Iterleukin (IL) 23 is a key cytokine that mediates the inflammatory state of the intestinal mucosa in UC.1 Mirikizumab is a humanized immunoglobulin G4 monoclonal antibody that attenuates inflammation by binding to subunit p19 of IL-23. The phase 3 LUCENT-1 study compared mirikizumab vs placebo as induction therapy in 1281 patients with UC.2 The trial met its primary endpoint, showing a superior rate of clinical remission at week 12 with mirikizumab vs placebo (24.2% vs 13.3%; 99.875% CI, 3.2-19.1; P=.00006).
LUCENT-2 was a double-blind phase 3 trial that evaluated mirikizumab maintenance therapy in patients with a clinical response to mirikizumab induction therapy at week 12 in LUCENT-1.3 Enrolled patients were randomly assigned in a 2:1 ratio to receive mirikizumab (200 mg) or placebo every 4 weeks through week 40 of LUCENT-2, for a total of 52 weeks of study treatment. Corticosteroid therapy was tapered starting at week 0 of LUCENT-2. The primary outcome was clinical remission at week 40 of maintenance therapy. Clinical remission was defined as a stool frequency score of 0, or a stool frequency score of 1 with a decrease of at least 1 point from baseline; a rectal bleeding score of 0; and an endoscopic subscore of 0 or 1, excluding friability. Secondary endpoints were also evaluated at week 40 of the trial.
The LUCENT-2 study randomly assigned 365 patients to treatment with mirikizumab and 179 to placebo. The patients’ median age was 42 years, and 59% were male. The baseline characteristics were well balanced between the mirikizumab and placebo arms, including disease duration (6.7 vs 6.9 years), median bowel urgency severity (6.0 for both arms), baseline corticosteroid use (37% vs 38%), and baseline immunomodulator use (21% vs 22%). Prior unsuccessful therapies included a biologic agent in 35% of patients and tofacitinib in 36% of patients.
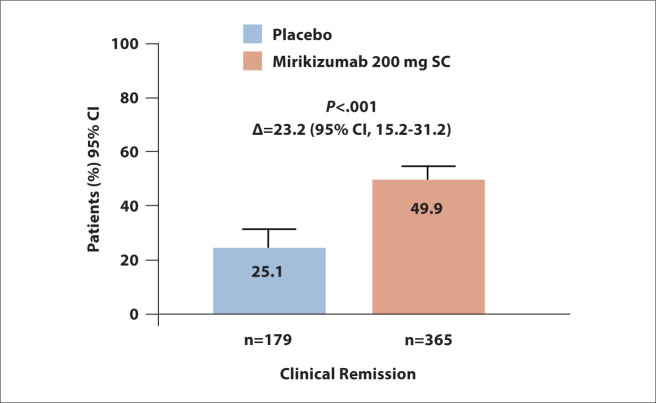
The LUCENT-2 trial met its primary endpoint. Clinical remission was achieved by 49.9% of the mirikizumab arm vs 25.1% of the placebo arm (P<.001; Figure 3). The rate of clinical remission at week 40 was 63.6% in the mirikizumab arm vs 36.0% in the placebo arm (95% CI, 10.4%-39.2%; P<.001). Moreover, 97.8% of patients in the mirikizumab arm who maintained clinical remission at week 40 were no longer receiving corticosteroids. The rate of corticosteroid-free remission was higher in the mirikizumab arm compared with the placebo arm (P<.001). The rate of endoscopic remission was superior with mirikizumab (P<.001), as was the rate of histologic-endoscopic mucosal remission (P<.001). Treatment with mirikizumab was superior to placebo in maintaining clinical remission at week 40 among patients who were naive to biologic therapy or tofacitinib (P<.001) and in patients who had previously received unsuccessful treatment with these agents (P<.001). Similarly, mirikizumab led to better endoscopic remission vs placebo in patients without prior exposure to biologic therapy or tofacitinib (P<.001) and in those who had received unsuccessful treatment with these agents (P<.001). The safety profile of mirikizumab was similar to that observed in prior studies.
Figure 3.
Clinical remission in the double-blind phase 3 LUCENT-2 trial, which evaluated mirikizumab maintenance therapy in patients with moderately to severely active ulcerative colitis who achieved a clinical response to mirikizumab induction therapy at week 12 in the LUCENT-1 trial. SC, subcutaneously. Adapted from Dubinsky MC et al. DDW abstract 867e. Gastroenterology. 2022;162(suppl 1).3
References
- 1.Hanzel J, Hulshoff MS, Grootjans J, D’Haens G. Emerging therapies for ulcerative colitis. Expert Rev Clin Immunol. 2022;18(5):513–524. doi: 10.1080/1744666X.2022.2069562. [DOI] [PubMed] [Google Scholar]
- 2.D’Haens G, Kobayashi T, Morris N et al. Efficacy and safety of mirikizumab as induction therapy in patients with moderately to severely active ulcerative colitis: results from the phase 3 LUCENT-1 study [DDW abstract 884]. Gastroenterology. 2022;162(suppl 1) [PMC free article] [PubMed] [Google Scholar]
- 3.Dubinsky MC, Irving PM, Li X et al. Efficacy and safety of mirikizumab as maintenance therapy in patients with moderately to severely active ulcerative colitis: results from the phase 3 LUCENT-2 study [DDW abstract 867e]. Gastroenterology. 2022;162(suppl 1) [PMC free article] [PubMed] [Google Scholar]