Abstract
Background
Screening for colorectal cancer (CRC) with faecal immunochemical test (FIT) is effective at reducing CRC mortality. Unfortunately, the COVID-19 pandemic has been associated with deferred care, especially screening for CRC.
Aim
We sought to develop a mailed FIT programme (MFP) to increase CRC screening and make recommendations for adoption across the Veterans Health Administration (VHA) and for other large healthcare systems.
Setting
2 regional VA medical centres in California and Washington state.
Participants
5667 average risk veterans aged 50–75 overdue or due within 90 days for CRC screening.
Programme description
A multidisciplinary implementation team collaborated to mail an FIT kit to eligible veterans. Both sites mailed a primer postcard, and one site added an automated reminder call.
Programme evaluation
We monitored FIT return and positivity rate, as well as impact of the programme on clinical staff. 34% of FIT kits were returned within 90 days and 7.8% were abnormal.
Discussion
We successfully implemented a population-based MFP at multiple regional VA sites and recommend that these efforts be spread across VA. Our model of regional leadership, facility champions and using centralised resources can be adaptable to other large healthcare systems. MFPs support catch-up from disrupted care by addressing access to CRC screening, unburden primary care visits and conserve limited procedural resources.
Keywords: COLORECTAL CANCER SCREENING, PRIMARY CARE, Ambulatory care, Quality improvement, Health Promotion
WHAT IS ALREADY KNOWN ON THIS TOPIC.
Mailed faecal immunochemical test (FIT) programmes are a population health approach used by healthcare systems to increase colorectal cancer (CRC) screening rates. In response to pandemic-related reductions in CRC screening, we sought to establish a protocol for a mailed FIT programme (MFP) at the Veterans Health Administration (VA), the largest integrated healthcare system in the USA.
WHAT THIS STUDY ADDS
We successfully implemented a large population-based MFP at multiple regional VA sites using dedicated teams and centralised resources. We offered screening to over 5000 veterans and found 34% returned a FIT within 90 days.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
We detail our MFP process, share lessons learnt and make recommendations to consider in establishing MFPs across VA and at other healthcare systems.
Introduction
Colorectal cancer (CRC) is the second leading cause of cancer death in the USA.1 Screening reduces CRC mortality and incidence. The faecal immunochemical test (FIT) is one of several recommended CRC screening options and can be performed at home.2 3 The standard of care for CRC screening in the Veterans Health Administration (VA) includes identifying individuals due for screening through an electronic health record (EHR) reminder during a primary care visit. Providers determine if the veteran is appropriate for screening and provide a FIT kit during the clinic visit, or order an alternative screening test (eg, colonoscopy) as clinically indicated.
At the start of the COVID-19 pandemic, the VA issued guidance to defer all non-urgent and elective procedures, including screening colonoscopy, and FIT was designated as the preferred CRC screening option.4 At the same time, a dramatic shift of primary care visits to telehealth modalities disrupted traditional CRC screening workflows.5 The USA as a whole experienced a decline in CRC diagnosis and screening during the pandemic.6 7 In response, the VA sought to identify an alternative approach to CRC screening.
Based on the urgent need to increase CRC screening, our teams in Washington state and California collaborated on a quality improvement initiative to establish a mailed FIT programme (MFP) in the VA. MFPs are a population health approach used by healthcare systems to increase CRC screening rates.8–11 These programmes proactively target an entire population of patients who are due for CRC screening, but do not rely on a traditional primary care visit. This helps unburden primary care teams who are responsible for numerous preventative health recommendations.12 MFPs have demonstrated increased screening rates across various populations including low-income and racially diverse groups, and among rural veterans.9 13–16
In this report, we describe the process for establishing an MFP at multiple regions in the VA. We describe initial results from large-scale implementation at pilot sites and make recommendations on ways to implement MFPs at VAs across the country.
Setting and participants
We implemented an MFP at two regional pilot sites: VA Central California Health Care System and VA Puget Sound Health Care System. VA Central California serves approximately 26 000 veterans across four sites of care and VA Puget Sound serves over 100 000 veterans across eight sites of care in Washington state. The MFP teams consisted of a clinical lead, programme manager and analysts who partnered with supply chain staff, laboratory staff and contracted mailing and logistics entities. The Central California team operated at the regional office level and the Puget Sound team was a local facility-based team. The pilot programme had the support of regional and national primary care and gastroenterology leadership.
Eligible veterans included individuals aged 50–75 years with at least one outpatient visit within the past 2 years (Puget Sound, n=20 090; Central California, n=12 167). The VA uses an EHR-based clinical reminder that prompts average risk screening for age-appropriate individuals unless prior screening (eg, prior colonoscopy, sigmoidoscopy or FIT) indicated that other action was appropriate (eg, repeat colonoscopy). This clinical reminder formed the basis of the average risk cohort identified for the MFP (Puget Sound, n=14 543; Central California, n=9186). Veterans were excluded if they were up to date with appropriate CRC screening (Puget Sound, n=9284; Central California, n=6196), scheduled for upcoming colonoscopy or sigmoidoscopy within 90 days (Puget Sound, n=36; Central California, n=n/a), enrolled in hospice (Puget Sound, n=29; Central California, n=11), lacked a mailing address (Puget Sound, n=167; Central California, n=2) or were newly started on clopidogrel within the past 6 months (Puget Sound, n=140; Central California, n=67). We defined a new clopidogrel start as a prescription filled within the last 180 days, but not the prior 180–365 days. A total of 4887 individuals at Puget Sound and 2912 at Central California were eligible for inclusion at the start of the programme. These inclusion and exclusion criteria were reapplied to eligible patients before each subsequent round of approximately 500 FIT kit mailings, capped by the availability of laboratory processing.
Programme description
Our MFP (figure 1) incorporated best practices from other reported MFPs,9 17 including a primer postcard mailed before the FIT kit and an automated reminder call.8 10 18 VA Central California followed this protocol for the pilot. To gain further insight into what components are necessary for an MFP, the VA Puget Sound team designed randomised controlled trials of programme elements.19 20 The data presented include the first 5 weeks of the VA Puget Sound pilot, during which primer postcards were sent to half of patients and no reminder calls were sent. The data presented include the first five rounds at Central California, which were conducted over a period of 3 months.
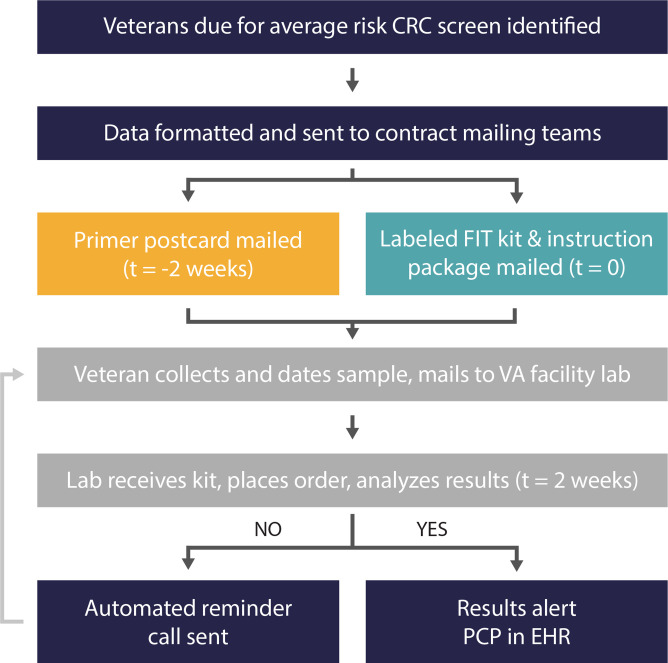
Figure 1.
The steps of the mailed FIT programme include cohort identification, use of contract mailing centres for FIT kit distribution and return processing by the local facility laboratory. CRC, colorectal cancer; EHR, electronic health record; FIT, faecal immunochemical test; PCP, primary care provider.
We used centralised contractors for printing, labelling and mailing FIT kits (US Government Printing Office and Western States Network Consortium Regional Reproduction Center). A primer postcard was mailed to participants introducing FIT for CRC screening (online supplemental figure 1). Two weeks later, veterans were mailed prelabelled FIT kits, instructions and a return envelope with prepaid postage. Veterans were instructed to collect a stool sample, record the date of collection and return the kit as soon as possible.
bmjoq-2022-001927supp001.pdf (556.9KB, pdf)
Returned FIT kits were analysed by laboratory staff, with results recorded in the EHR and alerted to the primary care provider (PCP). Tests received without a collection date or 15 days after the collection date have a comment to alert the PCP, as FIT kits processed >15 days after collection have a high false-negative rate. VA Central California patients who had not returned their kits within 2 weeks received an automated phone call reminder (AudioCARE Communicator, Wayne, Pennsylvania).
The MFP teams monitored for kits returned to sender and FIT sample laboratory errors. We communicated with contractors, laboratory staff and clinical teams via secure email and through staff meetings to identify and address any issue with the MFP.
The implementation of the MFP was operational and designated as a non-research activity.
Patient and public involvement
Prior to launch of the MFP, a primary care clinic advisory board that comprised patients provided feedback on the programme and mailed material at VA Puget Sound. Patients were not involved in the design, delivery or dissemination of the MFP.
Programme monitoring
Our MFP pilot was conducted between March and July 2021 at the VA Central California and between July and October 2021 at VA Puget Sound. Table 1 shows the demographic data for the cohort included in the MFP intervention.
Table 1.
Mailed FIT programme cohort demographics
| Central California | Puget Sound | |
| Mailed FIT total (n=3635) n (%)* |
Mailed FIT total (n=2022) n (%)* |
|
| Age | ||
| 50–64 | 1552 (43) | 1011 (50) |
| 65–75 | 2083 (57) | 1011 (50) |
| Sex | ||
| Male | 3415 (94) | 1806 (89) |
| Female | 220 (6) | 216 (11) |
| Race/ethnicity | ||
| Non-Hispanic white | 2046 (56) | 1354 (67) |
| Hispanic | 852 (23) | 79 (3.9) |
| Non-Hispanic black | 301 (8.3) | 359 (18) |
| Asian/Pacific Islander/Native Hawaiian | 106 (2.9) | 101 (5.0) |
| Multirace | 54 (1.5) | 44 (2.2) |
| American Indian/Alaska Native | 34 (0.9) | 23 (1.1) |
| Other | 18 (0.5) | 3 (0.1) |
| Rurality | ||
| Urban | 2673 (74) | 1521 (75) |
| Rural | 962 (26) | 454 (22) |
| Prior FIT completion in the VA | ||
| Completed ≥1 FIT† | 668 (18) | 1046 (52) |
| Never completed | 2967 (82) | 976 (48) |
*Numbers may not add to totals and percentages to 100% due to missing data (ie, unknown response).
†Completed ≥1 FIT kit within the past 5 years.
FIT, faecal immunochemical test; VA, Veterans Health Administration.
To capture routine care at each site, we measured the number of ordered and resulted FITs before implementation. In order to avoid overlap with the MFP cohort, preimplementation FIT data were from 3 to 6 months prior to the MFPs (ie, September through December for Central California, and January through April for Puget Sound). We implemented the programme and analysed the total return and positivity rates at 90 days after mailing the kits. FIT results were limited to one result per patient during the evaluation period and excluded kits returned before the mailed date. Table 2 shows the mean 90-day FIT return rates before and after MFP implementation at each site. During 4 months of routine care prior to the MFP implementation, 1259 FITs were ordered at Central California with a 39% (n=492) 90-day return rate, and 1510 FITs were ordered at Puget Sound with a 48% (n=725) 90-day return rate. After implementation of the MFP, 3635 FITs were mailed and the 90-day return rate was 35% (n=1277) at Central California, and 2022 FITs were mailed and 90-day return rate was 33% (n=672) at Puget Sound.
Table 2.
FIT return before and after mailed FIT programme implementation
| Intervention phase | Total ordered/mailed | Total returned 90 days n (%) |
Total positive n (%) |
|
| Central California | Premailed FIT | 1259 | 492 (39) | 49 (9.9) |
| Mailed FIT | 3635 | 1277 (35) | 88 (6.9) | |
| Puget Sound | Premailed FIT | 1510 | 725 (48) | 87 (12) |
| Mailed FIT | 2022 | 672 (33) | 58 (8.6) |
To capture routine care, we identified ordered and returned FIT kits from 3 to 6 months prior to the implementation of the MFPs (ie, premailed FIT above). 90-day return rates are shown for the pre-MFP and post-MFP implementation.
FIT, faecal immunochemical test; MFP, mailed FIT programme.
The Central California programme included automated reminder calls sent 2–4 weeks after the mailed FIT. Of the 3635 individuals included in the MFP, 3513 (96%) patients were successfully called by the automated phone system, of which 2989 patients (43.5%) were reached (ie, call answered or voice mail).
Discussion
We successfully implemented an MFP across two regional pilot sites to improve access to CRC screening for veterans. Our preliminary results found a veteran patient population with an average 34% FIT completion rate within 90 days across both sites. The return rate was slightly higher at the Central California VA than the Puget Sound VA (35% vs 33%), which may be attributable to the addition of an automated reminder call in the Central California programme. Despite baseline practice variation in use of FIT, with more patients having completed a prior FIT at the Puget Sound than the Central California VA (52% vs 18%), the MFP return rate was similar at both sites. Additionally, our MFP return rate was comparable to that seen in other MFPs, which range from 26% to 59%.10 Though FIT return rates were lower with the MFP than the preimplementation FIT screening, the MFP has several added benefits. The MFP: (1) systematically reaches more patients in a shorter time period, (2) saves time for busy primary care clinics, which carry the burden of preventative screening efforts,12 (3) includes individuals who may not seek in-person healthcare visits and (4) reduces demand for colonoscopies as the primary screening strategy, which is especially valuable given the deferral of procedures due to the COVID-19 pandemic.21
VA strives to be an ever-evolving learning health system (LHS), through leveraging research and a culture of innovation.22 An LHS approach promotes continuous, timely learning to improve the outcomes of individuals, populations and healthcare organisations. We adapted an LHS framework to describe the key components of our MFP, as well as our success, challenges and lessons learnt (box 1).23
Box 1. Lessons learnt from implementing the Veterans Health Administration (VA) mailed faecal immunochemical test (FIT) programme.
Individual level (relevant for patients)
Use clear, easy-to-understand patient materials.
Prelabel FIT kits and highlight need for sample date.
Monitor for equity and consider tailored outreach to harder-to-reach subpopulations.
Team/unit and mid-management level (relevant for front-line staff and managers)
Coordinate between primary care, gastroenterology and laboratory leadership.
Provide mechanism for communication and feedback between front-line staff and mailed FIT programme (MFP) team.
Maintain standard workflows for in-clinic colorectal cancer (CRC) screening.
Develop a process to batch automate FIT order entry.
Develop a process to alert providers to delayed or undated results.
Ensure standard workflow for positive FIT follow-up.
Organisational and operating level (relevant for executive leaders and organisations)
External conditions and national leadership support for FIT promote an environment conducive for an MFP.
Leadership should incentivise rapid adoption of MFP and support transitioning to a ‘FIT first’ model.
Establish a regional MFP team, which includes an analyst, administrator and clinical lead, to sustain the population health screening effort and to facilitate continuous quality improvement.
Create actionable data sets that are easy to use.
Ensure ongoing measurement of key outcome measures, that is, rate of FIT return over time.
Budget for outsourcing printing, labelling and mailing to save staff time and gain economies of scale.
Ensure FIT-positive patients have timely access to diagnostic colonoscopy.
At the individual level, we increased screening access and efficiently screened numerous veterans. However, similar to other MFPs, the majority still did not return their FIT kit. MFPs that incorporated advanced notifications and reminders have shown modest effects on increasing FIT completion rates.9 14 18 More work is needed to understand which strategies may increase MFP screening participation among veterans. In addition, we identified some logistical challenges. Despite incorporating best practices from other programmes, including a prelabelled kit,9 17 many veterans neglected to date their FIT kit, which can lead to unclear results and repeat testing.
At the team level, primary care and laboratory staff were familiar with processes for FIT. This enabled quicker programme implementation as no significant training was needed. Given that the programme is run by a separate dedicated team and not the individual PCP team, less workload fell onto primary care staff. While the MFP reduced primary care workload, it increased laboratory staff work due to manual FIT order entry. Work is underway to transition to a batch FIT ordering process. Early pilots used clinic staff for printing, labelling and mailing; however, we quickly determined that this was not sustainable for a large-scale effort. Moreover, we found it was important to establish clear communication channels between the MFP implementation team and front-line staff. This allowed staff to raise issues with the MFP, and to clarify workflows for in-clinic versus population health CRC screening. The communication channels with front-line staff were also essential for quality assurance. Early in the Puget Sound pilot, staff reported FIT results for some veterans who were not average risk, which identified an error in cohort identification that was quickly corrected.
At the organisational and operating level, primary care, gastroenterology and laboratory leadership were supportive of the MFP. Our MFPs were quickly implemented in response to reduced CRC screening due to the COVID-19 pandemic. However, we identified key steps to address with leaders. First, it was necessary for leadership to budget time and resources to create an MFP team—rather than use internal staff. Teams need protected time to manage the MFP, including working with logistics, contractors and health administration systems. Second, it was important for the MFP team to have experienced analysts. This allowed for continuous monitoring of outcomes and adaptation of the programme during implementation. Regional MFP teams should work in partnership with facility leadership, identify site champions and ensure local workflows allow proper follow-up of FIT results. Finally, implementation of the MFP required special agreements with leadership in several divisions. We had to have special agreements with laboratory staff to enter FIT orders. In addition, it was vital to have discussions with gastroenterology to address priority colonoscopy for veterans with a positive test result.
Given the known benefits of the MFP and the successes our team had in adapting the programme for the VA setting, we believe our pilot provides a blueprint for other VA primary care sites to implement MFP. Throughout this project, our team considered how our programme can be both implemented and sustained as a model that could be expanded nationally in the VA. At each step, we attempted to build a sustainable model, customised to the individual, team and organisational and operational factors, needed to develop an MFP. Moreover, we find that with leadership to champion the programme, appropriate staff for implementation, and funding support, this model could be sustainable for continuous operations of the MFP in the VA.
Limitations
We acknowledge several limitations. Importantly, we cannot account for the exact time and effort spent by primary care team members on screening efforts that may have occurred concurrently to the MFP efforts. Routine care continued throughout the MFP, and it is possible that veterans received kits and/or reminders from their clinical team at visits in addition to those from the MFP. Second, this pilot was conducted among veterans enrolled at the VA, a majority of whom are men, and results may not generalise to populations outside the VA. However, VA cares for over 6 million veterans in primary care and improving CRC screening among veterans is key priority.
One concern raised is that overscreening may happen when implementing an MFP. Overall, using an MFP results in only a small number of individuals who get duplicative screening. Overscreening can also happen in general practice when screening records are not up to date and unnecessary testing is performed.
Conclusions
We successfully implemented a large-scale population-based MFP at multiple regional VA sites. Our preliminary results show 34% of veterans completed CRC screening, and we were able to invite more patients to complete screening than through the usual care process. With pandemic-related care disruptions, it is more important than ever that healthcare systems seek new ways to provide needed preventive services. VA is primed for the opportunity to create a national MFP. Additional work is needed to determine if the MFP implementation will be as successful when spread to additional sites, and if the same model is viable. Further evaluations will assess the impact of the MFP on disparities and seek to test specific programmatic features that can enhance CRC screening rates among veterans.
Acknowledgments
This work was made possible through operational support and funding from VISN21 and VA Puget Sound Health Care System Leadership. We would like to thank the participants from the VA Puget Sound Primary Care Clinic Advisory Board that reviewed the programme materials.
Footnotes
Twitter: @StefanieDeeds
Contributors: All authors played a role in fulfilling all four criteria: (1) design, data, interpretation; (2) drafting and revising for intellectual content; (3, 4) final approval and will stand by the work.
Funding: This work was funded by the Primary Care Analytics team through the Veterans Health Administration Office of Primary Care.
Disclaimer: The funder had no role in the design and conduct of the study; collection, management, analysis and interpretation of the data; preparation, review or approval of the manuscript; and decision to submit the manuscript for publication. Author SD claims responsibility for the overall content as the guarantor, and accepts full responsibility for the work, had access to the data, and controlled the decision to publish. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the US government, the Department of Veterans Affairs and the University of Washington.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
No data are available.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.NIH National Cancer Institute Surveillance, Epidemiology, and End Result (SEER) Program . Cancer STAT facts: colorectal cancer. Available: https://seer.cancer.gov/statfacts/html/colorect.html [Accessed 13 Jan 2022].
- 2.US Preventive Services Task Force, Davidson KW, Barry MJ, et al. Screening for colorectal cancer: US preventive services task force recommendation statement. JAMA 2021;325:1965–77. 10.1001/jama.2021.6238 [DOI] [PubMed] [Google Scholar]
- 3.Rex DK, Boland CR, Dominitz JA, et al. Colorectal cancer screening: recommendations for physicians and patients from the U.S. multi-society task force on colorectal cancer. Gastroenterology 2017;153:307–23. 10.1053/j.gastro.2017.05.013 [DOI] [PubMed] [Google Scholar]
- 4.Department of Veterans Affairs . Guidance to avoid all routine or non-urgent face to face visits Memorandum; 2020. https://www.navao.org/wp-content/uploads/2020/04/Guidance-to-Avoid-All-Routine-or-Non-urgent-Face-to-Face-Visits-Signed.pdf [Accessed 10 Nov 2021]. [Google Scholar]
- 5.Reddy A, Gunnink E, Deeds SA, et al. A rapid mobilization of ‘virtual’ primary care services in response to COVID-19 at veterans health administration. Health Care 2020;8:100464. 10.1016/j.hjdsi.2020.100464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Periyanaayagam U, Dwter A, Kim J. New colorectal cancer diagnoses fall by one-third as colonoscopy screenings and biopsies grind to a halt during height of COVID-19 Komodohealth x Fight Colorectal Cancer. Research Brief; 2020. https://fightcolorectalcancer.org/wp-content/uploads/2020/05/COVID19-Impact-on-CRC-Patients_Research-Brief_Komodo-Health-Fight-CRC.pdf [Accessed 01 Nov 2021]. [Google Scholar]
- 7.Chen RC, Haynes K, Du S, et al. Association of cancer screening deficit in the United States with the COVID-19 pandemic. JAMA Oncol 2021;7:878–84. 10.1001/jamaoncol.2021.0884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gupta S, Coronado GD, Argenbright K, et al. Mailed fecal immunochemical test outreach for colorectal cancer screening: summary of a centers for disease control and prevention-sponsored summit. CA Cancer J Clin 2020;70:283–98. 10.3322/caac.21615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Selby K, Jensen CD, Levin TR, et al. Program components and results from an organized colorectal cancer screening program using annual fecal immunochemical testing. Clin Gastroenterol Hepatol 2022;20:145–52. 10.1016/j.cgh.2020.09.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jager M, Demb J, Asghar A, et al. Mailed outreach is superior to usual care alone for colorectal cancer screening in the USA: a systematic review and meta-analysis. Dig Dis Sci 2019;64:2489–96. 10.1007/s10620-019-05587-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dougherty MK, Brenner AT, Crockett SD, et al. Evaluation of interventions intended to increase colorectal cancer screening rates in the United States: a systematic review and meta-analysis. JAMA Intern Med 2018;178:1645–58. 10.1001/jamainternmed.2018.4637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Privett N, Guerrier S. Estimation of the time needed to deliver the 2020 USPSTF preventive care recommendations in primary care. Am J Public Health 2021;111:145–9. 10.2105/AJPH.2020.305967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jean-Jacques M, Kaleba EO, Gatta JL, et al. Program to improve colorectal cancer screening in a low-income, racially diverse population: a randomized controlled trial. Ann Fam Med 2012;10:412–7. 10.1370/afm.1381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davis MM, Freeman M, Shannon J, et al. A systematic review of clinic and community intervention to increase fecal testing for colorectal cancer in rural and low-income populations in the United States - how, what and when? BMC Cancer 2018;18:40. 10.1186/s12885-017-3813-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murphy CC, Halm EA, Zaki T. Colorectal cancer screening and yield in a mailed outreach program in a safety-net healthcare system. Dig Dis Sci 2021:1–7. 10.1007/s10620-021-07313-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Charlton ME, Mengeling MA, Halfdanarson TR, et al. Evaluation of a home-based colorectal cancer screening intervention in a rural state. J Rural Health 2014;30:322–32. 10.1111/jrh.12052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheng C, Ganz DA, Chang ET, et al. Reducing rejected fecal immunochemical tests received in the laboratory for colorectal cancer screening. J Healthc Qual 2019;41:75–82. 10.1097/JHQ.0000000000000181 [DOI] [PubMed] [Google Scholar]
- 18.Issaka RB, Avila P, Whitaker E, et al. Population health interventions to improve colorectal cancer screening by fecal immunochemical tests: a systematic review. Prev Med 2019;118:113–21. 10.1016/j.ypmed.2018.10.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schuttner L. Primers to improve adherence to annual CRC screening among veterans: a randomized control trial. ClinicalTrials.gov, 2021. Available: https://clinicaltrials.gov/ct2/show/NCT04923646 [Accessed 12 Jan 2022].
- 20.Schuttner L. Reminders for fit (fecal immunochemical test) kits via different modalities: a randomized controlled trial. ClinicalTrials.gov, 2021. Available: https://clinicaltrials.gov/ct2/show/NCT05012007 [Accessed 02 Feb 2022].
- 21.Gawron AJ, Kaltenbach T, Dominitz JA. The impact of the coronavirus disease-19 pandemic on access to endoscopy procedures in the Va healthcare system. Gastroenterology 2020;159:1216–20. 10.1053/j.gastro.2020.07.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Atkins D, Kilbourne AM, Shulkin D. Moving from discovery to system-wide change: the role of research in a learning health care system: experience from three decades of health systems research in the veterans health administration. Annu Rev Public Health 2017;38:467–87. 10.1146/annurev-publhealth-031816-044255 [DOI] [PubMed] [Google Scholar]
- 23.Harrison MI, Shortell SM. Multi-level analysis of the learning health system: integrating contributions from research on organizations and implementation. Learn Health Syst 2021;5:e10226. 10.1002/lrh2.10226 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjoq-2022-001927supp001.pdf (556.9KB, pdf)
Data Availability Statement
No data are available.