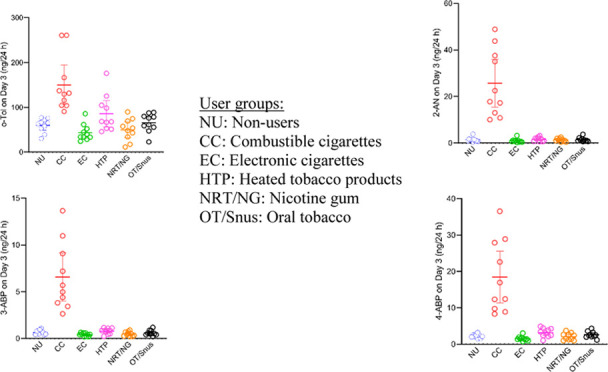
Since the biomarker data for Day 3 of the study can be regarded as those obtained under the most controlled conditions in terms of product use and other environmental factors as well as food intake, results for 24 h urine excretions of Day 3 were used for further evaluations. Descriptive statistics for the four aromatic amines are shown in Table 1. The amounts excreted by smokers (CC) on Day 3 were found to be significantly higher than in the non-CC groups, including non-users (NU). HTP users were found to excrete slightly but significantly higher amounts of o-Tol than vapers (EC) and NRT users, as well as higher amounts of 3-ABP and 4-ABP compared to vapers (EC). The difference in excretion rates between OT and EC users was statistically significant for o-Tol and 4-ABP. It should be noted that an appreciable number of samples (10–15 of 20) were found to be <LOD for 3-ABP for the non-CC groups (Table 1).
Table 1. Descriptive Statistics of Four Aromatic Amines Excreted in the 24 h Urine of Day 3 by User/Non-User Groupsa.
| biomarker | NU | CC | EC | HTP | NRT | OT | |
|---|---|---|---|---|---|---|---|
| o-Tol (ng/24 h) | <LOD, n of Nb | 0 of 20 | 0 of 20 | 0 of 20 | 0 of 20 | 0 of 20 | 0 of 20 |
| mean ± SD | 60.2 ± 15.4 | 150.2 ± 61.9 | 43.9 ± 18.6 | 85.8 ± 40.9 | 50.0 ± 24.8 | 65.7 ± 20.6 | |
| median (IQR)c | 64.2 (49.8–73.1) | 130.7 (104.5–190.1) | 37.2 (32.2–54.6) | 68.3 (52.7–109.8) | 51.1 (30.0–70.9) | 68.6 (53.6–81.9) | |
| min–max | 30.4–77.2 | 91.0–260.7 | 24.3–85.9 | 45.8–176.0 | 11.4–89.9 | 22.9–88.9 | |
| different fromd | CC | NU, EC, HTP, NRT, OT | CC, HTP, OT | CC, EC, NRT | CC, HTP | CC, EC | |
| 2-AN (ng/24 h) | <LOD, n of Nb | 8 of 20 | 0 of 20 | 9 of 20 | 7 of 20 | 3 of 20 | 6 of 20 |
| mean ± SD | 1.4 ± 1.0 | 25.7 ± 14.4 | 1.0 ± 0.8 | 1.7 ± 0.9 | 1.3 ± 0.7 | 1.5 ± 0.9 | |
| median (IQR)c | 1.1 (0.8–1.6) | 20.1 (12.5–39.1) | 0.8 (0.5–1.1) | 1.8 (0.7–2.2) | 1.2 (0.7–2.0) | 1.2 (0.8–1.9) | |
| min–max | 0.4–3.9 | 10.0–48.9 | 0.2–3.2 | 0.6–3.2 | 0.4–2.5 | 0.6–3.7 | |
| different fromd | CC | NU, EC, HTP, NRT, OT | CC | CC | CC | CC | |
| 3-ABP (ng/24 h) | <LOD, n of Nb | 12 of 20 | 0 of 20 | 15 of 20 | 10 of 20 | 12 of 20 | 11 of 20 |
| mean ± SD | 0.6 ± 0.3 | 6.6 ± 3.6 | 0.4 ± 0.2 | 0.8 ± 0.3 | 0.5 ± 0.2 | 0.6 ± 0.3 | |
| median (IQR)c | 0.6 (0.4–0.8) | 5.3 (3.7–9.5) | 0.4 (0.2–0.6) | 0.9 (0.5–1.1) | 0.5 (0.3–0.7) | 0.5 (0.4–0.9) | |
| min–max | 0.4–1.1 | 2.6–13.7 | 0.2–0.7 | 0.2–1.2 | 0.2–0.9 | 0.2–1.2 | |
| different fromd | CC | NU, EC, HTP, NRT, OT | CC, HTP | CC, EC | CC | CC | |
| 4-ABP (ng/24 h) | <LOD, n of Nb | 0 of 20 | 0 of 20 | 2 of 20 | 1 of 20 | 2 of 20 | 0 of 20 |
| mean ± SD | 2.3 ± 0.5 | 18.5 ± 9.9 | 1.5 ± 0.6 | 3.2 ± 1.2 | 2.1 ± 1.0 | 2.6 ± 0.9 | |
| median (IQR)c | 2.3 (1.9–3.2) | 14.7 (9.5–28.2) | 1.4 (1.2–1.7) | 3.2 (2.3–4.3) | 2.0 (1.2–2.8) | 2.5 (2.1–3.2) | |
| min–max | 1.5–3.2 | 8.3–36.5 | 0.8–3.0 | 1.0–4.9 | 1.0–3.7 | 1.2–4.3 | |
| different fromd | CC | NU, EC, HTP, NRT, OT | CC, HTP, OT | CC, EC | CC | CC, EC |
24 h Urine of Day 3: results were calculated from the last two urine fractions shown in the time courses of Figure 2.
n = number of samples <LOD of all evaluated samples (N) in a group; note that the 24 h urine of Day 3 is the sum of two urine fractions which were analyzed separately; for the group statistics, values <LOD were set to 0.5 × LOD.
IQR: interquartile range (25th–75th percentile).
Statistically significant differences (ANOVA, Kruskal–Wallis test, p < 0.05) to the other groups are indicated.
The correlations between the amounts of the aromatic amines excreted on Day 3 and three different dose markers for product use on that study day are shown in Table 2. The dose markers were the recorded consumption and amount of nicotine equivalents (Nequ) excreted on Day 3 as well as plasma cotinine concentrations at 5 pm on Day 3. For smokers (CC), all dose–response relationships were found to be highly significant (p < 0.01) with Spearman correlation coefficients in the range of 0.86–0.95. For the other user groups, no consistent associations between the dose markers and the excretion of aromatic amines were observed (Table 2).
Table 2. Spearman Correlation Coefficients between Four Aromatic Amines and Three Dose Markers on Day 3 for Users of five Tobacco/Nicotine Products (Statistically Significant Correlations Coefficients Are Highlighted in Gray).
| CC |
EC |
HTP |
NRT |
OT |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| biomarker | Consa | Nequb | CotPc | Consa | Nequb | CotPc | Consa | Nequb | CotPc | Consa | Nequb | CotPc | Consa | Nequb | CotPc |
| o-Tol (ng/day) | 0.94*** | 0.95*** | 0.87** | 0.22 | 0.01 | –0.44 | 0.48 | 0.72* | 0.20 | 0.15 | 0.49 | –0.15 | 0.43 | 0.33 | 0.06 |
| 2-AN (ng/day) | 0.92*** | 0.95*** | 0.93*** | –0.23 | –0.41 | –0.57 | 0.56(*) | 0.68* | 0.27 | 0.78* | 0.87** | 0.64 | 0.10 | 0.19 | –0.27 |
| 3-ABP (ng/day) | 0.86** | 0.93*** | 0.86** | 0.26 | 0.21 | 0.08 | 0.62(*) | 0.54 | 0.38 | 0.77* | 0.48 | 0.29 | –0.14 | 0.27 | –0.15 |
| 4-ABP (ng/day) | 0.92** | 0.92** | 0.90** | 0.37 | 0.22 | –0.25 | 0.50 | 0.36 | 0.16 | 0.58 | 0.69 | 0.39 | –0.05 | 0.10 | –0.21 |
Consumption recorded for Day 3: CC: cig/day, EC: mL of e-liquid/day (average of 3 study days), HTP: sticks/day, NRT: pieces of nicotine gum/day (N = 8) of 10, 2 subjects used nicotine inhalators, OT: g/day.
Nicotine equivalents (Nequ) excreted on Day 3, mg/day.
Cotinine in plasma, plasma sample derived from the blood samples drawn on Day 3 at 5 pm, ng/mL.
Statistical significance levels: *p < 0.05; **p < 0.01; ***p < 0.001; (*) borderline significance, p < 0.10.
Discussion
The presented results are part of a research project on biomarkers of exposure to be analyzed by targeted and untargeted methods in various biological matrices in users of five tobacco/nicotine products.27 The project aims to distinguish different nicotine user groups by either specific biomarkers or biomarker patterns. In this paper, results for urinary biomarkers of four aromatic amines (o-Tol, 2-AN, 3-ABP, 4-ABP) are presented. To date, results from this study for exposure and uptake of nicotine,28 tobacco-specific nitrosamines (TSNA),29 polycyclic aromatic hydrocarbons (PAH),35 benzo[a]pyrene,36 1,2-propylene glycol, and glycerol30 have been published. Results for 15 mercapturic acids have been submitted for publication. Furthermore, an evaluation of literature data on the intake and uptake of 38 chemicals (including o-Tol, 2-AN, and 4-ABP) in the same user/non-user groups as in this investigation has been published.12
The toxicological importance of the aromatic amines studied (o-Tol, 2-AN, and 4-ABP are human bladder carcinogens, 3-ABP has not been evaluated by the IARC)4,5 is out of question. Our results clearly show that smoking cigarettes (CC) dose-dependently increased exposure to these four aromatic amines. Users of other tobacco/nicotine products cannot be differentiated from NU in terms of urinary excretion of these aromatic amines (Figure 2 and Table 1). The slightly elevated levels observed of o-Tol, 3-ABP, and 4-ABP in users of HTP compared to some other non-CC groups require verification in studies with larger group sizes.
Our biomarkers results for aromatic amines are compatible with the release data of the five tobacco/nicotine products investigated, already briefly described in the Introduction section. Excretion of o-Tol in smokers (CC) on Day 3 was in the range of 91.0–260.7 (mean: 150.2) ng/24 h (Table 1). This is overall in good agreement with a series of other studies.31,37−46 NU of any tobacco/nicotine products in our study showed o-Tol excretion rates in the range of 30.4–77.2 (mean: 60.2) ng/24 h (Table 1). Agreement with reported o-Tol excretions in nonsmokers is acceptable.31,37−44 Urinary o-Tol levels in NU confirm the relatively high and diffuse background exposure of the general population to this aromatic amine.4,5 In some earlier studies, significantly higher o-Tol concentrations in urine (μg/L range) were reported in nonoccupationally exposed smokers and nonsmokers, with no significant differences observed between the two groups.47−49 Possibly, there were issues with the specificity of the analytical methods applied in these studies. Alkaline hydrolysis, used in some studies47,49 can also split the N-acetyl metabolites excreted in urine and thus increases the measurable level of aromatic amines in urine. Other factors were discussed elsewhere.31o-Tol released from HTP was reported to be in the range of 0.4–1.3 ng/stick.50−52 For the other non-CC products (EC, NRT, OT), no data for the release of o-Tol were available. The urinary o-Tol excretion rates, which we observed for HTP users (Figure 2 and Table 1), suggest that there might be some exposure to this chemical in this group. This, however, has to be verified in larger studies.
Excretion of 2-AN by smokers (CC) on Day 3 ranged from 10.0 to 48.9 (mean: 25.7) ng/24 h (Table 1). Corresponding values for NU were found to be 0.4–3.9 (mean: 1.4) ng/24 h. These levels are in line with the medians of 27.6 and 3.5 ng/24 h for smokers and nonsmokers, respectively, reported in a recent literature evaluation.12 Only trace amounts of 2-AN (<0.1 ng/unit) were found to be released by EC53 and HTP,50−52,54 whereas no data for the release of 2-AN were available for NRT and OT. The product release data are compatible with our observation that excretion of 2-AN in the users of non-CC products was not distinguishable from NU (Figure 2 and Table 1).
On Day 3 of the study, smokers (CC) excreted 8.3–36.5 (mean: 18.5) ng/24 h of 4-ABP, whereas excretion levels for NU were 1.5–3.2 (mean: 2.3) ng/24 h (Table 1). Again, the excretion rates were in good agreement with median levels of 20.9 and 2.4 ng/24 h for smokers and nonsmokers, respectively, obtained in a literature review.12 Only levels <LOD or trace amounts of 4-ABP were reported to be released by EC53 and HNB,50−52,54 whereas no data for the release of 4-ABP were available for NRT and OT. Product release data of 4-ABP are in line with our observation that excretion of 4-ABP in the users of non-CC products (EC, HTP, NRT, and OT) were not different from NU (Figure 2 and Table 1).
We found an excretion rate of 3-ABP in the range of 2.6–13.7 (mean: 6.6) ng/24 h on Day 3 in smokers (CC) and 0.4–1.1 (mean: 0.6) ng/24 h in NU (Table 1). In another study, mean excretion of 5.39 and 1.11 ng/24 h for smokers and nonsmokers, respectively, were reported, which is in good agreement with our findings.55 Release of 3-ABP from CC was reported to amount to 3.5–4.2 ng/cig.50,51,54 In HTP, 3-ABP release was found to be at or below the LOQ of about 0.03 ng/stick.50,51,54 No release data of 3-ABP for the other products were available. Our findings of urinary excretion of 3-ABP (Figure 2 and Table 1) are, therefore, in line with product release data of 3-ABP in the literature.
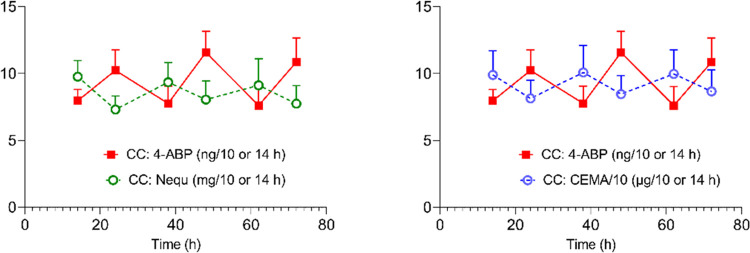
The unusual excretion pattern of 3- and 4-ABP in smokers (CC) was already mentioned in the Results section (Figure 2). All biomarkers which exhibited day versus night excretion rates in smokers (CC) showed higher levels in the fraction collected overnight compared to that collected during the day.28,29,35 We explained this by the fact that about 15% more cigarettes were consumed in the time period between 3 pm and midnight (which is mainly reflected in the amounts of biomarkers in the urine fraction collected overnight and completed with the first morning urine) than in the time period between 7 am until 5 pm (when smoking was allowed and is mainly reflected in the urine fractions collected during the day).28 This is illustrated in Figure 3, which shows the time patterns of 4-ABP in comparison to those of nicotine equivalents and 2-cyanoethyl-mercaturic acid (CEMA, a biomarker for exposure to acrylonitrile). How can this divergence, which is unique for 3- and 4-ABP, be explained?
Figure 3.
Time courses of urinary excretion of 4-aminobiphenyl (4-ABP) in smokers (CC) compared to nicotine equivalents (left) and 2-cyanoethyl-mercapturic acid (CEMA) over the confined study period of 31/2 days. Symbols and error bars in graphs represent means and standard errors of the means (SEMs), respectively. Note that the time course for 4-ABP is reverse to that of Nequ and CEMA. Time 0 h corresponds to about 5 pm or study Day −1 (admission to the clinic).
A possible explanation for this observation might be the fact that bladder content remained for longer in the overnight urine fraction than in the urine fraction collected during the day (because of more urine voids taking place during the day). The longer residence time in the overnight urine fraction increases the probability that the free 4-ABP is reabsorbed through the urothelial wall or N-hydroxylated by the urothelial cells.18,21 In addition, the N-glucuronide of 4-ABP might easily release the free form under acidic conditions. Since both the free and the N-glucuronidated forms are particularly assessed by our analytical method, this would lead to a lower level of measurable 4-ABP in the overnight urine fraction, as we observed. These considerations are compatible with a pharmacokinetic model to predict the exposure of the bladder epithelium to urinary N-hydroxyarylamine carcinogens as a function of urine pH, voiding interval, and resorption, published 40 years ago.56 This finding is also in line with a study, in which dogs were dosed with radio-labeled 4-ABP, showing that less frequent urination and, to a lesser extent, acidic pH in the bladder lumen, increased the level of 4-ABP-DNA adducts in the urothelium.21 To our knowledge, our data provide experimental evidence that these processes also are relevant for humans exposed to aromatic amines. We, however, can provide no explanation, why 2-NA and o-Tol did not show a similar urinary excretion pattern to 3- and 4-aminobiphenyl. If our hypothesis for explaining the unusual time course of 3- and 4-ABP is appropriate, this would imply that infrequent urination (occurring, for example, overnight) leads to an underestimation of the exposure by our method and, more critical, increases the bladder cancer risk since the formation of the procarcinogen (N-hydroxy-4-ABP) can increase. However, as long as no further evidence is available, these considerations have to be classified as speculative.
Conclusions
Our results derived from a clinical study with habitual users of CC, EC, HTP, NRT, and OT show significantly elevated urinary levels for the aromatic amines o-Tol, 2-AN, 3-ABP, and 4-ABP in smokers (CC) compared to all other groups. We observed a slight increase in o-Tol, 3-ABP, and 4-ABP excretion in HTP users compared to the other non-CC groups. This observation requires verification by larger studies. In contrast to other biomarkers determined in this study, excretion levels of 3- and 4-ABP in smokers (CC) were lower in the urine fraction collected overnight compared to the fraction collected during the day. We hypothesize that this is due to less frequent voiding in the former compared to the latter time period. We suggest that a lower frequency of urination can increase the risk of bladder cancer.
Acknowledgments
The authors thank Filip Sibul for helping organize and monitor the conductance of the clinical study and Marta Latawiec for her technical assistance in performing the analysis of aromatic amines. They also thank Janina Mütze for conducting the analysis of nicotine equivalents in urine and cotinine in plasma.
Author Contributions
M.S., G.S., and N.P. contributed to conceptualization. K.R. conducted formal analysis. G.S. involved in writing—original draft preparation. M.S., N.P., and K.R. contributed to writing—review and editing. M.S. and N.P. performed supervision. M.S. involved in project administration. M.S. and N.P. contributed to funding acquisition. All authors have read and agreed to the published version of the manuscript.
This study was funded with a grant from the Foundation for a Smoke-Free World (FSFW), a US nonprofit 501(c)(3) private foundation. FSFW had no role in the planning and execution of this study, data analysis, and publication of the results. The Foundation accepts charitable gifts from PMI Global Services Inc. (PMI); under the Foundation’s Bylaws and Pledge Agreement with PMI, the Foundation is independent of PMI and the tobacco industry.
The authors declare no competing financial interest.
Notes
The authors declare no conflict of interest. The funders had no role in the design of the study; collection, analyses, or interpretation of data; writing of the manuscript; and the decision to publish the results.
References
- US Department of Health and Human Services, 1986, The Healh Consequences of Using Smokeless Tobacco. A Report of the Surgeon General. http://resource.nlm.nih.gov/101584932X65.
- US Department of Health and Human Services . How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease. A Report of the Surgeon General; National Library of Medicine Cataloging in Publication: Rockville, MD, USA, 2010, https://www.ncbi.nlm.nih.gov/books/NBK53017/. [Google Scholar]
- International Agency for Research on Cancer (IARC) . Tobacco smoke and involuntary smoking; IARC Press: Lyon, France, 2004; Vol. 83. [Google Scholar]
- International Agency for Research of Cancer (IARC) . Some Aromatic Amines, Organic Dyes, and Related Exposures; IARC Press: Lyon, France, 2010; Vol. 99. [Google Scholar]
- International Agency for Research on Cancer (IARC) . Chemical Agents and Related OccupationsIARC Monographs. 100F; IARC Press: Lyon, France, 2012; Vol. 100F. [Google Scholar]
- Gaber K.; Harréus U. A.; Matthias C.; Kleinsasser N. H.; Richter E. Hemoglobin adducts of the human bladder carcinogen o-toluidine after treatment with the local anesthetic prilocaine. Toxicology 2007, 229, 157–164. 10.1016/j.tox.2006.10.012. [DOI] [PubMed] [Google Scholar]
- Stabbert R.; Schafer K. H.; Biefel C.; Rustemeier K. Analysis of aromatic amines in cigarette smoke. Rapid Commun. Mass Spectrom. 2003, 17, 2125–2132. 10.1002/rcm.1161. [DOI] [PubMed] [Google Scholar]
- Ji H.; Jin Z. Analysis of six aromatic amines in the mainstream smoke of tobacco products. Anal Bioanal Chem 2022, 414, 4227. 10.1007/s00216-022-04075-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klus H.; Kuhn H. Verteilung verschiedener Tabakrauchbestandteile auf Haupt- und Nebenstromrauch (Eine Übersicht). Beitr. Tabakforsch. Int. 1982, 11, 229–265. [Google Scholar]
- Palmiotto G.; Pieraccini G.; Moneti G.; Dolara P. Determination of the levels of aromatic amines in indoor and outdoor air in Italy. Chemosphere 2001, 43, 355–361. 10.1016/S0045-6535(00)00109-0. [DOI] [PubMed] [Google Scholar]
- Tricker A. R.; Schorp M. K.; Urban H. J.; Leyden D.; Hagedorn H. W.; Engl J.; Urban M.; Riedel K.; Gilch G.; Janket D.; Scherer G. Comparison of Environmental Tobacco Smoke (ETS) Concentrations Generated by an Electrically Heated Cigarette Smoking System and a Conventional Cigarette. Inhalation Toxicol. 2009, 21, 62–77. 10.1080/08958370802207334. [DOI] [PubMed] [Google Scholar]
- Scherer G.; Pluym N.; Scherer M. Intake and Uptake of Chemicals Upon Use of Various Tobacco/Nicotine Products: Can Users be Differentiated by Single or Combinations of Biomarkers?. Contrib. Tob. Nicotine Res. 2021, 30, 167–198. 10.2478/cttr-2021-0014. [DOI] [Google Scholar]
- Haziza C.; de La Bourdonnaye G.; Donelli A.; Poux V.; Skiada D.; Weitkunat R.; Baker G.; Picavet P.; Lüdicke F. Reduction in exposure to selected harmful and potentially harmful constituents approaching those observed upon smoking abstinence in smokers switching to the menthol tobacco heating system 2.2 for 3 months (Part 1). Nicotine Tob. Res. 2020, 22, 539–548. 10.1093/ntr/ntz013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosilkovska M.; Tran C. T.; de La Bourdonnaye G.; Taranu B.; Benzimra M.; Haziza C. Exposure to harmful and potentially harmful constituents decreased in smokers switching to Carbon-Heated Tobacco Product. Toxicol. Lett. 2020, 330, 30–40. 10.1016/j.toxlet.2020.04.013. [DOI] [PubMed] [Google Scholar]
- Yuki D.; Kikuchi A.; Suzuki T.; Sakaguchi C.; Huangfu D.; Nagata Y.; Kakehi A.. Assessment of the Exposure to Selected Smoke Constituents in Adult Smokers Using In-market Heated Tobacco Products: A Randomized, Controlled Study 2022 10.21203/rs.3.rs-1575780/v1. [DOI] [PMC free article] [PubMed]
- Gale N.; McEwan M.; Hardie G.; Proctor C. J.; Murphy J. Changes in biomarkers of exposure and biomarkers of potential harm after 360 days in smokers who either continue to smoke, switch to a tobacco heating product or quit smoking. Intern. Emerg. Med. 2022, 17, 2017–2030. 10.1007/s11739-022-03062-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter E.; Pfau W.. Aromatische Amine, Nitroaromaten und Beterozylische Aromatische Amine. In Lehrbuch der Toxikologie; Marquardt H.; Schäfer S., Eds.; Wissenschaftliche Verlagsgesellschft mbH: Stuttgart, 2004; pp 731–745. [Google Scholar]
- Talaska G.; Al Zoughool M. Aromatic amines and biomarkers of human exposure. J. Environ. Sci. Health, Part C 2003, 21, 133–164. 10.1081/GNC-120026234. [DOI] [PubMed] [Google Scholar]
- Sabbioni G.; Jones C. R. Biomonitoring of arylamines and nitroarenes. Biomarkers 2002, 7, 347–421. 10.1080/13547500210147253. [DOI] [PubMed] [Google Scholar]
- Babu S. R.; Lakshmi V. M.; Huang G. P.-W.; Zenser T. V.; Davis B. B. Glucuronide conjugates of 4-aminobiphenyl and its N-hydroxy metabolites: pH stability and synthesis by human and dog liver. Biochem. Pharmacol. 1996, 51, 1679–1685. 10.1016/0006-2952(96)00165-7. [DOI] [PubMed] [Google Scholar]
- Kadlubar F. F.; Dooley K. L.; Teitel C. H.; Roberts D. W.; Benson R. W.; Butler M. A.; Bailey J. R.; Young J. F.; Skipper P. W.; Tannenbaum S. R. Frequency of urination and its effects on metabolism, pharmacokinetics, blood hemoglobin adduct formation, and liver and urinary bladder DNA-adduct levels in beagle dogs given the carcinogen 4-aminobiphenyl. Cancer Res. 1991, 51, 4371–4377. [PubMed] [Google Scholar]
- Turesky R. J.; Le Marchand L. Metabolism and Biomarkers of Heterocyclic Aromatic Amines in Molecular Epidemiology Studies: Lessons Learned from Aromatic Amines. Chem. Res. Toxicol. 2011, 24, 1169–1214. 10.1021/tx200135s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiese M.Methemoglobinemia: A Comprehensive Treatise; CRC-Press: Cleveland, Ohio, 1974. [Google Scholar]
- Neumann H. G. Biomonitoring of aromatic amines and alkylating agents by measuring hemoglobin adducts. Int. Arch. Occup. Environ. Health 1988, 60, 151–155. 10.1007/BF00378690. [DOI] [PubMed] [Google Scholar]
- Green L. C.; Skipper P. L.; Turesky R. J.; Bryant M. S.; Tannenbaum S. R. In vivo dosimetry of 4-aminobiphenyl in rats via a cysteine adduct in hemoglobin. Cancer Res. 1984, 44, 4254–4259. [PubMed] [Google Scholar]
- Weiss T.; Angerer J. Simultaneous determination of various aromatic amines and metabolites of aromatic nitro compounds in urine for low level exposure using gas chromatography-mass spectrometry. J. Chromatogr. B 2002, 778, 179–192. 10.1016/S0378-4347(01)00542-4. [DOI] [PubMed] [Google Scholar]
- Sibul F.; Burkhardt T.; Kachhadia A.; Pilz F.; Scherer G.; Scherer M.; Pluym N. Identification of biomarkers specific to five different nicotine product user groups: Study protocol of a controlled clinical trial. Contemp. Clin. Trials Commun. 2021, 22, 100794 10.1016/j.conctc.2021.100794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer G.; Mutze J.; Pluym N.; Scherer M. Assessment of nicotine delivery and uptake in users of various tobacco/nicotine products. Curr. Res. Toxicol. 2022, 3, 100067 10.1016/j.crtox.2022.100067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer G.; Scherer M.; Mutze J.; Hauke T.; Pluym N. Assessment of the Exposure to Tobacco-Specific Nitrosamines and Minor Tobacco Alkaloids in Users of Various Tobacco/Nicotine Products. Chem. Res. Toxicol. 2022, 35, 684–693. 10.1021/acs.chemrestox.2c00020. [DOI] [PubMed] [Google Scholar]
- Burkhardt T.; Pluym N.; Scherer G.; Scherer M. 1,2-Propylene Glycol: A Biomarker of Exposure Specific to e-Cigarette Consumption. Separations 2021, 8, 180 10.3390/separations8100180. [DOI] [Google Scholar]
- Riedel K.; Scherer G.; Engl J.; Hagedorn H. W.; Tricker A. R. Determination of three carcinogenic aromatic amines in urine of smokers and nonsmokers. J. Anal. Toxicol. 2006, 30, 187–195. 10.1093/jat/30.3.187. [DOI] [PubMed] [Google Scholar]
- Piller M.; Gilch G.; Scherer G.; Scherer M. Simple, fast and sensitive LC-MS/MS analysis for the simultaneous quantification of nicotine and 10 of its major metabolites. J. Chromatogr. B 2014, 951–952, 7–15. 10.1016/j.jchromb.2014.01.025. [DOI] [PubMed] [Google Scholar]
- Scherer G.; Engl J.; Urban M.; Gilch G.; Janket D.; Riedel K. Relationship between machine-derived smoke yields and biomarkers in cigarette smokers in Germany. Regul. Toxicol. Pharmacol. 2007, 47, 171–183. 10.1016/j.yrtph.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Food and Drug Administration (FDA) . Bioanalytical Method Validation - Guidance for Industry, 2018. https://www.fda.gov/files/drugs/published/Bioanalytical-Method-Validation-Guidance-for-Industry.pdf.
- Scherer G.; Scherer M.; Rögner N.; Rögner N.; Pluym N. Assessment of the exposure to polycyclic aromatic hydrocarbons in users of various tobacco/nicotine products by suitable urinary biomarkers. Arch. Toxicol. 2022, 96, 3113–3126. 10.1007/s00204-022-03349-4. [DOI] [PubMed] [Google Scholar]
- Rögner N.; Hagedorn H.-W.; Scherer G.; Scherer M.; Pluym N. A Sensitive LC–MS/MS Method for the Quantification of 3-Hydroxybenzo[a]pyrene in Urine-Exposure Assessment in Smokers and Users of Potentially Reduced-Risk Products. Separations 2021, 8, 171 10.3390/separations8100171. [DOI] [Google Scholar]
- Gale N.; McEwan M.; Eldridge A. C.; Fearon I. M.; Sherwood N.; Bowen E.; McDermott S.; Holmes E.; Hedge A.; Hossack S.; Wakenshaw L.; Glew J.; Camacho O. M.; Errington G.; McAughey J.; Murphy J.; Liu C.; Proctor C. J. Changes in biomarkers of exposure on switching from a conventional cigarette to tobacco heating products: A randomized, controlled study in healthy Japanese subjects. Nicotine Tob. Res. 2019, 21, 1220–1227. 10.1093/ntr/nty104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krautter G. R.; Borgerding M. F. Comparison of consumption patterns, biomarkers of exposure and subjective effects in cigarette smokers switched to dissolvable Ttobacco (Camel Orbs), dual use, or tobacco abstinence. Nicotine Tob. Res. 2014, 16, 1336–1347. 10.1093/ntr/ntu082. [DOI] [PubMed] [Google Scholar]
- Krautter G. R.; Chen P. X.; Borgerding M. F. Consumption patterns and biomarkers of exposure in cigarette smokers switched to snus, various dissolvable tobacco products, dual use, or tobacco abstinence. Regul. Toxicol. Pharmacol. 2015, 71, 186–197. 10.1016/j.yrtph.2014.12.016. [DOI] [PubMed] [Google Scholar]
- Ogden M. W.; Marano K. M.; Jones B. A.; Morgan W. T.; Stiles M. F. Switching from usual brand cigarettes to a tobacco-heating cigarette or snus: Part 2. Biomarkers of exposure. Biomarkers 2015, 20, 391–403. 10.3109/1354750X.2015.1094134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar M.; Liu J.; Koval T.; Wang J.; Feng S.; Serafin R.; Jin Y.; Xie Y.; Newland K.; Roethig H. J. Evaluation of biomarkers of exposure in adult cigarette smokers using Marlboro Snus. Nicotine Tob. Res. 2010, 12, 105–116. 10.1093/ntr/ntp183. [DOI] [PubMed] [Google Scholar]
- Shepperd C. J.; Newland N.; Eldridge A.; Haswell L.; Lowe F.; Papadopoulou E.; Camacho O.; Proctor C. J.; Graff D.; Meyer I. Changes in levels of biomarkers of exposure and biological effect in a controlled study of smokers switched from conventional cigarettes to reduced-toxicant-prototype cigarettes. Regul. Toxicol. Pharmacol. 2015, 72, 273–291. 10.1016/j.yrtph.2015.04.016. [DOI] [PubMed] [Google Scholar]
- Theophilus E. H.; Coggins C. R. E.; Chen P.; Schmidt E.; Borgerding M. F. Magnitudes of biomarker reductions in response to controlled reductions in cigarettes smoked per day: A one-week clinical confinement study. Regul. Toxicol. Pharmacol. 2015, 71, 225–234. 10.1016/j.yrtph.2014.12.023. [DOI] [PubMed] [Google Scholar]
- Haziza C.; de La Bourdonnaye G.; Merlet S.; Benzimra M.; Ancerewicz J.; Donelli A.; Baker G.; Picavet P.; Lüdicke F. Assessment of the reduction in levels of exposure to harmful and potentially harmful constituents in Japanese subjects using a novel tobacco heating system compared with conventional cigarettes and smoking abstinence: A randomized controlled study in confinement. Regul. Toxicol. Pharmacol. 2016, 81, 489–499. 10.1016/j.yrtph.2016.09.014. [DOI] [PubMed] [Google Scholar]
- Round E. K.; Campbell L. R.; Stiles M. F.; Dixon M.; Borgerding M. F. Changes in biomarkers of exposure and subjective effects when smokers switch to dual use of cigarettes and either snus or a dissolvable tobacco product: A summary of three clinical studies. Beitr. Tabakforsch. Int. 2015, 26, 242–260. 10.1515/cttr-2015-0013. [DOI] [Google Scholar]
- Round E. K.; Chen P.; Taylor A. K.; Schmidt E. Biomarkers of tobacco exposure decrease after smokers switch to an e-cigarette or nicotine gum. Nicotine Tob. Res. 2019, 21, 1239–1247. 10.1093/ntr/nty140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Bayoumy K.; Donahue J. M.; Hecht S. S.; Hoffmann D. Identification and quantitative determination of aniline and toluidines in human urine. Cancer Res. 1986, 46, 6064–6067. [PubMed] [Google Scholar]
- Riffelmann M.; Muller G.; Schmieding W.; Popp W.; Norpoth K. Biomonitoring of urinary aromatic amines and arylamine hemoglobin adducts in exposed workers and nonexposed control persons. Int. Arch. Occup. Environ. Health 1996, 68, 36–43. 10.1007/BF01831631. [DOI] [PubMed] [Google Scholar]
- Ward E. M.; Sabbioni G.; DeBord D. G.; Teass A. W.; Brown K. K.; Talaska G. G.; Roberts D. R.; Ruder A. M.; Streicher R. P. Monitoring of aromatic amine exposures in workers at a chemical plant with a known bladder cancer excess. J. Natl. Cancer Inst. 1996, 88, 1046–1052. 10.1093/jnci/88.15.1046. [DOI] [PubMed] [Google Scholar]
- Forster M.; Fiebelkorn S.; Yurteri C.; Mariner D.; Liu C.; Wright C.; McAdam K.; Murphy J.; Proctor C. Assessment of novel tobacco heating product THP1.0. Part 3: Comprehensive chemical characterisation of harmful and potentially harmful aerosol emissions. Regul. Toxicol. Pharmacol. 2018, 93, 14–33. 10.1016/j.yrtph.2017.10.006. [DOI] [PubMed] [Google Scholar]
- Schaller J.-P.; Keller D.; Poget L.; Pratte P.; Kaelin E.; McHugh D.; Cudazzo G.; Smart D.; Tricker A. R.; Gautier L.; Yerly M.; Reis Pires R.; Le Bouhellec S.; Ghosh D.; Hofer I.; Garcia E.; Vanscheeuwijck P.; Maeder S. Evaluation of the Tobacco Heating System 2.2. Part 2: Chemical composition, genotoxicity, cytotoxicity, and physical properties of the aerosol. Regul. Toxicol. Pharmacol. 2016, 81, S27–S47. 10.1016/j.yrtph.2016.10.001. [DOI] [PubMed] [Google Scholar]
- Stabbert R.; Voncken P.; Rustemeier K.; Haussmann H. J.; Roemer E.; Schaffernicht H.; Patskan G. Toxicological evaluation of an electrically heated cigarette. Part 2: Chemical composition of mainstream smoke. J. Appl. Toxicol. 2003, 23, 329–339. 10.1002/jat.924. [DOI] [PubMed] [Google Scholar]
- Flora J. W.; Meruva N.; Huang C. B.; Wilkinson C. T.; Ballentine R.; Smith D. C.; Werley M. S.; McKinney W. J. Characterization of potential impurities and degradation products in electronic cigarette formulations and aerosols. Regul. Toxicol. Pharmacol. 2016, 74, 1–11. 10.1016/j.yrtph.2015.11.009. [DOI] [PubMed] [Google Scholar]
- Jaccard G.; Tafin Djoko D.; Moennikes O.; Jeannet C.; Kondylis A.; Belushkin M. Comparative assessment of HPHC yields in the Tobacco Heating System THS2.2 and commercial cigarettes. Regul. Toxicol. Pharmacol. 2017, 90, 1–8. 10.1016/j.yrtph.2017.08.006. [DOI] [PubMed] [Google Scholar]
- Mazumder S.; Ahamed R. A.; McGahee E.; Wang L.; Seyler T. H. A New Automated Method for the Analysis of Aromatic Amines in Human Urine by GC-MS/MS. J. Anal. Toxicol. 2018, 43, 25–35. 10.1093/jat/bky045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young J.; Kadlubar F. A pharmacokinetic model to predict exposure of the bladder epithelium to urinary N-hydroxyarylamine carcinogens as a function of urine pH, voiding interval, and resorption. Drug Metab. Dispos. 1982, 10, 641–644. [PubMed] [Google Scholar]