Abstract
Introduction
Percutaneous transfemoral access approach for the transcatheter aortic valve implantation (TAVI) is still associated with significant vascular complications. Hence, evaluation of best techniques for the reduction of vascular injury via the femoral access remains a key subject of research.
Aim
We report on a single centre’s experience with TAVI performed via the Femoral Artery Minimal Surgical Access (MSA) and percutaneous approach (PC). The primary endpoints were to evaluate the incidents of vascular complications by comparing the MSA versus the PC approach according to the VARC-2 criteria. The secondary endpoint included the impact of vascular complications on the in-hospital 30-day mortality and morbidity.
Material and methods
Between June 2010 and September 2020, two hundred and thirty-seven consecutive patients who underwent TAVI for severe symptomatic aortic stenosis in our department were divided into two groups: patients treated using the femoral artery minimal surgical access (n = 173), and patients treated using the percutaneous approach (n = 64).
Results
Overall rate of access site complications according the VARC-2 were significantly more frequent in the percutaneous cohort (n = 12/64, 18.8% vs n = 2/173, 1.1%, p = 0.0012). The minor access complications including haematoma, bleeding, aneurysm, dissection, stenosis, seroma and infection were more frequent in the PC group (n = 8/64, 12.5% vs n = 2/173, 1.1%, p < 0.001). There were no major access site complications and hospital deaths in the MSA group, which was statistically significant (p < 0.001). Major access complications (n = 4, 6.3%, p < 0.001) and hospital death (n = 2, 3.1%, p < 0.001) were found in the PC cohort.
Conclusions
The minimal surgical access approach provided direct and controlled access and significantly reduced the incidence of access site vascular complications in our TAVI patients. It also significantly reduced the in-hospital vascular-related mortality and morbidity. Though both approaches are complementary to each other, minimal surgical access approach would be a better choice for a calcified or tortuous femoral artery, and for a relatively small femoral artery diameter.
Keywords: Aortic stenosis, Vascular complications, TAVI, Aortic valve replacement
1. Background
Transcatheter aortic valve implantation (TAVI) has emerged as an essential therapeutic option in the management of severe, symptomatic aortic valve stenosis initially in patients at high risk for open surgical replacement. Since the PARTNER trial demonstrated that TAVI is not inferior to surgical aortic valve replacement (AVR) and showed similar mortality at 30 days and up to two years in surgical high-risk patients, the indication of TAVI progressed to include patients with lower surgical risk [1].
In the early days, there was no standardized criteria to define vascular complications (VC) until the Valve Academic Research Consortium (VARC-1 and VARC-2) established [2,3] and provided a standardized reference to researchers. Vascular complication rates were comparatively high in the early days of TAVI.
Despite the evolution of TAVI valves by (1) reducing their profile, (2) the improvement of the delivery system to reduce the sheath diameter sizing down from 22 to 24 to 14 French, (3) the increasing experience of the physicians’ implanting techniques, (4) the use of an assisting imaging tool to visualize the access site and (5) the improvement of vascular closure device, vascular complications remain a major concern for the operators. VC in TAVI are associated with increased in-hospital morbidity and mortality [4,5,10,12]. Mach et al. reported a higher hospital mortality in the percutaneous group (5.2% vs 1.9%, p = 0.075) and Olasińska-Wiśniewska et al. demonstrated similar results with a significantly higher in-hospital mortality (8.8% vs 1.5%, p = 0.1) for the PC group.
The transfemoral route remains the preferred access for the TAVI valve delivered either through percutaneous or surgical access. The risks and benefits of each access strategy were studied extensively. Recent studies reported surgical access provided controlled access and resulted in significantly lower minor VC even though there was no major VC difference [7,8,10,13]. However, Olasińska-Wiśniewska et al. reported that both minor and major access site complications were significantly decreased in the surgical cohort (35.5% vs 7.5%, p = 0.0012) and both minor and major access site complications were significantly less frequent in cut-down group (p = 0.04 and 0.016 respectively). A few studies reported that percutaneous approach complementary with surgical access [7,10,13] was less invasive with a shorter hospital length of stay [11].
The aim of this observational retrospective study is to report our single center experience and outcomes with TAVI performed through femoral access site by either percutaneous approach or minimal surgical access.
2. Method
2.1. Patient population
All patients undergoing elective transfemoral TAVI for severe aortic valve stenosis between June 2010 until September 2020 at the Department of Cardiac Science, King Abdulaziz Cardiac Centre, Riyadh, Saudi Arabia were retrospectively analyzed. Two hundred and thirty-seven (n = 237) patients were enrolled in this study; early-learning experience before June 2010 was excluded. The study population was divided into two groups according to the vascular access protocol: (Fig. 1) minimal surgical access (MSA) (n = 173) and percutaneous transfemoral access (PC) (n = 64). A multidisciplinary heart team consisting of a structural interventional cardiologist, a TAVI certified cardiac surgeon and an echocardiographer assessed all cases considering the calculated perioperative risk using the logistic EuroSCORE II as well as the patients’ characteristics for a consensus therapeutic strategy. The local Ethics Committee approved the study (RC19/425/R20191127) and written informed consent was obtained from all patients before the procedure.
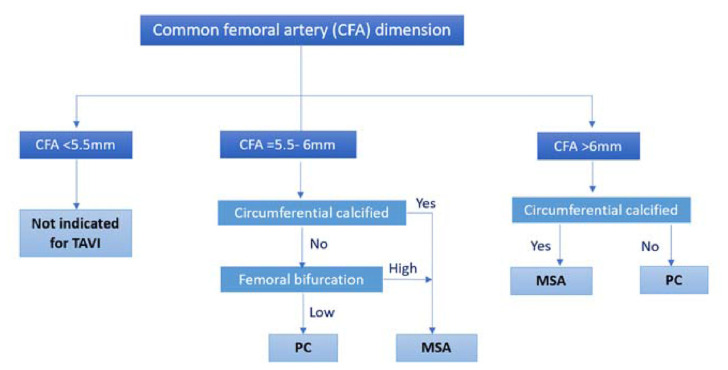
Fig. 1.
Flow diagram for patient selection to undergo percutaneous transfemoral access (PC) or femoral artery minimal surgical access (MSA) TAVR procedure.
2.2. Pre-operative screening imaging and vascular access protocol
The selection of vascular access was done by the heart team through detailed reviewing of all the routine pre-operative imaging. According to our vascular access protocol (Fig. 1), a minimum of 5.5 mm vessel diameter was considered suitable for TAVI enrollment.
The pre-operative screening consisted of the use of multidetector computed tomography (MDCT) and angiography examination which was performed using the dual Source CT (Somatom Definition, Siemens) to assess the accuracy of vascular anatomy. MDCT provides clear and complete three-dimensional assessment of the iliofemoral system, multiple plane reconstructions of aortic annulus measurements, extent of vessel calcification and tortuosity, and the morphology of the entire aorta. The vessel diameter was defined as the distance between the internal vessel walls. In the case of a heavily calcified vessel, the actual diameter was the vessel lumen.
In the percutaneous transfemoral access (PC) cohort, the common femoral artery (CFA) was the access site of choice for all the PC TAVI procedures. Circumferential calcified and high femoral bifurcations were excluded in this cohort. PC was performed in the standard fashion using the Seldinger technique. Hemostasis after sheath removal was achieved using the double Perclose Proglide 6F Suture-Mediated Closure System (Abbott Vascular, Santa Clara, CA, USA).
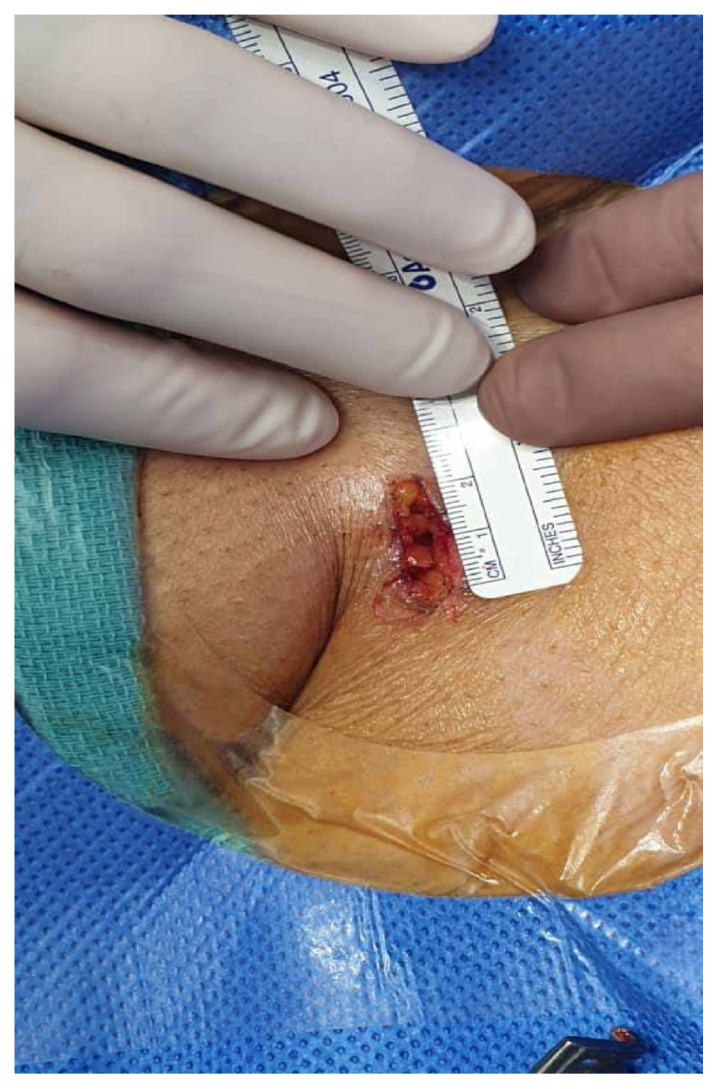
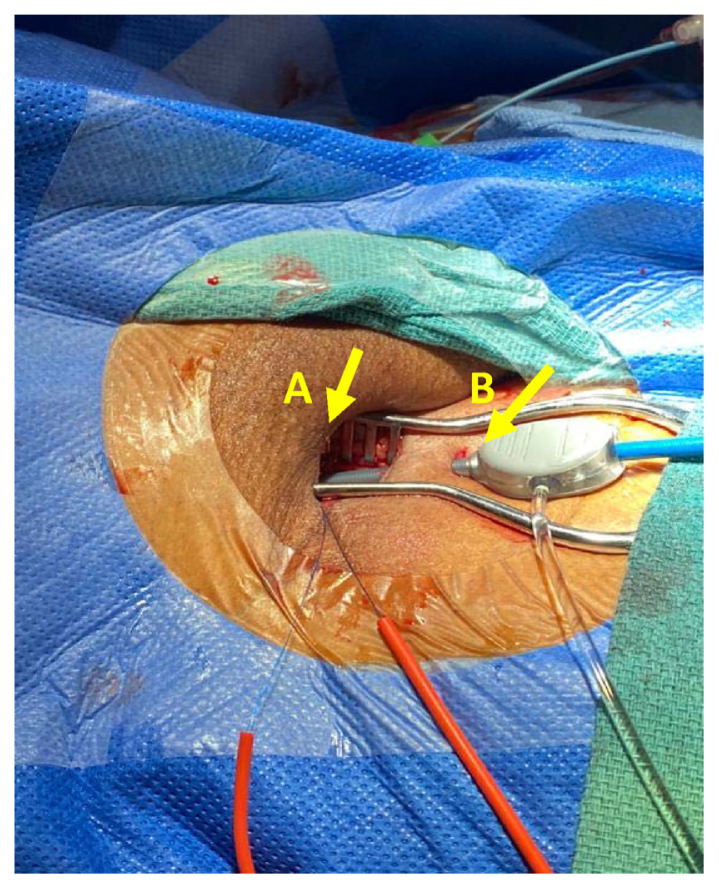
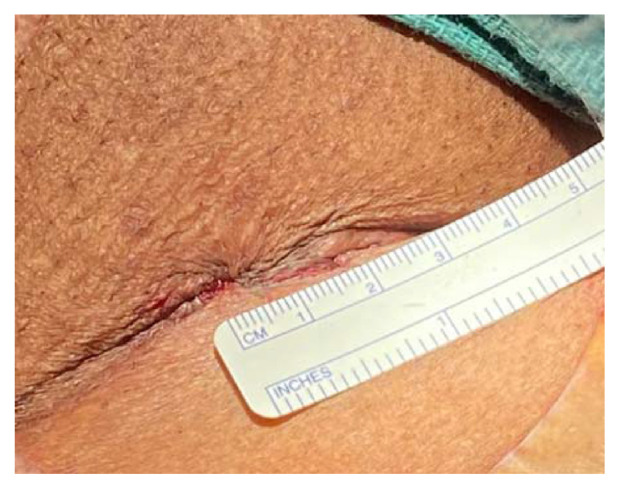
The femoral artery minimal surgical access (MSA) cohort was led by a cardiac surgeon. A 15–20 mm minimal incision was made starting distally at the inguinal ligament and directly over the femoral artery (Fig. 2). Subcutaneous tissue was carefully and minimally dissected, and the femoral artery was prepared by placing e a purse-string suture at a non-calcified site using 5/0 polypropylene suture as indicated in Fig. 3 site A. Vascular access was gained through a separate skin puncture as indicated in Fig. 3 site B and puncture of the femoral artery under direct visualization. The femoral artery puncture site was gradually dilated using vascular dilators 7, 9 and 12 French sequentially. A 14, 16 or 18 French Medtronic sheath then inserted. The sutures were tied after the removal of the Valve delivery system and haemostasis was secured (Fig. 4).
Fig. 2.
The view of the minimal incision dimension.
Fig. 3.
A. The minimal access incision (A) with a separate access (B) for the delivery system.
Fig. 4.
The final skin closure.
2.3. Prosthesis size selection
The final decision on implanted prosthesis size was left at the discretion of the physicians performing the procedure based on MDCT aortic annulus measurements.
Medtronic self-expanding CoreValve™ with sizes 26 & 29 mm, Evolut™ R with sizes 23, 26, 29 mm and Evolut™ PRO with sizes 23, 26, 29 mm (Medtronic, Minneapolis, MN, USA) transcatheter aortic valve implantation (TAVI) devices were implanted in our patient cohort.
An 18 French delivery system (n = 15, 5.5%) was used for CoreValve™ with sizes 26 & 29 mm. Only a 14 French delivery system (n = 214, 78.4%) was used for Evolut™ R with valve sizes 23, 26, 29 mm, and a 16 French delivery system (n = 8, 2.9%) was used for Evolut™ PRO with valve sizes 23, 26, 29mm(Table 1).
Table 1.
Prosthesis and sheath size used in TAVI group.
| Valve Brand | Patient number (%) | Sheath size (French) | Vascular access | |
|---|---|---|---|---|
|
| ||||
| MSA | PC | |||
| CoreValve® | 15 (5.5%) | 18 | 15 (100%) | 0 (0%) |
| Evolut™ R | 214 (78.4%) | 14 | 152 (71%) | 62 (29%) |
| Evolut™ PRO | 8 (2.9%) | 16 | 6 (75%) | 2 (25%) |
| Total number | 237 (100%) | 173 (73%) | 64 (27%) | |
2.4. Study endpoints
The clinical endpoints of this study were categorized using Valve Academic Research Consortium (VARC-2) criteria [3]. The primary endpoints were to evaluate the incidents of vascular complications by comparing MSA versus the PC approach according to the VARC-2 criteria. The secondary endpoint included the impact of vascular complications on the in-hospital 30 days mortality and morbidity.
2.5. Statistical analysis
Statistical analysis was undertaken using SPSS. Continuous variables, presented as means and standard deviation (±SD) or median and interquartile range, and continuous variables were analysed using the unpaired t-test. Categorical variables as absolute numbers and percentages and categorical variables were analysed with Fisher’s exact test. A p value of <0.05 was considered statistically significant for all analyses.
3. Results
The baseline characteristics of the study population are displayed in Table 2. The study population enrolled less patients in the percutaneous transfemoral access group compared to the minimal surgical access group (n = 173, 73% vs n = 64, 27%). The study cohort comprised an almost equal distribution between males (51.6% in PC and 46.2% in MSA) and females (48.4% in PC and 53.8% in MSA) with a mean age of 72 and 74 years for the PC and MSA group respectively.
Table 2.
Baseline patient characteristics for the PC and MSA group.
| Characteristics | PC Group (n = 64) | MSA Group (n = 173) | P value | |
|---|---|---|---|---|
| Mean age (Years) | 72 (68–77) | 74 (70–79) | 0.965 | |
| Gender | ||||
| Male no. (%) | 33 (51.6%) | 80 (46.2%) | 0.644 | |
| Female no. (%) | 31 (48.4%) | 93 (53.8%) | 0.633 | |
| BMI (Kg/m2) | 28.9 (24.8–33.6) | 35.2 (30.2–39.7) | 0.586 | |
| Diabetes Mellitus Type II no.(%) | 34 (53.1%) | 107 (61.8%) | 0.039 | |
| Hypertension no.(%) | 45 (70.3%) | 109 (63.0%) | 0.345 | |
| Prior CABG/PCI no.(%) | 15 (23.4%) | 37 (21.4%) | 0.696 | |
| CHF no.(%) | 7 (10.9%) | 40 (23.1%) | 0.430 | |
| NYHA Class no. (%) | I | 11 (17.1%) | 24 (13.8%) | 0.530 |
| II | 27 (42.2%) | 64 (37.0%) | 0.659 | |
| III | 18 (28.1%) | 69 (40.0%) | 0.046 | |
| IV | 8 (12.6%) | 16 (9.2%) | 0.582 | |
| Cerebro vascular event no.(%) | 2 (3.1%) | 9 (5.2%) | 0.960 | |
| Euroscore II (mean ± SD) | 6.86 ± 2.15 | 5.47 ± 1.48 | 0.988 |
BM:I body mass index ≥35 is considered as obese, CABG: coronary–artery bypass grafting, PCI: percutaneous coronary intervention, CHF: Congestive Heart Failure, NYHA: New York Heart Association.
The baseline characteristics of our patient groups were comparable. There were no significant differences between the two groups in obesity level, hypertension, prior coronary artery bypass grafting, prior percutaneous coronary intervention, congestive heart failure, cerebrovascular event and Euro-Score II. Incidence of diabetes mellitus and NYHA class III was higher in minimal surgical access group compared to the percutaneous transfemoral access group (61.8% vs 53.1%, p = 0.039) and (40% vs 28.1%, p = 0.046) respectively. Approximately 47% (n = 111/273) of patients were in New York Heart Association functional class III/IV. More patients in the MSA group were in NYHA class III/IV (49.2% in MSA vs 40.7% in PC, n = 85 vs 26). The comparison of baseline clinical characteristics revealed a significantly higher risk profile in the surgical group, which was expressed by higher NYHA class III/IV and higher number of diabetic patients.
A total of 237 valves were implanted; Evolut™ R valves were mainly (78.4%) used followed by CoreValve® (5.5%) and Evolut™ PRO (2.9%). 14 French sheath size (n = 214, 90.3%) were mainly used in both the MSA and PC cohort (n = 152, 87.9% vs n = 62, 96.9%). MSA (73% vs 27%) was a predominant strategy in our study.
Overall rate of access site complications according the VARC-2 were significantly more frequent in the percutaneous cohort (n = 12/64, 18.8% vs n = 2/173, 1.1%, p = 0.0012) (Table 3). The minor access complications including haematoma, bleeding, aneurysm, dissection, stenosis, seroma and infection were more frequent in the PC group (n = 8/64, 12.5% vs n = 2/173, 1.1%, p < 0.001). There were no major access site complications or hospital deaths in the MSA group, which was statistically significant (p < 0.001). However, major access complications (n = 4, 6.3%, p < 0.001) and hospital death (n = 2, 3.1%, p < 0.001) were found in the PC cohort.
Table 3.
Major and minor vascular complications (Valve Academic Research Consortium-2 definitions) in the PC and MSA group.
| Event number (%) | PC Group (n = 64) | MSA Group (n = 173) | P value |
|---|---|---|---|
| Access site complications | 12 (18.8%) | 2 (1.1%) | 0.0012 |
| Minor access complication (Haematoma, bleeding, aneurysm, dissection, stenosis, seroma, infection) | 8 (12.5%) | 2 (1.1%) | <0.001 |
| Major access complications retroperitoneal haematoma, ileo-femoral rupture | 4 (6.3%) | 0 | <0.001 |
| Hospital death | 2 (3.1%) | 0 | <0.001 |
4. Discussion
The PARTNER trial [1] found that patients treated with TAVR had 15.3% major VC and 11.9% minor vascular complications within 30 days of the procedure. Olasińska-Wiśniewska et al. (2017) reported access site complication rates significantly decreased from 35.3% to 7.5% between the percutaneous and the cut down group [4]. Genereux et al. reported a vascular complication rate of 11.9% in a meta-analysis with 3519 patients [5]. Czerwin’ska-Jelonkiewicz et al. (2013) found a rate of 53.01% early vascular complications in their cohort in which 20.48% were major and 32.53% were minor incidents [6]. A Polish group reported 20.8% VC in 745 patients (Kochman 2018) while minor VC was significantly higher in the PC cohort (16.8% vs. 9.7%, p < 0.01) [7].
After VARC-2 standardized the definition of reporting the VC outcomes and the newer generation TAVI valves and delivery system were available, VC significantly declined in the recent years. The Abdelaziz et al. group did a meta-analysis review with 5859 patients and reported a major vascular complication of 8.7% and 8.5% in the percutaneous vs cut down group respectively [8]. Pellegrini et al. reported a major vascular complication rate of 8% [9]. Mach et al. showed VARC-2 access complications occurred significantly more often in percutaneous group than the cut down group (20.4% vs 8.5%), and minor complications were significantly higher in the PC compared to cut-down (14.4% vs 2.5%) [10]. Gennari et al. reported 4.5–5.1% minor vascular complications and around 2.7% major complications in their study [11].
The minimal surgical access approach in our study significantly reduced the incidence of access site vascular complications in our TAVI patients, and significantly reduced the in-hospital vascular-related mortality and morbidity.
Our results are consistent with the previous studies that compared the two different vascular access techniques. Spitzer et al. reported that the overall rate of VARC-2 access site complications (20.6% vs 8.1%, P = 0.04) and bleeding complications (18.1% vs. 4.4%; P = 0.029) were significantly less frequent in the cut-down cohort. Minor complications were significantly more frequent in the PC group (p = 0.04).
Mach et al. showed that the overall VARC-2 access complications (p = 0.037) and bleeding complications (p = 0.01) were significantly lower in the cut-down group. Minor complications were significantly higher in the percutaneous group (p = 0.04).
In contrast to the Spitzer et al. and Mach et al. reports which showed that major complications and hospital deaths were not significantly different between two groups (p = 0.19, p = 0.341), the surgical group in our study had significantly lower rate of major complications in MSA (n = 0 vs n = 4, p < 0.001). This finding occurred despite having a higher proportion of patients with diabetes and NYHA class III/VI. The minimal surgical access technique in which the operator gains direct and controlled access to the femoral artery through a separate skin puncture is believed to be the main contributor in the reduction of major complications in our cohort. The small incision, which is less than 20 mm, provided a less invasive access than a typical 20–30 mm cut-down incision and allowed just enough room for the delivery system and the sheath to pass through, minimizing the risk of dissecting the femoral artery particularly, if it is calcified.
Hospital death was significantly higher in our PC cohort (p < 0.001). The two hospital deaths were related directly to the major dissection of the ileo-femoral artery. Direct access to the femoral artery that was obtained in the minimal surgical access technique group resulted in less rupture and bleeding.
The size of delivery sheaths is one of the major risk factors of access site complications. In our study population, approximately 78% (n = 214/237) of 14-French sheaths were used in both groups. Almost all the PC cohort (n = 62/64, 97%) used 14-French sheaths during the TAVI. Despite a relatively smaller sheath used in the PC, the overall vascular site complications are significantly higher (P = 0.0012). The larger 18-French sheaths (n = 15/173, 5.5%) were only used for the MSA cohort and did not result in any major access site complication. The direct controlled access by MSA is believed to have reduced the site complications.
5. Conclusion
The minimal surgical access approach provided a direct and controlled vascular access and significantly reduces the incidence of access site vascular complications in our TAVI patients. It also significantly reduced the in-hospital vascular-related mortality and morbidity. Though both approaches are complementary to each other, minimal surgical access approach would be a better choice for calcified, tortuous, and relatively small femoral artery diameter vessels.
5.1. Limitation
The limitations of our study are due to its retrospective, single center, non-randomized nature. The small number of patients enrolled over a long period of time may affect the operator’s learning experience. Even though the heart team members remained stable throughout the study, there was some bias and preference on the mode of access particularly for some more complex cases. The collected data did not include specific computed tomography findings such as the vessel calcification, diameter and tortuosity, sheath to femoral artery ratios and the post-operative morbidity data, which did not allow assessment of their influence on the outcome in both access strategies.
Abbreviations
- AVR
aortic valve replacement
- MDCT
multidetector computed tomography
- MSA
minimal surgical access
- PC
percutaneous approach
- TAVI
transcatheter aortic valve implantation
- VC
Vascular complication
- VARC
Valve Academic Research Consortium
Footnotes
Conflict of interest
There is no conflict of interest in present study.
Author contribution
Conception: AAA, MB, KA, FAM, HNAK, EM. Literature review: AAA, MB, KA, FAM, HNAK, EM. Methodology: AAA, MB, KA, FAM, HNAK, EM. Software: AAA, MB, KA, FAM, HNAK, EM. Analysis and/or interpretation: AAA, MB, KA, FAM, HNAK, EM. Investigation: AAA, MB, KA, FAM, HNAK, EM. Resources: AAA, MB, KA, FAM, HNAK, EM. Data collection and/or processing: AAA, MB, KA, FAM, HNAK, EM. Writer-original draft: AAA, MB, KA, FAM, HNAK, EM. Writing- review & editing: AAA, MB, KA, FAM, HNAK, EM. Visualization: AAA, MB, KA, FAM, HNAK, EM. Supervision: AAA, MB, KA, FAM, HNAK, EM. Project administration: AAA, MB, KA, FAM, HNAK, EM.
Funding
No funding was received to perform this study.
References
- 1. Généreux P, Webb JG, Svensson LG, Kodali SK, Satler LF, Fearon WF, et al. PARTNER Trial Investigators. Vascular complications after transcatheter aortic valve replacement: insights from the PARTNER (Placement of AoRTic TraNs-cathetER Valve) trial. J Am Coll Cardiol. 2012 Sep 18;60(12):1043–52. doi: 10.1016/j.jacc.2012.07.003. . Epub 2012 Aug 8. [DOI] [PubMed] [Google Scholar]
- 2. Leon MB, Piazza N, Nikolsky E, Blackstone EH, Cutlip DE, Kappetein AP, et al. Standardized endpoint definitions for transcatheter aortic valve implantation clinical trials: a consensus report from the Valve Academic Research Consortium. Eur Heart J. 2011 Jan;32(2):205–17. doi: 10.1093/eurheartj/ehq406. . Epub 2011 Jan 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kappetein AP, Head SJ, Généreux P, Piazza N, van Mieghem NM, et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium-2 consensus document. J Am Coll Cardiol. 2012 Oct 9;60(15):1438–54. doi: 10.1016/j.jacc.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 4. Olasińska-Wiśniewska A, Grygier M, Lesiak M, Araszkiewicz A, Trojnarska O, Komosa A, et al. Femoral artery anatomy-tailored approach in transcatheter aortic valve implantation. Adv Interventional Cardiol. 2017;13(48):150–6. doi: 10.5114/pwki.2017.68050.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Généreux P, Head SJ, Van Mieghem NM, Kodali S, Kirtane AJ, Xu K, et al. Clinical outcomes after transcatheter aortic valve replacement using valve academic research consortium definitions: a weighted meta-analysis of 3,519 patients from 16 studies. J Am Coll Cardiol. 2012 Jun 19;59(25):2317–26. doi: 10.1016/j.jacc.2012.02.022. . Epub 2012 Apr 11. [DOI] [PubMed] [Google Scholar]
- 6. Czerwińska-Jelonkiewicz K, Michałowska I, Witkowski A, Dąbrowski M, Księżycka-Majczyńska E, Chmielak Z, et al. Vascular complications after transcatheter aortic valve implantation (TAVI): risk and long-term results. J Thromb Thrombolysis. 2014 May;37(4):490–8. doi: 10.1007/s11239-013-0996-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kochman J, Kołtowski L, Huczek Z, Rymuza B, Wilimski R, Dąbrowski M, et al. Complete percutaneous approach versus surgical access in transfemoral transcatheter aortic valve implantation: results from a multicentre registry. Kardiol Pol. 2018;76(1):202–8. doi: 10.5603/KP.a2017.0205. . Epub 2017 Nov 13. [DOI] [PubMed] [Google Scholar]
- 8. Abdelaziz HK, Megaly M, Debski M, Rahbi H, Kamal D, Saad M, et al. Meta-analysis comparing percutaneous to surgical access in trans-femoral transcatheter aortic valve implantation. Am J Cardiol. 2020 Apr 15;125(8):1239–48. doi: 10.1016/j.amjcard.2020.01.021. . Epub 2020 Jan 28. [DOI] [PubMed] [Google Scholar]
- 9. Pellegrini C, Rheude T, Trenkwalder T, Mayr NP, Joner M, Kastrati A, et al. One year VARC-2-defined clinical outcomes after transcatheter aortic valve implantation with the SAPIEN 3. Clin Res Cardiol. 2019 Nov;108(11):1258–65. doi: 10.1007/s00392-019-01461-7. . Epub 2019 May 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mach M, Wilbring M, Winkler B, Alexiou K, Kappert U, Delle-Karth G, et al. Cut-down outperforms complete percutaneous transcatheter valve implantation. Asian Cardiovasc Thorac Ann. 2018 Feb;26(2):107–13. doi: 10.1177/0218492318759350. . Epub 2018 Feb 7. [DOI] [PubMed] [Google Scholar]
- 11. Gennari M, Rigoni M, Mastroiacovo G, Trabattoni P, Roberto M, Bartorelli AL, et al. Proper selection does make the difference: a propensity-matched analysis of percutaneous and surgical cut-down transfemoral TAVR. J Clin Med. 2021 Feb 25;10(5):909. doi: 10.3390/jcm10050909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Drafts BC, Choi CH, Sangal K, Cammarata MW, Applegate RJ, Gandhi SK, et al. Comparison of outcomes with surgical cut-down versus percutaneous transfemoral transcatheter aortic valve replacement: TAVR transfemoral access comparisons between surgical cut-down and percutaneous approach. Cathet Cardiovasc Interv. 2018 Jun;91(7):1354–62. doi: 10.1002/ccd.27377. . Epub 2017 Oct 10. [DOI] [PubMed] [Google Scholar]
- 13. Spitzer SG, Wilbring M, Alexiou K, Stumpf J, Kappert U, Matschke K. Surgical cut-down or percutaneous access-which is best for less vascular access complications in transfemoral TAVI? Cathet Cardiovasc Interv. 2016 Aug;88(2):E52–8. doi: 10.1002/ccd.26361. . Epub 2015 Dec 28. [DOI] [PubMed] [Google Scholar]