Abstract
Objective:
To study the association of Aspirin and other nonsteroidal anti-inflammatory drugs (NSAIDs) with bone mineral density (BMD).
Methods:
Spine BMD was evaluated in NSAIDs users (including aspirin), in a subset of 2028 participants from the Multi-Ethnic Study of Atherosclerosis (MESA) cohort who underwent both lumbar and thoracic imaging. MESA is a prospective cohort study that includes four ethnic groups (European descent, Asian, African-American, and Hispanic). Trabecular BMD was evaluated by quantitative computed tomography (QCT) based on cardiac CT images, which were obtained during coronary calcium scans.
Results:
After adjustment for potential confounders including age, sex, race, and traditional cardiovascular (CVD) risk factors, a small association between trabecular BMD and baseline use of COX-2 selective NSAID was observed. Cox-2 selective NSAID use was associated with 7.4 mg/cm3 (95% CI 1.6, 13.3, P=0. 013) higher trabecular BMD in thoracic spine and 10.6 mg/cm3 higher in lumbar spine (95% CI 5.1, 16.1, P<0.001). Among regular aspirin users, associations between medication use and trabecular BMD showed evidence of lower BMD. Considering all spine fractures together, the prevalence ratio (PR) of fractures among aspirin users was 1.0 (95%CI: 0.6. 1.6), and 1.1 (95%CI; 0.5, 2.3) among COX-2 selective NSAID users.
Conclusion:
Regular use of aspirin has no significant association with trabecular BMD in either the thoracic or lumbar spine, and no association with fracture prevalence. COX-2 selective NSAIDs may have modest positive association with BMD, but the mechanism is uncertain and the small effect size makes residual confounding a possible alternate explanation. Potential pathological mechanisms warrant further longitudinal exploration.
INTRODUCTION
Since the 1970s, aspirin has been extensively used to prevent heart attack, stroke, and other manifestations of CVD. In the United States alone, more than 36% adults reported regularly taking aspirin for primary CVD prevention (1, 2). Data revealed that aspirin with or without COX-2 selective NSAIDs may affect BMD and fracture risk by inhibiting the action of cyclooxygenase isozymes (both COX-1 and COX-2), but different studies have yielded divergent results (3-5).
Our aim was to investigate the association between vertebral BMD, which was evaluated through non-contrast cardiac computed tomography (CT) and NSAIDs use including aspirin. Given that BMD was the primary outcome of interest, an accurate and reliable BMD assessment was a key goal of our study. Several groups, including our own, have demonstrated that non-contrast cardiac CT can garner reliable information related to vertebral BMD (6, 7), compared to Dual energy X-ray absorptiometry (DXA), which is still the most common method to assess bone mineral density in lower back spine, hip or forearm. The latest generation of multidetector computed tomography (MDCT) has at least four unique advantages for BMD evaluation: 1) clear separation of the metabolically-active bone tissue (trabecular) in the spine for analysis, whereas other BMD exams combine both cortical and trabecular bone; 2) true volumetric density (in units of mg/cm³ ) contrary to the area density (in units of mg/cm2); 3) high-resolution three-dimensional (3D) digital images of bone morphometry; 4) providing an opportunity to evaluate bone health while assessing atherosclerotic risk with no extra cost or radiation. Notably, the data from clinical trials as well as other prospective studies suggest that trabecular bone mineral loss occurs first, but quickly responds to medical therapies, making it uniquely possible to reflect bone quality as well as density when compared to cortical bone(8-10). Based on the above information, we evaluated trabecular BMD using quantitative non-contrast CT images from MESA cohort
METHODS
Study Population
All images were taken from MESA, a prospective cohort study with the goal of identifying risk factors for subclinical atherosclerosis. MESA participants were enrolled from six clinical sites across the United States in between July 2000 and September 2002. The detailed study design has been reported previously (11).All MESA participants were free of clinical CVD at baseline, and the characteristics of participants including age, gender, race/ethnicity, comorbidities including diabetes, hypertension, hyperlipidemia, family history of CVD, smoking, drinking and socioeconomic factors including income and education were collected and analyzed resulting in a well-characterized cohort. In addition, medication inventories (12)were used to assess any use of bisphosphonate medications (Actonel, Alendronate and Fosamax) or hormones which were considered confounding variables, in review of their possible impact on BMD. CT scans were performed at Columbia University, Johns Hopkins University, Northwestern University; University of California Los Angeles, University of Minnesota, and Wake Forest University. All images were sent to LA BioMed CT Reading Center for evaluation.
The CT scan procedure and assessment of BMD
Images were examined on a PACS workstation. We adopted use of electron-beam computed tomography scanner with 130 kVp and 630 mA current (Chicago, Los Angeles and New York field imaging centers) or MDCT with 120–140 kVp, 200 to 600 mA current (Baltimore, Forsyth County, and St. Paul field image centers)to assess trabecular BMD on all chest and abdomen CT scans. Trabecular BMD was measured with the standard elliptical region of interest (ROI) positioned within the trabecular region utilizing Q5000 and NVivo workstation (QCT, Columbia, Kentucky). The thickness of the (ROI) was 10mm and 6mm in the center if the ROI was located in the center of the vertebrae, at least 2mm away from the cortical spinal edge. Major anterior portion of the vertebral body was segmented by the software, and all the bones densities were measured as a composite density of both cortical and trabecular bone components.
Assessment of vertebral fractures
Vertebral fracture status was assessed based on the Spinal Fracture Index (SFI), which was described by Genant (13) and Lenchik (14, 15). The degree of vertebral deformity was derived by comparing the ratio of heights with the adjacent upper and lower vertebral body when seen on visual inspection. Fractures were classified as follows: Grade I: Up to, 25% height reduction, Grade II 25%−40% height reduction, and Grade III more than 40% height reduction.
Assessment of data on aspirin and other NSAID use
A detailed medication inventory was performed for each participant using all medications that were either prescribed or taken over the counter. History of aspirin use and frequency was obtained via means of short questionnaire during patient visit which included the following questions:
Ever used regularly (Yes, No, Unknown)?
Currently using regularly (Yes/No); Age started regular use;
Days per week;
Age stopped regular use
For the purpose of our study, regular aspirin use was defined as taking aspirin at least three days per week. Since we used medication inventories, aspirin exposure assessment included both prescriptions for CVD prevention and over-the-counter (OTC) use. This study did not include the combination of non-steroidal anti-inflammatory agents with aspirin, and more detailed information (beyond the inventory) was not available for non-aspirin NSAIDs. Since all NSAIDs were available using medication inventory, we compared medication inventory estimates to survey estimates for aspirin, and found them quite similar.
Statistical Methods
Multivariable linear regression analysis was used to estimate the independent association of aspirin with vertebral trabecular BMD. Multiple logistic regression model was used to estimate odds ratios [OR] (as an estimate of the prevalence ratio) for the association between aspirin use and prevalent vertebral fractures. The covariates including age, gender, race/ethnicity, BMI (body mass index), hypertension (elevated blood pressure >=130/80), hyperlipidemia (elevated lipid (fat) levels in the blood), diabetes (abnormal metabolism of carbohydrates and elevated levels of glucose in the blood), smoking, drinking and socioeconomic factors (income and educational status). Model diagnostics were conducted via formal hypothesis testing as well as graphical methods to assess outliers. During analysis, an unadjusted model was adapted, followed by an age, gender and race adjusted model. A third model was adopted for the full list of covariates listed above, seen as the likely confounders.
Finally, sensitivity analysis was performed for age adjustment and unavailable BMD data, due to age effects and prevalent fractures. We tested polynomial transforms of age and looked at 40 imputations of spine segments that contained a fracture or were cut off in the CT scan (which could only occur in the lowest region of the spine). As an additional check, we did a frequency of use and dose analysis (≤ 100mg versus > 100mg pill use) for aspirin and a comparison with other medications in the NSAID class. All statistical analyses were performed using SAS 9.3 (SAS Institute, Cary, NC)
RESULTS
The characteristics of the participants
As described in figure 1, the final analysis data set included 2028 participants (49.9% women, mean age 62±10 years) with BMD scans including 654 (32.2%) participants reported regular aspirin use and 138 (6.8%) participants reported using COX-2 selective NSAIDs. For regular aspirin users, 75.1%were reported to be taking low dose aspirin daily, presumably for primary CVD prevention. Compared with women, men had a higher prevalence of aspirin use (35.3% versus 29.2%, p<0.003). However, women had an approximately 9.2% COX-2 selective NSAID use, which was more than twice as high as men (4. 4%).The median self-reported age of starting regular aspirin use was 58 years (range 58±13 years), and the median self-reported duration of aspirin use was 3.5 years (range 2.5–5.1 years). Our analysis also indicated that prescription was a major source of aspirin compared with nonprescription (OTC) Aspirin, (88.4% vs. 11.6%). In this cohort, a total of 138 (6.8%) participants used COX-2 selective NSAIDs. Additionally, the association of demographic factors and medication use (aspirin and COX-2 selective NSAIDs) with trabecular spine BMD (mg/cm) is shown in Table 1.
Figure 1.
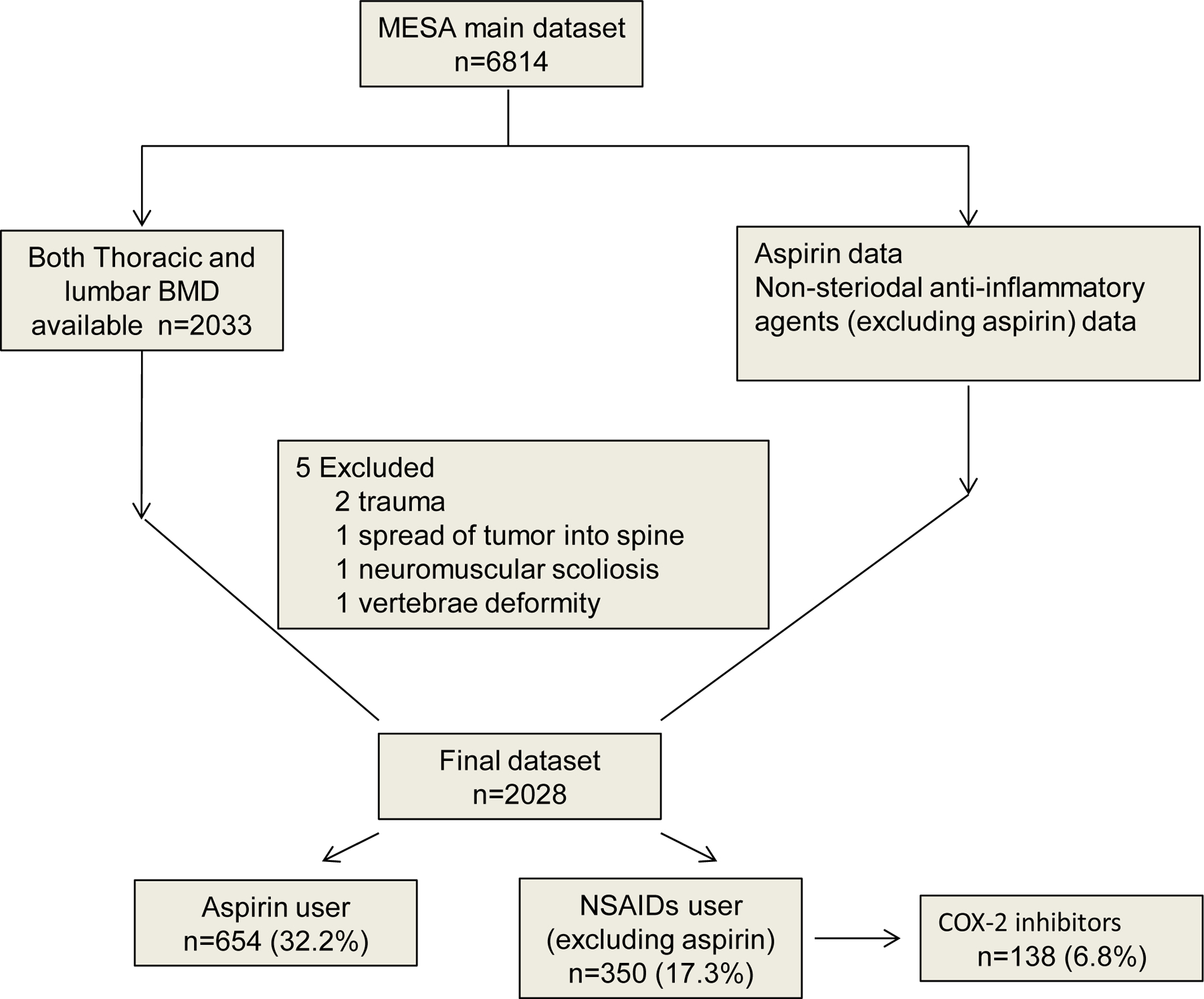
Inclusion/Exclusion In This Study
Table 1.
Selected Characteristics Of Study Population
| Aspirin |
Non-aspirin Non-selective NSAID |
COX-2 selective NSAID |
||||
|---|---|---|---|---|---|---|
| User (n=654) |
Nonuser (n=1374) |
User (n=350) |
Nonuser (n=1678) |
User (n=138) |
Nonuser (n=1888) |
|
|
| ||||||
| Mean age (SD), y | 64.6±9.3 | 60.6±9.7 | 60.6±9.1 | 62.2±9.8 | 66.5+9.1 | 66.5+9.1 |
| 45<age≤55 | 50.8±2.7 | 50.2±2.9 | 50.6+2.4 | 50.3+3.0 | 51.4+3.0 | 50.3+2.9 |
| 55<age≤65 | 59.7±2.8 | 59.2±2.8 | 59.6+2.9 | 59.3+2.8 | 59.3+2.8 | 60.2+2.8 |
| 65<age≤75 | 69.0±2.8 | 68.8±2.7 | 68.9+2.7 | 68.9+2.7 | 59.3+2.8 | 68.9+2.7 |
| 75<age≤85 | 77.9±2.5 | 77.9±2.4 | 77.8+2.4 | 78.0+2.6 | 78.0+2.1 | 77.9+2.6 |
| Female, % | 295(45.1) | 716(52.1) | 215(61.4) | 794(47.4) | 93(67.4) | 916(48.5) |
| Race/ethnicity, % | ||||||
| White | 349(53.4) | 488(35.5) | 196(56.0) | 640(38.2) | 72(52.2) | 764(40.5) |
| Asian | 46(7.0) | 212(15.4) | 7(2.0) | 251(15.0) | 13(9.4) | 245(13.0) |
| Black | 118(18.0) | 305(22.2) | 68(19.4) | 354(21.1) | 25(18.1) | 397(21.0) |
| Hispanic | 141(21.6) | 369(26.9) | 79(22.6) | 431(25.7) | 28(20.3) | 482(25.5) |
| BMI, % | ||||||
| Normal † | 147(22.5) | 428(31.1) | 78(22.3) | 496(29.6) | 23(16.7) | 551(29.2) |
| Overweight | 285(43.6) | 544(39.6) | 126(36.0) | 703(41.9) | 48(34.8) | 781(41.4) |
| Obesity | 222(33.9) | 402(29.3) | 146(41.7) | 477(28.5) | 67(48.6) | 556(29.4) |
| Diabetes mellitus | ||||||
| Normal fasting glucose | 461(70.5) | 1055(77.0) | 278(79.7) | 1237(73.9) | 94(68.1) | 1421(75.4) |
| Impaired fasting glucose | 107(16.4) | 177(12.9) | 41(11.7) | 243(14.5) | 27(19.6) | 257(13.6) |
| Untreated diabetes | 12(1.8) | 40(2.9) | 10(2.9) | 41(2.5) | 0(0.0) | 51(2.7) |
| Treated diabetes | 74(11.3) | 98(7.2) | 20(5.7) | 152(9.1) | 17(12.3) | 155(8.2) |
| Hypertension | 366(56.0) | 557(40.5) | 162(46.3) | 760(45.3) | 85(61.6) | 837(44.3) |
| Lipid-lowering medication | 145(22.2) | 177(12.9) | 53(15.1) | 269(16.1) | 37(26.8) | 285(15.1) |
| Coronary calcification | 384(58.7) | 624(45.4) | 160(45.7) | 847(50.5) | 84(60.9) | 923(48.9) |
Data are presented as mean±SD or number (%).
Normal weight: 18.5 kg/m2≤BMI<24.9 kg/m2, Overweight: 25.0 kg/m2≤BMI<29.9 kg/m2, Obesity: 30.0 kg/m2≤BMI
Coronary calcification: CAC score>0
BMD distribution among NSAID users
The association between drug use and trabecular BMD at the spine is reported in Table 2. There was no association of aspirin use with trabecular BMD of thoracic (β=1.2 mg/cm3, 95%CI −2.1,4.4, P=0. 48) or lumbar spine (β=1. 5 mg/cm3, 95%CI −1.6,4.5 mg/cm3, P=0.34) observed after adjustment of potential confounders including age, gender, BMI, exercise level, diabetes mellitus, hyperlipidemia, hypertension and coronary artery calcification status. However, COX-2 selective NSAID use was associated with higher trabecular spine BMD. After adjustment for the above covariantes, the mean thoracic vertebral BMD for COX-2 selective NSAID users was 7.4 mg/cm³ higher than non-users (95% CI 1.5, 13.3. P=0. 013). A similar result was observed in the lumbar spine for COX-2 selective NSAID users (10.6 mg/cm³ higher, 95%CI 5.1,16.1 mg/cm3 P<0.001). Non-aspirin non-selective NSAIDs, including such drugs as ibuprofen, had associations that were quite similar to aspirin as opposed to Cox-2 selective NSAIDs (Table 2).
Table 2.
Adjusted association between drug use and trabacular BMD at spine (mg/cm3)
| Aspirin | Non-aspirin non-selective NSAID | COX-2 selective NSAID | |||||
|---|---|---|---|---|---|---|---|
| β (SE) | 95% CI | β (SE) | 95% CI | 95% CI | 95% CI | ||
| Thoracic | Total levels | 1.2(1.7) | −2.1,4.4 | −0.9 (2.2) | −4.4,4.3 | 7.4(3.0) | 1.5,13.3 |
| Left main level thoracic | 1.1 (1.8) | −2.3,4.6 | −0.1(2.3) | −4.7,4.5 | 7.2(3.2) | 1.1,13.4 | |
| Upper thoracic spine | 1.4(1.7) | −2.3,4.6 | 0.3(2.3) | −4.2,4.8 | 7.5(3.1) | 1.5,13.6 | |
| Middle thoracic spine | 1.8(1.7) | −1.5,5.2 | −0.7(2.3) | −5.1,3.8 | 8.5(3.1) | 2.4,14.5 | |
| Lower thoracic spine | 0.1(2.4) | −4.7,4.9 | 2.4 (3.1) | −3.7, 8.6 | 9.8(4.3) | 1.3,18.2 | |
|
| |||||||
| Lumbar | Total levels | 1.5(1.6) | −1.6,4.5 | 1.8 (2.1) | −2.3, 5.8 | 10.6(2.8) | 5.1,16.1 |
| Upper lumbar spine | 1.7(1.7) | −1.6,4.9 | 1.2 (2.2) | −3.0, 5.5 | 10.1(2.9) | 4.4,15.9 | |
| Middle lumbar spine | 1.2(1.7) | −2.1,4.4 | 2.0 (2.2) | −2.1, 6.3 | 12.8(3.0) | 7.0,18.7 | |
| Lower lumbar spine | 2.1(1.6) | −1.1,5.3 | 2.1 (2.2) | −2.2, 6.3 | 10.9(3.0) | 5.1,16.7 | |
Abbreviations: BMD, bone mineral density; CI, confidence interval;
Adjusted for age category, gender and traditional CVD risk factors, thyroid agents, oral steroids, anti-osteoporosis drug, vitamin D
β stands for the absolute value change of thoracic BMD, SE stands for standard error of a beta.
When stratified by spinal level (or spinal segment), lower thoracic spinal level relative to the upper one was associated with higher trabecular BMD in COX selective NSAID users, in other words, a protective association between BMD and Cox-2 specific NSAIDs. For lumbar, COX selectivity seemed to be more strongly associated with lumbar spinal nerve 2 (L2) to L3 level (BMD difference12.8 mg/cm3, 95% CI 7.0 to 18.7 mg/cm3) versus L1 (β 10.1 mg/cm3, 95% CI 4.4 to 15.9 mg/cm3). The similar protective association was observed in all lumbar vertebrae beginning at the level of L1 to L4. Although the association between COX-2 selectivity and BMD was attenuated in the upper or lower lumbar spine, they still suggested the possibility of higher BMD. To our knowledge, it is the first time that the association of COX-2 selectivity with BMD has been reported across individual spinal vertebral levels, although previous reports have suggested that this association may exist3.
The association between NSAID use and fracture prevalence
Given that NSAIDS may be associated differentially with fracture prevalence based on Cox-2 selectivity, we performed further analysis to evaluate the association of these drugs with the prevalence of vertebral fracture. As shown in Table 3, compared to non-users, regular use of aspirin or selective COX-2 NSAID showed no statistically significant association with higher frequency of spinal fractures in this study population. Although a higher prevalence of lumbar fractures was observed among aspirin users (4.0% versus 2.5%), this was not statistically significant (P=0. 062). Analogous results were also observed among Cox-2 users. Compared with non-medicine users, Aspirin users (OR 1.1; 9%CI 0.6–1.7) and Cox-2 selective NSAID users (OR 1.1; 9%CI 0.5–2.2) did not show a significantly higher or lower prevalence of vertebral fractures.
Table 3.
Adjusted Odds Ratio (OR) between vertebral fractures comparing aspirin and cox-2 selective NSAIDs
| Aspirin |
COX-2 selective NSAID |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| No | Yes | Adjusted OR | 95% CI | No | Yes | Adjusted OR | 95% CI | ||
|
| |||||||||
| Thoracic fractures | No | 1351(98.3%) | 646(98.8%) | 1.0 (Ref) | 1861(98.6%) | 134(97.1%) | 1.0 (Ref) | ||
| Yes | 23(1.7%) | 8(1.2%) | 0.7 | 0.3,1.6 | 27(1.4%) | 4(2.9%) | 1.3 | 0.4,4.1 | |
| Lumbar fracture | No | 1340(97.5%) | 628(96.0%) | 1.0 (Ref) | 1834(97.1%) | 132(95.7%) | 1.0 (Ref) | ||
| Yes | 34(2.5%) | 26(4.0%) | 1.2 | 0.7,2.2 | 54(2.9%) | 6(3.4%) | 0.7 | 0.3,1.9 | |
| Total spine fracture | No | 1321(96.1%) | 621(95.0%) | 1.0 (Ref) | 1812(96.0%) | 128(92.8%) | 1.0 (Ref) | ||
| Yes | 53(3.9%) | 33(5.0%) | 1.1 | 0.6,1.7 | 76(4.0%) | 10(7.2%) | 1.1 | 0.5,2.2 | |
Adjusted for age category, gender and traditional CVD risk factors, thyroid agents, oral steroids, anti-osteoporosis drug, vitamin D.
Sensitivity analysis
As a final step, we evaluated aspirin use using a continuous age adjustment model and used multiple imputations to account for missing data due to fracture and/or age effects on the scans. In this sensitivity analysis aspirin use was not associated with decreased BMD, showing a non-significant increase of 0.59 mg/cm3 (95% CI −2.72, 3.89, P= 0.72), although there was a trend towards decreased BMD in occasional users (Table 4). Interestingly, baseline Cox 2 selective non-steroidal anti-inflammatory drug users showed an increased in BMD of 6.59 mg/cm3 (95% CI 0.83, 12.33, P= 0.0249), being almost entirely for participants taking Celecoxib and Rofecoxib.
Table 4.
Sensitivity analysis for Estimating the association between medication use and vertebra BMD (mg/cm3)
| β (Se) † | 95% CI | P-value | |
|---|---|---|---|
|
| |||
| No NSAID or ASA use | Reference | ||
| ASA User (all) | 0.59(1.69) | −2.72, 3.89 | 0.72 |
| Regular use, low dose ASA | 2.54(2.62) | −2.61, 7.68 | 0.33 |
| Occasional use ASA | −2.41(3.50) | −9.28,4.47 | 0.49 |
| Regular use, high dose ASA | 1.03(2.01) | −2.92, 4.97 | 0.61 |
| Non-selective NSAIDs use | −0.57(1.93) | −4.35, 3.21 | 0.77 |
| Cox 2 selective NSAID use | 6.58(2.93) | 0.83, 12.33 | 0.02 |
Sensitivity analysis using stronger age adjustments, multiple imputation for missing values in BMD reads, and looking at other medications in the non-steroidal anti-inflammatory drug (NSAID) class.
Abbreviations: BMD, bone mineral density; CI, confidence interval; NSAID, non-steroidal anti-inflammatory drug; ASA, aspirin; Std, Standard
β stands for the absolute value change of thoracic BMD
DISCUSSION
We found that the regular use of aspirin has no significant association with trabecular BMD in both thoracic and lumbar spines, while COX-2 selective NSAIDs may have modest positive correlation with BMD. Osteoporosis and CVD are distinct but highly interactive growing public health problems. Each year, there are approximately two million osteoporosis-related fractures, with a net cost to the US Medicare system of $19 billion (16, 17). Recent observations have raised concerns regarding the high prevalence of osteoporosis among CVD patients,(18) although the mechanism for any causal link remains unclear. Therefore, it is important to rule out unexpected adverse medication side-effects as a possible source of this association between CVD and osteoporosis. Aspirin, a commonly used therapeutic and preventive medicine for CVD, may have a modest effect on bone density and fracture risk due to the relation between fractures and inflammation (19). Given the pharmacodynamics of aspirin, inflammatory processes may be toxic to osteoblastic cells suggesting a mechanism for a possible protective effect. However, NSAIDs have also been implicated with slow healing of fractures(19) suggesting that there may be a competing mechanism that might attribute to an adverse effect. This clinical equipoise as to the risk of aspirin use makes understanding the population of aspirin users especially important.
As a nonsteroidal anti-inflammatory drug and a salicylate, aspirin is one of the most widely used medications. Today, in the United States alone, more than 36% of American adults take aspirin regularly for the prevention of CVD. Though some initial studies have found that aspirin or NSAIDs may have an effect on bone health and fracture healing (20, 21), the credibility of these results is compromised due to the following limitations: 1) none of these studies were performed in large multi-center, multi-ethnic population; 2) none of these studies used BMD evaluation based on trabecular bone. Again, it is important to recognize that trabecular bone can more accurately reflect the state of bone density. Our study attempts to overcome these limitations by evaluating trabecular bone in a large, multi-center population. Cardiac CT, especially the latest generation of Multi-Detector CT, makes it possible to evaluate trabecular BMD by providing high-resolution 3D imaging while providing additional CVD risk factors information (e.g. Coronary artery calcification) as well. MESA is an ideal epidemiological cohort for the following reasons; 1) MESA is a large-scale multi-ethnic longitudinal cohort study that includes high quality, uniform measurements of imaging and potential confounding factors, including diet, BMI, exercise, medications, and CVD risk factors; 2) All MESA participants underwent a CT scan, which included a calcium calibration phantom under each participant for each scan; 3) MESA collects participants’ CVD information and data regarding the use of various drugs.
In our study, unlike the lumbar spine, the thoracic spine did not demonstrate a significantly higher prevalence of fracture with aspirin use. This may be due to the relatively small number (n=7) of thoracic fractures among aspirin users, less pain (reducing the use for fractures), or different participant characteristics among this group (as, with only seven exposed cases, we could not properly adjust the estimates). However, our data did show that the prevalence of fracture is highest in the upper lumbar spine, and our study reports that the lumbar spine vertebrae had two times the prevalence of fracture compared to the thoracic spine (3.0% vs. 1.5%, respectively).
In prior studies, aspirin was shown to cause a delay in the healing of a variety of fractures for up to two weeks in the animal and human models (22, 23), but these differences may not influence bone remodeling in healthy bone tissue. It remains unclear if the healing delay has any clinical implications or if these associations are dictated by the same underlying cause of both disease states. However, bone mineral density testing may be prudent for older patients starting aspirin, as the association between cardiovascular disease and osteoporosis may suggest screening for the second disease given the first (24). This provides a second advantage of coronary calcium testing, as one can assess atherosclerosis risk and measure bone density with one scan (25-26).
Previous Studies
There are two previous prospective cohort studies that have considered this association, the Health ABC cohort (3)and Danish Osteoporosis Prevention Study (DOPS)(27). DOPS authors found no association between aspirin and BMD, despite the fact that that study was small and restricted to women. The results of the Health ABC study are also from a smaller and less diverse population (the Health ABC included only European- and African-American participants). They found the same association with increased BMD that we established in MESA, but only among aspirin users. MESA is better powered to detect an association of aspirin with BMD (28-30). Statistical adjustment in these studies were most similar to the sensitivity analysis, which might be the best comparator and is compatible with these associations.
Limitations
Some possible limitations of this study could be the following: First, the cross-sectional nature of our study does not allow to determine a cause-effect relationship. There is a serious concern that participants with prevalent fracture might use these medications for pain control – this could lead to a reverse causality issue with fracture assessment. Second, there were certain missing data in the participant’s osteoporosis medication record and a few segments of the spine (due to fractures or scan cuts) which we accounted for using multiple imputation. Third, the small effect size makes it possible that residual confounding and missing data might be enough to account for the statistically significant but slight association between Cox-2 selective NSAIDs and BMD. The divergence between these approaches and the lack of overwhelming effect sizes highlight the need for longitudinal (ideally randomized) data to better estimate these associations. Fourth, the small number of prevalent fractures made it difficult to fully adjust the statistical analysis and the possibility of confounding by traditional CVD risk factors cannot be dismissed. Finally, MESA included both OTC and prescribed NSAIDS. Most of the aspirin is low-dose (31) which may limit comparisons to the higher doses seen for other classes of NSAIDs. Finally, almost all of the non-aspirin non-selective NSAID use was ibuprofen and naproxen (31) limiting generalizability to other drugs in this class.
CONCLUSIONS
Regular use of aspirin has no significant association with trabecular BMD at either the thoracic or lumbar spine. COX-2 selective NSAID have a modest positive correlation with BMD. This study has provided key clinical epidemiology data, including the prevalence of aspirin use in MESA population, the trabecular vertebral BMD reference distribution, and the prevalence of fracture for investigating the clinical epidemiological characteristics of vertebral BMD.
Figure 2.
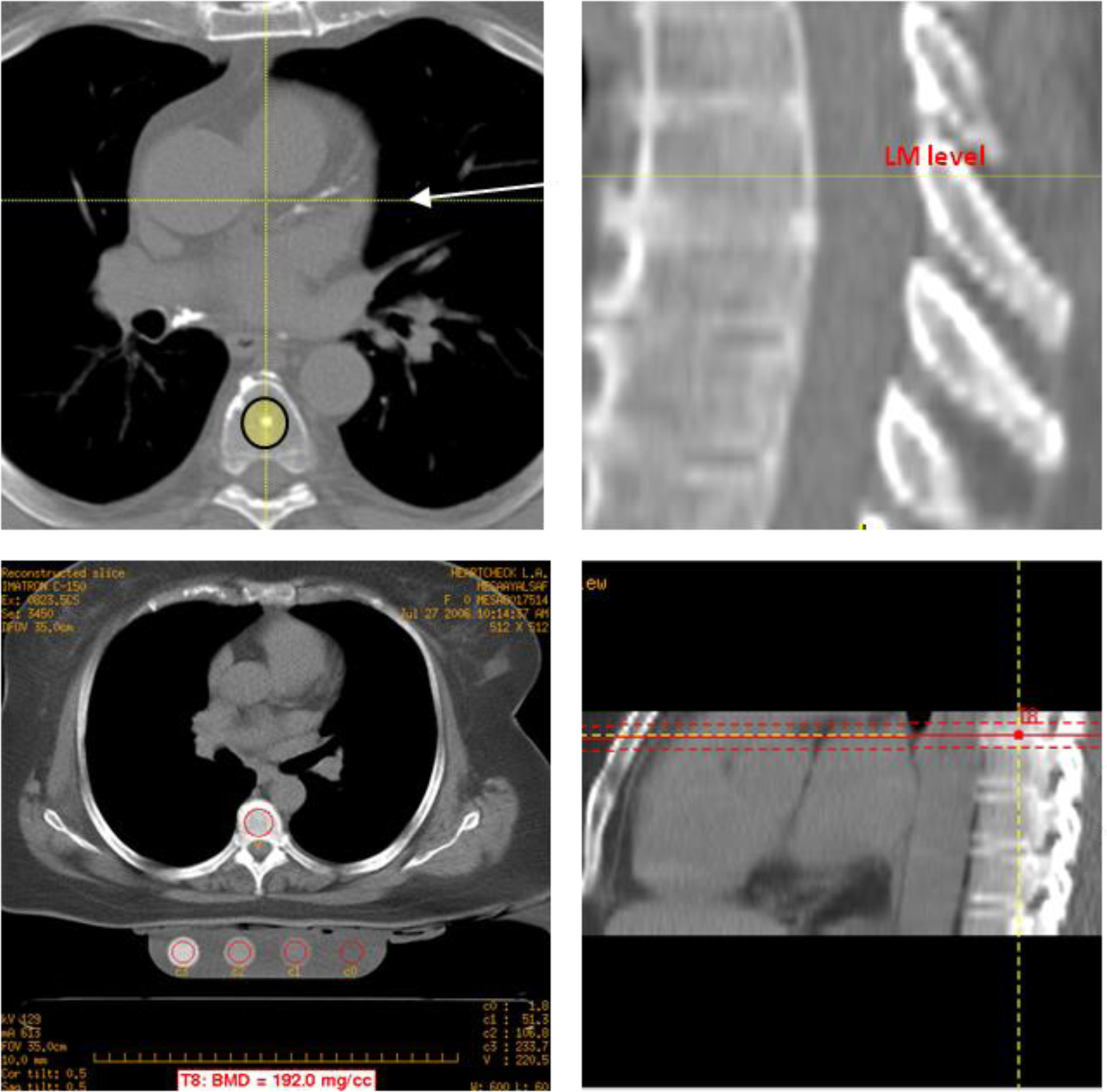
Trabecular BMD measurement using MDCT
Acknowledgments
This research was supported by contracts HHSN268201500003I, N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168 and N01-HC-95169 from the National Heart, Lung, and Blood Institute and by grants UL1-TR-000040 and UL1-RR-025005 from NCRR. The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org
References
- 1.Ajani UA, Ford ES, Greenland KJ, Giles WH, Mokdad AH. Aspirin use among U.S. adults: Behavioral Risk Factor Surveillance System. Am J Prev Med 2006;30(1):74–7. [DOI] [PubMed] [Google Scholar]
- 2.Campbell CL, Smyth S, Montalescot G, Steinhubl SR. Aspirin dose for the prevention of cardiovascular disease: a systematic review. JAMA 2007;297(18):2018–24. [DOI] [PubMed] [Google Scholar]
- 3.Carbone LD, Tylavsky FA, Cauley JA, Harris TB, Lang TF, Bauer DC, et al.Association between bone mineral density and the use of nonsteroidal anti-inflammatory drugs and aspirin: impact of cyclooxygenase selectivity. J Bone Miner Res 2003;18(10):1795–802. [DOI] [PubMed] [Google Scholar]
- 4.Bauer DC, Orwoll ES, Fox KM, Vogt TM, Lane NE, Hochberg MC, et al.Aspirin and NSAID use in older women: effect on bone mineral density and fracture risk. Study of Osteoporotic Fractures Research Group. J Bone Miner Res 1996;11(1):29–35. [DOI] [PubMed] [Google Scholar]
- 5.Lack WD, Fredericks D, Petersen E, Donovan M, George M, Nepola J, et al.Effect of aspirin on bone healing in a rabbit ulnar osteotomy model. J Bone Joint Surg Am 2013;95(6):488–96. [DOI] [PubMed] [Google Scholar]
- 6.Budoff MJ, Hamirani YS, Gao YL, Ismaeel H, Flores FR, Child J, et al.Measurement of thoracic bone mineral density with quantitative CT. Radiology 2010;257(2):434–40. [DOI] [PubMed] [Google Scholar]
- 7.Budoff MJ, Khairallah W, Li D, Gao YL, Ismaeel H, Flores F, et al.Trabecular bone mineral density measurement using thoracic and lumbar quantitative computed tomography. Acad Radiol 2012;19(2):179–83. [DOI] [PubMed] [Google Scholar]
- 8.Heaney RP. Skeletal remodeling physiology and its relation to metabolic bone disease. N Y State J Med 1975;75(10):1656–61. [PubMed] [Google Scholar]
- 9.Ito M [CT evaluation of trabecular and cortical bone mineral density of the lumbar spine in patients on hemodialysis]. Nihon Igaku Hoshasen Gakkai Zasshi 1989;49(11):1382–9. [PubMed] [Google Scholar]
- 10.Wasnich RD, Ross PD, Davis JW. Osteoporosis Current practice and future perspectives. Trends Endocrinol Metab 1991;2(2):59–62. [DOI] [PubMed] [Google Scholar]
- 11.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, et al.Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol 2002;156(9):871–81. [DOI] [PubMed] [Google Scholar]
- 12.Psaty BM, Lee M, Savage PJ, Rutan GH, German PS, Lyles M. Assessing the use of medications in the elderly: methods and initial experience in the Cardiovascular Health Study. The Cardiovascular Health Study Collaborative Research Group. J Clin Epidemiol 1992;45(6):683–92. [DOI] [PubMed] [Google Scholar]
- 13.Genant HK, Wu CY, van Kuijk C, Nevitt MC. Vertebral fracture assessment using a semiquantitative technique. J Bone Miner Res 1993;8(9):1137–48. [DOI] [PubMed] [Google Scholar]
- 14.Lenchik L, Rogers LF, Delmas PD, Genant HK. Diagnosis of osteoporotic vertebral fractures: importance of recognition and description by radiologists. AJR Am J Roentgenol 2004;183(4):949–58. [DOI] [PubMed] [Google Scholar]
- 15.Kotzki PO, Buyck D, Leroux JL, Thomas E, Rossi M, Blotman F. Measurement of the bone mineral density of the os calcis as an indication of vertebral fracture in women with lumbar osteoarthritis. Br J Radiol 1993;66(781):55–60. [DOI] [PubMed] [Google Scholar]
- 16.Lewiecki EM. Managing osteoporosis: challenges and strategies. Cleve Clin J Med 2009;76(8):457–66. [DOI] [PubMed] [Google Scholar]
- 17.Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025. J Bone Miner Res 2007;22(3):465–75. [DOI] [PubMed] [Google Scholar]
- 18.Piscitelli P, Iolascon G, Gimigliano F, Gimigliano A, Marinelli A, Di Nuzzo R, et al.Osteoporosis and cardiovascular diseases’ cosegregation: epidemiological features. Clin Cases Miner Bone Metab 2008;5(1):14–8. [PMC free article] [PubMed] [Google Scholar]
- 19.Cauley JA, Danielson ME, Boudreau RM, Forrest KY, Zmuda JM, Pahor M, et al.Inflammatory markers and incident fracture risk in older men and women: the Health Aging and Body Composition Study. J Bone Miner Res 2007;22(7):1088–95. [DOI] [PubMed] [Google Scholar]
- 20.Gilsanz V, Perez FJ, Campbell PP, Dorey FJ, Lee DC, Wren TA. Quantitative CT reference values for vertebral trabecular bone density in children and young adults. Radiology 2009;250(1):222–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goodman SB, Jiranek W, Petrow E, Yasko AW. The effects of medications on bone. J Am Acad Orthop Surg 2007;15(8):450–60. [DOI] [PubMed] [Google Scholar]
- 22.Reynolds JF, Noakes TD, Schwellnus MP, Windt A, Bowerbank P. Non-steroidal anti-inflammatory drugs fail to enhance healing of acute hamstring injuries treated with physiotherapy. S Afr Med J 1995;85(6):517–22. [PubMed] [Google Scholar]
- 23.Liu C, Tsai AL, Chen YC, Fan SC, Huang CH, Wu CC, et al.Facilitation of human osteoblast apoptosis by sulindac and indomethacin under hypoxic injury. J Cell Biochem 2012;113(1):148–55. [DOI] [PubMed] [Google Scholar]
- 24.Jensky NE, Hyder JA, Allison MA, Wong N, Aboyans V, Blumenthal RS, et al.The association of bone density and calcified atherosclerosis is stronger in women without dyslipidemia: the multi-ethnic study of atherosclerosis. J Bone Miner Res 2011;26(11):2702–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Budoff MJ, Malpeso JM, Zeb I, Gao YL, Li D, Choi TY, Dailing CA, Mao SS. Measurement of Phantomless Thoracic Bone Mineral Density on Coronary Artery Calcium CT Scans Acquired with Various CT Scanner Models. Radiology 2013;267(3):830–6. [DOI] [PubMed] [Google Scholar]
- 26.Li D, Mao SS, Khazai B, Hyder JA, Allison M, McClelland R, de Boer I, Carr JJ, Criqui MH, Gao Y,Budoff MJ. Noncontrast Cardiac Computed Tomography Image-Based Vertebral Bone Mineral Density: The Multi-Ethnic Study of Atherosclerosis (MESA). Acad Radiol 2013. May;20(5):621–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vestergaard P, Hermann P, Jensen JE, Eiken P, Mosekilde L. Effects of paracetamol, non-steroidal anti-inflammatory drugs, acetylsalicylic acid, and opioids on bone mineral density and risk of fracture: results of the Danish Osteoporosis Prevention Study (DOPS). Osteoporos Int 2012;23(4):1255–65. [DOI] [PubMed] [Google Scholar]
- 28.Mao SS, Li D, Luo Y, Syed YS, Budoff MJ. Application of quantitative computed tomography for assessment of trabecular bone mineral density, microarchitecture and mechanical property. Clin Imaging 2016;40: 330–338. [DOI] [PubMed] [Google Scholar]
- 29.Mao SS, Li D, Syed YS, Gao Y, Luo Y, Flores F, Child J, Cervantes M, Kalantar-Zadeh K, Budoff MJ. Thoracic Quantitative Computed Tomography (QCT) Can Sensitively Monitor Bone Mineral Metabolism: Comparison of Thoracic QCT vs Lumbar QCT and Dual-energy X-ray Absorptiometry in Detection of Age-relative Change in Bone Mineral Density. Acad Radiol 2017;24(12):1582–1587. [DOI] [PubMed] [Google Scholar]
- 30.Ahmadi N, Mao SS, Hajsadeghi F, Arnold B, Kiramijyan S, Gao Y, Flores F, Azen S, Budoff M. The relation of low levels of bone mineral density with coronary artery calcium and mortality. Osteoporos Int 2018;29(7):1609–1616. [DOI] [PubMed] [Google Scholar]
- 31.Delaney JAC, Biggs ML, Kronmal RA, Psaty BM. Demographic, medical, and behavioral characteristics associated with over the counter non-steroidal anti-inflammatory drug use in a population-based cohort: results from the Multi-Ethnic Study of Atherosclerosis. Pharmacoepidemiol Drug Saf 2011;20(1):83–9 [DOI] [PMC free article] [PubMed] [Google Scholar]


