Abstract
Background
Reducing the transmission of SARS-CoV-2 from asymptomatic and pre-symptomatic patients is critical in controlling the circulation of the virus.
Methods
This study evaluated the prevalence of Reverse transcription polymerase chain reaction (RT-PCR) positivity in serial tests in 429 asymptomatic health care workers (HCW) and its impact on absenteeism. HCW from a COVID-19 reference hospital were tested, screened, and placed on leave. A time-series segmented regression of weekly absenteeism rates was used, and cases of infection among hospitalized patients were analyzed. Viral gene sequencing and phylogenetic analysis were performed on samples from HCW who had a positive result.
Results
A significant decrease in absenteeism was detected 3–4 weeks after the intervention at a time of increased transmission within the city. The prevalence of RT-PCR positivity among asymptomatic professionals was 17.3%. Phylogenetic analyses (59 samples) detected nine clusters, two of them strongly suggestive of intrahospital transmission with strains (75% B.1.1.28) circulating in the region during this period.
Conclusions
Testing and placing asymptomatic professionals on leave contributed to control strategy for COVID-19 transmission in the hospital environment, and in reducing positivity and absenteeism, which directly influences the quality of care and exposes professionals to an extra load of stress.
Key Words: SARS-CoV-2 asymptomatic infection, SARS-CoV-2 surveillance, Infection control
Health care workers (HCW) comprise a group that is particularly affected by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and exposed to high viral loads, higher than the general population.1, 2, 3 Transmissibility between asymptomatic or presymptomatic individuals is a challenge for the control of the pandemic, especially among HCW.4
Absenteeism among HCWs is a major challenge for hospital management because it burdens the health services and promotes a decrease in the quality of care, exposing professionals to the extra load of stress, often associated with mental suffering.
A review identified different percentages (17%-54%) and an estimated average of 40% of asymptomatic individuals with positive molecular tests for SARS-CoV-2 in different population groups, with evidence of silent viral circulation.5 , 6
Universal testing of asymptomatic patients has been adopted in several centers as a strategy to control intrahospital infection of patients and HCWs with molecular tests.7 , 8
The objective of this study was to evaluate the impact of a extensive RT-PCR testing strategy for asymptomatic HCWs in a university hospital to reduce the circulation of SARS-CoV-2 among health care workers, absenteeism, and consequently, nosocomial transmission between professionals and patients.
Methods
This is a study of the prevalence of SARS-CoV-2 positivity and a time series of weekly rates of absenteeism due to COVID-19 among HCWs in a hospital during the first months of the epidemic, March- August 2020. The impact of implementing an extensive serial RT-PCR testing program for asymptomatic HCW, tracking of contacts, and absenteeism were evaluated. Through the phylogenetic reconstruction of some of the positive samples, potential transmission clusters were investigated and correlated with information on function and place of work.
This study was approved by the Medical Ethics Committee under CAE protocol no. 31042120.4.0000.5481.
Study location and period
The hospital has 325 beds, of which 196 are dedicated to public health, placing it as a regional clinical reference center (3 million inhabitants), and in the city of Campinas (1.2 million inhabitants), 100 km from the capital São Paulo. The hospital sectors enrolled in the research were the intensive care units (ICUs), COVID-19 wards, clinical and surgical wards, the adult emergency room, administrative, and cleaning and support sectors, with a total of 170 beds and 473 professionals.
The protocols for the management and prevention of intra-hospital Covid-19 transmission were first implemented in February 2020 and focused especially on measures related to patients, such as: reinforcement of hand hygiene and environment cleaning, contact and droplet isolation for suspected and confirmed cases, aerosol precaution in invasive procedures (mechanical ventilation, swab collection, noninvasive ventilation, among others) and cohort unites for confirmed cases. Universal mask use was recommended only in the second half of March 2020, following state and city guidelines.
Protocols for testing/tracking/absence from work on leave for symptomatic patients and personal protective equipment (PPE) were already implemented in the hospital according to the recommendations of the National Health Surveillance Agency (ANVISA)9 and Centers for Disease Control and Prevention (CDC).10
The hospital and the COVID-19 pandemic
The first imported case in Campinas was registered on March 12, 2020; the first patient with COVID-19 admitted to the PUC-Campinas Hospital was on March 15, and the first positive case in an HCW was on March 18. The increase in the incidence curves for COVID-19 in the city followed the transmission growth noted within the state of São Paulo, with a peak in epidemiological weeks 23-27 (June 2020), with a total of 8,523 cases accumulated up to week 27.11
The intervention: Extensive testing and placing on leave of asymptomatic HCW
On May 11 (epidemiological week 20), the intervention began by testing all asymptomatic health professionals who agreed to participate in the research, from all the hospital sectors enrolled in the study. The staff was personally invited to participate, when the first series of testing was carried out in their work unit. This invitation happened moments before or after the work shift. Only 2 invited HCWs did not agree to participate.
After signing the informed consent form, professionals were invited to answer a questionnaire and then a biological sample was collected using a nasopharynx swab to perform the RT-PCR Fleury test (Charité12 and CDC10 protocols) and/or Gene-X-pert (Cepheid-USA) for faster results. If positive, HCW were place on leave. Three serial collections were performed on each professional who had been negative in previous tests, with an interval of 20 days between them. Our testing team was scheduled to continuously perform tests 3-4 times a week, each day at a different unit. Symptomatic workers were not included in the study; that is, those who presented at least one of the following symptoms: headache, fever, cough, sore throat, anosmia, ageusia, myalgia, chills, cough, or runny nose.
Study variables and data source
Using a structured questionnaire, data on demographics (gender and age), workplace, and role performed in the hospital, as well as symptoms (in the last 14 days) were obtained.
RT-PCR test positivity rates were calculated according to the variables of interest (gender, age, professional category, work place in the hospital). The number of daily leaves and the total days of leave for any reason and for COVID-19 were obtained from the Department of Human Resources and Occupational Medicine of the hospital from January 1 2019, to September 12, 2019, and 2020 (epidemiological weeks 1-37).
The impact of the testing intervention in asymptomatic individuals was evaluated based on the temporal trend of weekly leave rates by COVID-19 (number of leaves per week/total number of employees scheduled for the week) from March 8, 2020, to September 12, 2020 (epidemiological weeks 11-37).
Data on the COVID-19 epidemic (confirmed cases of COVDI-19 severe acute respiratory syndrome) of the population of Campinas and HCW were obtained from publications of the Health Surveillance Department of the Municipal Health Department of Campinas, allowing for comparison of the incidence curves in the study institution and in the community (https://covid-19.campinas.sp.gov.br/).
Health care-associated COVID-19 was considered when the onset of symptoms occurred at least 7 days after admission.13
Viral genetic sequencing and phylogenetic analysis
Biological samples from professionals with positive RT-PCR results collected in different sectors of the hospital were sequenced to assess clusters and the lineage of SARS-CoV-2. Contact tracing was not performed in this sample and RT-PCR for patients was not available for the research.
After extraction of viral RNA, cDNA synthesis was performed using the Protoscript II First Strand transcription kit (New England Biolabs) and random hexamers (Thermo Fisher Scientific). Amplification of the total genome was carried o\ut using the multiplex PCR reaction with the primers designed for the amplification of the complete genome of SARS-CoV-2 (https://artic.network/ncov-2019), together with the Q5 High-Fidelity DNA Polymerase Kit (New England Biolabs). The PCR conditions have been described previously (https://artic.network/ncov-2019). Amplicons formed after the PCR reaction were purified using magnetic beads (1 × AMPure XP, Beckman Coulter). After purification, the product was quantified using fluorometry techniques with Qubit dsDNA high sensitivity reagents, and reading was performed using the Qubit 3.0 instrument (Life Technologies).
Complete genome sequencing of SARS-CoV-2
To achieve good coverage of the genome, only samples with more than 4 ng/µL of DNA were used to prepare the library for sequencing. The amplicons of each sample were normalized so that there was an equimolar amount of each of the samples in the reaction. After this process, the normalized amplicons were processed to continue preparing the library for sequencing according to the previously published protocol14 using the EXP-NBD104 (1-12) and EXPNBD114 (13-24) kits (Oxford Nanopore Technologies). When this process was completed, the libraries were loaded into a flow cell and sequenced by MinION for 8-24 hours using the SQK-LSK109 kit (Oxford Nanopore Technologies). To monitor the sequencing in real time and estimate the depth of coverage (200 times target), ARTIC Network RAMPART software (https://artic.network/ncov-2019) was used. Minimap2 v2.28.0 software was used to obtain the consensus sequence and structure the fast 5 files against the reference genome of the SARS-CoV-2 Wuhan-Hu 1 1 isolate (GenBank accession number MN908947).
Phylogenetic analysis
Consensus sequences were initially submitted to a quality control check using Nextclade. To retain the maximum amount of information possible, sequences with coverage above 80% were used for further analysis. Our final dataset consisted of 49 original SARS-CoV-2 complete genomes and nine sequences collected in Campinas available from GISAID as of July 31, 2020.
Fast multiple sequence alignment was applied in our dataset with MAFFT v. 7.450. A maximum likelihood tree was reconstructed using IQ-TREE version 1.6.1215 under the TIM+F+I nucleotide substitution model chosen as the best fitting model according to the Bayesian Information Criteria (BIC) through ModelFinder implementation. Statistical support was evaluated using 1,000 bootstrap replicates. Internal nodes with >90% statistical support were assigned on the tree. To infer the existence of potential transmission clusters, we used Phydelity v. 2.1, which determines pairwise patristic distance distribution of closely related tips in the phylogenetic tree. Nonsynonymous mutations were inferred through CovSurver implementation on GISAID (I210del, A520S, P561H, D614G, V615A, G946V, V1176F(C-term)).
SARS-CoV-2 lineages were identified using the Phylogenetic Assignment of Named Global Outbreak Lineages tool (https://github.com/cov-lineages/pangolin).
Statistical analysis
The prevalence of positivity and the respective 95% confidence intervals were calculated by the ratio of positive tests to the population tested at the 3 testing moments, according to the variables of interest. Proportions were compared using the χ² test with Yates’ correction, with a significance level of 5% (P < .05).
After verifying the assumption of homoscedasticity, absence of autocorrelation, and normality of residuals, a segmented regression of weekly absenteeism rates was adjusted. The percentage and mean variations in the rates and their statistical significance were obtained using the Joint Point Regression Program version 4.5.0.1.
The median and quartiles (first and third) of daily leaves for all causes of health care workers from January 1, 2019, to September 12, 2019, and 2020 were also evaluated. The daily averages of HCW sick leave from 2019 and 2020 were compared using analysis of variance analysis (ANOVA) and the F test, considering a significance level of 5% (P < .05).
Results
From May 2020 to August 2020, 429 asymptomatic HCWs were tested with RT-PCR 3 times at a mean interval of 20 days. The total prevalence of positive RT-PCR tests was 74 of 429 (17.3%; 95% CI, 13.7-20.3), with 11.9% (95% CI, 8.8-14.9) in the first collection, 6.9% (95% CI, 3.8-9.9) in the second, and 2.9% (95% CI, 0.4-5.4) in the third. The highest positivity rate occurred in the youngest HCW (P < .05). Physiotherapists, cleaning professionals (hygiene) and nursing technicians were the most prevalent categories and those who work in administrative sectors were the least affected, as expected (P < .05) (data on Supplementary Table S1)
Professionals from non-Covid-19 units (clinical and surgery wards, coronary care unit) were as affected as the Covid-19 ICU in the period.
Fifty-two RT-PCR positive nasopharyngeal secretion samples were sequenced. The analysis of the strains revealed that approximately 75% of the sequences belonged to the B.1.1.28 strain, 21.15% to the B.1.1.143 strain, and 3.84% to the B.1 strain. The most frequent amino acid substitutions were L3930F from the ORF 1ab region (open reading frame), D614G and V1176F from the Spike protein region, and R203K and G204R from the Nucleocapsid protein. According to PROVEAN analysis, all these substitutions can be classified as neutral.
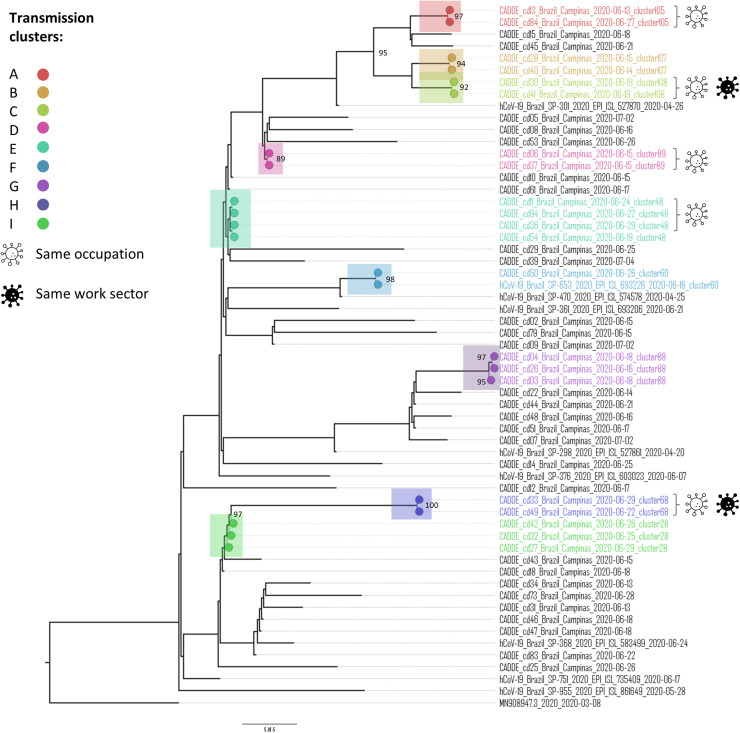
The phylogenetic tree shows potential putative transmission clusters among the health professionals (Fig 1 ). A total of 9 clusters were inferred through the patristic distance distribution on the tips of the tree. Among them, 5 clusters shared samples belonging to professionals who worked in the same occupation. Two clusters also shared the same workplace inside the hospital, strongly suggesting intrahospital transmission; cluster C was found in 2 nursing technicians in the COVID-19 ward and collected on the same day (June 19, 2020) and cluster H collected from 2 cleaning staff of the same unit, on different dates (June 22, 2020, and 29, 2020). The spatial distribution of HCW's address in Campinas (presented at supplemental material S1), shows that the vast majority of them are randomly distributed in the city. It is possible that HCW doesn't infect each other at the time of assistance, but during coffee and meal breaks, for example, where distancing and measures are not always respected.
Fig 1.
Phylogenetic tree of SARS-CoV-2 detected in samples of nasopharyngeal secretion (n = 59) from health care professionals at the PUC-Campinas hospital from May 2020, to September 2020. Cluster C, both nursing technicians in the COVID ward collected on the same day (June 19); cluster H, cleaning staff in the COVID ward collected on June 22 and 29.
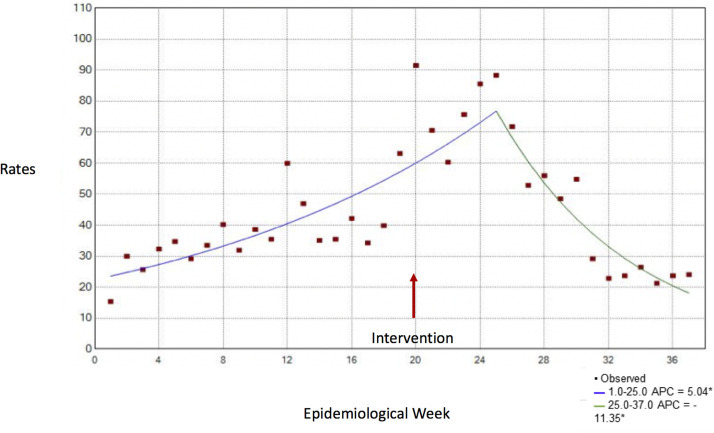
Figure 2 shows the temporal trend of weekly rates of work leave due to COVID-19 and their respective variations by time segment. From the intervention (week 20), at first there was an increase in HCW leave, still in segment 1; and after 3-4 weeks, there was a significant change in the trend and a decrease in absenteeism from the epidemiological week onward. (Weekly Percent Variation: P < .05)
Fig 2.
Temporal trend of weekly rates of absence from work by COVID-19. In PUC-Campinas Hospital, epidemiological weeks 1-37, 2020. *APC refers to weekly percent variation of rates, which are significantly different from zero at alpha = 0.05.
The epidemic curve of COVID-19 in Campinas (in the general population and among health professionals) does not present a decrease concomitant with the reduction of absenteeism in the hospital; on the contrary, this reduction occurs in the weeks of peak incidence of infection in the city (Supplementary Figure S1).
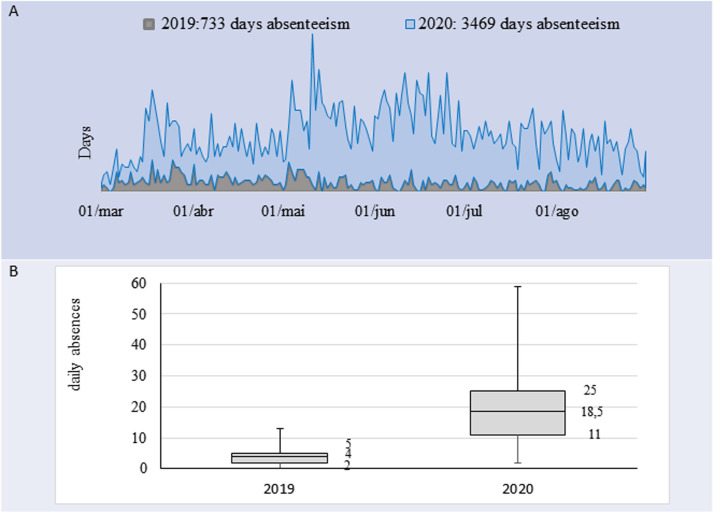
The total number of days of leave for all causes from January to August 2019 and 2020 were 733 days (daily average, 4; SD, 2.6) and 3,469 days (daily average, 18.9; SD, 9.7) (P < .001), respectively, which represented an increase of 473%. An excess of leave for all causes was observed when comparing the 2019 and 2020 (Fig 3 ).
Fig 3.
Absence of health professionals for all causes at Hospital PUCC, Campinas. (A) Temporal trend of absence from work from March 2019 to August 2019 and 2020. (B) Daily absenteeism (median and first and third quartiles) for all causes from January 1, 2019, to August 30, 2019, and 2020.
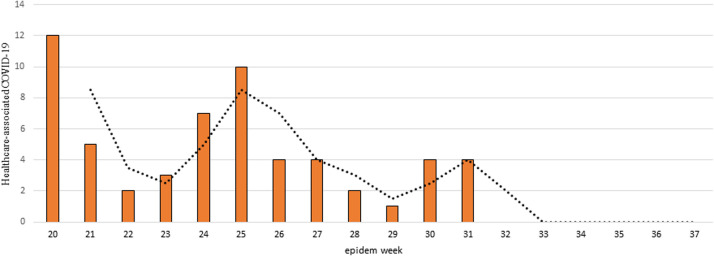
At the fourth week of the intervention (weeks 24-25), there was a decrease in the number of health care-associated patients with COVID-19 (Fig 4 ).
Fig 4.
Health care-related COVID-19 by epidemiological weeks from the intervention (week 20). At hospital PUC-Campinas, 2020. *Dotted line, moving average, interval 2 (weeks).
Discussion
Several studies report wide circulation of SARS-CoV-2 among health workers, identified as one of the most affected segments in the COVID-19 epidemic, both in developed countries and in poorer regions.2 , 3 , 16 We found a high prevalence of positive RT-PCR tests of 17.3% (95% CI, 13.7-20.3), decreasing in the successive tests of asymptomatic professionals. Surprisingly, the high prevalence in the non-COVID-19 clinical/surgical and ICU cardiology sectors of 20.3% (95% CI, 10.8-29.8) and 28.0% (95% CI, 17.8-38.2), respectively, highlight the importance of universal testing of professionals, regardless of their locations with supposedly less exposure to the virus. The phylogenetic identification of hospital clusters of cases with the same occupation and in the same work environment requires specific prevention and training measures, both of which occurred in COVID-19 units. The impact on reducing the risk of infection of hospitalized patients from transmission by pre- or asymptomatic health professionals is also expected.
The B.1.1.28 strain identified in the hospital, which came from Europe (clade 1) in the first months of the pandemic,16 was the most predominant strain in the state of São Paulo. In April 2020, SARS-CoV-2 with the D614G mutation in the Spike protein became dominant in the pandemic in Brazil and in various parts of the world, associated with higher transmissibility and a higher viral load, however, without a change in the pathogenicity.17, 18
Some reports of tracking symptomatic19 , 20 and asymptomatic HCW21 have identified areas of risk for infection in specific hospital care sectors for COVID-19 and in others. Particularly at the beginning of the epidemic, with less access to protective equipment and testing resources and with no available vaccines, HCW were especially exposed by SARS-CoV-2.1 Outbreaks, both among patients and among HCW, puts individuals with comorbidities who are hospitalized for other causes at risk and depletes the work teams in outpatient and inpatient services.22 In addition to investigating the molecular test positivity, genomic surveillance could qualify the epidemiological investigation allowing the identification of clusters, transmission sites, and timely actions for the control and prevention of hospital infection.23 In the UK, a study carried out on the general population,25 showed a variation in percentages of asymptomatic individuals of 15%-66% (on average, one-third of those detected by RT-PCR) depending on the timing in the pandemic.
Up to the start of the screening of asymptomatic HCW (week 20), the increase in leaves due to COVID-19 followed the growth of COVID-19 diagnoses among professionals and the general population in the municipality (outpatient clinics and admissions for severe acute respiratory syndrome). However, the temporal distribution of HCW leaves due to COVID-19 showed a significant reversal of the trend 3-4 weeks after the beginning of the testing of asymptomatic professionals, suggesting a reduction in hospital exposure after the intervention.
Absenteeism due to COVID-19, in addition to posing a risk to HCWs, worsens inpatient care, exposes the rest of the team to an exhaustive and, consequently, less safe workday.8 , 25, 26 Work overload in small teams causes insecurity and stress in health professionals and is related to a wide spectrum of mental health problems.24
Among the limitations of this study, the short period of postintervention analysis makes it difficult to obtain more robust estimates of the impact, although there is already a significant reduction in intrahospital transmission between professionals and patients. We used different databases to compare the temporal evolution of indicators in the city and in the hospital, however these were used as proxies for the local epidemiological situation in order to contextualize the downward trend in absenteeism in the institution. Besides, external influences due to community-related intervention measures, such as social isolation, may have interfered with viral circulation, reducing the community risk of transmission. However, the study was carried out in the weeks with the highest transmission peak during the city's first wave of the epidemic and when hospital occupancy rates were more than 80%.11
Conclusion
Screening and isolation of asymptomatic health care workers using RT-PCR possibly had an impact on intrahospital viral circulation, significantly reducing tests positivity and leave among HCWs due to COVID-19 at a time of increased community transmission in the city and in hospitalized patients. The reduction in absenteeism indirectly reflects a decrease in the circulation of SARS-CoV-2 in the hospital environment, detected in nine clusters within the institution. There was also a trend toward a reduction in the transmission of the infection to hospitalized patients.
Therefore, this is an important strategy for the prevention and control of outbreaks of COVID-19 in the hospital environment, given the insufficiency of screening based on symptoms. Antigen tests could also be used for this purpose, with lower cost and faster results, although with less sensitivity.
Acknowledgments
The authors would like to thank the PUC-Campinas Hospital's Board of Directors, as well as the Clinical Analysis Laboratory departments, the São Lucas Clinical Research Center (PUC-Campinas), and the Human Resources Department for all their help and support. The authors are also grateful to the health professionals of PUC-Campinas Hospital for agreeing to participate in this study.
Footnotes
Funding/support: The research received funding from National Council for Scientific and Technological Development – CNPq (401130/2020-7).
Conflict of interest: None to report.
Author contributions: Each author has substantially contributed to the study conducting the underlying research and drafting this manuscript. Authors have no conflict of interest, financial, or otherwise.
Availability of data and material: All the data analyzed in this study will be made available upon reasonable request to the corresponding author.
Supplementary material associated with this article can be found in the online version at https://doi.org/10.1016/j.ajic.2022.10.014.
Appendix. SUPPLEMENTARY MATERIALS
References
- 1.Boccia S, Ricciardi W, Ioannidis JP. What other countries can learn from Italy during the COVID-19 pandemic. JAMA Internal Med. 2020;180:927–928. doi: 10.1001/jamainternmed.2020.1447. [DOI] [PubMed] [Google Scholar]
- 2.Mutambudzi M, Niedwiedz C, Macdonald EB, et al. Occupation and risk of severe COVID-19: prospective cohort study of 120 075 UK Biobank participants. Occup Environ Med. 2020;78:307–314. doi: 10.1136/oemed-2020-106731. oemed-2020-106731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Houlihan CF, Vora N, Byrne T. Pandemic peak SARS-CoV-2 infection and seroconversion rates in London frontline health-care workers. Lancet. 2020;396:e6–e7. doi: 10.1016/S0140-6736(20)31484-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gandhi M, Yokoe DS, Havlir DV. Asymptomatic transmission, the Achilles’ heel of current strategies to control Covid-19. N Engl J Med. 2020;382:2158–2160. doi: 10.1056/NEJMe2009758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oran DP, Topol EJ. Prevalence of asymptomatic SARS-CoV-2 infection: a narrative review. Ann Intern Med. 2020;173:362–367. doi: 10.7326/M20-3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cevik M, Tate M, Lloyd O, et al. SARS-CoV-2, SARS-CoV, and MERS-CoV viral load dynamics, duration of viral shedding, and infectiousness: a systematic review and meta-analysis. Lancet Microbe. 2020;2:e13–e22. doi: 10.1016/S2666-5247(20)30172-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shield A, Faustini SE, Perez-Toledo M, et al. SARS-CoV-2 seroprevalence and asymptomatic viral carriage in healthcare workers: a cross-sectional study. Thorax. 2020;75:1089–1094. doi: 10.1136/thoraxjnl-2020-215414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Groenewold MR, Burrer SL, Ahmed F, et al. Increases in health-related workplace absenteeism among workers in essential critical infrastructure occupations during the COVID-19 pandemic — United States, March–April 2020. MMWR Morb Mortal Wkly Rep. 2020;69:853–858. doi: 10.15585/mmwr.mm6927a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Agência Nacional de Vigilância Sanitária (ANVISA). Nota Técnica GVIMS/GGTES/ANVISA no. 04/2020. Orientações para serviços de saúde: medidas de prevenção e controle que devem ser adotadas durante a assistência aos casos suspeitos ou confirmados de infecção pelo novo coronavírus (SARS-CoV-2) [Guidelines for health services: prevention and control measures that must be adopted during the care of suspected or confirmed cases of infection by the new coronavirus (SARS-CoV-2)]. Accessed February 25, 2021.https://www.gov.br/anvisa/pt-br/centraisdeconteudo/publicacoes/servicosdesaude/notas-tecnicas/nota-tecnica-gvims_ggtes_anvisa-04_2020-25-02-para-o-site.pdf
- 10.Centers for Disease Control and Prevention. Interim infection prevention and control recommendations for healthcare personnel during the coronavirus disease 2019 (COVID-19) pandemic. 2020. Accessed December 4, 2022. https://www.cdc.gov/coronavirus/2019-ncov/hcp/infection-control-recommendations.html.
- 11.Campinas, Secretaria Municipal de Saúde, Departamento de Vigilância em Saúde. Campinas city data, 2020.
- 12.Corman VM, Landt O, Kaiser M, et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020;25 doi: 10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.National Health Service (England). Healthcare associated COVID-19 infections – further action:https://www.england.nhs.uk/coronavirus/wp-content/uploads/sites/52/2020/06/Healthcare-associated-COVID-19-infections–further-action-24-June-2020.pdf. Accessed June 30, 2020.
- 14.Quick J, Grubaugh ND, Pullan ST, et al. Multiplex PCR method for MinION and Illumina sequencing of Zika and other virus genomes directly from clinical samples. Nat Protoc. 2017;12:1261–1276. doi: 10.1038/nprot.2017.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nguyen LT, Schmidt HA, von Haeseler A, et al. IQ-TREE: a fast and effective stochastic algorithm for estimating maximum likelihood phylogenies. Mol Biol Evol. 2015;32:268–274. doi: 10.1093/molbev/msu300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Costantino C, Cannizzaro E, Verso MG, et al. SARS-CoV-2 infection in healthcare professionals and general population during “first wave” of COVID-19 pandemic: a cross-sectional study conducted in Sicily, Italy. Front Public Health. 2021;13 doi: 10.3389/fpubh.2021.644008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Candido DS, Claro IM, De Jesus JG, et al. Evolution and epidemic spread of SARS-CoV-2 in Brazil. Science. 2020;369:1255–1260. doi: 10.1126/science.abd2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Korber B, Fischer WM, Gnanakaran S, et al. Tracking changes in SARS-CoV-2 spike: evidence that D614G increases infectivity of the COVID-19 virus. Cell. 2020;182:812–827. doi: 10.1016/j.cell.2020.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Van Loon N, Verbrugghe M, Cartuyvels R, et al. Diagnosis of COVID-19 based on symptomatic analysis of hospital healthcare workers in Belgium: observational study in a large Belgian tertiary care center during early COVID-19 outbreak. J Occup Environ Med. 2021;63:27–31. doi: 10.1097/JOM.0000000000002015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mani NS, Budak JZ, Lan KF, et al. Prevalence of coronavirus disease 2019 infection and outcomes among symptomatic healthcare workers in Seattle, Washington. Clin Infect Dis. 2020;71:2702–2707. doi: 10.1093/cid/ciaa761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rivas VF, Schwarz SN, Carreño JV, et al. Serological study of healthcare workers in four different hospitals in Madrid (Spain) with no previous history of COVID-19. Occup Environ Med. 2021;78:600–603. doi: 10.1136/oemed-2020-107001. [DOI] [PubMed] [Google Scholar]
- 22.Meredith LW, Hamilton WL, Warne B, et al. Rapid implementation of SARS-CoV-2 sequencing to investigate cases of health-care associated COVID-19: a prospective genomic surveillance study. Lancet Infect Dis. 2020;20:1263–1272. doi: 10.1016/S1473-3099(20)30562-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Long QX, Tang XJ, Shi QL, et al. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat Med. 2020;26:1200–1204. doi: 10.1038/s41591-020-0965-6. [DOI] [PubMed] [Google Scholar]
- 24.Wells PM, Doores KJ, Couvreur S, et al. Estimates of the rate of infection and asymptomatic COVID-19 disease in a population sample from SE England. J Infect. 2020;81:931–936. doi: 10.1016/j.jinf.2020.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Felice C, Di Tanna GL, Zanus G, et al. Impact of COVID-19 outbreak on healthcare workers in Italy: results from a national E-survey. J Community Health. 2020;45:675–683. doi: 10.1007/s10900-020-00845-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abbas M, Robalo Nunes T, Martischang R, et al. Nosocomial transmission and outbreaks of coronavirus disease 2019: the need to protect both patients and healthcare workers. Antimicrob Resist Infect Control. 2021;10:1–13. doi: 10.1186/s13756-020-00875-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.