ABSTRACT
TonB-dependent transporters (TDTs) are essential proteins for metal acquisition, an important step in the growth and pathogenesis of many pathogens, including Neisseria gonorrhoeae, the causative agent of gonorrhea. There is currently no available vaccine for gonorrhea; TDTs are being investigated as vaccine candidates because they are highly conserved and expressed in vivo. Transferrin binding protein A (TbpA) is an essential virulence factor in the initiation of experimental infection in human males and functions by acquiring iron upon binding to host transferrin (human transferrin [hTf]). The loop 3 helix (L3H) is a helix finger that inserts into the hTf C-lobe and is required for hTf binding and subsequent iron acquisition. This study identified and characterized the first TbpA single-point substitutions resulting in significantly decreased hTf binding and iron acquisition, suggesting that the helix structure is more important than charge for hTf binding and utilization. The tbpA D355P ΔtbpB and tbpA A356P ΔtbpB mutants demonstrated significantly reduced hTf binding and impaired iron uptake from Fe-loaded hTf; however, only the tbpA A356P ΔtbpB mutant was able to grow when hTf was the sole source of iron. The expression of tbpB was able to restore function in all tbpA mutants. These results implicate both D355 and A356 in the key binding, extraction, and uptake functions of gonococcal TbpA.
KEYWORDS: TonB-dependent transporter, transferrin binding protein A, transferrin, site-directed mutagenesis, N. gonorrhoeae, N. meningitidis, Neisseria gonorrhoeae, Neisseria meningitidis
INTRODUCTION
Neisseria gonorrhoeae is the human-specific pathogen that causes the sexually transmitted infection gonorrhea. The World Health Organization (WHO) reported 87 million new cases globally in 2016 (1), and the Centers for Disease Control and Prevention (CDC) estimates that approximately 1.6 million new cases occurred in 2018 in the United States alone (2). Uncomplicated gonorrhea presents as urethritis in men and cervicitis in women (3–6). An estimated 80% of gonococcal infections in women are asymptomatic (3, 7). Asymptomatic cases that are left untreated allow gonococcal infection to ascend upward in the reproductive tract and cause more severe secondary sequelae, such as pelvic inflammatory disease, ectopic pregnancy, and infertility (3). Previous gonococcal infection does not provide protective immunity (5, 8, 9), and there has been an increase in the incidence of antimicrobial resistance among gonococcal isolates (1, 10–16). Reports to the WHO in 2018 indicated that 7 out of 65 countries reported isolating gonococcal strains with decreased susceptibility or resistance to extended-spectrum cephalosporins (10). Drastic increases in antimicrobial resistance, including resistance to azithromycin, caused the CDC to modify recommendations for treatment as of late 2020; uncomplicated gonococcal infection should currently be treated with monotherapy of ceftriaxone without additional oral azithromycin (17). The availability of effective therapeutics has dwindled to the point that researchers are analyzing older antibiotics as potential alternative treatments for gonorrhea infection (15). The widespread prevalence of gonococcal infection, the increasing incidence of antibiotic resistance, and the lack of protective immunity add urgency to the development of an effective gonococcal vaccine.
Many of the neisserial outer membrane proteins, such as the Opas and pilin, are subject to high-frequency antigenic variation (18–21), allowing the gonococcus to effectively camouflage itself from an adaptive immune response. This genetic adaptability has presented quite a challenge for vaccine development. TonB-dependent metal transporters (TDTs) have been the focus of several vaccine studies (20, 22–28). TDTs are well-suited vaccine candidates because they are highly conserved, present in all pathogenic Neisseria strains (29), and expressed in vivo (30), and many are not subject to high-frequency antigenic variation (20, 31). The importance of transferrin binding proteins A and B (TbpAB) for gonococcal infection has been demonstrated in a human male infection study where a tbpAB double-knockout mutant was unable to elicit signs or symptoms of urethritis in human male volunteers (4). The mechanism for iron piracy through the interaction of human transferrin (hTf) with TbpA is crucial to understand as TbpA is being evaluated as a potential vaccine and therapeutic target.
Neisseria species can acquire iron through the TDT TbpA (25, 32). With the help of the lipoprotein TbpB, hTf binds to TbpA, and iron is internalized by a TonB-dependent mechanism powered by proton motive force (29, 32–35). TbpA is required for iron acquisition from hTf (36, 37), and TbpB increases the efficiency of hTf binding (38, 39). TbpA binds both apo- and saturated hTf with similar affinities, but TbpB preferentially binds to Fe-saturated hTf (40, 41).
The exact interaction between TbpA and hTf is still being elucidated. The cocrystal structure of TbpA and bound hTf demonstrates a helix finger in loop 3 (L3H) that projects into the cleft of the hTf C-lobe (29). TbpA interacts exclusively with the C-lobe for hTf acquisition, hinting at the importance of the L3H interaction for iron extraction (29). Three pH-sensing residues (K534, R632, and D634) reside in the C-lobe cleft to store iron (25, 29). Upon hTf binding, the TbpA L3H, which contains a positive charge at the penultimate residue (K359), is hypothesized to interact with the hTf C-lobe residue D634 to cause a conformational change in the C-lobe, resulting in the release and subsequent internalization of iron (25, 42). Mutation of D634A increased iron release by 83-fold, indicating that D634 neutralizes the positive charges of K359 and R632 (43). Upon hTf binding, the L3H K359 residue is inserted near the C-lobe residue D634, which causes a charge repulsion to release iron (44). A hemagglutinin (HA) epitope insertion into extracellular loop 3 (L3) and a deletion of the L3H were both disruptive enough to abrogate hTf binding and iron acquisition, demonstrating that the L3H is essential for hTf binding and iron acquisition from hTf (25, 45). Alanine or opposite-charge amino acid substitutions in the L3H failed to significantly abrogate hTf binding (25).
The current study aims to extend previous TbpA structure-function analyses by generating proline substitution mutants in the TbpA L3H. The structure of proline contains a ring, which is predicted to disrupt the helix motif. Characterization of 16 proline substitution mutants for hTf binding, iron uptake from hTf, and growth using hTf as the sole iron source helped to elucidate the details of the interaction between the L3H and hTf. Understanding the mechanism of binding is essential for developing therapeutics and vaccines. Drugs could be developed to block hTf binding for the treatment of infection (42). TbpA mutants unable to bind hTf with minimal protein conformational changes compared to the wild-type (WT) protein could be tested for immunogenicity, similar to protection studies using nonbinding TbpB and factor H binding protein (fHbp) as vaccine antigens (28, 46). Molecular dynamics (MD) simulations were utilized to predict protein conformational changes in lieu of an available crystal structure. In this study, two mutants with single proline substitutions in TbpA demonstrated significantly reduced hTf binding and iron internalization.
RESULTS
The TbpA L3H sequence is well conserved at the amino terminus, but residues are more variable at the carboxy-terminal end.
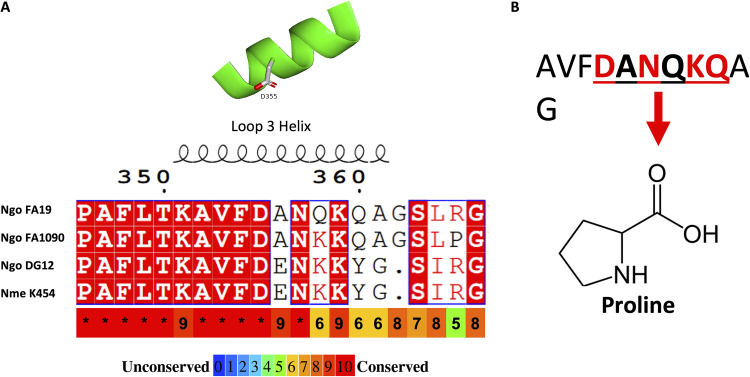
TbpA from Neisseria meningitidis strain K454 was previously crystalized (29) and shares 94% sequence identity with TbpA from N. gonorrhoeae strain FA19 (32). Using this similarity, we generated a homology model for TbpA from gonococcal strain FA19, which is shown in Fig. 1. Key components of the TbpA structure include the outer membrane-embedded β-barrel motif; the key hTf-interacting region, the L3H; and the TonB-interacting region, the plug domain (Fig. 1A and C, yellow, green, and cyan, respectively). A closer look at the L3H (Fig. 1B) shows the key residues that were mutated in this study in order to test structure-function relationships. Figure 1C shows TbpA from a top-down perspective to demonstrate the location of the plug domain. Both the FA19 model and the K454 TbpA structure share the L3H, previously shown to be essential for hTf binding (25, 29, 45). Interestingly, the helical structure is well conserved despite considerable variability at the carboxy-terminal end, the region that interacts with the iron in hTf during iron acquisition (Fig. 2A). In an attempt to identify residues critical for hTf binding, site-directed mutagenesis was conducted on several residues within the TbpA L3H region from D355 to Q360, replacing each residue with a proline (Fig. 2B).
FIG 1.
TbpA homology model from gonococcal strain FA19. (A) TbpA homology model predicted structure based on the N. meningitidis strain K454 TbpA crystal structure. The TonB-interacting plug domain is shown in cyan. The L3H is shown in green. (B) The residues of the L3H targeted for site-directed mutagenesis in this study. (C) Top-down view of TbpA demonstrating the plug domain situated in the β-barrel pore.
FIG 2.
L3H structure and amino acid sequence alignment of Neisseria strains. (A) Alignment of the TbpA L3H domain amino acid sequences for three N. gonorrhoeae (Ngo) strains (FA19, FA1090, and DG12) and one N. meningitidis (Nme) strain (K454). Conserved residues are highlighted in red. Secondary structure prediction is shown above the residues. Below the alignment is the conservation of amino acids among the closest 100 N. gonorrhoeae strains, according to the key, where blue is unconserved, and red is highly conserved. (B) The FA19 TbpA L3H sequence residues that were replaced with proline in this study (D355 to Q360). Conserved residues are shown in red, and variable residues are shown in black.
Mutagenized TbpA was surface exposed despite proline substitutions in the L3H.
A previous study characterized several FA19 tbpA L3H point mutants and did not identify any single residue that completely abrogated hTf binding (25). The current study extends those previous findings by replacing residues within the L3H with proline residues, which are proposed to be more impactful on the secondary structure of the helix. To evaluate the expression of TbpA in each mutant, strains were grown under iron-stressed conditions, and whole-cell lysates were prepared, run on SDS-PAGE gels, and transferred to nitrocellulose for Western blotting using a polyclonal anti-TbpA antibody (see Fig. S1 in the supplemental material). The Western blot analysis showed that the TbpA protein is produced by all mutants. Because mutagenesis has the potential to disrupt the three-dimensional (3D) protein conformation and localization to the outer membrane, each strain was evaluated for the proper surface exposure of TbpA using protease digestion. The trypsin digestion assay for TbpA was previously validated for digesting exclusively extracellular protein (40); therefore, a digestion pattern similar to that of wild-type cells would suggest proper extracellular localization. Each gonococcal strain was grown under iron-stressed conditions and then subjected to a time course of trypsin digestion before whole-cell lysates were collected for Western blot analysis (Fig. S2). As the cells were exposed to trypsin, full-length TbpA, approximately 100 kDa, was cleaved, producing 95-kDa and 55-kDa degradation products, as previously described (25, 40). Each mutant showed a pattern similar to that of FA19 (WT); Fig. S2 shows TbpA protein levels from FA19, FA6747, the D355P mutant, and the A356P mutant after protease digestion, which is representative of all strains tested. Taken together, these data suggest that the mutations did not disrupt TbpA production or wild-type surface exposure.
Selected mutants containing proline substitutions in the L3H are deficient in hTf binding.
Proline substitution mutants were evaluated for their ability to bind hTf in whole-cell binding assays. Since TbpB expressed by whole N. gonorrhoeae cells is also capable of binding to hTf, only tbpA mutant ΔtbpB strains were used for the hTf binding analyses. Whole-cell enzyme-linked immunosorbent assays (ELISAs) of strains lacking tbpB were conducted using horseradish peroxidase (HRP)-conjugated hTf as the ligand (Fig. 3). In these experiments, FA6905 (tbpA+ ΔtbpB) was used as a positive control (Fig. 3, WT), and two negative controls were used, FA6815 (ΔtbpA ΔtbpB) (Fig. 3, Neg) and competitive inhibition (Fig. 3, WT w/Comp and Neg w/Comp) conditions, which were generated by adding a 100-fold excess of unlabeled apo-hTf to both FA6905 and FA6815. A previous study showed that a mutant with a deletion of the L3H binds hTf at about 9% of the levels of a strain expressing WT TbpA, and mutants expressing tbpA with alanine or charge change point mutations reduced hTf binding to approximately 40 to 80% of WT FA6905 (tbpA+ ΔtbpB) levels (25). The current study found significantly reduced hTf binding in almost every tbpA mutant (Fig. 3). Significantly reduced levels of hTf binding in the mutants ranged from approximately 14 to 58% of those of FA6905 (tbpA+ ΔtbpB). Two mutants in particular, the tbpA D355P ΔtbpB and tbpA A356P ΔtbpB mutants (approximately 14% and 18% of WT HRP-hTf binding, respectively), showed the lowest levels of HRP-hTf binding of all tested mutants; the levels of hTf binding of the tbpA D355P ΔtbpB and tbpA A356P ΔtbpB mutants were not statistically different from those of the negative controls (Fig. 3).
FIG 3.
TbpA-hTf binding of tbpA mutants in a ΔtbpB background. Whole, iron-stressed gonococcal cells were applied to microtiter dishes for ELISAs using HRP-labeled hTf as a ligand. FA6905 (tbpA+ ΔtbpB) served as the positive control, and FA6815 (ΔtbpA ΔtbpB) and the excess competitor apo-hTf condition (Comp) served as the negative controls. The data represent the means ± standard errors from at least three independent experiments. All strains were compared to the positive-control strain FA6905. Statistics were calculated with Student’s t test. Significant differences are noted (*, P < 0.05; **, P < 0.005; ***, P < 0.0005).
L3H proline mutants in a ΔtbpB background are deficient in iron internalization from hTf.
Mutants with decreased hTf binding were hypothesized to demonstrate diminished levels of internalized iron. Internal iron pools were measured using inductively coupled plasma mass spectrometry (ICP-MS) in the WT tbpA strain and proline substitution mutants. In the ΔtbpB background, the tbpA D355P, A356P, N357P, Q358P, and double K359P Q360P mutants demonstrated statistically reduced levels of iron internalization compared to the WT tbpA strain (FA19) (Fig. 4). These same mutants in a tbpB+ background did not demonstrate statistically different levels of iron internalization compared to those of the WT tbpA strain, suggesting that the expression of TbpB is able to compensate for these proline substitutions in TbpA (Fig. 4). These data also indicate that the mutagenized TbpA proteins are not significantly impaired in membrane localization or structure, consistent with the data described above.
FIG 4.
Iron internalization by tbpA proline mutants in ΔtbpB background (A) and tbpB+ background (B) strains. Gonococcal cells were iron stressed and allowed to bind to 5 μM 30% Fe-saturated hTf for 1 h. Bacteria were pelleted and washed prior to being subjected to nitric acid digestion for ICP-MS analysis. Raw micrograms of Fe per gram of bacteria is plotted for each strain. FA19 (tbpA+ tbpB+) served as the positive control. FA6747 (tbpA::mTn3cat tbpB+) served as the negative control. The data represent the means ± standard errors from at least three independent experiments. All strains were compared to the positive control. Statistics were calculated with Student’s t test. Nonsignificance (ns) was defined as a P value of >0.05. Significant differences are noted (*, P < 0.05; **, P < 0.005; ***, P < 0.0005).
Several mutants with proline substitutions in the L3H of tbpA in a ΔtbpB background are unable to grow if hTf is the sole source of iron.
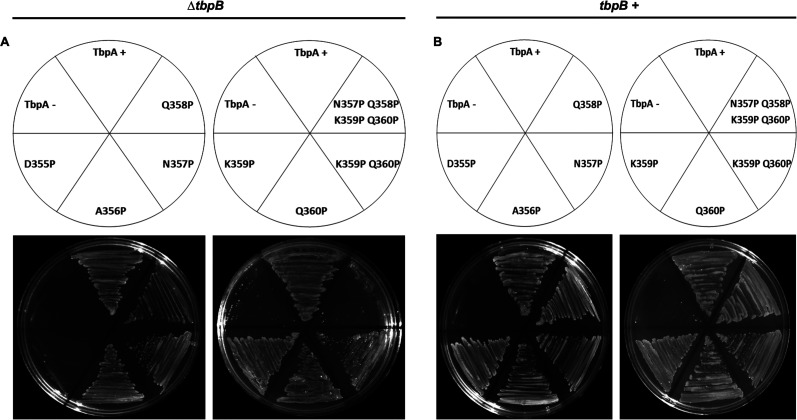
In a previous study, only the L3H deletion mutant showed any growth defect when employing hTf as the sole iron source, regardless of the presence of tbpB (25). Using the same methodology, the growth phenotype of the tbpA mutants was characterized by plating mutant gonococcal strains onto solid medium with hTf provided as the sole source of iron. Approximately equal inocula were applied to Fe-deficient chemically defined medium (CDM) agarose plates supplemented with 30% Fe-saturated hTf as the sole iron source (Fig. 5). FA19 (tbpA+ tbpB+) and FA6905 (tbpA+ ΔtbpB) served as the positive controls, and FA6747 (tbpA::mTn3cat tbpB+) and FA6815 (ΔtbpA ΔtbpB) served as the negative controls. As seen previously (25), all tbpB+ strains were able to grow like the WT tbpA strain (Fig. 5B). Both the tbpA D355P mutant and the tbpA N357P/Q358P/K359P/Q360P quadruple mutant in a ΔtbpB background were unable to grow on solid CDM with hTf as the sole source of iron (Fig. 5A).
FIG 5.
Growth of tbpA L3H proline substitution mutants on hTf plates. Strains were grown on solid CDM agarose plates supplemented with 30% Fe-saturated hTf as the sole source of iron. CDM agarose plates contained 2.5 μM 30% saturated hTf, and the growth phenotypes for ΔtbpB background (A) and tbpB+ background (B) strains were determined. Approximately equivalent amounts of bacteria were applied to each plate. (A) FA6905 (tbpA+ ΔtbpB) served as the positive control for tbpA ΔtbpB mutants, and FA6815 (ΔtbpA ΔtbpB) was the negative control for tbpA ΔtbpB mutants. (B) FA19 (tbpA+ tbpB+) was plated as the positive control for tbpA mutants in a ΔtbpB background, and FA6747 (tbpA::mTn3cat tbpB+) was the negative control for tbpA mutants in the tbpB+ background.
To assess the growth defect of the tbpA D355P ΔtbpB mutant further, a liquid CDM growth assay was developed. Iron-starved cells were transferred to a 96-well plate and supplemented with 5 μM 30% iron-saturated hTf and 5 μM deferoxamine mesylate (Desferal) to chelate free iron, and growth was monitored over 24 h. The tbpA D355P ΔtbpB strain demonstrated essentially no growth in the liquid growth assay (Fig. 6).
FIG 6.
Growth of tbpA L3H proline substitution ΔtbpB mutants on hTf as the sole source of iron in liquid CDM. Whole, iron-stressed gonococci were grown in liquid CDM supplemented with 5 μM 30% saturated hTf over 24 h. FA6905 (tbpA+ ΔtbpB) was used as a positive control, and FA6815 (ΔtbpA ΔtbpB) served as a negative control. Two-way ANOVA was used to determine significance in comparison to the positive control. Significant differences are noted (***, P < 0.0005).
The tbpA D355P ΔtbpB mutant demonstrates reduced hTf binding in serum.
To assess if the binding deficiency of the tbpA D355P ΔtbpB mutant could be replicated in a more biologically relevant environment, three samples of human serum were pooled and used as a source of hTf to assess hTf-TbpA binding on whole cells. FA6905 (tbpA+ ΔtbpB) served as a positive control for hTf binding, and FA6815 (ΔtbpA ΔtbpB) served as a negative control for hTf binding. The controls and the tbpA D355P ΔtbpB strain were grown in Fe-depleted liquid CDM. Whole cells were blocked with bovine serum albumin (BSA) prior to the addition of hTf or pooled human serum, and after a 20-min incubation, lysates were collected for Western blot analysis (Fig. 7). Approximately equivalent levels of TbpA were detected in the tbpA D355P ΔtbpB strains compared to FA6905 (tbpA+ ΔtbpB), but reduced binding was detected in the cases of hTf alone and hTf in the context of serum using the tbpA D355P ΔtbpB mutant. The small amount of background binding of FA6815 (ΔtbpA ΔtbpB) suggests that there may be components in the serum that allow hTf to interact with the gonococcus by a TbpAB-independent mechanism.
FIG 7.
The tbpA D355P ΔtbpB mutant shows reduced hTf binding compared to FA6905 (tbpA+ ΔtbpB). Iron-stressed gonococcal cells were incubated with 1 μM hTf or approximately 1 μM hTf from human serum for 20 min. Lysates were collected, subjected to SDS-PAGE, transferred to nitrocellulose, and probed with polyclonal anti-TbpA antibody and polyclonal anti-hTf antibody. FA6905 (tbpA+ ΔtbpB) was used as a positive control, and FA6815 (ΔtbpA ΔtbpB) was used as a negative control. Ponceau S staining was used to show even protein loading. TbpA is approximately 100 kDa, and hTf is approximately 80 kDa.
Molecular dynamics simulations suggest that D355P mutagenesis is disruptive to the TbpA L2 and L3 structures but not the L5 structure.
Because there is no experimentally determined structure of the D355P mutant, we instead used molecular dynamics (MD) simulations to model its effects on the structure and dynamics of the L3H and the interactions between TbpA and hTf. MD is a mature approach that provides atomistic-level insight into the microsecond-scale dynamics of proteins in native-like environments with typically high qualitative and quantitative agreement with experimental measurements (47). Here, MD simulations were performed on four systems, WT TbpA, WT TbpA plus hTf, TbpA D355P, and TbpA D355P plus hTf; in all systems, the protein was embedded in a realistic species-specific outer membrane model and solvated with water and ions (see Materials and Methods). Two replicas were run for each system in order to improve the reliability of our observations (48). The L3H begins at residue 351 and ends at residue 361, as shown by the α-helix (H) (orange) in Fig. 8. The presence of hTf stabilized the α-helix secondary structure, as demonstrated by fewer interruptions of the α-helix in WT TbpA plus hTf (Fig. 8B) than in WT TbpA (Fig. 8A). The α-helix structure of the L3H was interrupted by β-turns (T) (blue) after D355P mutagenesis (Fig. 8C); however, the presence of hTf appeared to be moderately stabilizing to the helical structure despite D355P mutagenesis (Fig. 8D). To assess whether the mutation in the L3H impacted the overall structure of the protein, two wild-type loops were assessed for their structure compared to WT TbpA. The predicted secondary structure of extracellular L2 demonstrated some variation between residues 262 and 276 (Fig. S4 and S5); however, the predicted secondary structure of extracellular L5 remained highly conserved (Fig. S6 and S7).
FIG 8.
TbpA L3 mutagenesis and secondary structure conservation. Shown are the secondary structures around TbpA L3 over the course of a 500-ns MD simulation for WT TbpA (A), WT TbpA plus hTf (B), TbpA D355P (C), and TbpA D355P plus hTf (D). L3H residue 355 is highlighted in red in each panel. The key at the bottom indicates the colors for the seven standard secondary structures as described in the Dictionary of Protein Secondary Structure (75). T, β-turn; E, β-sheet; B, β-bridge; H, α-helix; G, 310 helix; I, π-helix; C, unstructured coil. Plots for the second replica are provided in Fig. S3 in the supplemental material.
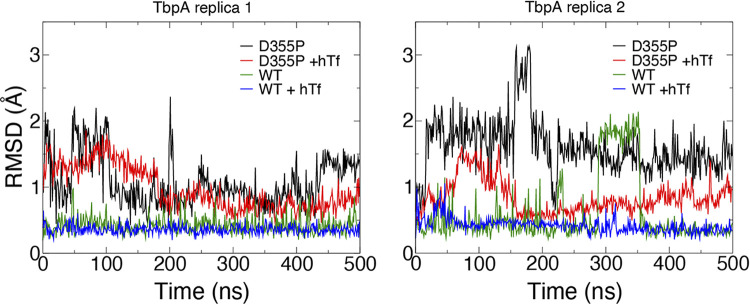
We also measured the root mean square deviation (RMSD) for TbpA L3H over time for each simulated system and replica. This metric illustrates how much the region diverges from the original structure over time after being superimposed. The RMSD values measured here demonstrate that there is more distance between that TbpA L3H in TbpA D355P and TbpA D355P plus hTf than in WT TbpA and WT TbpA plus hTf (Fig. 9). Consistent with the results shown in Fig. 8, the RMSD plots also demonstrated that the presence of hTf is more stabilizing to the secondary structure (Fig. 9). The numbers of hydrogen bonds between TbpA and hTf were also tracked for the WT and D355P mutant systems (Fig. 10). We find that for one of the two replicas, the number of hydrogen bonds in the D355P mutant drops precipitously after ~200 ns, from ~11 to 6. In contrast, both WT replicas maintain similar numbers of hydrogen bonds (~10 to 12) throughout the trajectories.
FIG 9.
Root mean square deviations (RMSDs) of TbpA L3H mutants bound and unbound to hTf. The RMSDs of the L3 helix (residues 351 to 361) after alignment for full-length WT TbpA, WT TbpA plus hTf, TbpA D355P, and TbpA D355P plus hTf over the course of two 500-ns MD simulations are shown (left, replica 1; right, replica 2).
FIG 10.

Hydrogen bonds of WT D355P and TbpA D355P over the course of 500-ns MD simulations. Shown are hydrogen bonds with hTf for WT TbpA (blue and green for replicas 1 and 2, respectively) and TbpA D355P (black and red for replicas 1 and 2, respectively) over the course of 500-ns MD simulations.
DISCUSSION
Prior to this study, no TbpA point mutation has been reported to completely abrogate hTf binding. The generation of such mutants was facilitated by the availability of the 3D crystal structure of TbpA from N. meningitidis (K454) bound to hTf (25, 49). Because the TbpA sequence from gonococcal strain FA19 shares 94% sequence identity with the meningococcal K454 strain, important structural inferences can be made to identify crucial binding domains (25). Initially, the K359 and Q360 residues were predicted to be key hTf-interacting and binding partners based on their charge and proximity to the C-lobe of hTf (25). The previous mutagenesis study demonstrates that alanine or opposite-charge point substitutions are insufficient in abolishing hTf binding and TbpA function. In the previous study, the most substantial decrease in hTf binding was observed with the substitution of residue 360Q to a negative charge, followed by the substitution of residue 359K with the opposite or no charge (25). The high level of conservation of the L3H secondary structure among Neisseria species and the data from this study suggest that the structure of the helix may be more important for function than the charges of L3H residues (50). MD simulations were used to elucidate the mechanism of TbpA binding to hTf (42). Prior to hTf binding, a conformational change occurs within TbpA, including L5, L8, and the L3H shifting from a “closed” to an “open” state, providing space for hTf to interact (42). In the closed state, the L3H is shifted downward, preventing hTf binding, whereas in the open state, the helix finger is positioned upward and can be inserted into the cleft of hTf (42). Based on previous findings, the reduced hTf binding observed in this study suggests that the proline mutations have caused the L3H to shift downward, thus inhibiting TbpA-hTf binding.
Both the tbpA D355P ΔtbpB and tbpA A356P ΔtbpB mutants showed the lowest levels of hTf binding of all tested mutants compared to the WT. Similarly, both the tbpA D355P ΔtbpB and tbpA A356P ΔtbpB mutants demonstrated significantly reduced iron internalization compared to the WT. Together, these findings suggest that proline substitutions at the beginning of the L3H are more disruptive to the structure and function than proline substitutions near the end of the L3H.
Interestingly, the tbpA A356P ΔtbpB mutant was able to grow with hTf as the sole source of iron, whereas the tbpA D355P ΔtbpB mutant could not. Without a functional TbpA protein, growth using hTf as the sole iron source would be impossible. These data suggest that the tbpA A356P ΔtbpB mutant is able to overcome the decreases in hTf binding and iron internalization by obtaining iron elsewhere to grow, such as residual cellular iron stores. All strains were grown on deferoxamine mesylate plates to deplete cellular iron stores; however, it is possible that there were some residual internal iron stores that allowed growth. Quantification of iron via ICP-MS is very sensitive; therefore, trace iron levels could account for the variation observed.
Iron internalization levels from all proline substitution tbpA L3H ΔtbpB mutants were significantly reduced compared to those of WT FA6905 (tbpA+ ΔtbpB), suggesting that despite some hTf binding, there is still a defect in iron internalization. These results are consistent with those of a previous study demonstrating that high-affinity hTf binding to TbpA L4, L5, and L8 is required for iron internalization (51). The deletion of TbpA extracellular L4 and L5 completely abolishes hTf binding, whereas the deletion of L8 inhibits hTf binding by 10-fold (51). With all three deletions, iron internalization is abolished (51). These findings are consistent with the results of this study: while hTf binding was not completely abrogated in each tbpA L3H ΔtbpB mutant, most mutants demonstrated reduced iron internalization.
As observed previously (25, 45), the expression of tbpB rescues TbpA function despite a decrease in TbpA-hTf binding in each tbpA mutant used in this study. Two mutants (the tbpA D355P ΔtbpB mutant and the quadruple tbpA D355P/A356P/N357P/Q358P ΔtbpB mutant) did not grow with hTf as the sole source of iron; however, the expression of tbpB restored function. The ability of tbpB to restore function suggests that the inhibition of hTf binding or iron internalization is likely caused by conformational changes small enough not to alter the overall protein conformation.
Nonbinding mutants for use in a vaccine should maintain the overall conformation of the WT protein, to stimulate the immune response and elicit antibodies that recognize WT epitopes. To evaluate how the D355P L3H proline substitution may affect the overall protein conformation, the predicted secondary structures of extracellular L2 and L5 were monitored over the course of 500-ns simulations. Both L2 and L5 have demonstrated roles in hTf binding (45, 51). The simulation results suggest that D335P mutagenesis affects the secondary structure of the L3H region and a short region in L2, but otherwise, the overall secondary structures of L2, L3, and L5 are maintained. Thus, the overall 3D conformation of the protein is predicted to be similar to that of the WT (45). MD simulation provides valuable insight into the hTf-TbpA interaction. The data from this study suggest that the TbpA D355P mutation is destabilizing to the α-helical structure of the L3H; furthermore, hydrogen bonding between TbpA and hTf is notably reduced in one of the two replicates of the TbpA D355P mutant when started in the hTf-bound state (Fig. 10). As described above, recent evidence suggests that TbpA extracellular L5 and L8 flex to an open state, opening a pocket for hTf to bind (42). This implies that the hTf C-lobe is in an open and unbound state as a result of the L3H D355P substitution. L3 is sandwiched between L2 and L5, which are the longest extracellular loops (52). In variants with higher levels of hTf binding but low levels of iron internalization, it is possible that L3H point mutations cause a small but impactful change to the TbpA structure that allows poor hTf binding or possible downstream conformational changes such as blocking the β-barrel pore or the TonB box, which is responsible for transporting iron into the β-barrel (53).
Proper WT TbpA function has been observed in tbpA L3H mutants with proper tbpB expression in previous studies, with the exception of the L3H deletion mutant (25, 45, 51). Because TbpB can bind to hTf and facilitate iron transfer, the incorporation of TbpB into a vaccine will also require mutagenesis or modification to inhibit binding to hTf, as binding to host proteins has been shown to inhibit a robust immune response (23, 28). Because TbpB is more immunogenic and sequence variable and TbpA is less immunogenic but shares more sequence conservation among strains, a combination of both Tbps is predicted to enhance protection and the immune response (30, 54, 55). Ideally, nonhost binding variants of TbpA, TbpB, and other TDTs would be included in a vaccine cocktail, which is hypothesized to increase immunogenicity and protection.
The ability of a vaccine antigen to bind to host proteins is hypothesized to inhibit the development of a robust immune response and may explain the weak immunogenicity of WT TbpA, WT TbpB, and WT fHbp (25, 29, 31, 56). The generation of mutants unable to bind host ligands has already been shown to be a viable option for creating a superior vaccine candidate using TbpB and fHbp (23, 28, 46, 57). This is the first study to identify a point mutation in tbpA that completely abrogates both binding and growth functions. Future studies will focus on elucidating the interaction between the L3H and iron extraction from hTf using structural analysis and comparing the immunization potentials using the TbpA D355P and TbpA A356P variants in a human transferrin transgenic mouse model.
MATERIALS AND METHODS
Strains, plasmids, and media.
All primers and plasmids used in this study are described in Table 1. Site-directed mutagenesis started with the plasmid pUNCH755 (38). All strains utilized in this study are described in Table 2. Plasmids were propagated in Escherichia coli Top10 cells (Invitrogen), E. coli XL-10 Gold cells (Agilent Technologies), or E. coli NEB 5-alpha cells (New England BioLabs). E. coli was cultured in Luria-Bertani broth (10 g of tryptone, 5 g of NaCl, and 5 g of yeast extract in 1 L of water) in the presence of ampicillin-carbenicillin (100 μg/mL) or chloramphenicol (34 μg/mL), where appropriate. Gonococcal strains FA6747, FA6815, and FA6905 are isogenic to FA19 and vary only in the tbpA and/or tbpB locus.
TABLE 1.
Mutagenesis primers used in this studya
| Generated plasmid | Generated TbpA mutation | Target plasmid | Primer name | Primer direction | Primer sequence (5′–3′) | Kit |
|---|---|---|---|---|---|---|
| pGSU001 | StuI insert | pUNCH755 | oVCU 905 | Fwd | GGCAACCACAAATACGGAGGCCTGTTTACCAGCGGC | Agilent |
| oVCU 906 | Rev | GCCGCTGGTAAACAGGCCTCCGTATTTGTGGTTGCC | ||||
| pGSU002 | D355P | pGSU001 | oVCU 927 | Fwd | AAGGCGGTTTTTCCTGCAAATCAAAAACAGGCG | Agilent |
| oVCU 928 | Rev | CGCCTGTTTTTGATTTGCAGGAAAAACCGCCTT | ||||
| pGSU003 | A356P | pGSU001 | oVCU 929 | Fwd | CTGACCAAGGCGGTTTTTGATCCAAATCAAAAACAGGCG | Agilent |
| oVCU 930 | Rev | CGCCTGTTTTTGATTTGGATCAAAAACCGCCTTGGTCAG | ||||
| pGSU004 | N357P | pGSU001 | oVCU 973 | Fwd | TTTTGATGCACCTCAAAAACAGGCGGGTTC | NEB Q5 |
| oVCU 974 | Rev | ACCGCCTTGGTCAGAAAT | ||||
| pGSU005 | Q358P | pGSU001 | oVCU 975 | Fwd | TGATGCAAATCCAAAACAGGCGG | NEB Q5 |
| oVCU 976 | Rev | AAAACCGCCTTGGTCAGA | ||||
| pGSU006 | K359P | pGSU001 | oVCU 977 | Fwd | TGCAAATCAACCACAGGCGGGTTC | NEB Q5 |
| oVCU 978 | Rev | TCAAAAACCGCCTTGGTC | ||||
| pGSU007 | K360P | pGSU001 | oVCU 913 | Fwd | GATGCAAATCAAAAACCGGCGGGTTCTTTGCGC | Agilent |
| oVCU 914 | Rev | CTACGTTTAGTTTTTGGCCGCCCAAGAAACGCG | ||||
| pGSU008 | K359P/Q360P | pGSU001 | oVCU 917 | Fwd | GATGCAAATCAACCACCGGCGGGTTCTTTGCGC | Agilent |
| oVCU 918 | Rev | CTACGTTTAGTTGGTGGCCGCCCAAGAAACGCG | ||||
| pGSU009 | N357P/Q358P/K359P/Q360P | pGSU008 | oVCU 933 | Fwd | GCGGTTTTTCCTCCACCTCCACCACCGGCGGGTTCTTTGCGC | Agilent |
| oVCU 934 | Rev | GCGCAAAGAACCCGCCGGTGGTGGAGGTGGAGGAAAAACCGC | ||||
Mutations are underlined. Fwd, forward; Rev, reverse.
TABLE 2.
Bacterial strains used in this study
| Strain | Description | Source or reference(s) |
|---|---|---|
| E. coli | ||
| Top10 | F mcrA (mrr-hsdRMS-mcrBC) 80lacZM15 lacX74 recA1 deoR araD139 (ara-leu)7697 galU galK rpsL(Strr) endA1 nupG | Invitrogen |
| XL10 Ultracompetent Gold | endA1 glnV44 recA1 thi-1 gyrA96 relA1 lac Hte (mcrA)183 (mcrCB-hsdSMR-mrr)173 Tetr F' [proAB lacIqZM15 Tn10(Tetr Amyr Cmr)] | Agilent Technologies |
| NEB 5-alpha competent | fhuA2 Δ(argF-lacZ)U169 phoA glnV44 ϕ80Δ(lacZ)M15 gyrA96 recA1 relA1 endA1 thi-1 hsdR17 | New England BioLabs |
| N. gonorrhoeae | ||
| FA19 | Wild type | 76 |
| FA6747 | TbpA− (tbpA::mTn3cat) | 36, 40 |
| FA6815 | TbpAB− (ΔtbpB::Ω) | 38 |
| FA6905 | TbpB− (ΔtbpB) | 40 |
| MCV 192 | TbpA L3HΔ (T350–A361) | 25 |
| RSC 100 | TbpA D355P TbpB− (ΔtbpB) | This study |
| RSC 101 | TbpA D355P | This study |
| RSC 102 | TbpA A356P TbpB− (ΔtbpB) | This study |
| RSC 103 | TbpA A356P | This study |
| RSC 104 | TbpA N357P TbpB− (ΔtbpB) | This study |
| RSC 105 | TbpA N357P | This study |
| RSC 106 | TbpA Q358P TbpB− (ΔtbpB) | This study |
| RSC 107 | TbpA Q358P | This study |
| RSC 108 | TbpA K359P TbpB− (ΔtbpB) | This study |
| RSC 109 | TbpA K359P | This study |
| RSC 110 | TbpA Q360P TbpB− (ΔtbpB) | This study |
| RSC 111 | TbpA Q360P | This study |
| RSC 112 | TbpA K369P/Q360P TbpB− (ΔtbpB) | This study |
| RSC 113 | TbpA K369P/Q360P | This study |
| RSC 114 | TbpA N357P/Q358P/K359P/Q360P TbpB− (ΔtbpB) | This study |
| RSC 115 | TbpA N357P/Q358P/K359P/Q360P | This study |
Site-directed mutagenesis.
Site-directed mutagenesis was performed using the QuikChange II site-directed mutagenesis kit (catalog number 200523; Agilent Technologies) or the Q5 site-directed mutagenesis kit (catalog number E0552S; New England BioLabs) according to the manufacturer’s instructions. NEB 5-alpha competent E. coli or XL10 Ultracompetent Gold E. coli cells were transformed with mutagenized pUNCH755. First, a StuI restriction site was inserted downstream of the tbpA L3H region in pUNCH755. The StuI insertion was used to identify gonococcal transformants that retained the WT tbpA sequence. PCR was used to amplify tbpA from transformant DNA, and PCR products were digested with StuI; WT tbpA does not contain the StuI site, but site-directed mutants retained the site. Following StuI restriction site insertion, proline mutations were directed to the L3H region of tbpA between D355 and Q360 as described in Table S1 in the supplemental material. Primers and plasmids are further described in Table 1. The sequences of the mutated plasmids were confirmed before transformation into gonococcal strain FA19. As described previously, gonococcal transformation with one plasmid yielded two genotypes: tbpA+ tbpB+ and tbpA+ ΔtbpB (25). Mutated FA19 strains were single-colony purified and then characterized as either tbpB+ or ΔtbpB by PCR, amplifying the wild-type or mutagenized tbpB gene.
Gonococcal growth.
Gonococci were propagated using GC medium base (GCB; Difco) with Kellogg’s supplement I (58) and 12 μM Fe(NO3)3 at 35°C to 37°C with 5% atmospheric CO2. To provide selection of gonococcal transformants, 1 μg/mL chloramphenicol was added to GCB agar plates. Gonococcal strains were iron stressed with 10 μM deferoxamine mesylate on GCB agar plates or cultured from GCB plates into liquid chemically defined medium (CDM) pretreated with Chelex 100 (Bio-Rad) to remove excess metals, as described previously (59).
Homology modeling of TbpA and alignment of the L3H.
The homology model of TbpA from strain FA19 was generated based on the known structure of TbpA from N. meningitidis strain K454. Phyre 2.0 was used to generate the PDB file, and PyMOL was used to generate Fig. 1. ESPript 3.0 was used to generate the multiple-strain alignment and secondary structure prediction of the TbpA L3H as previously described (60). Praline was used to assess conservation among the top 100 N. gonorrhoeae strains in the NCBI database (61). GenBank accession numbers are as follows: EEZ46093.1 for N. gonorrhoeae FA19, WP_010951283.1 for N. gonorrhoeae FA1090, EFE04786.1 for N. gonorrhoeae DG12, and AAF81744.1 for N. meningitidis K454.
Western blotting.
Gonococcal strains were grown under iron-stressed conditions using CDM, as described above. Cells were harvested 3 to 4 h after culturing at 36°C with 5% CO2 to a standardized density. Pellets were standardized by weight and lysed in 2× Laemmli solubilizing buffer (Bio-Rad) supplemented with 5% β-mercaptoethanol. Whole-cell lysates were boiled for 10 min. A total of 10 μL of the lysate per sample was loaded onto a 7.5% SDS-PAGE gel. Proteins were transferred to a 0.45-μm nitrocellulose membrane. Uniform protein loading was confirmed via Ponceau S staining after transfer and before blocking. Blots were blocked with 5% bovine serum albumin (BSA) in high-salt Tris-buffered saline (HS-TBS) (20 mM Tris, 500 mM NaCl [pH 7.5], 0.05% Tween 20) and probed for TbpA using polyclonal rabbit serum against full-length TbpA (36). Goat anti-rabbit IgG conjugated to horseradish peroxidase (HRP; Bio-Rad) or rabbit anti-goat IgG conjugated to HRP (Bio-Rad) was used as the secondary antibody. Goat anti-hTf (Sigma) was used to detect hTf. Opti-4CN (Bio-Rad) or West Femto (Thermo) was used as the substrate according to the manufacturer’s recommendations. Images were acquired using a Bio-Rad ChemiDoc system.
Protease accessibility assay.
Trypsin digest assays were completed as previously described (40). Briefly, gonococcal cells were grown in liquid CDM as described above, diluted to a standardized density, and grown for an additional 3 to 4 h at 36°C with 5% CO2. Whole, iron-stressed gonococci were treated with 2.5 μg trypsin (Sigma) per mL of culture for 0, 10, 20, or 30 min at 36°C with 5% CO2. Reactions were stopped by the addition of 0.6 trypsin-inhibiting units of aprotinin (Sigma). Whole-cell lysates were collected and subjected to Western blotting for TbpA detection, as described above.
hTf binding ELISA.
hTf (Sigma) was prepared as previously described (62). Strains were iron starved on GCB agar plates supplemented with 12.5 μM deferoxamine mesylate overnight to induce TbpA expression. MaxiSorp microtiter plates (Nunc), pretreated with 200 μL 0.01% polylysine (Sigma), were inoculated with whole gonococcal cells scraped from GCB-deferoxamine mesylate plates. Cells were resuspended to a standardized optical density at 600 nm (OD600) of 1.0, and 100 μL per strain was then inoculated into triplicate wells and allowed to incubate for 1 h at room temperature. Unbound cells in the suspension were removed, and plates were blocked with 200 μL 3% BSA in phosphate-buffered saline (PBS) for 1 h at room temperature. Blocking was followed by the addition of 1 μg/mL HRP-conjugated hTf (Jackson ImmunoResearch) dissolved in 1× PBS also containing 3% BSA for 1 h at room temperature. To show specificity, 100 μg/mL apo-hTf was added as a competitor to HRP-hTf in samples labeled “Comp” in Fig. 3. Wells were washed 3 times in 200 μL 1× PBS using a plate washer (BioTek). The colorimetric 1-Slow-step 3, 3′, 5, 5′-tetramethylbenzidine (TMB) ELISA substrate solution (Thermo) was applied until a sufficient color change was observed, and the reaction was quenched with 100 μL 1.8 N H2SO4. The results were read at 600 nm using a BioTek plate reader. Standard curves were generated for each assay using a range of HRP-hTf amounts ranging between 1 × 10−3 ng and 2 × 10−6 ng. Strains were analyzed in triplicate, and the standard deviations were plotted. Student’s t test was used to determine significance compared to the positive-control strain FA6905 (P < 0.05).
ICP-MS.
WT and mutant strains were grown under iron-stressed conditions in liquid CDM at 36°C with 5% CO2, as described above. Four hours after dilution to a standardized density, 5 μM hTf (30% Fe saturated) was added for 1 h. Cells were then pelleted in metal-free tubes at 3,570 × g for 5 min, washed once in 5 mL of Chelex-treated 10 mM HEPES–1 mM EDTA (pH 7.4), and washed twice in 5 mL of 10 mM HEPES (pH 7.4). The internalized Fe in micrograms was normalized to the wet mass of the cells (in milligrams). Digestions and analysis of frozen pellets were performed by the University of Georgia Center for Applied Isotope Studies Plasma Chemistry Laboratory.
Growth in iron-depleted CDM supplemented with hTf.
Strains were plated onto CDM agarose plates supplemented with 2.5 μM hTf (30% Fe saturated) and incubated for 48 h at 36°C with 5% CO2. Images were acquired with a Bio-Rad ChemiDoc system. For liquid growth assays, strains were plated onto GCB plates supplemented with 10 μM deferoxamine mesylate and incubated at 36°C with 5% CO2 for 12 to 16 h. Iron-starved strains were inoculated into liquid CDM as described above. After doubling, cells were standardized to 2 × 10−5 OD600 units and plated into 96-well dishes supplemented with 5 μM deferoxamine mesylate and 5 μM hTf. BioTek Synergy and Cytation plate readers were used to incubate the cells at 36°C with 5% CO2. The OD600 was plotted over 24 h.
Assessment of whole-cell binding to hTf in serum.
Strains were grown in liquid CDM as described above. Cells were blocked with 10 mg/mL BSA for 5 min after harvest, and the cells were then incubated with either 1 μM purified hTf or an estimated 1 μM hTf in human serum. Serum hTf was estimated to be approximately 50 μM based on the literature and preliminary tests (63–66). Cells were incubated with hTf or serum for 20 min at 36°C with 5% CO2. Lysates were washed twice with 500 μL 1× PBS, standardized, and subjected to SDS-PAGE and Western blotting as described above. Images were acquired with a Bio-Rad ChemiDoc system.
Molecular dynamic simulations.
The homology model of FA19 TbpA bound to hTf was used as a starting point for simulations. Cocrystallized water molecules as well as the two glycan chains attached to hTf were adopted from the TbpA structure from N. meningitidis (PDB accession number 3V8X) (32). A total of 3 disulfide bonds were formed in TbpA, along with 19 in hTf. An outer membrane model representing that of N. gonorrhoeae was created using the CHARMM-GUI Membrane Builder (67), and the TbpA:hTf complex was inserted. The membrane contained 77 lipopolysaccharide (LPS) molecules with inner and outer core glycans in the outer leaflet. The inner leaflet contained 174 1-palmitoyl-2-palmitoleoyl-sn-glycero-3-phosphoethanolamine (PYPE), 50 1-palmitoyl-2-palmitoleoyl-sn-glycero-3-phosphoglycerol (PYPG), and 25 1,2-dipalmitoyl-sn-glycero-3-phosphate (DPPA) lipids. Totals of 154 Ca2+ and 77 Mg2+ ions were added to neutralize the LPS. An additional 66,164 water molecules as well as 202 K+ and 158 Cl− ions were added to solvate the system and bring the system to a bulk KCl concentration of 150 mM using VMD (68). The final system size for the TbpA:hTf system was 290,178 atoms. The apo-TbpA system was created from the bound one by deleting hTf and repeating the solvation and KCl ionization steps. The final system size for the apo-TbpA system was 251,845 atoms.
MD simulations were run using NAMD (69, 70). A 2-fs time step was used for all simulations with long-range electrostatics calculation every other time step using the particle mesh Ewald method (71). A cutoff of 12 Å was used for van der Waals interactions with a force-based switching function beginning at 10 Å. A temperature of 310 K was maintained using Langevin dynamics; for all production simulations, constant pressure of 1 atm was enforced using an anisotropic Langevin piston. The CHARMM36 force field for lipids and glycans (72, 73) and the CHARMM36m force field for proteins (74) were used. The bound Fe3+ ion and the coordinating carbonate ion were covalently bound to one another, and additional bonds between Fe3+ and D392, Y426, Y517, and H585 of hTf were added to further enforce coordination, as done previously (32). Prior to production runs, the membrane was equilibrated for 50 ns with the protein backbone restrained. In total, four systems with two replicas each were run for 500 ns each (4 μs in aggregate): TbpA (WT) with and without hTf bound as well as TbpA (D355P) with and without hTf bound.
Statistics.
Statistical analyses were conducted by comparing positive-control to mutant strains utilizing Student’s t test. Pairwise differences with a P value of <0.05 were considered statistically significant. ELISA and ICP-MS values are shown as the means of multiple concentration points from the studies conducted in at least triplicate ± standard errors of the means. Statistical analysis for hTf-dependent gonococcal growth was completed using PRISM 9.0. Two-way analysis of variance (ANOVA) with Tukey’s post hoc test was performed on 3 independent biological replicates. The graph in Fig. 6 shows a representative growth curve.
ACKNOWLEDGMENTS
We acknowledge Sarah Jantzi and the University of Georgia Center for Applied Isotope Studies for assistance in the development of protocols and analysis of ICP-MS data.
This work was supported by funding from National Health Service grants R01 A1 AI125421, R01 AI125421, R01 AI127793, U19 AI144182, and R01 GM123169. Computational resources were provided through XSEDE (TG-MCB130173), which is supported by the U.S. National Science Foundation (NSF) (ACI-1548562). This work also used the Hive cluster, which is supported by the NSF (1828187) and is managed by PACE at Georgia Tech. The funders had no role in study design, data collection, data analysis, decision to publish, or preparation of the manuscript.
Footnotes
This article is a direct contribution from Cynthia Nau Cornelissen, a member of the Infection and Immunity Editorial Board, who arranged for and secured reviews by Eric Skaar, Vanderbilt University, and Kenneth Fields, University of kentucky.
Supplemental material is available online only.
Contributor Information
Cynthia Nau Cornelissen, Email: ccornelissen@gsu.edu.
Andreas J. Bäumler, University of California, Davis
REFERENCES
- 1.World Health Organization. 2018. Sexually transmitted infections (STIs). World Health Organization, Geneva, Switzerland. https://www.who.int/en/news-room/fact-sheets/detail/sexually-transmitted-infections-(stis). [Google Scholar]
- 2.Centers for Disease Control and Prevention. 2021. Incidence, prevalence and cost of sexually transmitted infections in the United States. Centers for Disease Control and Prevention, Atlanta, GA. https://www.cdc.gov/nchhstp/newsroom/2021/2018-STI-incidence-prevalence-estimates.html. [Google Scholar]
- 3.Walker CK, Sweet RL. 2011. Gonorrhea infection in women: prevalence, effects, screening, and management. Int J Womens Health 3:197–206. 10.2147/IJWH.S13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cornelissen CN, Kelley M, Hobbs MM, Anderson JE, Cannon JG, Cohen MS, Sparling PF. 1998. The transferrin receptor expressed by gonococcal strain FA1090 is required for the experimental infection of human male volunteers. Mol Microbiol 27:611–616. 10.1046/j.1365-2958.1998.00710.x. [DOI] [PubMed] [Google Scholar]
- 5.Schmidt KA, Schneider H, Lindstrom JA, Boslego JW, Warren RA, Van de Verg L, Deal CD, McClain JB, Griffiss JM. 2001. Experimental gonococcal urethritis and reinfection with homologous gonococci in male volunteers. Sex Transm Dis 28:555–564. 10.1097/00007435-200110000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Unemo M, Shafer WM. 2014. Antimicrobial resistance in Neisseria gonorrhoeae in the 21st century: past, evolution, and future. Clin Microbiol Rev 27:587–613. 10.1128/CMR.00010-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Portnoy J, Mendelson J, Clecner B, Heisler L. 1974. Asymptomatic gonorrhea in the male. Can Med Assoc J 110:169, 171. [PMC free article] [PubMed] [Google Scholar]
- 8.Liu Y, Liu W, Russell MW. 2014. Suppression of host adaptive immune responses by Neisseria gonorrhoeae: role of interleukin 10 and type 1 regulatory T cells. Mucosal Immunol 7:165–176. 10.1038/mi.2013.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu Y, Feinen B, Russell MW. 2011. New concepts in immunity to Neisseria gonorrhoeae: innate responses and suppression of adaptive immunity favor the pathogen, not the host. Front Microbiol 2:52. 10.3389/fmicb.2011.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organization. 2018. WHO gonococcal AMR surveillance programme. World Health Organization, Geneva, Switzerland. https://www.who.int/data/gho/data/themes/topics/who-gonococcal-amr-surveillance-programme-who-gasp. [Google Scholar]
- 11.Ohnishi M, Golparian D, Shimuta K, Saika T, Hoshina S, Iwasaku K, Nakayama S, Kitawaki J, Unemo M. 2011. Is Neisseria gonorrhoeae initiating a future era of untreatable gonorrhea? Detailed characterization of the first strain with high-level resistance to ceftriaxone. Antimicrob Agents Chemother 55:3538–3545. 10.1128/AAC.00325-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Unemo M, Golparian D, Nicholas R, Ohnishi M, Gallay A, Sednaoui P. 2012. High-level cefixime- and ceftriaxone-resistant Neisseria gonorrhoeae in France: novel penA mosaic allele in a successful international clone causes treatment failure. Antimicrob Agents Chemother 56:1273–1280. 10.1128/AAC.05760-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lahra MM, Ryder N, Whiley DM. 2014. A new multidrug-resistant strain of Neisseria gonorrhoeae in Australia. N Engl J Med 371:1850–1851. 10.1056/NEJMc1408109. [DOI] [PubMed] [Google Scholar]
- 14.Cámara J, Serra J, Ayats J, Bastida T, Carnicer-Pont D, Andreu A, Ardanuy C. 2012. Molecular characterization of two high-level ceftriaxone-resistant Neisseria gonorrhoeae isolates detected in Catalonia, Spain. J Antimicrob Chemother 67:1858–1860. 10.1093/jac/dks162. [DOI] [PubMed] [Google Scholar]
- 15.Fifer H, Livermore DM, Uthayakumaran T, Woodford N, Cole MJ. 2021. What’s left in the cupboard? Older antimicrobials for treating gonorrhoea. J Antimicrob Chemother 76:1215–1220. 10.1093/jac/dkaa559. [DOI] [PubMed] [Google Scholar]
- 16.Yan J, Chen Y, Yang F, Ling X, Jiang S, Zhao F, Yu Y, van der Veen S. 2021. High percentage of the ceftriaxone-resistant Neisseria gonorrhoeae FC428 clone among isolates from a single hospital in Hangzhou, China. J Antimicrob Chemother 76:936–939. 10.1093/jac/dkaa526. [DOI] [PubMed] [Google Scholar]
- 17.St Cyr S, Workowski KA, Bachmann LH, Pham C, Schlanger K, Torrone E, Weinstock H, Kersh EN, Thorpe P. 2020. Update to CDC’s treatment guidelines for gonococcal infection, 2020. MMWR Morb Mortal Wkly Rep 69:1911–1916. 10.15585/mmwr.mm6950a6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Virji M. 2009. Pathogenic neisseriae: surface modulation, pathogenesis and infection control. Nat Rev Microbiol 7:274–286. 10.1038/nrmicro2097. [DOI] [PubMed] [Google Scholar]
- 19.Zhu W, Chen C-J, Thomas CE, Anderson JE, Jerse AE, Sparling PF. 2011. Vaccines for gonorrhea: can we rise to the challenge? Front Microbiol 2:124. 10.3389/fmicb.2011.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Russell MW, Jerse AE, Gray-Owen SD. 2019. Progress toward a gonococcal vaccine: the way forward. Front Immunol 10:2417. 10.3389/fimmu.2019.02417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Criss AK, Kline KA, Seifert HS. 2005. The frequency and rate of pilin antigenic variation in Neisseria gonorrhoeae. Mol Microbiol 58:510–519. 10.1111/j.1365-2958.2005.04838.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cornelissen CN. 2008. Identification and characterization of gonococcal iron transport systems as potential vaccine antigens. Future Microbiol 3:287–298. 10.2217/17460913.3.3.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martínez-Martínez S, Frandoloso R, Rodríguez-Ferri E-F, García-Iglesias M-J, Pérez-Martínez C, Álvarez-Estrada Á, Gutiérrez-Martín C-B. 2016. A vaccine based on a mutant transferrin binding protein B of Haemophilus parasuis induces a strong T-helper 2 response and bacterial clearance after experimental infection. Vet Immunol Immunopathol 179:18–25. 10.1016/j.vetimm.2016.07.011. [DOI] [PubMed] [Google Scholar]
- 24.Martínez-Martínez S, Frandoloso R, Gutiérrez-Martín CB, Lampreave F, García-Iglesias MJ, Pérez-Martínez C, Rodríguez-Ferri EF. 2011. Acute phase protein concentrations in colostrum-deprived pigs immunized with subunit and commercial vaccines against Glässer’s disease. Vet Immunol Immunopathol 144:61–67. 10.1016/j.vetimm.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 25.Cash DR, Noinaj N, Buchanan SK, Cornelissen CN. 2015. Beyond the crystal structure: insight into the function and vaccine potential of TbpA expressed by Neisseria gonorrhoeae. Infect Immun 83:4438–4449. 10.1128/IAI.00762-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chan C, Andisi VF, Ng D, Ostan N, Yunker WK, Schryvers AB. 2018. Are lactoferrin receptors in Gram-negative bacteria viable vaccine targets? Biometals 31:381–398. 10.1007/s10534-018-0105-7. [DOI] [PubMed] [Google Scholar]
- 27.Martínez-Martínez S, Rodríguez-Ferri EF, Frandoloso R, Garrido-Pavón JJ, Zaldívar-López S, Barreiro C, Gutiérrez-Martín CB. 2016. Molecular analysis of lungs from pigs immunized with a mutant transferrin binding protein B-based vaccine and challenged with Haemophilus parasuis. Comp Immunol Microbiol Infect Dis 48:69–78. 10.1016/j.cimid.2016.08.005. [DOI] [PubMed] [Google Scholar]
- 28.Frandoloso R, Martínez-Martínez S, Calmettes C, Fegan J, Costa E, Curran D, Yu R-H, Gutiérrez-Martín CB, Rodríguez-Ferri EF, Moraes TF, Schryvers AB. 2015. Nonbinding site-directed mutants of transferrin binding protein B exhibit enhanced immunogenicity and protective capabilities. Infect Immun 83:1030–1038. 10.1128/IAI.02572-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Noinaj N, Buchanan SK, Cornelissen CN. 2012. The transferrin-iron import system from pathogenic Neisseria species. Mol Microbiol 86:246–257. 10.1111/mmi.12002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Price GA, Hobbs MM, Cornelissen CN. 2004. Immunogenicity of gonococcal transferrin binding proteins during natural infections. Infect Immun 72:277–283. 10.1128/IAI.72.1.277-283.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cornelissen CN, Anderson JE, Boulton IC, Sparling PF. 2000. Antigenic and sequence diversity in gonococcal transferrin-binding protein A. Infect Immun 68:4725–4735. 10.1128/IAI.68.8.4725-4735.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Noinaj N, Easley NC, Oke M, Mizuno N, Gumbart J, Boura E, Steere AN, Zak O, Aisen P, Tajkhorshid E, Evans RW, Gorringe AR, Mason AB, Steven AC, Buchanan SK. 2012. Structural basis for iron piracy by pathogenic Neisseria. Nature 483:53–58. 10.1038/nature10823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Noinaj N, Gumbart JC, Buchanan SK. 2017. The beta-barrel assembly machinery in motion. Nat Rev Microbiol 15:197–204. 10.1038/nrmicro.2016.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Noinaj N, Buchanan SK. 2014. Structural insights into the transport of small molecules across membranes. Curr Opin Struct Biol 27:8–15. 10.1016/j.sbi.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Noinaj N, Guillier M, Barnard TJ, Buchanan SK. 2010. TonB-dependent transporters: regulation, structure, and function. Annu Rev Microbiol 64:43–60. 10.1146/annurev.micro.112408.134247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cornelissen CN, Biswas GD, Tsai J, Paruchuri DK, Thompson SA, Sparling PF. 1992. Gonococcal transferrin-binding protein 1 is required for transferrin utilization and is homologous to TonB-dependent outer membrane receptors. J Bacteriol 174:5788–5797. 10.1128/jb.174.18.5788-5797.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Irwin SW, Averil N, Cheng CY, Schryvers AB. 1993. Preparation and analysis of isogenic mutants in the transferrin receptor protein genes, tbpA and tbpB, from Neisseria meningitidis. Mol Microbiol 8:1125–1133. 10.1111/j.1365-2958.1993.tb01657.x. [DOI] [PubMed] [Google Scholar]
- 38.Anderson JE, Sparling PF, Cornelissen CN. 1994. Gonococcal transferrin-binding protein 2 facilitates but is not essential for transferrin utilization. J Bacteriol 176:3162–3170. 10.1128/jb.176.11.3162-3170.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Renauld-Mongénie G, Poncet D, Mignon M, Fraysse S, Chabanel C, Danve B, Krell T, Quentin-Millet M-J. 2004. Role of transferrin receptor from a Neisseria meningitidis tbpB isotype II strain in human transferrin binding and virulence. Infect Immun 72:3461–3470. 10.1128/IAI.72.6.3461-3470.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cornelissen CN, Sparling PF. 1996. Binding and surface exposure characteristics of the gonococcal transferrin receptor are dependent on both transferrin-binding proteins. J Bacteriol 178:1437–1444. 10.1128/jb.178.5.1437-1444.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Retzer MD, Yu R, Zhang Y, Gonzalez GC, Schryvers AB. 1998. Discrimination between apo and iron-loaded forms of transferrin by transferrin binding protein B and its N-terminal subfragment. Microb Pathog 25:175–180. 10.1006/mpat.1998.0226. [DOI] [PubMed] [Google Scholar]
- 42.Duran GN, Özbil M. 2021. Structural rearrangement of Neisseria meningitidis transferrin binding protein A (TbpA) prior to human transferrin protein (hTf) binding. Turk J Chem 45:1146–1154. 10.3906/kim-2102-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Steere AN, Miller BF, Roberts SE, Byrne SL, Chasteen ND, Smith VC, MacGillivray RTA, Mason AB. 2012. Ionic residues of human serum transferrin affect binding to the transferrin receptor and iron release. Biochemistry 51:686–694. 10.1021/bi201661g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pogoutse AK, Moraes TF. 2017. Iron acquisition through the bacterial transferrin receptor. Crit Rev Biochem Mol Biol 52:314–326. 10.1080/10409238.2017.1293606. [DOI] [PubMed] [Google Scholar]
- 45.Yost-Daljev MK, Cornelissen CN. 2004. Determination of surface-exposed, functional domains of gonococcal transferrin-binding protein A. Infect Immun 72:1775–1785. 10.1128/IAI.72.3.1775-1785.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Beernink PT, Shaughnessy J, Braga EM, Liu Q, Rice PA, Ram S, Granoff DM. 2011. A meningococcal factor H binding protein mutant that eliminates factor H binding enhances protective antibody responses to vaccination. J Immunol 186:3606–3614. 10.4049/jimmunol.1003470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hollingsworth SA, Dror RO. 2018. Molecular dynamics simulation for all. Neuron 99:1129–1143. 10.1016/j.neuron.2018.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Knapp B, Ospina L, Deane CM. 2018. Avoiding false positive conclusions in molecular simulation: the importance of replicas. J Chem Theory Comput 14:6127–6138. 10.1021/acs.jctc.8b00391. [DOI] [PubMed] [Google Scholar]
- 49.Calmettes C, Alcantara J, Yu RH, Schryvers AB, Moraes TF. 2012. The structural basis of transferrin sequestration by transferrin-binding protein B. Nat Struct Mol Biol 19:358–360. 10.1038/nsmb.2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yadav R, Noinaj N, Ostan N, Moraes T, Stoudenmire J, Maurakis S, Cornelissen CN. 2019. Structural basis for evasion of nutritional immunity by the pathogenic Neisseriae. Front Microbiol 10:2981. 10.3389/fmicb.2019.02981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Boulton IC, Yost MK, Anderson JE, Cornelissen CN. 2000. Identification of discrete domains within gonococcal transferrin-binding protein A that are necessary for ligand binding and iron uptake functions. Infect Immun 68:6988–6996. 10.1128/IAI.68.12.6988-6996.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cornelissen CN. 2003. Transferrin-iron uptake by Gram-negative bacteria. Front Biosci 8:d836–d847. 10.2741/1076. [DOI] [PubMed] [Google Scholar]
- 53.Kenney CD, Cornelissen CN. 2002. Demonstration and characterization of a specific interaction between gonococcal transferrin binding protein A and TonB. J Bacteriol 184:6138–6145. 10.1128/JB.184.22.6138-6145.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.West D, Reddin K, Matheson M, Heath R, Funnell S, Hudson M, Robinson A, Gorringe A. 2001. Recombinant Neisseria meningitidis transferrin binding protein A protects against experimental meningococcal infection. Infect Immun 69:1561–1567. 10.1128/IAI.69.3.1561-1567.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Price GA, Russell MW, Cornelissen CN. 2005. Intranasal administration of recombinant Neisseria gonorrhoeae transferrin binding proteins A and B conjugated to the cholera toxin B subunit induces systemic and vaginal antibodies in mice. Infect Immun 73:3945–3953. 10.1128/IAI.73.7.3945-3953.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cornelissen CN, Anderson JE, Sparling PF. 1997. Characterization of the diversity and the transferrin-binding domain of gonococcal transferrin-binding protein 2. Infect Immun 65:822–828. 10.1128/iai.65.2.822-828.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rossi R, Granoff DM, Beernink PT. 2013. Meningococcal factor H-binding protein vaccines with decreased binding to human complement factor H have enhanced immunogenicity in human factor H transgenic mice. Vaccine 31:5451–5457. 10.1016/j.vaccine.2013.08.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kellogg DS, Jr, Peacock WL, Jr, Deacon WE, Brown L, Pirkle DI. 1963. Neisseria gonorrhoeae. Virulence genetically linked to clonal variation. J Bacteriol 85:1274–1279. 10.1128/jb.85.6.1274-1279.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.West SE, Sparling PF. 1987. Aerobactin utilization by Neisseria gonorrhoeae and cloning of a genomic DNA fragment that complements Escherichia coli fhuB mutations. J Bacteriol 169:3414–3421. 10.1128/jb.169.8.3414-3421.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Robert X, Gouet P. 2014. Deciphering key features in protein structures with the new ENDscript server. Nucleic Acids Res 42:W320–W324. 10.1093/nar/gku316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Heringa J. 1999. Two strategies for sequence comparison: profile-preprocessed and secondary structure-induced multiple alignment. Comput Chem 23:341–364. 10.1016/s0097-8485(99)00012-1. [DOI] [PubMed] [Google Scholar]
- 62.Maurakis S, Keller K, Maxwell CN, Pereira K, Chazin WJ, Criss AK, Cornelissen CN. 2019. The novel interaction between Neisseria gonorrhoeae TdfJ and human S100A7 allows gonococci to subvert host zinc restriction. PLoS Pathog 15:e1007937. 10.1371/journal.ppat.1007937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kasvosve I, Delanghe J. 2002. Total iron binding capacity and transferrin concentration in the assessment of iron status. Clin Chem Lab Med 40:1014–1018. 10.1515/CCLM.2002.176. [DOI] [PubMed] [Google Scholar]
- 64.Page MGP. 2019. The role of iron and siderophores in infection, and the development of siderophore antibiotics. Clin Infect Dis 69:S529–S537. 10.1093/cid/ciz825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ganz T, Nemeth E. 2015. Iron homeostasis in host defence and inflammation. Nat Rev Immunol 15:500–510. 10.1038/nri3863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Andrews NC, Ganz T. 2019. The molecular basis of iron metabolism, p 161–172. In Provan D, Gribben J (ed), Molecular hematology, 4th ed. John Wiley & Sons, Ltd, Oxford, United Kingdom. 10.1002/9781119252863.ch13. [DOI] [Google Scholar]
- 67.Wu EL, Cheng X, Jo S, Rui H, Song KC, Dávila-Contreras EM, Qi Y, Lee J, Monje-Galvan V, Venable RM, Klauda JB, Im W. 2014. CHARMM-GUI Membrane Builder toward realistic biological membrane simulations. J Comput Chem 35:1997–2004. 10.1002/jcc.23702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Humphrey W, Dalke A, Schulten K. 1996. VMD—visual molecular dynamics. J Mol Graph 14:33–38. 10.1016/0263-7855(96)00018-5. [DOI] [PubMed] [Google Scholar]
- 69.Phillips JC, Braun R, Wang W, Gumbart J, Tajkhorshid E, Villa E, Chipot C, Skeel RD, Kalé L, Schulten K. 2005. Scalable molecular dynamics with NAMD. J Comput Chem 26:1781–1802. 10.1002/jcc.20289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Phillips JC, Hardy DJ, Maia JDC, Stone JE, Ribeiro JV, Bernardi RC, Buch R, Fiorin G, Hénin J, Jiang W, McGreevy R, Melo MCR, Radak BK, Skeel RD, Singharoy A, Wang Y, Roux B, Aksimentiev A, Luthey-Schulten Z, Kalé LV, Schulten K, Chipot C, Tajkhorshid E. 2020. Scalable molecular dynamics on CPU and GPU architectures with NAMD. J Chem Phys 153:e044130. 10.1063/5.0014475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Darden TA, York DM, Pedersen LG. 1993. Particle mesh Ewald: an N log N method for Ewald sums in large systems. J Comput Phys 98:10089–10092. 10.1063/1.464397. [DOI] [Google Scholar]
- 72.Klauda JB, Venable RM, Freites JA, O’Connor JW, Tobias DJ, Mondragon-Ramirez C, Vorobyov I, MacKerell AD, Jr, Pastor RW. 2010. Update of the CHARMM all-atom additive force field for lipids: validation on six lipid types. J Phys Chem B 114:7830–7843. 10.1021/jp101759q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Guvench O, Hatcher ER, Venable RM, Pastor RW, MacKerell AD, Jr.. 2009. Additive empirical CHARMM force field for glycosyl linked hexopyranoses. J Chem Theory Comput 5:2353–2370. 10.1021/ct900242e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Huang J, Rauscher S, Nawrocki G, Ran T, Feig M, de Groot BL, Grubmüller H, MacKerell AD, Jr.. 2017. CHARMM36m: an improved force field for folded and intrinsically disordered proteins. Nat Methods 14:71–73. 10.1038/nmeth.4067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kabsch W, Sander C. 1983. Dictionary of protein secondary structure: pattern recognition of hydrogen-bonded and geometrical features. Biopolymers 22:2577–2637. 10.1002/bip.360221211. [DOI] [PubMed] [Google Scholar]
- 76.Mickelsen PA, Sparling PF. 1981. Ability of Neisseria gonorrhoeae, Neisseria meningitidis, and commensal Neisseria species to obtain iron from transferrin and iron compounds. Infect Immun 33:555–564. 10.1128/iai.33.2.555-564.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Movie A. Download iai.00414-22-s0001.mov, MOV file, 3.2 MB (3.3MB, mov)
Movie B. Download iai.00414-22-s0002.mov, MOV file, 3.5 MB (3.6MB, mov)
Movie C. Download iai.00414-22-s0003.mov, MOV file, 4.2 MB (4.4MB, mov)
Movie D. Download iai.00414-22-s0004.mov, MOV file, 3.5 MB (3.6MB, mov)
Fig. S1 to S7, Table S1, and legend for supplemental movies. Download iai.00414-22-s0005.pdf, PDF file, 1.9 MB (1.9MB, pdf)