Abstract
Chronic neurological diseases are the leading cause of disability globally. Yet, our health-care systems are not designed to meet the needs of many patients with chronic neurological conditions. Care is fragmented with poor interdisciplinary collaboration and lack of timely access to services and therapies. Furthermore, care is typically reactive, and complex problems are managed inadequately because of a scarcity of disease-specific expertise and insufficient use of non-pharmacological interventions. Treatment plans tend to focus on the disease rather than the individual living with it, and patients are often not involved in clinical decision making. By use of Parkinson's disease as a model condition, we show an integrated care concept with a patient-centred perspective that includes evidence-based solutions to improve health-care delivery for people with chronic neurological conditions. We anticipate that this integrated care model will improve the quality of life for patients, create a positive working environment for health-care professionals, and be affordable.
Introduction
Chronic neurological conditions are the leading cause of disability globally.1 Because of a growing ageing population, the worldwide prevalence and burden of chronic neurological diseases will rise further in the coming decades. To meet the needs of patients with chronic neurological diseases, health-care systems should reach the so-called Quadruple Aim: enhancing patient experience, improving population health, reducing costs (these originally formed the Triple Aim),2 and improving the work-life balance of clinicians.3 However, current health-care systems are often not designed to reach this Quadruple Aim because patient experience is far from optimal,4 patients often sustain avoidable disability, health-care costs are rising, and many clinicians face dissatisfaction or burnout.5 In this Personal View, we review the challenges of current neurology care from a patient's perspective, and introduce possible evidence-based solutions that can be combined within a model of integrated care. An integrated care model is defined as health services that are managed, discussed, and delivered so that patients can make various health-related and disease-related choices according to their needs throughout the life course.6 This integrated model takes a patient-centred approach and would be tailored around helping patients with chronic neurological conditions to minimise the effects of their disease—eg, supporting a patient to live independently at home and preventing escalation to expensive institutionalised care (figure 1 ). We illustrate our vision by using Parkinson's disease as a model condition for many other chronic neurological disorders (panel 1 ), assuming that improvements in the care for people with Parkinson's disease could be applied to other chronic neurological disorders. The model mostly entails solutions that are applicable across a wide range of chronic disorders and largely restricted to specific health systems, such as middle-income countries, high-income countries, or public insurance-based health-care systems.
Figure 1.
Challenges and strategies for achieving the Quadruple Aim
The central goal of health care is to reach the Quadruple Aim (central circle). Six important challenges must be addressed in order to reach this goal (second circle). We also identify four main strategies that can help to address this series of challenges (outer circle). The interplay between these four strategies and six challenges in reaching the Quadruple Aim is complex and multifaceted. For example, patient empowerment helps to achieve a more personalised care delivery, facilitates care delivery close to home, and makes care timelier with an improved patient experience, improved health outcomes, and reduced costs as important results. Similarly, professional empowerment ascertains that patients receive appropriate care, but well-trained clinicians are probably also better able to deploy precision medicine approaches. Each of the four strategies is facilitated by modern technological solutions (appendix p 2).
Panel 1. Reasons to regard Parkinson's disease as an ideal model condition for other chronic neurological disorders.
-
•
The clinical phenotype encompasses a wide range of non-motor and motor features, including cognitive decline, autonomic failure, and neuropsychiatric features
-
•
Optimal care of patients with Parkinson's disease requires involvement of staff from multiple (over 20 different) professional disciplines, who work in different health-care settings, including the community, regional hospitals, and specialised clinics
-
•
Management is multimodal, involving complex pharmacotherapy, neurosurgical procedures, and various non-pharmacological interventions
-
•
The disease duration is long, spanning up to decades for affected patients, plus a presumably lengthy prodromal phase
-
•
The disease is common, with an incidence and prevalence that are rising due to demographic changes and possibly other factors7
Challenges and solutions for health care
There are several barriers in modern health-care systems that prevent us reaching the Quadruple Aim (appendix p 1). However, here we discuss some solutions that can make systems better equipped to overcome these barriers (table ).
Table.
Aims and solutions to improve the management of patients with chronic neurological conditions such as Parkinson's disease
| Supportive evidence* | Minimum standard† | |
|---|---|---|
| Aim to organise care close to home | ||
| Remote monitoring | Passive monitoring of falls in home environment;10, 11 passive monitoring using mobile health technologies (eg, electronic device typing);12, 13 active monitoring (predefined tasks using smartphone to assess postural tremor or responsiveness to changes with medication);14, 15 other monitoring16 | No |
| Support patient empowerment | Use of online communities for patient communication17 | Yes |
| Online communication | Telemedicine visits by neurologists;18 interdisciplinary plan including home visits;19, 20 secure video-conferencing18, 21 | No |
| Aim to inform self-management | ||
| Focus on ability to adapt and self-manage | Education on daily life management22, 23 | Yes |
| Promote a healthy lifestyle | Encouragement of a healthy diet24 and exercise25, 26, 27 | Yes |
| Support for working capacity | Education on strategies and techniques to counteract symptoms of Parkinson's disease and enable longer workforce participation28 | Yes |
| Shared decision making | Tools for making informed shared decisions between available options for continuous dopaminergic stimulation (deep brain stimulation, intraduodenal levodopa, apomorphine)29 | Yes |
| Caregiver support | Peer-to-peer caregiver education30 | Yes |
| Aim to manage care proactively | ||
| Timely identification of specific complications | Active screening for precipitants of hospital admission such as near-falls | Yes |
| Aim to provide personalised or precision medicine | ||
| Focus on individual patient priorities | Consideration of differences between men and women in clinical presentation, treatment response, and health-care utilisation (eg, brain surgery for Parkinson's disease);31, 32, 33 consideration of racial34, 35, 36, 37 or cultural differences38, 39 | Yes |
| Big data and artificial intelligence | Personalised profiling and individualised prognostic or treatment advice40, 41, 42 | No |
| Aim to enable access to appropriate care | ||
| Parkinson-specific specialisation for all professional disciplines involved in Parkinson care, according to evidence-based guidelines | Training of commonly engaged disciplines, such as allied health professionals or specialised nurses;43, 44, 45, 46, 47, 48 training of less commonly recognised disciplines such as dentist or pulmonologist;49 inclusion of nursing home staff and clinicians involved in advanced care planning (issues at the end-of-life, palliative care)50, 51 | Yes |
| Concentration of care among trained experts (increase case load) | Dutch ParkinsonNet approach44 | Yes |
| Organising peer-to-peer networking | Implementation of interprofessional education for health-care professionals on evidence-based Parkinson's disease practices and working effectively in teams46 | Yes |
| Aim to provide coordinated care management | ||
| Coordination of care | Employment of personal care managers to coordinate care for people with Parkinson's disease52 | Yes |
| Establish links between Parkinson's disease specialists and generalists working in the community | Increased Parkinson's disease-specific knowledge among general practitioners53 | Yes |
| Telemedicine (peer-to-peer consultations) | More accurate clinical decision making in the field of acute stroke54 | No |
| To deliver integrated care and continuity of care | ||
| Breach silos by connecting all layers of health care and bundle into a model of integrated network care, both across professional disciplines and across all echelons | Some examples in the field of dementia;55 scarce examples available outside the field of neurology;56 models yet to be implemented for patients with chronic neurological conditions | Yes |
For each proposed solution, we provide supportive evidence, capitalising not only on the experience in the field of Parkinson's disease, but also on knowledge obtained for other conditions such as dementia8, 9 and other fields of medicine.
The minimum standard indicates whether a solution might be more readily available for wider scaling across other countries, health-care systems, or areas of medicine. No formal specific criteria for such minimum standards have been defined to date, so the suggestions offered here can only be used to offer some global guidance.
Care delivery close to home
Currently, chronic neurology care is delivered mainly in hospitals that are designed primarily for patients with acute diseases. Accessibility to such hospitals is difficult for those living at long distances.57 Furthermore, medical decisions are based almost exclusively on periodic in-clinic evaluations, but such short consultations cannot capture the effect on the patient's quality of life in their own home. An important aim is therefore to take neurological care away from medical centres and back into the patient's own environment.57 Being able to monitor and treat people at home is not only a service to patients, but also can lead to improved insights, care, and cost. Consequently, a patient's home could be considered a place where some hospital tasks could be implemented—a so-called homespital.58 For this shift in location of care to happen we need to consider two important factors: first, the importance of assessing patients during their normal activities; second, the value of delivering interventions as close to the home as possible. New developments in the field of digital health will be greatly supportive here (appendix p 2). Remote monitoring can obviate unnecessary routine appointments for patients who do not need in-person consultations and identify those patients who require medical attention. When care is required, the default should be to deliver care close to the patient's home whenever possible and only within institutions when necessary.
Patient empowerment and self-management
Patients demand more empowerment than they currently receive. Against their wishes,4, 59 most patients are not well informed about Parkinson's disease and are not counselled adequately to cope with the effects of the disease. Therefore, there needs to be a shift from paternalistic care—led by health-care professionals—to participatory health, with equality in attitude towards the contributions of patients, carers, and health-care professionals. The focus should be on self-management by well informed, empowered patients, with involvement of professional support when needed. The premise is that empowered patients will be less anxious, experience a better quality of life, and are less likely to seek medical support, thus helping to reduce health-care costs. This development fits with a new definition of health, which is no longer described as the complete absence of any physical, mental, or social disorders, but rather as the ability to adapt and self-manage.22
Patients can be empowered in various ways (table). Adequate patient counselling is essential, yet patients generally feel uninformed.4, 59 Patient education must extend beyond information about the neurological condition or its management to include lifestyle advice as part of counselling by health-care professionals, irrespective of the health system they work in.
An important part of patient empowerment is introducing tools for shared decision making, allowing patients and carers to participate in making optimal treatment decisions that are tailored to their specific needs. Formal procedures exist to develop validated shared decision tools that allow patients to make choices on the basis of reliable evidence (eg, information on treatment effects or risk of adverse effects). Shared decision tools are already available for several key choices in Parkinson's disease management. Both patients and clinicians feel that use of patient-reported outcome measures can help to support the shared decision process during consultations, although patients might need training to interpret the information correctly.60 Involvement of patients in developing these tools boosts face validity and increases their potential to reflect outcomes that are important to patients.61 Achieving shared decision making should be a universal priority among health-care professionals. A European survey showed that many people with Parkinson's disease feel insufficiently involved when it comes to making important treatment decisions, suggesting that improvements to the decision-making process are needed.62
Patient empowerment also implies caring for the individual's entire environment, with specific emphasis on immediate carers. Caring for people with Parkinson's disease is associated with high burden of stress, negative wellbeing and depression,63 and increased mortality risk.64 Other psychological effects include social isolation, loss of self-identity, and feelings of helplessness and lack of control.65 Carers often have their own health-care needs, and these requirements, coupled with the physical and economic burden of caring, frequently precipitate inpatient admissions for patients who can no longer be cared for at home. This effect on wellbeing is ample reason to consider not just the patient, but also the immediate carers.
Proactive and timely care
Current care for chronic neurological diseases is mainly reactive—ie, focuses on solving problems when they arise—which causes unnecessary burden for patients and leads to costly and potentially preventable hospital admissions. Optimal care involves not just responding to problems expressed by patients but also adopting a proactive approach that aims to detect early warning signs that might herald the onset of more debilitating (and costly) problems. People with Parkinson's disease are more likely to be admitted to hospital than their peers, and unplanned hospital admissions are more likely as the disease progresses.66, 67 Early recognition of patients at risk of hospital admission provides an opportunity to intervene. One example is timely detection of near-falls, which typically precede the onset of falls and fall-related injuries—the latter being a major cost in the care of patients with Parkinson's disease.68 Rather than waiting for injuries to occur, preventive measures can be taken, including medication adjustments, specialised physiotherapy, and optimisation of the home environment, to minimise the risk of falling.69
Proactive care becomes increasingly relevant in older people (typically older than 70 years) with Parkinson's disease because they are more likely to have neuropsychiatric manifestations, including apathy or dementia, which makes self-management difficult. Case management serves a crucial function to proactively identify these susceptible patients. This proactive thinking could pre-emptively optimise the patient's resilience and environment to minimise the risk of avoidable complications and prevent admission to hospital and care facilities. Yet, a reality is that hospital visits for patients with either a severe clinical phenotype of a single neurological disease, multiple comorbidities, or multiple neurological diseases can never be fully prevented.66, 67 As inpatients, older people have a longer length of hospital stay than age-matched controls with more deconditioning, loss of confidence, and exposure to iatrogenic risks (such as acquiring hospital related infections). They are also less likely to return to their normal place of residence and have higher in-hospital mortality.70 Delays in receiving medication can further exacerbate problems, such as having difficulty swallowing, reduced mobility, and falls. Inpatient care should therefore be optimised to minimise the risk of these complications, including education of ward nurses on the importance of administering medication at the appropriate time. Specific measures to enhance care of older patients include early review by a Parkinson's disease specialist to optimise medication and admissions to general wards.71, 72 This process should be supported by early flagging in electronic medical records to alert a dedicated inpatient team that someone with Parkinson's disease has been admitted. Unpublished experience at Struthers Parkinson's Centre, Minneapolis, MN, USA shows that such electronic flagging can markedly improve the timing of levodopa administrations and minimise missed doses in the hospital and emergency centre (Nance M, Wielinski C, Struthers Parkinson's Center, Minneapolis, MN, USA, personal communication). This example illustrates how proactive screening can be facilitated by developments in digital health (appendix p 2). Digital health could improve the quality and cost of care because proactive monitoring can prevent complications. However, the evidence for these benefits is in short supply, especially for beneficial effects on patient outcomes.
Precision medicine
Current treatments typically follow a generalised approach, but there is an urgent need for development of personalised or precision approaches, with care tailored to target each patient's personal needs. Delivery of care according to each patient's unique sociodemographic background, disease symptoms, genetic factors, and personal objectives is key. Current scientific evidence, especially insights derived from controlled clinical trials, is imperfect in this regard because the insights are based on relatively small and often highly selected study populations with brief follow-up periods, making it difficult to apply the outcomes to the care of individual patients in everyday practice. The generalised approach to treatment disregards specific issues related to age,73 gender,33, 74 racial,34, 35, 36, 37 or cultural differences.38, 39
Realisation of personalised medicine will greatly benefit from developments in the fields of big data and artificial intelligence,40, 41 in which insights derived from much larger and unselected real-life populations, or from smaller groups of patients for whom deep phenotyping has been done, can lead to development of personal disease profiles that represent the full complexity of individual patients. Such knowledge will allow clinicians to offer patients more detailed prognostic information and tailor their treatment advice to the unique profiles of their patients, which is particularly important for a disorder characterised by substantial heterogeneity such as Parkinson's disease.42 Progress in development of precision medicine for Parkinson's disease is being made, mainly in establishing refined prognosticators for specific endpoints at the group level, but reliable individual predictors have yet to be identified.75, 76, 77, 78, 79, 80 Importantly, big data approaches do not intend to replace existing information resources, but can be a complementary source of information in clinical decision making when used alongside scientific evidence, professional expertise, and the personal needs and preferences of patients. Combination of these four information sources then strengthens the process of decision making. Additionally insights derived from these information sources can be used to measure the effect of precision medicine approaches on the goals of the Quadruple Aim.81
Specialist care and professional training
Management of chronic neurological conditions, such as Parkinson's disease, has developed into a highly specialised field of medicine that requires expert skill to ensure that patients receive optimal care, in accordance with the latest scientific evidence. Such specialised professionals are more likely to adhere to guidelines for Parkinson's disease management than professionals with generic training, and are better aware of what fellow professionals can contribute to care.44 Trainings are delivered best to multidisciplinary teams, which improves knowledge specific to Parkinson's disease and leads to a better understanding of the role of other disciplines.46 Allocation of care preferentially to allied health professionals who have specialised in Parkinson's disease management is associated with improved patient outcomes and reduced costs in the Netherlands (panel 2 ).45, 82 There are also successful examples in other areas of neurology and medicine in which specialisation was associated with improvements in care, patient health, and cost savings.83, 84 Medical societies should always be involved in ascertaining the quality and nationwide implementation of the educational programmes. For example, all training programmes delivered by ParkinsonNet are done according to guidelines that have been ratified by the relevant national medical societies. The evidence from the Netherlands shows that the costs of such educational programmes are offset by subsequent cost savings.44
Panel 2. Key elements and outcomes of the ParkinsonNet model of care.
The ParkinsonNet model was introduced in 2004 in the Netherlands as an innovative treatment concept for patients with Parkinson's disease. Specifically, ParkinsonNet consists of regional community-based networks that encompass a restricted number of dedicated allied health therapists who have been trained specifically according to evidence-based guidelines. Key elements of the model are described in detail elsewhere44 and are summarised below. ParkinsonNet has reached full national coverage in the Netherlands, and currently includes 74 regional subnetworks with 3400 specifically trained health-care professionals, including—among others—physiotherapists, occupational therapists, speech-language therapists, dietitians, and Parkinson's disease nurses. Of note, ParkinsonNet is embedded within a not-for-profit organisation.
Guidelines *
-
•
Monodisciplinary—for physiotherapy, speech-language therapy, occupational therapy, dietary issues, and Parkinson's disease nurses
-
•
Multidisciplinary—includes a consensus-based model for regional and transmural organisation of multidisciplinary care
Preferred referral
-
•
Patients and physicians preferentially funnel their referrals towards ParkinsonNet experts to increase their caseload (using standardised referral forms with referral criteria)
Education
-
•
Baseline training of participating health-care professionals according to evidence-based guidelines (4 days)
-
•
Learning on the job: increase experience by treating many patients
-
•
Continuous interaction and information exchange between health-care professionals through an annual national conference, regional interdisciplinary meetings (at least twice a year), and participation in web-based national and regional communities
Information technology platform
- •
- •
-
•
Web-based communities for patients and professionals
Selection and re-certification
-
•
Inclusion of motivated and specifically trained health-care providers only; every 2 years a mandatory re-certification is required based on quality-of-care criteria
Commitment
-
•
Members of ParkinsonNet agree to work according to treatment guidelines and to collaborate with other professionals in multidisciplinary teams
Transparency about quality of services and health outcomes
-
•
Outcomes, costs, and average caseloads at the regional level published in the Parkinson Atlas
Patient-centred approach
-
•
Various approaches, including use of guidelines for patients, web-based communities for patients and web-based informative television programme for patients
Professional training might further help improve the quality of care for people with Parkinson's disease in nursing homes, where undertreatment with Parkinson's disease medication and overtreatment with sedatives are common.85 Another area in which training would improve care is palliative care interventions, which are traditionally equated with cancer management. People with Parkinson's disease can have moderate palliative care needs,86 but palliation is presently unavailable for most patients.50, 51 One study showed that a 1-year multidisciplinary palliative care programme improved quality of life for patients with moderate palliative care needs,87 emphasising the importance of training professionals to recognise these needs as part of integrated Parkinson's disease management.88
Finally, professional health-care training could increase intervention engagement for professionals and enhance their experience of delivering care. Complex and debilitating neurological conditions, such as Parkinson's disease, can be alarming to inexperienced clinicians, but can be gratifying to manage when their knowledge is adequate.89 Enhancement of the work-life balance of clinicians thus helps to achieve the Quadruple Aim by reducing burnout3 and possibly by motivating students to opt for a future career in medicine.
Care management
Current care is fragmented across different health-care providers and organisations, leading to a waste of resources. Unsurprisingly, people with Parkinson's disease—when asked to identify their top priorities for health-care improvement—identify having a single point of access (personal care manager) as their most urgent need.4 Having a personal care manager means questions can be answered immediately and problems can be identified early.4 It is neither feasible nor cost-effective to place a movement disorder specialist in this role. The reality is that there is already a shortage of specialist clinicians for the fast-growing population of people with Parkinson's disease.7, 90 Consequently, waiting lists are long, and movement disorder specialists have little time to see their patients. Nurses specialised in Parksinson's disease care are an excellent candidate to fulfil this role of being the first point of access for patient queries, with several specific tasks: triage, dedicated referral, and care coordination (appendix p 4). Nurses acting as a personal care manager might be based in community hospitals but, in less densely populated areas, nurses could also deliver services from a remote service desk (eg, a telephone call centre), acting as telehealth assistants. Such a model is recommended and partly in place in the UK,52 although many areas have insufficient numbers of specialised nurses to deliver ready access to all patients and to fully adopt the role as personal care manager.
An important part of care coordination is to establish links between Parkinson's disease specialists and generalists, including general practitioners who are optimally positioned to manage comorbidities and polypharmacy within the wider social context and domestic situation.53 Increases in depth of Parkinson's disease knowledge among general practitioners will augment their confidence in caring for patients with complex neurological illnesses and strengthen collaborative links with movement disorder specialists.53 To achieve this specific knowledge, general practitioners should be provided with easy access to a specialised professional when in need of referral for a Parkinson's disease health issue. Furthermore, general neurologists working in community hospitals can deliver better quality care for people with Parkinson's disease when supported by a remote expert via telemedicine (peer-to-peer consultations). This approach previously contributed to improved accuracy in clinical decision making in the field of acute stroke treatment.54
Delivery of integrated care
Each of the aforementioned solutions, when delivered in isolation, will help to improve the quality of care for patients with neurological illnesses. However, we anticipate that care delivery can be optimised further by seamlessly connecting the layers of health care and combining all solutions into an integrated network, across both professional disciplines and different health-care settings.56 Such an integrated model is referred to as population health management, in which responsibility is taken for an entire specific population, including cross-sector collaboration (eg, with coupling of health institutions to local social services), coordination with community services (eg, ascertaining adequate housing circumstances), and non-clinical interventions (eg, promoting a healthier lifestyle).2, 91 There is some initial evidence to suggest that this approach leads to better outcomes for the patient, while overall health-care spending remains the same.91
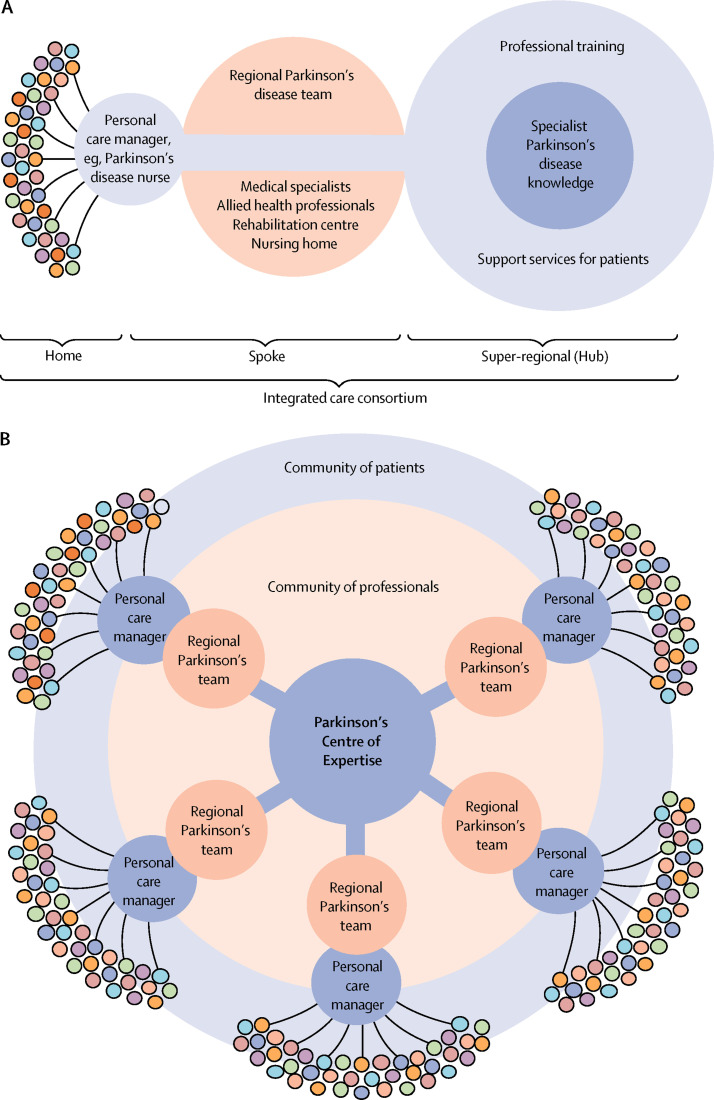
A so-called home-hub-and-spoke model is one way of structuring care services for a whole population (figure 2 ). The model features three components: a patient's home, a hub serving as an expert knowledge centre, and a spoke in between that contains the members of the regional Parkinson's disease team; the personal case manager connects these three components. The patient's own home can be the setting for patient education, promotion of self-management, and home-based monitoring. The movement disorder specialist in a community hospital, combined with specialised allied health professionals in the regional community, jointly form the second constituent (the spoke). Many services can be offered here. The role of digital health and academia (the hub) is not in physically seeing many patients, but in adding value to the entire network—eg, via peer-to-peer support for multiple spokes, educating patients and professionals, guideline development, and research. The hub can be located geographically close to its surrounding spokes, although many supportive services can be offered remotely via telemedicine, which would be classed as a virtual hub.
Figure 2.
The home-hub-and-spoke model
(A) Illustration of how a centre of expertise (the hub; services shown in light blue) can be coupled to a single spoke (services shown in red), consisting of a neighbouring community hospital (where regional care is delivered by specialist Parkinson's disease doctors), a Parkinson's disease nurse (acting as a personal care manager), and regional community-based professionals. (B) One centre of expertise can support many surrounding community hospitals (multiple spokes). The Parkinson's disease nurses working in the various spokes could collectively form a virtual service desk to provide easy access for patients with Parkinson's disease. The multicoloured circles represent individual patients, and the variations in colours are illustrative for their unique individual presentation and personal needs.
The need for integrated care increases when advanced, and probably expensive, new treatments become available, such as pharmacological and non-pharmacological disease-modifying strategies. In these situations, considerable Parksinson's disease-specific knowledge is required for optimal decision making. In the proposed model, regional movement disorder specialists would have a crucial role in informing decisions, supported when necessary by expertise and infrastructure at the hub (peer-to-peer expertise via telemedicine would be appropriate for this). Such remote hub support could also aid the diagnostic process (eg, reviewing videotaped neurological examinations) because the rate of diagnostic misclassification in early disease stages is higher among generalists than among experts.92
The experience with integrated care is thus far not unequivocally positive, although most studies suggest that reductions in hospital admissions, hospital readmissions, and emergency department visits can be achieved.56 Further work is now needed to show the actual value of integrated care for patients with neurological diseases. Additional research is also warranted on the implementation of the model in conditions in which cognition is notably affected, such as Alzheimer's disease or other primary dementias.
Financial considerations
The primary aim of integrated care should be improving quality of life for patients with chronic neurological conditions and enhancing population health. Cost containment is not a purpose in its own right,91 although integrated care might reduce costs, which is one component of the Quadruple Aim (figure 1).3 Cost savings can result from greater efficiency of care, prevention of disease complications, and reductions in unplanned hospital admissions (panel 3 ), although any cost savings are potentially offset by the necessary upfront investments in quality improvement of integrated care.91
Panel 3. Improved quality of care and cost savings expected from an integrated care approach.
Reduced outpatient visits
-
•
More self-management
-
•
Healthier lifestyle
-
•
Optimally timed consultations because of remote monitoring
-
•
Telemedicine consultations instead of physical visits to the hospital
Reduced inpatient admissions
-
•
Prevented disease complications including fractures
-
•
Fewer medication errors
Substitution of care
-
•
Nurse-led care reduces pressure on more expensive evaluations by movement disorder specialists
Optimal use of integrated multidisciplinary care
-
•
Personal care manager ascertains timely referral to specialised professionals
Seamless organisation of the entire health-care chain
-
•
Less financial waste caused by transitions between the various layers of health care: community services, regional hospitals, and tertiary centers
There is some evidence in the field of Parkinson's disease to support these assumptions. For example, various studies showed that professional specialisation, improved interdisciplinary collaboration, and patient education—as achieved via the ParkinsonNet approach (panel 2)—leads to considerable cost savings because of greater efficiency of care (specialised therapists provide substantially fewer treatment sessions) and fewer disease complications (injuries or pneumonia).43, 44, 45, 47 Taking the most conservative cost saving of US$439 per patient,93 the savings equated to around 5% of the expenditure on chronic Parkinson's disease care in the Netherlands (about €20–30 million saved annually). It is conceivable that adding further elements to this approach—eg, personalised care management—will lead to even greater cost savings. As such, integrated care models could help to ascertain an affordable care system for future generations. This model for network-based allied health care was originally developed in the Netherlands, which has a public insurance-based health-care system; however, it has been successfully transferred in health-care systems that have a different infrastructure (eg, accountable care organisation Kaiser Permanente, Oakland, CA, USA).94 We note that translation of this model and its possible implications for cost savings requires further study in other health-care systems.
Challenges in rural and low-resource settings
Four decades after the WHO declared Health for All by the year 2000, international and even regional differences in quality of care for patients with chronic diseases remain stark. We realise that implementing an integrated model of care, or even elements thereof, will pose tremendous challenges in sparsely populated or economically less-developed areas of the world. We therefore anticipate that the network by which integrated care is delivered will depend on local, regional, or national circumstances, including the geographical spread of the population across urban and rural areas, and on physical distances. In countries where health care for people with Parkinson's disease is largely hospital-based or delivered across substantial distances, care models must be adapted. For example, introduction of network care in California, USA, involved specialised education for health-care professionals, but not for allied health therapists working in the community—as was done in the Netherlands—but rather by training hospital-based teams.94 This approach, although different, resulted in the desired concentration of care among specifically trained professionals,94 which is a subsidiary intermediate for achieving good outcomes.44
The current and future provision of Parkinson's disease nurses, movement disorder specialists, and multidisciplinary expertise will also affect care structures. In countries where the role of the Parkinson's disease nurse specialist is well-established and successful, such as the UK and the Netherlands, nurses are based in both community and secondary care settings. However, most Parkinson's disease nurses in these countries work closely with movement disorder specialists who treat the same people with Parkinson's disease, suggesting that transference of this model might be challenging in countries where patients do not have regular follow ups by a movement disorder specialist. For example, 33% of people with Parkinson's disease in the USA do not receive regular care from a neurologist, let alone from a movement disorder specialist.36
Challenges will be even greater in in economically less-developed areas of the world (appendix p 5). In these countries, barriers to delivering care relate to human resources (eg, shortage of sufficiently trained health-care professionals), financial factors, and cultural differences in leadership or accountability.95 In many countries worldwide, many patients with Parkinson's disease remain undiagnosed,96 and essential medication such as levodopa is either poorly available or, when offered, unaffordable for many.38 Furthermore, people from several large regions (eg, the Western Pacific) are under-represented in health-care innovation research, despite substantial variability in the clinical presentation and comorbidity profiles of patients with Parkinson's disease across the world.97 The role of telemedicine will become particularly important in combating these challenges in clinical care and research. In countries such as China, readily available mobile phone applications are already widely used among medical professionals and patients for communication or consultations. A complementary strategy would be to ensure that health-care professionals in low-resource settings have access to health information technology systems that support clinical decision-making and enhance evidence-informed care. In the area of communicable diseases, this strategy substantially improved measures of population health, quality of care, and effective use of health-care services.95
Conclusions and future perspectives
By use of Parkinson's disease as an example, we have described a model of care for patients with chronic neurological conditions, including a patient-centred and proactive approach embedded within integrated networks where specifically trained professionals from multiple disciplines collaborate effectively (panel 4 ). We foresee an increasingly prominent and recognised role for specialised nurses, who can act as personal care managers for individual patients and as care coordinators for the network of different professional disciplines and health-care settings. Part of this vision is supported by empirical evidence, albeit mainly for the separate components of the integrated approach. There are also substantial gaps in knowledge, such as insufficient scientific evidence on the roles of Parkinson's disease nurses. Future work must therefore gather evidence for all the separate components of the model and their integration. A challenge here is that evaluations of such complex interventions require a spectrum of approaches to provide robust evidence. Alongside randomised clinical trials one approach could be to include additional methodologies, such as observational studies with analyses of medical claims data that compare regions with integrated care versus regions with care as usual.98
Panel 4. Take-home messages.
-
•
Whenever possible, care should be delivered near the patient's own home environment, both in terms of monitoring and care delivery57
-
•
This model of care educates patients, supports them in self-management, relieves anxiety, and alleviates pressure on the medical system4, 22, 59, 60, 61, 62, 63, 64, 65
-
•
The approach to neurology care should be proactive instead of reactive, thereby preventing disease burden and avoiding escalation to more expensive care (including avoidance of unplanned admissions)66, 67, 68, 69, 70, 71, 72
-
•
Proactive care can be supported by remote monitoring using sensors and online diaries, allowing for timely detection of medical problems99, 100, 101
-
•
Care should be delivered according to each patient's unique sociodemographic, disease-specific, and genetic factors, considered in tandem with their personal needs33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 73, 74, 75, 76, 77, 78, 79, 80, 81
-
•
Management of complex neurological conditions, such as Parkinson's disease, is beyond the expertise of generalists alone (despite generalists having a very important role in the management of these patients); patients benefit from having access to specialised professionals who attract a high case load of patients with a particular neurological condition44, 45, 46, 50, 51, 82, 83, 84, 85, 86, 87, 88, 89
-
•
Patients should have easy access to a single point of access (personal care manager) who can directly answer simple questions, refer patients to appropriate colleagues, and coordinate the multidisciplinary team advice4, 7, 52
-
•
Care delivery can be optimised further by seamlessly connecting the layers of health care and bundling all solutions into an integrated network, across both professional disciplines and different health-care settings2, 56, 91, 92
Most solutions proposed in the model could be readily implemented across various health systems and patient groups, including those in low-resource or remote settings; however, other solutions might not be universally applicable. In particular, the feasibility of implementing novel technology might be limited to high-income and middle-income countries. However, beyond the short term, technological developments could become available to people with Parkinson's disease in low-income countries, given the remarkable rise in smartphone ownership across less-developed regions—eg, in sub-Saharan Africa, smartphone ownership rose from 15% in 2014 to 33% in 2017.102
We anticipate that patients with other chronic and complex progressive neurological conditions, like dystonia or neuromuscular diseases, or those with a more paroxysmal symptoms, such as epilepsy or relapsing–remitting multiple sclerosis, also deserve an approach of networked care with a patient-centred proactive methodology delivered by specialised professionals treating a high case load. Modifications in care delivery might be required, depending on the nature and prevalence of each condition and on specific patient needs. For example, considerably fewer health-care professionals are needed for rare disorders, such as Huntington disease, so concentration of this specific knowledge within a small number of expert centres makes sense, whereas Parkinson's disease seems to benefit from community-based centres. The knowledge in some of these areas of neurology is growing, as exemplified by positive experiences with network care for patients with Alzheimer's disease.9 A key point will be to learn from contrasts between different networks, so the care model can be optimised. From a population health management perspective, it might be beneficial to concentrate expertise around a number of comparable chronic progressive neurological disorders within bundled specialised networks, which are characterised by a patient-centred proactive methodology delivered by specialised health-care professionals treating high case loads—including selected outreach clinics with connection to specialist hubs. We anticipate that research on bundled specialised networks will rapidly increase over the next 5 to 10 years. We therefore extend an open invitation to colleagues from other fields to share their experiences of integrated care for non-neurological chronic diseases, so that all our patients can benefit.
Search strategy and selection criteria
We searched for publications on health care and neurological conditions (in particular Parkinson's disease) published in MEDLINE from Jan 1, 1990, to Jan 28, 2020, using comprehensive electronic search strategies combining the MeSH and free text search terms “chronic”, “care”, “network”, “patient-centeredness”, “integrated”, “multidisciplinary”, “interdisciplinary”, “technology”, “health services”, “wearable sensors”, “telemedicine”, “Parkinson disease” and “Parkinson's disease”, without language restrictions. Selected articles were also obtained from the reference lists of papers identified by the PubMed search and from searches of our own files. We included both original studies and viewpoints.
Acknowledgments
Acknowledgments
The Centre of Expertise for Parkinson and Movement Disorders was supported by a grant from the Parkinson Foundation. The contributions by BRB, EJH, SKLD, and MM are part of the collaborative Proactive and Integrated Management and Empowerment in Parkinson's Disease (PRIME) project, which is financed by the Gatsby Foundation and Dutch Ministry of Economic Affairs by means of the PPP Allowance made available by the Top Sector Life Sciences & Health to stimulate public–private partnerships.
Contributors
BRB prepared the first draft of the manuscript. The remaining authors provided feedback on this first draft. EJH provided specific contributions to the sections on nursing home care palliative care and care management. MSO, SKLD, PC, and MM contributed to the integrated care model, ERD to the sections on remote monitoring, and NO and PC to the section on underserved countries. JA offered the patient perspective for all sections of the manuscript. All authors approved the final version of the manuscript.
Declaration of interests
BRB currently serves as Co-Editor in Chief for the Journal of Parkinson's Disease, serves on the editorial board of Practical Neurology and Digital Biomarker. BRB has received honoraria from serving on the scientific advisory board for AbbVie, Biogen, and Union Chimique Belge (UCB). BRB has also received fees for speaking at conferences from AbbVie, Zambon, Roche, GE Healthcare, and Bial, and has received research support from the Netherlands Organisation for Scientific Research, Michael J Fox Foundation, UCB, AbbVie, Stichting Parkinson Fonds, Hersenstichting Nederland, Parkinson's Foundation, Verily Life Sciences, Horizon 2020, and Parkinson Vereniging. EJH received funding from the UK National Institute of Health (NIH) Research, the Gatsby Foundation, British Geriatrics Society, and Parkinson's UK. EJH has also received fees for speaking and consultancy from Profile, Medicys, and Luye and received travel support from Bial AbbVie and Ever pharma. MSO serves as a consultant for the Parkinson's Foundation, and has received research grants from the US NIH, Parkinson's Foundation, the Michael J Fox Foundation, Parkinson Alliance, Smallwood Foundation, Bachmann-Strauss Foundation, Tourette Syndrome Association, and University of Florida Foundation. MSO's deep brain stimulation research is supported by NIH National Institute of Nursing Research (R01 NR014852) and NIH National Institute of Neurological Disorders and Stroke R01NS096008. MSO has received royalties for publications with Demos, Manson, Amazon, Smashwords, Books4Patients, Perseus, Robert Rose, Oxford, and Cambridge for movement disorder books. MSO is an Associate Editor for New England Journal of Medicine Journal Watch Neurology. MSO has participated in continuing medical education courses and educational activities on movement disorders sponsored by the Academy for Healthcare Learning, PeerView, Prime, QuantiaMD, WebMD–Medscape, Medicus, MedNet, Einstein, MedNet, Henry Stewart, American Academy of Neurology, Movement Disorders Society, and Vanderbilt University. The University of Florida and not MSO receives grants from Medtronic, AbbVie, Abbott, and Allergan. MSO has participated as a site principal investigator or co-investigator for several NIH, foundation, and industry sponsored trials over the years but has not received honoraria. Research projects at MSO's institution, the University of Florida, receive device and drug donations from Medtronic, Abbott, Boston Scientific, and Neuropace. ERD is a medical adviser to and holds stock options in Grand Rounds. Grand Rounds provide a second opinion on the diagnosis and management of health-care conditions of employees generally of large corporations. These second opinions are usually provided after review of medical records and addressing written questions of patients. This model is not discussed in this Personal View. ERD has also received honoraria for speaking at American Academy of Neurology courses; received compensation for consulting activities from 23andMe, Clintrex, GlaxoSmithKline, Lundbeck, MC10, MedAvante, Medico Legal services, US National Institute of Neurological Disorders and Stroke, Shire, Teva, and UCB; and research support from AMC Health, Burroughs Wellcome Fund, Davis Phinney Foundation, Duke University, GlaxoSmithKline, Great Lakes Neurotechnologies, Greater Rochester Health Foundation, Huntington Study Group, Michael J Fox Foundation, National Science Foundation, Patient-Centered Outcomes Research Institute, Prana Biotechnology, Raptor Pharmaceuticals, Roche, Saffra Foundation, and University of California Irvine. PC has received honoraria from serving on the scientific advisory board for Lundbeck, Teva, and Green Valley. PC has also received fees for speaking at conferences from Roche, Lundbeck, Sanofi, Eisai, Green Valley, Luye, GlaxoSmithKline, Medtronic, and Boehringer-Ingelheim; and has received research support from Chinese Organisations for Scientific Research, and Michael J Fox Foundation. SKLD has received a research grant from the Parkinson Foundation. MSO, JA, and MM declare no competing interests.
Footnotes
Evidence-based recommendations and consensus-based statements.
Supplementary Material
References
- 1.GBD 2016 Neurology Colloborators Global, regional, and national burden of neurological disorders, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18:459–480. doi: 10.1016/S1474-4422(18)30499-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berwick DM, Nolan TW, Whittington J. The triple aim: care, health, and cost. Health Aff. 2008;27:759–769. doi: 10.1377/hlthaff.27.3.759. [DOI] [PubMed] [Google Scholar]
- 3.Bodenheimer T, Sinsky C. From triple to quadruple aim: care of the patient requires care of the provider. Annals Fam Med. 2014;12:573–576. doi: 10.1370/afm.1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vlaanderen FP, Rompen L, Munneke M, Stoffer M, Bloem BR, Faber MJ. The voice of the Parkinson customer. J Parkinsons Dis. 2019;9:197–201. doi: 10.3233/JPD-181431. [DOI] [PubMed] [Google Scholar]
- 5.Levin KH, Shanafelt TD, Keran CM, et al. Burnout, career satisfaction, and well-being among US neurology residents and fellows in 2016. Neurology. 2017;89:492–501. doi: 10.1212/WNL.0000000000004135. [DOI] [PubMed] [Google Scholar]
- 6.WHO A69/39: framework on integrated, people-centred health services. April 15, 2016. https://apps.who.int/gb/ebwha/pdf_files/WHA69/A69_39-en.pdf
- 7.Dorsey ER, Bloem BR. The Parkinson pandemic—a call to action. JAMA Neurol. 2018;75:9–10. doi: 10.1001/jamaneurol.2017.3299. [DOI] [PubMed] [Google Scholar]
- 8.MacNeil Vroomen J, Bosmans JE, Eekhout I, et al. The cost-effectiveness of two forms of case management compared to a control group for persons with dementia and their informal caregivers from a societal perspective. PLoS One. 2016;11 doi: 10.1371/journal.pone.0160908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Richters A, Nieuwboer MS, Olde Rikkert MGM, Melis RJF, Perry M, van der Marck MA. Longitudinal multiple case study on effectiveness of network-based dementia care towards more integration, quality of care, and collaboration in primary care. PLoS One. 2018;13 doi: 10.1371/journal.pone.0198811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Silva de Lima AL, Smits T, Darweesh SK, et al. Home-based monitoring of falls using wearable sensors in Parkinson's disease. Mov Disord. 2020;35:109–115. doi: 10.1002/mds.27830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Silva de Lima AL, Hahn T, Evers LJW, et al. Feasibility of large-scale deployment of multiple wearable sensors in Parkinson's disease. PLoS One. 2017;12 doi: 10.1371/journal.pone.0189161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matarazzo M, Arroyo-Gallego T, Montero P, et al. Remote monitoring of treatment response in Parkinson's disease: the habit of typing on a computer. Mov Disord. 2019;34:1488–1495. doi: 10.1002/mds.27772. [DOI] [PubMed] [Google Scholar]
- 13.Espay AJ, Hausdorff JM, Sánchez-Ferro A, et al. A roadmap for implementation of patient-centered digital outcome measures in Parkinson's disease obtained using mobile health technologies. Mov Disord. 2019;34:657–663. doi: 10.1002/mds.27671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lipsmeier F, Taylor KI, Kilchenmann T, et al. Evaluation of smartphone-based testing to generate exploratory outcome measures in a phase 1 Parkinson's disease clinical trial. Mov Disord. 2018;33:1287–1297. doi: 10.1002/mds.27376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhan A, Mohan S, Tarolli C, et al. Using smartphones and machine learning to quantify Parkinson disease severity: the mobile Parkinson disease score. JAMA Neurol. 2018;75:876–880. doi: 10.1001/jamaneurol.2018.0809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klucken J, Krüger R, Schmidt P, Bloem BR. Management of Parkinson's disease 20 years from now: towards digital health pathways. J Parkinsons Dis. 2018;8:S85–S94. doi: 10.3233/JPD-181519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Visser LM, Bleijenbergh IL, Benschop YW, Van Riel AC, Bloem BR. Do online communities change power processes in healthcare? Using case studies to examine the use of online health communities by patients with Parkinson's disease. BMJ Open. 2016;6 doi: 10.1136/bmjopen-2016-012110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beck CA, Beran DB, Biglan KM, et al. National randomised controlled trial of virtual house calls for Parkinson disease. Neurology. 2017;89:1152–1161. doi: 10.1212/WNL.0000000000004357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fleisher J, Barbosa W, Sweeney MM, et al. Interdisciplinary home visits for individuals with advanced Parkinson's disease and related disorders. J Am Geriatr Soc. 2018;66:1226–1232. doi: 10.1111/jgs.15337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eggers C, Dano R, Schill J, Fink GR, Hellmich M, Timmermann L. Patient-centered integrated healthcare improves quality of life in Parkinson's disease patients: a randomised controlled trial. J Neurol. 2018;265:764–773. doi: 10.1007/s00415-018-8761-7. [DOI] [PubMed] [Google Scholar]
- 21.Dorsey ER, Glidden AM, Holloway MR, Birbeck GL, Schwamm LH. Teleneurology and mobile technologies: the future of neurological care. Nat Rev Neurol. 2018;14:285–297. doi: 10.1038/nrneurol.2018.31. [DOI] [PubMed] [Google Scholar]
- 22.Huber M, Knottnerus JA, Green L, et al. How should we define health? BMJ. 2011;343 doi: 10.1136/bmj.d4163. [DOI] [PubMed] [Google Scholar]
- 23.Hellqvist C, Dizdar N, Hagell P, Berterö C, Sund-Levander M. Improving self-management for persons with Parkinson's disease through education focusing on management of daily life: patients' and relatives' experience of the Swedish National Parkinson School. J Clin Nurs. 2018;27:3719–3728. doi: 10.1111/jocn.14522. [DOI] [PubMed] [Google Scholar]
- 24.Barichella M, Cereda E, Pinelli G, et al. Muscle-targeted nutritional support for rehabilitation in patients with parkinsonian syndrome. Neurology. 2019;93:e485–e496. doi: 10.1212/WNL.0000000000007858. [DOI] [PubMed] [Google Scholar]
- 25.Mirelman A, Rochester L, Maidan I, et al. Addition of a non-immersive virtual reality component to treadmill training to reduce fall risk in older adults (V-TIME): a randomised controlled trial. Lancet. 2016;388:1170–1182. doi: 10.1016/S0140-6736(16)31325-3. [DOI] [PubMed] [Google Scholar]
- 26.Schenkman M, Moore CG, Kohrt WM, et al. Effect of high-intensity treadmill exercise on motor symptoms in patients with de novo Parkinson disease: a phase 2 randomised clinical trial. JAMA Neurol. 2018;75:219–226. doi: 10.1001/jamaneurol.2017.3517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van der Kolk NM, de Vries NM, Kessels RPC, et al. Effectiveness of home-based and remotely supervised aerobic exercise in Parkinson's disease: a double-blind, randomised controlled trial. Lancet Neurol. 2019;18:998–1008. doi: 10.1016/S1474-4422(19)30285-6. [DOI] [PubMed] [Google Scholar]
- 28.Koerts J, König M, Tucha L, Tucha O. Working capacity of patients with Parkinson's disease—a systematic review. Parkinsonism Relat Disord. 2016;27:9–24. doi: 10.1016/j.parkreldis.2016.03.017. [DOI] [PubMed] [Google Scholar]
- 29.Nijhuis FAP, Elwyn G, Bloem BR, Post B, Faber MJ. Improving shared decision-making in advanced Parkinson's disease: protocol of a mixed methods feasibility study. Pilot Feasibility Stud. 2018;4:94. doi: 10.1186/s40814-018-0286-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Van der Marck M. GOUDMantel-Parkinson. 2017. https://www.parkinsonnext.nl/resultaten-goudmantel/
- 31.Hariz GM, Lindberg M, Hariz MI, Bergenheim AT. Gender differences in disability and health-related quality of life in patients with Parkinson's disease treated with stereotactic surgery. Acta Neurol Scand. 2003;108:28–37. doi: 10.1034/j.1600-0404.2003.00092.x. [DOI] [PubMed] [Google Scholar]
- 32.Haaxma C, Bloem B, Borm G, et al. Gender differences in Parkinson's disease. J Neurol Neurosurg Psychiatry. 2007;78:819–824. doi: 10.1136/jnnp.2006.103788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Georgiev D, Hamberg K, Hariz M, Forsgren L, Hariz GM. Gender differences in Parkinson's disease: a clinical perspective. Acta Neurol Scand. 2017;136:570–584. doi: 10.1111/ane.12796. [DOI] [PubMed] [Google Scholar]
- 34.Saadi A, Himmelstein DU, Woolhandler S, Mejia NI. Racial disparities in neurologic health care access and utilisation in the United States. Neurology. 2017;88:2268–2275. doi: 10.1212/WNL.0000000000004025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Willis AW, Schootman M, Evanoff BA, Perlmutter JS, Racette BA. Neurologist care in Parkinson disease: a utilisation, outcomes, and survival study. Neurology. 2011;77:851–857. doi: 10.1212/WNL.0b013e31822c9123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fullard ME, Thibault DP, Hill A, et al. Utilisation of rehabilitation therapy services in Parkinson disease in the United States. Neurology. 2017;89:1162–1169. doi: 10.1212/WNL.0000000000004355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mantri S, Fullard ME, Beck J, Willis AW. State-level prevalence, health service use, and spending vary widely among Medicare beneficiaries with Parkinson disease. NPJ Parkinsons Dis. 2019;5:1. doi: 10.1038/s41531-019-0074-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Okubadejo NU, Ojo OO, Wahab KW, et al. A nationwide survey of Parkinson's disease medicines availability and affordability in Nigeria. Mov Disord Clin Pract. 2019;6:27–33. doi: 10.1002/mdc3.12682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tran Ngoc C, Bigirimana N, Muneene D, et al. Conclusions of the digital health hub of the Transform Africa Summit (2018): strong government leadership and public-private-partnerships are key prerequisites for sustainable scale up of digital health in Africa. BMC Proc. 2018;12:17. doi: 10.1186/s12919-018-0156-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Beam AL, Kohane IS. Big data and machine learning in health care. JAMA. 2018;319:1317–1318. doi: 10.1001/jama.2017.18391. [DOI] [PubMed] [Google Scholar]
- 41.He J, Baxter SL, Xu J, Xu J, Zhou X, Zhang K. The practical implementation of artificial intelligence technologies in medicine. Nat Med. 2019;25:30–36. doi: 10.1038/s41591-018-0307-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Okun MS. Management of Parkinson disease in 2017: personalised approaches for patient-specific needs. JAMA. 2017;318:791–792. doi: 10.1001/jama.2017.7914. [DOI] [PubMed] [Google Scholar]
- 43.Munneke M, Nijkrake MJ, Keus SH, et al. Efficacy of community-based physiotherapy networks for patients with Parkinson's disease: a cluster-randomised trial. Lancet Neurol. 2010;9:46–54. doi: 10.1016/S1474-4422(09)70327-8. [DOI] [PubMed] [Google Scholar]
- 44.Bloem BR, Munneke M. Revolutionising management of chronic disease: the ParkinsonNet approach. BMJ. 2014;348 doi: 10.1136/bmj.g1838. [DOI] [PubMed] [Google Scholar]
- 45.Ypinga JHL, de Vries NM, Boonen L, et al. Effectiveness and costs of specialised physiotherapy given via ParkinsonNet: a retrospective analysis of medical claims data. Lancet Neurol. 2018;17:153–161. doi: 10.1016/S1474-4422(17)30406-4. [DOI] [PubMed] [Google Scholar]
- 46.Cohen EV, Hagestuen R, Gonzalez-Ramos G, et al. Interprofessional education increases knowledge, promotes team building, and changes practice in the care of Parkinson's disease. Parkinsonism Relat Disord. 2016;22:21–27. doi: 10.1016/j.parkreldis.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 47.Sturkenboom IH, Graff MJ, Hendriks JC, et al. Efficacy of occupational therapy for patients with Parkinson's disease: a randomised controlled trial. Lancet Neurol. 2014;13:557–566. doi: 10.1016/S1474-4422(14)70055-9. [DOI] [PubMed] [Google Scholar]
- 48.Ramig L, Halpern A, Spielman J, Fox C, Freeman K. Speech treatment in Parkinson's disease: randomized controlled trial (RCT) Mov Disord. 2018;33:1777–1791. doi: 10.1002/mds.27460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Radder DLM, de Vries NM, Riksen NP, et al. Multidisciplinary care for people with Parkinson's disease: the new kids on the block! Expert Rev Neurother. 2019;19:145–157. doi: 10.1080/14737175.2019.1561285. [DOI] [PubMed] [Google Scholar]
- 50.Boersma I, Miyasaki J, Kutner J, Kluger B. Palliative care and neurology: time for a paradigm shift. Neurology. 2014;83:561–567. doi: 10.1212/WNL.0000000000000674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bouça-Machado R, Lennaerts-Kats H, Bloem BR, Ferreira J. Why palliative care applies to Parkinson's disease. Mov Disord. 2018;33:750–753. doi: 10.1002/mds.27309. [DOI] [PubMed] [Google Scholar]
- 52.Rogers G, Davies D, Pink J, Cooper P. Parkinson's disease: summary of updated NICE guidance. BMJ. 2017;358 doi: 10.1136/bmj.j1951. [DOI] [PubMed] [Google Scholar]
- 53.Plouvier AOA, Olde Hartman TC, Verhulst CEM, Bloem BR, van Weel C, Lagro-Janssen ALM. Parkinson's disease: patient and general practitioner perspectives on the role of primary care. Fam Pract. 2017;34:227–233. doi: 10.1093/fampra/cmw115. [DOI] [PubMed] [Google Scholar]
- 54.Meyer BC, Raman R, Hemmen T, et al. Efficacy of site-independent telemedicine in the STRokE DOC trial: a randomised, blinded, prospective study. Lancet Neurol. 2008;7:787–795. doi: 10.1016/S1474-4422(08)70171-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Draper B, Low LF, Brodaty H. Integrated care for adults with dementia and other cognitive disorders. Int Rev Psychiatry. 2018;30:272–291. doi: 10.1080/09540261.2018.1564021. [DOI] [PubMed] [Google Scholar]
- 56.Damery S, Flanagan S, Combes G. Does integrated care reduce hospital activity for patients with chronic diseases? An umbrella review of systematic reviews. BMJ Open. 2016;6 doi: 10.1136/bmjopen-2016-011952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dorsey ER, Vlaanderen FP, Engelen LJ, et al. Moving Parkinson care to the home. Mov Disord. 2016;31:1258–1262. doi: 10.1002/mds.26744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Medium.com; Home care: the next frontier in healthcare innovation. Feb 1, 2019. https://medium.com/bridgeable/home-care-the-next-frontier-in-healthcare-innovation-4fe289083910
- 59.van der Eijk M, Faber MJ, Post B, et al. Capturing patients' experiences to change Parkinson's disease care delivery: a multicenter study. J Neurol. 2015;262:2528–2538. doi: 10.1007/s00415-015-7877-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Damman OC, Verbiest MEA, Vonk SI, et al. Using PROMs during routine medical consultations: the perspectives of people with Parkinson's disease and their health professionals. Health Expect. 2019;22:939–951. doi: 10.1111/hex.12899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Domecq JP, Prutsky G, Elraiyah T, et al. Patient engagement in research: a systematic review. BMC Health Serv Res. 2014;14:89. doi: 10.1186/1472-6963-14-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bloem BR, Stocchi F. Move for change part III: a European survey evaluating the impact of the EPDA Charter for People with Parkinson's Disease. Eur J Neurol. 2015;22:133–141. doi: 10.1111/ene.12544. [DOI] [PubMed] [Google Scholar]
- 63.Martinez-Martin P, Arroyo S, Rojo-Abuin JM, et al. Burden, perceived health status, and mood among caregivers of Parkinson's disease patients. Mov Disord. 2008;23:1673–1680. doi: 10.1002/mds.22106. [DOI] [PubMed] [Google Scholar]
- 64.Nielsen M, Hansen J, Ritz B, et al. Cause-specific mortality among spouses of Parkinson disease patients. Epidemiology. 2014;25:225–232. doi: 10.1097/EDE.0000000000000042. [DOI] [PubMed] [Google Scholar]
- 65.Hasson F, Kernohan WG, McLaughlin M, et al. An exploration into the palliative and end-of-life experiences of carers of people with Parkinson's disease. Pallia Med. 2010;24:731–736. doi: 10.1177/0269216310371414. [DOI] [PubMed] [Google Scholar]
- 66.Gerlach OHH, Winogrodzka A, Weber WEJ. Clinical problems in the hospitalised Parkinson's disease patient: systematic review. Mov Disord. 2011;26:197–208. doi: 10.1002/mds.23449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hassan A, Wu SS, Schmidt P, et al. High rates and the risk factors for emergency room visits and hospitalisation in Parkinson's disease. Parkinsonism Relat Disord. 2013;19:949–954. doi: 10.1016/j.parkreldis.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 68.Farag I, Sherrington C, Hayes A, et al. Economic evaluation of a falls prevention exercise program among people With Parkinson's disease. Mov Disord. 2016;31:53–61. doi: 10.1002/mds.26420. [DOI] [PubMed] [Google Scholar]
- 69.van der Marck MA, Klok MP, Okun MS, et al. Consensus-based clinical practice recommendations for the examination and management of falls in patients with Parkinson's disease. Parkinsonism Relat Disord. 2014;20:360–369. doi: 10.1016/j.parkreldis.2013.10.030. [DOI] [PubMed] [Google Scholar]
- 70.Low V, Ben-Shlomo Y, Coward E, Fletcher S, Walker R, Clarke CE. Measuring the burden and mortality of hospitalisation in Parkinson's disease: a cross-sectional analysis of the English Hospital Episodes statistics database 2009–2013. Parkinsonism Relat Disord. 2015;21:449–454. doi: 10.1016/j.parkreldis.2015.01.017. [DOI] [PubMed] [Google Scholar]
- 71.Chou KL, Zamudio J, Schmidt P, et al. Hospitalisation in Parkinson disease: a survey of National Parkinson Foundation Centers. Parkinsonism Related Disord. 2011;17:440–445. doi: 10.1016/j.parkreldis.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Oguh O, Videnovic A. Inpatient management of Parkinson disease: current challenges and future directions. Neurohospitalist. 2012;2:28–35. doi: 10.1177/1941874411427734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.McMurdo ME, Roberts H, Parker S, et al. Improving recruitment of older people to research through good practice. Age Ageing. 2011;40:659–665. doi: 10.1093/ageing/afr115. [DOI] [PubMed] [Google Scholar]
- 74.Tosserams A, Araujo R, Pringsheim T, et al. Underrepresentation of women in Parkinson's disease trials. Mov Disord. 2018;33:1825–1826. doi: 10.1002/mds.27505. [DOI] [PubMed] [Google Scholar]
- 75.Valmarska A, Miljkovic D, Konitsiotis S, Gatsios D, Lavrac N, Robnik-Sikonja M. Symptoms and medications change patterns for Parkinson's disease patients stratification. Artif Intell Med. 2018;91:82–95. doi: 10.1016/j.artmed.2018.04.010. [DOI] [PubMed] [Google Scholar]
- 76.Evers LJW, Krijthe JH, Meinders MJ, Bloem BR, Heskes TM. Measuring Parkinson's disease over time: the real-world within-subject reliability of the MDS-UPDRS. Mov Disord. 2019;34:1480–1487. doi: 10.1002/mds.27790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kelly MJ, Lawton MA, Baig F, et al. Predictors of motor complications in early Parkinson's disease: a prospective cohort study. Mov Disord. 2019;34:1174–1183. doi: 10.1002/mds.27783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schrag A, Siddiqui UF, Anastasiou Z, Weintraub D, Schott JM. Clinical variables and biomarkers in prediction of cognitive impairment in patients with newly diagnosed Parkinson's disease: a cohort study. Lancet Neurol. 2017;16:66–75. doi: 10.1016/S1474-4422(16)30328-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Latourelle JC, Beste MT, Hadzi TC, et al. Large-scale identification of clinical and genetic predictors of motor progression in patients with newly diagnosed Parkinson's disease: a longitudinal cohort study and validation. Lancet Neurol. 2017;16:908–916. doi: 10.1016/S1474-4422(17)30328-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Liu G, Locascio JJ, Corvol JC, et al. Prediction of cognition in Parkinson's disease with a clinical-genetic score: a longitudinal analysis of nine cohorts. Lancet Neurol. 2017;16:620–629. doi: 10.1016/S1474-4422(17)30122-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.van den Heuvel L, Dorsey RR, Prainsack B, et al. Quadruple decision making for Parkinson's disease patients: combining expert opinion, patient preferences, scientific evidence, and big data approaches to reach precision medicine. J Parkinsons Dis. 2019;10:223–231. doi: 10.3233/JPD-191712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Munneke M, Nijkrake MJ, Keus SH, et al. Efficacy of community-based physiotherapy networks for patients with Parkinson's disease: a cluster-randomised trial. Lancet Neurol. 2010;9:46–54. doi: 10.1016/S1474-4422(09)70327-8. [DOI] [PubMed] [Google Scholar]
- 83.Kimmel AD, Martin EG, Galadima H, et al. Clinical outcomes of HIV care delivery models in the US: a systematic review. AIDS Care. 2016;28:1215–1222. doi: 10.1080/09540121.2016.1178702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lange JF, Meyer VM, Voropai DA, et al. The role of surgical expertise with regard to chronic postoperative inguinal pain (CPIP) after Lichtenstein correction of inguinal hernia: a systematic review. Hernia. 2016;20:349–356. doi: 10.1007/s10029-016-1483-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Weerkamp NJ, Zuidema SU, Tissingh G, et al. Motor profile and drug treatment of nursing home residents with Parkinson's disease. J Am Geriatr Soc. 2012;60:2277–2282. doi: 10.1111/jgs.12027. [DOI] [PubMed] [Google Scholar]
- 86.Saleem TZ, Higginson IJ, Chaudhuri KR, Martin A, Burman R, Leigh PN. Symptom prevalence, severity and palliative care needs assessment using the Palliative Outcome Scale: a cross-sectional study of patients with Parkinson's disease and related neurological conditions. Palliat Med. 2013;27:722–731. doi: 10.1177/0269216312465783. [DOI] [PubMed] [Google Scholar]
- 87.Kluger BM, Miyasaki J, Katz M, et al. Integrated outpatient palliative care for persons affected by Parkinson's disease and related disorders: a pragmatic randomised comparative effectiveness trial. JAMA Neurol. 2020 doi: 10.1001/jamaneurol.2019.4992. published online Feb 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bloem BR, Darweesh SKL, Meinders MJ. Palliative programs for persons with parkinsonism—the next frontier. JAMA Neurol. 2020 doi: 10.1001/jamaneurol.2019.4697. published online Feb 10. [DOI] [PubMed] [Google Scholar]
- 89.Canoy M, Faber MJ, Munneke M, Oortwijn W, Nijkrake MJ, Bloem BR. Hidden treasures and secret pitfalls: application of the capability approach to ParkinsonNet. J Parkinson's Dis. 2015;5:575–580. doi: 10.3233/JPD-150612. [DOI] [PubMed] [Google Scholar]
- 90.GBD 2016 Parkinson's Disease Colloborators Global, regional, and national burden of Parkinson's disease, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018;17:939–953. doi: 10.1016/S1474-4422(18)30295-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jha AK. Population health management: saving lives and saving money? JAMA. 2019;322:390–391. doi: 10.1001/jama.2019.10568. [DOI] [PubMed] [Google Scholar]
- 92.Rizzo G, Copetti M, Arcuti S, Martino D, Fontana A, Logroscino G. Accuracy of clinical diagnosis of Parkinson disease: a systematic review and meta-analysis. Neurology. 2016;86:566–576. doi: 10.1212/WNL.0000000000002350. [DOI] [PubMed] [Google Scholar]
- 93.Bloem BR, Rompen L, Vries NM, Klink A, Munneke M, Jeurissen P. ParkinsonNet: a low-cost health care innovation with a systems approach from the Netherlands. Health Aff. 2017;36:1987–1996. doi: 10.1377/hlthaff.2017.0832. [DOI] [PubMed] [Google Scholar]
- 94.Rompen L, de Vries NM, Munneke M, et al. Introduction of network-based healthcare at Kaiser Permanente. J Parkinsons Dis. 2020;10:207–212. doi: 10.3233/JPD-191620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Thornicroft G, Ahuja S, Barber S, et al. Integrated care for people with long-term mental and physical health conditions in low-income and middle-income countries. Lancet Psychiatry. 2019;6:174–186. doi: 10.1016/S2215-0366(18)30298-0. [DOI] [PubMed] [Google Scholar]
- 96.Cubo E, Doumbe J, Mapoure Njankouo Y, et al. The burden of movement disorders in Cameroon: a rural and urban-based inpatient/outpatient study. Mov Disord Clin Pract. 2017;4:568–573. doi: 10.1002/mdc3.12474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lim SY, Tan AH, Ahmad-Annuar A, et al. Parkinson's disease in the western Pacific region. Lancet Neurol. 2019;18:865–879. doi: 10.1016/S1474-4422(19)30195-4. [DOI] [PubMed] [Google Scholar]
- 98.Bloem BR, Ypinga JHL, Willis A, et al. Using medical claims analyses to understand interventions for Parkinson patients. J Parkinsons Dis. 2018;8:45–58. doi: 10.3233/JPD-171277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Poushter J, Bishop C, Chwe H. Social media use continues to rise in developing countries but plateaus across developed ones. June 19, 2018. https://www.pewresearch.org/global/2018/06/19/social-media-use-continues-to-rise-in-developing-countries-but-plateaus-across-developed-ones/
- 100.Isaacson SH, Boroojerdi B, Waln O, et al. Effect of using a wearable device on clinical decision-making and motor symptoms in patients with Parkinson's disease starting transdermal rotigotine patch: a pilot study. Parkinsonism Relat Disord. 2019;64:132–137. doi: 10.1016/j.parkreldis.2019.01.025. [DOI] [PubMed] [Google Scholar]
- 101.Farzanehfar P, Woodrow H, Braybrook M, et al. Objective measurement in routine care of people with Parkinson's disease improves outcomes. NPJ Parkinsons Dis. 2018;4:10. doi: 10.1038/s41531-018-0046-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ginis P, Nieuwboer A, Dorfman M, et al. Feasibility and effects of home-based smartphone-delivered automated feedback training for gait in people with Parkinson's disease: a pilot randomised controlled trial. Parkinsonism Relat Disord. 2016;22:28–34. doi: 10.1016/j.parkreldis.2015.11.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.