Abstract
The structural characteristics and biological activity of human antibodies that are reactive with the capsular polysaccharides of most serotypes of Streptococcus pneumoniae, including serotype 8, are unknown. This paper describes the generation, molecular structure, and protective efficacy of a human monoclonal antibody (MAb) reactive with the capsular polysaccharide of serotype 8 Streptococcus pneumoniae. We generated the immunoglobulin M(κ) [IgM(κ)] MAb D11 by Epstein-Barr virus transformation of peripheral lymphocytes from a Pneumovax recipient. Nucleic acid sequence analysis revealed that MAb D11 uses V3-15/VH3 and A20/Vκ gene segments with evidence of somatic mutation. In vitro studies revealed MAb D11-dependent complement deposition on the capsule of serotype 8 organisms via either the classical or the alternative complement pathway. In vivo, MAb D11 prolonged the survival of both normal and C4-deficient mice with lethal serotype 8 S. pneumoniae infection. Our findings demonstrate that a serotype-specific human IgM with certain structural and functional characteristics was protective in mice lacking a functional classical complement pathway and show that alternative complement pathway activation is an important determinant of pneumococcal protection.
In the past decade, the importance of pneumococcal vaccination for individuals at high risk for infection has been underscored by the emergence of antibiotic resistance among pneumococcal isolates (7, 14) and the increased prevalence of invasive pneumococcal infections in patients with immune impairment caused by chemotherapy or immune suppression in the setting of organ transplantation or human immunodeficiency virus infection (40). Unfortunately, available pneumococcal capsular polysaccharide vaccines are poorly immunogenic in many patients at high risk for infection (40). The prevalence of pneumococcal strains with high-level antibiotic resistance (50) has increased the need for new approaches to treatment of pneumococcal infections and the potential value of monoclonal antibodies (MAbs) as therapeutic agents. Antibody therapy was used for pneumococcal pneumonia until the late 1930s but was abandoned after the introduction of antimicrobial therapy because of the toxicity of heterologous sera in humans (51). Hence, the prospect of returning to antibody-based therapies for pneumococcal infections (15, 44) demands a better understanding of the nature of the antibodies that mediate protection.
The importance of both serotype-specific antibodies and complement components for host protection against Streptococcus pneumoniae has been recognized since the early 20th century (32). In vitro studies have shown that opsonization of S. pneumoniae by human sera via the classical and the alternative complement pathways requires serotype-specific antibody (9). The importance of an intact complement system for antibody-mediated protection against S. pneumoniae was revealed by studies of experimental pneumococcal infection of guinea pigs showing that neither polyclonal serotype-specific immunoglobulin M (IgM) nor IgG was protective in guinea pigs with C4 deficiency (12, 24). These studies suggested that an intact classical complement pathway was necessary for antibody-mediated protection. C4-deficient mice have enhanced susceptibility to the encapsulated pathogen group B Streptococcus (52). However, the importance of an intact classical complement pathway for innate resistance or the protective efficacy of serotype-specific human antibodies has not been examined in experimental pneumococcal infections of mice.
To evaluate the role that antibody-dependent complement activation plays in pneumococcal protection, we determined the ability of a human IgM reactive with the pneumococcal capsular polysaccharide (PPS) of serotype 8 S. pneumoniae to protect mice with deficiencies of C4 (19) and C3 (52) against an infection with this serotype. The results showed that the MAb, D11, prolonged the survival of mice with a normal complement system and those with C4 deficiency.
(Part of the work described herein was presented at the 99th General Meeting of the American Society for Microbiology [57].)
MATERIALS AND METHODS
Bacteria.
S. pneumoniae serotype 8 (strain 6308; American Type Culture Collection [ATCC], Manassas, Va.) was grown in tryptic soy broth (TSB) (Difco Laboratories, Detroit, Mich.) to mid-log phase at 37°C in 5% CO2 as described previously (1), frozen in TSB in 10% glycerol, and stored at −80°C. Bacteria were taken from a frozen stock, streaked on a blood agar plate, and passaged once in TSB before use.
Generation of the D11 antibody, a PPS 8-specific human MAb.
The human MAb to PPS 8 (D11) was generated from the peripheral lymphocytes of a Pneumovax (Pneumovax 23; Merck, West Point, Pa.) recipient. Mononuclear cells were isolated from whole blood of a normal volunteer 7 days after vaccination with Pneumovax as described previously (41). The mononuclear cells were placed in 96-well tissue culture plates (Corning Glass Works, Corning, N.Y.) and infected with 200 μl of supernatant from an Epstein-Barr virus-infected marmoset cell line (B95-8; ATCC) as described previously (41). Wells with transformants visible by light microscopy were tested for binding to Pneumovax by enzyme-linked immunosorbent assay (ELISA) (see below). Cell lines were cloned by limiting dilution within 3 weeks of transformation. Cell lines were maintained in RPMI medium, 10% fetal calf serum (HyClone Laboratories Inc., Logan, Utah), 1% glutamine, 1% pyruvate, 1% nonessential amino acids, and 0.01 M HEPES. Unless otherwise specified, the reagents for cell culture were obtained from Gibco (Grand Island, N.Y.). These studies were performed in accordance with the guidelines of the Institutional Review Board of the Albert Einstein College of Medicine.
ELISAs to determine the isotype and PPS specificity of MAb D11.
All cell lines were tested for antibodies that reacted with Pneumovax and purified PPS from serotypes 4, 6B, 8, 14, 19F, and 23F (ATCC) as described previously (3). Briefly, plates coated with Pneumovax (11.4 μg/ml) or purified PPS (10 μg/ml) were incubated with culture supernatants from the transformants at 37°C for 1 h and washed, and separate duplicate wells were incubated with alkaline phosphatase-labeled goat antibodies to human IgM, IgA, and IgG κ and λ light chains (Southern Biotechnology, Birmingham, Ala.) at 37°C for 1 h. After washing, antibody binding was detected with p-nitrophenyl phosphate substrate (Sigma Chemical Co., St. Louis, Mo.). The absorbances of the wells were measured at an optical density (OD) of 405 nm with an MRX (DYNEX Technologies, Inc., Chantilly, Va.) ELISA reader. The positive control was day 28 postvaccination serum from a Pneumovax recipient (absorbed with cell wall polysaccharide [CWPS] [Statens Serum Institut, Copenhagen, Denmark] as described previously [3]); the negative controls were the human IgM MAb specific for cryptococcal polysaccharide (MAb 2E9 [41]) and an IgM myeloma antibody (Calbiochem, San Francisco, Calif.). Antibodies produced by the transformants were also tested for binding to CWPS and staphylococcal protein A (SPA) (Sigma) as described previously (36, 41) and for the expression of the variable-region determinants recognized by mouse MAbs to human variable-region determinants (3).
Nucleotide sequence analysis.
The nucleic acid sequence of the D11 antibody was determined by sequencing DNA amplified from RNA by PCR as described previously (41). Briefly, VH (heavy chain) and VL (light chain) cDNA was generated by reverse transcription of RNA with heavy and light chain constant region primers as described previously (41). The VH and VL were initially amplified with a set of sense primers complementary to human VH and VL sequences and the same antisense constant region primers for VH and VL (27). For the D11 MAb, the primers were as follows: VH sense, 5′-GAGTTTGGGCTGAGCTGG-3′; VH antisense, 5′-GGAATTCTCACAGGAGACGAG-3′; Vκ sense, 5′-GAA(CT)ATC(T)GAGCTCACC(GT)CAGTCTCCA-3′; and Vκ antisense, 5′-CCTGTTGAAGCTCTTTGTGAC-3′. Oligonucleotides were synthesized at the DNA Synthesis Facility of the Cancer Center of the Albert Einstein College of Medicine. VH and Vκ PCR products were gel purified and cloned into the PCR 1000 plasmid of the TA cloning system (Invitrogen, San Diego, Calif.) according to the manufacturer’s instructions. The products of two independent PCRs were cloned. Inserts containing the VH and Vκ were identified by restriction endonuclease analysis. Plasmid DNA was isolated by the Maxi plasmid protocol (Qiagen, Inc., Chatsworth, Calif.), and DNA sequencing was performed by the DNA Synthesis Facility of the Cancer Center of the Albert Einstein College of Medicine. In addition, direct sequencing of the VH PCR product was also performed. Variable-region sequences were compared to the database of human immunoglobulin sequences by using DNA PLOT (V Base Index; MRC Center for Protein Engineering, Cambridge, United Kingdom).
Antibodies and complement sources.
A human IgM myeloma (Calbiochem, Inc.) was used as an isotype-matched control in this study. This antibody, which uses a human VH3 gene segment and binds SPA, has been used as a negative control in other studies (3, 56). The D11 antibody was purified from culture supernatants by column chromatography with anti-human IgM–Sepharose (Pharmacia). The influence of the classical complement pathway was evaluated with factor B-deficient human serum (Calbiochem), which has an inactive alternative pathway, and the influence of the alternative complement pathway was evaluated with C4-deficient guinea pig serum (Sigma), which has an inactive classical pathway. A polyclonal goat antibody to human C3 (Calbiochem) was used to detect C3 by ELISA (see below), and a horseradish peroxidase (HRP)-labeled antibody to human C3 (Cappel, ICN Pharmaceuticals, Inc., Aurora, Ohio) was used in ultrastructure studies to detect C3 binding to S. pneumoniae by electron microscopy. These antibodies bind human and guinea pig C3.
ELISA to evaluate complement activation.
C3 binding to solid-phase PPS was determined by ELISA as described previously (56). Briefly, ELISA plates were coated with PPS (see above) and incubated for 1 h at 37°C with solutions consisting of 5% (by volume) complement-deficient serum with 1.25 to 5 μg of the MAb D11, the control IgM, or phosphate-buffered saline (PBS) per ml. The plates were then washed and incubated for 1 h at 37°C with a goat antibody to human C3 (Calbiochem), washed, incubated for 1 h at 37°C with alkaline phosphatase-labeled rabbit antibodies to goat IgG (Calbiochem), and developed with p-nitrophenylphosphate substrate (Sigma). Negative controls were the MAb 2E9 (an IgM to cryptococcal polysaccharide [41]), the control IgM, and detection reagents without primary and secondary antibodies. The OD of the wells was measured at 405 nm with an MRX ELISA reader. The OD of the wells containing the secondary antibody and the complement-deficient serum alone was subtracted from the average of paired duplicate wells containing the MAbs or PBS. The results are reported as the OD at 405 nm.
EM.
Electron microscopy (EM) was used to visualize C3 deposition on the capsule of S. pneumoniae. The procedure for these studies was adapted from the protocol of Brown et al. (13). S. pneumoniae (ATCC 6308 serotype 8) was heat-killed by placing a suspension of 108 bacteria at 65°C for 1 h. PBS or 50 μg of D11 or the control IgM per ml was added to the bacteria; the samples (consisting of 180 μl) were incubated for 30 min at 37°C; 20 μl of each complement-deficient serum or PBS was then added (i.e., to a final concentration of 10% by volume); and the samples were incubated for 30 min at 37°C, washed, and incubated for 1 h at 4°C with an HRP-labeled antibody to human C3 in a volume of 300 μl. The bacteria were then fixed with 300 μl of 1% glutaraldehyde in 0.08 M sodium cacodylate buffer (pH 7.4) and incubated for 30 min at room temperature in a saturated solution of 3,3′-diaminobenzidine (DAB) (Sigma) in 0.05 M Tris (hydroxymethyl) aminomethane-hydrochloride (pH 7.6) containing H2O2 (0.001%). After postfixing with 1% osmium tetroxide in 0.08 M sodium cacodylate buffer, the samples were dehydrated in graded alcohols. Finally, the bacteria were embedded in LX112 resin (Ladd Research Industries, Burlington, Vt.), and ultrathin sections were cut on a Reichert Ultracut E apparatus and viewed on a JEOL 1200 EX transmission electron microscope at 80 kV.
Mouse infection experiments.
The protective efficacy of MAb D11 was evaluated in mouse models of pneumococcal infection. C57BL/6 and (C57BL/6 × 129)F2 mice were obtained from Jackson Laboratories (Bar Harbor, Maine). Mice with targeted deletions in C3 (C3−/− mice) (52) and C4−/− mice (19) were bred in the Animal Facility of the Albert Einstein College of Medicine in sterile isolators. The bacteria used for the infection experiments were rapidly thawed and placed on ice, diluted to the desired concentration in TSB and immediately injected into the mice as described elsewhere (1). The number of live bacteria in each experiment was quantified by CFU on blood agar plates (Difco) immediately after the mouse inoculations were completed. The plates were incubated at 37°C for 18 h, and the number of colonies on each plate (one colony = 1 CFU) was counted. The susceptibility to ATCC 6308 of the mouse strains studied was determined with groups of 6 to 10 mice. Groups of mice received intraperitoneal (i.p.) inoculations of 2, 20, or 200 CFU, and their survival was monitored twice daily. The number of bacteria that killed 50% of mice by 48 h after infection was defined as the 50% lethal dose (LD50) for that group. The LD50 determinations were performed twice.
Two mouse infection models were used. The first model was selected because historically, sera that were protective in a similar model had therapeutic efficacy in humans against established infections (16). In this model (the single syringe model), a solution containing both the organisms and antibody was injected i.p. into the mice: one group received 100 CFU of serotype 8 S. pneumoniae organisms and PBS, another received the organisms combined with 1 μg of the control IgM, and another received the organisms and 1 μg of D11. The antibody and organisms were mixed in the same syringe and immediately injected into the mice. For experiments according to this model, C57BL/6 mice (10 per group) and C3−/−, C4−/−, and F2 mice (6 to 7 per group) were used. For the complement-deficient mice, both male and female mice were used, and for the other strains of mice only female mice were used. The second model consisted of i.p. injection of antibody 1 h prior to i.p. infection (the i.p./i.p. model) (35): one group was first given PBS, another group received 1 μg of myeloma IgM, and another received 1 μg of MAb D11; then, 1 h later, all groups received 100 CFU of serotype 8 S. pneumoniae except the F2 mice, which received 200 CFU. This model was selected to eliminate the possibility of antibody-antigen agglutination in the syringe being a variable in the experimental outcome. For experiments using the second model, C3−/−, C4−/−, and F2 mice (8 to 10 per group) were used. All mice were observed twice daily, and the number of surviving mice was recorded.
Determination of bacteremia in C4−/− mice.
To evaluate whether antibody treatment reduced the degree of bacteremia of serotype 8-infected C4−/− mice, we determined the serum bacterial burden. The mice (n = 5) were given 1 μg of MAb D11, IgM, or PBS i.p. and then 50 CFU of serotype 8 S. pneumoniae i.p. 1 h later. Blood was obtained from the tail veins of these mice 3, 6, 9, and 12 h after infection. Dilutions of the blood (1:10, 1:100, and 1:1,000) in TSB were plated in duplicate on blood agar plates and incubated for 18 h, and the number of CFU per milliliter of blood was counted.
Statistical analysis.
Mouse survival data was analyzed statistically by using the Kaplan-Meier log rank test. The degrees of bacteremia in different treatment groups of mice were compared by using Student’s t test. The statistical tests were performed with SPSS for Windows (release 7.5.1; SPSS, Inc.), with a P value of 0.05 taken to indicate significance.
RESULTS
Isotype and specificity of the D11 antibody.
The D11 antibody was produced by an IgM(κ) lymphoblastoid cell line which had specificity for Pneumovax and PPS 8 only. No reactivity was observed with the PPS of serotypes 3, 4, 6B, 14, 19F, or 23F; CWPS; or SPA (data not shown). D11 did not express the VH1, VH3, and VH4 determinants recognized by mouse MAbs G6 and G8, D12 and 16.84 and B6, and LC1, respectively. The negative control myeloma IgM did not bind Pneumovax or any of the purified PPS serotypes, but it did manifest SPA binding as previously described (41).
Nucleic acid sequence analysis.
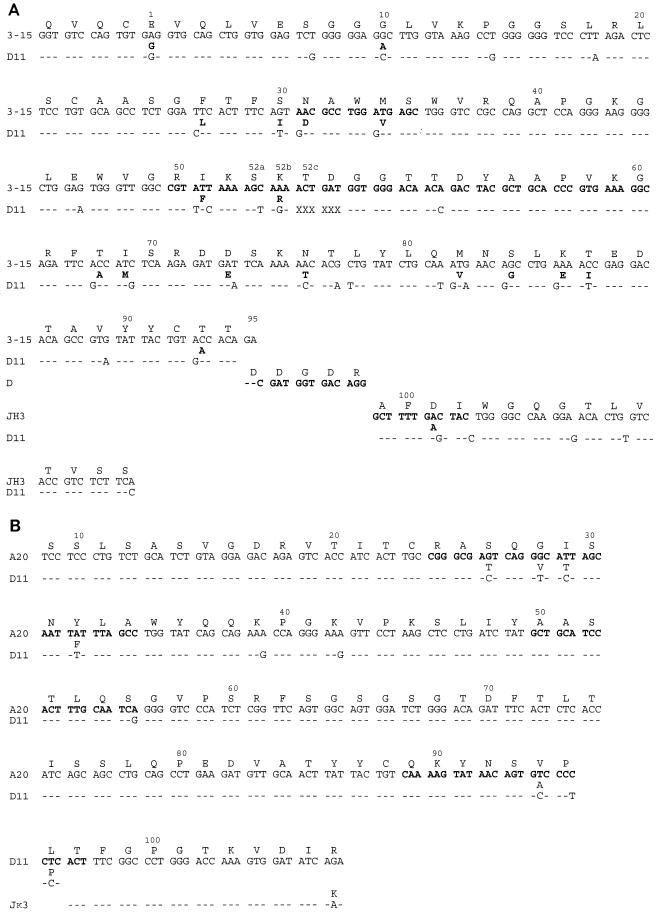
The MAb D11 VH was 90.5% homologous with the human germ line V3-15 gene segment (6) and 87% homologous with the germ line 9.1 III (DP 38) VH3 gene segment used by human antibodies to the Haemophilus influenzae type b polyribosyl-ribitol phosphate (4) (Fig. 1A). The MAb D11 VH sequence had an in-frame 6-base deletion in complementarity-determining region 2 (CDR 2) (Fig. 1A). The presence of this deletion was confirmed by two additional experiments, which revealed sequences identical to that shown in Fig. 1A: (i) direct sequencing of a PCR product generated with a different leader (sense) oligonucleotide and the same antisense oligonucleotide described above and (ii) sequencing of a PCR product obtained with a sense primer containing the MAb D11 framework 1 (FW 1) sequence (5′-GTCGGGGGGAGCCTTGGTAAA-3′) and an antisense primer containing the MAb D11 FW 3 sequence (5′-GTCCTCGATTTCCAGGCCGTT-3′) cloned into the TA vector. The MAb D11 JH was most homologous with JH3b, and the D region, used in reading frame 2, has homology with D4-17, D4-23, and D5-5 (6). The MAb D11 Vκ was 96.5% homologous with the germ line A20-Vκ1 gene segment (21), though the first 8 amino acids were not determined because the PCR primer included the bases encoding these residues (Fig. 1B). The MAb D11 Vκ uses the Jκ3 segment (6) (Fig. 1B). Based on the putative germ line genes of the MAb D11 VH and Vκ, the following replacement-to-silent (R/S) mutation ratios were calculated: VH FWs, 14/8 = 1.75 (counting the M-to-V change at amino acid 82 as two replacement changes); Vκ FWs, 0/2; VH CDRs, 5/2 = 2.5 (counting the F-to-I change at amino acid 51 as two replacement changes); and Vκ CDRs, 5/1 = 5.
FIG. 1.
(A) Amino acid (top) and nucleic acid (bottom) sequences of the D11 VH compared to its putative germ line sequence, V3-15. The deletion in the D11 CDR 2 is denoted by 6 Xs from amino acid 52c to 54. (B) Amino acid (top) and nucleic acid (bottom) sequences of the D11 Vκ compared to its putative germ line sequence, A 20. An FW 1 primer was used for PCR; thus, the nucleic acids of amino acids 1 to 10 were not determined. For both panels, single-letter designations are used for amino acids. Dashes represent homology, and letters in boldface type denote replacement amino acid changes. Numbering is according to VBase (MRC Center for Protein Engineering [6]).
ELISA to determine complement activation.
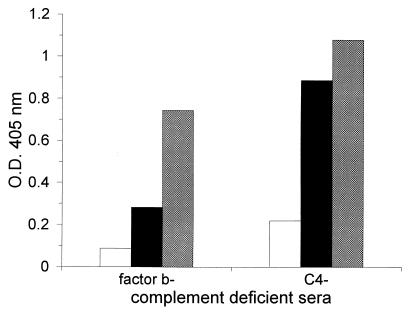
MAb D11 promoted deposition of C3 on solid-phase PPS 8 when either factor B- or C4-deficient sera were used as complement sources (Fig. 2). Based on the ODs observed, C3 deposition appeared to be greater in the presence of C4-deficient serum and a higher concentration of MAb D11 was required to promote C3 deposition when factor B-deficient serum was used as a complement source (Fig. 2). Although this may indicate that MAb D11 activation of the alternative pathway was more efficient, these results may also be explained by differences in the complement components or species of the sera used. Studies with EGTA-treated and untreated human serum (performed as described in reference 20) confirmed that MAb D11 mediated C3 deposition via the alternative pathway (data not shown). There was no C3 deposition detectable above background levels after incubation with the control IgM or PBS (data not shown).
FIG. 2.
ELISA-based detection of D11-mediated C3 deposition on solid-phase PPS 8. C3 deposition was assessed by the OD at 405 nm, as shown on the y axis, for the complement sources denoted on the x axis. The data shown represent the results when D11 was used at concentrations of 1.25 μg/ml (open bars), 2.5 μg/ml (black bars), and 5 μg/ml (grey bars). The level of C3 detected when PBS or the control IgM was used was background, and these results are not shown.
EM.
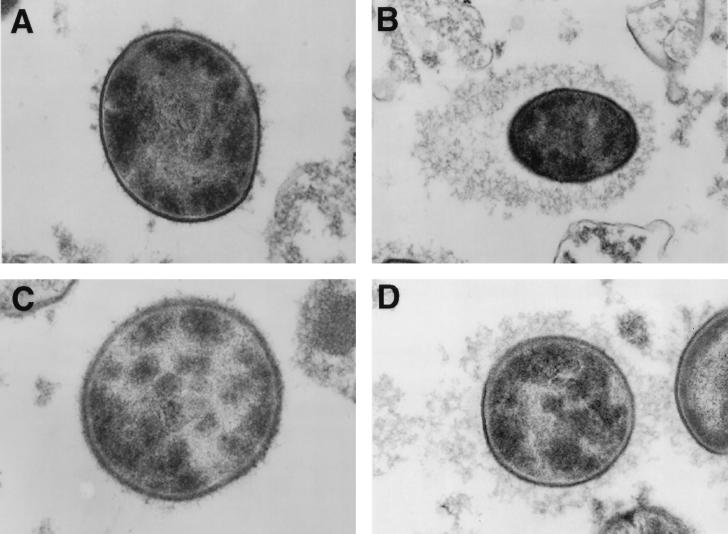
The pattern of C3 deposition on serotype 8 S. pneumoniae in the presence and absence of MAb D11 and different complement sources was examined by EM. In these experiments, C3 deposition was detected with an HRP-labeled antibody to human C3. The location of C3 deposition was determined by identifying the location of the precipitate which resulted after the addition of DAB. The cell wall of gram-positive organisms appears as an electron-lucent structure around the electron-dense cellular cytoplasm (37), and with the staining method used, capsular material is also electron lucent (37). When S. pneumoniae was incubated with PBS and factor B-deficient serum (which has an inactive alternative complement pathway) or C4-deficient serum (which has an inactive classical complement pathway), no detectable staining was observed (data not shown). In the presence of MAb D11, a precipitate which surrounded the bacteria and extended from the cell wall was observed when factor B- or C4-deficient serum was used (Fig. 3B and D). The staining pattern with C4-deficient serum appeared denser and was often associated with an asymmetric pattern in which the pattern of deposition was polar (Fig. 3B). Although the pattern observed with MAb D11 and factor B-deficient serum was very similar to that obtained with C4-deficient serum, the pattern of deposition appeared looser and less dense (Fig. 3D). In the presence of the control IgM and both complement sources, most organisms had no detectable staining, but some had small, discrete aggregated or hair-like projections located adjacent to the cell wall (Fig. 3A and C). The latter may represent small foci of C3 deposition. Hence, these studies revealed that MAb D11 was required for C3 deposition on the capsular surface, that C3 deposition could occur via the alternative or the classical complement pathway, and that subtle differences were found between the patterns of deposition with MAb D11 and each of the complement-deficient sera. The significance of the latter is unknown.
FIG. 3.
Transmission electron micrograph of serotype 8 S. pneumoniae treated with C4-deficient serum and a control IgM (A) or D11 (B) or with factor B-deficient serum and a control IgM (C) or D11 (D). All panels show representative cells (magnification, ×1,500). Experiments were performed as described in the text. (A) The cell wall and the capsule appear electron lucent in comparison to the electron-dense cytoplasm. The discrete, aggregated deposits emanating from the boundary of the cell represent C3 deposits. (B) The electron-lucent capsule is seen as a precipitate extending from the boundary of the cell. The precipitate, which represents C3 deposition detected with an antibody to C3 and DAB (see text) surrounds the entire cell and has an area of increased density, seen on the left. This asymmetry was seen on the majority of cells that were sectioned horizontally. (C) The fine, hair-like material emanating from the boundary of the cell represents capsular material. Most cells appeared unstained entirely, though some manifested infrequent, wispy C3 deposits which were sparse and difficult to distinguish from capsular material itself, as seen in the cell shown. (D) The precipitate extending from the boundary of the cell represents C3 deposits. In comparison to the deposits seen in panel B, the precipitate has a less apparent structure, which is looser and less dense.
Mouse protection experiments.
Inoculation of all mouse strains with ATCC 6308 resulted in lethal infection within 48 h. The mice appeared ill within 24 h of infection, which was manifested by poor feeding, unkempt fur, and reduced activity level. When the mice became moribund, they exhibited respiratory distress. The lethality of the strain, even at low inocula, prevented the determination of a precise LD50 in some of the mouse strains; e.g., the LD50 was <2 CFU for C57BL/6 and C4−/− mice and 20 CFU for F2 mice (data not shown). An LD50 was not determined for the C3−/− mice, because a limited number of these mice was available. An inoculum of 100 CFU was used for the protection experiments, except that 200 CFU was given to the F2 mice in the i.p./i.p. model.
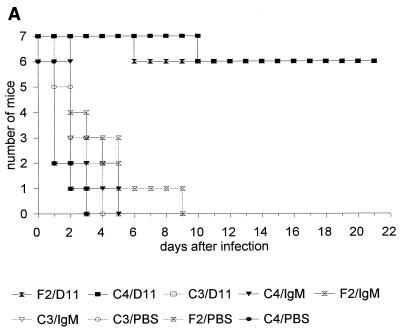
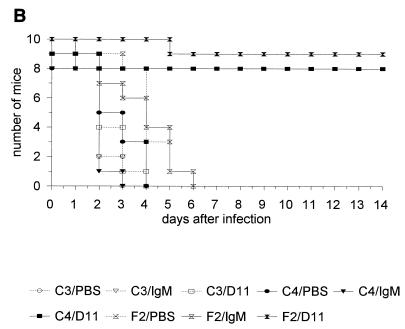
In both infection models, MAb D11 significantly enhanced survival of C57BL/6 (not shown), C4−/−, and F2 mice compared to PBS and the control IgM (P ≤ 5 × 10−4; Kaplan-Meier log rank survival test) (Fig. 4A and B; Tables 1 and 2). Administration of MAb D11 did not prolong survival of the C3−/− mice in either model (Fig. 4A and B; Tables 1 and 2). A small but statistically significant reduction in the survival of PBS-treated C4−/− mice was observed compared to IgM-treated control and C4−/− mice in the first infection model (Table 1), and in the second infection model there was a small but statistically significant prolongation of the survival of PBS-treated C4−/− mice compared to PBS- and IgM-treated C3−/− mice (Table 2).
FIG. 4.
Survival of control and D11-treated F2, C3−/−, and C4−/− mice after infection with serotype 8 S. pneumoniae in the single-syringe (A) or i.p./i.p. (B) model. (C) Survival of D11-treated and untreated C3−/− mice infected with serotype 8 S. pneumoniae and reconstituted with factor B- or C4-deficient serum in the single-syringe model. For all panels, see the text for details of the experiment. Symbols for groups of mice are explained below each panel (C4 designates C4−/− mice, and C3 designates C3−/− mice). Number of mice, number of mice surviving on the indicated day.
TABLE 1.
Statistical comparison of the numbers of mice surviving mice i.p. administration of a mixture of MAb and organismsa
| Mouse-treatment combination |
P for comparison with:
|
|||||||
|---|---|---|---|---|---|---|---|---|
| C3-PBS | C4-PBS | F2-PBS | C3-IgM | C4-IgM | F2-IgM | C3-D11 | C4-D11 | |
| C4-PBS | 0.06 | |||||||
| F2-PBS | 0.16 | 0.01 | ||||||
| C3-IgM | 0.39 | 9 × 10−3 | 0.45 | |||||
| C4-IgM | 0.68 | 0.01 | 0.30 | 0.63 | ||||
| F2-IgM | 0.18 | 5 × 10−3 | 0.61 | 0.65 | 0.37 | |||
| C3-D11 | 0.09 | 0.72 | 0.02 | 0.02 | 0.03 | 9 × 10−3 | ||
| C4-D11 | 2 × 10−4 | 3 × 10−4 | 2 × 10−4 | 2 × 10−4 | 1 × 10−4 | 2 × 10−4 | 1 × 10−4 | |
| F2-D11 | 2 × 10−4 | 3 × 10−4 | 5 × 10−4 | 2 × 10−4 | 1 × 10−4 | 2 × 10−4 | 1 × 10−4 | 0.9566 |
Mice with targeted deletions of C3 and C4 and F2 control mice were either treated with MAb D11 or a control IgM or left untreated. P values were determined by the Kaplan-Meier log rank survival test. Statistically significant values are shown in boldface type.
TABLE 2.
Statistical comparison of the numbers of mice surviving i.p. administration of MAb followed in 1 h by infectiona
| Mouse-treatment combination |
P for comparison with:
|
||||
|---|---|---|---|---|---|
| C3-PBS | C4-PBS | C3-IgM | C4-IgM | C3-D11 | |
| C4-PBS | 0.02 | ||||
| C3-IgM | 1.0 | 0.02 | |||
| C4-IgM | 0.55 | 0.13 | 0.55 | ||
| C3-D11 | 0.13 | 0.24 | 0.13 | 0.48 | |
| C4-D11 | 1 × 10−4 | 3 × 10−4 | 1 × 10−4 | 1 × 10−4 | 1 × 10−4 |
Mice with targeted deletions of C3 and C4 and F2 control mice were either treated with MAb D11 or a control IgM or left untreated. P values were determined by the Kaplan-Meier log rank survival test. Statistically significant values are shown in boldface type.
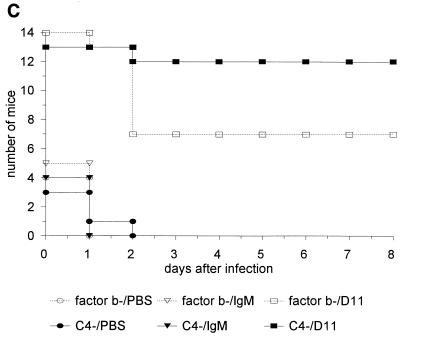
In another experiment using the single syringe model, the survival of C3−/− mice was determined following the administration of ATCC 6308 and 1 μg of MAb D11 or the control IgM per ml or PBS with 10% (by volume) factor B- or C4-deficient serum. The results showed that MAb D11 enhanced survival of C3−/− mice in the presence of both complement-deficient sera compared to IgM or PBS treatment (Fig. 4C). There was a trend towards increased survival of the mice receiving C4-deficient serum and MAb D11 compared to factor B-deficient serum and MAb D11, but the difference did not reach statistical significance (P = 0.07; Kaplan-Meier log rank survival test). However, when the results of this experiment were combined with a repeat study using the same protocol, the survival of the mice receiving C4-deficient serum and MAb D11 was significantly greater than that of the mice receiving factor B-deficient serum and MAb D11 (P < 0.01; Kaplan-Meier log rank survival test) (Fig. 4C).
Determination of bacteremia in C4−/− mice.
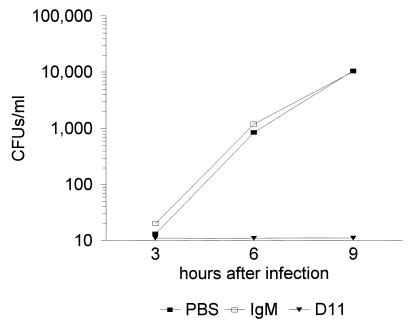
These studies confirmed that MAb D11-mediated protection of C4−/− mice was associated with a reduction in bacteremia. All mice infected with S. pneumoniae were bacteremic 3 h after infection, except those that received MAb D11 (Fig. 5). The inoculum for this experiment was confirmed to be 50 CFU, based on plating at the time of infection. The degree of bacteremia 3 h after infection in the mice that received IgM and PBS was 13 ± 14 and 20 ± 19 CFU/ml, respectively. The mice that received MAb D11 had no detectable S. pneumoniae in their blood at 3 h or any time after infection. No organisms were detected in any mouse that received D11, with a limit of detection of 10 CFU. The mice that received the control IgM and PBS manifested exponential increases in bacteremia and were all dead 12 h after infection. There were no significant differences in the degree of bacteremia between the group that received the control IgM and the group that received PBS (Student’s t test).
FIG. 5.
Bacteremia with serotype 8 S. pneumoniae in control and D11-treated C4−/− mice in the i.p./i.p. model. See the text for details of the experiment. The CFU were determined at the indicated times after infection. Symbols for groups of mice are explained below the graph. The results for D11-treated mice are shown as 10 CFU because this is the lower limit of detection for the assay. However, no CFU were detected at any time in blood at a 1:10 dilution from any of the five mice. All of the control mice died by 12 h after infection.
DISCUSSION
Serotype 8 S. pneumoniae is an important cause of adult pneumococcal infections, and it is the only serotype that increases in prevalence with age (45). Despite the medical importance of serotype 8, very little is known about the molecular structure of PPS 8-specific antibodies. To date, only one other human MAb to PPS 8 has been reported (48), but its molecular structure is unknown. The human IgM(κ) to PPS 8 reported here, MAb D11, uses a VH3 gene segment. The VH3 gene family is the largest of seven VH gene families. High levels of VH3 expression among circulating B cells (33) are thought to be a consequence of antigen selection, not family size (11), though the nature of the selecting antigen(s) or a mechanism to explain VH3 usage by antibodies to PPS has not been described. Although we did not isolate the germ line VH gene for MAb D11 from the lymphocyte donor, homology between the sequences of MAb D11 VH and the V3-15 gene (32) makes it unlikely that an unreported VH3 gene was used. V3-15 is also used by vaccine-elicited antibodies to the capsular polysaccharide of H. influenzae type b (4). Several groups have found that vaccine-elicited antibodies to PPS antigens express VH3 (3, 26, 31, 39). Our group showed previously that vaccine-elicited serum antibodies to PPS 8 express restricted VH3 determinants (3), but the specific gene segments used were not identified. Many VH3 gene segments bind SPA, including the germ line V3-15 gene segment (28), though the clinical significance of this phenomenon is uncertain. The lack of SPA binding to MAb D11 is probably due to sequence differences in regions important for SPA binding (23, 43): CDR 2 (where D11 has a 6-base deletion, though it has retained the T at position 57) and FW 3 (where D11 has four replacement changes between amino acids 82 and 87). Since MAb D11 prolonged the survival of mice infected with serotype 8 infection, molecular characteristics other than SPA binding are likely to be more important determinants of its protective efficacy.
The nucleic acid sequence of D11 revealed that it has molecular characteristics similar to those which are found among antigen-selected B cells, namely, somatic mutation, low FW R/S ratios, and CDR R/S ratios of >2 (17, 22). In comparison to its putative germ line sequence, six of seven amino acid changes in the D11 Vκ confer amino acid changes in the CDRs. Similarly, the D11 VH has a CDR R/S ratio of >2 and a truncated CDR 2 with an in-frame deletion of two codons compared to germ line V3-15 (Fig. 1A). CDR deletions and insertions have been reported to result from somatic hypermutation events (22, 53), and this feature has been shown to characterize memory B cells, including those expressing IgM (29). To our knowledge, MAb D11 is the first IgM with a known antigen specificity to manifest a CDR deletion. In addition, MAb D11 has additional molecular characteristics that indicate it may have originated from a memory cell precursor, e.g., multiple somatic mutations and a low FW R/S ratio. T-cell-independent type II antigens, such as PPS, are not thought to elicit memory B cells (34). However, IgM-expressing memory cells are found in the marginal zone of the spleen (22), which is the anatomic region where antibody responses to T-cell-independent antigens, including PPS, reportedly take place (38).
The virulence of pneumococcal serotypes 3, 4, and 8 has been proposed to be related to their ability to evade alternative complement pathway opsonization and phagocytosis (18, 25). Our data show that without MAb D11, there was little to no detectable C3 deposited on either solid-phase PPS 8 or serotype 8 organisms, whereas with MAb D11, pneumococcal opsonization was observed with both complement-deficient sera. These findings are consistent with previous studies showing that antibody is required for pneumococcal activation of the alternative pathway and opsonization by human serum via the alternative pathway (9, 18, 54, 55). Although IgM reactive with another serotype has been shown to promote pneumococcal opsonization via the alternative pathway (9), our study is the first to demonstrate that a monospecific IgM can promote opsonization of serotype 8 S. pneumoniae via the alternative pathway. Alternative pathway opsonization is mediated by the F(ab′)2 portion of the antibody molecule (8), and epitope specificity is a critical determinant of antibody opsonization of S. pneumoniae (13). Although the PPS 8 determinant that MAb D11 binds is unknown, the fact that it is protective whereas some PPS-specific human antibodies are not (2, 42) suggests that MAb D11 recognizes an antigenic determinant that elicits a protective antibody response. Determining the structure-function relationships of human antibodies to PPS that are protective is important for the identification of candidate antibodies for therapeutic use. Our studies with MAb D11 provide a useful framework which may lead to the rational design of antibody-based therapeutic reagents.
The amount of MAb D11 (1 μg) that was protective against serotype 8 infection of mice in our studies is similar to the amount of human antibody that conferred protection against other serotypes in different experimental models (35, 46). A previous study showed that an intact classical complement pathway was required for the protective efficacy of rabbit IgM against S. pneumoniae infection of C4-deficient guinea pigs (24). However, our studies show that MAb D11 was equally protective in mice which lack an intact classical pathway (C4−/−) and normal mice (F2). This discrepancy could be due to differences in antibody reagents (polyclonal serum versus MAb), serotype specificity (PPS 8 has not been studied previously), the species of the antibody used (rabbit versus human), or the animal model used (guinea pig versus mouse). Polyclonal sera may not contain sufficient levels of specific antibody (or the appropriate isotype) to mediate a biological effect (15), and antibody specificity is a critical determinant of alternative complement pathway activation (47). Our observation that the survival of S. pneumoniae-infected C3−/− mice was prolonged by treatment with MAb D11 and each complement-deficient serum shows that human and guinea pig C3 can interact with mouse complement receptors. Since our studies were performed with sera which may have different amounts of complement components and other serum factors which may contribute to alternative pathway activation (e.g., mannose binding protein[s] activating the lectin pathway [30, 49]), we cannot compare the relative importance of the classical versus the alternative pathway for MAb D11-mediated biological activity. More work is required to answer this question. Nevertheless, our data show that MAb D11 facilitated pneumococcal opsonization and mediated protection via the alternative pathway in the absence of an intact classical pathway, suggesting that this antibody reversed the serotype 8 organisms’ resistance to opsonization via the alternative pathway.
An earlier study reported that both C4−/− and C3−/− mice have enhanced susceptibility to the encapsulated pathogen group B Streptococcus (52). Similarly, our results show that these mice were at least 10 times more susceptible to pneumococcal infection with serotype 8 than mice without complement deficiency (control F2 mice). This is consistent with clinical reports of an association between C3 deficiency and pneumococcal susceptibility (5) and previous reports of the central role of complement opsonins for pneumococcal protection in experimental infection (12, 24). Our observation that MAb D11 was not protective in serotype 8-infected C3−/− mice reinforces the concept that opsonization with C3 is required for antibody (IgM)-dependent protection as well as innate resistance to infection. Since the survival of S. pneumoniae-infected C4−/− mice was decreased relative to mice with normal complement (F2 mice), an intact classical complement pathway might be important for innate resistance to pneumococcal infection. This notion is consistent with similar studies of group B Streptococcus infection in complement-deficient mice (52). Furthermore, our finding that the survival of IgM-treated mice was statistically greater than that of PBS-treated mice in the single-syringe model suggests that nonspecific IgM-mediated opsonization via the alternative pathway plays a role in innate resistance to S. pneumoniae in C4−/− mice. However, the biological significance of this relatively small difference in survival is uncertain, and we did not observe significant IgM-mediated C3 deposition in vitro. Nevertheless, another report has shown that passively administered, pooled mouse IgM protected IgM-deficient mice from sepsis, presumably by generating complement opsonins (10). Additional studies are necessary to determine if such nonspecific complement activation contributes to pneumococcal protection and define the relative importance of each complement pathway for antibody-dependent protection against S. pneumoniae.
ACKNOWLEDGMENTS
This work was supported by NIH grant AI 35370 (to L.P.), Microbial Pathogenesis Training grant AI 07576 (to T.B.), and a Howard Hughes Pilot Project Award for Medical Schools (to L.P.).
We thank Arturo Casadevall for critical review of the manuscript.
REFERENCES
- 1.Aaberge I S, Eng J, Lermark G, Lovik M. Virulence of Streptococcus pneumoniae in mice: a standardized method for preparation and frozen storage of the experimental bacterial inoculum. Microb Pathog. 1995;18:141–152. doi: 10.1016/s0882-4010(95)90125-6. [DOI] [PubMed] [Google Scholar]
- 2.Aaberge I S, Hvalbye B, Lovik M. Enhancement of Streptococcus pneumoniae serotype 6B infection in mice after passive immunization with human serum. Microb Pathog. 1996;21:125–137. doi: 10.1006/mpat.1996.0048. [DOI] [PubMed] [Google Scholar]
- 3.Abadi J, Friedman J, Jefferis R, Mageed R A, Pirofski L. Human antibodies elicited by a pneumococcal vaccine express idiotypic determinants indicative of VH3 gene segment usage. J Infect Dis. 1998;178:707–716. doi: 10.1086/515369. [DOI] [PubMed] [Google Scholar]
- 4.Adderson E E, Azmi F H, Wilson P M, Shackelford P G, Carroll W L. The human VH3b gene subfamily is highly polymorphic. J Immunol. 1993;151:800–809. [PubMed] [Google Scholar]
- 5.Alonso De Velasco E, Verheul A F, Verhoef J, Snippe H. Streptococcus pneumoniae: virulence factors, pathogenesis and vaccines. Microbiol Rev. 1995;59:591–603. doi: 10.1128/mr.59.4.591-603.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Altschul S F, Madden T L, Schaffer A A, Zhang J, Zhang Z, Miller W, Lipman D J. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bedos J, Chevret S, Chastang C, Geslin P, Regnier B French Cooperative Pneumococcus Study Group. Epidemiologic features of and risk factors for infection by Streptococcus pneumoniae strains with diminished susceptibility to penicillin: findings of a French survey. Clin Infect Dis. 1996;22:63–72. doi: 10.1093/clinids/22.1.63. [DOI] [PubMed] [Google Scholar]
- 8.Bjornson A B, Lobel J S. Lack of a requirement for the Fc region of IgG in restoring pneumococcal opsonization via the alternative complement pathway in sickle cell anemia. J Infect Dis. 1986;154:760–769. doi: 10.1093/infdis/154.5.760. [DOI] [PubMed] [Google Scholar]
- 9.Bjornson A B, Lobel J S. Direct evidence that decreased opsonization of Streptococcus pneumoniae via the alternative complement pathway in sickle cell disease is related to antibody deficiency. J Clin Investig. 1987;79:388–398. doi: 10.1172/JCI112824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boes M, Prodeus A P, Schmidt T, Carroll M C, Chen J. A critical role of natural immunoglobulin M in immediate defense against systemic bacterial infection. J Exp Med. 1999;188:2381–2386. doi: 10.1084/jem.188.12.2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brezinschek H P, Foster S J, Brezinschek R I, Dorner T, Domiati-Saad R, Lipsky P E. Analysis of the human VH gene repertoire. J Immunol. 1997;99:2488–2501. doi: 10.1172/JCI119433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brown E J, Hosea S W, Hammer C H, Burch G, Frank M M. A quantitative analysis of the interactions of antipneumococcal antibody and complement in experimental pneumococcal bacteremia. J Clin Investig. 1982;69:85–98. doi: 10.1172/JCI110444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brown E J, Joiner K A, Cole R M, Berger M. Localization of complement component 3 on Streptococcus pneumoniae: anti-capsular antibody causes complement deposition on the pneumococcal capsule. Infect Immun. 1983;39:403–409. doi: 10.1128/iai.39.1.403-409.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Butler J C, Hofmann J, Cetron M S, Elliott J A, Facklam R R, Breiman R F. The continued emergence of drug-resistant Streptococcus pneumoniae in the United States: an update from the Centers for Disease Control and Prevention’s Pneumococcal Sentinel Surveillance System. J Infect Dis. 1996;174:986–993. doi: 10.1093/infdis/174.5.986. [DOI] [PubMed] [Google Scholar]
- 15.Casadevall A, Scharff M D. Serum therapy revisited: animal models of infection and development of passive antibody therapy. Antimicrob Agents Chemother. 1994;38:1695–1702. doi: 10.1128/aac.38.8.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dochez A R. The occurrence and virulence of pneumococci in the circulating blood during lobar pneumonia and the susceptibility of pneumococcus strains to univalent antipneumococcus serum. J Exp Med. 1912;16:680–692. doi: 10.1084/jem.16.5.680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dorner T, Brezinschek H P, Brezinschek R I, Foster S J, Domiati-Saad R, Lipsky P E. Analysis of the frequency and pattern of somatic mutations within nonproductively rearranged human variable heavy chain genes. J Immunol. 1997;158:2779–2789. [PubMed] [Google Scholar]
- 18.Fine D P. Pneumococcal type-associated variability in alternate complement pathway activation. Infect Immun. 1975;12:772–778. doi: 10.1128/iai.12.4.772-778.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fisher M B, Ma M, Xhou Z X, Finco O, Han S, Kelsoe G, Howard R G, Rothstein T L, Kremmer E, Rosen F S, Carroll M C. Regulation of the B cell response to T-dependent antigens by classical pathway of complement. J Immunol. 1996;15:549–556. [PubMed] [Google Scholar]
- 20.Forsgren A, Quie P G. Opsonic activity in human serum chelated with ethylene glycoltetra-acetic acid. Immunology. 1974;26:1251–1256. [PMC free article] [PubMed] [Google Scholar]
- 21.Foster S J, Brezinschek H P, Brezinschek R I, Lipsky P E. Molecular mechanisms and selective influences that shape the kappa gene repertoire of IgM+ B cells. J Clin Investig. 1997;99:1614–1627. doi: 10.1172/JCI119324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goosens T, Klein U, Kuppers R. Frequent occurrence of deletions and duplications during somatic hypermutation: implications for oncogene translocations and heavy chain disease. Proc Natl Acad Sci USA. 1998;95:2463–2468. doi: 10.1073/pnas.95.5.2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hakoda M, Hayashimoto S, Yamanaka H, Terai C, Kamatani N, Kashiwazaki S. Molecular basis for the interaction between human IgM and staphylococcal protein A. Clin Immunol Immunopathol. 1994;72:394–401. doi: 10.1006/clin.1994.1158. [DOI] [PubMed] [Google Scholar]
- 24.Hosea S W, Brown E J, Frank M M. The critical role of complement in experimental pneumococcal sepsis. J Infect Dis. 1980;142:903–909. doi: 10.1093/infdis/142.6.903. [DOI] [PubMed] [Google Scholar]
- 25.Hostetter M K. Serotypic variations among virulent pneumococci in deposition and degradation of covalently bound C3b: implications for phagocytosis and antibody production. J Infect Dis. 1986;153:682–693. doi: 10.1093/infdis/153.4.682. [DOI] [PubMed] [Google Scholar]
- 26.Ibrahim S, Seppala I J, Sarvas H, Makela O. Proportion of protein A bindable molecules in human IgM and IgA antibodies to seven antigens. Microb Pathog. 1993;15:159–168. doi: 10.1006/mpat.1993.1066. [DOI] [PubMed] [Google Scholar]
- 27.Kang A, Burton D R, Lerner R A. Combinatorial immunoglobulin libraries in phage lambda. Methods Enzymol. 1991;2:111–118. [Google Scholar]
- 28.Karray S, Juompan L, Maroun R C, Isenberg G, Silverman G J, Zouali M. Structural basis of the gp 120 superantigen-binding site on human immunoglobulin. J Immunol. 1998;161:6681–6688. [PubMed] [Google Scholar]
- 29.Klein U, Rajewsky K, Kuppers R. Human immunoglobulin (Ig) M+D+ peripheral blood B cells expressing the CD27 cell surface antigen carry somatically mutated variable region genes: CD27 as a general marker for somatically mutated (memory) B cells. J Exp Med. 1998;188:1679–1689. doi: 10.1084/jem.188.9.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kuhlman M, Joiner K, Ezekowitz A B. The human mannose-binding protein functions as an opsonin. J Exp Med. 1989;169:1733–1745. doi: 10.1084/jem.169.5.1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lucas A H, Granoff D M, Mandrell R E, Connolly C C, Shah A S, Powers D C. Oligoclonality of serum immunoglobulin G antibody responses to Streptococcus pneumoniae capsular polysaccharide serotypes 6B, 14, and 23F. Infect Immun. 1997;65:5103–5109. doi: 10.1128/iai.65.12.5103-5109.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.MacLeod C M, Hodges R G, Heidelberger M, Bernhard W G. Prevention of pneumococcal pneumonia by immunization with specific capsular polysaccharides. J Exp Med. 1946;45:445–465. [PMC free article] [PubMed] [Google Scholar]
- 33.Matsuda F, Ishii K, Bourvagnet P, Kuma K-I, Hayashida H, Miyata T, Honjo T. The complete nucleotide sequence of the human immunoglobulin heavy chain variable region locus. J Exp Med. 1998;188:2151–2162. doi: 10.1084/jem.188.11.2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mond J J, Lees A, Snapper C M. T cell-independent antigens type 2. Annu Rev Immunol. 1995;13:655–692. doi: 10.1146/annurev.iy.13.040195.003255. [DOI] [PubMed] [Google Scholar]
- 35.Musher D M, Johnson B, Jr, Watson D A. Quantitative relationship between anticapsular antibody measured by enzyme-linked immunosorbent assay or radioimmunoassay and protection of mice against challenge with Streptococcus pneumoniae serotype 4. Infect Immun. 1990;58:3871–3876. doi: 10.1128/iai.58.12.3871-3876.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Musher D M, Luchi M J, Watson D A, Hamilton R, Baughn R E. Pneumococcal polysaccharide vaccine in young adults and older bronchitics: determination of IgG responses by ELISA and the effect of absorption of the serum with non-type-specific cell wall polysaccharide. J Infect Dis. 1990;161:728–735. doi: 10.1093/infdis/161.4.728. [DOI] [PubMed] [Google Scholar]
- 37.Payne C M. Electron microscopy in the diagnosis of infectious diseases. In: Connor D H, Chandler F W, Manz H J, Schwartz D A, Lack E E, editors. Pathology of infectious diseases. Stamford, Conn: Appleton and Lange; 1997. pp. 9–34. [Google Scholar]
- 38.Peset Llopis M J, Harms G, Hardonk M J, Timens W. Human immune response to pneumococcal polysaccharides: complement-mediated localization preferentially on CD21-positive splenic marginal zone B cells and follicular dendritic cells. J Allergy Clin Immunol. 1996;97:1015–1024. doi: 10.1016/s0091-6749(96)80078-9. [DOI] [PubMed] [Google Scholar]
- 39.Pirofski L, Casadevall A. Cryptococcus neoformans: paradigm for the role of antibody in immunity. Zentbl Bakteriol. 1996;284:475–495. doi: 10.1016/s0934-8840(96)80001-6. [DOI] [PubMed] [Google Scholar]
- 40.Pirofski L, Casadevall A. The use of licensed vaccines for active immunization of the immunocompromised host. Clin Microbiol Rev. 1998;11:1–26. doi: 10.1128/cmr.11.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pirofski L, Lui R, DeShaw M, Kressel A B, Zhong Z. Analysis of human monoclonal antibodies elicited by vaccination with a Cryptococcus neoformans glucuronoxylomannan capsular polysaccharide vaccine. Infect Immun. 1995;63:3005–3014. doi: 10.1128/iai.63.8.3005-3014.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ramisse F, Binder P, Szatanik M, Alonso J-M. Passive and active immunotherapy for experimental pneumococcal pneumonia by polyvalent human immunoglobulin or F(ab′)2 fragments administered intranasally. J Infect Dis. 1996;173:1123–1128. doi: 10.1093/infdis/173.5.1123. [DOI] [PubMed] [Google Scholar]
- 43.Randen I, Potter K N, Li Y, Thompson K M, Pascual V, Forre O, Natvig J B, Capra J D. Complementarity-determining region 2 is implicated in the binding of staphylococcal protein A to human immunoglobulin VHIII variable regions. Eur J Immunol. 1993;23:2682–2686. doi: 10.1002/eji.1830231044. [DOI] [PubMed] [Google Scholar]
- 44.Santosham M, Reid G R, Almeido-Hill J, Thompson C, Wolff M C, Siber G R. Efficacy of bacterial polysaccharide immune globulin (BPIG) for prevention of bacteremic pneumococcal infections in Apache children. Pediatr Res. 1992;31:178A. doi: 10.1093/infdis/165-supplement_1-s129. [DOI] [PubMed] [Google Scholar]
- 45.Scott J A G, Hall A J, Dagan R, Dixon J M S, Eykyn S J, Fenoll A, Hortal M, Jette L P, Jorgensen J H, Lamothe F, Latorre C, Macfarlane J T, Shlaes D M, Smart L E, Taunay A. Serogroup-specific epidemiology of Streptococcus pneumoniae: associations with age, sex, and geography in 7,000 episodes of invasive disease. Clin Infect Dis. 1996;22:973–981. doi: 10.1093/clinids/22.6.973. [DOI] [PubMed] [Google Scholar]
- 46.Stack A M, Malley R, Thompson C M, Kobzik L, Siber G R, Saladino R A. Minimum protective serum concentrations of pneumococcal anti-capsular antibodies in infant rats. J Infect Dis. 1998;177:986–990. doi: 10.1086/515259. [DOI] [PubMed] [Google Scholar]
- 47.Steele N P, Munson R S, Granoff D M, Cummins J E, Levine P R. Antibody-dependent alternative pathway killing of Haemophilus influenzae type b. Infect Immun. 1984;44:452–458. doi: 10.1128/iai.44.2.452-458.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Steinitz M, Tamir S, Ferne M, Goldfarb A. A protective human monoclonal IgA antibody produced in vitro: anti-pneumococcal antibody engendered by Epstein-Barr virus-immortalized cell line. Eur J Immunol. 1986;16:187–193. doi: 10.1002/eji.1830160214. [DOI] [PubMed] [Google Scholar]
- 49.Suankratay C, Zhang X-H, Zhang Y, Lint T F, Gewurz H. Requirement for the alternative pathway as well as C4 and C2 in complement-dependent hemolysis via the lectin pathway. J Immunol. 1998;160:3006–3013. [PubMed] [Google Scholar]
- 50.Tomasz A. Antibiotic resistance in Streptococcus pneumoniae. Clin Infect Dis. 1997;24(Suppl. 1):S85–S88. doi: 10.1093/clinids/24.supplement_1.s85. [DOI] [PubMed] [Google Scholar]
- 51.Watson D A, Musher D M, Jacobson J W, Verhoef J. A brief history of the pneumococcus in biomedical research: a panoply of scientific discovery. Clin Infect Dis. 1993;17:913–924. doi: 10.1093/clinids/17.5.913. [DOI] [PubMed] [Google Scholar]
- 52.Wessels M R, Butko R, Ma N, Warren H B, Lage A L, Carroll M C. Studies of group B streptococcal infection in mice deficient in complement component C3 or C4 demonstrate an essential role for complement in both innate and acquired immunity. Proc Natl Acad Sci USA. 1995;92:11490–11494. doi: 10.1073/pnas.92.25.11490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wilson P M, Bouteiller O, Liu Y J, Potter K, Banchereau J, Capra J D, Pascual V. Somatic hypermutation introduces insertions and deletions into immunoglobulin V genes. J Exp Med. 1998;187:59–70. doi: 10.1084/jem.187.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Winkelstein J A. Complement and the host’s defense against the pneumococcus. Crit Rev Microbiol. 1982;11:187–208. doi: 10.3109/10408418409105903. [DOI] [PubMed] [Google Scholar]
- 55.Winkelstein J A, Shin H S. The role of immunoglobulin in the interaction of pneumococci and the properdin pathway: evidence for its specificity and lack of requirement for the Fc portion of the molecule. J Immunol. 1974;112:1635–1642. [PubMed] [Google Scholar]
- 56.Zhong Z, Pirofski L. Antifungal activity of a human antiglucuronoxylomannan antibody. Clin Lab Diagn Immunol. 1998;5:58–64. doi: 10.1128/cdli.5.1.58-64.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhong Z, Burns T, Pirofski L. Abstracts of the 99th General Meeting of the American Society for Microbiology. Washington, D.C: American Society for Microbiology; 1999. A human IgM monoclonal antibody prolongs survival of mice with lethal pneumococcal infection, abstr. 617. [Google Scholar]