Abstract
Prolonged adolescent binge drinking can disrupt sleep quality and increase the likelihood of alcohol-induced sleep disruptions in young adulthood in rodents and in humans. Striking changes in spine density and morphology have been seen in many cortical and subcortical regions after adolescent alcohol exposure in rats. However, there is little known about the impact of alcohol exposure has on dendritic spines in the same motor and sensory cortices that EEG sleep is typically recorded from in rats. The aim of this study is to investigate whether an established model of chronic intermittent ethanol vapor in rats, that has been demonstrated to disrupt sleep during adolescence (AIE) or adulthood (CIE), also significantly alters cortical dendritic spine density and morphology. To this end, adolescent and adult Wistar rats were exposed to 5 weeks of ethanol vapor or control air exposure. After a 13-day withdrawal, primary motor cortex (M1) and primary/secondary visual cortex (V1/V2) layer V dendrites were analyzed for differences in spine density and morphology. Spines were classified into 4 categories (stubby, long, filopodia, and mushroom) based on the spine length and the width of the spine head and neck. The main results indicate an age-specific effect of AIE exposure decreasing spine density in the M1 cortex compared to age-matched controls. Reductions in the density of M1 long-shaped spine subclassifications were seen in AIE rats, but not CIE rats compared to their air-controls. Irrespective of age, there was an overall reduction produced by ethanol exposure on the density of filopodia and the length of long-shaped spines in V1/V2 cortex as compared to their air-exposed controls. Together, these data add to growing evidence that some cortical circuits are vulnerable to the effects of alcohol during adolescence and begin to elucidate potential mechanisms that may influence brain plasticity following early alcohol use.
Keywords: Adolescence, Alcohol, Dendrites, Sleep, Motor Cortex, Spines, Visual Cortex
Introduction
Sleep can play a significant role in psychological health during adolescence (Crowley, Wolfson, Tarokh, & Carskadon, 2018). Many lines of research have demonstrated that receiving adequate sleep is important for facilitating and maintaining spine dynamics and synaptic efficacy (Abel, Havekes, Saletin, & Walker, 2013; De Vivo et al., 2017; Frank, 2015; Tononi & Cirelli, 2014). Consequently, deviations from normal sleep can lead to disruptions in synaptic homeostasis potentially resulting in maladaptive learning, memory, and mood (De Vivo et al., 2014; Raven, Van der Zee, Meerlo, & Havekes, 2018; Spano et al., 2019; Tononi & Cirelli, 2014). Alcohol misuse is one factor that can produce long-term deviation in sleep, and this effect is particularly concerning during adolescence as it has the potential to impact adolescent brain development. If begun early in adolescence, heavy drinking can lead to a higher risk of alcohol-related problems in adulthood (Aiken et al., 2018; Patrick, Evans-Polce, & Terry-McElrath, 2019). Specifically, human and rodent research has shown that adolescent alcohol exposure can disrupt sleep quality and increase the likelihood of alcohol-induced sleep disruptions, such as insomnia in young adulthood (Amodeo, Wills, Sanchez‐Alavez, & Ehlers, 2020; Ehlers, Gilder, Criado, & Caetano, 2010; Ehlers, Wills, & Gilder, 2018; Sanchez-Alavez, Wills, Amodeo, & Ehlers, 2018). Recent research has begun to elucidate some of the cellular mechanisms by which deviations in sleep may alter synaptic plasticity, however, little is still known about how alcohol use may influence this relationship.
Our laboratory has established that intermittent ethanol exposure in rats can produce long-term changes in the sleep EEG, which mimics the human condition (Amodeo, Wills, Sanchez-Alavez, & Ehlers, 2020; Ehlers et al., 2018). Chronic intermittent ethanol in adolescent rats (AIE) using an established ethanol exposure model of vapor inhalation has resulted in lasting reductions in delta and theta during slow wave (SW) sleep (Amodeo et al., 2020; Ehlers, Sanchez-Alavez, & Wills, 2018), as well as decreases in synchrony between cortical neuronal networks (Ehlers, Wills, Desikan, Phillips, & Havstad, 2014; Sanchez-Alavez, Benedict, Wills, & Ehlers, 2019). Reduced delta and theta power during sleep has also been repeatedly demonstrated in clinical populations with alcohol use disorder (AUD) (Colrain, Turlington, & Baker, 2009; Irwin, Miller, Christian Gillin, Demodena, & Ehlers, 2000). Neocortical theta and delta oscillations are thought to originate exclusively from coordinated firing of interconnected excitatory networks in cortical layer V neurons (Chagnac-Amitai & Connors, 1989). These rhythms also appear to have an intimate relationship with sleep/wake cycles (Morikawa, Hayashi, & Hori, 1997) and specifically mature across adolescent development (Campbell & Feinberg, 2009). For instance, sleep deprivation in adolescence can lead to decreases in the activity of visual and frontal cortical layer V pyramidal cells and reductions in experience-dependent synapse elimination, both of which are important for neural refinement during development (Zhou et al., 2020).
Findings from both human postmortem studies and rodent models have also demonstrate that heavy or long-term drinking during adolescence can produce neuroadaptive changes in dendritic arborization and/or spines (Carpenter-Hyland & Chandler, 2007; Cui et al., 2013; Mulholland & Chandler, 2007; Mulholland, Chandler, & Kalivas, 2016). Chronic AIE can result in persistent changes in dendritic spine density and spine subpopulations in cortical and subcortical regions (Jury et al., 2017; Kyzar, Zhang, & Pandey, 2019; Mulholland et al., 2018; Pandey, Sakharkar, Tang, & Zhang, 2015; Risher et al., 2015; Trantham-Davidson et al., 2017). Subcortical regions such as the central and medial amygdala, but not basolateral amygdala, show reduced total dendritic spine density after adolescent AIE exposure (Jury et al., 2017; Pandey et al., 2015). Adolescent AIE also produces long-lasting decreases in dendritic spine density and changes in Fmr1 gene expression in the hippocampus, a possible mechanism underlying AIE-induced cognitive and affective deficits (Mulholland et al., 2018). Conversely, adolescent AIE increases spine density in the medial prefrontal cortex (Jury et al., 2017; Trantham-Davidson et al., 2017) and in the lateral orbitofrontal cortex of chronic intermittent ethanol in adult (CIE) mice after 7 days of withdrawal (McGuier, Padula, Lopez, Woodward, & Mulholland, 2015). Despite a small but significant area of cortical thinning in the prefrontal cortex, overall increases in cortical thickness (particularly in the posterior cingulate cortex) were shown in adolescent AIE rats (Vetreno, Yaxley, Paniagua, Johnson, & Crews, 2017).
Striking changes in spine density and morphology have been seen in many cortical and subcortical regions after AIE exposure. However, there is little known about the impact of ethanol exposure on dendritic spines in the same motor and sensory cortices at the sites of EEG sleep recordings. In this study, we targeted layer V in primary motor (M1) and primary/secondary visual (V1/V2) cortex. Cells in this layer have extensive apical and basal dendritic fields that allow them to integrate excitatory signals from all cortical layers (Schubert et al., 2001), including wide lateral intracortical connectivity (Kim, Juavinett, Kyubwa, Jacobs, & Callaway, 2015; Markram et al., 2015), which makes them well-suited to coordinate theta and delta activity within and between neocortical columns. Additionally, electrodes used to record EEG during sleep are positioned just above and record from this cortical layer. The aim of the current study was to use an established ethanol exposure model of vapor inhalation to investigate cortical dendritic spine density and morphology in M1 and V1/V2 cortices in adolescent (AIE) and adult (CIE) Wistar rats.
Materials and Methods
Animals
Eighteen male adolescent Wistar rats and 18 male adult Wistar rats (Charles River, USA) were used in the study. Adolescent rats arrived on postnatal day (PD) 21, whereas rats in the adult group were received on PD 90. Rats were housed in standard polycarbonate cages in sets of 3-4 rats with same-sex littermates at the Scripps Research Institute animal facilities. Temperature- (20°C) and humidity-controlled room with a 12-hour light/dark cycle (lights on 08:00). Food and water were available ad libitum. Subjects were cared for in accordance with the guidelines of the NIH Guide for the Care and Use of Laboratory Animals (NIH publication No. 80-23, revised 1996), and approved by the Institutional Animal Care and Use Committee of The Scripps Research Institute.
Intermittent Ethanol Exposure
Rats were randomly divided into chronic intermittent ethanol vapor exposure or air-control (Control) groups for adolescent rats (AIE), (Control = 9; AIE = 9) and adult rats (CIE), (Control = 8; CIE = 10). The ethanol exposed rats (AIE, CIE) were group-housed in sealed polycarbonate chambers, infused with vaporized 95% ethanol in a 14 hr-on/10 hr-off pattern (vapor beginning at 20:00) for 5 consecutive weeks beginning on PD 22-57 for AIE rats and on PD 91-126 for CIE rats. This intermittent ethanol inhalation procedure, in both adolescent and adult rats, has also been previously described (Ehlers et al., 2011; 2013; 2018a). To assess blood ethanol concentrations (BEC), blood samples (200 μL) were taken from the tip of the tail every 3 to 4 days during the 5-week exposure period. Samples were immediately centrifuged at 1500rpm for 15 minutes, with plasma extracted and stored at −80°C until further analysis using the Analox microstatAM1 (Analox Instr. Ltd., Lunenberg, MA). Adolescent and adult control rats experienced the same blood draw schedule as ethanol-treated animals but BEC’s were not analyzed. After the first three days of exposure in the ethanol vapor chambers, BECs stabilized and averaged 251.21 ± 9.94 mg/dL (ranging 200.31 – 287.61 mg/dL) for AIE rats and 232.61 ± 6.45 mg/dL (ranging 209.34 – 259.24 mg/dL) for CIE rats. The mean BECs were not significantly different between age groups [t(17)=1.60, p=0.128]. Following 5-weeks of ethanol exposure, rats were transferred to standard polycarbonate cages where they were group-housed for the duration of the experiment. Control animals were handled identically to ethanol rats, except they were not exposed to ethanol vapor and were maintained in standard cages throughout the experiment.
Dendritic spine analysis
Rats in each group (AIE, CIE) were exposed to a total of 5 weeks of intermittent vapor ethanol or air and euthanized 13 days following withdrawal (PD 70 for adolescent exposure and PD 139 for adult exposure animals). Animals were euthanized during a 3.5-hour period occurring between 9am-12:30pm in alternating groups so that ethanol and control tissue samples were represented across that time period. Following previously described methods (see Mulholland et al., 2018), rats were anesthetized with urethane (3 g/kg, i.p.) and perfused transcardially with room-temperature 0.1 M phosphate buffer (PB) and 1.5% paraformaldehyde (PFA) for 15-20 minutes. Brains were extracted and post-fixed in 1.5% PFA for 30 minutes and stored 0.1 M PB at 4°C. Fixed brains were shipped to the Medical University of South Carolina overnight. Coronal sections (150 μm) were sliced via vibratome (Leica VT1000S; Leica Biosystems) and then labeled with 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DiI)-coated tungsten particles (1.3 μm diameter) using a biolistic delivery system (Helios Gene Gun, Biorad) fitted with a polycarbonate filter (3.0 μm pore size; BD Biosciences; San Jose, CA). Slices were incubated in PB for overnight at 4°C before being post-fixed in 4% PFA in PB for 1 h at room temperature. After mounting with Prolong Gold Antifade mounting media (Life Technologies; Carlsbad, CA), second-order dendritic sections (~50-80 μm in length) that started >75 μm from the soma of pyramidal neurons in layers V of the primary motor cortex (M1) and primary visual cortex (V1/V2) were acquired with an Airyscan super-resolution detector on a confocal microscope (LSM 880; Carl Zeiss Microscopy). A series of optical sections (voxel size: 45 x 45 x 130 nm; 2 frame average) were captured for each dendritic section with a 63× water-immersion objective (Plan-Apochromat, Carl Zeiss, NA = 1.4, working distance = 190 μm) with 2.5× zoom. Raw Airyscan images were post-processed (linear Wiener filter-based deconvolution with pixel reassignment) in ZEN Black Software (version 14; Carl Zeiss) using the automatic filter strength setting. Imaris XT (version 9.1; Bitplane; Zurich, Switzerland) was used to generate a filament of the dendritic shaft and spines.
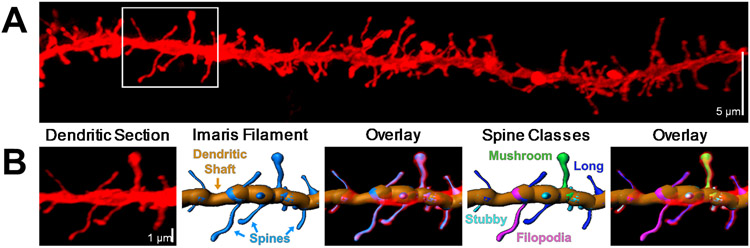
As previously described (Cannady et al., 2021; McGuier et al., 2015; Uys et al., 2016), spines were then classified into 4 categories (stubby, long, filopodia, and mushroom) based on the spine length and the width of the spine head and neck, where L is spine length, DH is spine head diameter, and DN is spine neck diameter. Long spines were identified as having a L ≥ 0.75 μm and L < 3 μm, mushroom spines had a L < 3.5 μm, DH > 0.35 μm and a DH > DN, stubby spines had a L < 0.75 μm, and filopodia were identified as having a L ≥ 3 μm. The density of multi-headed spines was not measured in this study. An example of a dendritic section and spine subclasses is shown in Fig 1. Data on dendritic spine parameters were averaged for each dendritic section and were collated from the Imaris output via custom scripts written in Python. The experimenter that acquired the confocal images and performed the Imaris image analyses was blind to the treatment groups.
Figure 1.
(A) Representative fluorescent labeling of a dendritic section in the visual cortex acquired using a super-resolution confocal microscope. (B) Imaris software was used to generate a 3D reconstruction of the dendritic shaft and spines from the fluorescent signal using the Filament function. An example of a dendritic section from the white box in Panel A shows the Imaris Filament and the four distinct morphological subtypes of dendritic protrusions: mushroom spines, long spines, stubby spines, and filopodia.
Data analysis
Dendritic spine data were analyzed as a general linear mixed model (SAS PROC MIXED; version 9.4) using Tukey post-hoc tests, when applicable, following our previous methods (McGuier et al., 2015; Mulholland et al., 2018). The dendritic spine variables were nested within each rat and were further nested across the sequential slices. Data are presented as mean ± standard error of the mean (SEM) and statistical significance was established with α = 0.05.
Results
Dendrites and Dendritic Spine Density in M1 cortex
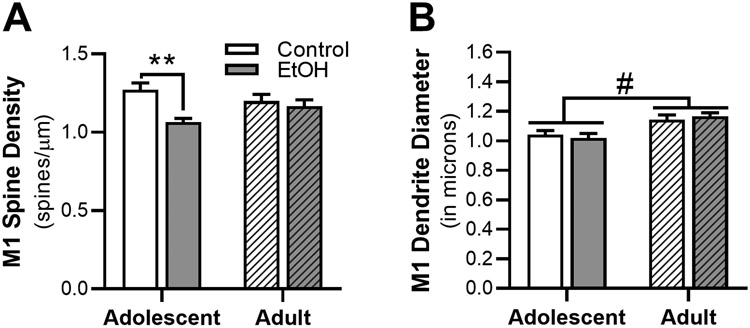
Ethanol-induced changes in morphology of dendrites and dendritic spines was assessed in the M1 cortex (n = 8 – 10 rats/group, n = 3 – 7 dendritic sections/rat, 38-50 sections/treatment group; 179 total dendritic sections; 15,283 total spines). There was a significant interaction between age and exposure in total spine density in M1 neurons [F(1,32) = 5.05, p = 0.032, Figure 2A]. Post-hoc analysis revealed that AIE vapor exposure significantly decreased total dendritic spine density compared to adolescent controls (p = 0.0031), an effect not seen in adults. While the mean diameter of the dendrites analyzed were not impact by ethanol exposure (Figure 2B), dendrite diameter was significantly affected by age [F(1,32) = 18.70, p < 0.001], with the dendritic diameter significantly smaller in adolescent rats compared to adults.
Figure 2.
Effects of ethanol vapor exposure in adolescent (AIE) and adult (CIE) rats on dendrite diameter and total dendritic spine density in primary motor (M1) cortical neurons. (A) Quantitation of total dendrite spine density and (B) dendrite diameter in M1 cortex from AIE and CIE rats or age-matched control. Ethanol exposure decreased M1 spine density in AIE rats compared to air-control rats, an effect not seen in CIE rats. Irrespective of exposure, M1 dendritic diameter was significantly increased in the adult rat group compared to adolescent group. **p > 0.05 compared to age-matched control, # p > 0.05 main effect of age.
Spine Subclass Density and Morphology in M1 cortex
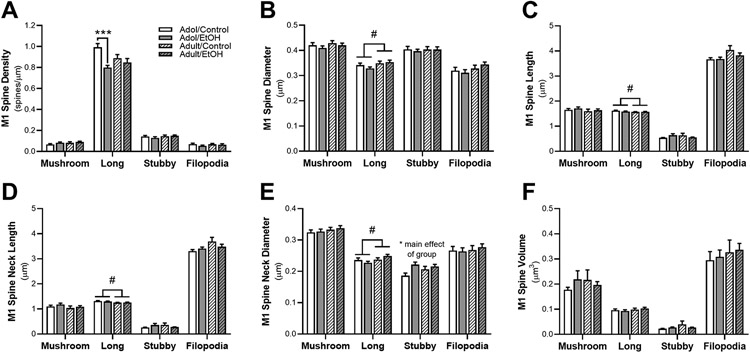
Spines were classified into four subclasses (i.e., filopodia, stubby, mushroom, and long) according to their length and head/neck diameters (Mulholland et al., 2018). For each of these subclassifications of spines, the density, diameter, length, volume, neck diameter and neck length of the spines were calculated. The results for these analyses are shown in Figure 3. The density of long-shaped spines were significantly impacted by exposure [F(1,32) = 12.80, p = 0.001] and an interaction between exposure x age F(1,32) = 5.56, p = 0.025; Figure 3A]. Adolescent AIE exposure significantly reduced the overall density of long-shaped spines compared to age-matched controls (p < 0.001). Stubby-shaped spine neck diameter was significant impacted by exposure [F(1,32) = 7.86, p = 0.009; Figure 3E], with an increase in neck diameter for AIE exposure compared to air-controls. There was also a significant effect of age on long-shaped spine subtype. Adolescent rats had significantly smaller long spine diameter [F(1,32) = 4.48, p = 0.042; Figure 3B] and smaller spine neck diameter [F(1,32) = 4.22, p = 0.048; Figure 3E] compared to adults. Long-shaped spines also exhibited overall longer spine length [F(1,32) = 4.65, p = 0.039; Figure 3C] and spine neck length [F(1,32) = 7.51, p = 0.001; Figure 3D] for adolescents compared to adults. Mushroom or filopodia spine subclasses were unaffected by ethanol exposure or age.
Figure 3.
Adaptations in dendritic morphology in M1 cortical neurons after almost two weeks of withdrawal from ethanol vapor in adolescent (AIE) and adult (CIE) rats or age-matched air-control exposure. Panel (A) depicts total dendrite spine density for mushroom, long, stubby and filopodia in primary motor cortex of AIE and CIE rats or air-control. The density of long spines in M1 was significantly reduced in AIE rats compared to age-matched controls. Quantification of M1 spine (B) diameter (C) length (D) neck length and (E) neck diameter resulted in a significant increase in the long spine subtype of CIE and AIE rats. Irrespective of age, ethanol exposure significantly increased stubby spine neck diameter compared to air-control. (F) M1 spine volume was not impacted by age or exposure group. ***p > 0.001 compared to age-matched control, # p > 0.05 main effect of age.
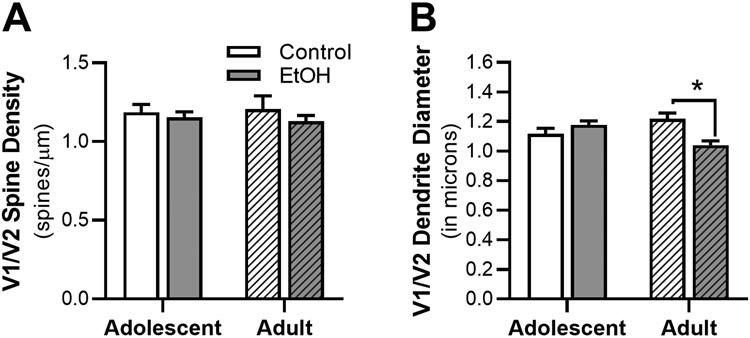
Ethanol-induced changes in morphology of dendrites and dendritic spines was assessed in the V1/V2 cortex (n = 5 – 10 rats/group, n = 3 – 6 dendritic sections/rat, 19 – 44 sections/treatment group; 140 total dendritic sections; 10,765 total spines). Ethanol exposure had no impact on total V1/V2 dendritic density (Figure 4A). However, there was a significant interaction between age and exposure for dendritic diameter [F(1,28) = 12.22, p = 0.002; Figure 4B], with CIE vapor in adulthood significantly reducing dendritic diameter compared to control-air exposure in adults rats, (p = 0.010).
Figure 4.
Effects of ethanol vapor exposure in adolescent (AIE) and adult (CIE) rats on dendrites and total dendritic spine density in adult V1/V2 cortical neurons. Quantitation of total (A) dendrite spine density and (B) dendrite diameter in AIE and CIE rats or age-matched control. Decreases in V1/V2 spine density were seen in CIE rats compared to air-controls, an effect not seen in AIE rats. **p > 0.05 compared to age-matched control.
Spine Subclass Density and Morphology in V1/V2 cortex
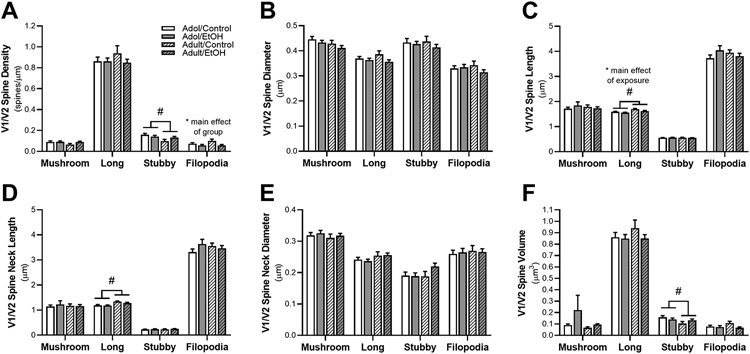
Filopodia spine density was significantly altered by exposure [F(1,28) = 9.20, p = 0.005; Figure 5A]. Overall, ethanol exposure in both adolescent and adult exposed reduced the density of filopodia spines in the V1/V2 cortex compared to air-controls. While long-shaped spine length significantly increased with age [F(1,28) = 13.37, p = 0.001; Fig. 5C], ethanol exposure significantly decreased spine length compared to controls [F(1,28) = 5.87, p = 0.022]. There was also a significant increase in the length of long spine neck from adolescence to adulthood [F(1,28) = 14.91, p = 0.001; Fig. 5D]. Stubby-shaped spines were impacted by the age of the animal with overall larger volume [F(1,28) = 5.24, p = 0.030, Figure 5F] and density [F(1,28) = 6.73, p = 0.015; Figure 5A] of these spines in adolescent V1/V2 cortex compared to adult. Total spine diameter (Fig. 5B) and spine neck diameter (Fig. 5E) were not impacted by ethanol exposure or age of the animal in any of the spine subclassifications. Mushroom spine subclasses were also unaffected by ethanol exposure or age.
Figure 5.
Adaptations in dendritic morphology in V1/V2 cortical neurons after withdrawal from ethanol vapor in adolescent (AIE) and adults (CIE) or air-control exposure. Figure depicts quantification of mushroom, long, stubby, and filopodia spine subtypes. Quantification of (A) spine density in V1/V2 resulted in a significant decrease in filopodia density in ethanol vapor rats compared to air-control. There was also an age-dependent effect in subby spines with a reduction in density in adult compared to adolescent exposed rats. (B) V1/V2 diameter of the spines and (E) diameter of the spine neck subtypes did not result in any differences. However, differences were seen in (C) spine length and (D) spine neck length subclassifications. Length of spine and spine neck for the long subclass were longer in adult rats compared to adolescent. There was a significant decrease in long spine length for ethanol animals compared to controls. Additionally, there is a main effect of age with greater long spine length in adult compared to adolescent rats. (E) Age of group did impact stubby spine volume with deceases with age. **p > 0.05 compared to age-matched control, # p > 0.05 main effect of age.
Discussion
High levels of ethanol exposure during adolescence or adulthood can have a significant impact on morphology of cortical dendrites (Crews et al., 2019; Frost, Peterson, Bird, McCool, & Hamilton, 2019; Mulholland et al., 2016). The current study compared the effects of chronic intermittent ethanol exposure during adolescence (AIE) and adulthood (CIE) on dendritic spine density and morphology in primary motor (M1) and primary/secondary visual (V1/V2) cortices. Adolescent exposure resulted in reduced spine density in the M1 cortex compared to age-matched controls, an effect not seen in adult-exposed spine morphology. Reductions in the density of long-shaped spine subclassifications were also seen after AIE in adolescence. Irrespective of age, ethanol exposure reduced the density of filopodia and the length of long-shaped spines in V1/V2 compared to air-exposed controls.
The impact that ethanol exposure has on adolescent and adult dendritic spines is complex, varying by brain region and duration of withdrawal (Cannady et al., 2021; Frost et al., 2019; Jones & Hassani, 2013; Jury et al., 2017; Kamarajan et al., 2015; Mulholland et al., 2018; Pandey et al., 2015; Trantham-Davidson et al., 2017). The current results expand on this literature to include reductions in the density of M1 basal dendrites in layer V cortex from ethanol-exposed adolescent, but not adult rats. While spine measurements were assessed 13 days post-ethanol, it is possible that adult exposure produces spine changes early in withdrawal that do not persist into extended abstinence. Additionally, these results suggest that changes in cortical EEG recordings from at least two weeks after withdrawal, could at least theoretically be attributed to altered synaptic dynamics and morphology. We did not directly record sleep EEG in this set of animals in order to allow the removal of the brains for image analyses to coincide exactly with when sleep changes following ethanol exposure are known to occur from previous studies (see Amodeo et al., 2020; Ehlers, Benedict, Wills, & Sanchez-Alavez, 2020; Ehlers et al., 2018). Additional longitudinal studies are needed to directly measure whether changes in spine density/morphology seen in AIE exposure animals correlate with sleep patterns in adulthood.
There is an abundant literature showing that sleep promotes molecular mechanisms of synaptic plasticity (Da Souza & Ribeiro, 2015; Navarro-Lobato & Genzel, 2019; Puentes-Mestril & Aton, 2017), and decreases in synaptic density have been shown to drive decline in SW sleep EEG power across adolescence (Campbell & Feinberg, 2009; Huttenlocher, 1979). Increased daytime sleepiness across adolescence has also been shown to be related to declining delta and theta power density in humans (Campbell, Higgins, Trinidad, Richardson, & Feinberg, 2007). Since EEG potentials are produced by large populations of interconnected cortical neurons that oscillate synchronously, declining synaptic density would be predicted to decrease the amplitude of these summed membrane potentials (Steriade, Nunez, & Amzica, 1993; Steriade, McCormick, & Sejnowski, 1993).
In the present study, the average dendritic diameter in M1 cortex increased from the end of adolescence (PD 70) to adulthood (PD 140). In particular, long-shaped spines in M1 decreased in length and increased in diameter with age. In the visual cortex, increases in the structure of long-shaped spines, and in the density of stubby-shaped spines, were found to occur at the end of adolescence and into late adulthood. These results expand on the current literature demonstrating changes in dendritic morphology across adult development. These maturational changes in spine morphology, including size, shape and density, are thought to reflect normal changes in glutamatergic synaptic transmission and plasticity that occur during aging (Dickstein, Weaver, Luebke, & Hof, 2013). It is plausible that these structural changes may lead to changes in the function of memory and learning, however a causal link is currently unsubstantiated (Dumitriu et al., 2010; Morrison & Baxter, 2012).
Studies have shown reduced spine density and retracted arborization in cortical motor areas in postmortem AUD individuals (Corbett & Harper, 1990; Ferrer, Fábregues, Rairiz, & Galofré, 1986), rodents (Charlton et al., 2019), and in macaque monkeys exposed to prenatal alcohol (Miller, 2006). Topography organized connections between motor cortex, basal ganglia, and cerebellum interact synergistically to influence the functioning of all related motor structures (Houk & Wise, 1995). Therefore, loss of M1 plasticity could potentially reflect a loss of coordination among cortical and subcortical inputs leading to abnormal plasticity of motor maps within M1 in individuals with AUD. While research is limited, ethanol exposure has also been shown to decreased number of dendritic spines in the mouse visual cortex and increased mean length of pyramidal neuron dendrites after prenatal ethanol (Cui et al., 2010). Repeated ethanol exposure in adult rodents can also reduce visual-evoked potentials in visual cortex neurons (Begleiter & Porjesz, 1977; O’Herron, Summers, Shih, Kara, & Woodward, 2020). In the current study, CIE significantly reduced dendrite diameter in the visual cortex. These results were specific to adulthood and were not seen in AIE rats perhaps suggesting a unique vulnerability of older adults to changes in dendritic morphology in the visual cortex.
In conclusion, intermittent ethanol exposure can significantly alter cortical dendritic spines with a unique impact on M1 neurons when exposure occurs during adolescence. Together, these data add to growing evidence that cortical circuits are vulnerable to the effects of alcohol during adolescence and began to elucidate that early alcohol use may alter sleep through morphological adaptations in motor and visual cortices. Further research is needed to better understand how cortical circuits are uniquely impacted by alcohol use and develop therapeutics to treat alcohol-induced sleep disorders.
Highlights:
Adolescent ethanol exposure reduced spine density in the primary motor cortex
Reductions in M1 spine morphology were age-specific
Ethanol exposure reduced spine morphology in primary/secondary visual cortex
Source of support:
This work was supported by National Institute of Health (NIH) grants, U01 AA019969; R01 AA006059 to Cindy L. Ehlers and U24 AA024603 to Patrick J. Mulholland from the National Institute on Alcohol Abuse and Alcoholism (NIAAA). This work was also supported by an S10 Shared Instrumentation Grant to Patrick J. Mulholland (OD021532) from the Office of the Director, National Institutes of Health.
References
- Abel T, Havekes R, Saletin JM, & Walker MP (2013, September 9). Sleep, plasticity and memory from molecules to whole-brain networks. Current Biology, Vol. 23. 10.1016/j.cub.2013.07.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aiken A, Clare PJ, Wadolowski M, Hutchinson D, Najman JM, Slade T, … Mattick RP (2018). Age of Alcohol Initiation and Progression to Binge Drinking in Adolescence: A Prospective Cohort Study. Alcoholism: Clinical and Experimental Research, 42(1), 100–110. 10.1111/acer.13525 [DOI] [PubMed] [Google Scholar]
- Amodeo LR, Wills DN, Sanchez-Alavez M, & Ehlers CL (2020). Effects of orexin-2 receptor antagonist on sleep and event-related oscillations in female rats exposed to chronic intermittent ethanol during adolescence. Alcoholism: Clinical and Experimental Research. 10.1111/acer.14361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begleiter H, & Porjesz B (1977). Persistence of brain hyperexcitability following chronic alcohol exposure in rats. Advances in Experimental Medicine and Biology, 85 B, 209–222. 10.1007/978-1-4615-9038-5_14 [DOI] [PubMed] [Google Scholar]
- Campbell IG, & Feinberg I (2009). Longitudinal trajectories of non-rapid eye movement delta and theta EEG as indicators of adolescent brain maturation. Proceedings of the National Academy of Sciences of the United States of America, 106(13), 5177–5180. 10.1073/pnas.0812947106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell IG, Higgins LM, Trinidad JM, Richardson P, & Feinberg I (2007). The increase in longitudinally measured sleepiness across adolescence is related to the maturational decline in low-frequency EEG power. Sleep, 30(12), 1677–1687. 10.1093/sleep/30.12.1677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannady R, Nguyen T, Padula AE, Rinker JA, Lopez MF, Becker HC, … Mulholland PJ (2021). Interaction of chronic intermittent ethanol and repeated stress on structural and functional plasticity in the mouse medial prefrontal cortex. Neuropharmacology, 182, 108396. 10.1016/j.neuropharm.2020.108396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter-Hyland EP, & Chandler LJ (2007, February). Adaptive plasticity of NMDA receptors and dendritic spines: Implications for enhanced vulnerability of the adolescent brain to alcohol addiction. Pharmacology Biochemistry and Behavior, Vol. 86, pp. 200–208. 10.1016/j.pbb.2007.01.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chagnac-Amitai Y, & Connors BW (1989). Synchronized excitation and inhibition driven by intrinsically bursting neurons in neocortex. Journal of Neurophysiology, 62(5), 1149–1162. 10.1152/jn.1989.62.5.1149 [DOI] [PubMed] [Google Scholar]
- Charlton AJ, May C, Luikinga SJ, Burrows EL, Hyun Kim J, Lawrence AJ, & Perry CJ (2019). Chronic voluntary alcohol consumption causes persistent cognitive deficits and cortical cell loss in a rodent model. Scientific Reports, 9(1). 10.1038/s41598-019-55095-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colrain IM, Turlington S, & Baker FC (2009). Impact of alcoholism on sleep architecture and EEG power spectra in men and women. Sleep, 32(10), 1341–1352. 10.1093/sleep/32.10.1341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett D, & Harper C (1990). Changes in the basal dendrites of cortical pyramidal cells from alcoholic patients-a quantitative Golgi study. Journal of Neurology, Neurosurgery and Psychiatry, 53(10), 856–861. 10.1136/jnnp.53.10.856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Robinson DL, Chandler LJ, Ehlers CL, Mulholland PJ, Pandey SC, … Vetreno RP (2019, September 1). Mechanisms of Persistent Neurobiological Changes Following Adolescent Alcohol Exposure: NADIA Consortium Findings. Alcoholism: Clinical and Experimental Research, Vol. 43, pp. 1806–1822. 10.1111/acer.14154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley SJ, Wolfson AR, Tarokh L, & Carskadon MA (2018, August 1). An update on adolescent sleep: New evidence informing the perfect storm model. Journal of Adolescence, Vol. 67, pp. 55–65. 10.1016/j.adolescence.2018.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui C, Noronha A, Morikawa H, Alvarez VA, Stuber GD, Szumlinski KK, … Wilcox MV (2013, April). New insights on neurobiological mechanisms underlying alcohol addiction. Neuropharmacology, Vol. 67, pp. 223–232. 10.1016/j.neuropharm.2012.09.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui ZJ, Zhao KB, Zhao HJ, Yu DM, Niu YL, Zhang JS, & Deng JB (2010). Prenatal alcohol exposure induces long-term changes in dendritic spines and synapses in the mouse visual cortex. Alcohol and Alcoholism, 45(4), 312–319. 10.1093/alcalc/agq036 [DOI] [PubMed] [Google Scholar]
- Da Souza AC, & Ribeiro S (2015). Sleep deprivation and gene expression. Current Topics in Behavioral Neurosciences, 25, 65–90. 10.1007/7854_2014_360 [DOI] [PubMed] [Google Scholar]
- De Vivo L, Bellesi M, Marshall W, Bushong EA, Ellisman MH, Tononi G, & Cirelli C (2017). Ultrastructural evidence for synaptic scaling across the wake/sleep cycle. Science, 355(6324), 507–510. 10.1126/science.aah5982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vivo L, Faraguna U, Nelson AB, Pfster-Genskow M, Klapperich ME, Tononi G, & Cirelli C (2014). Developmental patterns of sleep slow wave activity and synaptic density in adolescent mice. Sleep, 37(4), 689–700. 10.5665/sleep.3570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickstein DL, Weaver CM, Luebke JI, & Hof PR (2013, October 22). Dendritic spine changes associated with normal aging. Neuroscience, Vol. 251, pp. 21–32. 10.1016/j.neuroscience.2012.09.077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumitriu D, Hao J, Hara Y, Kaufmann J, Janssen WGM, Lou W, … Morrison JH (2010). Selective changes in thin spine density and morphology in monkey prefrontal cortex correlate with aging-related cognitive impairment. Journal of Neuroscience, 30(22), 7507–7515. 10.1523/JNEUROSCI.6410-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers CL, Benedict J, Wills D, & Sanchez-Alavez M (2020). PSPH-D-18-00526: Effect of a dual orexin receptor antagonist (DORA-12) on sleep and event-related oscillations in rats exposed to ethanol vapor during adolescence. Psychopharmacology, 237(10), 2917–2927. 10.1007/s00213-019-05371-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers CL, Gilder DA, Criado JR, & Caetano R (2010). Sleep quality and alcohol-use disorders in a select population of young-adult mexican americans. Journal of Studies on Alcohol and Drugs, 71(6), 879–884. 10.15288/jsad.2010.71.879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers CL, Wills D, & Gilder DA (2018). A history of binge drinking during adolescence is associated with poorer sleep quality in young adult Mexican Americans and American Indians. Psychopharmacology, 235(6), 1775–1782. 10.1007/s00213-018-4889-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers CL, Wills DN, Desikan A, Phillips E, & Havstad J (2014). Decreases in energy and increases in phase locking of event-related oscillations to auditory stimuli occur during adolescence in human and rodent brain. Developmental Neuroscience, 36(3–4), 175–195. 10.1159/000358484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers C, Sanchez-Alavez M, & Wills D (2018). Effect of gabapentin on sleep and delta and theta EEG power in adult rats exposed to chronic intermittent ethanol vapor and protracted withdrawal during adolescence. Psychopharmacology, 235(6), 1783–1791. 10.1007/s00213-018-4888-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrer I, Fábregues I, Rairiz J, & Galofré E (1986). Decreased numbers of dendritic spines on cortical pyramidal neurons in human chronic alcoholism. Neuroscience Letters, 69(1), 115–119. 10.1016/0304-3940(86)90425-8 [DOI] [PubMed] [Google Scholar]
- Frank MG (2015). Sleep and synaptic plasticity in the developing and adult brain. Current Topics in Behavioral Neurosciences, 25, 123–149. 10.1007/7854_2014_305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost ME, Peterson VL, Bird CW, McCool B, & Hamilton DA (2019). Effects of Ethanol Exposure and Withdrawal on Neuronal Morphology in the Agranular Insular and Prelimbic Cortices: Relationship with Withdrawal-Related Structural Plasticity in the Nucleus Accumbens. Brain Sciences, 9(8). 10.3390/BRAINSCI9080180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houk JC, & Wise SP (1995). Feature article: Distributed modular architectures linking basal ganglia, cerebellum, and cerebral cortex: Their role in planning and controlling action. Cerebral Cortex, 5(2), 95–110. 10.1093/cercor/5.2.95 [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR (1979). Synaptic density in human frontal cortex - Developmental changes and effects of aging. Brain Research, 163(2), 195–205. 10.1016/0006-8993(79)90349-4 [DOI] [PubMed] [Google Scholar]
- Irwin M, Miller C, Christian Gillin J, Demodena A, & Ehlers CL (2000). Polysomnographic and spectral sleep EEG in primary alcoholics: An interaction between alcohol dependence and African-American ethnicity. Alcoholism: Clinical and Experimental Research, 24(9), 1376–1384. 10.1111/j.1530-0277.2000.tb02106.x [DOI] [PubMed] [Google Scholar]
- Jones BE, & Hassani OK (2013). The role of Hcrt/Orx and MCH neurons in sleep-wake state regulation. Sleep, 36(12), 1769–1772. 10.5665/sleep.3188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jury NJ, Pollack GA, Ward MJ, Bezek JL, Ng AJ, Pinard CR, … Holmes A (2017). Chronic Ethanol During Adolescence Impacts Corticolimbic Dendritic Spines and Behavior. Alcoholism: Clinical and Experimental Research, 41(7), 1298–1308. 10.1111/acer.13422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamarajan C, Pandey AK, Chorlian DB, Manz N, Stimus AT, Anokhin AP, … Porjesz B (2015). Deficient Event-Related Theta Oscillations in Individuals at Risk for Alcoholism: A Study of Reward Processing and Impulsivity Features. PLOS ONE, 10(11), e0142659. 10.1371/journal.pone.0142659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim EJ, Juavinett AL, Kyubwa EM, Jacobs MW, & Callaway EM (2015). Three Types of Cortical Layer 5 Neurons That Differ in Brain-wide Connectivity and Function. Neuron, 88(6), 1253–1267. 10.1016/j.neuron.2015.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyzar EJ, Zhang H, & Pandey SC (2019). Adolescent Alcohol Exposure Epigenetically Suppresses Amygdala Arc Enhancer RNA Expression to Confer Adult Anxiety Susceptibility. Biological Psychiatry, 85(11), 904–914. 10.1016/j.biopsych.2018.12.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markram H, Muller E, Ramaswamy S, Reimann MW, Abdellah M, Sanchez CA, … Schürmann F (2015). Reconstruction and Simulation of Neocortical Microcircuitry. Cell, 163(2), 456–492. 10.1016/j.cell.2015.09.029 [DOI] [PubMed] [Google Scholar]
- McGuier NS, Padula AE, Lopez MF, Woodward JJ, & Mulholland PJ (2015). Withdrawal from chronic intermittent alcohol exposure increases dendritic spine density in the lateral orbitofrontal cortex of mice. Alcohol, 49(1), 21–27. 10.1016/j.alcohol.2014.07.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MW (2006). Effect of prenatal exposure to ethanol on glutamate and GABA immunoreactivity in macaque somatosensory and motor cortices: Critical timing of exposure. Neuroscience, 138(1), 97–107. 10.1016/j.neuroscience.2005.10.060 [DOI] [PubMed] [Google Scholar]
- Morikawa T, Hayashi M, & Hori T (1997). Auto power and coherence analysis of delta-theta band EEG during the waking-sleeping transition period. Electroencephalography and Clinical Neurophysiology, 103(6), 633–641. 10.1016/S0013-4694(97)00048-5 [DOI] [PubMed] [Google Scholar]
- Morrison JH, & Baxter MG (2012, April). The ageing cortical synapse: Hallmarks and implications for cognitive decline. Nature Reviews Neuroscience, Vol. 13, pp. 240–250. 10.1038/nrn3200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulholland PJ, & Chandler LJ (2007). The Thorny Side of Addiction: Adaptive Plasticity and Dendritic Spines. The Scientific World JOURNAL, 7, 9–21. 10.1100/tsw.2007.247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulholland PJ, Chandler LJ, & Kalivas PW (2016, July 1). Signals from the Fourth Dimension Regulate Drug Relapse. Trends in Neurosciences, Vol. 39, pp. 472–485. 10.1016/j.tins.2016.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulholland PJ, Teppen TL, Miller KM, Sexton HG, Pandey SC, & Swartzwelder HS (2018). Donepezil Reverses Dendritic Spine Morphology Adaptations and Fmr1 Epigenetic Modifications in Hippocampus of Adult Rats After Adolescent Alcohol Exposure. Alcoholism: Clinical and Experimental Research, 42(4), 706–717. 10.1111/acer.13599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro-Lobato I, & Genzel L (2019). The up and down of sleep: From molecules to electrophysiology. Neurobiology of Learning and Memory, 160, 3–10. 10.1016/j.nlm.2018.03.013 [DOI] [PubMed] [Google Scholar]
- O’Herron P, Summers PM, Shih AY, Kara P, & Woodward JJ (2020). In vivo two-photon imaging of neuronal and brain vascular responses in mice chronically exposed to ethanol. Alcohol, 85, 41–47. 10.1016/j.alcohol.2019.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey SC, Sakharkar AJ, Tang L, & Zhang H (2015). Potential role of adolescent alcohol exposure-induced amygdaloid histone modifications in anxiety and alcohol intake during adulthood. Neurobiology of Disease, 82, 607–619. 10.1016/j.nbd.2015.03.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick ME, Evans-Polce R, & Terry-McElrath YM (2019). Faster escalation from first drink to first intoxication as a risk factor for binge and high-intensity drinking among adolescents. Addictive Behaviors, 92, 199–202. 10.1016/j.addbeh.2019.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puentes-Mestril C, & Aton SJ (2017). Linking network activity to synaptic plasticity during sleep: Hypotheses and recent data. Frontiers in Neural Circuits, 11, 61. 10.3389/fncir.2017.00061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raven F, Van der Zee EA, Meerlo P, & Havekes R (2018, June 1). The role of sleep in regulating structural plasticity and synaptic strength: Implications for memory and cognitive function. Sleep Medicine Reviews, Vol. 39, pp. 3–11. 10.1016/j.smrv.2017.05.002 [DOI] [PubMed] [Google Scholar]
- Risher ML, Fleming RL, Risher WC, Miller KM, Klein RC, Wills T, … Swartzwelder HS (2015). Adolescent Intermittent Alcohol Exposure: Persistence of Structural and Functional Hippocampal Abnormalities into Adulthood. Alcoholism: Clinical and Experimental Research, 39(6), 989–997. 10.1111/acer.12725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Alavez M, Benedict J, Wills DN, & Ehlers CL (2019). Effect of suvorexant on event-related oscillations and EEG sleep in rats exposed to chronic intermittent ethanol vapor and protracted withdrawal. Sleep, 42(4). 10.1093/sleep/zsz020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Alavez M, Wills DN, Amodeo L, & Ehlers CL (2018). Effect of Gabapentin on Sleep and Event-Related Oscillations (EROs) in Rats Exposed to Chronic Intermittent Ethanol Vapor and Protracted Withdrawal. Alcoholism: Clinical and Experimental Research. 10.1111/acer.13588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert D, Staiger JF, Cho N, Kötter R, Zilles K, & Luhmann HJ (2001). Layer-specific intracolumnar and transcolumnar functional connectivity of layer V pyramidal cells in rat barrel cortex. Journal of Neuroscience, 21(10), 3580–3592. 10.1523/jneurosci.21-10-03580.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spano GM, Banningh SW, Marshall W, de Vivo L, Bellesi M, Loschky SS, … Cirelli C (2019). Sleep Deprivation by Exposure to Novel Objects Increases Synapse Density and Axon-Spine Interface in the Hippocampal CA1 Region of Adolescent Mice. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience, 39(34), 6613–6625. 10.1523/JNEUROSCI.0380-19.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M, Nunez A, & Amzica F (1993). Intracellular analysis of relations between the slow (<1 Hz) neocortical oscillation and other sleep rhythms of the electroencephalogram. Journal of Neuroscience, 13(8), 3266–3283. 10.1523/jneurosci.13-08-03266.1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade Mircea, McCormick DA, & Sejnowski TJ (1993). Thalamocortical oscillations in the sleeping and aroused brain. Science, 262(5134), 679–685. 10.1126/science.8235588 [DOI] [PubMed] [Google Scholar]
- Tononi G, & Cirelli C (2014, January 8). Sleep and the Price of Plasticity: From Synaptic and Cellular Homeostasis to Memory Consolidation and Integration. Neuron, Vol. 81, pp. 12–34. 10.1016/j.neuron.2013.12.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trantham-Davidson H, Centanni SW, Garr SC, New NN, Mulholland PJ, Gass JT, … Judson Chandler L (2017). Binge-Like Alcohol Exposure during Adolescence Disrupts Dopaminergic Neurotransmission in the Adult Prelimbic Cortex. Neuropsychopharmacology, 42(5), 1024–1036. 10.1038/npp.2016.190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uys JD, McGuier NS, Gass JT, Griffin WC, Ball LE, & Mulholland PJ (2016). Chronic intermittent ethanol exposure and withdrawal leads to adaptations in nucleus accumbens core postsynaptic density proteome and dendritic spines. Addiction Biology, 21(3), 560–574. 10.1111/adb.12238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetreno RP, Yaxley R, Paniagua B, Johnson GA, & Crews FT (2017). Adult rat cortical thickness changes across age and following adolescent intermittent ethanol treatment. Addiction Biology, 22(3), 712–723. 10.1111/adb.12364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Lai CSW, Bai Y, Li W, Zhao R, Yang G, … Gan WB (2020). REM sleep promotes experience-dependent dendritic spine elimination in the mouse cortex. Nature Communications, 11(1), 1–12. 10.1038/s41467-020-18592-5 [DOI] [PMC free article] [PubMed] [Google Scholar]