Dear editor
Growing evidence may have delighted us that the implementation of several non-pharmaceutical interventions (NPIs) such as social distancing, increased hand hygiene, mask-wearing, working from home, and school closures amid the global pandemic of coronavirus disease-2019 (COVID-19) has proven efficient not only to reduce COVID-19 but also many other infectious diseases including the acute gastroenteritis in all ages of people (AGE).1, 2, 3 Nevertheless, the recent re-emergence of these infections is worryings us again.4 Actually, the decline in infections was not confirmed by community-level investigations. As a result, whether these infections were truly decreased in the community yet remains vague. In this study, to our knowledge, we provided the first molecular evidence of the existence of infections in the community during the COVID-19 pandemic through wastewater-based epidemiology (WBE) which is considered a powerful public health surveillance tool to track infection trends within a community.
AGE still remains a major global public health problem that mainly affects children which represent about 70% of total AGE. Viruses including rotavirus (RV), norovirus (NoV), adenovirus (AdV), astrovirus (AstV), sapovirus (SV), and enterovirus (EV) remain the leading causes of viral AGE that account for more than 200,000 childhood deaths per year worldwide. In this study, we collected raw sewage water (SW) almost every month from August 2018 to March 2022 from a sewage treatment plant in the Kansai area of Japan. These raw SW were characterized as a pool of both black (wastewater from toilets) and gray water (all domestic wastewater except toilets) of about 245,000 inhabitants likely came from houses, schools, markets, farms, and hospitals (after initial treatment). The viruses in the SW were then concentrated by polyethylene-glycol (PEG)-precipitation method and investigated for these major enteric viruses by conventional reverse transcription polymerase chain reaction (RT-PCR) with specific primers as described previously.5 Namely, the partial regions of VP7 for RVA (primer pair: sBeg9-VP7–1′); capsid for NoV GI (G1SKF-G1SKR), GII (1st PCR: COG2F-G2SKR, nested PCR: G2SKF-G2SKR) and SV (SLV5317-SLV5749); hexon for AdV (Ad1-Ad2), ORF-1b for AstV (SF0073-SF0076), and 5′UTR for EV (F1-R1) were amplified for screening and sequenced for genotyping. The first PCR product (1 μl) was re-amplified using the same specific primers if the first PCR remained negative or faint. In addition, the major G-genotypes (G1, G2, human typical G3, equine-like G3 (G3e), G4, G8, G9, and G12) in RVA-positive samples were determined by nested monoplex PCR using primers as described by Fujii Y et al.6
We observed that most SW remained positive for several of these viruses as shown in Table 1 . The most frequently detected virus was SV (74.4%), followed by RVA (61.5%), AstV (59%), NoV GI (59%), NoV GII (56.4%), EV (48.7%), and AdV (46.2%). Although we reported, recently, an increasing trend of SV infection in Japanese children,7 the high detection rate of SV seems a particular problem for this community, because the SV detection rate of this treatment plant remained high (11/12; 91.7%) in our earlier study5 also, but such a high SV detection rate was not found in the SW of another treatment plant (data yet not published).
Table 1.
Detection of major AGE viruses in raw SW.
| Year | Month | RVA | NGI | NGII | SV | AdV | AstV | EV |
|---|---|---|---|---|---|---|---|---|
| 2018–2019 |
Aug | ++(G1,G3, G3e, G8,G9) | W | +++(F41) | ++ | +++(EV-A) | ||
| Sep | + | +++(F41) | ||||||
| Oct | +++ | +++(EV-A) | ||||||
| Nov | ++ | ++ | ++ | +++(EV-B) | ||||
| Mar | ++(G1,G2,G3, G3e,G8,G9) | ++ | W | ++ | ||||
| Apr | W | ++(GI.3) | ++(GII.1) | W | +++(EV-C) | |||
| May | ++ | W | ||||||
| Jun | + | +++(EV-A) | ||||||
| 2019–2020 |
Jul | W | +++(GII.3) | |||||
| Aug | +++(GII.3) | +++(EV-B) | ||||||
| Sep | ++(G2) | W | W | +++ | +++ | +++ | ||
| Oct | ++(G11) | W | +++ | +++ | ||||
| Nov | +++(G3,G8,G9) | ++(GII.17) | +++(F41) | +++(MLB1) | + | |||
| Dec | W | ++ | + | +++(F41) | +++(MLB1) | |||
| Jan | ++(G3) | + | ++(GII.2) | +++(GI.2) | +++ | +++(MLB3) | ||
| Feb | ++(G3) | + | +++(GII.3) | +++(F41) | ||||
| Mar | +++(G1,G3, G3e, G8,G9) | ++ | +++(GI.2) | W | ++(EV-C) | |||
| Apr | + | ++ | ||||||
| May | +(G3, G3e, G8,G9) | W | W | |||||
| 2020–2021 | Jul | + | ||||||
| Aug | +(G3) | + | W | ++ | ++(EV-B) | |||
| Sep | + | |||||||
| Oct | W | ++ | ||||||
| Nov | ++(G1,G3, G3e, G6, G8,G9) | ++(GII.6) | ++ | ++ | ||||
| Dec | ++(G3, G3e, G8,G9) | ++(GI.6) | ++ | ++ | ++ | +++(CAstV) | ||
| Jan | ++(G1,G3, G3e, G8,G9) | ++(GI.3) | ++ | + | ||||
| Feb | ++(G1,G3, G8) | ++ | ++ | +++(CAstV) | ||||
| Mar | ++(G1,G2,G3, G3e, G8,G9) | ++ | ++(GII.17) | +++ | +++(CAstV) | + | ||
| Apr | ++ (G1,G2,G3, G3e,G9) | ++ | ++(GII.2) | +++ | ||||
| May | + | ++(GII.2) | ++ | ++ | + | |||
| Jun | W | W | ++(GII.2) | ++ | ++ | |||
| 2021–2022 |
Jul | + | ++ | ++ | ||||
| Sep | ++(GII.2) | +++ | +++ | ++ | ||||
| Oct | +++(G2,G3, G3e,G9) | ++(GII.2) | +++ | +++(EV-A) | ||||
| Nov | + | ++(GII.2) | +++ | +++ | +++ | +++(EV-A) | ||
| Dec | ++(GI.3) | ++(GII.2) | +++(GI.1) | +++(F41) | +++(MLB1) | + | ||
| Jan | +++ (G2,G3, G3e, G8,G9) | ++(GI.3) | ++(GII.2) | +++(GI.1) | +++(F41) | +++(MLB1) | ||
| Feb | +++(G10) | ++(GI.1) | ++(GII.17) | +++(GI.1) | +++ | +++(CAstV) | ||
| Mar | +++(G3) | ++(GI.1) | ++(GII.2) | +++ | +++(F41) | +++(CAstV) | ||
| Total Positives (%) | 24 (61.5) | 23(59.0) | 22(56.4) | 29(74.4) | 18(46.1) | 23(59.0) | 19(48.7) | |
++/+++ Found in 1st PCR; Weak (W)/+ Found in 2nd PCR.
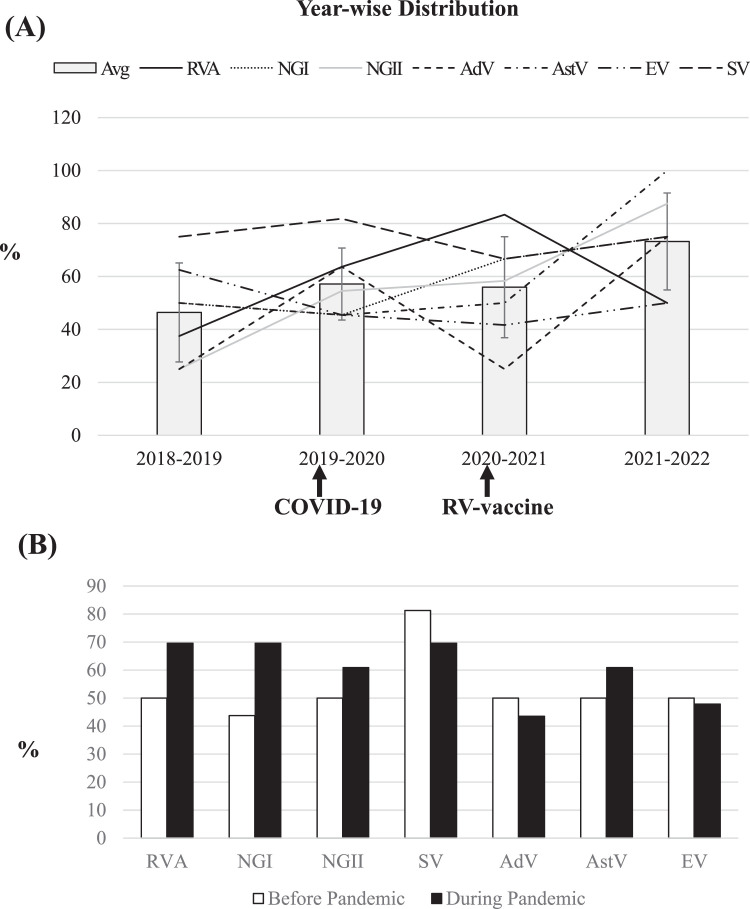
To understand the impact of NPIs in reducing viral AGE in the community, we analyzed year-wise distributions of AGE viruses in SW. In Japan, the first COVID-19 case was identified on 14 January 2020, though a series of containment measures including suspension of large-scale gatherings, campaigns for hygiene measures, and national school closures were implemented from early March 20208 which effectively reduced not only COVID-19 but also viral AGE in Japanese Children (data not shown). However, we found no decline in the detection of major diarrheal viruses in SW during the pandemic (Fig. 1 A). The average detection rates were observed: 46% in 2018–2019, 57% in 2019–2020, 56% in 2020–2021, and 73% in 2021–2022. The comparison of detection rates of individual enteric viruses before and during the COVID-19 pandemic revealed that these viruses remained similar or more abundant in SW during the pandemic than before (Fig. 1B). Furthermore, the genotypic distributions for every enteric virus also looked similar in pre- and post-COVID-19 emerging eras (Table 1). Our phylogenetic analyses suggest that although some of these stains appeared similar to animal strains (e.g., some RVA from bovine/dog/cat/porcine/equine, and some canine AstV), and some EVs remained associated with other diseases than AGE (e.g., HFMD, herpangina, and hemorrhagic conjunctivitis), yet the most of these viruses remained closely related to human strains causing AGE, suggesting that the AGE viruses existed similarly in the community during the pandemic as before.
Fig. 1.
Distribution of major diarrheal viruses in raw SW. (A) Year-wise distribution of each virus (line) and their averages (column) have been shown in percentage among the total number of samples investigated in that year. The emergence of COVID-19 and the introduction of the RV vaccine in the national immunization program have been indicated in the timeline with arrows. Error bars represent ± standard deviations. (B) The percentages of enteric viruses detected in SW before (Aug 2018-Feb 2020) and during (Mar 2020-Mar 2022) COVID-19 pandemic period.
Another vital observation of this study included no decrease of RVA in SW even after the introduction of RV vaccines in the Japanese national immunization program in October 2020 (Fig. 1A). In fact, RV vaccines remain successful in reducing disease severity, but cannot prevent the occurrence of RV infection.9 In addition, the diversity in RV genotypes that was found in the post-RV-vaccination era in clinical samples10 was also observed in raw sewages in both pre- and post-COVID-19 emerging eras (Table 1).
Finally, in this study, we detected a high abundance of diarrheal viruses in raw sewages during the COVID-19 pandemic, when viral AGE patients were decreased dramatically in clinics. It was thought that the precautious lifestyle adopted during the COVID-19 pandemic has decreased viral AGE sharply. But, indeed, the decline in the case number was probably due to less transmission because of school closure or activity restrictions; and/or uninterested medical care-seeking behavior of patients amid the pandemic resulting in less reporting which has already begun to disappear, and more disaster is ahead. Special preparedness is essential for rapid control of the re-emergence of infectious diseases before it attends another outbreak.
Declarations of Competing Interest
We do not have any association either directly or indirectly that might pose a conflict of interest.
Acknowledgments
The research was performed by an International Research Fellow of the Japan Society for the Promotion of Science (Invitational Fellowships for Research in Japan (Long-term)). This study was supported by Grants-in-Aid for Japan Agency for Medical Research and Development (AMED) [grant number JP22fk0108122 and JP22wm0225006 to S.S., T.K., and H.U]; and the Nihon University Research Grant for 2022. We express utmost gratitude to the former Director, Prof. Dr. M. A. Malek, and present Director, Prof. Dr. Ishtiaque M. Syed, of the Centre for Advanced Research in Sciences (CARS), the University of Dhaka, Bangladesh for allowing this collaborative study between the University of Dhaka, Bangladesh, and Nihon University School of Medicine, Japan. We gratefully acknowledge all members of the Division of Microbiology, Department of Pathology and Microbiology, Nihon University School of Medicine, for their generous cooperation throughout this work.
References
- 1.Eigner U., Verstraeten T., Weil J. Decrease in norovirus infections in Germany following COVID-19 containment measures. J Infect. 2021;82(6):276–316. doi: 10.1016/j.jinf.2021.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou J., et al. Changes of Haemophilus influenzae infection in children before and after the COVID-19 pandemic, Henan, China. J Infect. 2022 doi: 10.1016/j.jinf.2022.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Han X., et al. Incident changes in the prevalence of respiratory virus among children during COVID-19 pandemic in Hangzhou, China. J Infect. 2022;84(4):579–613. doi: 10.1016/j.jinf.2022.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu P., Xu J. Resurgence of influenza virus activity during COVID-19 pandemic in Shanghai, China. J Infect. 2022 doi: 10.1016/j.jinf.2022.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thongprachum A., et al. Detection of nineteen enteric viruses in raw sewage in Japan. Infect Genet Evol. 2018;63:17–23. doi: 10.1016/j.meegid.2018.05.006. [DOI] [PubMed] [Google Scholar]
- 6.Fujii Y., et al. Improvement of Rotavirus Genotyping Method by Using the Semi-Nested Multiplex-PCR With New Primer Set. Front Microbiol. 2019;10:647. doi: 10.3389/fmicb.2019.00647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoque S.A., et al. An increasing trend of human sapovirus infection in Japan, 2009 to 2019: an emerging public health concern. J Infect Public Health. 2022;15(3):315–320. doi: 10.1016/j.jiph.2022.01.019. [DOI] [PubMed] [Google Scholar]
- 8.Imai N., et al. COVID-19 in Japan, January-March 2020: insights from the first three months of the epidemic. BMC Infect Dis. 2022;22(1):493. doi: 10.1186/s12879-022-07469-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoque S.A., et al. Role of rotavirus vaccination on an emerging G8P[8]rotavirus strain causing an outbreak in central Japan. Vaccine. 2018;36(1):43–49. doi: 10.1016/j.vaccine.2017.11.056. [DOI] [PubMed] [Google Scholar]
- 10.Hoque S.A., et al. Distribution of rotavirus genotypes in Japan from 2015 to 2018: diversity in genotypes before and after introduction of rotavirus vaccines. Vaccine. 2020;38(23):3980–3986. doi: 10.1016/j.vaccine.2020.03.061. [DOI] [PubMed] [Google Scholar]