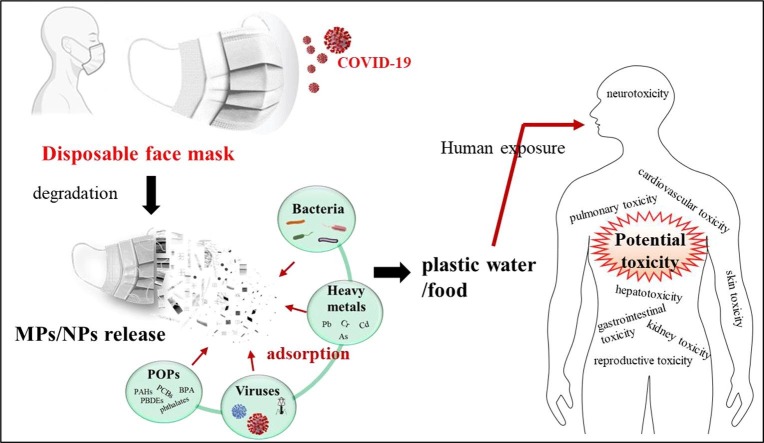
Graphical abstract
Keywords: Disposable face masks, Microplastics, Nanoplastics, Human health, Toxicity assessment
Abstract
With the global spread of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), disposable face masks (DFMs) have caused negative environmental impacts. DFMs will release microplastics (MPs) and nanoplastics (NPs) during environmental degradation. However, few studies reveal the release process of MPs/NPs from masks in the natural environment. This review presents the current knowledge on the abiotic and biotic degradation of DFMs. Though MPs and NPs have raised serious concerns about their potentially detrimental effects on human health, little attention was paid to their impacts on human health from DFM-derived MPs and NPs. The potential toxicity of mask-derived MPs/NPs, such as gastrointestinal toxicity, pneumotoxicity, neurotoxicity, hepatotoxicity, reproductive and transgenerational toxicity, and the underlying mechanism will be discussed in the present study. MPs/NPs serve as carriers of toxic chemicals and pathogens, leading to their bioaccumulation and adverse effects of biomagnification by food chains. Given human experiments are facing ethical issues and animal studies cannot completely reveal human characteristics, advanced human organoids will provide promising models for MP/NP risk assessment. Moreover, in-depth investigations are required to identify the release of MPs/NPs from discarded face masks and characterize their transportation through the food chains. More importantly, innovative approaches and eco-friendly strategies are urgently demanded to reduce DFM-derived MP/NP pollution.
1. Introduction
The global outbreak of Coronavirus Disease-2019 (COVID-19) has caused serious human health concerns. Globally, as of 22 July 2022, confirmed cases of COVID-19 have passed more than 565 million, including over 6 million deaths, were reported by World Health Organization (WHO) (WHO 2022). Since the wide spread of COVID-19, the demand for medical equipment is drastically increased, ultimately increasing medical and healthcare waste at an alarming rate (Al-Omran et al., 2021, Chowdhury et al., 2022). Wearing face masks in public transport and spaces is a feasible way to curb the infection and transmission of the virus, leading to increasing demand for face masks (Cheng et al., 2020, Tesfaldet et al., 2022). To date, the use and production of face masks have gradually increased to an unprecedented level. The WHO estimated that roughly 89 million medical masks will be required globally per month (WHO 2020). However, inadequate management of these used masks has posed threats to public health and environment, especially in developing countries (Chen et al., 2021b, Kwak and An, 2021, Liang et al., 2021, Morgana et al., 2021). Discarded masks have been widely observed in our surrounding environments, such as urban spaces (streets, parks, and gardens), fresh water (lakes and rivers), and marine environments (beaches and oceans) (Aragaw et al., 2022, Cordova et al., 2021, De-la-Torre et al., 2022, Gunasekaran et al., 2022, Kwak and An, 2021, Tesfaldet et al., 2022; Wang et al. 2021a). It was estimated that 10 million masks (equal to 30,000–40,000 kg) will be dispersed in the environment per day even considering only 1 % of disposable face masks (DFMs) (World Wildlife Fund 2020). Therefore, approximately 15 years would be needed to degrade half of marine masks (Chen et al. 2023). However, limited studies reported the degradation of DFMs in natural environment.
The materials commonly used in DFMs are plastic polymers such as polycarbonate (PC), polypropylene (PP), polystyrene (PS), polyurethane (PU), polyacrylonitrile (PAN), polyethylene (PE), or polyester (PES) (Fadare and Okoffo, 2020, Potluri and Needham, 2005). In the natural environment (weathering, temperature, solar ultraviolet (UV) radiation, pH, and biological degradation), discarded face masks can be gradually degraded into smaller plastics, such as microplastics (MPs) and nanoplastics (NPs), and give rise to a new environmental challenge (Chen et al., 2021b, Kwak and An, 2021). Moreover, plastic residue (tubes, pipet tips, falcon tubes, buffer bottles, and medical globes) wastes after polymerase chain reaction testing for COVID-19 diagnosis also are potential sources of plastic waste (Aragaw and Mekonnen 2022). MPs are often defined as plastic particles smaller than 5 mm in dimension, and NPs are ranging from 1 nm to 1 μm (Gigault et al. 2018). However, there is yet no universally accepted definition of the relevant plastic size range. DFM-derived MPs/NPs could readily get into waterways from where they were released and reach the aquatic ecosystem. Aquatic MP/NP issues are of particular concern since they can be transported over a long distance to diverse aquatic biota and ultimately transferred along the food chain (Chowdhury et al. 2021). For instance, MPs/NPs have already been observed in shellfish and fish consumption (Barboza et al., 2018, Clere et al., 2022, Smith et al., 2018). Thus, the bioaccumulation of and biomagnification of MPs in aquatic products could cause detrimental effects on human health. Not surprisingly, recently published studies detected MPs/NPs in human feces and revealed the level of MPs was closely associated with inflammatory bowel disease (IBD) status (Schwabl et al. 2019; Yan et al. 2020b; Yan et al. 2021).
Given the physical properties of plastics, the negative effects of MPs/NPs on organism are closely associated with their types, sizes, shapes, and concentrations (Qin et al., 2021, Schwarzer et al., 2022, Xu et al., 2021b, Zhang et al., 2022a). Moreover, various additives used in masks, such as plasticizers, pigments, and dyes, might also pose direct risks to environmental and public health (Lellis et al., 2019, Xie et al., 2022). The toxic effects of MPs/NPs also can be enhanced by their ability to adsorb chemical toxicants from the surrounding water (Bakir et al., 2014, He et al., 2022, Suzuki et al., 2020). MPs/NPs can not only act as carries of pollutants, such as persistent organic pollutants (POPs) (e.g. cpolycyclic aromatic hydrocarbons [PAHs], perfluorooctanoic acid [PFOA], polychlorinated biphenyls [PCBs], and polybrominated diphenyl ethers [PBDEs]) and heavy metals (e.g., arsenic [As], chromium [Cr], copper [Cu], cadmium [Cd], and lead [Pb]), but also transfer pathogenic bacteria and viruses through the food chain to humans (Bakir et al., 2014, Herrera et al., 2022, Li et al., 2022d; Qin et al. 2022; Wang et al. 2021a; Wang et al. 2021c; Zhang et al. 2022b; Zhang et al. 2022b; Zhou et al. 2022a). More recently, MPs/NPs also serve as an “antibiotic-resistant reef” providing an interface for the enrichment of antibiotic resistance genes (Liu et al. 2021b; Wang et al. 2022a; Zhou et al. 2022b). Although there are growing studies on DFM-derived MP/NP pollution, limited data on the potentially toxic effects they might have on aquatic animals, how they transfer through the food chain, and their direct or indirect impacts on human health.
In general, human exposure to face mask-derived MPs/NPs often happen through dermal contact (skin), inhalation (air), and ingestion (food and water). Therefore, it is urgent to estimate their potential adverse effects on humans, especially developing fetuses. Aiming at this case, our study provides an overview of the potential source of MPs/NPs from DFMs in detail about their types (surgical, N95, and cotton masks) and natural degradation (abiotic and biotic processes). Moreover, previously published reports about their toxic effects on different organs (gastrointestinal toxicity, pulmonary toxicity, neurotoxicity, hepatotoxicity, cardiovascular toxicity, kidney toxicity, skin toxicity, and reproductive toxicity) will be consulted and studied. The potential mechanism, directly or indirectly, is also needed to explain the impacts on humans brought by MPs/NPs. Given ethical issues and species difference between humans and animals, more studies combined with novel in vitro models need to be conducted to evaluate the potential risks of face mask-derived MPs/NPs. Moreover, in-depth investigations are demanded to identify the release of MPs/NPs from abandoned DFMs and characterize the transport of MPs/NPs in the food chains. At last, innovative methods and sustainable strategies to reduce DFM-derived MP/NP pollution will be discussed.
2. The release of MPs/NPs from disposable face masks
2.1. Diverse types of face masks
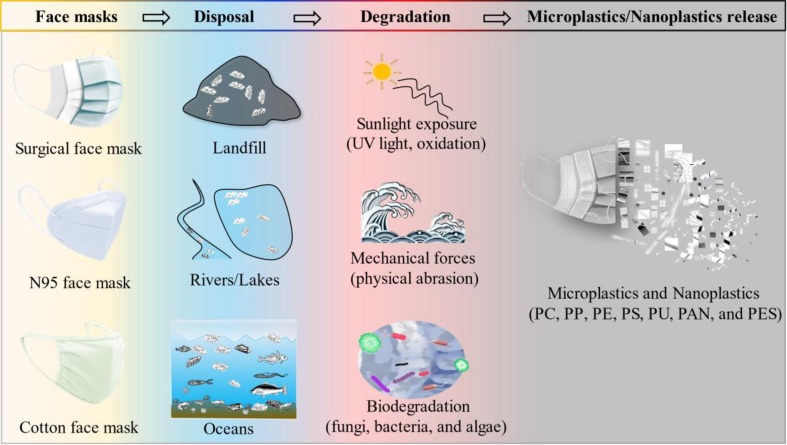
COVID-19 disease has made a profound impact across the world. The spread of the deadly coronavirus can be optimally prevented or avoided by using personal protective equipment (PPE) including protective clothing, helmets, gloves, face shields, goggles, and face masks. Face masks are the most widely used type of PPE due to their effectiveness and low cost. Governments and public health agencies across the world have passed legislation requiring the use of masks when taking public transportation and going to public areas. This has brought about a phenomenal rise in the worldwide production of face masks. Wearing face masks has gradually become a habit of people. The most commonly available face masks include medical or surgical masks, respirators, and cotton face masks (Fig. 1 ). However, controversy remains on the proper type of face masks and the situation in which they need to be used in community and health care settings to prevent SARS-CoV-2 infection (Qaseem et al. 2020).
Fig. 1.
Microplastics and nanoplastics are released from face masks. There are three commonly used face masks include surgical, N95, and cotton face masks. Improper waste management has caused used face masks to appear in landfill, rivers and lakes, and oceans. Once in the natural environment, face masks could release numerous MPs and NPs under sunlight exposure, mechanical forces, and biodegradation.
Medical or surgical masks are highly recommended by WHO for nurses, doctors, and patients to protect themselves from COVID-19 exposure. Increasing evidence suggests that SARS-CoV-2 can be transmitted via respiratory droplets and aerosols (Jayaweera et al., 2020, van Doremalen et al., 2020). Surgical face masks can protect healthcare workers against droplet communication. Although Milton et al. showed that surgical masks might not be able to effectively limit the emission of small droplets, more researchers have shown that no significant difference was found between the surgical masks and N95 masks in the protection from viral pathogens (Chua et al., 2020, Johnson et al., 2009, Milton et al., 2013). The common surgical masks consist of four parts (Long et al., 2020, Shen et al., 2021, Smith et al., 2016). The outer most superficial layer is composed of non-woven fibers (e.g., PES) that are water-resistant and normally colored. The middle layer is made up melt-blown filters (e.g., PP and PS), which are the core material for virus rejection. The inner layer and ear band are made of fiber materials. Therefore, MPs/NPs will be generated during the degradation of face masks (Aragaw, 2020, Fadare and Okoffo, 2020).
The N95 respirator is a respiratory protective device designed to achieve a very close facial fit and is the most common of the particulate filtering facepiece respirators (FFRs). The N95 designation is certified by the United States National Institute for Occupational Safety and Health (NIOSH), meaning that it removes at least 95 % of airborne particles. The N95 four-layer respirator consists of the inner, support layer, filter, and outer filter layer from inside to outside (Zhou et al. 2018). The innermost layer is constructed of non-woven PP which minimizes moisture from breathing into the mask materials. The support layer is composed of modacrylic that provides rigidity and adds thickness to the mask, giving it more structure and adding to the feel of comfort. The filter layer is made up non-woven PP and melt-blown to capture oil and non-oil based particles. The outer filter layer is constructed of hydrophobic non-woven PP that prevents external moisture from entering the mask. It was reported that the N95 respirator prevented air leakage more effectively than the surgical mask (Dbouk and Drikakis 2020).
Cloth face masks are made of cloth materials, including cotton, gauze, silk, or muslin. During the outbreak of SARS in 2002, cotton cloth masks were reported to effectively protect Health Care Workers and the general public in China (Pang et al. 2003). After the coronavirus outbreak, cloth masks might be the only viable option if the medical masks are unavailable in stock. However, cloth masks may provide a greater opportunity for air leakage around the sides, which may decrease their filtration efficiency, compared to surgical masks and N95 respirators (Jain et al. 2020). Ma et al. demonstrated that cloth homemade masks blocked 95.15 % of avian influenza virus, while surgical masks and N95 respirators were comparable (97.14 % and 99.98 %, respectively) (Ma et al. 2020). Although cloth masks exhibit less effective degree of protection than surgical masks and N95 respirators, to some extent, more research should be conducted to assess the effectiveness of cloth masks for virus prevention. Moreover, unlike surgical masks and N95 respirators, cloth masks can be reused after being sterilized and decontaminated contributing to pro-environment and sustainability. However, cloth mask users may put themselves at risk of contamination during washing and decontaminating of used masks (Chughtai et al., 2019, MacIntyre et al., 2017). Although the material of cloth masks may be unlike to degrade from normal degradation procedures, previous study also proved that fibers could be released from washing cloth masks (Chughtai et al., 2020, Das et al., 2021, Li et al., 2021a).
2.2. The degradation of disposable face masks
The massive use of face masks caused environmental disorder both in the terrestrial environment and aquatic medium during the COVID-19 scenario (Du et al. 2022). Once in the environment (disposal in landfill or discarded in public places, rivers, lakes, and oceans), discarded face masks can be gradually degraded or break down into numerous smaller plastic debris under environmental weathering, such as abiotic and biotic processes, becoming a new source of MPs/NPs (Fig. 1). The abiotic processes refer to the physicochemical and mechanical factors, such as sunlight exposure, temperature, and mechanical forces, while the biotic processes are related to the participation of animals, plants, fungi, and bacteria (Zhang et al. 2021c). Notably, biotic and abiotic processes often work in tandem in the natural environment.
Sunlight (UV-radiation) and oxygen are vital factors of abiotic plastic degradation in the environments (Chubarenko et al., 2020, Gewert et al., 2015, Mao et al., 2020). Plenty of synthetic polymers could absorb solar UV radiation and free radicals can react with oxygen to produce peroxy radicals that mediate oxidative reactions, leading to the initial degradation of polymers with C—C and C—H bonds (Chamas et al. 2020; Zhang et al. 2021c). Smaller polymer fragments formed by chain scission are more susceptible to degradation (Gewert et al., 2015, Wang et al., 2016). Light-induced oxidative degradation of polymers was found to be orders of magnitude faster compared to other types of degradation processes. However, the rate of degradation of plastics in the ocean was much slower than that of in the terrestrial environment due to the deficiency of oxygen and lower temperature (Anderson et al., 2016, Andrady, 2011, Chubarenko et al., 2020, Wang et al., 2016). Moreover, other factors include the differences in polymer structure and component are closely associated with the photodegradation processes (Min et al. 2020; Zhang et al. 2021c). A recent study showed that the middle layer (the melt-blown cloth) of masks was the most sensitive part to UV radiation and could release numerous MPs into the aqueous environment (Wang et al. 2021a). These data suggest that photo-oxidation of abandoned masks contributes to plastic degradation in the environment.
It has been widely recognized that mechanical degradation was the primary and critical process for the generation of MPs/NPs, especially on the beaches due to the wind and wave action, and tidal currents (Chubarenko et al., 2020, Song et al., 2017). Mechanical forces (wave action and abrasion with sand) can cause the flaking of weathered or oxidized surfaces, leading to ablation (Andrady, 2017, Chamas et al., 2020). To investigate the behavior of face masks in shoreline conditions, the masks were put into the water in the presence of sand (Wang et al. 2021a). The study showed that the physical abrasion from sand promoted the release of plastics in the aquatic environment. Coupled with UV radiation, physical abrasion could accelerate the fragmentation and photodegradation of abandoned masks (Song et al. 2017). A recent study demonstrated that MPs could be detected in artificial seawater with shaken continuously using a mechanical shaker for nine days (Sun et al. 2021a). Moreover, a surgical mask may release approximately 173,000 fibers/day in artificial seawater with 180 h of UV-light radiation combined with vigorous stirring (Saliu et al. 2021).
Biotic degradation of plastics refers to the deterioration of plastics caused by living microorganisms, such as fungi, bacteria, and algae (Gautam et al., 2007, Kyrikou and Briassoulis, 2007, Zeenat et al., 2021). Previous studies have shown that microbial strains could cause the weight loss of polymers and fractures and pores on the polymer surface (Harshvardhan and Jha, 2013, Wang et al., 2016, Zettler et al., 2013). Plastic biodegradation can be categorized as aerobic and anaerobic biodegradation based on the presence or absence of oxygen. In aerobic biodegradation, aerobic microbes break down large matter into smaller molecules with CO2 and water by using oxygen as an electron acceptor (Fesseha and Abebe, 2019, Priyanka and Archana, 2011, Zeenat et al., 2021). In anaerobic biodegradation, nitrate, iron, sulfate, manganese, and CO2 are used as electron acceptors by anaerobic bacteria to break down organic chemicals. Biodegradation of polymers entails three steps: (a) attachment of the microorganism to the surface of the polymer; (b) using the polymer as a carbon source for the growth of the microorganism; and (c) degradation of the polymer (Gu, 2003, Singh and Rawat, 2020). It was reported that the formation and development of biofilms onto MPs/NPs from microorganisms including pathogens, will influence plastic fragmentation and degradation (Jiang, 2018, Tu et al., 2020). Nonetheless, the biodegradation mechanism of plastics has yet to be fully elucidated. Furthermore, the biodegradation of plastics might be influenced by the physicochemical characteristics of plastics, including molecular types, weight, crystallinity, and hydrophobicity, as well as plasticizers or additives used in plastics (Lin et al., 2022, Yuan et al., 2020). Therefore, a full investigation is necessary to determine the biodegradation of discarded masks by microorganisms.
3. Potential toxicity of microplastics on human organs
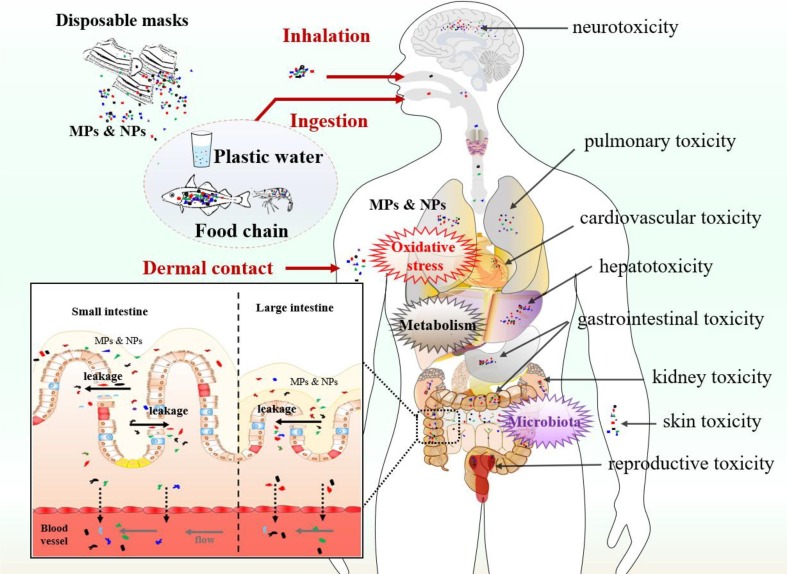
In recent years, increasing studies have reported that MPs/NPs accumulated in animal intestine, lung, brain, live, kidney, and testis after exposure, and induced cell apoptosis, acute or chronic inflammation, and functional abnormality in multi-organs (Kwon et al. 2022; Liu et al. 2022c; Xu et al. 2021a; Zheng et al. 2021). These studies provide evidence regarding the risk of human exposure to MPs/NPs and help us to predict the possible adverse effects of DFM-derived MPs/NPs on humans (Fig. 2 ).
Fig. 2.
Potential toxicity of disposable face mask-derived microplastics and nanoplastics on human organs. Human exposure to MPs/NPs may happen through dermal contact, inhalation, and ingestion (for instance, MPs/NPs cross the leakage of intestinal barriers and induce endothelial leakiness in vessels (Wei et al. 2022)). MPs/NPs could cause gastrointestinal toxicity, pulmonary toxicity, neurotoxicity, hepatotoxicity, cardiovascular toxicity, kidney toxicity, skin toxicity, and reproductive toxicity through oxidative stress, aberrant metabolism, and microbiota dysbiosis.
3.1. Gastrointestinal toxicity
The gut is one of the most important tissues which could be affected by DFM-derived MPs/NPs directly. Existing literature revealed that there was a median of 20 pieces of MPs per 10 g of human stool (Schwabl et al. 2019). Moreover, MPs were observed in human colectomy specimens, showing that PC was most abundant in colonic tissue samples (Ibrahim et al. 2021). However, it was different from Schwabl’s study, which found that PP was the most frequent polymer detected (Schwabl et al. 2019). The study indicated that the increased intestinal permeability, such as in IBD, may have a higher risk of MP translocation into deeper tissues and even the circulatory system which could lead to unpredictable adverse effects on human patients (Schmidt et al. 2013). Although studies in marine species showed that MPs induced aberrant intestinal permeability and dysbiosis, which may associate with intestinal inflammation, the relationship between MP exposure and intestinal toxicity in humans remains speculative due to the species differences (Qiao et al. 2019b). Obviously, gut microbiota plays a critical role in maintaining the host's metabolic homeostasis and health (Qin et al. 2022). Previous studies have shown that exposure to MPs caused intestinal microbiota dysbiosis in zebrafish and mice (Hu et al., 2022, Jin et al., 2018, Lu et al., 2018b). The decreased diversity of gut microbiota was closely correlated with the aberrant lipid metabolism (Karlsson et al., 2013, Qiao et al., 2019b). Therefore, exposure to DFM-derived MPs from food chains could cause human gastrointestinal toxicity.
3.2. Pneumotoxicity
Although studies speculated that humans may exposure to MPs/NPs through food and drinking water, MPs have been detected in human nasal mucus after wearing a mask (Ma et al., 2021, Nelms et al., 2018; Yan et al. 2020b). Similarly, MPs were detected in all regions of the human lung, indicating that MPs/NPs might translocate into the human body through inhalation (Jenner et al. 2022). Therefore, it is urgent to investigate whether DFM-derived MPs/NPs by inhalation cound cause adverse effects on lung tissues. For instance, Shi and colleagues showed that PS-MPs affected the interfacial properties of lung surfactants, including phase behavior, surface tension, and membrane structure (Shi et al. 2022). Moreover, PS-MP exposure accelerated conversion between ascorbic acid and deoxyascorbic acid, as well as increased the production of the level of hydroxyl radicals. Exposure to PS-MPs was also found to reduce the permeability of airway epithelium and impair lung barrier function (Dong et al. 2020). A similar result was observed by Yang’s group, showing that PS-NPs disrupted the alveolar epithelial barrier and lung function (Yang et al. 2021). PS-MPs induced alveolar destruction, bronchial epithelium disarrangement, and lung inflammation may be closely associated with altered expression of long noncoding RNAs and circular RNAs (Fan et al. 2022). Although a growing number of studies demonstrated the existence of MPs in the air, it remains unclear whether inhaled MPs/NPs are from DFMs following occupational exposure. There are limited studies reported human exposure to MPs/NPs through inhalation during the wearing of face masks (Li et al. 2021a). Furthermore, following intestinal absorption, research on the transportation of MPs/NPs into the circulation and finally deposited in the lung tissue is very scarce (Deng et al. 2017).
3.3. Neurotoxicity
The central nervous system has been identified as a sensitive target for nanoparticles (Prust et al., 2020, Teleanu et al., 2019). In recent studies, MPs/NPs absorbed by the gut can cross the blood–brain barrier (BBB) and exert neurotoxic effects (Kogel et al., 2020, Lusher et al., 2017, Prust et al., 2020). The accumulation of PS-MPs in mice brains was found after oral and intraperitoneal administration (Estrela et al., 2021, Kwon et al., 2022). In vitro study showed that the internalization of MPs/NPs was observed in neuronal cells (Hoelting et al., 2013, Murali et al., 2015). Existent evidence showed that short- and long-term PS exposure disrupted neuronal development in nematodes (Chen et al., 2021a, Qu et al., 2019, Qu and Wang, 2020). Recently, in the neurons, RNAi knockdown of eat-4 could induce resistance to PS-NPs toxicity in Caenorhabditis elegans (Wang et al. 2021b). Alteration regulation of cholinergic nervous transmission, particularly acetylcholinesterase (AchE) activity, was also found in fish after MP exposure (Bhagat et al. 2020). The inhibition of AchE caused by MPs/NPs could increase acetyl choline levels, leading to the disruption of the nervous system, such as motor dysfunction and behavioral abnormalities (Kim et al. 2021). Meanwhile, the visual system was also affected by MPs as illustrated by the upregulated visual-related gene expression (Chen et al. 2017a). Moreover, PS-NP exposure was found to induce defective neural tube morphogenesis during mice embryogenesis (Nie et al. 2021). However, whether DFM-derived MPs/NPs could cross the BBB by circulation and exert toxic effects on the nervous system of humans, remains to be determined.
3.4. Hepatotoxicity
The liver is a large and critical detoxifying organ in the human body. It was well accepted that MPs can accumulate in the living organism's liver and cause hepatotoxic effects (Araujo et al., 2020, Shen et al., 2022, Zitouni et al., 2022). For instance, PS-MPs were observed in zebrafish liver and induced oxidative stress (Lu et al. 2016). PS-MPs were also found to induce inflammation and lipid accumulation in the liver as illustrated by the high levels of necrosis, infiltration, and lipid droplets (Lu et al. 2016). Moreover, PS-MP exposure disrupted the lipid and energy metabolism in the liver (Deng et al. 2017; Lu et al., 2018b, Zhao et al., 2020, Zheng et al., 2021). The hepatotoxicity of MPs was closely associated with the activation of cGAS/STING (cyclic guanosine monophosphate-adenosine monophosphate synthase-stimulator of interferon gene) and Keap1-Nrf2 (kelch-like ECH-associated protein 1-nuclear factor-E2-related factor) signaling pathway (Li et al., 2021b, Shen et al., 2022). MP-induced liver injury was also evidenced by activating pyroptosis and ferroptosis, which were caused by intense oxidative stress and inflammation in mice (Mu et al. 2022). Furthermore, PS-MPs-induced the hepatotoxicity, such as hepatocyte apoptosis and abnormal glycolytic flux, could be caused by reactive oxygen species (ROS)-driven calcium overload (Li et al. 2021a). These findings provide new insights for uncovering the potential hepatotoxicity of DFM-derived MPs/NPs.
3.5. Others
The deposition and accumulation of MPs in the kidneys could induce kidney damage (Deng et al. 2017; Wang et al. 2021d; Yang et al. 2019). In vivo study, Meng et al. demonstrated that the intake and bioaccumulation of PS-MPs/NPs in the kidneys induced oxidative stress and kidney inflammation in mice (Meng et al. 2022). In vitro study, uptake of PS-MPs by human kidney proximal tubular epithelial cells (HK-2 cells) caused mitochondrial dysfunction, endoplasmic reticulum stress, and inflammation (Wang et al. 2021d). Moreover, it has been speculated that particles (< 100 nm) could penetrate the skin barrier and cause potential skin toxicity (Revel et al., 2018, Schirinzi et al., 2017).
The possible mechanisms on cardiovascular toxicity of NPs were investigated by Sun’s group (Sun et al. 2021b). Exposure to NPs caused severe pericardial edema and significantly decreased cardiac output and blood flow velocity, ultimately affecting thrombosis in zebrafish embryos. Similarly, MP exposure was found to cause cardiotoxicity in chicken and rats (Li et al. 2020c; Wei et al. 2021; Zhang et al. 2022d). Recently, plastic particles were detected in human blood and even accumulated in human thrombi (Leslie et al., 2022, Wu et al., 2022). These studies provide an essential clue for the potential toxicity of DFM-derived MPs/NPs on human cardiovascular systems.
More recently, researchers have explored the potential toxicity of DFM-derived MPs/NPs on reproductive toxic effects (Ferrante et al., 2022, Park et al., 2020, Sendra et al., 2022, Yan et al., 2020a). For instance, Sendra et al. found that face mask degradation fibers strongly impacted different steps of reproduction of Danio rerio, including gametogenesis, sperm-egg recognition and binding or fertilization (Sendra et al. 2022). Similarly, PS-MP exposure caused sperm deformity, promoted testicular inflammation, and disrupted the blood-testis barrier of male mice (Hou et al., 2021, Jin et al., 2021). In female mice, PS-MPs were found in uterus and decreased the levels of glutathione, mitochondrial membrane potential and endoplasmic reticulum calcium, and increased ROS in oocytes of mice (Li et al. 2022e). These studies indicated that DFM-derived MP/NP exposure could disrupt the function of the reproductive organ.
Recently, plastics were found in the placentas and could be maternally delivered to the next generation, causing detrimental effects on fetal development and offspring (Aghaei et al., 2022, Fournier et al., 2020, Huang et al., 2022, Pitt et al., 2018). It was found that maternal exposure to PS-MPs and PS-NPs brought about fetal growth restriction in mice (Aghaei et al. 2022). Jeong and colleagues proved that maternally ingested PS-NPs reached the developing brain of next-generation mice through maternal breast milk and caused brain dysfunction and impairment in cognition (Jeong et al. 2022). Similarly, Fournier et al. observed the transfer of PS from the maternal lung, crossed the placenta to the fetal circulation, and deposited in fetal tissues, such as the liver, lung, kidney, heart, and brain after maternal pulmonary exposure (Fournier et al. 2020). These data present a crucial first step toward evaluating the trans-generational toxicity of maternal exposure to DFM-derived MP/NPs.
4. The underlying toxic mechanism of discarded mask-derived MPs/NPs
4.1. Directly toxic effects
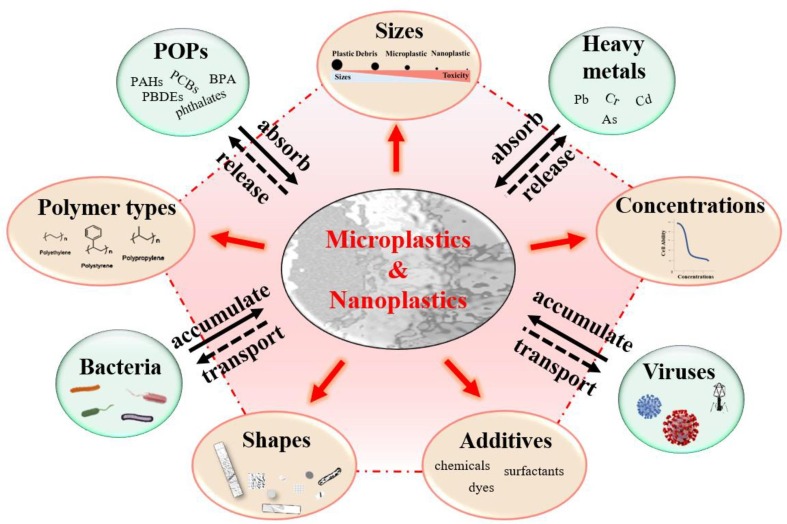
The directly detrimental effects of MPs/NPs can be categorized into physical and chemical injuries (Campanale et al. 2020). The former effects are associated with the type, size, shape, and level of MPs/NPs released from discarded masks, and the latter is associated with hazardous additives used in masks. Different types, sizes, shapes, and concentrations of MPs/NPs from DFMs have been characterized by atomic force microscopy, energy dispersive X-ray detector, scanning electron microscope, and fourier transform infrared spectroscopy (Chen et al., 2021b, Kwak and An, 2021, Liang et al., 2021, Ma et al., 2021, Morgana et al., 2021, Shen et al., 2021). It has been well accepted that MPs/NPs exerted toxicity in type-, size-, shape-, and dose-dependent manners (Fig. 3 ) (Qin et al., 2021, Schwarzer et al., 2022, Xu et al., 2021b, Zhang et al., 2022a). For instance, Brehm et al. conducted an in-depth characterization of fragments in the same level and size range, and explored the polymer type and chemical content-specific effects of MPs on Dreissena bugensis (Brehm et al. 2022). Lei et al. compared the adverse influences of five common types of MPs: polyamides (PA), PE, PP, polyvinyl chloride (PVC), and PS on Danio rerio (Lei et al. 2018). The study found that the intestinal toxicity of MPs is closely associated with their sizes, rather than their chemical constituents. However, different polymer types of MPs were reported to impact the distribution, accumulation, and toxic effects of pollutants on contamination (Sheng et al. 2021). Therefore, it is necessary to consider the influence of polymer types when evaluating the environmental risk of MPs.
Fig. 3.
The potential source of toxicity from microplastics and nanoplastics. The toxic influences of MPs/NPs are closely related to the polymer types, sizes, shapes, and concentrations of MPs/NPs and the usage of commercial additives. MPs/NPs can sorb environmental contaminants including POPs (persistent organic pollutants), heavy metals, bacteria, and viruses, and act as vectors to transport contaminants, resulting in the bioaccumulation and biomagnification of toxic substances.
As DFMs degrade in the natural environment, they are commonly decomposed into MPs/NPs with different sizes and concentrations, showing size- and concentration-dependent toxicity (Chen et al. 2020; Zhang et al. 2022a). The study conducted by Zhang’s group showed that the early developmental toxicity of PS-MPs exhibited a size-dependent manner, smaller MPs exerted more toxicity (Zhang et al. 2022a). Bigger plastics have lower bioreactivity than smaller plastics due to their lower surface-to-volume ratio, resulting in they cannot being internalized by cells (Deng et al., 2017, Gautam et al., 2022, Stock et al., 2019). Therefore, it was speculated that NPs could cross the barrier, such as the BBB, to some extent (Zhang et al. 2022a). Nonetheless, MPs with bigger sizes could directly interact with tissues or cells by surface cellular membrane contact (Hwang et al., 2019, Prata et al., 2020). Such size-dependent toxicity of MPs has also been reported in the Daphnia magna, showing that small spherical PS-MPs reduced neonatal body length and count compared to larger size PS-MPs (Schwarzer et al. 2022). Likewise, more studies have proved that the potential toxic effects of plastics could tend to increase with decreasing plastic size (An et al., 2021, Gonzalez-Soto et al., 2019, Rist et al., 2017). Except for shape-dependent PS-MPs toxicity, Zhang et al. demonstrated the concentration-dependent toxicity of MPs on hybrid snakeheads (Zhang et al. 2022a). This study showed that high levels of MPs (2 mg/L and 20 mg/L) induced more severe immune responses and shortened intestinal villi of hybrid snakeheads. Expectedly, the toxic effects were positively associated with the level of exposure, the higher level of MPs caused greater toxicity (Sun et al. 2021c; Tunali et al. 2020). Moreover, the accumulation of MPs in tissues was found to be closely associated with exposure time (Cappello et al., 2021, Dolar et al., 2022, Lackmann et al., 2022). Further studies are needed to focus on the toxic effects of MPs at environmentally relevant levels (Sun et al. 2021c). To better assess the size- and dose-dependent toxicity of MPs/NPs, the size and level ranges need to be determinated in diverse species of different developmental stages and different tissues (Kogel et al. 2020).
To elucidate whether the effects exhibit a shape-dependent manner, Qiao et al. conducted a study on zebrafish that were exposed to three main shapes (beads, fibers, and fragments) of MPs (Qiao et al. 2019a). The data showed that the accumulation of MPs in the gut exhibited shape-dependent manners with the order of fibers > fragments > beads. Similar results were also reported by Gray et al., where it was found that the shape of the MPs significantly affected the number of MPs ingested by the shrimp (Gray and Weinstein 2017). MP fibers caused more severe harm to the intestine than MP fragments and beads did (Qiao et al. 2019a). However, the shape-dependent toxicity of MPs is still a controversial issue. For instance, Schwarzer et al. pointed toward spherical particles likely to induce more toxicity on daphnids than PS fragments and fibers (Schwarzer et al. 2022). Moreover, studies compared the effects of spherically against irregularly shaped MPs, showing that spherical MPs caused fewer toxicity on daphnids than irregular MPs (Frydkjaer et al., 2017, Yokota and Mehlrose, 2020). Nevertheless, Jaikumar and colleagues demonstrated that spherical MPs exerted greater toxic potential as compared to irregular particles on daphnids (Jaikumar et al. 2019). Therefore, more studies are needed to be conducted to elucidate the shape-dependent toxicity of MPs.
Like commercial plastics, the additives and chemicals in face masks could be released into the environment during degradation due to additives are not chemically bound to the plastic polymers (Aragaw, 2020, Smith et al., 2018, Sullivan et al., 2021). Organic chemicals, surfactant molecules, dye-like molecules, plastic oligomers, and heavy metals (Pb, Cd, and antimony [Sb]) were identified in the leachates of DFMs (Sullivan et al. 2021). For instance, multiple phthalates, such as bis(2-ethylhexyl)phthalate (DEHP), dimethyl phthalate (DMP), diethyl phthalate (DEP), diisobutyl phthalate (DiBP), di-n-butyl phthalate (DBP), diamyl phthalate (DPP), diphenyl phthalate (DPhP), and dinonyl phthalate (DNP), have been detected in the face masks (Xie et al. 2022). The total phthalate level in the masks ranged from 115 ng/g to 37.700 μg/g (median 1.950 μg/g). Similarly, phthalate esters were frequently detected in a surgical mask and N95/P1/P2 masks, their levels summed up at 55 ± 35 ∼ 1700 ± 140 ng and 2300 ± 150 ∼ 5200 ± 800 ng per mask, respectively (Wang et al. 2022b). These studies suggest that face mask is a potential source of phthalate exposure. Moreover, previous studies have demonstrated that increased toxicity was found in the combination of MPs/NPs and associated chemicals (Lu et al., 2022b, Smith et al., 2018). However, the toxicity of 10 μg/mL perfluorooctane sulfonate (PFOS) was reduced in the presence of PS due to the decreased bioavailability of PFOS after absorption (Liu et al. 2022b). Therefore, a full investigation is necessary to determine the toxicity of MPs/NPs modified by the additives and chemicals.
4.2. Combined adverse effects of MPs/NPs and environmental contaminations
Given MPs/NPs can sorb environmental contaminants such as POPs, heavy metals, bacteria, and viruses, due to their physicochemical properties, specific surface area, and hydrophobic nature, DFM-derived plastics may act as vectors of these toxic substances (Fig. 3) (Brennecke et al., 2016, Kutralam-Muniasamy et al., 2021, Noro and Yabuki, 2021, Wang et al., 2021a). Adsorption is a reversible process; thus, the adsorbed toxics could be leached with time (Sharma et al. 2020). Therefore, the processes of absorption and release affect the toxicity and vectoring effects of MPs. Moreover, toxic contaminants may be concentrated and transferred into the human body through the food chain upon accidental ingestion by marine organisms, leading to unpredictable ecological effects for bioaccumulation and biomagnification of the toxic substances (Jiang, 2018, Noro and Yabuki, 2021).
MPs/NPs possess a vital property as they can adsorb POPs, such as PAHs, PCBs, PBDEs, phthalates, organochlorine pesticides, and bisphenol A (BPA) from the surrounding environment, and serve as vectors to transport these chemicals into aquatic animals and continuously release into the local tissues (Bakir et al., 2012, Bakir et al., 2014, Deng et al., 2020, Deng et al., 2021, Liao et al., 2022, Liu et al., 2022a; Wang et al. 2016; Wang et al. 2021a; Wang et al. 2020; Zhang et al. 2021a). Recently, the occurrence of carcinogenic PAHs in water and food has become a global problem (Sharma et al. 2020). A previous study showed that PAHs could be efficiently absorbed by MPs as illustrated that tissue localization of MPs was integrated with the measurement of PAHs bioaccumulation (Avio et al. 2015). This study showed that PAH-contaminated MPs entered the marine mussels and caused adverse effects. Benzo[a]pyrene (BaP) is one of the most toxic PAHs and was found to strongly sorb to MPs. Therefore, MPs with absorbed BaP exerted more toxicity than MPs alone as indicated by altered hemocyte viability and CAT activity (Gonzalez-Soto et al. 2019). Similarly, MPs/NPs can adsorb PBDEs and BPA and exacerbate their adverse effects on organisms (Chen et al., 2017b, Gu et al., 2020b).
Numerous studies have explored the role of MPs/NPs as carriers of heavy metals, including As, Cr, Cu, Cd, and Pb, in aquatic environments (Kutralam-Muniasamy et al., 2021, Naqash et al., 2020). Therefore, ingested MPs and NPs could exert more toxic than MP/NP or heavy metal exposure alone resulting from the combined effects of co-exposure (Cao et al., 2022, Eom et al., 2021, Jiang et al., 2022; Zhang et al. 2022a). For instance, MPs enhanced Cd accumulation in the livers, guts, and gills of zebrafish after MPs and Cd co-exposure for three weeks (Lu et al. 2018a). Therefore, MPs strengthened Cd toxicity on zebrafish as evidenced by increased oxidative damage and inflammation. Similarly, the combination of MPs and Pb enhanced Pb bioaccumulation in Chinese mitten crabs compared to Pb exposure alone and accelerated Pb toxicity on antioxidant performance and lipid metabolism (Yang et al. 2022). However, the level of adsorbed heavy metal in DFM-derived MPs/NPs and their re-release have not been well established yet. More studies are needed to establish a valid method to assess the combined effects of DFM-derived MPs/NPs and heavy metals.
Although face masks can effectively reduce the transmission of microbes, they could be rapidly contaminated during wear (Kalaiselvan et al. 2022). Highly bacterial contamination was found on the outside area of the used masks by hospital personnel (Luksamijarulkul et al. 2014). Bacteria and fungi have been found on the inside and outside areas of the used masks. Moreover, Gund et al. confirmed that surgical masks are contaminated after aerosol-producing dental treatment procedures (Gund et al. 2021). Compared with pathogenic bacteria and fungi, less attention has been paid to viruses on the discarded mask surface. Recently, viruses have been detected on plastic surfaces, and the used masks from the patients diagnosed with COVID-19 could be considered the primary transmission route of SARS-CoV-2 into water and wastewater (Chin et al., 2020, Tran et al., 2021, van Doremalen et al., 2020). Therefore, discarded face masks will be rapidly colonized by diverse groups of microbes including potentially pathogenic species after exposure to environment (Wright et al. 2020). Moreover, microbial contact transmission can occur by touching the tainted masks (Tunon-Molina et al. 2021). Released plastics provided a perfect habitat for microbes, such as algae, protists, viruses, fungi, and bacteria (Gkoutselis et al., 2021, Joo et al., 2021, Mammo et al., 2020, Meng et al., 2021, Oberbeckmann et al., 2015); thus, it is expected that DFM-derived MPs/NPs may act as reservoirs and carriers in pathogen transmission through food chains and enhance the detrimental influences of MPs/NPs. For example, a study of Banihashemi and colleagues showed that co-exposure to MPs and Yersinia ruckeri could synergistically affect infection in rainbow trout (Banihashemi et al. 2022). Therefore, discarded contaminated facemask-derived MPs/NPs may increase the risk of new infections in wildlife and humans. However, it is still unknown whether DFM-derived MPs/NPs would participate in pathogens transmission and increase the spread of diseases in humans due to the limited data.
4.3. Pathways of MP/NP toxicity
The generation of ROS, such as superoxide anion (O•- 2), hydrogen peroxide (H2O2) and hydroxyl (OH•), peroxyl (RO•2), and alkoxyl (RO•) radicals, has been proposed as the main mechanism of MPs- and NPs-induced toxicity (Hu and Palic 2020; Li et al. 2022c; Solomando et al. 2020). Reports showed that MPs/NPs can induce extracellular and intracellular ROS production (Hu and Palic 2020; Li et al. 2022c). The generation of extracellular ROS could be triggered by the weathering processes of plastic polymers and promote the degradation of environmental DFMs. Upon photo-oxidation and UV radiation, free radicals on plastic surfaces could be generated by chain scission of polymers. This could be one possible explanation as to why the aging process enhanced the oxidative stress after the cellular entrance of MPs (Wang et al. 2021b).
Intracellular ROS generation commonly occurs after the internalization of MPs. MPs and NPs enter the cell possibly through passive penetration or active endocytosis after adhesion to the cell membrane (Liu et al. 2021a). When intercellular oxidative stress occurs, endogenic antioxidant enzymes and molecules, including superoxide dismutase (SOD), catalase (CAT), glutathione-s-transferase (GST), glutathione (GSH), glutathione peroxidase (GPX), and glutathione reductase (GR), are synthesized to maintain the redox homeostasis. Although several studies have shown that low-dose MP exposure enhanced the activity of SOD, CAT, and GST, excessive production of ROS caused by MPs cannot be scavenged by the cellular antioxidant system resulting in redox imbalance (Huang et al., 2020, Kim et al., 2021; Li et al. 2022e; Lu et al., 2016, Solomando et al., 2020). Therefore, the imbalance between antioxidant defenses and ROS production could cause cellular oxidative injuries, such as protein oxidation, lipid peroxidation, and DNA damage. Moreover, since MPs act as a carrier for environmental contaminants, the combined effects of toxins and MPs could enhance oxidative injury.
In organisms, MPs and NPs are generally recognized as xenobiotics by the immune system and can directly affect the immune responses. Once MPs are absorbed into the body, innate immune cellular effectors can interact frequently with MPs/NPs (Browne et al., 2008, Hirt and Body-Malapel, 2020, Hu and Palic, 2020). Previous studies have shown that the immune system including lysozyme, neutrophil, phagocytosis, and immunoglobulin could be activated after fish exposure to MPs (Ahmadifar et al., 2021, Greven et al., 2016, Limonta et al., 2019). Lysozyme, a proteolytic enzyme contributing to innate immunity, plays bactericidal roles in vivo in fish and mammalians (Gu et al., 2020a, Luo et al., 2018, Saurabh and Sahoo, 2008). It was found that the expression of the lysozyme gene was upregulated in the fish after 28-day 9 μm PS-MP exposure (Ahmadifar et al. 2021). Lysozyme was observed to be present in human neutrophil granules (Lollike et al. 1995). Neutrophil activation is critical for resisting the invasion of various foreign substances. It has been shown that an increased number of neutrophils gathered in the larval blood after PS-MPs (50–350 μm) exposure, exhibit a dose-dependent manner (Qin et al. 2021). Furthermore, more neutrophils were observed after the 100 nm PS exposure than of micro-PS. The activated neutrophil function was found after exposure to both PE and PC in the fish model as evidenced by phagocytosis, degranulation, neutrophil extracellular trap release, and a slight increase in the oxidative burst (Greven et al. 2016). These data suggest that MPs are capable of phagocyte activation and act as a potential stressor of innate immunity.
Phagocytosis, the cellular ingestion and digestion of particulate matter, is the central effector mechanism of innate immunity (MacArthur and Fletcher 1985). In vitro study showed that PS-MPs could be absorbed by the intestinal epithelial Caco-2 cells via macropinocytosis and clathrin-mediated endocytosis, resulting in the tight junctions between cells being disrupted (Xu et al. 2021a). Oral small-sized PS-MP (< 2 μm) exposure could cause brain accumulation by microglial phagocytosis and activated microglia, leading to apoptosis (Kwon et al. 2022). The phagocytic capacity of microglia for PS-MPs corresponded with that of macrophages. Macrophages are the primary phagocytes that occur in the intestinal tract, lungs, and liver and act as a first-line defense against contaminants (Merkley et al. 2022). The internalization of MPs by murine macrophages did not cause MP degradation while inducing a rewriting of metabolism with a shift toward glycolysis and reduction of mitochondrial respiration (Merkley et al. 2022). The study indicated that the phagocytosis of MPs caused an immune-metabolic active state in macrophages. On the contrary, MP accumulation was found to suppress immune responses as illustrated by the reduction of lysozyme, immunoglobulin, and neutrophil levels (Banaee et al., 2019, Gu et al., 2020a, Hamed et al., 2019, Kim et al., 2021). These data suggest that MPs can cause the stimulation and suppression of immune responses by inducing immune toxicity, which exhibits gene expression variability across cells and species shape innate immunity (Hagai et al. 2018). However, considering the complexity of immune response and species differences, more precise studies on the human immune responses to MP exposure are urgently demanded.
It has been widely accepted that intestinal microorganism plays a critical role in maintaining host biological homeostasis and is a toxicity target due to plastics offer a habit for microbes and their interaction (Lu et al. 2019). Previous studies proved that PS-MP exposure altered gut microbiota composition, resulting in gut inflammation of zebrafish (Lu et al. 2019). Similarly, Kang et al. showed that MPs disrupted gut microbiota composition at both phylum and genus levels in the marine medaka (Kang et al. 2021). Alteration of gut microbiota was observed after mussels were exposed to MPs (Li et al. 2020b). These data suggest that digested MPs could cause adverse effects on gut microbiota within aquatic organisms (Li et al. 2022d). Moreover, MP exposure was found to induce gut microbiota dysbiosis in mammals (Djouina et al. 2022; Lu et al. 2018b). For instance, 5 μm PS-MPs induced gut microbiota dysbiosis in mice, as evidenced by the decreased content of Actinobacteria at the phylum level or the alteration of 15 types of bacteria at the genus level (Jin et al. 2019). Chronic exposure to PVC-MPs reduced the relative abundance of probiotics while increasing the abundance of conditionally pathogenic bacteria in mice (Chen et al. 2022b). According to existing studies, alterations or dysbiosis of the gut microbiota were closely related to the host metabolic disorders (Wu et al., 2018, Xia et al., 2018). The gut microbiota can directly impact the brain and behavior via the brain-gut axis (Cryan and Dinan, 2012, Valles-Colomer et al., 2019). Therefore, MPs/NPs might cause behavioral toxicities via the brain-gut-microbiota axis (Chen et al., 2022a, Guo et al., 2022). Although there is growing concern regarding the interaction between MPs and gut microorganisms, limited data show the relationship of MPs/NPs with human gut microbiota and their impacts on human health.
5. Challenges and future opportunities
The SARS-CoV-2 outbreak has increased face mask waste all around the world. Considering tremendous numbers of masks will be dispersed in the environment, further in-depth investigations are required to clear the degradation of abandoned face masks, the detection and characterization of MPs/NPs from abandoned DFMs, the transport of DFM-derived MPs/NPs in the food chains, and how they interact with human biological tissues. Moreover, possible strategies to reduce DFM-derived MP/NP pollution are urgently demanded.
5.1. Identification and characterization of MPs/NPs from abandon face masks
As mentioned above, discarded face masks can be broken down and decomposed into massive MPs and NPs, leading to the omnipresent presence of MPs/NPs in our surrounding environments. Although face masks are new sources of environmental MPs/NPs, the recognized sources of MPs and NPs are food containers, teabags, cigarette butts, and disposable cups (Hernandez et al. 2019; Xu et al. 2021c). Hence, it is imperative to clear the released characteristics of MPs and NPs from discarded face masks for potential risk assessment (Liang et al. 2021). However, few studies are available identifying the release kinetics of MPs from DFMs into the natural environment. Liang et al. revealed that the release process of MPs from DFMs was mainly divided into two steps, including the release of MPs on the surface of the masks and the migration of MPs inside the masks to the surface (Liang et al. 2021). The study also identified the length (fine groups: < 100, 500–1000, 1000–2000, and greater than 2000 μm), the shape (fibers and debris), and the color (transparent, black, blue, yellow, brown, and red) of released MPs. Among them, fiber and transparent MPs were the dominant MPs released from masks, and the number of released MPs increased with time (Liang et al. 2021). A previous study reported that the greatest difference between masks was in the middle layer (Chen et al. 2021b). Therefore, the number of MPs released did not differ from the type of mask (N95, surgical masks, and cotton masks) due to the broken state of the inner and outer layers (Liang et al. 2021). A previous study showed that N95 masks released more and smaller NPs than surgical masks (Ma et al. 2021). A potential reason for this may be two more layers of melt-blown fibers in N95 masks in comparison with the common surgical masks.
Nonetheless, the release process of MPs from DFMs was closely associated with the extent of DFM degradation due to complex environmental factors including sunlight exposure, temperature, mechanical forces, and microbe biofilm (Saliu et al. 2021). Currently, artificial weathering experiments have been carried out to recapitulate the environmental release process of MPs from DFMs. For instance, Saliu et al. reported that a surgical mask with UV-light exposure combined with stirring in artificial seawater released up to 173,000 fibers/day (Saliu et al. 2021). Consistently, a study by Wang’s group showed that DFMs released MPs to the aqueous environment could be exacerbated in the presence of sand (Wang et al. 2021a). Moreover, exposure to different levels of mechanical stress forces, mimicking conditions of realistic mechanical deterioration, has been demonstrated effective in breaking and fragmenting face mask nonwoven textiles into MPs and NPs (Morgana et al. 2021). These studies suggest that the release quantity of MPs from the weathered masks will be higher than that of virgin masks (Saliu et al. 2021; Wang et al. 2021a). Therefore, the combined effects of natural weathering are needed to be considered in the release of MPs/NPs in further studies. In addition, a previous study has proved that chronic low-level aged PS-MP exposure induced more severe neurotoxicity compared to original PS-MPs (Chen et al. 2021a). Compared with the pristine MPs, Qu’s team also showed that chemical modifications of MPs, such as sulfonate modification, leading to increased neurotoxicity on locomotion behaviors and sensory perception behaviors, as well as the development of dopaminergic neurons in exposed nematodes (Qu and Wang 2020).
Despite the ubiquitous distribution, the quantification and characterization of MPs and NPs in environmental and food matrices are still challenging (Ivleva 2021). Studies have suggested that the toxicity of MPs depends on exposure times, routes and concentrations, and plastic sizes, shapes, and types (Kogel et al. 2020). However, a precise study on the identification of MPs/NPs in the human body is rare because of ethical constraints and technical bottlenecks (Yan et al. 2020b). Recently, Yan et al. developed a feasible and efficient method for extracting MPs with a high recovery rate from feces of different species, including zebrafish, chicken, and humans (Yan et al. 2020b). With this method, different polymer types and shapes of MPs have been identified in chicken and IBD patient feces. Remarkably, MPs and NPs exerted dose- and time-dependent toxicity manners. However, current experimental exposure levels may higher as well as the exposure duration is shorter than human-relevant exposure (Prust et al. 2020). Moreover, most of the studies used plastic materials that were quite unlike those found in the environment (Guo et al., 2022, Lim, 2021). In the natural environment, organisms are commonly exposed to a mixture rather than one type of MPs, of a specific size and shape (Koelmans et al., 2019, Lim, 2021). Therefore, innovative methods are urgently required for the detection and characterization of MPs/NPs in environmental matrices. Foetisch et al. have developed a new method to extract and characterize NPs in environmental and food matrices by combining scanning transmission X-ray spectro-microscopy (STXM) with near-edge X-ray absorption fine-structure spectroscopy (NEXAFS) (Foetisch et al. 2022). STXM with a high spatial and spectral resolution will provide a promising tool to deliver a full chemical, size, and shape characterizations of the MPs and NPs in different types of matrices. More recently, the pyrolysis and gas chromatography-mass spectrometry (GC/MS) method was also established to identify the level of MPs in environmental samples (Wang et al. 2022a).
5.2. The transport of MPs/NPs in the food chains
Although the fate, transport, mechanistic behavior, and biological uptake of MPs/NPs can be investigated using artificial materials labeled with metals, fluorescent dyes, or enriched stable isotopes, they are not pre-labeled in the natural environment and transportation of DFM-derived MPs/NPs through the food chain is often difficult to trace (Foetisch et al. 2022; Li et al. 2020a; Mitrano et al., 2019, Wagner and Reemtsma, 2019). A previous study presented that MPs can be transported across trophic levels, from fish to a marine mammal top predator, which has implications for human health (Nelms et al. 2018). Moreover, it is not yet known the retention time of MPs/NPs in animals and the human body (Smith et al. 2018). To date, there are insufficient epidemiological studies that determined the human-relevant levels of MPs/NPs via food chains. It could be an efficient way to monitor the exposure level in human consumption, especially in aquatic foods. In fact, the detrimental influences of MPs/NPs on human health depend on exposure levels. Current approaches for toxicity assessment failed to capture the features of low-dose exposure or co-exposure of MPs/NPs. Moreover, chronic exposure could also produce cumulative effects. More recently, a harmonized in vitro static and dynamic gastrointestinal simgi® model was created to mimic the digestive process of polyethylene terephthalate (PET) in the gastrointestinal tract (Tamargo et al. 2022). The study showed that MPs presented structural differences during the biotransformation in the gastrointestinal tract. Therefore, it is indispensable to elucidate the process of MPs biodegradation in the human body.
Considering that plastics serve as carriers of toxins and cause the bioaccumulation and biomagnification of toxic substances, both adsorption capacity and adsorption/desorption rates of adverse substances to and from plastics may be influenced by several factors, such as the size of plastics (Gonzalez-Soto et al. 2019; Xu et al. 2021b). For instance, smaller plastics with a larger surface area and a shorter diffusion pathway were found to adsorb higher toxin levels and exchange with the surrounding environment more rapidly (Gonzalez-Soto et al., 2019, Velzeboer et al., 2014). Moreover, investigating the sorption and leachates of environmental toxins to/from MPs/NPs under diverse environmental conditions is also poorly understood. Therefore, determining the final distribution, impacts, and fate of DFM-derived MPs/NPs is an extremely arduous task.
5.3. Advanced human organoid models for MPs/NPs risk assessment
Zebrafish is s a successful model to evaluate the potentially adverse effects of MPs/NPs owing to the transparent embryo and larvae providing advantages for studying the localization by live imaging of fluorescent-labeled MPs/NPs (Bhagat et al. 2020). However, conventional animal models cannot mimic the human actual responses to MP/NP exposure due to the variations in species. Although animal models, such as Caenorhabditis elegans (C. elegans), have been commonly utilized to evaluate the neurotoxicity of MPs, it is hard to extrapolate the effects of plastics on human health owing to insufficient data demonstrating whether MPs/NPs could accumulate in human brains and differences in the nervous system (Chen et al., 2021a, Qu et al., 2019, Qu and Wang, 2020). Therefore, a proper model that enables a comprehensive assessment of possible human health risks caused by MPs/NPs remains to be developed.
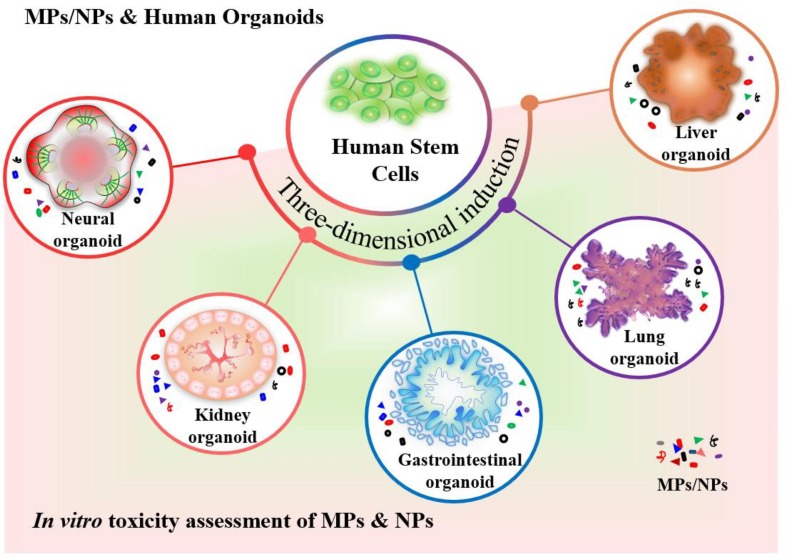
More recently, advanced human stem cell-derived three-dimensional (3D) organoids are of particular concern since they are sophisticated and multicellular organotypic models, which recapitulate organogenesis, morphogenesis, and cellular processes of human early development. To data, diverse 3D organoids have been successfully generated in vitro, such as neural, kidney, gastrointestinal, and lung organoids. With the rapid development of organoid technology, human organoids have been applied in toxicity assessment (Fig. 4 ) (Li et al. 2022a; Li et al. 2022b; Li et al. 2022c). In our previous study, human induced pluripotent stem cell (hiPSC)-derived intestinal organoids were established to analyze the uptake and toxic effects of PS-NPs on the human intestine (Hou et al. 2022). PS-NP accumulation was observed in the goblet, endocrine, Paneth, and enterocyte cells after 2-day PS-NP exposure (100 μg/mL) and induced cell apoptosis and triggered an inflammatory response (Hou et al. 2022). Recently, Cheng et al. developed human liver organoids to evaluate the hepatotoxicity of PS-MPs on humans due to previous studies showed that MP-caused hepatic disturbance in animal models (Cheng et al., 2022, Deng et al., 2017; Lu et al. 2018b; Zheng et al. 2021). Consistently, PS-MPs were found to disrupt lipid metabolism and caused hepatotoxicity in liver organoids (Cheng et al. 2022).
Fig. 4.
The novel human organoids can be applied in toxicity assessments of microplastics and nanoplastics. Human stem cells are induced into tissue-specific organoids, such as neural organoids, kidney organoids, gastrointestinal organoids, lung organoids, and liver organoids, providing a promising model for in-depth study to explore the possible adverse effects of MPs/NPs on humans and their underlying mechanism.
Given MPs have been detected in the human placenta and meconium, MPs/NPs might pose threats to embryonic development (Braun et al., 2021, Ragusa et al., 2021, Zhang et al., 2021b). Human stem cell-derived embryoid bodies (EBs) display a propensity to yield three primary germ layers, including ectoderm, mesoderm, and endoderm, which recapitulates human early embryogenesis (Itskovitz-Eldor et al. 2000). Hence, EBs have been applied to predict embryotoxicity and developmental toxicity of toxic substances in vitro (Faiola et al. 2015; Li et al., 2022a, Seiler and Spielmann, 2011). For instance, EB-derived forebrain cerebral spheroids were generated to investigate the potential influences of PS-MPs on the human developing brain owing to MPs were reported to be accumulated in the brain (Estrela et al., 2021, Hua et al., 2022, Kwon et al., 2022). The study showed the size- and dose-dependent manner of PS-MP toxicity on cortical layer differentiation (Hua et al. 2022).
Recently, Winkler et al. employed human airway organoids for toxicity assessment of inhaled and deposited MP fibers (MPFs) (Winkler et al. 2022). In our previous study, retinal organoids from human embryonic stem cell (hESC)-derived EBs were used to evaluate the adverse effects of low-level PBDE on human early retinal development (Li et al. 2022c). PBDE exposure was found to cause abnormal neural retinal development in human retinal organoid model. Considering the adverse effects on the visual system caused by MPs, human retinal organoids might be a promising tool for retinal toxicity assessment of DFM-derived MPs/NPs in the future (Chen et al. 2017a). Therefore, human organoids will provide ideal systems for the toxicity assessment of DFM-derived MPs/NPs on human health, even though the application of organoids for predictive toxicology is still in its infancy. Multi-organoids on a chip could be used to assess the systemic toxicity of MPs and NPs in vitro. Moreover, human organoids combined with single-cell RNA sequencing (scRNA-seq) would be a promising method for targeting cell population identification and corresponding adverse effects of DFM-derived MPs/NPs (Yu et al. 2022).
5.4. Possible strategies to reduce DFM-derived MP/NP pollution
Considering the high consumption rate of face masks that poses a severe environmental threat, it is critical to scale up innovation and technology to use bio-based and eco-friendly materials to produce biodegradable surgical face masks (Babaahmadi et al., 2021, Pandit et al., 2021). Furthermore, it is feasible to plan the sustainable management and treatment technology (e.g., recycling, recovery, and reuse) for used face masks to avoid entering the environment directly (Aragaw, 2021, Aragaw and Mekonnen, 2021, Mekonnen and Aragaw, 2021, Pourebrahimi, 2022). The controllable degradation process of DFMs is an environmentally safe action that could reduce the environmental contaminations of MPs/NPs. Moreover, advanced methods are required to decontaminate and sterilize the masks for multiple reuses (Lee et al., 2021, Pandit et al., 2021). However, it remains unclear whether MPs/NPs will be released from masks during the process of sterilization, disinfection, and antisepsis. Once in the environment, more efforts are needed to address the issues of the sorption capacity of discarded facemask-derived MPs/NPs to environmental toxins and their transportation through food chains.
Currently, great efforts have been made in developing innovative and promise approaches to promote the degradation of plastics (Conk et al., 2022, DelRe et al., 2021, Tournier et al., 2020). For instance, Conk et al. showed that mild catalysis with ethylene could be applied to deconstruct waste PE to form propylene (Conk et al. 2022). The catalytic latency can be regulated by thermal treatment and/or operation temperature (DelRe et al. 2021). More recently, a bacterial strain isolated from the gut of insect larvae can depolymerize PVC, which is potentially associated with catalase peroxidase (Zhang et al. 2022a). In chemical degradation, sodium dodecyl sulfate assisted electrochemical advanced oxidation process technology was found to cause obvious changes in the particle size, morphology, and functional groups of the PS MPs (Lu et al. 2022a). These studies suggest that abovementioned strategies could be applied to reduce DFM-derived MP/NP pollution.
6. Conclusions
Given global discarded face mask issues, aquatic face mask-derived MPs/NPs may pose a particular concern because they can be accumulated and retained in food consumption. To date, the influence of discarded face mask-derived MPs and NPs on human health has not been well understood yet. The novel human stem cell-derived 3D organoid will provide a promising model to evaluate the toxic effects of face mask-derived MPs/NPs on humans and reveal their potential mechanism. Moreover, the combination of environmental science and epidemiology is urgently needed in future studies. More importantly, innovative approaches and eco-friendly strategies are greatly demanded to reduce DFM-derived MP/NP pollution.
CRediT authorship contribution statement
Minghui Li: . Zongkun Hou: . Run Meng: . Shilei Hao: Supervision. Bochu Wang: .
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This study was supported by funding from the National Natural Science Foundation of China [grant numbers 82204083 and 11972099]; Natural Science Foundation of Chongqing [grant numbers cstc2021jcyj-msxmX0171].
Handling Editor: Xavier Querol
Data availability
No data was used for the research described in the article.
References
- Aghaei Z., Sled J.G., et al. Maternal exposure to polystyrene micro- and nanoplastics causes fetal growth restriction in mice. Environ. Sci. Technol. Lett. 2022;9:426–430. [Google Scholar]
- Ahmadifar E., Kalhor N., et al. Effects of polystyrene microparticles on inflammation, antioxidant enzyme activities, and related gene expression in nile tilapia (oreochromis niloticus) Environ. Sci. Pollut. Res. Int. 2021;28:14909–14916. doi: 10.1007/s11356-020-11731-x. [DOI] [PubMed] [Google Scholar]
- Al-Omran K., Khan E., et al. Estimation of covid-19 generated medical waste in the kingdom of bahrain. Sci. Total Environ. 2021;801 doi: 10.1016/j.scitotenv.2021.149642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An D., Na J., et al. Size-dependent chronic toxicity of fragmented polyethylene microplastics to daphnia magna. Chemosphere. 2021;271 doi: 10.1016/j.chemosphere.2021.129591. [DOI] [PubMed] [Google Scholar]
- Anderson J.C., Park B.J., et al. Microplastics in aquatic environments: implications for canadian ecosystems. Environ. Pollut. 2016;218:269–280. doi: 10.1016/j.envpol.2016.06.074. [DOI] [PubMed] [Google Scholar]
- Andrady A.L. Microplastics in the marine environment. Mar. Pollut. Bull. 2011;62:1596–1605. doi: 10.1016/j.marpolbul.2011.05.030. [DOI] [PubMed] [Google Scholar]
- Andrady A.L. The plastic in microplastics: A review. Mar. Pollut. Bull. 2017;119:12–22. doi: 10.1016/j.marpolbul.2017.01.082. [DOI] [PubMed] [Google Scholar]
- Aragaw T.A. Surgical face masks as a potential source for microplastic pollution in the covid-19 scenario. Mar. Pollut. Bull. 2020;159 doi: 10.1016/j.marpolbul.2020.111517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aragaw T.A. The macro-debris pollution in the shorelines of lake tana: First report on abundance, assessment, constituents, and potential sources. Sci. Total Environ. 2021;797 doi: 10.1016/j.scitotenv.2021.149235. [DOI] [PubMed] [Google Scholar]
- Aragaw T.A., De-la-Torre G.E., et al. Personal protective equipment (ppe) pollution driven by the covid-19 pandemic along the shoreline of lake tana, bahir dar, ethiopia. Sci. Total Environ. 2022;820 doi: 10.1016/j.scitotenv.2022.153261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aragaw T.A., Mekonnen B.A. Current plastics pollution threats due to covid-19 and its possible mitigation techniques: A waste-to-energy conversion via pyrolysis. Environ Syst Res (Heidelb) 2021;10:8. doi: 10.1186/s40068-020-00217-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aragaw T.A., Mekonnen B.A. Understanding disposable plastics effects generated from the pcr testing labs during the covid-19 pandemic. Journal of Hazardous Materials. Advances. 2022;7 doi: 10.1016/j.hazadv.2022.100126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araujo A., Gomes A.R., et al. Hepatotoxicity of pristine polyethylene microplastics in neotropical physalaemus cuvieri tadpoles (fitzinger, 1826) J. Hazard. Mater. 2020;386 doi: 10.1016/j.jhazmat.2019.121992. [DOI] [PubMed] [Google Scholar]
- Avio C.G., Gorbi S., et al. Pollutants bioavailability and toxicological risk from microplastics to marine mussels. Environ. Pollut. 2015;198:211–222. doi: 10.1016/j.envpol.2014.12.021. [DOI] [PubMed] [Google Scholar]
- Babaahmadi V., Amid H., et al. Biodegradable and multifunctional surgical face masks: a brief review on demands during covid-19 pandemic, recent developments, and future perspectives. Sci. Total Environ. 2021;798 doi: 10.1016/j.scitotenv.2021.149233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakir A., Rowland S.J., et al. Competitive sorption of persistent organic pollutants onto microplastics in the marine environment. Mar. Pollut. Bull. 2012;64:2782–2789. doi: 10.1016/j.marpolbul.2012.09.010. [DOI] [PubMed] [Google Scholar]
- Bakir A., Rowland S.J., et al. Enhanced desorption of persistent organic pollutants from microplastics under simulated physiological conditions. Environ. Pollut. 2014;185:16–23. doi: 10.1016/j.envpol.2013.10.007. [DOI] [PubMed] [Google Scholar]
- Banaee M., Soltanian S., et al. Evaluation of single and combined effects of cadmium and micro-plastic particles on biochemical and immunological parameters of common carp (cyprinus carpio) Chemosphere. 2019;236 doi: 10.1016/j.chemosphere.2019.07.066. [DOI] [PubMed] [Google Scholar]
- Banihashemi E.A., Soltanian S., et al. Effect of microplastics on yersinia ruckeri infection in rainbow trout (oncorhynchus mykiss) Environ. Sci. Pollut. Res. Int. 2022;29:11939–11950. doi: 10.1007/s11356-021-16517-3. [DOI] [PubMed] [Google Scholar]
- Barboza L.G.A., Dick Vethaak A., et al. Marine microplastic debris: An emerging issue for food security, food safety and human health. Mar. Pollut. Bull. 2018;133:336–348. doi: 10.1016/j.marpolbul.2018.05.047. [DOI] [PubMed] [Google Scholar]
- Bhagat J., Zang L., et al. Zebrafish: an emerging model to study microplastic and nanoplastic toxicity. Sci. Total Environ. 2020;728 doi: 10.1016/j.scitotenv.2020.138707. [DOI] [PubMed] [Google Scholar]
- Braun T., Ehrlich L., et al. Detection of microplastic in human placenta and meconium in a clinical setting. Pharmaceutics. 2021;13 doi: 10.3390/pharmaceutics13070921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brehm J., Wilde M.V., et al. In-depth characterization revealed polymer type and chemical content specific effects of microplastic on dreissena bugensis. J. Hazard. Mater. 2022;437 doi: 10.1016/j.jhazmat.2022.129351. [DOI] [PubMed] [Google Scholar]
- Brennecke D., Duarte B., et al. Microplastics as vector for heavy metal contamination from the marine environment. Estuar. Coast. Shelf Sci. 2016;178:189–195. [Google Scholar]
- Browne M.A., Dissanayake A., et al. Ingested microscopic plastic translocates to the circulatory system of the mussel, mytilus edulis (l) Environ. Sci. Technol. 2008;42:5026–5031. doi: 10.1021/es800249a. [DOI] [PubMed] [Google Scholar]
- Campanale C., Massarelli C., et al. A detailed review study on potential effects of microplastics and additives of concern on human health. Int. J. Environ. Res. Public Health. 2020;17 doi: 10.3390/ijerph17041212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J., Liao Y., et al. Enhanced microalgal toxicity due to polystyrene nanoplastics and cadmium co-exposure: From the perspective of physiological and metabolomic profiles. J. Hazard. Mater. 2022;427 doi: 10.1016/j.jhazmat.2021.127937. [DOI] [PubMed] [Google Scholar]
- Cappello T., De Marco G., et al. Time-dependent metabolic disorders induced by short-term exposure to polystyrene microplastics in the mediterranean mussel mytilus galloprovincialis. Ecotoxicol. Environ. Saf. 2021;209 doi: 10.1016/j.ecoenv.2020.111780. [DOI] [PubMed] [Google Scholar]
- Chamas A., Moon H., et al. Degradation rates of plastics in the environment. ACS Sustainable Chem. Eng. 2020;8:3494–3511. [Google Scholar]
- Chen J.C., Chen M.Y., et al. Microplastics negatively impact embryogenesis and modulate the immune response of the marine medaka oryzias melastigma. Mar. Pollut. Bull. 2020;158 doi: 10.1016/j.marpolbul.2020.111349. [DOI] [PubMed] [Google Scholar]
- Chen X., Chen X., et al. Used disposable face masks are significant sources of microplastics to environment. Environ. Pollut. 2021;285 doi: 10.1016/j.envpol.2021.117485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q., Gundlach M., et al. Quantitative investigation of the mechanisms of microplastics and nanoplastics toward zebrafish larvae locomotor activity. Sci. Total Environ. 2017;584–585:1022–1031. doi: 10.1016/j.scitotenv.2017.01.156. [DOI] [PubMed] [Google Scholar]
- Chen H., Hua X., et al. Chronic exposure to uv-aged microplastics induces neurotoxicity by affecting dopamine, glutamate, and serotonin neurotransmission in caenorhabditis elegans. J. Hazard. Mater. 2021;419 doi: 10.1016/j.jhazmat.2021.126482. [DOI] [PubMed] [Google Scholar]
- Chen J., Rao C., et al. Long-term exposure to polyethylene microplastics and glyphosate interferes with the behavior, intestinal microbial homeostasis, and metabolites of the common carp (cyprinus carpio l.) Sci. Total Environ. 2022;814(152681) doi: 10.1016/j.scitotenv.2021.152681. [DOI] [PubMed] [Google Scholar]
- Chen Q., Yin D., et al. Enhanced uptake of bpa in the presence of nanoplastics can lead to neurotoxic effects in adult zebrafish. Sci. Total Environ. 2017;609:1312–1321. doi: 10.1016/j.scitotenv.2017.07.144. [DOI] [PubMed] [Google Scholar]
- Chen C., Yu G., et al. Lifetime prediction of non-woven face masks in ocean and contributions to microplastics and dissolved organic carbon. J. Hazard. Mater. 2023;441 [Google Scholar]
- Chen X., Zhuang J., et al. Chronic exposure to polyvinyl chloride microplastics induces liver injury and gut microbiota dysbiosis based on the integration of liver transcriptome profiles and full-length 16s rrna sequencing data. Sci. Total Environ. 2022;839 doi: 10.1016/j.scitotenv.2022.155984. [DOI] [PubMed] [Google Scholar]
- Cheng K.K., Lam T.H., et al. Wearing face masks in the community during the covid-19 pandemic: Altruism and solidarity. The Lancet. 2020 doi: 10.1016/S0140-6736(20)30918-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng W., Li X., et al. Polystyrene microplastics induce hepatotoxicity and disrupt lipid metabolism in the liver organoids. Sci. Total Environ. 2022;806 doi: 10.1016/j.scitotenv.2021.150328. [DOI] [PubMed] [Google Scholar]
- Chin A.W.H., Chu J.T.S., et al. Stability of sars-cov-2 in different environmental conditions. The Lancet. Microbe. 2020;1 doi: 10.1016/S2666-5247(20)30003-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury H., Chowdhury T., et al. Estimating marine plastic pollution from covid-19 face masks in coastal regions. Mar. Pollut. Bull. 2021;168 doi: 10.1016/j.marpolbul.2021.112419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury T., Chowdhury H., et al. Estimation of the healthcare waste generation during covid-19 pandemic in bangladesh. Sci. Total Environ. 2022;811 doi: 10.1016/j.scitotenv.2021.152295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua M.H., Cheng W., et al. Face masks in the new covid-19 normal: Materials, testing, and perspectives. Research (Wash D C) 2020;2020:7286735. doi: 10.34133/2020/7286735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chubarenko I., Efimova I., et al. On mechanical fragmentation of single-use plastics in the sea swash zone with different types of bottom sediments: Insights from laboratory experiments. Mar. Pollut. Bull. 2020;150 doi: 10.1016/j.marpolbul.2019.110726. [DOI] [PubMed] [Google Scholar]
- Chughtai A.A., Seale H., et al. Effectiveness of cloth masks for protection against severe acute respiratory syndrome coronavirus 2. Emerg. Infect. Dis. 2020;26 doi: 10.3201/eid2610.200948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chughtai A.A., Stelzer-Braid S., et al. Contamination by respiratory viruses on outer surface of medical masks used by hospital healthcare workers. BMC Infect. Dis. 2019;19:491. doi: 10.1186/s12879-019-4109-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clere I.K., Ahmmed F., et al. Quantification and characterization of microplastics in commercial fish from southern new zealand. Mar. Pollut. Bull. 2022;184 doi: 10.1016/j.marpolbul.2022.114121. [DOI] [PubMed] [Google Scholar]
- Conk R.J., Hanna S., et al. Catalytic deconstruction of waste polyethylene with ethylene to form propylene. Science. 2022;377:1561–1566. doi: 10.1126/science.add1088. [DOI] [PubMed] [Google Scholar]
- Cordova M.R., Nurhati I.S., et al. Unprecedented plastic-made personal protective equipment (ppe) debris in river outlets into jakarta bay during covid-19 pandemic. Chemosphere. 2021;268 doi: 10.1016/j.chemosphere.2020.129360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryan J.F., Dinan T.G. Mind-altering microorganisms: The impact of the gut microbiota on brain and behaviour. Nat. Rev. Neurosci. 2012;13:701–712. doi: 10.1038/nrn3346. [DOI] [PubMed] [Google Scholar]
- Das S., Sarkar S., et al. A comprehensive review of various categories of face masks resistant to covid-19. Clin Epidemiol Glob Health. 2021;12 doi: 10.1016/j.cegh.2021.100835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dbouk T, Drikakis D. 2020. On respiratory droplets and face masks. Phys Fluids (1994) 32:063303. [DOI] [PMC free article] [PubMed]
- De-la-Torre G.E., Dioses-Salinas D.C., et al. Binational survey of personal protective equipment (ppe) pollution driven by the covid-19 pandemic in coastal environments: Abundance, distribution, and analytical characterization. J. Hazard. Mater. 2022;426 doi: 10.1016/j.jhazmat.2021.128070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DelRe C., Jiang Y., et al. Near-complete depolymerization of polyesters with nano-dispersed enzymes. Nature. 2021;592:558–563. doi: 10.1038/s41586-021-03408-3. [DOI] [PubMed] [Google Scholar]
- Deng Y., Yan Z., et al. Microplastics release phthalate esters and cause aggravated adverse effects in the mouse gut. Environ. Int. 2020;143 doi: 10.1016/j.envint.2020.105916. [DOI] [PubMed] [Google Scholar]
- Deng Y., Yan Z., et al. Enhanced reproductive toxicities induced by phthalates contaminated microplastics in male mice (mus musculus) J. Hazard. Mater. 2021;406 doi: 10.1016/j.jhazmat.2020.124644. [DOI] [PubMed] [Google Scholar]
- Deng Y., Zhang Y., et al. Tissue accumulation of microplastics in mice and biomarker responses suggest widespread health risks of exposure. Sci. Rep. 2017;7:46687. doi: 10.1038/srep46687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djouina M., Vignal C., et al. Oral exposure to polyethylene microplastics alters gut morphology, immune response, and microbiota composition in mice. Environ. Res. 2022;212 doi: 10.1016/j.envres.2022.113230. [DOI] [PubMed] [Google Scholar]
- Dolar A., Drobne D., et al. Time-dependent immune response in porcellio scaber following exposure to microplastics and natural particles. Sci. Total Environ. 2022;818 doi: 10.1016/j.scitotenv.2021.151816. [DOI] [PubMed] [Google Scholar]
- Dong C.D., Chen C.W., et al. Polystyrene microplastic particles: in vitro pulmonary toxicity assessment. J. Hazard. Mater. 2020;385 doi: 10.1016/j.jhazmat.2019.121575. [DOI] [PubMed] [Google Scholar]
- Du H., Huang S., et al. Environmental risks of polymer materials from disposable face masks linked to the covid-19 pandemic. Sci. Total Environ. 2022;815 doi: 10.1016/j.scitotenv.2022.152980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eom H.J., Haque M.N., et al. Exposure to metals premixed with microplastics increases toxicity through bioconcentration and impairs antioxidant defense and cholinergic response in a marine mysid. Comp. Biochem. Physiol. C: Toxicol. Pharmacol. 2021;249 doi: 10.1016/j.cbpc.2021.109142. [DOI] [PubMed] [Google Scholar]
- Estrela F.N., Guimaraes A.T.B., et al. Toxicity of polystyrene nanoplastics and zinc oxide to mice. Chemosphere. 2021;271 doi: 10.1016/j.chemosphere.2020.129476. [DOI] [PubMed] [Google Scholar]
- Fadare O.O., Okoffo E.D. Covid-19 face masks: A potential source of microplastic fibers in the environment. Sci. Total Environ. 2020;737 doi: 10.1016/j.scitotenv.2020.140279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faiola F., Yin N., et al. The rise of stem cell toxicology. Environ. Sci. Technol. 2015;49:5847–5848. doi: 10.1021/acs.est.5b01549. [DOI] [PubMed] [Google Scholar]
- Fan Z., Xiao T., et al. A study on the roles of long non-coding rna and circular rna in the pulmonary injuries induced by polystyrene microplastics. Environ. Int. 2022;163 doi: 10.1016/j.envint.2022.107223. [DOI] [PubMed] [Google Scholar]
- Ferrante MC, Monnolo A, et al. 2022. The pressing issue of micro- and nanoplastic contamination: Profiling the reproductive alterations mediated by oxidative stress. Antioxidants (Basel) 11. [DOI] [PMC free article] [PubMed]
- Fesseha H., Abebe F. Degradation of plastic materials using microorganisms: A review. Public Health – Open Journal. 2019;4:57–63. [Google Scholar]
- Foetisch A., Filella M., et al. Identification and characterisation of individual nanoplastics by scanning transmission x-ray microscopy (stxm) J. Hazard. Mater. 2022;426 doi: 10.1016/j.jhazmat.2021.127804. [DOI] [PubMed] [Google Scholar]
- Fournier S.B., D’Errico J.N., et al. Nanopolystyrene translocation and fetal deposition after acute lung exposure during late-stage pregnancy. Part. Fibre Toxicol. 2020;17 doi: 10.1186/s12989-020-00385-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frydkjaer C.K., Iversen N., et al. Ingestion and egestion of microplastics by the cladoceran daphnia magna: Effects of regular and irregular shaped plastic and sorbed phenanthrene. Bull. Environ. Contam. Toxicol. 2017;99:655–661. doi: 10.1007/s00128-017-2186-3. [DOI] [PubMed] [Google Scholar]
- Gautam R., Bassi A.S., et al. A review of biodegradation of synthetic plastic and foams. Appl. Biochem. Biotechnol. 2007;141:85–108. doi: 10.1007/s12010-007-9212-6. [DOI] [PubMed] [Google Scholar]
- Gautam R., Jo J., et al. Evaluation of potential toxicity of polyethylene microplastics on human derived cell lines. Sci. Total Environ. 2022;838 doi: 10.1016/j.scitotenv.2022.156089. [DOI] [PubMed] [Google Scholar]
- Gewert B., Plassmann M.M., et al. Pathways for degradation of plastic polymers floating in the marine environment. Environ Sci Process Impacts. 2015;17:1513–1521. doi: 10.1039/c5em00207a. [DOI] [PubMed] [Google Scholar]
- Gigault J., Halle A.T., et al. Current opinion: What is a nanoplastic? Environ. Pollut. 2018;235:1030–1034. doi: 10.1016/j.envpol.2018.01.024. [DOI] [PubMed] [Google Scholar]
- Gkoutselis G., Rohrbach S., et al. Microplastics accumulate fungal pathogens in terrestrial ecosystems. Sci. Rep. 2021;11:13214. doi: 10.1038/s41598-021-92405-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Soto N., Hatfield J., et al. Impacts of dietary exposure to different sized polystyrene microplastics alone and with sorbed benzo[a]pyrene on biomarkers and whole organism responses in mussels mytilus galloprovincialis. Sci. Total Environ. 2019;684:548–566. doi: 10.1016/j.scitotenv.2019.05.161. [DOI] [PubMed] [Google Scholar]
- Gray A.D., Weinstein J.E. Size- and shape-dependent effects of microplastic particles on adult daggerblade grass shrimp (palaemonetes pugio) Environ. Toxicol. Chem. 2017;36:3074–3080. doi: 10.1002/etc.3881. [DOI] [PubMed] [Google Scholar]
- Greven A.C., Merk T., et al. Polycarbonate and polystyrene nanoplastic particles act as stressors to the innate immune system of fathead minnow (pimephales promelas) Environ. Toxicol. Chem. 2016;35:3093–3100. doi: 10.1002/etc.3501. [DOI] [PubMed] [Google Scholar]
- Gu J.-D. Microbiological deterioration and degradation of synthetic polymeric materials: Recent research advances. Int. Biodeterior. Biodegrad. 2003;52:69–91. [Google Scholar]
- Gu H., Wang S., et al. Nanoplastics impair the intestinal health of the juvenile large yellow croaker larimichthys crocea. J. Hazard. Mater. 2020;397 doi: 10.1016/j.jhazmat.2020.122773. [DOI] [PubMed] [Google Scholar]
- Gu H., Wei S., et al. Microplastics aggravate the adverse effects of bde-47 on physiological and defense performance in mussels. J. Hazard. Mater. 2020;398 doi: 10.1016/j.jhazmat.2020.122909. [DOI] [PubMed] [Google Scholar]
- Gunasekaran K., Mghili B., et al. Personal protective equipment (ppe) pollution driven by the covid-19 pandemic in coastal environment, southeast coast of india. Mar. Pollut. Bull. 2022;180 doi: 10.1016/j.marpolbul.2022.113769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gund M., Isack J., et al. Contamination of surgical mask during aerosol-producing dental treatments. Clin Oral Investig. 2021;25:3173–3180. doi: 10.1007/s00784-020-03645-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X., Lv M., et al. The distinct toxicity effects between commercial and realistic polystyrene microplastics on microbiome and histopathology of gut in zebrafish. J. Hazard. Mater. 2022;434 doi: 10.1016/j.jhazmat.2022.128874. [DOI] [PubMed] [Google Scholar]
- Hagai T., Chen X., et al. Gene expression variability across cells and species shapes innate immunity. Nature. 2018;563:197–202. doi: 10.1038/s41586-018-0657-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamed M., Soliman H.A.M., et al. Assessment the effect of exposure to microplastics in nile tilapia (oreochromis niloticus) early juvenile: I. Blood biomarkers. Chemosphere. 2019;228:345–350. doi: 10.1016/j.chemosphere.2019.04.153. [DOI] [PubMed] [Google Scholar]
- Harshvardhan K., Jha B. Biodegradation of low-density polyethylene by marine bacteria from pelagic waters, arabian sea, india. Mar. Pollut. Bull. 2013;77:100–106. doi: 10.1016/j.marpolbul.2013.10.025. [DOI] [PubMed] [Google Scholar]
- He J., Fu X., et al. Quantitative assessment of interactions of hydrophilic organic contaminants with microplastics in natural water environment. Water Res. 2022;224 doi: 10.1016/j.watres.2022.119024. [DOI] [PubMed] [Google Scholar]
- Hernandez L.M., Xu E.G., et al. Plastic teabags release billions of microparticles and nanoparticles into tea. Environ. Sci. Technol. 2019;53:12300–12310. doi: 10.1021/acs.est.9b02540. [DOI] [PubMed] [Google Scholar]
- Herrera A., Acosta-Dacal A., et al. Bioaccumulation of additives and chemical contaminants from environmental microplastics in european seabass (dicentrarchus labrax) Sci. Total Environ. 2022;822 doi: 10.1016/j.scitotenv.2022.153396. [DOI] [PubMed] [Google Scholar]
- Hirt N., Body-Malapel M. Immunotoxicity and intestinal effects of nano- and microplastics: A review of the literature. Part. Fibre Toxicol. 2020;17:57. doi: 10.1186/s12989-020-00387-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoelting L., Scheinhardt B., et al. A 3-dimensional human embryonic stem cell (hesc)-derived model to detect developmental neurotoxicity of nanoparticles. Arch. Toxicol. 2013;87:721–733. doi: 10.1007/s00204-012-0984-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Z., Meng R., et al. Distinct accumulation of nanoplastics in human intestinal organoids. Sci. Total Environ. 2022;838 doi: 10.1016/j.scitotenv.2022.155811. [DOI] [PubMed] [Google Scholar]
- Hou B., Wang F., et al. Reproductive toxicity of polystyrene microplastics: in vivo experimental study on testicular toxicity in mice. J. Hazard. Mater. 2021;405 doi: 10.1016/j.jhazmat.2020.124028. [DOI] [PubMed] [Google Scholar]
- Hu M., Palic D. Micro- and nano-plastics activation of oxidative and inflammatory adverse outcome pathways. Redox Biol. 2020;37 doi: 10.1016/j.redox.2020.101620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu N., Zhang X., et al. Effects of oral exposure to leachate from boiled-water treated plastic products on gut microbiome and metabolomics. J. Hazard. Mater. 2022;439 doi: 10.1016/j.jhazmat.2022.129605. [DOI] [PubMed] [Google Scholar]
- Hua T., Kiran S., et al. Microplastics exposure affects neural development of human pluripotent stem cell-derived cortical spheroids. J. Hazard. Mater. 2022;435 doi: 10.1016/j.jhazmat.2022.128884. [DOI] [PubMed] [Google Scholar]
- Huang J.N., Wen B., Meng L.J., Li X.X., Wang M.H., Gao J.Z., et al. Integrated response of growth, antioxidant defense and isotopic composition to microplastics in juvenile guppy (poecilia reticulata) J. Hazard. Mater. 2020;399 doi: 10.1016/j.jhazmat.2020.123044. [DOI] [PubMed] [Google Scholar]
- Huang T., Zhang W., et al. Maternal exposure to polystyrene nanoplastics during gestation and lactation induces hepatic and testicular toxicity in male mouse offspring. Food Chem. Toxicol. 2022;160 doi: 10.1016/j.fct.2021.112803. [DOI] [PubMed] [Google Scholar]
- Hwang J., Choi D., et al. An assessment of the toxicity of polypropylene microplastics in human derived cells. Sci. Total Environ. 2019;684:657–669. doi: 10.1016/j.scitotenv.2019.05.071. [DOI] [PubMed] [Google Scholar]
- Ibrahim Y.S., Tuan Anuar S., et al. Detection of microplastics in human colectomy specimens. JGH Open. 2021;5:116–121. doi: 10.1002/jgh3.12457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itskovitz-Eldor J., Schuldiner M., et al. Differentiation of human embryonic stem cells into embryoid bodies compromising the three embryonic germ layers. Molecular medicine (Cambridge, Mass) 2000;6:88–95. [PMC free article] [PubMed] [Google Scholar]
- Ivleva N.P. Chemical analysis of microplastics and nanoplastics: Challenges, advanced methods, and perspectives. Chem. Rev. 2021;121:11886–11936. doi: 10.1021/acs.chemrev.1c00178. [DOI] [PubMed] [Google Scholar]
- Jaikumar G., Brun N.R., et al. Reproductive toxicity of primary and secondary microplastics to three cladocerans during chronic exposure. Environ. Pollution. 2019;249:638–646. doi: 10.1016/j.envpol.2019.03.085. [DOI] [PubMed] [Google Scholar]
- Jain M., Kim S.T., et al. Efficacy and use of cloth masks: a scoping review. Cureus. 2020;12:e10423. doi: 10.7759/cureus.10423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayaweera M., Perera H., et al. Transmission of covid-19 virus by droplets and aerosols: A critical review on the unresolved dichotomy. Environ. Res. 2020;188 doi: 10.1016/j.envres.2020.109819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenner L.C., Rotchell J.M., et al. Detection of microplastics in human lung tissue using muftir spectroscopy. Sci. Total Environ. 2022;831 doi: 10.1016/j.scitotenv.2022.154907. [DOI] [PubMed] [Google Scholar]
- Jeong B., Baek J.Y., et al. Maternal exposure to polystyrene nanoplastics causes brain abnormalities in progeny. J. Hazard. Mater. 2022;426 doi: 10.1016/j.jhazmat.2021.127815. [DOI] [PubMed] [Google Scholar]
- Jiang J.-Q. Occurrence of microplastics and its pollution in the environment: a review. Sustainable Production and Consumption. 2018;13:16–23. [Google Scholar]
- Jiang X., Yang Y., et al. Seasonal variations and feedback from microplastics and cadmium on soil organisms in agricultural fields. Environ. Int. 2022;161 doi: 10.1016/j.envint.2022.107096. [DOI] [PubMed] [Google Scholar]
- Jin Y., Lu L., et al. Impacts of polystyrene microplastic on the gut barrier, microbiota and metabolism of mice. Sci. Total Environ. 2019;649:308–317. doi: 10.1016/j.scitotenv.2018.08.353. [DOI] [PubMed] [Google Scholar]
- Jin H., Ma T., et al. Polystyrene microplastics induced male reproductive toxicity in mice. J. Hazard. Mater. 2021;401 doi: 10.1016/j.jhazmat.2020.123430. [DOI] [PubMed] [Google Scholar]
- Jin Y., Xia J., et al. Polystyrene microplastics induce microbiota dysbiosis and inflammation in the gut of adult zebrafish. Environ. Pollut. 2018;235:322–329. doi: 10.1016/j.envpol.2017.12.088. [DOI] [PubMed] [Google Scholar]
- Johnson D.F., Druce J.D., et al. A quantitative assessment of the efficacy of surgical and n95 masks to filter influenza virus in patients with acute influenza infection. Clin. Infect. Dis. 2009;49:275–277. doi: 10.1086/600041. [DOI] [PubMed] [Google Scholar]
- Joo S.H., Liang Y., et al. Microplastics with adsorbed contaminants: Mechanisms and treatment. Environmental Challenges. 2021;3 doi: 10.1016/j.envc.2021.100042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalaiselvan P., Tummanapalli S.S., et al. The ability of face masks to reduce transmission of microbes. Clinical & experimental optometry. 2022;105:214–221. doi: 10.1080/08164622.2021.1971050. [DOI] [PubMed] [Google Scholar]
- Kang H.M., Byeon E., et al. Different effects of nano- and microplastics on oxidative status and gut microbiota in the marine medaka oryzias melastigma. J. Hazard. Mater. 2021;405 doi: 10.1016/j.jhazmat.2020.124207. [DOI] [PubMed] [Google Scholar]
- Karlsson F., Tremaroli V., et al. Assessing the human gut microbiota in metabolic diseases. Diabetes. 2013;62:3341–3349. doi: 10.2337/db13-0844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.H., Yu Y.B., et al. Toxic effects on bioaccumulation, hematological parameters, oxidative stress, immune responses and neurotoxicity in fish exposed to microplastics: A review. J. Hazard. Mater. 2021;413 doi: 10.1016/j.jhazmat.2021.125423. [DOI] [PubMed] [Google Scholar]
- Koelmans A.A., Mohamed Nor N.H., et al. Microplastics in freshwaters and drinking water: Critical review and assessment of data quality. Water Res. 2019;155:410–422. doi: 10.1016/j.watres.2019.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogel T., Bjoroy O., et al. Micro- and nanoplastic toxicity on aquatic life: Determining factors. Sci. Total Environ. 2020;709 doi: 10.1016/j.scitotenv.2019.136050. [DOI] [PubMed] [Google Scholar]
- Kutralam-Muniasamy G., Perez-Guevara F., et al. Overview of microplastics pollution with heavy metals: Analytical methods, occurrence, transfer risks and call for standardization. J. Hazard. Mater. 2021;415 doi: 10.1016/j.jhazmat.2021.125755. [DOI] [PubMed] [Google Scholar]
- Kwak J.I., An Y.J. Post covid-19 pandemic: Biofragmentation and soil ecotoxicological effects of microplastics derived from face masks. J. Hazard. Mater. 2021;416 doi: 10.1016/j.jhazmat.2021.126169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon W., Kim D., et al. Microglial phagocytosis of polystyrene microplastics results in immune alteration and apoptosis in vitro and in vivo. Sci. Total Environ. 2022;807 doi: 10.1016/j.scitotenv.2021.150817. [DOI] [PubMed] [Google Scholar]
- Kyrikou I., Briassoulis D. Biodegradation of agricultural plastic films: a critical review. J. Polym. Environ. 2007;15:125–150. [Google Scholar]
- Lackmann C., Velki M., et al. Two types of microplastics (polystyrene-hbcd and car tire abrasion) affect oxidative stress-related biomarkers in earthworm eisenia andrei in a time-dependent manner. Environ. Int. 2022;163 doi: 10.1016/j.envint.2022.107190. [DOI] [PubMed] [Google Scholar]
- Lee L.Y., Chan I.C., et al. Reuse of face masks among adults in hong kong during the covid-19 pandemic. BMC Public Health. 2021;21:1267. doi: 10.1186/s12889-021-11346-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei L., Wu S., et al. Microplastic particles cause intestinal damage and other adverse effects in zebrafish danio rerio and nematode caenorhabditis elegans. Sci. Total Environ. 2018;619–620:1–8. doi: 10.1016/j.scitotenv.2017.11.103. [DOI] [PubMed] [Google Scholar]
- Lellis B., Fávaro-Polonio C.Z., et al. Effects of textile dyes on health and the environment and bioremediation potential of living organisms. Biotechnology Research and Innovation. 2019;3:275–290. [Google Scholar]
- Leslie H.A., van Velzen M.J.M., et al. Discovery and quantification of plastic particle pollution in human blood. Environ. Int. 2022;163 doi: 10.1016/j.envint.2022.107199. [DOI] [PubMed] [Google Scholar]
- Li L.L., Amara R., et al. Impacts of microplastics exposure on mussel (mytilus edulis) gut microbiota. Sci. Total Environ. 2020;745 doi: 10.1016/j.scitotenv.2020.141018. [DOI] [PubMed] [Google Scholar]
- Li Z., Chang X., et al. Is microplastic an oxidative stressor? Evidence from a meta-analysis on bivalves. J. Hazard. Mater. 2022;423 doi: 10.1016/j.jhazmat.2021.127211. [DOI] [PubMed] [Google Scholar]
- Li W., Chen X., et al. Microplastics as an aquatic pollutant affect gut microbiota within aquatic animals. J. Hazard. Mater. 2022;423 doi: 10.1016/j.jhazmat.2021.127094. [DOI] [PubMed] [Google Scholar]
- Li M., Gong J., et al. Advanced human developmental toxicity and teratogenicity assessment using human organoid models. Ecotoxicol. Environ. Saf. 2022;235 doi: 10.1016/j.ecoenv.2022.113429. [DOI] [PubMed] [Google Scholar]
- Li M., Gong J., et al. Development of human retinal organoid models for bisphenol toxicity assessment. Ecotoxicol. Environ. Saf. 2022;245 doi: 10.1016/j.ecoenv.2022.114094. [DOI] [PubMed] [Google Scholar]
- Li L., Luo Y., et al. Effective uptake of submicrometre plastics by crop plants via a crack-entry mode. Nat. Sustainability. 2020;3:929–937. [Google Scholar]
- Li S., Ma Y., et al. Polystyrene microplastics trigger hepatocyte apoptosis and abnormal glycolytic flux via ros-driven calcium overload. J. Hazard. Mater. 2021;417 doi: 10.1016/j.jhazmat.2021.126025. [DOI] [PubMed] [Google Scholar]
- Li M., Zeng Y., et al. Evaluation of the influences of low dose polybrominated diphenyl ethers exposure on human early retinal development. Environ. Int. 2022;163 doi: 10.1016/j.envint.2022.107187. [DOI] [PubMed] [Google Scholar]
- Li L., Zhao X., et al. Covid-19: Performance study of microplastic inhalation risk posed by wearing masks. J. Hazard. Mater. 2021;411 doi: 10.1016/j.jhazmat.2020.124955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Zhu S., et al. Polystyrene microplastics cause cardiac fibrosis by activating wnt/beta-catenin signaling pathway and promoting cardiomyocyte apoptosis in rats. Environ. Pollut. 2020;265 doi: 10.1016/j.envpol.2020.115025. [DOI] [PubMed] [Google Scholar]
- Liang H., Ji Y., et al. Release kinetics of microplastics from disposable face masks into the aqueous environment. Sci Total. 2021 doi: 10.1016/j.scitotenv.2021.151650. Environ:151650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao H., Liu S., et al. Di-(2-ethylhexyl) phthalate exacerbated the toxicity of polystyrene nanoplastics through histological damage and intestinal microbiota dysbiosis in freshwater micropterus salmoides. Water Res. 2022;219 doi: 10.1016/j.watres.2022.118608. [DOI] [PubMed] [Google Scholar]
- Lim X. Microplastics are everywhere - but are they harmful? Nature. 2021;593:22–25. doi: 10.1038/d41586-021-01143-3. [DOI] [PubMed] [Google Scholar]
- Limonta G., Mancia A., et al. Microplastics induce transcriptional changes, immune response and behavioral alterations in adult zebrafish. Sci. Rep. 2019;9:15775. doi: 10.1038/s41598-019-52292-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Z., Jin T., et al. Current progress on plastic/microplastic degradation: Fact influences and mechanism. Environ. Pollut. 2022;304 doi: 10.1016/j.envpol.2022.119159. [DOI] [PubMed] [Google Scholar]
- Liu Y., Liu W., et al. Microplastics are a hotspot for antibiotic resistance genes: Progress and perspective. Sci. Total Environ. 2021;773 doi: 10.1016/j.scitotenv.2021.145643. [DOI] [PubMed] [Google Scholar]
- Liu X., Ma J., et al. The combined effects of nanoplastics and dibutyl phthalate on streptomyces coelicolor m145. Sci. Total Environ. 2022;826 doi: 10.1016/j.scitotenv.2022.154151. [DOI] [PubMed] [Google Scholar]
- Liu Y., Shi Q., et al. Perfluorooctane sulfonate (pfos) enhanced polystyrene particles uptake by human colon adenocarcinoma caco-2 cells. Sci. Total Environ. 2022;848 doi: 10.1016/j.scitotenv.2022.157640. [DOI] [PubMed] [Google Scholar]
- Liu L., Xu K., et al. Cellular internalization and release of polystyrene microplastics and nanoplastics. Sci. Total Environ. 2021;779 doi: 10.1016/j.scitotenv.2021.146523. [DOI] [PubMed] [Google Scholar]
- Liu Z., Zhuan Q., et al. Polystyrene microplastics induced female reproductive toxicity in mice. J. Hazard. Mater. 2022;424 doi: 10.1016/j.jhazmat.2021.127629. [DOI] [PubMed] [Google Scholar]
- Lollike K., Kjeldsen L., et al. Lysozyme in human neutrophils and plasma. A parameter of myelopoietic activity. Leukemia. 1995;9:159–164. [PubMed] [Google Scholar]
- Long Y., Hu T., et al. Effectiveness of n95 respirators versus surgical masks against influenza: A systematic review and meta-analysis. J Evid Based Med. 2020;13:93–101. doi: 10.1111/jebm.12381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J., Hou R., et al. Surfactant-sodium dodecyl sulfate enhanced degradation of polystyrene microplastics with an energy-saving electrochemical advanced oxidation process (eaop) strategy. Water Res. 2022;226 doi: 10.1016/j.watres.2022.119277. [DOI] [PubMed] [Google Scholar]
- Lu L., Luo T., et al. Interaction between microplastics and microorganism as well as gut microbiota: A consideration on environmental animal and human health. Sci. Total Environ. 2019;667:94–100. doi: 10.1016/j.scitotenv.2019.02.380. [DOI] [PubMed] [Google Scholar]
- Lu K., Qiao R.X., et al. Influence of microplastics on the accumulation and chronic toxic effects of cadmium in zebrafish (danio rerio) Chemosphere. 2018;202:514–520. doi: 10.1016/j.chemosphere.2018.03.145. [DOI] [PubMed] [Google Scholar]
- Lu L., Wan Z., et al. Polystyrene microplastics induce gut microbiota dysbiosis and hepatic lipid metabolism disorder in mice. Sci. Total Environ. 2018;631–632:449–458. doi: 10.1016/j.scitotenv.2018.03.051. [DOI] [PubMed] [Google Scholar]
- Lu J., Wu J., et al. Combined toxicity of polystyrene microplastics and sulfamethoxazole on zebrafish embryos. Environ. Sci. Pollut. Res. Int. 2022;29:19273–19282. doi: 10.1007/s11356-021-17198-8. [DOI] [PubMed] [Google Scholar]
- Lu Y., Zhang Y., et al. Uptake and accumulation of polystyrene microplastics in zebrafish (danio rerio) and toxic effects in liver. Environ. Sci. Technol. 2016;50:4054–4060. doi: 10.1021/acs.est.6b00183. [DOI] [PubMed] [Google Scholar]
- Luksamijarulkul P., Aiempradit N., et al. Microbial contamination on used surgical masks among hospital personnel and microbial air quality in their working wards: A hospital in bangkok. Oman Med J. 2014;29:346–350. doi: 10.5001/omj.2014.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo C., Gwekwe B., et al. Bitter peptides from enzymatically hydrolyzed protein increase the number of leucocytes and lysozyme activity of large yellow croaker (larimichthys crocea) Fish Shellfish Immunol. 2018;81:130–134. doi: 10.1016/j.fsi.2018.07.013. [DOI] [PubMed] [Google Scholar]
- Lusher A.L., Welden N.A., et al. Sampling, isolating and identifying microplastics ingested by fish and invertebrates. Anal. Methods. 2017;9:1346–1360. [Google Scholar]
- Ma J., Chen F., et al. Face masks as a source of nanoplastics and microplastics in the environment: Quantification, characterization, and potential for bioaccumulation. Environ. Pollut. 2021;288 doi: 10.1016/j.envpol.2021.117748. [DOI] [PubMed] [Google Scholar]
- Ma Q.X., Shan H., et al. Potential utilities of mask-wearing and instant hand hygiene for fighting sars-cov-2. J. Med. Virol. 2020;92:1567–1571. doi: 10.1002/jmv.25805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacArthur JI, Fletcher TC. Phagocytosis in fish. 1985.
- MacIntyre C.R., Chughtai A.A., et al. The efficacy of medical masks and respirators against respiratory infection in healthcare workers. Influenza Other Resp. 2017;11:511–517. doi: 10.1111/irv.12474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mammo F.K., Amoah I.D., et al. Microplastics in the environment: Interactions with microbes and chemical contaminants. Sci. Total Environ. 2020;743 doi: 10.1016/j.scitotenv.2020.140518. [DOI] [PubMed] [Google Scholar]
- Mao R., Lang M., et al. Aging mechanism of microplastics with uv irradiation and its effects on the adsorption of heavy metals. J. Hazard. Mater. 2020;393 doi: 10.1016/j.jhazmat.2020.122515. [DOI] [PubMed] [Google Scholar]
- Mekonnen B.A., Aragaw T.A. In: Covid-19: Environmental sustainability and sustainable development goals. Muthu S.S., editor. Springer Singapore; Singapore: 2021. Environmental sustainability and covid-19 pandemic: An overview review on new opportunities and challenges; pp. 117–140. [Google Scholar]
- Meng J., Zhang Q., et al. Plastic waste as the potential carriers of pathogens. Curr. Opin. Food Sci. 2021;41:224–230. [Google Scholar]
- Meng X., Zhang J., et al. Effects of nano- and microplastics on kidney: Physicochemical properties, bioaccumulation, oxidative stress and immunoreaction. Chemosphere. 2022;288 doi: 10.1016/j.chemosphere.2021.132631. [DOI] [PubMed] [Google Scholar]
- Merkley S.D., Moss H.C., et al. Polystyrene microplastics induce an immunometabolic active state in macrophages. Cell Biol. Toxicol. 2022;38:31–41. doi: 10.1007/s10565-021-09616-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milton D.K., Fabian M.P., et al. Influenza virus aerosols in human exhaled breath: Particle size, culturability, and effect of surgical masks. PLoS Pathog. 2013;9:e1003205. doi: 10.1371/journal.ppat.1003205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min K., Cuiffi J.D., et al. Ranking environmental degradation trends of plastic marine debris based on physical properties and molecular structure. Nat. Commun. 2020;11:727. doi: 10.1038/s41467-020-14538-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitrano D.M., Beltzung A., et al. Synthesis of metal-doped nanoplastics and their utility to investigate fate and behaviour in complex environmental systems. Nat. Nanotechnol. 2019;14:362–368. doi: 10.1038/s41565-018-0360-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgana S., Casentini B., et al. Uncovering the release of micro/nanoplastics from disposable face masks at times of covid-19. J. Hazard. Mater. 2021;419 doi: 10.1016/j.jhazmat.2021.126507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu Y., Sun J., et al. Activation of pyroptosis and ferroptosis is involved in the hepatotoxicity induced by polystyrene microplastics in mice. Chemosphere. 2022;291 doi: 10.1016/j.chemosphere.2021.132944. [DOI] [PubMed] [Google Scholar]
- Murali K., Kenesei K., et al. Uptake and bio-reactivity of polystyrene nanoparticles is affected by surface modifications, ageing and lps adsorption: In vitro studies on neural tissue cells. Nanoscale. 2015;7:4199–4210. doi: 10.1039/c4nr06849a. [DOI] [PubMed] [Google Scholar]
- Naqash N., Prakash S., et al. Interaction of freshwater microplastics with biota and heavy metals: a review. Environ. Chem. Lett. 2020;18:1813–1824. [Google Scholar]
- Nelms S.E., Galloway T.S., et al. Investigating microplastic trophic transfer in marine top predators. Environ. Pollut. 2018;238:999–1007. doi: 10.1016/j.envpol.2018.02.016. [DOI] [PubMed] [Google Scholar]
- Nie J.H., Shen Y., et al. Polystyrene nanoplastics exposure caused defective neural tube morphogenesis through caveolae-mediated endocytosis and faulty apoptosis. Nanotoxicology. 2021;15:885–904. doi: 10.1080/17435390.2021.1930228. [DOI] [PubMed] [Google Scholar]
- Noro K., Yabuki Y. Photolysis of polycyclic aromatic hydrocarbons adsorbed on polyethylene microplastics. Mar. Pollut. Bull. 2021;169 doi: 10.1016/j.marpolbul.2021.112561. [DOI] [PubMed] [Google Scholar]
- Oberbeckmann S., Löder M.G.J., et al. Marine microplastic-associated biofilms – a review. Environ. Chem. 2015;12 [Google Scholar]
- Pandit P., Maity S., et al. Potential biodegradable face mask to counter environmental impact of covid-19. Clean Eng Technol. 2021;4 doi: 10.1016/j.clet.2021.100218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang X., Zhu Z., et al. Evaluation of control measures implemented in the severe acute respiratory syndrome outbreak in beijing, 2003. JAMA. 2003;290:3215–3221. doi: 10.1001/jama.290.24.3215. [DOI] [PubMed] [Google Scholar]
- Park E.J., Han J.S., et al. Repeated-oral dose toxicity of polyethylene microplastics and the possible implications on reproduction and development of the next generation. Toxicol. Lett. 2020;324:75–85. doi: 10.1016/j.toxlet.2020.01.008. [DOI] [PubMed] [Google Scholar]
- Pitt J.A., Trevisan R., et al. Maternal transfer of nanoplastics to offspring in zebrafish (danio rerio): A case study with nanopolystyrene. Sci. Total Environ. 2018;643:324–334. doi: 10.1016/j.scitotenv.2018.06.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potluri P., Needham P. In: Scott R.A., editor. Vol. 6. Woodhead Publishing; 2005. technical textiles for protection; pp. 151–175. (Textiles for protection). [Google Scholar]
- Pourebrahimi S. Upcycling face mask wastes generated during covid-19 into value-added engineering materials: a review. Sci. Total Environ. 2022;851 doi: 10.1016/j.scitotenv.2022.158396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prata J.C., da Costa J.P., et al. Environmental exposure to microplastics: an overview on possible human health effects. Sci. Total Environ. 2020;702 doi: 10.1016/j.scitotenv.2019.134455. [DOI] [PubMed] [Google Scholar]
- Priyanka N., Archana T. Biodegradability of polythene and plastic by the help of microorganism: A way for brighter future. Journal of Environmental & Analytical. Toxicology. 2011;01 [Google Scholar]
- Prust M., Meijer J., et al. The plastic brain: Neurotoxicity of micro- and nanoplastics. Part. Fibre Toxicol. 2020;17:24. doi: 10.1186/s12989-020-00358-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qaseem A., Etxeandia-Ikobaltzeta I., et al. Use of n95, surgical, and cloth masks to prevent covid-19 in health care and community settings: Living practice points from the american college of physicians (version 1) Ann. Intern. Med. 2020;173:642–649. doi: 10.7326/M20-3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao R., Deng Y., et al. Accumulation of different shapes of microplastics initiates intestinal injury and gut microbiota dysbiosis in the gut of zebrafish. Chemosphere. 2019;236 doi: 10.1016/j.chemosphere.2019.07.065. [DOI] [PubMed] [Google Scholar]
- Qiao R., Sheng C., et al. Microplastics induce intestinal inflammation, oxidative stress, and disorders of metabolome and microbiome in zebrafish. Sci. Total Environ. 2019;662:246–253. doi: 10.1016/j.scitotenv.2019.01.245. [DOI] [PubMed] [Google Scholar]
- Qin L., Duan Z., et al. Size-dependent impact of polystyrene microplastics on the toxicity of cadmium through altering neutrophil expression and metabolic regulation in zebrafish larvae. Environ. Pollut. 2021;291 doi: 10.1016/j.envpol.2021.118169. [DOI] [PubMed] [Google Scholar]
- Qin M., Gong J., et al. The role of microplastics in altering arsenic fractionation and microbial community structures in arsenic-contaminated riverine sediments. J. Hazard. Mater. 2022;433 doi: 10.1016/j.jhazmat.2022.128801. [DOI] [PubMed] [Google Scholar]
- Qu M., Kong Y., et al. Neuronal damage induced by nanopolystyrene particles in nematode caenorhabditis elegans. Environ. Sci. Nano. 2019;6:2591–2601. [Google Scholar]
- Qu M., Wang D. Toxicity comparison between pristine and sulfonate modified nanopolystyrene particles in affecting locomotion behavior, sensory perception, and neuronal development in caenorhabditis elegans. Sci. Total Environ. 2020;703 doi: 10.1016/j.scitotenv.2019.134817. [DOI] [PubMed] [Google Scholar]
- Ragusa A., Svelato A., et al. Plasticenta: First evidence of microplastics in human placenta. Environ. Int. 2021;146 doi: 10.1016/j.envint.2020.106274. [DOI] [PubMed] [Google Scholar]
- Revel M., Châtel A., et al. Micro(nano)plastics: A threat to human health? Current Opinion in Environmental Science & Health. 2018;1:17–23. [Google Scholar]
- Rist S., Baun A., et al. Ingestion of micro- and nanoplastics in daphnia magna - quantification of body burdens and assessment of feeding rates and reproduction. Environ. Pollut. 2017;228:398–407. doi: 10.1016/j.envpol.2017.05.048. [DOI] [PubMed] [Google Scholar]
- Saliu F., Veronelli M., et al. The release process of microfibers: From surgical face masks into the marine environment. Environmental. Advances. 2021;4 [Google Scholar]
- Saurabh S., Sahoo P.K. Lysozyme: an important defence molecule of fish innate immune system. Aquac. Res. 2008;39:223–239. [Google Scholar]
- Schirinzi G.F., Perez-Pomeda I., et al. Cytotoxic effects of commonly used nanomaterials and microplastics on cerebral and epithelial human cells. Environ. Res. 2017;159:579–587. doi: 10.1016/j.envres.2017.08.043. [DOI] [PubMed] [Google Scholar]
- Schmidt C., Lautenschlaeger C., et al. Nano- and microscaled particles for drug targeting to inflamed intestinal mucosa: a first in vivo study in human patients. J. Control. Release. 2013;165:139–145. doi: 10.1016/j.jconrel.2012.10.019. [DOI] [PubMed] [Google Scholar]
- Schwabl P., Koppel S., et al. Detection of various microplastics in human stool: a prospective case series. Ann. Intern. Med. 2019;171:453–457. doi: 10.7326/M19-0618. [DOI] [PubMed] [Google Scholar]
- Schwarzer M., Brehm J., et al. Shape, size, and polymer dependent effects of microplastics on daphnia magna. J. Hazard. Mater. 2022;426 doi: 10.1016/j.jhazmat.2021.128136. [DOI] [PubMed] [Google Scholar]
- Seiler A.E., Spielmann H. The validated embryonic stem cell test to predict embryotoxicity in vitro. Nat. Protoc. 2011;6:961–978. doi: 10.1038/nprot.2011.348. [DOI] [PubMed] [Google Scholar]
- Sendra M., Pereiro P., et al. Surgical face masks as a source of emergent pollutants in aquatic systems: Analysis of their degradation product effects in danio rerio through rna-seq. J. Hazard. Mater. 2022;428 doi: 10.1016/j.jhazmat.2021.128186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma M.D., Elanjickal A.I., et al. Assessment of cancer risk of microplastics enriched with polycyclic aromatic hydrocarbons. J. Hazard. Mater. 2020;398 doi: 10.1016/j.jhazmat.2020.122994. [DOI] [PubMed] [Google Scholar]
- Shen R., Yang K., et al. Accumulation of polystyrene microplastics induces liver fibrosis by activating cgas/sting pathway. Environ. Pollut. 2022;300 doi: 10.1016/j.envpol.2022.118986. [DOI] [PubMed] [Google Scholar]
- Shen M., Zeng Z., et al. Neglected microplastics pollution in global covid-19: Disposable surgical masks. Sci. Total Environ. 2021;790 doi: 10.1016/j.scitotenv.2021.148130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng C., Zhang S., et al. The influence of different polymer types of microplastics on adsorption, accumulation, and toxicity of triclosan in zebrafish. J. Hazard. Mater. 2021;402 doi: 10.1016/j.jhazmat.2020.123733. [DOI] [PubMed] [Google Scholar]
- Shi W., Cao Y., et al. Potential health risks of the interaction of microplastics and lung surfactant. J. Hazard. Mater. 2022;429 doi: 10.1016/j.jhazmat.2021.128109. [DOI] [PubMed] [Google Scholar]
- Singh S., Rawat P.S. In: Handbook of research on environmental and human health impacts of plastic pollution. Wani K.A., Ariana L., Zuber S.M., editors. USA:IGI Global; Hershey, PA: 2020. Biodegradation of plastic: An innovative solution to safe the human health and environment; pp. 435–461. [Google Scholar]
- Smith M., Love D.C., et al. Microplastics in seafood and the implications for human health. Curr Environ Health Rep. 2018;5:375–386. doi: 10.1007/s40572-018-0206-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J.D., MacDougall C.C., et al. Effectiveness of n95 respirators versus surgical masks in protecting health care workers from acute respiratory infection: a systematic review and meta-analysis. Can. Med. Assoc. J. 2016;188:567–574. doi: 10.1503/cmaj.150835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomando A., Capo X., et al. Long-term exposure to microplastics induces oxidative stress and a pro-inflammatory response in the gut of sparus aurata linnaeus, 1758. Environ. Pollut. 2020;266 doi: 10.1016/j.envpol.2020.115295. [DOI] [PubMed] [Google Scholar]
- Song Y.K., Hong S.H., et al. Combined effects of uv exposure duration and mechanical abrasion on microplastic fragmentation by polymer type. Environ. Sci. Technol. 2017;51:4368–4376. doi: 10.1021/acs.est.6b06155. [DOI] [PubMed] [Google Scholar]
- Stock V., Bohmert L., et al. Uptake and effects of orally ingested polystyrene microplastic particles in vitro and in vivo. Arch. Toxicol. 2019;93:1817–1833. doi: 10.1007/s00204-019-02478-7. [DOI] [PubMed] [Google Scholar]
- Sullivan G.L., Delgado-Gallardo J., et al. An investigation into the leaching of micro and nano particles and chemical pollutants from disposable face masks - linked to the covid-19 pandemic. Water Res. 2021;196 doi: 10.1016/j.watres.2021.117033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun M., Ding R., et al. Cardiovascular toxicity assessment of polyethylene nanoplastics on developing zebrafish embryos. Chemosphere. 2021;282 doi: 10.1016/j.chemosphere.2021.131124. [DOI] [PubMed] [Google Scholar]
- Sun J., Yang S., et al. Release of microplastics from discarded surgical masks and their adverse impacts on the marine copepod tigriopus japonicus. Environ. Sci. Technol. Lett. 2021;8:1065–1070. [Google Scholar]
- Sun T., Zhan J., et al. Environmentally relevant concentrations of microplastics influence the locomotor activity of aquatic biota. J. Hazard. Mater. 2021;414 doi: 10.1016/j.jhazmat.2021.125581. [DOI] [PubMed] [Google Scholar]
- Suzuki T., Hidaka T., et al. Environmental pollutants and the immune response. Nat. Immunol. 2020;21:1486–1495. doi: 10.1038/s41590-020-0802-6. [DOI] [PubMed] [Google Scholar]
- Tamargo A., Molinero N., et al. Pet microplastics affect human gut microbiota communities during simulated gastrointestinal digestion, first evidence of plausible polymer biodegradation during human digestion. Sci. Rep. 2022;12:528. doi: 10.1038/s41598-021-04489-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teleanu DM, Chircov C, Grumezescu AM, Teleanu RI. 2019. Neurotoxicity of nanomaterials: An up-to-date overview. Nanomaterials (Basel) 9. [DOI] [PMC free article] [PubMed]
- Tesfaldet Y.T., Ndeh N.T., et al. Assessing face mask littering in urban environments and policy implications: The case of bangkok. Sci. Total Environ. 2022;806 doi: 10.1016/j.scitotenv.2021.150952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tournier V., Topham C.M., et al. An engineered pet depolymerase to break down and recycle plastic bottles. Nature. 2020;580:216–219. doi: 10.1038/s41586-020-2149-4. [DOI] [PubMed] [Google Scholar]
- Tran H.N., Le G.T., et al. Sars-cov-2 coronavirus in water and wastewater: A critical review about presence and concern. Environ. Res. 2021;193 doi: 10.1016/j.envres.2020.110265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu C, Zhou Q, Zhang C, Liu Y, Luo Y. 2020. Biofilms of microplastics. In: Microplastics in terrestrial environments, 299-317.
- Tunali M., Uzoefuna E.N., et al. Effect of microplastics and microplastic-metal combinations on growth and chlorophyll a concentration of chlorella vulgaris. Sci. Total Environ. 2020;743 doi: 10.1016/j.scitotenv.2020.140479. [DOI] [PubMed] [Google Scholar]
- Tunon-Molina A., Takayama K., et al. Protective face masks: current status and future trends. ACS Appl. Mater. Interfaces. 2021;13:56725–56751. doi: 10.1021/acsami.1c12227. [DOI] [PubMed] [Google Scholar]
- Valles-Colomer M., Falony G., et al. The neuroactive potential of the human gut microbiota in quality of life and depression. Nat. Microbiol. 2019;4:623–632. doi: 10.1038/s41564-018-0337-x. [DOI] [PubMed] [Google Scholar]
- van Doremalen N., Bushmaker T., et al. Aerosol and surface stability of sars-cov-2 as compared with sars-cov-1. New Engl J Med. 2020;382:1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velzeboer I., Kwadijk C.J., et al. Strong sorption of pcbs to nanoplastics, microplastics, carbon nanotubes, and fullerenes. Environ. Sci. Technol. 2014;48:4869–4876. doi: 10.1021/es405721v. [DOI] [PubMed] [Google Scholar]
- Wagner S., Reemtsma T. Things we know and don't know about nanoplastic in the environment. Nat. Nanotechnol. 2019;14:300–301. doi: 10.1038/s41565-019-0424-z. [DOI] [PubMed] [Google Scholar]
- Wang Q., Bai J., et al. Effects of bisphenol a and nanoscale and microscale polystyrene plastic exposure on particle uptake and toxicity in human caco-2 cells. Chemosphere. 2020;254 doi: 10.1016/j.chemosphere.2020.126788. [DOI] [PubMed] [Google Scholar]
- Wang Y.L., Lee Y.H., et al. The kidney-related effects of polystyrene microplastics on human kidney proximal tubular epithelial cells hk-2 and male c57bl/6 mice. Environ. Health Perspect. 2021;129:57003. doi: 10.1289/EHP7612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Li H., et al. A neglected risk of nanoplastics as revealed by the promoted transformation of plasmid-borne ampicillin resistance gene by escherichia coli. Environ. 2022 doi: 10.1111/1462-2920.16178. Microbiol:e16178. [DOI] [PubMed] [Google Scholar]
- Wang L.C., Lin J.C., et al. The sorption of persistent organic pollutants in microplastics from the coastal environment. J. Hazard. Mater. 2021;420 doi: 10.1016/j.jhazmat.2021.126658. [DOI] [PubMed] [Google Scholar]
- Wang S., Liu H., et al. Response of tyramine and glutamate related signals to nanoplastic exposure in caenorhabditis elegans. Ecotoxicol. Environ. Saf. 2021;217 doi: 10.1016/j.ecoenv.2021.112239. [DOI] [PubMed] [Google Scholar]
- Wang L., Peng Y., et al. An in situ depolymerization and liquid chromatography-tandem mass spectrometry method for quantifying polylactic acid microplastics in environmental samples. Environ. Sci. Technol. 2022;56:13029–13035. doi: 10.1021/acs.est.2c02221. [DOI] [PubMed] [Google Scholar]
- Wang J., Tan Z., et al. The behaviors of microplastics in the marine environment. Mar. Environ. Res. 2016;113:7–17. doi: 10.1016/j.marenvres.2015.10.014. [DOI] [PubMed] [Google Scholar]
- Wang Y., Wang X., et al. Effects of exposure of polyethylene microplastics to air, water and soil on their adsorption behaviors for copper and tetracycline. Chem. Eng. J. 2021;404 [Google Scholar]
- Wei W., Li Y., et al. Anionic nanoplastic exposure induces endothelial leakiness. Nat. Commun. 2022;13:4757. doi: 10.1038/s41467-022-32532-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei J., Wang X., et al. The impact of polystyrene microplastics on cardiomyocytes pyroptosis through nlrp3/caspase-1 signaling pathway and oxidative stress in wistar rats. Environ. Toxicol. 2021;36:935–944. doi: 10.1002/tox.23095. [DOI] [PubMed] [Google Scholar]
- WHO. 2020. World health organization (who) shortage of personal protective equipment endangering health workers worldwide. Available: https://www.who.int/news/item/03-03-2020-shortage-of-personal-protective-equipment-endangering-health-workers-worldwide.
- WHO. 2022. Who coronavirus (covid-19) dashboard. Available: https://covid19.who.int/.
- Winkler A.S., Cherubini A., et al. Human airway organoids and microplastic fibers: a new exposure model for emerging contaminants. Environ. Int. 2022;163 doi: 10.1016/j.envint.2022.107200. [DOI] [PubMed] [Google Scholar]
- World Wildlife Fund. 2020. In the disposal of masks and gloves, responsibility is required. Available: www.wwf.it/scuole/?53500%2FNello-smaltimento-di-mascherinee-guanti-serve-responsabilita.
- Wright R.J., Erni-Cassola G., et al. Marine plastic debris: a new surface for microbial colonization. Environ. Sci. Technol. 2020;54:11657–11672. doi: 10.1021/acs.est.0c02305. [DOI] [PubMed] [Google Scholar]
- Wu D., Feng Y., et al. J. Adv Res; 2022. Pigment microparticles and microplastics found in human thrombi based on raman spectral evidence. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S., Jin C., et al. Exposure to the fungicide propamocarb causes gut microbiota dysbiosis and metabolic disorder in mice. Environ. Pollut. 2018;237:775–783. doi: 10.1016/j.envpol.2017.10.129. [DOI] [PubMed] [Google Scholar]
- Xia J., Jin C., et al. Chronic exposure to low concentrations of lead induces metabolic disorder and dysbiosis of the gut microbiota in mice. Sci. Total Environ. 2018;631–632:439–448. doi: 10.1016/j.scitotenv.2018.03.053. [DOI] [PubMed] [Google Scholar]
- Xie H., Han W., et al. Face mask—a potential source of phthalate exposure for human. J. Hazard. Mater. 2022;422 doi: 10.1016/j.jhazmat.2021.126848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J.L., Lin X., et al. Spectral imaging for characterization and detection of plastic substances in branded teabags. J. Hazard. Mater. 2021;418 doi: 10.1016/j.jhazmat.2021.126328. [DOI] [PubMed] [Google Scholar]
- Xu G., Liu Y., et al. Size effects of microplastics on accumulation and elimination of phenanthrene in earthworms. J. Hazard. Mater. 2021;403 doi: 10.1016/j.jhazmat.2020.123966. [DOI] [PubMed] [Google Scholar]
- Xu D., Ma Y., et al. Systematic toxicity evaluation of polystyrene nanoplastics on mice and molecular mechanism investigation about their internalization into caco-2 cells. J. Hazard. Mater. 2021;417 doi: 10.1016/j.jhazmat.2021.126092. [DOI] [PubMed] [Google Scholar]
- Yan W., Hamid N., et al. Individual and combined toxicogenetic effects of microplastics and heavy metals (cd, pb, and zn) perturb gut microbiota homeostasis and gonadal development in marine medaka (oryzias melastigma) J. Hazard. Mater. 2020;397 doi: 10.1016/j.jhazmat.2020.122795. [DOI] [PubMed] [Google Scholar]
- Yan Z., Liu Y., et al. Analysis of microplastics in human feces reveals a correlation between fecal microplastics and inflammatory bowel disease status. Environ. Sci. Technol. 2021 doi: 10.1021/acs.est.1c03924. [DOI] [PubMed] [Google Scholar]
- Yan Z., Zhao H., et al. An efficient method for extracting microplastics from feces of different species. J. Hazard. Mater. 2020;384 doi: 10.1016/j.jhazmat.2019.121489. [DOI] [PubMed] [Google Scholar]
- Yang Y.F., Chen C.Y., et al. Toxicity-based toxicokinetic/toxicodynamic assessment for bioaccumulation of polystyrene microplastics in mice. J. Hazard. Mater. 2019;366:703–713. doi: 10.1016/j.jhazmat.2018.12.048. [DOI] [PubMed] [Google Scholar]
- Yang S., Cheng Y., et al. In vitro evaluation of nanoplastics using human lung epithelial cells, microarray analysis and co-culture model. Ecotoxicol. Environ. Saf. 2021;226 doi: 10.1016/j.ecoenv.2021.112837. [DOI] [PubMed] [Google Scholar]
- Yang Z., Zhu L., et al. Polystyrene microplastics increase pb bioaccumulation and health damage in the chinese mitten crab eriocheir sinensis. Sci. Total Environ. 2022;829 doi: 10.1016/j.scitotenv.2022.154586. [DOI] [PubMed] [Google Scholar]
- Yokota K., Mehlrose M. Lake phytoplankton assemblage altered by irregularly shaped pla body wash microplastics but not by ps calibration beads. Water. 2020;12 [Google Scholar]
- Yu J., Chen L., et al. Heterogeneity effects of nanoplastics and lead on zebrafish intestinal cells identified by single-cell sequencing. Chemosphere. 2022;289 doi: 10.1016/j.chemosphere.2021.133133. [DOI] [PubMed] [Google Scholar]
- Yuan J., Ma J., et al. Microbial degradation and other environmental aspects of microplastics/plastics. Sci. Total Environ. 2020;715 doi: 10.1016/j.scitotenv.2020.136968. [DOI] [PubMed] [Google Scholar]
- Zeenat E.A., Bukhari D.A., et al. Plastics degradation by microbes: a sustainable approach. J. King Saud University – Sci. 2021;33 [Google Scholar]
- Zettler E.R., Mincer T.J., et al. Life in the “plastisphere”: Microbial communities on plastic marine debris. Environ. Sci. Technol. 2013;47:7137–7146. doi: 10.1021/es401288x. [DOI] [PubMed] [Google Scholar]
- Zhang K., Hamidian A.H., et al. Understanding plastic degradation and microplastic formation in the environment: a review. Environ. Pollut. 2021;274 doi: 10.1016/j.envpol.2021.116554. [DOI] [PubMed] [Google Scholar]
- Zhang C., Lei Y., et al. Sorption of organochlorine pesticides on polyethylene microplastics in soil suspension. Ecotoxicol. Environ. Saf. 2021;223 doi: 10.1016/j.ecoenv.2021.112591. [DOI] [PubMed] [Google Scholar]
- Zhang F., Li D., et al. Combined effects of polystyrene microplastics and copper on antioxidant capacity, immune response and intestinal microbiota of nile tilapia (oreochromis niloticus) Sci. Total Environ. 2022;808 doi: 10.1016/j.scitotenv.2021.152099. [DOI] [PubMed] [Google Scholar]
- Zhang C.N., Pan Z.K., et al. Size and concentration effects of microplastics on digestion and immunity of hybrid snakehead in developmental stages. Aquacult. Rep. 2022;22 [Google Scholar]
- Zhang J., Wang L., et al. Occurrence of polyethylene terephthalate and polycarbonate microplastics in infant and adult feces. Environ. Sci. Technol. Lett. 2021;8:989–994. doi: 10.1021/acs.est.9b03912. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Yin K., et al. Polystyrene microplastics-induced cardiotoxicity in chickens via the ros-driven nf-κb-nlrp3-gsdmd and ampk-pgc-1α axes. Sci. Total Environ. 2022;840 doi: 10.1016/j.scitotenv.2022.156727. [DOI] [PubMed] [Google Scholar]
- Zhao Y., Bao Z., et al. Polystyrene microplastic exposure disturbs hepatic glycolipid metabolism at the physiological, biochemical, and transcriptomic levels in adult zebrafish. Sci. Total Environ. 2020;710 doi: 10.1016/j.scitotenv.2019.136279. [DOI] [PubMed] [Google Scholar]
- Zheng H., Wang J., et al. Proinflammatory properties and lipid disturbance of polystyrene microplastics in the livers of mice with acute colitis. Sci. Total Environ. 2021;750 doi: 10.1016/j.scitotenv.2020.143085. [DOI] [PubMed] [Google Scholar]
- Zhou S.Y., Lin C., et al. Discarded masks as hotspots of antibiotic resistance genes during covid-19 pandemic. J. Hazard. Mater. 2022;425 doi: 10.1016/j.jhazmat.2021.127774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J., Liu X., et al. Antidote or trojan horse for submerged macrophytes: role of microplastics in copper toxicity in aquatic environments. Water Res. 2022;216 doi: 10.1016/j.watres.2022.118354. [DOI] [PubMed] [Google Scholar]
- Zhou S.S., Lukula S., et al. Assessment of a respiratory face mask for capturing air pollutants and pathogens including human influenza and rhinoviruses. J. Thorac Dis. 2018;10:2059–2069. doi: 10.21037/jtd.2018.03.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zitouni N., Cappello T., et al. Metabolomic disorders unveil hepatotoxicity of environmental microplastics in wild fish serranus scriba (linnaeus 1758) Sci. Total Environ. 2022;838 doi: 10.1016/j.scitotenv.2022.155872. [DOI] [PubMed] [Google Scholar]
Further reading
- Li S., Shi M., et al. Keap1-nrf2 pathway up-regulation via hydrogen sulfide mitigates polystyrene microplastics induced-hepatotoxic effects. J. Hazard. Mater. 2021;402 doi: 10.1016/j.jhazmat.2020.123933. [DOI] [PubMed] [Google Scholar]
- Wang Z., An C., et al. Disposable masks release microplastics to the aqueous environment with exacerbation by natural weathering. J. Hazard. Mater. 2021;417 doi: 10.1016/j.jhazmat.2021.126036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Fu D., et al. Aged microplastics decrease the bioavailability of coexisting heavy metals to microalga chlorella vulgaris. Ecotoxicol. Environ. Saf. 2021;217 doi: 10.1016/j.ecoenv.2021.112199. [DOI] [PubMed] [Google Scholar]
- Wang X., Okoffo E.D., et al. Phthalate esters in face masks and associated inhalation exposure risk. J. Hazard. Mater. 2022;423 doi: 10.1016/j.jhazmat.2021.127001. [DOI] [PubMed] [Google Scholar]
- Zhang Z., Peng H., et al. Polyvinyl chloride degradation by a bacterium isolated from the gut of insect larvae. Nat. Commun. 2022;13:5360. doi: 10.1038/s41467-022-32903-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F., Wang Z., et al. Theoretical investigation on the interactions of microplastics with a sars-cov-2 rna fragment and their potential impacts on viral transport and exposure. Sci. Total Environ. 2022;842 doi: 10.1016/j.scitotenv.2022.156812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z., Zulpiya M., et al. Occurrence and sources of microplastics in dust of the ebinur lake basin, northwest china. Environ. Geochem. Health. 2022 doi: 10.1007/s10653-022-01279-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.