Abstract
We purified three proline-rich antimicrobial peptides from elastase-treated extracts of sheep and goat leukocytes and subjected two of them, OaBac5α and ChBac5, to detailed analysis. OaBac5α and ChBac5 were homologous to each other and to bovine Bac5. Both exhibited potent, broad-spectrum antimicrobial activity under low-concentration salt conditions. While the peptides remained active against Escherichia coli, Pseudomonas aeruginosa, Bacillus subtilis, and Listeria monocytogenes in 100 mM NaCl, they lost activity against Staphylococcus aureus and Candida albicans under these conditions. ChBac5 was shown to bind lipopolysaccharide, a property that could enhance its ability to kill gram-negative bacteria. Proline-rich Bac5 peptides are highly conserved in ruminants and may contribute significantly to their innate host defense mechanisms.
Various antimicrobial peptides enhance the ability of mammalian neutrophils to overcome microbial incursions (5, 12). Among these are cathelicidins (31), propeptides containing a highly conserved N-terminal “cathelin” domain (17) and a C-terminal domain with antimicrobial properties. The secondary (specific) granules of human neutrophils contain a single cathelicidin, hCAP-18, the precursor of an α-helical antimicrobial peptide called LL-37 (7, 26). In contrast, bovine neutrophils contain many cathelicidins (31), including molecules whose antimicrobial domains encode a cyclic dodecapeptide (18), a tryptophan-rich tridecapeptide, indolicidin (2, 21), proline- and arginine-rich Bac5 and Bac7 peptides (4), and several α-helical peptides (20, 24, 29). Many of these peptides have been well studied at the peptide level.
There is cDNA evidence (1, 9, 13) for at least eight cathelin-associated peptides in the sheep; however, relatively little is known about their antimicrobial properties. We treated leukocyte extracts from sheep (Ovis aries) and goats (Capra hirca) with neutrophil elastase to generate antimicrobial moieties from their cathelicidin precursors. This report describes the purification, compositions, and antimicrobial properties of two proline-rich antimicrobial peptides, ovine (Oa)Bac5α and caprine (Ch)Bac5.
MATERIALS AND METHODS
Preparation of leukocytes.
With institutional approval, 250 to 300 ml of venous blood was obtained from healthy sheep and goats and anticoagulated with citrate. Leukocytes were prepared by lysing the erythrocytes with 0.83% ammonium chloride (2 cycles), followed by brief exposure to cold 0.22% saline. Goat leukocyte preparations contained 80.2% ± 2.4% neutrophils (mean ± standard error of the mean; n = 10), and sheep preparations contained 77.3% ± 4.0% neutrophils (n = 12). The final number of neutrophils obtained per collection was (1.18 ± 0.14) × 109 from goats and (0.74 ± 0.12) × 109 from sheep (mean ± standard error of the mean).
Purification of OaBac5α and ChBac5.
Initially, we attempted to purify neutrophil antimicrobial peptides from phorbol myristate acetate (PMA)-induced leukocyte secretions, but this approach yielded insufficient amounts of processed cathelicidins. Consequently, we generated the peptides described in this report by treating extracts of goat and sheep leukocytes with human neutrophil elastase (ART Biochemicals, Athens, Ga.), essentially as described by Panyutich et al. (15). Briefly, leukocytes were centrifuged at 225 × g for 10 min, resuspended in 10% acetic acid, sonicated, and extracted overnight at 0 to 4°C. The extracts were clarified at 3,000 × g for 30 min at 4°C, and the supernatants were lyophilized for storage. This material was dissolved in 0.1 M Tris-0.15 M NaCl buffer (pH 7.5) and treated with 1.5 to 2.0 μg of elastase/mg of protein for 30 min at 37°C. Proteolysis was stopped by adding acetic acid to a final concentration of 5%. After passage through a YM-10 filter (Amicon, Beverly, Mass.), the ultrafiltrates were concentrated by vacuum centrifugation and desalted on a Sep-Pak light C18 cartridge (Waters Millipore, Milford, Mass.). The recovered material was dried, resuspended in 1 ml of 0.5% acetic acid containing 3 M urea, and subjected to preparative continuous electrophoresis (8). Fractions containing 3- to 5-kDa peptides were identified by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), pooled, and purified by reversed-phase high-pressure liquid chromatography (RP-HPLC) on a Vydac C18 column by using linear gradients of 0 to 60% acetonitrile in 0.1% trifluoroacetic acid or 0.13% hexafluorobutyric acid.
Biochemical analyses.
Purified goat and sheep peptides or secretates were analyzed by MALDI and/or ESI mass spectrometry. Amino acid sequences were determined by gas-phase Edman degradation with a Porton Model 2090E instrument by using 300 to 500 pmols of purified peptide. Protein concentrations were measured by the bicinchoninic acid procedure (Pierce, Rockford, Ill.).
cDNA cloning.
Bone marrow was obtained from a young male goat, and a custom cDNA library was constructed in a Uni-ZAP XR vector (Stratagene, La Jolla, Calif.). The P1 sense primer 5′-GCTAATCTCTACCGCCTCCTGG-3′ (nucleotides [nt] 168 to 189 of BtBac5) and P2 antisense primer 5′-CCACACACTGTTTCACCAGCC-3′ (nt 319 to 339 of BtBac5) were derived from a conserved sequence of bovine Bac5 cDNA (32). To obtain 5′ side sequences, we used P2 and the vector SK primer to amplify goat bone marrow cDNA. To get 3′ side sequences, P1 and vector primer T7 were used. There was a 172-bp sequence overlap between the two PCR products. The amplified PCR products were cloned into PCR2.1-TOPO vector (Invitrogen, Carlsbad, Calif.), sequenced by the fluorescein-labeled dideoxynucleotide termination method, and analyzed on an ABI-373 Sequencer (Perkin-Elmer, Palo Alto, Calif.).
Western blots.
A rabbit polyclonal antibody against porcine cathelin (23) was obtained from Jishu Shi and Tomas Ganz of the University of California, Los Angeles, and was found to react with goat and sheep cathelins. Goat anti-rabbit immunoglobulin G conjugated to alkaline phosphatase was purchased from Bio-Rad (Hercules, Calif.). SDS-PAGE gels of neutrophil extracts were electrotransferred to Immobilon-P membranes (Millipore, Bedford, Mass.). The membranes were blocked for 1 h with 3% gelatin and 1% bovine serum albumin in 0.5 M NaCl and 20 mM Tris, pH 7.5, and then washed with 0.05% Tween 20 in 0.5 M NaCl and 20 mM Tris, pH 7.5. The membranes were probed with anticathelin antibody (1:1,000), washed, and then probed with a second antibody (1:500). A mixture of 5-bromo-4-chloro-3-indolylphosphate (BCIP) and nitroblue tetrazolium in a solution of 100 mM NaCl, 5 mM MgCl2, and 100 mM Tris (pH 9.8) was used for color development.
Antibacterial assays.
The peptides were tested for antimicrobial activity against Escherichia coli ML-35, Salmonella typhimurium 14028S, Pseudomonas aeruginosa MR 3007, Listeria monocytogenes EGD, Bacillus subtilis, Staphylococcus aureus 930918-3, and Candida albicans 820 by a two-stage radial diffusion technique (27). Briefly, approximately 4 × 106 CFU of mid-logarithmic-phase organisms were dispersed in a 10-ml volume of underlay gel that contained 10 mM sodium phosphate, 0.3 mg of trypticase soy broth powder (Difco, Detroit, Mich.) per ml, and 1% (wt/vol) agarose (Sigma A6013) with or without 100 mM NaCl. Peptide concentrations were established by quantitative amino acid analysis. Serial peptide dilutions were prepared in 0.01% acetic acid containing 0.1% human serum albumin, and 8-μl peptide samples were applied. Overlay gels (10 ml of trypticase soy agar, 60 g/liter) were poured 3 h after the peptide samples were added. The clear zones were measured to the nearest 0.1 mm after overnight incubation and were expressed in units (1 mm = 10 U) after subtracting the well diameter. Dose response studies were also performed by conventional colony counting.
In radial diffusion assays, the MIC is defined by the x intercept of a regression line through zone diameters obtained from a series of serially diluted peptide samples. An α-defensin, NP-1, purified from the leukocytes of rabbits was used as a control. The experiments were performed under low and high concentrations of salt. In low concentrations of salt, the underlay gels contained 10 mM sodium phosphate buffer without added NaCl (pH 7.4). For high concentrations of salt, underlay gels also contained 10 mM sodium phosphate buffer plus 100 mM NaCl.
LPS binding.
Peptides of interest were dried in 1.5-ml sterile polypropylene microcentrifuge tubes by vacuum centrifugation and were resuspended in endotoxin-free, 0.01% acetic acid. Polymyxin B (7,600 U/mg) (Sigma) was used as a positive control. Assays were performed in flat-bottom 96-well tissue culture plates (catalog no. 3596; Costar, Cambridge, Mass.) with a Quantitative Chromogenic Lysate kit (Bio Whittaker, Walkersville, Md.) as previously described (30). Standard curves generated with graded amounts of lipopolysaccharide (LPS) were linear between 0.5 and 0.0625 endotoxin units (EU)/assay (r = 0.997); consequently LPS binding was assumed to be proportional to the inhibition of procoagulant activation.
RESULTS
Peptide purification.
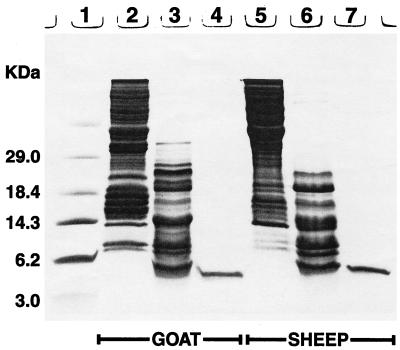
SDS-PAGE of acetic acid extracts of untreated goat and sheep leukocytes revealed a paucity of peptides smaller than 7 to 8 kDa (Fig. 1), suggesting that 3- to 4.5-kDa defensins were absent. Multiple 15- to 19-kDa peptide species that reacted with an anticathelin antibody were present in both species (data not shown). Because bovine cathelicidins are processed by neutrophil elastase (19), we used this enzyme (15) to treat goat and sheep cathelicidins in vitro. Figure 1 also shows the elastase-processed components of goat and sheep neutrophils and the purified Bac5 peptides described in this report. Figure 2 shows stages in the purification of OaBac5α, one of the peptides described in this report.
FIG. 1.
SDS-PAGE gel. The lanes contain the following: 1, mass standards; 2, acid extract of goat leukocytes (45 μg of protein); 3, goat leukocyte extract, post-elastase treatment (25 μg of protein); 4, purified ChBac5 (1 μg of protein); 5, acid extract of sheep leukocytes (45 μg of protein); 6, sheep leukocyte extract, post-elastase treatment (25 μg of protein); and 7, purified OaBac5α (1 μg of protein). The gel was stained with Coomassie blue.
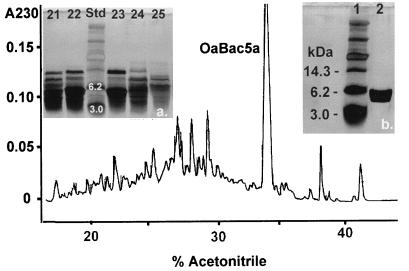
FIG. 2.
Purification of OaBac5α. The inset photographs are of silver-stained SDS-PAGE gels. Photograph a shows the composition of fractions 21 to 25, obtained after continuous preparative electrophoresis (8). The masses of two standards (3.0 and 6.2 kDa; Std) are shown. The main figure shows a chromatogram of the RP-HPLC purification of electrophoretic fractions 22 and 23. Note the prominent peak (OaBac5α) that emerged at approximately 34% acetonitrile. Photograph b shows this peak.
Quantitative aspects of the process were as follows. A crude acetic acid extract from 3 × 109 sheep leukocytes contained 31.2 mg of protein, representing 15.6% of the extract’s dry weight. After elastase cleavage, approximately two-thirds of the protein (19.8 mg) was recovered in the YM-10 filtrate. Of this, we recovered 3 mg of protein by eluting the Sep-Pak cartridge with 60% acetonitrile. Subjecting this material to preparative electrophoresis followed by three cycles of RP-HPLC yielded 45 to 50 μg of highly purified ovine OaBac5α. Handled in the same manner, 1.3 × 109 goat leukocytes (12.2 mg of total protein) yielded approximately 30 μg of highly purified ChBac5. Our largest-scale purification of goat Bac5 began with 407 mg of total protein and yielded 229 μg of highly purified ChBac5.
Characterization and cDNA cloning of ChBac5.
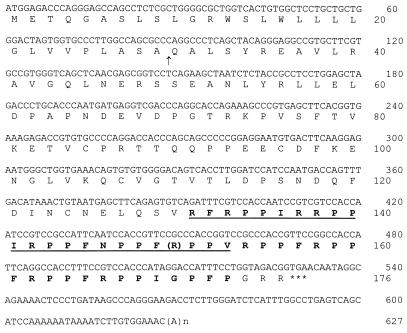
By using primers derived from bovine Bac5 cDNA and vector to amplify a goat bone marrow cDNA library, we obtained the full cDNA sequence of ChBac5 (Fig. 3). The 528-nt open reading frame predicted a 176-residue prepropeptide with a 29-residue signal sequence. The cDNA sequence corresponding to mature ChBac5 was at the 3′ end of the open reading frame. Its deduced sequence matched the amino acid sequence determined at the peptide level. The 46-residue (postcathelin) peptide encoded in goat cDNA had a calculated molecular mass of 5,531.6 Da. Since our purified peptide had a measured mass of 5,160.2 Da, we concluded that it was a 43-residue amidated form (calculated mass, 5,161.2 Da). Overall, 40 of the 46 (87%) residues in goat ChBac5-GRR were identical to those in bovine Bac5-GRR, and another 4 of the 46 (8.7%) represented conservative substitutions (Fig. 4).
FIG. 3.
cDNA and deduced amino acid sequences of ChBac5. All boldface and underlined amino acid residues were confirmed by peptide-level sequencing, with the exception of the arginine residue in parentheses. The arrow shows the putative cleavage site for the signal peptide. The predicted sequence of mature ChBac5 is shown in bold type, and the stop codon is marked by asterisks. Our peptide-level mass measurements indicated that the C-terminal GRR residues are removed, presumably when the peptide undergoes amidation.
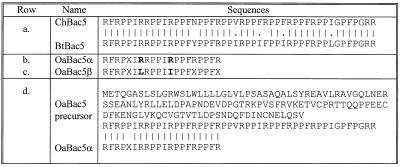
FIG. 4.
Amino acid sequences of Bac5 peptides. Row a compares the sequences of goat ChBac5 and bovine BtBac5 (4). Rows b and c show the N-terminal sequences of ovine Bac5α and -β, determined at the peptide level, with the differences in boldface. X signifies an unidentified residue. Row d shows the inferred sequence of prepro-OaBac5, as described by Huttner et al. (9). Below this is the N-terminal sequence of OaBac5α as determined in this study. Identical residues are connected with lines.
Figure 4 compares the N-terminal peptide sequence of ovine OaBac5α to the OaBac5 sequence established by cloning (9). All of the N-terminal residues identified by peptide sequencing corresponded to those encoded by the cDNA. The 46-residue postcathelin peptide encoded in ovine OaBac5 cDNA had a calculated mass of 5,539.7 Da, whereas an amidated peptide that contained 43 residues and lacked the C-terminal GRR should have a mass of 5,169.3 Da. Since the measured mass of purified OaBac5α peptide was 5,157.5 Da, OaBac5α was probably a 43-residue amidated variant of OaBac5 with a primary sequence that diverged from that encoded in the cDNA in at least one residue. We obtained experimental evidence for further heterogeneity of ovine Bac5 by isolating an additional peptide, OaBac5β, in the processed sheep leukocyte extracts (Fig. 4). OaBac5β was approximately 20 to 30% as abundant as OaBac5α, differed from it in 2 of 17 corresponding residues that were defined by N-terminal sequencing, and was 38 Da smaller. OaBac5β was antimicrobial, but we lacked sufficient quantities for extensive testing.
PMA-induced secretions.
We stimulated goat neutrophils (5 × 107 cells/ml) with 1 μg of PMA per ml and examined the supernatants for cathelicidin precursors and processed antimicrobial peptides. Precursors were recognized by their apparent molecular mass on SDS-PAGE gels and by their reactivity with antibody against cathelin. Consonant with earlier studies on bovine neutrophils (32), goat secretates contained mostly intact cathelicidin precursors. However, when the secreted material was subjected to RP-HPLC, we detected trace amounts of a 5,160-Da molecule by analyzing HPLC fractions that eluted at between 34 and 36% acetonitrile (data not shown).
Antimicrobial activity.
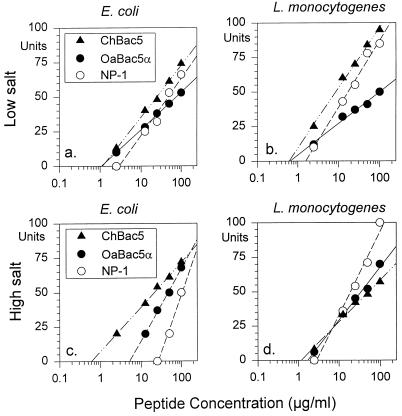
Because limited amounts of OaBac5α and ChBac5 were available for study, we used a two-stage radial diffusion assay for most studies (11, 27, 30). Under low concentrations of salt, the MICs of each peptide against E. coli ML-35p and L. monocytogenes EGD were less than 2.5 μg/ml (Fig. 5a and b). OaBac5α and ChBac5 appeared slightly more potent than rabbit defensin NP-1. High concentrations of salt reduced the activity of NP-1 against E. coli (MIC ≊ 25 μg/ml) without affecting its potency against L. monocytogenes (Fig. 5c and d). High concentrations of salt did not impair the activities of OaBac5α and ChBac5 against either organism.
FIG. 5.
Activity against E. coli and L. monocytogenes. The antimicrobial activities of OaBac5α, ChBac5, and rabbit defensin NP-1 were measured in radial diffusion assays against E. coli ML-35p (a and c) and L. monocytogenes EGD (b and d). The tests were performed under low (a and b) and high (c and d) concentrations of salt, as described in the text. The x intercepts of the least mean square regression lines through the respective data points define the minimal inhibitory concentration.
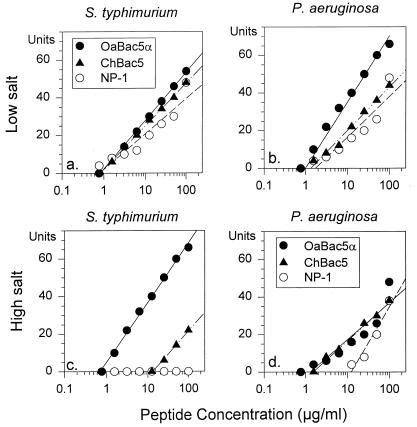
Under low concentrations of salt, OaBac5α, ChBac5, and NP-1 had approximately equal activity against P. aeruginosa, with MICs less than 2 μg/ml (Fig. 6b). The presence of 100 mM NaCl had little effect on OaBac5α or ChBac5 but decreased the activity of NP-1 considerably (Fig. 6d). ChBac5 was also active against two other strains of P. aeruginosa derived from patients with cystic fibrosis. The MICs for a highly mucoid strain were 1.2 and 5.2 μg/ml in the media containing low and high concentrations of salt, respectively. The MICs for the other strain were 0.5 and 3.6 μg/ml in these respective media.
FIG. 6.
Activity against S. typhimurium and P. aeruginosa. The antimicrobial activities of OaBac5α, ChBac5, and rabbit defensin NP-1 were measured in radial diffusion assays against S. typhimurium 14028S (a and c) and P. aeruginosa (b and d). The tests were performed under low (a and b) and high (c and d) concentrations of salt, as described in the text. The x intercepts of the least mean square regression lines through the data points define the minimal inhibitory concentration.
Figure 6a and c show that NP-1 was active against S. typhimurium 14028S under low concentrations of salt (MIC < 1 μg/ml) but lost efficacy in 100 mM NaCl (MIC > 100 μg/ml). In contrast, OaBac5α was equally active (MIC < 1 μg/ml) against S. typhimurium under both sets of conditions, but ChBac5 showed reduced activity (MIC = 10 μg/ml) in high concentrations of salt.
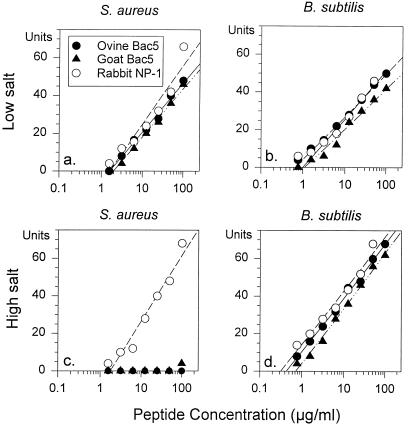
Figure 7 shows that NP-1 and both Bac5 peptides killed B. subtilis effectively under low (MIC = 1 to 2 μg/ml) and high concentrations of salt (MIC = 0.3 to 0.75 μg/ml). NP-1 retained activity (MIC = 1.5 μg/ml) against S. aureus in 100 mM NaCl, but OaBac5α and ChBac5 were inactive under these conditions. OaBac5α, ChBac5, and NP-1 were effective against C. albicans (MIC = 1.5 to 3 μg/ml) under low concentrations of salt but not in 100 mM NaCl (MIC > 100 μg/ml) (data not shown).
FIG. 7.
Activity against S. aureus and B. subtilis. The antimicrobial activities of OaBac5α, ChBac5, and rabbit defensin NP-1 were measured in radial diffusion assays against S. aureus (a and c) and B. subtilis (b and d). The tests were performed under low (a and b) and high (c and d) concentrations of salt, as described in the text. The x intercepts of the least mean square regression lines through the data points define the minimal inhibitory concentration.
LPS binding.
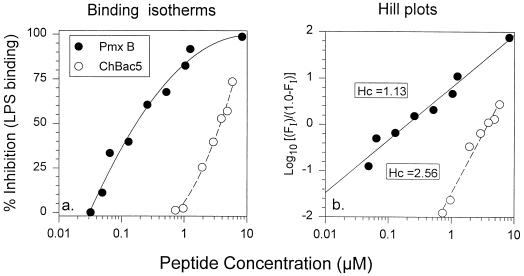
The ability of ChBac5 and polymyxin B to bind is shown in Fig. 8. Although polymyxin B had a higher affinity for E. coli 0111:B4 LPS than did ChBac5, the binding isotherm of ChBac5 was much steeper (Fig. 8a), consistent with positive cooperativity, an inference supported by the Hill plot, which gave a slope of 2.56 for the data (Fig. 8b). We did not test the ability of OaBac5 to bind LPS. More limited experiments performed with OaBac5 revealed that this peptide also bound LPS (data not shown).
FIG. 8.
LPS binding. The ability of ChBac5 and polymyxin B to bind E. coli LPS in a quantitative chromogenic Limulus assay. Note the different shapes of the binding curves in panel a. Panel b is a Hill plot of the binding data. Hc, Hill coefficient (slope).
DISCUSSION
Given the abundance of β-defensins in bovine neutrophils (22), we were surprised to see the lack of defensins in sheep and goat neutrophils (see Fig. 1). In this respect, ovine and caprine neutrophils resemble those of pigs (10) and mice (3), which also lack defensins. For the neutrophils of such animals, cathelicidins may constitute the principal repository of antimicrobial peptides, with β-defensins restricted to nonmyeloid tissues, such as epithelia and glands.
OaBac5α and ChBac5 were highly active against gram-negative bacteria (E. coli, S. typhimurium, and P. aeruginosa), exhibiting MICs of approximately 1 μg/ml under low concentrations of salt. At 100 mM concentration NaCl had little effect on their activities against E. coli and P. aeruginosa but reduced the efficacy of ChBac5 against S. typhimurium. Skerlavaj et al. reported that bovine Bac5 acted optimally against E. coli under low-ionic-strength conditions (25) and speculated that the early interaction of bovine Bac5 with gram-negative target cells involved its electrostatic binding to negatively charged surface molecules, such as LPS. Bac5 molecules may retain their activity against gram-negative bacteria in physiologic salt solutions by forming multiple hydrophobic interactions between LPS and the apolar residues in the repeated tetrameric motif.
Gennaro et al. (6) performed microdilution assays to examine the susceptibility of S. aureus, Staphylococcus epidermidis, and Streptococcus agalactiae to bovine Bac5 and reported that these gram-positive organisms were resistant to that protein (MIC > 200 μg/ml). We also tested three gram-positive organisms (S. aureus, L. monocytogenes, and B. subtilis) in the present studies. All were highly susceptible to OaBac5 and ChBac5 under low concentrations of salt (MIC < 2 μg/ml), but S. aureus was highly resistant under high concentrations of salt (100 mM NaCl). Since the Mueller-Hinton and Iso-Sensitest broths used by Gennaro et al. have high NaCl concentrations, our findings with S. aureus are consistent with theirs.
Of the eight cathelicidin genes previously described in sheep, four encoded the proline- and arginine-rich peptides designated OaBac5, OaBac6, OaBac7.5, and OaBac11 (9). The goat and sheep Bac5 peptides described in this report were very similar to each other and resembled the previously described bovine Bac5 peptide. ChBac5 and OaBac5α contained 10 and 11 arginine residues, respectively, and were highly cationic (pI > 13.0). They were also unusually proline rich, because nearly their entire length contained a repeated PPXR motif (with X representing an apolar amino acid—phenylalanine, isoleucine, or valine). This tetrameric motif is also found in bovine Bac5 (4).
Although we did not examine the structures or mechanisms of ovine and caprine Bac5, such information exists from studies of bovine Bac5 and related proline-rich antimicrobial peptides (14, 16, 28). The repeating tetrapeptide motif of Bac5 (also found in the goat and sheep peptides) assumes a polyproline II helical conformation when it interacts with acidic phospholipids (14, 16), and residues 7 to 22 of Bac5 (highly conserved in the goat and sheep peptides) appear to mediate its candidacidal activity (16). Gram-negative bacteria treated with bovine Bac5 displayed rapid decreases in respiration rate, transport, macromolecular syntheses, ATP content, and membrane integrity (25).
Although the precursor of OaBac5 was previously delineated at the nucleotide level (9), the corresponding peptide has not previously been purified and tested. While our experimental use of elastase to process cathelicidin precursors in vitro could be questioned, the in vitro-in vivo studies performed by Panyutich et al. with porcine protegrins offer strong support for this approach (15). The mass spectrometric measurements of elastase-generated ChBac5 indicate that its processing includes C-terminal trimming and amidation—features found in several other cathelin-associated antimicrobial peptides, including bovine indolicidin and porcine PR-39 and prophenin. It is noteworthy that the three residues (GKR) removed from the carboxy terminus of PR-39 propeptide when it is processed and amidated (see SwissProt accession no. P80054) are very similar to the GRR residues removed from the OaBac5 propeptide.
In summary, the present studies showed that Bac5 peptides from sheep or goats bind LPS and kill gram-negative bacteria in concentrations of NaCl similar to those found in extracellular fluids. Overall, these findings suggest that Bac5 peptides may contribute substantially to host defense against gram-negative bacterial infection in ruminants.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health grants AI 22839 and AI 40248 and by Fogarty award TW00355.
We thank Gwen Laird, Yoon Cho, Jeffrey Turner, and Jean Laufer for their expert technical assistance.
REFERENCES
- 1.Bagella L, Scocchi M, Zanetti M. cDNA sequences of three sheep myeloid cathelicidins. FEBS Lett. 1995;376:225–228. doi: 10.1016/0014-5793(95)01285-3. [DOI] [PubMed] [Google Scholar]
- 2.Del Sal G, Storici P, Schneider C, Romeo D, Zanetti M. cDNA cloning of the neutrophil bactericidal peptide indolicidin. Biochem Biophys Res Commun. 1992;187:467–472. doi: 10.1016/s0006-291x(05)81517-7. [DOI] [PubMed] [Google Scholar]
- 3.Eisenhauer P B, Lehrer R I. Mouse neutrophils lack defensins. Infect Immun. 1992;60:3446–3447. doi: 10.1128/iai.60.8.3446-3447.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frank R W, Gennaro G, Schneider K, Przybylski M, Romeo D. Amino acid sequences of two proline-rich bactenecins. Antimicrobial peptides of bovine neutrophils. J Biol Chem. 1990;265:18871–18874. [PubMed] [Google Scholar]
- 5.Ganz T, Weiss J. Antimicrobial peptides of phagocytes and epithelia. Semin Hematol. 1997;34:343–354. [PubMed] [Google Scholar]
- 6.Gennaro R, Skerlavaj B, Romeo D. Purification, composition, and activity of two bactenecins, antibacterial peptides of bovine neutrophils. Infect Immun. 1989;57:3142–3146. doi: 10.1128/iai.57.10.3142-3146.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gudmundsson G H, Agerberth B, Odeberg J, Bergman T, Olsson B, Salcedo R. The human gene FALL39 and processing of the cathelin precursor to the antibacterial peptide LL-37 in granulocytes. Eur J Biochem. 1996;238:325–332. doi: 10.1111/j.1432-1033.1996.0325z.x. [DOI] [PubMed] [Google Scholar]
- 8.Harwig S S L, Chen N P, Park A S K, Lehrer R I. Purification of cysteine-rich bioactive peptides from leukocytes by continuous acid-urea polyacrylamide gel electrophoresis (CAU-PAGE) Anal Biochem. 1993;208:382–386. doi: 10.1006/abio.1993.1065. [DOI] [PubMed] [Google Scholar]
- 9.Huttner K M, Lambeth M R, Burkin H R, Burkin D J, Broad T E. Localization and genomic organization of sheep antimicrobial peptide genes. Gene. 1998;206:85–91. doi: 10.1016/s0378-1119(97)00569-6. [DOI] [PubMed] [Google Scholar]
- 10.Kokryakov V N, Harwig S S L, Panyutich E A, Shevchenko A A, Aleshina G M, Shamova O V, Korneva H A, Lehrer R I. Protegrins: leukocyte antimicrobial peptides that combine features of corticostatic defensins and tachyplesins. FEBS Lett. 1993;327:231–236. doi: 10.1016/0014-5793(93)80175-t. [DOI] [PubMed] [Google Scholar]
- 11.Lehrer R I, Rosenman M, Harwig S S L, Jackson R, Eisenhauer P. Ultrasensitive assays for endogenous antimicrobial polypeptides. J Immunol Methods. 1991;137:167–173. doi: 10.1016/0022-1759(91)90021-7. [DOI] [PubMed] [Google Scholar]
- 12.Levy O. Antibiotic proteins of polymorphonuclear leukocytes. Eur J Haematol. 1996;56:263–277. doi: 10.1111/j.1600-0609.1996.tb00714.x. [DOI] [PubMed] [Google Scholar]
- 13.Mahoney M M, Lee A Y, Brezinski-Caliguri D J, Huttner K M. Molecular analysis of the sheep cathelin family reveals a novel antimicrobial peptide. FEBS Lett. 1995;377:519–522. doi: 10.1016/0014-5793(95)01390-3. [DOI] [PubMed] [Google Scholar]
- 14.Niidome T, Mihara H, Oka M, Hayashi T, Saiki T, Yoshida K, Aoyagi H. Structure and property of model peptides of proline/arginine-rich region in bactenecin 5. J Pept Res. 1998;51:337–345. doi: 10.1111/j.1399-3011.1998.tb01224.x. [DOI] [PubMed] [Google Scholar]
- 15.Panyutich A, Shi J, Boutz P L, Zhao C, Ganz T. Porcine polymorphonuclear leukocytes generate extracellular microbicidal activity by elastase-mediated activation of secreted proprotegrins. Infect Immun. 1997;65:978–985. doi: 10.1128/iai.65.3.978-985.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Raj P A, Marcus E, Edgerton M. Delineation of an active fragment and poly(l-proline) II conformation for candidacidal activity of bactenecin 5. Biochemistry. 1996;35:526–530. doi: 10.1021/bi951681r. [DOI] [PubMed] [Google Scholar]
- 17.Ritonja A, Kopitar M, Jerala R, Turk V. Primary structure of a new cysteine proteinase inhibitor from pig leukocytes. FEBS Lett. 1989;255:211–214. doi: 10.1016/0014-5793(89)81093-2. [DOI] [PubMed] [Google Scholar]
- 18.Romeo D, Skerlavaj B, Bolognesi M, Gennaro R. Structure and bactericidal activity of an antibiotic dodecapeptide purified from bovine neutrophils. J Biol Chem. 1988;263:9573–9575. [PubMed] [Google Scholar]
- 19.Scocchi M, Skerlavaj B, Romeo D, Gennaro R. Proteolytic cleavage by neutrophil elastase converts inactive storage proforms to antibacterial bactenecins. Eur J Biochem. 1992;209:589–595. doi: 10.1111/j.1432-1033.1992.tb17324.x. [DOI] [PubMed] [Google Scholar]
- 20.Scocchi M, Wang S, Zanetti M. Structural organization of the bovine cathelicidin gene family and identification of a novel member. FEBS Lett. 1997;417:311–315. doi: 10.1016/s0014-5793(97)01310-0. [DOI] [PubMed] [Google Scholar]
- 21.Selsted M E, Novotny M J, Morris W L, Tang Y Q, Smith W, Cullor J S. Indolicidin, a novel bactericidal tridecapeptide amide from neutrophils. J Biol Chem. 1992;267:4292–4295. [PubMed] [Google Scholar]
- 22.Selsted M E, Tang Y Q, Morris W L, McGuire P A, Novotny M J, Smith W, Henschen A H, Cullor J S. Purification, primary structures, and antibacterial activities of beta-defensins, a new family of antimicrobial peptides from bovine neutrophils. J Biol Chem. 1993;268:6641–6648. [PubMed] [Google Scholar]
- 23.Shi J, Ganz T. The role of protegrins and other elastase-activated polypeptides in the bactericidal properties of porcine inflammatory fluids. Infect Immun. 1998;66:3611–3617. doi: 10.1128/iai.66.8.3611-3617.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Skerlavaj B, Gennaro R, Bagella L, Merluzzi L, Risso A, Zanetti M. Biological characterization of two novel cathelicidin-derived peptides and identification of structural requirements for their antimicrobial and cell lytic activities. J Biol Chem. 1996;271:28375–28381. doi: 10.1074/jbc.271.45.28375. [DOI] [PubMed] [Google Scholar]
- 25.Skerlavaj B, Romeo D, Gennaro R. Rapid membrane permeabilization and inhibition of vital functions of gram-negative bacteria by bactenecins. Infect Immun. 1990;58:3724–3730. doi: 10.1128/iai.58.11.3724-3730.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sorensen O, Arnljots K, Cowland J B, Bainton D F, Borregaard N. The human antibacterial cathelicidin, hCAP-18, is synthesized in myelocytes and metamyelocytes and localized to specific granules in neutrophils. Blood. 1997;90:2796–2803. [PubMed] [Google Scholar]
- 27.Steinberg D, Lehrer R I. Designer assays for antimicrobial peptides: disputing the “one size fits all” theory. In: Shafer W M, editor. Methods in molecular biology. Totowa, N.J: Humana Press; 1997. pp. 169–187. [DOI] [PubMed] [Google Scholar]
- 28.Tani A, Lee S, Oishi O, Aoyagi H, Ohno M. Interaction of the fragments characteristic of bactenecin 7 with phospholipid bilayers and their antimicrobial activity. J Biochem. 1995;117:560–565. doi: 10.1093/oxfordjournals.jbchem.a124744. [DOI] [PubMed] [Google Scholar]
- 29.Tossi A, Scocchi M, Zanetti M, Storici P, Gennaro R. PMAP-37, a novel antibacterial peptide from pig myeloid cells. cDNA cloning, chemical synthesis and activity. Eur J Biochem. 1995;228:941–946. doi: 10.1111/j.1432-1033.1995.tb20344.x. [DOI] [PubMed] [Google Scholar]
- 30.Turner J, Cho Y, Dinh N N, Waring A J, Lehrer R I. Activities of LL-37, a cathelin-associated antimicrobial peptide of human neutrophils. Antimicrob Agents Chemother. 1998;42:2206–2214. doi: 10.1128/aac.42.9.2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zanetti M, Gennaro R, Romeo D. Cathelicidins: a novel protein family with a common proregion and a variable C-terminal antimicrobial domain. FEBS Lett. 1995;374:1–5. doi: 10.1016/0014-5793(95)01050-o. [DOI] [PubMed] [Google Scholar]
- 32.Zanetti M, Litteri L, Griffiths G, Gennaro R, Romeo D. Stimulus-induced maturation of probactenecins, precursors of neutrophil antimicrobial polypeptides. J Immunol. 1991;146:4295–4300. [PubMed] [Google Scholar]