Abstract
Recent connectome analyses of the entire synaptic circuit in the nervous system have provided tremendous insights into how neural processing occurs through the synaptic relay of neural information. Conversely, the extent to which ephaptic transmission which does not depend on the synapses contributes to the relay of neural information, especially beyond a distance between adjacent neurons and to neural processing remains unclear. We show that ephaptic transmission mediated by extracellular potential changes in female Drosophila melanogaster can reach >200 µm, equivalent to the depth of its brain. Furthermore, ephaptic transmission driven by retinal photoreceptor cells mediates light-evoked firing rate increases in olfactory sensory neurons. These results indicate that ephaptic transmission contributes to sensory responses that can change momentarily in a context-dependent manner.
SIGNIFICANCE STATEMENT Although extracellular field potential activities are commonly observed in many nervous systems, this activity has been generally considered as a side effect of synchronized spiking of neurons. This study, however, shows that field potential changes in retinae evoked by a sensory stimulus can control the excitability of distant neurons in vivo and mediates multimodal sensory integration in Drosophila melanogaster. As such ephaptic transmission is more effective at a short distance, the ephaptic transmission from the retinae may contribute significantly to firing rate changes in downstream neurons of the photoreceptor cells in the optic lobe.
Keywords: electric field, ephaptic, field potential, multimodal sensory integration, olfaction, vision
Introduction
Behavioral responses to a specific mode of sensory input varies with the context. For example, in Drosophila melanogaster, pairing a food odor with a visual stimulus enhanced the optomotor response (Chow et al., 2011), and light facilitated both establishment and recall of olfactory memory (Yarali et al., 2008). Sensory information from different modalities is generally integrated by sensory neurons expressing multimodal sensory receptors or by the convergence of synaptic relay of sensory information originating from distinct sensory organs onto individual neurons (van Atteveldt et al., 2014). This study demonstrates that ephaptic transmission also contributes to sensory integration.
Ephaptic transmission is a form of communication that does not depend on synapses. It is mediated by changes in extracellular field potential or electric field, evoked by neural activity (Weiss and Faber, 2010; Buzsáki et al., 2012; Anastassiou and Koch, 2015). Recent studies have reported that excitation of an olfactory sensory neuron (OSN) induces ephaptic inhibition in another OSN housed in the same sensillum in Drosophila antennae (Zhang et al., 2019), and that firing of a Purkinje cell promotes synchronous firing of nearby Purkinje cells by ephaptic coupling (Han et al., 2018). These findings reveal the existence of ephaptic transmission between neighboring neurons. However, the contribution of ephaptic transmission at a distance remains unclear in vivo, as it is not easy to separate the effect of ephaptic communication from synaptic communication. To study this further, we analyzed the integration of visual and olfactory inputs in Drosophila.
In Drosophila, light is primarily received by external photoreceptor cells in the compound eyes and ocelli. Moreover, internal photoreceptor cells in Hofbauer-Buchner eyelets and brain also contribute to sensing light to regulate the circadian rhythm (Yasuyama and Meinertzhagen, 1999; Malpel et al., 2002; Ni et al., 2017). In contrast, olfactory sensory input is received by OSNs in the antennae and maxillary palpi. OSNs send projections to the primary olfactory center, referred to as the antennal lobe (Wilson, 2013). Dye injection into the antennal lobe does not label the optic lobe (i.e., the primary visual center), indicating that there are no direct connections between the primary olfactory and visual centers (Tanaka et al., 2012). The mushroom body is considered as the site of integration of visual and olfactory information, since it is connected to the primary centers and its intrinsic neurons respond to light and odor stimulations (Vogt et al., 2016; Yagi et al., 2016). However, it remains unclear whether peripheral sensory neurons integrate visual and olfactory information.
In this study, we recorded odor and light responses from OSNs expressing the olfactory receptor Or67d, under various light conditions (see Fig. 1A). Or67d OSN is the sole OSN housed in the trichodia T1 sensillum, the largest spine-shaped sensillum distributed in the proximal part of the antenna (Shanbhag et al., 1999). Or67d OSN responds specifically to the male pheromone cis-vaccenyl acetate (cVA), and the spikes recorded from T1 sensilla are composed of a single unit of the Or67d OSN (van der Goes van Naters and Carlson, 2007), which enabled us to record the same type of neuron repeatedly from different individuals. Analyses of the firing patterns of Or67d OSNs revealed that a transient change in the light condition modified the odor response of Or67d OSNs, which was mediated by ephaptic transmission driven by retinal photoreceptor cells.
Figure 1.
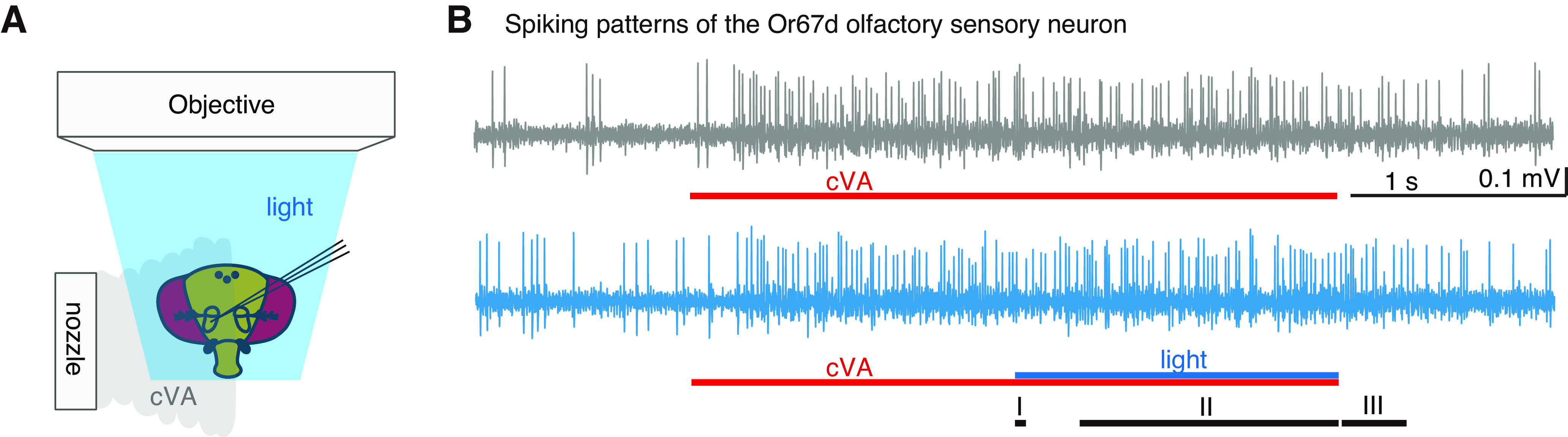
Transient changes in light condition modulated cVA response of Or67d OSNs in WT flies. A, Experimental setup. While recording spikes extracellularly from Or67d OSN, cVA and/or blue light stimulations were applied to the fly's head. B, Extracellular recording of spikes from Or67d OSNs. Top, The response to a sole cVA puff. Bottom, The response to simultaneous stimulation with cVA and blue light. Red lines indicate the timing of 3 s odor stimulations. Blue line indicates 1.5 s of lighting period. Periods I, II, and III represent the first 50 ms after the light condition was changed, 1.2 s while transient change in the light condition was sustained, and 300 ms just after the light condition was returned to the original condition, respectively.
Materials and Methods
Drosophila strains
Flies were reared on standard agar-cornmeal medium at 25°C with a 12 h light/12 h dark cycle. We used female flies between 3 and 7 d after eclosion.
We used Canton S as a WT and the following mutants for the single-sensillum recordings: eyes absent (eya2) (RRID:BDSC_2285; Choi and Benzer, 1994), histidine decarboxylase mutants (hdcJK910) (RRID:BDSC_64203; Burg et al., 1993; Melzig et al., 1996), and no receptor potential A mutant (norpA36) (RRID:BDSC_9048; Bloomquist et al., 1988) obtained from the Bloomington Drosophila Stock Center (BDSC). We prepared flies in which synaptic transmission of the photoreceptors in the ocelli, compound eyes, and eyelets was blocked by mating longGMR-GAL4 (RRID:BDSC_8121; Wernet et al., 2003) from BDSC with UAS-tetanus toxin (TNT; BDSC_28838; Sweeney et al., 1995) gifted by Aki Ejima. To label the photoreceptor cells, flies bearing both UAS-GFP obtained from Barry Dickson (Tanaka et al., 2009) and UAS-20XmCD8::GFP from BDSC (RRID:BDSC_32194) were crossed with longGMR-GAL4.
Single-sensillum recording from the antennal T1 sensillum
Each female fly was anesthetized in a vial on ice for <1 min and restrained in a custom-made plastic dish by fixing the appendages with wax and epoxy (Tanaka et al., 2009). The right antenna was then set on a tin foil to which the rear side of the antenna was bonded with epoxy to avoid movement. To exclude the light, the head except for the right antenna was painted with black acrylic paint. While the epoxy and paint were drying, the dish was placed in a moist chamber for 15-20 min in the dark. The lateral surface of the thorax was then gently heated using a wax melter to avoid muscle movement in the thorax. After applying Drosophila saline (in mm as follows: NaCl 103, KCl 3, N-tris(hydroxymethyl)methyl-2-aminoethanesulfonic acid 5, trehalose 10, glucose 10, sucrose 7, NaHCO3 26, NaH2PO4 1, CaCl2 1.5, MgCl2 4, adjusted to pH 7.25 with HCl) over the thorax, the cuticle of the thorax was partially removed. Surgical manipulations, such as transplantation of a compound eye, were performed using a window made on the top of the head in saline. After removing fat and air sacs over the brain through the window, saline was applied over the brain. For the transplantation experiment, a compound eye severed from a WT female fly by cutting the rim of the eye was set on the saline. The outer surface of the eye was kept dry. To amputate the antennal nerve, we inserted forceps through the window and cut the right antennal nerve. All dissection steps were performed with forceps under a dissection microscope.
The restrained fly was then placed under a Slicescope (Scientifica) equipped with a GSWH20x/12.5 ocular lens, LUCPlanFLN40x (NA 0.60) objective and U-ECA 2× magnification changers (Olympus). When recording spikes from the T1 sensillum, the recording and reference electrodes were inserted into the base of the sensillum and into saline over the thorax, respectively. We inserted the reference electrode into saline, instead of the head, to avoid recording light-evoked potentials through the reference electrode. To inject current, we additionally inserted a tungsten electrode that was connected to an AxoClamp-2B (Molecular Devices) into the right optic lobe area. We recorded neural responses from a single sensillum in each animal. The recording electrodes (impedance of ∼90 mΩ) were prepared by pulling quartz capillaries (QF100-70-7.5, Sutter Instrument) with a Sutter Instrument P2000 puller and filled with sensillum lymph ringer (in mm as follows: KCl 171.9, KH2PO4 9.2, K2HPO4 10.8, MgCl 3, CaCl2 1, glucose 22.5, NaCl 25, adjusted to pH 6.5 with HCl) (Kaissling and Thorson, 1980; Dobritsa et al., 2003; Olsson and Hansson, 2013).
Recorded signals were amplified 100-fold with a headstage (8024/7001 N = 1, Dagan) and DAGAN 8700 Cell Explorer amplifier, and were fed into a PC via an A/D converter Digidata 1322A or 1440A (Molecular Devices). Data were acquired at a sampling rate of 5 kHz using the Axoscope software (Molecular Devices). All recorded data were high-pass filtered at 65 Hz and analyzed using MATLAB 2019b (The MathWorks). Recordings under each light condition were repeated 3-5 times for each animal, and the firing rates were averaged for further analyses. In animals transplanted with a compound eye, data were excluded if the average firing rate in a 300 ms bin just before the light stimulations differed by >5 Hz between trials, with and without light stimulation.
Odor and light stimulations
For the odor stimulations, an air puff (3 s, 240 ml/min) from a pneumatic picopump (PV 820, World Precision Instruments) that passed through a tube inserted with filter paper (3.5 × 50 mm) containing 15 µl of either paraffin oil or 1% v/v cVA in paraffin oil was applied to a fly every 30 s. The airpuff speed was adjusted to 1 m/s. Odorized air was continuously removed using a vacuum tube set near the fly's body. The total amount of cVA in the oil was adjusted to 140 µg.
Flies were subjected to 1.5 s light-on or light-off stimulations simultaneously with odor stimulations. Emitted light from an Olympus U-HGLGPS mercury lamp was filtered by U-MNIBA3, which allows 470-495 nm light to pass, and the intensity was adjusted to 55 μmol m−2 s−1. We confirmed these values using a LI-250 light meter connected to an LI-190SA quantum sensor (LI-COR). The picopump and shutter were controlled using Master-8 (A.M.P.I.). We randomly alternated the order of different light conditions and counterbalanced the number of recordings of one light condition preceding the other, with those in the opposite sequence.
Antibody staining of the brain
Brains were dissected in PBS and fixed in 4% PFA in PBS for 60 min. After washing with PBS, the brains were shaken in a blocking solution: 10% goat serum in 0.2% Triton in PBS (PBST). They were subsequently incubated overnight in the blocking solution containing primary antibodies at room temperature with shaking. After washing with 0.2% PBST, the brains were incubated overnight in the blocking solution containing secondary antibodies at room temperature with shaking. Finally, the brains were washed with PBS and mounted in Vectashield mounting medium (Vector Laboratories).
The primary antibodies used were rabbit anti-GFP (A-11122, Invitrogen; RRID:AB_221569; diluted at 1:500) and mouse anti-choline acetyltransferase antibodies (Takagawa and Salvaterra, 1996) (4B1, Developmental Studies Hybridoma Bank at University of Iowa; RRID:AB_528122; 1:200). Goat anti-rabbit antibodies conjugated with AlexaFluor-488 (A11034, Invitrogen; RRID:AB_2576217; diluted at 1:200) and anti-mouse antibodies with AlexaFluor-568 (A11004, Invitrogen; RRID:AB_2534072; 1:200) were used as the secondary antibodies.
Reconstruction and analyses of confocal images
Confocal serial optical images were taken at 0.9-3.8 μm z intervals with a Carl Zeiss LSM 700 laser scanning confocal microscope equipped with Plan-Neofluar 20×/0.50 and C-Apochromat 40×/1.2W lenses. Three-dimensional reconstruction of confocal images was performed with Zeiss ZEN 2012. The brightness, color, and contrast of images were adjusted with Photoshop CS 5.1 (Adobe).
The confocal microscope was also used to measure the distance between the bottom of the transplanted eye and the top of the brain immediately after recording the odor responses from eya2 mutants transplanted with a compound eye from a WT fly.
Experimental design and statistical analysis
Data are presented as mean ± SEM. We performed Wilcoxon matched-pairs signed rank test and Mann–Whitney test using Prism 5 (GraphPad). We calculated r correlation coefficients as effect sizes on R 4.2.1 (https://www.R-project.org/) by dividing Z statistic by the square root of the total number of animals. We used G*Power 3.1 (Faul et al., 2007) to perform power analysis. We calculated the minimum sample sizes required to detect enough statistical power (0.8) during the Period II in Figures 2A, 3D,E, and 4A,D that showed firing rate increases during sustained lighting or current injection. The mean minimum sample size was ∼7. We thus considered that the sample size of 7 was enough to detect a positive result during the Period II. Statistical significance was set at p < 0.05. p values, r values, and sample sizes are shown in the figures and Table 1.
Figure 2.
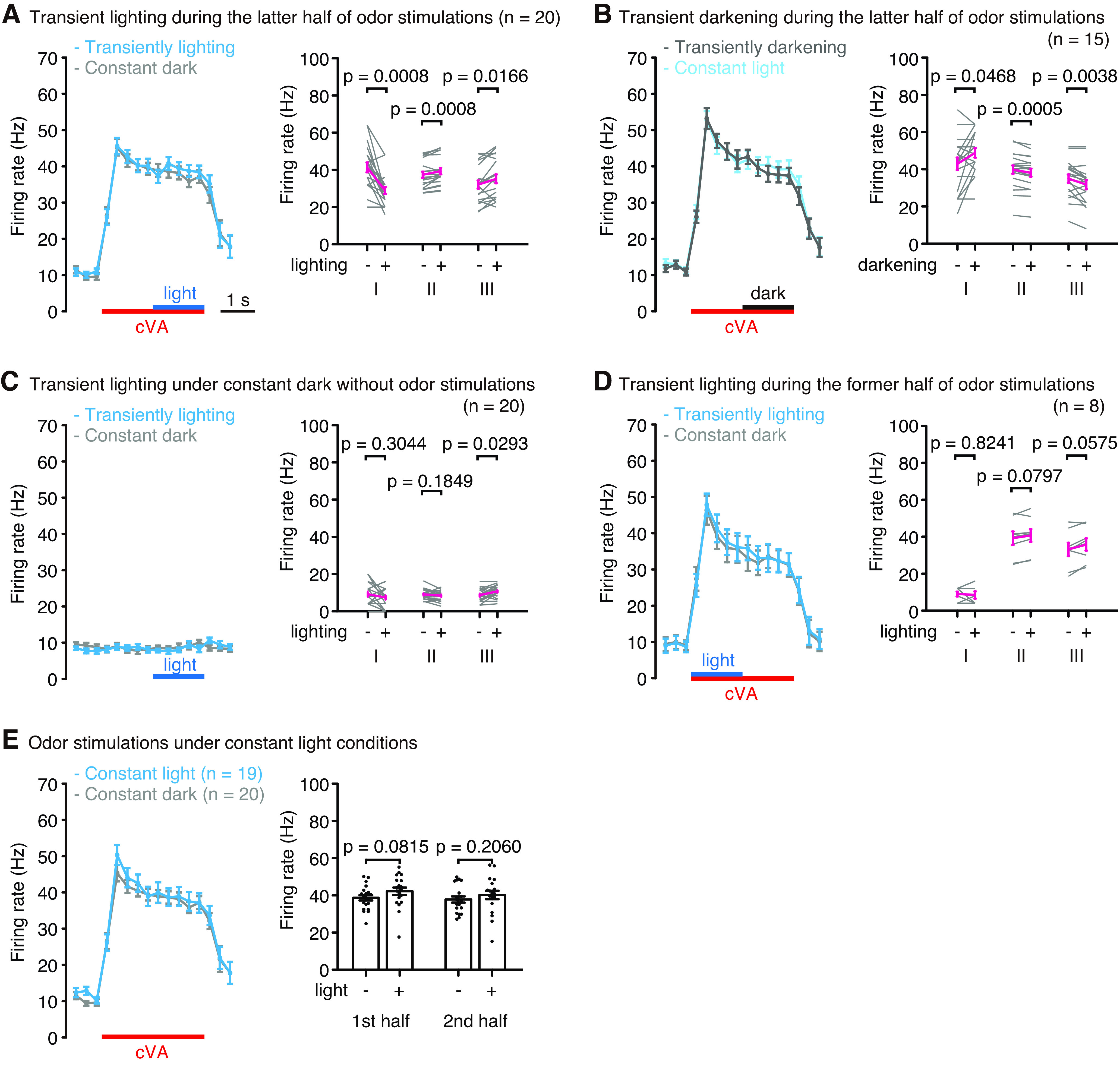
Transient changes in the light condition modulated cVA responses. A, B, Transient lighting (A) and darkening (B) during the latter half of odor stimulations changed the firing rates of OSNs. Left, The firing rates in each 300 ms bin. Blue and black lines indicate 1.5 s of the lighting and darkening periods, respectively. Right graphs represent the firing rates during Periods I, II, and III, as shown in Figure 1B. n indicates the number of animals recorded. C, Light responses of the OSNs without odor stimulations. D, Transient lighting was applied during the first half of odor stimulations. E, The cVA response under constant light or constant dark condition. Right, The firing rates during the first and second halves of the odor stimulations. For statistical analyses, Wilcoxon matched-pairs signed rank test (A–D) and Mann–Whitney test (E) were performed. Error bars indicate SEM.
Figure 3.
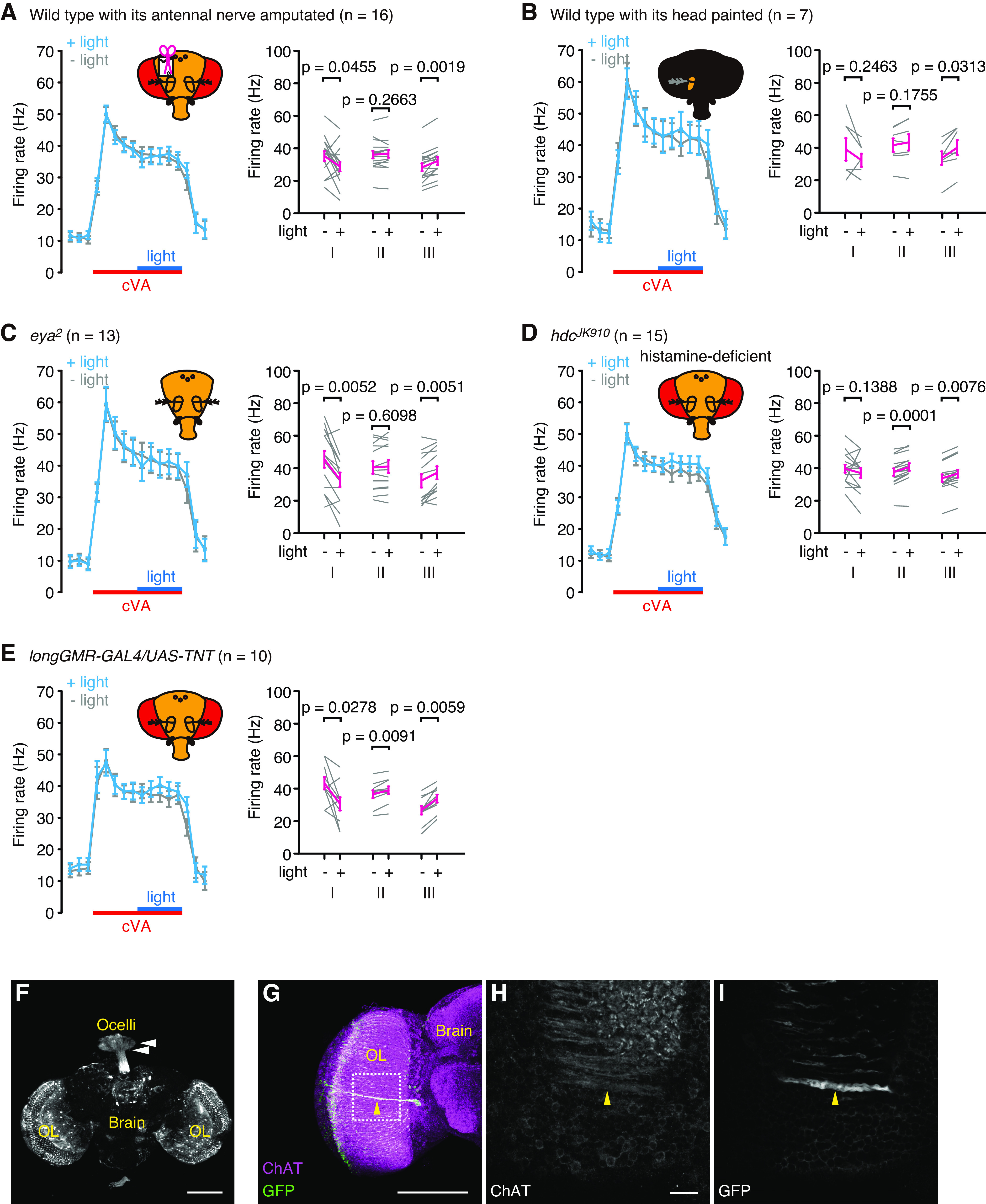
Transient lighting during the latter half of cVA stimulations modulated the responses of Or67d OSNs. A, WT with its antennal nerve amputated. B, WT whose head except for the antenna being recorded was painted black. C, eya2 mutant. D, hdcJK910 mutant. E, longGMR-GAL4/UAS-TNT. Left panels, The firing rates in each 300 ms bin. Blue lines indicate 1.5 s of the lighting period. Red lines indicate the timing of 3 s odor stimulations. Light blue lines indicate the firing rate when both odor and light stimulations were applied. Gray lines indicate those where odor stimulations were solely applied. Right panels, The firing rates during Periods I, II, and III, as shown in Figure 1B. For statistical analyses, Wilcoxon matched-pairs signed rank tests were performed. Error bars indicate SEM. F–I, Expression patterns of longGMR-GAL4. Photoreceptors in the ocelli (arrowheads in F) and eyelet (arrowheads in G–I) neurons as well as in the retinae are labeled. Magenta in G and white in H represent anti-choline acetyltransferase (ChAT) antibodies, whereas white in F and I and green in G represent the presence of anti-GFP antibodies. The area surrounded by dotted square in G is enlarged in H and I. Dorsal side is on the top, lateral is represented on the left (G–I). OL, Optic lobe. Single frontal confocal sections (H,I) and 3D-reconstructed images (F,G) are shown. Scale bars: H, 10 µm; F, G, 100 µm. Genotype: w; +/UAS-GFP; UAS-20XmCD8::GFP/longGMR-GAL4.
Figure 4.
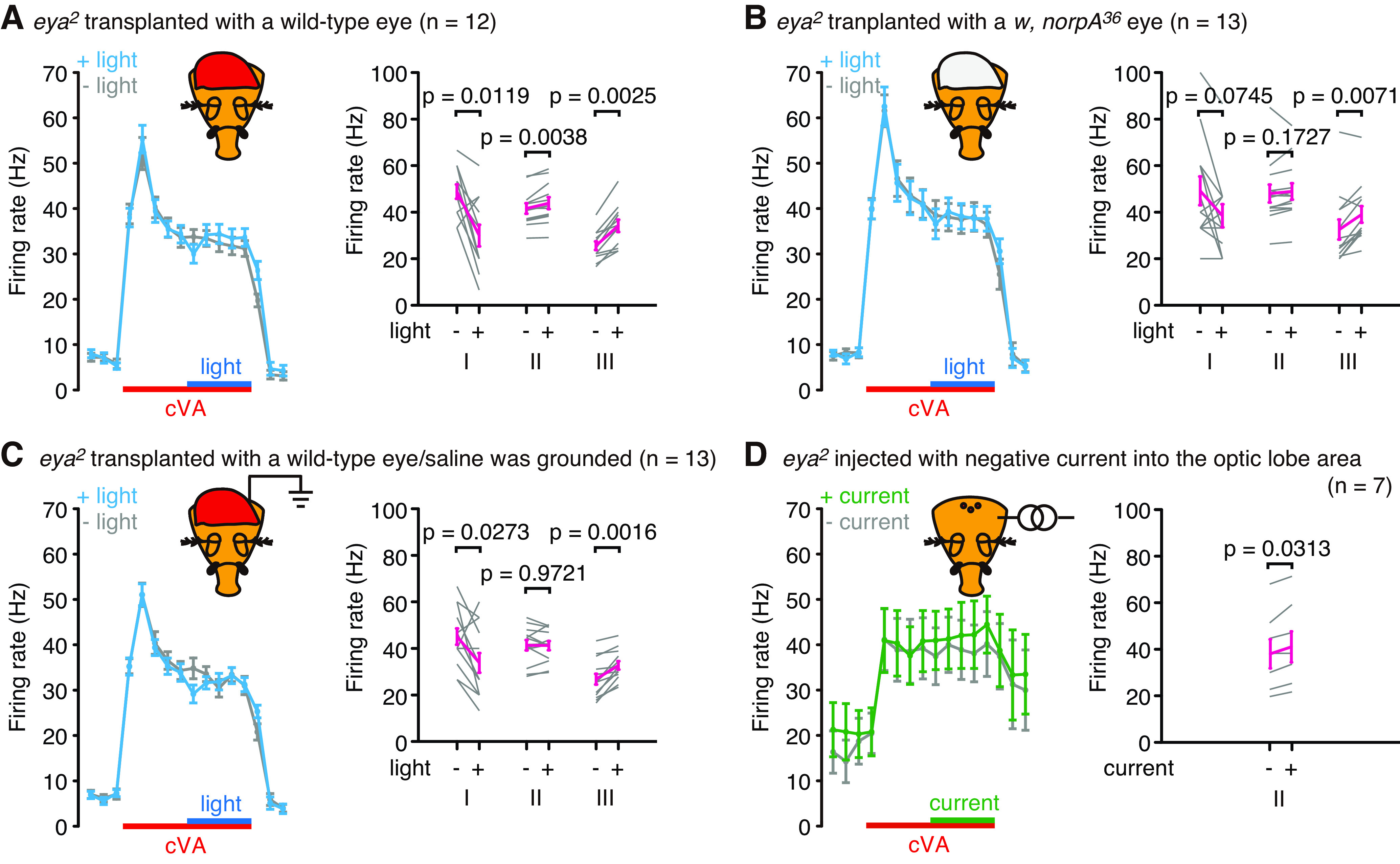
Ephaptic transmission mediates the light-evoked firing rate increase in the Or67d OSNs. A–C, Transient lighting was applied during the latter half of odor stimulations in eya2 mutants transplanted with a WT eye (A,C) or norpA36 mutant eye (B). C, Ground electrode was placed in the extracellular saline. Left panels, The firing rates in each 300 ms bin. Blue lines indicate 1.5 s of the lighting period. Red lines indicate the timing of 3 s odor stimulations. Right panels, The firing rates during Periods I, II, and III, as shown in Figure 1B. D, Negative current (−10 nA) injected into the optic lobe area caused firing rate increases of the OSNs during odor stimulations in eya2 mutants. For statistical analyses, Wilcoxon matched-pairs signed rank tests were performed. Error bars indicate SEM.
Table 1.
Detailed statistic values
| Figure | Period I | Period II | Period III | ||||
|---|---|---|---|---|---|---|---|
| Wilcoxon matched-pairs signed rank test | 2 A | W = 166 | Z = 3.3623 | W = −180 | Z = −3.3608 | W = −120 | Z = 2.4349 |
| (n = 20) | p = 0.0008 | r = 0.7518 | p = 0.0008 | r = 0.7515 | p = 0.0166 | r = 0.5445 | |
| 2 B | W = −64 | Z = −2.0193 | W = 161 | Z = 3.5062 | W = 123 | Z = 2.8905 | |
| (n = 15) | p = 0.0468 | r = 0.4633 | p = 0.0005 | r = 0.8044 | p = 0.0038 | r = 0.6631 | |
| 2 C | W = 43 | Z = 1.0514 | W = 72 | Z = 0.59753 | W = −101 | Z = −2.254 | |
| (n = 20) | p = 0.3044 | r = 0.2351 | p = 0.1849 | r = 0.1336 | p = 0.0293 | r = 0.5040 | |
| 2 D | W = 3 | Z = 0.3333 | W = −26 | Z = 1.6824 | W = −28 | Z = −1.8927 | |
| (n = 8) | p = 0.8241 | r = 0.1179 | p = 0.0797 | r = 0.5948 | p = 0.0575 | r = 0.6692 | |
| 3 A | W = 71 | Z = 2.029 | W = −44 | Z = −1.1376 | W = −121 | Z = −3.1326 | |
| (n = 16) | p = 0.0455 | r = 0.5073 | p = 0.2663 | r = 0.2844 | p = 0.0019 | r = 0.7832 | |
| 3 B | W = 12 | Z = 1.4393 | W = −17 | Z = −1.0142 | W = −21 | Z = −2.2014 | |
| (n = 7) | p = 0.2463 | r = 0.5440 | p = 0.1755 | r = 0.3833 | p = 0.0313 | r = 0.8321 | |
| 3 C | W = 72 | Z = 2.8317 | W = −14 | Z = −0.54934 | W = −81 | Z = −2.833 | |
| (n = 13) | p = 0.0052 | r = 0.7854 | p = 0.6098 | r = 0.1524 | p = 0.0051 | r = 0.7857 | |
| 3 D | W = 34 | Z = 1.5251 | W = −118 | Z = −3.351 | W = −86 | Z = −2.668 | |
| (n = 15) | p = 0.1388 | r = 0.3938 | p = 0.0001 | r = 0.8652 | p = 0.0076 | r = 0.6889 | |
| 3 E | W = 44 | Z = 2.2994 | W = −45 | Z = −2.6656 | W = −55 | Z = −2.8031 | |
| (n = 10) | p = 0.0278 | r = 0.7271 | p = 0.0091 | r = 0.8429 | p = 0.0059 | r = 0.8864 | |
| 4 A | W = 65 | Z = 2.5515 | W = −66 | Z = −2.9821 | W = −78 | Z = −3.0606 | |
| (n = 12) | p = 0.0119 | r = 0.7366 | p = 0.0038 | r = 0.8609 | p = 0.0025 | r = 0.8835 | |
| 4 B | W = 41 | Z = 1.8281 | W = −40 | Z = −1.3981 | W = −78 | Z = −2.6223 | |
| (n = 13) | p = 0.0745 | r = 0.5070 | p = 0.1727 | r = 0.3878 | p = 0.0071 | r = 0.7273 | |
| 4 4 | W = 57 | Z = 2.316 | W = 2 | Z = 0.0699 | W = −91 | Z = −3.1808 | |
| (n = 13) | p = 0.0273 | r = 0.6423 | p = 0.9721 | r = 0.0194 | p = 0.0016 | r = 0.8822 | |
| 4 D | W = 21 | Z = −2.2014 | |||||
| (n = 7) | p = 0.0313 | r = 0.8321 | |||||
| Figure | 1st half | 2nd half | |||||
| Mann–Whitney test |
2E (n = 19 to 20) |
U = 127.5 | Z = −1.7562 | U = 144.5 | Z = −1.2786 | ||
| p = 0.0815 | r = 0.2812 | p = 0.2060 | r = 0.2047 | ||||
Results
Effect of transient changes in light condition on the odor response pattern of Or67d OSNs
We first investigated whether light conditions affected the response to cVA in Or67d OSNs and found that 1.5 s blue light stimulations transiently applied in the dark during the latter half of 3 s odor stimulations decreased the firing rate by 12 Hz on an average, at the onset of light. The firing rate then increased by 2-3 Hz until the offset of light (Figs. 1B, 2A). Conversely, when the flies were set under constant blue light and subjected to transient darkening during the latter half of odor stimulation, we observed an increase in the firing rates at light offset and then a gradual decrease until the onset of light (Fig. 2B). When flies were subjected to blue light stimulation alone in the dark without odor stimulation, a significant change in the firing rate was observed only at the offset when the firing rate was increased (Fig. 2C). However, significant firing rate changes were not observed when blue light stimulation was applied transiently in the dark during the first half of cVA stimulation (Fig. 2D). This may be because the firing rate drastically changed during the first half of cVA stimulation, which may have obscured the effect of light stimulation. We also did not observe significant differences in the firing rates during cVA stimulation between constant light and constant dark conditions (Fig. 2E). These results suggest that transient changes in light conditions altered the odor response patterns of Or67d OSNs, especially when the firing rate in the OSNs was nearly constant. As a thermistor placed under the blue light did not show any temperature changes, the firing rate changes were caused by photoreception. The firing rates of Or67d OSNs during odor stimulation decreased at the onset of light and increased at the offset. Moreover, between the onset and offset, the firing rate increased during sustained lighting but decreased during sustained darkening.
Antennal photoreceptors contributing to the light-evoked firing rate changes in Or67d OSNs at the onset and offset of light
We next examined the neural mechanisms underlying light-evoked firing rate changes in the OSNs. We focused on the firing rate changes observed by blue light stimulations, which were transiently applied in the dark during the latter half of odor stimulations in which light-evoked firing rate changes were clearly observed. To determine whether antennal photoreceptors contribute to these changes, we recorded from WT flies whose antennal nerve which connects the antenna and brain was severed, and flies whose heads were covered with black paint, except for one antenna for recording (Fig. 3A,B). Neither fly exhibited an increase in firing rate during sustained light, indicating that the increase involves photoreceptors outside the antennae. However, we observed light-evoked firing rate changes at the onset and offset of light in these flies, although the change at the onset was not statistically significant in the painted flies (Fig. 3B). These results indicate that while the firing rate changes at light onset and offset occur autonomously within the antenna, the photoreceptors within the antennae contribute little to the firing rate increase between onset and offset.
Ephaptic transmission from the compound eyes contributes to the light-evoked firing rate increases in Or67d OSNs during sustained light
We next examined the contribution of photoreceptors outside the antennae to the firing rate increases during sustained light. We first analyzed eya2 mutants that lacked compound eyes, but not ocelli (Choi and Benzer, 1994), and did not observe an increase in firing rates in the eya2 mutants (Fig. 3C). We further analyzed two strains in which chemical transmission of photoreceptors in the compound eyes was blocked: hdcJK910 that lacked histamine, a neurotransmitter of the photoreceptor cells in the compound eyes (Burg et al., 1993; Melzig et al., 1996) (Fig. 3D), and longGMR-GAL4/UAS-tetanus toxin mutants, in which chemical synaptic transmission of cholinergic photoreceptor cells in Hofbauer-Buchner eyelets was blocked (Yasuyama and Meinertzhagen, 1999) as well as photoreceptor cells in the compound eyes and ocelli (Fig. 3E–I). These two strains exhibited normal light-evoked firing rate changes, except at light onset in hdcJK910. This result appears to contradict the findings in the eya2 mutants but also raises the possibility that, although photoreception by the photoreceptor cells in the compound eyes were indispensable, synaptic transmission from photoreceptor cells was not necessary for the light-evoked firing rate increases during sustained lighting.
We therefore verified whether synaptic relay of information from photoreceptor cells in the compound eyes was required for increasing the light-evoked firing rate. We prepared eya2 mutants in which a compound eye severed from a WT fly was placed over a window made at the top of the head cuticle. Synaptic relay of light information through both chemical and electrical synapses from the transplanted compound eye to the brain was completely missing in eya2 mutants that were operated on. The operated eya2 mutants showed the light-evoked firing rate increase comparable with that of the WT (Fig. 4A). This result indicates that the mechanisms that evoke firing rate changes in the OSNs are normal in the eya2 mutants except for photoreception in the compound eyes. The increase in firing rate was not induced by surgical manipulation, as the firing rate did not increase in eya2 mutants transplanted with the compound eye of norpA36, in which the phototransduction pathway in photoreceptor cells is disrupted because of dysfunction of phospholipase C-β gene (Bloomquist et al., 1988; Pearn et al., 1996) (Fig. 4B). Consistent with observations in hdcJK910 and longGMR-GAL4/UAS-tetanus toxin flies, this result demonstrates that synaptic relay of light information from the compound eyes to brain is not required to increase the firing rate. Furthermore, since we did not manipulate the brain of these operated eya2 mutants, the photoreceptors within the brain were unlikely to contribute to the increase in firing rate during sustained lighting.
We further investigated whether diffusion of chemical substances released from the transplanted compound eye induced an increase in firing rate in the OSNs. For this purpose, a tungsten wire connected to the ground was inserted into extracellular saline covering the eya2 brain, to minimize light-evoked extracellular field potential change near the transplanted compound eye, without interrupting the diffusion of chemical substances from the compound eye or the blue light path to the compound eye and brain. In such preparations, we did not observe an increase in the firing rate during sustained light (Fig. 4C), although we observed normal cVA responses in OSNs. This indicates that the light-evoked firing rate increase was not mediated by chemical substances released from the compound eye. We also examined whether negative filed potential deflections in the optic lobe area were also able to induce an increase in firing rate in the OSNs, as light evokes negative field potential deflections in the retina (Pearn et al., 1996). We found that a negative current (−10 nA) injected into the optic lobe area in eya2 mutants caused an increase in firing rate (Fig. 4D). Thus, we conclude that ephaptic transmission, but not synaptic transmission from the photoreceptor cells, mediated by field potential changes in the retina, induced light-evoked firing rate increase of OSNs in the antennae. The transplanted eye was separated from the brain and antennae by a distance of >200 µm, indicating that field potential change evoked by retinal activity has the potential to change the spiking patterns of distant neurons in the fly brain.
Discussion
We found that ephaptic transmission of light information from photoreceptor cells in the retina mediates the increase in firing rate in the OSNs during odor stimulations. This study has not revealed whether the ephaptic transmission directly changes the firing rate of the OSNs. We observed that amputation of the antennal nerve abolished the firing rate increases during sustained light (Fig. 3A), suggesting that once the light information might be received by neurons in the brain, the information would be relayed by the neurons through the antennal nerve to the antenna, resulting in the firing rate increases in the OSNs.
While ephaptic coupling has been reported earlier, such as between neighboring neurons within the same sensillum, or between Purkinje cells, which is at a distance of <100 µm (Su et al., 2012; Han et al., 2018), this study shows that ephaptic transmission reaches >200 µm in vivo, equivalent to the depth of the entire fly brain, beyond the distance between neighboring neurons. Light stimulations cause ∼−10 mV field potential deflections in a retina (Pearn et al., 1996). If we neglect endogenous fields in the brain, light stimulations may induce ∼33.3 mV/mm electric field between the retina and center of the brain (0 mV), since the distance between them is ∼300 µm. This electric field is strong enough to modulate neural activities, as even weaker electric fields (<0.5 mV/mm) changed the firing patterns of neurons in vitro (Weiss and Faber, 2010).
In rodents, the firing rate of cerebellar Purkinje cells either decreased or increased when a current was injected into the extracellular field around their axons, causing field potential changes of 0.2 mV (Blot and Barbour, 2014). In insects, odor-evoked field potential oscillations whose amplitude is comparable with that caused by the current injection in the rodents, are induced by synchronous firing of olfactory neurons in the antennal lobe which are mediated by GABAergic neurons forming reciprocal synapses with excitatory projection neurons (Stopfer et al., 1997; Tanaka et al., 2009). Changes in the extracellular field potential are commonly observed in many nervous systems (Buzsáki et al., 2012). While such extracellular field potential activities have been considered as a side effect of synchronized spiking of neurons, this study suggests that such field potential changes evoked by a sensory stimulus can control the excitability of distant neurons, in addition to adjacent neurons. As ephaptic transmission is more effective at a short distance, the ephaptic transmission from the retinae may contribute significantly to firing rate changes in downstream neurons of the photoreceptor cells in the optic lobe.
This study also revealed that odor responses of OSNs were clearly modulated when light conditions changed transiently. This mechanism may help flies switch attention to newly presented sensory cues or maintain attention toward those remaining after the change. Turning the light on, for example, reduces the firing rates of the OSNs, which may enable the flies to pay more attention to visual information, whereas turning the light off increases the firing rates of the OSNs, which may help them attend to olfactory sensory cues.
Recent connectome analyses have revealed the entire synaptic network in the CNS in Drosophila (Scheffer et al., 2020) and provides insight into how neural information is subject to synaptic relays to determine the behavioral output. In this study, we show that ephaptic relays also contribute to modulating the firing rate of distant neurons and modify the sensory responses that can change momentarily in a context-dependent manner. To build an integrated model of the fly brain, we should also consider ephaptic relay of neural information (Scheffer and Meinertzhagen, 2021). The compound eye-antenna model would be a suitable model to determine the role of ephaptic transmission in neural processing.
Footnotes
This work was supported by PRESTO, Japan Science and Technology Agency, and by Grant in-Aid for Scientific Research (#22770068, 24120509, 26830026, 17K07480, and 20K06733) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan to N.K.T. We thank Fumika Hamada, Akiko Sato, Takahiro Chihara, Kazuhiko Kume, Taro Ueno, Ryusuke Niwa, Tadao Usui, Leslie Vosshall, Barry Dickson, Sarah Certel, the Kyoto Drosophila Genetic Resource Center, and the Bloomington Drosophila Stock Center for providing fly strains; Ayumi Tanaka and Ryoichi Tanaka for measuring light intensities; Shuzo Sakata for critically reading the manuscript; and Makoto Mizunami, Joby Joseph, and members of the Hokto Kazama laboratory for helpful suggestions.
The authors declare no competing financial interests.
References
- Anastassiou CA, Koch C (2015) Ephaptic coupling to endogenous electric field activity: why bother? Curr Opin Neurobiol 31:95–103. 10.1016/j.conb.2014.09.002 [DOI] [PubMed] [Google Scholar]
- Bloomquist BT, Shortridge RD, Schneuwly S, Perdew M, Montell C, Steller H, Rubin GM, Pak WL (1988) Isolation of a putative phospholipase C gene of Drosophila, norpA, and its role in phototransduction. Cell 54:723–733. 10.1016/S0092-8674(88)80017-5 [DOI] [PubMed] [Google Scholar]
- Blot A, Barbour B (2014) Ultra-rapid axon-axon ephaptic inhibition of cerebellar Purkinje cells by the pinceau. Nat Neurosci 17:289–295. 10.1038/nn.3624 [DOI] [PubMed] [Google Scholar]
- Burg MG, Sarthy PV, Koliantz G, Pak WL (1993) Genetic and molecular identification of a Drosophila histidine decarboxylase gene required in photoreceptor transmitter synthesis. EMBO J 12:911–919. 10.1002/j.1460-2075.1993.tb05732.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsáki G, Anastassiou CA, Koch C (2012) The origin of extracellular fields and currents: EEG, ECoG, LFP and spikes. Nat Rev Neurosci 13:407–420. 10.1038/nrn3241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi KW, Benzer S (1994) Migration of glia along photoreceptor axons in the developing Drosophila eye. Neuron 12:423–431. 10.1016/0896-6273(94)90282-8 [DOI] [PubMed] [Google Scholar]
- Chow DM, Theobald JC, Frye MA (2011) An olfactory circuit increases the fidelity of visual behavior. J Neurosci 31:15035–15047. 10.1523/JNEUROSCI.1736-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobritsa AA, van der Goes van Naters W, Warr CG, Steinbrecht RA, Carlson JR (2003) Integrating the molecular and cellular basis of odor coding in the Drosophila antenna. Neuron 37:827–841. 10.1016/s0896-6273(03)00094-1 [DOI] [PubMed] [Google Scholar]
- Faul F, Erdfelder E, Lang AG, Buchner A (2007) G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 39:175–191. 10.3758/bf03193146 [DOI] [PubMed] [Google Scholar]
- Han KS, Guo C, Chen CH, Witter L, Osorno T, Regehr WG (2018) Ephaptic coupling promotes synchronous firing of cerebellar Purkinje cells. Neuron 100:564–578. 10.1016/j.neuron.2018.09.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaissling KE, Thorson J (1980) Insect olfactory sensilla: structural, chemical and electrical aspects of the functional organization. In: Receptors for neurotransmitters, hormones and pheromones in insects (Sattelle DB, Hall LM, Hildebrand JG, eds), pp 261–282. Amsterdam: Elsevier/North-Holland. [Google Scholar]
- Malpel S, Klarsfeld A, Rouyer F (2002) Larval optic nerve and adult extra-retinal photoreceptors sequentially associate with clock neurons during Drosophila brain development. Development 129:1443–1453. 10.1242/dev.129.6.1443 [DOI] [PubMed] [Google Scholar]
- Melzig J, Buchner S, Wiebel F, Wolf R, Burg M, Pak WL, Buchner E (1996) Genetic depletion of histamine from the nervous system of Drosophila eliminates specific visual and mechanosensory behavior. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 179:763–773. 10.1007/BF00207355 [DOI] [PubMed] [Google Scholar]
- Ni JD, Baik LS, Holmes TC, Montell C (2017) A rhodopsin in the brain functions in circadian photoentrainment in Drosophila. Nature 545:340–344. 10.1038/nature22325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson SB, Hansson BS (2013) Electroantennogram and single sensillum recording in insect antennae. Methods Mol Biol 1068:157–177. 10.1007/978-1-62703-619-1_11 [DOI] [PubMed] [Google Scholar]
- Pearn MT, Randall LL, Shortridge RD, Burg MG, Pak WL (1996) Molecular, biochemical, and electrophysiological characterization of Drosophila norpA mutants. J Biol Chem 271:4937–4945. 10.1074/jbc.271.9.4937 [DOI] [PubMed] [Google Scholar]
- Scheffer LK, Meinertzhagen IA (2021) A connectome is not enough: what is still needed to understand the brain of Drosophila? J Exp Biol 224:jeb242740. [DOI] [PubMed] [Google Scholar]
- Scheffer LK, et al. (2020) A connectome and analysis of the adult Drosophila central brain. Elife 9:e57443. 10.7554/eLife.57443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanbhag SR, Müller B, Steinbrecht RA (1999) Atlas of olfactory organs of Drosophila melanogaster: 1. Types, external organization, innervation and distribution of olfactory sensilla. Int J Insect Morph Embryol 28:377–397. 10.1016/S0020-7322(99)00039-2 [DOI] [Google Scholar]
- Stopfer M, Bhagavan S, Smith BH, Laurent G (1997) Impaired odour discrimination on desynchronization of odour-encoding neural assemblies. Nature 390:70–74. 10.1038/36335 [DOI] [PubMed] [Google Scholar]
- Su CY, Menuz K, Reisert J, Carlson JR (2012) Non-synaptic inhibition between grouped neurons in an olfactory circuit. Nature 492:66–71. 10.1038/nature11712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney ST, Broadie K, Keane J, Niemann H, O'Kane CJ (1995) Targeted expression of tetanus toxin light chain in Drosophila specifically eliminates synaptic transmission and causes behavioral defects. Neuron 14:341–351. 10.1016/0896-6273(95)90290-2 [DOI] [PubMed] [Google Scholar]
- Takagawa K, Salvaterra P (1996) Analysis of choline acetyltransferase protein in temperature sensitive mutant flies using newly generated monoclonal antibody. Neurosci Res 24:237–243. 10.1016/0168-0102(95)00999-x [DOI] [PubMed] [Google Scholar]
- Tanaka NK, Ito K, Stopfer M (2009) Odor-evoked neural oscillations in Drosophila are mediated by widely branching interneurons. J Neurosci 29:8595–8603. 10.1523/JNEUROSCI.1455-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka NK, Suzuki E, Dye L, Ejima A, Stopfer M (2012) Dye fills reveal additional olfactory tracts in the protocerebrum of wild-type Drosophila. J Comp Neurol 520:4131–4140. 10.1002/cne.23149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Atteveldt N, Murray MM, Thut G, Schroeder CE (2014) Multisensory integration: flexible use of general operations. Neuron 81:1240–1253. 10.1016/j.neuron.2014.02.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Goes van Naters W, Carlson JR (2007) Receptors and neurons for fly odors in Drosophila. Curr Biol 17:606–612. 10.1016/j.cub.2007.02.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt K, Aso Y, Hige T, Knapek S, Ichinose T, Friedrich AB, Turner GC, Rubin GM, Tanimoto H (2016) Direct neural pathways convey distinct visual information to Drosophila mushroom bodies. Elife 5:e14009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss SA, Faber DS (2010) Field effects in the CNS play functional roles. Front Neural Circuits 4:15. 10.3389/fncir.2010.00015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wernet MF, Labhart T, Baumann F, Mazzoni EO, Pichaud F, Desplan C (2003) Homothorax switches function of Drosophila photoreceptors from color to polarized light sensors. Cell 115:267–279. 10.1016/s0092-8674(03)00848-1 [DOI] [PubMed] [Google Scholar]
- Wilson RI (2013) Early olfactory processing in Drosophila: mechanisms and principles. Annu Rev Neurosci 36:217–241. 10.1146/annurev-neuro-062111-150533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagi R, Mabuchi Y, Mizunami M, Tanaka NK (2016) Convergence of multimodal sensory pathways to the mushroom body calyx in Drosophila melanogaster. Sci Rep 6:29481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarali A, Mayerle M, Nawroth C, Gerber B (2008) No evidence for visual context-dependency of olfactory learning in Drosophila. Naturwissenschaften 95:767–774. 10.1007/s00114-008-0380-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuyama K, Meinertzhagen IA (1999) Extraretinal photoreceptors at the compound eye's posterior margin in Drosophila melanogaster. J Comp Neurol 412:193–202. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Tsang TK, Bushong EA, Chu LA, Chiang AS, Ellisman MH, Reingruber J, Su CY (2019) Asymmetric ephaptic inhibition between compartmentalized olfactory receptor neurons. Nat Commun 10:1560. 10.1038/s41467-019-09346-z [DOI] [PMC free article] [PubMed] [Google Scholar]


