Abstract
Closed lipid bilayers in the form of giant unilamellar vesicles (GUVs) are commonly used membrane models. Various methods have been developed to prepare GUVs, however it is unknown if all approaches yield membranes with the same elastic, electric, and rheological properties. Here, we combine flickering spectroscopy and electrodefomation of GUVs to measure, at identical conditions, membrane capacitance, bending rigidity and shear surface viscosity of palmitoyloleoylphosphatidylcholine (POPC) membranes formed by several commonly used preparation methods: thin film hydration (spontaneous swelling), electroformation, gel assisted swelling using polyvinyl alcohol (PVA) or agarose, and phase-transfer. We find relatively similar bending rigidity value across all the methods except for the agarose hydration method. In addition, the capacitance values are similar except for vesicles prepared via PVA gel hydration. Intriguingly, membranes prepared by the gel assisted and phase-transfer methods exhibit much higher shear viscosity compared to electroformation and spontaneous swelling, likely due to remnants of polymers (PVA and agarose) and oils (hexadecane and mineral) in the lipid bilayer structure.
1. Introduction
Membranes play a central role in living systems: all cells are encapsulated by membranes; membranes divide the eukaryotic cell into compartments to sequester specific cellular functions; membranes are the sites where many cellular machineries carry out their tasks.1-5 In vitro membrane system such as the giant unilamellar vesicle (GUV), which is a cell-sized closed lipid bilayer provides a well-defined model to assay membrane properties and investigate the membrane biophysics at a fundamental level.6-8 Several methods exist to form GUVs.7,9,10 The oldest reported method, gentle hydration or spontaneous swelling,11 relies on the hydration and continuous swelling of dry lipid films deposited on a solid substrate (e.g glass or Teflon) to form GUVs. The method itself is simple but time consuming, requiring up to around 12-24 hours or more. The electroformation technique introduced by Angelova et al.12 significantly sped up the process by applying a uniform AC electric field, thus enabling GUVs formation in 1-2 hours. However, the difficulty to grow GUVs in highly saline conditions/buffer solutions,13,14 and the risk of lipid oxidation limits the use of this technique.15-17 With the advent of bottom-up approaches in synthetic biology,1 several other methods have been proposed to create complex membranes such as asymmetric bilayers: gel assisted methods, phase-transfer/inverted emulsion method, phase reverse evaporation, microfluidics, continuous droplet interface crossing encapsulation (cDICE) and fusion of liposomes.18-25 Given the library of available methods to form GUVs, it is unknown if all the methods yield lipid bilayers with the same material properties, or whether oxidation, residual oils, solvents, or polymers, modify the membrane thereby impacting biophysical studies.
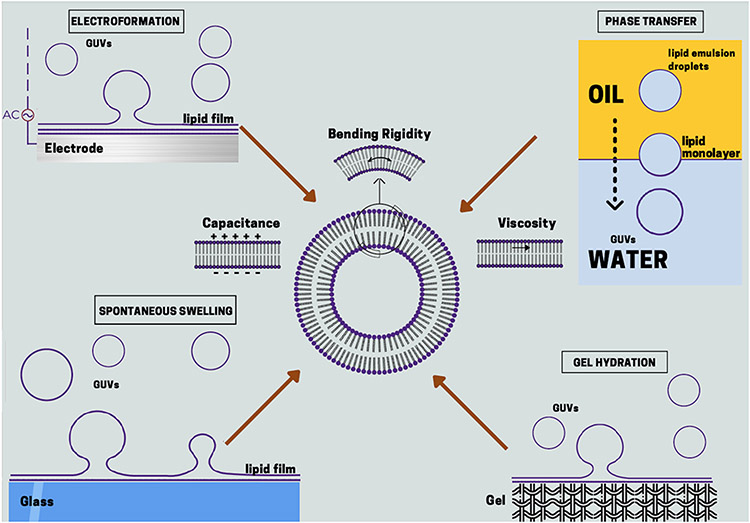
In this work, we compare three fundamental properties, namely bending rigidity, capacitance and shear viscosity, of lipid bilayers formed by four popular methods: spontaneous swelling, electroformation, gel assisted swelling and phase-transfer method. Our study is summarized in Figure 1. For each method we adopted the optimized protocol recommended in the literature and the solution conditions were kept identical for all methods in order to isolate the effect of the preparation approach. The aforementioned properties were measured using two popular non-invasive techniques, flickering spectroscopy and electrodeformation. 26-30
Figure 1:
Summary of the preparation methods chosen for this study. The methods are spontaneous swelling, electroformation, gel hydration and phase-transfer method. The material properties probed in this study are bending rigidity, capacitance and shear viscosity of bilayer membranes.
2. Materials and Methods
2.1. Materials
GUVs were formed from palmitoyloleoylphosphatidylcholine (POPC) purchased from Avanti Polar Lipids (Alabaster, AL). Polyvinyl alcohol (PVA), Mw = 145 kDa was purchased from Merck, Germany. Agarose (A5030, ultra low gelling temperature), hexadecane (H6703), mineral oil (M5904), sucrose and glucose were obtained from Sigma Aldrich, USA. HPLC water (22934 grade) was purchased from Fisher Scientific, USA.
2.2. Preparation methods
2.2.1. Spontaneous swelling (thin film hydration)
Thin film hydration or spontaneous swelling is one of the first methods developed to form GUVs.7,10,11,31,32 Vesicles grow from hydrated bilayer stacks due to a competition between osmotic and intermolecular forces. The method is popular due to the non-interference of external fields such as electric fields that limit preparation to low salinity conditions only. Here, we adopt the optimized preparation protocol suggested by Akashi et al.31 Initially, a 20 ml glass vial is rinsed with isopropanol, chloroform and water in the same order. 50 μl of 6 mM solution of POPC in chloroform is diluted in 200-300 μl of chloroform in a 20 ml vial. Nitrogen stream is blown over the lipid solution while it is mechanically swirled to facilitate solvent evaporation. The vial with deposited lipid film is stored under vacuum for 3 hours. The film is then hydrated with 2 ml 500 mM sucrose solution containing 0.3 mM NaCl and placed at 60 °C in an oven for 12 hours. 20 μl of harvested GUV solution is diluted in 100 μl 450 mM sucrose + 60 mM glucose solution and placed in a chamber assembled from cover slides. To avoid vesicle adhesion to the cover slides prior to introducing the vesicles the slides were incubated with 10 mg/ml bovine serum albumin (BSA, Sigma) for 15 mins and then rinsed with pure water.
2.2.2. Electroformation
In order to facilitate the swelling process, electric fields have been utilized to improve GUVs yield.12 In this approach, dried lipids are spread on two electrodes, and hydrated in the presence of electric fields to enhance the bilayers separation and closing into vesicles. The mechanism underlying the GUV formation using electroformation is still an active topic of research. Most commonly it is accepted that the alternating electric field induces electroosmotic flows to separate and bend the bilayers to form closed membranes.12,33-39 There are two choices for electrodes that are typically used: Indium Tin oxide (ITO) coated glass slides and Platinum (Pt) wires. Different material properties (adhesion forces to lipids, conductivity of electrodes, electrochemical properties) and geometry (flat glass ITO slides vs cylindrical Pt wires) in these two variations could potentially influence the GUV formation process.
Indium tin oxide electrodes
The glass slides (50 mm × 50 mm ITO slides with 50 Ohms resistance, Delta technologies, USA) are cleaned with acetone and isopropanol and triple rinsed with bi-distilled water. The stock solution of POPC in choloroform is diluted to 6 mM from which 7-10 μl of the solution is spread on the conductive sides of each slides using gas-tight glass syringe (Hamilton, USA). The lipid-coated slides are stored in dessicator for 3 hours to evaporate all organic solvents. Then the slides with facing conductive sides are assembled to sandwich a 2 mm thick Teflon spacer and are clipped together. Through a hole in the Teflon spacer, the chamber is gently filled with 2 ml of 500 mM sucrose solution in 0.3 mM of NaCl to avoid film disruption. Next, the conductive side of ITO is connected to a signal generator (Agilent Technology, USA) for 2 hours at 50 Hz and voltage 1.5 Vpp using copper tapes (3M, USA). After electroformation, the vesicles are smoothly aspirated using 1 ml micropipette tips (Eppendorf, Germany). The GUV harvest is diluted for analysis in a similar way like the spontaneous swelling method.
Platinum wires
For electroformation with Platinum (Pt) electrodes, the wires can be placed vertically or horizontally.35,36,38 Here, we utilize the horizontal configuration in order to monitor the vesicles during the electroformation process using a microscope. A home built device consisting of a PVC (polyvinyl chloride) chamber to house the Pt wires was used. The Pt wires are removable from the PVC chamber for cleaning purposes. Coverslips are attached at the bottom of chamber using vacuum grease. Slowly 5-10 μl of lipid solution is spread on both sides of the Pt wires. The device is placed in a dessicator for 3 hours to evaporate all solvents. The chamber is slowly filled with 2 ml of 500 mM sucrose in 0.3 mM of NaCl solution and the Pt wires are directly connected to signal generator (Agilent Technology, USA) for 2 hours at 50 Hz and voltage 1.5 Vpp. The vesicles are smoothly aspirated using 1 ml micropipette tips (Eppendorf, Germany). The GUV harvest is diluted for analysis in a similar way like the spontaneous swelling method.
2.2.3. Gel Assisted Methods
In these methods, polymer based substrate is used to accelerate vesicle growth. Vesicle formation is assisted by buffer influx below the bilayer through the porous polymer substrate to speed up hydration.7,35 We use agarose and polyvinyl alcohol (PVA), which are the two most popular polymer gel templates for vesicle formation.23,24
PVA gel:
This protocol is adapted and optimized from Weinberger et al.24 First, 22 mm × 50 mm microscope cover slides and Teflon spacers are cleaned with HPLC water, isopropyl alcohol and acetone in the same order. 5 % (w/w) solution of PVA is prepared by constantly stirring PVA in water at 90°C. Next, 300 μl of 5% PVA (w/v) in water solution is spread to form thin films without any bubbles. The coverslips are placed at 50°C in the oven for 45 mins to dry the film. Once the gel is dried, a Hamilton syringe, washed with chloroform, is used to spread 7-8 μl of POPC lipid solution evenly onto the surface of the PVA gel at room temperature. The slides are placed in a vacuum for 3 hours at room temperature. Clean Teflon spacers are placed onto the glass slide and secured. The chamber is filled with 2 ml of 500 mM sucrose solution containing 0.3 mM NaCl and sealed with a coverslip. After 30 minutes, the chamber is gently tapped few times and vesicles are smoothly aspirated using 1 ml pipette tips (Eppendorf, Germany). The vesicles are diluted for analysis in a similar way like the spontaneous swelling method.
Agarose:
The method of growing of GUVs from agarose films is adapted from Horger et al.23 22 mm × 50 mm microscope cover slides and Teflon spacers are cleaned with HPLC water and isopropyl alcohol. 1(% w/v) of agarose is prepared in HPLC water above the polymer melting temperature Tm, 52 °C. 300 μl of agarose solution is drawn up with a pipette and spread onto the glass surface. Slides are placed in an oven at 40°C for 2 hours. Once the gel is dried, a Hamilton syringe is cleaned with chloroform and used to spread 7-8 μl of POPC lipid solution onto the surface. Slides are placed in a vacuum for 3 hours. Clean Teflon spacers are then placed on top of the cover slides and secured. The chamber is filled with 2 ml of 500 mM sucrose solutions with 0.3 mM NaCl. The GUV harvest is diluted for analysis in a similar way like the spontaneous swelling method.
2.2.4. Phase-transfer method
The phase-transfer method is a two step process, which offers the possibility to encapsulate material inside the GUV. In the first step, a lipid monolayer (outer leaflet of the bilayer membrane) is formed between the aqueous solution, which would constitute the vesicle suspending medium, and oil. Next, water-in-oil emulsion droplets covered with a lipid monolayer, which would become the inner membrane leaflet, and containing the sugar solution that becomes the interior vesicle solution are passed through the first monolayer via gravitational or centrifugal forces.9
Several factors (sugar density gradients, centrifugal force, volume of inner solution, incubation time, type of oil, humidity) can influence the final yield of GUVs.40 Here, we utilize the optimized experimental protocol by Moga et al.40 The same procedure is used to prepare GUVs from either mineral oil or hexadecane.
Lipids in oil preparation:
Initially the lipid-oil mixture is formed by coating the dried lipid film on a 5 ml round bottom glass tube (Fisher Scientific, USA) by evaporating all the chloroform from 100 μl 6 mM POPC solution under Nitrogen gas. The tube is stored under vacuum for 1-2 hours to evaporate any leftover solvent. 1.5 ml of mineral oil/hexadecane is added to the tube under low humidity conditions (less than 10 %) inside Atmosbag to reach a final lipid in oil concentration of 400 μM. Note that for every lipid-oil preparation step, we open a new 5 ml oil bottle to minimize contamination from humidity. To improve the lipid solubilization in oil, the solution is sonicated for two hours and later incubated overnight at room temperature.
Surface treatment of micro-centrifuge tubes:
The 1.5 ml microcentrifuge tubes (Eppendorf Safe-Lock microcentrifuge tubes, T9661) are incubated for 1 hour with 200 μl of 2 mg/ml BSA solution. This is an important step to avoid adhesion and eventual bursting of GUVs. The tubes are then washed 3 times with glucose solution.
Phase-transfer process:
Initially 200 μl of 510 mM glucose solution is added to the surface treated microcentrifuge tubes. Next, 100 μl of lipids-oil solution is added on top of glucose solution. The entire setup is incubated for 1-2 hours for homogeneous formation of interfacial monolayer of lipids at the oil-water interface (outer vesicle leaflet). In another 1.5 ml microcentrifuge tube 250 μl of lipid-oil solution is added. To this tube, 10 μl of 500 mM sucrose solution in 0.3 mM NaCl is added and mechanically agitated using tube rack 2-3 times. Note that too much agitation can lead to small emulsion droplets, and eventually, small vesicles. An aliquot of 125 μl of the emulsion is pipetted on top of the lipid monolayer. The tubes are centrifuged for 3 mins at 1800 rpm. Next, we punch a hole at the bottom tip of microcentrifuge using a needle and harvest only 100 μl GUV solution with a 1 ml syringe (BD, USA) to prevent oil contamination from top layer. The vesicles are diluted on cover slips for analysis in a similar way like the spontaneous swelling method.
2.3. Characterization methods
2.3.1. Bending rigidity
Bending rigidity of membranes can be measured by a variety of methods.45 Here, we chose the flickering spectroscopy due to its non-invasive data collection and well developed statistical analysis. It can be implemented using either confocal or phase contrast microscopy. We select the latter to avoid the use of guest molecules in the bilayer such as fluorescent markers. A disadvantage of the method is that it requires visible fluctuations which means that very stiff or gel phase membranes cannot be probed. The details of the method can be found in Refs.26,27,30. In essence, using a camera at 60 frames per second (fps) (Photron SA1, USA) and optical microscope (phase contrast Zeiss A1, Germany) a time series of fluctuating vesicle contours imaged at the equatorial cross section is recorded. The fluctuating contour, r(ϕ), is decomposed in Fourier modes, r(ϕ) = R(1 + Σquq(t) exp(iqϕ)), where R is the average radius of the vesicle. The mean square amplitude of the fluctuating Fourier modes, uq, depends on the membrane bending rigidity κ and the tension σ, , where kBT is the thermal energy (kB is the Boltzmann constant and T is the temperature), and . The integration time effect of the camera is minimized by acquiring images at a high shutter speed of 200 μs per image. At least 10,000 images are recorded for each vesicle for good statistics. Since we are interested in bending rigidity measurements, only vesicles with low tension value in the range 10−8 − 10−10 N/m are chosen. This results in a small crossover mode, , to a regime where the shape fluctuations are dominated by bending rigidity.
2.3.2. Membrane Capacitance
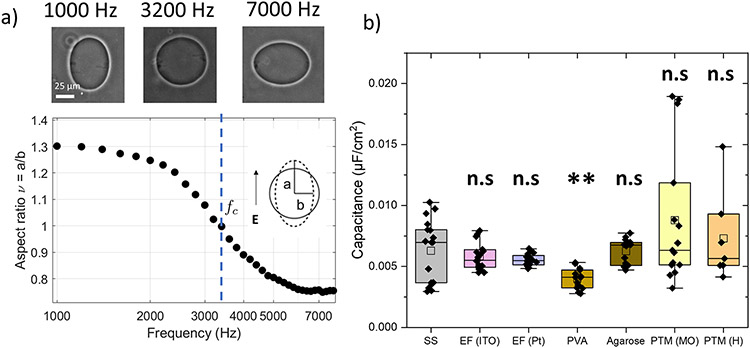
Membrane capacitance is measured using steady state electrodeformation as detailed in Refs.28,41 The vesicle shape with inside-outside conductivity ratio varies with the frequency (1-100 kHz) of an applied AC uniform electric field. At low frequencies, the shape is a prolate ellipsoid. As frequency increases, the ellipsoid aspect ratio ν decreases. At a frequency fc, the shape becomes a sphere (ν = 1). Further increase of the frequency results in oblate ellipsoidal shape ν < 1. The critical frequency, fc depends on the membrane capacitance Cm42,43
| (1) |
Hence, the membrane capacitance, Cm, can be determined from the experimentally measured critical frequency for the prolate-oblate transition during a frequency sweep. The electrodeformation method to measure membrane capacitance has the advantage of being non-invasive, high throughput, probe-free and able to measure membrane capacitance of a wide range of compositions and phase state.28,41 The only limitation with the method is that it cannot be used to measure capacitance for charged membranes (a detailed review can be found elsewhere7). The same pros and cons apply to the transient electrodeformation method for measuring membrane viscosity29.
2.3.3. Membrane Viscosity
We implement the transient electrodeformation of GUVs to measure membrane viscosity.29 To summarize, the method involves measuring the initial deformation rate of a vesicle as an AC electric field is applied at a particular frequency. High speed imaging of the increase of the vesicle aspect ratio, ν, is done at 1-2 kfps. The linear slope of the aspect ratio as a function of time depends on membrane viscosity as
| (2) |
where is the characteristic rate-of-strain imposed by the electric field and χm = ηm/ηR is the dimensionless surface viscosity ηm, η is the viscosity the solution inside and outside the vesicle, Eo is the electric field strength amplitude and p(ω) is forcing field function detailed out in Faizi et al.29 The apparent viscosities are measured at different frequencies in the range 0.1-1 kHz. The zero-frequency viscosity is obtained by extrapolating a linear ht of the viscosity vs frequency data. Electric field of 8 kV/m (strain rate 50 s−1) produces a good range of data in the linear initial slope.
2.3.4. Statistical Analysis
Statistical testing is performed using ANOVA testing for multiple comparison analyses. All data are expressed in terms of mean ± standard deviation, and the number of independent replicates is expressed in the figure captions. The following conventions for statistical significance are used throughout the paper: n.s, p > 0.05; *, p ≤ 0.05, **, p ≤ 0.01; ***, p ≤0.001; ****, p ≤ 0.0001.
3. Results and Discussion
GUVs were prepared under the same conditions to avoid effects on the bilayer properties due to variations in factors such as concentration and type of sugar and salt in the suspending solution, buffers, solution and lipid asymmetry, concentration of fluorescent lipids in the membrane, etc.26,44-50 At least 10-15 vesicles were analyzed for every GUV preparation divided in 2-3 batches. In all methods the vesicles were prepared such that the internal solution contains 500 mM sucrose and 0.3 mM NaCl. This high sugar concentration was imposed by the implementation of the phase transfer method which does not yield high amounts of good quality vesicles at low sugar concentrations40. After harvesting, 20 μl vesicle solution was diluted with 20 μl of a slightly higher osmolarity solution, e.g., 450 mM sucrose + 60 mM glucose, to deflate the vesicles for flickering spectroscopy and electrodeformation experiments. This combination of sugars minimizes effects of gravity on vesicle shape, thereby ensuring vesicles are quasispherical48. Note that with the phase-transfer method the vesicles are formed with 510 mM glucose outside. For every vesicle preparation method, the same vesicle population is utilized for material property measurements. For viscosity and bending rigidity measurements, the harvested vesicles are diluted without any salt outside. For capacitance measurements, 0.6 mM NaCl is added outside. Fluorescent markers were not added to label the membranes as these are known to modify membrane properties, e.g., some dyes cause photo induced lipid oxidation.27,49
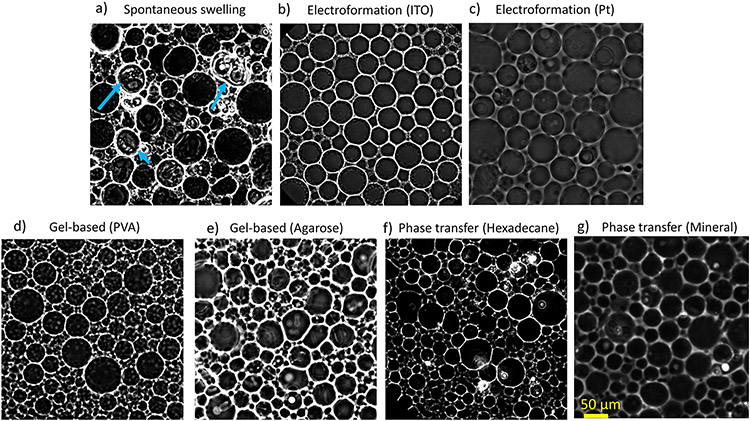
Figure 2 shows the GUVs formed by different methods. The GUVs were diluted with 510 mM glucose solution on cover slips. The vesicles were imaged using phase contrast microscopy after 2 hours to allow for sedimentation. As seen on Figure 2a, spontaneous swelling produces some GUVs with defects such as lipid clump and aggregation, while the other methods yield GUVs that appear defect-free. Some vesicles also had multilayer membranes or nested vesicles, see Figure 2a (blue arrows). These observations are consistent with the results from Refs.31,51 The spontaneous swelling method was chosen as a control experiment although electroformation can serve as an alternative control method.
Figure 2:
Phase contrast images of POPC GUVs produced from different preparation methods. 20 μl of harvested GUVs containing 500 mM sucorse and 0.3 mM NaCl inner solution were diluted in 510 mM glucose solution. The vesicles were imaged after 2 hours of sedimentation time. a) Spontaneous swelling. Blue arrows indicate lipid clumps or debris. b) Electroformation (ITO) c) Electroformation (Pt wire) d) Gel Assisted method (PVA) e) Gel assisted method (Agarose) f) Phase-transfer method (hexadecane) g) Phase-transfer method (mineral oil).
3.1. Bending Modulus
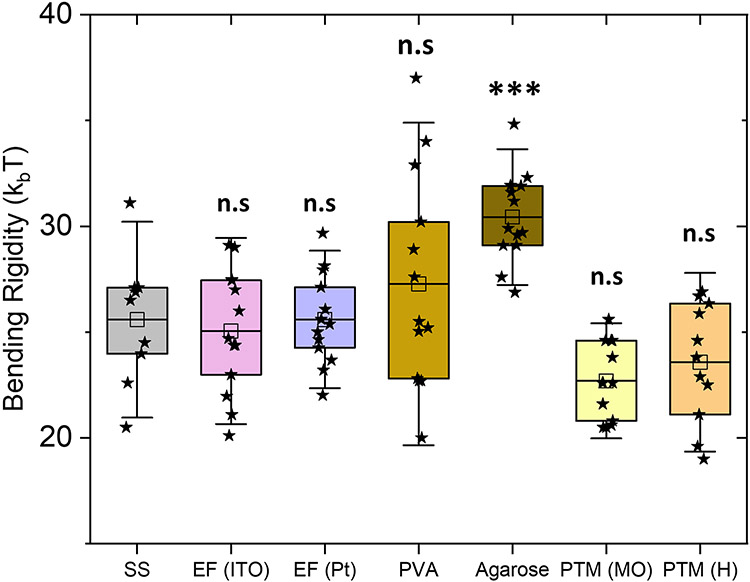
Bending rigidity of bilayers reflects the energy cost to change membrane curvature, which involves compression and expansion of the inner and outer monolayer leaflets, respectively.52,53 This physical property plays an instrumental role in cellular processes involving membrane remodeling.45,54 Figure 3 shows the box plot for bending rigidity of POPC bilayers measured with flickering spectroscopy. The bending rigidity obtained with electroformation (ITO) in this study is 25.5 ± 2.6 kBT in agreement with the literature values, 25 – 28 kBT,26,44,45,50 and in the lower region of this range, consistent with evidence for membrane softening by sugars46,55. We found similar bending rigidity values with four other preparation methods: spontaneous swelling, electroformation (Pt) and phase-transfer method (hexadecane and mineral oil) with statistically insignificant values as obtained by ANOVA test. The results with PVA show an average value 27.3±5.1kBT similar to the one obtained for the spontaneous swelling method, however, with a much wider spread, 20-37 kBT. This suggests that the PVA-gel assisted hydration leads to higher variability in membrane bending rigidity. These findings are consistent with the study from Dao et al56 where modification in another elastic property, the stretching modulus, was observed at similar hydrating conditions. Previously Moga et al.40 and Elani et al44 also found no differences between bending rigidity of GUVs formed from electroformation and phase-transfer method. This rules out effects of the oils used here in modifying elastic properties such as bending rigidity.
Figure 3:
Bending rigidity of bilayers measured with flickering spectroscopy of GUVs prepared by the seven GUV preparation methods. The box-plot represents the standardized distribution of data based on first quartile (Q1), mean, third quartile (Q3), and the error bars represent 1.5 Standard Deviation. The abbreviations in the figure are as follows, SS: spontaneous swelling, EF: electroformation, PVA: polyvinyl alcohol, PTM (MO): phase-transfer method (mineral oil) and PTM (H): phase-transfer method (hexadecane). The open squares represent the mean values. ANOVA comparisons test compared to spontaneous swelling which is set as control. n>10 vesicles were probed, ***p≤0.001, **p≤0.01, n.s p > 0.05.
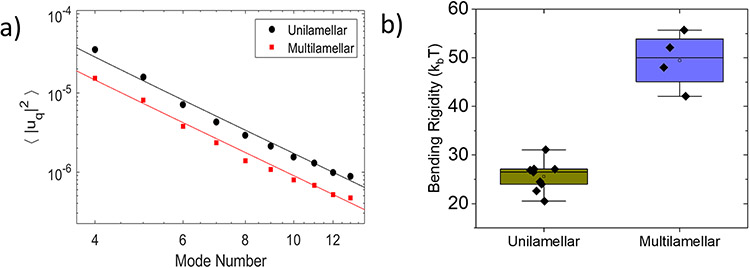
About 30% of the defect-free vesicles formed by spontaneous swelling demonstrated a reduced mean squared amplitude of the shape fluctuations, see Figure 4, and thus a higher bending rigidity (note that these data were not included in Figure 3). The bending rigidity values are almost twice the average value, see Figure 4b. The stiffening is likely due to formation of multilamellar membranes.
Figure 4:
a) Comparison of the mean squared amplitude of the membrane undulations of unilamellar and multilamellar vesicles prepared by spontaneous swelling b) Box-plot figure for bending rigidity values.
Agarose-prepared GUVs also exhibited a slightly higher bending rigidity, 30.2±2.2 kBT, compared to the rest of preparation methods, see Figure 3. Previously Lira et al.57 revealed encapsulation of agarose in GUVs in the form of gel-like network. The encapsulated agarose would arrest the thermally driven vesicle shape fluctuations and thus effectively increase the apparent bending rigidity.
3.2. Capacitance
Action potentials and electromotility depend on the bilayer capacitance, which controls ionic currents through the membrane.58,59 Knowledge of the capacitance value is thus needed in order to understand cell electrophysiology. The specific capacitance can be estimated from the permittivity, ϵ, and thickness, d, of the bilayer as Cm = ϵ/d. Changes in the dielectric properties or thickness of the bilayer, e.g., due to impurities introduced during membrane preparation, can thus affect the values of the measured capacitance. In fact, Vitkova et al.55 have demonstrated that sugars increase the membrane capacitance likely due to membrane thinning and changes in dielectric permittivity. Figure 5a shows the typical frequency dependent deformation of a GUV at fixed field strength in a uniform AC electric field. The GUV shape changes from a prolate to an oblate ellipsoid at a critical frequency related to the membrane capacitance, see Eq 1. Figure 5b shows the box plot for membrane capacitance obtained for POPC bilayers prepared from seven different methods. Membrane capacitance of bilayers prepared by spontaneous swelling and electroformation (ITO and Pt) are similar to each other suggesting similar dielectric constants and membrane thickness. Interestingly, the membrane capacitance obtained for bilayers formed by the PVA method is lower than the value for bilayers formed by spontaneous swelling/electroformation method. Given the similar bending rigidity found by us and the decrease in the stretching modulus observed by Dao et al.56 for membranes prepared by PVA method compared to electroformation (ITO), there is one plausible explanation of this observation. In order to satisfy the thin plate model,60,61 κ ~ Kd2, (where K is the stretching modulus) and the membrane capacitance relation, Cm = ϵ/d, a decrease in capacitance and stretching modulus at constant bending rigidity value suggests an increase in thickness of the membrane. Assuming a fixed electrical permittivity constant, a reduction from 58 μF/cm2 to 41 μF/cm2 (in this study) between electroformation and PVA method would mean a 41 % increase in membrane thickness (dPVA = 1.41dITO. Since we obtained same bending rigidity values, the expected stretching modulus ratio would be KPVA/KITO = (dITO/dPVA)2 ~ 0.5. This is in a reasonable agreement with the reported data of Dao et al56 from micropipette aspiration experiments where KITO ~ 160 mN/m and KPVA ~ 90 mN/m. Phase emulsion methods demonstrate a much wider spread in the data strongly indicating the effects of residual oil in the membrane. The presence of oil in the membrane could possibly be detected by means of fluorescent markers. However, such molecules might have different partitioning between the bulk and the membrane compared to that of the used oils, thus disproportionately reflecting the residual oil concentration in the membrane. A more suitable approach might be the use of bulk methods such as mass spectrometry (although oil droplets in the sample might jeopardize the measurement). Yet another approach, could be the application of polarity-sensitive dyes such as Laurdan as recently demonstrated for GUVs prepared PVA-assisted swelling56.
Figure 5:
a) Typical plot of membrane morphology in presence of AC electric field at different frequency at 8 kV/m and inner conductivity 40 muS/cm and outer conductivity 60-80 μS/cm. b) The box-plot represents the standardized distribution of membrane capacitance based on five numbers minimum value, first quartile (Q1), mean, third quartile (Q3), and maximum value. The abbreviations in the figure are as follows, SS: spontaneous swelling, EF: electroformation, PVA: polyvinyl alcohol, PTM (MO): phase-transfer method (mineral oil) and PTM (H): phase-transfer method (hexadecane). The open square represents the mean value. ANOVA comparisons test compared to spontaneous swelling which is set as control. n>10 vesicles were probed, ***p≤0.001, **p≤0.01, n.s p > 0.05.
3.3. Bilayer Viscosity
Lipids in bilayer membranes are held together by non-covalent bonds allowing for molecules to move freely in-plane (along the membrane). Membrane fluidity is essential for the lateral transport of biomolecules such as cholesterol, lipid rafts and proteins in physiological processes.4,62-64 To the best of our knowledge, there is no study comparing the viscosity of bilayer membranes with same lipid composition but produced by different methods. There is only a limited information about membrane fluidity from Dao et al,56 who compared molecular diffusivity in POPC and diblock copolymer bilayers prepared by PVA and electroformation method. This study found that PVA-formed membranes are more viscous than the electroformed ones, likely due to entrapment of PVA in the membranes. However, it is not trivial to deduce viscosity from diffusivity because of probe dependence.65
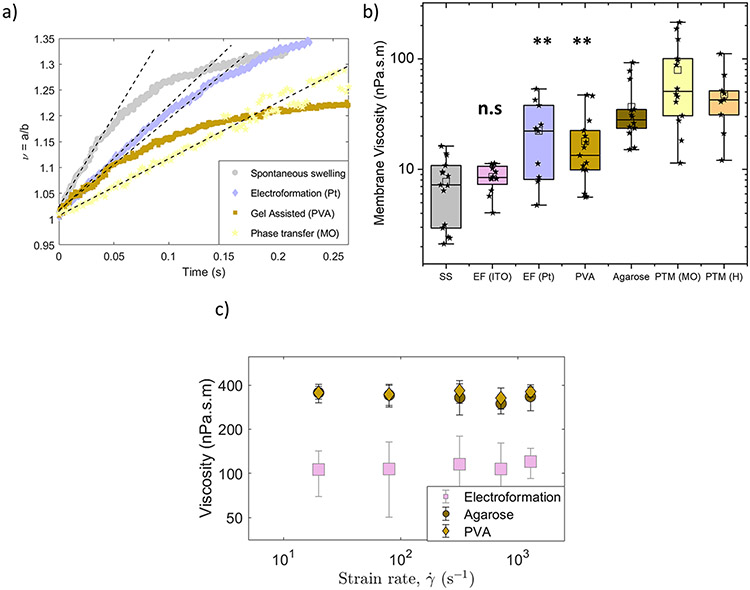
Here, we have utilized the recently developed non-invasive technique, the transient electrodeformation of GUVs,29 to directly measure shear viscosity. Figure 6a shows the deformation curves of POPC GUVs prepared by spontaneous swelling, electroformation, gel assisted method (PVA) and phase-transfer method (MO) with different initial rates. The membrane viscosity obtained from these deformation curves is given in Figure 6b and summarized in Table 1. We found no difference between membrane viscosity of lipid bilayers prepared from spontaneous swelling and electroformation, ηm ~8 nPa.s.m. However, membranes prepared by gel assisted methods (PVA and agarose) exhibit significantly higher membrane viscosities compared to the classical methods of electroformation (ITO) and spontaneously swelling. Bilayers of agarose-formed GUVs are more viscous (36.7 ± 23.9 nPa.s.m) compared to PVA-formed GUVs (17.9 ± 13.1 nPa.s.m) suggesting that remnants of agarose gel in the bilayer affect membrane rheological properties more severely. Leftover of agarose gel in the vesicle lumen compared to PVA GUVs can also lead to extra dissipation.
Figure 6:
a) Vesicles made of POPC by different preparation methods deform at a different rate indicating different membrane viscosity. The field strength and frequency are 8 kV/m and 0.1 kHz. The solid lines correspond to the theoretical fit with Eq. 2.b) Box-plot figure of shear viscosity values of bilayers obtained with transient electrodeformation for seven different GUV preparation methods. The box-plot represents the standardized distribution of data based on minimum value, first quartile (Q1), mean, third quartile (Q3), and maximum value. The open square represents the mean value. The abbreviations in the figure are as follows, SS: spontaneous swelling, EF: electroformation, PVA: polyvinyl alcohol, PTM (MO): phase-transfer method (mineral oil) and PTM (H): phase-transfer method (hexadecane). ANOVA comparisons test compared to spontaneous swelling which is set as control. ***p≤0.001, **p≤0.01, n.s p≥0.05 c) Viscosity dependence on electric field strength, or equivalently strain rate .
Table 1:
Membrane bending rigidity, capacitance and shear viscosity of POPC bilayers obtained from different preparation methods at 25 °C and determined in this study. Bending rigidity was measured with flickering spectroscopy and membrane capacitance and viscosity was measured with the electrodeformation method. The abbreviations in the table are as follows, SS: spontaneous swelling, EF: electroformation, PVA: polyvinyl alcohol, PTM (MO): phase-transfer method (mineral oil) and PTM (H): phase-transfer method (hexadecane).
| Method | κ (kBT) | Cm (μF/cm2) | ηm (nPa.s.m) |
|---|---|---|---|
| SS | 25.0 ± 3.1 | 0.63 ± 0.26 | 7.72 ± 4.6 |
| EF (ITO) | 25.5 ± 2.6 | 0.58 ±0.11 | 8.57 ± 2.3 |
| EF (Pt) | 25.5 ± 2.1 | 0.55 ±0.05 | 22.2 ± 16.3 |
| PVA | 27.3 ± 5.1 | 0.41 ±0.08 | 17.9 ± 13.2 |
| Agarose | 30.2 ± 2.2 | 0.63 ±0.11 | 36.7 ± 23.9 |
| PTM (MO) | 22.7 ± 1.7 | 0.87 ±0.55 | 79.11 ± 63.8 |
| PTM (H) | 23.6 ± 2.8 | 0.72 ±0.37 | 47.7 ± 29.8 |
Next, we examined more closely how the gel impurity modifies the membrane viscosity. Previously, it has been shown that aqueous solutions of agarose and PVA behave as shear thinning fluids: the viscosity decreases with increasing shear rate.66,67 The stress generated by the electric field shears the membrane with a characteristic rate . Modulating the field amplitude thus enables us to vary the shear rate in a wide range and to probe if bilayers behave as Newtonian (with shear-rate independent viscosity) or non-Newtonian fluids. Increasing E0 from 1 to 50 kV/m at a given frequency increases the effective shear rate from 1 s−1 to 2000 s−1. Figure 6c shows that the bilayers behave as Newtonian fluids, since viscosity does not change with the shear rate, even though the gel impurities increase the overall viscosity.
GUVs formed by the phase-transfer method (mineral oil and hexadecane) also demonstrated membrane viscosities higher by almost an order of magnitude, 70-80 nPa.s.m compared to the control methods. This strongly suggests that residual oil in the bilayer modifies the material’s rheology.68-70
4. Conclusions
In this work, we have compared the material properties of POPC bilayers in the form of GUVs prepared from commonly used protocols: spontaneous swelling, electroformation, gel assisted and phase-transfer method. The results are summarized in Table 1. Probing material properties provides a straightforward means to monitor any method based modulation. Using flickering spectroscopy and electrodeformation, we compared bending rigidity, membrane capacitance and shear viscosity to determine if all the methods yield membranes with same properties. We chose spontaneous swelling as a control method, however, with similar material properties obtained with electroformation, either could serve as a control method. Although the gel assisted and the phase-transfer methods offer unprecedented advantage to grow GUVs rapidly in physiological buffers, we found evidence that gel remnants or residual oil alter bilayer properties especially the shear surface viscosity. A higher sugar concentration was chosen for all GUV preparation methods primarily due to low yield obtained with phase-transfer method at a low sugar concentration (see Moga et al40). As a word of caution, sugars (i) can interdigitate in the membrane,71 (ii) might interfere with agarose (because of the ‘sugary’ structure of the polymer), and (iii) might be the source of impurities in the system72. However, since the final solution conditions across the membrane of the vesicles were identical in all the preparation methods, we believe the presence of sugars imposed by the phase-transfer method, does not change our comparative conclusions regarding the properties of the membranes prepared by the different methods. We also acknowledge that GUV preparation protocols vary slightly from lab to lab and we have adopted protocols suggested from literature that yield high quality vesicles without visible defects. The data presented in this study would help in more informed decision in the choice of preparation method for GUVs in respect to rheology of bilayer and mobility of biomolecules in synthetic cell studies.
Acknowledgements
P.M.V and H.A.F acknowledge financial support by NIGMS award 1R01GM140461.This research was also supported in part by the National Science Foundation under grant NSF PHY-1748958.
Footnotes
Conflicts of interest
The authors have declared no conflict of interest.
References
- (1).Szostak JW; Bartel DP; Luisi PL Synthesizing life. Nature 2001, 409, 387–390. [DOI] [PubMed] [Google Scholar]
- (2).Noireaux V; Libchaber A A vesicle bioreactor as a step toward an artificial cell assembly. Proceedings of the National Academy of Sciences 2004, 101, 17669–17674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Howard J Molecular motors: structural adaptations to cellular functions. Nature 1997, 389, 561–567. [DOI] [PubMed] [Google Scholar]
- (4).Stachowiak JC; Brodsky FM; Miller EA A cost-benefit analysis of the physical mechanisms of membrane curvature. Nature Cell Biology 2013, 15, 1019 EP -, Review Article. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Fenz SF; Sengupta K Giant vesicles as cell models. Integrative Biology 2012, 4, 982–995. [DOI] [PubMed] [Google Scholar]
- (6).Dimova R; Aranda S; Bezlyepkina N; Nikolov V; Riske KA; Lipowsky R A practical guide to giant vesicles. Probing the membrane nanoregime via optical microscopy. Journal of Physics: Condensed Matter 2006, 18, S1151–S1176. [DOI] [PubMed] [Google Scholar]
- (7).Dimova R Giant Vesicles and Their Use in Assays for Assessing Membrane Phase State, Curvature, Mechanics, and Electrical Properties. Annual Review of Biophysics 2019, 48, 93–119. [DOI] [PubMed] [Google Scholar]
- (8).Dimova R, Marques C, Eds. The Giant Vesicle Book; CRC Press, 2019. [Google Scholar]
- (9).Walde P; Cosentino K; Engel H; Stano P Giant Vesicles: Preparations and Applications. ChemBioChem 2010, 11, 848–865. [DOI] [PubMed] [Google Scholar]
- (10).Dimova R; Stano P; Marques C; Walde P In The Giant Vesicle Book; Dimova R, Marques C, Eds.; CRC Press, 2019; p Chapter 1. [Google Scholar]
- (11).Reeves JP; Dowben RM Formation and properties of thin-walled phospholipid vesicles. Journal of Cellular Physiology 1969. 73, 49–60. [DOI] [PubMed] [Google Scholar]
- (12).Angelova MI; Dimitrov DS Liposome electroformation. Faraday Discuss. Chem. Soc 1986, 81, 303–311. [Google Scholar]
- (13).Pott T; Bouvrais H; Méléard P Giant unilamellar vesicle formation under physiologically relevant conditions. Chemistry and Physics of Lipids 2008, 154, 115–119. [DOI] [PubMed] [Google Scholar]
- (14).Li Q; Wang X; Ma S; Zhang Y; Han X Electroformation of giant unilamellar vesicles in saline solution. Colloids and Surfaces B: Biointerfaces 2016, 147, 368–375. [DOI] [PubMed] [Google Scholar]
- (15).Drabik D; Doskocz J; Przybyło M Effects of electroformation protocol parameters on quality of homogeneous GUV populations. Chemistry and Physics of Lipids 2018, 212, 88–95. [DOI] [PubMed] [Google Scholar]
- (16).Sankhagowit S; Wu S-H; Biswas R; Riche CT; Povinelli ML; Malmstadt N The dynamics of giant unilamellar vesicle oxidation probed by morphological transitions. Biochimica et Biophysica Acta (BBA) - Biomembranes 2014, 1838, 2615–2624. [DOI] [PubMed] [Google Scholar]
- (17).Ayuyan AG; Cohen FS Lipid Peroxides Promote Large Rafts: Effects of Excitation of Probes in Fluorescence Microscopy and Electrochemical Reactions during Vesicle Formation. Biophysical Journal 2006, 91, 2172–2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).van Swaay D; deMello A Microfluidic methods for forming liposomes. Lab Chip 2013, 13, 752–767. [DOI] [PubMed] [Google Scholar]
- (19).Trantidou T; Friddin MS; Salehi-Reyhani A; Ces O; Elani Y Droplet microfluidics for the construction of compartmentalised model membranes. Lab Chip 2018, 18, 2488–2509. [DOI] [PubMed] [Google Scholar]
- (20).Stachowiak JC; Richmond DL; Li TH; Liu AP; Parekh SH; Fletcher DA Unilamellar vesicle formation and encapsulation by microfluidic jetting. Proceedings of the National Academy of Sciences 2008, 105, 4697–4702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Shum HC; Lee D; Yoon I; Kodger T; Weitz DA Double Emulsion Templated Monodisperse Phospholipid Vesicles. Langmuir 2008, 24, 7651–7653. [DOI] [PubMed] [Google Scholar]
- (22).Abkarian M; Loiseau E; Massiera G Continuous droplet interface crossing encapsulation (cDICE) for high throughput monodisperse vesicle design. Soft Matter 2011, 7, 4610–4614. [Google Scholar]
- (23).Horger KS; Estes DJ; Capone R; Mayer M Films of Agarose Enable Rapid Formation of Giant Liposomes in Solutions of Physiologic Ionic Strength. Journal of the American Chemical Society 2009, 131, 1810–1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Weinberger A; Tsai F-C; Koenderink G; Schmidt T; Itri R; Meier W; Schmatko T; Schröder A; Marques C Gel-Assisted Formation of Giant Unilamellar Vesicles. Biophysical Journal 2013, 105, 154–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Moscho A; Orwar O; Chiu DT; Modi BP; Zare RN Rapid preparation of giant unilamellar vesicles. Proceedings of the National Academy of Sciences 1996, 93, 11443–11447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Faizi HA; Frey SL; Steinkühler J; Dimova R; Vlahovska PM Bending rigidity of charged lipid bilayer membranes. Soft Matter 2019, 15, 6006–6013. [DOI] [PubMed] [Google Scholar]
- (27).Faizi HA; Reeves CJ; Georgiev VN; Vlahovska PM; Dimova R Fluctuation spectroscopy of giant unilamellar vesicles using confocal and phase contrast microscopy. Soft Matter 2020, 16, 8996–9001. [DOI] [PubMed] [Google Scholar]
- (28).Faizi HA; Dimova R; Vlahovska PM Electromechanical characterization of biomimetic membranes using electrodeformation of vesicles. Electrophoresis 2021, 42, 2027–2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Faizi HA; Dimova R; Vlahovska PM A vesicle microrheometer for high-throughput viscosity measurements of lipid and polymer membranes. Biophysical Journal 2022, 121, 910–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Gracia RS; Bezlyepkina N; Knorr RL; Lipowsky R; Dimova R Effect of cholesterol on the rigidity of saturated and unsaturated membranes: fluctuation and electrodeformation analysis of giant vesicles. Soft Matter 2010, 6, 1472–1482. [Google Scholar]
- (31).Akashi K; Miyata H; Itoh H; Kinosita K Preparation of giant liposomes in physiological conditions and their characterization under an optical microscope. Biophysical Journal 1996, 71, 3242–3250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Tsumoto K; Matsuo H; Tomita M; Yoshimura T Efficient formation of giant liposomes through the gentle hydration of phosphatidylcholine films doped with sugar. Colloids and Surfaces B: Biointerfaces 2009, 68, 98–105. [DOI] [PubMed] [Google Scholar]
- (33).Politano TJ; Froude VE; Jing B; Zhu Y AC-electric field dependent electroformation of giant lipid vesicles. Colloids and Surfaces B: Biointerfaces 2010, 79, 75–82. [DOI] [PubMed] [Google Scholar]
- (34).Micheletto YMS; Marques CM; Silveira NPD; Schroder AP Electroformation of Giant Unilamellar Vesicles: Investigating Vesicle Fusion versus Bulge Merging. Langmuir 2016, 32, 8123–8130. [DOI] [PubMed] [Google Scholar]
- (35).Rideau E; Wurm FR; Landfester K Self-Assembly of Giant Unilamellar Vesicles by Film Hydration Methodologies. Advanced Biosystems 2019, 3, 1800324. [DOI] [PubMed] [Google Scholar]
- (36).Stein H; Spindler S; Bonakdar N; Wang C; Sandoghdar V Production of Isolated Giant Unilamellar Vesicles under High Salt Concentrations. Frontiers in Physiology 2017, 8, 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Lefrançois P; Goudeau B; Arbault S Electroformation of phospholipid giant unilamellar vesicles in physiological phosphate buffer. Integr. Biol 2018, 10, 429–434. [DOI] [PubMed] [Google Scholar]
- (38).Witkowska A; Jablonski L; Jahn R A convenient protocol for generating giant unilamellar vesicles containing SNARE proteins using electroformation. Scientific Reports 2018, 8, 9422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Li W; Wang Q; Yang Z; Wang W; Cao Y; Hu N; Luo H; Liao Y; Yang J Impacts of electrical parameters on the electroformation of giant vesicles on ITO glass chips. Colloids and Surfaces B: Biointerfaces 2016, 140, 560–566. [DOI] [PubMed] [Google Scholar]
- (40).Moga A; Yandrapalli N; Dimova R; Robinson T Optimization of the Inverted Emulsion Method for High-Yield Production of Biomimetic Giant Unilamellar Vesicles. ChemBioChem 2019, 20, 2674–2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Salipante PF; Knorr R; Dimova R; Vlahovska PM Electrodeformation method for measuring the capacitance of bilayer membranes. Soft Matter 2012, 8, 3810–3816. [Google Scholar]
- (42).Vlahovska PM; Gracià RS; Aranda-Espinoza S; Dimova R Electrohydrodynamic Model of Vesicle Deformation in Alternating Electric Fields. Biophysical Journal 2009, 96, 4789–4803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Yamamoto T; Aranda-Espinoza S; Dimova R; Lipowsky R Stability of Spherical Vesicles in Electric Fields. Langmuir 2010, 26, 12390–12407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Elani Y; Purushothaman S; Booth PJ; Seddon JM; Brooks NJ; Law RV; Ces O Measurements of the effect of membrane asymmetry on the mechanical properties of lipid bilayers. Chem. Commun 2015, 51, 6976–6979. [DOI] [PubMed] [Google Scholar]
- (45).Dimova R Recent developments in the field of bending rigidity measurements on membranes. Advances in Colloid and Interface Science 2014, 208, 225–234, Special issue in honour of Wolfgang Helfrich. [DOI] [PubMed] [Google Scholar]
- (46).Vitkova V; Genova J; Mitov MD; Bivas I Sugars in the Aqueous Phase Change the Mechanical Properties of Lipid Mono- and Bilayers. Molecular Crystals and Liquid Crystals 2006, 449, 95–106. [Google Scholar]
- (47).Karimi M; Steinkühler J; Roy D; Dasgupta R; Lipowsky R; Dimova R Asymmetric Ionic Conditions Generate Large Membrane Curvatures. Nano Letters 2018, 18, 7816–7821. [DOI] [PubMed] [Google Scholar]
- (48).Henriksen J; Rowat AC; Ipsen JH Vesicle fluctuation analysis of the effects of sterols on membrane bending rigidity. European Biophysics Journal 2004, 33, 732–741. [DOI] [PubMed] [Google Scholar]
- (49).Bouvrais H; Pott T; Bagatolli LA; Ipsen JH; Méléard P Impact of membrane-anchored fluorescent probes on the mechanical properties of lipid bilayers. Biochimica et Biophysica Acta (BBA) - Biomembranes 2010, 1798, 1333–1337, Microscopy Imaging of Membrane Domains. [DOI] [PubMed] [Google Scholar]
- (50).Bouvrais H; Duelund L; Ipsen JH Buffers Affect the Bending Rigidity of Model Lipid Membranes. Langmuir 2014, 30, 13–16. [DOI] [PubMed] [Google Scholar]
- (51).Rodriguez N; Pincet F; Cribier S Giant vesicles formed by gentle hydration and electroformation: A comparison by fluorescence microscopy. Colloids and Surfaces B: Biointerfaces 2005, 42, 125–130. [DOI] [PubMed] [Google Scholar]
- (52).Helfrich W Elastic Properties of Lipid Bilayers: Theory and Possible Experiments. Z. Naturforsch. C 1973, 28, 693–703. [DOI] [PubMed] [Google Scholar]
- (53).Helfrich W In Structure and Dynamics of Membranes; Lipowsky R, Sackmann E, Eds.; Handbook of Biological Physics; North-Holland, 1995; Vol. 1; pp 691–721. [Google Scholar]
- (54).Bassereau P; Sorre B; Lévy A Bending lipid membranes: Experiments after W. Helfrich’s model. Advances in Colloid and Interface Science 2014, 208, 47–57, Special issue in honour of Wolfgang Helfrich. [DOI] [PubMed] [Google Scholar]
- (55).Vitkova V; Mitkova D; Antonova K; Popkirov G; Dimova R Sucrose solutions alter the electric capacitance and dielectric permittivity of lipid bilayers. Colloids and Surfaces A: Physicochemical and Engineering Aspects 2018, 557, 51–57, “A Collection of Papers Presented at the 31st ECIS Meeting, Madrid, Spain, 3-8 September, 2017”. [Google Scholar]
- (56).Dao T; Fauquignon M; Fernandes F; Ibarboure E; Vax A; Prieto M; Le Meins J Membrane properties of giant polymer and lipid vesicles obtained by electroformation and pva gel-assisted hydration methods. Colloids and Surfaces A: Physicochemical and Engineering Aspects 2017, 533, 347–353. [Google Scholar]
- (57).Lira R; Dimova R; Riske K Giant Unilamellar Vesicles Formed by Hybrid Films of Agarose and Lipids Display Altered Mechanical Properties. Biophysical Journal 2014, 107, 1609–1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (58).Bean B The action potential in mammalian central neurons. Nature Reviews: Neuroscience 2007, 8, 451–465. [DOI] [PubMed] [Google Scholar]
- (59).Brownell WE; Spector AA; Raphael RM; Popel AS Micro- and Nanomechanics of the Cochlear Outer Hair Cell. Annual Review of Biomedical Engineering 2001, 3, 169–194. [DOI] [PubMed] [Google Scholar]
- (60).Boal D Mechanics of the Cell, 2nd ed.; Cambridge University Press, 2012. [Google Scholar]
- (61).Rawicz W; Olbrich K; McIntosh T; Needham D; Evans E Effect of chain length and unsaturation on elasticity of lipid bilayers. Biophys. J 2000, 79, 328–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (62).Cohen AE; Shi Z Do Cell Membranes Flow Like Honey or Jiggle Like Jello? BioEssays 2020, 42, 1900142. [DOI] [PubMed] [Google Scholar]
- (63).Saffman PG; Delbrück M Brownian motion in biological membranes. PNAS 1975, 72, 3111–3113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (64).Singer SJ; Nicolson GL The Fluid Mosaic Model of the Structure of Cell Membranes. Science 1972, 175, 720–731. [DOI] [PubMed] [Google Scholar]
- (65).Adrien V; Rayan G; Astafyeva K; Broutin I; Picard M; Fuchs P; Urbach W; Taulier N How to best estimate the viscosity of lipid bilayers. Biophysical Chemistry 2022, 281, 106732. [DOI] [PubMed] [Google Scholar]
- (66).Song SI; Kim BC Characteristic rheological features of PVA solutions in water-containing solvents with different hydration states. Polymer 2004, 45, 2381–2386. [Google Scholar]
- (67).Ghebremedhin M; Seiffert S; Vilgis TA Physics of agarose fluid gels: Rheological properties and microstructure. Current Research in Food Science 2021, 4, 436–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (68).Tarun OB; Eremchev MY; Roke S Interaction of Oil and Lipids in Freestanding Lipid Bilayer Membranes Studied with Label-Free High-Throughput Wide-Field Second-Harmonic Microscopy. Langmuir 2018, 34, 11305–11310. [DOI] [PubMed] [Google Scholar]
- (69).Richens JL; Lane JS; Mather ML; O’Shea P The interactions of squalene, alkanes and other mineral oils with model membranes; effects on membrane heterogeneity and function. Journal of Colloid and Interface Science 2015, 457, 225–231. [DOI] [PubMed] [Google Scholar]
- (70).Morita M; Noda N Membrane Shape Dynamics-Based Analysis of the Physical Properties of Giant Unilamellar Vesicles Prepared by Inverted Emulsion and Hydration Techniques. Langmuir 2021, 37, 2268–2275. [DOI] [PubMed] [Google Scholar]
- (71).Andersen HD; Wang C; Arleth L; Peters GH; Westh P Reconciliation of opposing views on membrane-sugar interactions. Proceedings of the National Academy of Sciences 2011, 108, 1874–1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (72).Knorr RL; Steinkühler J; Dimova R Micron-sized domains in quasi single-component giant vesicles. Biochimica et Biophysica Acta (BBA) - Biomembranes 2018, 1860, 1957–1964, Emergence of Complex Behavior in Biomembranes. [DOI] [PubMed] [Google Scholar]