ABSTRACT
Purpose
This study aimed to identify and characterize joint profiles of sedentary time and physical activity among adults and to investigate how these profiles are associated with markers of cardiometabolic health.
Methods
The participants included 3702 of the Northern Finland Birth Cohort 1966 at age 46 yr, who wore a hip-worn accelerometer during waking hours and provided seven consecutive days of valid data. Sedentary time, light-intensity physical activity, and moderate- to vigorous-intensity physical activity on each valid day were obtained, and a data-driven clustering approach (“KmL3D”) was used to characterize distinct joint profiles of sedentary time and physical activity intensities. Participants self-reported their sleep duration and performed a submaximal step test with continuous heart rate measurement to estimate their cardiorespiratory fitness (peak heart rate). Linear regression was used to determine the association between joint profiles of sedentary time and physical activities with cardiometabolic health markers, including adiposity markers and blood lipid, glucose, and insulin levels.
Results
Four distinct groups were identified: “active couch potatoes” (n = 1173), “sedentary light movers” (n = 1199), “sedentary exercisers” (n = 694), and “movers” (n = 636). Although sufficiently active, active couch potatoes had the highest daily sedentary time (>10 h) and lowest light-intensity physical activity. Compared with active couch potatoes, sedentary light movers, sedentary exercisers, and movers spent less time in sedentary by performing more physical activity at light-intensity upward and had favorable differences in their cardiometabolic health markers after accounting for potential confounders (1.1%–25.0% lower values depending on the health marker and profile).
Conclusions
After accounting for sleep duration and cardiorespiratory fitness, waking activity profiles characterized by performing more physical activity at light-intensity upward, resulting in less time spent in sedentary, were associated with better cardiometabolic health.
Key Words: METABOLIC DISEASES, ADIPOSITY, INSULIN RESISTANCE, DYSLIPIDEMIAS, WAKING ACTIVITY BEHAVIORS
Adults spend their waking time undertaking three main movement behaviors—sedentary behavior, light-intensity physical activity (LPA), and moderate- to vigorous-intensity physical activity (MVPA) (1). Recent research indicates a complex interrelationship between these movement behaviors and cardiometabolic health because the time spent in each activity may modify the health-related influences of time spent in any of the other movement behaviors (1). For instance, increasing the time spent in MVPA may significantly reduce the negative effects of sedentary time, leading to better cardiometabolic health (2,3). With the availability of device-based methodologies for accurately measuring activity behaviors across the entire intensity continuum (4), it has been shown that activities at lighter intensities may also confer considerable cardiometabolic health benefits (5), especially when they replace sedentary time (2,3). Conversely, excessive sedentary time has been associated with poor cardiometabolic health (6). However, the optimal combination of LPA and MVPA required to minimize the health risks of excessive sedentary time remains unclear (1,7,8).
Sedentary time and physical activity intensities are influenced by occupation, environment, and physical capacity (e.g., fitness level), over which individuals have minimal control (9,10). Substantial variations may occur in diurnal levels of sedentary time and physical activity intensities across weekdays and weekend days (11), which are shown to be related to cardiometabolic health (12,13) in adults. Robust evidence exists that, regardless of accumulation patterns, any amount of MVPA confers benefits on several health outcomes (8,14). However, how the patterns and variations of sedentary time and LPA contribute to adults’ cardiometabolic health remains largely unknown (1,5,8). It is likely that those who consistently perform more LPA and MVPA on weekdays are less active and spend more time being sedentary on weekend days or vice versa. Further complicating this, there appears to be considerable variation in sedentary time, LPA, and MVPA between weekend days (Saturday and Sunday) (11), and this variation may potentially be related to cardiometabolic health.
Recently, a conceptual shift has occurred in analytical approaches used to assess associations with movement behaviors (15), moving away from exploring sedentary time, LPA, and MVPA as independent exposures toward using more advanced approaches to study the combined effects of these activities on various health markers (2,15–17). Among newer analytical approaches, isotemporal substitution modeling has been most frequently used for studying the joint associations of physical activity behaviors with health outcomes (15,18), whereas data-driven, person-centered approaches have recently gained momentum (15,16,19). Data-driven approaches have better capacity to handle multidimensional and correlated data (13,15,19) and are therefore appropriate candidates for understanding the complex interrelationship between sedentary time and physical activity intensities.
Few studies have recently used data-driven, person-centered statistical approaches, such as latent profile analysis and machine learning-based clustering methods (e.g., K-means), to identify groups of individuals with distinct activity profiles. Overall, those studies have consistently shown that, across a day, individuals spend time in a diverse set of activities (16,19–22). A wide range of sedentary and physical activity profiles have been so far identified in different study populations using person-centered approaches, such as prolonged sitters, breakers, and prolonged movers, which have been shown to be associated with cardiometabolic health (13,19), mortality risk (20), and other health indicators (22) in adults. For instance, a recent study using a clustering approach has shown that the individual accumulation patterns of sedentary time and sedentary breaks may explain the differences in cardiometabolic health among different groups of adults (19). Still, a major limitation of the existing data-driven studies in adults is that a subset of variables representing a proportion of movement behaviors have been used to create the activity behavior profiles, typically neglecting the potential interplay within the full waking activity behavior spectrum (22–25). This cross-sectional study applied a clustering-based approach to the trajectories of accelerometer-determined sedentary time, LPA, and MVPA over seven consecutive days in a large population-based sample of adults 1) to identify joint profiles of sedentary time and physical activity intensities and 2) to examine how these profiles of sedentary time, LPA, and MVPA were associated with cardiometabolic health, including adiposity level, blood glucose, and insulin and cholesterol levels.
MATERIALS AND METHODS
Study Population
Data for this study were from the population-based Northern Finland Birth Cohort 1966 study (NFBC1966). NFBC1966 (N = 12,058) is a life-course study involving participants whose date of birth was expected to be in 1966 in Northern Finland. The cohort members have been regularly monitored prospectively with a broad set of clinical measurements, interviews, and postal questionnaires. The participants have given written informed consent for participating in the NFBC1966 study. The study was carried out in conformance with the Declaration of Helsinki. It followed the legislation, decrees, and ethical principles concerning medical research on humans in Finland. Further information about the NFBC1966 study, recruitment, and follow-ups is available elsewhere (10). This cross-sectional study included members of the NFBC1966 who participated in the latest follow-up performed at the age of 46 yr (during 2012–2014) and who agreed to wear an accelerometer for measuring daily activity. The data collected in the 46-yr follow-up further included completion of postal questionnaires, attending a clinical examination day for collection of fasting blood samples and anthropometric measurements, and taking an oral glucose tolerance test on a separate day (26).
Measurements
Sedentary time and physical activity intensities
Movement behaviors were monitored with a hip-worn accelerometer (Hookie AM20; Traxmeet Ltd., Espoo, Finland). Participants were instructed to wear the accelerometer during all waking activities, except water-based activities, for 14 consecutive days. Raw acceleration signals were collected and stored at 100 Hz. The accelerometer data were first segmented into 6-s epochs, and the mean amplitude deviation values were computed (27). From the 6-s epochs, accelerometer nonwear intervals were detected and removed with a widely used approach for count-based data with a 30-s threshold to handle the artifactual acceleration (28). For this study, participants with seven consecutive valid days were considered eligible for the analyses, and each valid day was defined as ≥10 h of monitor wear time. The decision to analyze seven consecutive days for all participants was made to minimize the effects of differences in accelerometer wear time on the analyses. The 6-s epochs detected as wear-time intervals were classified as sedentary time (<1.5 METs), LPA (1.5–3.0 METs), or MVPA (≥3 METs) using a validated set of thresholds for mean amplitude deviation values (27), and the times spent in each of these movement behaviors on each of the seven consecutive valid days were obtained.
Cardiometabolic health
The participants attended the clinical examination after overnight fasting for 12 h and abstained from smoking and drinking coffee. Trained nurses measured height, weight, and waist circumference, and body mass index was calculated. Body composition was measured by bioelectrical impedance analysis (InBody720; InBody, Seoul, Korea), and body fat, fat mass, and visceral fat area were determined. Fasting blood samples were taken and analyzed for plasma glucose, serum insulin, total cholesterol, high-density lipoprotein (HDL) cholesterol, low-density lipoprotein (LDL) cholesterol, and triglycerides as described elsewhere (29). The ratios of total to HDL (total/HDL cholesterol ratio) and LDL to HDL (LDL/HDL cholesterol ratio) cholesterol levels were computed, as they could be better predictors of cardiovascular disease risk than lipid and lipoprotein levels alone (30). Participants who were not previously diagnosed with type 1 or type 2 diabetes were also asked to attend a 75-g oral glucose tolerance test on another fasted day. From the oral glucose tolerance test results, 2-h postload plasma glucose and insulin levels were obtained.
Confounders
Sex and birth weight were extracted from medical records. Cardiorespiratory fitness was estimated on the clinical examination day by a submaximal 4-min single-step test with continuous heart rate measurement (RS800CX; Polar Electro, Finland) and expressed as peak heart rate (29). The peak heart rate in a submaximal step test is an acceptable surrogate for estimating cardiorespiratory fitness in the general adult population (31). Participants self-reported their average sleep duration, education level, employment status, marital status, and household income. They provided information about lifestyles (smoking status and alcohol consumption), health-related quality of life, previous diagnosis of hypertension, heart problems, diabetes, and use of medication for hypertension, high cholesterol, and diabetes.
Statistical Analyses
Joint sedentary time and physical activity intensity profiles
All participants with valid accelerometry data were included in the clustering analysis to identify joint profiles of sedentary time, LPA, and MVPA. Clustering analysis was performed with the KmL3D clustering algorithm (32,33). KmL3D is an updated version of the popularly used K-means clustering algorithm, adapted to enable clustering analysis with several variables repeatedly measured over time (called joint trajectories) (33). Compared with the traditional K-means clustering algorithm, this method is shown to be appropriate for studying the joint evolution/variation of several variables over a certain period. Assuming that the trajectories are interconnected, KmL3D clusters the data based on the combined distances between the variable trajectories into K user-defined disjoint clusters so that participants within the same group exhibit maximum pairwise similarity scores and high dissimilarity scores with participants belonging to other cluster groups.
In the context of daily activity behaviors, sedentary time, LPA, and MVPA were presented as three repeated measures over 1 wk and considered as three joint and interdependent trajectories (Fig. 1). KmL3D was used to group the participants based on these input trajectories to simultaneously account for the interrelationships between sedentary time, LPA, and MVPA on each day, together with the interconnections between these activity behaviors across the 7 d of the week (Fig. 1).
FIGURE 1.
Schematic representation of trajectories of sedentary time, LPA, and moderate-to-vigorous physical activity forming the waking activity behaviors from Monday to Sunday (1 full week). The arrows indicate the potential interconnections between waking activity behavior compositions.
Before inclusion in the cluster analysis, sedentary time, LPA, and MVPA in each day were divided by the total accelerometer wear time in each corresponding day. Considering that the time budget is limited to a total sum (3,15), this was done to make the waking activity behaviors in each day add up to a total sum of 100% for all participants. The percentage values were then sorted from Monday to Sunday for all participants to have matching waking activity trajectories across the 7 d of the week. According to previous studies and guidelines for cluster analysis (19,34), the waking activity trajectories for all participants were standardized to have a range of 0–1 using the min–max method (34), so that all activity variables would be on a comparable scale for clustering analysis. Clustering analysis was performed in R version 3.6.2 (R Core Team, Vienna, Austria) using R package “KmL3D” (32).
Profile characteristics and their associations with cardiometabolic health outcomes
The optimal number of clusters was determined by iteratively repeating the clustering procedure. In each repetition, the number of clusters was increased by one (33), and the clustering solution was visualized. This procedure was repeated until the clusters were overlapping with each other, and no substantially different clusters were formed after increasing the number of clusters. The clustering solutions were compared with each other on the basis similarity among the identified clusters to select the optimal number of clusters, providing the most distinguishable cluster groups. The number of clusters providing the largest differences in activity trajectories between clusters was selected as the optimal clustering solution. For the final solution, the levels of sedentary time, LPA, and MVPA across the 7 d of the week were determined by cluster group. The mean values (95% confidence interval) of sedentary time, LPA, and MVPA within each profile for each of the 7 d were computed and shown. As in previous studies (35,36), the most distinguishing characteristics were used to name the profiles. The group with the unhealthiest profile (highest sedentary time and lowest LPA and MVPA) was selected as the referent group for regression analysis. The main clustering analysis was performed with all participants, regardless of missing values in health outcomes or confounders. To ensure that the cluster groups were reproducible and robust, the cluster analysis was repeated after randomly excluding 20% of participants.
Linear regression models were used to determine the associations (% difference) between the group/profile membership (included as categorical predictor) and each of the cardiometabolic health markers in separate models. All the cardiometabolic markers were log-transformed before inclusion in the regression analyses. The associations were examined with three incremental models (unadjusted, partially adjusted, and fully adjusted) for each cardiometabolic health marker. The unadjusted model included only profile membership and cardiometabolic markers. The partially adjusted model was adjusted for selected confounders, including age, sex, education, employment, and marital status, and the fully adjusted model was further adjusted for birth weight, medication use, health-related quality of life score, smoking, alcohol consumption, income, sleep duration, and cardiorespiratory fitness. Sleep duration was included as a continuous variable. The associations of sedentary and physical activity intensities are prone to the problem of reverse causality bias (37). To ascertain the robustness of the results, we conducted sensitivity analysis and repeated the regression analyses for the participants who slept according to current recommendations for sleep duration (7–9 h per night) (38) and had no hypertension, heart disease, or diabetes.
RESULTS
Participants
A total of 5840 NFBC1966 members participated in the 46-yr follow-up after completing the postal questionnaires. Of these, 3702 participants agreed to wear the accelerometer and provided valid data for seven consecutive days to be included in this study (Fig. 2). Descriptive statistics of the cohort members participating in the 46-yr follow-up and the subsample with valid accelerometry data are shown in Table 1. Compared with those participating in the follow-up, participants with valid accelerometry data had comparable cardiorespiratory fitness (147.6 vs 147.6 heartbeats per minute) and alcohol intake (10.7 vs 9.6 g·d−1). A comparable percentage of them were men (44.1% vs 40.7%), employed (88.2% vs 89.2%), married/cohabiting (78.8% vs 79.9%), nonsmokers (53.8% vs 55.2%), had polytechnic or university degrees (28.5% vs 29.8%), and slept 7–9 h per night (58.4% vs 60.5%). The total daily mean ± SD of the accelerometer wear time was 15.2 (2.0) h·d−1.
FIGURE 2.
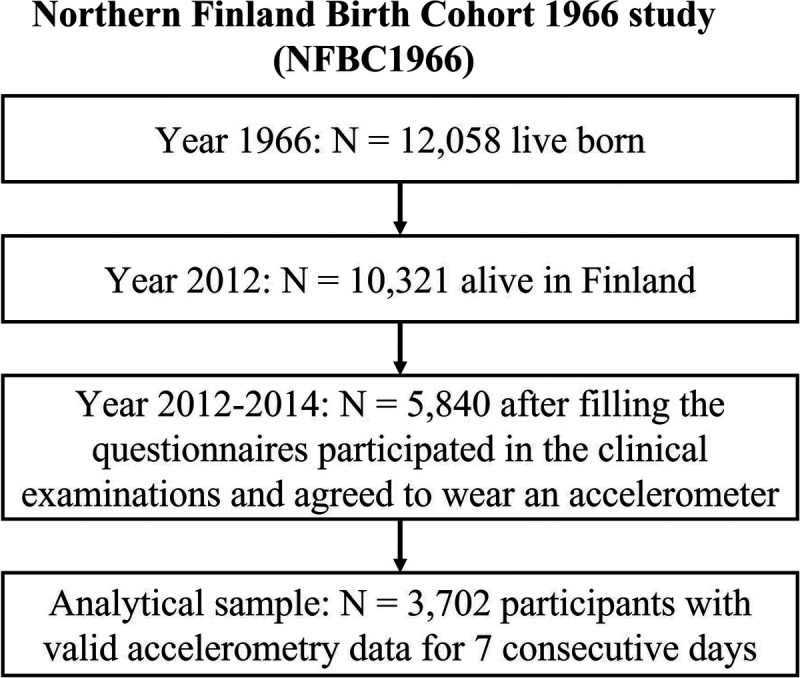
The selection of the study population from the Northern Finland Birth Cohort 1966 (NFBC1966).
TABLE 1.
Participant characteristics for the whole sample, the analytical sample with valid accelerometry data, and the four identified sedentary time and physical activity intensity profiles.
| Variable | Full Sample (n = 5822) | Analytical Sample (n = 3702) | Active Couch Potatoes (n = 1173) | Sedentary Light Movers (n = 1199) |
Sedentary Exercisers (n = 694) |
Movers (n = 636) |
|---|---|---|---|---|---|---|
| Demographics | ||||||
| Age, yr | 46.6 ± 0.6 | 46.6 ± 0.6 | 46.6 ± 0.6 | 46.6 ± 0.6 | 46.6 ± 0.5 | 46.6 ± 0.6 |
| Sex | ||||||
| Male | 2565 (44.1) | 1504 (40.7) | 485 (41.3) | 426 (35.6) | 305 (44.1) | 288 (45.4) |
| Female | 3257 (55.9) | 2190 (59.3) | 685 (58.4) | 772 (64.4) | 387 (55.9) | 346 (54.6) |
| Education | ||||||
| Polytechnic/university degree | 1530 (28.5) | 1037 (29.8) | 435 (39.5) | 258 (23.0) | 273 (41.3) | 71 (11.9) |
| Vocational/college level education | 3649 (68.1) | 2343 (67.3) | 649 (59.0) | 817 (72.8) | 378 (57.2) | 499 (83.7) |
| Comprehensive school | 183 (3.4) | 99 (2.8) | 16 (1.5) | 47 (4.2) | 10 (1.5) | 26 (4.4) |
| Employment status | ||||||
| Employed | 4672 (88.2) | 3089 (89.2) | 954 (86.3) | 995 (88.6) | 608 (92.5) | 532 (92.0) |
| Unemployed | 295 (5.5) | 183 (5.3) | 77 (7.0) | 61 (5.4) | 27 (4.1) | 18 (3.1) |
| Other (e.g., student, homemaker) | 333 (6.3) | 191 (5.5) | 74 (6.7) | 67 (6.0) | 22 (3.3) | 28 (4.8) |
| Marital status | ||||||
| Married/cohabiting | 4348 (78.8) | 2855 (79.9) | 844 (74.1) | 963 (83.5) | 526 (78.2) | 522 (86.1) |
| Divorced/Widowed | 555 (10.1) | 343 (9.6) | 136 (11.9) | 97 (8.4) | 65 (9.7) | 45 (7.4) |
| Unmarried | 615 (11.1) | 373 (10.4) | 159 (14.0) | 93 (8.1) | 82 (12.2) | 39 (6.4) |
| Household income (€ per year) | ||||||
| ≤50,000 | 2149 (42.8) | 1350 (41.2) | 415 (39.2) | 463 (43.9) | 210 (33.8) | 262 (48.6) |
| 50,001 to 100,000 | 2305 (45.9) | 1549 (47.3) | 482 (45.5) | 513 (48.7) | 311 (50.0) | 243 (45.1) |
| >100,000 | 564 (11.2) | 376 (11.5) | 163 (15.4) | 78 (7.4) | 101 (16.2) | 34 (6.3) |
| Birth weight, kg | 3.5 ± 0.5 | 3.5 ± 0.5 | 3.4 ± 0.5 | 3.4 ± 0.5 | 3.5 ± 0.5 | 3.4 ± 0.5 |
| Lifestyle factors, medication use, fitness, and health-related quality of life | ||||||
| Sleep | ||||||
| Duration, h per night | 7.5 ± 1.0 | 7.5 ± 0.9 | 7.5 ± 0.9 | 7.5 ± 0.9 | 7.5 ± 0.8 | 7.4=1.0 |
| Short (<7 h per night) | 2157 (39.1) | 1343 (37.7) | 400 (35.3) | 447 (38.8) | 248 (36.8) | 248 (40.8) |
| Appropriate (7–9 h per night) | 3219 (58.4) | 2158 (60.5) | 710 (62.7) | 684 (59.4) | 416 (61.7) | 348 (57.2) |
| Long (>9 h per night) | 138 (2.5) | 66 (1.9) | 23 (2.0) | 21 (1.8) | 10 (1.5) | 12 (2.0) |
| Smoking status | ||||||
| Nonsmoker | 2941 (53.8) | 1960 (55.2) | 647 (57.1) | 553 (48.4) | 420 (62.7) | 340 (56.2) |
| Former smoker | 1485 (27.1) | 969 (27.3) | 291 (25.7) | 352 (30.8) | 169 (25.2) | 157 (26.0) |
| Current smoker | 1045 (17.9) | 622 (17.5) | 195 (17.2) | 238 (20.8) | 81 (12.1) | 108 (17.9) |
| Diseases | ||||||
| Hypertension | 1103 (18.9) | 680 (18.3) | 265 (22.6) | 198 (16.5) | 114 (16.4) | 103 (16.2) |
| Heart diseases | 206 (3.5) | 125 (3.4) | 47 (4.0) | 41 (3.4) | 16 (2.3) | 21 (3.3) |
| Diabetes | 177 (3.0) | 105 (2.8) | 44 (3.7) | 34 (2.9) | 12 (1.7) | 15 (2.3) |
| Diabetes, cholesterol, and/or hypertension medication | ||||||
| Yes | 960 (21.7) | 591 (20.4) | 226 (23.9) | 188 (19.9) | 93 (17.3) | 84 (17.8) |
| No | 3458 (78.3) | 2308 (79.6) | 719 (76.1) | 755 (80.1) | 446 (82.7) | 388 (82.2) |
| Cardiorespiratory fitness, heart beats per minutea | 147.6 ± 15.3 | 147.6 ± 15.4 | 151.7 ± 14.7 | 149 ± 14.8 | 142 ± 15.1 | 143.4 ± 15.8 |
| Alcohol consumption, g·d−1 | 10.7 ± 17.3 | 9.6 ± 15.5 | 10.1 ± 16.7 | 9.4 ± 16.2 | 9.7 ± 13.1 | 9.3 ± 14.2 |
| Health-related quality of life score | 0.9 ± 0.1 | 0.9 ± 0.1 | 0.9 ± 0.1 | 0.9 ± 0.1 | 0.9 ± 0.1 | 0.9 ± 0.05 |
| Cardiometabolic biomarkers | ||||||
| Fasting insulin, pmol·L−1 | 9.8 ± 8.8 | 9.5 ± 8.0 | 11.0 ± 9.9 | 9.2 ± 6.1 | 8.0 ± 8.1 | 8.7 ± 7.1 |
| 2-h insulin, pmol·L−1 | 61.3 ± 58.4 | 59.9 ± 57.9 | 72.5 ± 72.1 | 62.2 ± 55.1 | 45.0 ± 38.1 | 49.1 ± 42.5 |
| Fasting glucose, mmol·L−1 | 5.5 ± 0.9 | 5.5 ± 0.8 | 5.5 ± 0.8 | 5.5 ± 0.9 | 5.5 ± 1.3 | 5.4 ± 0.6 |
| 2-h glucose, mmol·L−1 | 5.9 ± 1.7 | 5.8 ± 1.6 | 5.6 ± 1.8 | 5.8 ± 1.5 | 5.5 ± 1.3 | 5.7 ± 1.4 |
| Triglycerides, mmol·L−1 | 1.3 ± 0.8 | 1.2 ± 0.7 | 1.3 ± 0.7 | 1.2 ± 0.7 | 1.1 ± 0.7 | 1.1 ± 0.7 |
| Total/HDL cholesterol ratio | 3.6 ± 1.0 | 3.6 ± 1.0 | 3.8 ± 1.0 | 3.6 ± 1.0 | 3.4 ± 0.1 | 3.4 ± 0.1 |
| LDL/HDL cholesterol ratio | 2.4 ± 0.9 | 2.4 ± 0.9 | 2.5 ± 0.9 | 2.4 ± 0.9 | 2.2 ± 0.9 | 2.2 ± 0.9 |
| Adiposity measures | ||||||
| Body fat, % | 28.9 ± 9.3 | 28.9 ± 9.1 | 31.0 ± 9.4 | 29.7 ± 8.9 | 25.9 ± 7.9 | 26.6 ± 8.9 |
| Fat mass, kg | 23.1 ± 10.7 | 22.8 ± 10.4 | 25.5 ± 11.8 | 23.1 ± 10.2 | 19.6 ± 8.1 | 20.4 ± 8.8 |
| Visceral fat area, cm2 | 105.1 ± 41.6 | 103.3 ± 40.8 | 113.1 ± 43.1 | 105.9 ± 40.4 | 89.3 ± 34.1 | 95.9 ± 36.4 |
| BMI, kg·m−2 | 26.9 ± 4.9 | 26.6 ± 4.7 | 27.5 ± 5.3 | 26.6 ± 4.7 | 25.3 ± 3.8 | 26.1 ± 4.1 |
| Waist circumference, cm | 91.8 ± 13.6 | 90.7 ± 13.4 | 93.5 ± 14.1 | 90.6 ± 13.5 | 87.4 ± 11.8 | 89.5 ± 12.4 |
| Total daily volumes | ||||||
| Wear time, h·d−1 | – | 15.2 ± 1.0 | 15.1 ± 0.9 | 15.1 ± 1.0 | 15.2 ± 0.9 | 15.2 ± 1.0 |
| Sedentary time, % of waking time per day | – | 65.6 ± 9.1 | 75.3 ± 4.0 | 64.3 ± 3.5 | 64.3 ± 4.8 | 51.7 ± 5.4 |
| Sedentary time, min·d−1 | – | 597.4 ± 90.3 | 683.2 ± 55.0 | 585.0 ± 51.8 | 588.0 ± 58.8 | 471.9 ± 57.7 |
| LPA time, % of waking time per day | – | 29.1 ± 8.1 | 21.0 ± 3.8 | 31.7 ± 3.5 | 26.1 ± 4.4 | 41.3 ± 5.2 |
| LPA time, min·d−1 | – | 264.4 ± 77.9 | 189.7 ± 35.8 | 288.4 ± 37.3 | 244.5 ± 42.9 | 378.7 ± 56.5 |
| MVPA time, % of waking time per day | – | 5.2 ± 2.9 | 3.7 ± 1.7 | 3.9 ± 1.5 | 8.8 ± 2.5 | 6.9 ± 3.1 |
| MVPA time, min·d−1 | – | 47.8 ± 26.9 | 33.7 ± 15.8 | 35.4 ± 13.6 | 79.0 ± 23.0 | 63.4 ± 29.2 |
Values are presented as mean ± SD or n (%).
aMeasured during a submaximal 4-min single-step test with continuous heart rate measurement.
BMI, body mass index.
Cluster analysis and joint sedentary time and physical activity profiles
To find the appropriate number of clusters, the number of clusters was iteratively increased from 2 to 20, each time increasing the number of clusters by one. The most distinguishable clusters were found when the number of clusters was set to four. Increasing the number of clusters beyond four resulted in overlapping clusters with similar trajectories. The appropriate number of clusters was therefore set to four.
Figure 3 shows the trajectories of sedentary time, LPA, and MVPA by these four cluster groups across the 7 d of the week. According to the mean of sedentary time and physical activity trajectories, these four groups were named “active couch potatoes,” “sedentary light movers,” “sedentary exercisers,” and “movers.” The sedentary time for active couch potatoes (n = 1173, 32% of the sample) was highest and stable throughout the week, accumulating on average more than 600 min in sedentary behavior each day. Active couch potatoes had on average the lowest level of LPA throughout the whole week (less than 220 min in LPA each day) and were sufficiently active with a stable MVPA trajectory (~35 min) each day.
FIGURE 3.
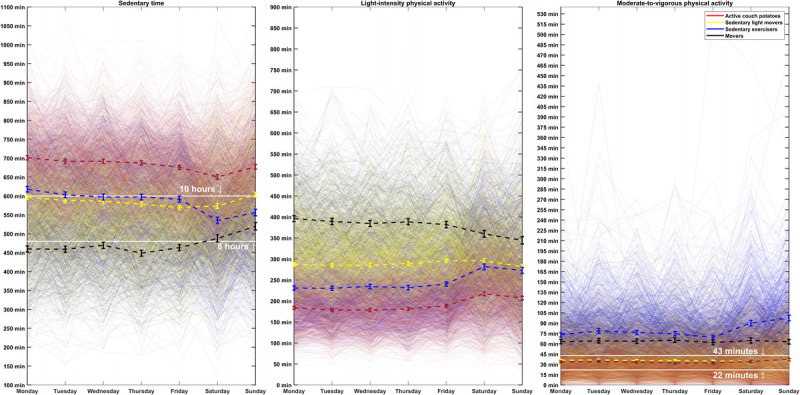
Trajectories of sedentary time, LPA, and moderate-to-vigorous physical activity (MVPA) in active couch potatoes, sedentary light movers, sedentary exercisers, and movers across the 7 d of the week (Monday to Sunday). The colored dashed lines and bars show the mean with a 95% confidence interval of sedentary time, LPA, and MVPA each day within the four profiles. The solid white lines on the sedentary graph mark 8 and 10 h of daily sedentary time, and the solid white lines on the MVPA graph mark 22 to 43 min, totaling approximately 150–300 min·wk−1. A larger version of this figure is presented in Supplemental Digital Content 1 (http://links.lww.com/MSS/C678).
Compared with active couch potatoes, sedentary light movers (n = 1199, 32% of the sample) and sedentary exercisers (n = 694, 19% of the sample) spent less time sedentary each day, although sedentary time was still a major part of their waking behaviors (an average of approximately 480–600 min·d−1). Hence, sedentary light movers had more LPA (an average of about 300 min each day), and sedentary exercisers had more MVPA (an average of >60 min each day). Movers (n = 636, 17% of the sample) spent the least time in sedentary behavior (an average of <540 min each day), whereas they spent more time in LPA and MVPA (an average of >350 and >60 min in LPA and MVPA, respectively).
Overall, the trajectories of sedentary time, LPA, and MVPA were stable across the weekdays (Monday to Friday) but changed substantially on the weekend days. The most substantial differences between weekday and weekend day levels of sedentary time and physical activity intensities were seen for sedentary exercisers and active couch potatoes. Sedentary exercisers further decreased their sedentary time on weekend days by performing more LPA and MVPA. Active couch potatoes slightly decreased their sedentary time on the weekend days by performing more LPA. Similar cluster groups with no substantial differences were identified, when the cluster analysis was repeated after randomly excluding 20% of the participants from the analysis (see Fig. S4, Supplemental Digital Content 2, Trajectories of sedentary time, light-intensity physical activity, and moderate-to-vigorous physical activity in active couch potatoes, sedentary light movers, sedentary exercisers, and movers, http://links.lww.com/MSS/C679).
Associations between joint profiles and cardiometabolic health markers
Tables 2 and 3 show the associations between the joint profiles and the cardiometabolic biomarkers and adiposity measures, respectively. In unadjusted regression models, sedentary light movers, sedentary exercisers, and movers had better cardiometabolic biomarkers and adiposity measures than active couch potatoes (range, 1.4%–33.4% lower values depending on the outcome). The only exception was that sedentary light movers did not differ from active couch potatoes in 2-h glucose.
TABLE 2.
Linear regression analysis of the association (percentage difference with 95% CI) between the four identified joint profiles of sedentary time and physical activity with cardiometabolic biomarkers.
| Sedentary Light Movers vs Active Couch Potatoes | Sedentary Exercisers vs Active Couch Potatoes | Movers vs Active Couch Potatoes | ||||||
|---|---|---|---|---|---|---|---|---|
| Cardiometabolic Outcome | Model | n | % Difference (95% CI) | P | % Difference (95% CI) | P | % Difference (95% CI) | P |
| 2-h insulin | Unadjusted | 3212 | −10.5 (−13.3 to −7.5) | 0.001 | −33.4 (−35.9 to −30.9) | <0.001 | −27.8 (−30.6 to −25.0) | <0.001 |
| Partially adjusted | 2913 | −13.8 (−16.7 to −10.8) | <0.001 | −32.7 (−35.3 to −30.1) | <0.001 | −32.3 (−35.1 to −29.4) | <0.001 | |
| Fully adjusted | 1851 | −11.4 (−14.8 to −7.9) | 0.002 | −22.7 (−26.1 to −19.2) | <0.001 | −18.3 (−22.2 to −14.1) | <0.001 | |
| Fasting serum insulin | Unadjusted | 3625 | −11.9 (−13.9 to −9.8) | <0.001 | −25.1 (−27.1 to −23.0) | <0.001 | −18.9 (−21.2 to −16.6) | <0.001 |
| Partially adjusted | 3261 | −12.9 (−15.0 to −10.7) | <0.001 | −24.3 (−26.4 to −22.1) | <0.001 | −21.6 (−23.9 to −19.2) | <0.001 | |
| Fully adjusted | 2036 | −9.3 (−11.8 to −6.7) | 0.001 | −9.5 (−12.4 to −6.6) | 0.002 | −8.6 (−11.8 to −5.3) | 0.012 | |
| Triglycerides | Unadjusted | 3675 | −7.2 (−9.0 to −5.4) | <0.001 | −16.1 (−18.0 to −14.1) | <0.001 | −15.2 (−17.2 to −13.2) | <0.001 |
| Partially adjusted | 3305 | −7.6 (−9.4 to −5.7) | <0.001 | −16.6 (−18.5 to −14.7) | <0.001 | −18.4 (−20.3 to −16.4) | <0.001 | |
| Fully adjusted | 2064 | −5.3 (−7.5 to −3.0) | 0.024 | −9.0 (−11.4 to −6.5) | <0.001 | −10.9 (−13.6 to −8.2) | <0.001 | |
| Total/HDL cholesterol | Unadjusted | 3675 | −4.1 (−5.1 to −3.0) | <0.001 | −9.3 (−10.5 to −8.1) | <0.001 | −8.9 (−10.1 to −7.6) | <0.001 |
| Partially adjusted | 3305 | −3.6 (−4.6 to −2.5) | 0.001 | −10.1 (−11.2 to −9.0) | <0.001 | −11.1 (−12.2 to −9.9) | <0.001 | |
| Fully adjusted | 2064 | −2.4 (−3.7 to −1.1) | 0.07 | −6.5 (−7.9 to −5.1) | <0.001 | −6.4 (−8.0 to −4.8) | <0.001 | |
| LDL/HDL cholesterol | Unadjusted | 3675 | −5.2 (−6.8 to −3.7) | 0.001 | −13.2 (−14.8 to −11.5) | <0.001 | −12.7 (−14.4 to −10.9) | <0.001 |
| Partially adjusted | 3305 | −4.7 (−6.2 to −3.1) | 0.003 | −14.1 (−15.7 to −12.5) | <0.001 | −15.8 (−17.5 to −14.1) | <0.001 | |
| Fully adjusted | 2064 | −3.0 (−4.9 to −1.0) | 0.136 | −9.3 (−11.4 to −7.2) | <0.001 | −9.9 (−12.2 to −7.6) | <0.001 | |
| 2-h glucose | Unadjusted | 3207 | −1.7 (−2.8 to −0.6) | 0.123 | −7.2 (−8.4 to −6.0) | <0.001 | −3.7 (−5.0 to −2.4) | 0.005 |
| Partially adjusted | 2899 | −1.8 (−3.0 to −0.7) | 0.121 | −7.0 (−8.2 to −5.7) | <0.001 | −5.1 (−6.5 to −3.7) | <0.001 | |
| Fully adjusted | 1846 | −0.3 (−1.7 to 1.1) | 0.839 | −3.7 (−5.2 to −2.2) | 0.018 | 1.1 (−0.7 to 2.9) | 0.547 | |
| Fasting plasma glucose | Unadjusted | 3606 | −1.4 (−1.8 to −0.9) | 0.005 | −2.7 (−3.3 to −2.2) | <0.001 | −1.6 (−2.2 to −1.0) | 0.007 |
| Partially adjusted | 3244 | −0.9 (−1.4 to −0.4) | 0.061 | −2.8 (−3.4 to −2.3) | <0.001 | −2.1 (−2.7 to −1.5) | <0.001 | |
| Fully adjusted | 2021 | −0.2 (−0.8 to 0.4) | 0.783 | −0.8 (−1.5 to −0.1) | 0.255 | 0.2 (−0.6 to 0.9) | 0.837 | |
Active couch potatoes were considered as the unhealthiest profile and selected as the referent group. Unadjusted models included only group membership. The partial models were adjusted for age, sex, education, employment, and marital status, and full models were further adjusted for medication use, health-related quality of life score, smoking, alcohol consumption, income, birth weight, cardiorespiratory fitness, and sleep duration. Significant associations (P < 0.05) are shown in bold.
CI, confidence interval.
TABLE 3.
Linear regression analysis of the association (percentage difference with 95% CI) between the four identified joint profiles of sedentary time and physical activity with adiposity measures.
| Sedentary Light Movers vs Active Couch Potatoes | Sedentary Exercisers vs Active Couch Potatoes | Movers vs Active Couch Potatoes | ||||||
|---|---|---|---|---|---|---|---|---|
| Adiposity Measure | Model | n | % Difference (95% CI) | P | % Difference (95% CI) | P | % Difference (95% CI) | P |
| Body fat | Unadjusted | 3628 | −4.2 (−5.5 to −2.9) | 0.002 | −16.5 (−17.8 to −15.1) | <0.001 | −15.2 (−16.6 to −13.8) | <0.001 |
| Partially adjusted | 3263 | −7.5 (−8.7 to −6.4) | <0.001 | −15.6 (−16.8 to −14.4) | <0.001 | −16.3 (−17.5 to −15.0) | <0.001 | |
| Fully adjusted | 2040 | −4.4 (−5.7 to −3.1) | 0.001 | −8.1 (−9.5 to −6.7) | <0.001 | −7.7 (−9.3 to −6.1) | <0.001 | |
| Fat mass | Unadjusted | 3628 | −8.7 (−10.3 to −7.0) | <0.001 | −22.0 (−23.6 to −20.4) | <0.001 | −19.4 (−21.1 to −17.7) | <0.001 |
| Partially adjusted | 3263 | −11.7 (−13.3 to −10.0) | <0.001 | −21.7 (−23.3 to −20.0) | <0.001 | −22.1 (−23.9 to −20.3) | <0.001 | |
| Fully adjusted | 2040 | −6.8 (−8.7 to −4.9) | <0.001 | −11.2 (−13.2 to −9.1) | <0.001 | −9.4 (−11.7 to −7.1) | <0.001 | |
| Visceral fat area | Unadjusted | 3628 | −6.3 (−7.9 to −4.7) | <0.001 | −21.2 (−22.7 to −19.6) | <0.001 | −15.3 (−17.0 to −13.6) | <0.001 |
| Partially adjusted | 3263 | −9.0 (−10.7 to −7.4) | <0.001 | −20.9 (−22.5 to −19.3) | <0.001 | −18.6 (−20.4 to −16.8) | <0.001 | |
| Fully adjusted | 2040 | −5.3 (−7.2 to −3.3) | 0.008 | −11.5 (−13.5 to −9.4) | <0.001 | −6.4 (−8.8 to −4.0) | 0.010 | |
| BMI | Unadjusted | 3690 | −2.8 (−3.5 to −2.1) | <0.001 | −7.1 (−7.8 to −6.4) | <0.001 | −4.3 (−5.1 to −3.5) | <0.001 |
| Partially adjusted | 3320 | −3.6 (−4.3 to −2.9) | <0.001 | −7.2 (−7.9 to −6.4) | <0.001 | −6.1 (−6.9 to −5.3) | <0.001 | |
| Fully adjusted | 2072 | −1.9 (−2.6 to −1.1) | 0.014 | −3.0 (−3.9 to −2.2) | <0.001 | −0.7 (−1.7 to 0.3) | 0.473 | |
| Waist circumference | Unadjusted | 3671 | −3.0 (−3.6 to −2.4) | <0.001 | −6.3 (−7.0 to −5.7) | <0.001 | −4.1 (−4.8 to −3.5) | <0.001 |
| Partially adjusted | 3302 | −3.1 (−3.6 to −2.5) | <0.001 | −6.6 (−7.2 to −5.9) | <0.001 | −5.8 (−6.5 to −5.2) | <0.001 | |
| Fully adjusted | 2057 | −1.8 (−2.4 to −1.2) | 0.003 | −3.1 (−3.7 to −2.4) | <0.001 | −1.7 (−2.5 to −0.9) | 0.025 | |
Active couch potatoes were considered as the unhealthiest profile and selected as the referent group. Unadjusted models included only group membership. The partial models were adjusted for age, sex, education, employment, and marital status, and full models were further adjusted for medication use, health-related quality of life score, smoking, alcohol consumption, income, birth weight, cardiorespiratory fitness, and sleep duration. Significant associations (P < 0.05) are shown in bold.
BMI, body mass index; CI, confidence interval.
The favorable associations in unadjusted models were all retained in partially adjusted models. In fully adjusted models, compared with active couch potatoes, sedentary light movers had lower 2-h insulin (11.4%), fasting serum insulin (9.3%), triglycerides (5.3%), and adiposity measures (range, 1.8%–6.8% lower values depending on the adiposity measure). Additionally, sedentary exercisers and movers had lower levels of 2-h insulin (22.7% and 18.3%), fasting serum insulin (9.5% and 8.6%), triglycerides (9.0% and 10.9%), total/HDL cholesterol (6.5% and 6.4%), LDL/HDL cholesterol (9.3% and 9.9%), body fat (8.1% and 7.7%), fat mass (11.2% and 9.4%), visceral fat area (11.5% and 6.4%), and waist circumference (3.1% and 1.7%). Sedentary exercisers also had significantly lower 2-h glucose (3.7%) and body mass index (3.0%) than active couch potatoes. Similar association patterns were observed when the analyses were repeated with participants with healthy sleep duration (7–9 h per night) and no hypertension, heart disease, or diabetes (see Table S1, Supplemental Digital Content 3, Linear regression analysis of the association between the four identified joint profiles of sedentary time and physical activity with cardiometabolic biomarkers, http://links.lww.com/MSS/C680; and Table S2, Supplemental Digital Content 4, Linear regression analysis of the association between the four identified joint profiles of sedentary time and physical activity with adiposity measures, http://links.lww.com/MSS/C681), but the associations were moderated in fully adjusted models.
DISCUSSION
The present cross-sectional study applied a clustering approach to the trajectories of sedentary time, LPA, and MVPA to create joint sedentary time and physical activity intensity profiles. Four distinct profiles of sedentary time and physical activity intensities were identified and named active couch potatoes, sedentary light movers, sedentary exercisers, and movers. Although meeting the minimum recommended MVPA duration on average, active couch potatoes were considered to have the unhealthiest profiles of these four groups because they had excessive sedentary time and the lowest daily levels of LPA during waking hours. Compared with active couch potatoes, sedentary light movers, sedentary exercisers, and movers all engaged in substantially less sedentary time by performing more physical activity at light-intensity upward and had favorable differences in the cardiometabolic health markers after accounting for potential confounders, sleep duration, and cardiorespiratory fitness. These results collectively suggest that having a waking activity behavior profile characterized by performing more physical activity from light-intensity upward in place of sedentary time may lead to better cardiometabolic health in adults, regardless of sleep duration and cardiorespiratory fitness level.
On average, all the four identified profiles had substantially differing trajectories of sedentary time, LPA, and MVPA across the 7 d of the week. With a few exceptions (13,16,20), previous studies using clustering methods have generally created either sedentary profiles or physical activity profiles in isolation (22–25). Isotemporal substitution modeling has been the most common statistical approach used to examine the combined associations of sedentary time and physical activity intensities with markers of cardiometabolic health (2,3,39) and mortality risk (17). Consistent with the findings of isotemporal substitution-based studies (2,3,17,39), the results of this observational study indicate that different combinations of sedentary time, LPA, and MVPA during waking hours explain the differences in cardiometabolic health, after accounting for sleep duration and cardiorespiratory fitness level.
The visualization trajectories of sedentary time indicate that many adults, even those meeting the minimum recommended amount of MVPA, may spend excessive time sedentary (i.e., >8 h) during weekdays and weekend days. Although no time-based limit exists for sedentary time, efforts have been made to provide more detailed recommendations for sedentary time (8,14,40), including encouraging adults to limit the daily time spent sedentary to 8 h·d−1 while accumulating at least 150–300 min of MVPA each week (40). However, sedentary time and physical activity intensities could be constrained by nonnegotiable factors such as occupation and environment (10,12,41). A few studies have recently argued that one-size-fits-all thresholds for sedentary time and physical activities may not always be adequate for promoting healthier daily activity behaviors (41,42). However, the focus should be on identifying an appropriate, healthy balance between the daily activity behaviors (17,41). Applying the 8-h time threshold for sedentary time, active couch potatoes, sedentary light movers, and sedentary exercisers were all on average noncompliant with the sedentary guideline. Even movers, individuals with the lowest level of sedentary time and highest level of LPA in this study population, spent on average more than 8 h in sedentary time on weekend days. These results reinforce the findings of these studies, indicating that recommendations for an optimal combination of sedentary time and physical activity intensities according to daily and individualized circumstances may be needed (8,41) to combat the escalating physical inactivity pandemic and sedentary lifestyle (43).
Active couch potatoes and sedentary light movers had similar average trajectories of MVPA time across all 7 d of the week, meeting the current recommendations for physical activity. However, sedentary light movers had better cardiometabolic health than active couch potatoes. Despite being sedentary for more than 8 h each day, sedentary light movers had substantially less sedentary time than active couch potatoes, which was achieved by replacing sedentary time with LPA. In recent years, several systematic reviews (5,44) and population-based (2,3) studies have reported that, in addition to MVPA, LPA is also a beneficial intensity of movement for cardiometabolic health in adults, especially when LPA replaces sedentary time. Adults may be more likely to replace sedentary time with LPA, given that LPA involves easier movement intensity than MVPA and is available in many situations as part of daily living activities. Consistent with previous studies examining the health outcomes associated with theoretical time reallocations among daily activity behaviors (2,3,5), our observational results indicate that having a waking activity behavior profile characterized by spending less time in sedentariness by replacing it with LPA could be associated with favorable differences in the markers of cardiometabolic health.
Sedentary exercisers and sedentary light movers spent a comparable amount of time being sedentary across the weekdays, although the latter had larger favorable differences in cardiometabolic health markers than the former compared with active couch potatoes. These larger favorable differences in sedentary exercisers could be attributed to trading LPA for MVPA. In accordance with these observational results, several studies examining how theoretical time reallocations among daily activity behaviors are associated with cardiometabolic health and mortality risk have shown that performing more MVPA in place of sedentary time and light-intensity activities could confer additional cardiometabolic health benefits (2,3,17,41).
Although substantially different in waking activity behaviors, sedentary exercisers and movers had comparable favorable differences in cardiometabolic health markers compared with active couch potatoes. Sedentary exercisers and movers exceeded the minimum recommended range for MVPA, accumulating more than 60–75 min on average each day at this intensity of movement. Similar to our results, but based on self-reported measures of sedentary time and daily activities that could be biased, a previous meta-analysis found that high levels of MVPA (about 60–75 min·d−1) appear to eliminate the increased mortality risk associated with high sedentary time (45). Our device-based study further supports these findings by demonstrating that having a waking activity behavior profile characterized by performing high daily levels of MVPA (60–75 min) may result in better cardiometabolic health, despite spending excessive time in sedentary behavior each day (8–10 h).
The strengths of this study include the large population-based sample of Finnish adults with a wide range of markers of cardiometabolic health. Additionally, we could account for a broad set of confounders, including sleep duration and cardiorespiratory fitness. This is a strength because previous studies performing profile analyses have not accounted for sleep duration or cardiorespiratory fitness (13,16,24,25), although both have been shown to be related to cardiometabolic health (46,47). Another strength was the clustering approach used for creating the joint profiles and proper visualization of trajectories of sedentary time, LPA, and MVPA across the identified groups. The KmL3D method (33) could simultaneously account for the interrelationships between the three parts of daily activity composition (sedentary time, LPA, and MVPA) on each given day together with the interconnections between the activity compositions across the 7 d of the week, potentially resulting in more representative joint sedentary time and physical activity profiles.
This study also has some inherent limitations. Because of the birth cohort setting, the study sample was homogeneous in age and ethnicity. Although beneficial for reducing the probability of confounding the associations, this may limit the generalizability of the results to more diverse populations. Peak heart rate from a submaximal step test was used as a surrogate measure for cardiorespiratory fitness, which is known as an acceptable method for estimating cardiorespiratory fitness but may not fully correspond to the direct measurement of maximal oxygen uptake (31). Sleep duration was not considered as part of the daily movement behavior compositions because it was not available on a daily basis as accelerometers were only worn during waking hours. Average sleep duration was self-reported and likely to be less accurate than the device-based measurements. However, we accounted for self-reported sleep duration in our regression models by including it as a covariate. Given that sleep behavior is also shown to be related to cardiometabolic health (47) and waking movement behaviors, future studies with 24-h accelerometry data are needed to further understand the role of sleep behavior as a component of 24-h day. Although different anatomical postures, such as standing still, sitting, or lying, are likely to have distinct effects on cardiometabolic health (35,48), our accelerometry data did not differentiate between these activity behaviors, which could be considered as a limitation. The subsample with valid accelerometry data had comparable demographics, cardiorespiratory fitness levels, and sleep durations compared with those participating in the 46-yr follow-up. Nevertheless, those who participated in the 46-yr follow-up may have been healthier, potentially resulting in a selection bias. Another limitation is the observational and cross-sectional design, which limits the inference about the causality of associations. Therefore, our findings must be further verified using prospective study designs. Similar profiles to those identified here, such as active couch potatoes, sedentary exercisers, and movers, have been previously theorized and identified (19,35). However, further studies with other populations are required to examine whether similar profiles can be observed and whether these profiles would similarly be associated with cardiometabolic health markers. Generally, the associations seen between the profiles of sedentary time and physical activity intensities are open to the possibility of a reverse causality pathway (37). To address this problem, we examined the associations after removing participants with short and long sleep duration, hypertension, diabetes, and heart problems. Although the magnitude of associations became less pronounced, the significant associations remained unchanged. Nonetheless, concerns about reverse causality cannot be completely excluded due to unmeasured and clinically undiagnosed diseases.
CONCLUSIONS
Regardless of sleep duration and cardiorespiratory fitness, accommodating more physical activity at light-intensity upward in the waking activity behavior composition could benefit cardiometabolic health in adults. Sufficiently active adults meeting the minimum recommendations for MVPA (150–300 min·wk−1) may gain additional cardiometabolic health benefits by performing more LPA in place of sedentary time. Adults accumulating excessive daily sedentary time may be encouraged to perform a higher level of MVPA (60–75 min·d−1) beyond the minimum recommended threshold for MVPA to reduce the potential cardiometabolic health risks of excessive sedentary time.
Supplementary Material
Acknowledgments
The present study is connected to the DigiHealth-project, a strategic profiling project at the University of Oulu. The project is supported by the Academy of Finland (project number 326291) and the University of Oulu. NFBC1966 received financial support from University of Oulu grant no. 24000692, Oulu University Hospital grant no. 24301140, and ERDF European Regional Development Fund grant no. 539/2010 A31592. This study has also received funding from the European Union’s Horizon 2020 research and innovation program under the Marie Skłodowska-Curie grant agreement no. 713645, and the Ministry of Education and Culture in Finland (grant nos. OKM/20/626/2022, OKM/86/626/2014, OKM/43/626/2015, OKM/17/626/2016, OKM 47/626/2017, OKM/78/626/2018, OKM/54/626/2019, OKM/85/626/2019, OKM/88/626/2019, and OKM/1096/626/2020). D. D. is supported by the Australian National Health and Medical Research Council (NHMRC) Early Career Fellowship APP1162166 and by the Centre of Research Excellence in Driving Global Investment in Adolescent Health funded by NHMRC APP1171981. The funders played no role in designing the study, or collecting, analyzing, and interpreting the data, or writing the manuscript.
The authors thank all cohort members and researchers who participated in the 46-yr study. They acknowledge the NFBC project center for their contribution in managing the NFBC1966 study and the UKK Institute for providing the accelerometers for the study. The authors declare no conflicts of interest. The results of the study are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation. The results of this study do not constitute endorsement by the American College of Sports Medicine.
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.acsm-msse.org).
Contributor Information
MEHRDAD ROSTAMI, Email: Mehrdad.Rostami@oulu.fi.
DOT DUMUID, Email: Dot.Dumuid@unisa.edu.au.
SEBASTIEN F. M. CHASTIN, Email: Sebastien.Chastin@gcu.ac.uk.
MAISA NIEMELÄ, Email: maisa.niemela@oulu.fi.
RAIJA KORPELAINEN, Email: Raija.Korpelainen@odl.fi.
TIMO JÄMSÄ, Email: timo.jamsa@oulu.fi.
MOURAD OUSSALAH, Email: Mourad.Oussalah@oulu.fi.
REFERENCES
- 1.Rosenberger ME Fulton JE Buman MP, et al. The 24-hour activity cycle: a new paradigm for physical activity. Med Sci Sports Exerc. 2019;51(3):454–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Farrahi V Kangas M Walmsley R, et al. Compositional associations of sleep and activities within the 24-h cycle with cardiometabolic health markers in adults. Med Sci Sports Exerc. 2021;53(2):324–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chastin SFM, Palarea-Albaladejo J, Dontje ML, Skelton DA. Combined effects of time spent in physical activity, sedentary behaviors and sleep on obesity and cardio-metabolic health markers: a novel compositional data analysis approach. PLoS One. 2015;10(10):e0139984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Farrahi V, Niemelä M, Kangas M, Korpelainen R, Jämsä T. Calibration and validation of accelerometer-based activity monitors: a systematic review of machine-learning approaches. Gait Posture. 2019;68:285–99. [DOI] [PubMed] [Google Scholar]
- 5.Chastin SFM De Craemer M De Cocker K, et al. How does light-intensity physical activity associate with adult cardiometabolic health and mortality? Systematic review with meta-analysis of experimental and observational studies. Br J Sports Med. 2019;53(6):370–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Powell C, Herring MP, Dowd KP, Donnelly AE, Carson BP. The cross-sectional associations between objectively measured sedentary time and cardiometabolic health markers in adults—a systematic review with meta-analysis component. Obes Rev. 2018;19(3):381–95. [DOI] [PubMed] [Google Scholar]
- 7.Chastin SFM, Egerton T, Leask C, Stamatakis E. Meta-analysis of the relationship between breaks in sedentary behavior and cardiometabolic health. Obesity. 2015;23(9):1800–10. [DOI] [PubMed] [Google Scholar]
- 8.DiPietro L Al-Ansari SS Biddle SJH, et al. Advancing the global physical activity agenda: recommendations for future research by the 2020 WHO physical activity and sedentary behavior guidelines development group. Int J Behav Nutr Phys Act. 2020;17(1):143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bauman AE, Reis RS, Sallis JF, Wells JC, Loos RJF, Martin BW. Correlates of physical activity: why are some people physically active and others not? Lancet. 2012;380(9838):258–71. [DOI] [PubMed] [Google Scholar]
- 10.Farrahi V Niemelä M Kärmeniemi M, et al. Correlates of physical activity behavior in adults: a data mining approach. Int J Behav Nutr Phys Act. 2020;17(1):94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doherty A Jackson D Hammerla N, et al. Large scale population assessment of physical activity using wrist worn accelerometers: the UK Biobank Study. PLoS One. 2017;12(2):e0169649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prince SA, Elliott CG, Scott K, Visintini S, Reed JL. Device-measured physical activity, sedentary behaviour and cardiometabolic health and fitness across occupational groups: a systematic review and meta-analysis. Int J Behav Nutr Phys Act. 2019;16(1):30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Niemelä M Kangas M Farrahi V, et al. Intensity and temporal patterns of physical activity and cardiovascular disease risk in midlife. Prev Med. 2019;124:33–41. [DOI] [PubMed] [Google Scholar]
- 14.Bull FC Al-Ansari SS Biddle S, et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br J Sports Med. 2020;54(24):1451–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Migueles JH Aadland E Andersen LB, et al. GRANADA consensus on analytical approaches to assess associations with accelerometer-determined physical behaviours (physical activity, sedentary behaviour and sleep) in epidemiological studies. Br J Sports Med. 2022;56(7):376–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gupta N Hallman DM Dumuid D, et al. Movement behavior profiles and obesity: a latent profile analysis of 24-h time-use composition among Danish workers. Int J Obes (Lond). 2020;44(2):409–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chastin S McGregor D Palarea-Albaladejo J, et al. Joint association between accelerometry-measured daily combination of time spent in physical activity, sedentary behaviour and sleep and all-cause mortality: a pooled analysis of six prospective cohorts using compositional analysis. Br J Sports Med. 2021;55(22):1271–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dumuid D Pedišić Ž Stanford TE, et al. The compositional isotemporal substitution model: a method for estimating changes in a health outcome for reallocation of time between sleep, physical activity and sedentary behaviour. Stat Methods Med Res. 2019;28(3):846–57. [DOI] [PubMed] [Google Scholar]
- 19.Farrahi V, Kangas M, Kiviniemi A, Puukka K, Korpelainen R, Jämsä T. Accumulation patterns of sedentary time and breaks and their association with cardiometabolic health markers in adults. Scand J Med Sci Sports. 2021;31(7):1489–507. [DOI] [PubMed] [Google Scholar]
- 20.von Rosen P, Dohrn I-M, Hagströmer M. Latent profiles analysis of physical activity and sedentary behaviour with mortality risk: a 15-year follow-up. Scand J Med Sci Sports. 2020;30(10):1949–56. [DOI] [PubMed] [Google Scholar]
- 21.Verswijveren SJJ Lamb KE Leech RM, et al. Activity accumulation and cardiometabolic risk in youth: a latent profile approach. Med Sci Sport Exerc. 2020;52(7):1502–10. [DOI] [PubMed] [Google Scholar]
- 22.Lee PH, Yu Y-Y, McDowell I, Leung GM, Lam TH. A cluster analysis of patterns of objectively measured physical activity in Hong Kong. Public Health Nutr. 2013;16(8):1436–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sabiston CM, Lacombe J, Faulkner G, Jones J, Trinh L. Profiling sedentary behavior in breast cancer survivors: links with depression symptoms during the early survivorship period. Psychooncology. 2018;27(2):569–75. [DOI] [PubMed] [Google Scholar]
- 24.Evenson KR, Wen F, Metzger JS, Herring AH. Physical activity and sedentary behavior patterns using accelerometry from a national sample of United States adults. Int J Behav Nutr Phys Act. 2015;12:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maddison R, Jiang Y, Foley L, Scragg R, Direito A, Olds T. The association between the activity profile and cardiovascular risk. J Sci Med Sport. 2016;19(8):605–10. [DOI] [PubMed] [Google Scholar]
- 26.University of Oulu Web site [Internet] . NFBC 1966 data. [cited 2022 Jan 10]. Available from: https://www.oulu.fi/nfbc/node/19663.
- 27.Vähä-Ypyä H Vasankari T Husu P, et al. Validation of cut-points for evaluating the intensity of physical activity with accelerometry-based mean amplitude deviation (MAD). PLoS One. 2015;10(8):e0134813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Choi L, Liu Z, Matthews CE, Buchowski MS. Validation of accelerometer wear and nonwear time classification algorithm. Med Sci Sports Exerc. 2011;43(2):357–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kiviniemi AM Perkiömäki N Auvinen J, et al. Fitness, fatness, physical activity, and autonomic function in midlife. Med Sci Sports Exerc. 2017;49(12):2459–68. [DOI] [PubMed] [Google Scholar]
- 30.Millán J Pintó X Muñoz A, et al. Lipoprotein ratios: physiological significance and clinical usefulness in cardiovascular prevention. Vasc Health Risk Manag. 2009;5:757–65. [PMC free article] [PubMed] [Google Scholar]
- 31.Bennett H, Parfitt G, Davison K, Eston R. Validity of submaximal step tests to estimate maximal oxygen uptake in healthy adults. Sports Med. 2016;46(5):737–50. [DOI] [PubMed] [Google Scholar]
- 32.Genolini C, Alacoque X, Sentenac M, Arnaud C. Kml and kml3d: R packages to cluster longitudinal data. J Stat Softw. 2015;65(4):1–34. [Google Scholar]
- 33.Genolini C Pingault J-B Driss T, et al. KmL3D: a non-parametric algorithm for clustering joint trajectories. Comput Methods Programs Biomed. 2013;109(1):104–11. [DOI] [PubMed] [Google Scholar]
- 34.Mohamad I, Usman D. Standardization and its effects on k-means clustering algorithm. Res J Appl Sci Eng Technol. 2013;6(17):3299–303. [Google Scholar]
- 35.Tremblay MS Aubert S Barnes JD, et al. Sedentary behavior research network (SBRN) - terminology consensus project process and outcome. Int J Behav Nutr Phys Act. 2017;14(1):75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dunstan DW, Healy GN, Sugiyama T, Owen N. Too much sitting and metabolic risk—has modern technology caught up with us. Eur Endocrinol. 2010;6(1):19–23. [Google Scholar]
- 37.Kujala UM. Is physical activity a cause of longevity? It is not as straightforward as some would believe. A critical analysis. Br J Sports Med. 2018;52(14):914–8. [DOI] [PubMed] [Google Scholar]
- 38.Watson NF Badr MS Belenky G, et al. Recommended amount of sleep for a healthy adult: a joint consensus statement of the American Academy of Sleep Medicine and Sleep Research Society. Sleep. 2015;38(6):843–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gupta N, Dumuid D, Korshøj M, Jørgensen MB, Søgaard K, Holtermann A. Is daily composition of movement behaviors related to blood pressure in working adults? Med Sci Sports Exerc. 2018;50(10):2150–5. [DOI] [PubMed] [Google Scholar]
- 40.Ross R Chaput J-P Giangregorio LM, et al. Canadian 24-hour movement guidelines for adults aged 18–64 years and adults aged 65 years or older: an integration of physical activity, sedentary behaviour, and sleep. Appl Physiol Nutr Metab. 2020;45(10 (Suppl 2)):S57–S102. [DOI] [PubMed] [Google Scholar]
- 41.Chastin SFM McGregor DE Biddle SJH, et al. Striking the right balance: evidence to inform combined physical activity and sedentary behavior recommendations. J Phys Act Health. 2021;18(6):631–7. [DOI] [PubMed] [Google Scholar]
- 42.Holtermann A, Rasmussen CL, Hallman DM, Ding D, Dumuid D, Gupta N. 24-hour physical behavior balance for better health for all: “The Sweet-Spot Hypothesis”. Sports Med Open. 2021;7(1):98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guthold R, Stevens GA, Riley LM, Bull FC. Worldwide trends in insufficient physical activity from 2001 to 2016: a pooled analysis of 358 population-based surveys with 1·9 million participants. Lancet Glob Health. 2018;6(10):e1077–86. [DOI] [PubMed] [Google Scholar]
- 44.Amagasa S Machida M Fukushima N, et al. Is objectively measured light-intensity physical activity associated with health outcomes after adjustment for moderate-to-vigorous physical activity in adults? A systematic review. Int J Behav Nutr Phys Act. 2018;15(1):65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ekelund U Steene-Johannessen J Brown WJ, et al. Does physical activity attenuate, or even eliminate, the detrimental association of sitting time with mortality? A harmonised meta-analysis of data from more than 1 million men and women. Lancet. 2016;388(10051):1302–10. [DOI] [PubMed] [Google Scholar]
- 46.van der Velde JHPM Schaper NC Stehouwer CDA, et al. Which is more important for cardiometabolic health: sedentary time, higher intensity physical activity or cardiorespiratory fitness? The Maastricht Study. Diabetologia. 2018;61(12):2561–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Knutson KL. Sleep duration and cardiometabolic risk: a review of the epidemiologic evidence. Best Pract Res Clin Endocrinol Metab. 2010;24(5):731–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pedišić Ž. Measurement issues and poor adjustments for physical activity and sleep undermine sedentary behaviour research—the focus should shift to the balance between sleep, sedentary behaviour, standing and activity. Kinesiology. 2014;46(1):135–46. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


