Abstract
The COVID-19 pandemic has resulted in societal-level changes to sleep and other behavioral patterns. Objective data would allow for a greater understanding of sleep-related changes at the population level. About 163,524 active Fitbit users from 6 major US cities contributed data, representing areas particularly hard-hit by the pandemic (Chicago, Houston, Los Angeles, New York, San Francisco, and Miami). Sleep variables extracted include nightly and weekly mean sleep duration and bedtime, and variability (standard deviation) of sleep duration and bedtime. Deviation from similar timeframes in 2018 and 2019 were examined, as were changes in these sleep metrics during the pandemic, relationships to changes in resting heart rate, and changes during re-opening in May and June. Overall, compared to 2019, mean sleep duration in 2020 was higher among nearly all groups, mean sleep phase shifted later for nearly all groups, and mean sleep duration and bedtime variability decreased for nearly all groups (owing to decreased weekday-weekend differences). Over the course of January to April 2020, mean sleep duration increased, mean bedtime shifted later, and mean sleep duration variability decreased. Changes in observed resting heart rate correlated positively with changes in sleep and negatively with activity levels. In later months (May and June), many of these changes started to drift back to historical norms.
Keywords: Sleep, Wearables, Coronavirus, Epidemiology
Introduction
Over the course of 2020, the global COVID-19 pandemic spread across the world. Daily schedules have changed in many ways. Many countries and municipalities around the world engaged social distancing measures and shelter-in-place orders. Most of these measures went into effect in March and continued into April. In some places, especially in the US, many of these orders were at least partially lifted in May and June. Millions of people have adapted to these changes, and this adaptation provides a unique opportunity to study how individuals responded to the recent social changes at a massive scale.
Sleep is socially driven, and environmental and societal factors can impact sleep.1 The social-ecological model of sleep and health suggests that sleep is determined by a combination of individual-level factors (eg, behaviors, beliefs, biology), which are embedded within social-level factors (eg, social networks, work/school, family/home), which themselves are embedded within societal-level factors (eg, technology, globalization, racism).2 , 3 The pandemic represents a rare opportunity to probe the impact on sleep by a societal-level factor that has impacted sleep experience in various ways, including schedule disruptions, changes to sleep opportunity, and changes to sleep-related symptoms and reports.4
In this study, a large number of Fitbit users from 6 major US cities variously impacted by the COVID-19 pandemic examined changes in their sleep behaviors and patterns. Specifically, this study examined the following exploratory questions:
-
(1)
Did sleep patterns during the pandemic reflect a departure from previous years?
-
(2)
Did sleep duration change over the course of the pandemic?
-
(3)
Did sleep timing shift later during the pandemic?
-
(4)
Did weekday-weekend discrepancies reduce during the pandemic?
-
(5)
Were changes in sleep reflected in changes to health (i.e., heart rate and physical activity)? And
-
(6)
How did sleep change in later months of the pandemic (May and June), compared to previous months, as stay-at-home orders were generally lifted?
Methods
Participants
Data were obtained from 163,524 Fitbit users who wore their heart rate enabled Fitbit device to sleep and had detected sleep stages at least 10 days in the month of January, the baseline period; and synced their devices at least once in the last 10 days of April. In addition, potential participants needed to reside in one of 6 target cities: Chicago, Illinois; Houston, Texas; Los Angeles, California; San Francisco, California; New York City, New York; and Miami, Florida. These cities were chosen because they represent large urban centers in the US. In addition, specific focus on New York and cities in California was intended to address the intensive lockdown procedures in those states, inclusion of cities in Florida and Texas were included because of more relaxed procedures in those states, and Chicago was chosen because it represents midwest and includes a very diverse user base. All of these cities were also selected because the Fitbit user base is sufficiently large to randomly aggregate a sizeable and diverse sample. Selection was completely random, and demographic grouping was a result of that random selection. No stratified or other complex sampling was used. Given the nature of these deidentified data, detailed information about residence and other geographic data are not available. However, every time a user synchronizes their data with the app, some general location data is provided. Based on this information, city of residence could be inferred. Participants who met these criteria were randomly selected.
All participants agreed to allow their deidentified data to be used for research purposes, as outlined in the Fitbit Terms and Conditions document applicable at the time of data collection.5 This research was performed internally by Fitbit and did not require separate IRB approval. All data were deidentified prior to analysis.
Measures
Sleep was recorded using Fitbit devices that were also enabled to record heart rate. Users of the following devices were included for analysis: Alta HR, Blaze, Charge 2, Charge 3, Charge 4, Inspire HR, Ionic, Versa, Versa 2, and Versa Lite. A complete breakdown is not possible since some users may have used different devices at different times. However, the sleep-wake detection across devices should be generally invariable (given hardware and software scoring parameters). These devices all used the same algorithm for sleep estimation during the study period. Regarding the sensors, there are slight variations in optics, which are not expected to affect accuracy of sleep estimation. Sleep-wake determinations are accomplished via an algorithm that uses information from movement (measured via accelerometer) and heart rate (measured through optical plethysmography).6 The devices in the field over the course of the assessment period did not receive any software or hardware update that would have impacted their sleep scoring. Although updates across various platforms may have occurred, the methods used in the devices included in this study did not experience any change that would have affected sleep scoring over the course of the study period. Fitbit data were uploaded to central servers every time users synched their device with the Fitbit app, which downloads and processes all the data. These data were accessed directly from Fitbit servers.
Active users were defined as those who synchronized their device at least once in the last 10 days. Absent any empirically validated number of nights of wearable data needed to establish a pattern, we chose 10 nights as a cutoff. Although some previous studies use as few as 3 nights of wearable data to estimate habitual sleep parameters,7 but based on current guidelines,8 we chose 10 nights as the minimum needed to establish habitual sleep. This was supported by visual inspection of frequency of use, which suggested that those with a minimum of 10 nights were likely to use their devices regularly. See Supplementary Fig. 1 for a graphical depiction of the frequency of number of nights of use for each of the 6 months included in the 2020 analyses.
To examine 2020 data compared to historical data, separate samples of Fitbit users from each month in 2018 and 2019 were aggregated. For each of these monthly historical samples, users were included if they contributed a minimum of 10 days of data, whose sleep duration was over 3 hours (in order to minimize invalid recordings). In addition, certain days were excluded, including New Year's Day, Thanksgiving, Daylight Savings days, and Christmas Day. Inclusion of an individual in one month did not bias their inclusion in another month (some individuals may have been included in multiple months), though the samples from Fitbit users in each city were random. Regarding the data from 2018 and 2019 included in these analyses, see Supplementary Table 1, which describes the characteristics of these normative samples, with averages across all 12 months of each year, stratified by age and gender grouping, as well as city.
Previous studies have validated these devices against polysomnography and have shown that accuracy for detecting sleep sleep/wake determinations is relatively high6 , 9 and is likely equivalent to or superior to standard actigraphy.10
Arousals were defined as 1 or more continuous epochs of wake during the sleep period (as determined by the Fitbit scoring) that may or may not have registered as a self-reported awakening by a user. This is differentiated from an awakening since these are infrequently recalled by users in the morning and it is unclear whether they represent complete or incomplete awakenings. Previous studies of polysomnbographic data suggest that there are dozens of such arousals on a typical night for most adults.11
Resting heart rate was defined as beats per minute, captured using the optical plethysmography device, reflecting baseline values characteristic of being still and rested. These values are determined by an algorithm that has been shown to agree with gold-standard measures.12 No adjustment for values was made beyond stratification by age and sex, since other variables were not available.
Sleep-related outcomes examined in this study included bedtime, time in bed, and sleep duration. Bedtime was determined as the clock time that the device estimated that the individual settled down and was sufficiently still to be potentially asleep. Time in bed was defined as the total time between bedtime and the device-estimated time out of bed. Sleep duration was computed as the difference between total time in bed and the sum of any wake minutes during the time in bed period. Thus, sleep duration was calculated based on the typical approach of calculating time in bed for the main sleep period, then subtracting any observed wake time such as sleep latency and wake after sleep onset.
Variability in bedtime, time in bed, and sleep duration were expressed as standard deviation of these variables. Using standard deviation as a measure of variability is an approach that has been utilized previously with wearable data13 but it does have limitations. First, it is highly correlated with the mean, and second, it does not account for temporal windows. Due to the nature of the data in this dataset, we chose to use the simpler metric of standard deviation as a broad indicator of spread. Future studies may improve on this approach by leveraging more complex longitudinal approaches.
Only sleep periods that included a consistent sleep bout of longer than 3 hours were considered valid sleep periods, and sleep periods longer than 12 hours were similarly excluded for the purpose of this study. Bedtime and bedtime variability calculations are based on the longest sleep periods that started between 8pm and 4am. Cohorts of users were defined based on their age, gender and the city in which they resided in April. Age cohorts include 18-29, 30-49, 50-65 and 65+. Since holidays may have an impact on sleep behavior, sleep data from New Year's Day and the day of the daylight saving time shift on March 8th were excluded. All sleep dates, as reported, correspond to the wake-up date. These selected criteria excluded several groups of people, including1 individuals who do not use their device at night,2 individuals who do not use their device regularly enough to establish a reliable estimate, and3 users who use their device at night but exhibit sleep characteristics that are outside of the selected window.
Data analysis
Mean values were calculated for bedtime and sleep duration for each subject during the following recording periods: January, February, March and April. Then, mean change for each cohort over the recording periods was compared to the baseline month. Descriptive statistics and plots summarize these differences over time. To discern changes in light of normal seasonal variation in sleep, changes in sleep of similar cohorts was examined, comparing values to 2018 and 2019. To determine whether sleep duration, bedtime, sleep duration variability (assessed as standard deviation of sleep duration), and bedtime variability (assessed as standard deviation of bedtime) differed from January to April, values were graphed, stratified by age group and gender. Statistical analyses included calculation of Cohen's d for effect size of the change. Changes in resting heart rate from January to April were computed, with Cohen's d quantifying effect sizes of the change. Then, correlations between change in heart rate and change in other Fitbit-derived values were computed, including change in sleep duration and timing, step count, and active minutes. To examine sleep in the subsequent months of the pandemic, changes in sleep duration and bedtime in May and June were compared to April. The focus on descriptive statistics is justified by the over-powered sample for basic hypothesis tests. All analyses were performed in the Python programming environment. Python libraries used for the analyses were Pandas, Numpy and Scipy. Python's Matplotlib and Seaborn libraries as well as Tableau were used for plotting and visualizations.
Results
Characteristics of the sample
Characteristics of the 2020 sample that provided data from January to April are reported in Table 1 . Only data regarding city of residence (reported in Table 1A) and gender and age group (reported in Table 1B). The following US cities were represented: Chicago, Houston, Los Angeles, New York, San Francisco, and Miami. For additional analyses of May and June, the same individuals were used, though some of the total was missing, due to no longer meeting inclusion criteria. Characteristics of the historical 2018 and 2019 samples are reported in Supplementary Table 1.
Table 1.
Characteristics of the sample, stratified by city and gender/age group
| A. City | ||
|---|---|---|
| City | % Participants | |
| Chicago | 38 | |
| Houston | 17 | |
| Los Angeles | 15 | |
| New York | 11 | |
| San Francisco | 9 | |
| Miami | 9 | |
| B. Gender/age group | ||
|---|---|---|
| Gender | Age group | % Participants |
| Female | 18-29 | 10 |
| 30-49 | 30 | |
| 50-64 | 18 | |
| 65+ | 9 | |
| Male | 18-29 | 4 |
| 30-49 | 15 | |
| 50-64 | 10 | |
| 65+ | 5 | |
Monthly patterns of sleep duration and variability, relative to prior years
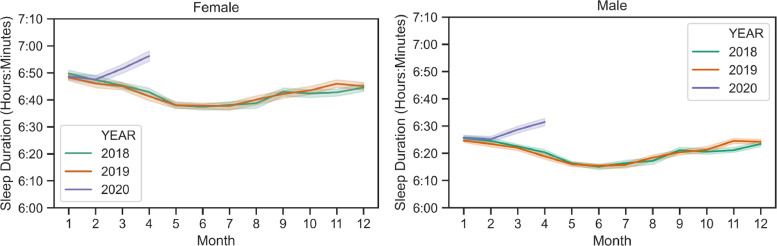
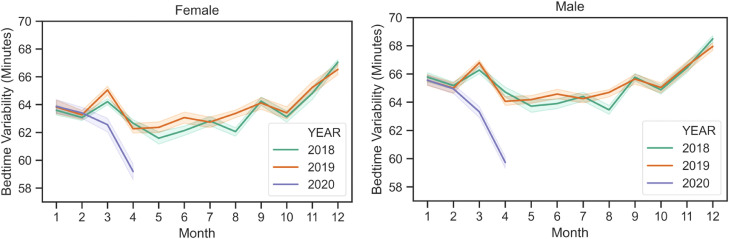
Fig. 1 shows the trajectory of sleep duration in 2020 from January to April, relative to historical sleep duration trends in 2018 and 2019. For both men and women, the change from January to February was similar in 2020, but then mean sleep duration started to increase relative to previous years. Supplementary Fig. 2 displays these values stratified by city. Fig. 2 shows that compared to 2018 and 2019, the first months of 2020 were similar in terms of bedtime variability as well, but this variability decreased through April, relative to previous years. Supplementary Fig. 3 displays these values stratified by city.
Fig. 1.
Monthly average sleep duration from 2018 to 2020, averaged across all age groups and cities. Average sleep duration increased across all these cities during March and April 2020, while it decreased during the same time in the previous years. Bands represent 95% confidence interval when averaged over cities.
Fig. 2.
Monthly average bedtime variability (assessed as standard deviation) from 2018 to 2020, averaged across all age groups and cities. Average sleep duration increased across all these cities during March and April 2020, while it decreased during the same time in the previous years. Bands represent 95% confidence interval when averaged over cities.
Categorical changes in sleep duration and timing
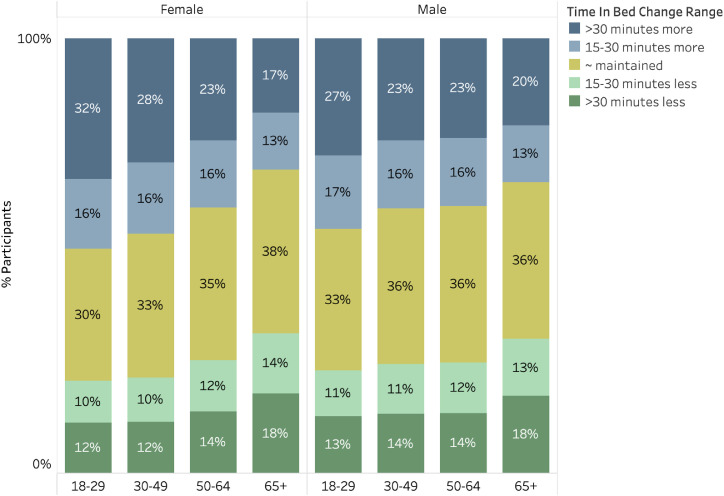
Fig. 3 displays the prevalence of participants who increased or decreased their sleep duration by at least 15 minutes or more than 30 minutes, stratified by gender and age group. As was reflected in Fig. 1, increasing sleep duration was more common than decreasing sleep duration. Further, younger age groups demonstrated more frequent lengthening of sleep by more than 30 minutes.
Fig. 3.
Prevalence of categorical changes in sleep duration from January to April. Prevalence of categorical changes in sleep duration from January to April. Stratified across gender and age groups, those whose mean sleep duration changed by <15 minutes in either direction are classified as “maintained” whereas those whose sleep duration increased by 15-30 minutes or >30 minutes are indicated. Increases in sleep duration were more common than decreases.
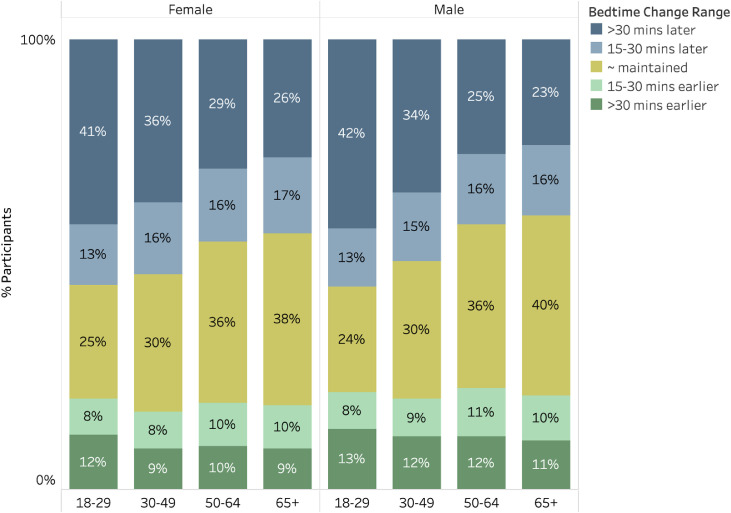
Fig. 4 displays the prevalence of participants who advanced or delayed their sleep by at least 15 minutes or more than 30 minutes, stratified by gender and age group. Sleep phase delay was more common than advance, in all age groups. Further, younger age groups demonstrated more pronounced tendencies towards phase delay, with the majority of those age 18-29 delaying bedtime by at least 15 minutes.
Fig. 4.
Prevalence of categorical changes in bedtime from January to April. Prevalence of categorical changes in bedtime from January to April. Stratified across gender and age groups, those whose mean bedtime changed by <15 minutes in either direction are classified as “maintained” whereas those whose bedtime was 15-30 minutes earlier or later or >30 minutes earlier or later are indicated. Bedtimes were more commonly shifted later than earlier.
Changes in sleep duration, timing, and variability from January to April
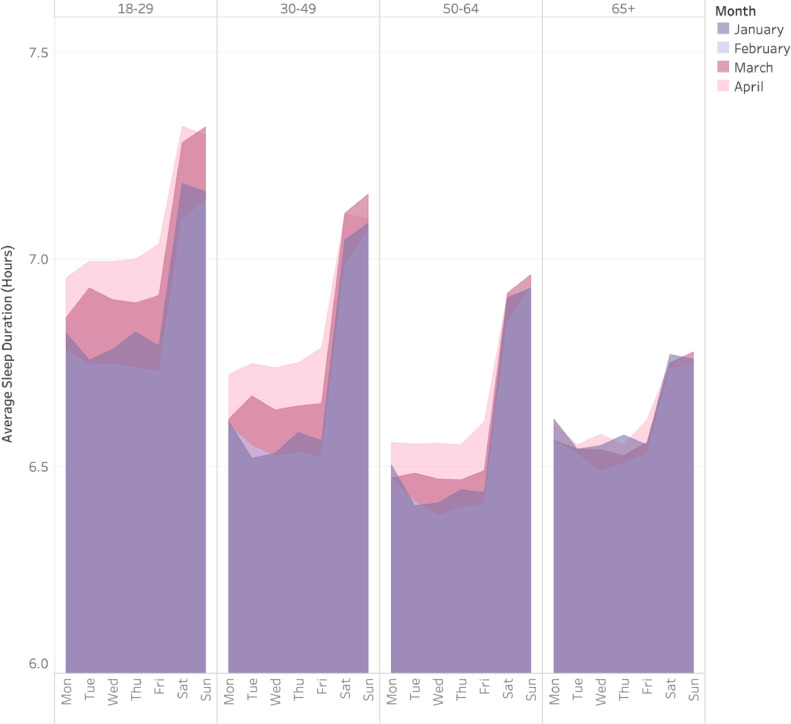
Fig. 5 displays nightly mean sleep duration across the week, for January through April, 2020, stratified by age group. This figure shows pronounced weekday-weekend discrepancies in real-world sleep duration, across all age groups (though most pronounced in younger groups). Further, this figure shows that sleep duration increased, especially on weeknights, among all age groups except for those 65 and older. In addition, this figure illustrates decreases in sleep duration variability, as increased sleep duration on weekdays more closely approximated the sleep of weekends. Supplementary Fig. 4 depicts these results stratified by gender, and Supplementary Fig. 5 depicts these results stratified by city.
Fig. 5.
Nightly sleep duration by age group across months. Comparison of sleep duration during different months by day of the week, stratified by age group.
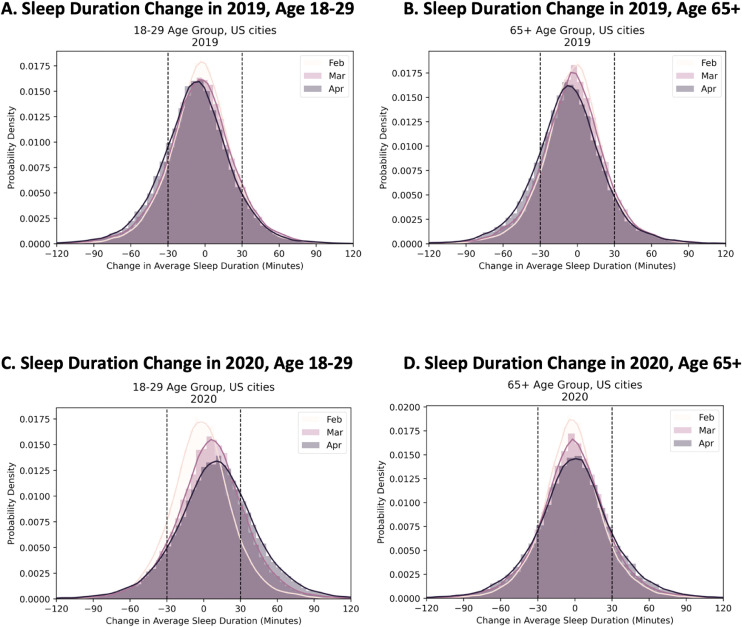
Fig. 6 examines change in sleep duration in February, March, and April, relative to January, for both 2020 and 2019, among the youngest18., 19., 20., 21., 22., 23., 24., 25., 26., 27., 28., 29. vs the oldest (65 and older) age groups. Although sleep duration did not change much across months in 2019—though sleep duration decreased slightly and increased in variability—more pronounced changes, especially for the 18-29 year-olds, could be seen in 2020.
Fig. 6.
Histogram of change (compared to January) in average sleep duration truncated at 120 minutes in February, March and April of 2019 and 2020, for age groups 18-29 and 65+ years old. The youngest and oldest age groups respectively experienced the largest and the least change in their sleep duration. (A) Age 18-29, 2019; (B) Age 65+, 2019; (C) Age 18-29, 2020; (D) Age 65+, 2020. Vertical lines are at 30 minutes change. During COVID-19 related measures, March and April of 2020, a higher percentage of younger users slept at least 30 minutes longer. For the 65+ age group, a subpopulation's sleep duration decreased more than 30 minutes in April and March than February.
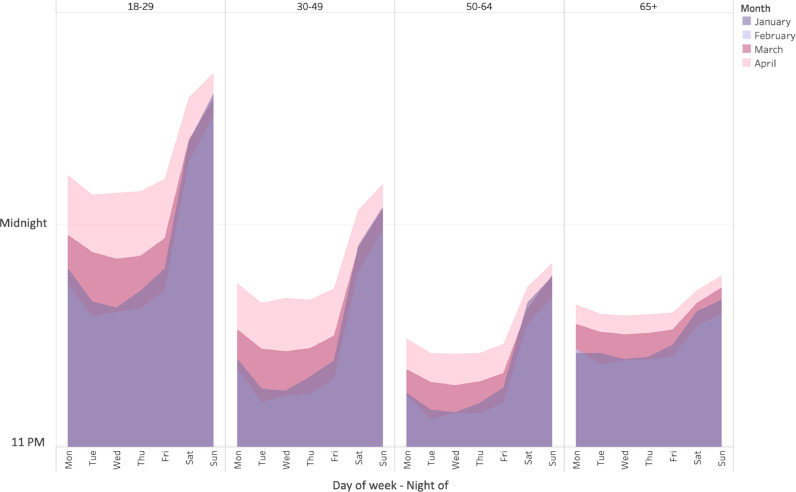
Fig. 7 displays nightly mean bedtime, stratified by age group, for the months of January, February, March, and April of 2020. It shows that all age groups delayed bedtime in March and April, though these were more pronounced in younger groups. Supplementary Fig. 6 depicts these results stratified by city. Fig. 8 shows the difference in the change in sleep duration variability from January to April in 2020, compared to 2019 among both genders age 18-29, where the largest such changes were seen.
Fig. 7.
Nightly bedtime by age group across months. Comparison of bedtime during different months by day of the week, stratified by age group.
Fig. 8.
Histogram of change in variability (standard deviation) of sleep duration between January and April in 2020 and 2019. Change reflected decreases in variability in 2020 than 2019.
Table 2 displays these results numerically, displaying for both 2019 and 2020 the mean values for bedtime, sleep duration, bedtime variability, and sleep duration variability for January and April. It also displays the computed change and Cohen's d values, documenting effect size. These results are stratified by gender and age group. In 2020, between January and April, 18-20 year-olds delayed their bedtime by about 24 minutes on average, 30-49 year-olds delayed bedtime by about 17-20 minutes on average, 50-64 year-olds delayed by 10-14 minutes on average, and those 65 and older delayed bedtime by 9-12 minutes on average. This is compared to changes of about 1-2 minutes across all age groups in 2019.
Table 2.
Change in bedtime (minutes from midnight), sleep duration (minutes), and variability (standard deviation) of bedtime and sleep minutes change from January to April, with size of the difference (delta) for 2019 and 2020 and effect size (Cohen's D) among both men and women, across age groups
| Metric | Age | Jan 2020 | April 2020 | Δ 2020 | D 2020 | Jan 2019 | April 2019 | Δ 2019 | D 2019 |
|---|---|---|---|---|---|---|---|---|---|
| Female | |||||||||
| Bedtime | 18-29 | 23:47 ± 69.6 | 00:10 ± 81.6 | 23.7 | −0.39 | 23:50 ± 67.6 | 23:47 ± 66.8 | −2.8 | 0.06 |
| 30-49 | 23:20 ± 64.4 | 23:40 ± 74.4 | 19.9 | −0.4 | 23:22 ± 63.2 | 23:20 ± 61.0 | −1.5 | 0.04 | |
| 50-64 | 23:15 ± 66.3 | 23:28 ± 74.7 | 13.3 | −0.31 | 23:13 ± 63.0 | 23:12 ± 61.0 | −0.6 | 0.02 | |
| 65+ | 23:25 ± 71.1 | 23:37 ± 78.3 | 11.8 | −0.3 | 23:25 ± 69.6 | 23:24 ± 67.8 | −0.6 | 0.02 | |
| Sleep minutes | 18-29 | 420.8 ± 42.0 | 431.9 ± 45.2 | 11.1 | −0.27 | 419.3 ± 41.1 | 411.1 ± 40.2 | −8.2 | 0.24 |
| 30-49 | 410.2 ± 45.4 | 419.4 ± 48.8 | 9.2 | −0.25 | 409.6 ± 44.0 | 403.3 ± 42.8 | −6.4 | 0.2 | |
| 50-64 | 401.3 ± 49.7 | 406.1 ± 52.7 | 4.8 | −0.14 | 400.3 ± 47.5 | 393.0 ± 46.1 | −7.3 | 0.25 | |
| 65+ | 403.0 ± 55.1 | 401.9 ± 57.4 | −1.1 | 0.03 | 401.0 ± 53.7 | 393.3 ± 51.8 | −7.8 | 0.25 | |
| Bedtime variability | 18-29 | 69.7 ± 23.8 | 62.4 ± 24.2 | −7.3 | 0.24 | 70.0 ± 23.1 | 67.6 ± 23.0 | −2.4 | 0.09 |
| 30-49 | 65.9 ± 24.7 | 61.2 ± 25.2 | −4.7 | 0.16 | 65.3 ± 24.4 | 63.7 ± 23.6 | −1.5 | 0.06 | |
| 50-64 | 63.6 ± 25.3 | 60.5 ± 26.4 | −3.2 | 0.11 | 62.8 ± 24.7 | 61.8 ± 24.0 | −1 | 0.04 | |
| 65+ | 63.7 ± 27.5 | 60.7 ± 28.4 | −2.9 | 0.11 | 63.1 ± 26.8 | 62.7 ± 26.5 | −0.4 | 0.02 | |
| Sleep minutes variability | 18-29 | 77.6 ± 25.5 | 71.8 ± 26.9 | −5.8 | 0.21 | 76.7 ± 25.1 | 74.5 ± 24.7 | −2.2 | 0.08 |
| 30-49 | 73.9 ± 24.4 | 70.3 ± 25.9 | −3.6 | 0.14 | 72.9 ± 23.9 | 70.5 ± 23.4 | −2.4 | 0.1 | |
| 50-64 | 73.5 ± 24.1 | 70.2 ± 25.3 | −3.2 | 0.14 | 72.2 ± 23.4 | 69.8 ± 22.8 | −2.4 | 0.11 | |
| 65+ | 73.0 ± 24.7 | 69.8 ± 25.4 | −3.2 | 0.14 | 72.4 ± 24.5 | 70.7 ± 23.5 | −1.7 | 0.08 | |
| Male | |||||||||
| Bedtime | 18-29 | 00:11 ± 77.6 | 00:35 ± 89.5 | 24 | −0.35 | 00:14 ± 75.4 | 00:12 ± 75.2 | −2.1 | 0.04 |
| 30-49 | 23:44 ± 72.1 | 24:01 ± 80.9 | 16.6 | −0.31 | 23:46 ± 69.9 | 23:44 ± 68.5 | −2 | 0.05 | |
| 50-64 | 23:24 ± 69.6 | 23:33 ± 77.4 | 9.4 | −0.21 | 23:24 ± 66.7 | 23:23 ± 65.5 | −0.8 | 0.02 | |
| 65+ | 23:26 ± 73.5 | 23:35 ± 79.8 | 8.4 | −0.21 | 23:26 ± 71.3 | 23:24 ± 70.0 | −1.4 | 0.04 | |
| Sleep minutes | 18-29 | 398.4 ± 41.5 | 406.1 ± 44.9 | 7.7 | −0.2 | 398.0 ± 40.4 | 391.4 ± 40.2 | −6.6 | 0.2 |
| 30-49 | 386.2 ± 44.5 | 391.6 ± 47.6 | 5.4 | −0.15 | 385.5 ± 43.7 | 380.2 ± 42.5 | −5.3 | 0.18 | |
| 50-64 | 381.3 ± 50.5 | 386.0 ± 53.5 | 4.7 | −0.14 | 380.5 ± 48.4 | 374.4 ± 47.2 | −6.1 | 0.21 | |
| 65+ | 387.6 ± 58.6 | 388.4 ± 61.0 | 0.8 | −0.02 | 385.6 ± 56.3 | 379.1 ± 54.3 | −6.5 | 0.2 | |
| Bedtime variability | 18-29 | 72.7 ± 25.5 | 65.2 ± 26.9 | −7.6 | 0.24 | 72.4 ± 24.4 | 70.0 ± 24.1 | −2.4 | 0.08 |
| 30-49 | 69.8 ± 26.1 | 63.7 ± 26.4 | −6.2 | 0.21 | 68.4 ± 25.2 | 66.7 ± 24.3 | −1.7 | 0.06 | |
| 50-64 | 65.1 ± 26.2 | 60.1 ± 27.8 | −5 | 0.18 | 64.1 ± 25.7 | 63.0 ± 24.9 | −1 | 0.04 | |
| 65+ | 61.4 ± 27.8 | 57.1 ± 28.7 | −4.3 | 0.16 | 61.4 ± 27.3 | 60.8 ± 26.7 | −0.6 | 0.02 | |
| Sleep minutes variability | 18-29 | 74.6 ± 25.1 | 69.7 ± 26.5 | −4.9 | 0.18 | 73.6 ± 24.5 | 71.8 ± 24.3 | −1.9 | 0.07 |
| 30-49 | 72.2 ± 23.6 | 68.1 ± 24.6 | −4.1 | 0.17 | 70.7 ± 23.0 | 68.9 ± 22.4 | −1.8 | 0.08 | |
| 50-64 | 71.2 ± 23.5 | 67.5 ± 24.3 | −3.8 | 0.16 | 69.8 ± 22.8 | 67.9 ± 22.0 | −1.9 | 0.09 | |
| 65+ | 70.4 ± 24.5 | 67.1 ± 25.2 | −3.4 | 0.15 | 69.4 ± 23.8 | 67.5 ± 22.9 | −1.9 | 0.09 |
In 2020, 18-29 year-olds increased their sleep duration by 9-13 minutes on average, 30-49 year-olds by 6-10 minutes, 50-64 year-olds by 5 minutes, and those 65 and older demonstrated sleep duration of about 1 minute more or less in April vs January. This is markedly different from 2019 data, which documents decreases in sleep duration of 7-11 minutes across all groups (Table 2).
Variability in sleep decreased as well. Regarding bedtime variability, this was reduced by between 3 and 9 minutes, depending on age and gender group, from January to April (Table 2). This is not very dissimilar to 2019 values which showed a decrease from January to April of 1-4 minutes in bedtime variability. Variability in sleep duration decreased between 4 and 6 minutes between January and April, depending on age and gender group. This is compared to decreases of about 3 minutes seen in the same time period in 2019.
Changes in sleep in relation to health
Table 3 documents changes in resting heart rate (RHR) across genders and age groups in 2019 and 2020. In 2020, from January to April, RHR decreased about 1bpm across all groups. This is in relation to a reduction of <0.5 bpm in all age and gender groups. This is supported by Cohen's d values documenting a larger effect size of this change.
Table 3.
Resting heart rate (RHR) of men and women across age groups in 2019-2020, with change from January to April (delta) and effect size (Cohen's D)
| Age | Jan 2020 | April 2020 | Δ 2020 | D 2020 | Jan 2019 | April 2019 | Δ 2019 | D 2019 |
|---|---|---|---|---|---|---|---|---|
| Male | ||||||||
| 18-29 | 63.3 ± 7.5 | 62.3 ± 7.6 | −1 | 0.25 | 63.2 ± 7.3 | 62.8 ± 7.3 | −0.4 | 0.13 |
| 30-49 | 65.1 ± 8.0 | 64.3 ± 8.2 | −0.9 | 0.24 | 64.8 ± 7.8 | 64.5 ± 7.9 | −0.4 | 0.11 |
| 50-64 | 65.4 ± 8.3 | 64.6 ± 8.4 | −0.8 | 0.24 | 65.1 ± 8.2 | 64.7 ± 8.2 | −0.4 | 0.13 |
| 65+ | 62.9 ± 7.8 | 62.0 ± 7.9 | −0.9 | 0.28 | 63.0 ± 7.9 | 62.8 ± 7.9 | −0.3 | 0.09 |
| Female | ||||||||
| 18-29 | 67.9 ± 7.8 | 66.7 ± 8.1 | −1.2 | 0.29 | 67.3 ± 7.7 | 66.9 ± 7.8 | −0.4 | 0.1 |
| 30-49 | 68.5 ± 8.0 | 67.8 ± 8.2 | −0.7 | 0.18 | 68.1 ± 7.9 | 67.9 ± 7.9 | −0.2 | 0.06 |
| 50-64 | 67.6 ± 7.8 | 67.0 ± 8.0 | −0.6 | 0.17 | 67.5 ± 7.8 | 67.2 ± 7.8 | −0.3 | 0.1 |
| 65+ | 65.7 ± 7.5 | 64.9 ± 7.5 | −0.7 | 0.24 | 65.8 ± 7.4 | 65.6 ± 7.4 | −0.2 | 0.08 |
To determine whether this change in RHR is related to change in other variables, correlations examined RHR change relative to change in sleep duration, bedtime, sleep duration variability, and physical activity measures including step count and active minutes. For the youngest age group,18., 19., 20., 21., 22., 23., 24., 25., 26., 27., 28., 29. increased sleep duration and active minutes and delayed bedtime were associated with decreased RHR, and increased sleep variability was associated with higher RHR. Step count was positively associated with RHR in women but negatively in men. These results are displayed in Table 4 . Table 4 also shows these associations in the oldest age group (65 and older). Similarly, increased sleep duration and active minutes and delayed bedtime were associated with decreased RHR, and increased sleep variability was associated with higher RHR.
Table 4.
Associations between change in resting heart rate (RHR) and change in sleep duration, active minutes, step count, bedtime, and standard deviation of sleep duration among adults 18-29 and 65+
| Female |
Male |
|||
|---|---|---|---|---|
| Correlate | Pearson r | P value | Pearson r | P value |
| Age 18-29 | ||||
| Δ Sleep minutes | −0.08 | 2.79 × 10−29 | −0.09 | 1.57 × 10−19 |
| Δ Active minutes | −0.07 | 1.39 × 10−28 | −0.07 | 8.10 × 10−14 |
| Δ Bedtime | −0.03 | 3.05 × 10−06 | −0.03 | 4.45 × 10−02 |
| Δ Step count | 0.04 | 3.57 × 10−08 | −0.02 | 1.42 × 10−01 |
| Δ Standard deviation of sleep minutes | 0.04 | 5.44 × 10−07 | 0.06 | 1.14 × 10−08 |
| Age 65+ | ||||
| Δ Sleep minutes | −0.10 | 2.41 × 10−44 | −0.09 | 1.89 × 10−24 |
| Δ Active minutes | −0.05 | 1.28 × 10−12 | −0.08 | 2.80 × 10−18 |
| Δ Bedtime | −0.04 | 1.61 × 10−08 | −0.06 | 2.43 × 10−10 |
| Δ Step count | −0.01 | 0.546 | −0.01 | 1.000 |
| Δ Standard deviation of sleep minutes | 0.06 | 4.81 × 10−13 | 0.06 | 4.48 × 10−10 |
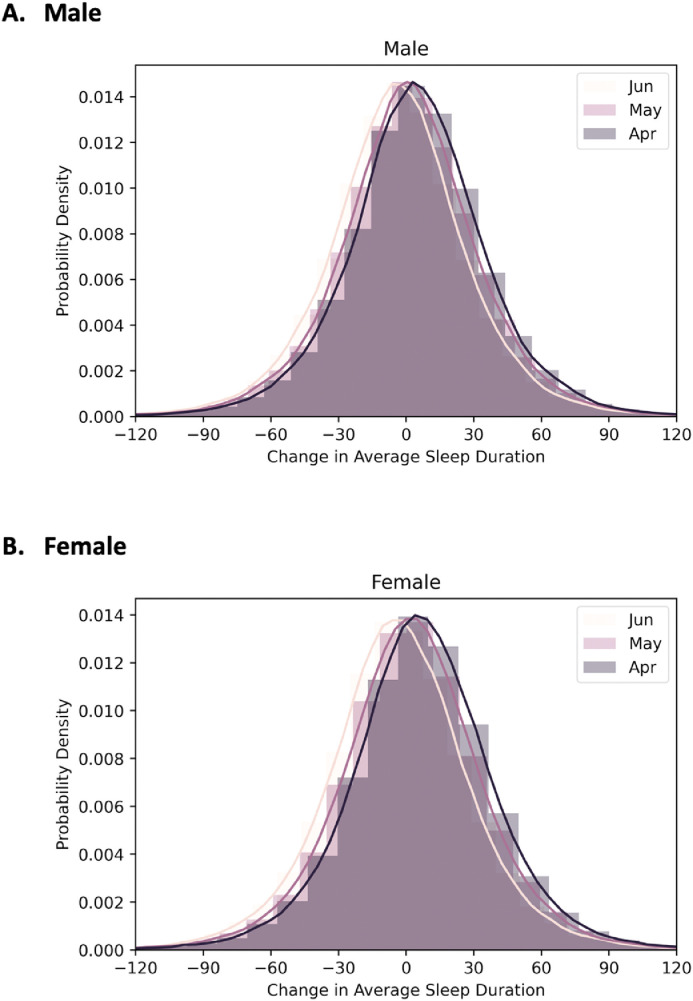
Sleep duration in May and June
Fig. 9 shows the histogram of sleep duration from April to June in 2020, stratified by gender. In both cases, a different pattern is evident, relative to the earlier (January through April) pattern. In May and June, in contrast, sleep duration decreased slightly for both men and women. Supplemental Fig. 4 expands this by showing comparisons between January and April, May, and June for males and females age 18-29 (who showed the most pronounced changes).
Fig. 9.
Histogram of change in mean sleep duration from January 2020 in April, May, and June 2020.
Discussion
This study examined changes in sleep duration, sleep timing, and regularity in 163,524 Fitbit users in 6 major US cities: New York, Los Angeles, Chicago, Houston, San Francisco, and Miami from January through April, 2020, during the COVID-19 pandemic, with additional analyses in May and June, 2020. The overall results of these analyses show that over the course of the pandemic, adults experienced changes in sleep—especially duration and timing.
Trajectories of sleep differed significantly from those seen in 2018 or 2019, suggesting that the pandemic had an influence on sleep patterns. Differences between 2020 and both 2018 and 2019 were evident for sleep duration, variability, and timing, suggesting that the values obtained in this study uniquely reflect the pandemic and not historical trends. Similarly, Ong and colleagues found similar patterns in Singaporean adults—that sleep duration, variability and timing differed in the early parts of 2020, compared to historical values.14 Unlike the Ong and colleagues, study, the present data evaluates relative to norms, and not the same individuals. Despite this, the fact that the findings were similar to the other study, which used data from the same individuals, suggests that these deviations are valid.
Overall, sleep duration increased in the US population. These results reflect a change from 2018 and 2019—in the first half of 2019, sleep durations gradually declined, yet in 2020, sleep duration increased from January through the assessment period. Of note, the average increase in sleep time depended on age and gender. Women experienced more of an increase in sleep duration during the pandemic than men, with the largest change seen in the younger adults (12 minutes in women and 8 minutes in men). This is in relation to 2019 values, which saw reductions in sleep time of 11 and 8 minutes for those groups, respectively. Thus, not only was the pandemic associated with increased sleep time, this increase was in contrast to these same months in 2019 when there was a net decrease in sleep time, and the increase seen in 2020 was most profound among younger adults, and especially among women. Sleep duration is increasingly recognized as a key indicator of health, and even modest increases in sleep duration may be meaningful and have physiologic benefits.
Bedtime variability (reflecting decreased weekday-weekend discrepancy) decreased during the pandemic. These also reflect a significant change from 2018 and 2019, where this decrease in variability was not seen. For example, in 2019, bedtime and bedtime variability did not change by more than 1-2 minutes during these months; yet, in 2020, bedtime was delayed by about 25 minutes in the younger adults, 17-20 minutes in adults age 30-49, and 9-10 minutes in men and about 12 minutes in women 50 or older. Decreased sleep variability is associated with improved health outcomes. Another possible explanation for the reduction in sleep variability during the pandemic is that changes to daytime and/or nocturnal social activities (eg, closed bars and restaurants, canceled events) may have contributed to the changes in sleep variability.
Comparisons to previous years of historical data are limited in that each comparison includes different individuals, rather than the same individual longitudinally. Although this is somewhat mitigated by the large, random sample and the similarity between 2018 and 2019 despite clear differences to 2020, results that compare such samples need to be interpreted with appropriate caution. Therefore, it is possible that the differences between 2020 and previous years reflect differences in the sample, and not historical changes in sleep-related behavior. Future studies that have such within-person, longitudinal data would be helpful to replicate these findings.
Resting heart rate, as estimated by the Fitbit devices, decreased across the pandemic. Despite the social and environmental stresses prevalent during this time, these results suggest that the decrease in RHR may be due to appreciable increases in both sleep duration and physical activity. As sleep and activity increased, RHR decreased. Previous studies have linked changes in sleep schedules to RHR, supporting the results of this study.15
The results showing relationships to RHR, in the context of relatively small changes to heart rate and sleep, may still be important for several reasons. First, these results serve to identify an objective health metric obtained at the population level that is systematically associated with even small changes in sleep-related behavior. This is, itself, an important finding; very few studies have been able to use such a big-data approach to demonstrate associations between sleep and any objective health metric at the population level. Second, changes in sleep may be reflecting changes in physiologic stress, which could be partially captured using RHR16 and this may add a mechanistic dimension to the discussion that, although tenuous, allows for further exploration. Third, showing that the changes in sleep were associated with changes in RHR helps to validate the physiologic impact of sleep changes by showing that they were related to changes in another system. Fourth, even very small changes in RHR at the population level may have significant impacts on population-level morbidity.16
During the pandemic, sleep duration increased on average and across age and gender groups, but this change was most visible in the youngest adults, who also experienced the greatest delay in bedtime. This is in line with the extensive data on school start times for adolescents and chronotype data for young adults.17., 18., 19. It suggests that younger adults may be living under increased circadian pressure to advance their sleep period in order to conform to social norms and work schedules. Yet, when the opportunity was presented, they went to bed later but slept more.
Another finding from this analysis is that when given the opportunity, the difference between weekdays and weekends becomes smaller. This is consistent with the Ong and colleagues, study in Singapore.14 Bedtime and sleep duration variability both decreased over the course of the pandemic, suggesting that individuals were more in control of their sleep patterns on average and required less of a discrepancy between weekday and weekend.
The observed magnitude of effects in the range of 10-12 likely represents a true difference. Although some previous studies found that older versions of wearables routinely differed from PSG by more than this amount (thereby leaving this amount of time within the margin of error), recent studies using the technology employed in this study found that, on average, the Fitbit device overestimated sleep by approximately 2.6 minutes vs polysomnography.10 Therefore, it is likely that this difference does, in fact reflect, a meaningful change. It is still possible, however, that a fluctuation of this magnitude simply reflects normal night-to-night measurement error. Unfortunately, no previous literature quantifies how much of a change this would be. Furthermore, many previous studies of wearable technology to detect sleep over time (eg, actigraphy studies) report changes in sleep duration around this magnitude and these effects have demonstrated utility whether or not measurement error exists in those devices as well. Finally, this study does not describe single-device changes in sleep duration, but rather sample-wide, systematic changes. Therefore, although any small effects observed using data collected by imperfect measures in large samples should be interpreted with appropriate caution, the findings in this study likely reflect meaningful changes rather than just measurement error.
Another important finding in this study is the documentation of changes to sleep as the stay-at-home orders have been lifted. These data show that from April to June, sleep patterns have somewhat reverted to pre-pandemic values. Of note, sleep duration in both May and June are still greater than that seen in prior years, reflecting a relative increase, but this difference is shrinking. It should be noted, though, that stay-at-home orders and other restrictions were variably enacted and/or enforced across these 6 cities. Therefore, externalities may have differentially impacted sleep-related behavioral patterns across the months of analysis. The interpretability of these results in the context of stay-at-home orders is limited, since these orders were variably initiated, followed, and/or enforced across all of these different locales, during this time period. In addition, some orders were geographically bound (eg, city limits only) even though users may or may not be living within those geographical bounds. Because of this, data are unavailable as to whether individuals were subject to or followed any such orders specifically.
The research landscape describing relationships between sleep-related parameters and aspects of the COVID-19 pandemic is rapidly evolving. Wright and colleagues showed that among young adults, there was a systematic phase delay of the sleep period.20 In addition, this study showed that there was a systematic increase in sleep duration moderated by baseline time in bed, such that those who spent the least amount of time in bed before the pandemic showed the greatest increase in sleep duration during the pandemic. A study by Ong and colleagues in Singapore also showed a general increase in sleep duration, as well as a decrease in the difference between weekday and weekend sleep (as work schedules were impacted).21 Lee and colleagues tracked sleep duration changes across 17 countries and showed that most showed a small but statistically significant increase in sleep duration of around 5-15 minutes.22 Blume and colleagues also showed a general slight increase in sleep duration in Europe, accompanied by a reduction in social jetlag.23
Several studies have explored relationships between sleep and pandemic-related mental health and stress. Pesonen and colleagues24 showed that those with the greatest increases in stress experiences during the pandemic experienced the greatest degree of pandemic-related shortened sleep duration, prolonged sleep latency, increased nightly awakenings, disturbed circadian rhythms, and increased nightmares. Kocevska and colleagues showed that there was an interaction between baseline sleep and sleep changes on pandemic-related mental health problems.25 They showed that the strongest relationship between worsening mental health and worsening sleep was among those who were good sleepers at the outset of the pandemic, suggesting that the general population may be at increased risk due to a lack of floor/ceiling effects. Bigalke and colleagues also showed that those who perceived overall worse sleep quality as a result of the pandemic reported worse insomnia scores and higher levels of stress.26 This is consistent with work by Killgore and colleagues, showing that the relationship between COVID-related stress and suicide ideation was mediated by insomnia symptoms.27
Other health issues have been implicated as well. Werneck and colleagues have shown that there is an overlap between worsening sleep as a result of the pandemic and increased behavioral health risk factors, including television-watching, physical inactivity, and high computer/tablet use.28 Further, a study in Italy showed that COVID patients with sleep apnea were 65% more likely to require hospitalization and 98% more likely to experience respiratory failure.29
This study had some important limitations. First, although Fitbit devices have been relatively well-validated to detect sleep relative to polysomnography and actigraphy, the accuracy of these devices in these real-world settings is still not completely clear. Another potential issue is the reliance on bed and wake time detection using the Fitbit device. This detection strategy has not been empirically validated and may misestimate time in and out of bed. Second, these data were not supplemented by subjective measures like sleep diaries, so insomnia was not well-characterized. Third, limited demographic data besides age, gender, and city of residence were available; this precludes analysis of sleep health disparities. Fourth, it is not known whether any of these individuals experienced major stressors such as job loss or contracting COVID during this period. Fifth, as this reflects a natural experiment, it is not clear which aspects of the pandemic had an influence on which aspects of sleep experience. Finally, it is unclear whether the differences observed in the present study reflect changes that would be evident with other sleep assessment modalities, such as polysomnography and/or sleep diary. The inclusion of estimates of variance should help assuage concerns regarding whether specific point estimates are made with confidence. By displaying means and standard deviations and showing that the standard deviations are usually quite small, it is probably safe to conclude that the estimates are reliable within the constraints of the study. Because this is largely a descriptive study that lacks assessment of many potential confounders or other explanatory variables, it is possible that these unmeasured factors play important roles in the relationships observed.
In conclusion, this study found that during the COVID-19 pandemic, sleep duration increased slightly and bedtime was delayed (especially among younger adults and women), bedtime and sleep variability reduced (reflecting decreased weekday-weekend discrepancy, and resting heart rate decreased (possibly due to increased sleep and physical activity). Despite limitations, this study represents one of the largest and most representative, objective analyses of sleep in general, as well as during the COVID-19 pandemic. These results provide important information about sleep and health during the COVID-19 pandemic and point to many future research questions. Future studies will be needed to examine additional sociodemographic and socioeconomic influences on sleep changes, relationships to other health outcomes, and the role of mental health. It is possible that these results might also be useful to those examining population sleep health, such as sleep and schedules among young adults, the role of sleep in impacting population cardiometabolic health, the role of technology in surveillance of sleep stage data, and other work.
Funding
MAG is supported by R01MD011600 and R01DA051321 from the National Institutes of Health. The work described herein is the sole responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or its institutes.
Disclosures
Dr. Rezeai is an employee of Fitbit, Inc. Over the past 36 months, Dr. Grandner has served as Principal Investigator on grants received from the National Institute on Minority Health and Health Disparities, the National Institute on Drug Abuse, the Military Suicide Research Consortium, Kemin Foods, Nexalin Technologies, Jazz Pharmaceuticals, and CereZ Technologies. He has received consulting fees in the past 36 months to (regardless of amount) the National Institutes of Health, New York University, Florida International University, University of Maryland, University of Evansville, University of Southern California, Fitbit, Natrol, Sunovion, Thrive Global, Smartypants Vitamins, Casper Sleep, Health and Wellness Partners, Athleta, Major League Baseball, Drug Free Sport, Clinical Care Solutions, DSM Supplements, and Idorsia. In addition, he serves as a scientific advisor (no monetary compensation) to Nightfood and Simple Habit.
Acknowledgment
We thank the members of the Fitbit Research team for illuminating discussions and the Fitbit users for providing data.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.sleh.2021.02.008.
Appendix. Supplementary materials
References
- 1.Grandner MA. Sleep, health, and society. Sleep Med Clin. 2017;12(1):1–22. doi: 10.1016/j.jsmc.2016.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grandner MA, Patel NP, Hale L, Moore M. Mortality associated with sleep duration: the evidence, the possible mechanisms, and the future. Sleep Med Rev. 2010;14:191–203. doi: 10.1016/j.smrv.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grandner MA. In: Sleep and Health. Grandner MA, editor. Academic Press; London: 2019. Social-ecological model of sleep health; pp. 45–53. [Google Scholar]
- 4.Crew EC, Baron KG, Grandner MA, et al. The Society of Behavioral Sleep Medicine (SBSM) COVID-19 Task Force: objectives and summary recommendations for managing sleep during a pandemic. Behav Sleep Med. 2020;18(4):570–572. doi: 10.1080/15402002.2020.1776288. [DOI] [PubMed] [Google Scholar]
- 5.Fitbit Inc. Fitbit; San Francisco, CA: 2018. Fitbit Terms of Service. [Google Scholar]
- 6.Beattie Z, Pantelopoulos A, Ghoreyshi A, Oyang Y, Statan A, Heneghan C. Estimation of sleep stages using cardiac and accelerometer data from a wrist-worn device. SLEEP. 2017;40(Abstract Supplement):A26. doi: 10.1088/1361-6579/aa9047. [DOI] [PubMed] [Google Scholar]
- 7.Lauderdale DS, Knutson KL, Rathouz PJ, Yan LL, Hulley SB, Liu K. Cross-sectional and longitudinal associations between objectively measured sleep duration and body mass index: the CARDIA Sleep Study. Am J Epidemiol. 2009;170(7):805–813. doi: 10.1093/aje/kwp230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ancoli-Israel S, Martin JL, Blackwell T, et al. The SBSM guide to actigraphy monitoring: clinical and research applications. Behav Sleep Med. 2015;13(Suppl 1):S4–S38. doi: 10.1080/15402002.2015.1046356. [DOI] [PubMed] [Google Scholar]
- 9.de Zambotti M, Baker FC, Willoughby AR, et al. Measures of sleep and cardiac functioning during sleep using a multi-sensory commercially-available wristband in adolescents. Physiol Behav. 2016;158:143–149. doi: 10.1016/j.physbeh.2016.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chinoy ED, Cuellar JA, Huwa KE, et al. Performance of seven consumer sleep-tracking devices compared with polysomnography. Sleep. In press. [DOI] [PMC free article] [PubMed]
- 11.Bonnet MH, Arand DL. EEG arousal norms by age. J Clin Sleep Med. 2007;3(3):271–274. [PMC free article] [PubMed] [Google Scholar]
- 12.Nazari G, MacDermid JC, Sinden KE, Richardson J, Tang A. Inter-instrument reliability and agreement of fitbit charge measurements of heart rate and activity at rest, during the modified Canadian aerobic fitness test, and in recovery. Physiother Can. 2019;71(3):197–206. doi: 10.3138/ptc.2018-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee E, Ramsey M, Malhotra A, et al. Links between objective sleep and sleep variability measures and inflammatory markers in adults with bipolar disorder. J Psychiatr Res. 2020;134:8–14. doi: 10.1016/j.jpsychires.2020.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ong JL, Lau TY, Massar SAA, et al. COVID-19 related mobility reduction: heterogeneous effects on sleep and physical activity rhythms. Archiv. 2020:2006.02100 [q-bio.QM]. In press. [DOI] [PMC free article] [PubMed]
- 15.Quer G, Gouda P, Galarnyk M, Topol EJ, Steinhubl SR. Inter- and intraindividual variability in daily resting heart rate and its associations with age, sex, sleep, BMI, and time of year: Retrospective, longitudinal cohort study of 92,457 adults. PLoS One. 2020;15(2) doi: 10.1371/journal.pone.0227709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Caetano J, Delgado Alves J. Heart rate and cardiovascular protection. Eur J Intern Med. 2015;26(4):217–222. doi: 10.1016/j.ejim.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 17.Minges KE, Redeker NS. Delayed school start times and adolescent sleep: a systematic review of the experimental evidence. Sleep Med Rev. 2016;28:86–95. doi: 10.1016/j.smrv.2015.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wheaton AG, Chapman DP, Croft JB. School start times, sleep, behavioral, health, and academic outcomes: a review of the literature. J Sch Health. 2016;86(5):363–381. doi: 10.1111/josh.12388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Troxel W, Wolfson A. Sleep science and policy: a focus on school start times. Sleep Health. 2016;2(3):186. doi: 10.1016/j.sleh.2016.07.001. [DOI] [PubMed] [Google Scholar]
- 20.Wright KP, Jr., Linton SK, Withrow D, et al. Sleep in university students prior to and during COVID-19 stay-at-home orders. Curr Biol. 2020;30(14):R797–R7R8. doi: 10.1016/j.cub.2020.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ong JL, Lau T, Massar SAA, et al. COVID-19 related mobility reduction: heterogenous effects on sleep and physical activity rhythms. Sleep. 2020 doi: 10.1093/sleep/zsaa179. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee PH, Marek J, Nalevka P. Sleep pattern in the US and 16 European countries during the COVID-19 outbreak using crowdsourced smartphone data. Eur J Public Health. 2020 doi: 10.1093/eurpub/ckaa208. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blume C, Schmidt MH, Cajochen C. Effects of the COVID-19 lockdown on human sleep and rest-activity rhythms. Curr Biol. 2020;30(14):R795–R7R7. doi: 10.1016/j.cub.2020.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pesonen AK, Lipsanen J, Halonen R, et al. Pandemic dreams: network analysis of dream content during the COVID-19 lockdown. Front Psychol. 2020;11 doi: 10.3389/fpsyg.2020.573961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kocevska D, Blanken TF, Van Someren EJW, Rosler L. Sleep quality during the COVID-19 pandemic: not one size fits all. Sleep Med. 2020;76:86–88. doi: 10.1016/j.sleep.2020.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bigalke JA, Greenlund IM, Carter JR. Sex differences in self-report anxiety and sleep quality during COVID-19 stay-at-home orders. Biol Sex Differ. 2020;11(1):56. doi: 10.1186/s13293-020-00333-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Killgore WDS, Cloonan SA, Taylor EC, Fernandez F, Grandner MA, Dailey NS. Suicidal ideation during the COVID-19 pandemic: the role of insomnia. Psychiatry Res. 2020;290 doi: 10.1016/j.psychres.2020.113134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Werneck AO, Silva DR, Malta DC, et al. The mediation role of sleep quality in the association between the incidence of unhealthy movement behaviors during the COVID-19 quarantine and mental health. Sleep Med. 2020;76:10–15. doi: 10.1016/j.sleep.2020.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maas MB, Kim M, Malkani RG, Abbott SM, Zee PC. Obstructive sleep apnea and risk of COVID-19 infection, hospitalization and respiratory failure. Sleep Breath. 2020 doi: 10.1007/s11325-020-02203-0. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.