Abstract
BACKGROUND:
Epidemiologic evidence reporting the role of frailty in survival among older adults with a prior cancer diagnosis is limited.
METHODS:
A total of 2050 older adults (≥60 years old) surviving for at least 1 year after a cancer diagnosis and 9474 older adults without a cancer history from the National Health and Nutrition Examination Survey (1999–2014) were included for analysis. The exposure variable, a 45-item frailty index (FI), was categorized on the basis of validated cutoffs (FI ≤ 0.10 [fit], 0.10 < FI ≤ 0.21 [prefrail], and FI > 0.21 [frail]). All-cause mortality was ascertained via the National Death Index. Multivariable Cox proportional hazards models were used to estimate adjusted hazard ratios (aHRs) and 95% confidence interval (CIs) for the FI, and this was followed by restricted cubic splines depicting dose-response curves.
RESULTS:
For older cancer survivors, the mean age at the baseline was 72.6 years (SD, 7.1 years); 5.9% were fit, 38.2% were prefrail, and 55.9% were frail. Older adults without a cancer history were slightly younger (mean age, 70.0 years) and less frail (47.9% were frail). At each level of the FI, cancer survivors (1.9 per 100 person-years for FI ≤ 0.10, 3.4 per 100 person-years for 0.10 < FI ≤ 0.21, and 7.5 per 100 person-years for FI > 0.21) had higher mortality than their cancer-free counterparts (1.4 per 100 person-years for FI ≤ 0.10, 2.4 per 100 person-years for 0.10 < FI ≤ 0.21, and 5.4 per 100 person-years for FI > 0.21). The multivariable model suggested a positive association between the FI and all-cause mortality for survivors (aHR for FI > 0.21 vs FI ≤ 0.10, 2.80; 95% CI, 1.73–4.53) and participants without a cancer history (aHR for FI > 0.21 vs FI ≤ 0.10, 2.75; 95% CI, 2.29–3.32). Restricted cubic splines indicated that all-cause mortality risk increased with the FI in a monotonic pattern.
CONCLUSIONS:
Frailty is associated with a higher risk of death in older cancer survivors and the elderly without a cancer history.
Keywords: cancer survivorship, epidemiology, frailty, geriatric oncology, gerontology, tertiary cancer prevention
INTRODUCTION
The proportion of the older adult population in the United States is growing at a rapid pace. Specifically, the US Census Bureau has estimated that approximately 25% of residents will be 65 years old or older by 2060,1 and they will include a large number of older adults living with comorbidities.2,3 Similar to many other chronic illnesses, cancer is an age-related disease.4 According to data from the Surveillance, Epidemiology, and End Results program, the median age of cancer diagnosis in the United States is 66 years.5 Moreover, with the development of antitumor treatment modalities, the prognosis of older patients with cancer has substantially improved. For example, the proportion of older adults among all cancer survivors is steadily rising in the United States: It increased from 50% in 1975 to 60% in 2010 and will reach more than 70% by 2040.6 In addition to treatment, other health-related factors should also be considered in health care management for older adults with prior cancer diagnoses, and frailty is an extremely fundamental one.
Frailty describes the status of physiological decline, vulnerability to diseases, and age-related disturbed homeostasis.7,8 It develops as a result of cumulative age-related decline across multiple organs and increases the risk of many adverse health outcomes, including death.9 A meta-analysis synthesizing data from 46 observational studies reported that the incidence rate of frailty is 43.4 per 1000 person-years among community-dwelling adults 60 years old or older.10 These data underscore the likelihood that many older adults are facing the consequences of frailty, such as slow recovery from chronic diseases, including cancer, and a reduction in life expectancy.
Studying the association between frailty and the risk of death in older adults with a prior cancer diagnosis can help cancer survivors and health care providers to better predict outcomes after treatment and improve survivorship. However, the existing studies reporting an association between frailty and death among older cancer survivors are limited and have some methodologic limitations. For example, these studies did not consider nutritional or lifestyle factors in their analyses or were performed solely in female cancer survivors.11–13 In addition, these studies did not compare effect measures of frailty between cancer survivors and counterparts without a cancer history, and this makes us unable to judge whether the impact of frailty is more substantial in cancer survivors. To remedy these gaps, we used data from the National Health and Nutrition Examination Survey (NHANES) to more thoroughly study the impact of frailty on the mortality of older cancer survivors and compare the estimates with those for older adults without a cancer history.
MATERIALS AND METHODS
Data Source and Study Population
The NHANES is a program led by the Centers for Disease Control and Prevention that assesses nutritional status, health-related behaviors, medical service utilization, and burdens of illnesses among adults and children in the United States. It uses interviews with physical examinations and laboratory testing to measure the aforementioned items.14 The National Center for Health Statistics has linked the 1999–2014 NHANES data with death certificate records from the National Death Index.15 To generate the cohort for analysis, we linked exposures and relevant covariates in the 1999–2014 NHANES to the vital status measured during the same period. We used 60 years as the cutoff to define the elderly as suggested by the World Health Organization.16 We referred to the definition used in the National Coalition for Cancer Survivorship17 and treated people who survived for a period of time after their cancer diagnosis as cancer survivors. People with the following characteristics were included in our study:
They were ≥60 years old at the interview.
They had less than 20% missing data18–21 for individual items incorporated into the frailty index (FI).
They had no missing data for covariates.
Cancer survivors should have survived for at least 1 year since their cancer diagnosis (because most patients with cancer will receive antitumor therapies during the first year of survivorship,22 which can substantially affect function and blood test results).
Older adults without cancer should have met criteria 1 to 3.
This yielded a total of 2050 older cancer survivors and 9474 older adults without a cancer history for analysis (Supporting Fig. 1).
Exposure and Outcome of Interest
To measure frailty, we used a cumulative index that was developed and validated in prior studies.18–21 Specifically, our study used a 45-item FI that incorporated comorbidities, functional status, clinical measures, and laboratory testing results in accordance with the published literature.18–21 At the baseline, health conditions (comorbidities [n = 15], functional impairments [n = 15], health services use [n = 3], and general health [n = 2]) were collected by self-report; clinical measures (n = 2) were obtained by trained examiners at a mobile examination center (MEC) at the baseline; and biomarkers (n = 8) were measured with the blood samples collected at the baseline.23 The FI was calculated as a proportion whose denominator was 45 and whose numerator was the sum of scores associated with each individual item (individual item scores ranged from 0 to 1); thus, the FI ranged from 0 to 1, with a larger value suggesting a higher burden of frailty. Scores for each individual item are presented in Supporting Table 1. On the basis of published literature using the same algorithm,18–21 participants with 20% or more missing data for individual items in the FI were excluded. The FI was categorized as an ordinal variable on the basis of cutoffs used in prior literature18–21 to reflect fitness (FI ≤ 0.10), prefrailty (0.10 < FI ≤ 0.21), and frailty (FI > 0.21).
All-cause death was identified by linkage to the National Death Index through December 31, 2015. The follow-up time was the interval from the baseline interview to the date of death or December 31, 2015, whichever occurred first. Participant were treated as censored if they were alive.
Other Covariates
Demographic factors (age, sex, and race) were self-reported at the baseline interview. Age was categorized as a 3-level variable (60–69, 70–79, or ≥80 years); sex was treated as female or male; and race was categorized as White, Black, or other. Education was classified as an ordinal variable (high school or less, attended college, or graduated from college). Participants who were never married, separated, divorced, or widowed were treated as not married in our study, and we pooled married individuals and individuals living with partners as 1 category. Smoking status was treated as a categorical variable (current, former, or never); in particular, participants who self-reported ever smoking at least 100 cigarettes in their life were treated as current smokers or former smokers if they had quit. The baseline body weight and height were measured by trained staff at the MEC. The body mass index (BMI) was calculated as the weight (kg) divided by the height squared (m2), and we categorized it as an ordinal variable (<25, 25–29.9, or ≥30 kg/m2) by using cutoffs recommended by the World Health Organization.24 At the baseline MEC, participants self-reported alcohol consumption during the past year; because some evidence suggested a potential for low to moderate alcohol consumption to lower frailty risk,25–28 we treated alcohol consumption as an ordinal variable that incorporated the following: nondrinker, low to moderate consumption (≤1 drink per day), and high consumption (>1 drink per day). We included the dietary intake of protein and energy measured by 24-hour food recall at the baseline MEC because population-based evidence suggested that they could affect frailty and mortality29–31; we used certain cutoffs to categorize them as ordinal variables to approximate quartiles in cancer survivors, and the same cutoffs were applied for participants without a cancer history. Self-reported cancer-related variables included the following: history of more than 1 cancer, age at cancer diagnosis (<60 vs ≥60 years), time elapsed since the cancer diagnosis (0–4, 5–9, or ≥10 years), and cancer type (breast cancer, prostate cancer, colorectal cancer, melanoma, or other). Because of the wide time span associated with the study population, we included the survey year in our analysis. Covariates were selected on the basis of a priori knowledge regarding their relationships with exposure and outcome.
Statistical Analysis
The overall analytical strategy was mainly based on an unweighted approach because the NHANES weight was generated to reflect the distribution of the general US population, whereas we focused on older adults who were heterogeneous in comparison with the general population.
First, we descriptively summarized distributions of the FI and other covariates in older cancer survivors and adults without a cancer history. Because prior studies32,33 suggested that age, sex, and race were associated with burdens of illnesses and function, we used a generalized linear model adjusting for age, sex, and race to investigate whether the distributions of the FI differed between older cancer survivors and those without a cancer history. Kaplan-Meier curves were used to visualize the risk of all-cause mortality by FI categories in these 2 groups. We used the follow-up in NHANES as the time scale, and log-rank tests were performed to examine whether the risk differed by FI categories. We then estimated the death rate by FI categories based on the numbers of deaths and person-years contributed by participants.
Univariate and multivariable Cox proportional hazards regression models were used to estimate hazard ratios (HRs) and 95% confidence intervals (CIs) for the FI, with FI ≤ 0.10 used as the reference. The multivariable model adjusted for age, sex, race, education, marital status, BMI, smoking, alcohol drinking, protein and energy consumption, and survey year; for cancer survivors, we additionally adjusted for the age at cancer diagnosis and a history of more than 1 cancer. The proportionality assumption of the Cox model was examined by visual inspections of log(−log[S]) plots, and there was no violation of the assumption.34 In addition, we corrected for the NHANES sampling weight in multivariable models adjusting for the same covariates to explore whether effect measures of the FI changed substantially. We conducted tests for trend by treating the FI as a continuous variable in the model. To depict the potential nonlinear dose-response relationship between the FI and the risk of death, we applied a restricted cubic spline35 in the multivariable Cox models, with FI = 0.21 used as the reference for the dose-response curve; we assessed the nonlinearity by contrasting the model fit using restricted cubic splines with a model fit assuming linearity for the FI via a likelihood ratio test.36
Subgroup analyses were based on age, sex, race, and nutrition-related factors (BMI, alcohol consumption, and dietary intake of protein and energy). Because of sample size considerations, we treated frailty as a binary variable in subgroup analyses (FI > 0.21 vs FI ≤ 0.21). An interaction term between the factor used for stratification and frailty was added to the model, and we used Wald tests to examine whether there were significant interactions between them.
In sensitivity analyses, multiple imputations fit with 5 replicates of chained equations were applied to examine whether missing data substantially affected the association pattern of the FI. We further applied the primary multivariable model to participants with breast cancer, prostate cancer, colorectal cancer, and melanoma separately and explored whether associations identified in the primary analysis changed for these specific types of cancer; we treated frailty as a binary variable (FI > 0.21 vs FI ≤ 0.21) in analyses for specific cancer types for sample size consideration.
Two-sided values of P < .05 were considered to be statistically significant. Statistical analyses were conducted with Stata 15.0 (StataCorp LLC, College Station, Texas) and SAS v9.4 (SAS Institute, Inc, Cary, North Carolina).
RESULTS
Table 1 presents distributions of study characteristics. Overall, for the 2050 older cancer survivors, the mean age at the baseline was 72.6 years (SD, 7.1 years); 5.9% were fit (FI ≤ 0.10), 38.2% were prefrail (0.10 < FI ≤ 0.21), and 55.9% were frail (FI > 0.21). Older adults without a cancer history were slightly younger than the cancer survivors, and their mean age was 70.0 years (SD, 7.3 years); the FI distribution in older adults without cancer (11.0% fit, 41.1% prefrail, and 47.9% frail) was different from that in cancer survivors, and the generalized linear model indicated that the difference was statistically significant (P < .01). Sex was almost evenly distributed regardless of prior cancer history. Compared with older adults without a cancer history, older cancer survivors had a higher proportion of White participants (cancer survivors, 74.9%; no cancer history, 50.8%). Detailed distributions of other covariates can be found in Table 1. In older cancer survivors and adults without a cancer history, the prevalence of frailty increased with age (Supporting Table 2); in each age category, cancer survivors (60–69 years, 45.4%; 70–79 years, 56.6%; and ≥80 years, 69.7%) had a higher prevalence of frailty than their cancer-free counterparts (60–69 years, 39.1%; 70–79 years, 51.8%; and ≥80 years, 67.7%), and this pattern was more substantial among participants younger than 80 years. More detailed distributions of frailty by study characteristics are present in Supporting Table 2.
TABLE 1.
Study Characteristics of Older Cancer Survivors and Adults Without a Cancer History
| Study Characteristic | Cancer Survivors (n = 2050), No. (%) | No Cancer History (n = 9474), No. (%) |
|---|---|---|
|
| ||
| FI | ||
| ≤0.10 | 121 (5.9) | 1045 (11.0) |
| >0.10 to 0.21 | 783 (38.2) | 3894 (41.1) |
| >0.21 | 1146 (55.9) | 4535 (47.9) |
| Age at interview | ||
| 60–69 y | 712 (34.7) | 4949 (52.2) |
| 70–79 y | 837 (40.8) | 2911 (30.7) |
| ≥80 y | 501 (24.4) | 1614 (17.1) |
| Sex | ||
| Female | 978 (47.7) | 4842 (51.1) |
| Male | 1072 (52.3) | 4632 (48.9) |
| Race | ||
| White | 1536 (74.9) | 4817 (50.8) |
| Black | 277 (13.5) | 1910 (20.2) |
| Other | 237 (11.6) | 2747 (29.0) |
| Education | ||
| High school or less | 995 (48.5) | 5795 (61.2) |
| Attended college | 546 (26.6) | 2100 (22.2) |
| Graduated from college | 509 (24.8) | 1579 (16.6) |
| Marital status | ||
| Not married | 764 (37.3) | 3897 (41.1) |
| Married or living with partner | 1286 (62.7) | 5577 (58.9) |
| BMI | ||
| <25.0 kg/m2 | 607 (29.6) | 2486 (26.2) |
| 25.0–29.9 kg/m2 | 768 (37.5) | 3555 (37.5) |
| ≥30.0 kg/m2 | 675 (32.9) | 3433 (36.3) |
| Smoking status | ||
| Never | 877 (42.8) | 4590 (48.4) |
| Current | 198 (9.7) | 1228 (13.0) |
| Former | 975 (47.5) | 3656 (38.6) |
| Alcohol consumption | ||
| No consumption | 689 (33.6) | 3460 (36.5) |
| ≤1 drink per day | 565 (27.6) | 2110 (22.3) |
| >1 drink per day | 796 (38.8) | 3904 (41.2) |
| Protein intake | ||
| <51.4 g/d | 505 (24.6) | 2732 (28.8) |
| 51.4–68.0 g/d | 511 (24.9) | 2415 (25.5) |
| 68.1–86.2 g/d | 521 (25.4) | 2103 (22.2) |
| ≥86.3 g/d | 513 (25.1) | 2224 (23.5) |
| Energy intake | ||
| <1351.0 kcal/d | 505 (24.6) | 2866 (30.2) |
| 1351.0–1735.4 kcal/d | 519 (25.3) | 2342 (24.7) |
| 1735.5–2180.9 kcal/d | 510 (24.9) | 2137 (22.6) |
| ≥2181.0 kcal/d | 516 (25.2) | 2129 (22.5) |
| History of more than 1 cancer | ||
| No | 1816 (88.6) | — |
| Yes | 234 (11.4) | — |
| Age at cancer diagnosis | ||
| <60 y | 808 (39.4) | — |
| ≥60 y | 1242 (60.6) | — |
| Time elapsed since cancer diagnosis | ||
| 1–4 y | 595 (29.0) | — |
| 5–9 y | 506 (24.7) | — |
| ≥10 y | 949 (46.3) | — |
| Cancer type | ||
| Breast cancer | 353 (17.2) | — |
| Prostate cancer | 414 (20.2) | — |
| Colorectal cancer | 170 (8.3) | — |
| Melanoma | 115 (5.6) | — |
| Other | 998 (48.7) | — |
| Survey year | ||
| 1999–2002 | 434 (21.1) | 2147 (22.7) |
| 2003–2006 | 475 (23.2) | 2272 (24.0) |
| 2007–2010 | 643 (31.4) | 2752 (29.0) |
| 2011–2014 | 498 (24.3) | 2303 (24.3) |
Abbreviations: BMI, body mass index; FI, frailty index.
Column percentages are reported.
A total of 738 older cancer survivors and 2521 participants without a cancer history died during the follow-up, and the former had a slightly shorter median follow-up (cancer survivors, 6.1 years; no cancer history, 6.9 years). The Kaplan-Meier curves suggested that the risk of death significantly increased with the FI in both populations (log-rank P values < .01; Fig. 1A,B), although the survival curves declined more drastically for older cancer survivors. The overall death rate of cancer survivors was 5.4 per 100 person-years, which was 50% higher than the death rate of adults without a cancer history (3.6 deaths per 100 person-years); similarly, at each level of the FI (Table 2), the death rate of older cancer survivors (1.9 per 100 person-years for FI ≤ 0.10, 3.4 per 100 person-years for 0.10 < FI ≤ 0.21, and 7.5 per 100 person-years for FI > 0.21) was higher than that of participants without a cancer history (1.4 per 100 person-years for FI ≤ 0.10, 2.4 per 100 person-years for 0.10 < FI ≤ 0.21, and 5.4 per 100 person-years for FI > 0.21). The multivariable Cox model (Table 2) suggested a positive association between the FI and all-cause mortality (adjusted hazard ratio [aHR] for FI > 0.21 vs FI ≤ 0.10, 2.80; 95% CI, 1.73–4.53; P trend < .01) in older cancer survivors; similar patterns were also observed for older adults without a cancer history (aHR for FI > 0.21 vs FI ≤ 0.10, 2.75; 95% CI, 2.29–3.32; P trend < .01). For older cancer survivors, effect measures of the FI obtained in the model correcting for sampling weight increased to some extent (aHR for FI > 0.21 vs FI ≤ 0.10, 3.41; 95% CI, 1.96–5.94; P trend < .01), but the corresponding 95% CIs largely overlapped that of the primary model. Correcting for sampling weight did not change the effect measures in older adults without a cancer history. Results from restricted cubic splines were in line with primary Cox models and suggested that the risk of death increased with the FI in a monotonic dose-response pattern (Fig. 2A,B); the likelihood ratio tests did not support nonlinearity of the dose-response curves (P nonlinearity for cancer survivors = .24; P nonlinearity for no cancer history = .08).
Figure 1.
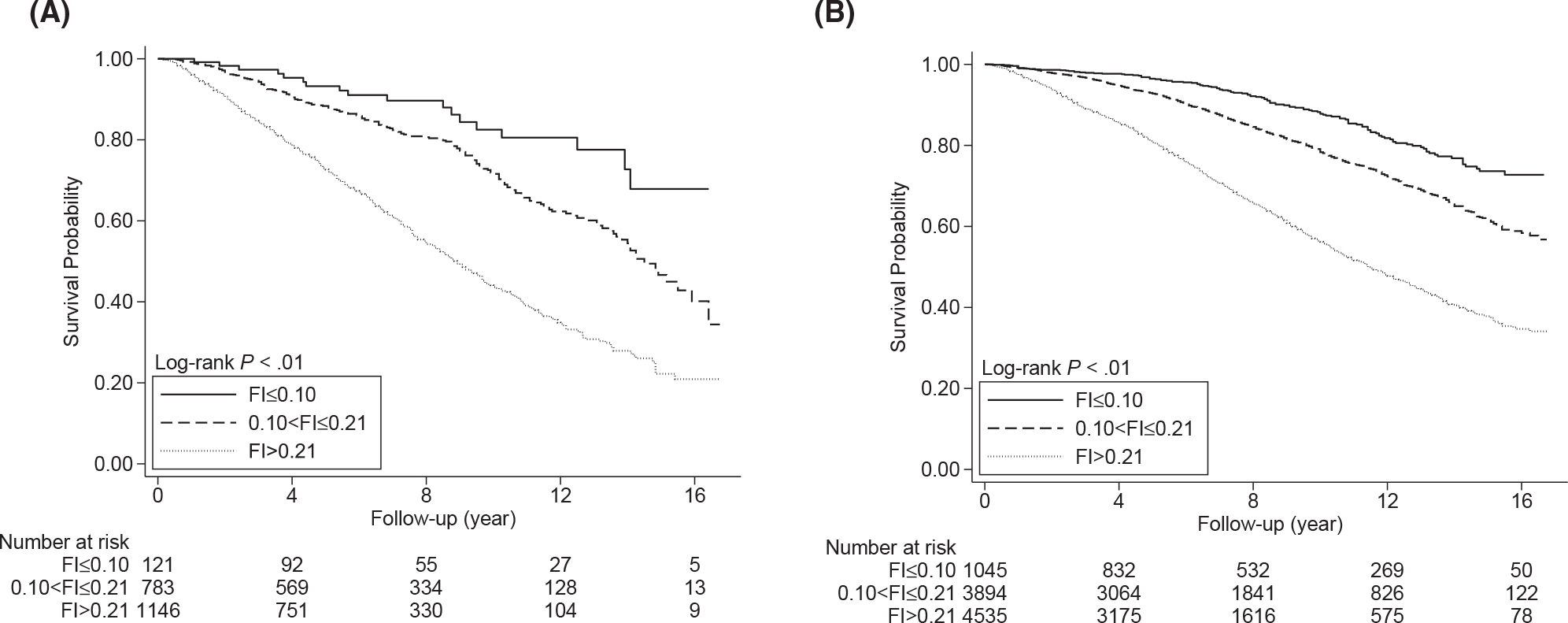
Kaplan-Meier curves for (A) older cancer survivors and (B) older adults without a cancer history. FI indicates frailty index.
TABLE 2.
Association Between the FI and Mortality in Older Cancer Survivors and Those Without a Cancer History
| FI | No. of Deaths/Total | Person-Years | Deaths per 100 Person-Years (95% CI) | cHR (95% CI) | aHR (95% CI)a | aHR (95% CI)b |
|---|---|---|---|---|---|---|
|
| ||||||
| Cancer survivors (n = 2050) | ||||||
| ≤0.10 | 18/121 | 963.8 | 1.9 (1.2–3.0) | REF | REF | REF |
| >0.10 to 0.21 | 198/783 | 5742.3 | 3.4 (3.0–4.0) | 1.92 (1.19–3.11) | 1.39 (0.86–2.27) | 1.58 (0.89–2.80) |
| >0.21 | 522/1146 | 6940.7 | 7.5 (6.9–8.2) | 4.49 (2.80–7.18) P trend < .01 |
2.80 (1.73–4.53) P trend < .01 |
3.41 (1.96–5.94) P trend < .01 |
| No cancer history (n = 9474) | ||||||
| ≤0.10 | 128/1045 | 8843.2 | 1.4 (1.2–1.7) | REF | REF | REF |
| >0.10 to 0.21 | 757/3894 | 31,088.5 | 2.4 (2.3–2.6) | 1.73 (1.43–2.09) | 1.38 (1.14–1.66) | 1.28 (1.05–1.56) |
| >0.21 | 1636/4535 | 30,577.3 | 5.4 (5.1–5.6) | 4.03 (3.37–4.83) P trend < .01 |
2.75 (2.29–3.32) P trend < .01 |
2.71 (2.23–3.29) P trend < .01 |
Abbreviations: aHR, adjusted hazard ratio; cHR, crude hazard ratio; CI, confidence interval; FI, frailty index; REF, reference.
The multivariable Cox model adjusted for the following: age, sex, race, education, marital status, body mass index, smoking, alcohol drinking, protein and energy consumption, age at cancer diagnosis (only for participants with a cancer history), history of more than 1 cancer (only for participants with a cancer history), and survey year.
The multivariable model corrected for the sampling weight and adjusted for the same set of covariates as the main model.
Figure 2.
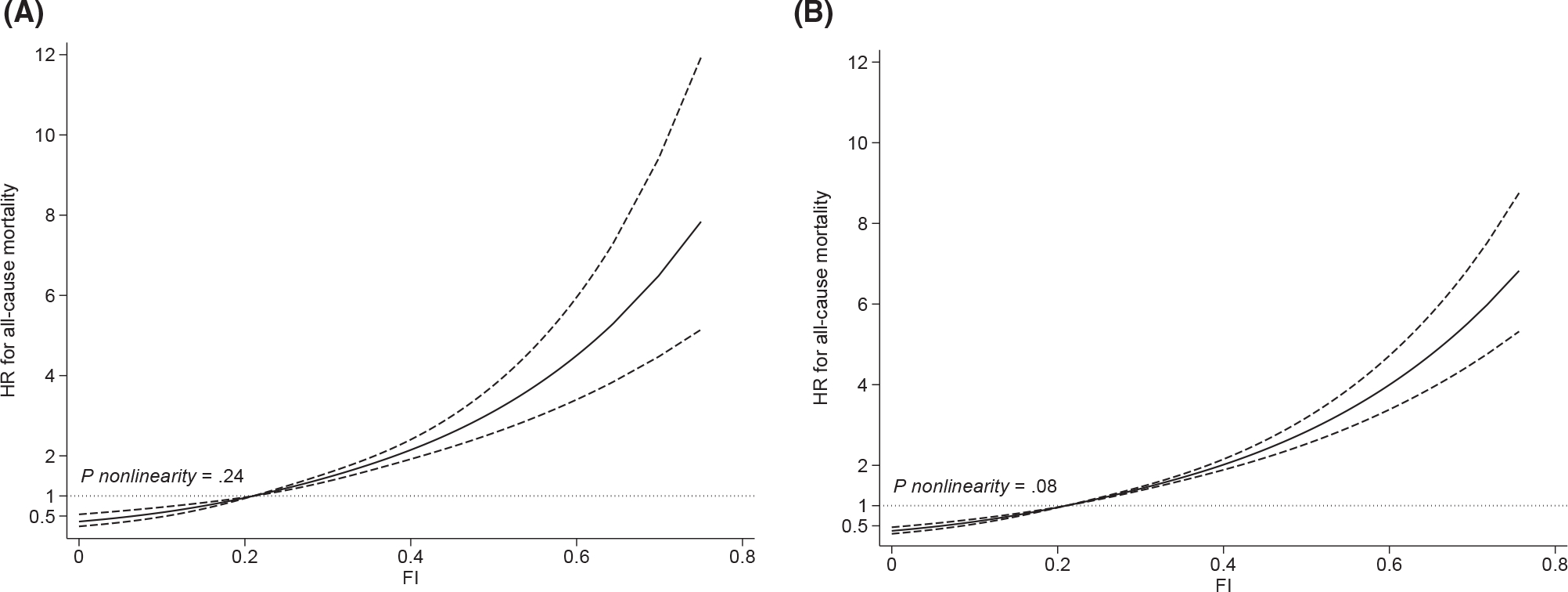
Restricted cubic splines depicting dose-response relationships between the FI and the risk of all-cause mortality in (A) older cancer survivors and (B) older adults without a cancer history. The model adjusted for the following: age, sex, race, education, marital status, body mass index, smoking, alcohol drinking, protein and energy consumption, age at cancer diagnosis (for cancer survivors), history of more than 1 cancer (for cancer survivors), and survey year. The solid lines are fitted lines, the dashed lines are the 95% confidence intervals, and the dotted lines are the reference lines. FI = 0.21 is the reference in the curve. FI indicates frailty index; HR, hazard ratio
In subgroup analyses (Table 3), frailty was associated with a higher risk of all-cause mortality in all subgroups. We did not observe any significant interaction in older adults without a cancer history (P interaction > .05). For older cancer survivors, a significant interaction was identified for age; specifically, in comparison with survivors 75 years old or older (aHR, 1.59; 95% CI, 1.27–1.97), the effect measure of frailty was much more substantial for survivors 60 to 74 years old (aHR, 2.62; 95% CI, 2.00–3.43; P interaction < .01). Although effect measures of frailty were largely different by BMI values in older cancer survivors (aHR for BMI < 30 kg/m2, 1.92; 95% CI, 1.59–2.33; aHR for BMI ≥ 30 kg/m2, 3.00; 95% CI, 2.04–4.40), the interaction was only marginally significant (P interaction = .06). No significant interactions were identified in other sets of subgroup analyses.
TABLE 3.
Association Between Frailty (FI > 0.21) and All-Cause Mortality in Subgroups Defined by Study Covariates
| Cancer Survivors (n = 2050) |
No Cancer History (n = 9474) |
|||
|---|---|---|---|---|
| No. of Deaths/Total | aHR (95% CI) | No. of Deaths/Total | aHR (95% CI) | |
|
| ||||
| Age | ||||
| <75 y | 274/1160 | 2.62 (2.00–3.43) | 1141/6707 | 2.25 (1.99–2.54) |
| ≥75 y | 464/890 | 1.59 (1.27–1.97) P interaction < .01 |
1380/2767 | 2.05 (1.82–2.31) P interaction = .13 |
| Sex | ||||
| Female | 305/978 | 2.46 (1.85–3.28) | 1120/4842 | 2.18 (1.91–2.49) |
| Male | 433/1072 | 1.89 (1.53–2.33) P interaction = .19 |
1401/4632 | 2.03 (1.82–2.27) P interaction = .46 |
| Race | ||||
| White | 581/1536 | 2.06 (1.70–2.50) | 1510/4817 | 2.12 (1.89–2.37) |
| Non-White | 157/514 | 2.00 (1.38–2.90) P interaction = .89 |
1011/4657 | 2.05 (1.79–2.34) P interaction = .67 |
| BMI | ||||
| <30 kg/m2 | 541/1375 | 1.92 (1.59–2.33) | 1773/6041 | 2.07 (1.88–2.29) |
| ≥30 kg/m2 | 197/675 | 3.00 (2.04–4.40) P interaction = .06 |
748/3433 | 2.16(1.83–2.55) P interaction = .85 |
| Alcohol drinking | ||||
| No | 256/689 | 2.08 (1.54–2.80) | 955/3460 | 2.30 (1.99–2.65) |
| Yes | 482/1361 | 2.15 (1.75–2.64) P interaction = .88 |
1566/6014 | 2.00 (1.80–2.23) P interaction = .10 |
| Protein intake | ||||
| <68.1 g/d | 413/1016 | 2.18 (1.73–2.76) | 1530/5147 | 2.10 (1.88–2.35) |
| ≥68.1 g/d | 325/1034 | 1.97 (1.54–2.51) P interaction = .68 |
991/4327 | 2.09 (1.83–2.38) P interaction = .93 |
| Energy intake | ||||
| <1735.5 kcal/d | 404/1024 | 2.08 (1.65–2.63) | 1508/5208 | 2.11 (1.88–2.36) |
| ≥1735.5 kcal/d | 334/1026 | 2.04 (1.60–2.60) P interaction = .96 |
1013/4266 | 2.09 (1.83–2.39) P interaction = .80 |
Abbreviations: aHR, adjusted hazard ratio; BMI, body mass index; CI, confidence interval; FI, frailty index.
The models adjusted for the same sets of covariates as those in Table 2 except for the ones used for stratification.
After multiple imputation, the effect measures of the FI in both study populations slightly increased, but their 95% CIs largely overlapped those estimated from primary multivariable models (Supporting Table 3). Positive associations between frailty and the risk of all-cause mortality were observed for individuals with a history of breast cancer, prostate cancer, colorectal cancer, or melanoma, although the effect measure for colorectal cancer was not statistically significant (Supporting Table 4).
DISCUSSION
Our study suggests that older cancer survivors have a higher prevalence of frailty than older adults without cancer, and this phenomenon may be caused by the effect of cancer-related pathogenesis (eg, chronic inflammation) accelerating aging processes and toxicities of antitumor treatment.37,38 The multivariable analyses suggest that frailty is associated with a higher risk of all-cause mortality in older adults regardless of prior cancer history. The magnitude of association between the burden of frailty and mortality is similar between older cancer survivors and adults without a cancer history, and it exists in a monotonic dose-response pattern. However, within the same level of the FI, older cancer survivors have a higher risk of death than their cancer-free counterparts, which could be caused by the synergistic effects between frailty and cancer-related burden.
We observed that the HR of frailty was less substantial for cancer survivors 75 years old or older than for younger survivors. One speculation is that, among older cancer survivors, the life expectancy of those 75 years old or older is much shorter than that of younger counterparts; this predisposes survivors 75 years old or older to an inherently high risk of death that is less likely to be affected by the frailty burden. Obesity has the potential to affect multiple biological pathways (eg, insulin signaling, inflammation, and apoptosis) that are relevant to cancer recurrence, progression, and long-term treatment toxicities,39–41 and this suggests that it can enhance the impact of frailty on the mortality of cancer survivors in a synergistic manner. Although we found that the point estimate of frailty was much larger in survivors with higher BMIs, the Wald test suggested only a marginally significant interaction, and this indicates that the potential modification effect of obesity should be further verified by larger studies in the future. On the other hand, we did not observe any interaction between frailty and age or BMI among older adults without a cancer history; one possibility is that negative effects of cancer treatment (eg, treatment toxicities) and preexisting factors relevant to carcinogenesis (eg, chronic inflammation) make cancer survivors heterogeneous from their cancer-free counterparts, and this leads to distinct outcomes when we are testing for interaction.
Our results are in line with previous studies investigating associations between frailty and the risk of death in older cancer survivors. A cohort study measured frailty by a 36-item index in 518 older adults with a cancer history (median age, 72 years) and followed them for 3.7 years on average; the multivariable analysis suggested that frailty was associated with a higher risk of all-cause mortality (HR, 2.36; 95% CI, 1.51–3 .68).11 Another cohort study used 35 illnesses and functional items to reflect frailty at the baseline in 1280 older patients with breast cancer (mean age, 72.4 years) and followed them for a maximum of 7 years; the results found that frailty was associated with a 1.4-fold relative increase in the risk of all-cause mortality.12 Moreover, a cohort study using a sample (median age, 63 years) from the Women’s Health Initiative measured frailty by a score derived from the Fried phenotype and reported that frail women (vs nonfrail women) had a 40% relative increase in their mortality risk after a cancer diagnosis.13 In contrast to the aforementioned studies, our analysis considered both sexes, used a more comprehensive frailty measure, considered nutritional and lifestyle factors, and compared the results between older adults with a cancer history and those without a cancer history; this makes the estimated association more robust and indicates a consistent prognostic role of frailty in older adults regardless of their prior cancer history.
Several mechanisms may partially explain the positive association between frailty and mortality identified in our analysis. First, previous studies indicated a link between frailty and chronic inflammation.42,43 A meta-analysis43 synthesized 32 cross-sectional studies and reported that frailty was associated with higher blood levels of C-reactive protein and interleukin 6, which have been found to be related to a higher risk of all-cause mortality in prior population-based studies.44–47 Second, frail adults have a higher rate of coexisting immunosenescence, although their causal relationship has not been fully uncovered48,49; this suggests that immune surveillance in older frail individuals with a cancer history can be compromised, and this increases the risk of cancer recurrence or an unfavorable prognosis.50–52 For example, Mima et al51 analyzed 729 patients with stage I to III colorectal cancer (46% were ≥75 years old; the median follow-up was 3.5 years); they reported that patients with frailty had a higher risk of recurrence than nonfrail patients (HR, 1.70; 95% CI, 1.25–2.31).
Our study has several notable strengths in its design and analysis. First, we used a 45-item FI that incorporated preexisting illnesses, functional status, clinical measures, and biomarkers; this made the exposure comprehensive.18,19 Second, using death certificates to measure mortality ensured the robustness of our outcome and largely reduced the risk of misclassification. Third, a model correcting for the sampling weight, a dose-response analysis, and an application of multiple imputation further validated the results obtained in the primary multivariable model. Fourth, comparing results between older cancer survivors and counterparts without a cancer history can make the conclusions more generalizable to older populations regardless of their cancer status. Fifth, compared with geriatric assessment (GA), using an FI in NHANES samples can better reflect the frailty burden of older cancer survivors in real-world settings. In oncology practices, clinicians use GA to identify frailty, and it is usually initiated before cancer treatment to improve communications, decision-making, and disease management.53–55 However, cancer survivors’ frailty levels can change after treatment; this indicates that pretreatment GA-based frailty may not accurately reflect the frailty burden in the survivorship period, and the nature of clinical settings can make a GA estimate the frailty burden with bias in older adults diagnosed with cancer.
In our study, several limitations should still be noted. Participants self-reported their comorbidities, and this is less accurate than a medical record review or measures from claims data. The health status of participants can change during the follow-up, whereas the FI can be established only on the basis of baseline measures; this makes it hard to perform a time-varying analysis. Because of the study design used for NHANES, a large proportion of the participants are not incident cancer cases. In our study sample, 46.3% were diagnosed 10 or more years before the baseline assessment, and 71% survived for at least 5 years since their cancer diagnosis. This raises the possibility of a survivorship bias that should be considered when one is interpreting the results because those with shorter survival times may already be deceased before the baseline assessment. In addition, the cancer treatment and the stage at diagnosis are very important factors that can affect survival, but the NHANES did not measure these variables at the interview; this leads to some residual confounding in the analysis of cancer survivors.
In light of the positive association between frailty and the risk of death, health interventions, either behavioral or nutritional, should be considered to reduce the impact of frailty among older cancer survivors because improving lifestyle behaviors has been found to have favorable effects on measures incorporated into the FI such as comorbidities, functional status, and other patient-reported outcomes, including quality of life.17,56–59
In conclusion, older cancer survivors will be at higher risk for mortality if they are living with substantial burdens of frailty. Understanding the frailty status is informative for developing long-term interventions for promoting the health of older cancer survivors.
Supplementary Material
FUNDING SUPPORT
This study was internally funded through the University of Florida Health Cancer Center.
We thank the National Health and Nutrition Examination Survey participants and the research staff for their contributions of the data and technical support.
Footnotes
CONFLICT OF INTEREST DISCLOSURES
The authors made no disclosures.
We used a publicly de-identified data set that does not require institutional review board approval.
Additional supporting information may be found in the online version of this article.
REFERENCES
- 1.Vespa J The U.S. joins other countries with large aging populations. US Census Bureau. Published March 13, 2018. Accessed February 9, 2021. https://www.census.gov/library/stories/2018/03/graying-america.html [Google Scholar]
- 2.Jindai K, Nielson CM, Vorderstrasse BA, Quinones AR. Multimorbidity and functional limitations among adults 65 or older, NHANES 2005–2012. Prev Chronic Dis. 2016;13:E151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.United Nations. World Population Ageing 2017—Highlights. United Nations; 2017. [Google Scholar]
- 4.White MC, Holman DM, Boehm JE, Peipins LA, Grossman M, Henley SJ. Age and cancer risk: a potentially modifiable relationship. Am J Prev Med. 2014;46(suppl 1):S7–S15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Age and cancer risk. National Cancer Institute. Accessed February 9, 2021. https://www.cancer.gov/about-cancer/causes-prevention/risk/age [Google Scholar]
- 6.Shapiro CL. Cancer survivorship. N Engl J Med. 2018;379:2438–2450. [DOI] [PubMed] [Google Scholar]
- 7.Wang MC, Li TC, Li CI, et al. Frailty, transition in frailty status and all-cause mortality in older adults of a Taichung community-based population. BMC Geriatr. 2019;19:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garcia-Garcia FJ, Carcaillon L, Fernandez-Tresguerres J, et al. A new operational definition of frailty: the Frailty Trait Scale. J Am Med Dir Assoc. 2014;15:371.e7–371.e13. [DOI] [PubMed] [Google Scholar]
- 9.Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. 2013;381:752–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ofori-Asenso R, Chin KL, Mazidi M, et al. Global incidence of frailty and prefrailty among community-dwelling older adults: a systematic review and meta-analysis. JAMA Netw Open. 2019;2:e198398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guerard EJ, Deal AM, Chang Y, et al. Frailty index developed from a cancer-specific geriatric assessment and the association with mortality among older adults with cancer. J Natl Compr Canc Netw. 2017;15:894–902. [DOI] [PubMed] [Google Scholar]
- 12.Mandelblatt JS, Cai L, Luta G, et al. Frailty and long-term mortality of older breast cancer patients: CALGB 369901 (Alliance). Breast Cancer Res Treat. 2017;164:107–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cespedes Feliciano EM, Hohensee C, Rosko AE, et al. Association of prediagnostic frailty, change in frailty status, and mortality after cancer diagnosis in the Women’s Health Initiative. JAMA Netw Open. 2020;3:e2016747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.About the National Health and Nutrition Examination Survey. Centers for Disease Control and Prevention. Accessed February 14, 2021. https://www.cdc.gov/nchs/nhanes/about_nhanes.htm [Google Scholar]
- 15.NCHS data linked to NDI mortality files. Centers for Disease Control and Prevention. Accessed February 14, 2021. https://www.cdc.gov/nchs/data-linkage/mortality.htm [Google Scholar]
- 16.Ageing. World Health Organization. Accessed February 14, 2021. https://www.who.int/health-topics/ageing#tab=tab_1 [Google Scholar]
- 17.Zhang D, Zhao Y, Kaushiva A, Zhu Z, Wang JH, Braithwaite D. Self-rated health in relation to fruit and vegetable consumption and physical activity among older cancer survivors. Support Care Cancer. 2021;29:2713–2722. [DOI] [PubMed] [Google Scholar]
- 18.Blodgett J, Theou O, Kirkland S, Andreou P, Rockwood K. Frailty in NHANES: comparing the frailty index and phenotype. Arch Gerontol Geriatr. 2015;60:464–470. [DOI] [PubMed] [Google Scholar]
- 19.Blodgett J, Theou O, Kirkland S, Andreou P, Rockwood K. The association between sedentary behaviour, moderate-vigorous physical activity and frailty in NHANES cohorts. Maturitas. 2015;80:187–191. [DOI] [PubMed] [Google Scholar]
- 20.Chen C, Winterstein AG, Fillingim RB, Wei YJ. Body weight, frailty, and chronic pain in older adults: a cross-sectional study. BMC Geriatr. 2019;19:143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller AJ, Theou O, McMillan M, Howlett SE, Tennankore KK, Rockwood K. Dysnatremia in relation to frailty and age in community-dwelling adults in the National Health and Nutrition Examination Survey. J Gerontol A Biol Sci Med Sci. 2017;72:376–381. [DOI] [PubMed] [Google Scholar]
- 22.Cone EB, Marchese M, Paciotti M, et al. Assessment of time-to-treatment initiation and survival in a cohort of patients with common cancers. JAMA Netw Open. 2020;3:e2030072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.National Health and Nutrition Examination Survey (NHANES): Laboratory Procedures Manual. Centers for Disease Control and Prevention. Published March 2011. Accessed February 16, 2021. https://www.cdc.gov/nchs/data/nhanes/nhanes_11_12/2011-12_laboratory_procedures_manual.pdf [Google Scholar]
- 24.Body mass index—BMI. World Health Organization. Accessed February 19, 2021. https://www.euro.who.int/en/health-topics/disease-prevention/nutrition/a-healthy-lifestyle/body-mass-index-bmi [Google Scholar]
- 25.DeClercq V, Duhamel TA, Theou O, Kehler S. Association between lifestyle behaviors and frailty in Atlantic Canadian males and females. Arch Gerontol Geriatr. 2020;91:104207. [DOI] [PubMed] [Google Scholar]
- 26.Kojima G, Jivraj S, Iliffe S, Falcaro M, Liljas A, Walters K. Alcohol consumption and risk of incident frailty: the English Longitudinal Study of Aging. J Am Med Dir Assoc. 2019;20:725–729. [DOI] [PubMed] [Google Scholar]
- 27.Woods NF, LaCroix AZ, Gray SL, et al. Frailty: emergence and consequences in women aged 65 and older in the Women’s Health Initiative Observational Study. J Am Geriatr Soc. 2005;53:1321–1330. [DOI] [PubMed] [Google Scholar]
- 28.Strandberg AY, Trygg T, Pitkala KH, Strandberg TE. Alcohol consumption in midlife and old age and risk of frailty: alcohol paradox in a 30-year follow-up study. Age Ageing. 2018;47:248–254. [DOI] [PubMed] [Google Scholar]
- 29.Schoufour JD, Franco OH, Kiefte-de Jong JC, et al. The association between dietary protein intake, energy intake and physical frailty: results from the Rotterdam Study. Br J Nutr. 2019;121:393–401. [DOI] [PubMed] [Google Scholar]
- 30.Yannakoulia M, Ntanasi E, Anastasiou CA, Scarmeas N. Frailty and nutrition: from epidemiological and clinical evidence to potential mechanisms. Metabolism. 2017;68:64–76. [DOI] [PubMed] [Google Scholar]
- 31.Naghshi S, Sadeghi O, Willett WC, Esmaillzadeh A. Dietary intake of total, animal, and plant proteins and risk of all cause, cardiovascular, and cancer mortality: systematic review and dose-response meta-analysis of prospective cohort studies. BMJ. 2020;370:m2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang D, Tailor TD, Kim C, Atkins MB, Braithwaite D, Akinyemiju T. Immunotherapy utilization among patients with metastatic NSCLC: impact of comorbidities. J Immunother. 2021;44:198–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang D, Abraham L, Demb J, et al. Function-related indicators and outcomes of screening mammography in older women: evidence from the Breast Cancer Surveillance Consortium Cohort. Cancer Epidemiol Biomarkers Prev. 2021;30:1582–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Robinson D Modelling survival data in medical research, 2nd edition. J R Stat Soc Series A Stat Soc. 2004;167:760. [Google Scholar]
- 35.Desquilbet L, Mariotti F. Dose-response analyses using restricted cubic spline functions in public health research. Stat Med. 2010;29:1037–1057. [DOI] [PubMed] [Google Scholar]
- 36.Shepherd BE, Rebeiro PF; Caribbean, Central and South America Network for HIV Epidemiology. Brief report: assessing and interpreting the association between continuous covariates and outcomes in observational studies of HIV using splines. J Acquir Immune Defic Syndr. 2017;74:e60–e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hurria A, Jones L, Muss HB. Cancer treatment as an accelerated aging process: assessment, biomarkers, and interventions. Am Soc Clin Oncol Educ Book. 2016;35:e516–e522. [DOI] [PubMed] [Google Scholar]
- 38.Smitherman AB, Wood WA, Mitin N, et al. Accelerated aging among childhood, adolescent, and young adult cancer survivors is evidenced by increased expression of p16(INK4a) and frailty. Cancer. 2020;126:4975–4983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Demark-Wahnefried W, Platz EA, Ligibel JA, et al. The role of obesity in cancer survival and recurrence. Cancer Epidemiol Biomarkers Prev. 2012;21:1244–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schmidt ME, Wiskemann J, Schneeweiss A, Potthoff K, Ulrich CM, Steindorf K. Determinants of physical, affective, and cognitive fatigue during breast cancer therapy and 12 months follow-up. Int J Cancer. 2018;142:1148–1157. [DOI] [PubMed] [Google Scholar]
- 41.Khandekar MJ, Cohen P, Spiegelman BM. Molecular mechanisms of cancer development in obesity. Nat Rev Cancer. 2011;11:886–895. [DOI] [PubMed] [Google Scholar]
- 42.Van Epps P, Oswald D, Higgins PA, et al. Frailty has a stronger association with inflammation than age in older veterans. Immun Ageing. 2016;13:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Soysal P, Stubbs B, Lucato P, et al. Inflammation and frailty in the elderly: a systematic review and meta-analysis. Ageing Res Rev. 2016;31:1–8. [DOI] [PubMed] [Google Scholar]
- 44.Bonaccio M, Di Castelnuovo A, Pounis G, et al. A score of low-grade inflammation and risk of mortality: prospective findings from the Moli-Sani study. Haematologica. 2016;101:1434–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kabagambe EK, Judd SE, Howard VJ, et al. Inflammation biomarkers and risk of all-cause mortality in the Reasons for Geographic and Racial Differences in Stroke cohort. Am J Epidemiol. 2011;174:284–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ko YJ, Kwon YM, Kim KH, et al. High-sensitivity C-reactive protein levels and cancer mortality. Cancer Epidemiol Biomarkers Prev. 2012;21:2076–2086. [DOI] [PubMed] [Google Scholar]
- 47.Retterstol L, Eikvar L, Bohn M, Bakken A, Erikssen J, Berg K. C-reactive protein predicts death in patients with previous premature myocardial infarction—a 10 year follow-up study. Atherosclerosis. 2002;160:433–440. [DOI] [PubMed] [Google Scholar]
- 48.Fulop T, McElhaney J, Pawelec G, et al. Frailty, inflammation and immunosenescence. Interdiscip Top Gerontol Geriatr. 2015;41:26–40. [DOI] [PubMed] [Google Scholar]
- 49.Yao X, Li H, Leng SX. Inflammation and immune system alterations in frailty. Clin Geriatr Med. 2011;27:79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim R, Emi M, Tanabe K. Cancer immunoediting from immune surveillance to immune escape. Immunology. 2007;121:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mima K, Miyanari N, Morito A, et al. Frailty is an independent risk factor for recurrence and mortality following curative resection of stage I-III colorectal cancer. Ann Gastroenterol Surg. 2020;4:405–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yamada S, Shimada M, Morine Y, et al. Significance of frailty in prognosis after hepatectomy for elderly patients with hepatocellular carcinoma. Ann Surg Oncol. 2021;28:439–446. [DOI] [PubMed] [Google Scholar]
- 53.Wildiers H, Heeren P, Puts M, et al. International Society of Geriatric Oncology consensus on geriatric assessment in older patients with cancer. J Clin Oncol. 2014;32:2595–2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Elsawy B, Higgins KE. The geriatric assessment. Am Fam Physician. 2011;83:48–56. [PubMed] [Google Scholar]
- 55.Dale W, Williams GR, MacKenzie AR, et al. How is geriatric assessment used in clinical practice for older adults with cancer? A survey of cancer providers by the American Society of Clinical Oncology. JCO Oncol Pract 2021;17:336–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang D, Feng Y, Li N, Sun X. Fruit and vegetable consumptions in relation to frequent mental distress in breast cancer survivors. Support Care Cancer. 2021;29:193–201. [DOI] [PubMed] [Google Scholar]
- 57.Chase JD, Phillips LJ, Brown M. Physical activity intervention effects on physical function among community-dwelling older adults: a systematic review and meta-analysis. J Aging Phys Act. 2017;25:149–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nomura SJO, Hwang YT, Gomez SL, et al. Dietary intake of soy and cruciferous vegetables and treatment-related symptoms in Chinese-American and non-Hispanic White breast cancer survivors. Breast Cancer Res Treat. 2018;168:467–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Morris MS, Jacques PF, Rosenberg IH, Selhub J. Folate and vitamin B-12 status in relation to anemia, macrocytosis, and cognitive impairment in older Americans in the age of folic acid fortification. Am J Clin Nutr. 2007;85:193–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


