Deucravacitinib is an allosteric inhibitor of tyrosine kinase 2 (TYK2), which plays a role in adaptive and innate immunity.1 This inhibitor has a mechanism of action that is distinct from the other Janus kinase (JAK) inhibitors. Deucravacitinib selectively impedes human immune cells from responding to IL-12, IL-23, or type I interferon. In a mouse model of inflammatory bowel disease, deucravacitinib demonstrated efficacy that was consistent with inhibition of autoimmunity. Deucravacitinib was effective in phase 2 and 3 trials of patients with psoriatic arthritis or plaque psoriasis.2,3
LATTICE-UC was a double-blind phase 2 study that investigated the safety and efficacy of deucravacitinib in patients with moderately to severely active UC who had experienced an inadequate response or loss of response or who were intolerant to 1 or more conventional or biologic therapies.4 After a 4-week screening period, 131 patients were randomly assigned to receive deucravacitinib (6 mg twice daily) or placebo. The primary endpoint was clinical remission at week 12. Clinical remission was defined as a modified Mayo score with a stool frequency subscore of 1 or less and with a decrease from baseline of at least 1 point, a rectal bleeding subscore of 0, and an endoscopy subscore of 1 or less.
The baseline characteristics were generally similar in the 2 arms. There were differences between the treatment arms for the patients’ weight (≤90 kg, 25.0% in the deucravacitinib arm vs 16.3% in the placebo arm), modified Mayo score (≤7, 64.8% vs 72.1%, respectively), and endoscopic subscore (median of 3.0 [range, 2-3] vs 2.0 [range, 1-3], respectively).
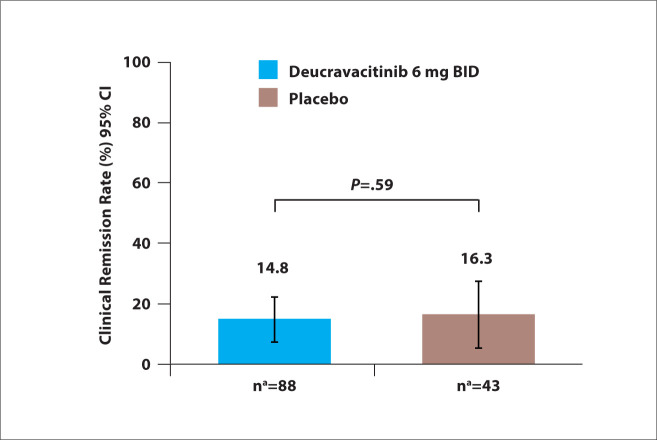
At week 12, the rates of clinical remission were 14.8% in the deucravacitinib arm vs 16.3% in the placebo arm (P=.59; Figure 5). Among patients without prior exposure to biologic therapy, the rates of clinical remission were 14.0% with deucravacitinib vs 25.9% with placebo. However, in patients with prior exposure to at least 1 biologic therapy, the rates of clinical remission were higher with deucravacitinib compared with placebo (16.1% vs 0.0%, respectively). The rates of endoscopic response were similar in the deucravacitinib arm and the placebo arm in the overall population (P=.88), as well as in subgroups with or without prior exposure to biologic therapy.
Figure 5.
Rates of clinical remission at 12 weeks among patients with moderately to severely active ulcerative colitis treated with deucravacitinib or placebo in the phase 2 LATTICE-UC study. aThe numbers of patients were based on the electronic case report form. BID, twice daily. Adapted from Danese S et al. DDW abstract 965. Gastroenterology. 2022;162(suppl 1).4
The trial failed to meet its primary and secondary endpoints. In the overall population, treatment with deucravacitinib led to a numerical improvement in the symptomatic Mayo score from baseline compared with placebo (–2.2 vs –1.6). Most AEs were mild to moderate in severity. Serious AEs occurred in 9.2% of patients in the deucravacitinib arm. A second phase 2 trial will evaluate a higher dose of deucravacitinib in patients with UC.
References
- 1.Burke JR, Cheng L, Gillooly KM et al. Autoimmune pathways in mice and humans are blocked by pharmacological stabilization of the TYK2 pseudokinase domain. Sci Transl Med. 2019;11(502):eaaw1736. doi: 10.1126/scitranslmed.aaw1736. [DOI] [PubMed] [Google Scholar]
- 2.Armstrong A, Gooderham M, Warren RB et al. Efficacy and safety of deucravacitinib, an oral, selective tyrosine kinase 2 (TYK2) inhibitor, compared with placebo and apremilast in moderate to severe plaque psoriasis: results from the phase 3 POETYK PSO-1 study [AAD abstract POS1042]. Ann Rheum Dis. 2021;80(suppl 1) [Google Scholar]
- 3.Mease PJ, Deodhar AA, van der Heijde D et al. Efficacy and safety of selective TYK2 inhibitor, deucravacitinib, in a phase II trial in psoriatic arthritis. Ann Rheum Dis. 2022;81(6):815–822. doi: 10.1136/annrheumdis-2021-221664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Danese S, Panaccione R, D’Haens G et al. Efficacy and safety of deucravacitinib, an oral, selective tyrosine kinase 2 inhibitor, in patients with moderately to severely active ulcerative colitis: 12-week results from the phase 2 LATTICE-UC study [DDW abstract 965]. Gastroenterology. 2022;162(suppl 1) [PMC free article] [PubMed] [Google Scholar]