Abstract
With the rapid development of biopharmaceuticals and the outbreak of COVID-19, the world has ushered in a frenzy to develop gene therapy. Therefore, therapeutic genes have received enormous attention. However, due to the extreme instability and low intracellular gene expression of naked genes, specific vectors are required. Viral vectors are widely used attributed to their high transfection efficiency. However, due to the safety concerns of viral vectors, nanotechnology-based non-viral vectors have attracted extensive investigation. Still, issues of low transfection efficiency and poor tissue targeting of non-viral vectors need to be addressed. Especially, pulmonary gene delivery has obvious advantages for the treatment of inherited lung diseases, lung cancer, and viral pneumonia, which can not only enhance lung targeting and but also reduce enzymatic degradation. For systemic diseases therapy, pulmonary gene delivery can enhance vaccine efficacy via inducing not only cellular, humoral immunity but also mucosal immunity. This review provides a comprehensive overview of nanocarriers as non-viral vectors of therapeutic genes for enhanced pulmonary delivery. First of all, the characteristics and therapeutic mechanism of DNA, mRNA, and siRNA are provided. Thereafter, the advantages and challenges of pulmonary gene delivery in exerting local and systemic effects are discussed. Then, the inhalation dosage forms for nanoparticle-based drug delivery systems are introduced. Moreover, a series of materials used as nanocarriers for pulmonary gene delivery are presented, and the endosomal escape mechanisms of nanocarriers based on different materials are explored. The application of various non-viral vectors for pulmonary gene delivery are summarized in detail, with the perspectives of nano-vectors for pulmonary gene delivery.
Keywords: Therapeutic genes, Pulmonary drug delivery, Non-viral vectors, Nanoparticles, Gene delivery
Graphical abstract
1. Introduction
With the fast development of biotechnology, a large number of biopharmaceuticals are available on the market, which are expected to be well applied in the clinical treatment of diseases. At present, the active ingredients of biopharmaceuticals include peptides, recombinant proteins, antibodies, therapeutic genes etc. [1]. With the outbreak of COVID-19, therapeutic genes have received enormous attention. Gene therapy is a strategy to deliver exogenous therapeutic genes to target cells. It can treat diseases that are untreatable via conventional treatments and offers a possibility to completely cure diseases [2]. In gene addition therapy, therapeutic genes, including plasmids DNA (pDNA) and messenger RNA (mRNA), can produce gene expression that restores normal protein levels, ultimately treating diseases that result from gene deletions. In gene inhibition therapy, therapeutic genes such as small interfering RNA (siRNA) can produce gene silencing, thereby inhibiting protein production, and ultimately treating diseases caused by gene overexpression. In genome editing, specific genome editing tools such as clustered regularly interspaced short palindromic repeats (CRISPR)-CRISPR-associated (Cas) systems can repair mutations in the genome [3].
Although therapeutic genes have shown great potential in vaccines and in the treatment of genetic diseases, a major bottleneck hindering their broad development is how to deliver therapeutic genes to the target site safely and effectively. Due to the extreme instability, low cellular uptake, and low transfection efficiency of naked genes, specific vectors are required for gene delivery. Currently, the vectors of therapeutic genes can be categorized into viral vectors and non-viral vectors. To avoid the safety problems of viral vectors, non-viral vectors have gained extensive attention [4]. However, the issues of low transfection efficiency and poor tissue targeting of non-viral vectors need to be solved urgently. With the fast development of nanotechnology, nanocarriers have become a research hotspot of non-viral vectors, and it has been demonstrated that targeted delivery and enhanced transfection in cells can be achieved by appropriate nanocarriers.
At present, gene delivery based on nanotechnology has achieved the transformation from basic research to clinical application. mRNA vaccines have been in development and clinical testing for the past 30 years. With the global pandemic of severe acute respiratory syndrome (SARS-CoV-2), many researchers around the world have developed mRNA vaccines at an unprecedented speed [5]. The US Food and Drug Administration (FDA) has granted emergency use authorization for Pfizer/BioNTech and Moderna mRNA vaccine for the treatment of COVID-19 [6]. After two decades of development, the FDA approved the world's first siRNA drug in 2018, ONPATTRO® (Patisiran), for the treatment of transthyretin (TTR)-type familial amyloid polyneuropathy [7]. The above three marketed products all use lipid nanoparticles (LNPs) as vectors of therapeutic genes. Although LNPs are currently the most successful gene vectors, they accumulate mainly in the liver after intravenous administration, so there is an urgent need to develop novel vectors or select appropriate methods of administration to address the challenge of extrahepatic targeting [8].
Recently, due to ambient/outdoor air pollution [9] and smoking [10], lung related diseases is increasing remarkably. Pulmonary delivery of therapeutic genes can provide obvious superiority in the treatment of inherited lung diseases (e.g., asthma and cystic fibrosis), lung cancer [11], and viral pneumonia (e.g., COVID-19) [12], while drugs can be directly delivered to lung targets with decreased dose and systemic exposure, and meanwhile leading to improved therapeutic effects. Additionally, the lung has abundant capillaries, large absorptive surface areas, ultrathin epithelial cells, and slow cell surface clearance, making the lung also a favorable site for delivering therapeutic genes to produce systemic effects [13] compared with oral administration. The non-invasiveness of pulmonary delivery can also improve patient compliance compared with injection. Moreover, inhalable gene-based vaccines can generate not only cellular and humoral immunity but also mucosal immunity, leading to enhanced vaccine efficacy [14].
The objective of this review is to provide a comprehensive overview of nanocarriers as a reference for the design of novel non-viral vectors for enhanced pulmonary gene delivery. First of all, the characteristic and therapeutic mechanism of DNA, mRNA, and siRNA are provided and compared. The advantages and challenges of pulmonary gene delivery in exerting local and systemic effects are systematically discussed. Thereafter, the inhalation dosage forms for nanoparticle-based drug delivery systems are introduced. The materials commonly used as the nanocarriers for pulmonary gene delivery are presented, with the endosomal escape mechanisms of nanocarriers based on different materials. Moreover, different nanocarriers, including but not limited to lipid-based nanocarriers, polymer-based nanocarriers, peptide-based nanocarriers, and hybrid nanocarriers, are described. Finally, the present development status and the perspectives of nanocarriers for pulmonary gene delivery are further explored and addressed.
2. Therapeutic mechanism of different genes
Therapeutic genes are biological macromolecular drugs that can correct the process of transcription or translation at the level of DNA or mRNA [4]. At present, pDNA, mRNA, and siRNA have been extensively studied as therapeutic genes for pulmonary delivery. Because negatively charged genes are unstable and cannot cross anionic cell membranes, the assistance of vectors is usually required. After therapeutic genes are delivered to target tissues and cells by appropriate vectors, they can enter cells via clathrin-mediated endocytosis, caveola-mediated endocytosis, or macro/micropinocytosis [15], followed by endosomal escape, resulting in the release of genes in the cytoplasm. Both pDNA and mRNA are used to produce therapeutic proteins, but the site of action of pDNA is the nucleus, while mRNA functions in the cytoplasm. Different from pDNA and mRNA, siRNA produces gene silencing via RNA interference (RNAi) mechanism (It is a natural defense mechanism for the invasion of exogenous genes [16]) in the cytoplasm, thereby preventing the production of target proteins [17]. Therefore, a full understanding of their therapeutic mechanisms is a prerequisite for the appropriate design of specific gene related delivery systems.
2.1. DNA
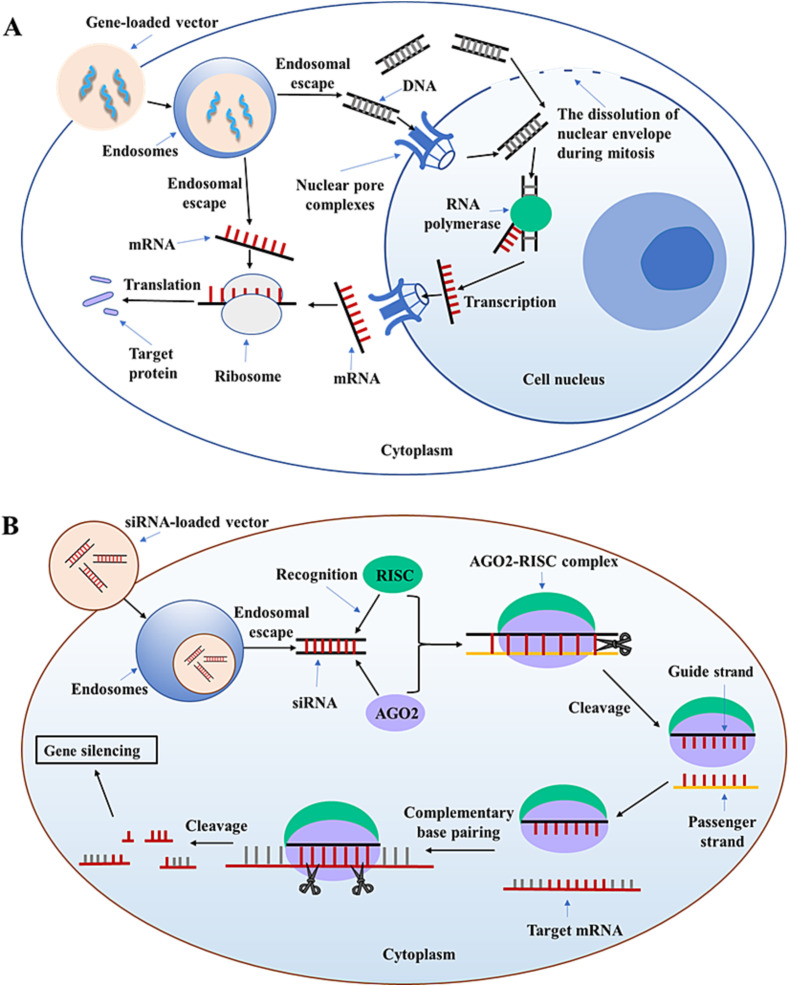
DNA is a double-stranded nucleic acid composed of deoxynucleotides that carries the genetic information used to synthesize RNA and proteins. As shown in Fig. 1(A), the therapeutic mechanism of DNA is as follows: DNA or DNA-loaded vectors are released into the cytoplasm via endosome escape and subsequently into the nucleus via the nuclear pore complexes (NPCs) or the dissolution of nuclear envelope during mitosis [15]. However, DNA may be separated from the vectors in the cytoplasm or nucleus, and the specific mechanism is not clear. Either way, DNA must enter the nucleus to function. Then mRNA is generated in the nucleus by transcription. Finally, mRNA enters the cytoplasm, and then target proteins are produced by translation [18]. Hence, DNA can be used to restore the level of endogenous proteins, introduce new cell functions, and produce immunogenic antigens for vaccine [19].
Fig. 1.
Schematic illustration of the therapeutic mechanism of (A) DNA, mRNA, and (B) siRNA.
In the past decades, DNA vaccines have been widely investigated for the prevention and treatment of a variety of diseases, such as infectious diseases, cancer, autoimmune diseases, and allergies [20]. pDNA is formed by inserting gene fragments encoding immunogenic antigens into a bacterial plasmid and the expression of pDNA produces the desired antigens in the host [20], resulting in the induction of a strong cellular immunity with a preference to cytotoxic T lymphocyte (CTL) and T helper type 1 (Th1) immune response [21]. To ensure the stability of vaccines, refrigeration is usually required, while DNA vaccines are highly stable and rarely require refrigeration, which is very beneficial for storage and transportation [22]. However, DNA have the following disadvantages: (1) Easily degraded by enzymes; (2) DNA supercoiled structure is easily destroyed and loses its biological activity after destruction; (3) Cellular uptake is poor due to the dual barrier of cell membrane and nuclear membrane [11,23,24]. Thus, a major challenge is how to deliver large, fragile, and negatively charged DNA efficiently to the nucleus without degradation [19,23]. Furthermore, DNA may integrate into the host genome, causing a potential risk of serious mutations and new diseases for which there is currently no solution [24].
2.2. mRNA
mRNA (300–5000 kDa) is a single-stranded nucleic acid composed of ribonucleotides that carries the genetic information for protein synthesis and is ∼1–15 kilobase (kb) in length [25]. Therapeutic mechanism of mRNA is as follows (Fig. 1(A)): After endosomal escape, the release of mRNA is occurred in the cytoplasm. Then non-replicating mRNA creates target proteins by translation. Self-amplifying mRNA (SAM) directs RNA-dependent RNA-polymerase (RDRP) complex and generates multiple copies of the antigen-encoding mRNA, which can also produce target proteins [26,27]. In contrast to pDNA, mRNA directs the synthesis of proteins in the cytoplasm without the barrier of nuclear membrane and is degraded naturally during antigen expression without the risk of mutation and integration [28]. The transfection of mRNA is rapid, whereas the transfection of pDNA takes several hours or days [18]. Hence, mRNA can be used as an alternative to pDNA in gene therapy.
Over the past two decades, mRNA vaccines have been widely used in the prevention of infectious diseases, including SARS-CoV-2, influenza A virus, rabies virus, respiratory syncytial virus (RSV), zika virus (Zika), human immunodeficiency virus (HIV-1), cytomegalovirus (CMV), and Ebola virus (EBOV), and in the prevention or treatment of cancer [28]. For infectious diseases, the antigens of infectious pathogens can be produced in vivo by mRNA vaccines, and subsequently robust cellular immunity and humoral immunity are induced by the antigens. For cancer, mRNA vaccines are designed to express tumor associated antigens and then stimulate a cell-mediated immune response to clear or suppress cancer cells [24]. However, compared with DNA vaccines, the stability of mRNA vaccines is poor. Currently, mRNA-based COVID-19 vaccines must be stored at low temperatures. To address instability, mRNAs can be chemically modified, which may also reduce immunostimulatory responses [29]. Additionally, negatively charged mRNA has poor cellular uptake and is easily phagocytosed by immune cells or degraded by nucleases [28].The core issue that needs to be addressed for delivery is to achieve efficient intracellular gene expression while ensuring the in vivo stability of mRNA after administration [29]. Moreover, unmodified mRNA can activate various Toll-like receptors, increase cytokine levels, and produce related toxicity [26].
2.3. siRNA
siRNA (∼13 kDa) is generally a double-stranded RNA with a length of 21 to 23 base pairs (bp) [17,30]. As shown in Fig. 1(B), the therapeutic mechanism of siRNA is different from DNA and mRNA. After siRNA is released into the cytoplasm via endosomal escape, it is recognized by Argonaute-2 (AGO2) protein and RNA-induced silencing complex (RISC). When AGO2-RISC complex is activated, one strand of the siRNA (a passenger strand) is degraded, and the other strand (a guide strand and mostly antisense) recognizes target mRNA by complementary base pairing. Finally, mRNA is cleaved by AGO2, resulting in gene silencing [19,30,31]. In contrast to DNA, the site of action of siRNA is the cytoplasm, so there is no barrier of nuclear membrane in siRNA delivery. However, siRNA has the challenge of off-target effects, which can degrade an unknown amount of unintended mRNA, and it may also have immunostimulatory effects [30]. Moreover, it is easily degraded by enzymes and rapidly excreted from the kidney, resulting in a short half-life in the systemic circulation. The cellular uptake of siRNA is poor due to its negative charge. Currently, the main issue of siRNA delivery is how to successfully release the siRNA into the cytoplasm of target cells for efficient gene silencing [4,11,19].
3. Advantages and challenges of pulmonary gene delivery
3.1. Advantages
For respiratory diseases therapy, gene delivery via inhalation can be more targeted, resulting in efficient local effects. Inhalation therapy achieves high local concentrations in lung lesions, lower systemic exposure, and rapid clinical response. Hence, pulmonary administration is the preferred route for first-line treatment of asthma, CF, and COPD, etc. [32,33]. The disorders of autosomal recessive or dominant can be treated by the exogenous delivery of wild-type genes, so DNA and mRNA can be used to treat inherited lung diseases with single-gene mutations [34]. Furthermore, because many lung diseases are caused by over-transcription of genes, including lung cancer, idiopathic pulmonary fibrosis (IPF), asthma, respiratory syncytial virus (RSV) infection, and influenza infection, there is a great potential to directly deliver siRNA to lung lesions by inhalation [19]. For instance, there are more than 1900 identified cystic fibrosis transmembrane conductance regulator (CFTR) mutations to cause CF, but many of these mutations cannot be treated with existing drugs [35]. While mRNA can be used to replace or edit CFTR and has the potential to treat any CF patient [36]. siRNA can be used as co-adjuvant therapy for CF by silencing α-epithelial Na+ channel (ENaC) gene [37]. For non-small cell lung cancer (NSCLC) therapy, surgery is limited by the location and number of lung lesions and the condition of patients [38]. Although chemotherapy and radiotherapy are now relatively standardized and the development of precision medicine has facilitated the application of small molecule inhibitors in targeted therapy, the 5-year survival rate of patients is still low (∼16%) [39,40]. Conventional treatments neglect tumor specificity, which has a decisive influence on tumor resistance and immune escape, while RNA therapy has the advantage of high specificity and a wide range of targets [41]. Meanwhile, acquired and inherent drug-resistance in tumors are major challenges in cancer treatment. To overcome drug resistance caused by mutations and overexpression of oncogenes, therapeutic strategies that combine RNAi technology with chemotherapy or immunotherapy have become a hot research topic [42]. Targets such as epidermal growth factor receptor (EGFR), vascular endothelial growth factor (VEGF), kristen rat sarcoma viral oncogene homolog (KRAS), anexelekto (AXL) kinase [40], and yes-associated protein (YAP) [43] are considered to be the key to the development of innovative approaches for the treatment of NSCLC. For the above targets, gene inhibition therapy can be adopted: After delivering specific siRNA to cancer cells, the siRNA in the cytoplasm can have a gene silencing effect on the mRNA of targets, thus inhibiting the overexpression and mutation of specific oncogenes, preventing the production of cancer-related receptors or oncoproteins, and ultimately stopping the growth and proliferation of cancer cells. Huang et al. [43] used nanotechnology to co-deliver EGFR-tyrosine kinase inhibitor (EGFR-TKI) gefitinib (Gef) and YAP-siRNA, and successfully transfected Gef-resistant NSCLC cells. This illustrates the potential ability of siRNA to address the resistance of targeted anti-cancer drug, EGFR-TKI, in the clinic. Garbuzenko et al. [44] delivered EGFR-siRNA- and paclitaxel (TAX)-loaded nanostructured lipid carriers (NLC) for the treatment of NSCLC and found that nanoparticles delivered via inhalation significantly inhibited tumor growth in orthotopic NSCLC mouse model compared to that of intravenous injection.
For systemic diseases therapy, the analysis of lung physiology can clearly reveal the advantages of pulmonary gene delivery. The adult human lung has over 300 million alveoli with a surface area of about 70–140 square meters (m2), thus providing an enormous absorption area for drugs, which is conducive to systemic effects [45,46]. Furthermore, more than 280 billion capillaries are arranged on the alveoli, forming a huge capillary network. The distance between capillaries and alveoli is only about 0.5 μm, and the thickness of monolayer alveolar epithelium cells, including flattened alveolar type I (ATI) and cuboidal alveolar type II (ATII) cells, are about 0.1–0.5 μm, so the proteins produced by gene transfection in alveolar cells can easily enter capillaries, and then enter the blood circulation with systemic effects [33,46]. For vaccines with systemic effect, antigen-presenting cells (APCs) are ideal target, while the lung has a large number of APCs, making the lung an excellent site for vaccine delivery [47]. Intramuscular vaccines are typically used in the clinic, but intramuscular injection produces a poor mucosal immune response, while inhalable vaccines have the unique advantage of not only inducing highly efficient humoral immunity but also mucosal immunity [48]. Inhaled vaccines follow the natural route of infection and may best mimic the induction of immunity by pathogens in the respiratory tract [14]. The mucosa is the body's first line of defense, and inhaled vaccines stimulate the production of antigen-specific immunoglobulin (Ig) A antibodies. IgA is secreted from epithelial cells into the mucus of the mucosal surface and subsequently forms complexes with pathogens, thus preventing the cells from being infected [49,50]. Moreover, the various mucosa-associated lymphoid tissues (MALT) presented on the mucosal surface, including bronchus-associated lymphoid tissue (BALT), nasopharynx-associated lymphoid tissue (NALT), rectum-associated lymphoid tissue (RALT), vagina-associated lymphoid tissue (VALT), and gut-associated lymphoid tissue (GALT), form an immune network. Thus, instead of inducing mucosal immunity only in the lung, inhalable vaccines can induce mucosal immunity in mucous membranes throughout the body [50]. The specific process of mucosal immunity in the respiratory tract is shown in Fig. 2 . Currently, inhaled therapy for COVID-19 is being extensively investigated due to the advantages of pulmonary delivery as described above and the fact that the lung is the primary target of infection for SARS-CoV-2. Afkhami et al. [51] demonstrated the single-dose intranasal inhalation of adenoviral-vectored trivalent COVID-19 vaccine was superior to intramuscular immunization in induction of the tripartite protective immunity consisting of local and systemic antibody responses, mucosal tissue-resident memory T cells and mucosal trained innate immunity. Compared to inactivated or live-attenuated virus vaccines with time-consuming and demanding production procedures, DNA and mRNA vaccines can avoid the complex production procedures of traditional vaccines, and are fast to produce, easy to scale up, and capable of encoding almost any type of protein. In addition to directly delivering antigens via viral vectors, DNA and mRNA can be delivered to the lung via non-viral vectors to produce specific antigenic proteins that imitate parts of the target bacteria or viruses. Currently, nebulized delivery of mRNA-loaded LNPs has been studied, but there are few reports of pulmonary delivery of other non-viral vector-based DNA or mRNA vaccines [20].
Fig. 2.
Schematic illustration of mucosal immunity in the respiratory tract. (1) After antigens are inhaled and deposited on the respiratory tract, they are mainly taken up by microfold cells (M cells) and then by dendritic cells (DCs). Additionally, CD103+-expressing lung alveolar DCs can extend their dendrites and then cross the tight junctions between epithelial cells to take up antigens [52]; (2) DCs enter bronchus-associated lymphoid tissue (BALT) and stimulate CD4+ T cells to form T helper 2 cell (Th2). Subsequently, Th2 cells activate B cells [50]; (3) B cells enter the bloodstream via regional lymph nodes and the thoracic duct, and can subsequently reach not only the respiratory tract but also other mucosal sites, including gut, nasal, and genitourinary-associated lymphoid tissues; (4) B lymphocytes differentiate into plasma cells and produce IgA [21,53]; (5) IgA binds to the polymeric immunoglobulin receptor (pIgR) on the basolateral surface of epithelial cells, and then the complex is endocytosed and delivered to the surface of cells [54].
3.2. Challenges
Despite of the many advantages of pulmonary gene delivery, there are still many challenges to be overcome. To protect genes and achieve efficient transfection, it is generally necessary to efficiently concentrate genes into nanoparticles using high biosafety materials. However, the selection of materials is a challenge because they must achieve efficient encapsulation while being able to be taken up by the target cells and successfully deliver the genes to the intracellular site of action.
When particles are inhaled, the first challenge is anatomical barriers. The human respiratory tract consists of 23 generations. The first 16 generations are the conducting region (trachea, bronchi, bronchioles, and terminal bronchioles), and the 17–23 generations are the respiratory region (respiratory bronchioles, alveolar ducts, and alveolar sacs). Among them, the respiratory region is the absorption site of drugs [55]. The barrier created by the highly branching with different diameters and lengths in the respiratory tract must be overcome for inhaled drugs to deposit in specific locations in the lung [56]. The aerodynamic diameter (Da) of the particles is the main factor deciding the site of deposition. Particles with Da > 5 μm are mainly deposited in the upper respiratory tract via inertial impaction mechanism. Particles with Da in the range of 1–5 μm are deposited in the respiratory region via the gravitational settling mechanism. Particles with Da < 1 μm are mostly exhaled [55]. Because nanoparticles have nanoscale dimensions, they are readily exhaled. Hence, certain methods must be used (e.g., mixing nanoparticles with excipients) so as to confer excellent inhalation properties to nanoparticles. In addition to particle size, shape is also an important parameter affecting deposition and fate during pulmonary delivery. The aspect ratio can be used to describe the geometry of inhalable particles [57]. Particles with a high aspect ratio have an elongated geometry and are deposited at the main bronchial bifurcation, or on airway walls via interception [58].
The second challenge is the natural clearance mechanism of the lung, including mucociliary clearance (MCC) and phagocytosis by alveolar macrophages (AMs). Mucociliary clearance is the major defense mechanism naturally present in airways, and its functional components include the protective mucus layer, periciliary layer (PCL), pulmonary surfactants, and cilia. In inhalation therapy, inhalable particles are deposited in the mucus layer, preventing particles from entering the cells. The low viscosity periciliary layer can lubricate airway surfaces, while pulmonary surfactants can prevent the entanglement between mucus and cilia, thus leading to the promotion of ciliary motion mediated by the motor activity of axonemal dynein. Next, cilia beat in a coordinated fashion and transport particles from the airways to the throat. Finally, particles are excreted by coughing or swallowed into the digestive tract [59]. The primary clearance mechanism of particles in the respiratory region is phagocytosis by alveolar macrophages. Alveolar macrophages account for more than 90% of airway immune cells, and there are 8–12 alveolar macrophages around each alveolar cell. When inhalable particles deposited at the alveolar-blood interface, they are phagocytosed by macrophages and then degraded by phagosomes, especially those with a size of 1.5–3 μm [60,61]. Furthermore, the aspect ratio also affects the clearance efficiency of macrophages [57]. Moehwald et al. [57] prepared pDNA-loaded cylindrical nanostructured microparticles (The aspect ratio is approximately 3) and found that the aspherical microparticles have the ability to target and transfect alveolar macrophages.
Intracellular barriers have been considered as another major challenge. Generally, gene-loaded nanoparticles enter cells by endocytosis, and then they are encapsulated by membrane vesicles. After decapsulation, particles transport into the early endosome (The vesicular organelles). The early endosomes have ATP-driven proton pumps that can pump hydrogen ions into the endosomes and lower the pH from 7.4 to 6.6. Afterwards, the pH is reduced to 6.0, and the late endosomes are formed. Eventually, the late endosomes merge with lysosomes, and the pH is reduced to 5.0 by acidification. Next, the degradative enzymes in the lysosomes are activated, resulting in the destruction of genes. To prevent destruction, nanoparticles must escape from the endosomes or lysosomes, or bypass the endosomal pathway entirely [[62], [63], [64]]. Because transfection efficiency is definitely affected by endosome escape, DNA, mRNA, and siRNA must be released from the endosomes to the cytoplasm. However, even the most advanced gene vector, LNPs, have a limited capacity for endosomal escape with less than 2–3% of the intracellular siRNA being visualized in the cytoplasm [8]. For DNA, after entering the cytoplasm, there is a further challenge of being transported to the nucleus. It was evidenced that positively charged nanoparticles could bind to anionic microtubules or molecular motor proteins and move to the nuclear membrane along with the cytoskeletal network, enhancing the transport of DNA from the cytoplasm to the nucleus [65].
The above challenges collectively affect the fate of drugs in vivo and must be overcome simultaneously for successful pulmonary gene delivery. Among them, anatomical barriers and natural clearance mechanisms of the lung are specific challenges for pulmonary delivery, while intracellular barriers are specific challenges for genes. At present, many nanocarriers have been developed as non-viral vectors of genes. It is anticipated that non-viral vectors with appropriate physicochemical property (e.g., particle size, superficial charge, aerodynamic diameter, hydrophilicity, and pH sensitivity) can ensure the stability of genes, evade the clearance of mucociliary or macrophages, facilitate cellular uptake, and achieve endosomal escape.
4. Inhalation dosage forms for nanoparticle-based drug delivery systems
For inhalation therapy, inhalation dosage forms are critical because they can affect storage stability, efficacy of delivery, and even patient compliance. Currently, pulmonary delivery of gene-loaded nanocarriers can be achieved by using nebulizers for liquid formulations, and pressurized metered dose inhaler (pMDI) and dry powder inhaler (DPI) can also be used when appropriate. For nanoparticles-based inhalation therapy, nanoparticles can be administered in liquid form by using nebulizers or pMDI and in solid form by using DPI [66].
The nebulizers have been widely used in clinical and have become the foundation of inhalation therapy in acute and critical care settings [67], mainly including air-jet nebulizer, ultrasonic nebulizer, vibrating-mesh nebulizer, and surface acoustic wave microfluidic atomization [68]. As an example, the marketed amikacin liposome inhalation suspension (ALIS) is based on eFlow® technology [69] (Vibrating membrane nebulizers [70]). Inhalation therapy via nebulizers does not require the patient to master any special breathing techniques, which is particularly beneficial for patients with physical or cognitive impairments [71]. After preparation, nanoparticles are typically dispersed in a liquid medium to form suspensions, and nanosuspensions can be directly aerosolized by nebulizers, resulting in aerosols with an aerodynamic diameter of 1–5 μm. However, nanoparticles have problems with physical (aggregation) and chemical (degradation) instability in water [72]. To enhance long-term stability of nanoparticles, Beck-Broichsitter et al. [72] added common excipients (lyoprotectants) to PLGA nanosuspensions to entrap the nanoparticles in the excipient matrix, and subsequently prepared dry powders by freeze-drying. They demonstrated that lyoprotectant/nanoparticles ratios above 5/1 were necessary to preserve the physical stability of polymeric nanoparticles and facilitate their redispersion. They also investigated the effects of different lyoprotectants on the performance of air-jet, ultrasonic, and vibrating-mesh nebulization, and found that only the vibrating mesh could effectively aerosolize rehydrated nanocomposites, and the changes in temperature, concentration, surface tension, and dynamic viscosity of suspensions were less pronounced during nebulization. Additionally, air-jet and ultrasonic nebulizer can impair the physical stability and integrity of naked pDNA and some gene delivery systems, resulting in a marked reduction in their transfection efficiency [73], while Luo et al. [73] demonstrated that vibrating-mesh nebulizer (Aeroneb Pro nebulizer) exhibited excellent performance in delivering pDNA or RNA-loaded polymeric nanoparticles. These suggest that vibrating-mesh nebulizer is suitable for the delivery of inhalable nanoparticles.
DPI consist of dry powders and a special inhalation device. It has been used to deliver biomacromolecules such as inhaled human insulin products: Exubera® and Afrezza® [74]. In contrast to pMDI, the dry powders of nanocomposites do not have to be dispersed in a medium to form suspensions, but are stored in capsules or the reservoir of special inhalation devices. Dry powders have higher storage stability and better sterility compared with suspensions [62]. The physical properties of particles (e.g., size, shape, density, surface charge, and moisture content) directly influence the aerosolization of dry powders [75]. The aerosolization behavior of DPI depends not only on dry powders, but also on inhaler devices. DPI devices can be divided into four categories including multi-unit, multi-dose reservoir, reusable single dose, and single-use devices. The choice of device depends on dose, dosing frequency, and powder properties [76]. However, some common errors (e.g., no exhalation before activation, no forceful and deep inhalation, no breath-hold, and failure to breathe out slowly) in the usage of DPI can greatly affect the efficacy of inhalation, so proper usage by patients is crucial [77]. For application, once the intended nanocarriers have been successfully designed, safe water-soluble excipients (e.g., lactose, mannitol, sucrose, and PEG etc.) need to be added and mixed with nanocarriers to form nano-embedded microparticles/nanocomposites so that the aerodynamic diameter of inhalable particles is in the range of 1–5 μm, thus obtaining optimal deposition in the respiratory region [78,79]. The nanocomposites are then formed into dry powders by freeze-drying (FD), spray-drying (SD), spray-freeze-drying (SFD), thin-film freeze-drying (TFFD), or supercritical fluid‑carbon dioxide drying technique [75]. This method not only increases the particle size but also improves the long-term stability of nanoparticles [72]. Patients then require appropriate inhalation devices to inhale nanocomposites into the lung. When nanocomposites reach the alveolar surface, the excipients disintegrate in alveolar fluids, thereby releasing the drug-loaded nanoparticles [80]. Additionally, DPI must satisfy the following conditions for gene delivery: (1) Dry powders need to have excellent characteristics for inhalation, and they are not toxic in the respiratory zone after inhalation; (2) The stability of genes must be ensured when preparing dry powders; (3) The stability of vectors in the inhaler needs to be guaranteed, such as preventing particles from agglomeration and keeping them dry [81,82].
pMDI has been used in the clinic for the treatment of asthma and COPD since its introduction in 1956 [76], and it is the cheapest and most widely used portable inhalation devices [83]. pMDI is composed of a pressure-resistant container with a special valve system and a solution, suspension, or emulsion formed by drugs and liquid propellants. pMDI needs to be applied with the pressure provided by propellants to eject the contents, followed by the evaporation of propellants and formation of drug aerosols. Currently, propellants are hydrofluoroalkanes (HFAs), including HFA-134a and HFA-227. In contrast to chlorofluorocarbons (CFCs), which have ozone-depleting properties and have been deprecated, HFAs are non-toxic, non-flammable, and chemically stable gas without carcinogenic or mutagenic effects [84]. For gene delivery, suspension based pressurized metered dose inhaler is mainly used. The excipients need to be added to the prepared nanosuspensions, and then the nanocomposites are dried to form dry powders with excellent aerodynamic behavior. Finally, the solid particles are uniformly dispersed in liquid propellants to reform suspensions [85,86]. Special methods are required for filling the propellants into pressure-resistant containers, including cold filling and pressure filling [87]. Similar to DPI, the specific inhalation skills of patients are also required in the usage of pMDI, including coordination between inhalation and actuation, emptying the lung before inhalation, slow and deep inhalation, and holding the breath after inhalation [71]. However, the application of pMDI for gene delivery is less and limited because it is difficult to stabilize the dispersion of vectors in propellants [68]. In addition, the force of nebulization tends to destroy gene-loaded vectors, so it is indispensable to conduct stability tests [88].
5. Commonly used materials as nanocarriers for pulmonary gene delivery
For the design of non-viral vectors, it is firstly essential to select appropriate material as the core of nanoparticles, followed by functional modification of the core. Typically, positively charged materials are combined with negatively charged genes to form electrostatic nanocomplexes. Alternatively, genes can also be embedded in the materials during the formation of nanoparticles, but often with low encapsulation efficiency. Moreover, the combined usage of several different materials can also have a coordinated effect, allowing nanoparticles to break through multiple challenges. The choice of materials is critical to the design of novel drug delivery systems. Therefore, an understanding of common materials is required before introducing nano-drug delivery systems for therapeutic genes.
5.1. Lipids
5.1.1. Cationic lipids
Cationic lipids are amphiphilic small molecules that are easy to design and synthesize [89]. Broadly speaking, cationic lipids consist of a cationic headgroup covalently bound to a hydrophobic tail through a linker. All three components affect the properties of lipid-based nanocarriers. Positively charged polar headgroups are prominent because electrostatic interactions are required for drug loading, and they include quaternary ammonium salts, amines (Primary, secondary, and tertiary), guanidine, heterocyclic compounds, and a combination thereof. Furthermore, polar headgroups need to be charged by protonation, so the pH of the solution is very important during preparation. Linkers affect the stability, biodegradability, cytotoxicity, and transfection efficiency, and they include ethers, esters, carbamates, and amides. Non-polar hydrophobic tails affect fluidity, overall stability, and cytotoxicity. Depending on the structure, hydrophobic tails are classified as aliphatic chains or cyclic (steroid-based) domains [90]. Commonly used cationic lipids for gene delivery are N-[1-(2,3-Dioleyloxy)propyl]-N,N,N-trimethylammonium chloride (DOTMA), 1, 2-Dioleoyloxypropyl-3(trimethylammonio)-propane (DOTAP) (Fig. 4), 2,3-Dioleoyloxy-N-[2(sperminecarboxamido)ethyl]-N,N′-dimethyl-1-propana-minium trifluoroacetate (DOSPA), 1,2-Dimyristyloxypropyl-3-dimethyl-hydroxyethylammonium bromide (DMRIE, cytofectin), Dioctadecylamidoglycylspermine (DOGS, transfectam), and (3β)-[N-(N′,N″-Dimethylaminoethyl) carbamoyl]cholesterol (DC-Chol) [91].
Fig. 4.
Chemical structures of commonly used lipids and polymers as nanocarriers for pulmonary gene delivery.
5.1.2. Ionizable lipids
The head of the cationic lipid was replaced with an ionizable moiety, resulting in the first ionizable lipid: 1,2-dioleoyl-3-dimethylammonium propane (DODAP) [8] (Fig. 4). Ionizable lipids are distinct from permanently charged cationic lipids. At physiological pH (i.e., in blood), the surface of the ionizable lipid nanocarriers is essentially neutral, thus reducing toxicity [92]. After cellular uptake, the nanocarriers are positively charged by protonation of free amines following acidification in endosomes [25], and then electrostatic interactions between LNPs and the negatively charged endosome membranes will facilitate endosome escape [93]. Like cationic lipids, positively charged ionizable lipids bind to negatively charged genes via electrostatic interactions, so the solution pH during preparation can affect drug loading. Currently, ionizable lipids have become the mainstream materials in the study of lipid-based vectors, mainly including 1,2-dilinoleyloxy-N,N-dimethyl-3-aminopropane (DLin-DMA), 2,2-dilinoleyl-4-(2-dimethylaminoethyl)-[1,3]-dioxolane (DLin-KC2-DMA), and (6Z,9Z,28Z,31Z)-heptatriacont-6,9,28,31-tetraene-19-yl-4-(dimethylamino)butanoate (DLin-MC3-DMA) (Fig. 4). Among them, DLin-MC3-DMA is the gold standard ionizable lipid for gene silencing in the liver [7]. Qiu et al. [94] found that ionizable lipid with an amide bond in the tail can predispose LNPs to deliver mRNA to the mouse lung, whereas LNPs exhibit liver targeting when the tail of the ionizable lipid contain an ester bond.
5.1.3. Neutral lipids
Neutral lipids are generally a component of lipid-based nanocarriers such as cationic liposomes (CLs) and LNPs, which act as assistant in formulations to improve transfection efficiency, including cholesterol and dioleyl phosphatidylethanolamine (DOPE) [95] (Fig. 4). Cholesterol is a natural waxy steroid found in all animal cell membranes. Not only can it improve the stability of vectors, but it may also facilitate the interaction of vectors with cell membranes and endosomal membranes [90]. As a fusogenic lipid, DOPE fuses with the lipid bilayer membrane and achieves an unstable geometry at acidic pH, thereby destabilizing the endosomal membranes and ultimately leading to the release of genes [90,95].
5.2. Polymers
5.2.1. Chitosan (CS)
Chitosan is a natural material extracted from the shells of crustaceans and obtained by deacetylation of chitin. It has the advantages of biodegradability, low immunogenicity, and good biocompatibility. Furthermore, its mucoadhesion and mucosal permeability make it particularly beneficial for pulmonary administration [96]. Every deacetylated subunit of chitosan contains a primary amine with a pKa of approximately 6.5. At acidic pH, below the pKa, the primary amines in the chitosan backbone become positively charged, so chitosan can form complexes with negatively charged nucleic acids via electrostatic interactions. However, the transfection efficiency of complexes was affected by the molecular weight of chitosan, degree of chitosan deacetylation (DD), N/P ratio (charge ratio of amine (chitosan) to phosphate (DNA/RNA)), chitosan salt form, DNA/RNA concentration, pH of the culture medium, presence of serum, additives, preparation techniques, and route of administration. Among them, molecular weight affects particle size, and particle size impacts pulmonary deposition, transfection efficiency, and cellular uptake, hence chitosan with appropriate molecular weight should be selected to achieve suitable particle size for pulmonary delivery [65]. However, chitosan-based vectors have the issues of low cell specificity and low transfection efficiency. Ligand modification, stimuli-response modification, or penetrating modification can be used to solve these issues [97].
Conti et al. [83] prepared pressurized metered dose inhaler (pMDI) of CS-DNA NPs using HFA-227 as a propellant and CS (Mw = 31 kDa) as a vector material, and successfully transfected A549 cells. The mass median aerodynamic diameter (MMAD) was 2 μm and the fine particle fraction (FPF) was 63%, which showed excellent inhalation performance of pMDI. They demonstrated the feasibility of gene-loaded polymeric nanoparticles for pulmonary delivery.
5.2.2. Hyaluronic acid (HA)
HA is a glycosaminoglycan that is naturally found in the lung, and it can protect pulmonary elastin from inflammation. HA has the advantages of biocompatibility, biodegradability, and non-immunogenicity, so it is widely used as a material for nanocarriers [98]. For gene delivery, HA is negatively charged, so it can be used for the surface modification of positively charged vectors to shield excessive positive charges, resulting in reduced cytotoxicity [99]. For instance, Fukushige et al. [100] modified liposome-protamine-DNA nano-complex (LPD) with HA, and prepared dry powders by spray-freeze-drying (SFD). They not only demonstrated that surface modification with HA reduced the cytotoxicity of LPD, but also proved that the cellular uptake and gene silencing of HA-modified LPD was higher than that of unmodified LPD in A549 cells. The increase in cellular uptake may be due to HA promoting the specific binding of LPD to CD44 receptor on the surface of A549 cells, and the increase in gene silencing may be due to the improved stability of vectors.
5.2.3. Polyethyleneimine (PEI)
PEI is a gold standard for in vitro gene delivery [101]. Positively charged PEI can form polyplexes with negatively charged nucleic acids [102], and PEI-based polyplexes and PEI-liposome polyplexes (lipopolyplexes) can effectively deliver DNA or siRNA in vitro and in vivo [103]. According to the proton sponge effect, the high buffering capacity of PEI leads to rupture of endosomes, thereby promoting endosome escape [102,104]. Therefore, PEI-based NPs have high intracellular release efficiency. However, the high charge density of PEI causes extensive damage to cell membranes and leads to apoptosis, necrosis, and inhibition of ATP synthesis [101]. Because PEI has safety problems, it is usually used as a reference at present, and PEI is rarely used alone as a gene vector.
Keil et al. [105] prepared dry powders (MMAD = 3.17 μm) using PEI (Mw = 25 kDa)-DNA NPs and 10% trehalose. The results showed that the cellular uptake and transfection efficiency of spray-dried and redispersed microparticles in A549 cells was similar to that of non-spray-dried microparticles, which demonstrated the feasibility of dry powder inhaler of DNA-loaded PNPs for inhalation therapy.
5.2.4. Poly (lactic-co-glycolic acid) (PLGA)
PLGA has been approved by the FDA. It has excellent biocompatibility, and its biodegradability can be adjusted by changing its composition, molecular weight and chemical structure, so it is widely used in the research of inhaled vectors [106]. PLGA NPs can achieve sustained release by controlling the degradation rate of PLGA [96]. Unlike the gene-loading mechanism of cationic polymers, genes are encapsulated by PLGA to form NPs, which can protect genes from degradation [96], and PLGA NPs have been shown to achieve endosomal escape [107]. Generally, PLGA NPs are prepared by the double emulsion solvent evaporation method. Specifically, gene-containing buffer is mixed with PLGA-containing organic solvent, followed by sonication, resulting in the formation of a water-in-oil (w/o) emulsion. Then an additive (e.g., Pluronic F-68 or polyvinyl alcohol (PVA)) is added to the primary emulsion, followed by sonication again, resulting in a water-in-oil-in-water (w/o/w) double emulsion. Finally, the organic solvent is evaporated to obtain NPs [[108], [109], [110]].
One key problem of DNA vaccines is the insufficient transfer of DNA to antigen-presenting cells (APCs), resulting in insufficient antigen expression [111], while PLGA NPs can be taken up by APCs such as macrophages and DCs [107], so PLGA can be used as a vector material for DNA vaccines. Additionally, the particle size of PLGA NPs affects their biodistribution and interaction with immune cells. Particles of 20–200 nm can effectively induce cellular immune responses by endocytosis or pinocytosis of DCs, while larger particles of 0.5–5 μm can elicit humoral immune responses mainly by phagocytosis or micropinocytosis [111]. Dalirfardouei et al. [111] prepared a NP-based DNA vaccine using PLGA (Mw = 50 kDa) for the treatment of infection caused by mycobacterium tuberculosis. The results indicated that the particle size of DNA-loaded NPs was 181 nm, encapsulation efficiency was 80%, and PLGA-DNA NPs produced higher concentrations of interferon-γ (IFN-γ) in BALB/c mice compared to naked DNA vaccines, demonstrating that PLGA-DNA NPs could stimulate strong T helper type 1 (Th1) immune response.
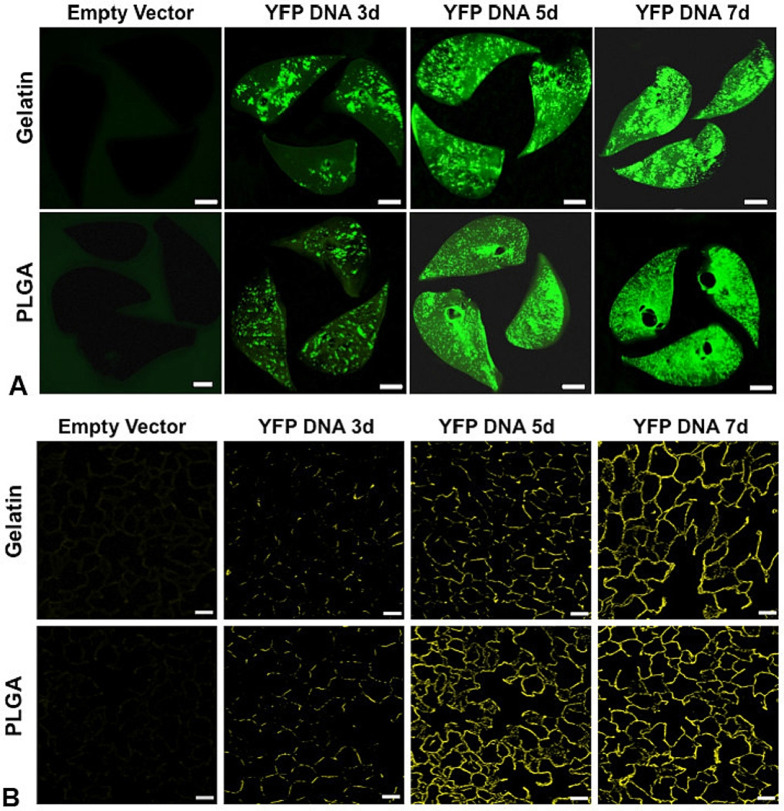
Menon et al. [112] screened six polymer materials (Gelatin, chitosan, sodium alginate, PLGA, PLGA-CS, and PLGA-PEG) of NPs for pulmonary delivery of proteins or DNA. The in vivo and in vitro studies of NPs and the physicochemical properties of NPs suggested that gelatin and PLGA NPs had the most prospect for inhalation delivery of proteins or DNA. The polydispersity index (PDI) of PLGA NPs (164 nm) was 0.14, which indicated that it had good particle size uniformity. PLGA NPs were stable for more than 5 days in deionized water, 10% fetal bovine serum (FBS), 0.9% normal saline, and simulated lung fluid (Gamble's solution) without significant aggregation or change in size, but their burst release reached 48% within 2 days. The viability of human ATI cells was greater than 90% at all concentrations of PLGA NPs, demonstrating the high biocompatibility of PLGA. Furthermore, they encapsulated pDNA encoding yellow fluorescent protein (YFP) in PLGA NPs and then delivered the suspension of pDNA-loaded PLGA NPs to the lung of rat via a pediatric mesh nebulizer (Aeroneb™), resulting in widespread and increasing fluorescence throughout lung tissue and the protein expression was increased in the lung within 7 days, and PLGA NPs had more persistent uniform protein expression compared to gelatin NPs (Fig. 3 ). This study is a good demonstration of the feasibility of delivering gene-loaded polymeric nanoparticles via inhalation for the transfection of cells.
Fig. 3.
A: Biofluorescence of rat lung slices fixed at 3, 5 and 7 d following nebulization of gelatin or PLGA based NPs loaded with YFP cDNA, compared to control lung following nebulization of the corresponding NPs loaded with empty vector (bar = 0.5 cm). The panels show increasing YFP expression up to 7 d following nebulization; expression was greater and more uniform using PLGA than gelatin NPs. B: Confocal fluorescence microscopy of histological sections taken from the corresponding lung shows increasing and widespread YFP expression up to 7 d post-inhalation compared to the respective controls (bar = 50 μm) [112]. Reproduced with the permission from Ref. 112. Copyright © 2014 Elsevier.
Although PLGA NPs have many advantages, they suffer from insufficient endosome escape and low cellular uptake. To solve the above problems, PLGA can be combined with other polymers (e.g., PEG, poly-l-lysine (PLL), polyethyleneimine, and chitosan) to form NPs. Compared with PLGA NPs, PEGylated PLGA NPs can increase the solubility, stability, and circulatory half-life, and reduce immunogenicity, aggregation, and recognition of NPs by the reticuloendothelial system (RES) [113].
5.2.5. Polyamidoamine (PAMAM) dendrimers
PAMAM dendrimers are highly branched, spheroidal, cascade polymers. The surface groups (Primary amines) of PAMAM dendrimers are protonated under physiological conditions, and their size and surface charge can be controlled by varying the number of ‘generations’ during the synthesis. For gene delivery, positively charged PAMAM can form NPs with negatively charged nucleic acids, and PAMAM dendrimers are also considered to have the proton sponge effect due to the secondary and tertiary amines in the branch. However, the high density of cationic groups on the surface of PAMAM dendrimers may lead to significant cytotoxicity and the clinical application of PAMAM dendrimers is limited due to their low degradability [85,114]. To reduce cytotoxicity, the positively charged PAMAM dendrimer-based NPs can be surface-modified with functional anionic polymers (e.g., hyaluronic acid) [99,115].
Conti et al. [85] prepared HFA-227-based pMDI of PAMAM G4NH2-siRNA complexes, and demonstrated that the complexes could transfect A549 cells with low to moderate gene silencing in vitro. To improve transfection efficiency and reduce cytotoxicity of PAMAM dendrimers, Bielski et al. [86] modified PAMAM G4NH2 with (3-carboxypropyl) triphenylphosphonium bromide (TPP), and delivered G4NH2/12TPP (12 TPP molecules on the surface)-siRNA NPs to the lung. The results showed that the increase of TPP density and N/P ratio increased gene silencing, and in vitro gene silencing was the highest (45 ± 5%) when the N/P ratio was 30. Additionally, the FPF of pMDI and DPI of G4NH2/12TPP-siRNA NPs were 50% and 39%, respectively, suggesting their preferable pulmonary deposition. Furthermore, Bohr et al. [116] prepared siRNA-loaded NPs using generation 3 PAMAM dendrimer for the treatment of acute lung inflammation. The results illustrated that the NPs achieved gene silencing in the mouse lung via pulmonary administration. As can be seen from the findings above, PAMAM dendrimers can be used for pulmonary gene delivery.
5.2.6. Poly (β-amino esters) (PBAEs)
PBAEs are cationic polymers, and the ester bonds in backbones can be hydrolyzed by intracellular esterases, thus significantly improving their biocompatibility and biodegradability. Moreover, their degradation rate is highly dependent on the hydrophilicity of polymers, and when the hydrophilicity is high, PBAEs chains swell and the ester bonds are easily affected by water, thus increasing degradation rate. Additionally, PBAEs can be easily synthesized by Michael addition reaction of diacrylate with primary or bis(secondary amine) [117]. However, positively charged PBAEs inevitably interact with biomacromolecules, leading to rapid clearance by RES and reduced transfection efficiency [118]. To solve this problem, Guo et al. [118] modified the surface of branched PBAEs with dopamine (DA)-grafted hyaluronic acid (HA, Mw = 7 kDa), and prepared HA/DA/PBAEs-DNA NPs via electrostatic attraction. The results indicated that the HA-DA/PBAEs/DNA weight ratio of 5:40:1 could ensure sufficient surface modification, and the surface charge became negative, which was beneficial to escape from the recognition and capture of RES, thereby prolonging circulatory half-life. They also found that the transfection efficiency of the NPs with a 43% DA grafting degree was superior to PEI NPs.
Patel et al. [119] synthesized hyperbranched poly (β-amino esters) (hPBAEs) for the preparation of stable and nontoxic inhalable NPs. Hyperbranching can improve physical properties associated with nebulization (e.g., particle size and zeta potential) without the changes of chemical composition. The results illustrated that luciferase mRNA was evenly distributed in all five lobes of the mouse lung, and the gene expression was the largest after inhalation using a vibrating mesh nebulizer for 24 h. After a single transfection using the Ai14 tdTomato reporter mice, 24.6% of lung epithelial cells were transfected. At a 72-h dosing interval, target proteins were detected in the lung with 3 doses, and there was no local or systemic toxicity with repeated dosing.
5.3. Peptides
5.3.1. Cell-penetrating peptides (CPPs)
CPPs consist of 4–40 amino acids and are able to enter the interior of cells via different mechanisms, including energy-independent (direct penetration) and endocytotic pathways [120]. More than 100 peptides with cell-penetrating abilities have been identified and the amino acid sequence, length, and polarity of different peptides are highly variable [121]. Cationic CPPs can form nanocomplexes with genes, but the transfection efficiency is lower when only using CPPs as vectors of genes, so they are commonly used for the surface modification of NPs to enhance cellular uptake. Moreover, CPPs suffer from poor targeting and may be taken up by almost all cells [120].
Gomes et al. [122] reported DNA-loaded NPs using CPPs (They derived from lactoferrin and composed by 22 amino acids) and mannitol, and the internalization efficiencies of CPPs-based NPs in A549 cells and Calu-3 cells (adenocarcinomic human bronchial epithelial cells) were 77.13 ± 9.1% and 44.5 ± 16.9%, respectively. However, CPPs-based NPs were not enough to facilitate pDNA transfection. Osman et al. [123] modified octa-arginine (R8), a CPP, with a peptide derived from fibroblast growth factor 2 (FGF2) and an amphipathic sequence (LK15), and then formed NPs with DNA via electrostatic interactions. After NIH3T3 cells were transfected in vitro, the transfection efficiency of unPEGylated NPs was similar to that of Lipofectamine 2000. Next, the surface of NPs was modified with PEG (Mw = 5 kDa), and 40% PEGylated NPs were uniformly distributed in the lung and showed colloidal stability in bronchoalveolar lavage fluid (BALF). Furthermore, after intratracheal administration, the in vivo gene expression of 40% PEGylated NPs was approximately 2 times that of PEI NPs and 6.5 times that of non-PEGylated NPs.
5.3.2. KL4 peptide
KL4 peptide (KLLLLKLLLLKLLLLKLLLLK−NH2) is a synthetic 21-residue cationic peptide containing repeating KLLLL sequences that can form complexes with negatively charged nucleic acids. It is a mimic of positively charged surfactant protein B (SP-B) in pulmonary surfactants [124]. Qiu et al. [124] reported that KL4-siRNA nanocomplexes could transfect A549 cells and Beas-2B cells in vitro with low risk of toxicity and inflammatory response for pulmonary administration. Moreover, Qiu et al. [125] prepared PEG12KL4 by attaching the KL4 peptide to a monodisperse linear PEG of 12-mers (PEG12, Mw = 600 Da). The dry powders of PEG12KL4-mRNA nanocomplexes were prepared by SD and spray-freeze-drying (SFD). Next, the 5 μg mRNA-50 μg PEG12KL4 nanocomplexes were delivered to the mouse lung via intratracheal administration, resulting in gene expression with high transfection efficiency and low toxicity, and the transfection efficiency was superior to lipofectamine 2000.
5.3.3. LAH peptides
LAH peptides (KKLAHALHLLALLWLHLAHALKKA-NH2) are cationic amphipathic histidine-rich peptides and can form complexes with genes via electrostatic interactions at physiological pH. During endosomal acidification, histidine residues are protonated, leading to an increase in their positive charges, which facilitates the release of peptides from gene-peptide complexes. Subsequently, the positively charged peptide interacts with the negatively charged lipid membranes, resulting in membrane destabilization and the release of genes, thereby enabling endosomal escape, and enhancing intracellular release efficiency and transfection efficiency [126]. Liang et al. [62,127] prepared pDNA or siRNA-loaded complexes using LAH or LADap (2–3-diaminopropionic acid (Dap)-rich peptides) as cationic amphipathic pH responsive peptides (Each peptide contains 4 or 6 pH responsive residues). They also prepared the dry powder inhaler of complexes using mannitol as protective agent.
5.3.4. GALA peptide
GALA peptide (WEAALAEALAEALAEHLAEALAEALEALAA) is an amphiphilic synthetic peptide consisting of 30 amino acids with a glutamic acid-alanine-leucine-alanine repeat. It has the advantages of good biocompatibility and high synthetic purity. Because glutamate (Glu) can provide a pH-dependent negatively charged side-chain, GALA cannot directly form complexes with negatively charged genes. At pH 5, GALA peptide adopts an amphipathic α-helical conformation in an acidic endosome environment, thereby forming a hydrophobic surface. The GALA peptide then binds to the hydrophobic region of lipid bilayers and interacts with the endosomal membranes (The exact mechanism is unknown). Next, the hydrophilic surface of the α-helix generates hydrophilic pores (0.5–1 nm) in the membranes. The pores promote endosomal destabilization, and damage the lipid membranes, thereby causing endosomal escape [128].
GALA peptide is usually used as a modification material on the surface of vectors. Kusumoto et al. [129] prepared liposomes using egg phosphatidylcholine (EPC) and cholesterol, and then surface-modified the liposomes with GALA peptide and stearyl-polyethylene glycol 2000 (STR-mPEG). They demonstrated that GALA peptides could target the sialic acid-terminated sugar chains on the pulmonary endothelium. To deliver siRNA, they added DOTMA and PEI to the prescription, and used CD31 as an endothelial cell-specific marker gene. Ultimately, lung-specific gene silencing in mice was observed following intravenous injection of GALA-modified liposomes.
6. Endosomal escape mechanism of non-viral vectors
Endosomal escape is one of the major bottlenecks in gene delivery. After cellular uptake, it determines the fate of non-viral vectors in cells and the release of genes. Although advances have been made in nanotechnology for gene delivery, the ability of non-viral vectors to induce endosomal escape remains low. Therefore, it is absolutely essential to understand the endosomal escape mechanism for the design of better novel delivery systems. The definitive endosome escape mechanism has not yet been discovered, but some widely accepted hypotheses have been proposed.
There are two models used to explain the endosomal escape of cationic lipid-based vectors. One is the fusion pore model (Fig. 5(A)): After fusion between the cationic lipid and the endosomal membranes via electrostatic interactions, genes are released into the cytoplasm. The other is the transient pore model (Fig. 5(B)): After electrostatic interactions between the cationic lipids and the endosomal membranes, transient pores are formed in endosomal membranes, and then genes are finally released [130]. For ionizable lipids, the interaction of protonated lipids with anionic lipids in acidic endosomes results in an inverted cone shape between the lipids, leading to the formation of hexagonal phases (HII). Ultimately, endosome escape is achieved by membrane fusion [8].
Fig. 5.
Endosomal escape mechanism of lipid and polymer-based nano-vectors. A: The fusion pore model for cationic lipid-mediated endosomal escape of therapeutic genes. B: The transient pore model for cationic lipid-mediated endosomal escape of therapeutic genes. C: Schematic diagram of the proton sponge effect. (1) During acidification, protons and chloride ions enter endosomes; (2) Water enters endosomes, causing the swelling of endosomes; (3) Endosomes rupture with the release of genes.
Polymeric vectors are generally considered to achieve endosomal escape by the proton sponge effect (Fig. 5(C)). Due to the high buffering capacity of polymers, when the pH of environment in which the polymer vectors are located keeps decreasing, the polymers can bind protons and limit the acidification of endosomes. At the same time, more protons are transported into the endosome. To maintain charge balance and osmotic pressure, chloride ions and water enter the endosomes, respectively. Due to the double functions of the increase of water and the internal charge repulsion of polymers, endosomes swell and eventually rupture with the releasing of genes [104,131]. However, the proton sponge effect has been highly controversial [131].
Peptide-based nanocarriers mainly lead to endosomal escape via the special properties of the peptide itself, such as GALA peptide (pH-sensitive peptide), which can generate hydrophilic pores and lead to the destruction of the endosomal membranes [128]. Another example is melittin, the endosomal membranes are destroyed due to its special ability of membrane fusion [132]. For hybrid nanocarriers, specific mechanisms are lacking, possibly because of synergistic effects of different materials.
Currently, it is difficult to compare the efficiency of endosomal escape in different studies because there is no standard quantitative method for direct measurement. Generally, endosomal escape is mainly measured indirectly by the expression of reporter proteins [63]. For DNA and mRNA, a greater fluorescence intensity of intracellular reporter proteins after transfection indicates that significant endosomal escape has occurred. In contrast, siRNA has a silencing effect on mRNA, so the lower amount of a specific protein that is stably expressed in cells, the more efficient escape is indicated. Genes and endosomes can be labelled with fluorescent dyes. After transfection, fluorescence is measured via confocal microscopy and the two fluorescent images are subsequently co-localized, indicating that endosome escape has occurred if genes are dispersed in the cytoplasm and not just in the endosomes. Furthermore, late endosomes are multivesicular and highly enriched in bis(monoacylglycerol)phosphate (BMP, also known as lysobisphosphatidic acid (LBPA)), with BMPs accounting for approximately 15–20 mol% of total phospholipid concentration. BMP-containing liposomes can be used to mimic late endosomes, and the endosome escape can be measured based on the leakage of calcein (Fluorescent indicator) from liposomes after contact with the material of vectors [133]. However, this approach is also qualitative and does not measure the efficiency of endosome escape [63].
7. Nanocarriers as non-viral vectors for gene delivery
To ensure efficient gene delivery, viral vectors (e.g., retrovirus, lentivirus, vaccinia virus, adenovirus, adeno-associated virus, cytomegalovirus, and bacteriophages) and non-viral vectors (e.g., endogenous, lipid-based, polymer-based, peptide-based, inorganic materials-based, and hybrid vectors) have been developed [4,134]. In pulmonary gene therapy, adenovirus (AdV) is the most widely used due to its high transfection efficiency. Currently, the FDA has approved adeno-associated virus (AAV)-based therapeutic genes, Luxturna and Zolgensma, for the treatment of congenital blindness and spinal muscular atrophy, respectively [135]. However, viral vectors have safety issues such as high immunogenicity, high risk of mutation, infectivity, and parasitism [4]. FDA-approved viral gene therapies require package insert warnings of the side effect of possible cytokine release storm, a potentially lethal overactive immune response [135]. Furthermore, clinical trials have shown that AdV and AAV can reduce the effectiveness of drugs after multiple doses due to the immune response, so they are not suitable for repeated dosing [136]. Therefore, the development of safe and efficient non-viral vectors is the key to solve the above problems. Compared with viral vectors, non-viral vectors have the advantages of low immunogenicity, high safety, low production costs, high drug loading, and effective combination with pulmonary epithelial cells, but the low transfection efficiency of non-viral vectors is a tremendous shortcoming preventing its application in the clinic [4,136]. Based on the sources, non-viral gene vectors are divided into endogenous vectors and synthetic vectors. Endogenous vectors mainly include exosomes and ferritin [4]. However, there are few reports on their use in pulmonary gene delivery, and their potential remains to be further explored. On the contrary, various synthetic nanocarriers (1–1000 nm) have been extensively investigated as gene-loaded vectors for pulmonary delivery, including liposomes, lipid nanoparticles, micelles, polymeric nanoparticles, inorganic nanoparticles, solid lipid nanoparticles, hybrid nanoparticles [137], and niosomes [138]. The non-viral vectors highlighted in this chapter are shown in Fig. 6. Moreover, cell membrane coating strategy has shown potential in the construction of biomimetic drug delivery systems, and red blood cell membranes, platelet membranes, natural killer (NK) cell membranes, and cancer cell membranes have been applied for biomimetic functionalization of nanoparticles [139].
Fig. 6.
Schematic illustration of different non-viral vectors for gene delivery.
7.1. Lipid-based nanocarriers
Lipid-based nanocarriers include liposomes, lipid nanoparticles, solid lipid nanoparticles, self-nano and microemulsifying drug delivery systems, nanoemulsions, and nanocapsules [140]. In gene delivery, lipid-based nanocarriers are the most widely reported alternative to viral vectors [90], especially lipid nanoparticles, which are currently the most successful non-viral gene vectors for clinical translation. This section describes vectors that are primarily used for pulmonary gene delivery, including liposomes, lipid nanoparticles, and solid lipid nanoparticles.
7.1.1. Liposomes
Liposomes were first reported in 1960s. The clinical application of liposomes has been very successful, and a range of products have been approved, such as antitumor nanodrugs: Doxil®, DaunoXome®, Myocet®, DepoCyt®, Marqibo®, and Onivyde® [141]. Liposomes are generally closed vesicles with the structure of phospholipid bilayer and are composed of amphiphilic lipids (neutral, cationic, or anionic lipids) and helper lipids (e.g., cholesterol) (Fig. 6(A)). They have excellent biodegradability and are highly biocompatible and safe, making them suitable for pulmonary delivery [142]. Inhaled liposomal antimicrobials have been widely reported for the treatment of pulmonary infections, which can reduce drug toxicity and improve tolerability [143]. In 2018, the FDA approved amikacin liposome inhalation suspension (ALIS, Arikayce™), and it is the first marketed nanoparticles-based inhalation product [69].
The most widely applied method for preparing liposomes is thin film hydration. Specifically, lipids are first dissolved in an organic solvent. After evaporation, a thin film of lipids is formed on the inner wall of the rotary evaporator flask, and then the film is hydrated with a water or buffer solution. Finally, the film can be peeled off to form liposomes via vigorous shaking or ultrasonication [144]. During drug loading, the addition of negatively charged genes to cationic liposome suspensions resulted in positively charged complexes, while the reverse order of addition resulted in negatively charged complexes [145]. Other conventional and common methods include reverse phase evaporation, solvent (Ethanol/ether) injection, and detergent dialysis. In addition, there are new methods for scale production, including microfluidic hydrodynamic focusing (MHF), supercritical fluid processing, spray-drying (SD), freeze-drying (FD), membrane contactor, and cross-flow injection technique [146].
Cationic liposomes (CLs) can interact with negatively charged genes, so they can be used as vectors of genes [147]. CLs have the advantages of efficient in vitro transfection, high loading capacity, structural flexibility, and easy large-scale production [148]. However, CLs have serious toxicity issues [149] and potentially adverse interactions with negatively charged macromolecules in serum and on cell surfaces [147]. Moreover, CLs are unstable in lung microenvironment, leading to increased alveolar macrophage uptake, thus making CLs unfavorable for pulmonary delivery [150]. Hybrid lipid formulation may enhance the toxicity of CLs, such as the combined use of DOTAP and cholesterol causes stronger non-specific cell killing effects than the DOTAP only nanocarrier. To reduce toxicity, the surface charge of CLs needs to be reduced, but such an approach will lead to a decrease in encapsulation efficiency. Liposomes can also be formulated with nonionic or anionic lipids, but encapsulation efficiency, transfection efficiency, and stability may be reduced by this approach [149].
Because of the high cytotoxicity of CLs and the successful application of LNPs, CLs are currently more often used as cationic controls, such as high-efficiency transfection reagents: Lipofectamine [151]. There are also liposomes prepared using novel materials for pulmonary gene delivery. For example, Li et al. [152] prepared liposomes using cationic 6-lauroxyhexyl lysinate (LHLN) and demonstrated that the CLs not only had lower cytotoxicity but also could transfect A549 cells (Human non-small cell lung cancer cells). Other applications of CLs in pulmonary delivery are summarized in Table 1 .
Table 1.
Representative lipid-based nanocarriers for the pulmonary delivery of DNA, mRNA, and siRNA.
| Nanocarriers | Composition | Size (nm) | Zeta potential (mV) | Mass median aerodynamic diameter (μm) | Encapsulation efficiency (w/w) (%) | Internalization efficiency/Transfection efficiency | Refs |
|---|---|---|---|---|---|---|---|
| pDNA | |||||||
| Liposomes | 6-lauroxyhexyl lysinate (LHLN) Dioleyl phosphatidylethanolamine (DOPE) |
226.3 | +20.5 | – | – | A549 and HepG2 cells were transfected in vitro. The transfection efficiency of CLs was similar to that of Lipofectamine 2000. After intratracheal administration to rats, the transfection efficiency of CLs was approximately 1.8 times that of Lipofectamine 2000 after 36 h and 2.3 times that of Lipofectamine 2000 after 48 h. | [152] |
| SLNs | Mannan L-α-phosphatidylethanolamine (PE) Soya lecithin (For injection) |
125.7 | +4.37 | – | – | RAW 264.7 cells (Mouse macrophages) were transfected. After 24 h, the transfection efficiency of SLNs was similar to that of Lipofectamine 2000. After 48 h, the transfection efficiency of SLN was higher than that of Lipofectamine 2000. After intratracheal administration to rats, the transfection efficiency of SLNs was greater than that of Lipofectamine 2000 (Neither quantified). | [179] |
| SLNs | Chitosan (100 kDa) Glyceryl dibehenate Glyceryl tristearate |
200 | +15 | 4.85–7.01 | – | Calu-3 cells and A549 cells were transfected in vitro. The transfection efficiency of NPs was similar to that of Lipofectamine 2000. | [173] |
| mRNA | |||||||
| LNPs | Dlin-MC3-DMA (ionizable lipids) DSPC Cholesterol DMG-PEG DSPE-PEG Fab-C4 (αPV1 antibody for targeting caveolin) |
70/160 | −4.42−−6.34 | – | 95.5% (70 nm) 84.8–91.2% (160 nm) |
When the particle size was 70 nm, the transfection efficiency of αPV1 LNPs in the lung was 24-fold higher than that of LNPs. Although the accumulation of αPV1 LNPs in the liver was lower than that in the lung, their transfection efficiency was 2-fold higher in the liver than in the lung. When the particle size was 160 nm, the transfection efficiency of αPV1 LNPs in the lung was 50-fold higher than that of LNPs. The transfection efficiency of αPV1 LNPs with a weight ratio (mRNA: total lipid) of 3 was 10-fold higher than that of αPV1 LNPs with a weight ratio of 10. |
[180] |
| siRNA | |||||||
| SLNs | Phosphatidylcholine Cholesterol Mannitol DOTAP PEG (2 kDa)-hydrazone-stearic acid (C18) derivative (PHC) |
164.5 | −34 | 3.96 | – | J774A.1 cells (Mouse mononuclear macrophages) were transfected in vitro, and the transfection efficiency of SLNs was 1/2 of that of Lipofectamine RNAiMAX. | [177] |
| Liposomes | Hyaluronic acid (1600 kDa) DOPE [2-(2-3didodecyloxypropyl) hydroxyethyl] ammonium bromide, (DE) |
233 | −42 | – | 96 | A549 cells were transfected in vitro, and the gene silencing was 81% when the charge ratio (+/−) of DE to siRNA was 2. After intravenous administration in female nude mice, the expression of PGL3-luc transcript in the lung was reduced by 54% compared with the saline control group. |
[181] |
| Liposomes | DOTMA DOPE E peptide (K16GACSERSMNFCG) Y peptide (K16GACYGLPHKFCG) |
192 | +23.6 | – | – | 1HAEo cells (Bronchial epithelial cells) were transfected in vitro, and the gene silencing of E peptide-based liposomes was 74.8%, and the gene silencing of Y peptide-based liposomes was 78.5% after nebulization. | [37] |
| Liposomes | GALA peptide PEG (2 kDa) Egg phosphatidylcholine Cholesterol |
145–169 | −6.9−−10.2 | – | 70–72 | When GALA peptide was 5 mol%, the internalization efficiency of GALA/PEG liposomes in HMVEC-L cells was about 70% after 1 h. After injection of GALA/PEG liposomes into C57BL/6 N mice, gene silencing in the lung was approximately 68–74% after 24 h. | [182] |
| Liposomes | DPPE-PEG (5 kDa) Hyaluronic acid (1.5–2.2 million Da) DOPE DOTAP |
200 | close to 0 | – | – | Under optimal hybrid formulation, the internalization efficiency of unmodified liposomes, HA liposomes, and HA/PEG liposomes in A549 cells after 2–3 h were approximately 97%. | [183] |
1,2-dimyristoyl-rac-glycero-3-methoxypolyethylene glycol (DMG-PEG); 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-polyethylene glycol (DSPE-PEG); N-(carbonyl-methoxypolyethylene glycol 5000)-1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine (DPPE-PEG 5000).
The nature of the cationic and helper lipids, the stoichiometry of cationic lipids and DNA, the method of preparation, and the nature of the medium used in preparation can all affect gene delivery by liposomes [147]. For inhalation, the deposition site of liposomes in the lung is affected by the composition, size, and charge [46]. During pulmonary administration, liposomal bilayers may be disrupted by nebulization, resulting in the fusion or fragmentation of vesicles, which further affects the stability of drugs and the encapsulation efficiency and size of liposomes [153]. If liposomes have multilamellar bilayer, the outer lipid layer protects the inner lipid layer from damage during the nebulization [154]. Additionally, liposomal dry powder inhaler (DPI) can be selected to avoid the disruption of lipid layer, thereby reducing leakage [155].
7.1.2. Lipid nanoparticles (LNPs)
LNPs are nanosized lipid systems made of two or more (generally four) lipids at varying ratios [156]. Currently, they are mainly composed of ionizable cationic lipids, neutral helper lipids, cholesterol, and PEGylated phospholipids (Fig. 6(B)), among which ionizable lipids are the most important constituents of LNPs, they can form LNPs with negatively charged genes [28,92]. They are quite different from conventional liposomes, because they have a phospholipid monolayer structure without a hydrophilic core, and their core structure depends on the saturation and charges of the ionizable lipids. LNPs are safer than cationic liposomes and are well tolerated for multiple dosing [92,157], making them suitable for pulmonary delivery. However, the large number of lipids and lipid-like materials makes the screening of formulation parameters extremely difficult [92,157]. In addition, because cell membranes are mainly composed of phospholipids, LNPs can interact well with cell membranes, which facilitates cellular uptake [149,158].
At present, the preparation method of LNPs is ethanol loading technique via microfluidics. Microfluidics is a bottom-up synthesis approach that is reproducible and scalable. Because it allows fine control of process parameters, it can be used for the optimization of product quality [159]. The parameters of microfluidics affect the physicochemical properties of LNPs. For example, an increase in flow rate ratio (FRR) or total flow rate (TFR) can reduce the particle size, but the particle size cannot be reduced continuously and eventually a minimum particle size occurs with an increase in TFR [160]. The preparation process is as follows: All lipid materials are dissolved in ethanol as the organic phase and genes are placed in aqueous buffer (pH 4) as the aqueous phase. The two phases are added to microfluidic device at an appropriate FRR (Usually aqueous phase: organic phase = 3:1) and an appropriate TFR, and then gene-loaded LNPs are spontaneously formed after rapid mixing in a microfluidic chip [156,161]. The resulting LNPs have high encapsulation efficiency (>90%), low polydispersity index, and reproducible physicochemical properties [156]. After the LNPs dispersion is formed, the purification of LNPs is required. There are two main methods for purification, one is dialysis: Residual ethanol is first removed with a pH 4 buffer and then the pH is raised to physiological values with a pH 7.4 buffer [161], and the other is tangential flow filtration (TFF) [162].
With the FDA approval of ONPATTRO® (patisiran), LNPs are increasingly used in gene therapy, protein replacement therapy, and mRNA vaccines [163]. LNPs-mRNA vaccines have been applied against COVID-19, and the successful application of Pfizer-BioNTech and Moderna mRNA vaccine has drawn extensive attention to LNPs by researchers. Suzuki et al. [164] summarized the differences between the three approved LNPs-based drugs. Although there are few reports on the delivery of LNPs by inhalation at present, it is undoubtedly a good attempt to use the already successful LNPs as inhalable vectors for gene delivery. Pardi et al. [165] delivered mRNA-loaded LNPs intratracheally and found high levels of protein production in the lung. Zhang et al. [158] optimized the formulation of LNPs using the Design of Experiments (DOE) strategy, and delivered mRNA-loaded LNPs to the lung of mice via a vibrating mesh nebulizer. Finally, they found that the protein expression was successfully produced. Likewise, Lokugamage et al. [166] optimized the formulation of LNPs for efficient mRNA delivery via nebulization and customized a nose-only exposure system to deliver LNPs to the lung of mice via nasal inhalation.
Formulation ingredients are factors that affect the gene delivery of LNPs. The endosomal escape of LNPs is mainly determined by ionizable lipids, and the gene expression of LNPs is closely related to the apparent acid-dissociation constant (pKa) of ionizable lipids [7], and the optimal pKa of ionizable lipids is ∼6.4 for siRNA delivery and 6.6–6.8 for mRNA delivery [160]. PEGylated phospholipids, cholesterol, and neutral helper lipids also affect the properties of LNPs. During the preparation of LNPs, an increase in the content of PEGylated lipids leads to a decrease in particle size [167], and in the absence of PEGylated lipids, the low pH and ethanolic environment during the preparation promote fusion between nanoparticles, resulting in the failure of the preparation [92]. PEGylated lipids are located on the surface of LNPs, and they prevent the proximity and fusion of positively charged LNPs with the endosomal bilayer, which affects endosomal escape, so their content is generally kept to a minimum [163], typically at ∼1.5 mol% [29,92]. Cholesterol fills the voids between phospholipids in lipid membranes [93] and modulates membrane integrity and rigidity, thereby enhancing the stability of LNPs [168]. Currently, LNPs for RNA delivery typically use phosphatidylcholines (PCs), the primary natural components of biological membranes, as neutral helper lipids, especially 1, 2-distearoyl-sn-glycero-3-phosphocholine (DSPC). DSPC has high melting temperatures (Tms), which improves the stability of LNPs [93,156]. The three previously mentioned LNPs-based products all contain 10 mol% DSPC [29]. Recently, LoPresti et al. [169] found that the replacement of neutral helper lipids to different charged lipids with different concentrations could alter the targeting of LNPs.
7.1.3. Solid lipid nanoparticles (SLNs)
SLNs are solid core lipid nanocarriers (Fig. 6(C)), which generally consist of biodegradable and safe lipidic components, emulsifiers/ stabilizers, and water [170]. As a colloidal drug delivery system, they are applied to deliver hydrophilic or lipophilic small-molecule drugs and various macromolecules [171]. SLNs were developed as alternative to liposomes and nanoemulsions in the 1990s [172]. Compared with liposomes, SLNs have better biocompatibility and stability in nebulization. Compared with polymeric nanoparticles, SLNs are easy to scale up for production [60]. The solid matrix of SLNs can protect drugs from chemical degradation and modulate the release properties of drugs that make them suitable for pulmonary delivery [173]. Furthermore, SLN can achieve controlled release in the lung, depending on lipid matrix, surfactant concentration, and production parameters [174]. However, the agglomeration or coagulation of SLNs may occur, resulting in the burst release of drugs [175]. Commonly used techniques for the preparation of SLNs include high-pressure homogenization (Hot or cold homogenization), high-shear homogenization, microemulsion-based technique, solvent emulsification/evaporation, double emulsion method, spray-drying (SD), and supercritical fluid technology [155,171].
Since the early 2000s, cationic SLNs have been applied to gene delivery [176]. For gene delivery, positively charged surfactants are used as emulsifiers/stabilizers that allow the positively charged SLNs to bind negatively charged genes [172]. Alternatively, genes can also be complexed with positively charged lipids [177]. Gene-loaded SLNs have been shown to transfect cells in vitro and in vivo with good biocompatibility and low cytotoxicity [178]. Currently, SLN-based dry powder inhaler has been widely reported. For pulmonary gene delivery, there are reports of using SLNs as non-viral vectors of pDNA and siRNA (Table 1). Wang et al. [177] prepared siRNA-loaded SLNs by solvent evaporation and demonstrated that thin-film freeze-drying (TFFD) is a promising method for the preparation of SLNs dry powder inhaler.
7.2. Polymer-based nanocarriers
Polymeric nanoparticles (PNPs) have a wide range of applications, such as drug delivery, imaging, therapy, and theranostic applications [184]. They are nanocarriers composed of natural or synthetic polymeric materials (Fig. 6(D)) and can be loaded with active compounds entrapped within or surface-adsorbed onto the polymeric core [185]. The bioadhesive materials can prolong the pulmonary retention time of vectors, resulting in controlled release [186], so PNPs are preferred as a controlled pulmonary delivery system [112]. The preparation methods of PNPs are top-down and bottom-up. Currently, top-down has no application in the preparation of gene-loaded PNPs. In contrast, bottom-up is the widely used method in gene delivery, which involve the coalescence or assembly of small molecules to form larger complexes [187]. Microfluidics can also be applied to the preparation of PNPs, which can overcome the limitations of scale-up production in traditional methods. Similar to the preparation of LNPs, the polymer-containing organic solution and aqueous solution are mixed in a microfluidic chip, and due to the low solubility of polymers in aqueous phase, the polymers precipitates at the aqueous/organic interface, leading to the formation of NPs [188]. For gene delivery, PNPs can also be formed by mixing negatively charged genes with positively charged polymers. However, poly (lactic-co-glycolic acid) (PLGA) is an exception. Genes are encapsulated by PLGA, which does not form NPs via electrostatic interactions [96].
Polymeric nanocarriers have the advantage that their physicochemical properties can be precisely tuned by changing molecular weight or chemical structure of the polymeric materials, thereby obtaining the desired carrier properties [186]. Although PNPs have been developed as gene vectors for many years, their clinical translation is not as good as lipid-based nanocarriers. One of the important reasons is the issue of biocompatibility. Many non-biodegradable polymers are not eliminated in a defined way from cells and tissues after administration, so there is a risk of toxicity due to the potential accumulation of substances during long-term administration. Furthermore, positively charged materials may disrupt cell membranes via electrostatic interactions, leading to apoptosis. To solve the above problems, biodegradable polymers can be selected, especially polymers that can be degraded into non-toxic natural metabolites in vivo, thereby enhancing biocompatibility [101]. At present, there are many studies of NPs based on natural polymers (e.g., chitosan (CS) and hyaluronic acid (HA)) and synthetic polymers (e.g., polyethyleneimine (PEI), PLGA, polyamidoamine (PAMAM) dendrimers, polyethylene glycol (PEG), and poly (β-amino esters) (PBAEs)) (Fig. 4 ) for the delivery of DNA, mRNA, and siRNA. The application of the above materials in pulmonary gene delivery is shown in Table 2 .
Table 2.
Representative polymer-based nanocarriers for the pulmonary delivery of DNA, mRNA, and siRNA.
| Nanocarriers | Composition | Size (nm) | Zeta potential (mV) | Mass median aerodynamic diameter (μm) | Encapsulation efficiency (w/w) (%) | Internalization efficiency/Transfection efficiency | Refs |
|---|---|---|---|---|---|---|---|
| pDNA | |||||||
| PNPs | PLGA (17 kDa) PEI (25 kDa) DSPE-PEG2000 |
190 | +16 | 3.83 | 99.90 | CFBE41o cells (Human cystic fibrosis bronchial epithelial cells) were transfected in vitro. The transfection efficiency of NPs was about 2/3 of that of Lipofectamine plus after 24 h, about 3/2 of that of Lipofectamine plus after 96 h, and about 5 times higher than that of Lipofectamine plus after 144 h. | [110] |
| PNPs | PEI (25 kDa) | 225 | – | 4.7 | – | BEAS-2B cells and 16HBE14o cells were transfected in vitro. Gene expression was obtained in the lung of BALB/c mice by nebulization, but no transfection agent was used as a positive control. | [192] |
| PNPs | Chitosan (31 kDa, 80% DDA) | 180 | +21 − +22 | 2.0–2.2 | > 90 | A549 cells were transfected in vitro. The transfection efficiency of NPs was higher than that of free pDNA, but lower than that of Transfast™ transfection reagent (Without quantification). | [83] |
| MPPs | mPEG-NHS (5 kDa) PEI (25 kDa) |
56 | +3 | – | – | The mean luciferase activity of PEI-MPPs in mice was 230 times higher than that of the saline control group. | [191] |
| MPPs | PBAEs PEG (5 kDa) |
50 | +2 | – | – | The transfection efficiency of MPPs in mice was approximately 33 times higher than that of PEI NPs. | [193] |
| Micelles | Diethylenetriamine (DET) PEG (12 kDa) β-Benzyl-l-aspartate N-carboxyanhydride (BLA-NCA) |
70–90 | +8 | – | – | After intratracheal administration to the lung of BALB/c mice for 48 h, the transfection efficiency of micelle was approximately 75-fold higher than that of PEI NPs. | [194,195] |
| PNPs | Spermine (SPE) (202.34 Da) PEG diacrylate (258 Da) |
130 | +18.61 | – | – | A549 cells were transfected in vitro. The transfection efficiency of PNP was approximately 10 times that of PEI NPs and 2.5 times that of Lipofectamine. After nebulized administration to BALB/c mice for 48 h, the transfection efficiency of PNP was greater than that of PEI NPs (Without quantification). | [196] |
| mRNA | |||||||
| PNPs | hPBAEs | 146 | +50 | – | – | NPs were delivered to Ai14 tdTomato reporter mice via nebulization, and 24.6% of total lung epithelial cells were transfected after a single dose. | [119] |
| PNPs | Poly(amine-co-ester) (PACE) PEG (5 kDa) |
160–360 | −10−−14 | – | – | After local intratracheal administration to BALB/c mice for 24 h, the transfection efficiency of PEG/PACE NPs was approximately 33-fold higher than that of non-PEGylated PACE NPs when the content of PACE-PEG was 5 wt%. | [197] |
| PNPs | 2-Pyridyldithiol-tetraoxaoctatriacontane-N-hydroxysuccinimide (PEG12-SPDP) PEI (22 kDa) |
46.0–82.2 | +2 − +20 | – | 90 | DC2.4 cells (mouse dendritic cells) were transfected in vitro. When the PEG terminal group was −S(CH2)2COOH, and the PEG grafting ratio was 0.5%, the in vitro transfection efficiency of PEI/PEG NPs was 628-fold and 214-fold higher than that of PEI NPs and Lipofectamine 3000 NPs, respectively. NPs was intravenously injected to BALB/c mice. When the PEG graft ratio was 0.5% and the PEG terminal groups were − S(CH2)2NH2 or − SCH2CHNH2COOH, the transfection efficiency of PEI/PEG NPs in the lung was 5.4-fold or 4.6-fold higher than that of PEI NPs after 24 h, respectively. The above two PEI/PEG NPs were intravenously injected to Ai14 Cre reporter mice, and about 7% of lung immune cells were transfected after 24 h. |
[198] |
| siRNA | |||||||
| PNPs | Generation 4 amine-terminated polyamidoamine dendrimer (PAMAM G4NH2) Chitosan (CS) (100–300 kDa) Lactide (LA) d-mannitol (98%) |
258 | +32 − +36 | 1.9 (Using CSLA) 2.6 (Using mannitol) |
97 | A549 cells were transfected in vitro, and the gene silencing of PAMAM NPs was 22%–37%, which was about 1/2 of that of Lipofectamine 2000. At an N/P ratio of 20, the gene silencing of PAMAM NPs was 22% after two months of storage in hydrofluoroalkane (HFA)-227. | [85] |
| PNPs | PAMAM G4NH2 Triphenylphosphonim (TPP) d-mannitol (98%) |
363 | +27 | 3.6/4.8 | – | A549 cells were transfected in vitro, and the transfection efficiency of PAMAM/12TPP NPs was 2/3 of that of Lipofectamine 2000. The gene silencing of PAMAM NPs was 49%. | [86] |
| PNPs | Chitosan (50 kDa) Cyanamide Salbutamol (β2-adrenoceptor agonist) |
100 | +15 | – | – | After intratracheal administration to the lung of EGFP transgenic mice for 24 h, the gene silencing of chitosan NPs was about 8%, the gene silencing of guanidinylated chitosan NPs was about 20%, and the gene silencing of guanidinylated chitosan NPs with salbutamol as a ligand was about 38%. | [73] |
Among PNPs, muco-inert particles (MIPs)/mucus-penetrating particles (MPPs) have shown potential in pulmonary delivery. MIPs/MPPs are surface-modified PNPs with a highly dense mucus-inert coating. They can achieve uniform distribution of drugs on the mucosal surface and prevent particles from being eliminated by mucociliary clearance, thereby prolonging the residence time of particles in the lung, and enhancing drug delivery [189]. MIPs with the above characteristics must satisfy two requirements: (1) The size is small enough to avoid clogging when passing through the dense network of mucin fibers; (2) The coating needs to be muco-inert enough to avoid binding between particles and mucus [190]. Generally, PEG is used as a mucus-inert coating, because neutral particles with no charge or high density of positive and negative charges have better permeability of mucus, while dense hydrophilic PEG coatings can make NPs close to electroneutrality. Meanwhile, hydrophilic NPs have better mucus-penetrating ability compared with hydrophobic NPs [61]. Currently, PS/PEG NPs, PLGA/PEG NPs, and PLGA/F127 NPs have been shown to reduce mucociliary clearance and increase drug retention time in the lung [189]. Chai et al. [189] prepared MIPs using methoxy-terminated PEG (PEG-NH2, Mw = 5 kDa) and carboxylate-modified polystyrene (PS), and then produced spray-dried micrometer-sized dry powders (∼2 μm) using mannitol, L-leucine, and poloxamer 188. They demonstrated that PEG-PS NPs could diffuse rapidly in human airway mucus and cystic fibrosis sputum. Suk et al. [191] prepared pDNA-loaded MIPs using methoxypolyethylene glycol-N-hydroxysuccinimide (mPEG-NHS, Mw = 5 kDa) as a muco-inert coating and PEI (Mw = 25 kDa) as a cationic vector material. The results demonstrated that MIPs uniformly distributed in the airway epithelium of the mouse lung at 1 h post-administration, and over 70% of MPPs initially deposited in the lung were retained in the airways at 6 h post-administration. MIPs successfully transfected airway epithelial cells, and their transfection efficiency did not decrease with repeated dosing at 2- and 4-week intervals.
7.3. Peptide-based nanocarriers
Peptide-based nanocarriers (Fig. 6(E)) have shown potential in gene delivery due to the advantages of versatility, ease of synthesis, biocompatibility, and low cytotoxicity. There are three types of peptide usage in gene delivery (Fig. 7). The first is that peptides can be used alone as materials of non-viral vectors, which encapsulate genes by covalent conjugation or noncovalent complexation. For covalent conjugation, a variety of methods are available, including thioether, thiol-maleimide, ester or disulphide bridge formation, and “click” chemistry. For noncovalent complexation, positively charged peptides and negatively charged genes can form nanocarriers via electrostatic interactions [199]. Because peptides with low molecular weight and low charge density are not suitable for use alone, or to endow vectors with special capabilities (e.g., promoting cellular uptake or endosome escape), peptides can also be used for the surface modification of non-viral vectors such as liposomes or cationic PNPs [38]. Moreover, peptides have the advantage of variability in primary structure and secondary structure (α-helices, β-sheets, and β-turns), so they can serve as building blocks for self-assembly. For instance, peptide building blocks can be attached to polymers to enable self-assembly of nanocarriers [[200], [201], [202]]. Nano-assemblies may respond to different external stimuli, such as temperature, pH, redox state, enzymes, or light, and peptides with specific responses have advantages in gene delivery, such as pH-responsive peptides can facilitate endosomal escape. However, peptide-based nanocarriers are sensitive to proteases, which can lead to off-target gene release following proteolysis [202]. Moreover, they lack sufficient information on biodistribution, clearance, immunocompatibility, and specific organ toxicity, as well as data on human clinical trials [199]. Currently, peptides being applied in pulmonary gene delivery include cell-penetrating peptides, KL4 peptide, LAH peptides, and GALA peptide, details of which are discussed in Part 5. However, peptide-based nanocarriers have not received as much attention as lipid-based nanocarriers and polymer-based nanocarriers.
Fig. 7.
Schematic illustration of three types of peptide usage in gene delivery.
7.4. Hybrid nanocarriers (HNCs)
HNCs are novel nano-vectors prepared by mixing two or more materials, including lipids, polymers, and peptides, which exhibit complementary properties of different materials (Fig. 6(F)). Therefore, they can produce synergistic effects, such as improving biocompatibility, vector-gene affinity, encapsulation efficiency, cellular internalization efficiency, stability, and blood circulation time. They can also reduce immunogenicity and alter the biodistribution of vectors. However, HNCs have difficulty in screening and combining different materials. The understanding of the mechanism of HNCs is still in its infancy [148,203]. At present, more and more attention has been paid to HNCs, and many novel vectors for pulmonary gene delivery have been reported, including lipid-polymer nanoparticles, polymer-peptide nanoparticles, and lipid-peptide nanoparticles. Their research progress is summarized in Table 3 .
Table 3.
Representatives of hybrid nanocarriers for the pulmonary delivery of DNA, mRNA, and siRNA.
| Nanocarriers | Composition | Size (nm) | Zeta potential (mV) | Mass median aerodynamic diameter (μm) | Encapsulation efficiency (w/w) (%) | Internalization efficiency/Transfection efficiency | Refs |
|---|---|---|---|---|---|---|---|
| pDNA | |||||||
| Polymer-peptide nanoparticles | Chitosan (126 kDa/mol) Fibronectin attachment protein of mycobacterium bovis (FAP-B) |
250 | −1 | – | – | After administration of FAP-B/CS-DNA NPs to BALB/c mice using an air-jet nebulizer, the transfection efficiency in the mouse lung was increased 16-fold compared with unmodified Chitosan NPs. | [211] |
| Polymer-peptide nanoparticles | PLGA (7–17 kDa) CPPs (They derived from lactoferrin and composed by 22 amino acids) |
167.9 | −0.13 | – | 96.7 | The internalization efficiencies of NPs were 83.85 ± 1.2% in Beas-2B cells and 96.76 ± 1.7% in A549 cells. | [207] |
| Lipid-polymer nanoparticles | Triolein PEI (1.2 kDa) DOPE |
228.1 | +11.92 | – | – | SPC-A1 cells (Human lung adenocarcinoma cells) were transfected in vitro. When N/P (The ratio of nitrogen in PEI to phosphate in DNA) was 10, the transfection efficiency of NPs was twice that of Lipofectamine 2000. | [212] |
| Polymer-peptide nanoparticles | DSPE-PEG2000 PBAEs PLGA CPP (mTAT/bPrPp/MPG) |
< 200 | +9.1 − +30.2 | – | – | CFBE41o cells were transfected in vitro. After 3 h, the transfection efficiency of Lipofectamine 2000 was approximately 4.5 times that of PBAEs/PLGA NPs. After 7 h, the transfection efficiency of bPrPp- or MPG-modified NPs was similar to that of Lipofectamine 2000. However, the transfection efficiency of mTAT-modified NPs was lower than that of PBAEs/PLGA NPs and Lipofectamine 2000. | [109] |
| Lipid-polymer nanoparticles | DOTAP DOPE Protamine sulfate salt Hyaluronic acid sodium salt |
207.8 | −13.1 | – | – | In A549 cells, the cellular uptake of HA-modified liposome-protamine-DNA nano-complex (LPD) was approximately 1.2 times that of LPD, and the gene silencing of HA-modified LPD was 2.5 times that of LPD. | [100] |
| mRNA | |||||||
| Polymer-peptide nanoparticles | KL4 peptide PEG12 (600 Da) |
432 (SD) 375 (SFD) |
+27.58 (SD) +30.58 (SFD) |
4.45/5.54 (SD) 1.53/2.13 (SFD) |
– | In vitro transfection of A549 cells, the transfection efficiency of HNPs was similar to that of Lipofectamine 2000. After intratracheal administration to the lung of BALB/c mice for 24 h, the transfection efficiency of PEG12KL4 NPs was 12-fold higher than that of Lipofectamine 2000 at a ratio of PEG12KL4 to mRNA of 10:1 (w/w). | [125] |
| siRNA | |||||||
| Lipid-polymer nanoparticles | PLGA (20 kDa) DOTAP (15 w/w) |
216 | +33.1 | 3.69 | – | H1299/EGFP cells (Human non-small cell lung carcinoma cell line stably expressing the reporter gene EGFP) were transfected in vitro. The gene silencing of HNPs was about 1/6 of that of Lipofectamine 2000. | [213] |
| Lipid-polymer nanoparticles | PLGA (7–17 kDa) DPPC |
141 | −29.1 | 3.96 | 74.8 | In vitro transfection of A549 cells resulted in gene silencing, and DPPC/PLGA NPs reduced the protein expression of aENaC and bENaC by 60% and 40%, respectively. | [204] |
| Polymer-peptide nanoparticles | Melittin (It inserts into lipid membranes and induces pores) p(OEGMA-DMAEMA) (Hydrophilic cation polymer) p(DIPAMA-PDSEMA) (pH-sensitive polymer that changes from hydrophobic to hydrophilic under acidic conditions) |
62 | +40.5 | – | – | When the N/P ratio was 8, the internalization efficiency of virus-inspired polymer for endosomal release (VIPER) in T lymphocytes was twice that of Lipofectamine 2000. H1299/EGFP cells were transfected in vitro. When the N/P ratio was 8 and 10, the gene silencing of VIPER was 4 times that of Lipofectamine 2000. When the N/P ratio was 8, the gene silencing was 76% after intratracheal administration to the lung of BALB/c mice for 24 h, which was approximately twice that of PEI. | [132,214] |
| Lipid-polymer nanoparticles | Lipidoid 5 (L5) (It is a novel cationic lipid consisting of an alkylated tetraamine backbone) PLGA (20 kDa) Cholesterol DOTAP N-palmitoyl-sphingosine-1-succinyl(methoxypolyethylene glycol 2000) (C16-PEG2000 ceramide) |
60–100 | 5–10 | – | 80–90 | The internalization efficiency of L5/C16-PEG2000/DOTAP/PLGA NPs in THP-1 cells (human macrophages) was approximately 4 times that of L5/DOTAP/PLGA NPs and 6 times that of DOTAP/PLGA NPs. However, the internalization efficiency of DOTAP/PLGA NPs in hAELVi cells (human ATI cells) was approximately 8.5 times higher than that of L5/C16-PEG2000/PLGA NPs. | [215] |
7.4.1. Lipid-polymer nanoparticles
Lipid-polymer nanoparticles are a hybrid delivery system in which a polymer core is encapsulated in a lipid layer [81]. They combine the good biocompatibility and low immunogenicity of lipids with the high affinity of polymers for RNA [148], so they can solve the problems of poor structural stability of lipids and poor biocompatibility of polymers [137]. The polymer core is used for drug loading and the lipid shell helps to impart special surface properties of vectors. For instance, using endogenous phospholipids can not only effectively increase the compatibility of nanoparticles with the lung [204], but also affect the in vivo fate of nanoparticles [205]. Liu et al. [205] modified PLGA NPs with biomimetic endogenous pulmonary surfactant phospholipids, and the results showed that dipalmitoylphosphatidylcholine (DPPC), dipalmitoylphosphatidylamine (DPPE), and dipalmitoylphosphatidylglycerol (DPPG) could improve the mucoadhesive ability, while dipalmitoylphosphatidylserine (DPPS) could improve the mucus permeability of PLGA NPs.
The methods of lipid-polymer nanoparticles preparation include the single-step method and the two-step method. In the single-step method, when polymer-containing organic solutions are added to lipid-containing aqueous solutions, the polymer precipitates, and then lipids can self-assemble on the polymer core to form a monolayer. In the two-step method, the prepared gene-loaded PNPs are directly added to dried lipid films or prepared lipid vesicles, and then HNCs can be formed by vortexing and/or ultrasonication at a temperature above the phase transition temperature of lipids [206].
Gabriella et al. [204] demonstrated that DPPC/PLGA-siRNA hybrid nanoparticles could transfect A549 cells in vitro and produce long-term inhibition of epithelial sodium channel (ENaC) protein expression. Moreover, hybrid nanoparticles did not produce cytotoxic or inflammatory responses to airway epithelial cells.
7.4.2. Polymer-peptide nanoparticles
Polymer-peptide nanoparticles are mostly surface-modified PNPs with peptides. The main purpose of polymer-peptide conjugation is to enhance cellular uptake [148]. Gomes et al. [207] prepared pDNA-loaded nanoparticles using PLGA and cell-penetrating peptides (CPPs), and proved that PLGA nanoparticles could effectively encapsulate pDNA and protect genes. The cellular internalization efficiencies of nanoparticles in Beas-2B cells (Human bronchial epithelial cells) and A549 cells were 83.85 ± 1.2% and 96.76 ± 1.7%, respectively. These data suggest the ability of CPPs to increase cellular internalization efficiency.
7.4.3. Lipid-peptide nanoparticles
Lipid-peptide nanoparticles are lipid vectors that use peptides for surface modification [208] or incorporating peptides into lipid bilayers to impart the specific response mechanisms of vectors [209]. Hagino et al. [208] studied bilayer LNPs with inner layer consisting of CPP (Stearyl-octa-arginine, STR-RRRRRRRR (R8)) or DOPE, and outer layer was composed of YSK05 (It's a pH-sensitive lipid obtained by replacing quaternary ammonium groups in DOTAP with tertiary amine groups, and YSK05-modified nanocarriers exhibited high membrane fusion at pH = 5.5 [210]) or DOTMA. Furthermore, the bilayer LNPs was surface-modified with DMG-PEG and cholesteryl-GALA (See below for details on GALA peptides). The results indicated that YSK05, R8, and GALA enhanced the endosomal escape of nanoparticles in vitro and in vivo, and GALA peptide promoted the lung targeting of vectors. Moreover, the bilayer LNPs showed high gene expression (>107 RLU/mg protein) in human lung microvascular endothelial cells (HMVEC-L). When the amount of GALA was 2 mol% of total lipids and 1280 nmol of lipid, GALA showed the best lung targeting, and their gene expression in the lung was higher than that in the liver and spleen (∼80-fold and 12-fold, respectively).
8. Expert opinion and perspectives
With the rapid development of biotechnology, the emergence of gene therapy provides hope for the treatment or even cure of many genetic diseases, and with the outbreak of COVID-19, LNPs- based mRNA vaccines have become a hot research topic, which undoubtedly increases the focus on non-viral vectors. In addition to the appropriate vectors, the route of administration is also crucial to ensure successful transfection of target cells. Pulmonary delivery is more targeted in the treatment of lung diseases, including asthma, CF, COPD, lung infection, and lung cancer, and leads to effective topical gene therapy with reduced side effects. For systemic effects, inhaled mRNA vaccines have shown unique advantages in inducing mucosal immunity. Therefore, pulmonary delivery of non-viral vectors for gene therapy holds great promise.
Based on the current research progress, non-viral vectors have solved the problems of gene instability and drug loading. For lipid-based nanocarriers, ionizable lipids avoid the toxicity of cationic lipids while addressing the challenge of low transfection efficiency. Although LNPs have been a great success in gene delivery, they have difficulties in extrahepatic targeting, and pulmonary delivery of LNPs is certainly one of the good options to solve this problem. However, even the most advanced drug delivery systems in the clinic currently, LNPs, still have the bottleneck of low efficiency of endosome escape. For polymer-based nanocarriers, degradable natural polymeric materials have greatly improved safety, but there are still issues of endosomal escape and low transfection efficiency, and the real mechanism of endosomal escape is still unclear. Although there are many studies on pulmonary delivery of PNPs, there is still no marketed product. Surface modification of non-viral vectors with peptide materials increases endosomal escape and even enables vectors to acquire the ability of active targeting, but there is a problem that clinical translation is difficult due to the complicated modification. In summary, to achieve the transformation of gene-loaded nanoparticles from basic research to clinical application, the design and construction of vectors should be simple and the materials of vectors must have high biosafety with clear enhanced endosome escape ability. To solve many difficulties and achieve breakthroughs, it cannot be limited to the modification and amelioration of existing vectors using a series of materials, while novel materials or drug delivery systems are urgently required.
Facing the challenge of pulmonary delivery, the suitable aerodynamic diameter (1–5 μm) enables successful deposition of vectors in the respiratory region. The appropriate particle size (Less than 200 nm) can reduce or even avoid macrophage phagocytosis and penetrate mucus preferably [216]. The near-neutral surface charge and hydrophilicity enable vectors to acquire the ability to penetrate mucus. Furthermore, state of lung diseases can affect the effectiveness of pulmonary delivery, and vectors can be designed according to the pathological characteristics of the lung. For example, in obstructive pulmonary diseases such as CF, COPD, and asthma, the airway mucus of patients becomes thick, which is not conducive to the cellular internalization of nanocarriers, and the strategy of mucus-penetrating particles may solve this problem [217]. After the appropriate design of nanocarriers, the preparation technology is equally important. The successful application of microfluidics solves the problems of the encapsulation efficiency, uniform particle size, reproducibility, and scale-up production of non-viral vectors.
Moreover, it should be noted that the stability of nanocarrier is not only affected by aerosolization or drying, but also by airway mucins and pulmonary surfactants after inhalation [12]. For example, lung surfactant protein SP-A (630 kDa) may better bind lipophilic inhalable vectors, while SP-D (520 kDa) may interact with hydrophilic inhalable vectors and subsequently trigger macrophage phagocytosis [61,218]. Therefore, the stability of vectors in a simulated lung environment should not be ignored. Additionally, to improve the safety of vectors, the interaction between nanocarriers and pulmonary surfactants should be reduced to avoid breaking the original component balance of the lung. Hydrophilic modification and bio-inspired surface coatings of NPs can be used to reduce this interaction [219]. It should be noted that there is a positive side of pulmonary surfactants in pulmonary gene delivery, and it has been reported that pulmonary surfactant-modified nanoparticles effectively increase transfection efficiency to lung cells compared to unmodified nanoparticles [220,221]. Due to the complexity of the lung microenvironment, there may be discrepancies between the results of in vitro and in vivo studies. Therefore, the selection of in vitro models is crucial. To narrow the differences, human air-liquid-interface (ALI) organotypic airway tissue models can be chosen, which more closely resemble native epithelial tissues than traditional submerged cultures [222]. Three-dimensional (3D) human lung cell-based culture models can also be considered, which can overcome the limitations of traditional two-dimensional (2D) culture models [223]. In addition, PEI and Lipofectamine have high transfection efficiency, but they have low safety due to toxicity issues, and are widely used as reference in experiments. Therefore, during the research, even if the vector shows excellent transfection ability, even higher than that of the reference, cytotoxicity must be considered, which is often overlooked. For in vivo experiments, a perfect fit between the non-viral vector and the inhalation device needs to be considered. Nanocarriers are an intermediate, so appropriate dosage forms need to be selected. Numerous high-performance nebulizers have emerged that can enhance the efficacy of inhalation therapy. Dry powder inhaler solves the damage to vectors and genes caused by high shear force during nebulization, but it has high technical barriers. All in all, progressive nebulizers and dry powder inhaler seem to be excellent choices for pulmonary gene delivery, both of which can not only deliver nanoparticles to the respiratory region with an appropriate aerodynamic diameter, but also maintain a high physical stability of vectors. Finally, storage stability can be considered from two aspects: Modification of genes and optimization of vector formulation. In summary, with all the above mentioned challenged addressed, nanocarriers as non-viral vector may play a pivotal role in enhancing the pulmonary delivery of therapeutic genes, for both local and systemic diseases therapy.
Acknowledgement
This work is financially supported by the Key Research Funding of Education Department of Liaoning Province (2022) and the National Key R&D Program of China (No. 2020YFE0201700).
Data availability
No data was used for the research described in the article.
References
- 1.Kesik-Brodacka M. Progress in biopharmaceutical development. Biotechnol. Appl. Biochem. 2018;65:306–322. doi: 10.1002/bab.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhao Z.M., Anselmo A.C., Mitragotri S. Viral vector-based gene therapies in the clinic. Bioeng Transl. Med. 2022;7 doi: 10.1002/btm2.10258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sainz-Ramos M., Gallego I., Villate-Beitia I., Zarate J., Maldonado I., Puras G., Pedraz J.L. How far are non-viral vectors to come of age and reach clinical translation in gene therapy? Int. J. Mol. Sci. 2021;22 doi: 10.3390/ijms22147545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pan X., Veroniaina H., Su N., Sha K., Jiang F., Wu Z., Qi X. Applications and developments of gene therapy drug delivery systems for genetic diseases. Asian J. Pharm. Sci. 2021;16:687–703. doi: 10.1016/j.ajps.2021.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chung Y.H., Beiss V., Fiering S.N., Steinmetz N.F. COVID-19 vaccine frontrunners and their nanotechnology design. ACS Nano. 2020;14:12522–12537. doi: 10.1021/acsnano.0c07197. [DOI] [PubMed] [Google Scholar]
- 6.Meo S.A., Bukhari I.A., Akram J., Meo A.S., Klonoff D.C. COVID-19 vaccines: comparison of biological, pharmacological characteristics and adverse effects of Pfizer/BioNTech and Moderna vaccines. Eur. Rev. Med. Pharmacol. Sci. 2021;25:1663–1669. doi: 10.26355/eurrev_202102_24877. [DOI] [PubMed] [Google Scholar]
- 7.Yonezawa S., Koide H., Asai T. Recent advances in siRNA delivery mediated by lipid-based nanoparticles. Adv. Drug Deliv. Rev. 2020;154-155:64–78. doi: 10.1016/j.addr.2020.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schlich M., Palomba R., Costabile G., Mizrahy S., Pannuzzo M., Peer D., Decuzzi P. Cytosolic delivery of nucleic acids: the case of ionizable lipid nanoparticles. Bioeng Transl. Med. 2021;6 doi: 10.1002/btm2.10213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adamkiewicz G., Liddie J., Gaffin J.M. The respiratory risks of ambient/outdoor air pollution. Clin. Chest Med. 2020;41:809–824. doi: 10.1016/j.ccm.2020.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dawod Y.T., Cook N.E., Graham W.B., Madhani-Lovely F., Thao C. Smoking-associated interstitial lung disease: update and review. Expert Rev. Respir. Med. 2020;14:825–834. doi: 10.1080/17476348.2020.1766971. [DOI] [PubMed] [Google Scholar]
- 11.Chen J., Tang Y., Liu Y., Dou Y. Nucleic acid-based therapeutics for pulmonary diseases. AAPS PharmSciTech. 2018;19:3670–3680. doi: 10.1208/s12249-018-1183-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baldassi D., Ambike S., Feuerherd M., Cheng C.-C., Peeler D.J., Feldmann D.P., Porras-Gonzalez D.L., Wei X., Keller L.-A., Kneidinger N., Stoleriu M.G., Popp A., Burgstaller G., Pun S.H., Michler T., Merkel O.M. Inhibition of SARS-CoV-2 replication in the lung with siRNA/VIPER polyplexes. J. Control. Release. 2022;345:661–674. doi: 10.1016/j.jconrel.2022.03.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shen A.M., Minko T. Pharmacokinetics of inhaled nanotherapeutics for pulmonary delivery. J. Control. Release. 2020;326:222–244. doi: 10.1016/j.jconrel.2020.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marasini N., Kaminskas L.M. Subunit-based mucosal vaccine delivery systems for pulmonary delivery - are they feasible? Drug Dev. Ind. Pharm. 2019;45:882–894. doi: 10.1080/03639045.2019.1583758. [DOI] [PubMed] [Google Scholar]
- 15.Graceffa V. Physical and mechanical cues affecting biomaterial-mediated plasmid DNA delivery: insights into non-viral delivery systems. J. Genet. Eng. Biotechnol. 2021;19:90. doi: 10.1186/s43141-021-00194-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu B., Zhong L.P., Weng Y.H., Peng L., Huang Y.Y., Zhao Y.X., Liang X.J. Therapeutic siRNA: state of the art. Signal Transduct. Target Ther. 2020;5 doi: 10.1038/s41392-020-0207-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dong Y.Z., Siegwart D.J., Anderson D.G. Strategies, design, and chemistry in siRNA delivery systems. Adv. Drug Deliv. Rev. 2019;144:133–147. doi: 10.1016/j.addr.2019.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mohammadinejad R., Dehshahri A., Madamsetty V.S., Zahmatkeshan M., Tavakol S., Makvandi P., Khorsandi D., Pardakhty A., Ashrafizadeh M., Afshar E.G., Zarrabi A. In vivo gene delivery mediated by non-viral vectors for cancer therapy. J. Control. Release. 2020;325:249–275. doi: 10.1016/j.jconrel.2020.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ruigrok M.J.R., Frijlink H.W., Hinrichs W.L.J. Pulmonary administration of small interfering RNA: the route to go? J. Control. Release. 2016;235:14–23. doi: 10.1016/j.jconrel.2016.05.054. [DOI] [PubMed] [Google Scholar]
- 20.Tang J., Cai L.R.Y., Xu C.F., Sun S., Liu Y.H., Rosenecker J., Guan S. Nanotechnologies in delivery of DNA and mRNA vaccines to the nasal and pulmonary mucosa. Nanomaterials. 2022;12 doi: 10.3390/nano12020226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu D., Hickey A.J. Pulmonary vaccine delivery. Expert Rev. Vaccines. 2007;6:213–226. doi: 10.1586/14760584.6.2.213. [DOI] [PubMed] [Google Scholar]
- 22.Silveira M.M., Moreira G.M. Schmidt Garcia, Mendonca M. DNA vaccines against COVID-19: perspectives and challenges. Life Sci. 2021;267 doi: 10.1016/j.lfs.2020.118919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ibraheem D., Elaissari A., Fessi H. Gene therapy and DNA delivery systems. Int. J. Pharm. 2014;459:70–83. doi: 10.1016/j.ijpharm.2013.11.041. [DOI] [PubMed] [Google Scholar]
- 24.Zhang C.L., Maruggi G., Shan H., Li J.W. Advances in mRNA vaccines for infectious diseases. Front. Immunol. 2019;10 doi: 10.3389/fimmu.2019.00594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kowalski P.S., Rudra A., Miao L., Anderson D.G. Delivering the messenger: advances in technologies for therapeutic mRNA delivery. Mol. Ther. 2019;27:710–728. doi: 10.1016/j.ymthe.2019.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guan S., Rosenecker J. Nanotechnologies in delivery of mRNA therapeutics using nonviral vector-based delivery systems. Gene Ther. 2017;24:133–143. doi: 10.1038/gt.2017.5. [DOI] [PubMed] [Google Scholar]
- 27.Nitika J., Wei A.M. Hui. The development of mRNA vaccines for infectious diseases: recent updates. Infect Drug Resist. 2021;14:5271–5285. doi: 10.2147/IDR.S341694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li M., Li Y., Li S., Jia L., Wang H., Li M., Deng J., Zhu A., Ma L., Li W., Yu P., Zhu T. The nano delivery systems and applications of mRNA. Eur. J. Med. Chem. 2021;227 doi: 10.1016/j.ejmech.2021.113910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schoenmaker L., Witzigmann D., Kulkarni J.A., Verbeke R., Kersten G., Jiskoot W., Crommelin D.J.A. mRNA-lipid nanoparticle COVID-19 vaccines: structure and stability. Int. J. Pharm. 2021;601 doi: 10.1016/j.ijpharm.2021.120586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ozcan G., Ozpolat B., Coleman R.L., Sood A.K., Lopez-Berestein G. Preclinical and clinical development of siRNA-based therapeutics. Adv. Drug Deliv. Rev. 2015;87:108–119. doi: 10.1016/j.addr.2015.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Youngren-Ortiz S.R., Gandhi N.S., España-Serrano L., Chougule M.B. Aerosol delivery of siRNA to the lungs. Part 1: rationale for gene delivery systems. Kona. 2016;33:63–85. doi: 10.14356/kona.2016014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Labiris N.R., Dolovich M.B. Pulmonary drug delivery. Part I: physiological factors affecting therapeutic effectiveness of aerosolized medications. Br. J. Clin. Pharmacol. 2003;56:588–599. doi: 10.1046/j.1365-2125.2003.01892.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ibrahim M., Garcia-Contreras L. Mechanisms of absorption and elimination of drugs administered by inhalation. Ther. Deliv. 2013;4:1027–1045. doi: 10.4155/tde.13.67. [DOI] [PubMed] [Google Scholar]
- 34.Katz M.G., Fargnoli A.S., Gubara S.M., Fish K., Weber T., Bridges C.R., Hajjar R.J., Ishikawa K. Targeted gene delivery through the respiratory system: rationale for intratracheal gene transfer. J. Cardiovasc. Dev. Dis. 2019;6 doi: 10.3390/jcdd6010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim N., Duncan G.A., Hanes J., Suk J.S. Barriers to inhaled gene therapy of obstructive lung diseases: a review. J. Control. Release. 2016;240:465–488. doi: 10.1016/j.jconrel.2016.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sanchez A.D., Paunovska K., Cristian A., Dahlman J.E. Treating cystic fibrosis with mRNA and CRISPR. Hum. Gene Ther. 2020;31:940–955. doi: 10.1089/hum.2020.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Manunta M.D.I., Tagalakis A.D., Attwood M., Aldossary A.M., Barnes J.L., Munye M.M., Weng A., McAnulty R.J., Hart S.L. Delivery of ENaC siRNA to epithelial cells mediated by a targeted nanocomplex: a therapeutic strategy for cystic fibrosis. Sci. Rep. 2017;7:700. doi: 10.1038/s41598-017-00662-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee A.Y., Cho M.-H., Kim S. Recent advances in aerosol gene delivery systems using non-viral vectors for lung cancer therapy. Expert Opin. Drug Deliv. 2019;16:757–772. doi: 10.1080/17425247.2019.1641083. [DOI] [PubMed] [Google Scholar]
- 39.Suster D.I., Mino-Kenudson M. Molecular pathology of primary non-small cell lung cancer. Arch. Med. Res. 2020;51:784–798. doi: 10.1016/j.arcmed.2020.08.004. [DOI] [PubMed] [Google Scholar]
- 40.Kumar V., Yadavilli S., Kannan R. A review on RNAi therapy for NSCLC: opportunities and challenges. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2021;13 doi: 10.1002/wnan.1677. [DOI] [PubMed] [Google Scholar]
- 41.Liang X., Li D., Leng S., Zhu X. RNA-based pharmacotherapy for tumors: from bench to clinic and back. Biomed. Pharmacother. 2020;125 doi: 10.1016/j.biopha.2020.109997. [DOI] [PubMed] [Google Scholar]
- 42.Khan P., Siddiqui J.A., Lakshmanan I., Ganti A.K., Salgia R., Jain M., Batra S.K., Nasser M.W. RNA-based therapies: a cog in the wheel of lung cancer defense. Mol. Cancer. 2021;20 doi: 10.1186/s12943-021-01338-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huang J., Zhuang C., Chen J., Chen X., Li X., Zhang T., Wang B., Feng Q., Zheng X., Gong M., Gong Q., Xiao K., Luo K., Li W. Targeted drug/gene/photodynamic therapy via a stimuli-responsive dendritic-polymer-based nanococktail for treatment of EGFR-TKI-resistant non-small-cell lung cancer. Adv. Mater. 2022;34 doi: 10.1002/adma.202201516. [DOI] [PubMed] [Google Scholar]
- 44.Garbuzenko O.B., Kuzmov A., Taratula O., Pine S.R., Minko T. Strategy to enhance lung cancer treatment by five essential elements: inhalation delivery, nanotechnology, tumor-receptor targeting, chemo- and gene therapy. Theranostics. 2019;9:8362–8376. doi: 10.7150/thno.39816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Groneberg D.A., Witt C., Wagner U., Chung K.F., Fischer A. Fundamentals of pulmonary drug delivery. Respir. Med. 2003;97:382–387. doi: 10.1053/rmed.2002.1457. [DOI] [PubMed] [Google Scholar]
- 46.El-Sherbiny I.M., El-Baz N.M., Yacoub M.H. Inhaled nano- and microparticles for drug delivery. Glob. Cardiol. Sci. Pract. 2015;2015:2. doi: 10.5339/gcsp.2015.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tang W., Zhang Y., Zhu G. Pulmonary delivery of mucosal nanovaccines. Nanoscale. 2022;14:263–276. doi: 10.1039/d1nr06512b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hellfritzsch M., Scherliess R. Mucosal vaccination via the respiratory tract. Pharmaceutics. 2019;11 doi: 10.3390/pharmaceutics11080375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shakya A.K., Chowdhury M.Y.E., Tao W.Q., Gill H.S. Mucosal vaccine delivery: current state and a pediatric perspective. J. Control. Release. 2016;240:394–413. doi: 10.1016/j.jconrel.2016.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Masjedi M., Montahaei T., Sharafi Z., Jalali A. Pulmonary vaccine delivery: an emerging strategy for vaccination and immunotherapy. J. Drug Deliv. Sci. Technol. 2022;69 [Google Scholar]
- 51.Afkhami S., D’Agostino M.R., Zhang A., Stacey H.D., Marzok A., Kang A., Singh R., Bavananthasivam J., Ye G., Luo X., Wang F., Ang J.C., Zganiacz A., Sankar U., Kazhdan N., Koenig J.F.E., Phelps A., Gameiro S.F., Tang S., Jordana M., Wan Y., Mossman K.L., Jeyanathan M., Gillgrass A., Medina M.F.C., Smaill F., Lichty B.D., Miller M.S., Xing Z. Respiratory mucosal delivery of next-generation COVID-19 vaccine provides robust protection against both ancestral and variant strains of SARS-CoV-2. Cell. 2022;185:896–915. doi: 10.1016/j.cell.2022.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sato S., Kiyono H. The mucosal immune system of the respiratory tract. Curr. Opin. Virol. 2012;2:225–232. doi: 10.1016/j.coviro.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 53.Li M., Wang Y., Sun Y., Cui H.Y., Zhu S.J., Qiu H.J. Mucosal vaccines: strategies and challenges. Immunol. Lett. 2020;217:116–125. doi: 10.1016/j.imlet.2019.10.013. [DOI] [PubMed] [Google Scholar]
- 54.Gohy S., Moeremans A., Pilette C., Collin A. Immunoglobulin a mucosal immunity and altered respiratory epithelium in cystic fibrosis. Cells. 2021;10 doi: 10.3390/cells10123603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Guo Y., Bera H., Shi C., Zhang L., Cun D., Yang M. Pharmaceutical strategies to extend pulmonary exposure of inhaled medicines. Acta Pharm. Sin. B. 2021;11:2565–2584. doi: 10.1016/j.apsb.2021.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bardoliwala D., Patel V., Javia A., Ghosh S., Patel A., Misra A. Nanocarriers in effective pulmonary delivery of siRNA: current approaches and challenges. Ther. Deliv. 2019;10:311–332. doi: 10.4155/tde-2019-0012. [DOI] [PubMed] [Google Scholar]
- 57.Moehwald M., Pinnapireddy S.R., Wonnenberg B., Pourasghar M., Jurisic M., Jung A., Fink-Straube C., Tschernig T., Bakowsky U., Schneider M. Aspherical, nanostructured microparticles for targeted gene delivery to alveolar macrophages. Adv. Healthc. Mater. 2017;6 doi: 10.1002/adhm.201700478. [DOI] [PubMed] [Google Scholar]
- 58.Asgharian B., Owen T.P., Kuempel E.D., Jarabek A.M. Dosimetry of inhaled elongate mineral particles in the respiratory tract: the impact of shape factor. Toxicol. Appl. Pharmacol. 2018;361:27–35. doi: 10.1016/j.taap.2018.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bustamante-Marin X.M., Ostrowski L.E. Cilia and mucociliary clearance. Cold Spring Harb. Perspect. Biol. 2017;9 doi: 10.1101/cshperspect.a028241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liang Z., Ni R., Zhou J., Mao S. Recent advances in controlled pulmonary drug delivery. Drug Discov. Today. 2015;20:380–389. doi: 10.1016/j.drudis.2014.09.020. [DOI] [PubMed] [Google Scholar]
- 61.Liu Q., Guan J., Qin L., Zhang X., Mao S. Physicochemical properties affecting the fate of nanoparticles in pulmonary drug delivery. Drug Discov. Today. 2020;25:150–159. doi: 10.1016/j.drudis.2019.09.023. [DOI] [PubMed] [Google Scholar]
- 62.Liang W., Kwok P.C.L., Chow M.Y.T., Tang P., Mason A.J., Chan H.-K., Lam J.K.W. Formulation of pH responsive peptides as inhalable dry powders for pulmonary delivery of nucleic acids. Eur. J. Pharm. Biopharm. 2014;86:64–73. doi: 10.1016/j.ejpb.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Smith S.A., Selby L.I., Johnston A.P.R., Such G.K. The endosomal escape of nanoparticles: toward more efficient cellular delivery. Bioconjug. Chem. 2019;30:263–272. doi: 10.1021/acs.bioconjchem.8b00732. [DOI] [PubMed] [Google Scholar]
- 64.Torres-Vanegas J.D., Cruz J.C., Reyes L.H. Delivery systems for nucleic acids and proteins: barriers, cell capture pathways and nanocarriers. Pharmaceutics. 2021;13 doi: 10.3390/pharmaceutics13030428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mao S., Sun W., Kissel T. Chitosan-based formulations for delivery of DNA and siRNA. Adv. Drug Deliv. Rev. 2010;62:12–27. doi: 10.1016/j.addr.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 66.Di Cristo L., Maguire C.M., Mc Quillan K., Aleardi M., Volkov Y., Movia D., Prina-Mello A. Towards the identification of an in vitro tool for assessing the biological behavior of aerosol supplied nanomaterials. Int. J. Environ. Res. Public Health. 2018;15 doi: 10.3390/ijerph15040563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Barjaktarevic I.Z., Milstone A.P. Nebulized therapies in COPD: past, present, and the future. Int. J. Chron. Obstruct. Pulmon. Dis. 2020;15:1665–1677. doi: 10.2147/COPD.S252435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.dos Reis L.G., Svolos M., Hartwig B., Windhab N., Young P.M., Traini D. Inhaled gene delivery: a formulation and delivery approach. Expert Opin. Drug Deliv. 2017;14:319–330. doi: 10.1080/17425247.2016.1214569. [DOI] [PubMed] [Google Scholar]
- 69.Osman N.M., Sexton D.W., Saleem I.Y. Toxicological assessment of nanoparticle interactions with the pulmonary system. Nanotoxicology. 2020;14:21–58. doi: 10.1080/17435390.2019.1661043. [DOI] [PubMed] [Google Scholar]
- 70.Scherer T., Geller D.E., Owyang L., Tservistas M., Keller M., Boden N., Kesser K.C., Shire S.J. A technical feasibility study of dornase alfa delivery with eFlow® vibrating membrane nebulizers: aerosol characteristics and physicochemical stability. J. Pharm. Sci. 2011;100:98–109. doi: 10.1002/jps.22231. [DOI] [PubMed] [Google Scholar]
- 71.Usmani O.S. Choosing the right inhaler for your asthma or COPD patient. Ther. Clin. Risk Manag. 2019;15:461–472. doi: 10.2147/TCRM.S160365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Beck-Broichsitter M., Kleimann P., Schmehl T., Betz T., Bakowsky U., Kissel T., Seeger W. Impact of lyoprotectants for the stabilization of biodegradable nanoparticles on the performance of air-jet, ultrasonic, and vibrating-mesh nebulizers. Eur. J. Pharm. Biopharm. 2012;82:272–280. doi: 10.1016/j.ejpb.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 73.Luo Y., Zhai X., Ma C., Sun P., Fu Z., Liu W., Xu J. An inhalable β₂-adrenoceptor ligand-directed guanidinylated chitosan carrier for targeted delivery of siRNA to lung. J. Control. Release. 2012;162:28–36. doi: 10.1016/j.jconrel.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 74.Ferrati S., Wu T., Kanapuram S.R., Smyth H.D.C. Dosing considerations for inhaled biologics. Int. J. Pharm. 2018;549:58–66. doi: 10.1016/j.ijpharm.2018.07.054. [DOI] [PubMed] [Google Scholar]
- 75.Chaurasiya B., Zhao Y.-Y. Dry powder for pulmonary delivery: a comprehensive review. Pharmaceutics. 2021;13 doi: 10.3390/pharmaceutics13010031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhou Q., Tang P., Leung S.S.Y., Chan J.G.Y., Chan H.-K. Emerging inhalation aerosol devices and strategies: where are we headed? Adv. Drug Deliv. Rev. 2014;75:3–17. doi: 10.1016/j.addr.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 77.Saha T., Quinones-Mateu M.E., Das S.C. Inhaled therapy for COVID-19: considerations of drugs, formulations and devices. Int. J. Pharm. 2022;624:122042. doi: 10.1016/j.ijpharm.2022.122042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Agnoletti M., Bohr A., Thanki K., Wan F., Zeng X., Boetker J.P., Yang M., Foged C. Inhalable siRNA-loaded nano-embedded microparticles engineered using microfluidics and spray drying. Eur. J. Pharm. Biopharm. 2017;120:9–21. doi: 10.1016/j.ejpb.2017.08.001. [DOI] [PubMed] [Google Scholar]
- 79.Xu Y., Liu H., Song L. Novel drug delivery systems targeting oxidative stress in chronic obstructive pulmonary disease: a review. J. Nanobiotechnol. 2020;18 doi: 10.1186/s12951-020-00703-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Abdelaziz H.M., Gaber M., Abd-Elwakil M.M., Mabrouk M.T., Elgohary M.M., Kamel N.M., Kabary D.M., Freag M.S., Samaha M.W., Mortada S.M., Elkhodairy K.A., Fang J.-Y., Elzoghby A.O. Inhalable particulate drug delivery systems for lung cancer therapy: nanoparticles, microparticles, nanocomposites and nanoaggregates. J. Control. Release. 2018;269:374–392. doi: 10.1016/j.jconrel.2017.11.036. [DOI] [PubMed] [Google Scholar]
- 81.Muralidharan P., Malapit M., Mallory E., Hayes D., Jr., Mansour H.M. Inhalable nanoparticulate powders for respiratory delivery. Nanomedicine. 2015;11:1189–1199. doi: 10.1016/j.nano.2015.01.007. [DOI] [PubMed] [Google Scholar]
- 82.Ito T., Fukuhara M., Okuda T., Okamoto H. Naked pDNA/hyaluronic acid powder shows excellent long-term storage stability and gene expression in murine lungs. Int. J. Pharm. 2020;574 doi: 10.1016/j.ijpharm.2019.118880. [DOI] [PubMed] [Google Scholar]
- 83.Conti D.S., Bharatwaj B., Brewer D., da Rocha S.R.P. Propellant-based inhalers for the non-invasive delivery of genes via oral inhalation. J. Control. Release. 2012;157:406–417. doi: 10.1016/j.jconrel.2011.09.089. [DOI] [PubMed] [Google Scholar]
- 84.Andrade F., Rafael D., Videira M., Ferreira D., Sosnik A., Sarmento B. Nanotechnology and pulmonary delivery to overcome resistance in infectious diseases. Adv. Drug Deliv. Rev. 2013;65:1816–1827. doi: 10.1016/j.addr.2013.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Conti D.S., Brewer D., Grashik J., Avasarala S., da Rocha S.R.P. Poly(amidoamine) dendrimer nanocarriers and their aerosol formulations for siRNA delivery to the lung epithelium. Mol. Pharm. 2014;11:1808–1822. doi: 10.1021/mp4006358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bielski E., Zhong Q., Mirza H., Brown M., Molla A., Carvajal T., da Rocha S.R.P. TPP-dendrimer nanocarriers for siRNA delivery to the pulmonary epithelium and their dry powder and metered-dose inhaler formulations. Int. J. Pharm. 2017;527:171–183. doi: 10.1016/j.ijpharm.2017.05.046. [DOI] [PubMed] [Google Scholar]
- 87.Vallorz E., Sheth P., Myrdal P. Pressurized metered dose inhaler technology: manufacturing. AAPS PharmSciTech. 2019;20 doi: 10.1208/s12249-019-1389-9. [DOI] [PubMed] [Google Scholar]
- 88.Zarogouldis P., Karamanos N.K., Porpodis K., Domvri K., Huang H., Hohenforst-Schimdt W., Goldberg E.P., Zarogoulidis K. Vectors for inhaled gene therapy in lung cancer. Application for nano oncology and safety of bio nanotechnology. Int. J. Mol. Sci. 2012;13:10828–10862. doi: 10.3390/ijms130910828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhi D., Bai Y., Yang J., Cui S., Zhao Y., Chen H., Zhang S. A review on cationic lipids with different linkers for gene delivery. Adv. Colloid Interf. Sci. 2018;253:117–140. doi: 10.1016/j.cis.2017.12.006. [DOI] [PubMed] [Google Scholar]
- 90.Ponti F., Campolungo M., Melchiori C., Bono N., Candiani G. Cationic lipids for gene delivery: many players, one goal. Chem. Phys. Lipids. 2021;235 doi: 10.1016/j.chemphyslip.2020.105032. [DOI] [PubMed] [Google Scholar]
- 91.Nicolazzi C., Garinot M., Mignet N., Scherman D., Bessodes M. Cationic lipids for transfection. Curr. Med. Chem. 2003;10:1263–1277. doi: 10.2174/0929867033457467. [DOI] [PubMed] [Google Scholar]
- 92.Samaridou E., Heyes J., Lutwyche P. Lipid nanoparticles for nucleic acid delivery: current perspectives. Adv. Drug Deliv. Rev. 2020;154:37–63. doi: 10.1016/j.addr.2020.06.002. [DOI] [PubMed] [Google Scholar]
- 93.Cheng X., Lee R.J. The role of helper lipids in lipid nanoparticles (LNPs) designed for oligonucleotide delivery. Adv. Drug Deliv. Rev. 2016;99:129–137. doi: 10.1016/j.addr.2016.01.022. [DOI] [PubMed] [Google Scholar]
- 94.Qiu M., Tang Y., Chen J., Muriph R., Ye Z., Huang C., Evans J., Henske E.P., Xu Q. Lung-selective mRNA delivery of synthetic lipid nanoparticles for the treatment of pulmonary lymphangioleiomyomatosis. Proc. Natl. Acad. Sci. U. S. A. 2022;119 doi: 10.1073/pnas.2116271119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhang S.B., Xu Y.M., Wang B., Qiao W.H., Liu D.L., Li Z.S. Cationic compounds used in lipoplexes and polyplexes for gene delivery. J. Control. Release. 2004;100:165–180. doi: 10.1016/j.jconrel.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 96.Lam J.K.-W., Liang W., Chan H.-K. Pulmonary delivery of therapeutic siRNA. Adv. Drug Deliv. Rev. 2012;64:1–15. doi: 10.1016/j.addr.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Jiang H.-L., Cui P.-F., Xie R.-L., Cho C.-S. Chemical modification of chitosan for efficient gene therapy. Adv. Food Nutr. Res. 2014;73:83–101. doi: 10.1016/B978-0-12-800268-1.00006-8. [DOI] [PubMed] [Google Scholar]
- 98.Huang G., Huang H. Hyaluronic acid-based biopharmaceutical delivery and tumor-targeted drug delivery system. J. Control. Release. 2018;278:122–126. doi: 10.1016/j.jconrel.2018.04.015. [DOI] [PubMed] [Google Scholar]
- 99.Chen D., Zhang P., Li M., Li C., Lu X., Sun Y., Sun K. Hyaluronic acid-modified redox-sensitive hybrid nanocomplex loading with siRNA for non-small-cell lung carcinoma therapy. Drug Deliv. 2022;29:574–587. doi: 10.1080/10717544.2022.2032874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Fukushige K., Tagami T., Naito M., Goto E., Hirai S., Hatayama N., Yokota H., Yasui T., Baba Y., Ozeki T. Developing spray-freeze-dried particles containing a hyaluronic acid-coated liposome-protamine-DNA complex for pulmonary inhalation. Int. J. Pharm. 2020;583 doi: 10.1016/j.ijpharm.2020.119338. [DOI] [PubMed] [Google Scholar]
- 101.Piotrowski-Daspit A.S., Kauffman A.C., Bracaglia L.G., Saltzman W.M. Polymeric vehicles for nucleic acid delivery. Adv. Drug Deliv. Rev. 2020;156:119–132. doi: 10.1016/j.addr.2020.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mao S., Neu M., Germershaus O., Merkel O., Sitterberg J., Bakowsky U., Kissel T. Influence of polyethylene glycol chain length on the physicochemical and biological properties of poly(ethylene imine)-graft-poly(ethylene glycol) block copolymer/SiRNA polyplexes. Bioconjug. Chem. 2006;17:1209–1218. doi: 10.1021/bc060129j. [DOI] [PubMed] [Google Scholar]
- 103.Schulze J., Kuhn S., Hendrikx S., Schulz-Siegmund M., Polte T., Aigner A. Spray-dried nanoparticle-in-microparticle delivery systems (NiMDS) for gene delivery, comprising polyethylenimine (PEI)-based nanoparticles in a poly(vinyl alcohol) matrix. Small. 2018;14 doi: 10.1002/smll.201701810. [DOI] [PubMed] [Google Scholar]
- 104.Shete H.K., Prabhu R.H., Patravale V.B. Endosomal escape: a bottleneck in intracellular delivery. J. Nanosci. Nanotechnol. 2014;14:460–474. doi: 10.1166/jnn.2014.9082. [DOI] [PubMed] [Google Scholar]
- 105.Keil T.W.M., Feldmann D.P., Costabile G., Zhong Q., da Rocha S., Merkel O.M. Characterization of spray dried powders with nucleic acid-containing PEI nanoparticles. Eur. J. Pharm. Biopharm. 2019;143:61–69. doi: 10.1016/j.ejpb.2019.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ungaro F., d’Angelo I., Miro A., La Rotonda M.I., Quaglia F. Engineered PLGA nano- and micro-carriers for pulmonary delivery: challenges and promises. J. Pharm. Pharmacol. 2012;64:1217–1235. doi: 10.1111/j.2042-7158.2012.01486.x. [DOI] [PubMed] [Google Scholar]
- 107.Bivas-Benita M., Romeijn S., Junginger H.E., Borchard G. PLGA-PEI nanoparticles for gene delivery to pulmonary epithelium. Eur. J. Pharm. Biopharm. 2004;58:1–6. doi: 10.1016/j.ejpb.2004.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jensen D.M.K., Cun D., Maltesen M.J., Frokjaer S., Nielsen H.M., Foged C. Spray drying of siRNA-containing PLGA nanoparticles intended for inhalation. J. Control. Release. 2010;142:138–145. doi: 10.1016/j.jconrel.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Fields R.J., Cheng C.J., Quijano E., Weller C., Kristofik N., Duong N., Hoimes C., Egan M.E., Saltzman W.M. Surface modified poly(beta amino ester)-containing nanoparticles for plasmid DNA delivery. J. Control. Release. 2012;164:41–48. doi: 10.1016/j.jconrel.2012.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kolte A., Patil S., Lesimple P., Hanrahan J.W., Misra A. PEGylated composite nanoparticles of PLGA and polyethylenimine for safe and efficient delivery of pDNA to lungs. Int. J. Pharm. 2017;524:382–396. doi: 10.1016/j.ijpharm.2017.03.094. [DOI] [PubMed] [Google Scholar]
- 111.Dalirfardouei R., Tafaghodi M., Meshkat Z., Najafi A., Gholoobi A., Nabavinia M.S., Sajedifar S., Meshkat M., Badiee A., Ramezani M., Varasteh A.-R., Naderinasab M. A novel formulation of Mtb72F DNA vaccine for immunization against tuberculosis. Iran J. Basic Med. Sci. 2020;23:826–832. doi: 10.22038/ijbms.2020.41806.9881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Menon J.U., Ravikumar P., Pise A., Gyawali D., Hsia C.C.W., Nguyen K.T. Polymeric nanoparticles for pulmonary protein and DNA delivery. Acta Biomater. 2014;10:2643–2652. doi: 10.1016/j.actbio.2014.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mokhtarzadeh A., Alibakhshi A., Yaghoobi H., Hashemi M., Hejazi M., Ramezani M. Recent advances on biocompatible and biodegradable nanoparticles as gene carriers. Expert. Opin. Biol. Ther. 2016;16:771–785. doi: 10.1517/14712598.2016.1169269. [DOI] [PubMed] [Google Scholar]
- 114.Luo K., He B., Wu Y., Shen Y., Gu Z. Functional and biodegradable dendritic macromolecules with controlled architectures as nontoxic and efficient nanoscale gene vectors. Biotechnol. Adv. 2014;32:818–830. doi: 10.1016/j.biotechadv.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 115.Zhang X., Pan J., Yao M., Mendes L.P., Sarisozen C., Mao S., Torchilin V.P. Charge reversible hyaluronic acid-modified dendrimer-based nanoparticles for siMDR-1 and doxorubicin co-delivery. Eur. J. Pharm. Biopharm. 2020;154:43–49. doi: 10.1016/j.ejpb.2020.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bohr A., Tsapis N., Foged C., Andreana I., Yang M., Fattal E. Treatment of acute lung inflammation by pulmonary delivery of anti-TNF-α siRNA with PAMAM dendrimers in a murine model. Eur. J. Pharm. Biopharm. 2020;156:114–120. doi: 10.1016/j.ejpb.2020.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Cordeiro R.A., Serra A., Coelho J.F.J., Faneca H. Poly(β-amino ester)-based gene delivery systems: from discovery to therapeutic applications. J. Control. Release. 2019;310:155–187. doi: 10.1016/j.jconrel.2019.08.024. [DOI] [PubMed] [Google Scholar]
- 118.Guo M., Meng Y., Qin X., Zhou W. Dopamine-grafted hyaluronic acid coated hyperbranched poly(β-amino esters)/DNA nano-complexes for enhanced gene delivery and biosafety. Crystals. 2021;11 [Google Scholar]
- 119.Patel A.K., Kaczmarek J.C., Bose S., Kauffman K.J., Mir F., Heartlein M.W., DeRosa F., Langer R., Anderson D.G. Inhaled nanoformulated mRNA polyplexes for protein production in lung epithelium. Adv. Mater. 2019;31 doi: 10.1002/adma.201805116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Langel U. Cell-penetrating peptides and transportan. Pharmaceutics. 2021;13 doi: 10.3390/pharmaceutics13070987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tai W., Gao X. Functional peptides for siRNA delivery. Adv. Drug Deliv. Rev. 2017;110:157–168. doi: 10.1016/j.addr.2016.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gomes Dos Reis L., Svolos M., Moir L.M., Jaber R., Windhab N., Young P.M., Traini D. Delivery of pDNA polyplexes to bronchial and alveolar epithelial cells using a mesh nebulizer. Pharm. Res. 2018;36:14. doi: 10.1007/s11095-018-2542-y. [DOI] [PubMed] [Google Scholar]
- 123.Osman G., Rodriguez J., Chan S.Y., Chisholm J., Duncan G., Kim N., Tatler A.L., Shakesheff K.M., Hanes J., Suk J.S., Dixon J.E. PEGylated enhanced cell penetrating peptide nanoparticles for lung gene therapy. J. Control. Release. 2018;285:35–45. doi: 10.1016/j.jconrel.2018.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Qiu Y., Chow M.Y.T., Liang W., Chung W.W.Y., Mak J.C.W., Lam J.K.W. From pulmonary surfactant, synthetic KL4 peptide as effective siRNA delivery vector for pulmonary delivery. Mol. Pharm. 2017;14:4606–4617. doi: 10.1021/acs.molpharmaceut.7b00725. [DOI] [PubMed] [Google Scholar]
- 125.Qiu Y., Man R.C.H., Liao Q., Kung K.L.K., Chow M.Y.T., Lam J.K.W. Effective mRNA pulmonary delivery by dry powder formulation of PEGylated synthetic KL4 peptide. J. Control. Release. 2019;314:102–115. doi: 10.1016/j.jconrel.2019.10.026. [DOI] [PubMed] [Google Scholar]
- 126.Kichler A., Mason A.J., Bechinger B. Cationic amphipathic histidine-rich peptides for gene delivery. Biochim. Biophys. Acta. 2006;1758:301–307. doi: 10.1016/j.bbamem.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 127.Liang W., Chow M.Y.T., Lau P.N., Zhou Q.T., Kwok P.C.L., Leung G.P.H., Mason A.J., Chan H.-K., Poon L.L.M., Lam J.K.W. Inhalable dry powder formulations of siRNA and pH-responsive peptides with antiviral activity against H1N1 influenza virus. Mol. Pharm. 2015;12:910–921. doi: 10.1021/mp500745v. [DOI] [PubMed] [Google Scholar]
- 128.Li W., Nicol F., Szoka F.C. GALA: a designed synthetic pH-responsive amphipathic peptide with applications in drug and gene delivery. Adv. Drug Deliv. Rev. 2004;56:967–985. doi: 10.1016/j.addr.2003.10.041. [DOI] [PubMed] [Google Scholar]
- 129.Kusumoto K., Akita H., Ishitsuka T., Matsumoto Y., Nomoto T., Furukawa R., El-Sayed A., Hatakeyama H., Kajimoto K., Yamada Y., Kataoka K., Harashima H. Lipid envelope-type nanoparticle incorporating a multifunctional peptide for systemic siRNA delivery to the pulmonary endothelium. ACS Nano. 2013;7:7534–7541. doi: 10.1021/nn401317t. [DOI] [PubMed] [Google Scholar]
- 130.Degors I.M.S., Wang C.F., Rehman Z.U., Zuhorn I.S. Carriers break barriers in drug delivery: endocytosis and endosomal escape of gene delivery vectors. Acc. Chem. Res. 2019;52:1750–1760. doi: 10.1021/acs.accounts.9b00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Vermeulen L.M.P., De Smedt S.C., Remaut K., Braeckmans K. The proton sponge hypothesis: fable or fact? Eur. J. Pharm. Biopharm. 2018;129:184–190. doi: 10.1016/j.ejpb.2018.05.034. [DOI] [PubMed] [Google Scholar]
- 132.Feldmann D.P., Cheng Y., Kandil R., Xie Y., Mohammadi M., Harz H., Sharma A., Peeler D.J., Moszczynska A., Leonhardt H., Pun S.H., Merkel O.M. In vitro and in vivo delivery of siRNA via VIPER polymer system to lung cells. J. Control. Release. 2018;276:50–58. doi: 10.1016/j.jconrel.2018.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Brock D.J., Kondow-McConaghy H., Allen J., Brkljaca Z., Kustigian L., Jiang M., Zhang J., Rye H., Vazdar M., J.-P. Pellois, mechanism of cell penetration by permeabilization of late endosomes: interplay between a multivalent TAT peptide and bis(monoacylglycero)phosphate. Cell Chem. Biol. 2020;27:1296–+. doi: 10.1016/j.chembiol.2020.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Patil S., Gao Y.-G., Lin X., Li Y., Dang K., Tian Y., Zhang W.-J., Jiang S.-F., Qadir A., Qian A.-R. The development of functional non-viral vectors for gene delivery. Int. J. Mol. Sci. 2019;20 doi: 10.3390/ijms20215491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Baliga U.K., Dean D.A. Pulmonary gene delivery-realities and possibilities. Exp. Biol. Med. 2021;246:260–274. doi: 10.1177/1535370220965985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Geiger J., Aneja M.K., Rudolph C. Vectors for pulmonary gene therapy. Int. J. Pharm. 2010;390:84–88. doi: 10.1016/j.ijpharm.2009.10.010. [DOI] [PubMed] [Google Scholar]
- 137.Chow M.Y.T., Qiu Y., Lam J.K.W. Inhaled RNA therapy: from promise to reality. Trends Pharmacol. Sci. 2020;41:715–729. doi: 10.1016/j.tips.2020.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ge X.M., Wei M.Y., He S.N., Yuan W.E. Advances of non-ionic surfactant vesicles (niosomes) and their application in drug delivery. Pharmaceutics. 2019;11 doi: 10.3390/pharmaceutics11020055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Li Z., Cai H., Li Z., Ren L., Ma X., Zhu H., Gong Q., Zhang H., Gu Z., Luo K. A tumor cell membrane-coated self-amplified nanosystem as a nanovaccine to boost the therapeutic effect of anti-PD-L1 antibody. Bioact. Mater. 2023;21:299–312. doi: 10.1016/j.bioactmat.2022.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Plaza-Oliver M., Jesus Santander-Ortega M., Victoria Lozano M. Current approaches in lipid-based nanocarriers for oral drug delivery. Drug Deliv. Transl. Res. 2021;11:471–497. doi: 10.1007/s13346-021-00908-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Shah S., Dhawan V., Holm R., Nagarsenker M.S., Perrie Y. Liposomes: advancements and innovation in the manufacturing process. Adv. Drug Deliv. Rev. 2020;154:102–122. doi: 10.1016/j.addr.2020.07.002. [DOI] [PubMed] [Google Scholar]
- 142.Antimisiaris S.G., Marazioti A., Kannavou M., Natsaridis E., Gkartziou F., Kogkos G., Mourtas S. Overcoming barriers by local drug delivery with liposomes. Adv. Drug Deliv. Rev. 2021;174:53–86. doi: 10.1016/j.addr.2021.01.019. [DOI] [PubMed] [Google Scholar]
- 143.Bassetti M., Vena A., Russo A., Peghin M. Inhaled liposomal antimicrobial delivery in lung infections. Drugs. 2020;80:1309–1318. doi: 10.1007/s40265-020-01359-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sturm L., Ulrih N.P. Basic methods for preparation of liposomes and studying their interactions with different compounds, with the emphasis on polyphenols. Int. J. Mol. Sci. 2021;22 doi: 10.3390/ijms22126547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.de Lima M.C.P., Neves S., Filipe A., Duzgunes N., Simoes S. Cationic liposomes for gene delivery: from biophysics to biological applications. Curr. Med. Chem. 2003;10:1221–1231. doi: 10.2174/0929867033457430. [DOI] [PubMed] [Google Scholar]
- 146.Koynova R., Tenchov B. Recent progress in liposome production, relevance to drug delivery and nanomedicine. Rec. Pat. Nanotechnol. 2015;9:86–93. doi: 10.2174/187221050902150819151721. [DOI] [PubMed] [Google Scholar]
- 147.Simoes S., Filipe A., Faneca H., Mano M., Penacho N., Duzgunes N., de Lima M.P. Cationic liposomes for gene delivery. Expert Opin. Drug Deliv. 2005;2:237–254. doi: 10.1517/17425247.2.2.237. [DOI] [PubMed] [Google Scholar]
- 148.Zoulikha M., Xiao Q., Boafo G.F., Sallam M.A., Chen Z., He W. Pulmonary delivery of siRNA against acute lung injury/acute respiratory distress syndrome. Acta Pharm. Sin. B. 2022;12:600–620. doi: 10.1016/j.apsb.2021.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Xue H.Y., Guo P., Wen W.-C., Wong H.L. Lipid-based nanocarriers for RNA delivery. Curr. Pharm. Des. 2015;21:3140–3147. doi: 10.2174/1381612821666150531164540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Zhao J., Qin L., Song R., Su J., Yuan Y., Zhang X., Mao S. Elucidating inhaled liposome surface charge on its interaction with biological barriers in the lung. Eur. J. Pharm. Biopharm. 2022;172:101–111. doi: 10.1016/j.ejpb.2022.01.009. [DOI] [PubMed] [Google Scholar]
- 151.Dalby B., Cates S., Harris A., Ohki E.C., Tilkins M.L., Price P.J., Ciccarone V.C. Advanced transfection with Lipofectamine 2000 reagent: primary neurons, siRNA, and high-throughput applications. Methods. 2004;33:95–103. doi: 10.1016/j.ymeth.2003.11.023. [DOI] [PubMed] [Google Scholar]
- 152.Li P., Liu D., Sun X., Liu C., Liu Y., Zhang N. A novel cationic liposome formulation for efficient gene delivery via a pulmonary route. Nanotechnology. 2011;22 doi: 10.1088/0957-4484/22/24/245104. [DOI] [PubMed] [Google Scholar]
- 153.Cipolla D., Gonda I., Chan H.-K. Liposomal formulations for inhalation. Ther. Deliv. 2013;4:1047–1072. doi: 10.4155/tde.13.71. [DOI] [PubMed] [Google Scholar]
- 154.Chattopadhyay S. Aerosol generation using nanometer liposome suspensions for pulmonary drug delivery applications. J. Liposome Res. 2013;23:255–267. doi: 10.3109/08982104.2013.802332. [DOI] [PubMed] [Google Scholar]
- 155.Jaafar-Maalej C., Elaissari A., Fessi H. Lipid-based carriers: manufacturing and applications for pulmonary route. Expert Opin. Drug Deliv. 2012;9:1111–1127. doi: 10.1517/17425247.2012.702751. [DOI] [PubMed] [Google Scholar]
- 156.Pilkington E.H., Suys E.J.A., Trevaskis N.L., Wheatley A.K., Zukancic D., Algarni A., Al-Wassiti H., Davis T.P., Pouton C.W., Kent S.J., Truong N.P. From influenza to COVID-19: lipid nanoparticle mRNA vaccines at the frontiers of infectious diseases. Acta Biomater. 2021;131:16–40. doi: 10.1016/j.actbio.2021.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Moss K.H., Popova P., Hadrup S.R., Astakhova K., Taskova M. Lipid nanoparticles for delivery of therapeutic RNA oligonucleotides. Mol. Pharm. 2019;16:2265–2277. doi: 10.1021/acs.molpharmaceut.8b01290. [DOI] [PubMed] [Google Scholar]
- 158.Zhang H.R., Leal J., Soto M.R., Smyth H.D.C., Ghosh D. Aerosolizable lipid nanoparticles for pulmonary delivery of mRNA through design of experiments. Pharmaceutics. 2020;12:16. doi: 10.3390/pharmaceutics12111042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Garg S., Heuck G., Ip S., Ramsay E. Microfluidics: a transformational tool for nanomedicine development and production. J. Drug Target. 2016;24:821–835. doi: 10.1080/1061186X.2016.1198354. [DOI] [PubMed] [Google Scholar]
- 160.Roces C.B., Lou G., Jain N., Abraham S., Thomas A., Halbert G.W., Perrie Y. Manufacturing considerations for the development of lipid nanoparticles using microfluidics. Pharmaceutics. 2020;12 doi: 10.3390/pharmaceutics12111095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Cullis P.R., Hope M.J. Lipid nanoparticle systems for enabling gene therapies. Mol. Ther. 2017;25:1467–1475. doi: 10.1016/j.ymthe.2017.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Sakurai Y., Hada T., Harashima H. Scalable preparation of poly(ethyleneglycol)-grafted siRNA-loaded lipid nanoparticles using a commercially available fluidic device and tangential flow filtration. J. Biomater. Sci. Polym. Ed. 2017;28:1086–1096. doi: 10.1080/09205063.2017.1291297. [DOI] [PubMed] [Google Scholar]
- 163.Aldosari B.N., Alfagih I.M., Almurshedi A.S. Lipid nanoparticles as delivery systems for RNA-based vaccines. Pharmaceutics. 2021;13 doi: 10.3390/pharmaceutics13020206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Suzuki Y., Ishihara H. Difference in the lipid nanoparticle technology employed in three approved siRNA (Patisiran) and mRNA (COVID-19 vaccine) drugs. Drug Metab. Pharmacokinet. 2021;41 doi: 10.1016/j.dmpk.2021.100424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Pardi N., Tuyishime S., Muramatsu H., Kariko K., Mui B.L., Tam Y.K., Madden T.D., Hope M.J., Weissman D. Expression kinetics of nucleoside-modified mRNA delivered in lipid nanoparticles to mice by various routes. J. Control. Release. 2015;217:345–351. doi: 10.1016/j.jconrel.2015.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Lokugamage M.P., Vanover D., Beyersdorf J., Hatit M.Z.C., Rotolo L., Echeverri E.S., Peck H.E., Ni H., Yoon J.-K., Kim Y., Santangelo P.J., Dahlman J.E. Optimization of lipid nanoparticles for the delivery of nebulized therapeutic mRNA to the lungs. Nat. Biomed. Eng. 2021;5:1059–1068. doi: 10.1038/s41551-021-00786-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Kulkarni J.A., Witzigmann D., Chen S., Cullis P.R., van der Meel R. Lipid nanoparticle technology for clinical translation of siRNA therapeutics. Acc. Chem. Res. 2019;52:2435–2444. doi: 10.1021/acs.accounts.9b00368. [DOI] [PubMed] [Google Scholar]
- 168.Hou X.C., Zaks T., Langer R., Dong Y.Z. Lipid nanoparticles for mRNA delivery. Nat. Rev. Mater. 2021;6:1078–1094. doi: 10.1038/s41578-021-00358-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.LoPresti S.T., Arral M.L., Chaudhary N., Whitehead K.A. The replacement of helper lipids with charged alternatives in lipid nanoparticles facilities targeted mRNA delivery to the spleen and lungs. J. Control. Release. 2022;345:819–831. doi: 10.1016/j.jconrel.2022.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Paliwal R., Paliwal S.R., Kenwat R., Kurmi B.D., Sahu M.K. Solid lipid nanoparticles: a review on recent perspectives and patents. Expert Opin. Ther. Pat. 2020;30:179–194. doi: 10.1080/13543776.2020.1720649. [DOI] [PubMed] [Google Scholar]
- 171.Dolatabadi J. Ezzati Nazhad, Valizadeh H., Hamishehkar H. Solid lipid nanoparticles as efficient drug and gene delivery systems: recent breakthroughs. Adv. Pharm. Bull. 2015;5:151–159. doi: 10.15171/apb.2015.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.del Pozo-Rodriguez A., Solinis M.A., Rodriguez-Gascon A. Applications of lipid nanoparticles in gene therapy. Eur. J. Pharm. Biopharm. 2016;109:184–193. doi: 10.1016/j.ejpb.2016.10.016. [DOI] [PubMed] [Google Scholar]
- 173.Gaspar D.P., Vital J., Leiva M.C., Goncalves L.M.D., Taboada P., Remunan-Lopez C., Vitor J., Almeida A.J. Transfection of pulmonary cells by stable pDNA-polycationic hybrid nanostructured particles. Nanomedicine. 2019;14:407–429. doi: 10.2217/nnm-2018-0270. [DOI] [PubMed] [Google Scholar]
- 174.Nassimi M., Schleh C., Lauenstein H.D., Hussein R., Hoymann H.G., Koch W., Pohlmann G., Krug N., Sewald K., Rittinghausen S., Braun A., Mueller-Goymann C. A toxicological evaluation of inhaled solid lipid nanoparticles used as a potential drug delivery system for the lung. Eur. J. Pharm. Biopharm. 2010;75:107–116. doi: 10.1016/j.ejpb.2010.02.014. [DOI] [PubMed] [Google Scholar]
- 175.Puri A., Loomis K., Smith B., Lee J.-H., Yavlovich A., Heldman E., Blumenthal R. Lipid-based nanoparticles as pharmaceutical drug carriers: from concepts to clinic. Crit. Rev. Ther. Drug Carrier Syst. 2009;26:523–580. doi: 10.1615/critrevtherdrugcarriersyst.v26.i6.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.De Jesus M.B., Zuhorn I.S. Solid lipid nanoparticles as nucleic acid delivery system: properties and molecular mechanisms. J. Control. Release. 2015;201:1–13. doi: 10.1016/j.jconrel.2015.01.010. [DOI] [PubMed] [Google Scholar]
- 177.Wang J.L., Hanafy M.S., Xu H.Y., Leal J., Zhai Y.F., Ghosh D., Williams R.O., Smyth H.D.C., Cui Z.R. Aerosolizable siRNA-encapsulated solid lipid nanoparticles prepared by thin-film freeze-drying for potential pulmonary delivery. Int. J. Pharm. 2021;596 doi: 10.1016/j.ijpharm.2021.120215. [DOI] [PubMed] [Google Scholar]
- 178.Marc S.P., Silvia P.S., Younes E.Y., Sofía B., Anna N.R., Isaac N.R., Pilar P.L., Encarna G.M., Montserrat M.C., Ramón T. Cholesteryl oleate-loaded cationic solid lipid nanoparticles as carriers for efficient gene-silencing therapy. Int. J. Nanomedicine. 2018;13:3223–3233. doi: 10.2147/IJN.S158884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Yu W., Liu C., Liu Y., Zhang N., Xu W. Mannan-modified solid lipid nanoparticles for targeted gene delivery to alveolar macrophages. Pharm. Res. 2010;27:1584–1596. doi: 10.1007/s11095-010-0149-z. [DOI] [PubMed] [Google Scholar]
- 180.Li Q., Chan C., Peterson N., Hanna R.N., Alfaro A., Allen K.L., Wu H., Dall’Acqua W.F., Borrok M.J., Santos J.L. Engineering caveolae-targeted lipid nanoparticles to deliver mRNA to the lungs. ACS Chem. Biol. 2020;15:830–836. doi: 10.1021/acschembio.0c00003. [DOI] [PubMed] [Google Scholar]
- 181.Leite Nascimento T., Hillaireau H., Vergnaud J., Rivano M., Deloménie C., Courilleau D., Arpicco S., Suk J.S., Hanes J., Fattal E. Hyaluronic acid-conjugated lipoplexes for targeted delivery of siRNA in a murine metastatic lung cancer model. Int. J. Pharm. 2016;514:103–111. doi: 10.1016/j.ijpharm.2016.06.125. [DOI] [PubMed] [Google Scholar]
- 182.Santiwarangkool S., Akita H., Nakatani T., Kusumoto K., Kimura H., Suzuki M., Nishimura M., Sato Y., Harashima H. PEGylation of the GALA peptide enhances the lung-targeting activity of nanocarriers that contain encapsulated siRNA. J. Pharm. Sci. 2017;106:2420–2427. doi: 10.1016/j.xphs.2017.04.075. [DOI] [PubMed] [Google Scholar]
- 183.Almeida A.P.B., Damaceno G.B.R., Carneiro A.F., Bohr A., Gonçalves H.R., Valadares M.C., Nascimento T.L., Lima E.M. Mucopenetrating lipoplexes modified with PEG and hyaluronic acid for CD44-targeted local siRNA delivery to the lungs. J. Biomater. Appl. 2019;34:617–630. doi: 10.1177/0885328219863291. [DOI] [PubMed] [Google Scholar]
- 184.Banik B.L., Fattahi P., Brown J.L. Polymeric nanoparticles: the future of nanomedicine. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2016;8:271–299. doi: 10.1002/wnan.1364. [DOI] [PubMed] [Google Scholar]
- 185.Zielinska A., Carreiro F., Oliveira A.M., Neves A., Pires B., Venkatesh D.N., Durazzo A., Lucarini M., Eder P., Silva A.M., Santini A., Souto E.B. Polymeric nanoparticles: production, characterization, toxicology and ecotoxicology. Molecules. 2020;25 doi: 10.3390/molecules25163731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Beck-Broichsitter M., Merkel O.M., Kissel T. Controlled pulmonary drug and gene delivery using polymeric nano-carriers. J. Control. Release. 2012;161:214–224. doi: 10.1016/j.jconrel.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 187.Subjakova V., Oravczova V., Hianik T. Polymer nanoparticles and nanomotors modified by DNA/RNA aptamers and antibodies in targeted therapy of cancer. Polymers. 2021;13 doi: 10.3390/polym13030341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Streck S., Hong L., Boyd B.J., McDowell A. Microfluidics for the production of nanomedicines: considerations for polymer and lipid-based systems. Pharm. Nanotechnol. 2019;7:423–443. doi: 10.2174/2211738507666191019154815. [DOI] [PubMed] [Google Scholar]
- 189.Chai G.H., Hassan A., Meng T., Lou L.H., Ma J., Simmers R., Zhou L., Rubin B.K., Zhou Q., Longest P.W., Hindle M., Xu Q.G. Dry powder aerosol containing muco-inert particles for excipient enhanced growth pulmonary drug delivery. Nanomedicine. 2020;29 doi: 10.1016/j.nano.2020.102262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Huckaby J.T., Lai S.K. PEGylation for enhancing nanoparticle diffusion in mucus. Adv. Drug Deliv. Rev. 2018;124:125–139. doi: 10.1016/j.addr.2017.08.010. [DOI] [PubMed] [Google Scholar]
- 191.Suk J.S., Kim A.J., Trehan K., Schneider C.S., Cebotaru L., Woodward O.M., Boylan N.J., Boyle M.P., Lai S.K., Guggino W.B., Hanes J. Lung gene therapy with highly compacted DNA nanoparticles that overcome the mucus barrier. J. Control. Release. 2014;178:8–17. doi: 10.1016/j.jconrel.2014.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Pfeifer C., Hasenpusch G., Uezguen S., Aneja M.K., Reinhardt D., Kirch J., Schneider M., Claus S., Friess W., Rudolph C. Dry powder aerosols of polyethylenimine (PEI)-based gene vectors mediate efficient gene delivery to the lung. J. Control. Release. 2011;154:69–76. doi: 10.1016/j.jconrel.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 193.Mastorakos P., da Silva A.L., Chisholm J., Song E., Choi W.K., Boyle M.P., Morales M.M., Hanes J., Suk J.S. Highly compacted biodegradable DNA nanoparticles capable of overcoming the mucus barrier for inhaled lung gene therapy. Proc. Natl. Acad. Sci. U. S. A. 2015;112:8720–8725. doi: 10.1073/pnas.1502281112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Kanayama N., Fukushima S., Nishiyama N., Itaka K., Jang W.-D., Miyata K., Yamasaki Y., Chung U.-I., Kataoka K. A PEG-based biocompatible block catiomer with high buffering capacity for the construction of polyplex micelles showing efficient gene transfer toward primary cells. ChemMedChem. 2006;1:439–444. doi: 10.1002/cmdc.200600008. [DOI] [PubMed] [Google Scholar]
- 195.Uchida S., Itaka K., Chen Q., Osada K., Ishii T., Shibata M.-A., Harada-Shiba M., Kataoka K. PEGylated polyplex with optimized PEG shielding enhances gene introduction in lungs by minimizing inflammatory responses. Mol. Ther. 2012;20:1196–1203. doi: 10.1038/mt.2012.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Kim Y.K., Cho C.S., Cho M.H., Jiang H.L. Spermine-alt-poly(ethylene glycol) polyspermine as a safe and efficient aerosol gene carrier for lung cancer therapy. J. Biomed. Mater. Res. A. 2014;102:2230–2237. doi: 10.1002/jbm.a.34905. [DOI] [PubMed] [Google Scholar]
- 197.Grun M.K., Suberi A., Shin K., Lee T., Gomerdinger V., Moscato Z.M., Piotrowski-Daspit A.S., Saltzman W.M. PEGylation of poly(amine-co-ester) polyplexes for tunable gene delivery. Biomaterials. 2021;272 doi: 10.1016/j.biomaterials.2021.120780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Ke X., Shelton L., Hu Y., Zhu Y., Chow E., Tang H., Santos J.L., Mao H.Q. Surface-functionalized PEGylated nanoparticles deliver messenger RNA to pulmonary immune cells. ACS Appl. Mater. Interfaces. 2020;12:35835–35844. doi: 10.1021/acsami.0c08268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Hadianamrei R., Zhao X. Current state of the art in peptide-based gene delivery. J. Control. Release. 2022;343:600–619. doi: 10.1016/j.jconrel.2022.02.010. [DOI] [PubMed] [Google Scholar]
- 200.Zhao Z.-X., Gao S.-Y., Wang J.-C., Chen C.-J., Zhao E.-Y., Hou W.-J., Feng Q., Gao L.-Y., Liu X.-Y., Zhang L.-R., Zhang Q. Self-assembly nanomicelles based on cationic mPEG-PLA-b-Polyarginine(R-15) triblock copolymer for siRNA delivery. Biomaterials. 2012;33:6793–6807. doi: 10.1016/j.biomaterials.2012.05.067. [DOI] [PubMed] [Google Scholar]
- 201.Ibaraki H., Kanazawa T., Owada M., Iwaya K., Takashima Y., Seta Y. Anti-metastatic effects on melanoma via intravenous administration of anti-NF-kappa B siRNA complexed with functional peptide-modified nano-micelles. Pharmaceutics. 2020;12 doi: 10.3390/pharmaceutics12010064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Tarvirdipour S., Skowicki M., Schoenenberger C.-A., Palivan C.G. Peptide-assisted nucleic acid delivery systems on the rise. Int. J. Mol. Sci. 2021;22 doi: 10.3390/ijms22169092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Soares D.C.F., Domingues S.C., Viana D.B., Tebaldi M.L. Polymer-hybrid nanoparticles: current advances in biomedical applications. Biomed. Pharmacother. 2020;131 doi: 10.1016/j.biopha.2020.110695. [DOI] [PubMed] [Google Scholar]
- 204.d’Angelo I., Costabile G., Durantie E., Brocca P., Rondelli V., Russo A., Russo G., Miro A., Quaglia F., Petri-Fink A., Rothen-Rutishauser B., Ungaro F. Hybrid lipid/polymer nanoparticles for pulmonary delivery of siRNA: development and fate upon in vitro deposition on the human epithelial airway barrier. J. Aerosol Med. Pulm. Drug Deliv. 2018;31:170–181. doi: 10.1089/jamp.2017.1364. [DOI] [PubMed] [Google Scholar]
- 205.Liu Q., Xue J., Zhang X., Chai J., Qin L., Guan J., Zhang X., Mao S. The influence of a biomimetic pulmonary surfactant modification on the in vivo fate of nanoparticles in the lung. Acta Biomater. 2022;147:391–402. doi: 10.1016/j.actbio.2022.05.038. [DOI] [PubMed] [Google Scholar]
- 206.Mukherjee A., Waters A.K., Kalyan P., Achrol A.S., Kesari S., Yenugonda V.M. Lipid-polymer hybrid nanoparticles as a next-generation drug delivery platform: state of the art, emerging technologies, and perspectives. Int. J. Nanomedicine. 2019;14:1937–1952. doi: 10.2147/IJN.S198353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Gomes Dos Reis L., Lee W.-H., Svolos M., Moir L.M., Jaber R., Engel A., Windhab N., Young P.M., Traini D. Delivery of pDNA to lung epithelial cells using PLGA nanoparticles formulated with a cell-penetrating peptide: understanding the intracellular fate. Drug Dev. Ind. Pharm. 2020;46:427–442. doi: 10.1080/03639045.2020.1724134. [DOI] [PubMed] [Google Scholar]
- 208.Hagino Y., Khalil I.A., Kimura S., Kusumoto K., Harashima H. GALA-modified lipid nanoparticles for the targeted delivery of plasmid DNA to the lungs. Mol. Pharm. 2021;18:878–888. doi: 10.1021/acs.molpharmaceut.0c00854. [DOI] [PubMed] [Google Scholar]
- 209.Al-Ahmady Z.S., Al-Jamal W.T., Bossche J.V., Bui T.T., Drake A.F., Mason A.J., Kostarelos K. Lipid-peptide vesicle nanoscale hybrids for triggered drug release by mild hyperthermia in vitro and in vivo. ACS Nano. 2012;6:9335–9346. doi: 10.1021/nn302148p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Hyodo M., Sakurai Y., Akita H., Harashima H. “Programmed packaging” for gene delivery. J. Control. Release. 2014;193:316–323. doi: 10.1016/j.jconrel.2014.04.023. [DOI] [PubMed] [Google Scholar]
- 211.Mohammadi Z., Dorkoosh F.A., Hosseinkhani S., Gilani K., Amini T., Najafabadi A.R., Tehrani M.R. In vivo transfection study of chitosan-DNA-FAP-B nanoparticles as a new non viral vector for gene delivery to the lung. Int. J. Pharm. 2011;421:183–188. doi: 10.1016/j.ijpharm.2011.09.029. [DOI] [PubMed] [Google Scholar]
- 212.Zhang Z., Fang X., Hao J., Li Y., Sha X. Triolein-based polycation lipid nanocarrier for efficient gene delivery: characteristics and mechanism. Int. J. Nanomedicine. 2011;6:2235–2244. doi: 10.2147/IJN.S24720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Jensen D.K., Jensen L.B., Koocheki S., Bengtson L., Cun D.M., Nielsen H.M., Foged C. Design of an inhalable dry powder formulation of DOTAP-modified PLGA nanoparticles loaded with siRNA. J. Control. Release. 2012;157:141–148. doi: 10.1016/j.jconrel.2011.08.011. [DOI] [PubMed] [Google Scholar]
- 214.Cheng Y., Yumul R.C., Pun S.H. Virus-inspired polymer for efficient in vitro and in vivo gene delivery. Angew. Chem. Int. Ed. Eng. 2016;55:12013–12017. doi: 10.1002/anie.201605958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Thanki K., van Eetvelde D., Geyer A., Fraire J., Hendrix R., Van Eygen H., Putteman E., Sami H., de Souza Carvalho-Wodarz C., Franzyk H., Nielsen H.M., Braeckmans K., Lehr C.M., Ogris M., Foged C. Mechanistic profiling of the release kinetics of siRNA from lipidoid-polymer hybrid nanoparticles in vitro and in vivo after pulmonary administration. J. Control. Release. 2019;310:82–93. doi: 10.1016/j.jconrel.2019.08.004. [DOI] [PubMed] [Google Scholar]
- 216.Deng Z.C., Kalin G.T., Shi D.L., Kalinichenko V.V. Nanoparticle delivery systems with cell-specific targeting for pulmonary diseases. Am. J. Respir. Cell Mol. Biol. 2021;64:292–307. doi: 10.1165/rcmb.2020-0306TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Song D., Cahn D., Duncan GA. Mucin biopolymers and their barrier function at airway surfaces. Langmuir. 2020;36:12773–12783. doi: 10.1021/acs.langmuir.0c02410. [DOI] [PubMed] [Google Scholar]
- 218.Ruge C.A., Schaefer U.F., Herrmann J., Kirch J., Cañadas O., Echaide M., Pérez-Gil J., Casals C., Müller R., Lehr C.M. The interplay of lung surfactant proteins and lipids assimilates the macrophage clearance of nanoparticles. PLoS One. 2012;7 doi: 10.1371/journal.pone.0040775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Liu Q., Guan J., Song R., Zhang X., Mao S. Physicochemical properties of nanoparticles affecting their fate and the physiological function of pulmonary surfactants. Acta Biomater. 2022;140:76–87. doi: 10.1016/j.actbio.2021.11.034. [DOI] [PubMed] [Google Scholar]
- 220.Nguyen J., Reul R., Betz T., Dayyoub E., Schmehl T., Gessler T., Bakowsky U., Seeger W., Kissel T. Nanocomposites of lung surfactant and biodegradable cationic nanoparticles improve transfection efficiency to lung cells. J. Control. Release. 2009;140:47–54. doi: 10.1016/j.jconrel.2009.07.017. [DOI] [PubMed] [Google Scholar]
- 221.De Backer L., Braeckmans K., Stuart M.C.A., Demeester J., De Smedt S.C., Raemdonck K. Bio-inspired pulmonary surfactant-modified nanogels: a promising siRNA delivery system. J. Control. Release. 2015;206:177–186. doi: 10.1016/j.jconrel.2015.03.015. [DOI] [PubMed] [Google Scholar]
- 222.Cao X., Coyle J.P., Xiong R., Wang Y., Heflich R.H., Ren B., Gwinn W.M., Hayden P., Rojanasakul L. Invited review: human air-liquid-interface organotypic airway tissue models derived from primary tracheobronchial epithelial cells-overview and perspectives. In Vitro Cell Dev. Biol. Anim. 2021;57:104–132. doi: 10.1007/s11626-020-00517-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Tan Q., Choi K.M., Sicard D., Tschumperlin D.J. Human airway organoid engineering as a step toward lung regeneration and disease modeling. Biomaterials. 2017;113:118–132. doi: 10.1016/j.biomaterials.2016.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.