Abstract

Fatty acid methyl ester (FAME) from oil seeds is conventionally produced via a two/three-process-step method: extraction of oil and subsequent esterification/transesterification to fatty FAME (biodiesel). However, in the present study, we investigated the production of castor kernel oil (CKO) FAME by reactive extraction for extraction and transesterification in a single process using a heterogeneous catalyst. The content of oil that can be extracted was checked by investigating several nonreactive extraction parameters such as solvent type (polar, nonpolar, and mixture), the solvent to kernel ratio, and extraction time. Maximum oil was extracted using methanol as a solvent with a methanol-to-seed ratio of 6.25:1 for 6 h extraction time. The viscosity of CKO obtained by nonreactive extraction was reduced from 288.83 to 19.04 mm2/s by reactive extraction using a 4.09 wt % catalyst concentration (BaO) and a 330.9:1 methanol-to-oil molar ratio for 6 h reaction time at 64 °C. Reactive extraction for transesterification of CKO was performed using BaO, CaO, and ZnO heterogeneous catalysts. BaO results in the increased yield of CKO FAME compared to other catalysts. Central composite design (CCD) using the response surface methodology (RSM) was implemented to design the experimental matrix, process parameter optimization, maximize the yield of CKO FAME, and investigate interaction effects of parameters such as reactive extraction temperature (55–65 °C), catalyst concentration (3–5 wt %), and methanol-to-oil molar ratio (175:1–350:1) on the yield of CKO FAME. A second-order model equation with a p-value < 0.05 and an R2 value near 1.0 was obtained to predict the yield using the input parameters. The maximum yield CKO FAME of 96.13 wt % with 94.4% purity of produced CKO FAME was obtained at a catalyst concentration of 4.09 wt % and a methanol-to-oil molar ratio of 330.9:1 for 6 h with a reaction temperature of 64 °C. Therefore, a comparable conversion of castor seed oil triglyceride (96.13 wt %) was obtained in a single step directly from castor seeds. Furthermore, the rheological behavior investigation of castor kernel oil and castor methyl ester revealed that the dynamic viscosity of both samples was found to be dependent on triglyceride content and temperature.
Introduction
The current increase in demand, price, and utilization of diesel due to the expansion of generators, automobiles and agricultural machinery, as well as the resulting depletion rate and environmental impact by mineral fuels drives the search for substitute renewable energy sources such as biodiesel.1 Biodiesel has been proven to be the best diesel fuel because of (1) the renewability and availability of the raw materials; (2) significant reductions in CO, aldehydes, SO2, and unburned hydrocarbons; (3) less toxicity and biodegradability nature; and (4) safety to handle and use.2−4 Biodiesel, like petroleum diesel, has a feasible ignite ability when used to fuel unmodified diesel engines. Biodiesel can also serve to mitigate the effects of the transportation sector on climate change and offer a more stable energy strategy to the country.5−7
Biodiesel is a fatty acid alkyl ester generated from oil containing renewable biological resources such as vegetable oils, animal fats, waste cooking oil, and algal oil using a transesterification reaction in the presence of a catalyst.8−12 Both edible and nonedible vegetable oils can be utilized as feedstock for biodiesel production.1 Nonedible vegetable oils are gaining special interest since they are very inexpensive, have low production costs, and are now negotiating the “food versus energy” argument.3 Castor is chosen as a prospective nonedible feedstock for this study because of its high yearly seed output; it can thrive in marginal terrain, i.e., in less fertile soil and in uncomfortably weather conditions; and it has high oil content.13
Transesterification, thermal cracking, microemulsification, and blending can be used to reduce the acidity and viscosity of vegetable oils, preventing engine damage. Among these, transesterification is a potential method for producing a clean and environmentally safe fuel from vegetable oils. Transesterification converts highly viscous triglycerides into long-chain monoesters with lower viscosity and a greater combustion rate.14,15 Reactive extraction is a transesterification technology that eliminates the need for oil extraction and purification, lowering production costs, and saving process time. The effectiveness of the reactive extraction process is primarily determined by the raw material utilized, type and amount of alcohol, catalyst employed, reaction temperature and time, free fatty acids (FFAs), and moisture content of the feedstock.16−18
Polar solvents are preferred and more effective for the reactive extraction of castor seeds because castor contains ricinoleic acid, which renders it more polar than other vegetable oils.8 Among polar solvents, methanol is preferred for the production of castor biodiesel. Reactive extraction is facilitated by an appropriate catalyst, which can be homogeneous or heterogeneous. The amount of free fatty acid in the feedstock influences the choice of the catalyst.19 Reactive extraction transesterification is preferable for feedstocks with low free fatty acid contents (<1 wt %). Because castor contains lesser free fatty acid, its methanolysis requires homogeneous or heterogeneous base catalysts. Homogeneous catalysis is speedy and produces a higher yield, but disposal of wastewater during product purification and the issue of purification influence the process cost.20,21 However, heterogeneous catalyst-based transesterification is a green technology due to easier separation of the product and reusability of the catalyst and a reduction in corrosion problems because acid sites are chemically bound with the solid catalyst.7,22−25 Attari et al.26 reported a 98.62% yield of biodiesel from waste cooking oil using 6.04 wt % CaO as a catalyst. Singh et al.27 and Amesho et al.28 conducted Jatropha curcas oil transesterification and obtained 86.1 wt % and 91.1% yields of biodiesel using 5 wt % CaO as a catalyst within a 3 h transesterification reaction time catalyst, respectively. Krishnamurthy et al.29 synthesized a snail shell-based CaO catalyst for the Hydnocarpus wightiana oil transesterification reaction to obtain a 98.93 wt % yield of Hydnocarpus wightiana oil fatty acid methyl ester with a reaction condition of a 0.892 wt % catalyst loading at a 61 °C reaction temperature for 2.4 h. In a similar way, Hanif et al.30 investigated the catalytic performance of BaO as a catalyst for the Toona ciliata oil transesterification reaction and obtained a 94 wt % yield of Toona ciliata oil biodiesel at 90 °C for 2.5 h with a methanol-to-oil molar ratio of 9:1 and an amount of about 0.39 wt %. Beef tallow fatty was transesterified using BaO as a heterogeneous catalyst, and about 94.95 wt % biodiesel yield was reported by Fard et al.31 The finding by Salim et al.32 shows that ZnO catalyst’s catalytic performance in biodiesel production from sunflower oil was 71 wt % with respect to biodiesel yield under reaction conditions, i.e., a methanol-to-oil molar ratio of 20:1 and a catalyst amount of 4.7 wt % at a reaction temperature of 70 °C for 3 h. With this regard, the present study is trying to evaluate the performance of reactive extraction transesterification for biodiesel production directly from castor seeds using heterogeneous metal oxide catalysts such as BaO, CaO, and ZnO previously used by several researchers for the three/and two-step biodiesel production process (i.e., extraction, esterification, and transesterification).
Various researchers reported on reactive extraction for biodiesel production from different seeds using NaOH and KOH as homogeneous catalysts.33−36 The use of homogeneous catalysts has generated a huge amount of wastewater during product purification during reactive extraction for biodiesel production. Further, the purification step and wastewater generation result in increased production costs and environmental pollution. However, such an issue can be resolved by utilizing heterogeneous alkali catalysts. But, to the best of our knowledge, no study reports on heterogeneous catalysis reactive extraction for the production of castor seed base FAME (biodiesel). Hence, the current study focused on exploiting heterogeneous base catalysts for the reactive extraction of castor seeds by screening the most active and appropriate heterogeneous catalysts for castor seed base FAME (biodiesel) production. Further, the flow behaviors of extracted castor kernel oil and its biodiesel (FAME) obtained via a reactive extraction process at various shear rates and temperatures were investigated, and also their flow was tested using a power law model.
Materials and Methods
Materials
The raw material, castor seeds, were purchased from a local market in Addis Ababa, Ethiopia. Chemicals such as methanol (99.8%), n-hexane (99%), zinc oxide (97% purity), calcium oxide (99% purity), and barium oxide (98% purity) were purchased from local suppliers in Addis Ababa, Ethiopia.
Methods
Nonreactive Extraction and Characterization of Castor Kernel Oil
Castor kernel oil was extracted using a nonreactive process (solvent extraction) using a soxhlet extractor, and the extracted oil was characterized because it is a necessary step in the reactive extraction procedure. As a result, this technique is utilized to determine the oil content of the kernel and to select the potential subjected catalyst based on the acid value (free fatty acid content) of the oil.
The effect of three major parameters such as the solvent-to-seed ratio (2.5:1 to 8:1 mL/g), extraction time (2–7 h), and solvent type (polar, nonpolar, mixture of the two) on nonreactive oil yield was explored at the boiling point of the solvent while maintaining a consistent particle size range (less than 2 mm). The oil was extracted from the deshelled kernel using the soxhlet apparatus with n-hexane, methanol, and a combination of the two solvents. After the desired extraction time, the extracted oil was concentrated, and the solvent was recovered by a rotary evaporator (ML-E14-2050). The extracted oil was then cooled and weighed to calculate the yield. The oil physiochemical properties (acid value, density, kinematic viscosity, iodine value, saponification value, heating value, cetane number, and molecular weight) were then determined using their standard test methods (Table 1). FTIR analysis, NMR analysis, and its rheological properties were also studied for the extracted CKO.
Table 1. Comparisons of Physiochemical Properties of Vegetable Oils Used for Biodiesel Production.
| present study |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| property | unit | CKO | test method | CSO8 | CoSO37 | RSO38 | JSO39 | NSO40 | ASTM ranges of CSO |
| SGa | 0.981 | Hydrometer | 0.9076 | 0.91 | 0.932 | ||||
| ρb | g/cm3 | 0.965 | Hydrometer | 0.961 | 0.92 | 0.957–0.968 | |||
| μb | mm2/s | 288.83 | Empirically41 | 241 | 29.22 | 13.13 | 32a | 26.09 | 6.3–8.9a |
| AV | mg KOH/g oil | 1.122 | AOAC 969.17 | 0.91 | 0.24 | 24 | 3.76 | 8.36 | 2.0 max |
| FFA | wt % | 0.564 | Empirically42 | 0.12 | 12 | 1.89 | 4.18 | 0.3–0.7 | |
| SV | mg KOH/g oil | 182.35 | ASTM D-1962 | 185 | 187.94 | 235.28 | 203.4 | 205.5 | 175–187 |
| IV | g I2/100 g of oil | 49.35 | Titration42 | 89 | 68.91 | 113 | 104.9 | 71.4 | 83-88 |
| MW | g/mol | 928.66 | Empirically43 | 870 | |||||
| HV | MJ/kg | 41.21 | Empirically44 | 41.25 | 39.34 | ||||
| CN | 65.13 | Empirically45 | 44 | ||||||
| FP | °C | 255 | 273 | ||||||
Represents the measurement value at 20 °C.
Represents a measurement at 40 °C, CKO: castor kernel oil, CSO: castor seed oil, CoSO: cottonseed oil, RSO: rubber seed oil, JSO: jartopha seed oil, NSO: neem seed oil, SG: specific gravity, ρ: density in g/cm3, μ: kinematic viscosity in mm2/s, AV: acid value in mg KOH/g oil, FFA: free fatty acid (wt %), SV: saponification value in mg KOH/g oil, IV: iodine value in g I2/100 g of oil, MW: molecular weight in g/mol, HV: heating value in MJ/kg, CN: cetane number, and FP: flash point (°C).
Experimental Procedure of the Reactive Extraction Process
Three heterogeneous base catalysts (BaO, CaO, and ZnO) were selected for screening for reactive extractions to produce castor kernel oil fatty acid methyl esters using a modified soxhlet extractor (Figure 1). Reactive extraction was performed in a three-neck round-bottom flask outfitted with a condenser, Soxhlet chamber, thermometer, and magnetic stirrer (Figure 1). Generally, the amount of the methanol-to-oil ratio in a conventional transesterification reaction varies from 3:1 to 15:1 in the molar ratio.7,38,46−48 Methanol was used as an extraction solvent and a transesterification reactant in a reactive extraction process from the castor kernel. Hence, a larger amount of methanol is required as compared to the amount of methanol required for oil transesterification to produce biodiesel in the conventional method.8,33,49,50 During the experiment, the known amounts of the catalyst (BaO, CaO, and ZnO) and methanol were initially put in a three-neck round-bottom flask based on the methanol-to-oil molar ratio and the catalyst concentration range and then spun to mix. Following that, a measured amount of the sample (crushed kernel) was placed in a thimble and then in the Soxhlet chamber depending on the chosen methanol to oil molar ratio. The chamber was then outfitted with a flask holding methanol, a catalyst at the bottom, and a condenser at the top (Figure 1.). The solution was then heated to vaporize methanol. Some methanol formed methoxide with the catalyst while the rest was vaporized to extract and rinsed out the oil into the reaction flask.
Figure 1.

Experimental setup of the reactive extraction process.
After the reaction time was reached, the reaction mixture was promptly chilled with an ice bath and centrifuged (Pro-Analytical C2004) at 4000 rpm to recover the catalyst. A rotary evaporator was then used to recover the excess methanol. The product mixture was then transferred into a separating funnel and placed in the funnel for 24 h to separate methyl ester from glycerol. Following glycerol separation, the product was washed with hot distilled water (heated to 40 °C) to remove any leftover glycerol and to clear up castor FAME. The washing procedure was continued until the pH of the water was detected to be neutral (∼7.0). After the distilled water was removed, the product was finally dried in an oven at 110 °C for 0.5 h to remove moisture. The yield was then calculated by weighing the dried FAME (eq 1).
| 1 |
Further, the purity produced FAME and the conversion triglycerides of castor kernel oil were calculated using 1H NMR spectra by eq 2.33,51,52
| 2 |
where X (%) is the percentage conversion of castor kernel oil triglycerides into its fatty acid methyl ester, AME is the integration value of the methoxy group (methyl esters), while ACH2 is the integration value of α-methylene protons (methylene value adjacent to the carboxy group).
Design of Experiment Reactive Extraction for FAME Production
The response surface methodology (RSM) model with central composite design (CCD) was used to investigate the effects of the reactive extraction process variables on the yield of castor FAME and optimize the parameter for maximum FAME and oil yield. Design expert software version 12.0 was used for the experimental design. In general, for three factors with three levels, about 20 experiment combinations with replicate were conducted by varying process parameters: reaction temperature (55–65 °C), methanol-to-oil molar ratio (175:1–350:1), and BaO catalyst concentration (3–5 wt % per oil content of the kernel), a 6 h reactive extraction time, which was the optimum condition where maximum oil was extracted during the oil extraction process, and an agitation speed of 600 rpm. The analysis of variance (ANOVA) was used to determine the significance of the model.
Catalyst Reusability Study
The reusability of the catalyst was studied by recovering and reusing the catalyst used in the preliminary methyl ester production performed at the optimum conditions that gave the highest yield of castor kernel oil FAME. To reuse the catalyst, it was extensively washed with methanol to remove the glycerol and any unwanted components adhered to the surface of the catalyst and then filtered with filter paper. The catalyst was then dried in an oven at 100 °C for 17 h. Finally, the dried catalyst was subjected as a reactive extraction catalyst for castor kernel fatty acid methyl ester production under the same process conditions as the previous cycle. The cycle for the reaction was repeated until there was a considerable drop in the yield of castor kernel oil FAME.
Product Characterization
Characterization of Castor Kernel Oil Fatty Acid Methyl Ester
The obtained methyl ester was characterized to determine whether it fulfilled the standard biodiesel criteria. Physiochemical properties of the produced methyl ester, such as density, kinematic viscosity,41 acid value, heating value,44 saponification value, iodine value,42 cetane number,45 molecular weight,43 and flash point, were tested according to ASTM standards.
Fourier Transform Infrared (FTIR) Analysis
Infrared (IR) spectra were recorded using an FTIR spectrometer (iS50 ABX) for the qualitative analysis of nonreactively extracted castor oil and its FAME produced through the reactive extraction process. The spectra were used to determine the functional groups present in the sample. FTIR spectra were recorded with scans 32 and a resolution of 16 cm–1 over a wavenumber range of 4000–500 cm–1. The functional groups were identified by comparing the obtained spectra to the previously reported spectra of castor seed oil and its methyl ester.
Nuclear Magnetic Resonance (NMR) Analysis
Nuclear magnetic resonance spectroscopy (NMR) (Make: Bruker, 400 MHz) analyses were performed to determine the conversion of castor kernel oil triglyceride to its FAME (eq 2). A liquid sample of 40 μL was taken in a 5 mm NMR tube and mixed with 500 μL of a deuterated chloroform (CDCl3) solvent. The chemical shift peak of deuterated chloroform at 7.26 ppm was taken as an internal reference. The proton shift peaks of methyl ester and methylene were taken at around 3.65 and 2.31 ppm, respectively. Further, 13C NMR analyses were performed for both extracted castor kernel oil from nonreactive extraction and for castor kernel oil FAME obtained from reactive extraction for conformation of conversion of castor kernel oil triglycerides into its FAME.
Rheological Experiments
Castor kernel oil and its fatty acid methyl ester have different viscosities as the FAME is obtained through chemical modification by the transesterification reaction. The rheological properties of both the oil and its methyl ester were analyzed using an Anton Paar MCR 102 Rheometer (Austria) at temperatures ranging from 20 to 80 °C and shear rates ranging from 0.1 to 100 s–1. The flow test was conducted at 25 °C, and the shear rate ranged from 0.1 to 100 s–1 for the power law model (eq 3).53
| 3 |
where τ, γ, n, and k are the shear stress (Pa), the shear rate (s–1), the non-Newtonian flow behavior index (dimensionless), and the flow consistency index (Pa·sn), respectively.
Both the power law parameters (n and k) are determined from the linearization of the power law equation (eq 3), where the plot of ln τ versus ln γ should be a straight line with slope n and intercept ln k if the fluid flow fit with the power law model.
Results and Discussion
Nonreactive Extraction and the Characterization of Castor Oil
Figure 2a–c presents the effect of the solvent-to-kernel ratio, extraction time, and type of solvents on the yield of nonreactive castor kernel oil extraction. The effect of the solvent-to-seed ratio was investigated using nonpolar solvent n-hexane for 4 h extraction time (Figure 2a). Figure 2a shows that the oil yield increased from 34.3 ± 2.1 to 53.2 ± 0.39 wt % as the solvent-to-castor kernel ratio increased from 2.3 to 6.5 mL/g. However, an increase in the ratio beyond 6.5 mL/g has no significant change in the yield of castor kernel oil. Hence, the solvent-to-castor kernel ratio of 6.25 mL/g is considered at an optimum condition.
Figure 2.
Effect of nonreactive extraction parameters on the yield of castor kernel oil: (a) solvent-to-kernel ratio for 4 h extraction time using hexane, (b) extraction time at the solvent-to-kernel ratio of 6.25 mL/g using hexane, and (c) solvent type at the solvent-to-kernel ratio of 6.25 mL/g and 6 h extraction time.
Figure 2b shows the effect of extraction time (2–7 h) on the yield of castor kernel oil at a fixed solvent-to-castor kernel ratio (6.5 mL/g) using n-hexane. At this ratio, the yield was increased with extraction time to a maximum of 54.79 ± 0.26 wt % oil yield at 6 h, and no significant change in the oil yield was observed at a 6 h extraction time (Figure 2b). Further study of the influence of solvent type reveals that pure methanol extracts more oil than n-hexane and their mixture (Figure 2c). The oil yield of the castor kernel was found to be the maximum (59.7 ± 0.31 wt %) using pure methanol as compared to n-hexane as a solvent. This result is attributed to castor oil containing 85–90% ricinoleic acid, which makes it polar and soluble in methanol. From Figure 2a–c, it was concluded that the maximum possible castor kernel oil (59.7 ± 0.31 wt %) could be extracted at a 6.25 mL/g of solvent-to-castor kernel ratio for a reaction time of 6 h using polar solvent methanol.
Nonreactive castor kernel oil physiochemical properties were measured and compared to ASTM specifications for quality castor oil, and the results are also compared with other vegetable oils such as castor seed oil,8 cottonseed oil,37 rubber seed oil,38 and Jartopha carcus L.41 Castor kernel oil extracted with methanol was red-brown in color. As shown in Table 1, the density of castor kernel oil obtained from the nonreactive extraction process at room temperature was 0.981 g/cm3, which was close to the prescribed limit of castor seed oil used for biodiesel.54−58 However, it has a higher density than other vegetable oils such as neem seed oil (0.92 g/cm3),40 rubber seed oil (0.91 g/cm3),38 and Jartopha carcus L. (0.932 g/cm3).39 This result may be due to the larger viscosity nature of castor kernel oil (i.e., 288.83 mm2/s at 20 °C). The result of kinematic viscosity shows that the castor kernel oil is much higher than that of jatropha carcus seed oil (32 mm2/s), cottonseed oil (29.22 mm2/s), neem seed oil (26.09 mm2/s), and rubber seed oil (13.13 mm2/s).37−40 This is perhaps attributed to the presence of hydroxyl groups of fatty acid as ricinoleic acid in the castor kernel oil, which contribute to higher viscosity.59 However, the kinematic viscosity at 40 °C was found to be 288.83 mm2/s, which is also significantly higher than the viscosity of other vegetable oils. This result suggests that the oil is too viscous and difficult to consume directly as a fuel because it causes poor fuel atomization, incomplete combustion, fuel injector choking, and ring carbonization.60 As a result, the extracted oil suffered a chemical modification, reactive extraction, which reduced its viscosity for the suitability of fuel application. Castor kernel oil has a higher viscosity than other vegetable oils due to the presence of a hydrogen bond in its hydroxyl group (−OH) or the presence of ricinoleic acid.56 The acid value of the extracted castor kernel oil was found to be 1.122 mg KOH/g. This shows the free fatty acid (FFA) content is lesser than 1 wt %. Hence, due to the lower value of FFA, the oil castor kernel is suitable feedstock for the production of methyl ester with an alkali catalyst using the reactive extraction technique. The extracted castor kernel oil acid value and FFA content were comparable to those reported by other researcher’s report.8,61 Extracted castor kernel oil had a saponification value of 182.35 mg KOH/g. The iodine value of the oil extracted using the nonreactive technique was found to be low (49.35 gI2/100 g oil), which was lower than the value of other vegetable oils. This result implies that the lower unsaturation in the fatty acid62,63 results in higher viscosity.64
Catalyst Screening and Reactive Extraction for Transesterification
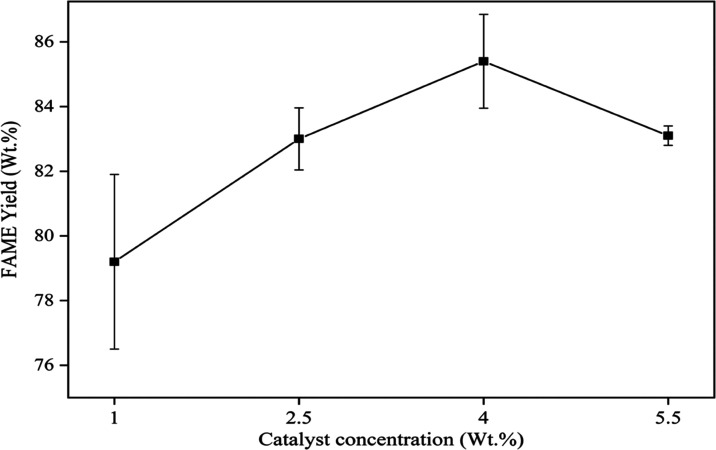
Since the free fatty acid content of the castor kernel oil was <1%, a reactive extraction of the castor kernel was performed using a heterogeneous base catalyst. Three heterogeneous base catalysts such as BaO, CaO, and ZnO were selected to identify the catalyst that provides a high yield of castor kernel oil FAME by reactive extraction. The maximum yield of castor kernel oil FAME was obtained using BaO with similar transesterification reaction conditions of a methanol-to-oil molar ratio of 250:1, a catalyst concentration of 4 wt %, and an agitation speed of 600 rpm for 6 h at a reaction temperature of 65 °C (Figure 3). This result is probably attributed to higher pore volume and basicity nature.65−67 Hence, the BaO catalyst was selected for further investigation in the present study. Figure 4 shows the preliminary transesterification reaction at various BaO catalyst concentrations at a fixed methanol-to-oil molar ratio of 250:1 and an agitation speed of 600 rpm for 6 h at a reaction temperature of 65 °C. It was observed that the maximum yield was obtained at a concentration of 4 wt %; hence, a range of 3–5 wt % was chosen for the reactive extraction process (Figure 4).
Figure 3.
Yield of castor kernel oil FAME using heterogeneous base catalysts at reactive extraction conditions of a methanol-to-oil molar ratio of 250:1, a catalyst loading of 4 wt %, and an agitation speed of 600 rpm for 6 h at a reaction temperature of 65 °C.
Figure 4.
Effect of the catalyst concentration on the yield of castor kernel oil FAME using the reactive extraction process at a methanol-to-oil molar ratio of 250:1 and an agitation speed of 600 rpm for 6 h at a reaction temperature of 65 °C.
Optimization of Reactive Extraction Process Parameters
Model Fitting and Statistical Analysis of Variance (ANOVA)
A total of 20 reactive extraction experiment matrices were formulated using RSM-CCD for three parameters (BaO catalyst concentration, methanol to oil ratio, and reaction temperature) at an optimum nonreactive extraction time of 6 h. To predict the yield of FAME, the design experimental data were used to generate a quadratic polynomial equation based on code and actual values of the parameters (eqs 4 and 5). The statistical significance of the regression mathematical model was evaluated using analysis of variance (ANOVA), and the results are presented in Table 2. The model F-value was found to be 2280.92, which indicates that the model is highly significant and has a low probability value (p < 0.0001). Individual effects of A (methanol to oil molar ratio), B (catalyst concentration), and C (temperature); interaction effects of AB, AC, and BC; and quadratic effects of A2, B2, and C2 all had a considerable effect on the yield of castor kernel oil FAME within the experimental range.
Table 2. ANOVA for the Response Surface Quadratic Model Obtained for the Reactive Extraction of Castor Kernel Oil.
| source | sum of squares | df | mean square | F-value | p-value | |
|---|---|---|---|---|---|---|
| model | 5041.61 | 9 | 560.18 | 2280.92 | <0.0001 | significant |
| A—methanol to oil molar ratio | 2695.82 | 1 | 2695.82 | 10976.78 | <0.0001 | |
| B—catalyst concentration | 166.88 | 1 | 166.88 | 679.51 | <0.0001 | |
| C—temperature | 586.98 | 1 | 586.98 | 2390.04 | <0.0001 | |
| AB | 6.18 | 1 | 6.18 | 25.15 | 0.0005 | |
| AC | 87.85 | 1 | 87.85 | 357.70 | <0.0001 | |
| BC | 5.33 | 1 | 5.33 | 21.70 | 0.0009 | |
| A2 | 1195.72 | 1 | 1195.72 | 4868.69 | <0.0001 | |
| B2 | 75.73 | 1 | 75.73 | 308.35 | <0.0001 | |
| C2 | 406.33 | 1 | 406.33 | 1654.47 | <0.0001 | |
| residual | 2.46 | 10 | 0.2456 | |||
| lack of fit | 2.05 | 5 | 0.4098 | 5.03 | 0.0503 | not significant |
| pure error | 0.4071 | 5 | 0.0814 | |||
| Cor total | 5044.07 | 19 | ||||
| R2= 0.9995, R2 adj = 0.9991 | predicted R2 = 0.9968, CV = 6.588% | |||||
The value of the regression coefficient (R2) was used to evaluate the quality of the model fit. The obtained model has an R2 value of 0.9995, indicating that 99.95% of the experimental results are explained by the obtained model equation in the form of the coded and actual factor (eqs 4 and 5). Figure 5a also shows that the model-predicted and actual yield of castor kernel oil FAME was similar, with an average error value of 0.05%. Further, the adjusted coefficient of determination (R2adj) was 0.9991, which validates the significance of the model.8 A high predicted R2 (0.9968) value also indicates that the fitted model is reasonably accurate.50 The predicted R2 value is reasonably close to the adjusted R2 value, with a difference of less than 0.2. The coefficient of variation (CV) is lower (6.6%), reflecting the reliability of the results of the fitted model. The regression coefficient and the corresponding 95% high and low confidence interval (CI) are used to investigate the effect of the reactive extraction process variables on the yield of castor methyl ester. The higher the regression coefficient, the stronger the effect on the reactive extraction process and, consequently, on the yield of castor oil FAME (Table 3).8 Thus, the reactive extraction parameters such as the methanol-to-oil molar ratio (A), catalyst concentration (B), and temperature (C), as well as the interaction parameters, were found to be significant for the yield of castor kernel oil FAME.
| 4 |
| 5 |
Figure 5.
Response surface plots: (a) predicted yield versus actual yield of castor kernel oil FAME, (b) interaction effect of the methanol-to-oil molar ratio and catalyst concentration, (c) interaction effect of the methanol-to-oil molar ratio and reaction temperature, and (d) interaction effect of the catalyst concentration and reaction temperature on the yield of castor kernel oil FAME.
Table 3. Regression Coefficients and the Corresponding 95% CI (Low and High) and Parameter Optimization.
| factor | coefficient estimate | df | standard error | 95% CI low | 95% CI high | VIF |
|---|---|---|---|---|---|---|
| intercept | 86.64 | 1 | 0.2021 | 86.19 | 87.09 | |
| A—methanol to oil molar ratio | 14.05 | 1 | 0.1341 | 13.75 | 14.35 | 1.0000 |
| B—catalyst concentration | 3.50 | 1 | 0.1341 | 3.20 | 3.79 | 1.0000 |
| C—temperature | 6.56 | 1 | 0.1341 | 6.26 | 6.85 | 1.0000 |
| AB | –0.8787 | 1 | 0.1752 | –1.27 | –0.4884 | 1.0000 |
| AC | 3.31 | 1 | 0.1752 | 2.92 | 3.70 | 1.0000 |
| BC | –0.8162 | 1 | 0.1752 | –1.21 | –0.4259 | 1.0000 |
| A2 | –9.11 | 1 | 0.1305 | –9.40 | –8.82 | 1.02 |
| B2 | –2.29 | 1 | 0.1305 | –2.58 | –2.00 | 1.02 |
| C2 | –5.31 | 1 | 0.1305 | –5.60 | –5.02 | 1.02 |
| Parameter Optimization and Validation of the Predicted
Value with the Experimental Result | ||||||
|---|---|---|---|---|---|---|
| yield of FAME (wt %) | ||||||
| parameter | constraint | optimum condition | predicted value | experimental value | ||
| methanol to oil molar ratio | 175:1–350:1 | 330.9:1 | 96.15 | 96.16 | ||
| catalyst concentration (wt %) | 3–5 | 4.09 | ||||
| temperature (°C) | 55–65 | 64 | ||||
Process Optimization
Following the regression analysis by ANOVA, numerical optimization was carried out for reactive extraction for castor kernel oil FAME production using within a range as a constraint for the independent parameters and maximization as a goal for the response and desirability function. The predicted optimal values for the reactive extraction of castor kernel to produce FAME from the model equation (eq 5) are a 330.9:1 methanol to oil molar ratio, a 4.09 wt % catalyst concentration, and a reaction temperature of 64 °C (Table 3). The model-predicted optimum yield of castor kernel oil FAME was found to be 96.15 wt % under optimal process conditions. An experiment at the model-predicted optimum condition was carried out to validate the model-predicted yield of castor kernel oil FAME (Table 3). The result shows that the model-predicted value and the experimental result were proximity similar with a 0.02% error. Hence, the interaction effects of reactive parameters were further investigated and presented in the Interaction Effect section. Furthermore, the present study result for the yield of biodiesel (96.15 wt %) obtained using the reactive extraction transesterification reaction method was comparable with mustard oil transesterification (97.5 wt %),46 used frying mustard oil (96.85 wt %),68 rubber seed oil (97 wt %),38 castor seed oil (95 wt %,50 88.2 wt %8), and castor oil (95.2 wt %).57 Hence, the following method and heterogeneous catalyst (BaO) can minimize the processing time and wastewater disposal for transesterification to produce biodiesel and product purifications, respectively (Table 4).
Table 4. Comparison of the Yield of FAME (Biodiesel) from Various Feedstocks Using Three Process Steps Such as Extraction, Esterification, and Transesterification and Reactive Extraction Transesterification as a Single Processa.
| time required
for each process (h) |
||||||||
|---|---|---|---|---|---|---|---|---|
| feedstock | oil extraction | extracted oil purification | esterification | transesterification | TSET (h) | catalyst type | TTMP | yield of FAME (wt %) |
| rubber seed | 8 | unknown | 2 | 0.25 | H2SO4 and Ba(OH)2·8H2O | 10.25 | ∼9738 | |
| sea mango (Cerbera odollam) | 4 | unknown | no esterification | 3 | sulfated zirconia | 7 | 94.147 | |
| Croton macrostachyus seed | 6 | unknown | 2 | NaOH | 8 | 95.5269 | ||
| castor seed | NR | NR | NR | NR | 3 | KOH | 3 | 88.48 |
| castor seed | NR | NR | NR | NR | 4 | NaOH | 4 | 9333 |
| Jatropha curcas L. seed | NR | NR | NR | NR | 10 | H2SO4 | 10 | 98.149 |
| castor seed | NR | NR | NR | NR | 6 | BaO | 6 | 93.13PS |
NR: not required, PS present study, TSET: time for simultaneous extraction and transesterification, and TTMP: total time for main process.
Interaction Effect of Reactive Extraction Parameters on the Yield of Castor Fatty Acid Methyl Ester at Optimum Conditions
The interaction effect of the methanol-to-oil molar ratio and catalyst concentration was investigated using a response surface (3D) plot of eq 5 at optimum reaction temperature (64 °C) (Figure 5b). As observed in the plot, the yield of castor kernel oil FAME increased with an increase in the methanol-to-oil molar ratio and catalyst concentration. This also demonstrates that both the methanol-to-oil molar ratio and catalyst concentration are significant parameters for reactive extraction. However, the yield was reduced beyond a molar ratio of 330.9:1 and a catalyst concentration of 4.09 wt %. A similar observation was reported by Tarigan et al.25 during waste passion fruit peel oil transesterification.
The interaction effect of the methanol-to-oil molar ratio and reaction temperature on the yield at an optimum catalyst loading of 4.09 wt % is shown in Figure 5c. Thus, the yield increased with an increase in the methanol-to-oil molar ratio and reaction temperature and then reduced above 64°C reactive extraction temperature and a molar ratio of 330.9:1. In addition, the effect of catalyst concentration combined with reaction temperature at an optimum methanol-to-oil molar ratio of 330.9:1 is presented in Figure 5d. At a lower reaction temperature, FAME yield increased with an increase in the catalyst concentration, and the same is true for the yield increase with a less amount of the catalyst. Hence, a simultaneous increase in the combined variables increases the yield.
Reusability of the BaO Catalyst
The reusability of BaO for the reactive extraction of castor kernel oil for FAME production was tested at optimum reaction conditions. It was observed that the yield of castor FAME using a fresh catalyst was 96.1 wt %, which was marginally reduced to 85.8 wt % in the 3rd recycle and then gradually reduced to 74.4 wt % in the 4th cycle (Figure 6). This was probably due to leaching formation, catalyst loss in each recycling phase, and/or carbon deposit formation on the active sites of the catalyst.65 Furthermore, the result also revealed that the BaO catalyst could be easily recovered and reused for three consecutive cycles with a 6.63 wt % reduction of yield of castor kernel oil FAME. However, after the 3rd recycle stage, the catalyst performance was reduced by more than 10 wt %. Hence, the BaO catalyst is required to regenerate before being used for the reactive extraction of castor kernel oil for FAME production.
Figure 6.
Reusability of BaO for the reactive extraction of the transesterification process at optimum conditions of a methanol-to-oil molar ratio of 330.9:1, a catalyst concentration of 4.09 wt %, and a reaction temperature of 64 °C.
Characterization of Nonreactive (CKO) and Reactive Extraction (CKO FAME) Products
FTIR Analysis
The results of FTIR analysis of castor kernel, CKO, and CKO FAME are presented in Figure 7. The results showed that the spectra of the castor seed kernel and castor kernel oil obtained from the nonreactive extraction process and its methyl ester obtained through the reactive extraction process were identical to a large extent (Figure 7). The spectra agreed with the results reported by Hernández-Sierra et al.70 In the spectra of castor kernel oil, the band at around 3369 cm–1 indicates the presence of −OH stretching due to the presence of hydroxylated ricinoleic acid in the samples. Similarly, the band was also observed with some shift to the left at 3333 cm–1 and to the right at 3426 cm–1 in castor seed kernel and castor kernel oil FAME, respectively (Table 5). The bands at around 2923 and 2854 cm–1 are typical of asymmetrical and symmetrical vibrations of aliphatic −CH2 fatty acid hydrocarbon chains, respectively.58,71 The presence of alkene groups (=CH−) in castor kernel oil and its FAME were observed with stretching vibration at wavenumbers of 3010 and 3005 cm–1, respectively.58,72 The presence of ester carbonyl functional groups (C=O) and (C–O) were observed at 1741 and 1162 cm–1. This ester group (C–O) was observed at about 1159 cm–1 in the castor seed kernel, but it was shifted to 1170 cm–1 in the case of castor kernel methyl ester. This signature signifies that the oil extracted from the kernel using the reactive extraction process was converted into FAME.48,72,73 Further, the presence of a band at around 1439 cm–1 for the methyl group (−O–CH3) and 1170 cm–1 for the ester group simultaneously indicated the formation of castor kernel oil fatty acid methyl ester (Figure 7).54
Figure 7.
FTIR spectra of castor seed kernel, castor kernel oil obtained at optimum nonreactive extraction conditions, and castor kernel oil FAME obtained at optimum reactive extraction conditions.
Table 5. FTIR Spectra Summary for Castor Kernel Oil and Castor Kernel Oil FAME.
| frequency (cm–1) |
||||
|---|---|---|---|---|
| reported value | experimental value | |||
| functional groups | castor kernel oil | castor kernel oil FAME | vibration mode | |
| alcohol (−OH) | 3600–3200 | 3369 | 3426 | stretching |
| alkene (=CH) | 3010 | 3005 | stretching | |
| aliphatic [alkane (−CH2)] | 2975–2840 | 2923 and 2854 | 2924 and 2854 | stretching |
| carbonyl [ester (C=O)] | 1750–1730 | 1741 | 1738 | stretching |
| methoxy [ester (C–O)] | 1300–1000 | 1162 | 1170 and 1244 | stretching |
| alkenes (C=C) | 1500–1400 | 1459 | 1439 | bending |
| aromatics (C–H) | 960–690 | 722 | 723 | out of plane |
NMR Analysis
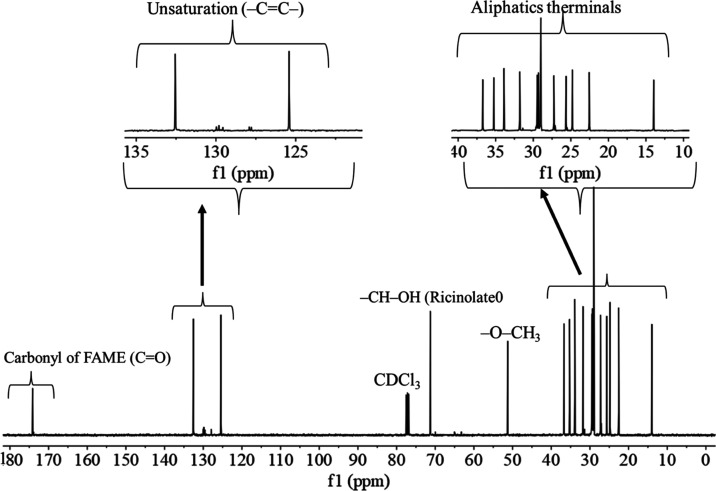
1H NMR and 13C NMR spectra of the castor kernel oil obtained by a nonreactive extraction process and castor methyl ester produced by a reactive extraction process obtained at optimum conditions are shown in Figures 8, 9, and 10, respectively. NMR spectra can be used to know the functional groups present in the sample and the conversion status of triglycerides into biodiesel.73Figure 8 shows the 1H NMR spectra of virgin castor kernel oil and the final product after transesterification (castor kernel oil FAME) using reactive extraction methods, respectively. In the 1H NMR spectra, the peak at around 3.7 ppm corresponds to the characteristic peak of FAME. In castor kernel oil, the peak corresponds to methyl ester, a proton of biodiesel is absent (Figure 8). The peak at around 3.3 ppm corresponds to −CH–OH for the presence of ricinoleic fatty acid alkyl groups in both samples (Figure 8). This was also confirmed in 13C NMR at the 70.9 ppm chemical shift in the form of ricinoleic fatty acid alkyl groups of triglycerides in CKO (Figure 9) and ricinolate fatty acid esters in CKO FAME (Figure 10). The presence of a prominent methyl ester proton peak at 3.7 ppm in the transesterified product (castor kernel oil FAME) confirms the progress of transesterification by reactive extraction with the formation of large quantities of fatty acid methyl esters (FAME). Hence, the conversion of triglycerides of castor kernel oil triglycerides into castor kernel oil methyl ester via reactive extraction using BaO as a heterogeneous catalyst was found to be 94.4 wt % (eq 2). This result is comparable with the previously reported methyl esters produced by reactive extraction using homogeneous catalysts50 and heterogeneous catalysis with conventional transesterification.57,74 The 13C NMR spectra of castor kernel oil and its methyl esters are depicted in Figures 9 and 10. As the free fatty acid content of castor kernel oil is too less, the carbonyl (−C=O) signal for free fatty acids did not appear at 179 ppm (i.e., carbonyl of free fatty acids (C=O)) in 13C NMR, whereas the ester signal in triglycerides of castor kernel oil appeared at the 172–174 ppm chemical shift (i.e., carbonyl of triglycerides (C=O)). The signals at 69 ppm and 62 ppm in the 13C NMR spectrum of castor kernel oil are due to the carbonyl methylene groups of triglycerides (69 ppm (H–C–O−) and 62 ppm (CH2–C–O−)) (Figure 9), while the signals are proximity absent in the castor kernel oil FAME (Figure 10). It can be clearly seen that the glyceride backbone of the triglyceride is almost absent in the castor kernel oil FAME sample (Figure 10). The methoxy carbon of methyl esters of castor kernel oil fatty acid methyl esters illustrates the signal at 51.49 ppm. The unsaturation signal (−C=C−) obtained between 133 and 120 ppm in 13C NMR is due to the presence of polyunsaturated and monounsaturated fatty acids and ester in castor kernel oil (Figure 9) and castor kernel oil FAME (Figure 10), respectively.
Figure 8.
1H NMR spectra of castor kernel oil and castor kernel oil FAME obtained at optimum nonreactive and reactive extraction conditions, respectively.
Figure 9.
13C NMR spectra of castor kernel oil obtained at the optimum nonreactive extraction condition.
Figure 10.
13C NMR spectra of castor kernel oil FAME obtained at the optimum reactive extraction condition.
Physiochemical Properties of Castor Fatty Acid Methyl Ester
The determined physiochemical properties of the castor kernel oil fatty acid methyl ester obtained at optimum reactive extraction conditions are presented in Table 6. The acid value of produced FAME by reactive extraction was found to be 0.29 mg KOH/g and lesser than that of nonreactive extractive products (i.e., castor kernel oil) 1.122 mg KOH/g. This result may be due to the conversion of free fatty acid by a side reaction during reactive extraction. The kinematic viscosity of castor kernel oil was reduced from 288.83 mm2/s (castor kernel oil) to 19.04 mm2/s (castor kernel oil FAME) upon transesterification by the reactive extraction method. The obtained viscosity of castor kernel oil methyl esters showed a higher value than that of castor seed oil biodiesel reported by Dasari and Goud.50 This variation of the result is attributed to the genotype of the seed. Further, the reported values for the viscosity are higher as compared to fatty acid obtained from transesterification reaction products of rubber seed FAME (3.81 mm2/s)38 and palm seed FAME (4.2 mm2/s).34 However, the result was lower than that of the used frying soybean oil FAME viscosity (12 mm2/s).75 The result is attributed to the variation of the fatty acid composition and unsaturation in the sample.3,7,38 Further, the results were confirmed by measuring the density of castor kernel oil fatty acid methyl ester at 20 and 40 °C (Table 6). The density of castor kernel oil was reduced from 0.981 to 0.871 g/cm3 at 20 °C and 0965 to 0.819 g/cm3 at 40 °C by reactive extraction. The reduction of density and viscosity by reactive extraction demonstrates the chemical modification of castor kernel oil and conversion of triglycerides in the castor kernel oil to fatty acid methyl esters via the transesterification reaction with methanol in the presence of a BaO catalyst.55,64,76 However, the obtained values of the viscosity of castor kernel oil FAME are still higher than the prescribed standard limit (Table 6); it is unsuitable for use in engines unless blended with mineral diesel fuel.77,78
Table 6. Comparison of the Physiochemical Properties of FAMEs Produced from CKO and Different Seed Oils.
| property name | unit | present study FAME | castor FAME50 | jatropha FAME36 | rubber FAME38 | palm FAME34 | ASTM standard ranges |
|---|---|---|---|---|---|---|---|
| densitya | g/cm3 | 0.871 | 0.886 | 0.8824 | 0.883 | 0.869 | 0.86–0.9 |
| densityb | g/cm3 | 0.819 | |||||
| viscosityb | mPa·s | 15.595 | 1.6–3.5 cp | ||||
| kinematic viscosityb | mm2/s | 19.04 | 10.39 | 4.05 | 3.81 | 4.2 | 1.9–6.0 |
| acid value | mg KOH/g | 0.29 | 0.64 | 0.38 | 0.4 | 0.5 max | |
| FFA | wt % | 0.145 | 0.32 | 0.19 | 0.2 | 0.25 max | |
| saponification value | mg KOH/g | 152.1 | 103.2 | 190 | |||
| iodine value | g I2/100 g | 52.57 | 83.24 | 114 | 58 | 82–88 | |
| heating value | MJ/kg | 42.4 | 39.43 | 39.53 | 37.5–42.8 | ||
| cetane number | 70.36 | 52 | 49.9 | 47 min | |||
| flash point | °C | 216 | 202 | 135 | 131 | >120 | 130 min |
| ash content | wt % | 0.01 max | |||||
| moisture content | wt % | 0.16 | 0.287 | 0.33 |
Represents the measurement value at 20 °C.
Represents the measurement at 40 °C.
Similarly, during the reactive extraction process, the saponification value of castor kernel oil was decreased from 182.35 to 152.1 mg KOH/g. This result is attributed to the reduction of the acid value during the conversion of triglycerides into FAME. The iodine value of castor kernel oil FAME was found to be 52.57 gI2/100 g. The lower iodine value result suggests that the castor kernel oil fatty acid methyl ester is less sensitive to oxidation during storage.79 The obtained heating value (42.4 MJ/kg) of FAME was slightly higher than FAME produced from used frying soybean oil (39.8 MJ/kg).75 Further, the cetane number (70.36) and flash point (216 °C) were found to be within the ASTM standard (Table 6). The heating value indicated that the obtained methyl ester (biodiesel) is promising to be used as fuel.15 The flash point of biodiesel is also positively correlated with its viscosity; the higher the viscosity, the higher the boiling point and, consequently, the higher the flash point.80 The measured value of the flash point for castor kernel oil FAME suggests that the castor methyl ester produced can be handled and used safely.6,38,53
Rheological Behavior of Castor Kernel Oil and Its Methyl Ester
Internal flow behaviors for biodiesel and it feedstocks are important as fuel flow properties and exert a great influence on fuel circulation, the mechanism of atomization of the fuel spray, and injection during diesel engine operation.64,78 The plot of shear stress versus the shear rate at 25°C shows a linear relationship with a zero intercept and R2 values of 1 and 0.9996 for CKO and CKO FAME, respectively (Figure 11). The dynamic viscosity of CKO and its FAME shows approximately a similar flow pattern within the shear rate range of 01–100 s–1. Further, the values of the zero intercept with the R2 value close to unity indicate that both the samples have Newtonian flow behavior with the studied range of the shear rate. Similar flow behavior observations were reported for Moringa oleifera oil,81 waste cooking oil, rubber seed oil, castor seed oil, and their FAME.53,64 Additionally, the flow behaviors of the two samples were analyzed using a commonly used model to estimate flow consistency (k) and non-Newtonian flow behavior indices (n) at 25°C from the linearization of the power law equation (eq 3). The higher deviation of the n value from unity (1) results in the higher deviation of the fluid from Newtonian behavior.78,82 The non-Newtonian flow behavior indices (n) for CKO and its FAME were found to be 1 ± 0.001 and 1 ± 0.01, respectively (Table 7). The variation of dynamic viscosity of the samples within the studied shear rate at 25 °C obtained from the experiment and the power law model (flow consistency index or viscosity) (eq 3) were found to be in good agreement with 0.14 and 2.78% variations for CKO and its FAME, respectively. The results are attributed to the Newtonian fluid behavior of the tested samples.83 The power law model was well fitted with R2 values near unity for both CKO (0.999) and its FAME (0.996). Chemical modification of CKO by reactive extraction reduces the flow consistency index (k) from 583.4 ± 0.59 to 23.78 ± 0.78 mPa.s (Table 7). This is due to the reduction of high-molecular-weight esters of glycerol present in oil samples in castor kernel into straight chain methyl esters by a reactive extraction process. Further, the result also shows the conversion of castor kernel oil triglycerides into castor kernel FAME.
Figure 11.
Shear stress versus shear rate profile of castor kernel oil and castor kernel oil FAME at 25°C.
Table 7. Rheological Behavior Parameters for the Experiment and the Power Law Model for Castor Kernel Oil and Its FAME at 25°Ca.
| properties | unit | castor kernel oil | castor kernel oil FAME |
|---|---|---|---|
| shear rate | 1/s | 0.1–100 | 0.1–100 |
| k | mPa·sn | 583.4 ± 0.59 | 23.78 ± 0.87 |
| kexp | mPa·s | 584.21 ± 5.7 | 24.46 ± 2.05 |
| n | 1 ± 0.001 | 1 ± 0.01 | |
| R2 | 0.999 | 0.996 |
k is the flow consistency index obtained from the power law model, kexp is the dynamic viscosity obtained from the experiment, and n is the non-Newtonian flow behavior index.
The effects of temperature (20°C–80°C) on the dynamic viscosity of CKO and its FAME are presented in Figure 12. It can be seen from Figure 12 that the dynamic viscosity was higher for CKO FAME over the temperature range of 20°C to 80°C. The temperature effect of the dynamic viscosity has a similar pattern within the temperature range for both samples. The viscosity nonlinearly decreased from 848.78 to 38.41 mPa·s and 32.22 to 4.36 mPa·s as the temperature increased from 20 to 80 °C in similar fashions. This is attributed to the reduction of the intermolecular interaction at a higher temperature. Similar observations were reported for castor oil, rubber seed oil, and waste cooking oil.64
Figure 12.
Effect of temperature on the dynamic viscosity of castor kernel oil and castor kernel oil FAME obtained at optimum nonreactive and reactive extraction conditions, respectively.
Conclusions
In this study, FAME has been produced by simultaneous extraction and transesterification directly from castor kernel using a heterogeneous catalyst and also optimized the process conditions using RSM-CCD. A maximum yield of 59.7 wt % of castor kernel oil was extracted with a solvent-to-seed ratio of 6:1 mL/g and an extraction time of 6 h using polar solvent methanol and with its boiling point. The role of n-hexane for the nonreactive extraction of castor kernel oil was less significant as compared to methanol, and hence only a polar solvent (methanol) was used for the reactive extraction. The acid value of nonreactive castor kernel oil was obtained at 1.122 mg KOH/g; thus, it is suitable for reactive extraction by a heterogeneous base catalysis reaction carried out for its FAME production. The reactive extraction performance for castor kernel oil production was higher in BaO as compared to CaO and ZnO. The catalyst reusability test indicated that the BaO catalyst could be easily recovered and reused for three successive cycles with a 6.37% reduction in its activity. Furthermore, three process parameters such as BaO catalyst concentration, reactive extraction reaction temperature, and methanol-to-oil molar ratio were studied for the processes. The optimum values of parameters for the highest yield of biodiesel (96.13 wt %) using the reactive extraction method have been determined as follows: a methanol-to-oil molar ratio of 330.9:1 and a catalyst concentration of 4.09 wt % at a 64 °C temperature using a BaO catalyst. The developed second-order model equation was able to predict the response function (i.e., the yield of castor kernel oil FAME) based on the input parameters with less than a 0.05% error. The obtained model can be employed for large-scale synthesis of castor kernel oil fatty acid methyl ester to predict the conversion of castor kernel oil before reactive extraction for the transesterification process to save time and maximize the yield of FAME at various conditions within the range studied. The rheological behavior investigation shows that both CKO and its FAME are Newtonian fluids and their flows fit well by the power law model with an R2 value greater than 0.99. Further, the physiochemical properties of castor kernel oil FAME obtained at optimum conditions were tested and found within the ASTM standards specifications except for the viscosity, which could be further reduced by blending it with conventional diesel fuel. Hence, the obtained FAME was found to be suitable for blending with diesel fuel for use in diesel engines. Based on the obtained results, simultaneous castor seed oil extraction and transesterification through reactive extraction using a heterogenous catalyst (i.e., BaO) was an efficient and time-saving approach to produce castor kernel fatty acid methyl ester directly from a raw castor kernel pre-extraction process followed by an esterification/transesterification process. Further, the following method and using a heterogeneous catalyst eliminated the oil extraction step and reduced the usage of n-hexane and wastewater generation during product purification. Finally, it can be concluded that the simultaneous extraction and transesterification process is a feasible process for biodiesel production from low free fatty acid-containing oil seed (<1%) in a single step.
Acknowledgments
The authors would like to acknowledge the Chemical and Food Engineering Laboratories under Chemical Engineering Department, Addis Ababa Science and Technology University, for providing the characterization instruments to conduct the sample analyses and laboratory facilities.
This study was funded by Addis Ababa Science and Technology University as master’s student thesis work.
The authors declare no competing financial interest.
References
- Sriharikota C. S.; Karuppasamy K.; Nagarajan V.; Sathyamurthy R.; Ramani B.; Muthu V.; Karuppiah S. Experimental investigation of the emission and performance characteristics of a DI diesel engine fueled with the vachellia nilotica seed oil methyl ester and diesel blends. ACS Omega 2021, 6, 14068–14077. 10.1021/acsomega.1c00437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D.-S.; Hanifzadeh M.; Kumar A. Trend of biodiesel feedstock and its impact on biodiesel emission characteristics. Environ. Prog. Sustainable Energy 2018, 37, 7–19. 10.1002/ep.12800. [DOI] [Google Scholar]
- Lim S.; Teong L. K. Recent trends, opportunities and challenges of biodiesel in Malaysia: an overview. Renewable Sustainable Energy Rev. 2010, 14, 938–954. 10.1016/j.rser.2009.10.027. [DOI] [Google Scholar]
- Encinar J. M.; González J. F.; Martínez G.; Sánchez N.; Pardal A. Soybean oil transesterification by the use of a microwave flow system. Fuel 2012, 95, 386–393. 10.1016/j.fuel.2011.11.010. [DOI] [Google Scholar]
- Liu W.; Yin P.; Liu X.; Zhang S.; Rongjun Q. Biodiesel production from the esterification of fatty acid over organophosphonic acid. J. Ind. Eng. Chem. 2015, 21, 893–899. 10.1016/j.jiec.2014.04.029. [DOI] [Google Scholar]
- Silitonga A. S.; Ong H. C.; Mahlia T. M. I.; Masjuki H. H.; Chong W. T. Characterization and production of Ceiba pentandra biodiesel and its blends. Fuel 2013, 108, 855–858. 10.1016/j.fuel.2013.02.014. [DOI] [Google Scholar]
- Mootabadi H.; Salamatinia B.; Bhatia S.; Abdullah A. Z. Ultrasonic-assisted biodiesel production process from palm oil using alkaline earth metal oxides as the heterogeneous catalysts. Fuel 2010, 89, 1818–1825. 10.1016/j.fuel.2009.12.023. [DOI] [Google Scholar]
- Pradhan S.; Madankar C. S.; Mohanty P.; Naik S. N. Optimization of reactive extraction of castor seed to produce biodiesel using response surface methodology. Fuel 2012, 97, 848–855. 10.1016/j.fuel.2012.02.052. [DOI] [Google Scholar]
- Velasquez-Orta S.; Lee J. G. M.; Harvey A. Alkaline in situ transesterification of Chlorella vulgaris. Fuel 2012, 94, 544–550. 10.1016/j.fuel.2011.11.045. [DOI] [Google Scholar]
- Moazami N.; Ranjbar R.; Ashori A.; Tangestani M.; Nejad A. S. Biomass and lipid productivities of marine microalgae isolated from the Persian Gulf and the Qeshm Island. Biomass Bioenergy 2011, 35, 1935–1939. 10.1016/j.biombioe.2011.01.039. [DOI] [Google Scholar]
- Xie W.; Hongyan W.; Li H. Silica-supported tin oxides as heterogeneous acid catalysts for transesterification of soybean oil with methanol. Ind. Eng. Chem. Res. 2012, 51, 225–231. 10.1021/ie202262t. [DOI] [Google Scholar]
- Tang Y.; Xu J.; Zhang J.; Lu Y. Biodiesel production from vegetable oil by using modified CaO as solid basic catalysts. J. Cleaner Prod. 2013, 42, 198–203. 10.1016/j.jclepro.2012.11.001. [DOI] [Google Scholar]
- Carrino L.; Visconti D.; Fiorentino N.; Fagnano M. Biofuel production with castor bean: A win–win strategy for marginal land. Agronomy 2020, 10, 1690–1712. 10.3390/agronomy10111690. [DOI] [Google Scholar]
- Yimer S.; Sahu O. Optimization of biodiesel production from waste cooking oil. Sustainable Energy 2014, 2, 81–84. [Google Scholar]
- Parawira W. Biodiesel production from Jatropha curcas: A review. Sci. Res. Essays 2010, 5, 1796–1808. [Google Scholar]
- Sendzikiene E.; Santaraite M.; Makareviciene V. Lipase-catalysed in situ transesterification of waste rapeseed oil to producedDiesel-biodiesel blends. Processes 2020, 8, 1–13. [Google Scholar]
- Farooq M.; Ramli A.; Subbarao D. Biodiesel production from waste cooking oil using bifunctional heterogeneous solid catalysts. J. Cleaner Prod. 2013, 59, 131–140. 10.1016/j.jclepro.2013.06.015. [DOI] [Google Scholar]
- Xie W.; Hu L.; Yang X. Basic ionic liquid supported on mesoporous SBA-15 silica as an efficient heterogeneous catalyst for biodiesel production. Ind. Eng. Chem. Res. 2015, 54, 1505–1512. 10.1021/ie5045007. [DOI] [Google Scholar]
- Helwani Z.; Othman M. R.; Aziz N.; Fernando W. J. N.; Kim J. Technologies for production of biodiesel focusing on green catalytic techniques: a review. Fuel Process. Technol. 2009, 90, 1502–1514. 10.1016/j.fuproc.2009.07.016. [DOI] [Google Scholar]
- Pasupulety N.; Rempel G. L.; Ng F. T. T. Studies on Mg-Zn mixed oxide catalyst for biodiesel production. Appl. Catal., A 2015, 489, 77–85. 10.1016/j.apcata.2014.10.015. [DOI] [Google Scholar]
- Wang Y.; Li D.; Zhao D.; Fan Y.; Bi J.; Shan R.; Yang J.; Luo B.; Yuan H.; Ling X.; Huhe T.; Chen Y. Calcium-Loaded Municipal Sludge-Biochar as an Efficient and Stable Catalyst for Biodiesel Production from Vegetable Oil. ACS Omega 2020, 5, 17471–17478. 10.1021/acsomega.0c01970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jitputti J.; Kitiyanan B.; Rangsunvigit P.; Bunyakiat K.; Attanatho L.; Jenvanitpanjakul P. Transesterification of crude palm kernel oil and crude coconut oil by different solid catalysts. Chem. Eng. J. 2006, 116, 61–66. 10.1016/j.cej.2005.09.025. [DOI] [Google Scholar]
- Wan Omar W. N. N.; Amin N. A. S. Optimization of heterogeneous biodiesel production from waste cooking palm oil via response surface methodology. Biomass Bioenergy 2011, 35, 1329–1338. 10.1016/j.biombioe.2010.12.049. [DOI] [Google Scholar]
- Xie W.; Fan M. Biodiesel production by transesterification using tetraalkylammonium hydroxides immobilized onto SBA-15 as a solid catalyst. Chem. Eng. J. 2014, 239, 60–67. 10.1016/j.cej.2013.11.009. [DOI] [Google Scholar]
- Tarigan J. B.; Singh K.; Sinuraya J. S.; Supeno M.; Sembiring H.; Tarigan K.; Rambe S. M.; Karo-karo J. A.; Sitepu E. K. Waste passion fruit peel as a heterogeneous catalyst for room-temperature biodiesel production. ACS Omega 2022, 7, 7885–7892. 10.1021/acsomega.1c06785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attari A.; Abbaszadeh-Mayvan A.; Taghizadeh-Alisaraei A. Process optimization of ultrasonic-assisted biodiesel production from waste cooking oil using waste chicken eggshell-derived CaO as a green heterogeneous catalyst. Biomass Bioenergy 2022, 158, 106357 10.1016/j.biombioe.2022.106357. [DOI] [Google Scholar]
- Singh A.; Sinha S.; Choudhary A. K.; Panchal H.; Elkelawy M.; Sadasivuni K. K. Optimization of performance and emission characteristics of CI engine fueled with Jatropha biodiesel produced using a heterogeneous catalyst (CaO). Fuel 2020, 280, 118611 10.1016/j.fuel.2020.118611. [DOI] [Google Scholar]
- Amesho K. T. T.; Lin Y.-C.; Chen C.-E.; Cheng P.-C.; Shangdiar S. Kinetics studies of sustainable biodiesel synthesis from Jatropha curcas oil by exploiting bio-waste derived CaO-based heterogeneous catalyst via microwave heating system as a green chemistry technique. Fuel 2022, 323, 123876 10.1016/j.fuel.2022.123876. [DOI] [Google Scholar]
- Krishnamurthy K. N.; Sridhara S. N.; Ananda Kumar C. S. Optimization and kinetic study of biodiesel production from Hydnocarpus wightiana oil and dairy waste scum using snail shell CaO nano catalyst. Renewable Energy 2020, 146, 280–296. 10.1016/j.renene.2019.06.161. [DOI] [Google Scholar]
- Hanif S.; Alsaiari M.; Ahmad M.; Sultana S.; Zafar M.; Rozina; Harraz F. A.; Alharbi A. F.; Abahussain A. A. M.; Ahmad Z. Membrane reactor based synthesis of biodiesel from Toona ciliata seed oil using barium oxide nano catalyst. Chemosphere 2022, 308, 136458 10.1016/j.chemosphere.2022.136458. [DOI] [PubMed] [Google Scholar]
- Ghanbari Zadeh Fard R.; Jafari D.; Palizian M.; Esfandyari M. Biodiesel production from beef tallow using the barium oxide catalyst. React. Kinet., Mech. Catal. 2019, 128, 723–738. 10.1007/s11144-019-01672-z. [DOI] [Google Scholar]
- Salim S. M.; Izriq R.; Almaky M. M.; Al-Abbassi A. A. Synthesis and characterization of ZnO nanoparticles for the production of biodiesel by transesterification: Kinetic and thermodynamic studies. Fuel 2022, 321, 124135 10.1016/j.fuel.2022.124135. [DOI] [Google Scholar]
- Dasari S. R.; Borugadda V. B.; Goud V. V. Reactive extraction of castor seeds and storage stability characteristics of produced biodiesel. Process Saf. Environ. Prot. 2016, 100, 252–263. 10.1016/j.psep.2016.01.019. [DOI] [Google Scholar]
- Jairurob P.; Phalakornkule C.; Na-udom A.; Petiraksakul A. Reactive extraction of after-stripping sterilized palm fruit to biodiesel. Fuel 2013, 107, 282–289. 10.1016/j.fuel.2013.01.051. [DOI] [Google Scholar]
- Sulaiman S.; Aziz A. R. A.; Aroua M. K. Reactive extraction of solid coconut waste to produce biodiesel. J. Taiwan Inst. Chem. Eng. 2013, 44, 233–238. 10.1016/j.jtice.2012.10.008. [DOI] [Google Scholar]
- Kumar G. Ultrasonic-assisted reactive-extraction is a fast and easy method for biodiesel production from Jatropha curcas oilseeds. Ultrason. Sonochem. 2017, 37, 634–639. 10.1016/j.ultsonch.2017.02.018. [DOI] [PubMed] [Google Scholar]
- Onukwuli D. O.; Emembolu L. N.; Ude C. N.; Aliozo S. O.; Menkiti M. C. Optimization of biodiesel production from refined cotton seed oil and its characterization. Egypt. J. Pet. 2017, 26, 103–110. 10.1016/j.ejpe.2016.02.001. [DOI] [Google Scholar]
- Reshad A. S.; Panjiara D.; Tiwari P.; Goud V. V. Two-step process for production of methyl ester from rubber seed oil using barium hydroxide octahydrate catalyst: Process optimization. J. Cleaner Prod. 2017, 142, 3490–3499. 10.1016/j.jclepro.2016.10.118. [DOI] [Google Scholar]
- Abdullah B. M.; Yusop R. M.; Salimon J.; Yousif E.; Salih N. Physical and chemical properties analysis of Jatropha curcas L. seed oil for industrial applications. World Acad. Sci. Eng. Technol. 2013, 84, 475–479. [Google Scholar]
- Oladipo A. S.; Ajayi O. A.; Oladipo A. A.; Azarmi S. L.; Nurudeen Y.; Atta A. Y.; Ogunyemi S. S. Magnetic recyclable eggshell-based mesoporous catalyst for biodiesel production from crude neem oil: Process optimization by central composite design and artificial neural network. C. R. Chim. 2018, 21, 684–695. 10.1016/j.crci.2018.03.011. [DOI] [Google Scholar]
- Martinez-Herrera J.; Siddhuraju P.; Francis G.; Davila-Ortiz G.; Becker K. Chemical composition, toxic/antimetabolic constituents, and effects of different treatments on their levels, in four provenances of Jatropha curcas L. from Mexico. Food Chem. 2006, 96, 80–89. 10.1016/j.foodchem.2005.01.059. [DOI] [Google Scholar]
- Shimamoto G. G.; Aricetti J. A.; Tubino M. A simple, fast, and green titrimetric method for the determination of the iodine value of vegetable oils without wijs solution (ICl). Food Anal. Methods 2016, 9, 2479–83. 10.1007/s12161-016-0401-1. [DOI] [Google Scholar]
- Cheng J.; Li Y.; He S.; Shen W.; Liu Y.; Song Y. Reaction kinetics of transesterification between vegetable oil and methanol under supercritical conditions. Energy Sources, Part A 2008, 30, 681–688. 10.1080/15567030601082084. [DOI] [Google Scholar]
- Demirbaş A. Fuel properties and calculation of higher heating values of vegetable oils. Fuel 1998, 77, 1117–1120. 10.1016/S0016-2361(97)00289-5. [DOI] [Google Scholar]
- Bose P. K. Empirical approach for predicting the cetane number of biodiesel. Int. J. Automot. Technol. 2009, 10, 421–429. 10.1007/s12239-009-0048-7. [DOI] [Google Scholar]
- Chakraborty R.; Das S.; Pradhan P.; Mukhopadhyay P. Prediction of optimal conditions in the methanolysis of mustard oil for biodiesel production using cost-effective Mg-solid catalysts. Ind. Eng. Chem. Res. 2014, 53, 19681–19689. 10.1021/ie501084z. [DOI] [Google Scholar]
- Kansedo J.; Lee K. T. Process optimization and kinetic study for biodiesel production from non-edible sea mango (Cerbera odollam) oil using response surface methodology. Chem. Eng. J. 2013, 214, 157–164. 10.1016/j.cej.2012.10.048. [DOI] [Google Scholar]
- Mumtaz M. W.; Adnan A.; Anwar F.; Mukhtar H.; Raza M. A.; Ahmad F.; Rashid U. Response Surface Methodology: An Emphatic Tool for Optimized Biodiesel Production Using Rice Bran and Sunflower Oils. Energies 2012, 5, 3307–3328. 10.3390/en5093307. [DOI] [Google Scholar]
- Shuit S. H.; Lee K. T.; Kamaruddin A. H.; Yusup S. Reactive extraction of Jatropha curcas L. seed for production of biodiesel: Process optimization study. Environ. Sci. Technol. 2010, 44, 4361–4367. 10.1021/es902608v. [DOI] [PubMed] [Google Scholar]
- Dasari S. R.; Goud V. V. Simultaneous extraction and transesterification of castor seeds for biodiesel production: Assessment of biodegradability. Process Saf. Environ. Prot. 2017, 107, 373–387. 10.1016/j.psep.2017.03.002. [DOI] [Google Scholar]
- Naureen R.; Tariq M.; Yusoff I.; Chowdhury A. J. K.; Ashraf M. A. Synthesis, spectroscopic and chromatographic studies of sunflower oil biodiesel using optimized base catalyzed methanolysis. Saudi J. Biol. Sci. 2015, 22, 332–339. 10.1016/j.sjbs.2014.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma Y. C.; Bhaskar S.; Korstad J. High yield and conversion of biodiesel from a nonedible feedstock (Pongamia pinnata). J. Agric. Food Chem. 2010, 58, 242–247. 10.1021/jf903227e. [DOI] [PubMed] [Google Scholar]
- Borugadda V. B.; Goud V. V. Physicochemical and rheological characterization of waste cooking oil epoxide and their blends. Waste Biomass Valorazation 2016, 7, 23–30. 10.1007/s12649-015-9434-8. [DOI] [Google Scholar]
- Ahmed A. S.; Ismail S.; Rahman R.; Hamdan S. In Experimental Investigation of Castor Oil as an Alternative Fuel for Biodiesel, Proceedings of the International Engineering Conference, 2014; pp 53–61.
- Demirbas A.; Bafail A.; Ahmad W.; Sheikh M. Biodiesel production from non-edible plant oils. Energy Explor. Exploit. 2016, 34, 290–318. 10.1177/0144598716630166. [DOI] [Google Scholar]
- Ogunniyi D. S. Castor oil: a vital industrial raw material. Bioresour. Technol. 2006, 97, 1086–1091. 10.1016/j.biortech.2005.03.028. [DOI] [PubMed] [Google Scholar]
- Baskar G.; Selvakumari I. A. E.; Aiswarya R. Biodiesel production from castor oil using heterogeneous Ni doped ZnO nanocatalyst. Bioresour. Technol. 2018, 250, 793–798. 10.1016/j.biortech.2017.12.010. [DOI] [PubMed] [Google Scholar]
- Panhwar T.; Mahesar S. A.; Kandhro A. A.; Sheerazi S. T. H.; Kori A. H.; Laghari Z. H.; Memon J.-u.-R. Physicochemical composition and FTIR characterization of castor seed oil. Ukr. Food J. 2019, 8, 778–787. 10.24263/2304-974X-2019-8-4-9. [DOI] [Google Scholar]
- Okullo A. A.; Temu A. K.; Ogwok P.; Ntalikwa J. W. Physico-chemical properties of biodiesel from jatropha and castor oils. Int. J. Renewable Energy Res. 2012, 2, 47–52. [Google Scholar]
- Ramezani K.; Rowshanzamir S.; Eikani M. H. Castor oil transesterification reaction: a kinetic study and optimization of parameters. Energy 2010, 35, 4142–4148. 10.1016/j.energy.2010.06.034. [DOI] [Google Scholar]
- Zhang A.; Wang Q.; He Y.; Lai P.; Miu Y.; Xiao Z. Preparation of biodiesel based on alkaline ionic liquid [Bmim]OH catalyzed castor oil. IOP Conf. Ser.: Mater. Sci. Eng. 2020, 729, 012048 10.1088/1757-899X/729/1/012048. [DOI] [Google Scholar]
- Yalcin H.; Toker O. S.; Dogan M. Effect of oil type and fatty acid composition on dynamic and steady shear rheology of vegetable oils. J. Oleo Sci. 2012, 61, 181–187. 10.5650/jos.61.181. [DOI] [PubMed] [Google Scholar]
- Santos J. C. O.; Santos I. M. G.; Souza A. G. Effect of heating and cooling on rheological parameters of edible vegetable oils. J. Food Eng. 2005, 67, 401–405. 10.1016/j.jfoodeng.2004.05.007. [DOI] [Google Scholar]
- Paul A. K.; Borugadda V. B.; Reshad A. S.; Bhalerao M. S.; Tiwari P.; Goud V. V. Comparative study of physicochemical and rheological property of waste cooking oil, castor oil, rubber seed oil, their methyl esters and blends with mineral diesel fuel. Mater. Sci. Energy Technol. 2021, 4, 148–155. 10.1016/j.mset.2021.03.004. [DOI] [Google Scholar]
- Babak S.; Iman H.; Abdullah A. Z. Alkaline earth metal oxide catalysts for Biodiesel production from Palm Oil: Elucidation of process behaviors and modeling using Response Surface Methodology. Iran. J. Chem. Chem. Eng. 2013, 32, 113–126. [Google Scholar]
- Yoo S. J.; Lee H.-s.; Veriansyah B.; Kim J.; Kim J.-D.; Lee Y.-W. Synthesis of biodiesel from rapeseed oil using supercritical methanol with metal oxide catalysts. Bioresour. Technol. 2010, 101, 8686–8689. 10.1016/j.biortech.2010.06.073. [DOI] [PubMed] [Google Scholar]
- Umukoro E. H.; Peleyeju M. G.; Idris A. O.; Ngila J. C.; Mabuba N.; Rhyman L.; Ramasami P.; Arotiba O. A. Photoelectrocatalytic application of palladium decorated zinc oxide-expanded graphite electrode for the removal of 4-nitrophenol: experimental and computational studies. RSC Adv. 2018, 8, 10255–10266. 10.1039/C8RA00180D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradhan P.; Chakraborty R. Optimal efficient biodiesel synthesis from used oil employing low-cost ram bone supported Cr catalyst: Engine performance and exhaust assessment. Energy 2018, 164, 35–45. 10.1016/j.energy.2018.08.181. [DOI] [Google Scholar]
- Zamba Z. Z.; Reshad A. S. Synthesis of fatty acid methyl ester from Croton macrostachyus (bisana) kernel oil: parameter optimization, engine performance, and emission characteristics for Croton macrostachyus kernel oil fatty acid methyl ester blend with mineral diesel fuel. ACS Omega 2022, 7, 20619–20633. 10.1021/acsomega.2c00682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández-Sierra M.; Aguilera-Camacho L. D.; Báez-García J. E.; García-Miranda J. S.; Moreno K. J. Thermal stability and lubrication properties of biodegradable castor oil on AISI 4140 Steel. Metals 2018, 8, 428–443. 10.3390/met8060428. [DOI] [Google Scholar]
- Sherazi S. T. H.; Kandhro A.; Mahesar S. A.; Bhanger M. I.; Talpur M. Y.; Sarfraz A. Application of transmission FT-IR spectroscopy for the trans fat determination in the industrially processed edible oils. Food Chem. 2009, 114, 323–327. 10.1016/j.foodchem.2008.09.058. [DOI] [Google Scholar]
- Thangarasu V.; Anand R.. Physicochemical Fuel Properties and Tribological Behavior of Aegle Marmelos Correa Biodiesel. In Advances in Eco-Fuels for a Sustainable Environment; Azad K., Ed.; Woodhead Publishing Series in Energy, 2019; pp 309–336. [Google Scholar]
- Doudin K. I. Quantitative and qualitative analysis of biodiesel by NMR spectroscopic methods. Fuel 2021, 284, 119114 10.1016/j.fuel.2020.119114. [DOI] [Google Scholar]
- Ismail S.; Ahmed A. S.; Anr R.; Hamdan S. Biodiesel production from castor oil by using calcium oxide derived from mud clam shell. J. Renewable Energy 2016, 2016, 1–8. 10.1155/2016/5274917. [DOI] [Google Scholar]
- Chakraborty R.; Banerjee A. Prediction of fuel properties of biodiesel produced by sequential esterification and transesterification of used frying soybean oil using statistical analysis. Waste Biomass Valorization 2010, 1, 201–208. 10.1007/s12649-010-9016-8. [DOI] [Google Scholar]
- Demirbas A. Biodiesel production from vegetable oils by supercritical methanol. J. Sci. Ind. Res. 2005, 64, 858–865. [Google Scholar]
- Demirbas A.Energy Convers Manage, 2009. In Progress and Recent Trends in Biodiesel Fuels; Elsevier, 2009; pp 14–34. [Google Scholar]
- Conceição M. M.; Candeia R. A.; Dantas H. J.; Soledade L. E. B.; Fernandes V. J.; Souza A. G. Rheological behavior of castor oil biodiesel. Energy Fuel 2005, 19, 2185–2188. 10.1021/ef050016g. [DOI] [Google Scholar]
- Barabás I.; Todorut I.. Biodiesel Quality, Standards and Properties; IntechOpen, 2011. [Google Scholar]
- Ismail S.; Abu S. A.; Rezaur R.; Sinin H. Biodiesel production from castor oil and its application in Diesel Engine. ASEAN J. SciTechnol Devel 2014, 31, 90–100. 10.29037/ajstd.18. [DOI] [Google Scholar]
- Domínguez Y. D.; García D. T.; Pérez L. G.; Fernández-Santana E.; Macias M. R.; Fischer T.; Piloto-Rodríguez R. Rheological behavior and properties of biodiesel and vegetable oil from Moringa oleifera Lam. Afinidad 2019, 587, 204–212. [Google Scholar]
- Tangsathitkulchai C.; Sittichaitaweekul Y.; Tangsathitkulchai M. Temperature effect on the viscosities of palm oil and coconut oil blended with diesel oil. J. Am. Oil Chem. Soc. 2004, 81, 401–405. 10.1007/s11746-004-0913-8. [DOI] [Google Scholar]
- Giwa S. O.; Chuah L. A.; Adam N. M. Fuel properties and rheological behavior of biodiesel from egusi (Colocynthis citrullus L.) seed kernel oil. Fuel Process. Technol. 2014, 122, 42–48. 10.1016/j.fuproc.2014.01.014. [DOI] [Google Scholar]