Abstract
Objectives
The purpose of this meta-analysis was to investigate the efficacy and safety of mesenchymal stem cells (MSCs) combined with platelet-rich plasma (PRP) in the treatment of knee osteoarthritis (KOA).
Design
Systematic review and meta-analysis.
Participants
Patients with KOA.
Interventions
Use of MSCs+PRP.
Primary and secondary outcomes
Visual Analogue Scale (VAS) score, Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) score, Knee Injury and Osteoarthritis Outcome Score (KOOS) and adverse reactions.
Data sources
PubMed, Cochrane Library, Embase and China National Knowledge Infrastructure were searched from inception to 15 July 2021.
Measures
The OR or weighted mean difference (WMD) of relevant outcome indicators was calculated. Study quality was evaluated using the risk-of-bias assessment tool version 2.0. Heterogeneity among studies was evaluated by calculating I2. If I2<50%, a fixed-effect model was applied; conversely, if I2 ≥50%, a random-effect model was applied.
Results
Six controlled clinical trials with 493 cases were included. The meta-analysis results showed that in terms of the VAS score 3 months after treatment, MSCs+PRP had no significant effect on the reduction of the VAS score in patients with KOA compared with the control (p=0.09), hyaluronic acid (HA) (p=0.15) or PRP alone (p=0.07). MSCs+PRP was more effective in reducing the VAS score at 6 and 12 months after treatment than the control (WMD=−0.55, 95% CI −0.87 to −0.22, p<0.001), HA (WMD=−1.20, 95% CI −2.28 to −0.13, p=0.03) or PRP alone (WMD=−0.54, 95% CI −0.89 to −0.18, p=0.003). Regarding the decrease in the total WOMAC score at 3 and 6 months after treatment, MSCs+PRP showed better clinical efficacy than the control or HA alone (p<0.01). Compared with the control, MSCs+PRP exhibited no significant difference in reducing the total WOMAC score 12 months after treatment (p=0.39). There was no significant difference between MSCs+PRP and the control in terms of improvement of the KOOS 12 months after treatment (p=0.16). Compared with MSCs alone, MSCs+PRP exhibited no significant difference in the incidence of adverse reactions (p=0.22) 12 months after treatment.
Conclusions
Treatment with MSCs+PRP showed good clinical efficacy in improving pain and joint function in patients with KOA. Compared with MSCs alone, there was no significant difference in the incidence of adverse reactions with MSCs+PRP.
PROSPERO registration number
CRD 42021275830.
Keywords: Knee, Adult surgery, Health informatics
STRENGTHS AND LIMITATIONS OF THIS STUDY.
This is the first meta-analysis to investigate the efficacy and safety of mesenchymal stem cells (MSCs) combined with platelet-rich plasma (PRP) in the treatment of knee osteoarthritis (KOA).
Most of the included studies were of high quality with a low risk of bias.
The number of studies and sample sizes included in this meta-analysis was limited.
The follow-up time of the included studies was short, which is not conducive to deducing the long-term efficacy of MSCs combined with PRP for the treatment of KOA.
Introduction
With the increase in the ageing population worldwide, the incidence rate of knee osteoarthritis (KOA) has increased annually.1 Epidemiological investigations have shown that KOA is more common in middle and old age groups, especially in the population over 65 years old, in which the incidence rate is approximately 50%.2 The effective prevention and treatment of KOA has become the key to improving the quality of life of middle-aged and elderly individuals.2 The purpose of non-surgical treatment for KOA is to reduce pain, improve function and avoid complications of surgical treatment as much as possible. Conservative pain control methods include drug treatment, increased daily activity and periarticular analgesia, but the optimal method is still controversial.3 4
In recent years, cell therapy has shown good potential for the treatment of many diseases, including osteoarthritis, and studies have preliminarily shown that bone marrow mesenchymal stem cells (MSCs) can promote the repair of articular cartilage and be used to treat osteoarthritis.5 6 In addition, adipose-derived stem cells can be directionally differentiated into chondrocytes and osteoblasts, playing a role in repairing and treating osteoarthritis.7 8 Platelet-rich plasma (PRP) contains a large number of growth factors, such as platelet-derived growth factor and vascular endothelial growth factor, which play an important role in cartilage repair.9 10 Previous studies have shown that PRP has good safety and efficacy in the treatment of KOA, but it shows only short-term clinical effects.11 12 Interestingly, in vitro studies have shown that PRP can promote the proliferation and differentiation of mesenchymal stem cells (MSCs).13 Coculture of MSCs with PRP in vitro can promote the proliferation and chondrogenic differentiation of MSCs.14 In addition, in vivo animal experiments have confirmed that the combination of PRP and MSCs can promote the healing of rabbit radial bone defects more than MSCs alone.15 Moreover, we believe that the injection of MSCs and PRP into the knee can theoretically promote chondrocyte regeneration, eliminate nonbacterial inflammation of the synovium, and alleviate symptoms through the aetiological treatment of osteoarthritis.16 17
PRP has shown good safety and efficacy in treating KOA, but the therapeutic effect decreases with increasing patient age.18 19 In addition, studies have shown that MSCs can promote the repair of articular cartilage to treat KOA.20 Whether there is a synergistic effect of PRP combined with MSCs in the treatment of KOA has attracted the attention of researchers. However, the efficacy and safety of MSCs+PRP in the treatment of KOA are still controversial, and there is a lack of higher-level clinical evidence. This study will use meta-analysis and quantitative evaluation to explore the efficacy and safety of MSCs+PRP in the treatment of KOA to provide an evidence-based foundation for clinical application and basic research.
Materials and methods
This meta-analysis was conducted in strict accordance with the Preferred Reporting Items for Systematic reviews and Meta-Analyses statement.21
Data source and retrieval strategy
Two researchers conducted a comprehensive search of four databases: PubMed, Cochrane Library, Embase and China National Knowledge Infrastructure (CNKI). The retrieval time limit was from the establishment of each database to 15 July 2021. A combination of subject words and free words was used to establish a retrieval model to search the above four databases. The search terms included “mesenchymal stem cell”, “stem cell”, “platelet-rich plasma”, “knee osteoarthritis” and “osteoarthritis”. The search strategy for each database can be found in online supplemental material 1.
bmjopen-2022-061008supp001.pdf (610KB, pdf)
Inclusion and exclusion criteria
The inclusion criteria were as follows: (1) All the included patients had KOA, and clear diagnostic criteria for KOA were described in the study. (2) The experimental group was treated with MSCs combined with PRP, and the control group was treated with MSCs, PRP or hyaluronic acid (HA) alone. It should be noted that the source of MSCs could be bone marrow, adipose tissue or umbilical cord tissue. (3) The type of study was a clinical controlled trial. (4) The relevant outcome indicators of clinical efficacy or safety were reported in the literature. To better evaluate the changes after treatment in patients with KOA, the Visual Analogue Scale (VAS) and Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) scores were selected as primary outcomes, and the Knee Injury and Osteoarthritis Outcome Score (KOOS) and adverse reactions were selected as secondary outcomes. (5) There were no restrictions regarding the language of the published studies.
The exclusion criteria were as follows: (1) incomplete original data; (2) results of repeated studies in the same population and (3) interventions involving surgical treatment.
Data extraction and literature quality evaluation
Two researchers independently conducted literature screening and data extraction according to the inclusion and exclusion criteria. If there were different opinions, they were discussed and resolved with a third researcher. The researchers standardised the relevant data included in the literature. The extracted data included basic information of the study (first author, year of publication, sample size, patient age, etc), outcome indicators and information related to the literature quality evaluation.
Study quality was evaluated using the risk-of-bias assessment tool version 2.0 (ROB 2.0) recommended by Cochrane.22 The evaluation tool evaluates the risk of bias in five areas. If the evaluation results in five areas indicate low risk, the overall risk of bias is low. If the assessment result of any one of these areas indicates high risk or the assessment result of multiple areas indicates possible risk, the overall risk is high. If neither of the above two conditions is met, the clinical control trial is judged as having a possible risk of bias.
Data analysis
Review Manager V.5.3 software (Cochrane Collaboration, UK) was used for data analysis in this meta-analysis. Continuous variables are represented by the weighted mean difference (WMD) and 95% CI, and classified variables are represented by the OR and 95% CI. The WMD and OR were calculated in our study referring to the method in the Cochrane system evaluation manual.23 A p<0.05 was considered statistically significant. We used the Cochrane Q test to evaluate the heterogeneity among studies, which was judged according to the I2 value. I2<50% was considered to indicate low heterogeneity, and the data were analysed by a fixed-effect model; I2≥50% was considered to indicate high heterogeneity, and the data were corrected by a random-effect model and interpreted carefully. In addition, if more than 10 articles were included, we created a funnel chart to evaluate publication bias; if the number of included studies was less than 10, we did not analyse publication bias.
Patient and public involvement
Neither the patients nor the public substantially participated in any stage of this study.
Results
Literature search results
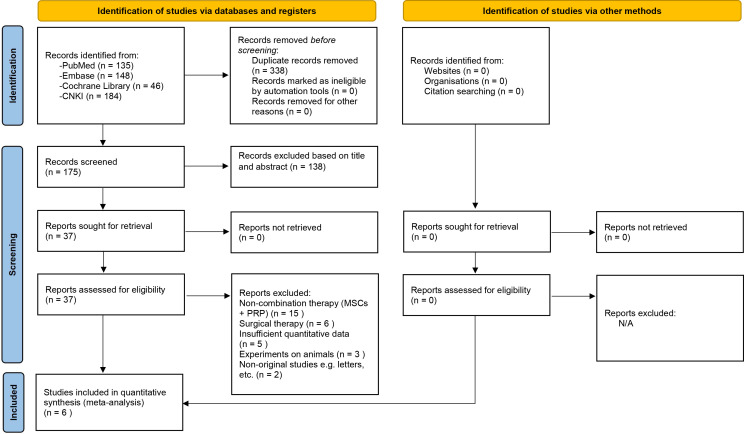
A total of 513 studies were identified, including 135 in PubMed, 148 in Embase, 46 in the Cochrane Library and 184 in CNKI. After removing duplicate studies and reading the full text, six studies24–29 were finally included, including two from Portugal,24 26 three from China27–29 and one from Spain.26 The document retrieval process and reasons for elimination are shown in figure 1. The six studies included in this study were clinical controlled trials, and the level of clinical evidence was 1–2. One study was a four-arm trial,29 and the control groups were treated with MSCs, PRP or HA alone, without a blank group or placebo among the control groups. A total of 493 cases were included, including 204 cases in the experimental group and 289 cases in the control group. Five studies were conducted with MSCs derived from bone marrow,24–28 and one study was conducted with stem cells derived from umbilical cord tissue.29 All the included studies24–29 used MSCs combined with PRP in the experimental groups. The average age of the patients included in each study was higher than 54 years. One included study27 did not report the OA stage of the included patients. The other five studies24–26 28 29 reported the OA stage of the included patients (using the Kellgren-Lawrence (K-L) staging method), and the stage ranged from 1 to 4. The follow-up time was 6–12 months. The basic characteristics of the studies included in this analysis are shown in table 1.
Figure 1.
Flow chart of the included studies.
Table 1.
Characteristics of the included studies
| First author | Year | Country | Level of evidence | No of participants (M/F) | Intervention method in CG | Age, years (mean or mean±SD) | BMI, kg/m2 (mean or mean±SD) | Follow-up period, months | Stem cell source | K-L grade | |||
| MSCs+PRP | CG | MSCs+PRP | CG | MSCs+PRP | CG | ||||||||
| Bastos24 | 2019 | Portugal | II | 14 (5/9) | 16 (10/6) | MSCs | 60.8±9.9 | 55.7±7.8 | 28.9±4.9 | 30.6±4.5 | 12 | Bone marrow | 1–4 |
| Lamo-Espinosa25 | 2020 | Spain | I | 24 (17/7) | 26 (16/10) | PRP | 56 | 54.6 | 27 | 25.3 | 12 | Bone marrow | 2–4 |
| Bastos26 | 2018 | Portugal | II | 9 (4/5) | 9 (5/4) | MSCs | 60.4±11.3 | 54.7±7.2 | NR | NR | 12 | Bone marrow | 2–4 |
| Hou27 | 2016 | China | I | 92 (40/52) | 88 (38/50) | HA | 57±8.3 | 55±9.2 | NR | NR | 6 | Bone marrow | NR |
| Cheng28 | 2019 | China | I | 20 (8/12) | 20 (9/11) | HA | 54.6±6.2 | 52.9±5.3 | 22.1±1.6 | 21.5±1.5 | 6 | Bone marrow | 1–3 |
| Ha29 | 2018 | China | I | 45 (15/30) | 44 (14/30) 43 (12/31) 43 (11/32) |
PRP MSCs HA |
56.8±6.1 | 55.6±3.6 57±3.2 56.2±6.7 |
25.5±2 | 25.4±0.8 25.8±3.3 24.9±1.6 |
12 | Umbilical cord | 1–3 |
BMI, body mass index; CG, control group; F, female; HA, hyaluronic acid; KL, Kellgren-Lawrence; M, male; MSCs, mesenchymal stem cells; NR, not reported; PRP, platelet-rich plasma.
Study quality evaluation
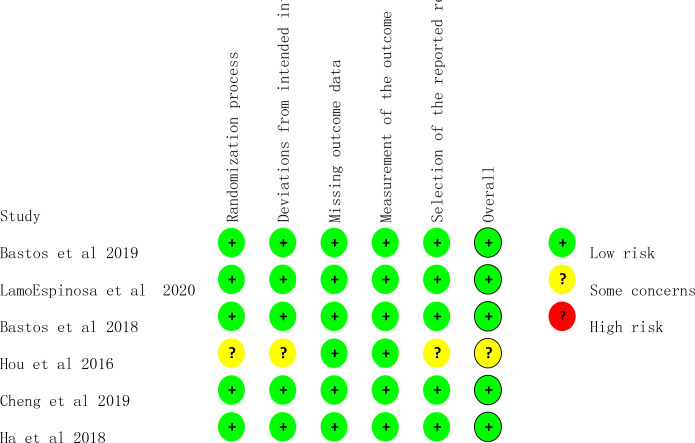
In this study, the Cochrane ROB 2.0 for randomised controlled trials was used to evaluate the quality of the six included studies. Overall, five studies were evaluated as having low risk.24–27 29 In terms of the blinding method, five studies all described the random allocation method used in the study,24–27 29 and only one study28 did not specify the specific method of randomization. In terms of data integrity, six studies24–29 were reported according to the research scheme, and the follow-up protocol and excluded cases were accurately explained. In terms of selective reporting, one article was not clear about the risk due to the lack of necessary information for selective reporting evaluation.28 The above literature quality evaluation results show that the methodological quality of the literature included in this study is generally high, which is of high value for excluding clinical heterogeneity. The risk-of-bias results of each study are shown in figure 2.
Figure 2.
Risk of bias assessment.
Meta-analysis results
VAS score
VAS score three months after treatment
Three studies25 28 29 reported a comparison of VAS scores 3 months after treatment. There was no heterogeneity among the studies (p=0.67, I2=0%); thus, a fixed-effect model was used. The results of the meta-analysis showed that there was no significant difference in the VAS scores of patients with KOA 3 months after treatment with MSCs+PRP compared with the VAS scores of patients in the control group (WMD −0.30, 95% CI −0.64 to 0.04). Similarly, compared with HA alone (WMD −0.57, 95% CI - 1.34 to 0.20, p=0.15) or PRP alone (WMD −0.34, 95% CI - 0.72 to 0.03, p=0.07), MSCs+PRP did not have an advantage in reducing the degree of pain (online supplemental figure 1).
VAS score 6 months after treatment
Three studies25 28 29 reported a comparison of VAS scores 6 months after treatment. The results of the meta-analysis showed that compared with the control, MSCs+PRP could reduce the VAS score of patients with KOA after 6 months (WMD −0.40, 95% CI −0.72 to 0.07), and the difference was statistically significant (p=0.02). Compared with HA alone (WMD −0.99, 95% CI −1.75 to −0.23, p=0.01) or PRP alone (WMD −0.38, 95% CI −0.73 to −0.03, p=0.03), MSCs+PRP showed more advantages in reducing the VAS score of patients with KOA (online supplemental figure 2).
VAS score 12 months after treatment
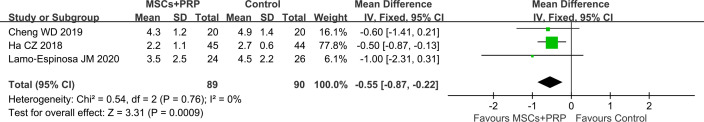
The results of the meta-analysis showed that compared with the control (WMD −0.55, 95% CI −0.87 to −0.22, p=0.0009), HA alone (WMD −1.20, 95% CI −2.28 to −0.13, p=0.03) and PRP alone (WMD −0.54, 95% CI −0.89 to −0.18, p=0.003), MSCs+PRP could significantly reduce the VAS score of patients with KOA 12 months after treatment (figure 3 and online supplemental figure 3).
Figure 3.
Forest plot of VAS score 12 months after treatment: MSCs+PRP versus control group (PRP and HA in control group). HA, hyaluronic acid; MSCs, mesenchymal stem cells; PRP, platelet-rich plasma; VAS, Visual Analogue Scale.
WOMAC score
WOMAC score 3 months after treatment
Meta-analysis showed that compared with the control (WMD −5.20, 95% CI −8.55 to −1.86, p=0.002) and HA alone (WMD −6.65, 95% CI −8.06 to −5.05, p<0.00001), MSCs+PRP could significantly reduce the total WOMAC score 3 months after treatment (online supplemental figure 4).
WOMAC score 6 months after treatment
The results of this study showed that MSCs+PRP could reduce the total WOMAC score 6 months after treatment compared with the control (WMD −7.65, 95% CI - 12.38 to −2.92, p=0.002) or HA alone (WMD −9.11, 95% CI - 13.43 to −4.80, p<0.0001) (online supplemental figure 5).
WOMAC score 12 months after treatment
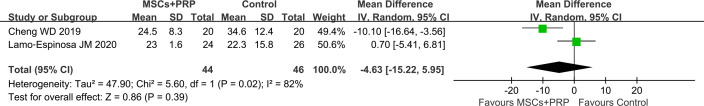
The results of the meta-analysis showed that there was no significant difference between MSCs+PRP and the control in terms of the reduction of the total WOMAC score 12 months after treatment (WMD −4.63, 95% CI - 15.22 to 5.95, p=0.39) (figure 4).
Figure 4.
Forest plot of WOMAC score 12 months after treatment: MSCs+PRP versus control group (PRP and HA in control group). HA, hyaluronic acid; MSCs, mesenchymal stem cells; PRP, platelet-rich plasma; WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index.
KOOS 12 months after treatment
The heterogeneity among the studies was low (p=0.42, I2=0%); thus, a fixed-effect model was adopted. Meta-analysis showed that there was no significant difference between MSCs+PRP and the control in terms of improvement of the KOOS 12 months after treatment (WMD 10.08, 95% CI −4.13 to 24.30, p=0.16) (online supplemental figure 6).
Adverse reactions
Only two studies26 29 reported the incidence of adverse reactions. The specific manifestations of adverse reactions were aggravation of knee joint pain, low back pain, low fever and swelling, and the above adverse reactions were relatively mild. There was no heterogeneity among the studies (p=0.38, I2=0%); thus, a fixed-effect model was used. Meta-analysis showed that there was no significant difference in the incidence of adverse reactions between MSCs+PRP and the control (OR 0.59, 95% CI 0.25 to 1.36, p=0.22) (online supplemental figure 7).
Discussion
Early KOA is generally treated with drugs, but drug treatment has limitations, including failure to repair damaged cartilage and serious gastrointestinal and renal adverse reactions.30 31 Therefore, it is of great significance to explore safer and more effective treatments or combination therapies for KOA. The results of this meta-analysis showed that compared with the control, HA and PRP alone, MSCs+PRP had more advantages in reducing the VAS score of patients with KOA at 6 and 12 months after treatment; MSCs+PRP also showed better clinical efficacy than the control and HA in improving the total WOMAC score of patients with KOA 3 months and 6 months after treatment. There was no significant difference in the incidence of adverse reactions between patients treated with MSCs alone and MSCs+PRP. It is worth noting that some statistically significant results were found in this meta-analysis, but the statistically significant differences do not necessarily represent significant differences in clinical efficacy; in additiony, the number of randomised controlled trials included in this systematic review was small, which may lead to false-positive statistical results. Therefore, we suggest that users should consider the KOA stage and the tolerance of patients to treatment with PRP or MSCs in combination with our research conclusions when considering the best treatment strategy.
According to this meta-analysis, we believe that MSCs+PRP has potential advantages in improving pain and joint activity in patients with KOA. Although many studies have indicated that MSCs can repair joint surface damage, the function of MSCs depends more on the local microenvironment of the joint.32 The inflammatory and apoptotic environment formed by the loss of intra-articular dynamic balance will affect the proliferation and differentiation of MSCs.33 The addition of PRP can improve the intra-articular microenvironment and promote the proliferation and differentiation of MSCs, which is also consistent with the conclusion that PRP can promote the proliferation and differentiation of MSCs confirmed by in vitro experiments.14 Combined with the conclusions of this study and previous basic studies,14 32 33 we believe that MSCs combined with PRP can improve the intra-articular microenvironment and promote the proliferation and differentiation of MSCs to repair damaged chondrocytes, inhibit the synovial inflammatory response, improve the intra-articular microenvironment and treat KOA. This may be the physiological mechanism through which MSCs+PRP improves VAS scores and total WOMAC scores in patients with KOA after treatment. In addition, due to the small number of included studies, we did not compare the efficacy of MSCs+PRP among different age, body mass index and sex subgroups. Previous studies34 35 of PRP for KOA have shown a shorter time of pain reappearance among patients with K-L stage 3 KOA than among patients with K-L stage I KOA and considered that age was negatively correlated with efficacy. Due to differences in age, sex and K-L stage, among other factors, different treatment schemes may show differences in efficacy in KOA patients. The study population should also be limited in future studies to clarify the therapeutic effect of MSCs+PRP.
The limitations of this study are as follows: (1) Although the quality of the included studies was generally high, the total number of studies and sample size were small; thus, the results still need to be confirmed by more studies in the future. (2) The follow-up time reported in the studies included in this meta-analysis was short, no more than 12 months, which is not conducive to deducing the long-term efficacy of MSCs combined with PRP for the treatment of KOA. (3) The outcome indicators of this study lack an objective test index and imaging indicators, which is not conducive to the multidimensional evaluation of the efficacy of MSCs combined with PRP for the treatment of KOA.
Conclusions
MSCs+PRP has a good clinical effect in improving pain and joint function in patients with KOA. Compared with MSCs alone, there was no significant difference in the incidence of adverse reactions with the clinical application of MSCs+PRP. Future research should focus on changes in the efficacy of MSCs+PRP over short-term, medium-term and long-term follow-up, which would be very valuable for clinicians in selecting treatment prescriptions. Due to the limited number of studies included, the conclusions of the above meta-analysis still need to be confirmed by larger multicentre, clinical, randomised controlled trials with longer follow-up periods and evaluations of more objective physical, chemical and imaging indexes.
Supplementary Material
Footnotes
Contributors: JL and L-FZ conceived of the project, acting as guarantor, wrote the article and critically revised it for methodological and intellectual substance. JZ, GL and YH wrote the first draft of the manuscript. The search strategy, data extraction and literature search were all done by NX, WY, ML and JP who also critically revised the work for methodological and intellectual content. The final paper was approved by all authors.
Funding: This work was supported by the the National Natural Science Foundation of China (No. 81873314, No. 82004386), Natural Science Foundation of Guangdong Province (No.2022A1515010385, No.2022A1515011700), Research Fund for Bajian Talents of Guangdong Provincial Hospital of Chinese Medicine (No.BJ2022KY01), Project of Philosophy and Social Science Planning of Guangzhou in 2022 (No.2022GZQN42), the Project of Administration of Traditional Chinese Medicine of Guangdong Province (No.20225025, No.20231109), the Project of Guangdong Provincial Department of Finance (No.[2018]8), the National key research and development program (2021YFC1712804) and the Science and Technology Research Project of Guangdong Provincial Hospital of Chinese Medicine (No.YN2019ML08, YN2015MS15).
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
All data relevant to the study are included in the article or uploaded as online supplemental information. No data are available. The authors confirm that the data supporting the findings of this study are available within the article and its online supplemental materials.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.Hartley A, Gregson CL, Paternoster L, et al. Osteoarthritis: insights offered by the study of bone mass genetics. Curr Osteoporos Rep 2021;19:115–22. 10.1007/s11914-021-00655-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Geusens PP, van den Bergh JP. Osteoporosis and osteoarthritis. Curr Opin Rheumatol 2016;28:97–103. 10.1097/BOR.0000000000000256 [DOI] [PubMed] [Google Scholar]
- 3.Lo CWT, Tsang WWN, Yan CH, et al. Risk factors for falls in patients with total hip arthroplasty and total knee arthroplasty: a systematic review and meta-analysis. Osteoarthritis Cartilage 2019;27:979–93. 10.1016/j.joca.2019.04.006 [DOI] [PubMed] [Google Scholar]
- 4.Farpour HR, Fereydooni F. Comparative effectiveness of intra-articular prolotherapy versus peri-articular prolotherapy on pain reduction and improving function in patients with knee osteoarthritis: a randomized clinical trial. Electron Physician 2017;9:5663–9. 10.19082/5663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Soler R, Orozco L, Munar A, et al. Final results of a phase I-II trial using ex vivo expanded autologous mesenchymal stromal cells for the treatment of osteoarthritis of the knee confirming safety and suggesting cartilage regeneration. Knee 2016;23:647–54. 10.1016/j.knee.2015.08.013 [DOI] [PubMed] [Google Scholar]
- 6.Bruyère O, Cooper C, Pelletier J-P, et al. A consensus statement on the European society for clinical and economic aspects of osteoporosis and osteoarthritis (ESCEO) algorithm for the management of knee osteoarthritis-from evidence-based medicine to the real-life setting. Semin Arthritis Rheum 2016;45:S3–11. 10.1016/j.semarthrit.2015.11.010 [DOI] [PubMed] [Google Scholar]
- 7.ter Huurne M, Schelbergen R, Blattes R, et al. Antiinflammatory and chondroprotective effects of intraarticular injection of adipose-derived stem cells in experimental osteoarthritis. Arthritis Rheum 2012;64:3604–13. 10.1002/art.34626 [DOI] [PubMed] [Google Scholar]
- 8.Park M-J, Moon S-J, Baek J-A, et al. Metformin augments anti-inflammatory and chondroprotective properties of mesenchymal stem cells in experimental osteoarthritis. J Immunol 2019;203:127–36. 10.4049/jimmunol.1800006 [DOI] [PubMed] [Google Scholar]
- 9.Fusco G, Gambaro FM, Di Matteo B, et al. Injections in the osteoarthritic knee: a review of current treatment options. EFORT Open Rev 2021;6:501–9. 10.1302/2058-5241.6.210026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bennell KL, Hunter DJ, Paterson KL. Platelet-rich plasma for the management of hip and knee osteoarthritis. Curr Rheumatol Rep 2017;19:24. 10.1007/s11926-017-0652-x [DOI] [PubMed] [Google Scholar]
- 11.Karasavvidis T, Totlis T, Gilat R, et al. Platelet-rich plasma combined with hyaluronic acid improves pain and function compared with hyaluronic acid alone in knee osteoarthritis: a systematic review and meta-analysis. Arthroscopy 2021;37:1277–87. 10.1016/j.arthro.2020.11.052 [DOI] [PubMed] [Google Scholar]
- 12.Zhao J, Huang H, Liang G, et al. Effects and safety of the combination of platelet-rich plasma (PrP) and hyaluronic acid (HA) in the treatment of knee osteoarthritis: a systematic review and meta-analysis. BMC Musculoskelet Disord 2020;21:224. 10.1186/s12891-020-03262-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ramezanifard R, Kabiri M, Hanaee Ahvaz H. Effects of platelet rich plasma and chondrocyte co-culture on MSC chondrogenesis, hypertrophy and pathological responses. Excli J 2017;16:1031–45. 10.17179/excli2017-453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu F-T, Li H-M, Yin Q-S, et al. Effect of activated autologous platelet-rich plasma on proliferation and osteogenic differentiation of human adipose-derived stem cells in vitro. Am J Transl Res 2015;7:257–70. [PMC free article] [PubMed] [Google Scholar]
- 15.Kasten P, Beverungen M, Lorenz H, et al. Comparison of platelet-rich plasma and VEGF-transfected mesenchymal stem cells on vascularization and bone formation in a critical-size bone defect. Cells Tissues Organs 2012;196:523–33. 10.1159/000337490 [DOI] [PubMed] [Google Scholar]
- 16.Woodell-May J, Matuska A, Oyster M, et al. Autologous protein solution inhibits MMP-13 production by IL-1β and TNFα-stimulated human articular chondrocytes. J Orthop Res 2011;29:1320–6. 10.1002/jor.21384 [DOI] [PubMed] [Google Scholar]
- 17.Tang X-B, Dong P-L, Wang J, et al. Effect of autologous platelet-rich plasma on the chondrogenic differentiation of rabbit adipose-derived stem cells in vitro. Exp Ther Med 2015;10:477–83. 10.3892/etm.2015.2528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dório M, Pereira RMR, Luz AGB, et al. Efficacy of platelet-rich plasma and plasma for symptomatic treatment of knee osteoarthritis: a double-blinded placebo-controlled randomized clinical trial. BMC Musculoskelet Disord 2021;22:822. 10.1186/s12891-021-04706-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun S-F, Hsu C-W, Lin H-S, et al. A single intraarticular platelet-rich plasma improves pain and function for patients with early knee osteoarthritis: analyses by radiographic severity and age. J Back Musculoskelet Rehabil 2022;35:93–102. 10.3233/BMR-200193 [DOI] [PubMed] [Google Scholar]
- 20.Li J, Shao Q, Zhu X, et al. Efficacy of autologous bone marrow mesenchymal stem cells in the treatment of knee osteoarthritis and their effects on the expression of serum TNF-α and IL-6. J Musculoskelet Neuronal Interact 2020;20:128–35. [PMC free article] [PubMed] [Google Scholar]
- 21.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sterne JAC, Savović J, Page MJ, et al. Rob 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019;366:l4898. 10.1136/bmj.l4898 [DOI] [PubMed] [Google Scholar]
- 23.Cumpston M, Li T, Page MJ, et al. Updated guidance for trusted systematic reviews: a new edition of the cochrane handbook for systematic reviews of interventions. Cochrane Database Syst Rev 2019;10:ED000142. 10.1002/14651858.ED000142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bastos R, Mathias M, Andrade R, et al. Intra-articular injection of culture-expanded mesenchymal stem cells with or without addition of platelet-rich plasma is effective in decreasing pain and symptoms in knee osteoarthritis: a controlled, double-blind clinical trial. Knee Surg Sports Traumatol Arthrosc 2020;28:1989–99. 10.1007/s00167-019-05732-8 [DOI] [PubMed] [Google Scholar]
- 25.Lamo-Espinosa JM, Blanco JF, Sánchez M, et al. Phase II multicenter randomized controlled clinical trial on the efficacy of intra-articular injection of autologous bone marrow mesenchymal stem cells with platelet rich plasma for the treatment of knee osteoarthritis. J Transl Med 2020;18:356. 10.1186/s12967-020-02530-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bastos R, Mathias M, Andrade R, et al. Intra-articular injections of expanded mesenchymal stem cells with and without addition of platelet-rich plasma are safe and effective for knee osteoarthritis. Knee Surg Sports Traumatol Arthrosc 2018;26:3342–50. 10.1007/s00167-018-4883-9 [DOI] [PubMed] [Google Scholar]
- 27.Hou JZ, Bao HW, Xiao HX. Effect of intra-articular injection of bone marrow PrP on knee osteoarthritis. Med J of Communications 2016;30:73–5. [Google Scholar]
- 28.Cheng WD, SL X, XS W. Autologous bone marrow mesenchymal stem cells combined with platelet-rich plasma treats knee osteoarthritis. Chinese Journal of General Practice 2019;17:1652–5. 10.16766/j.cnki.issn.1674-4152.001020 [DOI] [Google Scholar]
- 29.CZ H, Li W, Ren SD. Effect of platelet rich plasma combined with mesenchymal stem cells in treatment of knee osteoarthritis. Chin J Joint Surg 2018;12:644–52. [Google Scholar]
- 30.Cao P, Li Y, Tang Y, et al. Pharmacotherapy for knee osteoarthritis: current and emerging therapies. Expert Opin Pharmacother 2020;21:797–809. 10.1080/14656566.2020.1732924 [DOI] [PubMed] [Google Scholar]
- 31.Jones BQ, Covey CJ, Sineath MH. Nonsurgical management of knee pain in adults. Am Fam Physician 2015;92:875–83. [PubMed] [Google Scholar]
- 32.Emadedin M, Ghorbani Liastani M, Fazeli R, et al. Long-term follow-up of intra-articular injection of autologous mesenchymal stem cells in patients with knee, ankle, or hip osteoarthritis. Arch Iran Med 2015;18:336. doi:015186/AIM.003 [PubMed] [Google Scholar]
- 33.Cui G-H, Wang YY, Li C-J, et al. Efficacy of mesenchymal stem cells in treating patients with osteoarthritis of the knee: a meta-analysis. Exp Ther Med 2016;12:3390–400. 10.3892/etm.2016.3791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kon E, Mandelbaum B, Buda R, et al. Platelet-rich plasma intra-articular injection versus hyaluronic acid viscosupplementation as treatments for cartilage pathology: from early degeneration to osteoarthritis. Arthroscopy 2011;27:1490–501. 10.1016/j.arthro.2011.05.011 [DOI] [PubMed] [Google Scholar]
- 35.Jang S-J, Kim J-D, Cha S-S. Platelet-rich plasma (PrP) injections as an effective treatment for early osteoarthritis. Eur J Orthop Surg Traumatol 2013;23:573–80. 10.1007/s00590-012-1037-5 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2022-061008supp001.pdf (610KB, pdf)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as online supplemental information. No data are available. The authors confirm that the data supporting the findings of this study are available within the article and its online supplemental materials.