Abstract
Previously we reported that the consecutive injection of lipopolysaccharide (LPS) into LPS-sensitized mice for the generalized Shwartzman reaction (GSR) appeared to induce the injury of renal tubular epithelial cells via apoptosis. The aim of this study was to characterize the mechanism of renal tubular epithelial cell injury in GSR. The expression of Fas and Fas ligand was immunohistochemically detected on renal tubular epithelial cells from GSR-induced mice, although neither Fas nor Fas ligand was found in cells from untreated control mice or in cells from mice receiving a single injection of LPS. GSR-induced renal tubular epithelial cell injury was produced in neither Fas-negative MRL-lpr/lpr mice nor Fas ligand-negative MRL-gld/gld mice. The administration of anti-gamma interferon antibody together with a preparative injection of LPS prevented the expression of Fas and Fas ligand and the apoptosis of renal tubular epithelial cells. A provocative injection of tumor necrosis factor alpha into LPS-sensitized mice augmented Fas and Fas ligand expression and the apoptosis of renal tubular epithelial cells. The administration of tumor necrosis factor alpha to interleukin-12-sensitized mice resulted in Fas and Fas ligand expression and the apoptosis. Sensitization with interleukin-12 together with anti-gamma interferon antibody did not cause the apoptosis of renal tubular epithelial cells. It was suggested that the Fas/Fas ligand system probably plays a critical role in the development of renal tubular epithelial cell injury through apoptotic cell death.
Bacterial lipopolysaccharide (LPS) is present on the outer membranes of all gram-negative bacteria and causes the systemic inflammatory response syndrome, endotoxic shock and disseminated intravascular coagulation (DIC) (1). The generalized Shwartzman reaction (GSR) is a potentially lethal shock reaction and is induced by two consecutive injections of LPS (called a preparative injection and a provocative injection, respectively) into animals at a 24-h interval (2, 8, 14, 21, 29). GSR is characterized by vascular occlusion, hemorrhage, perivascular accumulation of leukocytes, and necrosis (14, 29) and is known as an experimental DIC model (1, 21). It has been reported that GSR and DIC are due to systemic injuries of vascular endothelial cells (VEC) (1, 14). Previously we reported that the administration of LPS into LPS-sensitized mice induced acute injury of VEC and renal tubular epithelial cells (RTC) in GSR-induced mice (9). Further, it has been suggested that the injury of VEC is caused by apoptotic cell death and that gamma interferon (IFN-γ) and adhesion molecules play a critical role in the apoptosis of VEC (9, 10). On the other hand, the detailed mechanism of RTC injury in GSR remained unclear, although it seemed to be due to apoptotic cell death on the basis of morphological studies (9).
A series of signaling molecules can regulate apoptotic events. One potential candidate is the Fas and Fas ligand (FasL) system. Fas (Apo-1, CD95), a type I membrane protein, is a member of a family of cell surface receptors that include tumor necrosis factor (TNF) receptor, nerve growth factor receptor, CD40, CD27, CD30, and others (15, 32). FasL, a type II membrane protein, is a member of the TNF family which includes TNF-α, α- and β-chains of lymphotoxin, CD40 ligand, and CD30 ligand (16, 28). Fas induces apoptosis of various cell types, including RTC (12, 13, 23, 25–27, 33), when cross-linked with FasL. Thus, the Fas/FasL system plays an important role in signaling apoptosis. In this study, we investigated the participation of the Fas/FasL system in order to clarify the mechanism of RTC injury in GSR. Here we report the expression of Fas and FasL on RTC in GSR and its participation in the apoptotic cell death of RTC.
MATERIALS AND METHODS
Mice.
Male BALB/c, MRL/MpJ lpr/lpr (MRL-lpr), MRL/MpJ gld/gld (MRL-gld), and MRL/MpJ +/+ (MRL) mice were purchased from SLC (Hamamatsu, Japan) and used at about 6 weeks of age.
Antibodies.
Rabbit polyclonal antibody to mouse Fas and FasL were purchased from Wako Pure Chemicals, Osaka, Japan. Recombinant tumor necrosis factor alpha (TNF-α), gamma interferon (IFN-γ), interleukin-12 (IL-12), IL-1β, mouse anti-IFN-γ antibody, anti-IL-2 antibody, anti-TNF-α antibody, and anti-IL-1β antibody were obtained from Genzyme, Cambridge, Mass. Goat polyclonal antibody to mouse TNF receptor 1 (TNF-R1) and Bax were purchased from Santa Cruz Biotechnology, Santa Cruz, Calif. Hamster monoclonal antibody to mouse Fas, which can induce apoptosis in vivo (17), was purchased from MBL, Nagoya, Japan. These materials were used according to the manufacturers’ instructions.
Development of GSR.
LPS was extracted from Klebsiella pneumoniae O3 LEN-1 by the phenol-water method (34, 36). GSR was induced in mice by two consecutive injections of LPS (8, 18, 21). The optimal dose of LPS (5 μg) was injected intradermally into the footpads of mice as a preparative injection for priming of GSR. A provocative injection of LPS (400 μg) was administered intravenously 18 to 24 h after a preparative injection. Three to four mice were used in each experimental group. In preliminary experiments, more than 80% of the mice were dead within 12 h of the provocative injection of LPS.
In situ specific labeling of fragmented DNA.
Apoptotic cells were detected 5 h after LPS injection and increased up to 7 h. Mice were sacrificed 7 h after challenge of LPS unless otherwise stated, and the kidneys were collected. The tissues were fixed with formalin and cut serially into 4- to 6-μm sections. The sections were deparafinized for the in situ nick end labeling specific for fragmented DNA. The technique reported originally by Gavrieli et al. (6) was used as described previously (37).
Immunohistochemical staining.
A part of kidney removed was fixed in formalin, and the other part was frozen immediately in ACT compound in liquid N2. Paraffin sections of the kidneys were deparafinized, and the endogenous peroxidase activity was blocked with methanol containing 0.3% hydrogen peroxide for 10 min at room temperature. The sections were washed in 0.01 M phosphate-buffered saline (PBS) at pH 7.2 containing 10% normal horse serum and incubated overnight at 4°C with a 1:300 dilution of anti-Fas antibody or with a 1:200 dilution of anti-FasL antibody. Horseradish-conjugated goat anti-rabbit immunoglobulin (Ig) antibody was used at 1:200 after washing. Immune complexes were detected with a solution of 3,-3-diaminobenzidine (0.2 mg/ml) and hydrogen peroxide in 0.05 M Tris-HCl buffer. Sections were counterstained with methyl green. Similarly, the frozen sections were incubated with a 1:200 dilution of anti-TNF-R1 or Bax antibody and then treated with horseradish-conjugated second antibody as described above. In negative control sections, an irrelevant antibody was used.
Immunoblot analysis.
Kidneys were homogenized at 4°C in PBS containing 10−4 M phenylmethylsulfonyl fluoride (PMSF) and 1 μg of aprotinin (Sigma, St. Louis, Mo.)/ml by a homogenizer. The homogenate was diluted by suspension buffer (0.01 M Tris, 0.1 M NaCl, 1 mM EDTA, 10−4 M PMSF) containing 1% protease inhibitor mix (Sigma). The homogenate was centrifuged at 3,000 rpm for 20 min at 4°C, and the supernatant was used as the kidney homogenate. The kidney homogenate was diluted with an equal volume of 2× sample buffer containing 0.1 M Tris, 20% glycerol, 4% sodium dodecyl sulfate (SDS), 200 mM dithiothreitol, and 0.2% bromophenol blue and boiled for 5 min. Equal amounts which were measured by Coomassie plus protein assay reagent (Pierce, Rockford, Ill.) were loaded and separated by SDS-polyacrylamide gel electrophoresis (PAGE) by using 5 to 20% gradient gel. Proteins separated by SDS-PAGE were transferred to a membrane filter (Immunobillon; Nihon Millipore, Tokyo, Japan) by electroblotting (30). The filters were blocked with 5% skim milk in PBS. After being washed in PBS containing 0.05% Tween 20 (PBS-T), the blots were treated with a 1:200 dilution of anti-Fas antibody or anti-TNF-R1 antibody and then washed with PBS-T three times. Resulting immune complexes were reacted with a 1:1,000 dilution of horse radish peroxidase-conjugated goat IgG or rabbit IgG antibody (Nippon Bio-Rad Laboratories, Tokyo, Japan) in PBS-T. Finally, labeled antigen bands were detected by an ECL Western blotting detection reagent (Amersham, Buckinghamshire, United Kingdom). A prestained molecular weight standard kit from Nippon Bio-Rad was used as a reference.
Analysis of tissue mRNA by reverse transcription-PCR.
At various times after LPS injection, kidneys were removed from mice and frozen at −70°C. Total RNA was isolated by using Isogen (Nippon Gene, Toyama, Japan) according to the manufacturer’s instructions. Total RNA was analyzed by RT-PCR by using the Titan one-tube RT-PCR system (Boehringer, Mannheim, Germany). TNF-R1 cDNA was amplified by using the primers (5′-CAGGGAGTGAAAAGGGCAC-3′ and 5′-GTAGCGTTGGAACTGGTTCTC-3′) (24). The mouse GAPDH, a housekeeping transcript, was amplified by using the primers (5′-AGATCCACAACGGATACATT-3′ and 5′-TCCCTCAAGATTGTCAGCAA-3′) for semiquantitative comparison of TNF-R1 transcripts. The products were confirmed by the presence of the TNF-R1 band (441 bp) and the GAPDH band (309 bp) on an ethidium bromide-stained gel.
RESULTS
Expression of Fas and FasL molecules on RTC in GSR-induced mice.
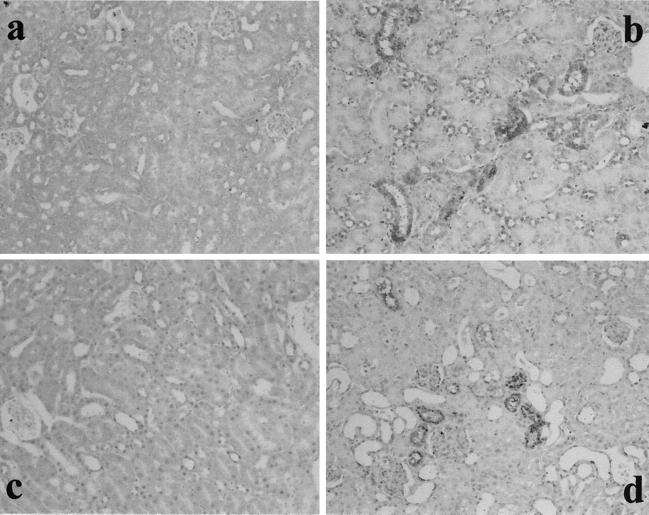
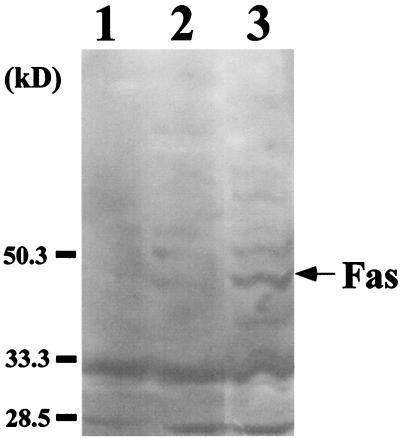
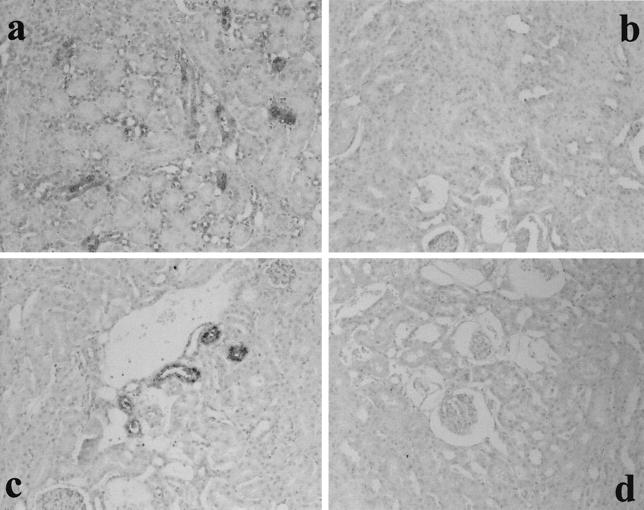
To determine whether the Fas/FasL system affected the development of RTC injury in GSR-induced mice, Fas and FasL expression were studied by immunohistochemical analysis. The result of the experiment is shown in Fig. 1. In GSR-induced mice, proximal RTC in the cortex showed positive stainings for Fas and FasL. Fas and FasL were expressed exclusively on RTC and not on mesangial cells or VEC. Previously we demonstrated that proximal RTC underwent apoptosis in GSR (9). Fas and FasL expression were not detected in mice injected with saline or with a single injection of LPS (5 or 400 μg, respectively). The appearance of Fas in GSR was also confirmed by immunoblotting (Fig. 2).
FIG. 1.
Fas and FasL expression in RTC from GSR-induced mice. The expression of Fas (a and b) and FasL (c and d) was immunohistochemically stained in RTC from GSR-induced mice (b and d) but not in RTC from saline-treated control mice (a and c). Magnification, ×200.
FIG. 2.
Detection of Fas expression in kidney extract from GSR-induced mice by immunoblotting. The expression of Fas was examined in kidney extract from saline-treated mice (lane 1), mice receiving a single injection of LPS (lane 2), and GSR-induced mice (lane 3). Quantitative analysis indicated that the intensity of Fas expression in GSR was approximately 5.2 times higher than that in mice receiving a single injection.
Failure of induction of RTC apoptosis in MRL-lpr and MRL-gld mice.
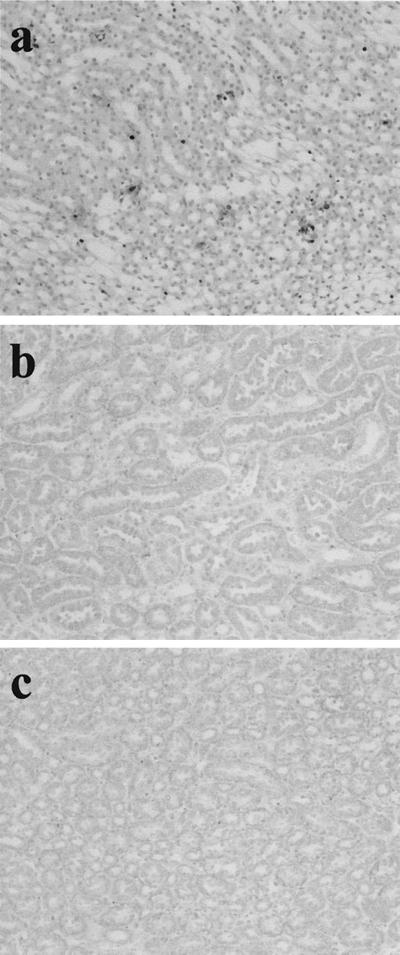
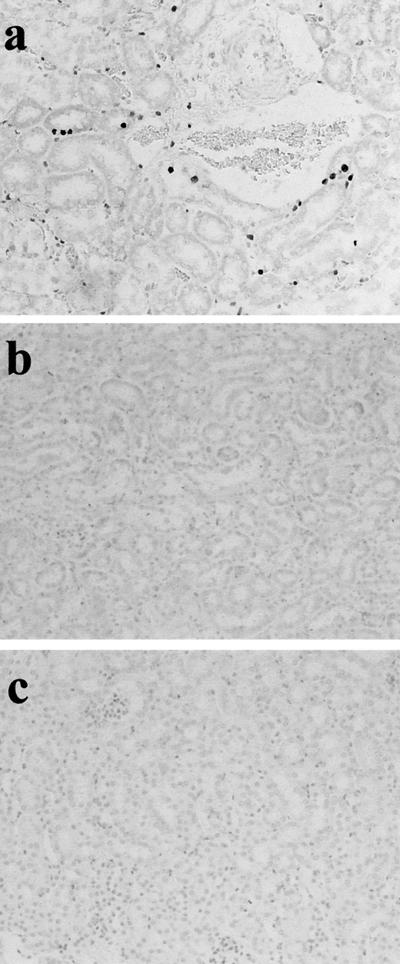
To confirm the participation of the Fas/FasL system in RTC injury, GSR was induced in Fas-negative MRL-lpr mice and FasL-negative MRL-gld mice. The results of the experiment are shown in Fig. 3. RTC of GSR-induced MRL-lpr and MRL-gld mice were not morphologically damaged. The specific labeling of fragmented DNA did not detect any apoptotic cells in RTC of those mice, and MRL-lpr and MRL-gld mice did not die after GSR treatment. However, apoptotic cells were detected in GSR-induced MRL control mice.
FIG. 3.
Failure of induction of RTC apoptosis in MRL-lpr mice and MRL-gld mice receiving two consecutive injections of LPS. Apoptotic cells in renal tubules from MRL control mice (a), MRL-lpr mice (b), and MRL-gld mice (c) were stained by the specific labeling of fragmented DNA. Magnification, ×200.
Augmentation of Fas and FasL expression and RTC apoptosis by the administration of TNF-α into LPS-sensitized mice.
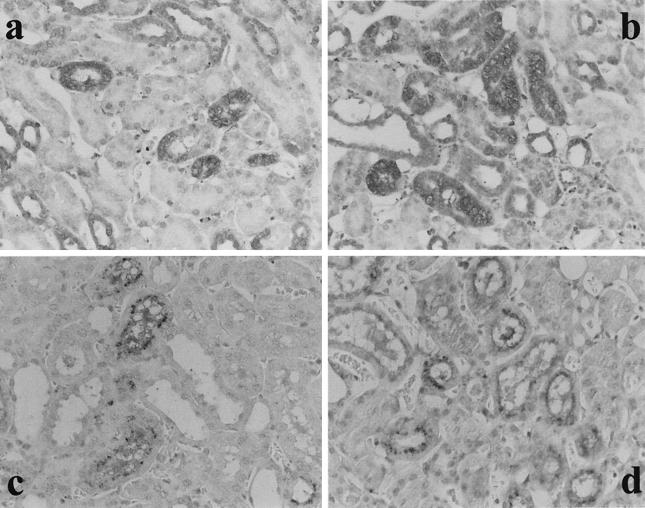
Previously we reported that the administration of TNF-α in place of LPS into LPS-primed mice (TNF-induced GSR) caused much more marked RTC apoptosis than in GSR-induced mice (9). To determine if and how Fas and FasL expression were related to augmented RTC apoptosis in TNF-induced GSR, we compared Fas and FasL expression between GSR and TNF-induced GSR. Immunohistochemical results suggested that Fas and FasL expression were augmented in mice with TNF-induced GSR compared to GSR (Fig. 4), suggesting quantitative correlation between the expression of Fas and FasL and RTC apoptosis in TNF-induced GSR.
FIG. 4.
Augmentation of Fas and FasL expression in RTC in TNF-induced GSR. LPS (a and c) or TNF-α (b and d) was injected into LPS-primed mice, and the expression of Fas (a and b) and FasL (c and d) in RTC was stained immunohistochemically. Note the augmented expression of Fas and FasL on renal tubular cells in TNF-induced GSR. Magnification, ×400.
Participation of IFN-γ in the expression of Fas and FasL on RTC.
As discussed in the preceding paragraph, immunohistochemical results showed that Fas and FasL expression were augmented by TNF-α. We sought to determine whether cytokines participate in Fas and FasL expression in GSR. First, mice were primed with LPS plus the neutralizing antibody against IFN-γ, IL-1β, or IL-2, and then the provocative injection of LPS was carried out to induce Fas and FasL expression. The simultaneous administration of anti-IFN-γ antibody (100 μg) in a preparative injection of LPS induced neither the expression of Fas and FasL on RTC (Fig. 5) nor the apoptosis of RTC. However, anti-IL-1β or IL-2 antibody (100 μg) did not affect Fas and FasL expression.
FIG. 5.
Inhibition of Fas and FasL expression in RTC of GSR-induced mice by anti-IFN-γ antibody. LPS was administered to mice primed with LPS alone (a and c) or with LPS and anti-IFN-γ antibody (b and d). The expression of Fas (a and b) and FasL (c and d) was stained immunohistochemically. Magnification, ×200.
Reconstitution of GSR with cytokines in Fas and FasL-mediated apoptosis of RTC.
IFN-γ and TNF-α were suggested to play a critical role in sensitization and induction of Fas and FasL expression, respectively. We tried to reconstitute Fas and FasL expression and RTC apoptosis with cytokines alone. Since IL-12 is known as a potent inducer of IFN-γ (19, 22, 35), TNF-α was injected into IL-12-primed mice. The successive treatment with IL-12 (250 ng) and TNF-α (1.5 μg) resulted in Fas and FasL expression and apoptosis on RTC. However, the degree of Fas and FasL expression and RTC apoptosis was slightly lower compared to that in GSR-induced mice. The administration of IL-12 together with anti-IFN-γ antibody (100 μg) significantly inhibited RTC apoptosis (Fig. 6), suggesting a critical role for IFN-γ.
FIG. 6.
Induction of RTC apoptosis in IL-12-primed mice by challenge with TNF-α and its inhibition by anti-IFN-γ antibody. IL-12-primed mice were injected with TNF-α alone (a), TNF-α and anti-IFN-γ antibody (b), or anti-IFN-γ antibody alone (c). The kidneys were subjected to the specific labeling of fragmented DNA. Magnification, ×200.
Role of TNF/TNF-R system in GSR-induced apoptosis of RTC.
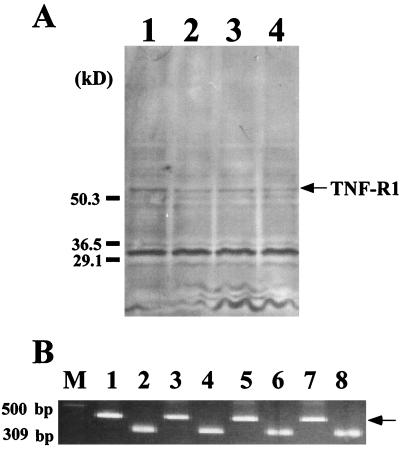
Because TNF-α is critical for the induction of RTC apoptosis in GSR, we examined whether TNF-R might affect the development of RTC injury. The expression of TNF-R1 was examined in GSR-induced mice and untreated mice by immunoblotting and RT-PCR (Fig. 7). TNF-R1 was detected in untreated control mice and GSR-induced mice by using these methods. There was no significant difference in TNF-R1 expression between these groups of mice. Further, the results of an immunohistochemical analysis suggested the same conclusion (data not shown).
FIG. 7.
No change of TNF-R1 expression in kidneys of mice treated with GSR. Expression of TNF-R1 was examined by immunoblotting (A) and RT-PCR (B). Mice were treated with PBS (lane 1), LPS alone (lane 2), GSR (lane 3), and TNF-induced GSR (lane 4). TNF-R1 bands defined by the antibody are shown in panel A. TNF-R1 mRNA products of 441 bp were analyzed by RT-PCR (B) with total RNA from mice treated with PBS (lanes 1 and 2), LPS alone (lanes 3 and 4), GSR (lanes 5 and 6), and TNF-induced GSR (lanes 7 and 8). Lanes 1 to 8, mRNA products of TNF-R1 (lanes 1, 3, 5, and 7) and the housekeeping gene GAPDH (lanes 2, 4, 6, and 8). The arrow indicates the size of the expected amplification product of mRNA for TNF-R1. M, DNA molecular size marker.
Detection of Bax on RTC of GSR-induced mice.
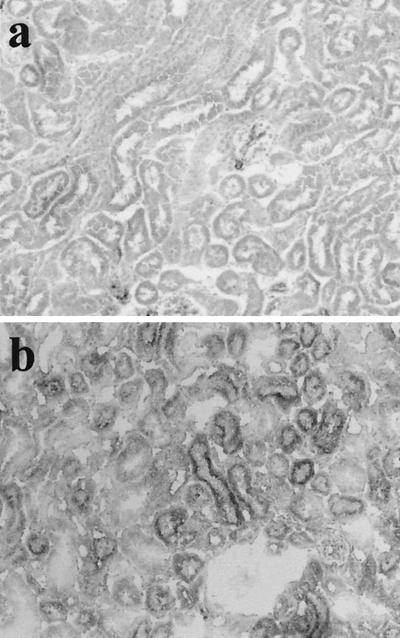
The findings of the present study strongly suggest that RTC injury in GSR is due to apoptotic cell death via the Fas/FasL system. Since the Bcl-2 family is known to be involved in Fas/FasL-dependent apoptosis (5), we studied the expression of Bcl-2 and Bax on the RTC of GSR-induced mice to confirm the participation of Fas/FasL in this study. The expression of Bax was immunohistochemically detected in the RTC of TNF-induced GSR mice, and marginal expression of Bax was observed in the RTC of GSR-induced mice but not in the RTC of untreated mice (Fig. 8). In contrast, the expression of Bcl-2 was not detected in mice with GSR or TNF-induced GSR or in untreated mice (data not shown).
FIG. 8.
Detection of Bax product in RTC of mice treated with TNF-induced GSR. Bax product was stained immunohistochemically by the antibody. Mice were treated with PBS alone (a) or TNF-induced GSR (b). Magnification, ×200.
DISCUSSION
In this study we demonstrated that the expression of Fas and FasL was induced in the RTC of GSR-induced mice and that Fas and FasL expression might play an important role in their apoptotic cell death. Several lines of evidence suggest that the Fas and FasL expression in RTC are involved in renal tubular apoptosis in GSR-induced mice. First, Fas and FasL were exclusively expressed in RTC undergoing apoptosis; second, the apoptosis of RTC was not produced in Fas-negative or FasL-negative mutant mice; third, Fas and FasL expression was related to the apoptosis of RTC. Once again, LPS-induced Fas and FasL expression was closely associated with the apoptosis of RTC. The expression pattern of Fas and FasL might suggest the production of RTC apoptosis with the juxtacrine interaction. It was reported previously that a single injection of LPS induced the expression of Fas on RTC (20). In our system, however, Fas and FasL expression required consecutive injections of LPS. Consecutive injections of LPS might be necessary for the induction of RTC apoptosis in GSR. Previously we reported that LPS-induced injury of RTC appeared to be mediated with apoptosis (9). This finding was supported by the participation of apoptosis-related molecules, such as the Fas/FasL system and Bax, in this study.
IFN-γ and TNF-α may play a critical role in the expression of Fas and FasL. The importance of IFN-γ in the sensitization for Fas and FasL expression was supported by the experiments with anti-IFN-γ antibody and IL-12. On the other hand, TNF-induced GSR augmented both the expression of Fas and FasL and the induction of RTC apoptosis. TNF-α seemed to play an important role in the induction of Fas and FasL. However, the administration of TNF-α alone to normal mice did not affect Fas and FasL expression (data not shown). It was therefore suggested that successive collaboration of IFN-γ and TNF-α might be essential for the expression of Fas and FasL in GSR. The administration of TNF-α to IFN-γ-primed mice did not induce apoptosis of RTC (data not shown), whereas that of TNF-α to IL-12-primed mice did. Although the exact mechanism of Fas and FasL expression is still unclear, other molecules such as IFN-γ might play a role.
Fas is reported to be expressed in RTC even under normal conditions (3, 5, 25). However, the immunochemical staining and immunoblotting could not detect Fas expression in the RTC of normal mice in the present study. These methods might not be sensitive enough to detect the faint expression of Fas. In fact, we could detect mRNA for Fas in normal mice by RT-PCR with renal extracts (data not shown), although it was unclear whether mRNA for Fas was derived from RTC. Further, the injection of anti-Fas antibody into normal mice or LPS-sensitized mice did not cause the apoptosis of RTC (data not shown). The effects of Fas expression in LPS-sensitized mice and normal mice might be negligible or absent, even though expression was marginal.
The mechanism in the apoptosis of VEC and RTC in GSR-induced mice might be different. GSR led to the injury of VEC and RTC, and both injuries were essentially dependent on apoptotic cell death. However, the Fas and FasL system appeared to be involved in RTC apoptosis. On the other hand, adhesion molecules seemed to participate in the injury of VEC (10). Interestingly, both IFN-γ and TNF-α were essential for both injuries in GSR-induced mice. The critical role of IFN-γ in sensitization was common to VEC and RTC. Moreover, TNF-α was critical for the induction of apoptosis in RTC and VEC. Thus, IFN-γ and TNF-α might regulate apoptosis of RTC and VEC through different mechanisms.
It was unlikely that the TNF/TNF-R system might induce the apoptosis of RTC in GSR-induced mice. First, the injection of TNF-α into normal mice did not cause the apoptosis of RTC. Second, treatments with GSR and TNF-induced GSR could not induce the apoptosis of RTC in MRL-gld and MRL-lpr mice carrying TNF-R (38). Third, there was no significant difference in TNF-R1 expression between the untreated control mice and the GSR-induced mice. However, we could not exclude the possibility that the TNF/TNF-R system might be partly involved in this apoptosis.
GSR is known as an experimental DIC model. Clinical DIC is frequently accompanied by renal tubular necrosis and nephropathy (4, 7, 11, 31). In this study, it was demonstrated that RTC underwent apoptosis in GSR-induced mice and that it might be mediated by the Fas-FasL system. Therefore, it is of particular interest to determine whether the apoptosis via the Fas-FasL system might be involved in the injury of RTC in clinical DIC.
ACKNOWLEDGMENTS
This work was supported by a grant from the Ministry of Education, Science and Culture of Japan.
We are grateful to K. Takahashi for excellent technical assistance.
REFERENCES
- 1.Archer L T. Pathologic manifestations of septic shock. In: Proctor R A, editor. Handbook of endotoxins. Vol. 4. Amsterdam, The Netherlands: Elsevier Science Publishers; 1986. pp. 18–54. [Google Scholar]
- 2.Billiau A, Heremans H, Vandekerckhove F, Dillen C. Anti-interferon-γ antibody protects mice against the generalized Shwartzman reaction. Eur J Immunol. 1987;17:1851–1854. doi: 10.1002/eji.1830171228. [DOI] [PubMed] [Google Scholar]
- 3.Boonstra J G, van der Woude F J, Wever P C, Laterveer J C, Daha M R, van Kooten C. Expression and function of Fas (CD95) on human renal tubular epithelial cells. J Am Soc Nephrol. 1997;8:1517–1524. doi: 10.1681/ASN.V8101517. [DOI] [PubMed] [Google Scholar]
- 4.Dahmash N S, Chowdhury N H, Fayed D F. Septic shock in critically ill patients: aetiology, management and outcome. J Infect. 1993;26:159–70. doi: 10.1016/0163-4453(93)92815-e. [DOI] [PubMed] [Google Scholar]
- 5.Eischen C M, Leibson J L. The Fas pathway in apoptosis. In: Kaufmann S H, editor. Advances in pharmacology. 41. Apoptosis. San Diego, Calif: Academic Press; 1997. pp. 107–130. [DOI] [PubMed] [Google Scholar]
- 6.Gavrieli Y, Sherman Y, Ben-Sasson S A. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992;119:493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hale D J, Robinson J A, Loeb H S, Gunnar R M. Pathophysiology of endotoxin shock in man. In: Proctor R A, editor. Handbook of endotoxins. Vol. 4. Amsterdam, The Netherlands: Elsevier Science Publishers; 1986. pp. 1–17. [Google Scholar]
- 8.Heremans H, van Damme J, Dillen C, Dijkmans R, Billiau A. Interferon γ, a mediator of lethal lipopolysaccharide-induced Shwartzman-like shock reactions in mice. J Exp Med. 1990;171:1853–1869. doi: 10.1084/jem.171.6.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koide N, Abe K, Narita K, Kato Y, Sugiyama T, Jiang G Z, Yokochi T. Apoptotic cell death of vascular endothelial cells and renal tubular cells in the generalized Shwartzman reaction. FEMS Immunol Med Microbiol. 1996;16:205–211. doi: 10.1111/j.1574-695X.1996.tb00137.x. [DOI] [PubMed] [Google Scholar]
- 10.Koide N, Abe K, Narita K, Kato Y, Sugiyama T, Yoshida T, Yokochi T. Expression of intracellular adhesion molecule (ICAM-1) on vascular endothelial cells and renal tubular cells in the generalized Shwartzman reaction as an experimental disseminated intravascular coagulation model. FEMS Immunol Med Microbiol. 1997;18:67–74. doi: 10.1111/j.1574-695X.1997.tb01029.x. [DOI] [PubMed] [Google Scholar]
- 11.Kreger B E, Craven D E, McCabe W R. Gram-negative bacteremia. IV. Re-evaluation of clinical features and treatment in 612 patients. Am J Med. 1980;68:344–355. doi: 10.1016/0002-9343(80)90102-3. [DOI] [PubMed] [Google Scholar]
- 12.Lieberthal W, Levine J S. Mechanism of apoptosis and its potential role in renal tubular epithelial cell injury. Am J Physiol. 1996;271:477–488. doi: 10.1152/ajprenal.1996.271.3.F477. [DOI] [PubMed] [Google Scholar]
- 13.Miyawaki T, Uehara T, Nibu R, Tsuji T, Yachie A, Yonehara S, Taniguchi N. Differential expression of apoptosis-related Fas antigen on lymphocyte subpopulations in human peripheral blood. J Immunol. 1992;149:3753–3758. [PubMed] [Google Scholar]
- 14.Movat H Z, Burrowes C E. The local Shwartzman reaction: endotoxin-mediated inflammatory and thrombo-hemorrhagic lesions. In: Berry L J, editor. Handbook of endotoxins. Vol. 3. Amsterdam, The Netherlands: Elsevier Science Publishers; 1985. pp. 260–302. [Google Scholar]
- 15.Nagata S, Golstein P. The Fas death factor. Science. 1995;267:1449–1456. doi: 10.1126/science.7533326. [DOI] [PubMed] [Google Scholar]
- 16.Nagata S, Suda T. Fas and Fas ligand: lpr and gld mutations. Immunol Today. 1995;16:39–43. doi: 10.1016/0167-5699(95)80069-7. [DOI] [PubMed] [Google Scholar]
- 17.Nishimura Y, Hirabayashi Y, Matsuzaki Y, Musette P, Ishii A, Nakauchi H, Inoue T, Yonehara S. In vivo analysis of Fas antigen-mediated apoptosis: effects of agonistic anti-mouse Fas mAb on thymus, spleen and liver. Int Immunol. 1996;9:307–316. doi: 10.1093/intimm/9.2.307. [DOI] [PubMed] [Google Scholar]
- 18.Ogasawara K, Takeda K, Hashimoto W, Satoh M, Okuyama R, Yanai N, Obinata M, Kumagai K, Takada H, Hiraide H, Seki S. Involvement of NK1+ T cells and their IFN-gamma production in the generalized Shwartzman reaction. J Immunol. 1998;160:3522–3527. [PubMed] [Google Scholar]
- 19.Orange J S, Biron C A. An absolute and restricted requirement for IL-12 in natural killer cell IFN-gamma production and antiviral defense. Studies of natural killer and T cell responses in contrasting viral infection. J Immunol. 1996;156:1138–1142. [PubMed] [Google Scholar]
- 20.Ortiz-Arduan A, Danoff T M, Kalluri R, Gonzalez-Cuadrado S, Karp S L, Elkon K, Egido J, Neilson E G. Regulation of Fas and Fas ligand expression in cultured murine renal cells and in the kidney during endotoxemia. Am J Physiol. 1996;271:1193–1201. doi: 10.1152/ajprenal.1996.271.6.F1193. [DOI] [PubMed] [Google Scholar]
- 21.Ozmen L, Pericin M, Hkimi J, Chionirw R H, Wysocka M, Trichieri G, Gately M, Garotta G. Interleukin 12, interferon γ, and tumor necrosis factor α are key cytokines of the generalized Shwartzman reaction. J Exp Med. 1994;180:907–915. doi: 10.1084/jem.180.3.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Puddu P, Fantuzzi L, Borghi P, Varano B, Rainaldi G, Guillemard E, Malorni W, Nicaise P, Wolf S F, Belardelli F, Gessani S. IL-12 induces IFN-gamma expression and secretion in mouse peritoneal macrophages. J Immunol. 1997;159:3490–3497. [PubMed] [Google Scholar]
- 23.Robertson M J, Manley T J, Pichert G, Cameron C, Cochran K J, Levine H, Ritz J. Functional consequences of APO-1/Fas (CD95) antigen expression by normal and neoplastic hematopoietic cells. Leuk Lymphoma. 1995;17:51–61. doi: 10.3109/10428199509051703. [DOI] [PubMed] [Google Scholar]
- 24.Salkowski C A, Detore G, McNally R, van Rooijen N, Vogel S N. Regulation of inducible nitric synthase messenger RNA expression and nitric oxide production by lipopolysaccharide in vivo: the roles of macrophages, endogenous IFN-gamma, and TNF receptor-1-mediated signaling. J Immunol. 1997;158:905–912. [PubMed] [Google Scholar]
- 25.Schelling J R, Nkemere N, Kopp J B, Ronald Cleveland P. Fas-dependent fratricidal apoptosis is a mechanism of tubular epithelial cell deletion in chronic renal failure. Lab Investig. 1998;78:813–824. [PubMed] [Google Scholar]
- 26.Schulze-Osthoff K, Ferrari D, Los M, Wesselborg S, Peter M E. Apoptosis signaling by death receptors. Eur J Biochem. 1998;254:439–459. doi: 10.1046/j.1432-1327.1998.2540439.x. [DOI] [PubMed] [Google Scholar]
- 27.Shoji N, Miyazaki M, Kobayashi N, Saito T, Abe K, Saito H, Nakane P K, Nakanishi Y, Koji T. Induction of apoptosis in ischemia-reperfusion model of mouse kidney: possible involvement of Fas. J Am Soc Nephrol. 1998;9:620–631. doi: 10.1681/ASN.V94620. [DOI] [PubMed] [Google Scholar]
- 28.Suda T, Okazaki T, Naito Y, Yokota T, Arai N, Ozaki S, Nakao K, Nagata S. Expression of the Fas ligand in cells of T cell lineage. J Immunol. 1995;154:3806–3813. [PubMed] [Google Scholar]
- 29.Thomas L, Good R A. Studies on the generalized Shwartzman reaction. I. General observations concerning the phenomenon. J Exp Med. 1952;96:605–624. doi: 10.1084/jem.96.6.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Towbin H, Staehelin T, Gordon J. Electric transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wardle E N. Endotoxin and acute renal failure. Nephron. 1975;14:321–332. doi: 10.1159/000180463. [DOI] [PubMed] [Google Scholar]
- 32.Watanabe-Fukunaga R, Brannan C I, Itoh N, Yonehara S, Copeland N G, Jenkins N A, Nagata S. The cDNA structure, expression, and chromosomal assignment of the mouse Fas antigen. J Immunol. 1992;148:1274–1279. [PubMed] [Google Scholar]
- 33.Wesselborg S, Janssen O, Kabelitz D. Induction of activation-driven death (apoptosis) in activated but not resting peripheral blood T cells. J Immunol. 1993;150:4338–4345. [PubMed] [Google Scholar]
- 34.Westphal O, Jann K. Bacterial lipopolysaccharides—extraction with phenol water and further application of the procedure. Methods Carbohydr Chem. 1965;5:83–91. [Google Scholar]
- 35.Wysocka M, Kubin M, Vieira L Q, Ozmen L, Garotta G, Scott P, Trinchieri G. Interleukin-12 is required for interferon-gamma production and lethality in lipopolysaccharide-induced shock in mice. Eur J Immunol. 1995;25:672–676. doi: 10.1002/eji.1830250307. [DOI] [PubMed] [Google Scholar]
- 36.Yokochi T, Inoue Y, Yokoo J, Kimura Y, Kato N. Retention of bacterial lipopolysaccharide at the site of subcutaneous injection. Infect Immun. 1989;57:1786–1791. doi: 10.1128/iai.57.6.1786-1791.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang X M, Morikawa A, Takahashi K, Jiang G Z, Kato Y, Sugiyama T, Kawai M, Fukada M, Yokochi T. Localization of apoptosis (programmed cell death) in mice by administration of lipopolysaccharide. Microbiol Immunol. 1994;38:669–671. doi: 10.1111/j.1348-0421.1994.tb01838.x. [DOI] [PubMed] [Google Scholar]
- 38.Zhou T, Edwards III C K, Yang P, Wang Z, Bluethmann H, Mountz J D. Greatly accelerated lymphadenopathy and auto immune disease in lpr mice lacking tumor necrosis factor receptor 1. J Immunol. 1996;156:2661–2665. [PubMed] [Google Scholar]