Abstract
Disruption of intestinal integrity and barrier function due to tissue inflammation has negative implications on overall growth and well-being in young pigs. In this study, we investigated the effects of oral gamma-cyclodextrin-encapsulated tributyrin (TBCD) in young pigs experiencing dextran sodium sulfate (DSS)-induced colitis. Pigs (n = 32 boars) were weaned from the sow at postnatal day (PND) 2, allotted to treatment based on the litter of origin and body weight (BW), and reared artificially over a 26-d feeding period. Treatment groups included: 1) nutritionally adequate (control) milk replacer, no DSS (Control n = 8), 2) control milk replacer plus oral DSS (DSS, n = 7), and 3) control diet supplemented with 8.3 g of TBCD per kg of reconstituted milk replacer plus oral DSS (TBCD + DSS, n = 8). Colitis was induced by administering DSS at 1.25 g of DSS/kg BW daily in a reconstituted milk replacer from PND 14–18. Milk replacer and water were provided ad libitum throughout the 26-d study. All the data were analyzed using a one-way ANOVA using the MIXED procedure of SAS. Control and DSS pigs had similar BW throughout the study, while TBCD + DSS pigs exhibited decreased (P < 0.05) BW starting at approximately PND 15. Additionally, average daily gain (ADG) before and after initiation of DSS dosing, along with over the total study duration, was decreased (P < 0.05) in pigs receiving TBCD + DSS compared with the Control. Milk disappearance was decreased (P < 0.05) in TBCD + DSS pigs when compared with Control and DSS groups. Both the concentration and molar ratio of cecal butyrate concentrations were increased (P < 0.05) in TBCD + DSS pigs compared with the Control group. The DSS and TBCD + DSS treatments also increased (P < 0.05) butyrate concentrations in the luminal contents with the proximal colon compared with Control. TBCD + DSS and DSS pigs had increased (P < 0.05) mucosal width in the distal colon compared with Control, thereby indicating heightened intestinal inflammation. Overall, oral supplementation of encapsulated tributyrin increased the concentration of butyrate in the colon, but was unable to mitigate the negative effects of DSS-induced colitis.
Keywords: colitis, dextran sodium sulfate, encapsulated tributyrin, inflammation, pig
Whereas oral supplementation of encapsulated tributyrin increased luminal butyrate concentrations in the colon, provision of this encapsulated short-chain fatty acid negatively affected growth performance in weanling pigs undergoing dextran sodium sulfate induced-colitis.
Introduction
Colitis is defined as large intestinal inflammation in pigs with an unknown etiology and specifically affecting the structure and function of the colon. Colitis in pigs leads to diarrhea, which can be a result from factors including infection, immune disruption, genetics, dysbiosis, psychological stress, and others (Sartor, 2006; Thomson, 2009; Lackeyram et al., 2012; Ren et al., 2014; Gajendran et al., 2019; Panah et al., 2021). Colitis presents as bloody diarrhea, without or with mucus present, a marked reduction in body weight (BW), general loss of appetite, fatigue, low-grade fever, and anemia (Head and Jurenka, 2003; Feuerstein and Cheifetz, 2014; Gajendran et al., 2019). Additionally, the colonic epithelial barrier of pigs experiencing colitis is damaged, thereby allowing passage of microbes and antigens from the intestinal lumen and into the body (Head and Jurenka, 2003; Ordás et al., 2012). Through efforts to identify strategies to ameliorate the negative effects of intestinal inflammation, supplemental butyrate has emerged as a potential practical intervention.
Butyrate is a short-chain fatty acid (SCFA) that is produced through microbial fermentation (Segain et al., 2000; Kotunia et al., 2004; Weber and Kerr, 2006; Sotira et al., 2020). Butyrate is the primary energy source for colonocytes (Segain et al., 2000; Leonel et al., 2013; Sotira et al., 2020). Butyrate also participates in supporting immunity, prompting the expression of host defense peptides and reducing of pro-inflammatory cytokine expression in response to bacterial and viral pathogens that are commonly experienced in young pigs expressing weaning stress (Sunkara et al., 2011; Ma et al., 2012; Wang et al., 2018, 2019). Lastly, butyrate inhibits enteric pathogens from passing through the epithelial barrier (Wang et al., 2019), along with accelerating the rate of tissue repair and regeneration and improving immune defenses and intestinal barrier function (Piva et al., 2002; Leonel et al., 2013, 2016; Shi and Lee, 2020). However, butyrate is challenging to incorporate into diets due to its bitter taste and foul smell, but tributyrin, where 3 moles of butyrate are bound to a glycerol backbone, may overcome some of these limitations (Leonel et al., 2013, 2016; Dong et al., 2016; Wang et al., 2019; Tugnoli et al., 2020).
One strategy to ensure tributyrin reaches the colon is microencapsulation, which is a chemical or physical process to trap the core material within a matrix (Donovan et al., 2016, 2017; Li et al., 2021). Microencapsulation allows for targeted release, which is crucial when utilizing tributyrin to alleviate inflammation in the colon (Donovan et al., 2016; Shi et al., 2020). Gamma-cyclodextrin is a microencapsulating agent that is suitable for targeted release to the colon as it is indigestible by the mammalian hydrolytic enzymes, but fermentable by the colonic microorganisms (Augustin et al., 2011; Donovan et al., 2016). Based on previous literature indicating the beneficial effects of oral butyrate supplementation, our objective was to investigate the effects of gamma-cyclodextrin encapsulated tributyrin (TBCD) in pigs experiencing dextran sodium sulfate (DSS)-induced colitis. Our hypothesis was that the supplementation of TBCD will support intestinal health of pigs and ameliorate the effects of DSS, therefore allowing pigs receiving TBCD and DSS to exhibit better growth performance and intestinal morphology than those receiving DSS alone.
Materials and Methods
Prior to study initiation, all animal care and experimental procedures were approved by the University of Illinois Institutional Animal Care and Use Committee.
Animal husbandry
A total of 32 boars [2.06 ± 0.21 kg initial body weight (BW)] were obtained from a commercial farrowing facility (PIC Line 3 dams × Line 2 sires) on postnatal d (PND) 2 and transported to the University of Illinois Piglet Nutrition and Cognition Laboratory. Upon arrival, pigs received a 5-mL dose of Clostridium perfringens antitoxins C and D (Colorado Serum Company, Denver, CO) subcutaneously plus a 3-mL dose orally. Pigs were assigned to experimental treatments based on initial BW and litter of origin and housed individually in stainless steel cages (1.03 m deep × 0.77 m wide × 0.81 m high) with clear, acrylic facades and side walls bearing a grid of 2.54-cm holes to allow for adequate ventilation. Ambient room temperature was maintained between 27 °C and 29 °C throughout the study. A 12:12 (L:D) h cycle was maintained with light from 0800 hours to 2000 hours as described in Fil et al. (2021). Milk replacer (ProNurse Multi Species Milk Replacer; Land O’Lakes, Arden Hills, MN) was provided ad libitum using an automated milk replacer delivery system from 1000 hours to 0600 hours the next day (i.e., a 20-h daily feeding cycle). Pigs were assigned to 1 of 2 dietary treatments (described below) and remained on those diets throughout the duration of the 28-d feeding study.
At study start (i.e., PND 2), pigs were assigned to 1 of 3 treatment groups: 1) control diet (nutritionally adequate) and no induction of colitis (Control), 2) control diet plus provision of oral DSS to induce colitis (DSS), and 3) control diet supplemented with 8.3 g of TBCD per kg reconstituted milk replacer (TBCD) plus oral DSS to induce colitis (TBCD + DSS). TBCD utilized in the experimental treatments was manufactured as according to the procedures in Shi et al. (2020). TBCD dosage was determined based off prior research conducted by Bartholome et al. (2004). From PNDs 14 to 18, pigs assigned to the DSS and TBCD + DSS groups were orally administered DSS at 1.25 g/kg BW daily by delivering DSS in the milk bowl 30 min prior to the initial delivery of milk replacer each day, thereby allowing adequate time for ingestion to induce intestinal inflammation (i.e., colitis). Health checks were performed thrice daily on pigs to visually track body condition, generalized home–cage activity, fecal consistency, and incidence of vomiting, lethargy, and any abnormal behaviors as clinical indicators of sickness. When loose stool was observed in pigs prior to DSS dosing, an electrolyte solution (Swine BlueLite; TechMix, Stewart, MN) was provided. In addition to electrolytes, antibiotics were administered as recommended by the attending veterinarian. A single administration was provided to one pig in the TBCD + DSS treatment, however, this treatment did not preclude the pig from the study. Pigs were removed from the study prior to DSS dosing if they displayed 3 or more of the following clinical signs: lethargy, delayed awareness of human observer, vomiting, diarrhea, or weight loss exceeding 10%.
Growth performance
Individual pig BW was recorded daily, along with the weight of any remaining milk replacer from the previous feeding cycle. The remaining milk weight was subtracted from the initial weight provided to quantify milk disappearance over the −20 h feeding period, which will hereafter be referred to as milk intake. Pig weight along with milk disappearance was utilized to calculate ADG, average daily feed intake (ADFI), and feed efficiency or the gain-to-feed ratio (G:F). Water was offered ad libitum to all pigs using a commercially available drinker (Suevia Model 90; Kirchheim am Neckar, Germany).
Blood and tissue collection
Prior to the collection of blood and tissue samples at study conclusion, pigs were subjected to standard anesthesia and neuroimaging procedures associated with a complementary study. Pigs were provided 24 h to recover from the anesthesia prior to being humanely euthanized on PND 27 or 28. To permit sample collection at study conclusion, pigs were anesthetized via an intramuscular injection of a telzaol:ketamine:xylazine solution [50 mg of zolazepam reconstituted with 2.50 mL ketamine (100 g/L) and 2.50 mL xylazine (100 g/L); Fort Dodge Animal Health, Overland Park, KS] at 0.06 mL/kg BW. After sedation, blood was collected via cardiac puncture and allowed to clot for up to 45 min before being placed on ice. Blood samples were centrifuged at 4 °C for 20 min at 1,160 × g, at which point serum was collected, aliquoted into cryovials, and stored at −80 °C pending analysis. Once blood collection was complete, pigs were euthanized using sodium pentobarbital (Euthasol Euthanasia Solution CIIIN; Patterson Veterinary Supply, St. Paul, MN) at 1 mL per 4.5 kg of BW via cardiac puncture. Subsequently, pigs were dissected to collect luminal contents and tissues from the midpoint of the ileum, tip of the cecum, along with the midpoint of both the ascending, and descending colon.
Serum cytokine concentrations
Serum samples were analyzed to quantify circulating pro-inflammatory cytokine concentrations using porcine-specific enzyme-linked immunosorbent assay (ELISA) kits. Following the manufacturer’s instructions, interleukin (IL)-1β, IL-6, and tumor necrosis factor-α (TNF-α) concentrations were determined utilizing a sandwich ELISA kit (Thermo Fisher Scientific; Waltham, MA). Serum cytokines were analyzed using individual kits with the following technical details: IL-1β: standard curve of 8.23 to 6,000 pg/mL, assay sensitivity of 6 pg/mL, and inter- and intra-assay CV of 12% and 10%, respectively; IL-6: standard curve of 40.96 to 10,000 pg/mL, assay sensitivity of 45 pg/mL, and inter- and intra-assay CV of 12% and 10%, respectively; and TNF-α: standard curve of 23.4 to 1,500 pg/mL, sensitivity of 3 pg/mL, and inter- and intra-assay CV of 7.3% and 6.0%, respectively.
Volatile fatty acids and dry matter analysis
Luminal contents were collected from the cecum, ascending, and descending colon for volatile fatty acid analysis (VFA) [i.e., SCFA plus branched-chain fatty acids (BCFA)]. Freshly-collected luminal samples were weighed and preserved using a 1 wt:1 vol ratio of 2 N HCl. Concentrations of individual VFA concentrations were determined utilizing gas chromatography (model 5890A series 99; Hewlett-Packard, Palo Alto, CA) equipped with a 100 m × 0.25 mm i.d. × 0.2 µm film thickness capillary column (model SP-2560; Supelco, Bellefonte, PA). Dry matter (DM) was determined after drying samples in a 105 °C oven for a minimum of 48 h.
Histopathology
For histopathology outcomes, 2.5-cm long rings of tissue sampled from the ileum, proximal colon, and distal colon were flushed with phosphate-buffered saline, then put in a 10% solution of neutral buffer formalin pending histomorphology and histopathology analyses that were performed by a board-certified veterinary pathologist (Veterinary Diagnostic Pathology, LLC; Fort Valley, VA). For these analyses, tissues were paraffin embedded and 5-µm sections were prepared and stained with hematoxylin and eosin. Stained tissue sections were evaluated and reported by a certified histopathologist who remained blinded to treatment identity. From the ileal sections, 10 villi and corresponding crypts were measured per pig and used to calculate villi height-to-crypt depth ratios. For the proximal and distal colon tissues, 10 mucosal widths were measured and recorded per pig, with only these tissues analyzed to determine histopathological lesion scores. There were approximately 20 different lesion measures that were identified and were recorded as no lesions present or lesions present to create a binary relationship. This was then analyzed as the percent of total pigs within each treatment that had a lesion present.
Statistical analysis
Data were subjected to an ANOVA using the MIXED procedure of SAS (version 9.4; SAS Institute, Cary, NC). A 1-way ANOVA was used to determine whether the overall model was significant, and in those instances, means separation was conducted assuming an alpha level of 0.05. Results are presented as least squares means with their respective SEM. Outliers were identified as having an absolute Studentized residual value of 3 or greater and were removed from the final dataset. Histopathology lesion scores were processed using the FREQ procedure of SAS (version 9.4; SAS Institute, Cary, NC) to produce F-test comparisons. Lesion scores were separated into binary datasets (no lesions present vs. lesions present) prior to statistical analysis. After creating the binary datasets per type of tissue lesion, a frequency analysis was applied to the data due to the data lacking a normal distribution of errors and percent of pigs expressing lesions as described previously (Knauer et al., 2007).
Results
Pigs generally remained healthy throughout the study, despite the induction of colitis in some treatment groups. However, there were pigs that were removed from the study based on objective removal criteria, with 2, 3, and 4 pigs removed from the Control, DSS, and TBCD + DSS treatments, respectively.
Growth performance
Results for growth performance can be found in Table 1. Prior to dosing with DSS, TBCD +DSS pigs exhibited decreased (P < 0.05) ADG compared with the Control and DSS treatments. Pigs receiving TBCD exhibited numerically lower (P = 0.075) ADFI prior to DSS administration when compared with the DSS and Control treatments. There were no differences in ADG, average daily feed intake in powder (ADFIP), and average daily feed intake in liquid (ADFIL) when comparing the Control and DSS treatments before DSS dosing. When comparing all treatments prior to DSS dosing, there were no differences in G:F when calculated based on the intake of powder or liquid. After dosing with DSS, there was a decrease in ADG, ADFIP, and ADFIL in the TBCD + DSS treatment compared with the Control and DSS treatments. There were no differences in G:F, either when calculated based on intake of powder or liquid, after DSS dosing when comparing all treatments. There were no differences in ADG, ADFIP, and ADFIL when comparing the Control and DSS treatments after DSS administration.
Table 1.
Effects of orally supplemented TBCD and DSS-induced colitis on growth performance of young pigs1
| Outcome per study period | Treatment | Pooled SEM | P-value | ||
|---|---|---|---|---|---|
| Control | DSS | TBCD + DSS | |||
| Pigs, n | 8 | 7 | 8 | ||
| Before DSS (PND 2–13) | |||||
| ADG, g/d | 190.8a | 189.5a | 137.5b | 22.13 | 0.016 |
| ADFIP, g/d | 232.0 | 231.5 | 194.0 | 27.34 | 0.075 |
| ADFIL, g/d | 1,160 | 1,157 | 969.9 | 136.72 | 0.075 |
| G:F powder, g:g | 0.83 | 0.81 | 0.74 | 0.04 | 0.298 |
| G:F liquid, g:g | 0.16 | 0.16 | 0.15 | 0.01 | 0.289 |
| After DSS (PND 14–27) | |||||
| ADG, g/d | 402.7a | 357.6a | 212.2b | 20.72 | < 0.001 |
| ADFIP, g/d | 463.0a | 411.2a | 248.0b | 21.95 | < 0.001 |
| ADFIL, g/d | 2,315a | 2,056a | 1,240b | 109.72 | < 0.001 |
| G:F powder, g:g | 0.87 | 0.87 | 0.83 | 0.03 | 0.536 |
| G:F liquid, g:g | 0.18 | 0.18 | 0.17 | 0.01 | 0.473 |
| Overall (PND 2–28) | |||||
| ADG, g/d | 333.7a | 301.5a,b | 225.2b | 34.36 | 0.009 |
| ADFIP, g/d | 349.6a | 320.6a | 221.5b | 22.61 | 0.001 |
| ADFIL, g/d | 1,748a | 1,603a | 1,108b | 113.01 | 0.001 |
| G:F powder, g:g | 0.92 | 0.92 | 0.83 | 0.03 | 0.093 |
| G:F liquid, g:g | 0.18 | 0.18 | 0.16 | 0.01 | 0.093 |
1Pigs were artificially reared from postnatal days 2–28, dosed with DSS on postnatal days 14–18, and euthanized on postnatal days 27 or 28 to permit sample collection.
a,bMeans lacking a common superscript letter within a row differ (P < 0.05).
Abbreviations: ADG, average daily body weight gain; ADFIL, average daily feed intake liquid; ADFIP, average daily feed intake powder; DSS, dextran sodium sulfate; G:F, gain-to-feed or feed efficiency; PND, postnatal day; TBCD, gamma-cyclodextrin-encapsulated tributyrin.
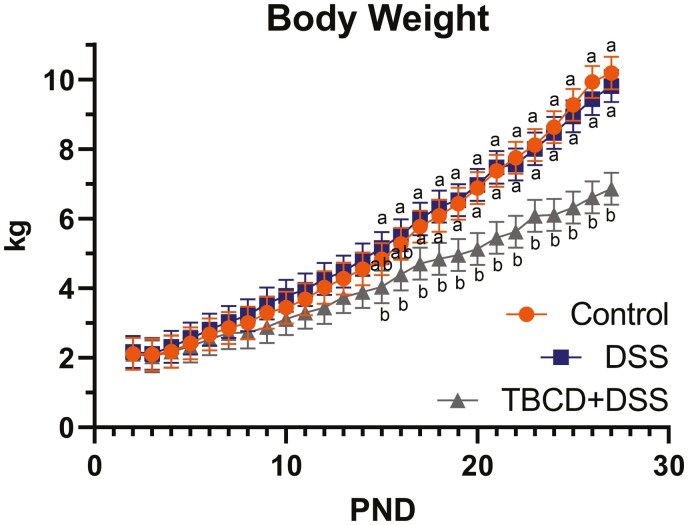
For the total study duration, TBCD + DSS pigs exhibited decreased (P < 0.05) ADG compared with Control treatment, with DSS pigs being intermediary. Decrease (P < 0.05) in ADFIP and ADFIL were observed when comparing TBCD + DSS with the DSS and Control treatments. Between the Control and DSS treatments, there were no overall differences in ADG, ADFIP, ADFIL, or G:F. Overall, the BW of the DSS and Control pigs did not differ, however, the BW of the TBCD + DSS pigs was decreased (P < 0.05) compared with the DSS and Control pigs starting on days 15 and 17, respectively, and continuing to study conclusion (Fig. 1).
Figure 1.
Daily pig body weights throughout the study duration. Pigs were artificially reared from postnatal days 2–28, dosed with DSS on postnatal days 14–18, and euthanized on postnatal days 27 or 28 to permit sample collection. Abbreviations: DSS, dextran sodium sulfate; PND, postnatal day; TBCD, gamma-cyclodextrin-encapsulated tributyrin. a,bMeans lacking a common superscript letter within a given day differ (P < 0.05).
Serum proinflammatory cytokines
No differences in circulating TNF-α concentrations were detected 10 d after cessation of DSS dosing (i.e., study conclusion; Fig. 2), and neither IL-1β nor IL-6 were detectable at study conclusion (data not shown).
Figure 2.
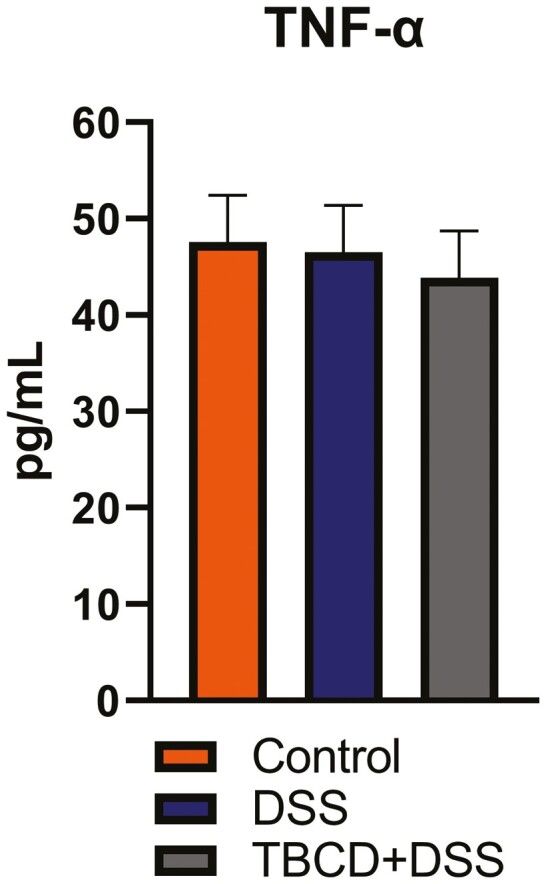
Serum concentrations of the pro-inflammatory cytokine, TNF-α, in pigs 10 d after the 5-d administration of DSS ended. Pigs were artificially reared from postnatal days 2–28, dosed with DSS on postnatal days 14–18, and euthanized on postnatal days 27 or 28 to permit sample collection. Serum IL-1β and IL-6 concentrations were also measured, but neither was detectable in any sample at PND 28. Abbreviations: DSS, dextran sodium sulfate; TBCD, gamma-cyclodextrin-encapsulated tributyrin; TNF-α, tumor necrosis factor-alpha.
Volatile compounds in luminal contents
At study conclusion, no treatment differences were detected for DM, acetate or propionate concentrations, or molar percentages of SCFA in cecal contents (Table 2). Increased (P < 0.05) butyrate concentrations were observed in DSS and TBCD + DSS pigs when compared with Control pigs. Molar percentages of acetate were decreased (P < 0.05) in pigs receiving TBCD, compared with the Control and DSS pigs. Pigs receiving TBCD had increased (P < 0.05) molar percentages of butyrate compared with the Control however, DSS pigs remained intermediary.
Table 2.
Effects of orally supplemented TBCD- and DSS-induced colitis on colonic VFA of young pigs1
| Outcome per intestinal segment | Treatment | Pooled SEM | P-value | ||
|---|---|---|---|---|---|
| Control | DSS | TBCD + DSS | |||
| Cecum | |||||
| Pigs, n | 7 | 7 | 6 | ||
| DM, % | 19.58 | 16.29 | 19.63 | 1.786 | 0.303 |
| SCFA absolute, μmol/g DM | |||||
| Acetate | 360.00 | 483.89 | 354.35 | 81.010 | 0.246 |
| Propionate | 105.42 | 127.71 | 121.96 | 19.508 | 0.673 |
| Butyrate | 60.84a | 99.90b | 100.55b | 18.287 | 0.044 |
| Total SCFA2 | 529.75 | 711.57 | 577.79 | 112.35 | 0.291 |
| SCFA relative3, % of total | |||||
| Acetate | 66.96a | 67.79a | 59.96b | 2.311 | 0.020 |
| Propionate | 21.32 | 18.02 | 22.55 | 1.779 | 0.128 |
| Butyrate | 11.72a | 14.14a,b | 17.56b | 1.611 | 0.018 |
| Total BCFA4 | 34.46 | 62.43 | 49.32 | 9.691 | 0.124 |
| Proximal Colon | |||||
| Pigs, n | 8 | 6 | 7 | ||
| DM, % | 22.01 | 17.93 | 21.74 | 1.862 | 0.212 |
| SCFA absolute, μmol/g DM | |||||
| Acetate | 186.74a | 314.81b | 200.85a | 22.885 | 0.001 |
| Propionate | 61.55a | 82.75a,b | 95.44b | 10.281 | 0.026 |
| Butyrate | 35.26a | 69.82b | 62.37b | 8.867 | 0.009 |
| Total SCFA2 | 281.81a | 464.02b | 341.77a | 33.109 | 0.001 |
| SCFA relative3, % of total | |||||
| Acetate | 65.92a | 67.80a | 58.80b | 1.273 | < 0.001 |
| Propionate | 22.54a | 17.56b | 24.27a | 1.526 | 0.012 |
| Butyrate | 11.75a | 14.60a | 15.91b | 1.136 | 0.031 |
| Total BCFA4 | 24.60a | 43.88b | 40.84b | 4.211 | 0.006 |
| Distal colon | |||||
| Pigs, n | 7 | 5 | 3 | ||
| DM, % | 29.65 | 27.84 | 20.87 | 3.756 | 0.186 |
| SCFA absolute, μmol/g DM | |||||
| Acetate | 76.80a | 129.49b | 120.50a | 30.557 | 0.045 |
| Propionate | 27.77 | 42.66 | 10.17 | 13.124 | 0.061 |
| Butyrate | 13.61 | 24.47 | 24.27 | 6.752 | 0.101 |
| Total SCFA2 | 117.97 | 195.72 | 214.46 | 47.844 | 0.065 |
| SCFA relative3, % of total | |||||
| Acetate | 65.958 | 65.198 | 60.50 | 3.581 | 0.449 |
| Propionate | 23.40 | 22.88 | 27.58 | 2.987 | 0.442 |
| Butyrate | 10.78 | 12.27 | 11.83 | 1.688 | 0.635 |
| Total BCFA4 | 14.44 | 24.35 | 29.89 | 6.746 | 0.106 |
1Pigs were artificially reared from postnatal days 2–28, dosed with DSS on postnatal days 14–18, and euthanized on postnatal days 27 or 28 to permit sample collection.
2Total SCFA calculated as the sum of all absolute concentrations (μmol/g) for acetate, propionate, and butyrate.
3SCFA relative percent values calculated by dividing absolute concentrations of each SCFA (μmol/g) by the total SCFA concentration (μmol/g) then multiplying by 100.
4Total BCFA calculated as the sum of all absolute concentrations (μmol/g) for isovalerate, valerate, and isobutyrate.
a,bMeans lacking a common superscript letter within a row differ (P < 0.05).
Abbreviations: BCFA, branched-chain fatty acids; DM, dry matter; DSS, dextran sodium sulfate; SCFA, short-chain fatty acids; TBCD, gamma-cyclodextrin-encapsulated tributyrin; VFA, volatile fatty acid.
No difference in DM content was observed in proximal colon luminal contents at study conclusion due to treatment. Pigs in the DSS treatment had increased (P < 0.05) acetate and total SCFA concentrations when compared with the Control and TBCD + DSS pigs. Propionate concentrations in proximal colon contents were increased (P < 0.05) in TBCD + DSS compared with the Control, while DSS remained intermediate. Both treatments that included oral DSS dosing elicited increased (P < 0.05) butyrate concentrations in proximal colon contents when compared with the Control treatment. Pigs receiving TBCD had decreased (P < 0.05) molar percentages of acetate in proximal colon contents compared with DSS and Control pigs. Oral supplementation of TBCD increased (P < 0.05) the molar percentage of butyrate in the proximal colon when compared with the Control and DSS treatments. Pigs in both treatments that included oral DSS dosing exhibited increased (P < 0.05) total BCFA when compared with Control; however, there were no differences between DSS and TBCD + DSS treatments.
No treatment differences in distal colon luminal contents were observed for DM, butyrate concentration, total SCFA or BCFA concentrations, or any molar percentages of SCFA. Pigs in the DSS treatment had increased (P < 0.05) acetate concentrations when compared with Control and TBCD + DSS.
Intestinal morphology and pathology
No treatment differences in histomorphology (e.g., villus height, crypt depth, or the villus height-to-crypt depth ratio) were observed in the ileum of pigs at study conclusion (Table 3). There was an increase (P < 0.05) in mucosal width in the proximal colon of pigs receiving TBCD compared with Control treatment. In the distal colon, the mucosal width was increased (P < 0.05) in both the DSS and TBCD + DSS treatments when compared with the Control treatment.
Table 3.
Effects of orally supplemented TBCD- and DSS-induced colitis on intestinal histomorphology of young pigs1
| Outcome per tissue | Treatment | Pooled SEM | P-value | ||
|---|---|---|---|---|---|
| Control | DSS | TBCD + DSS | |||
| Ileum | |||||
| Villi height, µm | 531.38 | 434.86 | 492.64 | 42.089 | 0.271 |
| Crypt depth, µm | 236.72 | 225.98 | 272.66 | 20.130 | 0.203 |
| V:C ratio | 2.28 | 2.20 | 1.88 | 0.218 | 0.193 |
| Proximal colon | |||||
| Mucosal width, µm | 342.23a | 388.93a,b | 586.57b | 70.277 | 0.042 |
| Distal colon | |||||
| Mucosal width, µm | 377.79a | 646.82b | 714.44b | 42.499 | < 0.001 |
1Pigs were artificially reared from postnatal days 2–28, dosed on postnatal days 14–18, and euthanized on postnatal days 27 or 28 to permit sample collection.
a,bMeans lacking a common superscript letter within a row differ (P < 0.05).
Abbreviations: DSS, dextran sodium sulfate; TBCD, gamma-cyclodextrin-encapsulated tributyrin; V:C, villus height-to-crypt depth.
For histopathological lesion scoring, the percentage of total pigs within each treatment that had lesions present was recorded for both proximal and distal colon tissues. Within the proximal colon, there were varying results across treatments (Table 4). Based on lesions pertaining to necrosis and apoptosis within the proximal colon lamina propria, DSS + TBCD pigs exhibited an increased (P < 0.05) incidence compared with Control and DSS treatments. Within the distal colon, there were no differences in the percentage of pigs exhibiting bacterial adherence, apoptosis, or mucus being present. There were increased (P < 0.05) percentages of pigs in the DSS and DSS + TBCD treatments exhibiting crypt abscesses, cystic crypts, neutrophil infiltration, presence of lamina propria gut-associated lymphoid tissue, erosions/ulcers, and fibrosis when compared with Control.
Table 4.
Effects of orally supplemented TBCD- and DSS-induced colitis on the frequency of intestinal histopathology scores exhibited in young pigs1
| Outcome per tissue | Treatment | P-value | ||
|---|---|---|---|---|
| Control | DSS | DSS + TBCD | ||
| Proximal colon | ||||
| LP fibrosis | 0.00 | 14.29 | 12.50 | 0.221 |
| LP necrosis/apoptosis | 37.50 | 27.14 | 87.50 | 0.019 |
| Neutrophil/eosinophil infiltration | 37.50 | 42.86 | 50.00 | 0.120 |
| Congestion | 50.00 | 71.43 | 50.00 | 0.090 |
| Crypt hyperplasia | 0.00 | 14.29 | 25.00 | 0.111 |
| Cystic crypts | 0.00 | 14.29 | 12.50 | 0.221 |
| Edema | 0.00 | 0.00 | 12.50 | 0.348 |
| Epithelial necrosis | 12.50 | 14.29 | 25.00 | 0.177 |
| Excessive mucus | 0.00 | 28.57 | 25.00 | 0.066 |
| Crypt microabscess | 0.00 | 14.29 | 25.00 | 0.111 |
| Distal colon | ||||
| Bacterial adherence | 12.50 | 14.29 | 0.00 | 0.221 |
| Crypt microabscess | 0.00 | 85.71 | 75.00 | <0.001 |
| Cystic crypt | 0.00 | 71.43 | 87.50 | <0.001 |
| LP GALT | 25.00 | 71.43 | 57.50 | 0.006 |
| Neutrophil/eosinophil infiltration | 12.50 | 71.43 | 100.00 | <0.001 |
| Apoptosis | 12.50 | 0.00 | 0.00 | 0.348 |
| Erosion/ulcers | 0.00 | 71.43 | 87.50 | <0.001 |
| LP fibrosis | 0.00 | 57.14 | 62.50 | 0.002 |
| Excessive mucus | 12.50 | 42.86 | 12.50 | 0.067 |
1Values presented as the percentage of pigs within each treatment that exhibited lesions as confirmed by a certified histopathologist who remained blinded to treatment identity. Pigs were artificially reared from postnatal days 2–28, dosed with DSS on postnatal days 14–18, and euthanized on postnatal days 27 or 28 to permit sample collection.
Abbreviations: DSS, dextran sodium sulfate; GALT, gut-associated lymphoid tissue; LP, lamina propria; TBCD, gamma-cyclodextrin-encapsulated tributyrin.
Discussion
Large intestinal inflammation with an unknown etiology is commonly referred to as colitis and this can be caused by infection, psychological stress, immune disruption, and myriad of other factors (Sartor, 2006; Panah et al., 2021). Clinical signs of colitis in pigs include bloody diarrhea, reduction in growth, loss of appetite, and physical damage to the epithelial barrier (Head and Jurenka, 2003; Gajendran et al., 2019), all of which contribute to pig morbidity and mortality (Thomson, 2009). The relatively immature state of digestive physiology and immunity in newly weaned pigs necessitates mitigation strategies, and dietary interventions that stimulate the production of SCFA by the resident microbiota have previously been proven to benefit intestinal health and overall well-being of pigs.
Of the 3 SCFA (acetate, propionate, and butyrate), butyrate is typically found at the lowest concentration, but it also serves as the main energy source for colonocytes, potentially making it a good candidate to mitigate intestinal stress (Hou et al., 2014; Sotira et al., 2020). However, due to poor organoleptic and high-volatility properties, free butyrate cannot be used as a dietary supplement, but conjugation and encapsulation of butyrate may serve to alleviate such issues. Tributyrin contains 3 moles of butyrate bound to a glycerol backbone, and when fed directly, tributyrin releases free butyrate within the small intestine and enhances the activation of pathways known to be stimulated by colonic microbe-derived butyrate (Leonel et al., 2013; Tugnoli et al., 2020). Primary benefits of increased intestinal butyrate concentrations include antioxidant, anti-inflammatory, anticarcinogenic, and trophic effects (Hou et al., 2014; Leonel et al., 2016; Tugnoli et al., 2020). The enhancement of these effects through oral butyrate supplementation has the potential to mitigate the effects of intestinal inflammation. To that end, the objective of this study was to investigate the effects of TBCD on weaned pigs experiencing acute colitis induced through oral DSS administration.
Counter to our hypothesis, the inclusion of oral TBCD negatively influenced growth performance of young pigs, even prior to the onset of colitis. Induction of colitis with DSS exacerbated the reduction in ADG for pigs receiving TBCD, while pigs dosed with DSS and consuming the control diet did not differ in ADG or ADFI from the Control group. These results are not in agreement with previous findings in mice regarding growth performance during colitis without or with tributyrin supplementation. For example, mice given DSS lost weight compared with sham-dosed controls (Leonel et al., 2013), but those effects were not mitigated by TBCD supplementation, while in the present study DSS did not cause a reduction in BW. The difference between the current study and Leonel et al. (2013) could be attributed to the different animal models used, as there is little research done on TBCD supplementation and DSS-induced colitis in pigs; however, results reported herein also contradict other studies that use pigs as a model. Pigs undergoing weaning stress, but not disease challenge, had an increase in ADG when supplemented with tributyrin (Wang et al., 2019). Additionally, tributyrin had no impact on ADFI when compared with the control, indicating that tributyrin did not increase feed intake above normal. These conflicting effects on ADG and ADFI may be due to differences in the age of the pigs or the TBCD preparation method that was used. Pigs in the current study were analyzed prior to weaning, specifically receiving TBCD from PNDs 2 to 28, while pigs used by Wang et al. (2019) were analyzed from PNDs 24 to 38. Pigs weaned on or after 21 d of age have a greater fermentation capacity and ability to utilize fermentation end-products due to a more diverse microbiota (Pluske, 2016; Guevarra et al., 2019). Therefore, the reduction in ADG reported here might be due to the young pig’s inability to effectively utilize tributyrin, or that the gamma-cyclodextrin encapsulation could not be effectively fermented due to the immature microbiota within the piglet colon. Furthermore, pigs receiving supplemental TBCD exhibited a similar efficiency of BW gain (i.e., G:F) compared with Control and DSS pigs, which appeared to result from synchronized decreases in ADG and ADFI. This correlates with Weber and Kerr (2008), who reported that supplemental sodium butyrate in weaning pigs undergoing a lipopolysaccharide challenge exhibited no changes in G:F compared with the control but did show decreased ADFI. In this study, TBCD did not ameliorate negative growth performance outcomes; therefore, other outcomes such as blood cytokine levels were investigated to understand the inflammatory response.
Although colitis is widely recognized as an inflammatory disease (Sartor, 2006; Ren et al., 2014; Nesterova et al., 2020; Panah et al., 2021), serum TNF-α in our study did not differ between treatments, along with undetectable concentrations of IL-1β and IL-6. Previous literature in pigs at 21-d-old or 8–10-wk-old have indicated that the baseline TNF-α concentration may be approximately 50 pg/mL (Kruse et al., 2008; Yu et al., 2019), which corresponds to the range of TNF-α concentrations (43–47.5 pg/mL) observed in our study. However, the lack of increased TNF-α in pigs receiving DSS may be explained by timing of sample collection. In mice with DSS-induced colitis, serum TNF-α levels 7 d after cessation of DSS dosing were elevated compared with the control treatment, and butyrate supplementation mitigated this effect (Simeoli et al., 2017). Thus, differences in the timing of sample collection, age of animal, and chosen animal model may all influence the circulating inflammatory response to DSS-induced colitis. In our pig study, we concluded that circulating TNF-α concentrations no longer indicate an inflammatory state following DSS administration, but it remains plausible that localized inflammation within the colon remained, so further research is warranted to characterize the DSS-induced inflammatory profile of young pigs.
To get a better understanding of where TBCD was released in the intestinal tract, VFA was analyzed in the cecum, proximal colon, and distal colon. Relative to the control group, increased butyrate concentrations in the proximal colon in both treatments receiving DSS may have resulted from decreased butyrate oxidation that is typically observed during colitis (Thibault et al., 2010; De Preter et al., 2012). As such, a decrease in butyrate oxidation has been shown to occur when luminal butyrate concentrates are decreased, colonocyte uptake of butyrate is reduced, and/or when irregularities occur within the β-oxidation pathway (Ahmad et al., 2000; Thibault et al., 2010; De Preter et al., 2012). Thus, a reduction in butyrate oxidation could mean that colonocytes were in an energy deficient state, thereby, restricting mucin secretion and sodium absorption, and ultimately resulting in further intestinal barrier dysfunction and permeability (De Preter et al., 2012; Cornick et al., 2015). An increase in the butyrate concentration as a proportion of total SCFA in the proximal colon for the TBCD treatment compared with the DSS treatment was expected, as these pigs were receiving supplemental butyrate in addition to that expected to be produced by the microbiota. Mallo et al. (2012) reported that encapsulated sodium butyrate increased colonic butyrate concentrations in pigs when compared with orally supplemented monobutyrate, but it should be noted that a negative control treatment was not included. Based on increased luminal concentrations, we conclude that the exogenous delivery of butyrate via TBCD was successful, but it remains to be determined whether this resulted from a lack of butyrate absorption and/or subsequent oxidation.
In contrast to effects observed in the proximal colon, the distal colon had no major differences in VFA concentrations or molar percentages. This was somewhat expected, as colitis alone has not been reported to influence VFA concentrations throughout the colon (Grosu et al., 2020). Of greater interest is the lack of differences in butyrate molar percentages between TBCD + DSS and DSS groups. In both the cecal and proximal colon contents, TBCD + DSS pigs had greater proportions of butyrate compared with DSS pigs, which we attributed to exogenous TBCD supplementation. However, by the time digestive contents reached the distal colon the butyrate concentration had decreased by approximately 37% across all treatments. This may indicate that by the time butyrate reached the distal colon, it had already been utilized by the colonocytes in the proximal colon as fuel (Velazquez et al., 1996).
Ileum histology did not differ between treatments, but phenotypic differences were observable in the proximal and distal colon. This result aligns with the known pathogenesis of colitis, as the intestinal damage occurs largely in the distal colon with the ability to progress proximally (Head and Jurenka, 2003; Ordás et al., 2012; Gajendran et al., 2019). Pigs in the DSS and TBCD + DSS treatments had increased mucosal width in the distal colon compared with the Control, which matches observations by Biton et al. (2018) and Kang et al. (2021) who reported mice undergoing DSS-induced colitis had increased mucosal thickness. Further evidence supporting the successful induction of intestinal inflammation by oral DSS was the clear increases in the percentage of DSS-treated pigs experiencing tissue lesions in the distal colon, specifically increases in crypt abscesses, lamina propria ulceration, cystic crypts, fibrosis, and presence of neutrophils. This evidence coincides with findings by Vieira et al. (2012), who reported mice receiving DSS had increased infiltration of eosinophils and neutrophils associated with tissues exhibiting inflammation. Increased mucosal width and tissue damage as indicated by elevated lesion scores have also been associated with colitis-related inflammation (Weinstein and Sajewicz, 2013; Xu et al., 2021), implying that pigs in our study had endured DSS-induced colitis with lasting indicators of inflammation more than a week after DSS dosing ceased.
In conclusion, these results suggest that oral supplementation of TBCD was successful at increasing the concentration of butyrate in proximal colon luminal contents. However, oral TBCD supplementation in pigs both prior to and after DSS-induced colitis did not ameliorate negative growth outcomes or tissue-level indicators of inflammation. Whereas increased butyrate concentrations were observed in the proximal colon, butyrate concentrations in the distal colon had normalized between treatments at study conclusion. Despite the observed elevation in butyrate concentration of colonic contents, there was clear evidence that supplementation of TBCD to pigs shortly after birth and continuing for approximately 4-wk negatively affected the rates of BW gain and milk intake, though the efficiency of gain was not affected. These TBCD-associated effects were exacerbated by DSS-induced colitis. It remains possible that organoleptic properties of the TBCD precluded adequate ingestion of liquid milk replacer in the supplemented group, but further research is warranted. In terms of intestinal morphology, oral TBCD supplementation in DSS-treated piglets increased mucosal width in the colon, suggesting it failed to mitigate inflammation-related remodeling. Collectively, these results indicate that the inclusion of TBCD does not appear to support the growth or intestinal health of young pigs experiencing an intestinal inflammatory challenge.
Acknowledgments
We would like to thank Dr. Marcia Monaco for her advice during the conduct of the study. Dr. Frederic Hoerr for performing histopathology on the tissue samples provided and Adam Jones for managing the swine facility along with helping care for the pigs in this study.
Glossary
Abbreviations
- ADG
average daily gain
- ADFI
average daily feed intake
- ADFIL
average daily feed intake liquid
- ADFIP
average daily feed intake powder
- BCFA
branched-chain fatty acid
- BW
body weight
- DM
dry matter
- DSS
dextran sodium sulfate
- ELISA
enzyme-linked immunosorbent assay
- G:F
gain to feed
- IL
interleukin
- PND
postnatal day
- SCFA
short-chained fatty acid
- TBCD
gamma-cyclodextrin encapsulated tributyrin; TNF-α, tumor necrosis factor-α
- VFA
volatile fatty acid
Contributor Information
Kaitlyn M Sommer, Department of Animal Sciences, University of Illinois, Urbana, IL, USA.
Julianna C Jespersen, Department of Animal Sciences, University of Illinois, Urbana, IL, USA.
Loretta T Sutkus, Department of Animal Sciences, University of Illinois, Urbana, IL, USA.
Youngsoo Lee, Department of Food Science and Human Nutrition, University of Illinois, Urbana, IL, USA.
Sharon M Donovan, Department of Animal Sciences, University of Illinois, Urbana, IL, USA.
Ryan N Dilger, Department of Animal Sciences, University of Illinois, Urbana, IL, USA.
Funding
This research was supported by the United States Department of Agriculture National Institute of Food and Agriculture, Hatch Project ILLU-698-330.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
References
- Ahmad, M. S., Krishnan S., Ramakrishna B. S., Mathan M., Pulimood A. B., and Murthy S. N.. . 2000. Butyrate and glucose metabolism by colonocytes in experimental colitis in mice. Gut. 46:493–499. doi: 10.1136/gut.46.4.493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustin, M. A., Abeywardena M. Y., Patten G., Head R., Lockett T., De Luca A., and Sanguansri L.. . 2011. Effects of microencapsulation on the gastrointestinal transit and tissue distribution of a bioactive mixture of fish oil, tributyrin and resveratrol. J. Funct. Foods 3:25–37. doi: 10.1016/j.jff.2011.01.003 [DOI] [Google Scholar]
- Bartholome, A. L., Albin D. M., Baker D. H., Holst J. J., and Tappenden K. A.. . 2004. Supplementation of total parenteral nutrition with butyrate acutely increases structural aspects of intestinal adaptation after an 80% jejunoileal resection in neonatal piglets. J. Parenter. Enter. Nutr. 28:210–223. doi: 10.1177/0148607104028004210 [DOI] [PubMed] [Google Scholar]
- Biton, I. E., Stettner N., Brener O., Erez A., Harmelin A., and Garbow J. R.. . 2018. Assessing mucosal inflammation in a DSS-induced colitis mouse model by MR colonography. Tomogr. (Ann Arbor, Mich.) 4:4–13. doi: 10.18383/j.tom.2017.00021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornick, S., Tawiah A., and Chadee K.. . 2015. Roles and regulation of the mucus barrier in the gut. Tissue Barriers. 3: 1–2. doi: 10.4161/21688370.2014.982426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Preter, V., Arijs I., Windey K., Vanhove W., Vermeire S., Schuit F., Rutgeerts P., and Verbeke K.. . 2012. Impaired butyrate oxidation in ulcerative colitis is due to decreased butyrate uptake and a defect in the oxidation pathway. Inflamm. Bowel Dis. 18:1127–1136. doi: 10.1002/ibd.21894 [DOI] [PubMed] [Google Scholar]
- Dong, L., Zhong X., He J., Zhang L., Bai K., Xu W., Wang T., and Huang X.. . 2016. Supplementation of tributyrin improves the growth and intestinal digestive and barrier functions in intrauterine growth-restricted piglets. Clin. Nutr. 35:399–407. doi: 10.1016/j.clnu.2015.03.002 [DOI] [PubMed] [Google Scholar]
- Donovan, J. D., Cadwallader K. R., and Lee Y.. . 2016. Volatile retention and morphological properties of microencapsulated tributyrin varied by wall material and drying method. J. Food Sci. 81:E643–E650. doi: 10.1111/1750-3841.13243 [DOI] [PubMed] [Google Scholar]
- Donovan, J. D., Bauer L., Fahey G. C., and Lee Y.. . 2017. In vitro digestion and fermentation of microencapsulated tributyrin for the delivery of butyrate. J. Food Sci. 82:1491–1499. doi: 10.1111/1750-3841.13725 [DOI] [PubMed] [Google Scholar]
- Fil, J. E., Joung S., Hayes C. A., and Dilger R. N.. . 2021. Influence of rearing environment on longitudinal brain development, object recognition memory, and exploratory behaviors in the domestic pig (Sus scrofa). Front. Neurosci. 15:1–16. doi: 10.3389/fnins.2021.649536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feuerstein, J. D., and Cheifetz A. S.. . 2014. Ulcerative colitis: epidemiology, diagnosis, and management. Mayo Clin. Proc. 89:1553–1563. doi: 10.1016/j.mayocp.2014.07.002 [DOI] [PubMed] [Google Scholar]
- Gajendran, M., Loganathan P., Jimenez G., Catinella A. P., Ng N., Umapathy C., Ziade N., and Hashash J. G.. . 2019. A comprehensive review and update on ulcerative colitis. Dis. Mon 65:100851. doi: 10.1016/j.disamonth.2019.02.004 [DOI] [PubMed] [Google Scholar]
- Grosu, I. A., Pistol G. C., Marin D. E., Cişmileanu A., Palade L. M., and Ţăranu I.. . 2020. Effects of dietary grape seed meal bioactive compounds on the colonic microbiota of weaned piglets with dextran sodium sulfate-induced colitis used as an inflammatory model. Front. Vet. Sci. 7:1–14. doi: 10.3389/fvets.2020.00031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guevarra, R. B., Lee J. H., Lee S. H., Seok M. J., Kim D. W., Kang B. N., Johnson T. J., Isaacson R. E., and Kim H. B.. . 2019. Piglet gut microbial shifts early in life: causes and effects. J. Anim. Sci. Biotechnol. 10:1–10. doi: 10.1186/s40104-018-0308-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Head, K., and Jurenka J.. . 2003. Inflammatory bowel disease part I: ulcerative colitis - pathophysiology and conventional and alternative treatment options. Altern. Med. Rev. 8:247–283. [PubMed] [Google Scholar]
- Hou, Y., Wang L., Yi D., Ding B., Chen X., Wang Q., Zhu H., Liu Y., Yin Y., Gong J., . et al. 2014. Dietary supplementation with tributyrin alleviates intestinal injury in piglets challenged with intrarectal administration of acetic acid. Br. J. Nutr. 111:1748–1758. doi: 10.1017/S0007114514000038 [DOI] [PubMed] [Google Scholar]
- Kang, S. H., Song Y. J., Jeon Y. D., Kim D. K., Park J. H., Soh J. R., Lee J. H., Kitalong C., Kim W., An H. J., . et al. 2021. Comparative study of anti-inflammatory effect on DSS-induced ulcerative colitis between novel glycyrrhiza variety and official compendia. Appl. Sci. 11:1–14. doi: 10.3390/app11041545 [DOI] [Google Scholar]
- Knauer, M., Stalder K. J., Karriker L., Baas T. J., Johnson C., Serenius T., Layman L., and McKean J. D.. . 2007. A descriptive survey of lesions from cull sows harvested at two Midwestern U.S. facilities. Prev. Vet. Med. 82:198–212. doi: 10.1016/j.prevetmed.2007.05.017 [DOI] [PubMed] [Google Scholar]
- Kotunia, A., Woliński J., Laubitz D., Jurkowska M., Romé V., Guilloteau P., and Zabielski R.. . 2004. Effect of sodium butyrate on the small intestine development in neonatal piglets fed [correction of feed] by artificial sow. J. Physiol. Pharmacol. 55:59–68 [PubMed] [Google Scholar]
- Kruse, R., Essén-Gustavsson B., Fossum C., and Jensen-Waern M.. . 2008. Blood concentrations of the cytokines IL-1beta, IL-6, IL-10, TNF-alpha and IFN-gamma during experimentally induced swine dysentery. Acta Vet. Scand. 50:1–7. doi: 10.1186/1751-0147-50-32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lackeyram, D., Mine Y., Archbold T., and Fan M. Z.. . 2012. The small intestinal apical hydrolase activities are decreased in the piglet with bowel inflammation induced by dextran sodium sulfate. J. Anim. Sci. 90:287–289. doi: 10.2527/jas.54010 [DOI] [PubMed] [Google Scholar]
- Leonel, A. J., Teixeira L. G., Oliveira R. P., Santiago A. F., Batista N. V., Ferreira T. R., Santos R. C., Cardoso V. N., Cara D. C., Faria A. M. C., . et al. 2013. Antioxidative and immunomodulatory effects of tributyrin supplementation on experimental colitis. Br. J. Nutr. 109:1396–1407. doi: 10.1017/S000711451200342X [DOI] [PubMed] [Google Scholar]
- Leonel, A. J., Silva E. L., Aguilar E. C., Teixeira L. G., Oliveira R. P., Faria A. M. C., Cara D. C., Ferreira L. A. M., and Alvarez-Leite J. I.. . 2016. Systemic administration of a nanoemulsion with tributyrin reduces inflammation in experimental colitis. Eur. J. Lipid Sci. Technol. 118:157–164. doi: 10.1002/ejlt.201400359 [DOI] [Google Scholar]
- Li, C., Li Z., Liu T., Gu Z., Ban X., Tang X., Hong Y., Cheng L., and Li Z.. . 2021. Encapsulating tributyrin during enzymatic cyclodextrin synthesis improves the solubility and bioavailability of tributyrin. Food Hydrocoll. 113:1–7. doi: 10.1016/j.foodhyd.2020.106512 [DOI] [Google Scholar]
- Ma, X., Fan P. X., Li L. S., Qiao S. Y., Zhang G. L., and Li D. F.. . 2012. Butyrate promotes the recovering of intestinal wound healing through its positive effect on the tight junctions. J. Anim. Sci. 90:266–268. doi: 10.2527/jas.50965 [DOI] [PubMed] [Google Scholar]
- Mallo, J. J., Balfagón A., Gracia M. I., Honrubia P., and Puyalto M.. . 2012. Evaluation of different protections of butyric acid aiming for release in the last part of the gastrointestinal tract of piglets. J. Anim. Sci. 90:227–229. doi: 10.2527/jas.53959 [DOI] [PubMed] [Google Scholar]
- Nesterova, A. P., Klimov E., Zharkova M., Sozin S., Sobolev V., Ivanikova N., Shkrob M., and Yuryev A.. . 2020. Diseases of the digestive system. In: Nesterova, A. P., Klimov E., Zharkova M., Sozin S., Sobolev V., Ivanikova N., Shkrob M., and Yuryev A., edtiors. Disease pathways. 1st ed. Elsevier, Cambridge, MA; p 443–491. [Google Scholar]
- Ordás, I., Eckmann L., Talamini M., Baumgart D. C., and Sandborn W. J.. . 2012. Ulcerative colitis. Lancet. 380:1606–1619. doi: 10.1016/S0140-6736(12)60150-0 [DOI] [PubMed] [Google Scholar]
- Panah, F. M., Lauridsen C., Højberg O., and Nielsen T. S.. . 2021. Etiology of colitis-complex diarrhea in growing pigs: a review. Animals. 11:1–22. doi: 10.3390/ani11072151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piva, A., Morlacchini M., Casadei G., Gatta P. P., Biagi G., and Prandini A.. . 2002. Sodium butyrate improves growth performance of weaned piglets during the first period after weaning. Ital. J. Anim. Sci. 1:35–41. doi: 10.4081/ijas.2002.35 [DOI] [Google Scholar]
- Pluske, J. R. 2016. Invited review: Aspects of gastrointestinal tract growth and maturation in the pre- and postweaning period of pigs. J. Anim. Sci. 94:399–411. doi: 10.2527/jas2015-9767 [DOI] [Google Scholar]
- Ren, W., Yin J., Wu M., Liu G., Yang G., Xion Y., Su D., Wu L., Li T., Chen S., . et al. 2014. Serum amino acids profile and the beneficial effects of L-arginine or L-glutamine supplementation in dextran sulfate sodium colitis. PloS One. 9:e88335. doi: 10.1371/journal.pone.0088335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartor, R. B. 2006. Mechanisms of disease: pathogenesis of Crohn’s disease and ulcerative colitis. Nat. Clin. Pract. Gastroenterol. Hepatol. 3:390–407. doi: 10.1038/ncpgasthep0528 [DOI] [PubMed] [Google Scholar]
- Segain, J. P., Galmiche J. P., Raingeard De La Blétière D., Bourreille A., Leray V., Gervois N., Rosales C., Ferrier L., Bonnet C., and Blottière H. M.. . 2000. Butyrate inhibits inflammatory responses through NFκB inhibition: implications for Crohn’s disease. Gut. 47:397–403. doi: 10.1136/gut.47.3.397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi, X., Monaco M. H., Donovan S. M., and Lee Y.. . 2020. Encapsulation of tributyrin by gamma-cyclodextrin: Complexation, spray drying, and in vitro fermentation. J. Food Sci. 85:2986–2993. doi: 10.1111/1750-3841.15440 [DOI] [PubMed] [Google Scholar]
- Shi, X., and Lee Y.. . 2020. Encapsulation of tributyrin with whey protein isolate (WPI) by spray-drying with a three-fluid nozzle. J. Food Eng. 281:109992. doi: 10.1016/j.jfoodeng.2020.109992. [DOI] [Google Scholar]
- Simeoli, R., Mattace Raso G., Pirozzi C., Lama A., Santoro A., Russo R., Montero-Melendez T., Berni Canani R., Calignano A., Perretti M., . et al. 2017. An orally administered butyrate-releasing derivative reduces neutrophil recruitment and inflammation in dextran sulphate sodium-induced murine colitis. Br. J. Pharmacol. 174:1484–1496. doi: 10.1111/bph.13637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotira, S., Dell’Anno M., Caprarulo V., Hejna M., Pirrone F., Callegari M. L., Tucci T. V., and Rossi L.. . 2020. Effects of tributyrin supplementation on growth performance, insulin, blood metabolites and gut microbiota in weaned piglets. Animals. 10:1–16. doi: 10.3390/ani10040726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunkara, L. T., Achanta M., Schreiber N. B., Bommineni Y. R., Dai G., Jiang W., Lamont S., Lillehoj H. S., Beker A., Teeter R. G., . et al. 2011. Butyrate enhances disease resistance of chickens by inducing antimicrobial host defense peptide gene expression. PLoS One. 6. doi: 10.1371/journal.pone.0027225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibault, R., Blachier F., Darcy-Vrillon B., De Coppet P., Bourreille A., and Segain J. P.. . 2010. Butyrate utilization by the colonic mucosa in inflammatory bowel diseases: a transport deficiency. Inflamm. Bowel Dis. 16:684–695. doi: 10.1002/ibd.21108 [DOI] [PubMed] [Google Scholar]
- Thomson, J. 2009. Feed-associated colitis of growing pigs and its interaction with enteric infections. Acta Sci. Vet. 37:1–10 [Google Scholar]
- Tugnoli, B., Piva A., Sarli G., and Grilli E.. . 2020. Tributyrin differentially regulates inflammatory markers and modulates goblet cells number along the intestinal tract segments of weaning pigs. Livest. Sci. 234:103996. doi: 10.1016/j.livsci.2020.103996 [DOI] [Google Scholar]
- Velazquez, O. C., Lederer H. M., and Rombeau J. L.. . 1996. Butyrate and the colonocyte. In: Kritchevsky, D. and Bonfield C., editors. Advances in experimental biology. 427th ed.New York: Plenum Press; p. 123–134. [Google Scholar]
- Vieira, E. L. M., Leonel A. J., Sad A. P., Beltrão N. R. M., Costa T. F., Ferreira T. M. R., Gomes-Santos A. C., Faria A. M. C., Peluzio M. C. G., Cara D. C., . et al. 2012. Oral administration of sodium butyrate attenuates inflammation and mucosal lesion in experimental acute ulcerative colitis. J. Nutr. Biochem. 23:430–436. doi: 10.1016/j.jnutbio.2011.01.007 [DOI] [PubMed] [Google Scholar]
- Wang, C. C., Wu H., Lin F. H., Gong R., Xie F., Peng Y., Feng J., and Hu C. H.. . 2018. Sodium butyrate enhances intestinal integrity, inhibits mast cell activation, inflammatory mediator production and JNK signaling pathway in weaned pigs. Innate Immun. 24:40–46. doi: 10.1177/1753425917741970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, C., Shen Z., Cao S., Zhang Q., Peng Y., Hong Q., Feng J., and Hu C.. . 2019. Effects of tributyrin on growth performance, intestinal microflora and barrier function of weaned pigs. Anim. Feed Sci. Technol. 258:1–7. doi: 10.1016/j.anifeedsci.2019.114311 [DOI] [Google Scholar]
- Weber, T. E., and Kerr B. J.. . 2006. Butyrate differentially regulates cytokines and proliferation in porcine peripheral blood mononuclear cells. Vet. Immunol. Immunopathol. 113:139–147. doi: 10.1016/j.vetimm.2006.04.006 [DOI] [PubMed] [Google Scholar]
- Weber, T. E., and Kerr B. J.. . 2008. Effect of sodium butyrate on growth performance and response to lipopolysaccharide in weanling pigs. J. Anim. Sci. 86:442–450. doi: 10.2527/jas.2007-0499. [DOI] [PubMed] [Google Scholar]
- Weinstein, S., and Sajewicz A.. . 2013. Colon and rectum inflammatory and infectious diseases. In: Hamm, B. and Pablo R. R., editors. Abdominal imaging. Heidelberg, Berlin: Springer, Berlin; p. 797–816. [Google Scholar]
- Xu, H. M., Huang H. L., Di Liu Y., Zhu J. Q., Zhou Y. L., Chen H. T., Xu J., Zhao H. L., Guo X., Shi W., . et al. 2021. Selection strategy of dextran sulfate sodium-induced acute or chronic colitis mouse models based on gut microbial profile. BMC Microbiol. 21:1–14. doi: 10.1186/s12866-021-02342-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, K., Canalias F., Solà-Oriol D., Arroyo L., Pato R., Saco Y., Terré M., and Bassols A.. . 2019. Age-related serum biochemical reference intervals established for unweaned calves and piglets in the post-weaning period. Front. Vet. Sci. 6:1–12. doi: 10.3389/fvets.2019.00123 [DOI] [PMC free article] [PubMed] [Google Scholar]